Abstract
It is becoming clear that nervous system development and adult functioning are highly coupled with other physiological systems. Accordingly, neurological and psychiatric disorders are increasingly being associated with a range of systemic co-morbidities including, most prominently, impairments in immunological and bio-energetic parameters as well as in the gut microbiome. Here, we discuss various aspects of the dynamic crosstalk between these systems that underlies nervous system development, homeostasis, and plasticity. We believe a better definition of this underappreciated systems physiology will yield important insights into how nervous system diseases with systemic co-morbidities arise and potentially identify novel diagnostic and therapeutic strategies.
Keywords: cytokine, enterotype, hypothalamic neurogenesis, immune surveillance, immunoglobulin superfamily, inflammatory reflex, leptin, microbiome, microvesicle, neural stem cell, niche, tolerance
Why focus on systems physiology?
Long-standing clinical observations and recent epidemiological and scientific studies suggest that many diseases classically thought to be nervous system-specific disorders actually have more complex phenotypes, including manifestations in other physiological systems and at brain-systemic interfaces (Box 1), profound in some cases and more subtle in others. Most, if not all, major neurological and psychiatric disorders display immunological abnormalities, such as high levels of inflammation and aberrant profiles of innate and adaptive immune system activity [1, 2]. Many nervous system disorders also exhibit a failure to maintain energy homeostasis, occurring not only at a cellular and sub-cellular level (i.e., mitochondrial dysfunction) but also in select brain regions and at an organismal level with overt signs of metabolic deregulation (i.e., alterations in body weight and composition and in glucose, amino acid and lipid homeostasis) [3-5]. A spectrum of other, less well characterized, impairments in additional organ systems are also emerging as features of disorders classically considered nervous system-specific. These include, as one exceptionally interesting example, manifestations vis-à-vis the gut microbiome (Glossary) that are likely to be as pervasive and important as—and intimately linked to—immunological and bio-energetic abnormalities [6-8]. Considering the significance of these observations calls for taking a whole-organism or ‘systems’ level view.
Box 1. Brain-systemic interfaces mediate systems physiology.
Brain-systemic interfaces mediate dynamic crosstalk between brain and other organ systems that underpins neural development, homeostasis, and plasticity and the pathophysiology of neurological and psychiatric diseases including their systemic co-morbidities.
These include interfaces engaged in local signaling (circumventricular organs, blood-brain barrier, blood-cerebrospinal fluid (CSF) barrier, and choroid plexus). The neural stem cell niche (Glossary) is a more recently recognized structure where local neurovascular, neuroimmune, and other interactions occur via signaling from perivascular factors, endothelial cells, chemokines, blood, and CSF [114].
Complementary interfaces include those widely distributed and responsible for long-distance brain-systemic communication via peripheral innervation and humoral mechanisms. The autonomic nervous system (ANS) innervates peripheral structures and modulates homeostasis and stress responses. Recent studies have uncovered novel ANS regulatory mechanisms. For example, autonomic innervation of bone marrow and associated circadian oscillations modulate the hematopoietic stem cell niche and, in turn, influence the maturation and migration and supply and activity of cells participating in innate and adaptive immunity in the brain and elsewhere [18, 115]. Blood-borne mediators also play roles in communication between the nervous system and other organ systems. Additionally, microvesicles (exosomes) are novel components secreted by donor cells (neural, immune and other cells) into the extracellular space, CSF, and peripheral circulation that release their contents (functional DNA, RNA, protein molecules) into selectively targeted recipient cells to promote cellular reprogramming [116].
Lastly, signaling between the nervous system and specific organ systems occurs via specialized organ-specific interfaces and mechanisms. For example, the interplay between the vascular, immune and nervous systems is partly mediated by gasotransmitters (nitric oxide, hydrogen sulfide and carbon monoxide) [117]. Similarly, functional interconnections between the nervous system and gut are mediated by gut intrinsic and extrinsic mechanisms including the enteric division of the ANS, hypothalamic-pituitary axis and sympatho–adrenal axis, immune cells, enteroendocrine cells, neurotransmitters, and gut peptides/hormones [6-8].
These perspectives suggest that better understanding brain disorders requires interrogation of functional and structural alterations in brain-systemic interfaces.
The tools and techniques of systems biology (e.g., network analysis, non-linear dynamics, control theory, and computational modeling) have been employed widely in recent years to analyze complex biological systems; and, the closely associated concept of network medicine (Glossary) has surfaced as a paradigm for understanding how human disease states result from perturbations of molecular and cellular networks and their emergent properties. Applying these methods to study the nervous system and its disorders has delivered valuable insights, such as identifying candidate genes responsible for various neuropsychiatric diseases. However, the majority of these inquiries have focused on evaluating only a limited subset of ‘omics’ data (i.e., genomic, transcriptomic, proteomic, metabolomic, or other phenomic) derived from individual cell types or tissues. Studies that account for multiple subsets of omics data across many cell types from different organ systems during development and adult life under evolving environmental conditions represent the future of systems biology and network medicine. Ongoing efforts, including the International Physiome Project and Virtual Physiological Human Initiative, have only just begun to develop frameworks for this type of integrative systems physiology.
We eagerly anticipate the maturation of these more formal approaches. However, there is an urgent need to better understand the nervous system and its disorders at a systems level. Thus, in this review, we draw attention to recent evidence illustrating the profound but often unanticipated interconnections that exist between the nervous system and the immune system, energy homeostasis, and the gut microbiome (Figures 1-2). We highlight the complex multidimensional relationships that are present between brain and these other systems, focusing on their relevance to neural development, adult homeostasis and plasticity, and disease. We discuss some of the potential mechanistic underpinnings for this crosstalk including, for example, how the nervous system and these other systems exploit common sets of molecules for diverse and overlapping functional purposes including intra- and inter-cellular signaling.
Figure 1. Multidimensional organization schema of the dynamic crosstalk that occurs between the nervous system and other physiological systems.
