Abstract
The lipoprotein receptor ligand Reelin is important for the processes of normal synaptic plasticity, dendritic morphogenesis, and learning and memory. Heterozygous reeler mice (HRM) show many neuroanatomical, biochemical, and behavioral features that are associated with schizophrenia. HRM show subtle morphological defects including reductions in dendritic spine density, altered synaptic plasticity and behavioral deficits in associative learning and memory and pre-pulse inhibition. The present studies test the hypothesis that in vivo elevation of Reelin levels can rescue synaptic and behavioral phenotypes associated with HRM. We demonstrate that a single in vivo injection of Reelin increases GAD67 expression and alters dendritic spine morphology. In parallel we observed enhancement of hippocampal synaptic function and associative learning and memory. Reelin supplementation also increases pre-pulse inhibition. These results suggest that characteristics of HRM, similar to those observed in schizophrenia, are sensitive to Reelin levels and can be modified with Reelin supplementation in male and female adults.
Keywords: Schizophrenia, low-density lipoprotein receptors, hippocampus, long-term potentiation, learning and memory, glutamic acid decarboxylase 67, sensorimotor gating, associative memory
Introduction
The extracellular glycoprotein Reelin is produced by Cajal–Retzius cells of the cortex and cerebellar granule cells during embryonic development (Pesold et al., 1998, 1999). In the adult, Reelin continues to be expressed by GABAergic interneurons in the cortex and hippocampus (D’arcangelo et al., 1997; Kubo et al., 2002; Pappas et al., 2001; Pesold et al., 1998, 1999; Rodriguez et al., 2000). Reelin signals through two lipoprotein receptors: very-low density lipoprotein receptor (VLDLR) and Apolipoprotein E receptor 2 (ApoER2) (Beffert et al., 2005, 2006; Hiesberger et al., 1999; Hoe et al., 2006). Receptor binding activates a number of neuronal signal transduction pathways in the central nervous system (CNS) that subsequently modulate synaptic function and ultimately learning and memory (Arnaud et al., 2003; Ballif et al., 2003; Beffert et al., 2005, 2006; Qiu et al., 2006). Furthermore, Reelin co-localizes with integrin receptors at the dendritic postsynaptic densities in the cortex of non-human primates (Rodriguez et al., 2000). Mice lacking Reelin or both ApoER2 and VLDLR exhibit disrupted cytoarchitecture in the cortex, cerebellum and hippocampus, and have impaired motor coordination, resting tremor and ataxia (D’arcangelo et al., 1995; Lambert De Rouvroit et al., 1998; Ogawa et al., 1995).
Despite a 50% reduction of Reelin in heterozygous reeler mice (HRM), they lack appreciable developmental abnormalities (Ballmaier et al., 2002; Liu et al., 2001; Pappas et al., 2001; Qiu et al., 2006). However, HRM have very subtle neuroanatomical, physiologic and behavioral deficits. These include a decrease in dendritic spine density in the parietal-frontal cortex pyramidal neurons and basal dendrites of hippocampal CA1 pyramidal neurons (Liu et al., 2001). At 3 months of age, these mice have a 16% deficit in the number of Purkinje cells and a 24% reduction at 16 months of age in the cerebellum (Hadj-Sahraoui et al., 1996; Maloku et al., 2010). This deficit is only present in the HRM males, while the females are spared (Hadj-Sahraoui et al., 1996). HRM have decreased PV+ interneuron density in the dorsomedial and ventromedial intermediate striatum and caudal striatum (Ammassari-Teule et al., 2009). Adult HRM also have impaired short-term and long-term plasticity in the hippocampal area CA1. Long-term potentiation (LTP) induced with theta-burst stimulation and long-term depression (LTD) induced with paired-pulse low-frequency stimulation (LFS) are disrupted. Behaviorally, HRM have deficits in pre-pulse inhibition (PPI), motor impulsivity, attentional-set shifting, motor stereotypies and contextual fear conditioning (CFC) (Laviola et al., 2009; Macri et al., 2010; Ognibene et al., 2007; Qiu et al., 2006; Tueting et al., 1999).
Previous research suggests that impairments of the Reelin pathway may be involved in the etiology of schizophrenia. Specifically, there is a 50% reduction of Reelin mRNA and protein levels in schizophrenic post-mortem brains (Guidotti et al., 2000; Impagnatiello et al., 1998). Numerous polymorphisms in the RELN gene are associated with schizophrenia (Chen et al., 2002; Costa et al., 2002a; Gregorio et al., 2009; Persico et al., 2006). In addition, hypermethylation of the RELN promoter, which leads to decreased Reelin expression, is the proposed mechanism for reduced Reelin expression in the schizophrenic brain (Abdolmaleky et al., 2005; Grayson et al., 2006, 2009; Guidotti et al., 2000; Persico et al., 2006). The correlation between reduced Reelin levels and schizophrenia is supported by observed decreases in glutamic acid decarboxylase 67 (GAD67) expression, abnormal acoustic PPI, motor impulsivity, perseverative behavior and reduced dendritic spine density in HRM and also observed in schizophrenic patients (Akbarian and Huang, 2006; Laviola et al., 2009; Liu et al., 2001; Macri et al., 2010; Ognibene et al., 2007; Qiu et al., 2006; Tueting et al., 2006). Schizophrenic patients have disrupted hippocampal-dependent associative learning and memory, reduced self-control behavior and changes in anxiety-related patterns similar to HRM (Hanlon et al., 2006; Ognibene et al., 2007; Qiu et al., 2006; Shohamy et al., 2010).
Presently it is unclear whether decreased synaptic plasticity and disruption of learning and memory in HRM are due to reduced Reelin function during development and/or in the adult. To address these unknowns, the present study determined whether acute in vivo elevation of Reelin in adult HRM can improve their biochemical, physiological or behavioral phenotype.
Materials and methods
Animals
All HRM (B6C3Fe a/a-Relnrl/+ strain) and wild-type littermate control mice were 12–16 weeks of age, and the original colony was obtained from Jackson Labs (Bar Harbor, Maine, USA). Breeding pairs consisted of two HRM and all offspring were genotyped by PCR. Animals were maintained at the University of South Florida and were housed in standard 12 hr light-dark cycle and supplied food pellets and water ad libitum. Following surgical procedures, all mice were singly housed in a cage. All animal care protocols used were in accordance with the Institutional Animal Care and Use Committee of the University of South Florida.
Reelin purification
Recombinant Reelin was produced using HEK293 cells that were stably transfected with the full-length Reelin pCrl vector as previously described (Weeber et al., 2002). Mock control media was obtained from untransfected HEK293 cells and purified in an identical manner.
Cannulations
Mice were anesthetized with isoflurane and placed on a stereo-taxic surgery apparatus (Stoelting Co, Wood Dale, IL, USA) and cannulas were inserted. Cannulas (Plastics One Inc., Roanoke, VA, USA) were bilaterally placed into the lateral ventricles (A.P −0.35 mm, L +/− 0.75 mm, and V −2.5 mm from bregma). The cannula was secured with dental cement. Cannulated mice were allowed to recover for 5 days prior to injection of 1 µL of mock or Reelin (300 nM).
Direct bilateral ventricular injections
Mice were anesthetized and placed on the stereotax as described above. Mice were bilaterally injected with 0.5 µL mock or Reelin (300 nM) into the ventricles (A.P. −.25 mm, L +/− 0.75 mm, and V −2.5 mm from bregma). Mice were injected at a rate of 1.0 µL/min and the needle was left in place for 30 s. We previously showed that perfusion of 5 nM Reelin onto acute wild-type hippocampal slices increased LTP (Weeber et al., 2002). Thus, the injection volume of Reelin for these studies was determined to produce an average total hemisphere concentration of 5 nM. The 5-day time point was chosen to be consistent with our previous work in wild-type mice. Reelin enhanced synaptic plasticity, associative and spatial memory formation 5 days post-injection in wild-type mice (Rogers et al., 2011).
