SUMMARY
Our objective in the present study was to examine 5-HT1A receptor function in prefrontal cortex and hippocampus of GR+/− mice, which appear to be an appropriate murine model of depression. 5-HT1A receptor function was determined by measuring [35S]GTPγS binding stimulated by the 5-HT1A receptor agonist 8-OH-DPAT (1 μM), an indication of the capacity of the receptor to activate G proteins. 5-HT1A receptor expression was determined by measuring the binding of [3H]8-OH-DPAT (2 nM). We observed no effect of the constitutive reduction in GR on 5-HT1A receptor-stimulated [35S]GTPγS binding or 5-HT1A receptor binding sites. Corticosterone treatment (10 mg/kg, sc once daily for 21 days) of wild-type mice resulted in a decrease in 5-HT1A receptor function in prefrontal cortex [8-OH-DPAT-stimulated [35S]GTPγS binding (% above basal), vehicle-treated: 39±4.9; corticosterone-treated: 17±2.8], but not in hippocampus. The constitutive reduction in GR expression prevented the down-regulation of 5-HT1A receptor function in frontal cortex by chronic corticosterone administration. In contrast, corticosterone treatment of GR+/− mice resulted in an increase in 5-HT1A receptor function in hippocampus which reached statistical significance in CA2/3 region [8-OH-DPAT-stimulated [35S]GTPγS binding (% above basal), vehicle-treated: 41±9.7; corticosterone-treated: 94±23]. These changes seem to be evoked by a combined effect of high corticosterone levels and GR deficiency. Although GR+/− mice do not exhibit changes in baseline corticosterone, the constitutive deficiency in GR appears to have unmasked regulatory effects of elevated corticosterone in the maintenance of 5-HT1A receptor function in prefrontal cortex and hippocampus.
Keywords: glucocorticoid receptor, quantitative autoradiography, serotonin, corticosterone, major depression, [35S]GTPγS binding
INTRODUCTION
Neurotransmitter and hormonal responses associated with acute stress are adaptive in the short-term in that they are necessary to promote homeostasis. However, repeated exposure to stress over time may lead to pathophysiological changes due to chronic elevations in glucocorticoid levels. The deleterious effects of prolonged exposure to elevated glucocorticoid levels, which include neurochemical and morphological changes in forebrain structures, are associated with cognitive deficits and affective disorders, specifically major depression (Herman et al., 2003; Sapolsky, 2003; McEwen, 2007).
Hyperactivity of the hypothalamic-pituitary-adrenal (HPA) system and elevated plasma cortisol levels associated with major depressive disorder may be due to diminished glucocorticoid receptor (GR) function or expression, and as a consequence, deficient feedback regulation of cortisol (Holsboer, 2000; Pariante, 2006). GR heterozygous (GR+/−) mice, with a 50% reduction in GR expression, are indistinguishable from wild-type control mice at baseline, but exhibit increased sensitivity to stress and dysregulation of the HPA axis. GR+/− mice display increased helplessness after stress exposure, a behavioral correlate of depression in mice, and increased stress-induced plasma corticosterone levels (Ridder et al., 2005). GR+/− mice also exhibit abnormal responses in the dexamethasone suppression test and dexamethasone/corticotropin-releasing hormone test (DEX/CRH test), similar to what is observed clinically in severely depressed patients (Ridder et al., 2005). Consistent with the neurotrophin hypothesis of depression, GR+/− mice exhibit a down regulation of brain-derived neurotrophic factor (BDNF) protein in hippocampus (Ridder et al., 2005).