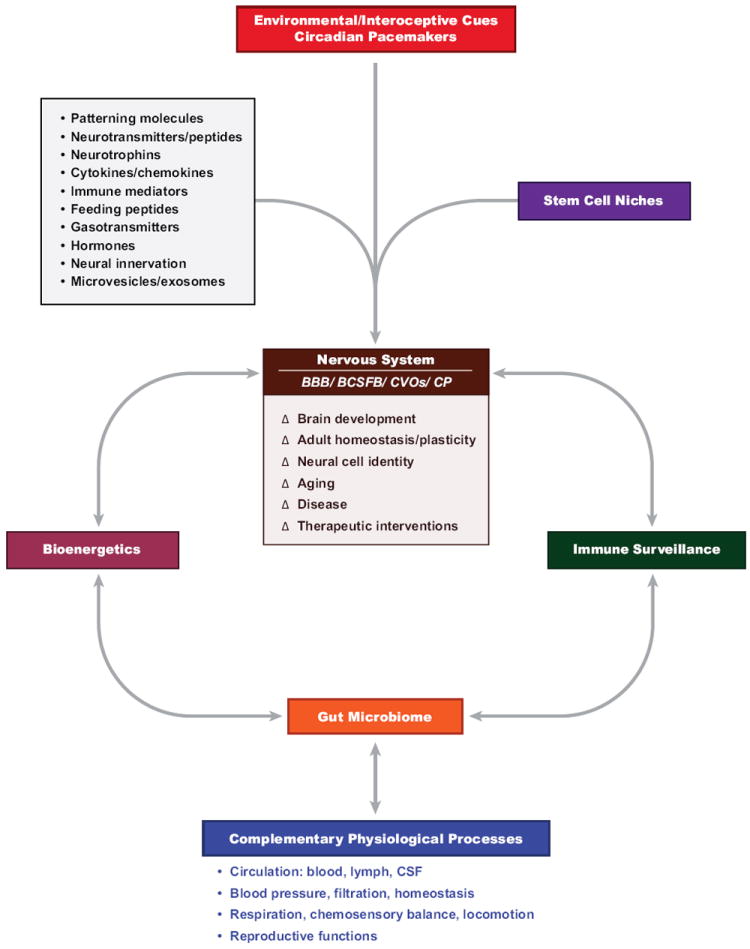
The nervous system participates in these ‘systems’ level processes through the actions of its major subdivisions, including the central nervous system (brain, spinal cord and integrative control systems), peripheral nervous system (sensory afferent and motor efferent divisions), somatic and autonomic (sympathetic and parasympathetic divisions) nervous system, enteric nervous system and interrelated components of the neuroendocrine system. The central nervous system forms topological interfaces with peripheral organ systems and associated communications routes via the blood-brain-barrier (BBB), blood-cerebrospinal fluid (CSF)-barrier (BCSFB), circumventricular organs (CVOs) and the choroid plexus (CP). Dynamic changes (Δ) in brain development, adult homeostasis and plasticity, neural cell identity, aging, disease and potential therapeutic interventions are mediated by the continuous interplay of environmental and interoceptive cues and central and peripheral circadian pacemakers; organ-specific stem cell niches; common signaling molecules, system-wide vesicular trafficking of bioactive substances (DNA, RNA, proteins, lipids) and peripheral neural innervation; and interrelated systems involving bioenergetics, immune surveillance, the gut microbiome and complementary physiological processes orchestrated by employing every organ system in an evolving spectrum of nervous system-peripheral context-specific homeostatic, adaptive and instrumental functions.
Figure 2. Complex temporal and spatial profiles of brain-systemic crosstalk encompass interrelated components of immune surveillance, bioenergetics and the gut microbiome.
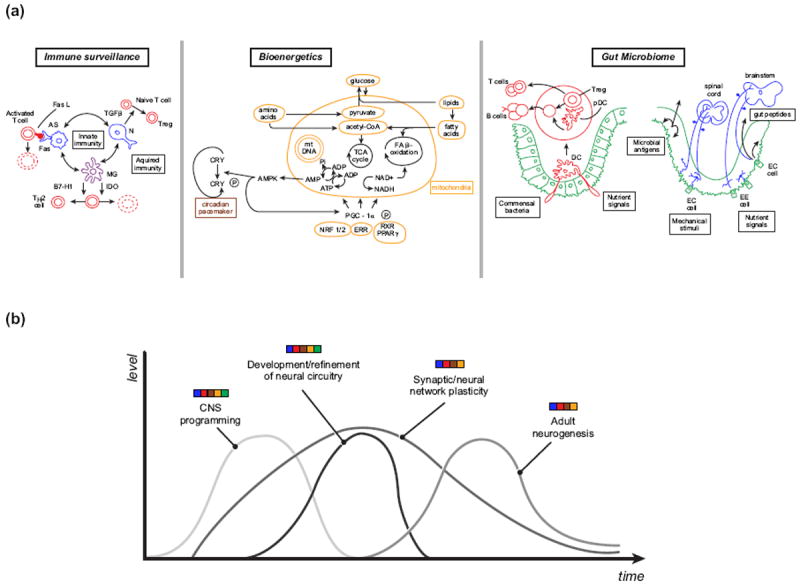
(a) Immune surveillance: astrocytes (AS, blue), microglia (MG, purple) and neurons (N, blue) are components of the innate immune system of the brain (blue, purple) that modulate acquired immune responses (red) by promoting death of activated T cells through Fas/Fas ligand (FasL) interactions, death or conversion of T cells to TH2 cells through indoleamine 2, 3-dioxygenase (IDO) biochemical reactions and co-inhibitory ligand (B7-H1) mediated actions, respectively, and/or conversion of naïve T cells to T regulatory cells (Tregs) through transforming growth factor-β (TGF-β) signaling. Bioenergetics: Within the complex cellular ecosystem of mitochondria (yellow), amino acids, glucose and lipids are metabolized directly and via metabolic intermediates through the tricarboxylic (TCA) cycle and the fatty acid (FA) β-oxidation cascade. NAD+/NADH are involved in mediating a spectrum of interrelated processes including energy homeostasis and immunological functions. Reducing equivalents generated by the Krebs cycle and by the β-oxidation pathway are subsequently shuttled through the electron transport chain to generate energy through a tripartite series of energy-generating and - conserving reactions. Within this energy cascade, adenosine monophosphate (AMP) gives rise to AMP-activated protein kinase (AMPK) and this factor, in turn, phosphorylates and activates both peroxisome proliferation-activated receptor gamma co-activation-1-beta (PGC-1α) and cryptochrome (CRY), thereby linking components of mitochondrial biogenesis (mitochondrial DNA [mtDNA]) and function, through the mediation of specific co-factors (nuclear respiratory factor ½ [NRF1/2], estrogen-related receptor [ERR], retinoid X receptor [RXR], peroxisome proliferation-activated receptor gamma [PPARγ]), with the circadian pacemaker (brown), respectively. Gut microbiome: Within the gastrointestinal system (green), immunological (red), endocrine and neuronal afferent signals respond to commensal bacteria and infectious pathogens, nutrient signals and gut-associated stimuli and these cues are transmitted to the peripheral and central nervous systems (blue) and non-neural organ systems. Dendritic cells (DC) sample commensal bacteria and nutrient signals through luminal sensing and convey their signals to adjacent Peyer’s patches where associated dendritic cells (pDC), B cells and Tregs interact to generate appropriate degrees of tolerance and protective immunological reactions that are disseminated through humoral mechanisms. Specific forms of gut-associated signals are conveyed through spinal afferents and extrinsic vagus afferents to spinal cord and brainstem regions. Multimodal gastrointestinal cues can activate networks of enteric, myenteric, spinal and vagal afferent nervous system elements. A spectrum of diverse immune-associated molecules, feeding peptides, neuropeptides and additional hormonal cues are released by immune cells within Peyer’s patches and the gut epithelium to activate corresponding receptors on spinal and vagal afferents and related signals are also released from enteroendocrine cells (EE cells) in response to luminal antigens, toxins and nutrients through humoral/circumferential organ and receptor-mediated paracrine mechanisms, respectively. Moreover, enterochromaffin (EC cells) convey complementary gut-related signals to enteric nervous system circuits and via vagal efferents to the brainstem. (b) Representation of how the levels of key nervous system processes dynamically evolve over time and are mediated by the interplay of signals (color bars) derived not only from brain (blue) but also from immune surveillance (red), bioenergetic (yellow) and circadian (brown) processes and diverse gut-associated cues (green).
This contemporary, constructionist approach—centered on understanding the dynamics of the whole-organism in a more integrated manner—will not only provide novel insights into how neurological and psychiatric disease states and their co-morbidities arise; it will also help to predict and explain the range of effects associated with modulating molecular targets that are shared by the nervous system and these other systems and will serve as the basis for developing innovative diagnostic and treatment modalities that complement and enhance existing approaches.
