Abstract
Death ligands and their tumor necrosis factor receptor (TNFR) family receptors are the best-characterized and most efficient inducers of apoptotic signaling in somatic cells. In this study, we analyzed whether these prototypic activators of apoptosis are also expressed and able to be activated in human pluripotent stem cells. We examined human embryonic stem cells (hESC) and human-induced pluripotent stem cells (hiPSC) and found that both cell types express primarily TNF-related apoptosis-inducing ligand (TRAIL) receptors and TNFR1, but very low levels of Fas/CD95. We also found that although hESC and hiPSC contain all the proteins required for efficient induction and progression of extrinsic apoptotic signaling, they are resistant to TRAIL-induced apoptosis. However, both hESC and hiPSC can be sensitized to TRAIL-induced apoptosis by co-treatment with protein synthesis inhibitors such as the anti-leukemia drug homoharringtonine (HHT). HHT treatment led to suppression of cellular FLICE inhibitory protein (cFLIP) and Mcl-1 expression and, in combination with TRAIL, enhanced processing of caspase-8 and full activation of caspase-3. cFLIP likely represents an important regulatory node, as its shRNA-mediated down-regulation significantly sensitized hESC to TRAIL-induced apoptosis. Thus, we provide the first evidence that, irrespective of their origin, human pluripotent stem cells express canonical components of the extrinsic apoptotic system and on stress can activate death receptor-mediated apoptosis.
Introduction
Human embryonic stem cells (hESC) originating from the inner cell mass of human blastocysts and human-induced pluripotent stem cells (hiPSC) produced by forced reprogramming of somatic cells by gene expression represent two types of human pluripotent stem cells with tremendous potential in various biomedical applications, including cell therapy, disease modeling, and drug development [1–4]. Although these types of human pluripotent stem cells can indefinitely proliferate in culture, unlike transformed cancer cells, they are prone to demise by apoptosis [5–7]. Both hESC and hiPSC express, and if necessary also employ, key canonical components and regulators of apoptotic signaling [8,9]. DNA damage, ectopic expression of oncogenes such as c-Myc, heat shock, viral infection, or even cell dissociation can trigger intrinsic apoptotic signaling that is largely dependent on pro-apoptotic proteins from the Bcl-2 family [5–7,10–12]. However, hESC and hiPSC can be at least partially protected against stress-induced apoptosis by a number of treatment modalities, such as addition of growth factors and/or inhibitors of ROCK kinase to culture media or by ectopic expression of anti-apoptotic Bcl-2 proteins [13–18]. Another level of anti-apoptotic protection in hESC involves increased expression of survivin, an anti-apoptotic member of the inhibitor of apoptosis (IAP) family that also contributes to teratoma formation [19,20]. In summary, elements of the intrinsic apoptotic pathway are clearly active in both hESC and hiPSC and are employed to regulate their homeostasis.
In addition, in virtually all somatic cells, apoptosis can also be mediated by the extrinsic pathway that is triggered by so-called death ligands from the tumor necrosis factor (TNF) family [TNFα, FasL, and TNF-related apoptosis-inducing ligand (TRAIL)] and their corresponding death receptors present on the cell surface [21,22]. Apoptotic signaling from death receptors relies on ligand-triggered clustering of receptors via their intracellular protein–protein interaction region called the death domain, followed by formation of the Death-Inducing Signaling Complex (DISC), a multiprotein platform that is critical for the proximity-based auto-processing and activation of the main initiator caspase-8 (recently reviewed in [23,24]). Activated caspase-8, and in some cases also caspase-10, then cleaves its cellular targets, most notably the effector caspase-3, the mitochondrial apoptotic signaling activator Bid (into truncated Bid or tBid), and the caspase-8 antagonist cellular FLICE inhibitory protein (cFLIP), resulting in cleavage of poly (ADP-ribose) polymerase (PARP), a well-established marker of ongoing apoptosis [25,26]. In addition to caspase-dependent apoptosis, under certain circumstances, death receptors can trigger a specific receptor-interacting protein (RIP)1/RIP3-dependent form of programmed necrosis called necroptosis [27,28]. Importantly, normal mesenchymal stem cells, progenitor cells, and terminally differentiated cells are resistant to death receptor-induced pro-death signaling [29–31]. In these cells, ligand-activated receptors may induce a number of other signaling events, for example, activation of the canonical NFκB pathway, mitogen-activated protein (MAP) and stress kinases, and the P3K/Akt axis, and can even enhance macroautophagy [32–34]. Considering the ultimate outcome of death receptor-induced pro-apoptotic signaling, both its initial and follow-up steps should be delicately regulated. At the proximal DISC node, expression levels of the caspase-8 antagonist cFLIP and also the efficacy of caspase-8 clustering and its stability have a pronounced impact on the robustness of pro-apoptotic signaling from the activated Fas/CD95 or TRAIL receptors [35–39]. More distally, efficient activation of effector caspases can be blunted at the mitochondria by blocking tBid-mediated amplification of apoptotic signaling or by competitive inhibitors of activated caspases from the IAP family [20,40,41].
As indicated earlier, in general, only damaged, transformed, or unneeded cells are induced to undergo apoptosis by death ligands, and TRAIL was brought to the forefront for its potential use in anti-tumor therapy [42,43]. hESC, and particularly hiPSC, may possess and/or develop characteristics that are typical of damaged or transformed cells. Though the human pluripotent stem cells express all canonical components of the extrinsic apoptotic signaling, they are, as we document, resistant to TRAIL. However, we show that on stress such as proteo-synthesis inhibition, both hESC and hiPSC become sensitized to TRAIL-induced apoptosis and we point to cFLIP as an essential molecule conferring TRAIL resistance in hESC.
Materials and Methods
Cultivation and treatment of hESC and hiPSC
Two hESC lines (CCTL 12 and CCTL 14) [44] between passages 25–80, and one hiPSC cell line (clone 4) [45] between passages 50–80, were used for these experiments. Colonies of hESC and hiPSC were either co-cultivated with mitotically inactivated mouse embryonic fibroblasts (MEFs; mouse strain-CF1; density 24,000 cells/cm2) as previously described [46] or grown on an MEF-derived extracellular matrix (ECM). Cultivation on ECM required MEF-conditioned hESC medium (CM) and was used during various treatments of the stem cells to avoid the bystander effect of MEFs.
ECM was prepared as follows: MEFs (mouse strain CF1, density 24,000 cells/cm2) were seeded on gelatin-coated dishes and grown for 5 days. Cells were then lysed on the plates by 0.5% deoxycholate in 10 mM Tris-HCl, pH 8.0, and washed five times with phosphate-buffered saline (PBS) without Mg2+ and Ca2+ (pH 7.4) at 4°C. Isolated ECM was stored in PBS at 4°C for approximately 1 week. For preparation of MEF-conditioned hESC medium, hESC medium was incubated with mitotically inactivated MEFs for 24 h, collected, supplemented with 10 ng/mL FGF2 (#100-18B; Pepro Tech) and 2 mM L-glutamine (#25030-24; GIBCO), filtered, and stored for approximately 1 week at 4°C.
