Abstract
Stable-isotope probing was previously used to identify bacterial anthracene-degraders in untreated soil from a former manufactured gas plant site. However, subsequent pyrosequence analyses of total bacterial communities and quantification of 16S rRNA genes indicated that relative abundances of the predominant anthracene-degrading bacteria (designated Anthracene Group 1) diminished as a result of biological treatment conditions in lab-scale, aerobic bioreactors. This study identified Alphaproteobacterial anthracene-degrading bacteria in bioreactor-treated soil which were dissimilar to those previously identified. The largest group of sequences was from the Alterythrobacter genus while other groups of sequences were associated with bacteria within the order Rhizobiales and the genus Bradyrhizobium. Conditions in the bioreactor enriched for organisms capable of degrading anthracene which were not the same as those identified as dominant degraders in the untreated soil. Further, these data suggest that identification of polycyclic aromatic hydrocarbon-degrading bacteria in contaminated but untreated soil may be a poor indicator of the most active degraders during biological treatment.
Key words: anthracene, bioremediation, polycyclic aromatic hydrocarbons, stable-isotope probing
Introduction
Anthracene is a three-ring polycyclic aromatic hydrocarbon (PAH) that is considered a priority pollutant by the United States Environmental Protection Agency (U.S. Environmental Protection Agency, 1993) and can be found in significant concentrations in soils at industrial sites contaminated with hydrocarbons, such as those of former manufactured gas plants (MGPs) (Haeseler et al., 1999; Benhabib et al., 2010). Similar to other low molecular weight (LMW) PAHs such as naphthalene and phenanthrene, anthracene can be degraded by a wide variety of microorganisms. Isolated bacteria capable of anthracene metabolism include organisms from both Gram-positive (Dean-Ross et al., 2001; Khan et al., 2002; Zeinali et al., 2007; Zhang et al., 2009; Zeng et al., 2010; Ling et al., 2011) and Gram-negative genera (Story et al., 2004; Jacques et al., 2005; Baboshin et al., 2008; Arulazhagan and Vasudevan, 2011; Jin et al., 2012), and several fungi have been described that are capable of transforming anthracene (Wu et al., 2010; Acevedo et al., 2011). However, only recently have culture-independent methods been applied to directly link uncultivated or uncharacterized bacteria to the metabolism of anthracene in contaminated environments.
Stable-isotope probing (SIP) is a culture-independent method in which an isotopically-enriched growth substrate is incubated in the presence of a bacterial community or environmental sample to identify microorganisms able to assimilate the labeled atoms from that substrate (Radajewski et al., 2000). SIP has been used to identify phylogenetically diverse bacteria capable of growth on PAHs ranging from 2 to 4 rings, including naphthalene (Jeon et al., 2003; Singleton et al., 2005; Yu and Chu, 2005; Gutierrez et al., 2011; Jones et al., 2011a; Uhlik et al., 2012), anthracene (Jones et al., 2011b; Zhang et al., 2011b, 2012), phenanthrene (Singleton et al., 2005, 2007; Cébron et al., 2011; Jones et al., 2011a; Martin et al., 2012), pyrene (Singleton et al., 2006, 2007; Jones et al., 2008; Jones et al., 2011a), fluoranthene (Jones et al., 2011a), and benz[a]anthracene (Jones et al., 2011a). A previous SIP study was conducted in this lab to identify aerobic anthracene-degrading bacteria in PAH-contaminated soil sampled from the site of a former manufactured gas plant (Jones et al., 2011b). Sequences from an uncharacterized clade within the Erythrobacteraceae family of the Sphingomonadales, designated “Anthracene Group 1” (AG1), and from within the Variovorax and Pigmentiphaga genera were identified as anthracene-degraders (Jones et al., 2011b). However, subsequent pyrosequence analyses of biologically treated samples of that soil indicated that sequences associated with those groups did not appear to favorably respond to biostimulation under both simulated ex situ (Singleton et al., 2011) and in situ bioremediation schemes (Singleton et al., 2013), which suggested the possibility of an alternate consortium of anthracene-degraders selected under biological treatment conditions.
Use of molecular biological tools in a predictive capacity for environmental engineering applications has been of interest for a number of years. However, based on our earlier pyrosequencing results, it was clear that the predominant anthracene-degrading bacteria in untreated soil were not selected under conditions in the bioreactor. We hypothesized that removal of anthracene in the bioreactor by different bacteria than in the untreated soil might represent functional redundancy for anthracene-degrading activity in the soil bacterial community. The goals of this study were to identify the response of the dominant anthracene degrader in the untreated soil (AG1) to incubation conditions similar to biological treatment in a lab-scale slurry phase bioreactor, to identify the most active anthracene-degrading bacteria in the bioreactor-treated soil by DNA-SIP, and to compare those organisms to the anthracene degraders found in the untreated soil.
Experimental Protocols
Soil samples
Soils used in this experiment were acquired from the site of a former MGP located in Salisbury, NC. Soil properties and details of samples can be found in Richardson, et al. (2011). The concentration of solvent-extractable anthracene in untreated and bioreactor-treated soil was 15 and 2 mg/kg, respectively (Singleton et al., 2011). Untreated soil was stored at 4°C prior to use.
Contaminated soils were treated in an aerobic, semi-continuous (draw-and-fill), lab-scale, 2.5 L slurry-phase bioreactor whose operation has been described previously (Singleton et al., 2011). Briefly, standard operation consisted of treatment of a 10% soil slurry (w/v) created using a buffer comprising 5 mM phosphate (pH 7.5) and 5 mM NH4NO3 (“reactor buffer”). The contents of the bioreactor were continuously mixed and amended with air at room temperature (∼23°C). Every 7 days 20% of the bioreactor volume was removed and replaced with an equivalent volume of untreated soil suspended in reactor buffer. At the time of this experiment, the bioreactor had been operating for over 3 years. Soil samples for this experiment were taken directly from the bioreactor during operation or from the stored untreated soil.
