Abstract
While it is well established that 17β-estradiol (E2) protects the rodent brain from ischemia-induced damage, it has been unclear how this neuroprotective effect is mediated. Interestingly, convincing evidence has also demonstrated that maintaining or increasing the expression of the oxidative stress response and DNA repair protein apurinic endonuclease 1 (Ape1) is instrumental in reducing ischemiainduced damage in the brain. Since E2 increases expression of the oxidative stress response proteins Cu/Zn superoxide dismutase and thioredoxin in the brain, we hypothesized that E2 may also increase Ape1 expression and that this E2-induced expression of Ape1 may help to mediate the neuroprotective effects of E2 in the brain. To test this hypothesis, we utilized three model systems including primary cortical neurons, brain slice cultures, and whole animals. Although estrogen receptor α and Ape1 were expressed in primary cortical neurons, E2 did not alter Ape1 expression in these cells. However, immunofluorescent staining and quantitative Western blot analysis demonstrated that estrogen receptor α and Ape1 were expressed in the nuclei of cortical neurons in brain slice cultures and that E2 increased Ape1 expression in the cerebral cortex of these cultures. Furthermore, Ape1 expression was increased and oxidative DNA damage was decreased in the cerebral cortices of ovariectomized female C57Bl/6J mice that had been treated with E2 and exposed to hypoxia. Taken together, our studies demonstrate that the neuronal microenvironment may be required for increased Ape1 expression and that E2 enhances expression of Ape1 and reduces oxidative DNA damage, which may in turn help to reduce ischemia-induced damage in the cerebral cortex and mediate the neuroprotective effects of E2.
Keywords: Apurinic endonuclease 1, estrogen, estrogen receptor, reactive oxygen species, neuroprotection, hypoxia
1. Introduction
The human brain utilizes 20% of the oxygen consumed, but accounts for only 2% of total body mass, making it the most metabolically active organ in the human body [1]. Because reactive oxygen species (ROS) are produced as byproducts of normal cellular metabolism, the massive consumption of oxygen by the brain can lead to substantial ROS production. ROS play a role in cellular signaling such as MAP kinase activation and tyrosine phosphorylation [2–5]. However, if not effectively dissipated, ROS can accumulate and the ability of the cell to maintain a reduced intracellular environment is compromised, which can result in oxidative stress and damage to resident proteins, lipids, and DNA.
Cells rely on a variety of proteins to dissipate ROS, reduce oxidative stress, and, if damage does occur, to repair ROS-induced damage to cellular macromolecules. The oxidative stress response protein apurinic endonuclease (Ape1) is a multifunctional protein involved in DNA repair and redox regulation. Ape1 is the primary mammalian endonuclease and plays an essential role in repairing the most common DNA lesions, apurinic and 8-hydroxydeoxyguanosine (8-OHG) sites [6–11]. In addition to its role in DNA repair, Ape1 is required for the reduction of oxidized cellular proteins and is especially important in maintaining numerous transcription factors in a reduced, active state [12–15]. Thus, Ape1 is required to maintain DNA integrity and protein structure and function in the brain.
Ape1 is also instrumental in repairing cellular damage caused after blood vessel occlusion as might occur during a stroke [16–18]. While the hypoxia resulting from blood vessel occlusion is deleterious, reoxygenation can be even more damaging as the oxygen supply is reestablished and ROS production rapidly escalates [19–21]. Studies in rodents have demonstrated that Ape1 levels decline following middle cerebral artery (MCA) occlusion leading to DNA damage and cell death in the infarct region [16,22,23]. However, by simply maintaining Ape1 levels, cell death and DNA damage can be reduced [16,17]. Furthermore, if Ape1 is overexpressed, DNA damage and cerebral infarct volume resulting from MCA occlusion is significantly reduced [18].
While best known for its role in female fertility, the steroid hormone 17β-estradiol (E2) also alters the expression of proteins involved in oxidative stress response, anti-inflammatory processes, and programmed cell death in the brain [24–35]. A number of laboratories have demonstrated that E2 diminishes neuronal injury associated with cerebral ischemia and brain trauma in rodents [36–40]. This E2-induced neuroprotection is most apparent in the cerebral cortex, which is particularly vulnerable to ischemia-induced injury [17,18,24–27,41]. An elegant series of studies by Wise and coworkers demonstrated that exposing ovariectomized female rodents to physiological levels of E2 reduces the infarct volume and cell death that occurs following MCA occlusion [24–27], but that this neuroprotective effect was only observed when the E2 was administered prior to artery occlusion [28]. Furthermore, the fact that E2 pretreatment decreases infarct volume in estrogen receptor β (ERβ), but not in estrogen receptor α (ERα) null mice that have been subjected to MCA occlusion, demonstrates that ERα, not ERβ, is involved in this E2-mediated neuroprotection [29,30].
We previously demonstrated that E2 increases expression of the oxidative stress response protein Cu/Zn superoxide dismutase in brain slice cultures and that this E2-induced expression helps to limit protein and DNA damage of cerebral cortical neurons [42]. From these studies we hypothesized that E2 might also increase Ape1 expression in the brain and that this E2-induced expression of Ape1 could help to protect the cerebral cortex from hypoxia-induced cell damage.
2. Materials and Methods
2.1. Mice
C57BL/6J breeding pairs for the generation of mouse pups for isolation of primary cortical neurons and brain slice cultures and female mice (12–15 weeks) were obtained from Jackson Laboratory (Bar Harbor, ME). All mice were maintained on a 12 h light/dark schedule with access to water and food ad libitum. Ovariectomized mice were maintained on phytoestrogen-free chow. Primary cortical neurons were prepared from P0 female mouse pups, non-neuronal cultures for the preparation of conditioned media were prepared from P0–P3 female mouse pups, and brain slice cultures were prepared from P7 to P9 day old female pups. Female pups were identified by the smaller anogenital distance and the absence of an adjacent pigmented region. All procedures were performed in accordance with guidelines of the University of Illinois at Urbana-Champaign Institutional Animal Care and Use Committee and Division of Animal Resources.
2.2. Primary cortical neurons
The isolation of primary cortical neurons was performed as described [43] with the following modifications. Conditioned plating medium (Neurobasal-A with 0.5 mM GlutaMAX, B27 supplement without antioxidants [Life Technologies, Grand Island, NY], and antibiotics) and conditioned maintenance medium (Neurobasal-A with 0.5 mM GlutaMAX, custom-formulated media supplement [Table 1], and antibiotics) were prepared by incubating non-neuronal cultures with media for 24 h. Cortical pieces were triturated in conditioned plating media, cells were counted and seeded on poly-D-lysine coated 60 mm petri dishes at 115,000 cells/cm2 or on poly-D-lysine and fibronectin pre-coated German glass coverslips (EMS, Hatfield, PA) at 50,000 cells/cm2. Neurons were incubated overnight and the conditioned plating media was removed and replaced with conditioned maintenance media. Primary cortical neurons were treated with 1 µM cytosine ß-D-arabinofuranoside once a week to reduce glial cell proliferation. Media was removed from the primary cortical neurons and replaced with conditioned maintenance media twice a week. Ethanol or 20 nM E2 was added 24 h prior to cell harvest. All cells were maintained in a 5% CO2 incubator at 36°C.
Table 1.
Final concentrations of our custom-formulated, serum-free media supplement. Ingredients and concentrations are based on Price and Brewer (74) and Roth et al (75) with some modifications.
| H2O soluble | Ethanol soluble | ||
| D-galactose | 15 µg/ml | Linolenic acid | 0.1 µg/ml |
| Putrescine dihydrochloride | 16.1 µg/ml | Linolenic acid | 0.1 µg/ml |
| Sodium Selenite (Na2SeO3) | 0.016 µg/ml | Retinyl acetate | 0.1 µg/ml |
| Albumin, bovine | 2.5 mg/ml | D,L-a-Lipoic acid | 0.047 µg/ml |
| Holo-Transferrin | 5 µg/ml | ||
| L-carnitine hydrochloride | 2 µg/ml | Antioxidant, ethanol soluble | |
| Ethanolamine | 1 µg/ml | D,L-a-Tocopherol | 1 µg/ml |
| CuSO4 | 5.2 nM | D,L-a-Tocopherol acetate | 1 µg/ml |
| ZnSO4 | 1.4 mM | ||
| MnSO4 | 0.3 nM | HCl soluble | |
| Insulin (bovine) | 4 µg/ml | ||
| Antioxidant, H2O soluble | |||
| L-Glutathione reduced | 1 ug/ml | NaOH soluble | |
| 3,3`,5-Triiodo-L-thryronine | 2 ng/ml (3.1nM) | ||
| NH4OH soluble | |||
| Biotin | 0.1 µg/ml |
Superoxide dismutase (2.5 µg/ml), catalase (2.5 µg/ml), progesterone (20 nM), and corticosterone (20 ng/ml), which are included in Complete B27 media supplement, were omitted in our custom-formulated media supplement.
2.3. Brain slice cultures
Brain slice cultures were prepared essentially as described [44] with modifications. Pups were decapitated and whole brains were quickly removed, mounted, and sectioned with a Leica VT1200 vibratome (Leica Microsystems, Nussloch, Germany). 300 µm coronal sections were sliced into chilled slicing solution (1.25 mM NaH2PO4, 2.5 mM KCl, 10 mM MgSO4, 0.5 mM CaCl2, 234 mM sucrose, 11 mM glucose and 26 mM NaHCO3). Slices were immediately placed on a sterile Millicell culture plate insert (Millipore, Billerica, MA) in each well of a chilled 12-well plate. Excess slicing solution was removed following sectioning and replaced with 1.2 ml of Neurobasal-A medium (Gibco, Carlsbad, CA) containing 0.5 mM GlutaMAX (Gibco), antibiotics (penicillin, streptomycin, and gentamycin) and 10% charcoal dextran-treated fetal bovine serum with ethanol or 20 nM E2. Slices were maintained in a 5% CO2 incubator at 36 °C for 24 h.