For treatment with human recombinant TRAIL (P-008; Apronex), homoharringtonine (HHT) (H0635; Sigma-Aldrich), pan caspase inhibitor Z-VAD-FMK (C2105; Sigma-Aldrich), caspase-8 inhibitor Z-IETD-FMK (550380; BD Pharmingen), cycloheximide (CHX) (C1988; Sigma-Aldrich), and necrostatin-1 (N9037; Sigma-Aldrich), hESC or hiPSC were allowed to grow on ECM in CM medium for 3–5 days after passage. The medium was changed 24 h before treatment, and the appropriate reagents were added to the media at the desired concentrations. To harvest the treated cells, colonies were washed with PBS and incubated for 2 min with 0.5 mM EDTA in PBS at 37°C. Cells were detached from the surface by gentle pipetting and collected in cold PBS. For detection of apoptosis, both adherent and floating cells were collected.
Flow cytometric analysis of death receptor expression
Adherent cells were harvested and incubated in PBS containing 20% human AB serum (Faculty Hospital, Brno, Czech Republic) and 0.2% cold water fish gelatin (G7765; Sigma-Aldrich) for 10 min. Cells were then washed in PBS-G buffer (PBS+0.2% cold fish gelatin and 0.1% NaN3) and incubated on ice for 30 min with primary antibodies specific for DR4 (DR-4-02; Exbio Praha), DR5 (DR5-01-1; Exbio Praha), DcR1 (HS301; Enzo LS), DcR2 (HS402; Enzo LS), TNFR1 (#16803; RnD systems), and Fas (DX2; ENZO LS), or isotype control (P3×, kindly provided by Prof. Peter W. Andrews [Department of Biomedical Science, University of Sheffield]). Cells were then washed twice with PBS-G and incubated with R-phycoerythrin-conjugated secondary antibody (1070-09; Southern Biotech) for 30 min on ice. Cells were washed twice with PBS-G, and receptor expression was analyzed by flow cytometry (FACS Canto II; BD Biosciences). We analyzed 20,000–30,000 cells for each sample. The level of receptor expression was expressed as the ratio of the median fluorescence index (MFI) of specific antibody to the MFI of isotype-stained control using FlowJo software (www.flowjo.com).
Detection and quantification of apoptosis and cell death by flow cytometry
Floating and adherent cells were harvested, washed with PBS, and permeabilized in 90% methanol for 30 min at 4°C. Cells were washed in incubation buffer (0.5% BSA in PBS) and then incubated with the incubation buffer containing RNAse-A (0.02 mg/mL) (Boehringer) at 37°C for 30 min. After washing with incubation buffer, cells were incubated with a primary antibody that was specific for the cleaved form of PARP (#9541; Cell Signaling Technologies) at room temperature for 1 h. Cells were then washed twice in the incubation buffer and incubated with secondary Alexa Fluor 488-conjugated antibody (A11008; Invitrogen) and propidium iodide in the dark at room temperature for 30 min to allow simultaneous detection of DNA content and the protein of interest. The fluorescence intensity of the population of mononuclear cells (gated using FSC, SSC) was assessed by flow cytometry (FACS Canto II; BD Biosciences). Cell death was assessed by DNA content analysis, by measuring the population of subG1 DNA content. Apoptosis induction was determined as the percentage of cells positive for the cleaved form of PARP. More than 10,000 cells were analyzed for each sample using FlowJo software. Specificity of the antibody recognizing the cleaved form of PARP was verified using western blotting (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/scd).
Western blotting
Harvested cells were washed thrice with PBS, lysed in lysis buffer [50 mM Tris-HCl (pH 6.8), 1% sodium dodecyl sulfate (SDS), and 10% glycerol], adjusted to a protein concentration of 1 mg/mL, and stored at −70°C until use. Western blot analysis was performed as previously described [46]. The following primary antibodies were used: caspase-3 (#9662), caspase-8 (#9746), phosphorylated NFκB p65 (Ser536) (#3033), phosphorylated Erk1/2 (MAPK) (Thr202/Tyr204) (#9101), Bid (#2002), Bcl-xL (#2764) (all from Cell Signaling Technologies), Caspase-10 (#M059-3; MBL International Corporation), FADD (#556402; BD Pharmigen), cFLIP (alx-804–428; Alexis), Bax (2281-MC-100; Trevigen), Mcl-1 (M8434; Sigma-Aldrich), and XIAP (48-hILP-XIAP; BD Transduction Laboratories). A protein ladder was used to identify the molecular weights of the analyzed proteins (#26619; Thermo Scientific).
Preparation of hESC with down-regulated expression of cFLIP or Mcl-1
HEK293T cells were transfected with the lentivirus packaging plasmids pMD2G, psPAX (Addgene), and pLKO.1 expressing short hairpin RNA (shRNA) against cFLIP (TRCN0000007229, shFLIP1; TRCN0000007230, shFLIP2), Mcl-1 (TRCN0000005515, shMcl-1-2; TRCN0000005517, shMcl-1-4; TRCN0000005518, shMcl-1-5), or nontargeting shRNA SHC002 (all from Sigma-Aldrich). After 2 days, the lentiviral particles were purified from the supernatant using PEG/it Virus Precipitation Solution (LV810A-1, SBI). The hESC (CCTL14) were transduced at a multiplicity of infection (MOI) of 5, selected in medium containing puromycin (3 μg/mL) for 4 days, and analyzed by western blotting for expression of the target gene protein. Survival, expression of pluripotency markers (SSEA-3, SSEA-4, and TRA 2–54), and expression of TRAIL receptors were monitored for approximately 20 passages and were identical to wild-type cells. The cells in passages 5–15 were used for all analyses.
Statistical analysis of FACS data
Data obtained for MFIs and the frequencies of apoptotic cells containing cleaved PARP are presented as means±standard error of means (SEM). Each bar represents at least three independent experiments. Statistical significance of the data was assessed by Student's unpaired t-test using Graph Pad Prism. Values of *p<0.05 were considered significant, **p<0.01 very significant, and ***p<0.001 extremely significant.
Results
Human pluripotent stem cells express TRAIL receptors, but are resistant to TRAIL-induced apoptosis
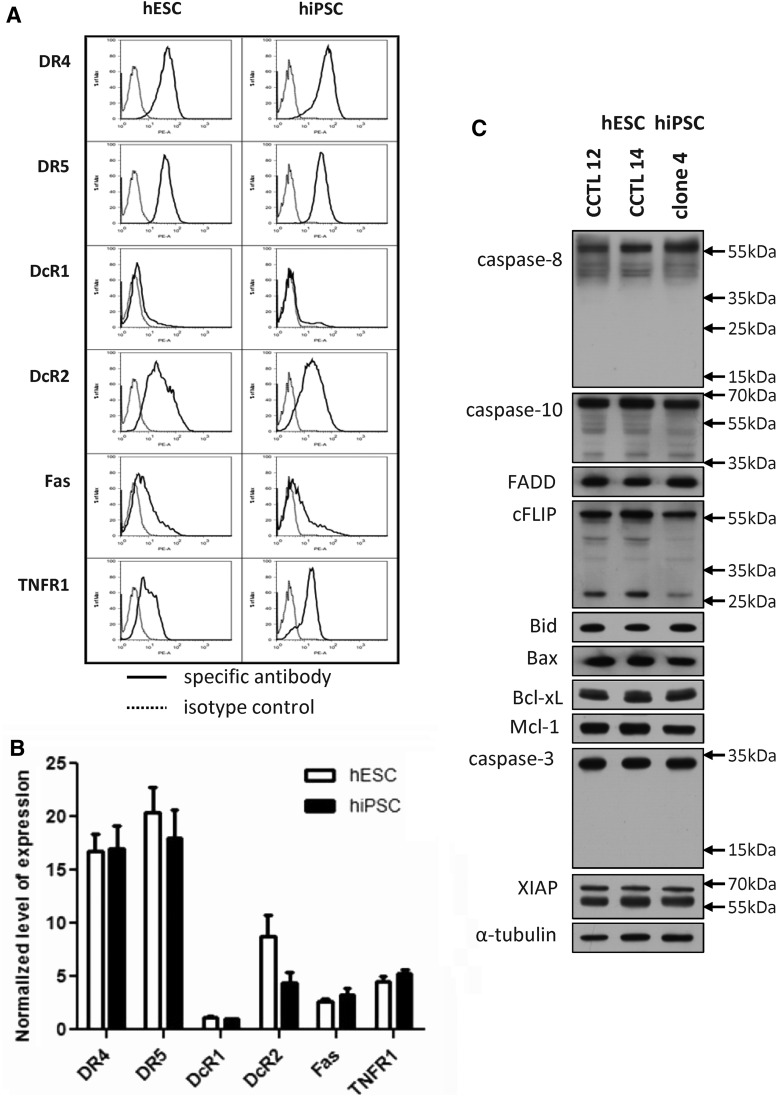
Death receptors from the TNFR family, namely TNFR1, Fas/CD95, and the death domain-containing TRAIL receptors TRAIL-R1/DR4 and TRAIL-R2/DR5, are major inducers of both extrinsic apoptosis and necroptosis in mammalian cells, and their expression levels and regulation of their pro-apoptotic activation represent a fine balance between cell survival and death. Differentiated and somatic stem cells and their progenitors are known to express various levels of these receptors, but no such information is available for human pluripotent stem cells.