Effect of source inoculum on pH and abundance of AG1 in anthracene-amended soils
We tested whether the composition of the aqueous phase of soil slurries might influence the abundance of AG1 genes in incubations containing untreated and bioreactor-treated soil. One gram (dry weight) of either untreated soil or soil sampled from the bioreactor was used as inoculum in triplicate incubations, each containing 12.5 mg anthracene (“scintillation grade”; Kodak, Rochester, NY) and 30 mL of either simulated groundwater amended with 0.07 mM inorganic nitrogen and 0.01 mM phosphorus (Richardson et al., 2012) or reactor buffer. Simulated groundwater was used in the previous SIP experiment on untreated soil with 13C-labeled anthracene (Jones et al., 2011b) and during the operation of previously examined in situ column systems (Singleton et al., accepted for publication), while the “reactor buffer” was used during regular operation of the ex situ bioreactor (Singleton et al., 2011). The pH of the slurries was measured daily using an Expandable Ion Analyzer EA920 (Orion Research, Cambridge, MA).
To ascertain how sequences associated with AG1 were affected by the source of soil inoculum, DNA was extracted from 1 mL of slurry from one flask for each condition after 114 h of shaking at 25°C and 250 rpm (∼33 mg of soil) using the FastDNA Spin Kit for Soil (MP Biomedicals, Solon, OH). DNA from the start of the incubations (0 h time point) was similarly extracted from the samples suspended in simulated groundwater. To determine the response of the previously identified dominant anthracene-degrading group (AG1) to various incubation conditions, quantitative real-time PCR with specific primers targeting the 16S rRNA gene of AG1 (primers AG1.1F and AG1.1R) and general bacterial 16S rRNA genes were performed in duplicate as described previously to obtain a relative abundance of group AG1 (Jones et al., 2011b). A bacterial standard curve generated from the same template used to create the AG1-specific standard curve was used to estimate total bacterial gene copy number.
Mineralization and disappearance of anthracene
To determine the length of time for subsequent SIP experiments, the mineralization of [1,2,3,4,4a,9a-14C]anthracene (Sigma, St. Louis, MO; specific activity 17.3 mCi/mmol) by microbial communities in bioreactor-treated soil was performed as described previously (Singleton et al., 2008). Briefly, 625 μg of unlabeled anthracene in dichloromethane (DCM) and ∼57,000 dpm of 14C-labeled anthracene in toluene were added to 125 mL Erlenmeyer flasks and the solvents allowed to evaporate. One gram (dry weight) of bioreactor-treated slurry washed and suspended in 30 mL reactor buffer (containing 5, 2.5, or 1 mM NH4NO3; pH 7.5) was then added to duplicate flasks containing anthracene for each condition. Labeled 14CO2 was captured on KOH-saturated filter paper suspended in test tubes within each flask. The extent of mineralization was determined using a Packard Tri-Carb 1900 TR Liquid Scintillation Analyzer. The concentration of anthracene in duplicate, parallel flasks for each condition that contained unlabeled anthracene but not 14C-anthracene or KOH traps was also determined by high performance liquid chromatography (HPLC) analyses as previously described (Jones et al., 2011b).
Stable-isotope probing
Duplicate 125-mL glass, screw-top flasks containing 1 g (dry weight) of bioreactor-treated soil washed and resuspended in 30 mL of a modified reactor buffer comprising 5 mM phosphate (pH 7.5) and 2.5 mM NH4NO3, and 625 μg of either [U-13C]anthracene (Zhang et al., 2011c) or unlabeled anthracene were incubated for 5 days with shaking at 225 rpm and 25°C in the dark. The anthracene was added to the flasks in 1 mL DCM and the solvent was allowed to evaporate prior to adding the soil suspension.
One milliliter of soil slurry (∼33 mg soil, dry weight) was sampled from each of the duplicate flasks containing unlabeled anthracene at 0, 24, 54, 76, and 99 h for DNA extraction using a single extraction with the FastDNA Spin Kit for Soil. An additional 1 mL of slurry was sampled at the same time points for HPLC determination of anthracene concentration. All of the incubations were terminated at 124 h. After additional aliquots were taken for the measurement of anthracene concentration, DNA was extracted from the remainder of the soil slurry containing unlabeled anthracene and the entirety of the soil samples containing [U-13C]anthracene using a FastDNA Spin Kit for Soil. To maximize DNA recovery and bacterial diversity a total of four aliquots (containing ∼250 mg soil) were each extracted in duplicate for each flask (for a total of eight DNA extractions; DNA was resuspended in 800 μL Tris-EDTA buffer [TE]) (Feinstein et al., 2009; Jones et al., 2011b).
Separation of light and heavy DNA was performed as described previously (Martineau et al., 2008; Jones et al., 2011b) using 600 μL of the extracted DNA and 150 ng of spiked Escherichia coli DNA added to each ultracentrifuge tube (Singleton et al., 2005). After 40 h of ultracentrifugation at 175,800 g at 20°C in a TV-1665 vertical rotor (Sorvall), 250-μL fractions were collected through the bottom of the ultracentrifuge tubes via displacement from the top with water and a syringe pump, DNA purified and concentrated through ethanol precipitation, and the DNA from each fraction resuspended in 50 μL of TE buffer.
Analyses of SIP fractions
The range of collected ultracentrifuge fractions containing DNA was identified through a combination of methods. DNA concentration was measured using a NanoDrop ND-3300 fluorospectrometer with the Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen, Eugene, OR). Conventional PCR to determine fractions with PCR-amplifiable concentrations of DNA was performed with both general bacterial primers (341F/517R) and E. coli–specific primers using a Mastercycler Gradient Thermocycler (Eppendorf, Hauppauge, NY) as previously described (Singleton et al., 2005). Finally, denaturing gradient gel electrophoresis (DGGE) was performed using primers 341FGC-517R and a 10% acrylamide gel with a linear denaturing gradient of 35–65% on a DCode Universal Mutation Detection System (Bio-rad Laboratories, Hercules, CA) to identify shifts in the bacterial community patterns due to isotopic enrichment.
16S rRNA gene clone libraries for each of the incubations containing [U-13C]anthracene were constructed from four combined fractions identified as containing 13C-enriched DNA using amplicons derived from general bacterial PCR primers 8F and 1492R and a Topo-TA cloning kit (Invitrogen, Carlsbad, CA). A total of 48 clones (24 from each of the replicate incubations) were selected and sequenced using vector-specific M13 primers by Eton Biosciences (Research Triangle Park, NC). Sequences were trimmed and assembled using Sequencher 5.0 (Gene Codes Corp., Ann Arbor, MI) and grouped into operational taxonomic units (OTUs) at a threshold of 97% sequence similarity. Representative sequences for each OTU were determined using software from the Ribosomal Database Project (RDP-II) pyrosequencing pipeline (Cole et al., 2009). Relatives of recovered sequences were determined by BLASTN searches of public databases (Altschul et al., 1990) and neighbor-joining phylogenetic trees were constructed by ClustalX using 1000 replicates and not considering gaps in the alignment (Thompson et al., 2002). The “classifier” software of RDP-II was utilized to classify sequences using a confidence threshold of 80%.