2.4. Ovariectomy
12–15 week old female mice were anesthetized by inhalation of 4% isoflurane, bilaterally ovariectomized, and implanted subcutaneously with silastic tubing (0.062 in/0.125 in, inner/outer diameter, 1 in length; Dow Corning, Midland, MI) plugged at both ends with medical adhesive (Dow Corning). Tubing contained 35 µl cottonseed oil (vehicle) or 35 µl of 180 µg/ml E2 in cottonseed oil, which produces a low physiological level of circulating E2 (~25 pg/ml) [29] that is equivalent to estrous levels of E2 in mice [45–47].
2.5. Hypoxia treatment
7 days after ovariectomy and implantation of silastic tubing, oil- and E2-treated mice were placed in cages inside a hypoxia chamber (BioSpherix, Lacona, NY) that was equilibrated to 7% O2 and 93% N2 for 3 h, conditions which have been shown to induce substantial changes in mRNA expression [48]. Oxygen concentration was monitored continuously throughout the experiments. Oil- and E2-treated mice were also maintained at normoxic conditions for 3 h. After hypoxia, animals were allowed to reoxygenate for 1 h (RT-PCR) or 3 h (protein expression and DNA damage). Following hypoxia and re-oxygenation, all animals were anesthetized with isoflurane, decapitated, and cerebral cortices were harvested for quantitative real time-PCR, immunofluorescent staining, Western blot, or DNA damage analyses.
2.6. Western blot analysis
Primary neurons, brain slice cultures, or cerebral cortical hemispheres were combined with 400, 250, or 800 µl RIPA buffer (Thermo Scientific, Rockford, IL), respectively, with 1× Protease Inhibitor Cocktail (Sigma) and homogenized for 10 seconds at high speed with a Pro Homogenizer (ProScientific Inc., Oxford, CT). The buffer was adjusted to 400 mM NaCl with 5 M NaCl, placed on ice, and vortexed every 5 min for 15 min. The extract was spun at 20,800×g in a 4°C microfuge, the supernatant was removed, and protein assays were performed with bicinchoninic acid using BSA as the protein standard (Thermo Scientific). Whole cell lysates (25µg) were loaded onto each lane of a denaturing gel and fractionated. Proteins were transferred to a nitrocellulose membrane and blots were probed with an Ape1- (1:2000, ab194, Abcam Inc., Cambridge, MA), β-tubulin-(1:5000, sc9104, Santa Cruz Biotechnologies, Santa Cruz, CA) or α-tubulin- (1:100,000, T6199, Sigma) specific antibody followed by a secondary antibody covalently linked to infrared fluorophores (IRDye 800CW donkey anti-mouse IgG [1:5,0000, LI-COR Biosciences, Lincoln, NE] or IRDye 800CW donkey anti-rabbit IgG [1:5,0000, LI-COR Biosciences]). The membranes were scanned with an Odyssey infrared imager (LI-COR Biosciences) to quantitate the level of protein present. The integrated intensity function with the automated median background correction method was used since this system provides a wide dynamic range and reduces error to improve quantitative accuracy [49,50].
2.7. Immunofluorescence imaging
Brain slices from cultures and whole animals were rinsed 2× with PBS, fixed in PBS with 4% formaldehyde for 1 h, washed 3× with PBS, permeabilized with PBS containing 1% Triton X-100 for 30 min, and incubated in blocking solution (PBS with 0.05% Tween-20 and 5% normal donkey serum) for 1–2 h. Slices were then incubated in blocking solution with an ERα-, (1:600, ab31312, Abcam Inc.), Ape1- (1:100, sc9919, Santa Cruz Biotechnologies), or NeuN- (1:500, MAB377, Millipore, Temecula, CA) specific antibody for 2 h at room temperature. Slices were washed 3× with PBS containing 0.1% Tween-20 (PBS-T) and incubated with DyLight 549-, 649- or 488-conjugated anti-rabbit, anti-goat or anti-mouse IgG (Jackson ImmunoResearch Laboratories Inc., West Grove, PA), respectively, for 1 h in the dark at room temperature, washed 3× with PBS-T, incubated with DAPI nucleic acid stain for 30 min at room temperature, washed 3× with PBS, and mounted with Pro-Long Gold antifade reagent (Life Technologies). 4′,6-diamidino-2-phenylindole (DAPI) co-staining was included with each treatment to identify nuclei and ensure that similar numbers of cells were present. Control slices, which had not been exposed to primary antibody, were processed in parallel.
Primary cortical neurons were stained as described for the brain slices with the following modifications. After primary neurons were rinsed 2× with PBS, they were fixed in PBS with 4% formaldehyde for 15 min, washed 3× with PBS, permeabilized with PBS containing 0.5% Triton X-100 for 10 min, and incubated in blocking solution for 30 min. DAPI staining time was reduced to 10 min.
2.8. Image collection and quantitation
All images were obtained with a 40× oil-immersion objective using the Leica DM 4000 B confocal microscope and Leica TCS SPE system and Application Suite Advanced Fluorescence software (Leica Microsystems, Inc., Bannockburn, IL). Detector gain and offset, laser power, and bandwidth of emission collection were kept constant for all treatments in each experiment and adjusted so that images had a full range of pixel intensities (0–255) and saturation was minimized. Images from 8 independent experiments were collected from primary cortical neurons to examine effect of E2 on Ape1 expression.
Image Pro Plus software (Media Cybernetics, Bethesda, MD) was used for quantitative immunofluorescent analysis of Ape1 staining in brain slice cultures. Analysis included 477 (ethanol) or 742 (E2) z-stack images from 6 (ethanol) or 7 (E2) individual brain slice cultures. The perimeter and area were adjusted so that only specifically stained cells were detected and recorded in a script designed to analyze the individual images in an entire z-stack. Data were exported into Excel and the mean density/intensity of specifically stained cells in each z-stack image was quantitated for each treatment.
2.9. RT-PCR
Total RNA was isolated from mouse cortices using RNAqueous reagents (Ambion, Life Technologies, Austin, TX) according to the manufacturer’s instructions. RNA concentrations were measured and cDNA was synthesized using the iScript kit (Bio-Rad, Hercules, CA) as described by the manufacturer. 1µl of cDNA was combined with iQ SYBR Green Supermix (Bio-Rad, Hercules, CA), forward and reverse primers for HIF3α (5’- GGACTCAGACTCAGGCTACAG-3’ and 5’- TCAGGAAGTGGACGCAGATG-3’) and VefgA (5’- GGCTGCTGTAACGATGAAG-3’ and 5’- TCTGCTGTGCTGTAGGAAG-3’) and real-time PCR was carried out using a Bio-Rad iQ5 multicolor Real-Time PCR Detection System. Standard curves were created using cDNA equivalents of 0.125, 1.25 and 12.5 ng of RNA and were run in triplicate with each primer set for each experiment.
2.10. 8-hydroxydeoxyguanosine quantitation
Genomic DNA was isolated by digesting each cerebral cortex in 500 µl of DNA isolation buffer (10 mM Tris-HCl, pH 8.0, 100 mM NaCl, 25 mM EDTA, pH 8.0, 0.5% SDS, 1 mg/ml proteinase K [Sigma], 200 µg/ml RNase A [Sigma]) overnight at 50°C in a rotating incubator. After tissue digestion, 500 µL of phenol-chloroform-isoamyl alcohol (25:24:1) was added and samples were vortexed and centrifuged at 15,000×g for 5 min at room temperature. The top, aqueous phase was transferred to a fresh tube. DNA was precipitated, resuspended in Tris-EDTA and 8-hydroxydeoxyguanosine (8-OHG) oxidative DNA damage was quantitated using the OxiSelect Oxidative DNA Damage ELISA kit (Cell Biolabs, San Diego, CA). A 96 well plate was coated with 8-OHG conjugate overnight. Sample DNA was denatured, digested, and treated with alkaline phosphatase. Individual samples or 8-OHG standards were added to the coated plate and 8-OHG antibody was added to each well for 1 hr. Wells were rinsed with wash buffer 3× and secondary antibody was added. After 1 hr, wells were washed 3× and the substrate solution was added. Stop solution was added to each well after the color had developed and the absorbance was read at 450 nm using a SpectraMax Plus 384 plate reader.
2.11. Statistics
Combined data are expressed as the mean ± SEM. SAS version 9.2 (SAS Institute Inc., Cary, NC) was used for statistical analysis. Ape1 protein expression in brain slice cultures was analyzed using Student’s t-test. Statistical analyses of HIF3α and VegfA mRNA expression, Ape1 protein expression, and 8-OHG levels among all groups of animals were compared using a two-way analysis of variance (ANOVA). A p value of <0.05 was considered statistically significant (95% confidence interval).
3. Results
3.1. Ape1 expression in primary cortical neurons
Since neuronal ERα is essential for E2-mediated neuroprotection [29,30,36] and the cerebral cortex is particularly susceptible to ischemia-induced damage [17,18,24–27,41], we examined the expression of ERα and Ape1 in primary cultures of cortical neurons. Primary neurons were isolated from the cerebral cortices of newborn C57Bl/6J female mouse pups and cultured for 9 or 10 days in vitro. When an ERα antibody was preincubated with purified ERα and used in immunofluorescence assays, no staining was detected (Data not shown). In contrast, antibody that had not been preincubated with receptor detected robust ERα staining, thus confirming the specificity of the antibody (Fig. 1A). Quantitative real-time PCR demonstrated that ERα transcripts were present in primary neurons that had been cultured for 9 days (data not shown). Furthermore, immunofluorescent staining demonstrated that the expression of Ape1 was quite robust in these cells as well suggesting that they might be an appropriate model system to study the potential effects of E2 on Ape1 expression.
