Abstract
Aims: Hydrogen sulfide (H2S) is suggested to act as a gaseous signaling molecule in a variety of physiological processes. Its molecular mechanism of action was proposed to involve protein S-sulfhydration, that is, conversion of cysteinyl thiolates (Cys-S−) to persulfides (Cys-S-S−). A central and unresolved question is how H2S—that is, a molecule with sulfur in its lowest possible oxidation state (−2)—can lead to oxidative thiol modifications. Results: Using the lipid phosphatase PTEN as a model protein, we find that the “H2S donor” sodium hydrosulfide (NaHS) leads to very rapid reversible oxidation of the enzyme in vitro. We identify polysulfides formed in NaHS solutions as the oxidizing species, and present evidence that sulfane sulfur is added to the active site cysteine. Polysulfide-mediated oxidation of PTEN was induced by all “H2S donors” tested, including sodium sulfide (Na2S), gaseous H2S, and morpholin-4-ium 4-methoxyphenyl(morpholino) phosphinodithioate (GYY4137). Moreover, we show that polysulfides formed in H2S solutions readily modify PTEN inside intact cells. Innovation: Our results shed light on the previously unresolved question of how H2S leads to protein thiol oxidation, and suggest that polysulfides formed in solutions of H2S mediate this process. Conclusion: This study suggests that the effects that have been attributed to H2S in previous reports may in fact have been mediated by polysulfides. It also supports the notion that sulfane sulfur rather than sulfide is the actual in vivo agent of H2S signaling. Antioxid. Redox Signal. 19, 1749–1765.
Introduction
For 300 years, hydrogen sulfide (H2S) gas has been known for both its malodorous smell and toxicity, the latter being primarily related to its potent ability to inhibit cytochrome c oxidase (36, 44). Interestingly, recent research has revealed H2S to act as a signaling molecule involved in various physiological processes, including inflammation, apoptosis, vasorelaxation, and neuromodulation (44). While more functions of H2S are being uncovered, its molecular mechanism of action remains unclear.
H2S has a pKa1 of 6.77 at 37°C, which is why it exists as both H2S (∼20%) and HS− (∼80%) at pH 7.4 (32). Due to its second pKa2 of>12, the concentration of the completely deprotonated S2− is extremely low at physiological pH (32). For simplicity, H2S will be used henceforth to refer to the total sulfide pool (H2S+HS−+S2−). While some of the observed (patho-) physiological effects induced by H2S have been attributed to its antioxidative capacity or to the inhibition of cytochrome c oxidase, H2S appears to predominantly act via S-sulfhydration of target proteins (34). This concept describes the addition of H2S-derived sulfur to a cysteinyl thiolate (Cys-S−) to yield a cysteinyl persulfide (Cys-S-S−), which then leads to either activation or inhibition of protein activity (20, 28). Numerous proteins have been reported to be S-sulfhydrated by H2S, both in vitro and/or in intact cells, including actin, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), nuclear factor κB (NF-κB), ATP-sensitive potassium channel (KATP), protein tyrosine phosphatase 1B (PTP1B), and Kelch-like ECH-associated protein-1 (Keap1) (11, 20, 28, 29, 38, 47). Surprisingly, none of these original publications have experimentally addressed the paradox that H2S, with its sulfur in the lowest possible oxidation state (−2), causes oxidation of thiols to persulfides, although by itself it should only be able to act as a reductant. The aim of this study was thus to elucidate the mechanism of H2S-induced oxidation of protein thiols.
Innovation.
The signaling mechanism of hydrogen sulfide (H2S) has been hypothesized to involve S-sulfhydration of target proteins. The underlying biochemistry has remained obscure, as chemically, H2S cannot oxidize protein thiols. Here we show that H2S solutions prepared from commonly used H2S sources contain and/or generate polysulfides, which in turn, rapidly oxidize protein thiols in vitro. Moreover, polysulfides in the cell culture supernatant are able to oxidize the lipid phosphatase PTEN in intact cells. Our work further elucidates the mechanism of H2S-induced protein thiol oxidation, by suggesting that it is mediated by polysulfides.
The lipid phosphatase and tensin homolog (PTEN) was chosen as a model protein to conduct these investigations. As the main antagonist of the phosphoinositide 3-kinase (PI3K)–Akt pathway, PTEN acts as a tumor suppressor, and is well established to be redox regulated in the course of growth factor signaling (21, 23). Specifically, upon oxidative challenge, PTEN was shown to form a disulfide bond between the active site cysteine Cys-124 and Cys-71, resulting in its immediate and reversible inhibition (22).
By monitoring the activity of PTEN in a newly developed real-time assay, we were able to continuously assess its redox state in response to H2S solutions. Our data demonstrate that dissolved sodium hydrosulfide (NaHS) leads to very rapid oxidation of the PTEN active site cysteine in vitro, surpassing even hydrogen peroxide (H2O2) in its oxidative efficiency. We identify polysulfides formed in neutral solutions of NaHS as the oxidizing species. Notably, we find evidence for the formation of polysulfides in solutions of all “H2S donors” tested, including sodium sulfide (Na2S), gaseous H2S, as well as morpholin-4-ium 4-methoxyphenyl(morpholino) phosphinodithioate (GYY4137). Moreover, we show that polysulfides in the culture medium lead to oxidative PTEN modification in intact cells. Our study highlights the phenomenon of polysulfide formation in neutral and weakly alkaline H2S solutions and suggests that at least some of the previously reported findings in H2S research may have been mediated by polysulfides.