Primers suitable for qPCR of 16S rRNA genes associated with putative anthracene-degrading bacteria identified in this study were developed and standard curves for quantification generated as previously described (Singleton et al., 2006). qPCR reactions using OTU-specific primers were performed as described above using template DNA extracted from the unlabeled-anthracene SIP time course samples or from the separated SIP fractions. The number of 16S rRNA genes from select OTUs were normalized by gram (dry weight) of soil (for SIP incubations) or volume of purified DNA (for SIP fractions). General bacterial qPCR primers were not used in these analyses. Queries of the previously constructed pyrosequence libraries derived from bioreactor-treated soil (Singleton et al., 2011) were performed by creating a local BLAST+ database of the pyrosequences, and the representative sequences from the SIP clonal OTUs were queried against the database (Camacho et al., 2009). Pyrosequence reads with ≥97% sequence similarity over at least 250 bp to a representative SIP sequence were considered putative members of that OTU. Representative sequences from each OTU were deposited into GenBank with the accession numbers KC138516-KC138525.
Results
Influence of soil source and slurry conditions on AG1 gene abundance
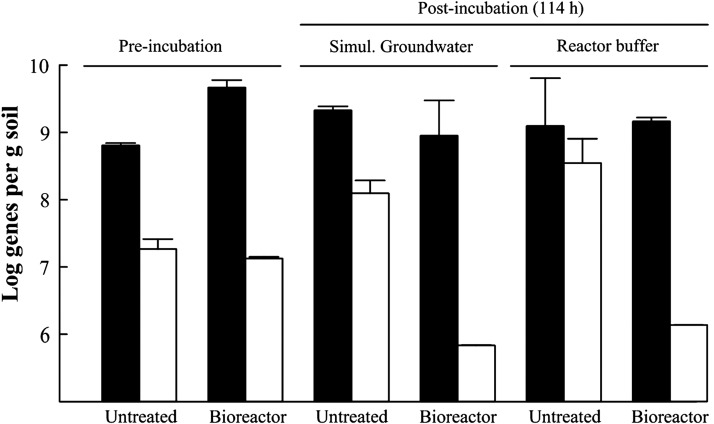
To test the response of previously identified AG1 organisms to a biostimulated environment, flasks containing either untreated, PAH-contaminated soil or the soil treated in a lab-scale, slurry-phase bioreactor were incubated with supplemental anthracene in either reactor buffer (which contained high levels of phosphate and nitrogen) or a simulated groundwater containing much lower inorganic nutrient concentrations. Samples in which untreated soil was the inoculum displayed a significant increase in the number of AG1-associated 16S rRNA genes regardless of the aqueous medium used; both simulated groundwater and the reactor buffer produced a similar, positive response (Fig. 1). In contrast, the number of AG1 16S rRNA genes significantly decreased in abundance when treated soil from the bioreactor was used as inoculum, and the aqueous phase of the slurry had no influence on this outcome. After 114 h the incubations of bioreactor-treated soil in reactor buffer displayed a terminal pH of 5.4±0.1 (from an initial pH of 7.0±0.0). All other tested conditions, including bioreactor-treated soil in simulated groundwater, maintained a neutral pH over the same time frame (data not shown). The response of AG1 to incubation appeared to be primarily determined by the source of soil inoculum (and associated microbial communities) rather than pH or the composition of the aqueous component of the soil slurry.
FIG. 1.
Effect of soil inoculum and batch incubation conditions on total bacteria and AG1-associated 16S rRNA genes. Solid bars represent total bacterial genes and open bars are AG1-associated genes. Bars are the mean of duplicate reactions and error bars the range of the values. Simul., simulated; AG1, Anthracene Group 1.
Optimization of conditions for SIP
To minimize the possible effects of pH variance during batch incubation of bioreactor-treated slurry during SIP, the effects of ammonium nitrate concentration on pH, mineralization of anthracene, and disappearance of anthracene were examined. In contrast to the base reactor buffer, formulations with reduced concentrations of ammonium nitrate (2.5 or 1 mM) maintained a neutral pH over 117 h of incubation (Supplementary Fig. S1). While assays showed reduced rates and extent of 14C-anthracene mineralization with reduced concentrations of ammonium nitrate compared with the base reactor buffer (Supplementary Fig. S2), the nitrogen concentration had little effect on the disappearance of anthracene (Supplementary Fig. S3) and <20% was recovered after 5 days under all tested conditions. Based on these preliminary data, SIP with bioreactor-treated soil was performed in a modified reactor buffer containing 2.5 mM ammonium nitrate over a period of 5 days.
Stable-isotope probing
DNA enriched with 13C derived from labeled anthracene was successfully recovered from batch incubations containing bioreactor-treated soil after 5 days. Fractions containing the 13C-enriched DNA (“heavy DNA”) were identified through a combination of DNA quantification, community analyses by DGGE, and the location of unlabeled E. coli DNA that had been spiked into the DNA samples (Supplementary Fig. S4 and data not shown).