Golgi staining and analysis of dendritic morphology in vivo
Whole brains were processed for Golgi staining using the FD Rapid Golgi Stain kit (FD NeuroTechnologies) as previously described (Hoe et al., 2009). Brains were sliced using a Vibratome (VT1000S, Leica) at a thickness of 150 µm. Bright-field microscopy (Axioplan 2; Zeiss) images (63× magnification) were taken of CA1 pyramidal neurons (n = 10 neurons/brain/group). The images were coded and dendritic spines were counted using the Neurolucida software (MicroBrightField). The average spine number, width and length were statistically compared between groups. Experimenters were blinded to treatment groups.
Immunoblotting
Brain tissue samples were homogenized in RIPA Lysis buffer (Millipore, Tris/HCl pH 7.4, 1 mM EDTA, 150 mM NaCl, 1% NP40, 0.25% sodium deoxycholate, 1× phosphatase inhibitors I and II (Sigma), 1× PMSF (Sigma) and 1× complete protease inhibitors (Sigma)). Protein concentrations were measured by a standard BCA assay (Pierce, Rockford, IL, USA). Lysates were heated in Laemmli buffer, and equal amounts of protein were loaded into 18-well 10% Tris-HCL gels (Bio-Rad). After transfer, blots were blocked with Blotto (5% nonfat dry milk in TBS) for 1 h at room temperature, and antibody against GAD67 (Millipore) was applied at 1:5000 dilution in Blotto overnight at 4°C. Membranes were washed three times for 10 min in TBS with 0.1% Tween 20 and incubated with HRP-conjugated secondary antibody (GE) for 1 h. Membranes were then washed three times for 10 min, and protein expression was visualized by electrochemiluminescence (ECL) treatment and exposure to film. Bands were quantified using Scion (Frederick, MD, USA) Image by analyzing pixel density. A semi-quantitative analysis was performed by densitometry, and standardized to beta-actin levels.
Extracellular recordings
Five days post-single direct injections, HRM were euthanized and hippocampi were dissected out for extracellular recordings as previously described (Beffert et al., 2005; Peters et al., 2006; Weeber et al., 2002). Briefly, field excitatory post-synaptic potentials (fEPSPs) were obtained from area CA1 stratum radiatum. Pairedpulse facilitation (PPF) was induced with the use of two pulses delivered with an inter-pulse interval of 20 ms. Incremental increases of 20 ms were given until a final inter-pulse interval of 300 ms was reached. LTP was induced using theta-burst stimulation consisting of five trains of four pulse bursts at 200 Hz separated by 200 ms, repeated six times with an inter-train interval of 10 s. LTD was induced by LFS which consisted of 15 min 1 Hz paired pulses with an inter-pulse interval of 50 ms. Post-tetanic potentiation (PTP) was induced using high-frequency stimulation (HFS) consisting of 1 sec, 100 Hz pulses. For PTP experiments, all hippocampal slices were bathed in artificial cerebrospinal fluid containing 50 µM APV for at least 15 min prior to and during recording. Potentiation was measured as the increase or decrease of the mean fEPSP descending slope following HFS, theta-burst stimulation or LFS normalized to the mean fEPSP descending slope of baseline recording.
Fear conditioning
Five days post-injection, HRM were tested for contextual fear-conditioned learning as previously described (Weeber et al., 2002). Briefly, animals were placed in the fear conditioning apparatus and given two 30 s acoustic conditioned stimulus (CS) paired with a 0.5 mA shock (unconditioned stimulus; US). For CFC, mice were placed in the chamber and monitored for freezing to the context 24 hrs following CS–US pairing. For cued fear conditioning, mice were placed in a novel environment and presented with the CS 24 hrs following CS–US pairing. Learning was assessed by measuring freezing behavior consisting of lack of motion for at least 1 s. Freezing behavior was recorded and processed by Freezing V1.2.00 software (Panlab, Barcelona, Spain).
Pre-pulse inhibition
The mice were placed in a restrainer (Panlab, Barcelona Spain) inside a sound-attenuation chamber. After a 5 min acclimation, mice were presented with seven trial types in a pseudorandom order with an inter-trial interval of 20 s: 1) a 40 ms, 120 dB sound burst (startle); 2–6) five different dB acoustic pre-pulses 100 msec before startle (74, 78, 82, 86, and 90 dB); 7) no stimulus. Startle response was measured as the peak startle observed within 120 ms after presentation of the startle.
Statistical analyses
Data obtained from immunoblotting and spine density were analyzed using the student’s 2-tailed t-tests and significance was assigned at p<0.05 (Prism Software). For all other data, an ANOVA was used to determine significance and are reported as ANOVA table p-value, F-values, genotype post hoc p-value, treatment post hoc p-value and genotype/treatment interaction post hoc p-values. All post hoc tests are Tukey’s multiple comparison test.
Results and discussion
Impairments in Reelin signaling have been implicated in several human cognitive disorders including autism spectrum disorders, schizophrenia and Alzheimer’s disease (Andres, 2002; Ballmaier et al., 2002; Botella-Lopez et al., 2006; Chin et al., 2007; Costa et al., 2002b; Durakoglugil et al., 2009; Impagnatiello et al., 1998). Investigations of HRM reveal striking similarities to schizophrenia. It is unclear if these deficits are due to the absence of Reelin during development or in adulthood. The present study determined the in vivo effects of increased Reelin on synaptic plasticity and memory function in adult male and female HRM. To reduce caveats associated with long-term Reelin application such as receptor internalization and unknown effects on downstream targets, a single Reelin-injection strategy was used. This strategy provided a time point from which to compare specific changes in dendritic morphology, synaptic function and behavior to mock-injected controls. Because both male and female mice were used in behavioral and electrophysiological experimentation, sex differences were first examined. There were no intragroup sex differences between wild-type littermate controls or treatment groups in either behavioral or electrophysiological experiments to report (data not shown).
Recently, we demonstrated that a single ventricular injection of Reelin increased Reelin immunoreactivity in the hippocampus of wild-type mice post-injection (Rogers et al., 2011). Reelin is a large 400 kDa protein in the full-length form, and it is unusual for proteins of this size to diffuse very far through the extracellular space. To determine if injected Reelin diffuses from the ventricles to the hippocampus of HRM, mice were injected with a Reelin-GFP and GFP was probed and visualized using immunohistochemistry. We found GFP immunoreactivity throughout the hippocampus 1 h post-injection (Supplementary data, Figure 1). These results demonstrate that Reelin can diffuse away from the ventricles and to the targeted hippocampus of HRM.