Results
A novel in vitro activity assay allows monitoring of the PTEN redox state in real time
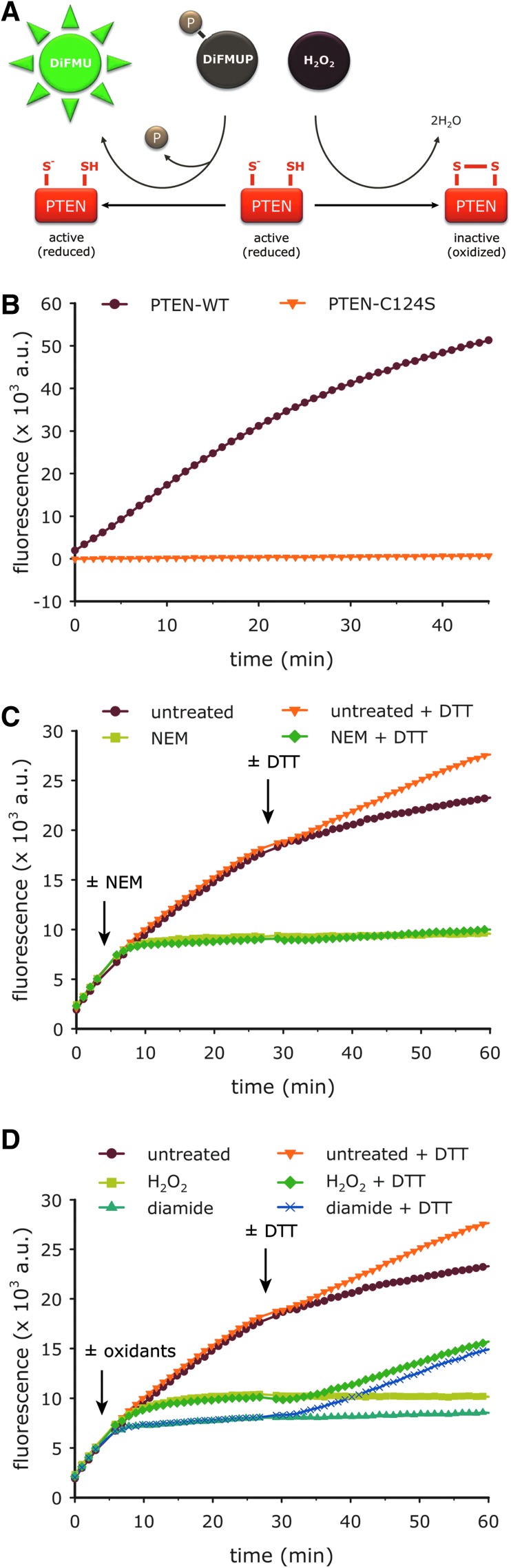
As PTEN activity strictly depends on the free thiol of Cys-124, oxidative modification of this thiol leads to inhibition of PTEN. To follow redox changes of the active site thiol on a second-to-minute time scale, we established an in vitro real-time assay for the PTEN activity based on recombinant PTEN and the fluorogenic substrate 6,8-difluoro-4-methylumbelliferyl phosphate (DiFMUP) (Fig. 1A). As expected, mutagenesis of the active site cysteine to serine (C124S) led to a complete loss of PTEN activity (Fig. 1B). Likewise, the thiol-reactive alkylating agent N-ethyl maleimide (NEM) inhibited the activity of wild type PTEN (PTEN-WT) rapidly and irreversibly (Fig. 1C). Both H2O2 and diamide (N,N,N′,N′-tetramethylazodicarboxamide) inhibited PTEN-WT in a dithiothreitol (DTT) reversible manner (Fig. 1D), suggesting formation of the previously described intramolecular disulfide bond between Cys-124 and Cys-71 (22). Gel mobility analysis confirmed H2O2-induced disulfide bond formation in PTEN-WT, which is abolished in PTEN mutants lacking either Cys-124 or Cys-71 (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/ars). In summary, we developed a novel assay that allows for the real-time detection of rapid and subtle changes in the activity, and thus, the Cys-124 redox state of PTEN.
FIG. 1.