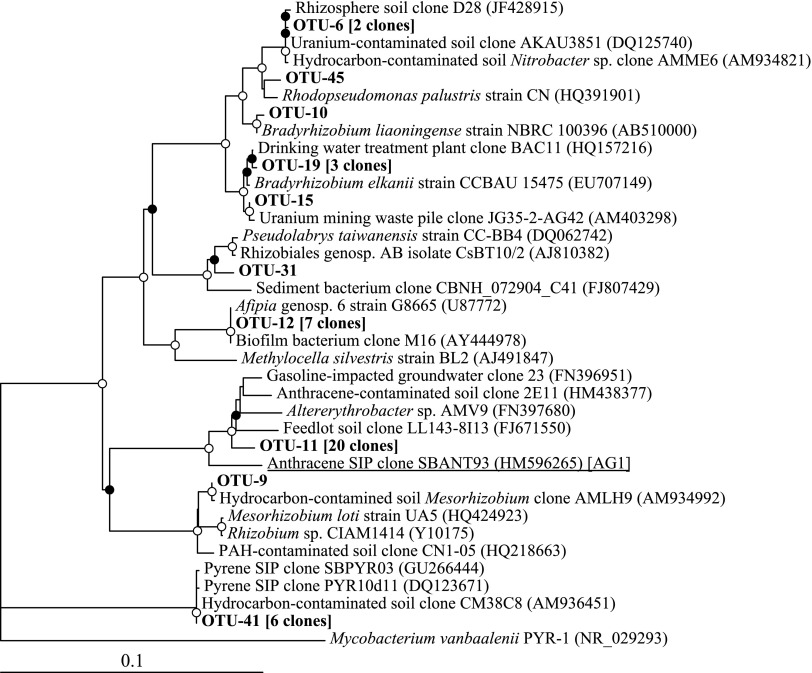
Clone libraries of 16S rRNA genes were constructed from heavy DNA fractions from each of the duplicate [U-13C]anthracene incubations. After removing chimeric and low-quality sequences, a total of 43 nearly complete 16S rRNA genes were recovered (20 and 23 from the first and second replicates, respectively). The sequences represented 10 OTUs (at 97% sequence similarity), nine of which were associated with members of the Alphaproteobacteria and one with the Gammaproteobacteria (Fig. 2). Five of these OTUs were singleton sequences and were not considered in further analyses. The largest group of sequences (OTU-11) contained 20 clones that were most closely related to the genus Alterythrobacter within the order Sphingomonadales. OTU-11 was also the phylogenetic clade most closely related to the previously identified AG1 (Fig. 2), although the OTU-11 and AG1 representative sequences shared only 96.3% 16S rRNA gene sequence similarity over 1,450 bp. The second most abundant group of sequences (OTU-12; seven clones) was most closely related to members of the Alphaproteobacterial family Beijerinckiaceae. Sequences with high similarity to members of the uncharacterized “pyrene group 2” (PG2), previously associated with the degradation of benz[a]anthracene, fluoranthene, and pyrene in the untreated soil (although not anthracene) comprised the third most abundant cluster of sequences (OTU-41; six clones). An OTU containing three sequences (OTU-19) was most closely related to members of the Bradyrhizobium genus, and another containing two sequences (OTU-6) possessed high similarity to bacteria within the family Bradyrhizobiaceae. Three of the singleton sequences also clustered within the family Bradyrhizobiaceae. OTUs containing at least three sequences were recovered in nearly equal distribution from clone libraries constructed from each the replicate 13C-anthracene incubations (data not shown).
FIG. 2.
16S rRNA gene neighbor-joining tree showing relationship of representative sequences from SIP-derived OTUs in this study (in bold) to nearest relatives. The total number of clones represented by each sequence is indicated in brackets. GenBank accession numbers are displayed in parentheses. A sequence representing AG1 from SIP of the untreated soil is underlined. The tree was bootstrapped 1,000 times and circles at nodes indicate either ≥95% (open circles) or ≥50% (closed circles) bootstrap support. The 16S rRNA gene sequence of Mycobacterium vanbaalenii was used as an outgroup. OTUs, operational taxonomic units; SIP, stable-isotope probing.
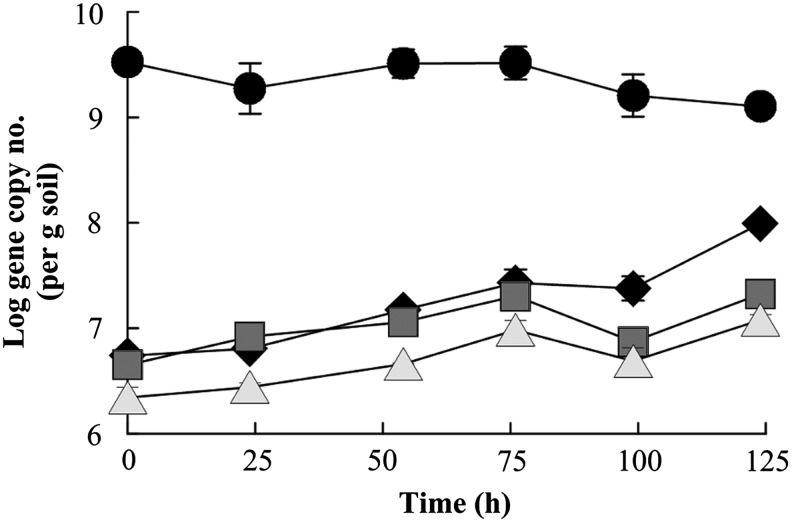
Quantitative real-time PCR primers targeting regions within the 16S rRNA gene sequences comprising the more abundant OTUs (OTU-6, -11, -12, -19, and -41) were developed to verify that the genes associated with those groups both increased in abundance during incubation with anthracene (as a surrogate for growth) and were located in significant numbers in heavy DNA fractions (as an indicator of growth on 13C-anthracene or a 13C-anthracene derived metabolite; Table 1). 16S rRNA sequences associated with three of the four most abundant OTUs displayed increased copy numbers over the 5-day incubation (OTU-11, -12, and -19; Fig. 3). In contrast, 16S rRNA genes associated with OTU-41 did not appear to increase in response to incubation with anthracene, although genes from this OTU began the experiment at a significantly higher abundance than those from the other quantified OTUs (approximately three orders of magnitude). Sequences associated with OTU-6 were below the quantification limit of the assay.
Table 1.
Quantitative Real-Time Polymerase Chain Reaction Primers Used in This Study
| Target | Primer name | Sequence (5′→3′) | Amplicon size (bp) | Annealing temp (°C) | Amp. eff.a | No. seqs.b | Ref. |
|---|---|---|---|---|---|---|---|
| OTU-6 | ANT-6F | AAC AAC TGA GGG AAA CTT CA | 113 | 57.5 | 1.70 | 1172 | This study |
| ANT-6R | TTG GTG AGC CAT TAC CTC | ||||||
| OTU-11 | ANT-11F | CGG ATT ACA GAG ATG TTT TC | 149 | 56 | 2.19 | 0 | This study |
| ANT-11R | TAG AGT TCC CAA CTG AAT GA | ||||||
| OTU-12 | ANT-12F | AAA TCC CAG AGC TCA ACT CT | 75 | 58 | 2.09 | 5 | This study |
| ANT-12R | CGC AGT TCC ACT TAC CTC TT | ||||||
| OTU-19 | ANT-19F | ACA TCC CGG TCG CGG ACT | 63 | 60 | 1.85 | 1782 | This study |
| ANT-19R | ACC YGT CTC CGG TCC AG | ||||||
| OTU-41 | PG2.4F | CCA AGC CGA CGA CGG GTA G | 94 | 59 | 1.99 | 742 | (Jones et al., 2011a) |
| PG2.4R | TTC CCC ACT GCT GCC TC |
Amplification efficiency.
