Abstract
Investigations into cell therapies for application in organ transplantation have grown. Here, we describe the ex vivo generation of donor bone marrow–derived dendritic cells (BMDCs) and glucocorticoid-treated BMDCs with potent immunomodulatory properties for application in allogeneic transplantation. BMDCs were treated with dexamethasone (Dexa) to induce an immature, maturation-resistant phenotype. BMDC and Dexa BMDC phenotype, antigen presenting cell function, and immunomodulatory properties were fully characterized. Both populations display significant immunomodulatory properties, including, but not limited to, a significant increase in mRNA expression of programmed death-ligand 1 and indoleamine 2,3-dioxygenase. BMDCs and Dexa BMDCs display a profound impaired capacity to stimulate allogeneic lymphocytes. Moreover, in a fully MHC I/II mismatched rat corneal transplantation model, injection of donor-derived, untreated BMDC or Dexa BMDCs (1 × 106 cells, day −7) significantly prolonged corneal allograft survival without the need for additional immunosuppression. Although neovascularization was not reduced and evidence of donor-specific alloantibody response was detected, a significant reduction in allograft cellular infiltration combined with a significant increase in the ratio of intragraft FoxP3-expressing regulatory cells was observed. Our comprehensive analysis demonstrates the novel cellular therapeutic approach and significant effect of donor-derived, untreated BMDCs and Dexa BMDCs in preventing corneal allograft rejection.
INTRODUCTION
Dendritic cell (DC) biology has greatly evolved since DCs were first identified and described by Steinman et al. in 1973.1 It is now well accepted that DCs derived from bone marrow hematopoietic stem cells are not only potent immune inducers, linking innate and adaptive immunity but they are also essential for the induction and maintenance of tolerance.2 DCs are important modulators of T-cell phenotype and function, providing the signals required for T cells to become fully activated effector or regulatory cells.3 All of these DC functions are dependent on the phenotypical state and the immunological environment within which the DCs find themselves.2 In recent years, manipulation of DC maturation, by altering the expression level of MHCII and costimulatory molecules, has been investigated by treating DCs with various cytokines or pharmaceutical agents,4,5,6,7,8 resulting in the generation of immature or regulatory DC phenotypes. This has generated considerable interest within the field of transplantation immunology as presently immunosuppressive drug therapy is the main rejection prophylaxis, with which harmful toxic side effects are associated. Accordingly, modified DC therapies for the promotion of allograft survival are an attractive and promising alternative. Administration of immature regulatory DCs has been investigated with varying degrees of efficacy in multiple transplantation models and it has become evident that the role of DCs in the immune response and allograft rejection is complex and dependant on a variety of factors including the source (recipient or donor) of the DCs, the nature of the DCs, the level of maturation, the environment in which the DCs become activated, and the model within which the DCs are administered.9,10,11,12 Recently, in models of islet and skin transplantation, it has been demonstrated that pretreatment with donor-derived tolerogenic DCs may be linked to an increased risk of sensitization of the recipient immune system rather than tolerance induction.9,13 Although autologous tolerogenic DCs are being investigated14,15 and preclinical studies with an aim to develop tolerogenic monocyte-derived DC for a clinical application have begun16 a comprehensive analysis of the mechanisms involved and characterization of both autologous and donor-derived, DC-mediated tolerance induction is required.
DC application in corneal transplantation remains relatively uninvestigated and only recently it was demonstrated that regulatory donor DCs suppress the indirect pathway of allosensitization in corneal transplantation, an important observation for the development of cell therapies for corneal transplantation as the cornea is the most commonly transplanted tissue.8,17 The eye is described as an immune privileged organ, though this privilege is in a dynamic rather than a static state as not all corneal allografts succeed in humans or in experimental animals.18 Although corneal transplantation is a relatively risk-free, uncomplicated procedure with 90% survival within the first year after transplantation, the 5-year prognosis is similar to that of renal, liver, or cardiac allografts with rejection remaining as the main cause of allograft failure.19 The application of ex vivo–generated dexamethasone (Dexa)-treated bone marrow–derived dendritic cells (BMDCs) has not been demonstrated, to our knowledge, in corneal transplantation.
We hypothesized that administration of donor-derived Dexa BMDCs will promote corneal allograft survival. To test this hypothesis in the present study, we have fully characterized the phenotype and immunomodulatory properties of Dexa BMDCs in both quiescent and inflammatory conditions with untreated BMDCs serving as a control. We also investigated the administration of both donor untreated BMDCs and Dexa-treated BMDCs to promote corneal allograft survival, obviating the need for immunosuppression. We aimed to describe and characterize in detail the local immune environment at the level of both the graft and the draining lymph nodes (LNs) as a result of these cell therapies.
In our allogeneic corneal transplant model, our results clearly demonstrate that both donor untreated BMDCs and Dexa BMDCs significantly prolong corneal allograft survival, which appears to be mediated by the generation of a protective, regulatory microenvironment within the graft but, significantly, also in the draining LNs. In contrast, recipient-derived, alloantigen-pulsed BMDCs do not promote graft survival. Although unresponsiveness to donor antigen in the periphery appears not to be induced after treatment with donor untreated BMDCs or Dexa BMDCs, nevertheless the corneal allograft remains protected and is not rejected. To our knowledge, our results collectively demonstrate for the first time, the efficacy of donor untreated BMDC and Dexa BMDC treatment in a fully MHC mismatched rodent corneal allograft model and details the level and phenotype of infiltrating immune cell populations and immune microenvironment within the graft and draining LNs of corneal allograft accepting recipients.
Results
Phenotypical and functional characterization of ex vivo–generated BMDCs and Dexa BMDCs
Bone marrow cells were differentiated in the presence of rat GM-CSF and interleukin (IL)-4 (5 ng/ml respectively). For Dexa-treated cultures, a final concentration of 10−6 mol/l of the glucocorticoid was added to the culture every other day from day 4. The phenotype of BMDCs was analyzed by flow cytometry on day 10. Gating on the CD11b/c+ population, the percentage expression levels of MHCII and the costimulatory molecules CD80 and CD86 indicated a semimature BMDC phenotype (Figure 1a). Treatment of BMDCs with Dexa resulted in a significant reduction in the expression level of these maturation markers (Figure 1a). To investigate expression of costimulatory molecules under inflammatory conditions, semimature BMDC cultures were stimulated for 24 hours with lipopolysaccharide (LPS, 1 µg/ml) and analyzed for expression of MHCII, CD80, and CD86. Results illustrated that LPS-stimulated Dexa BMDCs had a significantly lower level of expression of these maturation molecules compared with LPS-stimulated BMDC cultures. Expression of these maturation markers did not change significantly from unstimulated Dexa BMDCs, indicating that this DC population was maturation resistant (Figure 1a). Supernatants from day 10 cultures of unstimulated and LPS-stimulated (24 hours) BMDC and Dexa BMDC cultures were analyzed for the presence of the cytokines TNF-α and IL-10 (Figure 1b). LPS-stimulated Dexa BMDCs produced significantly higher amounts of IL-10 and lower amounts of TNF-α compared with BMDCs (Figure 1b). Supernatants from both BMDC and Dexa BMDC cultures demonstrated activity of the immunosuppressive molecule nitric oxide (NO) as measured by NO2− levels, with Dexa BMDC supernatants containing significantly higher levels of NO2− (Figure 1b). A detailed examination of BMDC and Dexa BMDC immunomodulatory molecule, cytokine, chemokine, and TLR mRNA expression profile by RT-PCR for day 10 unstimulated and LPS-stimulated cultures revealed that unstimulated and stimulated BMDCs express significantly higher levels of programmed death-ligand 1 (PD-L1) and inducible nitric oxide synthase (iNOS) compared with that of Dexa BMDCs (Figure 1c and Supplementary Figure S1b respectively). mRNA expression of indoleamine 2,3-dioxygenase (IDO), a tryptophan degrading enzyme, was significantly higher in stimulated Dexa BMDC cultures compared with BMDCs (Figure 1c). In summary, these results indicate that ex vivo–generated BMDCs display a semimature phenotype and only fully mature under inflammatory conditions. In contrast, Dexa BMDCs display an immature, maturation-resistant phenotype which is retained even under inflammatory conditions. Our results also demonstrate that BMDCs and Dexa BMDCs not only differ significantly in phenotype and expression of immunomodulatory molecules but they may use different mechanisms of immunosuppression. To evaluate the functional properties of ex vivo–generated BMDCs and Dexa BMDC, we analyzed their capacity to phagocytose and process antigen using a DQ OVA assay (Supplementary Figure S1e). We also assessed the allostimulatory capacity of BMDCs and Dexa BMDCs in an allogeneic setting. T-cell proliferation assays which compared freshly isolated mature donor (Dark Agouti, DA) Ox62+ DCs with both BMDCs and Dexa BMDCs demonstrated that BMDCs and Dexa BMDCs have a reduced capacity to induce allogeneic (Lewis, LEW) lymphocyte proliferation (Figure 1d). Moreover, there was a trend towards reduced expression of the T-cell activation marker CD25 and higher FoxP3 expression in BMDC- and Dexa BMDC–stimulated cultures (Supplementary Figure S1f). Finally, the immunosuppressive potential of BMDCs and Dexa BMDCs was examined in LEW lymphocyte cultures stimulated with allogeneic DA Ox62+ DCs, which were significantly suppressed with the addition of allogeneic DA BMDCs or Dexa BMDCs (Figure 1d no significant difference between BMDCs and Dexa BMDCs). Taken together, our results indicate that ex vivo–generated BMDC and Dexa BMDCs are functionally active antigen presenting cells (APCs) with a profound capacity to modulate allogeneic immune responses.