Neuroimmune interactions
The central nervous system (CNS) is subject to active immune surveillance (Glossary) throughout life by the innate and adaptive immune systems. There is increasing evidence that this form of immune surveillance is linked not only to pathology but it is also important for promoting normal brain development and adult activity.
CNS development, homeostasis, and plasticity
One key mechanism responsible for this crosstalk is that the nervous system and immune system express and secrete common sets of molecules, which are implicated in a diverse range of system-specific and interrelated functions (Table 1). These include factors with roles traditionally ascribed to the immune system or to the nervous system as well as novel mediators with emerging and conjoint immunological and neural roles. Indeed, many so-called immune molecules are found in specific regional, cellular and sub-cellular distributions in the CNS and their expression levels are modulated by neural activity. On the other hand, these factors can, themselves, have roles in regulating neural development and synaptic function and morphology [9, 10]. For example, components of the complement system, immunoglobulin superfamily proteins (e.g., major histocompatibility complex proteins), and cytokines and chemokines have well characterized immunological roles, including the mediation of cell migration, antigen presentation, cell-cell interactions, and signaling. It is now clear that many of these molecules also modulate CNS development through effects on cellular migration, axonal and dendritic targeting, and synapse formation and its adult activity by regulating synaptic plasticity and de novo neurogenesis. These factors are also increasingly being linked to the susceptibility to and clinical phenotypes of neurological disorders [11-15].
Table 1.
Examples of common factors mediating immune and nervous system crosstalk.
| Factor | Description | Ref. |
|---|---|---|
| Complement receptor 2 | Co-receptor for the B-lymphocyte antigen receptor, which is also expressed by adult neural progenitor cells of the dentate gyrus and involved in regulating hippocampal neurogenesis. | [101] |
| C1q | Initiating factor of the classical complement system, which is also involved in eliminating inappropriate synapses during neural development. | [102] |
| Down syndrome cell adhesion molecule | IgSF protein that mediates the establishment of neural circuitry during development, including processes such as self-avoidance, axon guidance, dendrite patterning, and synapse formation and plasticity. | [103] |
| Protogenin | IgSF expressed during neural development that modulates neurogenesis. | [104] |
| Toll-like receptors 3 | Mediators of innate and adaptive immune responses, which are also involved in neural lineage commitment and differentiation and in learning and memory. | [105, 106] |
| Tumor necrosis factor- α | Pro-inflammatory cytokine that also modulates the development and adult function of the brain through effects on neural cell fate specification and maturation, metabolism, stress responses, synaptic plasticity and neuronal-glial transmission and through effects on neural circuits that regulate motor activity, motivation, and mood and anxiety. | [107- 109] |
Likewise, neurotransmitters, neuropeptides and their receptors, canonically thought to subserve neural signaling and associated functions, have roles in the immune system. For example, T-cells express neurotransmitter receptors, and they can be activated or suppressed in a context dependent manner by various neurotransmitters. These factors do not simply mediate neural to immune signaling as T-cells produce many neurotransmitters and they can be found in immune organs, such as the thymus.
In addition, the nervous system affects the composition, mobilization and activity of the immune system. For example, the sympathetic division of the autonomic nervous system (ANS) mediates the activity and numbers of distinct subsets of T regulatory cells (Tregs), which are involved in orchestrating central and peripheral tolerance, via a transforming growth factor-β-dependent mechanism [16]. Further, the ANS modulates hematopoietic stem and progenitor cell (HSPC) proliferation, mobilization, peripheral migration and differentiation into lymphoid and myeloid cellular elements in a circadian fashion through the actions of adrenergic signaling [17-20]. Disease states that perturb the ANS, such as diabetes mellitus, which leads to abnormalities in sympathetic nerve termini, impair HSPC mobilization [21]. In addition, neural circuits regulate cytokine production in health and disease. For example, the ANS controls innate immunity through innervation of the spleen, regulation of T-cell-mediated production of acetylcholine, and modulation of the “inflammatory reflex” associated with pro-inflammatory cytokine production [22]. Correspondingly, post-stroke systemic immunosuppression is, at least in part, mediated by noradrenergic signaling acting upon hepatic invariant natural killer T-cells [23].
CNS disease and clinical implications
While immune surveillance plays a role in maintaining neural cell identity, homeostasis, connectivity and plasticity, diverse CNS pathologies are associated with abnormalities in immune surveillance [24]. Specifically, the onset and the progression of CNS disease states is often characterized by deregulation of systemic and CNS-specific T- and B-cells and microglia, CNS resident mononuclear phagocytes, and associated inflammatory cascades [25-30]. One particularly intriguing study recently highlighted the importance of proper microglial functioning in the brain. It reported that, in a mouse model of Rett syndrome, engraftment of brain parenchyma from wild-type bone-marrow-derived microglia or targeted expression of wild-type methyl-CpG binding protein 2 (Mecp2) in myeloid cells ameliorates disease symptoms and pathology [31]. These effects are dependent upon microglial phagocytic activity. Similarly, missense mutations in the triggering receptor expressed on myeloid cells 2 gene, which encodes an anti-inflammatory signaling protein expressed on dendritic cells, macrophages and microglia, impart significant risk for developing Alzheimer’s disease (AD) [32, 33]. In some instances, these immune responses can be protective [34]. For example, after injury, monocyte-derived macrophages exhibit neuroprotective effects in the retina and spinal cord [35, 36], and T-cells secrete factors that promote neuronal survival by modulating astrocyte functions [37, 38]. Alternatively, these impairments can be mechanistically linked to known pathogenic factors. For example, in Huntington’s disease (HD), the mutant huntingtin protein is known to impair the migration of immune cells [39]. Moreover, there are observations that are notable, but whose significance is yet to be determined. For example, Down syndrome (DS) is associated with deregulation of AIRE, which mediates central and peripheral tolerance, and thymic dysplasia [40, 41].
Overall, these observations indicate that, while our understanding of neuroimmune interactions is advancing, it remains incomplete. It is clear that immune surveillance plays a central role in promoting nervous system health. One interesting hypothesis is that, through strategically placed molecules that serve as substrates for neuroimmune crosstalk, the immune systems monitors the functional integrity of neural pathways and responds actively to changes in their fidelity. Subtle impairments in these homeostatic processes may even represent sentinel events in pre-clinical stages of disease, suggesting novel therapeutic windows. Further investigations are necessary to more precisely define these cellular mechanisms (e.g., the roles of microglia) and intracellular communications (e.g., at the stem cell niche) and the corresponding effects of brain aging and disease states on these processes.
The nervous system and energy homeostasis
Multiple organs, from the gut microbiome and immune system to the brain, are involved in a highly integrated manner in maintaining energy homeostasis by modulating energy intake, storage, and expenditure. In turn, energy balance and metabolic signals serve as key regulators of the development, programming, and function of these different organ systems. For example, changes in the gut microbiome, such as those associated—perhaps even causally—with obesity, increase the efficiency of harvesting energy from the diet [42]. Conversely, altering dietary fat and sugar content rapidly shifts the composition of the gut microbiome [43]. Likewise, immune system functioning and energy homeostasis are interdependent, evidenced by long-standing observations regarding the immunosuppressive effects of malnutrition and the recent emergence of the field of immunometabolism, which is focused on studying crosstalk between these systems [44]. These links include, for example, (i) the confluence of immune and bio-energetic signaling pathways in quiescent and activated immune cells, (ii) the role of metabolic stress responses, such as autophagy, in innate and adaptive immune system activity, (iii) immune surveillance in traditional metabolic tissues, and (iv) immune system activation and inflammation in metabolic diseases. Bidirectional relationships between nervous system processes and energy homeostasis are similarly complex and now being investigated.