In our initial experiments, we measured cell surface expression of these receptors in two hESC lines and one hiPSC line. Representative histograms for hESC (line CCTL14) and hiPSC (clone 4) are shown in Fig. 1A. Figure 1B shows the average of normalized median fluorescence intensity (MFI) obtained from at least three independent repeats for each cell line. hESC and hiPSC exhibited the same pattern of receptor expression, with relatively strong expression of both pro-apoptotic TRAIL receptors, lower expression of TNFR1, and minimal to no expression of the Fas/CD95 receptor. Among inhibitory TRAIL decoy receptors, only TRAIL-R4/DcR2 was significantly expressed on both hESC and hiPSC (Fig. 1A, B). Expression of mRNAs for the respective receptors as determined by qRT PCR (data not shown) was in full consonance with the flow cytometry findings cited earlier. The expression of death receptors is a key prerequisite for effective apoptotic signaling, but the DISC-forming proteins and other down-stream molecules are also required. Figure 1C shows that both hESC and hiPSC expressed significant quantities of DISC components: the adapter protein FADD, the initiator caspases 8 and 10, and their antagonist cFLIP. Similarly, both cell types expressed the BH3-only protein Bid, which transduces pro-apoptotic death receptor signaling to mitochondria, effector caspase-3, and pro- and anti-apoptotic members of the Bcl-2 family (Fig. 1C). It should be noted that levels of initiator caspase-8 and -10 in hESC and hiPSC were similar to those in cultured adult human dermal fibroblasts (AHDF), whereas levels of anti-apoptotic Bcl-2 family proteins and cFLIP were lower (data not shown).
FIG. 1.
Expression of extrinsic apoptotic pathway components in human embryonic stem cells (hESC) and human-induced pluripotent stem cells (hiPSC). (A) Representative histograms of death receptor expression in hESC (line CCTL14) and hiPSC (clone 4) as determined by flow cytometry. Solid line, specific antibody; dashed line, isotype control. (B) Quantification of levels of expression of death receptors in hESC (CCTL 14) and hiPSC (CCTL12) as determined by flow cytometry. Medians of fluorescent intensity were normalized to the isotype control. Each bar represents the mean of n>3 experiments. Error bars show standard error of mean (SEM). (C) Western blot analysis of the expression of intracellular components of the extrinsic apoptotic pathway and selected regulators of apoptosis in two hESC cell lines (CCTL12, CCTL14) and one hiPSC line (clone 4). Alpha tubulin was used as a loading control.
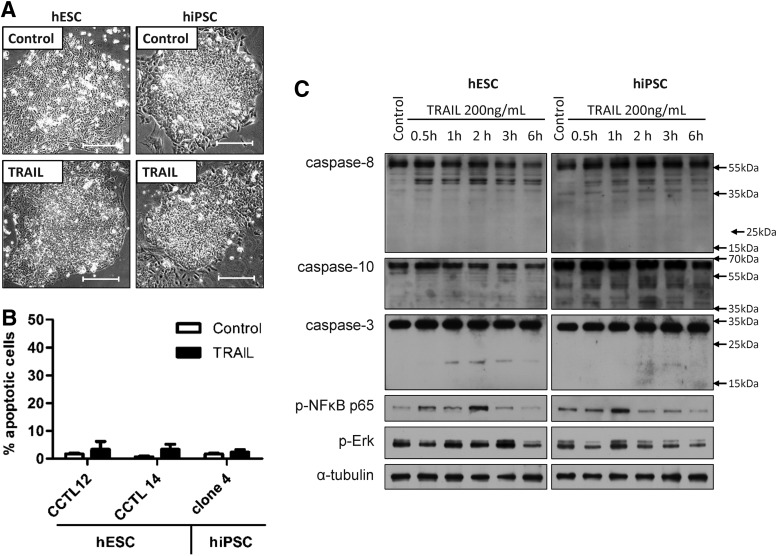
To evaluate the functionality of the extrinsic apoptotic pathway, we first exposed hESC and hiPSC to a wide range of concentrations (0–1 μg/mL) of human recombinant TRAIL for 24 h. Both cell types seemed to be refractory to even the highest concentrations of TRAIL, as demonstrated by their unchanged morphology (data not shown). For further experiments, we used a single concentration of TRAIL (200 ng/mL), which was able to induce massive apoptosis in TRAIL-sensitive cells such as colorectal cancer cell lines DLD-1 or Colo206F after 6 h of treatment [47]. This 6-h treatment did not evoke apoptosis in either cell type, as determined by the unchanged cell morphology (Fig. 2A) and the absence of cleavage of poly (ADP-ribose) polymerase (PARP) (Fig. 2B). This was also true when exposure to TRAIL was prolonged to 24 and 48 h (Supplementary Fig. S2). However, the activation of TRAIL receptors in the first hours of treatment seemed to be partially functional, because in TRAIL-treated cells, the initiator caspase-8 became preactivated and was cleaved to its p43/41 interforms. We also observed caspase-3 cleavage to the p20 preactive fragment, but the p16 and p18 active fragments were not produced (Fig. 2C). In addition to the partial processing of caspases, we also observed time-dependent changes in phosphorylation of the p65 subunit of NFκB and MAP kinases Erk1/2, indicating that nonapoptotic signaling from the activated TRAIL receptors is functional in human pluripotent stem cells (Fig. 2C).
FIG. 2.
TRAIL does not induce apoptosis in hESC and hiPSC. The hESC and hiPSC were treated with 200 ng/mL human recombinant TRAIL (TRAIL) or left untreated (control). (A) The morphology of colonies and cell detachment were observed by light microscopy after 24 h of treatment. Bar, 200 μm. (B) Induction of apoptosis was determined by staining with an antibody that was specific to cleaved poly (ADP-ribose) polymerase (PARP). The percentage of cells that stained positive for cleaved PARP (% apoptotic cells) in control and TRAIL-treated cells after 6 h of treatment was determined by flow cytometry. Each bar represents the mean of n=3 experiments; error bars show±SEM. (C) Western blot analysis of activation of the extrinsic apoptotic pathway, NFκB pathway, MAPK/ERK pathway, and caspase 3 after 30 min and 1, 2, 3, and 6 h of TRAIL treatment. Alpha tubulin was used as a loading control.