Figure 1.
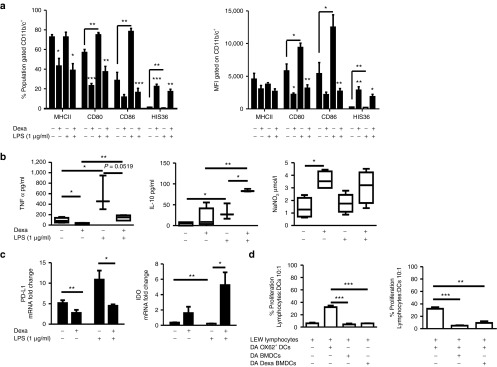
Phenotypic and functional characterization of immunomodulatory ex vivo–generated BMDCs and Dexa BMDCs. BMDCs were differentiated with GMCSF and IL-4 (5 ng/ml respectively) for 10 days in the absence or presence of Dexa (10−6 mol/l added to the culture on day 4). (a) Mean percentage and MFI of MHCII, CD80, CD86, and HIS36 expression (examined to monitor the presence of contaminating macrophage but known to be increased with glucocorticoid treatment within the CD11b/c+ population),50 BMDCs cultures were stimulated with LPS (1 µg/ml, 24 hours) (mean ± SEM n = 3 experiments *P ≤ 0.05 two-tailed Student's t-test). (b) Supernatants of BMDCs and Dexa BMDC were analyzed by ELISA for production of TNF-α, IL-10 and by Griess assay to measure nitrite (NO2−) production, before and after LPS stimulation (mean ± SEM *P ≤ 0.05 and **P ≤ 0.01 two-tailed Student's t-test n = 3–6). (c) mRNA expression (normalized to β-actin and fold change relative to Ox62+ DCs) of immunomodulatory molecules PDL-1, IDO (mean ± SEM *P ≤ 0.05, **P ≤ 0.01 and ***P ≤ 0.001 two-tailed Student's t-test n = 3). (d) DA BMDCs and Dexa BMDCs cocultured with allogeneic LEW lymphocytes have a reduced stimulatory capacity and an immunosuppressive capacity, illustrated by the reduction in percentage proliferating CFSE-labeled lymphocytes stimulated (10:1) with Ox62+ DC in the presence of (10:1 lymphocyte:BMDC) BMDCs or Dexa BMDCs (representative graph of four independent experiments mean ± SEM *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001 two-tailed Student's t-test). BMDC, bone marrow–derived dendritic cell; GM-CSF, granulocyte-macrophage colony-stimulating factor; LPS, lipopolysaccharide.
Ex vivo–generated, donor-derived, untreated BMDC and Dexa BMDC therapies prolong corneal allograft survival
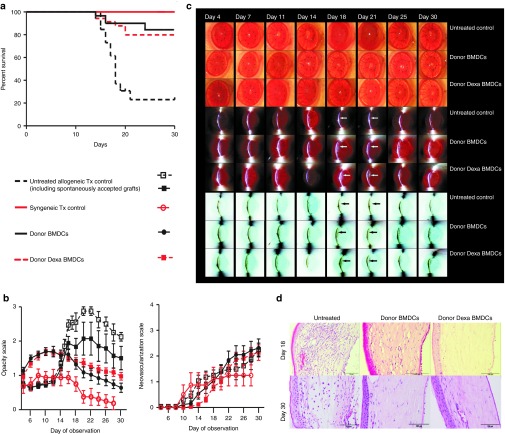
Next, we assessed the capacity of Dexa BMDCs (and control untreated BMDCs) to modulate allogeneic immune responses in vivo in a fully allogeneic corneal transplantation model (DA (donor)/LEW (recipient)). Before corneal transplantation (day −7), recipient LEW rats received an intravenous (i.v.) injection of donor untreated BMDCs or Dexa BMDCs (1 × 106 cells). In untreated animals receiving allogeneic corneal grafts, transplants were rejected uniformly with a mean survival time (MST) ± SD of 18 ± 1.57 days. In contrast, significant prolongation of corneal allograft survival was observed in transplanted animals receiving donor Dexa BMDC (MST ≥ 30 days). Interestingly, this was also achieved in animals receiving donor untreated BMDCs (Figure 2a). Although both BMDC treatments resulted in a significant reduction of corneal opacity, corneal neovascularization was not affected by either BMDC injection (Figure 2b). Clinical evaluation of the corneal allografts by light and slit lamp microscopy followed by histological analysis confirmed a significant reduction in the level of infiltration of inflammatory cells on day 18 (average day of rejection) and on day 30 after transplantation for both treated groups (Figure 2c,d). Evidence of reduced corneal thickness was observed at day 30 for both treatments (Supplementary Figure S2b). In contrast to the therapeutic effect achieved with donor BMDCs, application of Dexa-treated syngeneic (recipient derived) BMDCs pulsed with donor alloantigen did not prolong corneal allograft survival (MST 14 ± 7.16 days, Supplementary Figure S1g). Our results, therefore, indicate that single i.v. administration of donor-derived, untreated BMDCs or Dexa BMDCs, without additional immunosuppressive therapies, is sufficient to promote corneal allograft survival.
Figure 2.
Prolongation of corneal allograft survival with donor-derived, untreated BMDCs or Dexa BMDCs administration. (a) Graft survival curves of allogeneic transplantation (Tx) controls (n = 26), syngeneic Tx controls (n = 8), donor BMDCs (1 × 106 cells/ml PBS i.v. n = 30) and donor Dexa BMDCs (1 × 106 cells/ml PBS i.v. n = 34) (Kaplan–Meier survival analysis, n numbers include animals used for experiments in addition to the illustrated survival and opacity analysis above). (b) Opacity scores and neovascularization scores day 4–day 30 of control groups, donor BMDCs- and Dexa BMDCs–treated groups. (c) Every other day after transplantation, corneal allograft opacity was evaluated by light microscopy, slit lamp, and contrast slit lamp images for all groups, arrows indicate slit lamp reflection in iris visible only in donor BMDCs- and Dexa BMDCs–treated groups. (d) H&E-stained section of the cornea also illustrate a reduction in corneal allograft cell infiltration which was evident for both BMDC- and Dexa BMDC–treated groups at day 18 and day 30 points (n = 2–5 per group). BMDC, bone marrow–derived dendritic cell.