PTEN activity can be monitored in real time using DiFMUP as substrate. (A) Schematic illustrating the real time PTEN activity assay used throughout this study. PTEN dephosphorylates the fluorogenic substrate DiFMUP, leading to an increase in the fluorescence signal, unless it is inhibited via oxidation to the disulfide-bonded state by oxidants such as H2O2. (B) Recombinant PTEN-WT or -C124S was left to react with DiFMUP in the presence of 5 mM DTT following the procedure described in Materials and Methods, and fluorescence emission was measured every minute. (C, D) The activity of PTEN-WT was measured under nonreducing conditions upon injection of buffer (untreated) or 100 μM of NEM (C), diamide or H2O2 (D) (indicated by first arrow). Ca. 25 min later, 10 mM DTT (or buffer) was added to half of the samples to demonstrate (ir)reversibility of the reaction (injection indicated by second arrow). Note that slight inactivation of untreated PTEN-WT was always observed, most of which was due to oxidation by atmospheric oxygen and could be reversed by addition of DTT. Curves represent means of duplicate (B) or triplicate (C, D) wells. (C, D) are representative of three independent experiments. DiFMUP, 6,8-difluoro-4-methylumbelliferyl phosphate; DTT, dithiothreitol; H2O2, hydrogen peroxide; NEM, N-ethyl maleimide; PTEN, phosphatase and tensin homolog; WT, wild type. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
NaHS rapidly oxidizes the PTEN active site cysteine
Using the real-time PTEN activity assay, we asked if NaHS, which is widely used to generate H2S solutions, affects the activity of PTEN. Exposure to NaHS led to dose-dependent inhibition of the PTEN-WT activity, which could be recovered with DTT (Fig. 2A), thus indicating reversible oxidation of the active site cysteine. We then compared the oxidative efficiency of NaHS with that of other biologically relevant oxidants, namely, H2O2, peroxynitrite (ONOO−), S-nitrosoglutathione (GSNO), and glutathione disulfide (GSSG). Notably, on an equimolar basis, NaHS exhibited the fastest oxidative effect of all compounds tested (Fig. 2B, B′).
FIG. 2.
NaHS rapidly oxidizes the PTEN active site cysteine. (A) PTEN activity was measured under nonreducing conditions in response to buffer (untreated) or 5–50 μM NaHS (from Sigma; injection indicated by first arrow). Ca. 30 min later, samples received 20 mM DTT or buffer to demonstrate reversibility of the reaction (injection indicated by second arrow). (B) PTEN activity was measured under nonreducing conditions upon injection of buffer (untreated) or 50 μM GSSG, GSNO, ONOO−, ONOO− vehicle (DMF), H2O2, or NaHS (from Sigma; injection indicated by arrow). (B′) As a measure of PTEN activity, the fluorescence intensity of the product at the time of oxidant addition was subtracted from the fluorescence signal at 60 min, and was normalized to the untreated control. Of all oxidants tested, NaHS inhibits the PTEN activity most efficiently. Bars denote means±SD of triplicate wells. All curves represent means of triplicate wells. (A) is representative of three, (B/B′) of two independent experiments. GSSG, glutathione disulfide; GSNO, S-nitrosoglutathione; ONOO−, peroxynitrite; NaHS, sodium hydrosulfide. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
NaHS solutions facilitate oxidation of high pKa thiols and do not reduce disulfide bonds
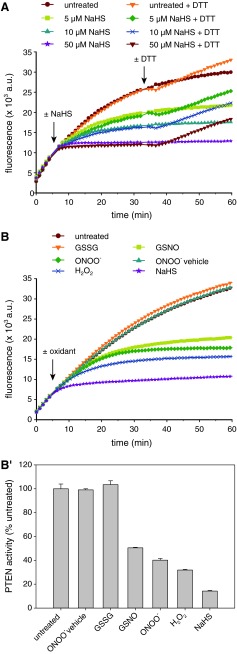
Considering that the PTEN activity is based on a cysteine with relatively low pKa (∼5) (7, 46), we asked if NaHS-mediated oxidation would also occur with more typical protein thiols of higher pKa (∼8–9). We chose a redox-sensitive variant of the enhanced green fluorescent protein (roGFP2) as a model protein containing two oxidizable cysteines of typical pKa (∼9). This protein allows real-time monitoring of its thiol–disulfide state by ratiometric fluorescence measurements (10). As observed previously, the uncatalyzed reaction of the roGFP2 thiols with H2O2 was very slow (Fig. 3A): 5 mM H2O2 did not fully oxidize 1 μM of roGFP2 within 40 min, reflecting the low intrinsic reactivity of the roGFP2 thiols toward H2O2. In contrast, NaHS led to the formation of the roGFP2 disulfide bond within 10 min, even at much lower concentrations (Fig. 3A′). As observed with PTEN, both H2O2- and NaHS-induced oxidation of roGFP2 were fully reversible by addition of DTT (Fig. 3A, A′).
FIG. 3.
NaHS solutions facilitate oxidation of high pKa thiols, but not disulfide bond reduction. (A) The fluorescence emission ratio of prereduced recombinant roGFP2 excited at 390 and 480 nm was determined continuously and used to calculate the degree of roGFP2 oxidation as described in Materials and Methods. Arrows indicate injection of 0.1–5 mM H2O2 (A) or NaHS (from Cayman; A′), followed by addition of 20 mM DTT to all samples ca. 40 min later to show reversibility. Data are representative of three independent experiments. (B) The activity of PTEN-WT was measured in the absence (B) or presence (B′) of 5 mM glutathione (GSH), which was added to wells as indicated. Subsequently, buffer (untreated) or 5–500 μM NaHS (from Cayman) were added (injection indicated by arrow). Quantification of the effect of NaHS on PTEN activity in the absence or presence of GSH was done as described for Figure 2B (B′′). Curves represent means, and bars denote mean±range, of duplicate wells. Data are representative of two independent experiments. (C) The degree of oxidation of preoxidized recombinant roGFP2 was determined in response to 0.01–1 mM DTT or NaHS (from Cayman) to test for their reducing effect (injection indicated by arrow). roGFP2, reduction-oxidation-sensitive GFP2. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
These observations indicated that NaHS exerts its oxidative effect not only on low pKa thiols, but also on less acidic thiols. Thus, reduced glutathione (GSH; pKa ∼9) should also be oxidized in the presence of NaHS. In fact, the presence of 5 mM GSH greatly diminished NaHS-mediated inactivation of PTEN-WT (Fig. 3B–B′′), suggesting that GSH and the active site thiol of PTEN compete for the oxidative equivalents contained in the NaHS solution. Diminished PTEN inactivation was not due to reduction of oxidized PTEN by GSH, as this reaction turned out to be kinetically insignificant (Supplementary Fig. S2). These results highlight the ability of NaHS to facilitate oxidation of both high and low pKa thiols.