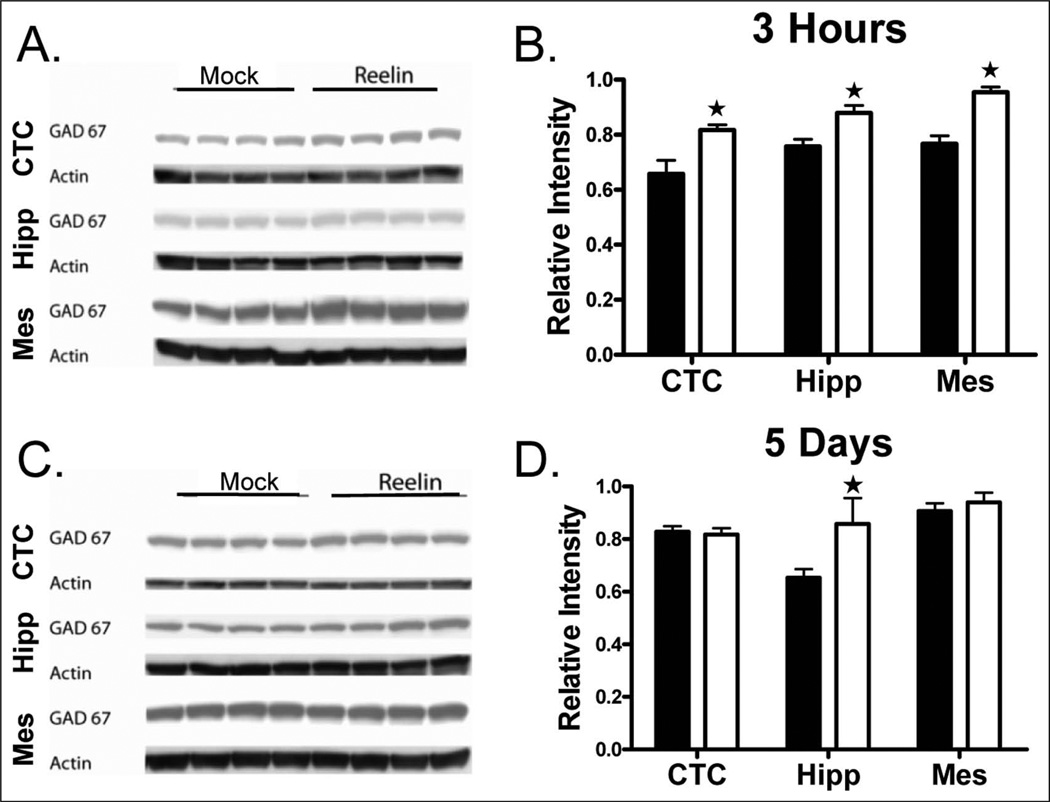
HRM have decreased levels of GAD67, the enzyme that catalyzes the decarboxylation of glutamate to yield GABA, in the frontoparietal cortex and hippocampus (Liu et al., 2001; Qiu et al., 2006). Thus, we determined if Reelin supplementation increases GAD67 levels. We observed increased GAD67 expression in the cortex, hippocampus and mesencephalon 3 h post-injection compared with mock-injected HRM (Figure 1(a), 1(b); p<0.05). However, only the hippocampus had increased GAD67 expression 5 days post-injection (Figure 1(c), 1(d); p<0.05). These results suggest that reduced Reelin may underlie the decrease in GAD67 expression in HRM which can be increased through Reelin supplementation.
Figure 1.
Reelin increases GAD67 expression in the cortex, hippocampus and mesencephalon. HRM received a single bilateral ventricular injection of either mock (black; n = 4) or Reelin (white; n = 4). Brains were harvested and the cortex, hippocampus and mesencephalon were separated through dissection at 3 h and 5 days post-injection. GAD67 protein expression was determined by Western blot analysis. (a) Representative western blots of GAD67 and actin. (b) Reelin increased GAD67 expression in the cortex, hippocampus and mesencephalon 3 h post-injection. (c) Reelin increased GAD67 expression in the hippocampus only 5 days post-injection (n = 4 mice/group; *, p<0.05). (d) Reelin increased GAD67 expression in the hippocampus only 5 days post-injection (n = 4 mice/group; *, p<0.05).
Previous studies in our laboratory revealed that adult HRM do not have detectable differences in activity, motor coordination, anxiety, or environmental perception when compared with wildtype littermate control mice, although adolescent HRM display lower levels of anxiety- and risk assessment-related behaviors in the elevated plus-maze (Ognibene et al., 2007; Qiu et al., 2006). HRM have a distinct hippocampal-dependent deficit in associative learning and impulsivity–anxiety-related behavior (Qiu et al., 2006). In the hippocampus, HRM CA1 pyramidal neurons receive significantly less inhibitory GABAergic innervations and have reduced spontaneous inhibitory postsynaptic current (sIPSC) amplitude and frequency (Qiu et al., 2006). These results in combination with decreased GAD67 expression indicate that Reelin-producing GABAergic interneurons are sensitive to the actions of Reelin, particularly those of the hippocampus when compared with the cortex and mesencephalon. It is important to note that most of what we know about the actions of Reelin on cognitive behavioral domain comes from studies of the hippocampus (for review, see Rogers and Weeber, 2009).
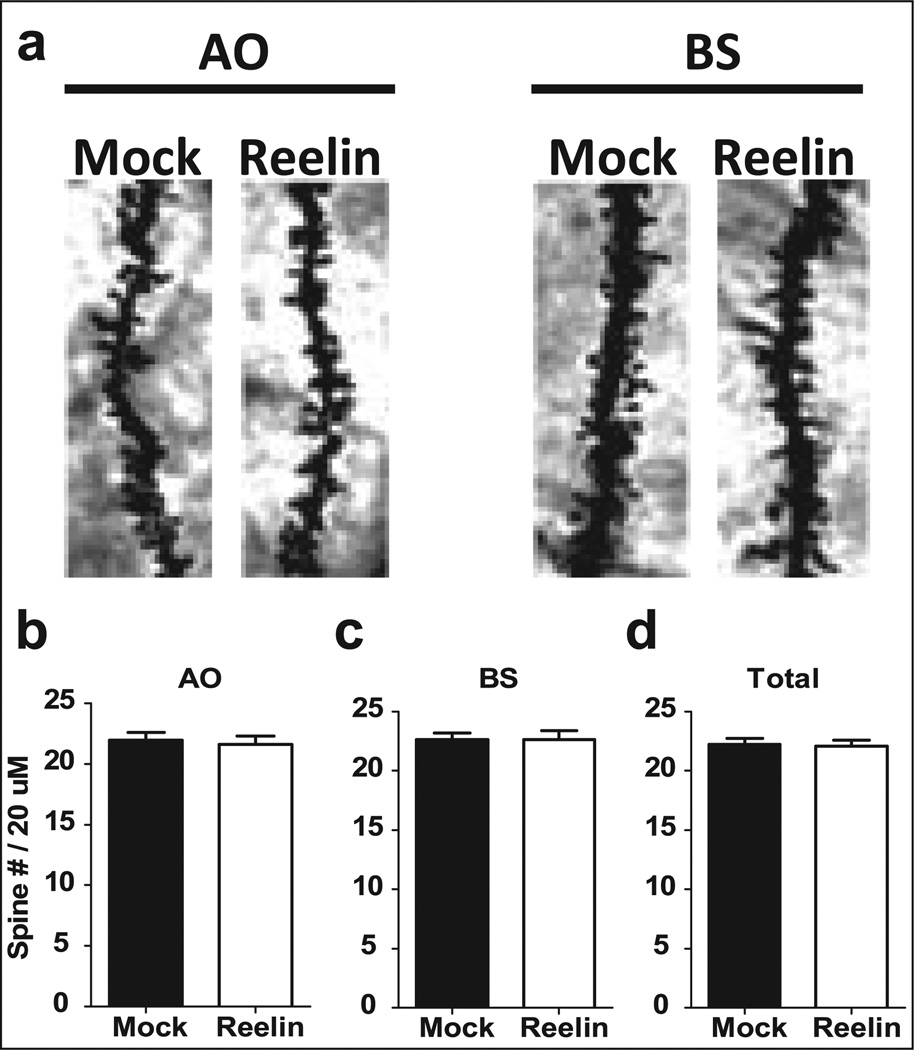
HRM have decreased spine density in cortical and hippocampal neurons when compared with wild-type mice (Liu et al., 2001; Niu et al., 2008; Pappas et al., 2001). We determined if a single Reelin supplementation alters dendritic spine density 5 days postinjection in the hippocampus. Spine density in the apical oblique (AO) and basal shaft (BS) dendrites of CA1 pyramidal neurons, as well as total spine density was quantified. There were no significant alterations in spine density in the hippocampus between Reelin and mock-injected HRM (Figure 2; p>0.05). Although spine density was unaltered, recent evidence suggests that Reelin can also affect spine morphology (Pujadas et al., 2010).
Figure 2.