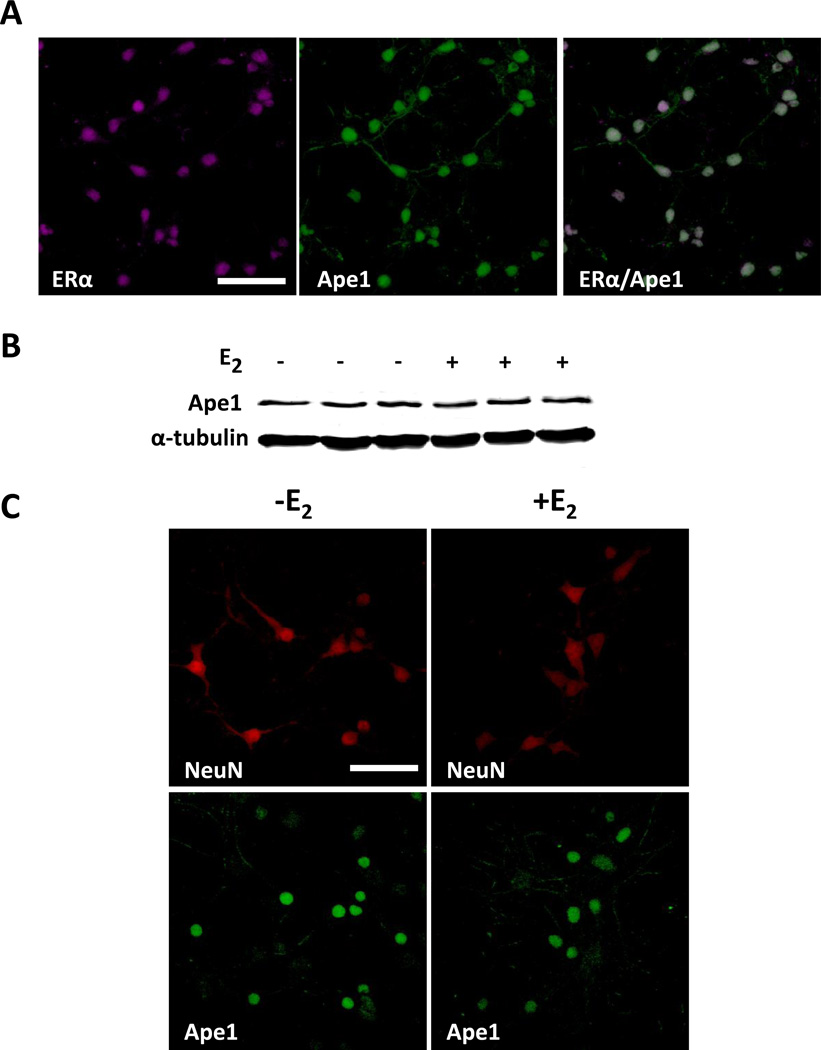
Fig. 1. Ape1 and ERα are expressed in primary cortical neurons.
Cerebral cortical neurons were isolated from P0 mouse pups and cultured in vitro for 10 days. (A) Immunofluorescent staining was performed using ERα- and Ape1-specific antibodies. Neurons were treated with ethanol or 20 nM E2 for 24 h and whole cell extracts were prepared for (B) Western blot analysis using Ape1- and α-tubulin-specific antibodies or (C) immunofluorescent staining using Ape1- and NeuN-specific antibodies. Scale bars indicate 25 µm.
When primary cerebral cortical neurons were treated with ethanol vehicle or 20 nM E2 for 24 h, no changes in Ape1 expression were detected using Western blot (Fig. 1B) or immunofluorescence (Fig. 1C) analyses. Ape1 staining was confined to the nuclear compartment of the primary neurons (Fig. 1C, compare Ape1 and NeuN staining). Varying the concentration (10–100 nM) or time (3–48 h) of E2 treatment did not alter Ape1 expression (data not shown). Even after eliminating the superoxide dismutase, catalase, corticosterone, and progesterone from the B27 media supplement, which are typically used for primary neuronal cultures (Table 1), we were still unable to detect an E2-induced expression of Ape1. Thus, in spite of the fact that ERα was present and would presumably have been able to respond to E2 treatment, no changes in Ape1 expression were observed suggesting that the primary neurons may require other cell types and/or the organizational features and structural architecture present in the intact brain.
3.2. Ape1 expression in brain slice cultures
To more closely recapitulate the neuronal environment present in the brain, we used brain slice cultures, which maintain much of the spatial architecture and many of the organizational features and local synaptic connections present in the brain [44]. Since the cellular environment can be carefully defined and manipulated, brain slice cultures have been used extensively to study electrophysiological properties, angiogenesis, dendritic growth, neural cell migration, and the responsiveness of neural cells to various drugs and treatments including E2 [42,51–55].
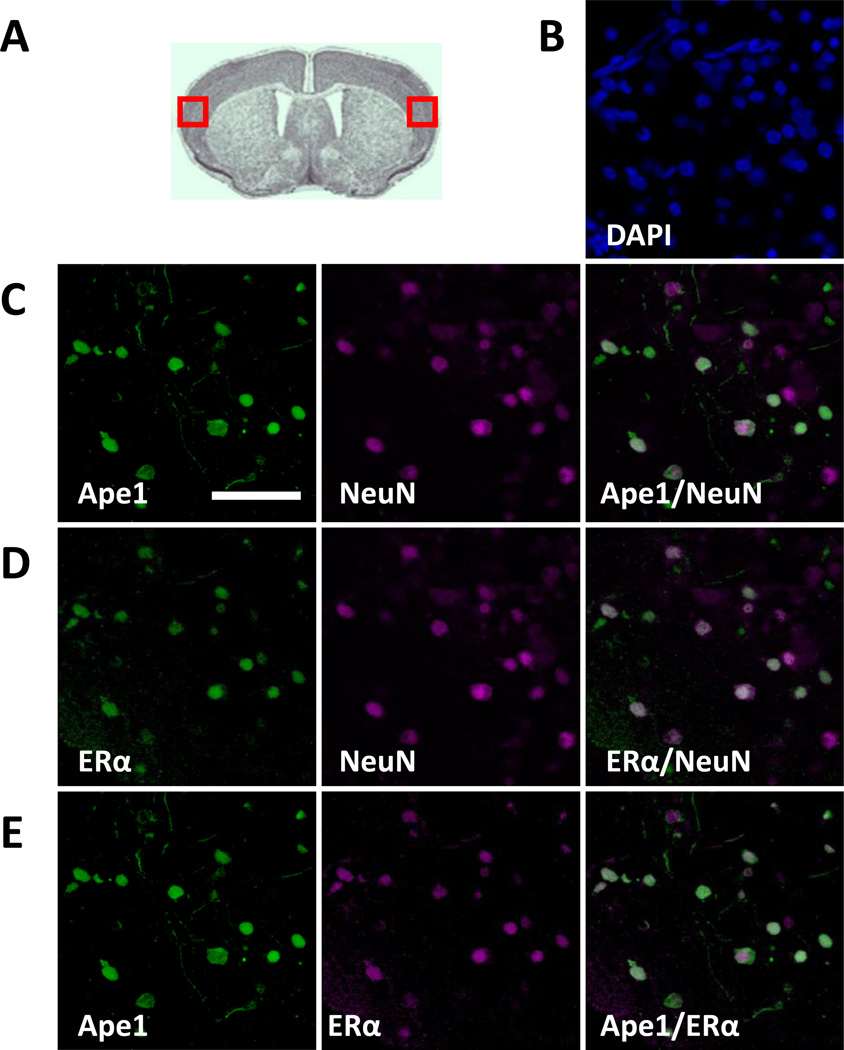
Brain slice cultures were prepared from 7–9 day old C57Bl/6J mouse pups. Immunofluorescent staining was used to characterize the expression of Ape1 and ERα in the cerebral cortex. In order to decrease variation and limit bias, the same regions of the cerebral cortex (Fig. 2A, red boxed regions) were examined in each of the experiments described herein. Ape1 was expressed in the nuclei of cerebral cortical neurons as shown by co-staining with DAPI and the neuronal marker NeuN (Fig. 2B and 2C). Similarly, ERα was expressed in the nuclei of cerebral cortical neurons (Fig. 2D). In contrast, no staining was observed when the Ape1- or ERα-specific antibody was omitted (data not shown). The co-expression of ERα and Ape1 in the nuclei of the same cerebral cortical neurons was evident when the ERα and Ape1 channels were merged (Fig. 2E).
Fig. 2. Ape1 and ERα are expressed in cortical neurons in brain slice cultures.
(A) Red boxes indicate the regions of the cerebral cortex examined by immunofluorescence. Immunofluorescent staining was performed with brain slice cultures using (B) DAPI to identify cortical cell nuclei, (C) Ape1- and NeuN-, (D) ERα- and NeuN-, or (E) Ape1- and ERα-specific antibodies. Scale bar indicates 25 µm.
When brain slice cultures were treated with ethanol or 20 nM E2 for 24 h and stained with an Ape1-specific antibody, Ape1-staining was observed in the absence of E2, but when brain slice cultures were treated with E2, Ape1 staining was increased (Fig. 3A). Immunofluorescence image analysis of ethanol- or E2-treated brain slice cultures demonstrated that Ape1 expression was significantly increased in the cerebral cortex when cultures were treated with E2 compared to cultures that were treated with ethanol vehicle (Fig. 3B).