Investigation into the mechanism of untreated BMDC- and Dexa BMDC–mediated prolongation of corneal allograft survival
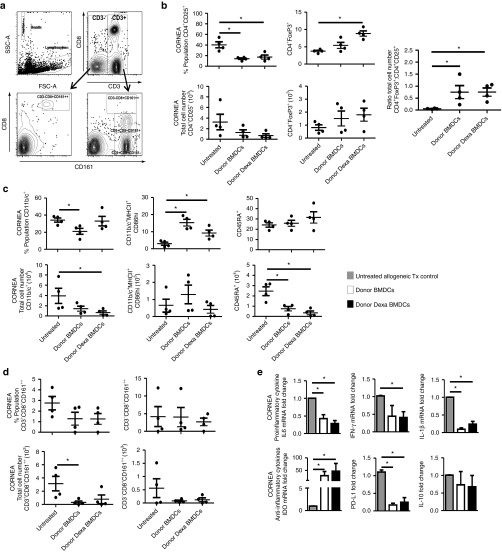
To further characterize untreated BMDC and Dexa BMDCs mechanism to promote survival of corneal allografts, we examined the phenotype of the cell populations infiltrating the allograft and in secondary lymphoid organs by flow cytometry and RT-PCR. As expected, the significantly reduced corneal opacity levels correlated with a significant reduction in the absolute number of cells isolated from corneal allografts for both treatments (Supplementary Figure S2a). A significant reduction in the frequency of activated T cells (CD4+CD25+) was observed in both treated groups (Figure 3b). There was also a significant increase in the percentage of intragraft regulatory CD4+FoxP3+ cells within the Dexa BMDC–treated group and an overall significant increase in the ratio of FoxP3+ regulatory T cells to CD4+CD25+ activated T cells in both treated groups (Figure 3b). The absolute numbers of CD11b/c+ cells (monocyte/macrophage/DCs) were reduced in BMDC and Dexa BMDC groups; however, both treatments resulted in a significant increase in the frequency (percentage population) of CD11b/c+ MHCII+CD86+ DCs present in the graft (Figure 3c). A significant reduction in the total number of B cells (CD45RA+) in the cornea and a trend towards a reduced frequency and total cell number of activated natural killer T (NKT) (CD3+CD8+CD161++) and activated NK cells (CD3−CD8+CD161++) for both treated groups was also observed (Figure 3d). Results of cytokine RT-PCR analysis revealed a significant reduction in the mRNA expression levels of IL-6 and IL-1β for both treated groups within the corneal graft (Figure 3e). IFN-γ mRNA expression was also significantly reduced in Dexa BMDC group (Figure 3e). We also detected a profound increase in the level of IDO mRNA expression in the corneal graft. Interestingly, PD-L1 mRNA expression was significantly reduced and no detectable changes in IL-10 mRNA levels for both BMDC- and Dexa BMDC–treated groups (Figure 3e) were observed. The secondary lymphoid organs (ipsilateral draining LNs and the spleen (data not shown)) were collected from grafted animals and analyzed (Supplementary Figure S2d–f). Results indicated that there was a trend towards a reduction in CD4+CD25+ T cells in the draining LNs and an increased ratio of regulatory CD4+FoxP3+ cells (Supplementary Figure S2e). Upon investigating the mRNA expression of immunomodulatory molecules in the draining LN, a profound increase in the level of IDO mRNA expression and a significant increase in FoxP3 mRNA was also detected for both treated groups (Supplementary Figure S2f). In summary, our results indicate that administration of donor untreated BMDCs and Dexa BMDCs promotes an immunoregulatory microenvironment within the corneal allograft itself and the draining LNs resulting in prolongation of graft survival.
Figure 3.
Both untreated BMDC- and Dexa BMDC–administration result in a reduction in percentage and absolute number of graft infiltrating cells and an increased ratio of intragraft FoxP3+–expressing cells. (a) Gating strategy for corneal cell–infiltrating analysis. (b) The corneal infiltrating population of activated T cells (CD4+CD25+) and regulatory T cells (CD4+FoxP3+) were analyzed looking at percentage cell population, total cell number. The intragraft ratio of regulatory CD4+FoxP3+ T cells to activated CD4+CD25+ T cells was also analyzed (mean ± SEM *P ≤ 0.05 two-tailed Mann–Whitney test n = 4 per group). (c) Infiltrating population of APCs (CD11b/c+), DCs (CD11b/c+MHCII+CD86hi), and B cells (CD45RA) were evaluated, as were (d) activated NKT (CD3+CD8+CD161++), NK (CD3−CD8+CD161++) (mean ± SEM *P ≤ 0.05 two-tailed Mann–Whitney test n = 4 per group). (e) mRNA analysis of intragraft cytokine expression (normalized to β-actin, fold change relative to untreated allogeneic Tx controls) for proinflammatory cytokines IL-6, IFN-γ, and IL-1β and IDO, PD-L1, and IL-10 expression (mean ± SEM *P ≤ 0.05 two-tailed Mann–Whitney test n = 4 per group). BMDC, bone marrow–derived dendritic cell.
Evaluation of peripheral donor-specific unresponsiveness and alloantibody production after donor untreated BMDC and Dexa BMDC administration
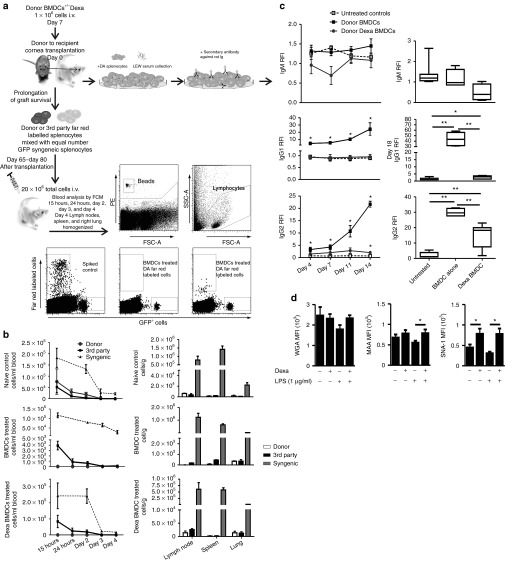
To examine the induction of peripheral donor-specific unresponsiveness following BMDC and Dexa BMDCs treatment, a strategy was devised where grafted animals day 65–80 after transplantation were rechallenged with donor antigen (Figure 4a). Injection of complete mismatched cells to donor and recipient (3rd party Sprague Dawley (CD) rats) which mimics first-time exposure to an antigen was used as a control. Detection of fluorescently labeled cells within the circulation was examined for all groups between 15 hours and 4 days after injection (Figure 4a,b). Both control 3rd party and syngeneic splenocytes were detected in the blood at similar frequencies in all groups. In naïve groups (recipients without corneal transplantation) donor (DA) splenocytes could be detected at similar frequencies to that of 3rd party splenocytes. However, results from recipients treated with donor-derived BMDCs and Dexa BMDCs at day 65–80 after transplantation which were injected with syngeneic and donor-derived splenocyte cell mix, revealed that only the syngeneic cells were detectable 15 hours after injection (Figure 4b). This indicated that donor cells were removed from the circulation at a faster rate than that of 3rd party or syngeneic cells in both treated groups compared with that of naïve recipients, thereby showing donor responsiveness. Analysis of cell distribution within LNs, spleen and lung on day 4 after injection was performed (Figure 4b). It is important to note that 4 days after rechallenge of grafted animals with donor splenocytes the graft itself remained clear and did not reject (Supplementary Figure S2g). The rechallenge and subsequent rapid loss of detectable donor splenocytes in the circulation of treated groups indicated the possible presence of donor-specific alloantibodies. Analysis of the serum from untreated and BMDC-treated, transplanted groups (day 4–14 and day 18 after transplantation) for the presence of donor-specific alloantibodies revealed significantly higher levels of IgG1 and IgG2 antibodies recognizing donor antigen detected in the serum from BMDC-treated animals compared with untreated transplanted controls (IgM response for all groups did not significantly differ, Figure 4c). The Dexa BMDC response was significantly lower compared with untreated BMDC-treated animals, with the detectable IgG1 and IgG2 response of Dexa BMDC–treated animals remaining similar to that of untreated transplanted animals until day 14/18 at which time a significant increase was observed (Figure 4c). To characterize the differences in the donor alloantibody response, we further examined the phenotype of untreated BMDCs and Dexa BMDCs looking at the modifications in the glycome profile of Dexa BMDC cultures compared with untreated BMDCs. We found that Dexa BMDCs (Figure 4d) had significantly higher expression of α-2,3 linked sialic acids compared with untreated BMDCs after LPS stimulation. Moreover, the α-2,6 sialic linked acid expression, characteristic of a tolerogenic, immature DC, was significantly higher in Dexa BMDC cultures before and after LPS stimulation compared with untreated BMDCs. The differential glycome profile may in part explain the difference observed in antibody production. Furthermore, the donor alloantibody results may explain the results observed in peripheral clearance of donor splenocytes after administration of donor-derived BMDCs and Dexa BMDCs. Although there were detectable levels of donor-specific alloantibodies and a clearance of the injected donor splenocytes, crucially, the grafts themselves remained clear and did not reject suggesting a level of local graft protection (Supplementary Figure S2g).