Spine density in area CA1 of the hippocampus was unaffected by Reelin administration. All HRM mice received a bilateral ventricular injection of either mock (black) or Reelin (white). Brains were harvested 5 days post-injection and changes in spine density were determined in apical oblique (AO) and basal shaft (BS) dendrites in area CA1 of the hippocampus (n = 10 neurons/brain; 3 brains/group). (a) Representative AO and BS dendrites for both mock and Reelin-injected animals. There was no observed change in AO (b), BS (c) or total dendritic (d) spine density in area CA1 between Reelin or mock-injected animals.
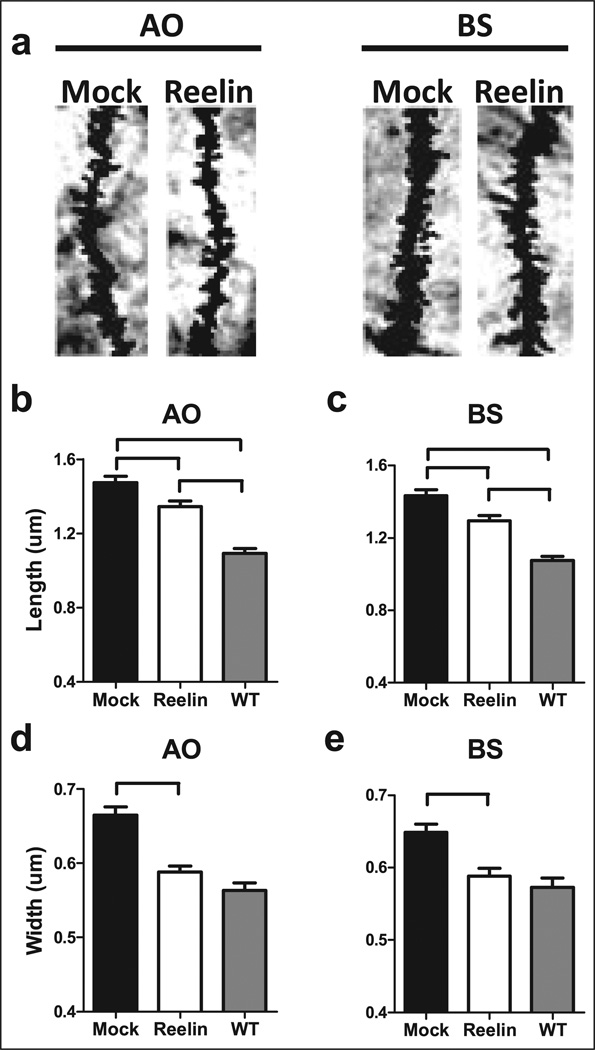
Changes in dendritic spine morphology may play a key role in synaptic plasticity. Live imaging studies reveal that spines are dynamic, changing length and width over a time period of seconds to days (Alvarez and Sabatini, 2007; Holtmaat and Svoboda, 2009). These changes in spine morphology are dependent on glutamate receptor activation and neuronal activity (Kasai et al., 2003; Matsuzaki et al., 2001). Furthermore, transient changes in spine morphology correlate with alterations in synaptic plasticity; specifically, enlargement of spine volume occurs after induction of LTP and reduced volume after induction of LTD (Desmond and Levy, 1988; Engert and Bonhoeffer, 1999; Maletic-Savatic et al., 1999; Zhou et al., 2004). We determined if Reelin injection altered the morphology of spines on CA1 pyramidal neurons in HRM. Reelin injection reduced the average spine length (Figure 3(a), 3(b); treatment/genotype, p<0.01, F(2,321) = 42.11) and width (Figure 3(c), treatment/genotype, p<0.01, F(2,320) = 38.09; 3(d), treatment/genotype, p<0.001, F(2,459) = 26.15) in both the AO and BS dendrites. Importantly, we found a full recovery of both AO and BS spine width, but only partial recovery of AO spine length in HRM with Reelin administration compared with wild-type littermate control mice (Supplementary data, Figure 2; cumulative percent representation). There are two novel findings from these experiments: 1) HRM had perturbed spine morphology; and 2) these morphological alterations were reversible with Reelin supplementation. Interestingly, mice that conditionally overexpress Reelin in the forebrain have hypertrophic spines with large mushroom-like heads, similar to the observed spine morphology of HRM we report, when compared with wild-type mice (Pujadas et al., 2010). When both studies are considered, these findings suggest that normal dendritic spine morphology is dependent upon optimal Reelin levels, with decreased or increased Reelin concentrations altering spine morphology away from the wild-type phenotype. Reelin co-localizes with integrin receptors at the dendritic post-synaptic densities in the cortex of non-human primates (Rodriguez et al., 2000). A decrease in co-localization of integrin receptors with Reelin may also contribute to the synaptic deficits observed in HRM. How Reelin levels and integrin interactions are determined and regulated will be explored in future studies.
Figure 3.
Dendritic spine morphology in area CA1 is altered toward the wild-type phenotype 5 days following a single injection of Reelin. All HRM mice received a single bilateral ventricular injection of either mock or Reelin. Brains were harvested 5 days post-injection and changes in both spine length and spine width were determined in apical oblique (AO) and basal shaft (BS) dendrites in area CA1 of the hippocampus (n = 10 neurons/brain; 3 brains/group). (a) Representative AO and BS dendrites for both mock and Reelin-injected animals. (b) HRM had increased spine length in AO dendrites compared with wild-type littermate control mice that received a bilateral ventricular injection of mock (black). Reelin-injected mice had longer AO spine length when compared with wild-type littermate control mice. Reelin decreased spine length in AO dendrites of HRM when compared with mock-injected mice. (c) HRM had increased spine length in BS dendrites compared with wild-type littermate control mice. Reelin-injected mice had longer spine length in BS dendrites when compared with wild-type littermate control mice. Reelin decreased spine length in BS dendrites of HRM compared with mock-injected animals. (d) HRM had increased spine width in AO dendrites compared with wild-type littermate control mice. A single injection of Reelin decreased spine width in AO dendrites of HRM compared with mock-injected. (e) HRM had increased spine width in BS dendrites compared with wild-type littermate control mice. Reelin decreased spine width in AO dendrites of HRM. Data are represented as means ± SEM.
HRM have alterations in short-term and long-term synaptic plasticity. Specifically, PTP, LTP and LTD in area CA1 of the hippocampus are impaired (Qiu et al., 2006). Since changes in spine morphology are intimately involved in synaptic plasticity, we determined if Reelin supplementation could recover synaptic plasticity deficits observed in HRM.