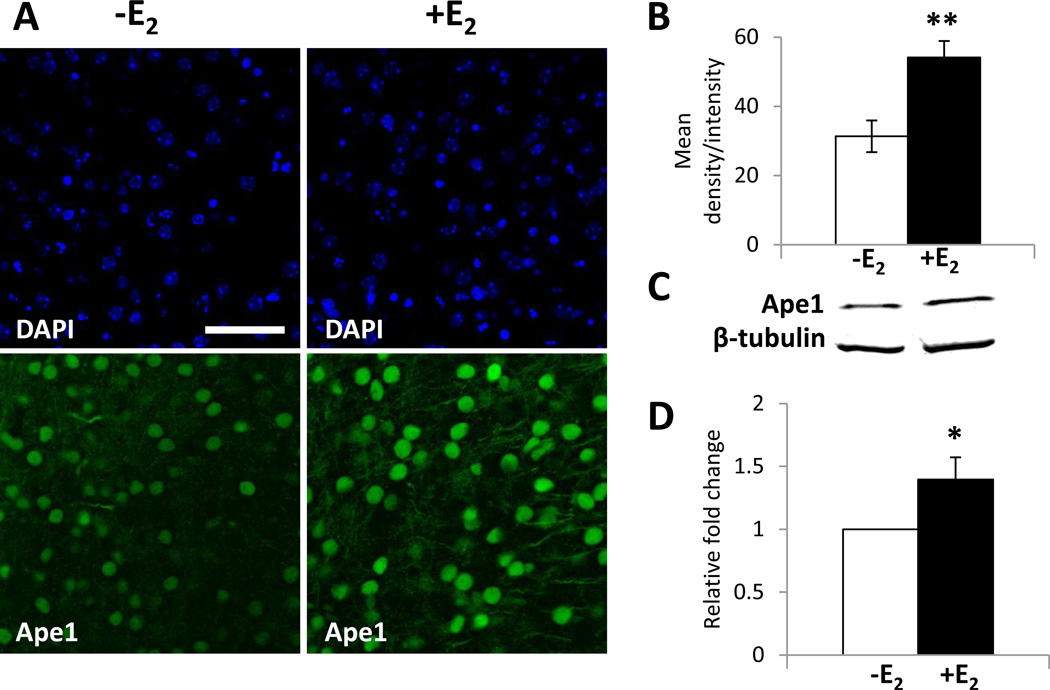
Fig. 3. E2 increases Ape1 expression in brain slice cultures.
Brain slice cultures were treated with ethanol or 20nM E2 for 24 h. (A) Immunofluorescent staining was performed using an Ape1-specific antibody. DAPI staining was included to identify cortical cell nuclei. Scale bar indicates 25 µm. (B) Image analysis of Ape1 expression from 6 individual ethanol- or 7 individual E2-treated brain slice cultures were combined and are expressed as the mean density/intensity ± SEM. (C) Whole cell extracts from brain slice cultures were prepared and quantitative Western blot analysis was performed using Ape1- and β-tubulin-specific antibodies. Ape1 expression was normalized to β-tubulin expression. (D) Data from 8 individual brain slice cultures were combined and are expressed as the normalized expression ± SEM. Ape1 expression from ethanol- or E2-treated brain slice cultures were compared using Student’s t test to determine statistical significance (* p < 0.05, ** p < 0.001).
Whole cell extracts were prepared from brain slice cultures that had been treated with ethanol or 20 nM E2 for 24 h and Western blot analysis was performed to examine the effect of E2 on Ape1 expression using another independent method (Fig. 3C). Combined data from 8 individual brain slice cultures demonstrated that E2 significantly increased Ape1 expression (Fig. 3D). These findings confirmed that E2 increased Ape1 expression in cortical neurons and demonstrated that brain slice cultures provide a valuable model system to study estrogen action in the brain.
3.3. Effect of hypoxia on Ape1 expression and DNA damage in the cerebral cortex of mice
Previous studies have demonstrated that significantly less neural damage is observed when ovariectomized female rodents are treated with E2 and subjected to MCA occlusion than when animals have been treated with oil [24–27]. Thus, MCA occlusion experiments have been extremely informative in demonstrating the neuroprotective effects of E2. However, because the infarct region contains dead or dying cells using this experimental paradigm, the amount of information that can be gathered from these cells is limited. Thus, rather than use an artery occlusion method, we examined the effect of hypoxia, a hallmark of ischemia, in ovariectomized female mice that had or had not been treated with E2.
C57Bl/6J female mice were ovariectomized and silastic tubing containing oil or E2 was implanted. After 7 days, a time when E2-mediated neuroprotection is observed in rodents [25,28–30,56,57], mice were placed in a hypoxia chamber that was equilibrated to 7% oxygen to mimic the decrease in oxygen that can occur with ischemia. The animals were allowed to recover in normoxic conditions and then sacrificed. Control mice that had not been exposed to hypoxia were processed in parallel.
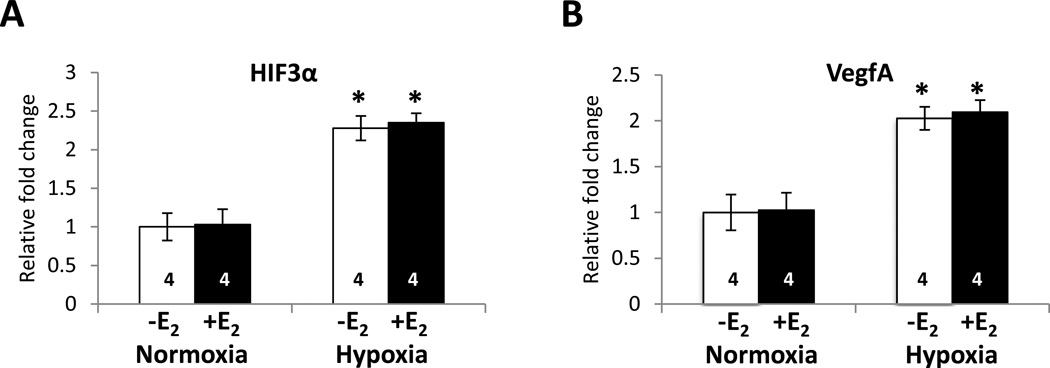
Previous studies have shown that hypoxia induces the expression of several genes in the brain including HIF3α and VegfA [58–62]. Quantitative real-time PCR demonstrated that when animals were exposed to hypoxia for 3 h and allowed to recover for 1 h, HIF3α and VegfA transcript levels were significantly increased (Figs. 4A and 4B, respectively).
Fig. 4. Hypoxia significantly increases HIF3α and VegfA transcript levels.
Ovariectomized female mice were treated with oil or E2 for 7 days and subjected to normoxic or hypoxic conditions. Cortices were dissected and total RNA was isolated. cDNA was synthesized and quantitiative real-time PCR was carried out with (A) HIF3α- or (B) VegfA-specific primers. The relative fold change was calculated using the delta-delta Ct method with ribosomal protein L7 (RPL7) as a control. The mean relative fold change in each group is shown ± SEM. Two-way analysis of variance (ANOVA) was used to detect a significant difference in mRNA levels from mice that had been exposed to hypoxia compared with mice that had been exposed to normoxia (* p < 0.0001). The number of animals in each treatment group is indicated at the base of each bar.
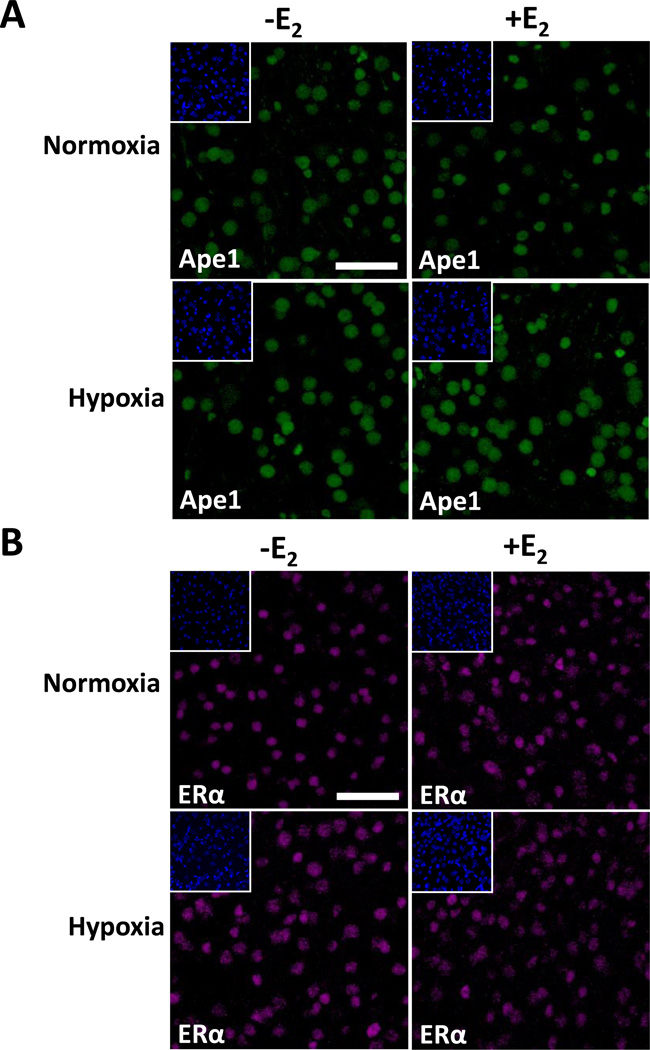
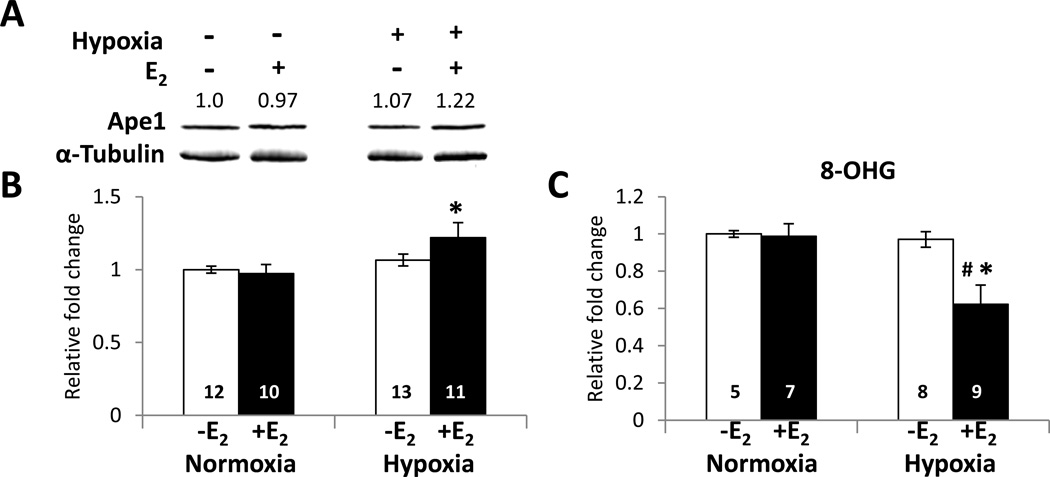
Immunofluorescent staining of the cerebral cortex from ovariectomized female mice that had been treated with oil or E2 for 7 days, exposed to hypoxia for 3 h, and allowed to recover to normoxic conditions for 3 h indicated that Ape1 (Fig. 5A) and ERα (Fig. 5B) were present in the nuclei of the cerebral cortical neurons. Whole cell extracts were prepared from cerebral cortices to determine whether E2 altered Ape1 expression during hypoxia using Western blot analysis (Fig. 6A). As seen in Fig. 6B, a modest but significant increase in Ape1 expression was observed when mice were treated with E2 and exposed to hypoxia. Thus, E2 treatment and hypoxia together increased Ape1 expression in the cerebral cortex of ovariectomized female mice.