Figure 4.
Effects of donor untreated BMDC and Dexa BMDC administration on peripheral donor-specific unresponsiveness. (a) Illustration of experimental design for evaluation of peripheral donor-specific unresponsiveness (including gating strategy) and alloantibody detection. (b) Day 65–day 80 after transplantation, long-term allograft surviving and naïve ungrafted LEW rats were injected i.v. with a total of 20 × 106–labeled Far Red donor (DA) + GFP syngeneic (LEW) splenocytes or Far Red 3rd party (CD) + GFP syngeneic cells. Blood samples were collected from naïve ungrafted, BMDC- and Dexa BMDC–treated grafted groups 15 hours–day 4 after injections and analyzed for the detection of labeled cell populations. On day 4 after injection of labeled cells, LNs (submandibular, cervical, and deep cervical), spleen, and lung were also harvested (mean ± SEM n = 2–4 per group). (c) Differential levels of antidonor antibodies detectable in both BMDC- and Dexa BMDC–treated groups on day 4–day 14 after transplantation and day 18 (average time point of rejection) (mean ± SEM *P ≤ 0.05, **P ≤ 0.01 two-tailed Mann–Whitney test n = 3–7 per group). (d) Differences in the expression of cell surface glycans, N-acetylglucosamine, α2,3 linked sialic acids, and α2,6 sialic linked acids were analyzed using plant lectins WGA, MAA, and SNA-I (respectively), was also analyzed (mean ± SEM n = 3 experiments *P ≤ 0.05 one-tailed Student's t-test). BMDC, bone marrow–derived dendritic cell; MAA, Maackia amurensis; SNA-I, Sambucus nigra; WGA, wheat germ agglutinin.
Discussion
It is well accepted that the immune response may be customized to the organ in which the response is initiated, and being specialized for the region in which it has to function.20 Others have demonstrated, with mixed efficacy, that the application of donor-derived DCs alone/modified in combination with/without additional immunosuppressive therapies results in prolongation and tolerance of allografts, others have also reported priming of the recipient immune response.9,10,13,17,21,22 However, the eye is well defined as an immune-privileged organ,23,24,25 and thus, there may be differences in immune responses within the cornea and draining LNs to donor antigen after i.v. administration of donor-derived BMDCs and Dexa BMDCs compared with other transplantation models. We show that application of donor BMDCs leads to prolongation of corneal allograft survival by inducing an intragraft immunoregulatory environment which favors graft acceptance. To our knowledge, there has not been an indepth analysis in this model of graft infiltrating cell populations after treatment with donor-derived BMDCs or Dexa BMDCs. We used flow cytometric analysis of graft infiltrating cells26 and described the local immune cell populations within the allograft and of the draining LNs after BMDC and Dexa BMDC treatment. We chose to examine, as prototypic therapeutic DCs, ex vivo–generated, donor-derived BMDCs treated with Dexa. This treatment protocol resulted in cells with a maturation-resistant phenotype (ensuring BMDCs remain in a continued immature state upon injection) capable of modulating an allogeneic lymphocyte response with an efficacy comparable with previously described immature, tolerogenic DCs.4,5,7,17 Our lymphocyte assay results also demonstrated that BMDCs, even without additional Dexa treatment, have a reduced capacity to stimulate allogeneic lymphocytes. On further investigation of the immunomodulatory properties of Dexa BMDCs, it became clear that untreated BMDCs themselves had significant immunomodulatory properties allowing them to inhibit T-cell proliferation and/or modify T-cell differentiation independently of their maturation status.22,27 The molecular basis for BMDCs and Dexa BMDCs profound immunomodulatory properties may be due to BMDC expression of NO and PD-L1 and in the case of Dexa BMDCs IL-10, NO, PD-L1, and IDO. Expression of these molecules, such as the well-characterized PD-L1,28 are significantly increased in both unstimulated and stimulatory conditions relative to ex vivo mature Ox62+ DCs for both untreated BMDCs and Dexa BMDCs. Recently, it has been suggested that PD-L1 is not essential to inhibit lymphocyte proliferation, NO which we have also shown to be expressed by both BMDCs and Dexa BMDCs, was found to be the key modulator of proliferation inhibition.29 It has been demonstrated that unlike freshly isolated DCs, ex vivo–generated BMDCs secrete NO, which may explain our observed in vitro results which demonstrated that not only do Dexa BMDCs significantly inhibit lymphocyte proliferation but so too do the untreated BMDCs.30 The catabolism of essential amino acids, such as arginine by iNOS or tryptophan by IDO may result in a localized, immune-privileged microenvironment in which naïve T cells that would normally become activated proliferating T cells, are instead kept in an anergic, unproliferative state.27,31 Our results show clear evidence that not only maturation-resistant, immature Dexa BMDCs have significantly reduced immunogenicity but also ex vivo–generated, semimature, untreated BMDCs and in an inflammatory environment these cells express significant levels of molecules such as PD-L1 and NO.
We were interested in examining the effect of both donor-derived, untreated BMDC and Dexa BMDC administration in an in vivo transplantation model. For this, a corneal allograft transplantation model using the high-responder allogeneic strain combination of LEW recipients to DA donors was selected.9,32 Treatment of allograft recipients with donor-derived Dexa BMDCs significantly prolonged corneal allograft survival and interestingly, significance was also observed in groups treated with unmodified donor-derived untreated BMDCs. One contributing factor for the promotion of allograft survival is the maturation status of BMDCs at the time of injection, which is a key determinant of transplantation outcomes. It is therefore important to note that allograft recipients received these untreated BMDCs in a semimature phenotypic state and not in a fully (LPS treated) matured state, as in other studies which subsequently leads to the rejection of the allograft.17 Our results suggest that in addition to Dexa BMDCs having a strong in vivo immunomodulatory potential, untreated BMDCs also display a significant immunomodulatory capacity sufficient to promote corneal graft survival. Notably, we were unable to prolong corneal allograft survival with a systemic injection of syngeneic donor antigen-pulsed Dexa BMDCs. Recently, it has been suggested that it is in fact the recipient DC processing of donor DC cell therapies and immunomodulation of both in-direct and semi-direct pathways that play a significant role in the induction of allograft survival.33,34,35 This may be a potential explanation for the failed induction of graft survival with syngeneic donor antigen-pulsed Dexa BMDCs as insufficient quantities of donor MHC antigen are available to promote corneal allograft survival. However, it is likely that the intact donor MHC antigen expression on donor-derived, untreated BMDC and Dexa BMDC in combination with their expression of immunomodulatory molecules (e.g., PD-L1, NO, or IL-10) are what is required to induce corneal allograft survival.