As GR+/− mice appear to be an appropriate murine model of depression (Ridder et al., 2005), our objective in the present study was to examine serotonin (5-hydroxytryptamine, 5-HT) 5-HT1A receptor function in GR+/− mice. 5-HT1A receptors are present in high density in cortical and limbic areas, where they are post-synaptic to serotonergic neurons. The distribution of 5-HT1A receptors in brain is consistent with a role for this 5-HT receptor in cognitive or integrative functions, as well as in emotional states. We have found that 5-HT1A receptor function in hippocampus, but not in frontal cortical areas, is attenuated in BDNF+/− mice and mice with a forebrain-specific reduction in BDNF (Hensler et al., 2003, 2007). As hippocampal BDNF protein levels are also diminished in GR+/− mice, we hypothesized that hippocampal 5-HT1A receptor function would be attenuated in GR+/− mice.. Moreover, we were interested in examining the effect of chronic corticosterone treatment, a means to mimic chronic stress, on 5-HT1A receptor function and expression. 5-HT1A receptor function was determined by measuring 5-HT1A receptor agonist 8-OH-DPAT stimulated [35S]GTPγS binding to G proteins, an indication of the capacity of the receptor to activate G proteins. 5-HT1A receptor expression was determined by measuring the binding of the agonist radioligand [3H]8-OH-DPAT to 5-HT1A receptor sites. As cortical and limbic structures play a key role in the integration and association of stressful stimuli with previous experiences, and therefore are brain regions of particular interest when examining the long-term effects of repeated exposure to stress, we focused our attention in the current study on prefrontal cortex and dorsal hippocampus.
MATERIALS AND METHODS
Mice
GR-heterozygous mice (GR+/−) were generated as previously described to obtain F1 hybrid mice with the same background as used in behavioral and neurochemical studies (Ridder et al., 2005; Schulte-Herbrüggen et al., 2007; Trajkovska et al., 2009). Male GR+/− mice and wildtype littermate controls were 4–5 months old at the beginning of experiments. Mice were housed individually under a reverse phase 12:12 hour light/dark cycle, with ad libitum access to food and water. German animal welfare authorities approved all experiments.
Corticosterone Treatment
Mice were injected subcutaneously with either sesame oil vehicle or corticosterone (10 mg/kg) once daily for 21 days. This corticosterone treatment of mice results in attenuation of 5-HT1A receptor function in the dorsal raphe, consistent with the effects of chronic stress on somatodendritic autoreceptor function (Hensler et al., 2007).
Tissue Preparation
Mice were sacrificed 24 hours after the last injection of vehicle or corticosterone. Brains were rapidly removed and stored at −80°C. Coronal sections of 20 μm thickness were cut in a cryostat microtome at the level of the frontal cortex (plates 13–14) and dorsal hippocampus (plates 46–47) according to the atlas of the mouse brain (Paxinos and Franklin, 1997), and thaw-mounted onto gelatin-coated glass slides. After desiccation at 4°C, sections were stored at −80°C.
[35S]GTPγS Autoradiography
Autoradiography of 8-OH-DPAT-stimulated [35S]GTPγS binding in brain sections was performed as previously described (Hensler et al., 2007). Sections were incubated in assay buffer containing 40 pM [35S]GTPγS (1250 Ci/mmol, Dupont/NEN) either in the absence or in the presence of (±)8-OH-DPAT (1 μM) (Tocris). Basal [35S]GTPγS binding was defined in the absence of 8-OH-DPAT. Nonspecific [35S]GTPγS binding was defined in the absence of 8-OH-DPAT and in the presence of 10 μM GTPγS (Roche/Boehringer-Manheim). The incubation was stopped by two washes for 5 minutes each in ice-cold HEPES buffer, followed by a brief immersion in ice-cold de-ionized water. Dried sections were exposed to Kodak Biomax MR film (Amersham) for 48 hours.
[3H]8-OH-DPAT Autoradiography
Autoradiography of the binding of [3H]8-OH-DPAT to 5-HT1A receptors in brain sections was performed as described with slight modification (Hensler et al., 1991). Sections incubated in assay buffer containing 2 nM [3H]8-OH-DPAT (210 Ci/mmol, Amersham). Nonspecific binding was defined by incubating adjacent sections in the presence of 10 μM WAY-100635 (Sigma/RBI, St. Louis, MO). Incubation was terminated by two washes for 5 minutes each in ice-cold buffer, followed by a dip in ice-cold de-ionized water. Dried sections exposed to Kodak BioMax MR Film (Amersham, Piscataway, NJ) for 9 weeks.