Chemically, NaHS is expected to have reducing, rather than oxidizing, properties. We therefore tested whether NaHS was able to reduce protein disulfide bonds. We found that, in contrast to DTT, NaHS was not able to reduce either oxidized roGFP2 (Fig. 3C) or oxidized PTEN (Supplementary Fig. S3). Surprisingly, but consistent with previous reports (28, 29), NaHS appears to act solely as a mediator of thiol oxidation.
The oxidative properties of NaHS are due to polysulfide formation
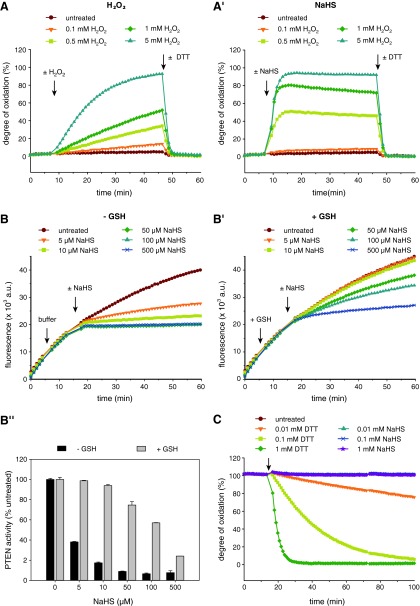
We next aimed to elucidate the cause for the pro-oxidative effect exerted by NaHS solutions. As HS− was previously reported to autoxidize to polysulfides in the presence of molecular oxygen and trace metals (2), we tested for the existence of polysulfides in our NaHS solution. Polysulfides are molecular chains of a variable number of sulfur atoms (Sn2−, n=2–8), which appear yellow in solution and are in continuous equilibrium with each other (17). Their formation occurs via transition metal-catalyzed autoxidation of HS− and is most rapid at mildly alkaline pH (2). Notably, when preparing NaHS solutions for experiments, we observed the development of a weak yellow color within seconds after NaHS was dissolved at high concentrations in buffers of mildly alkaline pH, that is, between pH 7 and 9 (not shown). Polysulfides are known to show distinct absorption peaks at ∼300 and ∼372 nm (6). Therefore, to test for the presence of polysulfides, we measured the UV/Vis-absorbance spectrum of the NaHS solutions and compared it to that of a positive control, namely, potassium (poly)sulfide (K2Sx). K2Sx is a mixture of ∼40% K2S and potassium polysulfides of different chain lengths, which is why neither a molecular weight nor a molar concentration can be specified. As expected, a solution of K2Sx strongly absorbed at 300 and 372 nm (Fig. 4A). Notably, the UV/Vis spectrum of freshly prepared NaHS (Sigma) also showed substantial absorbance peaks at these wavelengths, indicating the presence of polysulfides (Fig. 4A). These peaks were also observed when using NaHS from a different supplier (Cayman), although at a lower intensity (Fig. 4A). We also measured the UV/Vis spectrum of a solution of the alternative H2S source Na2S, whose purity is reported as 96% by the supplier (Alfa Aesar), and which has been recommended by other authors concerned about the purity of sulfide salts (13). Even Na2S from Alfa Aesar showed a weak absorbance peak at 300 nm (Fig. 4A).
FIG. 4.