We also examined how administration of BMDC populations affects the immune cell populations within the graft and secondary lymphoid organs. Our results indicated a significant reduction in the percentage population of intragraft-activated CD4+CD25+ T cells which was accompanied by a profound reduction in the total number of CD4+CD25+ T cells and a significantly increased ratio of CD4+Foxp3+ cells to activated CD4+CD25+ T cells in both treated groups. It is likely that these CD4+FoxP3+ cells, along with the significantly increased expression of IDO, which is known to promote and maintain a regulatory T-cell phenotype,36,37 play a key role in inducing and promoting survival of the corneal allografts. A profound reduction in the numbers of NK and NKT cells after BMDC and Dexa BMDC treatment was also detected. Evidence now shows that the cells of innate immunity such as NK and NKT cells play a key role during corneal allograft rejection.32 Prolongation of graft survival has been established in models where treatment significantly impacts the level of NK- and NKT-cell infiltration, as was observed in work also from our group, where corneal allograft overexpression of PD-L1 resulted in a reduction in graft infiltrating NK- and NKT-cell populations.32 The observation of coincidental increases of CD11b/c+MHCII+CD86+ and IDO expression within the allografts of both treatment groups is strengthened by a recent study which demonstrated that IDO-expressing DCs are required for promotion of graft survival in various transplantation models.36,38,39,40 We believe similar to what has been described for other transplantation models, the immunomodulatory microenvironment created within the corneal allograft of BMDC- and Dexa BMDC–treated groups, allows FoxP3+ cell interaction with DCs, inducing expression of IDO that can locally deplete tryptophan and may therefore play an important role in limiting T-cell proliferation and effector function within the graft.31,41,42 The observed increased ratio of CD4+FoxP3+ cells in the draining LNs and also the significant increase in the level of FoxP3 mRNA expression for both BMDC- and Dexa-treated groups is an important indicator of graft acceptance.43
Despite inducing corneal allograft survival, donor BMDC and Dexa BMDC treatment was not effective at inducing peripheral donor-specific unresponsiveness. Recent reports have described sensitization of the recipient to donor antigen with pretreatment of donor-derived Dexa BMDCs.9,13 Our data indicating a donor-specific response in the form of detectable levels of donor alloantibodies with both donor BMDC treatments support these recent observations.9,13 The alloresponse was, however, significantly reduced with Dexa BMDC treatment, which may be due to the immunomodulatory phenotype and glycome profile of the BMDCs after Dexa treatment. Unlike the aforementioned reports which demonstrate accelerated rejection of the allografts,9,13 in our corneal allograft model, the allografts remained protected and were not rejected. Although cell-mediated immunity is believed to play the dominant role in corneal graft rejection, the role of antibody-mediated rejection is controversial.19,44,45 The immunomodulatory environment generated by treatment with donor BMDCs or donor Dexa BMDCs may protect the corneal allograft from cell-mediated immunity which in turn may promote the prevention of rejection of the cornea by complement-fixing alloantibodies.
In conclusion, we have clearly demonstrated that ex vivo–generated, donor Dexa BMDCs have sufficient immunomodulatory properties to significantly prolong corneal allograft survival. Interestingly, donor-derived, untreated BMDCs have similar effects in this model. Although both cell therapies failed to induce peripheral donor-specific unresponsiveness, they did induce a local immunoregulatory milieu within the allograft and draining LNs resulting in protection of the corneal allograft. These results demonstrate a novel therapeutic application for donor-derived BMDCs with and without glucocorticoid treatment for the prevention of corneal allograft rejection but also highlight the potentially contrasting results associated with DC therapies in different models of transplantation.
Materials and Methods
Animals and corneal transplantation. All procedures performed on animals were approved by the Animals Care Research Ethics Committee of the National University of Ireland (Galway, Ireland) and conducted under license from the Department of Health, Ireland. In addition, animal care and management followed the Standard Operating Procedures of the Animal Facility at the National Centre for Biomedical Engineering Science (Galway, Ireland). A well-established, fully allogeneic MHC I/II disparate cornea transplant model was applied for these studies. Male LEW (RT-1l) rats served as recipients of male DA (RT-1avl) grafts. DA and LEW rats were obtained from Harlan Laboratories (Bicester, UK). The outbred strain Sprague Dawley (CD) rats used as a 3rd party cell source were obtained from Charles River (Bicester, UK) and the LEW GFP transgenic rats were a gift from Naoto Kawakami, Max Planck Institute of Biochemistry (Munich, Germany). All animals were 8–14 weeks old and housed with food and water ad lib. Orthotopic corneal transplantation was performed as reported previously.26,32 Briefly, isoflurane was administered systemically at 2%– 2.5% in medical oxygen (BOC, Galway, Ireland) with a flow rate of 2 l/minute. Tetracaine 1% (Chauvin Pharmaceuticals, Kingston upon Thames, UK) was used as a local anesthesia and iris dilation was performed with atropine 1%, tropicamide 1% and phenylephrine 2.5% (all Chauvin Pharmaceuticals). A 2.5-mm graft bed was prepared and a 3 mm full thickness graft was fixed in place with 8–10 interrupted 10–0 Ethilon sutures (Ethicon, Livingston, Scotland) and covered with chloramphenicol antibiotic ointment. Alcon BSS (Alcon, Hemel Hempstead, UK) was used for irrigation of cornea tissue. Eyelids stayed open after operation and the sutures were not removed.26 Graft transparency as an indicator of rejection was evaluated every second day by light and slit lamp microscopy and graded as follows: 0—completely transparent cornea; 0.5—slight corneal opacity, iris structure easily visible; 1.0—low opacity with visible iris details; 1.5—modest corneal opacity, iris vessels still visible; 2.0—moderate opacity, only some iris details visible; 2.5—high corneal opacity, only pupil margin visible; 3.0—complete corneal opacity, anterior chamber not visible. Grafts were considered rejected based on an opacity score of 2.5 for three or more consecutive days or an opacity score of 3, in combination with edema and correlating changes of transplant geometry (degree of convex contour, shrinking and surface roughness of graft).32,46 Animals with surgical complications were excluded.
Generation of BMDCs. BMDCs were generated as previously described for the rat47,48 with some modifications. Briefly, male DA BM was flushed from both the femur and tibia; the cell suspension was collected and pelleted then resuspended in ACK buffer to lyse the red blood cells. BM cells were washed in complete medium consisting of RPMI-1640 (Gibco, Grand Island, NY) supplemented with heat inactivated 10% fetal bovine serum (FBS), 2 mmol/l l-glutamine, 0.1 mol/l nonessential amino acids, 1 mmol/l sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin, and 55 μmol/l 2β-ME (Gibco). BM cells were then seeded in a six-well plate at a concentration of 4.5 × 106 cells/3 ml per well. The culture medium was supplemented with 5 ng/ml rat granulocyte-macrophage colony-stimulating factor (GM-CSF) (Invitrogen, Paisley, UK) and 5 ng/ml rat IL-4 (Peprotech EC, London, UK). Cells were then incubated at 37 °C at 5% CO2. On the 3rd day of culture, half of the medium from each well was harvested and cells were resuspended in fresh medium supplemented with rat GM-CSF and IL-4 and added back into the culture. On the 5th day of culture, all medium was removed (to remove dead lymphocytes and granulocytes) and replaced with fresh complete medium supplemented with GM-CSF and IL-4. For the generation of Dexa BMDCs, 10−6 mol/l Dexa was added to the culture. On day 7, half the medium was replaced with fresh medium supplemented with GM-CSF, IL-4, and Dexa if required. For the preparation of mature BMDCs, cultures were subsequently stimulated with LPS (1 µg/ml; Sigma-Aldrich, Dublin, Ireland) for 24 hours. Cultures were maintained until day 10 when they were harvested for in vitro assays or in vivo applications.
Cytokine and NO analysis. TNF-α and IL-10 cytokine determination for BMDC supernatants, were quantified using enzyme-linked immunosorbent assay (ELISA; R&D Systems, Abingdon, UK), using the manufacturers protocols. NO release was assessed using a standard Griess Assay protocol. Briefly, 100 µl of supernatant from 1 × 106 unstimulated BMDCs/Dexa BMDCs and LPS (1 µg/ml)-stimulated cells in addition to the required standards (NaNO2 first standard 100 µmol/l in culture media) were added to the appropriate wells of a 96-well, flat-bottom plate. Solution A (sulfanilamide, phosphoric acid, H2O) of 50 µl was added to each well and then 50 µl of solution B (n-(1-naphthyl)-ethylenediamine dihydrochloride, phosphoric acid, H2O). Absorbance was read at 550 nm.
Allogeneic lymphocyte assays. Isolation of DA Ox62+ DCs was carried out by MACs bead sorting as follows. Briefly, a rat splenocyte and thymocyte cell mix (2 × 108 cells) was resuspended in 80 µl of MACs buffer per 107 total cells and 20 µl of Ox62 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) per 107 total cells were added and the protocol carried out according to the manufacturer's instructions using a LS column (Miltenyi Biotec). LEW lymphocytes were stained with CFSE (Molecular Probes, Invitrogen), according to the manufacturer's instructions; lymphocytes were washed and resuspended in the appropriate volume of complete medium (1.5 × 105 cells/125µl) and plated in a 96-well, round-bottom plate. Gamma irradiated (20Gy) DA Ox62+, BMDCs, and Dexa BMDCs were added to the appropriate wells in a ratio 10:1, lymphocyte:DC. Proliferation and activation of lymphocytes was measured on day 5.