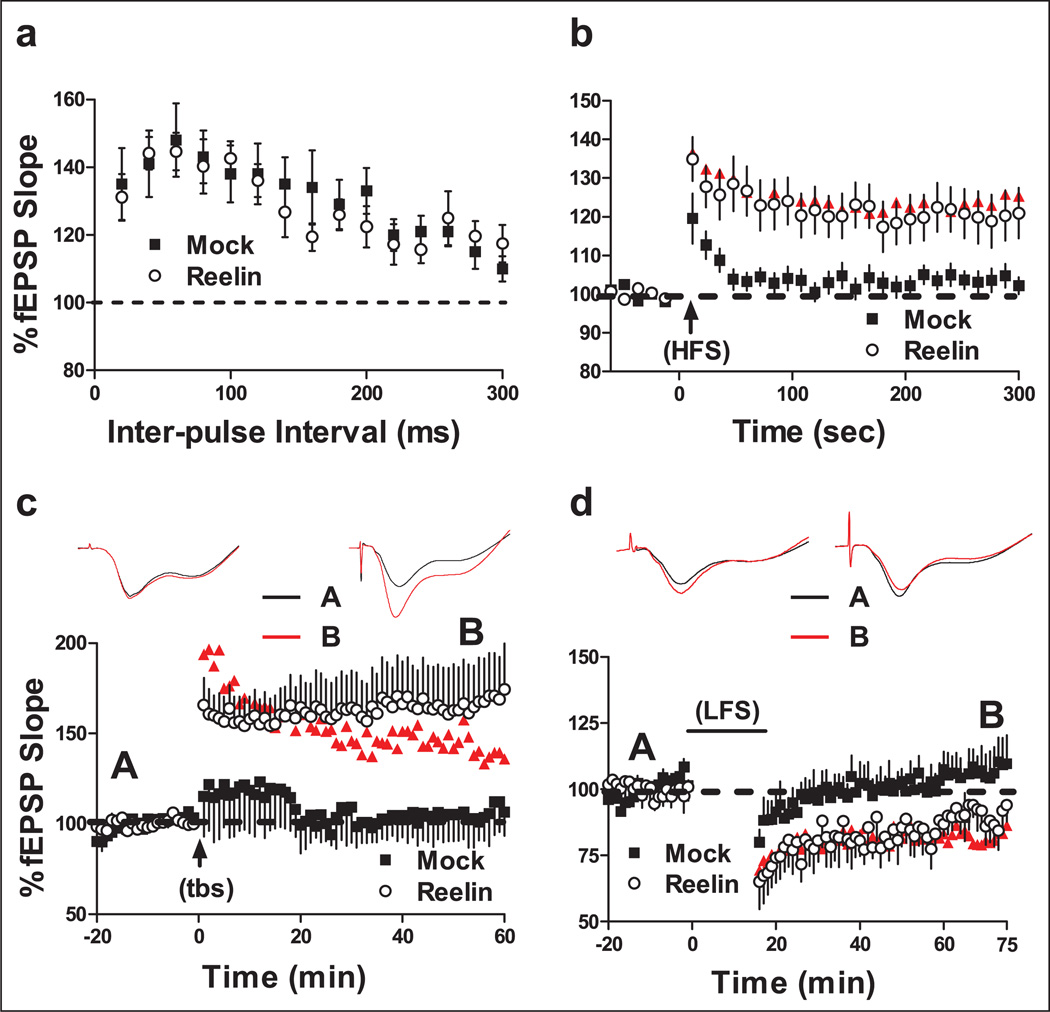
Proteins that control presynaptic neurotransmitter release are reduced in Reelin deficient mice. Expression of SNAP25, a protein component of the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex, is reduced in these mice (Hellwig et al., 2011). The decrease in SNAP25 expression is rescued with recombinant Reelin application, which is independent of ApoER2 and VLDLR, and is mediated through integrin signaling. PTP is a form of short-term plasticity that is associated with neurotransmitter release and is reduced in area CA1 of Reelin-deficient mice when compared with wild-type mice (Hellwig et al., 2011). These findings suggest that Reelin signaling can act presynaptically and alter neurotransmitter release. We determined if Reelin supplementation could recover impaired PTP, which is dependent on presynaptic function, in HRM. Examination of basal synaptic transmission revealed no significant differences of fEPSP slope in response to increased fiber volley amplitude (Supplementary data, Figure 3). PPF is a form of short-term plasticity that is dependent upon presynaptic calcium-induced calcium release, and is another measure of presynaptic function. There were also no significant differences observed in short-term plasticity evaluated by PPF (Figure 4(a)). However, Reelin injection significantly enhanced PTP induced by HFS (Figure 4(b)). In fact, PTP expression following Reelin injection was almost indistinguishable from PTP levels observed in wild-type littermate control mice (Figure 4(b), ANOVA p<0.01, F(2,47) = 4.76 mock vs. Reelin p<0.05, mock vs. wild-type p<0.05, Reelin vs. wild-type, p>0.05). Although it is widely accepted that PTP is dependent on presynaptic function, the underlying mechanisms are not well understood. The ability of Reelin to recover PTP further suggests that established deficits observed in HRM are recoverable with Reelin supplementation in the adult.
Figure 4.
Reelin recovered synaptic plasticity deficits found in HRM. Mice were sacrificed 5 days following a single bilateral ventricular injection of either mock (white) or Reelin (black) for extracellular recordings. Normal wild-type littermate controls observations are shown in red (n = 11). Changes in fEPSP slope are expressed as a percentage of baseline. (a) Paired-pulse facilitation (PPF) was induced with the use of paired- pulses given with an initial delay of 20 ms and the time to the second pulse was increased 20 ms incrementally until a final delay of 300 ms was reached. There was no significant difference in PPF between experimental groups. (b) Post-tetanic potentiation (PTP) was performed in the presence of 50 µM APV and was induced with HFS (1 sec, 100 Hz bursts; arrow) after 1 min of baseline recording. Reelin rescues the PTP deficit in hippocampi taken from HRM (n = 18) when compared with mock-injected mice (n = 17) in area CA1. (C) Long-term potentiation (LTP) was induced with theta-burst stimulation (5 bursts of 200 Hz separated by 200 ms, repeated six times with 10 s between the 6 trains; arrow) after 20 min of baseline recording. Inserts are representative fEPSP traces taken before (black) and after (red) theta-burst stimulation of either mock (left; n = 8) or Reelin (right; n = 10). Reelin enhanced LTP in HRM when compared with mock-injected mice. (D) Long-term depression (LTD) was induced with low-frequency stimulation (LFS) (2 bursts separated by 20 ms, given at 1 Hz for 15 min; bar) after 20 min of baseline recording. Inserts are representative fEPSP traces taken before (black) and after (red) LFS from hippocampi of HRM injected with either mock (left; n = 9) or Reelin (right; n = 10). Reelin injection significantly decreased LTD in area CA1 when compared with mock-injected mice.
We previously determined that Reelin perfusion significantly elevates LTP in acute hippocampal slices (Weeber et al., 2002). However, Reelin-dependent enhancement of LTP does not occur in VLDLR and ApoER2 knockout mice. Increased tyrosine phosphorylation of the NMDAR subunits in parallel with increased Ca2+ conductance through NMDARs is associated with Reelin-mediated enhancement of LTP (Beffert et al., 2005). HRM have reductions in both theta-burst stimulation-induced LTP and whole-cell pairing LTP in area CA1, both of which are NMDAR dependent (Qiu et al., 2006). Thus, we determined if LTP in HRM is altered following Reelin injection. Reelin supplementation enhanced LTP in area CA1 of HRM to levels observed in wildtype littermate control mice (Figure 4(c), ANOVA p<0.05, F(2,31) = 4.4; mock vs. Reelin p<0.05; mock vs. wild-type p>0.05; Reelin vs. wild-type p>0.05). These data indicate that the LTP deficit observed in HRM is not due to subtle neurodevelopmental abnormalities, but can be rescued through Reelin administration in the adult. However, long-term consequences of reduced Reelin during development cannot be completely excluded on other endpoints, which have not been specifically investigated here.
LTD is a form of inhibitory plasticity in the hippocampus. Reduced inhibition in the hippocampus is hypothesized to play a role in the etiology of schizophrenia and other neuropsychiatric disorders (Benes, 1999; Keverne, 1999). In agreement with this hypothesis, HRM have decreased LTD and sIPSCs in the hippocampus (Qiu et al., 2006). We determined if Reelin supplementation recovers the LTD deficits observed in HRM. We found that Reelin enhanced LTD in area CA1 of hippocampi from HRM to levels that resembled that of wild-type mice (Figure 4(d), ANOVA p<0.01, F(2,20) = 5.49; mock vs. Reelin p>0.05; mock vs. wild-type p<0.05; Reelin vs. wild-type p>0.05 ). The ability for Reelin to recover LTD deficits is in agreement with the above data suggesting that the morphological, biochemical and physiological deficits observed in HRM are capable of recovery.