Number of sequences in the RDP-II database (release 10, update 29; June 1, 2012) that match both primers with no mismatches as determined by the ProbeMatch function (Cole et al., 2009).
OTU, operational taxonomic unit.
FIG. 3.
16S rRNA gene copy number per gram soil (dry weight) over the time of the SIP experiment as determined from DNA extracted from aliquots of unlabeled anthracene incubations. Points represent the mean of qPCRs (n=2) and error bars the range of values. Groups of sequences quantified were OTU-41 (●), OTU-11 (♦), OTU-12 (■), and OTU-19 (△).
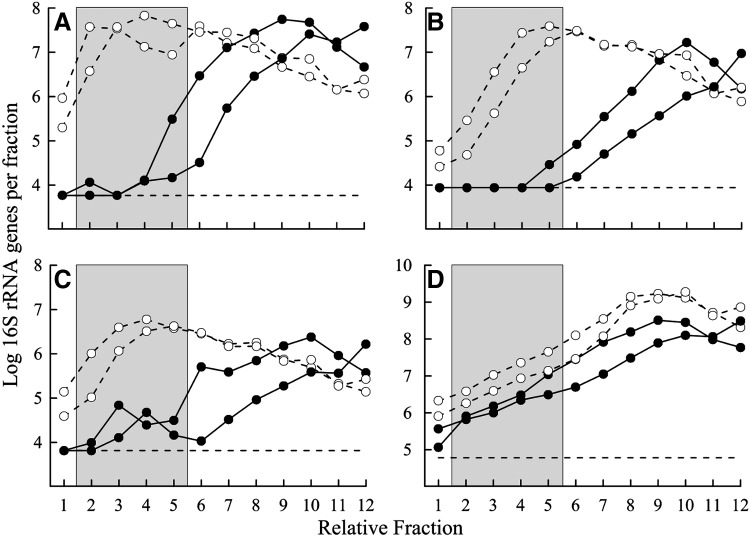
A range of 12 collected DNA fractions spanning regions determined to contain either heavy or unlabeled DNA were analyzed by qPCR using primers specific for members of each OTU to determine the distribution of DNA from each group in the separated SIP fractions (Fig. 4). In incubations with 13C-anthracene, fractions representing heavy DNA (relative fractions 2–5) contained an average of 57.8%, 36.0%, and 58.7% of the total quantified 16S rRNA genes from OTU-11, -12, and -19, respectively. The equivalent fractions from incubations with unlabeled anthracene contained 0.1%, 0.2%, and 2.4% of genes from the same OTUs, confirming 13C-enrichment of the DNA for a significant percentage of those populations. In contrast to those OTUs, sequences associated with OTU-41 comprised nearly equal percentages of the designated heavy fractions from the 13C-anthracene incubations and the equivalent fractions from the unlabeled anthracene incubations (1.0% and 1.3%, respectively) indicating little or no 13C-enrichment of DNA from those organisms.
FIG. 4.
16S rRNA gene copy number associated with abundant OTUs in recovered SIP fractions; (A) OTU-11, (B) OTU-12, (C) OTU-19, and (D) OTU-41. -○-, fractions from incubations with 13C-anthracene; —●—, fractions from incubations with unlabeled anthracene. Each point represents a single qPCR and separate lines are for duplicate incubations. The horizontal dashed line in each panel indicates the quantification limit of the assay and qPCR values below that threshold were increased to that limit for the purposes of presenting the data. The shaded area indicates fractions identified as containing heavy DNA and combined to generate clone libraries.
Discussion
Bacterial 16S rRNA gene sequences recovered during DNA-based SIP of bioreactor-treated soil with 13C-anthracene differed from sequences recovered from a similar SIP experiment using the same soil prior to biological treatment. These differences were attributable to the source material (a bacterial community conditioned within a long-running bioreactor) and the environment within the bioreactor (and by extension, within the SIP incubations), which contained a much higher concentration of amended inorganic nutrients compared with the prior study (Jones et al., 2011b). Biostimulation as part of a remediation scheme is expected to result in significant bacterial community shifts, including selecting for organisms capable of degrading a particular substrate that might not be in high abundance under more oligotrophic conditions.
Sequences associated with a group of uncharacterized bacteria designated AG1 were the most frequently encountered in 13C-enriched DNA derived from untreated soil (Jones et al., 2011b). As attempts to isolate members of AG1 in our lab using traditional methods were unsuccessful, molecular methods were used to ascertain the response of these organisms to specific environmental conditions. While sequences from this group were abundant in the untreated soil, ranging from 4–10% of total bacterial 16S rRNA genes in pyrosequence libraries (Singleton et al., accepted for publication; Singleton et al., 2011), operation of the bioreactor resulted in a declining relative abundance of AG1 sequences, with a relative abundance after 140 days of 0.3% (Singleton et al., 2011). Anthracene-degrading Variovorax and Pigmentiphaga sequences in the untreated soil similarly declined in relative abundance during bioreactor operation, decreasing from <1% of sequences in the untreated soil to negligible or undetectable levels (Singleton et al., 2011; Jones et al., 2011b).
The most commonly encountered sequences in clone libraries derived from 13C-enriched DNA in this study of bioreactor-treated soil were associated with the genus Altererythrobacter (Fig. 2); although they shared a relatively high degree of 16S rRNA gene sequence similarity to members of AG1 (96%), the extent of sequence divergence implied two separate groups of anthracene-degrading organisms. Members of the Altererythrobacter genus have been only rarely associated with PAH degradation, with one strain isolated from Indonesian seawater shown to grow on the 3-ring PAHs phenanthrene and fluorene; growth on anthracene was not tested in that study (Teramoto et al., 2010). Members of this genus were undetectable at most time points in pyrosequence libraries of the untreated soil and bioreactor-treated soil over the first several months of operation (Singleton et al., 2011), so their dominant presence in 13C-enriched DNA clone libraries coupled with their low relative abundance in bioreactor slurry (estimated 0.1% of total 16S rRNA genes) may indicate a specific affinity for anthracene as a growth substrate.