Flow cytometry. The following monoclonal antibodies (mAbs) were used for the characterization of BMDCs: CD11b/c APC, CD80-PE, CD86-PE, the macrophage marker HIS36-PE (BioLegend, San Diego, CA), and MHCII-PE (Serotec, Oxford, UK). Appropriate APC- or PE-conjugated Ig isotype controls were also used (BioLegend). For glycome analysis of BMDC, the following FITC-labeled lectins were used: wheat germ agglutinin (WGA), Maackia amurensis (MAA), and Sambucus nigra (SNA-I) (1.5 µg/ml, gift from Jared Gerlach and Lokesh Joshi) prepared in lectin staining buffer (PBS containing 1% FBS, 1 mmol/l CaCl2 and 2 mmol/l MgCl2). Controls were used by incubating BMDCs with lectins in FBS-only buffer. Cells were stained on ice for 30 minutes in lectin staining buffer and washed and resuspended in FACS buffer (PBS containing 2% fetal calf serum and 0.01% NaN3, all from Sigma-Aldrich) for analysis using a FACS Canto (BD Biosciences, Oxford, UK).
For the analysis of lymphocytes isolated from transplanted corneas, ipsilateral submandibular, and cervical LNs, the following mAbs were used: CD3−FITC, CD8−PE, CD161−AF647, CD4−APC, CD11b/c−APC, CD86−PE, CD45RA−PE (BioLegend), CD25−FITC, FoxP3−PE (eBioscience, San Diego, CA), and MHCII-FITC (BD Biosciences, Franklin Lakes, NJ). For staining, cells were washed with FACS buffer. mAbs were diluted in 50 µl FACS buffer, added to the cells, and incubated for 30 minutes at 4 °C. Finally, unbound antibody was removed by washing twice with FACS buffer. A commercial kit (eBioscience) was used to detect the transcription factor FoxP3. After samples were stained with appropriate cell surface stains the permeabilization and staining of FoxP3 was performed using the manufacturer's protocol as a guideline. Cells were resuspended in FACS buffer for analysis. Compensation parameters were established on the FACS Canto using appropriately single stained cells and fluorescence minus one (FMO) controls. Data were analyzed using FlowJo software (Tree Star, Ashland, OR). Results are presented as percentage of cell population or as absolute cell number in graft. Unlabeled beads (CaliBRITE unlabeled beads; BD Biosciences, Oxford, UK) were added to FACS samples for detection of absolute number of cells. Absolute number of graft-infiltrated cells was calculated as a function from number of FACS counted beads and cells and number of beads which were added into the probe.
DQ OVA assay. Ox62+ DCs, BMDCs, and Dexa BMDCs were seeded in a 96-well, round-bottom plate at a concentration of 1 × 105 cells/250 µl. DQ OVA (Molecular Probes, Invitrogen) was added to the DCs at a final concentration of 50 µg/ml. Cells were collected at various time points washed and resuspended in FACS buffer for analysis by flow cytometry.
Isolation of lymphocytes from transplanted corneas and lymph nodes. Single-cell suspensions from individual transplanted corneas were prepared from the excised graft. The corneal graft was excised using a 3-mm trephine and vannas scissors ensuring that the graft was free of iris pigments. The graft was then stored in sterile PBS on ice. and then incubated and digested with 2.5 µg/ml Collagenase D (Sigma-Aldrich) in a 1.5-ml eppendorf and placing it into a tube shaker/heater (50× rcf; 90 minutes; 37 °C). Digestion was stopped and all liquid and tissue poured into a 100-µm cell strainer and placed into a 6-cm Petri dish. The cornea graft was further disintegrated by mashing with the head of a syringe plunger. The cell suspension was collected in a 15-ml falcon tube and the cell strainer and Petri dish thoroughly rinsed and added to cell suspension. The sample was centrifuged (400× rcf; 3 minutes; 4 °C) and resuspended in 1.2 ml of FACS buffer for counting. Ipsilateral submandibular and cervical LNs were also homogenized with the syringe plunger and passed through a 100 µmol/l cell strainer. Cell suspensions were transferred into 15 ml tubes, spun at 400× rcf for 5 minutes, and washed again with PBS. Cell suspensions from individual corneas and LNs were resuspended in FACS buffer and used for subsequent flow cytometry.
RNA-isolation and RT-PCR. Total RNA from Ox62+ DCs, BMDCs, Dexa BMDCs, corneas, and LNs was isolated using TRIzol reagent (Invitrogen) according to the manufacturer's protocol. PCR array on BMDCs and Dexa BMDCs was performed using a detection kit according to the manufacturer's protocol (PARN-011; Qiagen, SA Biosciences, Crawley, UK). cDNA was synthesized using RevertAid H Minus Reverse Transcriptase (Fermentas, Thermo Scientific, MA, USA) with random primers. Two step qRT-PCR based on BMDC and Dexa BMDC RNA was performed on day 10 and on day 18 for grafted cornea and draining LNs. For primer sequences of PD-L1, IL-10, IDO, iNOS, CXCL9, CXCL10, CCR2, TLR2, TLR4, TLR7, TLR9, IFNγ, IL-6, IL-1β. IL-2R, FoxP3 and eNOS (Supplementary Table S1). All samples were normalized to expression of the house-keeping gene β-actin and relative expression in the case of BMDC and Dexa BMDCs was to Ox62+ DC and for treated groups cornea and LN analysis it was to untreated allogeneic controls. All quantitative real-time PCR was performed according to the standard program on the Applied Biosystems StepOne Plus Real Time PCR System.
Histology and histochemistry. For histological analysis, rat eyes were enucleated at day 18 after transplantation for all groups and at the end of the observation period for graft survival on day 30. Briefly, the eyes embedded in paraffin wax were cut for 5 µm thick sections, dried overnight at 56 °C and then deparaffinized twice in xylene for 10 minutes, followed by hydration through graded alcohols. Slides were incubated for 40 seconds in Harris hematoxylin, washed in tap water for 2 minutes, then stained in eosin for 7 minutes, washed again in water for 2 minutes, and dehydrated through graded alcohols. Next, sections were cleared twice for 10 minutes in xylene and mounted in DPX (Sigma-Aldrich).
In vivo cell trace experiment. Spleens and thymi were harvested from LEW (syngeneic) GFP transgenic, DA (donor), and CD (3rd party) rats, organs were homogenized and treated with ACK as previously described. Cells from donor and 3rd party origins were stained using CellTrace Far Red DDAO-SE (as per manufacturers' recommendations, Molecular Probes, Invitrogen), washed and resuspended at 20 × 106 cell/ml PBS. Far Red–labeled donor or 3rd party cells were mixed with equal numbers of syngeneic GFP cells. Naïve (ungrafted control), BMDC- and Dexa BMDC–treated, grafted animals received an i.v. injection of a total 20 × 106 cells/ml PBS of Far Red donor/3rd party and syngeneic GFP cell mix. Blood from naive control and treated groups was withdrawn from the tail vein using a 25G needle and transferred into a sterile 1.5 ml eppendorf tube containing 100 µl PBS and heparin (2 U/ml) at various time points. The blood was then treated with ACK buffer, washed, centrifuged (400× rcf; 5 minutes; 4 °C), and resuspended in FACS buffer. Fluorescent beads (CaliBRITE-PerCP beads; BD Biosciences, Oxford, UK) were added to FACS samples for detection of absolute number of Far Red– and GFP-labeled cells. Absolute number of circulating fluorescently labeled cells was calculated as a function from number of FACS-counted beads and cells and number of beads which were added into the probe. On day 4 after injection, right lung (digested as described for spleen), spleen, submandibular, and cervical LNs were harvested and homogenized for localization of fluorescently labeled cells within these tissues. Aliquots of homogenized tissues were resuspended in FACS buffer and fluorescent beads were added for cell enumeration as previously described and samples were analyzed on FACS Canto.