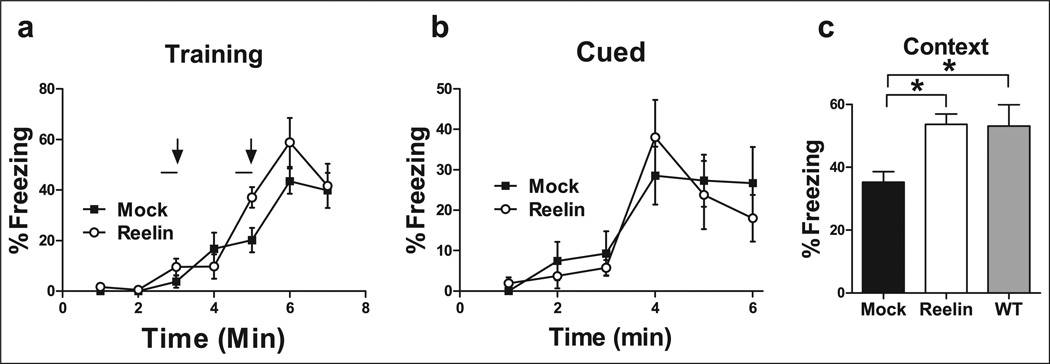
Our lab has previously established that HRM have a deficit in associative leaning when compared with wild-type mice (Qiu et al., 2006). Thus, we injected mice with Reelin and performed the identical behavioral test 5 days post injection. HRM were trained with an aversive US paired with an auditory CS within a novel environment. During training, both sets of animals showed similar levels of freezing after the presentation of the US (Figure 5(a)). Changes in fear memory to the cue were not altered in injected HRM (Figure 5(b)). To assess if increased Reelin levels alter contextual conditioning, HRM were placed back into the context 24 h following training. HRM that received a Reelin-injection had a significant enhancement of associative memory as demonstrated by increased freezing behavior (Figure 5(c), ANOVA p<0.01, F(2,23) = 5.35; mock vs. Reelin p<0.05; mock vs. wild-type p<0.05; Reelin vs. wild-type p>0.05). Taken together, our results reveal that a single Reelin injection can enhance contextual fear conditioned learning in HRM.
Figure 5.
Reelin recovers the associative learning deficit observed in HRM. HRM received a bilateral ventricular injection of either mock (black; n = 9) or Reelin (white; n = 9). HRM were trained with a standard 2-shock protocol (bars and arrows) 5 days post-injection. (a) There were no significant differences in freezing between experimental groups during training. (b) Freezing to the cue was assessed 24 h post-training. There were no significant differences in freezing to the cue between experimental groups. (c) Freezing to the context was assessed for 3 min 24 h post-training. Reelin supplementation increased the average context-dependent freezing in HRM to wild-type littermate control levels (grey; n = 8) when compared with mock-injected animals.
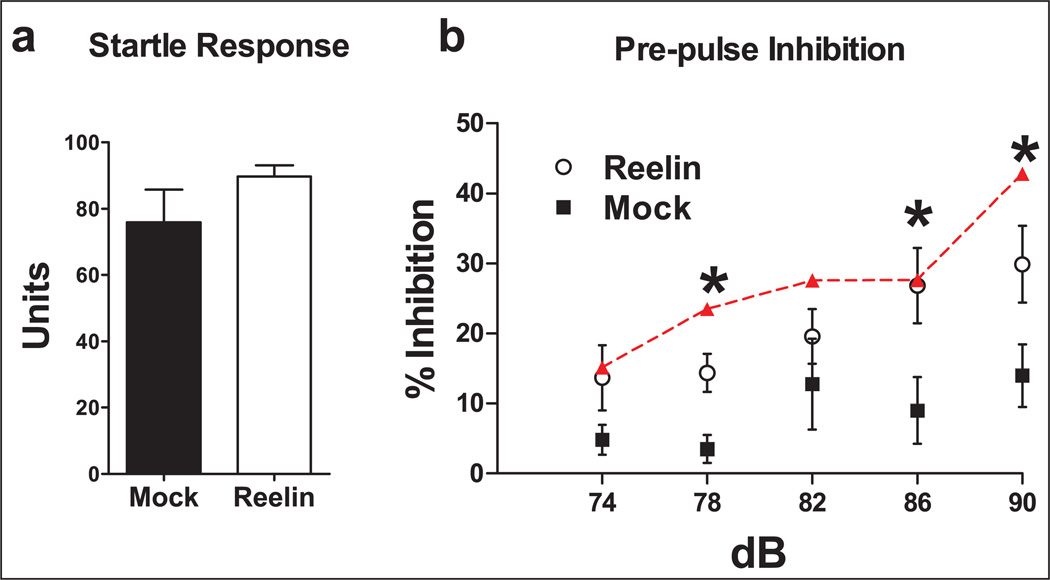
Schizophrenic patients have diminished sensorimotor gating as tested by acoustic PPI (Akbarian and Huang, 2006; Braff et al., 1992, 1999; Geyer and Braff, 1987 Geyer et al., 1990; Mcdowd et al., 1993). Previous research with HRM has revealed inconsistencies in certain behavioral phenotypes, particularly in PPI (Qiu et al., 2006; Salinger et al., 2003; Tueting et al., 1999). The inconsistencies between these studies may be attributed to differences in age, training or background strain. Previously, we have shown that HRM have decreased PPI (Qiu et al., 2006). We determined if Reelin supplementation could increase PPI in HRM 5 days postinjection. There were no significant differences in acoustic startle reflex between groups (Figure 6(a)). However, Reelin-injected mice showed a significant increase in the percent inhibition to 78, 86 and 90 dB pre-pulse (Figure 6(b), 78 ANOVA p<0.05, F(2,25) = 3.584; mock vs. Reelin p>0.05; mock vs. wild-type p<0.05; wildtype vs. Reelin p>0.05; 86 ANOVA p=0.05, F(2,25) = 3.266; mock vs. Reelin p>0.05; mock vs. wild-type p<0.05; wild-type vs. Reelin p>0.05; 90 ANOVA p<0.01 F(2,25) = 3.266; mock vs. Reelin p>0.05; mock vs. wild-type p<0.01; wild-type vs. Reelin p>0.05).
Figure 6.
Reelin rescues the sensorimotor gating deficit observed in HRM as tested by acoustic pre-pulse inhibition (PPI). Mice received a single bilateral ventricular injection of either mock (black; n = 10) or Reelin (white; n = 9). Normal wild-type littermate control observations are shown in red (n = 8). Acoustic startle and PPI to acoustic startle were given to determine sensorimotor gating ability 5 days post-injection. (a) There were no significant differences of acoustic startle reflex in HRM given a 120 dB pulse between experimental groups. (b) Reelin increased the percent inhibition to 78, 86 and 90 dB pre-pulse compared with mock-injected mice.
We demonstrate that a single in vivo injection of Reelin can recover biochemical, morphological and physiological deficits in adult HRM. Even more compelling is the recovery of HRM-related behavioral and cognitive impairments. Neuromorphological, biochemical and behavioral alterations occurring in HRM during development due to the reduction in Reelin levels are persistent and can result in long-term effects on the adult phenotype. However, these data suggest that deficits associated with both HRM and schizophrenic patients may be due to tonic reductions in Reelin signaling in the adult and not exclusively a carry-over result of neurodevelopmental abnormalities. Future therapeutic strategies for the treatment of schizophrenia may benefit by focusing on the Reelin signaling pathway.
Supplementary Material
Acknowledgments
Funding
This work was supported by the National Institute of Health (P01AG030128).
Footnotes
Conflict of interest
The authors declare that they do not have any conflict of interest.