The oxidative properties of NaHS and Na2S solutions are due to polysulfide formation. (A) UV/Vis spectra of NaHS (from Sigma or Cayman) and Na2S (from Alfa Aesar) dissolved freshly to 150 mM in 200 mM Tris-HCl, pH 8.0, were recorded and compared to that of 0.83 mg/ml K2Sx dissolved in 200 mM Tris-HCl, pH 9.2. Absorbance peaks at 300 and 372 nm (indicated by arrows) are characteristic of polysulfides. (B) The stock solution of K2Sx used in (A) was subjected to cyanolysis as described in Materials and Methods to determine sulfane sulfur (S0) levels. Data are given for 13.2 mg/ml K2Sx. Preincubation of K2Sx with 200 mM DTT for 10 min abolished the signal due to polysulfide degradation. Bars denote mean±range of duplicate wells. (B′) Activity of PTEN-WT (380 nM) was recorded upon injection of dilutions of the same K2Sx stock solution. S0 concentrations given in parentheses are calculated from the cyanolysis data in (B). Curves represent means of triplicate wells. (C) Sulfane sulfur (S0) levels of the same NaHS and Na2S solutions as in (A) were determined by cyanolysis. Data are given for 120 mM stock solutions. Bars denote mean±range of duplicate wells. (C′) PTEN activity was measured upon addition of buffer (untreated) or 10 μM of these NaHS and Na2S solutions (injection indicated by arrow). Curves represent means of triplicate samples. Quantification of the effect of these agents on PTEN activity (expressed as PTEN inhibition) was done as for Figure 2B and is shown in (C′′). Bars denote means±SD of triplicate wells. The experiment shown in (A–C′′) was repeated twice. (D) 5 mM of NaHS (Cayman) or Na2S, or 0.55 mg/ml of K2Sx were pretreated with (D′) or without (D) 50 mM cyanide (KCN) for 1 h before they were injected into a running PTEN activity assay to give a final concentration of 50 μM NaHS/Na2S±500 μM KCN or 5.5 ng/ml K2Sx±500 μM KCN. Curves represent means of triplicate samples. The same protective effect by KCN was observed for roGFP2 (not shown). K2Sx, potassium (poly)sulfide; Na2S, sodium sulfide. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
To further confirm and quantitate polysulfide levels in NaHS and Na2S solutions, we performed a cold cyanolysis assay (45). Polysulfides contain sulfane sulfur, which is characterized by an oxidation number of zero (i.e., six valence electrons; represented by S0) and through a proposed thiosulfoxide tautomerization step readily attaches to nucleophilic acceptors such as thiolate anions (R-S−) or cyanide (CN−) (42). The reaction with CN− at alkaline pH to form thiocyanate (SCN−) is a defining characteristic of sulfane sulfur; it is thus often referred to as cyanolyzable sulfur. In the presence of excess iron chloride (FeCl3), SCN− produces iron (III) thiocyanate ([Fe(SCN)(H2O)5]2+), which can readily be detected by measuring its absorbance at 460 nm. As expected, when K2Sx was subjected to cyanolysis, [Fe(SCN)(H2O)5]2+ was formed (Fig. 4B). A potassium thiocyanate (KSCN) standard curve was used to quantitate [Fe(SCN)(H2O)5]2+ levels, which are a direct measure of the concentration of S0 atoms in the solution. Typically, stock solutions of 13.2 mg/ml K2Sx were found to contain ∼60 mM of sulfane sulfur. Formation of [Fe(SCN)(H2O)5]2+ was almost completely prevented by pretreatment with DTT, which is known to reduce polysulfides to H2S (Fig. 4B). Importantly, polysulfides added as K2Sx oxidized PTEN very efficiently, in a near-stoichiometric manner (Fig. 4B′). Supporting the results obtained by UV/Vis spectroscopy (Fig. 4A), all NaHS and Na2S solutions gave rise to the formation of [Fe(SCN)(H2O)5]2+ (Fig. 4C). Their levels ranged from 0.49 mM S0 to 2.3 mM S0 in 120 mM solutions of Na2S and NaHS (Sigma), respectively, and paralleled the intensity of the polysulfide absorbance peaks of the respective solutions (Fig. 4A). Notably, the polysulfide and H2S levels of K2Sx, NaHS, and Na2S solutions were inversely correlated (Supplementary Fig. S4). These observations indicate the existence of polysulfides in freshly prepared mildly alkaline solutions of sulfide salts such as NaHS and Na2S. To investigate whether the presence of polysulfides correlates with oxidative inactivation of PTEN, PTEN-WT was exposed to the same NaHS and Na2S solutions that were tested for polysulfide levels in Figure 4C. The PTEN activity was lost most rapidly after treatment with NaHS from Sigma, followed by NaHS from Cayman and Na2S (Fig. 4C′, C′′), thus more closely mirroring polysulfide rather than H2S concentrations. These observations are consistent with the idea that PTEN is oxidized by polysulfides that formed in solutions of H2S (or on the surface of sulfide salt crystals). Although these experiments were performed at pH 8.0, very similar results were obtained at pH 7.4 (Supplementary Fig. S5).
To further confirm polysulfides as the oxidizing species in solutions of sulfide salts, PTEN-WT was exposed to K2Sx, NaHS, or Na2S solutions that had been preincubated with or without cyanide. As shown in Figure 4D and D′, all solutions completely lost their oxidative effect on PTEN after pretreatment with cyanide, consistent with the efficient degradation of polysulfides through cyanolysis. It should be stressed that under the given conditions, cyanide specifically degrades sulfane sulfur species, it does not cleave protein disulfide bonds, nor does it reactivate PTEN that is already oxidized (data not shown).
Together, these results strongly suggest that oxidation of PTEN induced by solutions of different sulfide salts, such as NaHS and Na2S, is attributable to polysulfides.