The protein synthesis inhibitor HHT sensitizes human pluripotent stem cells to TRAIL-mediated apoptosis
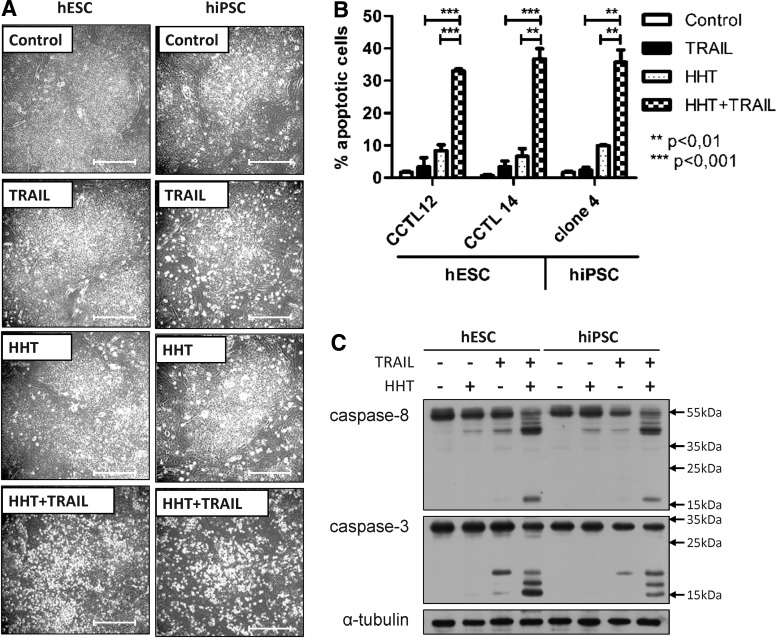
TRAIL-induced apoptosis can be regulated at several levels: the activated receptors, the mitochondria, and postmitochondrial signaling. Most nontransformed cells can be sensitized to TRAIL-induced apoptosis by various agents that, in principle, bring down either one or several of the safeguards and/or shift the cell status toward apoptosis. We recently discovered that the natural alkaloid HHT, which inhibits translation, is such a sensitizer [48]. When applied to hESC and hiPSC, HHT alone had almost no effect on cell survival as evidenced by no change in cell morphology (Fig. 3A) and only a minimal increase in the proportion of cells containing cleaved PARP (Fig. 3B), similar to the effects of TRAIL. However, both parameters of ongoing apoptosis, cell detachment, and number of cells with cleaved PARP increased dramatically when these compounds were applied in combination (Fig. 3A, B). In full consonance with these findings, western blot analysis revealed that, although individually, TRAIL and HHT induce detectable processing of the initiator caspase-8 to the p43/41 interforms and the effector caspase-3 to its p20 interform, and only co-treatment unleashes full activation of caspase-8 (monitored by its cleaved p18 form) and caspase-3 (monitored by active p18 and p16 forms) (Fig. 3C). Since one of the previously observed effects of HHT is inhibition of protein synthesis [48], we repeated the same set of analyses in cells, in which protein synthesis was inhibited by the classic protein inhibitor, CHX. The resulting data that are summarized in Supplementary Fig. S3A–C fully match those obtained with HHT.
FIG. 3.
The protein-synthesis inhibitor homoharringtonine (HHT) sensitizes hESC and hiPSC to TRAIL-induced apoptosis. HESC and hiPSC were left untreated (control), treated with 50 nM HHT or 200 ng/mL human recombinant TRAIL, or pretreated with 50 nM HHT for 1 h followed by 200 ng/mL human recombinant TRAIL treatment (HHT+TRAIL). (A) The morphology of colonies and cell detachment of control, HHT-, TRAIL-, or HHT+TRAIL-treated cells was observed by light microscopy after 3 h of treatment. Bar, 200 μm. (B) Induction of apoptosis was determined by staining with an antibody that was specific to cleaved PARP. The percentage of cells that stained positive for cleaved PARP (% apoptotic cells) in control, HHT-, TRAIL-, and HHT+TRAIL-treated cells after 6 h of treatment was determined by flow cytometry. Each bar represents the mean of n=3 experiments; error bars show±SEM. (C) Western blot analysis of activation of the extrinsic apoptotic pathway in control, HHT-, TRAIL-, and HHT+TRAIL-treated cells after 3 h of treatment. Alpha tubulin was used as a loading control.
It is of note that both the pancaspase inhibitor Z-VAD-FMK and the specific caspase-8 inhibitor Z-IETD-FMK suppressed cell death (with Z-IETD-FMK being less efficient) in HHT and TRAIL co-treated hESC, indicating its dependence on caspase-8 (Supplementary Fig. S4A, B). At the molecular level, treatment with both caspase inhibitors led to reduced cleavage of initiator caspase-8 to its p43/41 interforms and abolished production of its p18 form. Cleavage of caspase-3 to its active p18 and p16 forms was also significantly reduced (Supplementary Fig. S4C), indicating that execution of cell death induced by TRAIL in HHT-sensitized cells is caspase-8 dependent.
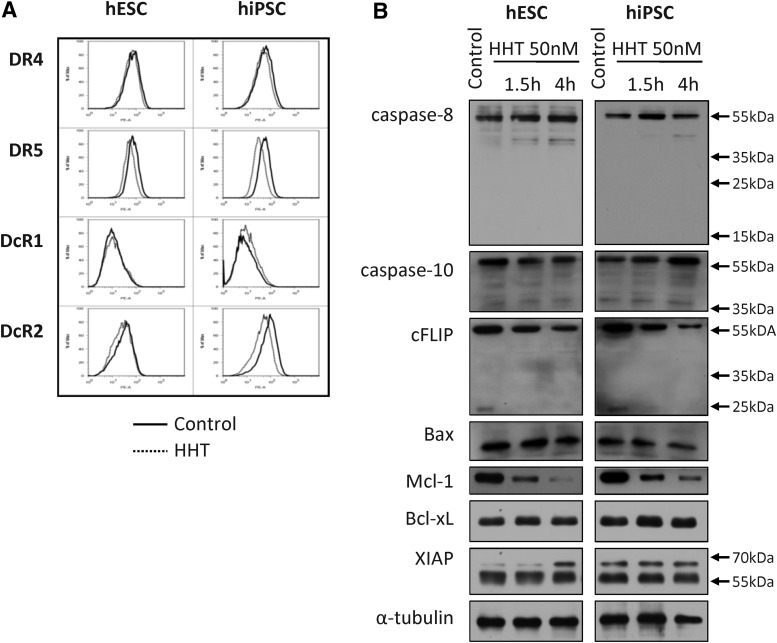
Taken together, our findings demonstrate that both types of human pluripotent stem cells can be made sensitive to TRAIL-induced apoptosis by a single compound, a protein synthesis inhibitor. We took advantage of this observation to probe for factors that inhibit the extrinsic pathway under normal conditions. We first analyzed whether HHT treatment affected cell surface expression of the TRAIL receptors. As shown in Fig. 4A, exposure of both hESC and hiPSC to 50 nM HHT for 4 h resulted in subtle changes to TRAIL receptor expression with only weak up-regulation of DR5 being reproducible. Next, we examined whether HHT treatment modified the quantity of major apoptosis-activating or apoptosis-regulating proteins expressed in hESC or hiPSC. Treatment of the cells with 50 nM HHT for approximately 4 h did not change the expression of major initiator caspases-8 and -10, pro- and anti-apoptotic proteins Bax and Bcl-xL, and the caspase-3/-9 competitive inhibitor XIAP (Fig. 4B). On the other hand, in both hESC and hiPSC, HHT treatment led to rapid and strong down-regulation of Mcl-1, an important anti-apoptotic member of the Bcl-2 family, as well as a less pronounced, but reproducible, suppression of cFLIP-L expression (Fig. 4B). Importantly, the potent short anti-apoptotic variant of cFLIP (cFLIP-S) was not detected after 1.5 h from the start of HHT treatment (Fig. 4B). It is of note that down-regulation of cFLIP and Mcl-1 was also achieved in cells exposed to CHX instead of HHT (Supplementary Fig. S3D).