Fig. 5. E2 increases Ape1 expression after hypoxia in the mouse cerebral cortex.
Ovariectomized female mice were treated with oil or E2 for 7 days, subjected to normoxic or hypoxic conditions, and allowed to recover for 3h. Immunofluorescent staining was performed using an (A) Ape1- or (B) ERα-specific antibody. DAPI staining is shown in the inserts. Scale bars indicate 25 µm.
Fig. 6. E2 and hypoxia increase Ape1 protein expression and decrease 8-OHG damage in the mouse cerebral cortex.
Ovariectomized female mice were treated with oil or E2 for 7 days and subjected to hypoxic conditions. (A) Quantitative Western blot analysis using whole cell extracts from cerebral cortices was performed using Ape1- and α-tubulin-specific antibodies. (B) Ape1 expression was normalized to α-tubulin expression and data are expressed as the relative fold change ± SEM. The value above each band indicates the relative fold change for each condition. (C) Genomic DNA was isolated from each cerebral cortex and 8-OHG concentrations were measured. Data represent the normalized mean ± SEM. Two-way analysis of variance (ANOVA) was used to detect significant differences in Ape1 protein expression or 8-OHG level in response to hypoxia (* p < 0.05) or E2 (# p < 0.05). Relative fold change on the Y-axis indicates the normalized mean value for each treatment with the value of oil-treated mice maintained at normoxic conditions set at 1. The number of animals in each treatment group is indicated at the base of each bar.
Guanine is particularly susceptible to oxidation and is the most common target of oxidative DNA damage. 8-hydroxydeoxyguanosine (8-OHG) mispairs with adenine and results in a guanine to thymine transversion and perturbation in DNA conformation [63]. To determine whether E2 altered DNA damage during hypoxia, the level of 8-OHG was examined. Ovariectomized female mice were treated with oil or E2 for 7 days, exposed to hypoxia for 3 h, recovered at normoxic conditions for 3 h, and then sacrificed. Control mice that had not been exposed to hypoxia were processed in parallel. Genomic DNA was isolated from cerebral cortices and 8-OHG levels was measured and reported as the normalized mean ± SEM (Fig. 6C). E2 significantly reduced 8-OHG levels in the cerebral cortex of mice that had been exposed to hypoxia.
4. Discussion
Although it has been known for some time that E2 decreases ischemia-induced damage in the rodent brain, the mechanisms mediating this neuroprotective effect have remained unclear [24–27]. We now provide evidence for a link between E2 treatment, Ape1 expression, and neuroprotection. Our studies demonstrate that E2 increased Ape1 expression in the cerebral cortex of brain slice cultures and that combined hypoxia and E2 treatment increased Ape1 expression and decreased 8-OHG oxidative DNA damage in the mouse cerebral cortex.
4.1. Ape1-induced neuroprotection
Previous studies have convincingly demonstrated that Ape1 protects the brain from ischemia-induced injury by decreasing DNA damage and cell death [16–18,22,23]. One way Ape1 might protect the cerebral cortex from ischemia-induced damage is by repairing DNA. In fact, results from our studies demonstrate that when E2 was administered prior to a hypoxic event, the levels of the oxidative DNA damage marker 8-OHG were significantly decreased.
Ape1 is an essential enzyme in the base excision repair pathway. Ape1 recognizes and repairs apurinic sites and also enhances the activity of 8-oxoguanine-DNA glycosylase, the enzyme responsible for removing 8- OHG lesions [6,9–11]. Furthermore, Ape1 activity in base excision repair is especially vital in post-mitotic cells such as neurons that rely predominantly on this pathway to maintain DNA integrity [64–66]. When Ape1 expression declines after MCA occlusion, extensive DNA and cellular damage is observed [16,17]. However, if Ape1 expression is maintained or elevated, the number of DNA lesions significantly decline [17,18,67,68]. Our work demonstrates that even the modest elevation of Ape1 protein expression observed in mice that had been treated with E2 and then exposed to hypoxia is sufficient to decrease oxidative DNA damage. Thus, the E2-induced increase in Ape1 expression may help to protect the cerebral cortex from ischemia-induced DNA damage.
Another mechanism by which Ape1 can reduce ischemic injury is by maintaining numerous proteins in an active, reduced state. When ROS accumulate, cellular proteins can become oxidized and are no longer functional. Ape1 reduces a number of transcription factors including p53, NFκB, Fos, Jun, and HIF1α [12–15]. The ability of these transcription factors to modulate expression of numerous genes could have widespread effects on gene expression, cellular function, and neuronal survival [7,69]. Thus, Ape1 is required to maintain protein structure and function and preserve cellular homeostasis that is critical in the central nervous system. The overall importance of Ape1 is evident in the embryonic death of Ape1 null mice [70,71].
4.2. Combined data from the three models
At first glance the findings from our cell-based, brain slice, and whole animal studies may seem inconsistent. However, a more careful inspection highlights the fact that each model system provides clues about the requirements for Ape1 expression in the cerebral cortex. The fact that no increase in Ape1 expression was observed in primary cortical neurons seems contradictory to the brain slice and whole animal studies where E2 increased Ape1 expression. However, it seems plausible that the failure of E2 to alter Ape1 expression in primary neurons might be attributed to the lack of architectural and organizational features present in the brain microenvironment and/or the absence of other cells such as astroglia, microglia, or oligodendrocytes. Recently, E2 has been shown to increase glial cell release of growth factors that promote neuronal survival [72]. The presence of these other cells and/or organizational features in the brain slice cultures may be required for E2-induced expression of Ape1 as observed in the brain slice cultures. When the neuronal environment was more closely recapitulated in brain slice cultures, E2 treatment was sufficient to enhance Ape1 expression. However, both hypoxia and E2 treatment were required for increased Ape1 expression in the whole animal. If one considers that brain slice cultures are exposed to significant stress during harvesting and slicing, this stress combined with E2 treatment may be sufficient to increase Ape1 expression in brain slice cultures and supports the idea that E2 alone may not be sufficient to increase Ape1 expression.
4.3. Role of E2-induced proteins in neuroprotection
Although our studies focused on the E2-induced expression of a single oxidative stress response protein, Ape1, E2 enhances the expression of other oxidative stress response proteins including Cu/Zn superoxide dismutase and thioredoxin, which are known to reduce ischemia-induced damage [42,73–75]. Superoxide dismutase reduces superoxide levels and decreases protein and DNA damage in neurons [42]. Thioredoxin, like Ape1, reduces oxidized cellular proteins and enhances ischemia-induced neuronal survival [76,77]. Overexpression of Ape1, Cu/Zn superoxide dismutase, or thioredoxin confers a neuroprotective effect following MCA occlusion [18,77,78]. Furthermore, intravenous administration of thioredoxin in mice following ischemia reduces infarct volume, protein damage, and cell death [79]. Together these studies emphasize the important role that oxidative stress response proteins have, as a whole, in sustaining cell viability after an ischemic event [16,17,23,77].
Although an E2-mediated increase in Bcl-2 expression has also been implicated in inhibiting apoptosis after an ischemic event [34,35], it seems unlikely that simply limiting apoptosis in neurons with extensive DNA damage would be beneficial. However, if the E2-mediated increase in Bcl-2 expression was coupled with increased expression of oxidative stress response and DNA repair proteins, together these proteins could reduce protein and DNA damage and enhance neuronal cell survival.
In addition to reducing oxidative stress and apoptosis by mediating the induction of oxidative stress response proteins and Bcl-2 in the brain, E2 attenuates inflammation by reducing pro-inflammatory molecules, decreases cytokine and chemokine release following administration of a neuroinflammatory endotoxin, and reduces microglial activation and peripheral monocyte recruitment [31–33].
While it is clear that ERα plays a role in E2-mediated neuroprotection [29,30], other studies have implicated a role for ERβ in neuroprotection [80]. Both ERα and ERβ are involved in E2-mediated neurogenesis in the subventricular zone, which generates neural stem cells that migrate to the site of injury. [81]. In addition, estrogen binding sites in the mitochondria and plasma membranes have also been implicated in conferring estrogen responsiveness. [82,83] [82,83].