CNS development, homeostasis, and plasticity
The brain senses, integrates, and responds to fluxes in energy states throughout life via a range of complementary mechanisms. The central regulation of energy balance is complex and mediated by distributed neural networks, such as those in the limbic system and cerebral cortex underpinning reward and motivation, food anticipatory circadian rhythms, and other feeding behaviors [45, 46]. The hypothalamus and brainstem are essential centers for controlling these processes, with various nuclei and subpopulations of neurons having specific roles in regulating feeding behavior and satiety, lipid and glucose levels, body weight, and related metabolic parameters [47-60]. The best characterized is the arcuate nucleus (ARC) of the hypothalamus, which contains subpopulations of anorexigenic pro-opiomelanocortin (POMC)-expressing neurons and orexigenic agouti-related peptide (AgRP)- and neuropeptide Y (NPY)-expressing neurons.
Correspondingly, nutrient levels, gut- and adipose-derived peptides and hormones, and sundry metabolic signaling pathways influence CNS development, homeostasis, and plasticity [61]. Most notably, hypothalamic development and functioning are regulated by factors, which mediate feeding behavior and satiety, energy balance, and metabolism. During developmental critical periods, for example, the adipocyte-derived anorexigenic hormone, leptin (Glossary), promotes the programming of metabolism and establishment of feeding circuitry through activation of POMC and AgRP/NPY cell type-specific developmental signaling pathways [62-65]. Leptin and the gut-derived orexigenic hormone, ghrelin (Glossary), also promote synaptic plasticity in the ARC of adult mice [66, 67]. Interestingly, one of the potential mechanisms by which hypothalamic energy balance circuits undergo remodeling during adult life is through ongoing neurogenesis [68], and it has been suggested that leptin and ghrelin can modulate neurogenesis, in these and other contexts [69-71]. In addition, these and other factors involved in mediating feeding behavior and satiety, energy homeostasis, and metabolism are implicated in regulating learning and memory, reward and motivation, anxiety and depression via extra-hypothalamic actions, underscoring the highly integrated but widely distributed effects of energy balance and nutrition on the brain [72, 73].
Furthermore, like neuroimmune interactions, a common set of signaling pathways effects energy homeostasis within the nervous system and in other organ systems (Table 2). These common molecules play diverse roles in nutrient sensing, lipid and glucose homeostasis, and mitochondrial biogenesis and activity; and, evidence suggests they simultaneously regulate aspects of neural development, synaptic plasticity, and stress responses [4, 74-77].
Table 2.
Examples of key molecules linking energy homeostasis with nervous system development and functioning.
| Factor | Functions in energy homeostasis | Emerging functions in brain | Ref. |
|---|---|---|---|
| AMP-activated kinase |
|
|
[76, 110] |
| Insulin |
|
|
[111] |
| Sirtuins |
|
|
[112, 113] |
CNS disease and clinical implications
Not surprisingly, hypothalamic abnormalities, such as inflammation, autophagy, neuronal injury, and aberrant circuitry, are associated with disorders of energy homeostasis, such as obesity. For example, a recent study reported that, within 1 to 3 days of consuming a high fat diet (HFD), rodents exhibit increased expression levels of inflammation-related genes, reactive gliosis, neuronal injury, and autophagy in the ARC [78]. These very early changes do not occur peripherally in liver and adipose tissues, and are transient. With chronic consumption of a HFD and the development of obesity, however, these abnormalities recur both in the ARC and peripherally. This time course raises the possibility that the early hypothalamic changes lead to impairments in the regulation of energy balance and ultimately to obesity. A related study found that obese mice have defects in the normal profiles of dynamic cellular remodeling within the ARC [71]. The authors observed that hypothalamic neuronal turnover is suppressed in a HFD-induced obesity model, with a decrease in proliferating NPCs and neurogenesis and an increase in apoptosis of newborn neurons. Similarly, they found that levels of hypothalamic neurogenesis are decreased in a leptin deficiency obesity model (ob/ob mice) as a result of a depleted pool of hypothalamic neural stem cells (NSCs). Another report suggests that hypothalamic neurogenesis acts as a compensatory mechanism for maintaining energy balance in response to environmental and physiologic insults (and in the context of neurodegeneration) [79]. In a related study with implications for developing treatments, it was found that transplanting dissociated developmentally appropriate leptin-responsive hypothalamic cells into analogous regions of a leptin receptor-deficient obesity model (db/db mice) leads to their functional integration into the hypothalamic circuitry and mitigates disease processes [80]. Specifically, the transplanted cells survive and differentiate into multiple hypothalamic neuronal subtypes with appropriate electrophysiological and ultrastructural features and responsiveness to leptin, glucose and insulin, leading to a decrease in adiposity. Leptin also modulates T-cell subsets and promotes a pro-inflammatory milieu, and ob/ob mice are resistant to the induction of EAE [81, 82]. These findings link energy homeostasis signals with immune system activity and CNS disease.
Deregulation of bio-energetic signaling pathways is also implicated in the pathogenesis of CNS disorders, particularly those associated with age-related neurodegeneration, such as Alzheimer’s, Huntington’s, and Parkinson’s disease and amyotrophic lateral sclerosis, which clearly each exhibit distinctive metabolic phenotypes [83, 84]. These same pathways are often deregulated in metabolic disorders. Thus, an important question to be answered is whether there is crosstalk when metabolic disorders are co-morbid with these neurological diseases and, if so, then what is its precise nature. Is it protective, detrimental, or somehow more complex over the courses of the different diseases?
Importantly, many bio-energetic pathways can be targeted with existing and emerging therapeutic modalities for treating metabolic disorders [85], and it has been suggested they might also be beneficial in nervous system diseases. In fact, studies suggest that PPAR agonists, such as bezafibrate and thiazolidinediones (e.g., ciglitazone, pioglitazone, and troglitazone), have neuroprotective effects in neurodegenerative disease models. Similarly, analogs of GLP-1 (exendin-4) and GLP-1 receptor agonists (liraglutide) have neurotrophic and neuroprotective effects. The molecular and cellular mechanisms for the apparent benefits of these agents are currently a matter of debate and include putative effects on microglia, inflammation, mitochondria, and oxidative stress. Nevertheless, these preliminary observations imply that both dietary interventions and FDA approved and emerging drugs for metabolic disorders, such as insulin sensitizers and secretagogues and related agents, can modify CNS disease processes, including potentially during pre-clinical stages of disease. Clinical trials evaluating these treatments are underway (NCT01280123, NCT00811681, NCT01174810, NCT01255163).
The brain-gut microbiome axis
Microbiota (bacteria, viruses and fungi) are important mediators of health and disease. Commensal microbial populations are associated with various tissues (gut, skin, and vagina). These communities engage in quorum sensing–intercellular communication among bacteria–and in complex interactions with the host.