FIG. 4.
Inhibition of protein synthesis in hESC and hiPSC rapidly reduces levels of endogenous cFLIP and Mcl-1. The hESC (CCTL 14) and hiPSC (clone 4) cells were left untreated (control) or treated with 50 nM HHT for 1.5 and 4 h. (A) The expression of TRAIL receptors in untreated (control, solid line) and HHT-treated (HHT, dashed line) hESC and hiPSC after 4 h of treatment was compared by flow cytometry. Representative histograms of three independent experiments are shown. (B) Western blot analysis of the expression of extrinsic apoptotic pathway components in control and HHT-treated cells (1.5 and 4 h). Alpha tubulin was used as a loading control.
cFLIP represents a safeguard protecting human pluripotent stem cells against TRAIL-induced apoptosis
The data obtained from sensitization experiments indicated that a small number of molecular players, namely cFLIP and Mcl-1, might underlie the resistance of hESC and hiPSC to activators of the external apoptotic pathway. To address the significance of cFLIP and Mcl-1 as regulatory elements, we generated recombinant lentiviruses expressing shRNA specific to each of these proteins and prepared clones of transduced hESC from the CCTL14 cell line. However, on selection with puromycin and subsequent analysis of target protein expression, we noticed that we could effectively down-regulate expression of cFLIP but not Mcl-1 (Fig. 5A and data not shown). Although we tested three different shRNAs targeting Mcl-1, the surviving cells showed either no change or only a very modest decrease in Mcl-1 protein expression.
FIG. 5.
Knock-down of cFLIP sensitizes hESC to TRAIL-induced apoptosis. Stable cell lines were established following transduction of the hESC line CCTL14 with a lentivirus expressing nontargeting, nonsilencing shRNA (NS shRNA) or shRNA targeting cFLIP mRNA (shFLIP1). (A) Western blot showing a decrease in cFLIP levels in shFLIP1-transduced cells compared with NS shRNA-transduced cells. Alpha tubulin was used as a loading control. (B) NS shRNA and shFLIP1 cells were left untreated (control) or treated with 200 ng/mL TRAIL. The morphology of colonies and cell detachment were observed by light microscopy after 6 h of treatment. (C) Nontransduced (WT), NS shRNA-transduced, and shFLIP1-transduced cells were left untreated (control) or treated with 200 ng/mL TRAIL for 6 h. Induction of apoptosis was determined as in Figure 3B. Each bar represents the mean of n=3 experiments, error bars show±SEM. (D) Western blot analysis of the activation of the extrinsic apoptotic pathway in NS shRNA and shFLIP1-transduced cells after 30 min and 1, 2, 3, and 6 h of treatment with 200 ng/mL TRAIL. Alpha tubulin was used as a loading control.
Cells with down-regulated cFLIP expression (achieved using two different shRNA-expressing lentiviruses) and mock-transduced cells expressing nontargeting shRNA retained their basic stem cell phenotype and were used for all subsequent experiments. Initial experiments analyzed the behavior of the mock- and shFLIP-transduced cells after treatment with TRAIL by light microscopy. Untreated cells, TRAIL-treated, and mock-transduced cells appeared normal without any significant signs of cell death (Fig. 5B). In sharp contrast, cultures of hESC with knocked-down cFLIP (by either of the two shRNAs, only shFLIP1 is shown) typically showed massive cell detachment, clearly indicating execution of the apoptotic process (Fig. 5B). Consistent with the morphological observations, quantification of this phenomenon using an antibody against cleaved PARP revealed a significant increase in apoptotic cells from approximately 2%–3% in untreated cells to 25%–30% in TRAIL-treated cells with down-regulated expression of cFLIP (Fig. 5C).The number of cells positive for cleaved PARP also increased by approximately 10% in TRAIL-treated mock-transduced cells. In TRAIL-treated cells with down-regulated expression of cFLIP, but not in mock-transduced cells, we also observed pronounced cleavage of initiator caspase-8 and -10, as well as effector caspase-3 (Fig. 5D).
To further investigate the functionality of such caspase cleavage, we exposed these cells to a combination of TRAIL with pan caspase inhibitor Z-VAD-FMK and caspase-8 inhibitor Z-IETD-FMK, respectively. As shown in Supplementary Fig. S5, both inhibitors significantly reduced apoptosis and cell death, in accordance with reduced activation of initiator caspase-8 and effector caspase-3. Recently, cFLIP was found to also prevent cells from undergoing necroptosis [49]. To test the possibility that necroptosis may contribute to the pool of dying cells in TRAIL-treated shFLIP cells, the necroptotic pathway was inhibited by necrostatin-1. In this experiment, no change in cell death was observed (Supplementary Fig. S5A, B).
Discussion
hESCs and induced pluripotent stem cells are highly vulnerable to various adverse situations and respond to such stresses by activation of the intrinsic apoptotic pathway [6,50]. However, virtually nothing is known about the other main branch of canonical apoptotic signaling—induction of extrinsic apoptosis via activated death receptors in the TNFR family. In this communication, we describe for the first time the expression of the major death domain-containing receptors, TNFR1 and Fas/CD95, and the functional state of TRAIL receptors, in ICM-derived hESC and hiPSC obtained by fibroblast reprogramming. We found that Fas/CD95 expression is virtually undetectable in either cell type (Fig. 1A, B) but increases dramatically (by more than an order of magnitude) during differentiation of hESC into neural progenitors (unpublished data). The expression of TNFR1 on human pluripotent stem cells is also rather low, especially when compared with the TRAIL receptors DR4 and DR5. Analysis of the expression and function of these two death receptors in mouse embryonic stem cells (mESCs), however, provides contradictory results. While earlier reports argue for functional Fas/CD95 and TNFR expression on mESCs [51–53], more recent publications document a lack, or very low expression, of either Fas/CD95 or p55 TNFR in these cells [54,55], in agreement with our data for hESC and hiPSC, at least regarding the Fas/CD95 receptor. Notably, in their study, Kim and colleagues reported that Fas/CD95 expression is induced by environmental cell stress-inducing pollutants [56]. In contrast to these death receptors, the pro-apoptotic TRAIL receptors DR4 and DR5 are highly expressed in both types of human pluripotent stem cells evaluated in our study, and their expression levels are similar to those measured on adult human fibroblasts and hESC-derived neural progenitors (unpublished data). Interestingly, mouse blastomeres and trophectoderm cells were shown to express a death domain-containing TRAIL receptor and its ligand, supporting our findings for ICM-derived hESC and engineered hiPSC [57].