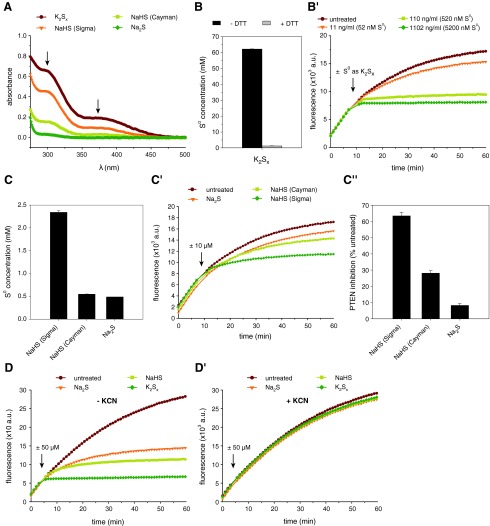
Polysulfides also form in solutions of gaseous H2S and GYY4137
We next asked if the potentially purest source of H2S, H2S gas, allows the preparation of H2S solutions devoid of polysulfides. To this end, we passed H2S gas through ultrapure water and tested the effect on PTEN activity. Of note, H2S(aq) prepared from gaseous H2S also led to PTEN oxidation, again suggesting the formation of polysulfides (Fig. 5A, A′).
FIG. 5.
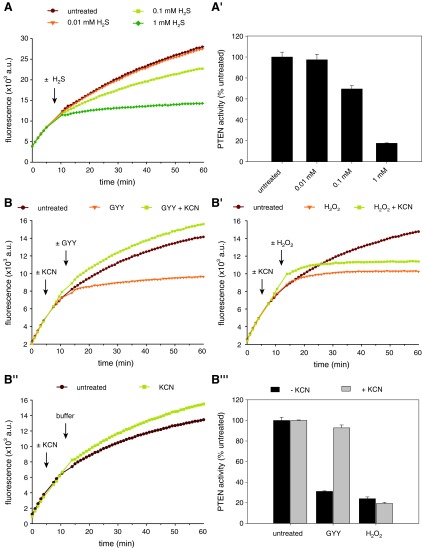
Polysulfides also form in solutions of gaseous H2S and GYY4137. (A) PTEN activity was recorded upon injection of 0.01–1 mM H2S(aq) (indicated by arrow) prepared by passing H2S gas through ultrapure water. (A′) The effect of these H2S(aq) solutions on PTEN activity was quantified as described for Figure 2B. Bars denote means±SD of triplicate wells, and are representative of two independent experiments. (B) Activity of PTEN-WT was measured in response to 1 mM of the slow-releasing H2S donor GYY4137 (GYY; B) or 50 μM H2O2 (B′) (injection indicated by second arrow), in the presence or absence of 45 mM KCN (injection indicated by first arrow). The KCN-only control sample is shown in (B′′). (B′′′) depicts the effects of GYY or H2O2 on PTEN activity in the absence of presence of KCN, quantified as for Figure 2B, but normalized to either the untreated sample or the KCN-only control. Bars denote means±SD of triplicate wells. All curves represent means of triplicate wells. GYY4137, morpholin-4-ium 4-methoxyphenyl(morpholino) phosphinodithioate; H2S, hydrogen sulfide. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
An alternative H2S donor is GYY4137 (24), which slowly releases H2S and thus may better mimic physiological conditions. For this reason, GYY4137 has been suggested to be superior to NaHS and Na2S, as well as H2S gas (24). We hence investigated the effect of GYY4137 solutions on PTEN. Importantly, even GYY4137 inhibited PTEN activity, and inhibition was completely prevented in the presence of cyanide (Fig. 5B, B′′′). In contrast, cyanide did not protect PTEN against H2O2-mediated inhibition (Fig. 5B′, B′′′); thus, cyanide does not cleave the PTEN disulfide bond. Together, these results imply that polysulfides form, even when using H2S gas or slow-releasing H2S donors.
Polysulfides inhibit PTEN by sulfane sulfur addition
We next asked about the nature of the cysteine modification caused by polysulfides. Thiol chemistry would predict the addition of one or more sulfur atoms to the PTEN active site cysteine, depending on the length of the polysulfide sulfur chain and whether thiosulfoxide tautomerization occurs. To describe this process, we will, henceforth, use the term sulfane sulfur addition (with S-sulfhydration, i.e., addition of one sulfur atom, being one particular case). The resulting modification (Cys-Sx−) may subsequently convert to a disulfide, or potentially a trisulfide bond, if a second cysteine is nearby. Using gel mobility analysis, we investigated if NaHS treatment leads to disulfide bond formation in PTEN (Supplementary Fig. S6). While neither H2O2 nor NaHS produced a mobility shift of the C124S and C71S mutants, H2O2 completely shifted PTEN-WT to the high mobility (disulfide) form as expected. NaHS also converted PTEN-WT to the high mobility form, but only partially (∼50%), despite using conditions (100 μM for 15′) that led to total inhibition of the enzyme (Fig. 6A′). These findings suggest that all PTEN molecules become inhibited by sulfane sulfur addition to Cys-124, which then only partially reacts to give the high mobility form, that is, the disulfide (and/or trisulfide) between Cys-124 and Cys-71.
FIG. 6.