Including the genus Altererythrobacter, all of the sequences identified in this study associated with anthracene metabolism were members of the Alphaproteobacteria, with a particular association of the remaining OTUs with the order Rhizobiales and genus Bradyrhizobium. While not commonly identified as dominant members of PAH-impacted soils, sequences identified with this particular order have been fairly frequently associated with anthracene contamination. Bacteria associated with the Rhizobiales have been found in abundance in anthracene-impacted soils and sediments from the United Kingdom, China, and Mexico (Long et al., 2009; Zhang et al., 2011a, 2012; Núñez et al., 2012).
Representative sequences of the three dominant OTUs recovered in this study were compared to previously pyrosequenced communities from the untreated soil and bioreactor (Singleton et al., 2011). None of the OTUs representing putative anthracene-degraders represented >0.3% of the total 16S rRNA genes in the untreated soil (note that OTU-41, or “PG2” was not included among this group because it did not appear to be associated with anthracene). OTU-19 represented 1.0% of the sequences after an initial equilibration phase, but that percentage declined to 0.3% after 140 d of operation. Neither sequences from OTU-11 nor OTU-12 represented more than 0.1% of the community at any examined time point in the bioreactor study. The low relative abundance of bacteria associated with these OTUs may be a reflection of the relatively low concentration of anthracene in this particular soil (compared to other PAHs). While sequences from OTU-41 were recovered in fractions containing heavy DNA from the SIP incubations, genes associated with the group did not increase during anthracene enrichment and were primarily associated with SIP fractions containing unenriched DNA. The occurrence of OTU-41 sequences in SIP clone libraries was attributed to nonspecific migration of unlabeled DNA into the heavy fractions during separation due to the high concentration of DNA from these organisms in the original sample.
Results of this study demonstrate functional redundancy for anthracene degradation in the natural community found in aged, PAH-contaminated soil. Functional redundancy for pollutant degradation has previously been observed during biological treatment of other contaminated soils (Korotkevych et al., 2011; Pilloni et al., 2011) and wastewater (Curtis and Sloan, 2006), and such redundancy has also been specifically shown for the degradation of the LMW PAH naphthalene (Guazzaroni et al., 2013). This and other works demonstrate the possibility that organisms identified as degraders of a particular compound (either through cultivation or molecular techniques) based solely on analyses of a contaminated but untreated sample may fail to accurately predict the most relevant organisms within a biological treatment scheme.
Supplementary Material
Acknowledgments
We thank our funding source, the U.S. National Institute of Environmental Health Sciences (NIEHS) Superfund Research Program, grant number 5 P42 ES005948. We also acknowledge Dr. Jing Hu, who provided assistance with the HPLC analyses performed in this work.
Author Disclosure Statement
No competing financial interests exist.
References
- Acevedo F. Pizzul L. Castillo Mdel P. Cuevas R. Diez M.C. Degradation of polycyclic aromatic hydrocarbons by the Chilean white-rot fungus Anthracophyllum discolor. J. Hazard. Mater. 2011;185:212. doi: 10.1016/j.jhazmat.2010.09.020. [DOI] [PubMed] [Google Scholar]
- Altschul S.F. Gish W. Miller W. Myers E.W. Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Arulazhagan P. Vasudevan N. Biodegradation of polycyclic aromatic hydrocarbons by a halotolerant bacterial strain Ochrobactrum sp. VA1. Mar. Pollut. Bull. 2011;62:388. doi: 10.1016/j.marpolbul.2010.09.020. [DOI] [PubMed] [Google Scholar]
- Baboshin M. Akimov V. Baskunov B. Born T. Khan S. Golovleva L. Conversion of polycyclic aromatic hydrocarbons by Sphingomonas sp. VKM B-2434. Biodegradation. 2008;19:567. doi: 10.1007/s10532-007-9162-2. [DOI] [PubMed] [Google Scholar]
- Benhabib K. Faure P. Sardin M. Simonnot M.-O. Characteristics of a solid coal tar sampled from a contaminated soil and of the organics transferred into water. Fuel. 2010;89:352. [Google Scholar]
- Camacho C. Coulouris G. Avagyan V. Ma N. Papadopoulos J. Bealer K. Madden T.L. BLAST plus: Architecture and applications. BMC Bioinform. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cébron A. Louvel B. Faure P. France-Lanord C. Chen Y. Murrell J.C. Leyval C. Root exudates modify bacterial diversity of phenanthrene degraders in PAH-polluted soil but not phenanthrene degradation rates. Environ. Microbiol. 2011;13:722. doi: 10.1111/j.1462-2920.2010.02376.x. [DOI] [PubMed] [Google Scholar]
- Cole J.R. Wang Q. Cardenas E. Fish J. Chai B. Farris R.J. Kulam-Syed-Mohideen A.S. McGarrell D.M. Marsh T. Garrity G.M. Tiedje J.M. The Ribosomal Database Project: Improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009;37:D141. doi: 10.1093/nar/gkn879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis T.P. Sloan W.T. Towards the design of diversity: Stochastic models for community assembly in wastewater treatment plants. Water Sci. Technol. 2006;54:227. doi: 10.2166/wst.2006.391. [DOI] [PubMed] [Google Scholar]
- Dean-Ross D. Moody J.D. Freeman J.P. Doerge D.R. Cerniglia C.E. Metabolism of anthracene by a Rhodococcus species. FEMS Microbiol. Lett. 2001;204:205. doi: 10.1111/j.1574-6968.2001.tb10886.x. [DOI] [PubMed] [Google Scholar]