Image Analysis
Analysis of the digitized autoradiograms was performed using the image analysis program NIH Image, version 1.47 (NIH, Bethesda, MD) as previously described (see Hensler et al., 1991; Hensler et al., 2007).
Data Analysis
Statistical comparisons were made by two-way ANOVA with genotype and treatment as factors. F values reaching significance (p<0.05) were evaluated further by post hoc analysis using the Newman-Keuls test. Statistical tests were performed using Statistica software (version 4.1, Statsoft, Tulsa, OK).
RESULTS
Differences in 5-HT1A Receptor Function: wild-type versus GR+/− Mice
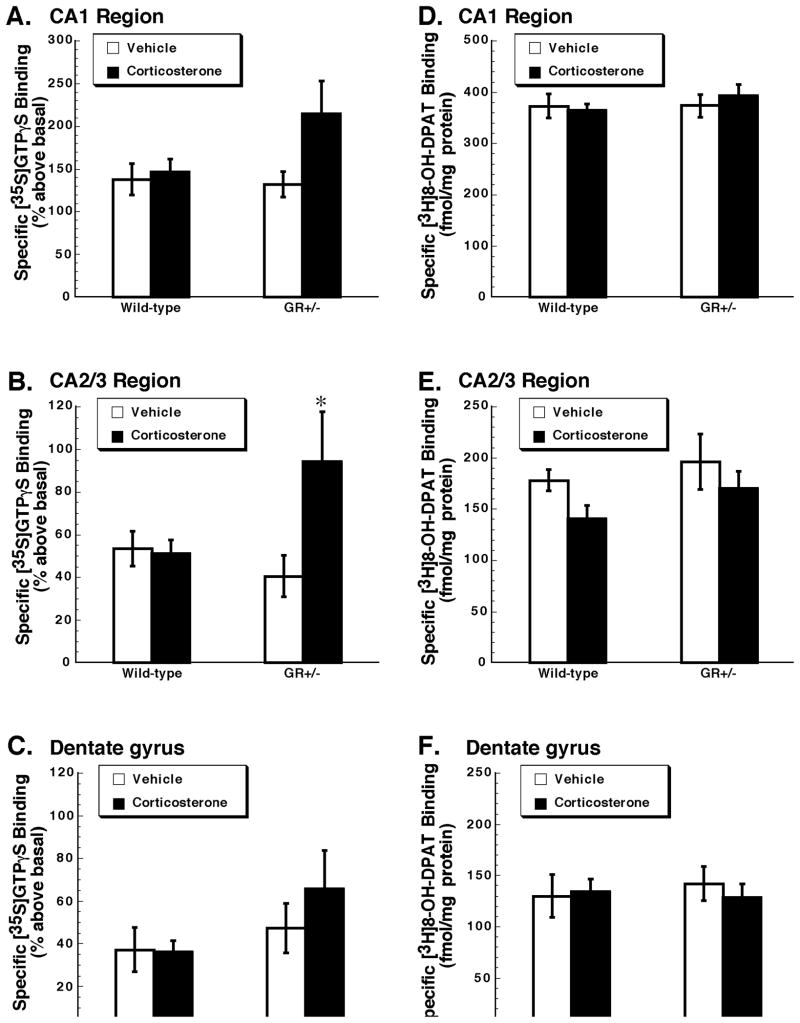
[35S]GTPγS binding stimulated by the 5-HT1A receptor agonist 8-OH-DPAT was assessed in the dorsal hippocampus (Figure 1, panels A, B, C). In CA1, dentate gyrus and CA2/3 regions we found no significant main effect of genotype (CA1: F1,22=1.71, p=0.20; CA2/3: F1,22=1.35, p=0.26; dentate gyrus: F1,22=1.83, p=0.19). There was also no difference between wild-type and GR+/− mice in the number of 5-HT1A receptor binding sites as measured by the binding of [3H]8-OH-DPAT [main effect of genotype, CA1: F1,22=0.134, p=0.72; CA2/3: F1,22=1.74, p=0.20; dentate gyrus: F1,22=0.033, p=0.86 (Figure 1, panels D, E, F). Thus the deficiency in GR did not alter 5-HT1A receptor binding or function, specifically the capacity of 5-HT1A receptors to activate G proteins, in dorsal hippocampus.