The dynamic equilibrium that exists between gut microbiota and their associated genomes, host-related factors (age, gender, and pregnancy), and environmental influences (diet) is termed the gut microbiome. Although it is thought to play a primary role in digestion and energy metabolism in the gut lumen, the gut microbiome also modulates development and maturation of the immune system, including effects on both the innate and adaptive immune responses [86]. For example, the gut is colonized after birth with a skin- or vagina-like composition that evolves into a relatively stable community through the induction of tolerance to particular bacteria, mediated by recognition of symbiotic bacterial molecules, such as those affecting TLR signaling, and the generation of bacterial antigen-specific populations of Treg cells [87]. Emerging evidence suggests the gut microbiome plays a similar instructive role in the CNS, either directly or indirectly through immune regulation, neuroendocrine signaling and other processes. Indeed, there are efforts underway aimed at elucidating functional interconnections between the gut microbiome and the nervous system, mediated by gut intrinsic and extrinsic mechanisms including the enteric and autonomic nervous systems, the HPA axis and sympatho–adrenal axes, gut-associated lymphoid tissue, immune cells, enteroendocrine cells, neurotransmitters, and gut peptides and hormones [6, 8].
CNS development, homeostasis, stress, and behavioral responses
It is becoming clear that the gut microbiome can modulate CNS development and homeostasis, stress and behavioral responses, and disease processes. Specifically, observations suggest that, during a developmental critical period, the gut microbiome plays a role in the programming of regional neural gene expression levels, signaling pathways, and behavioral repertoires present later in life [6, 8]. One seminal study demonstrated that adult mice raised in germ-free conditions (GF) exhibit higher levels of motor activity and lower levels of anxiety-like behavior compared to specific pathogen free (SPF) mice, which have a normal gut microbiota [88]. This phenotype is associated with increased rates of striatal neurotransmitter turnover and differential expression in various brain regions of genes involved in synaptic plasticity, cyclic adenosine monophosphate signaling, and other pathways. Furthermore, exposing GF mice early in life, but not in adulthood, to microbiota obtained from SPF mice, results in a phenotype similar to that of SPF mice. The mechanisms by which these processes are mediated are emerging. A recent study found that male GF animals have increased 5-hydroxytryptamine and 5-hydroxyindoleacetic acid in the hippocampus and higher concentrations of their precursor, tryptophan, in plasma, suggesting that the microbiome impacts hippocampal serotonergic neurotransmission via a humoral mechanism [89]. Another study showed that chronic ingestion of a particular Lactobacillus strain modulates regional expression profiles of gamma aminobutyric acid (GABA) receptor subtypes in the brain and associated behavioral phenotypes in adult mice and that these effects are abrogated by vagotomy [90]. These observations imply that the vagus nerve serves as a key mediator of gut-to-brain signaling and, further, that the effects of the gut microbiome are not simply restricted to the developing brain but are also involved in adult brain functions.
CNS disease and clinical implications
It is intriguing to speculate that the gut microbiome influences susceptibility to and pathogenesis of CNS diseases, as it does for other organ systems. In fact, one study found that the composition of the fecal microbiota is more diverse in autistic children with gastrointestinal symptoms compared to controls [91]. Although these findings are correlative, an important study utilizing a mouse model for multiple sclerosis [92] supports a more causal relationship [93]. It reported that commensal microbiota are necessary for the development of spontaneous relapsing-remitting experimental autoimmune encephalomyelitis (EAE). While 80% of mice raised under SPF conditions develop this form of EAE within 3-8 months of age, those raised under GF conditions exhibit impaired differentiation of pro-inflammatory T helper 17 cells and do not develop EAE. However, exposing GF mice to conventional commensal microbiota leads to the rapid development of EAE. Corresponding studies have demonstrated that strategies aimed at modifying the composition of the gut microbiota can influence the course of EAE (and other disorders), likely mediated by microbiota-induced changes in the milieu of cytokines with pro- and anti-inflammatory effects and the balance between different T-cell subsets [7, 94, 95].
The gut microbiome may have an impact on the pathogenesis of a broader range of CNS disorders directly or through indirect effects (1) on the immune system, given how neuroimmune interactions are responsible for mediating CNS health and disease; (2) on energy metabolism, given the complex interrelationships that exist between the brain and energy balance; and (3) on other physiological processes. Conversely, the CNS may exert effects on the gut microbiome through these same interconnections. For example, in the example of autism, above, it is reasonable to conjecture that the CNS pathology is responsible for giving rise to the abnormal profile of fecal microbiota by inducing impairments in the activity of the immune system or the gut. Indeed, stress during development and adulthood can alter the composition of gut microbial populations [96, 97].
These findings imply that defining personal enterotypes (Glossary), interrogating host-gut microbiome interactions, and identifying dysbiosis might yield insights into CNS disease states and have important therapeutic implications [98]. Approaches used to modify gut microbiota, including dietary interventions, pre- and pro-biotic agents, antibiotics, fecal transplants, and other modalities might impact neurobiological programming during developmental critical periods and brain function throughout the lifespan. Further, the effects of drugs for neurological and psychiatric diseases, including specific therapeutic responses and side effects, can potentially be mediated by the microbiome. For example, chronic treatment of rats with olanzapine induces changes in the gut microbiome that might influence the weight gain and metabolic dysfunction associated with this atypical antipsychotic agent [99].
Concluding remarks
The traditional view is that brain acts as a central regulator of homeostatic processes. However, this brain-body connection is not unidirectional. Brain development and functioning, along with disease onset and progression, occur within the context of the whole organism. Seminal neural processes are highly responsive to environmental and interoceptive cues, including those derived from circadian pacemakers. Recent studies have started elucidating how these are also mediated, at a mechanistic level, by dynamic crosstalk that takes place between the nervous system and other organ systems. Herein, we have highlighted emerging roles for immune surveillance, bio-energetic factors, and the gut microbiome. Further interrelationships between the brain and various other organ systems are also now being recognized. These encompass complex signals propagated across a broad range of local, widely distributed, and specialized organ-specific brain-systemic interfaces through both existing and novel mechanisms (intercellular trafficking of exosomes), representing intriguing areas for future study.
Collectively, these evolving insights raise many interesting questions (Box 2). For example, it is known that predispositions to a subset of neurological and psychiatric diseases—as well as metabolic phenotypes, cancers, and other systemic disorders—can be programmed during developmental critical periods, but these states are difficult to assess functionally since they are either subtle, relatively inaccessible, or their biological substrates unknown. Might it be possible to better characterize these pre-clinical vulnerabilities or frank disease states by interrogating elements of brain-systemic crosstalk, especially because these complex disorders often have manifestations in multiple organ systems? If so, can diagnostic and therapeutic modalities targeting these signals be developed, perhaps as extensions of ‘systems’ approaches for biomarker discovery and pharmacology that are currently in vogue [100]?
Box 2. Outstanding questions.
How can shared sets of common molecules be better exploited for diagnosing and treating nervous system disorders associated with systemic co-morbidities (and systemic disorders with manifestations in brain)?
What are the precise molecular and cellular mechanisms by which the gut microbiota modulate CNS development and homeostasis, stress and behavioral responses, and disease processes?
What roles do brain-systemic interfaces play, specifically, in mediating nervous system disease pathophysiology?