Harvest of autologous serum and detection of alloantibodies. Blood from untreated controls, treated groups, and naïve controls, was withdrawn from tail vein using a 25G needle and transferred into a sterile 1.5 ml eppendorf tube containing 100 µl PBS and heparin (2 U/ml). The blood was then centrifuged (500× rcf; 10 minutes; 4 °C) and the resulting serum fraction was harvested with a sterile pipette and transferred into 1.5 ml micro reaction tubes. The serum was stored at −20 °C for later use. Alloantibody analysis was performed as reported previously.49 Recipient serum was diluted (1:2 in FACS buffer) and incubated with 1 × 106 DA splenocytes for 45 minutes on ice in a total volume of 50 µl per test. Samples were washed twice with FACS buffer and pelleted (400× rcf for 5 minutes at 4 °C). In the fashion of a secondary FACS stain, samples were then labeled with either antirat IgM-PE, IgG1-FITC, or IgG2a-FITC (all from Antibodies, online, Germany). In the case of anti-IgM-PE staining, anti-CD45RA−FITC (BD Biosciences) was added to later allow exclusion of B cells from analysis. Splenocytes were incubated further for 45 minutes on ice, washed and resuspended in FACS buffer for analysis using a FACS Canto.
Statistics. Statistical analysis was performed by GraphPad Prism software (La Jolla, CA) using nonparametric Mann–Whitney or two-tailed parametric Student's t-test where appropriate, unless otherwise stated in text. Survival data were compared using the Mantel-Cox log rank test. Differences were considered significant if P ≤ 0.05.
SUPPLEMENTARY MATERIAL Figure S1. Additional in vitro characterization of BMDC and Dexa BMDC cultures and application of syngeneic donor antigen-pulsed Dexa BMDCs in corneal transplantation. Figure S2. Additional cell population analysis within the corneal allograft and draining LNs after BMDC and Dexa BMDC treatment. Table S1. RT PCR primer design.
Acknowledgments
The authors thank Francesca Odoardi from Institute for Multiple-Sclerosis Research, Department of Neuroimmunology, University Medicine, Göttingen, Germany, for her cytokine and chemokine primer designs. The authors also thank Juliana Riesselmann for her contribution to the histological studies of grafted corneas and Gerry Fahy, consultant ophthalmologist, University Hospital Galway for helpful discussions. This material is based upon works supported by the Science Foundation Ireland (www.sfi.ie) under Grant No. [07/IN.1/B925], 09/SRC-B1794 and by the European Regional Development Fund (ERDF). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors declare no conflict of interest.
Supplementary Material
References
- Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nat Rev Immunol. 2006;6:127–135. doi: 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]
- Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7:610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- Unger WW, Laban S, Kleijwegt FS, van der Slik AR, Roep BO. Induction of Treg by monocyte-derived DC modulated by vitamin D3 or dexamethasone: differential role for PD-L1. Eur J Immunol. 2009;39:3147–3159. doi: 10.1002/eji.200839103. [DOI] [PubMed] [Google Scholar]
- Fischer R, Turnquist HR, Taner T, Thomson AW. Use of rapamycin in the induction of tolerogenic dendritic cells. Handb Exp Pharmacol. 2009. pp. 215–232. [DOI] [PubMed]
- Boks MA, Kager-Groenland JR, Haasjes MS, Zwaginga JJ, van Ham SM, ten Brinke A. IL-10-generated tolerogenic dendritic cells are optimal for functional regulatory T cell induction–a comparative study of human clinical-applicable DC. Clin Immunol. 2012;142:332–342. doi: 10.1016/j.clim.2011.11.011. [DOI] [PubMed] [Google Scholar]
- Khan A, Fu H, Tan LA, Harper JE, Beutelspacher SC, Larkin DFP, et al. Dendritic cell modification as a route to inhibiting corneal graft rejection by the indirect pathway of allorecognition. Eur J Immunol. 2013;43:734–746. doi: 10.1002/eji.201242914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kort H, Crul C, van der Wal AM, Schlagwein N, Stax AM, Bruijn JA, et al. Accelerated antibody-mediated graft loss of rodent pancreatic islets after pretreatment with dexamethasone-treated immature donor dendritic cells. Transplantation. 2012;94:903–910. doi: 10.1097/TP.0b013e31826acd01. [DOI] [PubMed] [Google Scholar]
- Stax AM, Gelderman KA, Schlagwein N, Essers MC, Kamerling SW, Woltman AM, et al. Induction of donor-specific T-cell hyporesponsiveness using dexamethasone-treated dendritic cells in two fully mismatched rat kidney transplantation models. Transplantation. 2008;86:1275–1282. doi: 10.1097/TP.0b013e31818a6682. [DOI] [PubMed] [Google Scholar]
- Alawieh M, Rifle G, Bouchot O, Malapert G, Mousson C, Martin L. Injection of donor-derived OX62+ splenic dendritic cells with anti-CD4 monoclonal antibody generates CD4+CD25+FOXP3+ regulatory T cells that prolong allograft skin survival indefinitely and abrogate production of donor-specific antibodies in a rat model. Transplant Proc. 2009;41:3363–3366. doi: 10.1016/j.transproceed.2009.08.038. [DOI] [PubMed] [Google Scholar]
- Luo X, Pothoven KL, McCarthy D, DeGutes M, Martin A, Getts DR, et al. ECDI-fixed allogeneic splenocytes induce donor-specific tolerance for long-term survival of islet transplants via two distinct mechanisms. Proc Natl Acad Sci USA. 2008;105:14527–14532. doi: 10.1073/pnas.0805204105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth LA, Ratnasothy K, Moreau A, Alcock S, Sagoo P, Meader L, et al. Tolerogenic Donor-Derived Dendritic Cells Risk Sensitization In Vivo owing to Processing and Presentation by Recipient APCs. J Immunol. 2013;190:4848–4860. doi: 10.4049/jimmunol.1200870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M, Thebault P, Segovia M, Louvet C, Bériou G, Tilly G, et al. Cell therapy with autologous tolerogenic dendritic cells induces allograft tolerance through interferon-gamma and epstein-barr virus-induced gene 3. Am J Transplant. 2011;11:2036–2045. doi: 10.1111/j.1600-6143.2011.03651.x. [DOI] [PubMed] [Google Scholar]
- Pêche H, Trinité B, Martinet B, Cuturi MC. Prolongation of heart allograft survival by immature dendritic cells generated from recipient type bone marrow progenitors. Am J Transplant. 2005;5:255–267. doi: 10.1111/j.1600-6143.2004.00683.x. [DOI] [PubMed] [Google Scholar]
- Moreau A, Varey E, Bériou G, Hill M, Bouchet-Delbos L, Segovia M, et al. Tolerogenic dendritic cells and negative vaccination in transplantation: from rodents to clinical trials. Front Immunol. 2012;3:218. doi: 10.3389/fimmu.2012.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori T, Saban DR, Emami-Naeini P, Chauhan SK, Funaki T, Ueno H, et al. Donor-derived, tolerogenic dendritic cells suppress immune rejection in the indirect allosensitization-dominant setting of corneal transplantation. J Leukoc Biol. 2012;91:621–627. doi: 10.1189/jlb.1011500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada J, Kurimoto I, Streilein JW. Role of CD4+ T cells in immunobiology of orthotopic corneal transplants in mice. Invest Ophthalmol Vis Sci. 1999;40:2614–2621. [PubMed] [Google Scholar]
- Niederkorn JY. Cornea: Window to Ocular Immunology. Curr Immunol Rev. 2011;7:328–335. doi: 10.2174/157339511796196593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein-Streilein J. Immune regulation and the eye. Trends Immunol. 2008;29:548–554. doi: 10.1016/j.it.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Alawieh M, Malapert G, Bouchot O, Rifle G, Mousson C, Martin L. Injection of donor-derived splenic dendritic cells plus a nondepleting anti-CD4 monoclonal antibody to prolong primary skin graft survival indefinitely and abrogate the production of donor-specific antibodies in the Fischer-to-Lewis rat combination. Transplant Proc. 2010;42:4347–4349. doi: 10.1016/j.transproceed.2010.09.128. [DOI] [PubMed] [Google Scholar]