References
- Abdolmaleky HM, Cheng KH, Russo A, et al. Hypermethylation of the Reelin (Reln) promoter in the brain of schizophrenic patients: a preliminary report. Am J Med Genet B Neuropsychiatr Genet. 2005;134B:60–66. doi: 10.1002/ajmg.b.30140. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Huang HS. Molecular and cellular mechanisms of altered Gad1/Gad67 expression in schizophrenia and related disorders. Brain Res Rev. 2006;52:293–304. doi: 10.1016/j.brainresrev.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci. 2007;30:79–97. doi: 10.1146/annurev.neuro.30.051606.094222. [DOI] [PubMed] [Google Scholar]
- Ammassari-Teule M, Sgobio C, Biamonte F, et al. Reelin haploinsufficiency reduces the density of Pv+ neurons in circumscribed regions of the striatum and selectively alters striatal-based behaviors. Psychopharmacology (Berl) 2009;204:511–521. doi: 10.1007/s00213-009-1483-x. [DOI] [PubMed] [Google Scholar]
- Andres C. Molecular genetics and animal models in autistic disorder. Brain Res Bull. 2002;57:109–119. doi: 10.1016/s0361-9230(01)00642-6. [DOI] [PubMed] [Google Scholar]
- Arnaud L, Ballif BA, Cooper JA. Regulation of protein tyrosine kinase signaling by substrate degradation during brain development. Mol Cell Biol. 2003;23:9293–9302. doi: 10.1128/MCB.23.24.9293-9302.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballif BA, Arnaud L, Cooper JA. Tyrosine phosphorylation of Disabled-1 is essential for reelin-stimulated activation of Akt and Src family kinases. Brain Res Mol Brain Res. 2003;117:152–159. doi: 10.1016/s0169-328x(03)00295-x. [DOI] [PubMed] [Google Scholar]
- Ballmaier M, Zoli M, Leo G, et al. Preferential alterations in the mesolimbic dopamine pathway of heterozygous reeler mice: an emerging animal-based model of schizophrenia. Eur J Neurosci. 2002;15:1197–1205. doi: 10.1046/j.1460-9568.2002.01952.x. [DOI] [PubMed] [Google Scholar]
- Beffert U, Durudas A, Weeber EJ, et al. Functional dissection of Reelin signaling by site-directed disruption of Disabled-1 adaptor binding to Apolipoprotein E Receptor 2: distinct roles in development and synaptic plasticity. J Neurosci. 2006;26:2041–2052. doi: 10.1523/JNEUROSCI.4566-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beffert U, Weeber EJ, Durudas A, et al. Modulation of synaptic plasticity and memory by Reelin involves differential splicing of the lipoprotein receptor ApoER2. Neuron. 2005;47:567–579. doi: 10.1016/j.neuron.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Benes FM. Evidence for altered trisynaptic circuitry in schizophrenic hippocampus. Biol Psychiatry. 1999;46:589–599. doi: 10.1016/s0006-3223(99)00136-5. [DOI] [PubMed] [Google Scholar]
- Botella-Lopez A, Burgaya F, Gavin R, et al. Reelin expression and glycosylation patterns are altered in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2006;103:5573–5578. doi: 10.1073/pnas.0601279103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL, Grillon C, Geyer MA. Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiatry. 1992;49:206–215. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- Braff DL, Swerdlow NR, Geyer MA. Symptom correlates of prepulse inhibition deficits in male schizophrenic patients. Am J Psychiatry. 1999;156:596–602. doi: 10.1176/ajp.156.4.596. [DOI] [PubMed] [Google Scholar]
- Chen Y, Sharma RP, Costa RH, et al. On the epigenetic regulation of the human Reelin promoter. Nucleic Acids Res. 2002;30:2930–2939. doi: 10.1093/nar/gkf401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J, Massaro CM, Palop JJ, et al. Reelin depletion in the entorhinal cortex of human amyloid precursor protein transgenic mice and humans with Alzheimer’s disease. J Neurosci. 2007;27:2727–2733. doi: 10.1523/JNEUROSCI.3758-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa E, Chen Y, Davis J, et al. Reelin and schizophrenia: a disease at the interface of the genome and the epigenome. Mol Interv. 2002a;2:47–57. doi: 10.1124/mi.2.1.47. [DOI] [PubMed] [Google Scholar]
- Costa E, Davis J, Pesold C, et al. The heterozygote reeler mouse as a model for the development of a new generation of antipsychotics. Curr Opin Pharmacol. 2002b;2:56–62. doi: 10.1016/s1471-4892(01)00121-7. [DOI] [PubMed] [Google Scholar]
- D’arcangelo G, Miao GG, Chen SC, et al. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- D’arcangelo G, Nakajima K, Miyata T, et al. Reelin is a secreted glycoprotein recognized by the Cr-50 monoclonal antibody. J Neurosci. 1997;17:23–31. doi: 10.1523/JNEUROSCI.17-01-00023.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond NL, Levy WB. Synaptic interface surface area increases with long-term potentiation in the hippocampal dentate gyrus. Brain Res. 1988;453:308–314. doi: 10.1016/0006-8993(88)90171-0. [DOI] [PubMed] [Google Scholar]
- Durakoglugil MS, Chen Y, White CL, et al. Reelin signaling antagonizes beta-amyloid at the synapse. Proc Natl Acad Sci U S A. 2009;106:15,938–15,943. doi: 10.1073/pnas.0908176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Braff DL. Startle habituation and sensorimotor gating in schizophrenia and related animal models. Schizophr Bull. 1987;13:643–668. doi: 10.1093/schbul/13.4.643. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Swerdlow NR, Mansbach RS, et al. Startle response models of sensorimotor gating and habituation deficits in schizophrenia. Brain Res Bull. 1990;25:485–498. doi: 10.1016/0361-9230(90)90241-q. [DOI] [PubMed] [Google Scholar]
- Grayson DR, Chen Y, Costa E, et al. The human reelin gene: transcription factors (+), repressors (−) and the methylation switch (+/−) in schizophrenia. Pharmacol Ther. 2006;111:272–286. doi: 10.1016/j.pharmthera.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Grayson DR, Chen Y, Dong E, et al. From trans-methylation to cytosine methylation: evolution of the methylation hypothesis of schizophrenia. Epigenetics. 2009;4:144–149. doi: 10.4161/epi.4.3.8534. [DOI] [PubMed] [Google Scholar]
- Gregorio SP, Sallet PC, Do KA, et al. Polymorphisms in genes involved in neurodevelopment may be associated with altered brain morphology in schizophrenia: preliminary evidence. Psychiatry Res. 2009;165:1–9. doi: 10.1016/j.psychres.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, et al. Decrease in Reelin and Glutamic Acid Decarboxylase67 (Gad67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Pesold C, Costa E. New neurochemical markers for psychosis: a working hypothesis of their operation. Neurochem Res. 2000;25:1207–1218. doi: 10.1023/a:1007635927069. [DOI] [PubMed] [Google Scholar]
- Hadj-Sahraoui N, Frederic F, Delhaye-Bouchaud N, et al. Gender effect on Purkinje cell loss in the cerebellum of the heterozygous reeler mouse. J Neurogenet. 1996;11:45–58. doi: 10.3109/01677069609107062. [DOI] [PubMed] [Google Scholar]
- Hanlon FM, Weisend MP, Hamilton DA, et al. Impairment on the hippocampal-dependent virtual morris water task in schizophrenia. Schizophr Res. 2006;87:67–80. doi: 10.1016/j.schres.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Hellwig S, Hack I, Kowalski J, et al. Role for Reelin in neurotransmitter release. J Neurosci. 2011;31:2352–2360. doi: 10.1523/JNEUROSCI.3984-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiesberger T, Trommsdorff M, Howell BW, et al. Direct binding of Reelin to Vldl receptor and ApoE receptor 2 induces tyrosine phosphorylation of Disabled-1 and modulates tau phosphorylation. Neuron. 1999;24:481–489. doi: 10.1016/s0896-6273(00)80861-2. [DOI] [PubMed] [Google Scholar]