The expression of death receptors is a necessary, but not sufficient, prerequisite for full execution of the extrinsic apoptotic pathway. Despite the expression of both pro-apoptotic TRAIL receptors and all other proteins that are necessary for the efficient transduction of TRAIL-induced apoptotic signaling (see Fig. 1C), human pluripotent stem cells were resistant to TRAIL-induced apoptosis, similar to mESCs [57]. This resistance can be imposed by a number of signaling pathways, but, in principle, these mechanisms cluster into two regulatory nodes—a proximal one related to DISC-mediated activation of the initiator caspase-8 and a distal one relying on mitochondrial and postmitochondrial regulation of apoptotic signaling [39,58]. One of the essential regulatory proteins at the DISC node is cFLIP, which can competitively block caspase-8 self-processing. In particular, its shorter splice variants, cFLIP-R and -S, are very effective inhibitors [59,60]. In hESC and hiPSC, cFLIP-S, cFLIP-L (although to a lesser extent), and the anti-apoptotic Bcl-2 family protein Mcl-1 rapidly responded to HHT-mediated sensitization to TRAIL-induced apoptosis. We were able to confirm the importance of the first node's regulatory role by shRNA-mediated cFLIP-L/S knock-down in hESC, which significantly and strongly sensitized the cells to TRAIL-induced apoptosis.
The question remains as to why mouse and, as we document, also human pluripotent stem cells would need to express activatable TRAIL receptors. In mice, gene targeting of TNFRSF10B (coding for the only pro-apoptotic TRAIL receptor DR5/Killer) or its ligand does not hamper embryonic development, although in adult mice it can accelerate both spontaneous and chemically induced tumorigenesis [61,62]. However, in the context of recent knowledge on the relatively high sensitivity of human pluripotent stem cells to DNA damage and/or virus-induced apoptosis, our findings are not so surprising [6,57,63]. Recent studies document that hESC are, in general, equipped to undergo fast apoptosis because of elevated levels of several BH3-only pro-apoptotic Bcl-2 family members and the presence of active Bax at the Golgi that can rapidly translocate to mitochondria [8,9]. Importantly, DR4 was recently shown to be instrumental for activation of poised Bax after DNA damage [64], and TRAIL and its receptors are among the death ligands/receptors that are generally considered stress sensors. From this perspective, the expression of functional TRAIL receptors in addition to preactivated Bax and higher expression of the pro-apoptotic BH3-only protein could represent another level of protection of human pluripotent stem cells against malicious and potentially genotoxic extracellular stresses such as radiation or chemicals.
Supplementary Material
Acknowledgments
This research was supported by a grant from the Czech Science Foundation no. P301/10/1971 and partly by institutional grants AV0Z50520514 (LA), MSM0021622430, and CZ.1.05/1.1.00/02.0123. The authors thank Majlinda Lako (University of Newcastle, UK) for providing the clone 4 line of hiPSC.
Author Disclosure Statement
The authors state that no competing financial interests exist.
References
- 1.Takahashi K. Tanabe K. Ohnuki M. Narita M. Ichisaka T. Tomoda K. Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 2.Thomson JA. Itskovitz-Eldor J. Shapiro SS. Waknitz MA. Swiergiel JJ. Marshall VS. Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 3.Drews K. Jozefczuk J. Prigione A. Adjaye J. Human induced pluripotent stem cells—from mechanisms to clinical applications. J Mol Med (Berl) 2012;90:735–745. doi: 10.1007/s00109-012-0913-0. [DOI] [PubMed] [Google Scholar]
- 4.Vazin T. Freed WJ. Human embryonic stem cells: derivation, culture, and differentiation: a review. Restor Neurol Neurosci. 2010;28:589–603. doi: 10.3233/RNN-2010-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alekseenko LL. Zemelko VI. Zenin VV. Pugovkina NA. Kozhukharova IV. Kovaleva ZV. Grinchuk TM. Fridlyanskaya II. Nikolsky NN. Heat shock induces apoptosis in human embryonic stem cells but a premature senescence phenotype in their differentiated progeny. Cell Cycle. 2012;11:3260–3269. doi: 10.4161/cc.21595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desmarais JA. Hoffmann MJ. Bing ham G. Gagou ME. Meuth M. Andrews PW. Human embryonic stem cells fail to activate CHK1 and commit to apoptosis in response to DNA replication stress. Stem Cells. 2012;30:1385–1393. doi: 10.1002/stem.1117. [DOI] [PubMed] [Google Scholar]
- 7.Sumi T. Tsuneyoshi N. Nakatsuji N. Suemori H. Apoptosis and differentiation of human embryonic stem cells induced by sustained activation of c-Myc. Oncogene. 2007;26:5564–5576. doi: 10.1038/sj.onc.1210353. [DOI] [PubMed] [Google Scholar]
- 8.Dumitru R. Gama V. Fagan BM. Bower JJ. Swahari V. Pevny LH. Deshmukh M. Human embryonic stem cells have constitutively active Bax at the Golgi and are primed to undergo rapid apoptosis. Mol Cell. 2012;46:573–583. doi: 10.1016/j.molcel.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madden DT. Davila-Kruger D. Melov S. Bredesen DE. Human embryonic stem cells express elevated levels of multiple pro-apoptotic BCL-2 family members. PLoS One. 2011;6:e28530. doi: 10.1371/journal.pone.0028530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Momcilovic O. Navara C. Schatten G. Cell cycle adaptations and maintenance of genomic integrity in embryonic stem cells and induced pluripotent stem cells. Results Probl Cell Differ. 2011;53:415–458. doi: 10.1007/978-3-642-19065-0_18. [DOI] [PubMed] [Google Scholar]
- 11.Ohgushi M. Matsumura M. Eiraku M. Murakami K. Aramaki T. Nishiyama A. Muguruma K. Nakano T. Suga H, et al. Molecular pathway and cell state responsible for dissociation-induced apoptosis in human pluripotent stem cells. Cell Stem Cell. 2010;7:225–239. doi: 10.1016/j.stem.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 12.Romorini L. Scassa ME. Richardson GV. Bluguermann C. de Giusti CJ. Questa M. Fernandez Espinosa DD. Gomez RM. Sevlever GE. Miriuka SG. Activation of apoptotic signalling events in human embryonic stem cells upon Coxsackievirus B3 infection. Apoptosis. 2012;17:132–142. doi: 10.1007/s10495-011-0668-z. [DOI] [PubMed] [Google Scholar]