Figure 1. Chronic corticosterone treatment increases 5-HT1A receptor function in the hippocampus of GR+/− but not wild-type mice.
[35S]GTPγS binding (A, B, C) was stimulated by the 5-HT1A receptor agonist 8-OH-DPAT (1 μM), and is expressed as % above basal. Specific binding of [3H]8-OH-DPAT (2 nM) (D, E, F) is expressed as fmol/mg protein. Panels A and B show data for the CA1 region. Panels C and D show corresponding data in the CA2/3 region. Panels E and F show data for the dentate gyrus. Presented are the mean ± S.E.M. n= 6, wild-type mice treated with vehicle; n = 8, wild-type mice treated with corticosterone; n = 6, GR+/− mice treated with vehicle; n = 6, GR+/− mice treated with vehicle. Data were analyzed by two-way ANOVA. *p<0.05 when compared with vehicle-treated GR+/− mice, post hoc Newman-Keuls test.
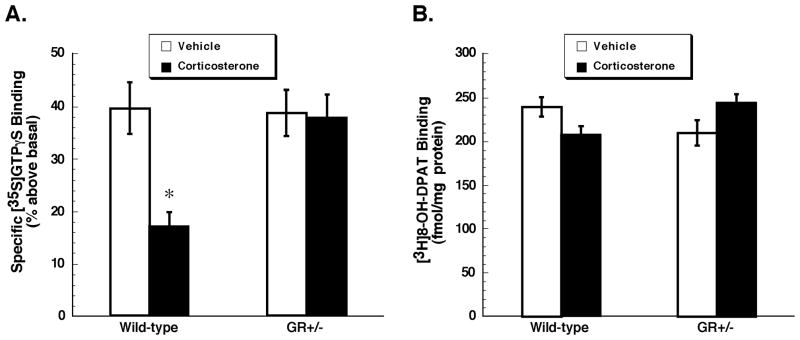
We also assessed 8-OH-DPAT-stimulated [35S]GTPγS binding in the prefrontal cortex (Figure 2A). Here we found a significant main effect of genotype (F1,22=5.86, p=0.02). Post hoc analysis, however, revealed no significant difference between vehicle-treated wild-type and GR+/− mice in this brain region (p=0.88). There was also no difference between genotypes in the number of 5-HT1A receptors. As observed for the hippocampus, the deficiency in GR did not alter 5-HT1A receptor binding or function in the prefrontal cortex.
Figure 2. Chronic corticosterone treatment decreases 5-HT1A receptor function in prefrontal cortex of wild-type but not GR+/− mice.
[35S]GTPγS binding (A) was stimulated by the 5-HT1A receptor agonist 8-OH-DPAT (1 μM), and is expressed as % above basal. Specific binding of [3H]8-OH-DPAT (2 nM) (B) is expressed as fmol/mg protein. Presented are mean ± S.E.M., n= 6 wild-type mice treated with vehicle; n = 8, wild-type mice treated with corticosterone; n = 6, GR+/− mice treated with vehicle; n = 6, GR+/− mice treated with vehicle. Data were analyzed by two-way ANOVA. *p<0.05 when compared with vehicle-treated wild-type mice, post hoc Newman-Keuls test.