- Hoe HS, Lee JY, Pak DT. Combinatorial morphogenesis of dendritic spines and filopodia by Spar and Alpha-Actinin2. Biochem Biophys Res Commun. 2009;384:55–60. doi: 10.1016/j.bbrc.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoe HS, Tran TS, Matsuoka Y, et al. Dab1 and Reelin effects on amyloid precursor protein and ApoE Receptor 2 trafficking and processing. J Biol Chem. 2006;281:35,176–35,185. doi: 10.1074/jbc.M602162200. [DOI] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- Impagnatiello F, Guidotti AR, Pesold C, et al. A decrease of Reelin expression as a putative vulnerability factor in schizophrenia. Proc Natl Acad Sci U S A. 1998;95:15,718–15,723. doi: 10.1073/pnas.95.26.15718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H, Matsuzaki M, Noguchi J, et al. Structure–stability–function relationships of dendritic spines. Trends Neurosci. 2003;26:360–368. doi: 10.1016/S0166-2236(03)00162-0. [DOI] [PubMed] [Google Scholar]
- Keverne EB. Gaba-ergic neurons and the neurobiology of schizophrenia and other psychoses. Brain Res Bull. 1999;48:467–473. doi: 10.1016/s0361-9230(99)00025-8. [DOI] [PubMed] [Google Scholar]
- Kubo K, Mikoshiba K, Nakajima K. Secreted Reelin molecules form homodimers. Neurosci Res. 2002;43:381–388. doi: 10.1016/s0168-0102(02)00068-8. [DOI] [PubMed] [Google Scholar]
- Lambert De Rouvroit C, Goffinet AM. The reeler mouse as a model of brain development. Adv Anat Embryol Cell Biol. 1998;150:1–106. [PubMed] [Google Scholar]
- Laviola G, Ognibene E, Romano E, et al. Gene–environment interaction during early development in the heterozygous reeler mouse: clues for modelling of major neurobehavioral syndromes. Neurosci Biobehav Rev. 2009;33:560–572. doi: 10.1016/j.neubiorev.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Liu WS, Pesold C, Rodriguez MA, et al. Down-regulation of dendritic spine and glutamic acid decarboxylase 67 expressions in the Reelin haploinsufficient heterozygous reeler mouse. Proc Natl Acad Sci U S A. 2001;98:3477–3482. doi: 10.1073/pnas.051614698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macri S, Biamonte F, Romano E, et al. Perseverative responding and neuroanatomical alterations in adult heterozygous reeler mice are mitigated by neonatal estrogen administration. Psychoneuroendocrinology. 2010;35:1374–1387. doi: 10.1016/j.psyneuen.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Maletic-Savatic M, Malinow R, Svoboda K. Rapid dendritic morphogenesis in Ca1 hippocampal dendrites induced by synaptic activity. Science. 1999;283:1923–1927. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- Maloku E, Covelo IR, Hanbauer I, et al. Lower number of cerebellar Purkinje neurons in psychosis is associated with reduced Reelin expression. Proc Natl Acad Sci U S A. 2010;107:4407–4411. doi: 10.1073/pnas.0914483107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Ellis-Davies GC, Nemoto T, et al. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal Ca1 pyramidal neurons. Nat Neurosci. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcdowd JM, Filion DL, Harris MJ, et al. Sensory gating and inhibitory function in late-life schizophrenia. Schizophr Bull. 1993;19:733–746. doi: 10.1093/schbul/19.4.733. [DOI] [PubMed] [Google Scholar]
- Niu S, Yabut O, D’arcangelo G. The Reelin signaling pathway promotes dendritic spine development in hippocampal neurons. J Neurosci. 2008;28:10,339–10,348. doi: 10.1523/JNEUROSCI.1917-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M, Miyata T, Nakajima K, et al. The Reeler gene-associated antigen on Cajal-Retzius neurons is a crucial molecule for laminar organization of cortical neurons. Neuron. 1995;14:899–912. doi: 10.1016/0896-6273(95)90329-1. [DOI] [PubMed] [Google Scholar]
- Ognibene E, Adriani W, Granstrem O, et al. Impulsivity– anxiety-related behavior and profiles of morphine-induced analgesia in heterozygous reeler mice. Brain Res. 2007;1131:173–180. doi: 10.1016/j.brainres.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Pappas GD, Kriho V, Pesold C. Reelin in the extracellular matrix and dendritic spines of the cortex and hippocampus: a comparison between wild type and heterozygous reeler mice by immunoelectron microscopy. J Neurocytol. 2001;30:413–425. doi: 10.1023/a:1015017710332. [DOI] [PubMed] [Google Scholar]
- Persico AM, Levitt P, Pimenta AF. Polymorphic Ggc repeat differentially regulates human Reelin gene expression levels. J Neural Transm. 2006;113:1373–1382. doi: 10.1007/s00702-006-0441-6. [DOI] [PubMed] [Google Scholar]
- Pesold C, Impagnatiello F, Pisu MG, et al. Reelin is preferentially expressed in neurons synthesizing gamma-aminobutyric acid in cortex and hippocampus of adult rats. Proc Natl Acad Sci U S A. 1998;95:3221–3226. doi: 10.1073/pnas.95.6.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesold C, Liu WS, Guidotti A, et al. Cortical bitufted, horizontal, and Martinotti cells preferentially express and secrete Reelin into perineuronal nets, nonsynaptically modulating gene expression. Proc Natl Acad Sci U S A. 1999;96:3217–3222. doi: 10.1073/pnas.96.6.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters MM, Hill KE, Burk RF, et al. Altered hippocampus synaptic function in Selenoprotein P deficient mice. Mol Neurodegener. 2006;1:12. doi: 10.1186/1750-1326-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujadas L, Gruart A, Bosch C, et al. Reelin regulates postnatal neurogenesis and enhances spine hypertrophy and long-term potentiation. J Neurosci. 2010;30:4636–4649. doi: 10.1523/JNEUROSCI.5284-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S, Korwek KM, Pratt-Davis AR, et al. Cognitive disruption and altered hippocampus synaptic function in Reelin haploinsufficient mice. Neurobiol Learn Mem. 2006;85:228–242. doi: 10.1016/j.nlm.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Qiu S, Zhao LF, Korwek KM, et al. Differential Reelin-induced enhancement of NMDA and AMPA receptor activity in the adult hippocampus. J Neurosci. 2006;26:12,943–12,955. doi: 10.1523/JNEUROSCI.2561-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez MA, Pesold C, Liu WS, et al. Colocalization of integrin receptors and Reelin in dendritic spine postsynaptic densities of adult nonhuman primate cortex. Proc Natl Acad Sci U S A. 2000;97:3550–3555. doi: 10.1073/pnas.050589797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JT, Rusiana I, Trotter J, et al. Reelin supplementation enhances cognitive ability, synaptic plasticity, and dendritic spine density. Learn Mem. 2011;18:558–564. doi: 10.1101/lm.2153511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JT, Weeber EJ. Reelin and ApoE actions on signal transduction, synaptic function and memory formation. Neuron Glia Biol. 2009;4:259–270. doi: 10.1017/S1740925X09990184. [DOI] [PubMed] [Google Scholar]
- Salinger WL, Ladrow P, Wheeler C. Behavioral phenotype of the reeler mutant mouse: effects of RELN gene dosage and social isolation. Behav Neurosci. 2003;117:1257–1275. doi: 10.1037/0735-7044.117.6.1257. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Mihalakos P, Chin R, et al. Learning and generalization in schizophrenia: effects of disease and antipsychotic drug treatment. Biol Psychiatry. 2010;67:926–932. doi: 10.1016/j.biopsych.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tueting P, Costa E, Dwivedi Y, et al. The phenotypic characteristics of heterozygous reeler mouse. Neuroreport. 1999;10:1329–1334. doi: 10.1097/00001756-199904260-00032. [DOI] [PubMed] [Google Scholar]
- Tueting P, Doueiri MS, Guidotti A, et al. Reelin down-regulation in mice and psychosis endophenotypes. Neurosci Biobehav Rev. 2006;30:1065–1077. doi: 10.1016/j.neubiorev.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Weeber EJ, Beffert U, Jones C, et al. Reelin and ApoE Receptors Cooperate to Enhance Hippocampal Synaptic Plasticity and Learning. J Biol Chem. 2002;277:39,944–39,952. doi: 10.1074/jbc.M205147200. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Homma KJ, Poo MM. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44:749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.