- Feinstein L.M. Sul W.J. Blackwood C.B. Assessment of bias associated with incomplete extraction of microbial DNA from soil. Appl. Environ. Microbiol. 2009;75:5428. doi: 10.1128/AEM.00120-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guazzaroni M.-E. Herbst F.-A. Lores I. Tamames J. Pelaez A.I. Lopez-Cortes N. Alcaide M. Del Pozo M.V. Vieites J.M. von Bergen M. Gallego J.L.R. Bargiela R. Lopez-Lopez A. Pieper D.H. Rossello-Mora R. Sanchez J. Seifert J. Ferrer M. Metaproteogenomic insights beyond bacterial response to naphthalene exposure and bio-stimulation. ISME J. 2013;7:122. doi: 10.1038/ismej.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez T. Singleton D.R. Aitken M.D. Semple K.T. Stable isotope probing of an algal bloom to identify uncultivated members of the Rhodobacteraceae associated with low-molecular-weight polycyclic aromatic hydrocarbon degradation. Appl. Environ. Microbiol. 2011;77:7856. doi: 10.1128/AEM.06200-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeseler F. Blanchet D. Durelle V. Werner P. Vandecasteele J.-P. Analytical characterization of contaminated soils from former manufactured gas plants. Environ. Sci. Technol. 1999;33:825. [Google Scholar]
- Jacques R.J.S. Santos E.C. Bento F.M. Peralba M.C.R. Selbach P.A. Sá E.S. Camargo F.A.O. Anthracene biodegradation by Pseudomonas sp. isolated from a petrochemical sludge landfarming site. Int. Biodeterior. Biodegrad. 2005;56:143. [Google Scholar]
- Jeon C.O. Park W. Padmanabhan P. DeRito C. Snape J.R. Madsen E.L. Discovery of a bacterium, with distinctive dioxygenase, that is responsible for in situ biodegradation in contaminated sediment. Proc. Natl. Acad. Sci. USA. 2003;100:13591. doi: 10.1073/pnas.1735529100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H.M. Kim J.M. Lee H.J. Madsen E.L. Jeon C.O. Alteromonas as a key agent of polycyclic aromatic hydrocarbon biodegradation in crude oil-contaminated coastal sediment. Environ. Sci. Technol. 2012;46:7731. doi: 10.1021/es3018545. [DOI] [PubMed] [Google Scholar]
- Jones M.D. Crandell D.W. Singleton D.R. Aitken M.D. Stable-isotope probing of the polycyclic aromatic hydrocarbon-degrading bacterial guild in a contaminated soil. Environ. Microbiol. 2011a;13:2623. doi: 10.1111/j.1462-2920.2011.02501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M.D. Singleton D.R. Carstensen D.P. Powell S.N. Swanson J.S. Pfaender F.K. Aitken M.D. Effect of incubation conditions on the enrichment of pyrene-degrading bacteria identified by stable-isotope probing in an aged, PAH-contaminated soil. Microb. Ecol. 2008;56:341. doi: 10.1007/s00248-007-9352-9. [DOI] [PubMed] [Google Scholar]
- Jones M.J. Singleton D.R. Sun W. Aitken M.D. Multiple DNA extractions coupled to stable-isotope probing of anthracene-degrading bacteria in contaminated soil. Appl. Environ. Microbiol. 2011b;77:2984. doi: 10.1128/AEM.01942-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A.A. Kim S.J. Paine D.D. Cerniglia C.E. Classification of a polycyclic aromatic hydrocarbon-metabolizing bacterium, Mycobacterium sp. strain PYR-1, as Mycobacterium vanbaalenii sp. nov. Int. J. Syst. Evol. Microbiol. 2002;52:1997. doi: 10.1099/00207713-52-6-1997. [DOI] [PubMed] [Google Scholar]
- Korotkevych O. Josefiova J. Praveckova M. Cajthaml T. Stavelova M. Brennerova M.V. Functional adaptation of microbial communities from jet fuel-contaminated soil under bioremediation treatment: simulation of pollutant rebound. FEMS Microbiol. Ecol. 2011;78:137. doi: 10.1111/j.1574-6941.2011.01169.x. [DOI] [PubMed] [Google Scholar]
- Ling J. Zhang G. Sun H. Fan Y. Ju J. Zhang C. Isolation and characterization of a novel pyrene-degrading Bacillus vallismortis strain JY3A. Sci. Total Environ. 2011;409:1994. doi: 10.1016/j.scitotenv.2011.02.020. [DOI] [PubMed] [Google Scholar]
- Long R. Lappin-Scott H. Stevens J. Enrichment and identification of polycyclic aromatic compound-degrading bacteria enriched from sediment samples. Biodegradation. 2009;20:521. doi: 10.1007/s10532-008-9241-z. [DOI] [PubMed] [Google Scholar]
- Martin F. Torelli S. Le Paslier D. Barbance A. Martin-Laurent F. Bru D. Geremia R. Blake G. Jouanneau Y. Betaproteobacteria dominance and diversity shifts in the bacterial community of a PAH-contaminated soil exposed to phenanthrene. Environ. Pollut. 2012;162:345. doi: 10.1016/j.envpol.2011.11.032. [DOI] [PubMed] [Google Scholar]
- Martineau C. Whyte L.G. Greer C.W. Development of a SYBR safe™ technique for the sensitive detection of DNA in cesium chloride density gradients for stable isotope probing assays. J. Microbiol. Methods. 2008;73:199. doi: 10.1016/j.mimet.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Núñez E.V. Valenzuela-Encinas C. Alcántara-Hernández R.J. Navarro-Noya Y.E. Luna-Guido M. Marsch R. Dendooven L. Modifications of bacterial populations in anthracene contaminated soil. Appl. Soil Ecol. 2012;61:113. [Google Scholar]
- Pilloni G. von Netzer F. Engel M. Lueders T. Electron acceptor-dependent identification of key anaerobic toluene degraders at a tar-oil-contaminated aquifer by Pyro-SIP. FEMS Microbiol. Ecol. 2011;78:165. doi: 10.1111/j.1574-6941.2011.01083.x. [DOI] [PubMed] [Google Scholar]
- Radajewski S. Ineson P. Parekh N.R. Murrell J.C. Stable-isotope probing as a tool in microbial ecology. Nature. 2000;403:646. doi: 10.1038/35001054. [DOI] [PubMed] [Google Scholar]