Effect of Corticosterone Treatment
There was no significant main effect of corticosterone treatment on 8-OH-DPAT-stimulated [35S]GTPγS binding in subregions of dorsal hippocampus (CA1: F1,22=3.73, p=0.07; CA2/3: F1,22=3.87, p=0.06; dentate gyrus: F1,22=1.63 p = 0.21) (see Figure 1). The interaction between factors (genotype and treatment), although not significant for CA1 (F1,22=2.47, p=0.13) or dentate gyrus (F1,22=1.63, p=0.18), was significant for the CA2/3 region (F1,22=4.59, p=0.04). Post hoc analysis indicated that repeated corticosterone treatment of GR+/− mice resulted in a significant increase in 8-OH-DPAT-stimulated [35S]GTPγS binding in CA2/3 region (Figure 1, panel B). The effect of corticosterone treatment on the binding of [3H]8-OH-DPAT to 5-HT1A receptors sites was also measured (Figure 1, panel D, E, F). There was no significant main effect of treatment (CA1: F1,22=0.018, p=0.89; CA2/3: F1,22=3.29, p=0.08; dentate gyrus: F1,22=0.09, p=0.76) or interaction between factors (genotype and treatment) (CA1: F1,22=0.129, p=0.72; CA2/3: F1,22=0.11, p=0.74; dentate gyrus: F1,22=0.33, p=0.57). Thus although chronic corticosterone treatment of GR+/− mice resulted in an increase in the capacity of 5-HT1A receptors in dorsal hippocampus to activate G proteins, this was not accompanied by an increase in 5-HT1A receptor binding sites.
In the prefrontal cortex, we found a significant main effect of corticosterone treatment on 8-OH-DPAT stimulated [35S]GTPγS binding (F1,22=8.28, p=0.01) and a significant interaction between factors (genotype and treatment) (F1,22=6.92, p=0.02). Post hoc analysis indicated that corticosterone treatment of wild-type, but not of GR+/− mice resulted in a significant attenuation in 5-HT1A receptor-stimulated [35S]GTPγS binding in this brain region (Figure 2A). We observed no significant main effect of corticosterone treatment on [3H]8-OH-DPAT binding to 5-HT1A receptors sites (F1,22=0.018, p=0.89). Although the interaction between factors (genotype and treatment) was significant (F1,22=9.58, p=0.01), post hoc analysis indicated no significant difference between groups (p>0.05) (Figure 2B). Thus although chronic corticosterone administration resulted in a significant attenuation in 5-HT1A receptor function in prefrontal cortex of wild-type mice, this was not accompanied by a down-regulation of 5-HT1A receptor binding sites.
DISCUSSION
In the present study we found no effect of the constitutive reduction in GR on 5-HT1A receptor function or binding sites in hippocampus or in prefrontal cortex. The lack of effect of genotype on 5-HT1A receptor-stimulated [35S]GTPγS binding in hippocampus was somewhat unexpected given the reduction in hippocampal BDNF in GR+/− mice (Ridder et al., 2005) and our previous findings of reduced hippocampal 5-HT1A receptor-stimulated [35S]GTPγS binding in mice deficient in BDNF (Hensler et al., 2003, 2007). Our previous observations in BDNF deficient mice have led us to speculate that BDNF may support or promote 5-HT1A receptor function in the hippocampus. However, in GR+/− mice the hypothesized effect of reduced BDNF to decrease 5-HT1A receptor function appears to be countered or opposed by the constitutive reduction in GR.
Chronic administration of corticosterone to wild-type mice resulted in an attenuation of 5-HT1A receptor-stimulated [35S]GTPγS binding in prefrontal cortex. Interestingly, the constitutive reduction in GR expression prevented the down-regulation of 5-HT1A receptor function in frontal cortex by chronic corticosterone administration. Thus, the negative regulatory effect of corticosterone administration on 5-HT1A function in frontal cortex appears to be lost in GR+/− mice. In hippocampus, corticosterone treatment of GR+/− mice resulted in an increase in 5-HT1A receptor-stimulated [35S]GTPγS binding in hippocampus. These functional changes in 5-HT1A receptors seem to be evoked by a combined effect of high corticosterone levels and GR deficiency. The constitutive deficiency in GR may have unmasked regulatory effects of corticosterone in the maintenance of 5-HT1A receptor function in prefrontal cortex and hippocampus.