4.4. Biological relevance of E2-induced Ape1 expression
The protective effects of E2 have been documented in numerous animal studies but have also been reported in humans as well [24–30,84,85]. It has been suggested that the decline in production of ovarian hormones contributes to the increased incidence of stroke in postmenopausal women [24,86]. In fact, a number of observational and epidemiological studies have reported that E2 replacement therapy in postmenopausal women reduces stroke as well as coronary heart disease and neurodegenerative diseases [87–93]. Importantly, recent analysis of data from the Women’s Health Initiative indicates that women who initiate E2 replacement therapy before 60 years of age or within ten years of menopause have reduced rates of coronary heart disease and mortality [84,85].
5. Conclusions
Taken together, our studies demonstrate that E2 treatment increases Ape1 expression in the cerebral cortex and suggest that E2 mediates its neuroprotective effects in the brain in part by increasing expression of oxidative stress response proteins and reducing oxidative DNA damage. Because the maintenance or overexpression of Ape1 results in decreased ischemia-induced damage [16–18] and the decline of Ape1 expression leads to an increase in ischemic damage [16,22,23], we believe that the E2-mediated increase in Ape1 expression enables neurons to repair DNA lesions, reduce oxidized cellular proteins, and more effectively regulate transcription.
Highlights.
Apurinic endonuclease (Ape1) is required to maintain protein and DNA integrity.
17β-estradiol (E2) increases Ape1 protein expression in cerebral cortical neurons.
E2 reduces hypoxia-induced oxidative DNA damage in cortical neurons.
The E2-induced expression of Ape1 helps to reduce hypoxia-induced neuronal damage.
These studies identify a mechanism by which E2 neuroprotection may be conferred.
Acknowledgements
We thank L. Raetzman, Y. Ziegler, L. Yuan, and C. Scavuzzo for providing technical expertise and helpful discussions.
This research was supported by NIH grant R01DK 053884 (to AMN). AKD was supported by a predoctoral fellowship from the NIEHS Reproductive Toxicology Training Grant T32 ES007326.
Abbreviations
- DAPI
4′,6-diamidino-2-phenylindole
- 8-OHG
8-hydroxydeoxyguanosine
- Ape1
Apurinic endonuclease 1
- E2
17β-estradiol
- ERα
estrogen receptor α
- ERβ
estrogen receptor β
- ROS
reactive oxygen species
- RPL7
ribosomal protein L7
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT: The authors have nothing to disclose.
References
- 1.Tuma R. The two faces of oxygen. Sci Aging Knowledge Environ. 2001;(1):oa5. doi: 10.1126/sageke.2001.1.oa5. [DOI] [PubMed] [Google Scholar]
- 2.Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312(5782):1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 3.Fialkow L, Chan CK, Rotin D, Grinstein S, Downey GP. Activation of the mitogen-activated protein kinase signaling pathway in neutrophils. Role of oxidants. J. Biol. Chem. 1994;269(49):31234–31242. [PubMed] [Google Scholar]
- 4.Stevenson MA, Pollock SS, Coleman CN, Calderwood SK. X-irradiation, phorbol esters, and H2O2 stimulate mitogen-activated protein kinase activity in NIH-3T3 cells through the formation of reactive oxygen intermediates. Cancer Res. 1994;54(1):12–15. [PubMed] [Google Scholar]
- 5.Bauskin AR, Alkalay I, Ben-Neriah Y. Redox regulation of a protein tyrosine kinase in the endoplasmic reticulum. Cell. 1991;66(4):685–696. doi: 10.1016/0092-8674(91)90114-e. [DOI] [PubMed] [Google Scholar]
- 6.Sancar A, Sancar GB. DNA repair enzymes. Annu. Rev. Biochem. 1988;57:29–67. doi: 10.1146/annurev.bi.57.070188.000333. [DOI] [PubMed] [Google Scholar]
- 7.Wilson SH, Kunkel TA. Passing the baton in base excision repair. Nat. Struct. Biol. 2000;7(3):176–178. doi: 10.1038/73260. [DOI] [PubMed] [Google Scholar]
- 8.Hegde ML, Hazra TK, Mitra S. Early steps in the DNA base excision/single-strand interruption repair pathway in mammalian cells. Cell Res. 2008;18(1):27–47. doi: 10.1038/cr.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill JW, Hazra TK, Izumi T, Mitra S. Stimulation of human 8-oxoguanine-DNA glycosylase by AP-endonuclease: potential coordination of the initial steps in base excision repair. Nucleic Acids Res. 2001;29(2):430–438. doi: 10.1093/nar/29.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saitoh T, Shinmura K, Yamaguchi S, Tani M, Seki S, Murakami H, Nojima Y, Yokota J. Enhancement of OGG1 protein AP lyase activity by increase of APEX protein. Mutat. Res. 2001;486(1):31–40. doi: 10.1016/s0921-8777(01)00078-7. [DOI] [PubMed] [Google Scholar]
- 11.Vidal AE, Hickson ID, Boiteux S, Radicella JP. Mechanism of stimulation of the DNA glycosylase activity of hOGG1 by the major human AP endonuclease: bypass of the AP lyase activity step. Nucleic Acids Res. 2001;29(6):1285–1292. doi: 10.1093/nar/29.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jayaraman L, Murthy KG, Zhu C, Curran T, Xanthoudakis S, Prives C. Identification of redox/repair protein Ref-1 as a potent activator of p53. Genes Dev. 1997;11(5):558–570. doi: 10.1101/gad.11.5.558. [DOI] [PubMed] [Google Scholar]
- 13.Nishi T, Shimizu N, Hiramoto M, Sato I, Yamaguchi Y, Hasegawa M, Aizawa S, Tanaka H, Kataoka K, Watanabe H, Handa H. Spatial redox regulation of a critical cysteine residue of NF-kappa B in vivo. J. Biol. Chem. 2002;277(46):44548–44556. doi: 10.1074/jbc.M202970200. [DOI] [PubMed] [Google Scholar]
- 14.Xanthoudakis S, Curran T. Identification and characterization of Ref-1, a nuclear protein that facilitates AP-1 DNA-binding activity. EMBO J. 1992;11(2):653–665. doi: 10.1002/j.1460-2075.1992.tb05097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lando D, Pongratz I, Poellinger L, Whitelaw ML. A redox mechanism controls differential DNA binding activities of hypoxia-inducible factor (HIF) 1 alpha and the HIF-like factor. J. Biol. Chem. 2000;275(7):4618–4627. doi: 10.1074/jbc.275.7.4618. [DOI] [PubMed] [Google Scholar]
- 16.Fujimura M, Morita-Fujimura Y, Narasimhan P, Copin JC, Kawase M, Chan PH. Copper-zinc superoxide dismutase prevents the early decrease of apurinic/apyrimidinic endonuclease and subsequent DNA fragmentation after transient focal cerebral ischemia in mice. Stroke. 1999;30(11):2408–2415. doi: 10.1161/01.str.30.11.2408. [DOI] [PubMed] [Google Scholar]
- 17.Narasimhan P, Sugawara T, Liu J, Hayashi T, Noshita N, Chan PH. Overexpression of human copper/zinc-superoxide dismutase in transgenic animals attenuates the reduction of apurinic/apyrimidinic endonuclease expression in neurons after in vitro ischemia and after transient global cerebral ischemia. J. Neurochem. 2005;93(2):351–358. doi: 10.1111/j.1471-4159.2005.03039.x. [DOI] [PubMed] [Google Scholar]
- 18.Kim H, Cho K, Park S, Kim H, Kim GW. The adenoviral vector-mediated increase in apurinic/apyrimidinic endonuclease inhibits the induction of neuronal cell death after transient ischemic stroke in mice. Brain Res. 2009;1274:1–10. doi: 10.1016/j.brainres.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Jean WC, Spellman SR, Nussbaum ES, Low WC. Reperfusion injury after focal cerebral ischemia: the role of inflammation and the therapeutic horizon. Neurosurgery. 1998;43(6):1382–1396. doi: 10.1097/00006123-199812000-00076. [DOI] [PubMed] [Google Scholar]
- 20.Edwards M, Kent TA, Rea HC, Wei J, Quast M, Izumi T, Mitra S, Perez-Polo JR. APE/Ref-1 responses to ischemia in rat brain. Neuroreport. 1998;9(18):4015–4018. doi: 10.1097/00001756-199812210-00005. [DOI] [PubMed] [Google Scholar]
- 21.Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J. Cereb. Blood Flow Metab. 2001;21(1):2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Fujimura M, Morita-Fujimura Y, Kawase M, Chan PH. Early decrease of apurinic/apyrimidinic endonuclease expression after transient focal cerebral ischemia in mice. J. Cereb. Blood Flow Metab. 1999;19(5):495–501. doi: 10.1097/00004647-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Kawase M, Fujimura M, Morita-Fujimura Y, Chan PH. Reduction of apurinic/apyrimidinic endonuclease expression after transient global cerebral ischemia in rats: implication of the failure of DNA repair in neuronal apoptosis. Stroke. 1999;30(2):441–448. doi: 10.1161/01.str.30.2.441. [DOI] [PubMed] [Google Scholar]