Does the presence of classical immune signaling molecules intimately associated with stem cell niches, synaptic terminals and related structures during brain development and adult life suggest that a novel form of immune surveillance occurs within the CNS in which subtle functional alterations in stem cell maintenance and maturation and synaptic efficacy and plasticity, for example, are sensed and acted upon to attempt to maintain or reestablish homeostasis during latent phases of neurological disease states?
Also, as the molecular and cellular mechanisms by which the gut microbiota influence brain development and homeostasis are uncovered, how can these be targeted for diagnostic, prognostic and therapeutic purposes? Specifically, is it possible to define enterotypes, through high-throughput metagenomic and other types of studies, which are associated with clinically relevant patterns of neural circuitry and behavioral responses and linked to disease risk, onset and progression and to drug responsiveness and toxicities? What is the interplay between these enterotypes and other sets of omics data from the host, particularly metabolomic profiles? Can these types of information be used to develop novel treatment modalities such as individualized dietary interventions; vitamins and supplements; pre-, pro- and anti-biotics; and fecal transplants?
Continuing to study aspects of ‘systems physiology’ with an emphasis on brain, by utilizing emerging tools and techniques from systems biology and network medicine, therefore, has important implications for understanding the nervous system and diagnosing and perhaps even treating brain disorders and their systemic co-morbidities in a predictive, preventative, personalized, and participatory fashion.
Highlights.
Unanticipated interconnections exist between the nervous system and the immune system, energy homeostasis, and gut microbiome.
Crosstalk between these systems mediates nervous system development, homeostasis, and plasticity.
Defining these mechanisms will provide insights into neuropsychiatric diseases and their co-morbidities and promote development of innovative diagnostics and therapeutics.
Acknowledgments
We regret that space constraints have prevented the citation of many relevant and important references. M.F.M. is supported by grants from the National Institutes of Health (NS071571, HD071593, MH66290), as well as by the F.M. Kirby, Alpern Family, Harold and Isabel Feld and Roslyn and Leslie Goldstein Foundations.
Glossary
- Enterotypes
variants of gut microbial communities that, in humans, are largely dominated by Fermicutes, Bacteroides, Prevotella, or Ruminococcus. Their composition is thought to influence disease pathogenesis and be subject to modification by longer-term dietary interventions.
- Immune surveillance
process by which the host immune system deploys innate and adaptive immune cell types, effector molecules, and related signaling pathways to protect from pathology.
- Leptin
adipocyte-derived anorexigenic hormone.
- Ghrelin
gut-derived orexigenic hormone.
- Gut microbiome
complex ecosystem arising from the symbiotic relationship between the commensal intestinal microbial community and the host.
- Network medicine
non-reductionistic paradigm for understanding how complex human diseases arise from the disruption of molecular and cellular network topologies and dynamics, relevant for identifying novel disease mechanisms, biomarkers, and therapeutic targets.
- Stem cell niches
highly specialized microenvironments found in the developing and adult brain (and in other organ systems) responsible for maintenance, activation and differentiation of tissue-specific stem and progenitor cell subtypes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.El Khoury J. Neurodegeneration and the neuroimmune system. Nat Med. 2010;16:1369–1370. doi: 10.1038/nm1210-1369. [DOI] [PubMed] [Google Scholar]
- 2.Drexhage RC, et al. Immune and neuroimmune alterations in mood disorders and schizophrenia. Int Rev Neurobiol. 2011;101:169–201. doi: 10.1016/B978-0-12-387718-5.00007-9. [DOI] [PubMed] [Google Scholar]
- 3.Kapogiannis D, Mattson MP. Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer’s disease. Lancet Neurol. 2011;10:187–198. doi: 10.1016/S1474-4422(10)70277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rafalski VA, Brunet A. Energy metabolism in adult neural stem cell fate. Prog Neurobiol. 2011;93:182–203. doi: 10.1016/j.pneurobio.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Farooqui AA, et al. Metabolic syndrome as a risk factor for neurological disorders. Cell Mol Life Sci. 2012;69:741–762. doi: 10.1007/s00018-011-0840-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. 2011;12:453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ochoa-Reparaz J, et al. Gut, bugs, and brain: role of commensal bacteria in the control of central nervous system disease. Ann Neurol. 2011;69:240–247. doi: 10.1002/ana.22344. [DOI] [PubMed] [Google Scholar]
- 8.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 9.Goddard CA, et al. Regulation of CNS synapses by neuronal MHC class I. Proc Natl Acad Sci U S A. 2007;104:6828–6833. doi: 10.1073/pnas.0702023104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Datwani A, et al. Classical MHCI molecules regulate retinogeniculate refinement and limit ocular dominance plasticity. Neuron. 2009;64:463–470. doi: 10.1016/j.neuron.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sawcer S, et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476:214–219. doi: 10.1038/nature10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baranzini SE, et al. Genome-wide association analysis of susceptibility and clinical phenotype in multiple sclerosis. Hum Mol Genet. 2009;18:767–778. doi: 10.1093/hmg/ddn388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rioux JD, et al. Mapping of multiple susceptibility variants within the MHC region for 7 immune-mediated diseases. Proc Natl Acad Sci U S A. 2009;106:18680–18685. doi: 10.1073/pnas.0909307106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khankhanian P, et al. Genetic variation in the odorant receptors family 13 and the mhc loci influence mate selection in a multiple sclerosis dataset. BMC Genomics. 2010;11:626. doi: 10.1186/1471-2164-11-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McElroy JP, et al. SNP-based analysis of the HLA locus in Japanese multiple sclerosis patients. Genes Immun. 2011;12:523–530. doi: 10.1038/gene.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhowmick S, et al. The sympathetic nervous system modulates CD4(+)FoxP3(+)regulatory T cells via a TGF-beta-dependent mechanism. J Leukoc Biol. 2009;86:1275–1283. doi: 10.1189/jlb.0209107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katayama Y, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 18.Mendez-Ferrer S, et al. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 19.Mendez-Ferrer S, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mercier FE, et al. The bone marrow at the crossroads of blood and immunity. Nat Rev Immunol. 2011;12:49–60. doi: 10.1038/nri3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferraro F, et al. Diabetes impairs hematopoietic stem cell mobilization by altering niche function. Sci Transl Med. 2011;3:104ra101. doi: 10.1126/scitranslmed.3002191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosas-Ballina M, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong CH, et al. Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science. 2011;334:101–105. doi: 10.1126/science.1210301. [DOI] [PubMed] [Google Scholar]
- 24.Romo-Gonzalez T, et al. Central nervous system: A modified immune surveillance circuit? Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 25.Trotta MB, et al. Inflammatory and Immunological parameters in adults with Down syndrome. Immunity & ageing : I & A. 2011;8:4. doi: 10.1186/1742-4933-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Budingen HC, et al. B cell exchange across the blood-brain barrier in multiple sclerosis. J Clin Invest. 2012;122:4533–4543. doi: 10.1172/JCI63842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Black JA, Waxman SG. Sodium channels and microglial function. Exp Neurol. 2012;234:302–315. doi: 10.1016/j.expneurol.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 28.Odoardi F, et al. T cells become licensed in the lung to enter the central nervous system. Nature. 2012;488:675–679. doi: 10.1038/nature11337. [DOI] [PubMed] [Google Scholar]