GR, with low affinity for glucocorticoids, have been implicated in the negative feedback regulation of the HPA axis when glucocorticoid levels are high (de Kloet 2007). Mineralocorticoid receptors (MR), with high affinity for glucocorticoids, are believed to play a role in homeostasis as they are activated when glucocorticoid levels are low (de Kloet 2007). In the hippocampus, 5-HT1A receptor number and function are under stringent regulation by MR and GR. Occupancy of MR and GR by high levels of corticosterone results in the potentiation of 5-HT1A receptor-mediated responses in CA1 pyramidal neurons. By contrast, the strict inhibitory regulation of hippocampal 5-HT1A receptor expression and function by corticosterone is predominantly mediated by MR (Kuroda et al., 1994; Meijer and de Kloet, 1995; Hesen and Joëls, 1996). Human imaging and postmortem studies indicate that hippocampal and cortical 5-HT1A receptors are down regulated in major depressive disorder (see Drevets et al., 2007, and references therein), an effect believed to be mediated predominantly through MR as a result of elevated basal corticosteroid levels (Meijer et al., 1997). Hippocampal MR expression in cytosol and nuclear fractions, as determined by Western blot analysis, is not altered in GR+/− mice (Riva and Gass, unpublished observations). That 5-HT1A receptors are unchanged in GR+/− mice suggests that MR function in these mice, at least with regard to the regulation of 5-HT1A receptors, is not altered.
MRs and GRs function together in a complementary manner to promote adaptive responses to stress, eg. facilitation of memory storage, behavioral adaptation, and preparation for future events. 5-HT1A receptors in cortical and limbic structures may play an important role in these adaptive processes. Perturbation of the MR/GR balance, specifically decreased GR function, is implicated in major depressive disorder, and appears to be involved in increased vulnerability to stress (Ridder et al., 2005; de Kloet et al., 2007). However, in the present study, the constitutive reduction in GR appears to have unmasked a positive regulatory effect of corticosterone, most likely through activation of both MR and GR, in the maintenance of 5-HT1A receptor function in hippocampus and prefrontal cortex. Future experiments have to clarify by which molecular mechanisms this regulation is mediated.
Acknowledgments
The authors gratefully acknowledge the excellent technical assistance of Mrs. Teresa Burke, Mrs. Christiane Brandwein, Mrs. Natascha Pfeiffer and Mr. Christof Dormann.
ROLE OF FUNDING SOURCE
Funding for this study was provided by NARSAD (JGH) and NIMH grant MH 071488 (JGH). NARSAD and NIMH had no further role in study design, in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication. This work was also supported by grants from the Deutsche Forschungsgemeinschaft (SFB636/B3 and GA 427/9-1 to P.G.). MAV received a scholarship from the GK791 of the University of Heidelberg.
Footnotes
CONTRIBUTORS
PG designed the study and wrote the protocol. MAV performed the animal experiments. JGH supervised the collection of data and undertook the statistical analyses. JGH wrote the first draft of the manuscript. All authors managed literature searches, and contributed to and have approved the final manuscript.