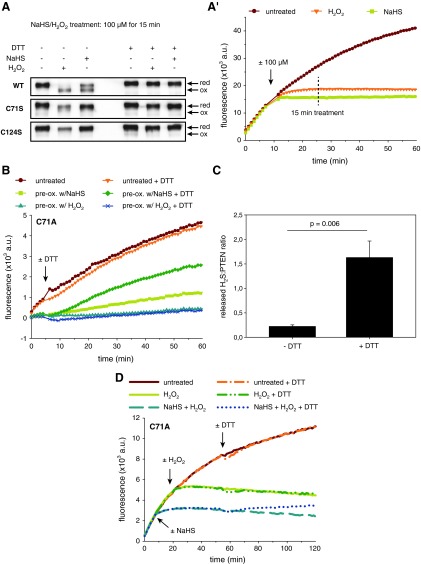
Polysulfides inhibit PTEN by sulfane sulfur addition. (A) Recombinant PTEN-WT was prereduced with 20 mM DTT for 10 min, followed by buffer exchange into a nonreducing PTEN assay buffer and exposure to 100 μM H2O2 or NaHS (Cayman) for 15 min. The reaction was stopped with an excess of NEM to block free thiols for 15 min. Samples were then treated with or without 50 mM DTT for reduction and subjected to SDS-PAGE and immunoblotting for PTEN. Noncropped immunoblot images are shown in Supplementary Fig. S6. Data are representative of two independent experiments. (A′) depicts a representative PTEN activity assay showing complete inactivation of PTEN-WT within 15 min of exposure to 100 μM H2O2 or NaHS (Cayman). (B) PTEN-C71A (2.5 μM) was pretreated with buffer (untreated), 5 mM H2O2 or 5 mM NaHS for 15 min, then desalted, and subjected to the PTEN activity assay under nonreducing conditions (at a final protein concentration of 380 nM). DTT at 20 mM (or buffer) was added to half of the samples to demonstrate (ir)reversibility of the reaction (injection indicated by arrow). Data are representative of three independent experiments. (C) Prereduced PTEN-WT (10 μM) was exposed to 2 mM polysulfides (prepared by mixing hypochlorous acid and Na2S solution) for 30 min, followed by buffer exchange and concentration of the protein. The sample was split in half and treated±1 mM DTT for 30 min to liberate protein-bound H2S, which was detected using monobromobimane derivatization as described in Materials and Methods. Bars denote the released H2S/PTEN ratio as means±SEM of four independent experiments. The Student's t-test yielded a two-tailed p-value of 0.006. (D) Activity of PTEN-C71A (380 nM) was measured under nonreducing conditions upon addition of 100 μM NaHS (or buffer), followed by injection of 400 μM H2O2 (or buffer), and then 10 mM DTT (or buffer), as indicated by arrows. The same results were observed for PTEN-C71S (not shown). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
To corroborate these results, we made use of the Cys71-to-alanine (C71A) mutant of PTEN. It should be pointed out that the C71A and C71S mutants behave identically in displaying a markedly reduced activity (∼35%) relative to the WT enzyme (Supplementary Fig. S7A). This appears to be due to an effect on the pKa and/or nucleophilicity of the active site thiol, as the sensitivity of Cys-124 to both alkylation (by NEM) and oxidation (by either H2O2 or polysulfides) is diminished (Supplementary Fig. S7B, C). Therefore, higher concentrations of H2O2 and NaHS were needed to achieve inhibition of PTEN-C71A within the same time frame as used for PTEN-WT. To investigate whether sulfane sulfur addition to the active site cysteine of PTEN occurs, we tested if PTEN-C71A can be reactivated by DTT after exposure to NaHS or H2O2. As expected, pretreatment of PTEN-C71A with H2O2 led to irreversible inactivation of the enzyme due to overoxidation of the active site cysteine (Fig. 6B). In contrast, PTEN-C71A pretreated with NaHS was reactivated by DTT, indicating a reversible thiol-based modification such as sulfane sulfur addition/S-sulfhydration (Fig. 6B).
If polysulfides indeed add sulfane sulfur to the active site thiol of PTEN, subsequent reduction of PTEN with DTT should release protein-bound sulfane sulfur as H2S. To test this notion, we started out with air-oxidized PTEN-WT, which was either prereduced with DTT or not (thus, to make the Cys-124 thiol available or to keep it in the disulfide form). PTEN-WT was then exposed to polysulfides, desalted, and again incubated with or without DTT. Using monobromobimane (MBB) derivatization and HPLC to quantitate released H2S, we found that H2S was specifically released by DTT with a H2S-to-protein stoichiometry of 1.6±0.3 (Fig. 6C). Moreover, if the PTEN disulfide was not reduced before polysulfide addition, no H2S release was detectable (Supplementary Table S1), suggesting that Cys-124 and Cys-71 are the predominant targets of sulfane sulfur addition. This notion is further supported by the observation that each mutant (C124S and C71A) released less H2S upon DTT treatment (Supplementary Table S1).
To further investigate PTEN thiol modifications in response to NaHS, we performed mass spectrometry. Treatment of PTEN-WT with NaHS clearly induced formation of the C124-C71 disulfide bond (Supplementary Fig. S8), while the corresponding trisulfide bond could not be detected. This may be an indication that the high-mobility form induced by NaHS (Fig. 6A) represents the disulfide rather than trisulfide. We did not directly detect sulfane sulfur addition to Cys-124, most likely due to instability of sulfane sulfur–sulfur bonds under our conditions of sample preparation that required double digestion using AspN and trypsin to detect the Cys-124 peptide. However, differential alkylation confirmed that Cys-124 becomes reversibly oxidized by NaHS treatment especially in the C71S mutant, that is, independently of disulfide formation, thus implicating sulfane sulfur addition to Cys-124 (Supplementary Fig. S9).