- 29.Zhao P, et al. Extracellular signal-regulated kinase-regulated microglia-neuron signaling by prostaglandin E2 contributes to pain after spinal cord injury. J Neurosci. 2007;27:2357–2368. doi: 10.1523/JNEUROSCI.0138-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao P, et al. Modulation of thalamic nociceptive processing after spinal cord injury through remote activation of thalamic microglia by cysteine cysteine chemokine ligand 21. J Neurosci. 2007;27:8893–8902. doi: 10.1523/JNEUROSCI.2209-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Derecki NC, et al. Wild-type microglia arrest pathology in a mouse model of Rett syndrome. Nature. 2012;484:105–109. doi: 10.1038/nature10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guerreiro R, et al. TREM2 Variants in Alzheimer’s Disease. N Engl J Med. 2012 doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jonsson T, et al. Variant of TREM2 Associated with the Risk of Alzheimer’s Disease. N Engl J Med. 2012 doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saab CY, et al. Alarm or curse? The pain of neuroinflammation. Brain Res Rev. 2008;58:226–235. doi: 10.1016/j.brainresrev.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 35.London A, et al. Neuroprotection and progenitor cell renewal in the injured adult murine retina requires healing monocyte-derived macrophages. J Exp Med. 2011;208:23–39. doi: 10.1084/jem.20101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shechter R, et al. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med. 2009;6:e1000113. doi: 10.1371/journal.pmed.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garg SK, et al. IFN-gamma and IL-4 differentially shape metabolic responses and neuroprotective phenotype of astrocytes. J Neurochem. 2009;108:1155–1166. doi: 10.1111/j.1471-4159.2009.05872.x. [DOI] [PubMed] [Google Scholar]
- 38.Garg SK, et al. Neuroprotective immunity: T cell-derived glutamate endows astrocytes with a neuroprotective phenotype. J Immunol. 2008;180:3866–3873. doi: 10.4049/jimmunol.180.6.3866. [DOI] [PubMed] [Google Scholar]
- 39.Kwan W, et al. Mutant huntingtin impairs immune cell migration in Huntington disease. J Clin Invest. 2012 doi: 10.1172/JCI64484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lima FA, et al. Decreased AIRE expression and global thymic hypofunction in Down syndrome. J Immunol. 2011;187:3422–3430. doi: 10.4049/jimmunol.1003053. [DOI] [PubMed] [Google Scholar]
- 41.Wekerle H. Breaking ignorance: the case of the brain. Curr Top Microbiol Immunol. 2006;305:25–50. doi: 10.1007/3-540-29714-6_2. [DOI] [PubMed] [Google Scholar]
- 42.Turnbaugh PJ, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 43.Turnbaugh PJ, et al. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1:6ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nikolajczyk BS, et al. The outliers become a stampede as immunometabolism reaches a tipping point. Immunol Rev. 2012;249:253–275. doi: 10.1111/j.1600-065X.2012.01142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Faulconbridge LF, Hayes MR. Regulation of energy balance and body weight by the brain: a distributed system prone to disruption. Psychiatr Clin North Am. 2011;34:733–745. doi: 10.1016/j.psc.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mistlberger RE. Neurobiology of food anticipatory circadian rhythms. Physiol Behav. 2011;104:535–545. doi: 10.1016/j.physbeh.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 47.Lam TK, et al. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med. 2005;11:320–327. doi: 10.1038/nm1201. [DOI] [PubMed] [Google Scholar]
- 48.Lam TK, et al. Hypothalamic sensing of fatty acids. Nat Neurosci. 2005;8:579–584. doi: 10.1038/nn1456. [DOI] [PubMed] [Google Scholar]
- 49.Pocai A, et al. Hypothalamic K(ATP) channels control hepatic glucose production. Nature. 2005;434:1026–1031. doi: 10.1038/nature03439. [DOI] [PubMed] [Google Scholar]
- 50.He W, et al. Molecular disruption of hypothalamic nutrient sensing induces obesity. Nat Neurosci. 2006;9:227–233. doi: 10.1038/nn1626. [DOI] [PubMed] [Google Scholar]
- 51.Kitamura T, et al. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med. 2006;12:534–540. doi: 10.1038/nm1392. [DOI] [PubMed] [Google Scholar]
- 52.Buettner C, et al. Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat Med. 2008;14:667–675. doi: 10.1038/nm1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kokorovic A, et al. Hypothalamic sensing of circulating lactate regulates glucose production. J Cell Mol Med. 2009;13:4403–4408. doi: 10.1111/j.1582-4934.2008.00596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ross RA, et al. Differential effects of hypothalamic long-chain fatty acid infusions on suppression of hepatic glucose production. Am J Physiol Endocrinol Metab. 2010;299:E633–639. doi: 10.1152/ajpendo.00190.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Knight CM, et al. Mediobasal hypothalamic SIRT1 is essential for resveratrol’s effects on insulin action in rats. Diabetes. 2011;60:2691–2700. doi: 10.2337/db10-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ono H, et al. Activation of hypothalamic S6 kinase mediates diet-induced hepatic insulin resistance in rats. J Clin Invest. 2008;118:2959–2968. doi: 10.1172/JCI34277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin HV, et al. Divergent regulation of energy expenditure and hepatic glucose production by insulin receptor in agouti-related protein and POMC neurons. Diabetes. 2010;59:337–346. doi: 10.2337/db09-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu Y, et al. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate insulin sensitivity in liver. Nat Neurosci. 2010;13:1457–1459. doi: 10.1038/nn.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hill JW, et al. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab. 2010;11:286–297. doi: 10.1016/j.cmet.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muse ED, et al. Hypothalamic resistin induces hepatic insulin resistance. J Clin Invest. 2007;117:1670–1678. doi: 10.1172/JCI30440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dietrich MO, Horvath TL. Hypothalamic control of energy balance: insights into the role of synaptic plasticity. Trends Neurosci. 2013 doi: 10.1016/j.tins.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 62.Bouret SG, et al. Distinct roles for specific leptin receptor signals in the development of hypothalamic feeding circuits. J Neurosci. 2012;32:1244–1252. doi: 10.1523/JNEUROSCI.2277-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Varela L, Horvath TL. Leptin and insulin pathways in POMC and AgRP neurons that modulate energy balance and glucose homeostasis. EMBO Rep. 2012;13:1079–1086. doi: 10.1038/embor.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Steculorum SM, Bouret SG. Maternal diabetes compromises the organization of hypothalamic feeding circuits and impairs leptin sensitivity in offspring. Endocrinology. 2011;152:4171–4179. doi: 10.1210/en.2011-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bouyer K, Simerly RB. Neonatal leptin exposure specifies innervation of presympathetic hypothalamic neurons and improves the metabolic status of leptin-deficient mice. J Neurosci. 2013;33:840–851. doi: 10.1523/JNEUROSCI.3215-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pinto S, et al. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–115. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- 67.Yang Y, et al. Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell. 2011;146:992–1003. doi: 10.1016/j.cell.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kokoeva MV, et al. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science. 2005;310:679–683. doi: 10.1126/science.1115360. [DOI] [PubMed] [Google Scholar]
- 69.Garza JC, et al. Leptin increases adult hippocampal neurogenesis in vivo and in vitro. J Biol Chem. 2008;283:18238–18247. doi: 10.1074/jbc.M800053200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moon M, et al. Ghrelin regulates hippocampal neurogenesis in adult mice. Endocr J. 2009;56:525–531. doi: 10.1507/endocrj.k09e-089. [DOI] [PubMed] [Google Scholar]