- Richardson S.D. Jones M.D. Singleton D.R. Aitken M.D. Long-term simulation of in situ biostimulation of polycyclic aromatic hydrocarbon-contaminated soil. Biodegradation. 2012;23:621. doi: 10.1007/s10532-012-9538-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson S.D. Lebron B. Miller C.T. Aitken M.D. Recovery of phenanthrene-degrading bacteria after simulated in situ persulfate oxidation in contaminated soil. Environ. Sci. Technol. 2011;45:719. doi: 10.1021/es102420r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton D.R. Hunt M. Powell S.N. Frontera-Suau R. Aitken M.D. Stable-isotope probing with multiple growth substrates to determine substrate specificity of uncultivated bacteria. J. Microbiol. Methods. 2007;69:180. doi: 10.1016/j.mimet.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Singleton D.R. Jones M.D. Richardson S.D. Aitken M.D. Pyrosequence analyses of bacterial communities during simulated in situ bioremediation of polycyclic aromatic hydrocarbon-contaminated soil. Appl. Microbiol. Biotechnol. 2013;97:8381. doi: 10.1007/s00253-012-4531-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton D.R. Powell S.N. Sangaiah R. Gold A. Ball L.M. Aitken M.D. Stable-isotope probing of bacteria capable of degrading salicylate, naphthalene, or phenanthrene in a bioreactor treating contaminated soil. Appl. Environ. Microbiol. 2005;71:1202. doi: 10.1128/AEM.71.3.1202-1209.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton D.R. Richardson S.D. Aitken M.D. Effects of enrichment with phthalate on polycyclic aromatic hydrocarbon biodegradation in contaminated soil. Biodegradation. 2008;19:577. doi: 10.1007/s10532-007-9163-1. [DOI] [PubMed] [Google Scholar]
- Singleton D.R. Richardson S.D. Aitken M.D. Pyrosequence analysis of bacterial communities in aerobic bioreactors treating polycyclic aromatic hydrocarbon-contamined soil. Biodegradation. 2011;22:1061. doi: 10.1007/s10532-011-9463-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton D.R. Sangaiah R. Gold A. Ball L.M. Aitken M.D. Identification and quantification of uncultivated proteobacteria associated with pyrene degradation in a bioreactor treating PAH-contaminated soil. Environ. Microbiol. 2006;10:1736. doi: 10.1111/j.1462-2920.2006.01112.x. [DOI] [PubMed] [Google Scholar]
- Story S.P. Kline E.L. Hughes T.A. Riley M.B. Hayasaka S.S. Degradation of aromatic hydrocarbons by Sphingomonas paucimobilis strain EPA505. Arch. Environ. Contam. Toxicol. 2004;47:168. doi: 10.1007/s00244-004-3069-2. [DOI] [PubMed] [Google Scholar]
- Teramoto M. Suzuki M. Hatmanti A. Harayama S. The potential of Cycloclasticus and Altererythrobacter strains for use in bioremediation of petroleum-aromatic-contaminated tropical marine environments. J. Biosci. Bioeng. 2010;110:48. doi: 10.1016/j.jbiosc.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Thompson J.D. Gibson T.J. Higgins D.G. Multiple sequence alignment using ClustalW and ClustalX. Current Protocols in Bioinformatics. 2002:2.3.1–2.3.22. doi: 10.1002/0471250953.bi0203s00. [DOI] [PubMed] [Google Scholar]
- Uhlik O. Wald J. Strejcek M. Musilova L. Ridl J. Hroudova M. Vlcek C. Cardenas E. Mackova M. Macek T. Identification of bacteria utilizing biphenyl, benzoate, and naphthalene in long-term contaminated soil. PLoS One. 2012;7:e40653. doi: 10.1371/journal.pone.0040653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. Provisional Guidance for Quantitative Risk Assessment of Polycyclic Aromatic Hydrocarbons. Environmental Criteria and Assessment Office, Office of Health and Environmental Assessment; Washington D.C.: 1993. [Google Scholar]
- Wu Y.-R. Luo Z.-H. Vrijmoed L.L.P. Biodegradation of anthracene and benz[a]anthracene by two Fusarium solani strains isolated from mangrove sediments. Bioresour. Technol. 2010;101:9666. doi: 10.1016/j.biortech.2010.07.049. [DOI] [PubMed] [Google Scholar]
- Yu C.P. Chu K.H. A quantitative assay for linking microbial community function and structure of a naphthalene-degrading microbial consortium. Environ. Sci. Technol. 2005;39:9611. doi: 10.1021/es051024e. [DOI] [PubMed] [Google Scholar]
- Zeinali M. Vossoughi M. Ardestani S.K. Characterization of a moderate thermophilic Nocardia species able to grow on polycyclic aromatic hydrocarbons. Lett. Appl. Microbiol. 2007;45:622. doi: 10.1111/j.1472-765X.2007.02241.x. [DOI] [PubMed] [Google Scholar]
- Zeng J. Lin X. Zhang J. Li X. Isolation of polycyclic aromatic hydrocarbons (PAHs)-degrading Mycobacterium spp. and the degradation in soil. J. Hazard. Mater. 2010;183:718. doi: 10.1016/j.jhazmat.2010.07.085. [DOI] [PubMed] [Google Scholar]
- Zhang G.-Y. Ling J.-Y. Sun H.-B. Luo J. Fan Y.-Y. Cui Z.-J. Isolation and characterization of a newly isolated polycyclic aromatic hydrocarbons-degrading Janibacter anophelis strain JY11. J. Hazard. Mater. 2009;172:580. doi: 10.1016/j.jhazmat.2009.07.037. [DOI] [PubMed] [Google Scholar]
- Zhang S.-Y. Wang Q.-F. Wan R. Xie S.-G. Changes in bacterial community of anthracene bioremediation in municipal solid waste composting soil. J. Zhejiang. Univ. Sci. B. 2011a;12:760. doi: 10.1631/jzus.B1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S. Wan R. Wang Q. Xie S. Identification of anthracene degraders in leachate-contaminated aquifer using stable isotope probing. Int. Biodeterior. Biodegrad. 2011b;65:1224. [Google Scholar]
- Zhang S. Wang Q. Xie S. Stable isotope probing identifies anthracene degraders under methanogenic conditions. Biodegradation. 2012;23:221. doi: 10.1007/s10532-011-9501-1. [DOI] [PubMed] [Google Scholar]
- Zhang Z. Sangaiah R. Gold A. Ball L.M. Synthesis of uniformly 13C-labeled polycyclic aromatic hydrocarbons. Org. Biomol. Chem. 2011c;9:5431. doi: 10.1039/c0ob01107j. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.