- 13.Ardehali R. Inlay MA. Ali SR. Tang C. Drukker M. Weissman IL. Overexpression of BCL2 enhances survival of human embryonic stem cells during stress and obviates the requirement for serum factors. Proc Natl Acad Sci U S A. 2011;108:3282–3287. doi: 10.1073/pnas.1019047108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bai H. Chen K. Gao YX. Arzigian M. Xie YL. Malcosky C. Yang YG. Wu WS. Wang ZZ. Bcl-xL enhances single-cell survival and expansion of human embryonic stem cells without affecting self-renewal. Stem Cell Res. 2012;8:26–37. doi: 10.1016/j.scr.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eiselleova L. Matulka K. Kriz V. Kunova M. Schmidtova Z. Neradil J. Tichy B. Dvorakova D. Pospisilova S. Hampl A. Dvorak P. A complex role for FGF-2 in self-renewal, survival, and adhesion of human embryonic stem cells. Stem Cells. 2009;27:1847–1857. doi: 10.1002/stem.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gauthaman K. Fong CY. Bongso A. Effect of ROCK inhibitor Y-27632 on normal and variant human embryonic stem cells (hESCs) in vitro: its benefits in hESC expansion. Stem Cell Rev. 2010;6:86–95. doi: 10.1007/s12015-009-9107-8. [DOI] [PubMed] [Google Scholar]
- 17.Li Y. Shelat H. Geng YJ. IGF-1 prevents oxidative stress induced-apoptosis in induced pluripotent stem cells which is mediated by microRNA-1. Biochem Biophys Res Commun. 2012;426:615–619. doi: 10.1016/j.bbrc.2012.08.139. [DOI] [PubMed] [Google Scholar]
- 18.Wang X. Lin G. Martins-Taylor K. Zeng H. Xu RH. Inhibition of caspase-mediated anoikis is critical for basic fibroblast growth factor-sustained culture of human pluripotent stem cells. J Biol Chem. 2009;284:34054–34064. doi: 10.1074/jbc.M109.052290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blum B. Bar-Nur O. Golan-Lev T. Benvenisty N. The anti-apoptotic gene survivin contributes to teratoma formation by human embryonic stem cells. Nat Biotechnol. 2009;27:281–287. doi: 10.1038/nbt.1527. [DOI] [PubMed] [Google Scholar]
- 20.Filion TM. Qiao M. Ghule PN. Mandeville M. van Wijnen AJ. Stein JL. Lian JB. Altieri DC. Stein GS. Survival responses of human embryonic stem cells to DNA damage. J Cell Physiol. 2009;220:586–592. doi: 10.1002/jcp.21735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aggarwal BB. Gupta SC. Kim JH. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood. 2012;119:651–665. doi: 10.1182/blood-2011-04-325225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grewal IS. Overview of TNF superfamily: a chest full of potential therapeutic targets. Adv Exp Med Biol. 2009;647:1–7. doi: 10.1007/978-0-387-89520-8_1. [DOI] [PubMed] [Google Scholar]
- 23.Dickens LS. Powley IR. Hughes MA. MacFarlane M. The “complexities” of life and death: death receptor signalling platforms. Exp Cell Res. 2012;318:1269–1277. doi: 10.1016/j.yexcr.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Lavrik IN. Krammer PH. Regulation of CD95/Fas signaling at the DISC. Cell Death Differ. 2012;19:36–41. doi: 10.1038/cdd.2011.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kischkel FC. Lawrence DA. Tinel A. LeBlanc H. Virmani A. Schow P. Gazdar A. Blenis J. Arnott D. Ashkenazi A. Death receptor recruitment of endogenous caspase-10 and apoptosis initiation in the absence of caspase-8. J Biol Chem. 2001;276:46639–46646. doi: 10.1074/jbc.M105102200. [DOI] [PubMed] [Google Scholar]
- 26.Tewari M. Quan LT. O'Rourke K. Desnoyers S. Zeng Z. Beidler DR. Poirier GG. Salvesen GS. Dixit VM. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995;81:801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- 27.Hitomi J. Christofferson DE. Ng A. Yao J. Degterev A. Xavier RJ. Yuan J. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135:1311–1323. doi: 10.1016/j.cell.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christofferson DE. Yuan J. Necroptosis as an alternative form of programmed cell death. Curr Opin Cell Biol. 2010;22:263–268. doi: 10.1016/j.ceb.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lluis JM. Nachbur U. Cook WD. Gentle IE. Moujalled D. Moulin M. Wong WW. Khan N. Chau D, et al. TAK1 is required for survival of mouse fibroblasts treated with TRAIL, and does so by NF-kappaB dependent induction of cFLIPL. PLoS One. 2010;5:e8620. doi: 10.1371/journal.pone.0008620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ricci-Vitiani L. Pedini F. Mollinari C. Condorelli G. Bonci D. Bez A. Colombo A. Parati E. Peschle C. De Maria R. Absence of caspase 8 and high expression of PED protect primitive neural cells from cell death. J Exp Med. 2004;200:1257–1266. doi: 10.1084/jem.20040921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szegezdi E. O'Reilly A. Davy Y. Vawda R. Taylor DL. Murphy M. Samali A. Mehmet H. Stem cells are resistant to TRAIL receptor-mediated apoptosis. J Cell Mol Med. 2009;13:4409–4414. doi: 10.1111/j.1582-4934.2008.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Falschlehner C. Emmerich CH. Gerlach B. Walczak H. TRAIL signalling: decisions between life and death. Int J Biochem Cell Biol. 2007;39:1462–1475. doi: 10.1016/j.biocel.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 33.Herrero-Martin G. Hoyer-Hansen M. Garcia-Garcia C. Fumarola C. Farkas T. Lopez-Rivas A. Jaattela M. TAK1 activates AMPK-dependent cytoprotective autophagy in TRAIL-treated epithelial cells. Embo J. 2009;28:677–685. doi: 10.1038/emboj.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siegmund D. Klose S. Zhou D. Baumann B. Roder C. Kalthoff H. Wajant H. Trauzold A. Role of caspases in CD95L- and TRAIL-induced non-apoptotic signalling in pancreatic tumour cells. Cell Signal. 2007;19:1172–1184. doi: 10.1016/j.cellsig.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Dickens LS. Boyd RS. Jukes-Jones R. Hughes MA. Robinson GL. Fairall L. Schwabe JW. Cain K. Macfarlane M. A death effector domain chain DISC model reveals a crucial role for caspase-8 chain assembly in mediating apoptotic cell death. Mol Cell. 2012;47:291–305. doi: 10.1016/j.molcel.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzalvez F. Lawrence D. Yang B. Yee S. Pitti R. Marsters S. Pham VC. Stephan JP. Lill J. Ashkenazi A. TRAF2 Sets a Threshold for Extrinsic Apoptosis by Tagging Caspase-8 with a Ubiquitin Shutoff Timer. Mol Cell. 2012;48:888–899. doi: 10.1016/j.molcel.2012.09.031. [DOI] [PubMed] [Google Scholar]
- 37.Jin Z. Li Y. Pitti R. Lawrence D. Pham VC. Lill JR. Ashkenazi A. Cullin3-based polyubiquitination and p62-dependent aggregation of caspase-8 mediate extrinsic apoptosis signaling. Cell. 2009;137:721–735. doi: 10.1016/j.cell.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 38.Schleich K. Warnken U. Fricker N. Ozturk S. Richter P. Kammerer K. Schnolzer M. Krammer PH. Lavrik IN. Stoichiometry of the CD95 death-inducing signaling complex: experimental and modeling evidence for a death effector domain chain model. Mol Cell. 2012;47:306–319. doi: 10.1016/j.molcel.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 39.Shirley S. Morizot A. Micheau O. Regulating TRAIL receptor-induced cell death at the membrane: a deadly discussion. Recent Pat Anticancer Drug Discov. 2011;6:311–323. doi: 10.2174/157489211796957757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaufmann T. Strasser A. Jost PJ. Fas death receptor signalling: roles of Bid and XIAP. Cell Death Differ. 2012;19:42–50. doi: 10.1038/cdd.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Llambi F. Moldoveanu T. Tait SW. Bouchier-Hayes L. Temirov J. McCormick LL. Dillon CP. Green DR. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol Cell. 2011;44:517–531. doi: 10.1016/j.molcel.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abdulghani J. El-Deiry WS. TRAIL receptor signaling and therapeutics. Expert Opin Ther Targets. 2010;14:1091–1108. doi: 10.1517/14728222.2010.519701. [DOI] [PubMed] [Google Scholar]