- 24.Wise PM, Dubal DB, Wilson ME, Rau SW, Bottner M, Rosewell KL. Estradiol is a protective factor in the adult and aging brain: understanding of mechanisms derived from in vivo and in vitro studies. Brain Res. Rev. 2001;37(1–3):313–319. doi: 10.1016/s0165-0173(01)00136-9. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki S, Brown CM, Dela Cruz CD, Yang E, Bridwell DA, Wise PM. Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and antiinflammatory actions. Proc. Natl. Acad. Sci. U. S. A. 2007;104(14):6013–6018. doi: 10.1073/pnas.0610394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wise PM, Dubal DB, Wilson ME, Rau SW. Estradiol is a neuroprotective factor in in vivo and in vitro models of brain injury. J. Neurocytol. 2000;29(5–6):401–410. doi: 10.1023/a:1007169408561. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki S, Brown CM, Wise PM. Neuroprotective effects of estrogens following ischemic stroke. Front. Neuroendocrinol. 2009;30(2):201–211. doi: 10.1016/j.yfrne.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dubal DB, Kashon ML, Pettigrew LC, Ren JM, Finklestein SP, Rau SW, Wise PM. Estradiol protects against ischemic injury. J. Cereb. Blood Flow Metab. 1998;18(11):1253–1258. doi: 10.1097/00004647-199811000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, Kindy MS, Wise PM. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc. Natl. Acad. Sci. U. S. A. 2001;98(4):1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubal DB, Rau SW, Shughrue PJ, Zhu H, Yu J, Cashion AB, Suzuki S, Gerhold LM, Bottner MB, Dubal SB, Merchanthaler I, Kindy MS, Wise PM. Differential modulation of estrogen receptors (ERs) in ischemic brain injury: a role for ERalpha in estradiol-mediated protection against delayed cell death. Endocrinology. 2006;147(6):3076–3084. doi: 10.1210/en.2005-1177. [DOI] [PubMed] [Google Scholar]
- 31.Brown CM, Mulcahey TA, Filipek NC, Wise PM. Production of proinflammatory cytokines and chemokines during neuroinflammation: novel roles for estrogen receptors alpha and beta. Endocrinology. 2010;151(10):4916–4925. doi: 10.1210/en.2010-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cerciat M, Unkila M, Garcia-Segura LM, Arevalo MA. Selective estrogen receptor modulators decrease the production of interleukin-6 and interferon-gamma-inducible protein-10 by astrocytes exposed to inflammatory challenge in vitro. Glia. 2010;58(1):93–102. doi: 10.1002/glia.20904. [DOI] [PubMed] [Google Scholar]
- 33.Vegeto E, Belcredito S, Etteri S, Ghisletti S, Brusadelli A, Meda C, Krust A, Dupont S, Ciana P, Chambon P, Maggi A. Estrogen receptor-alpha mediates the brain antiinflammatory activity of estradiol. Proc. Natl. Acad. Sci. U. S. A. 2003;100(16):9614–9619. doi: 10.1073/pnas.1531957100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dubal DB, Shughrue PJ, Wilson ME, Merchenthaler I, Wise PM. Estradiol modulates bcl-2 in cerebral ischemia: a potential role for estrogen receptors. J. Neurosci. 1999;19(15):6385–6393. doi: 10.1523/JNEUROSCI.19-15-06385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nilsen J, Diaz Brinton R. Mechanism of estrogen-mediated neuroprotection: regulation of mitochondrial calcium and Bcl-2 expression. Proc. Natl. Acad. Sci. U. S. A. 2003;100(5):2842–2847. doi: 10.1073/pnas.0438041100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elzer JG, Muhammad S, Wintermantel TM, Regnier-Vigouroux A, Ludwig J, Schutz G, Schwaninger M. Neuronal estrogen receptor-alpha mediates neuroprotection by 17beta-estradiol. J. Cereb. Blood Flow Metab. 2010;30(5):935–942. doi: 10.1038/jcbfm.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arevalo MA, Santos-Galindo M, Bellini MJ, Azcoitia I, Garcia-Segura LM. Actions of estrogens on glial cells: Implications for neuroprotection. Biochim. Biophys. Acta. 2010;1800(10):1106–1112. doi: 10.1016/j.bbagen.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM. Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications. Steroids. 2007;72(5):381–405. doi: 10.1016/j.steroids.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raval AP, Bramlett H, Perez-Pinzon M. Estrogen preconditioning protects the hippocampal CA1 against ischemia. Neuroscience. 2006;141(4):1721–1730. doi: 10.1016/j.neuroscience.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 40.Zhao L, Brinton RD. Select estrogens within the complex formulation of conjugated equine estrogens (Premarin) are protective against neurodegenerative insults: implications for a composition of estrogen therapy to promote neuronal function and prevent Alzheimer's disease. BMC Neurosci. 2006;7:24. doi: 10.1186/1471-2202-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fukuda K, Yao H, Ibayashi S, Nakahara T, Uchimura H, Fujishima M, Hall ED. Ovariectomy exacerbates and estrogen replacement attenuates photothrombotic focal ischemic brain injury in rats. Stroke. 2000;31(1):155–160. doi: 10.1161/01.str.31.1.155. [DOI] [PubMed] [Google Scholar]
- 42.Rao AK, Dietrich AK, Ziegler YS, Nardulli AM. 17beta-Estradiol-mediated increase in Cu/Zn superoxide dismutase expression in the brain: a mechanism to protect neurons from ischemia. J. Steroid Biochem. Mol. Biol. 2011;127(3–5):382–389. doi: 10.1016/j.jsbmb.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hilgenberg LG, Smith MA. Preparation of dissociated mouse cortical neuron cultures. J. Vis. Exp. 2007;(10):562. doi: 10.3791/562. (10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J. Neurosci. Methods. 1991;37(2):173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- 45.Wise PM, Camp-Grossman P, Barraclough CA. Effects of estradiol and progesterone on plasma gonadotropins, prolactin, and LHRH in specific brain areas of ovariectomized rats. Biol. Reprod. 1981;24(4):820–830. doi: 10.1095/biolreprod24.4.820. [DOI] [PubMed] [Google Scholar]
- 46.Nelson JF, Felicio LS, Osterburg HH, Finch CE. Differential contributions of ovarian and extraovarian factors to age-related reductions in plasma estradiol and progesterone during the estrous cycle of C57BL/6J mice. Endocrinology. 1992;130(2):805–810. doi: 10.1210/endo.130.2.1733727. [DOI] [PubMed] [Google Scholar]
- 47.Couse JF, Curtis SW, Washburn TF, Lindzey J, Golding TS, Lubahn DB, Smithies O, Korach KS. Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Mol. Endocrinol. 1995;9(11):1441–1454. doi: 10.1210/mend.9.11.8584021. [DOI] [PubMed] [Google Scholar]
- 48.Xu H, Lu A, Sharp FR. Regional genome transcriptional response of adult mouse brain to hypoxia. BMC Genomics. 2011;12:499. doi: 10.1186/1471-2164-12-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang YV, Wade M, Wong E, Li YC, Rodewald LW, Wahl GM. Quantitative analyses reveal the importance of regulated Hdmx degradation for p53 activation. Proc. Natl. Acad. Sci. U. S. A. 2007;104(30):12365–12370. doi: 10.1073/pnas.0701497104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomson TM, Benjamin KR, Bush A, Love T, Pincus D, Resnekov O, Yu RC, Gordon A, Colman-Lerner A, Endy D, Brent R. Scaffold number in yeast signaling system sets tradeoff between system output and dynamic range. Proc. Natl. Acad. Sci. U. S. A. 2011;108(50):20265–20270. doi: 10.1073/pnas.1004042108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sundstrom L, Morrison B, 3, Bradley M, Pringle A. Organotypic cultures as tools for functional screening in the CNS. Drug Discov. Today. 2005;10(14):993–1000. doi: 10.1016/S1359-6446(05)03502-6. [DOI] [PubMed] [Google Scholar]
- 52.Shima Y, Kengaku M, Hirano T, Takeichi M, Uemura T. Regulation of dendritic maintenance and growth by a mammalian 7-pass transmembrane cadherin. Dev Cell. 2004;7(2):205–216. doi: 10.1016/j.devcel.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 53.Moser KV, Schmidt-Kastner R, Hinterhuber H, Humpel C. Brain capillaries and cholinergic neurons persist in organotypic brain slices in the absence of blood flow. Eur. J. Neurosci. 2003;18(1):85–94. doi: 10.1046/j.1460-9568.2003.02728.x. [DOI] [PubMed] [Google Scholar]
- 54.Raval AP, Dave KR, Mochly-Rosen D, Sick TJ, Perez-Pinzon M. Epsilon PKC is required for the induction of tolerance by ischemic and NMDA-mediated preconditioning in the organotypic hippocampal slice. J. Neurosci. 2003;23(2):384–391. doi: 10.1523/JNEUROSCI.23-02-00384.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakayama AY, Harms MB, Luo L. Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J. Neurosci. 2000;20(14):5329–5338. doi: 10.1523/JNEUROSCI.20-14-05329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wise P. Estradiol exerts neuroprotective actions against ischemic brain injury: insights derived from animal models. Endocrine. 2003;21(1):11–15. doi: 10.1385/endo:21:1:11. [DOI] [PubMed] [Google Scholar]
- 57.Rau SW, Dubal DB, Bottner M, Gerhold LM, Wise PM. Estradiol attenuates programmed cell death after stroke-like injury. J. Neurosci. 2003;23(36):11420–11426. doi: 10.1523/JNEUROSCI.23-36-11420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heidbreder M, Fröhlich F, Jöhren O, Dendorfer A, Qadri F, Dominiak P. Hypoxia rapidly activates HIF-3α mRNA expression. The FASEB Journal. 2003;17(11):1541–1543. doi: 10.1096/fj.02-0963fje. [DOI] [PubMed] [Google Scholar]
- 59.Greer SN, Metcalf JL, Wang Y, Ohh M. The updated biology of hypoxia-inducible factor. EMBO J. 2012;31(11):2448–2460. doi: 10.1038/emboj.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schoch HJ, Fischer S, Marti HH. Hypoxia - induced vascular endothelial growth factor expression causes vascular leakage in the brain. Brain. 2002;125(11):2549–2557. doi: 10.1093/brain/awf257. [DOI] [PubMed] [Google Scholar]