CONFLICT OF INTEREST
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- de Kloet ER, Derijk RH, Meijer OC. Therapy Insight: is there an imbalanced response of mineralocorticoid and glucocorticoid receptors in depression? Nat Clin Pract Endocrinol Metab. 2007;3:168–179. doi: 10.1038/ncpendmet0403. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Thase ME, Moses-Kolko EL, Price J, Frank E, Kupfer DJ, Mathis C. Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nuclear Medicine and Biology. 2007;34:865–877. doi: 10.1016/j.nucmedbio.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KB, Paxinos G. The mouse brain in stereotaxic coordinates. Academics Press; Sidney: 1997. [Google Scholar]
- Hensler JG, Advani T, Monteggia LM. Regulation of 5-HT1A receptor function in inducible BDNF knock-out mice following administration of corticosterone. Biol Psychiatry. 2007;62:521–529. doi: 10.1016/j.biopsych.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Hensler JG, Kovachich GB, Frazer A. Quantitative autoradiographic study of serotonin1A receptor regulation: Effect of 5,7-dihydroxytryptamine and antidepressant treatments. Neuropsychopharmacol. 1991;4:131–144. [PubMed] [Google Scholar]
- Hensler JG, Ladenheim EE, Lyons WE. Ethanol consumption and serotonin-1A (5-HT1A) receptor function in heterozygous BDNF (+/−) mice. J Neurochem. 2003;85:1139–1147. doi: 10.1046/j.1471-4159.2003.01748.x. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Hesen W, Joëls M. Modulation of 5-HT1A responsiveness in CA1 pyramidal neurons by in vivo activation of corticosteroid receptors. J Neuroendocrinol. 1996;8:433–438. doi: 10.1046/j.1365-2826.1996.04724.x. [DOI] [PubMed] [Google Scholar]
- Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacol. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- Kuroda Y, Watanabe Y, Albeck DS, Hastings NB, McEwen BS. Effects of adrenalectomy and Type I or Type II glucocorticoid receptor activation on 5-HT1A and 5-HT2 receptor binding and 5-HT transporter mRNA expression in rat brain. Brain Res. 1994;648:157–161. doi: 10.1016/0006-8993(94)91916-x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Meijer OC, de Kloet ER. A role for the mineralocorticoid receptor in a rapid and transient suppression of hippocampal 5-HT1A receptor mRNA by corticosterone. J Neuroendocrinol. 1995;7:653–657. doi: 10.1111/j.1365-2826.1995.tb00804.x. [DOI] [PubMed] [Google Scholar]
- Meijer OC, Van oosten RV, de Kloet ER. Elevated basal trough levels of corticosterone suppress hippocampal 5-hydroxytryptamine1A receptor expression in adrenally intact rats: implications for the pathogenesis of depression. Neuroscience. 1997;80:419–426. doi: 10.1016/s0306-4522(97)00008-0. [DOI] [PubMed] [Google Scholar]
- Pariante CM. The glucocorticoid receptor: part of the solution or part of the problem? J Psycopharmacol. 2006;20:79–84. doi: 10.1177/1359786806066063. [DOI] [PubMed] [Google Scholar]
- Ridder S, Chourbaji S, Hellweg R, Urani A, Zacher C, Schmid W, Zink M, Hörtnagl H, Flor H, Henn FA, Schütz G, Gass P. Mice with genetically altered glucocorticoid receptor expression show altered sensitivity for stress-induced depressive reactions. J Neurosci. 2005;25:6243–6250. doi: 10.1523/JNEUROSCI.0736-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Stress and plasticity in the limbic system. Neurochem Res. 2003;28:1735–1742. doi: 10.1023/a:1026021307833. [DOI] [PubMed] [Google Scholar]
- Schulte-Herbrüggen O, Hellweg R, Chourbaji S, Ridder S, Brandwein C, Gass P, Hörtnagl H. Differential regulation of neurotrophins and serotonergic function in mice with genetically reduced glucocorticoid receptor expression. Exp Neurol. 2007;204:307–316. doi: 10.1016/j.expneurol.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Trajkovska V, Kirkegaard L, Krey G, Marcussen AB, Thomsen MS, Chourbaji S, Brandwein C, Ridder S, Halldin C, Gass P, Knudsen GM, Aznar S. Activation of glucocorticoid receptors increases 5-HT(2A) receptor levels. Exp Neurol. 2009 Apr 18; doi: 10.1016/j.expneurol.2009.04.008. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]