- 71.McNay DE, et al. Remodeling of the arcuate nucleus energy-balance circuit is inhibited in obese mice. J Clin Invest. 2012;122:142–152. doi: 10.1172/JCI43134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cowley MA, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 73.Bouret SG. Neurodevelopmental actions of leptin. Brain Res. 2010;1350:2–9. doi: 10.1016/j.brainres.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maiese K, et al. mTOR: on target for novel therapeutic strategies in the nervous system. Trends Mol Med. 2013;19:51–60. doi: 10.1016/j.molmed.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.O’Neill C, et al. Insulin and IGF-1 signalling: longevity, protein homoeostasis and Alzheimer’s disease. Biochem Soc Trans. 2012;40:721–727. doi: 10.1042/BST20120080. [DOI] [PubMed] [Google Scholar]
- 76.Amato S, Man HY. Bioenergy sensing in the brain: the role of AMP-activated protein kinase in neuronal metabolism, development and neurological diseases. Cell Cycle. 2011;10:3452–3460. doi: 10.4161/cc.10.20.17953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Donmez G. The neurobiology of sirtuins and their role in neurodegeneration. Trends Pharmacol Sci. 2012;33:494–501. doi: 10.1016/j.tips.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 78.Thaler JP, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122:153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pierce AA, Xu AW. De novo neurogenesis in adult hypothalamus as a compensatory mechanism to regulate energy balance. J Neurosci. 2010;30:723–730. doi: 10.1523/JNEUROSCI.2479-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Czupryn A, et al. Transplanted hypothalamic neurons restore leptin signaling and ameliorate obesity in db/db mice. Science. 2011;334:1133–1137. doi: 10.1126/science.1209870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Matarese G, et al. Leptin as a metabolic link to multiple sclerosis. Nature reviews Neurology. 2010;6:455–461. doi: 10.1038/nrneurol.2010.89. [DOI] [PubMed] [Google Scholar]
- 82.Galgani M, et al. Leptin modulates the survival of autoreactive CD4+ T cells through the nutrient/energy-sensing mammalian target of rapamycin signaling pathway. J Immunol. 2010;185:7474–7479. doi: 10.4049/jimmunol.1001674. [DOI] [PubMed] [Google Scholar]
- 83.Olsson B, et al. Biomarker-based dissection of neurodegenerative diseases. Prog Neurobiol. 2011;95:520–534. doi: 10.1016/j.pneurobio.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 84.Pinto M, et al. Regional susceptibilities to mitochondrial dysfunctions in the CNS. Biol Chem. 2012;393:275–281. doi: 10.1515/hsz-2011-0236. [DOI] [PubMed] [Google Scholar]
- 85.Vetter ML, et al. Behavioral and pharmacologic therapies for obesity. Nat Rev Endocrinol. 2010;6:578–588. doi: 10.1038/nrendo.2010.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Clemente JC, et al. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148:1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Round JL, et al. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332:974–977. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Heijtz RD, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Clarke G, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- 90.Bravo JA, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Finegold SM, et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe. 2010;16:444–453. doi: 10.1016/j.anaerobe.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 92.Pollinger B, et al. Spontaneous relapsing-remitting EAE in the SJL/J mouse: MOG-reactive transgenic T cells recruit endogenous MOG-specific B cells. J Exp Med. 2009;206:1303–1316. doi: 10.1084/jem.20090299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Berer K, et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479:538–541. doi: 10.1038/nature10554. [DOI] [PubMed] [Google Scholar]
- 94.Ochoa-Reparaz J, et al. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 2010;3:487–495. doi: 10.1038/mi.2010.29. [DOI] [PubMed] [Google Scholar]
- 95.Ochoa-Reparaz J, et al. Central nervous system demyelinating disease protection by the human commensal Bacteroides fragilis depends on polysaccharide A expression. J Immunol. 2010;185:4101–4108. doi: 10.4049/jimmunol.1001443. [DOI] [PubMed] [Google Scholar]
- 96.O’Mahony SM, et al. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. 2009;65:263–267. doi: 10.1016/j.biopsych.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 97.Bailey MT, et al. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun. 2011;25:397–407. doi: 10.1016/j.bbi.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu GD, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Davey KJ, et al. Gender-dependent consequences of chronic olanzapine in the rat: effects on body weight, inflammatory, metabolic and microbiota parameters. Psychopharmacology (Berl) 2012;221:155–169. doi: 10.1007/s00213-011-2555-2. [DOI] [PubMed] [Google Scholar]
- 100.Iyengar R, et al. Merging systems biology with pharmacodynamics. Sci Transl Med. 2012;4:126ps127. doi: 10.1126/scitranslmed.3003563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moriyama M, et al. Complement receptor 2 is expressed in neural progenitor cells and regulates adult hippocampal neurogenesis. J Neurosci. 2011;31:3981–3989. doi: 10.1523/JNEUROSCI.3617-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stevens B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 103.Blank M, et al. The Down syndrome critical region regulates retinogeniculate refinement. J Neurosci. 2011;31:5764–5776. doi: 10.1523/JNEUROSCI.6015-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wong YH, et al. Protogenin defines a transition stage during embryonic neurogenesis and prevents precocious neuronal differentiation. J Neurosci. 2010;30:4428–4439. doi: 10.1523/JNEUROSCI.0473-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fathi A, et al. Comprehensive gene expression analysis of human embryonic stem cells during differentiation into neural cells. PLoS ONE. 2011;6:e22856. doi: 10.1371/journal.pone.0022856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Okun E, et al. Toll-like receptor 3 inhibits memory retention and constrains adult hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2010;107:15625–15630. doi: 10.1073/pnas.1005807107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. 2009;64:61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 108.Boulanger LM. Immune proteins in brain development and synaptic plasticity. Neuron. 2009;64:93–109. doi: 10.1016/j.neuron.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 109.Montgomery SL, Bowers WJ. Tumor necrosis factor-alpha and the roles it plays in homeostatic and degenerative processes within the central nervous system. J Neuroimmune Pharmacol. 2012;7:42–59. doi: 10.1007/s11481-011-9287-2. [DOI] [PubMed] [Google Scholar]
- 110.Hardie DG, et al. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nature reviews Molecular cell biology. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ghasemi R, et al. Insulin in the brain: sources, localization and functions. Molecular neurobiology. 2013;47:145–171. doi: 10.1007/s12035-012-8339-9. [DOI] [PubMed] [Google Scholar]
- 112.Coppari R. Metabolic actions of hypothalamic SIRT1. Trends in endocrinology and metabolism: TEM. 2012;23:179–185. doi: 10.1016/j.tem.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang F, et al. Protective effects and mechanisms of sirtuins in the nervous system. Progress in neurobiology. 2011;95:373–395. doi: 10.1016/j.pneurobio.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Goldman SA, Chen Z. Perivascular instruction of cell genesis and fate in the adult brain. Nat Neurosci. 2011;14:1382–1389. doi: 10.1038/nn.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lucas D, et al. Mobilized hematopoietic stem cell yield depends on species-specific circadian timing. Cell Stem Cell. 2008;3:364–366. doi: 10.1016/j.stem.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Skog J, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mustafa AK, et al. Signaling by gasotransmitters. Sci Signal. 2009;2:re2. doi: 10.1126/scisignal.268re2. [DOI] [PMC free article] [PubMed] [Google Scholar]