- 43.Yerbes R. Palacios C. Lopez-Rivas A. The therapeutic potential of TRAIL receptor signalling in cancer cells. Clin Transl Oncol. 2011;13:839–847. doi: 10.1007/s12094-011-0744-4. [DOI] [PubMed] [Google Scholar]
- 44.Dolezalova D. Mraz M. Barta T. Plevova K. Vinarsky V. Holubcova Z. Jaros J. Dvorak P. Pospisilova S. Hampl A. MicroRNAs regulate p21(Waf1/Cip1) protein expression and the DNA damage response in human embryonic stem cells. Stem Cells. 2012;30:1362–1372. doi: 10.1002/stem.1108. [DOI] [PubMed] [Google Scholar]
- 45.Armstrong L. Tilgner K. Saretzki G. Atkinson SP. Stojkovic M. Moreno R. Przyborski S. Lako M. Human induced pluripotent stem cell lines show stress defense mechanisms and mitochondrial regulation similar to those of human embryonic stem cells. Stem Cells. 2010;28:661–673. doi: 10.1002/stem.307. [DOI] [PubMed] [Google Scholar]
- 46.Barta T. Vinarsky V. Holubcova Z. Dolezalova D. Verner J. Pospisilova S. Dvorak P. Hampl A. Human embryonic stem cells are capable of executing G1/S checkpoint activation. Stem Cells. 2010;28:1143–1152. doi: 10.1002/stem.451. [DOI] [PubMed] [Google Scholar]
- 47.Horova V. Hradilova N. Jelinkova I. Koc M. Svadlenka J. Brazina J. Klima J. Slavik I. Hyrslova Vaculova A. Andera L. Inhibition of vacuolar ATPase attenuates the TRAIL-induced activation of caspase-8 and modulates the trafficking of TRAIL receptosomes. FEBS J. 2013;280:3436–3450. doi: 10.1111/febs.12347. [DOI] [PubMed] [Google Scholar]
- 48.Beranova L. Pombinho AR. Spegarova J. Koc M. Klanova M. Molinsky J. Klener P. Bartunek P. Andera L. The plant alkaloid and anti-leukemia drug homoharringtonine sensitizes resistant human colorectal carcinoma cells to TRAIL-induced apoptosis via multiple mechanisms. Apoptosis. 2013;18:739–750. doi: 10.1007/s10495-013-0823-9. [DOI] [PubMed] [Google Scholar]
- 49.He MX. He YW. CFLAR/c-FLIPL: a star in the autophagy, apoptosis and necroptosis alliance. Autophagy. 2013;9:791–793. doi: 10.4161/auto.23785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lan ML. Acharya MM. Tran KK. Bahari-Kashani J. Patel NH. Strnadel J. Giedzinski E. Limoli CL. Characterizing the radioresponse of pluripotent and multipotent human stem cells. PLoS One. 2012;7:e50048. doi: 10.1371/journal.pone.0050048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kohchi C. Tanabe Y. Noguchi K. Mizuno D. Soma G. Induction of differentiation in embryonic stem cells by 26-kD membrane-bound tumor necrosis factor (TNF) and 17-kD free TNF. In Vivo. 1996;10:19–27. [PubMed] [Google Scholar]
- 52.Wuu YD. Pampfer S. Vanderheyden I. Lee KH. De Hertogh R. Impact of tumor necrosis factor alpha on mouse embryonic stem cells. Biol Reprod. 1998;58:1416–1424. doi: 10.1095/biolreprod58.6.1416. [DOI] [PubMed] [Google Scholar]
- 53.Zou GM. Reznikoff-Etievant MF. Leon A. Verge V. Hirsch F. Milliez J. Fas-mediated apoptosis of mouse embryo stem cells: its role during embryonic development. Am J Reprod Immunol. 2000;43:240–248. doi: 10.1111/j.8755-8920.2000.430409.x. [DOI] [PubMed] [Google Scholar]
- 54.Brunlid G. Pruszak J. Holmes B. Isacson O. Sonntag KC. Immature and neurally differentiated mouse embryonic stem cells do not express a functional Fas/Fas ligand system. Stem Cells. 2007;25:2551–2558. doi: 10.1634/stemcells.2006-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zampetaki A. Zeng L. Xiao Q. Margariti A. Hu Y. Xu Q. Lacking cytokine production in ES cells and ES-cell-derived vascular cells stimulated by TNF-alpha is rescued by HDAC inhibitor trichostatin A. Am J Physiol Cell Physiol. 2007;293:C1226–C1238. doi: 10.1152/ajpcell.00152.2007. [DOI] [PubMed] [Google Scholar]
- 56.Kim SK. Kim BK. Shim JH. Gil JE. Yoon YD. Kim JH. Nonylphenol and octylphenol-induced apoptosis in human embryonic stem cells is related to Fas-Fas ligand pathway. Toxicol Sci. 2006;94:310–321. doi: 10.1093/toxsci/kfl114. [DOI] [PubMed] [Google Scholar]
- 57.Riley JK. Heeley JM. Wyman AH. Schlichting EL. Moley KH. TRAIL and KILLER are expressed and induce apoptosis in the murine preimplantation embryo. Biol Reprod. 2004;71:871–877. doi: 10.1095/biolreprod.103.026963. [DOI] [PubMed] [Google Scholar]
- 58.Dimberg LY. Anderson CK. Camidge R. Behbakht K. Thorburn A. Ford HL. On the TRAIL to successful cancer therapy? Predicting and counteracting resistance against TRAIL-based therapeutics. Oncogene. 2013;32:1341–1350. doi: 10.1038/onc.2012.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Golks A. Brenner D. Fritsch C. Krammer PH. Lavrik IN. c-FLIPR, a new regulator of death receptor-induced apoptosis. J Biol Chem. 2005;280:14507–14513. doi: 10.1074/jbc.M414425200. [DOI] [PubMed] [Google Scholar]
- 60.Irmler M. Thome M. Hahne M. Schneider P. Hofmann K. Steiner V. Bodmer JL. Schroter M. Burns K, et al. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 61.Finnberg N. Gruber JJ. Fei P. Rudolph D. Bric A. Kim SH. Burns TF. Ajuha H. Page R, et al. DR5 knockout mice are compromised in radiation-induced apoptosis. Mol Cell Biol. 2005;25:2000–2013. doi: 10.1128/MCB.25.5.2000-2013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grosse-Wilde A. Voloshanenko O. Bailey SL. Longton GM. Schaefer U. Csernok AI. Schutz G. Greiner EF. Kemp CJ. Walczak H. TRAIL-R deficiency in mice enhances lymph node metastasis without affecting primary tumor development. J Clin Invest. 2008;118:100–110. doi: 10.1172/JCI33061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hirsch ML. Fagan BM. Dumitru R. Bower JJ. Yadav S. Porteus MH. Pevny LH. Samulski RJ. Viral single-strand DNA induces p53-dependent apoptosis in human embryonic stem cells. PLoS One. 2011;6:e27520. doi: 10.1371/journal.pone.0027520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Henry RE. Andrysik Z. Paris R. Galbraith MD. Espinosa JM. A DR4:tBID axis drives the p53 apoptotic response by promoting oligomerization of poised BAX. Embo J. 2012;31:1266–1278. doi: 10.1038/emboj.2011.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.