- Lutz MB, Suri RM, Niimi M, Ogilvie AL, Kukutsch NA, Rössner S, et al. Immature dendritic cells generated with low doses of GM-CSF in the absence of IL-4 are maturation resistant and prolong allograft survival in vivo. Eur J Immunol. 2000;30:1813–1822. doi: 10.1002/1521-4141(200007)30:7<1813::AID-IMMU1813>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Hori J, Vega JL, Masli S. Review of ocular immune privilege in the year 2010: modifying the immune privilege of the eye. Ocul Immunol Inflamm. 2010;18:325–333. doi: 10.3109/09273948.2010.512696. [DOI] [PubMed] [Google Scholar]
- Masli S, Vega JL. Ocular immune privilege sites: suppression and regulation of immune responses. Methods Mol Biol. 2011;677:449–458. doi: 10.1007/978-1-60761-869-0_28. [DOI] [PubMed] [Google Scholar]
- Niederkorn JY, Stein-Streilein J. History and physiology of immune privilege. Ocul Immunol Inflamm. 2010;18:19–23. doi: 10.3109/09273940903564766. [DOI] [PubMed] [Google Scholar]
- Maenz M, Morcos M, Ritter T. A comprehensive flow-cytometric analysis of graft infiltrating lymphocytes, draining lymph nodes and serum during the rejection phase in a fully allogeneic rat cornea transplant model. Mol Vis. 2011;17:420–429. [PMC free article] [PubMed] [Google Scholar]
- Mellor AL, Chandler P, Baban B, Hansen AM, Marshall B, Pihkala J, et al. Specific subsets of murine dendritic cells acquire potent T cell regulatory functions following CTLA4-mediated induction of indoleamine 2,3 dioxygenase. Int Immunol. 2004;16:1391–1401. doi: 10.1093/intimm/dxh140. [DOI] [PubMed] [Google Scholar]
- Amarnath S, Costanzo CM, Mariotti J, Ullman JL, Telford WG, Kapoor V, et al. Regulatory T cells and human myeloid dendritic cells promote tolerance via programmed death ligand-1. PLoS Biol. 2010;8:e1000302. doi: 10.1371/journal.pbio.1000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rössner S, Voigtländer C, Wiethe C, Hänig J, Seifarth C, Lutz MB. Myeloid dendritic cell precursors generated from bone marrow suppress T cell responses via cell contact and nitric oxide production in vitro. Eur J Immunol. 2005;35:3533–3544. doi: 10.1002/eji.200526172. [DOI] [PubMed] [Google Scholar]
- Powell TJ, Jenkins CD, Hattori R, MacPherson GG. Rat bone marrow-derived dendritic cells, but not ex vivo dendritic cells, secrete nitric oxide and can inhibit T-cell proliferation. Immunology. 2003;109:197–208. doi: 10.1046/j.1365-2567.2003.01639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34:137–143. doi: 10.1016/j.it.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosov M, Wilk M, Morcos M, Cregg M, O'Flynn L, Treacy O, et al. Role of lentivirus-mediated overexpression of programmed death-ligand 1 on corneal allograft survival. Am J Transplant. 2012;12:1313–1322. doi: 10.1111/j.1600-6143.2011.03948.x. [DOI] [PubMed] [Google Scholar]
- Divito SJ, Wang Z, Shufesky WJ, Liu Q, Tkacheva OA, Montecalvo A, et al. Endogenous dendritic cells mediate the effects of intravenously injected therapeutic immunosuppressive dendritic cells in transplantation. Blood. 2010;116:2694–2705. doi: 10.1182/blood-2009-10-251058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheradmand T, Wang S, Bryant J, Tasch JJ, Lerret N, Pothoven KL, et al. Ethylenecarbodiimide-fixed donor splenocyte infusions differentially target direct and indirect pathways of allorecognition for induction of transplant tolerance. J Immunol. 2012;189:804–812. doi: 10.4049/jimmunol.1103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Divito SJ, Shufesky WJ, Sumpter T, Wang H, Tkacheva OA, et al. Dendritic cell therapies in transplantation revisited: deletion of recipient DCs deters the effect of therapeutic DCs. Am J Transplant. 2012;12:1398–1408. doi: 10.1111/j.1600-6143.2012.04060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucher R, Fischler K, Oberhuber R, Kronberger I, Margreiter C, Ollinger R, et al. IDO and regulatory T cell support are critical for cytotoxic T lymphocyte-associated Ag-4 Ig-mediated long-term solid organ allograft survival. J Immunol. 2012;188:37–46. doi: 10.4049/jimmunol.1002777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baban B, Chandler PR, Johnson BA, 3rd, Huang L, Li M, Sharpe ML, et al. Physiologic control of IDO competence in splenic dendritic cells. J Immunol. 2011;187:2329–2335. doi: 10.4049/jimmunol.1100276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Kheradmand T, Bryant J, Wang S, Tasch J, Wang JJ, et al. Intragraft CD11b(+) IDO(+) cells mediate cardiac allograft tolerance by ECDI-fixed donor splenocyte infusions. Am J Transplant. 2012;12:2920–2929. doi: 10.1111/j.1600-6143.2012.04203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook CH, Bickerstaff AA, Wang JJ, Nadasdy T, Della Pelle P, Colvin RB, et al. Spontaneous renal allograft acceptance associated with “regulatory” dendritic cells and IDO. J Immunol. 2008;180:3103–3112. doi: 10.4049/jimmunol.180.5.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Gong ZJ, Wang ZW, Li T, Zhang JY, Sun HC, et al. IDO-competent-DCs induced by IFN-γ attenuate acute rejection in rat liver transplantation. J Clin Immunol. 2012;32:837–847. doi: 10.1007/s10875-012-9681-4. [DOI] [PubMed] [Google Scholar]
- McGrath MM, Najafian N. The role of coinhibitory signaling pathways in transplantation and tolerance. Front Immunol. 2012;3:47. doi: 10.3389/fimmu.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold SP, Adams E, Nolan KF, Regateiro FS, Waldmann H. Connecting the mechanisms of T-cell regulation: dendritic cells as the missing link. Immunol Rev. 2010;236:203–218. doi: 10.1111/j.1600-065X.2010.00913.x. [DOI] [PubMed] [Google Scholar]
- Chauhan SK, Saban DR, Lee HK, Dana R. Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation. J Immunol. 2009;182:148–153. doi: 10.4049/jimmunol.182.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde S, Mellon JK, Hargrave SL, Niederkorn JY. Effect of alloantibodies on corneal allograft survival. Invest Ophthalmol Vis Sci. 2002;43:1012–1018. [PubMed] [Google Scholar]
- Holán V, Vítová A, Krulová M, Zajícová A, Neuwirth A, Filipec M, et al. Susceptibility of corneal allografts and xenografts to antibody-mediated rejection. Immunol Lett. 2005;100:211–213. doi: 10.1016/j.imlet.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Matoba AY, Peeler JS, Niederkorn JY. T cell subsets in the immune rejection of murine heterotopic corneal allografts. Invest Ophthalmol Vis Sci. 1986;27:1244–1254. [PubMed] [Google Scholar]
- Horibe EK, Sacks J, Unadkat J, Raimondi G, Wang Z, Ikeguchi R, et al. Rapamycin-conditioned, alloantigen-pulsed dendritic cells promote indefinite survival of vascularized skin allografts in association with T regulatory cell expansion. Transpl Immunol. 2008;18:307–318. doi: 10.1016/j.trim.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Taieb A, Breitinger JJ, Unadkat JV, Shufesky WJ, Morelli AE, Thomson AW, et al. Intrinsic ability of GM+IL-4 but not Flt3L-induced rat dendritic cells to promote allogeneic T cell hyporesponsiveness. Clin Immunol. 2007;123:176–189. doi: 10.1016/j.clim.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Schu S, Nosov M, O'Flynn L, Shaw G, Treacy O, Barry F, et al. Immunogenicity of allogeneic mesenchymal stem cells. J Cell Mol Med. 2012;16:2094–2103. doi: 10.1111/j.1582-4934.2011.01509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulahian TH, Högger P, Wahner AE, Wardwell K, Goulding NJ, Sorg C, et al. Human monocytes express CD163, which is upregulated by IL-10 and identical to p155. Cytokine. 2000;12:1312–1321. doi: 10.1006/cyto.2000.0720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.