- 61.Marti HJH, Bernaudin M, Bellail A, Schoch H, Euler M, Petit E, Risau W. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. The American Journal of Pathology. 2000;156(3):965–976. doi: 10.1016/S0002-9440(10)64964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stein I, Neeman M, Shweiki D, Itin A, Keshet E. Stabilization of vascular endothelial growth factor mRNA by hypoxia and hypoglycemia and coregulation with other ischemia-induced genes. Molecular and Cellular Biology. 1995;15(10):5363–5368. doi: 10.1128/mcb.15.10.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sidorenko VS, Nevinsky GA, Zharkov DO. Mechanism of interaction between human 8-oxoguanine-DNA glycosylase and AP endonuclease. DNA Repair (Amst) 2007;6(3):317–328. doi: 10.1016/j.dnarep.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 64.Lan J, Li W, Zhang F, Sun FY, Nagayama T, O'Horo C, Chen J. I nducible repair of oxidative DNA lesions in the rat brain after transient focal ischemia and reperfusion. J. Cereb. Blood Flow Metab. 2003;23(11):1324–1339. doi: 10.1097/01.WCB.0000091540.60196.F2. [DOI] [PubMed] [Google Scholar]
- 65.Li W, Luo Y, Zhang F, Signore AP, Gobbel GT, Simon RP, Chen J. Ischemic preconditioning in the rat brain enhances the repair of endogenous oxidative DNA damage by activating the base-excision repair pathway. J. Cereb. Blood Flow Metab. 2006;26(2):181–198. doi: 10.1038/sj.jcbfm.9600180. [DOI] [PubMed] [Google Scholar]
- 66.Fishel ML, Vasko MR, Kelley MR. DNA repair in neurons: so if they don't divide what's to repair? Mutat. Res. 2007;614(1–2):24–36. doi: 10.1016/j.mrfmmm.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 67.Stetler RA, Gao Y, Zukin RS, Vosler PS, Zhang L, Zhang F, Cao G, Bennett MV, Chen J. Apurinic/apyrimidinic endonuclease APE1 is required for PACAP-induced neuroprotection against global cerebral ischemia. Proc. Natl. Acad. Sci. U. S. A. 2010;107(7):3204–3209. doi: 10.1073/pnas.1000030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cho KJ, Kim HJ, Park SC, Kim HW, Kim GW. Decisive role of apurinic/apyrimidinic endonuclease/Ref-1 in initiation of cell death. Mol. Cell. Neurosci. 2010;45(3):267–276. doi: 10.1016/j.mcn.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 69.Evans AR, Limp-Foster M, Kelley MR. Going APE over ref-1. Mutat. Res. 2000;461(2):83–108. doi: 10.1016/s0921-8777(00)00046-x. [DOI] [PubMed] [Google Scholar]
- 70.Xanthoudakis S, Smeyne RJ, Wallace JD, Curran T. The redox/DNA repair protein, Ref-1, is essential for early embryonic development in mice. Proc. Natl. Acad. Sci. U. S. A. 1996;93(17):8919–8923. doi: 10.1073/pnas.93.17.8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Izumi T, Brown DB, Naidu CV, Bhakat KK, Macinnes MA, Saito H, Chen DJ, Mitra S. Two essential but distinct functions of the mammalian abasic endonuclease. Proc. Natl. Acad. Sci. U. S. A. 2005;102(16):5739–5743. doi: 10.1073/pnas.0500986102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arevalo M, Santos-Galindo M, Bellini M, Azcoitia I, Garcia-Segura LM. Actions of estrogens on glial cells: Implications for neuroprotection. Biochimica et Biophysica Acta (BBA) - General Subjects. doi: 10.1016/j.bbagen.2009.10.002. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 73.Rao AK, Ziegler YS, McLeod IX, Yates JR, Nardulli AM. Effects of Cu/Zn superoxide dismutase on estrogen responsiveness and oxidative stress in human breast cancer cells. Molecular Endocrinololgy. 2008;22(5):1113–1124. doi: 10.1210/me.2007-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rao AK, Ziegler YS, McLeod IX, Yates JR, Nardulli AM. Thioredoxin and thioredoxin reductase influence estrogen receptor alpha-mediated gene expression in human breast cancer cells. J. Mol. Endocrinol. 2009;43(6):251–261. doi: 10.1677/JME-09-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee SY, Andoh T, Murphy DL, Chiueh CC. 17beta-estradiol activates ICI 182,780-sensitive estrogen receptors and cyclic GMP-dependent thioredoxin expression for neuroprotection. FASEB J. 2003;17(8):947–948. doi: 10.1096/fj.02-0807fje. [DOI] [PubMed] [Google Scholar]
- 76.Hirota K, Matsui M, Iwata S, Nishiyama A, Mori K, Yodoi J. AP-1 transcriptional activity is regulated by a direct association between thioredoxin and Ref-1. Proc. Natl. Acad. Sci. U. S. A. 1997;94(8):3633–3638. doi: 10.1073/pnas.94.8.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Takagi Y, Mitsui A, Nishiyama A, Nozaki K, Sono H, Gon Y, Hashimoto N, Yodoi J. Overexpression of thioredoxin in transgenic mice attenuates focal ischemic brain damage. Proc. Natl. Acad. Sci. U. S. A. 1999;96(7):4131–4136. doi: 10.1073/pnas.96.7.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kinouchi H, Epstein CJ, Mizui T, Carlson E, Chen SF, Chan PH. Attenuation of focal cerebral ischemic injury in transgenic mice overexpressing CuZn superoxide dismutase. Proc. Natl. Acad. Sci. U. S. A. 1991;88(24):11158–11162. doi: 10.1073/pnas.88.24.11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hattori I, Takagi Y, Nakamura H, Nozaki K, Bai J, Kondo N, Sugino T, Nishimura M, Hashimoto N, Yodoi J. Intravenous administration of thioredoxin decreases brain damage following transient focal cerebral ischemia in mice. Antioxid. Redox Signal. 2004;6(1):81–87. doi: 10.1089/152308604771978372. [DOI] [PubMed] [Google Scholar]
- 80.Zhao L, Wu TW, Brinton RD. Estrogen receptor subtypes alpha and beta contribute to neuroprotection and increased Bcl-2 expression in primary hippocampal neurons. Brain Res. 2004;1010(1–2):22–34. doi: 10.1016/j.brainres.2004.02.066. [DOI] [PubMed] [Google Scholar]
- 81.Suzuki S, Gerhold LM, Bottner M, Rau SW, Dela Cruz C, Yang E, Zhu H, Yu J, Cashion AB, Kindy MS, Merchenthaler I, Gage FH, Wise PM. Estradiol enhances neurogenesis following ischemic stroke through estrogen receptors alpha and beta. J. Comp. Neurol. 2007;500(6):1064–1075. doi: 10.1002/cne.21240. [DOI] [PubMed] [Google Scholar]
- 82.Singh M, Dykens JA, Simpkins JW. Novel mechanisms for estrogen-induced neuroprotection. Exp. Biol. Med. (Maywood) 2006;231(5):514–521. doi: 10.1177/153537020623100505. [DOI] [PubMed] [Google Scholar]
- 83.Yang SH, Liu R, Perez EJ, Wen Y, Stevens SM, Jr, Valencia T, Brun-Zinkernagel AM, Prokai L, Will Y, Dykens J, Koulen P, Simpkins JW. Mitochondrial localization of estrogen receptor beta. Proc. Natl. Acad. Sci. U. S. A. 2004;101(12):4130–4135. doi: 10.1073/pnas.0306948101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL, Stefanick ML. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297(13):1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 85.Hodis HN, Collins P, Mack WJ, Schierbeck LL. The timing hypothesis for coronary heart disease prevention with hormone therapy: past, present and future in perspective. Climacteric. 2012;15(3):217–228. doi: 10.3109/13697137.2012.656401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alonso de Lecinana M, Egido JA. Estrogens as neuroprotectants against ischemic stroke. Cerebrovasc. Dis. 2006;21(Suppl 2):48–53. doi: 10.1159/000091703. [DOI] [PubMed] [Google Scholar]
- 87.Bush TL, Barrett-Connor E, Cowan LD, Criqui MH, Wallace RB, Suchindran CM, Tyroler HA, Rifkind BM. Cardiovascular mortality and noncontraceptive use of estrogen in women: results from the Lipid Research Clinics Program Follow-up Study. Circulation. 1987;75(6):1102–1109. doi: 10.1161/01.cir.75.6.1102. [DOI] [PubMed] [Google Scholar]
- 88.Adams MR, Kaplan JR, Manuck SB, Koritnik DR, Parks JS, Wolfe MS, Clarkson TB. Inhibition of coronary artery atherosclerosis by 17-beta estradiol in ovariectomized monkeys. Lack of an effect of added progesterone. Arteriosclerosis. 1990;10(6):1051–1057. doi: 10.1161/01.atv.10.6.1051. [DOI] [PubMed] [Google Scholar]
- 89.Rijpkema AH, van der Sanden AA, Ruijs AH. Effects of post-menopausal oestrogen-progestogen replacement therapy on serum lipids and lipoproteins: a review. Maturitas. 1990;12(3):259–285. doi: 10.1016/0378-5122(90)90007-s. [DOI] [PubMed] [Google Scholar]
- 90.Stampfer MJ, Colditz GA. Estrogen replacement therapy and coronary heart disease: a quantitative assessment of the epidemiologic evidence. Prev. Med. 1991;20(1):47–63. doi: 10.1016/0091-7435(91)90006-p. [DOI] [PubMed] [Google Scholar]
- 91.Paganini-Hill A, Ross RK, Henderson BE. Postmenopausal oestrogen treatment and stroke: a prospective study. BMJ. 1988;297(6647):519–522. doi: 10.1136/bmj.297.6647.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Prog. Neurobiol. 2001;63(1):29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- 93.Asthana S, Craft S, Baker LD, Raskind MA, Birnbaum RS, Lofgreen CP, Veith RC, Plymate SR. Cognitive and neuroendocrine response to transdermal estrogen in postmenopausal women with Alzheimer's disease: results of a placebo-controlled, double-blind, pilot study. Psychoneuroendocrinology. 1999;24(6):657–677. doi: 10.1016/s0306-4530(99)00020-7. [DOI] [PubMed] [Google Scholar]