Sulfhydration of proteins has been hypothesized to protect single cysteines from irreversible overoxidation (20, 34). To address this idea experimentally, we exposed PTEN-C71A to NaHS, followed by H2O2. Consistent with a protective function of NaHS-induced sulfane sulfur addition/S-sulfhydration, DTT was only able to reactivate H2O2-treated PTEN-C71A, which had been pretreated with NaHS (Fig. 6D).
In summary, our data demonstrate that NaHS treatment of PTEN promotes rapid oxidation of its active site cysteine, almost certainly via the reversible addition of sulfane sulfur that (partially) converts to an intramolecular disulfide bond.
Polysulfides modify PTEN in intact cells
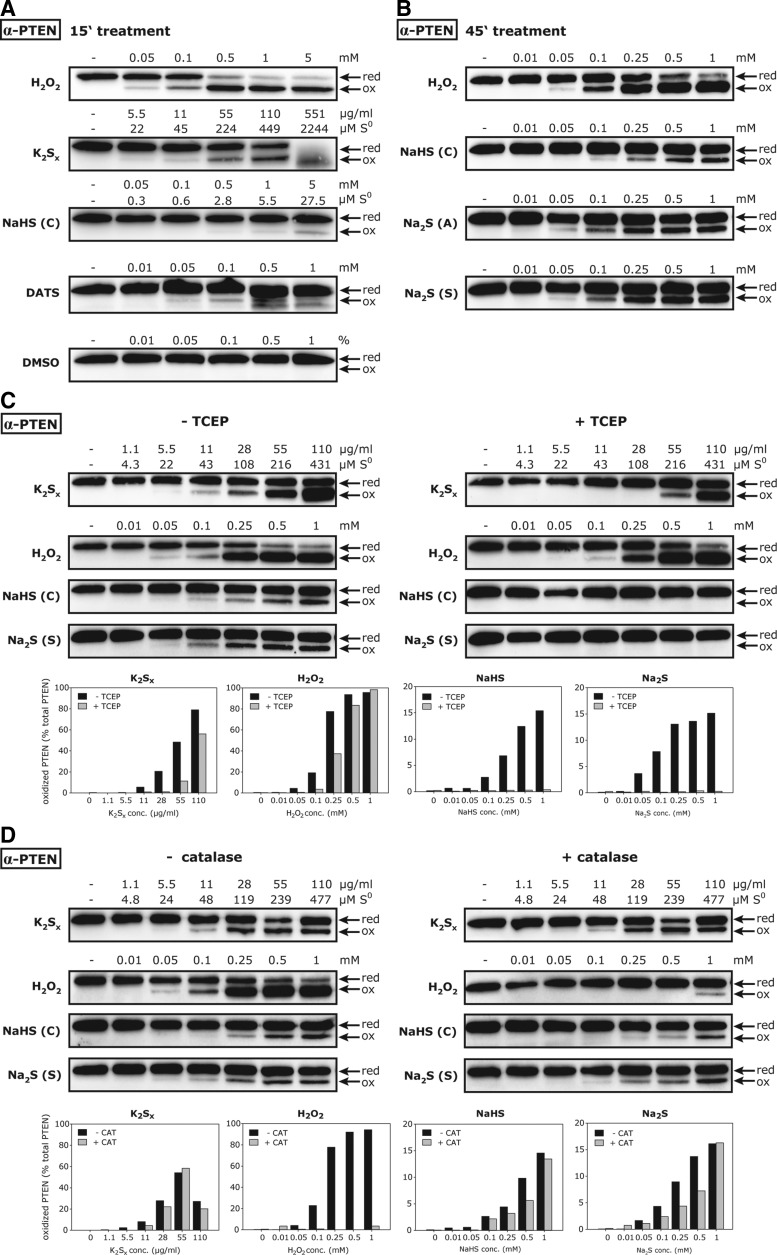
The oxidation of proteins in intact cells in response to H2S treatment has been reported in a number of studies (11, 20, 28, 29, 38, 47). Based on our observation that all tested “H2S donors” either contained polysulfides from the outset or formed polysulfides upon dissolution, we hypothesized that polysulfides may also be the cause for oxidative effects previously observed in cell culture. To test whether polysulfides would be able to enter cells and modify PTEN in situ, we added H2O2, K2Sx, NaHS, or the organic polysulfide diallyl trisulfide (DATS) to the culture medium of human embryonic kidney HEK293T cells for 15 min, and analyzed the influence on PTEN gel mobility. DATS, like inorganic polysulfide species, efficiently inhibits PTEN in vitro (Supplementary Fig. S10). As shown in Figure 7A, all four stimuli, but not the DATS vehicle DMSO, caused the PTEN gel mobility shift, implying the formation of a disulfide bond, as observed after exposure of recombinant PTEN to H2O2 or NaHS (Fig. 6A). Analogous to the correlation between S0 levels and PTEN inhibition observed in vitro (see Fig. 4C–C′′), the proportion of shifted PTEN paralleled the S0 concentration to which the cells were exposed, as determined by cyanolysis of the stock solutions of the respective compounds (see S0 levels indicated below compound concentrations in Fig. 7A). It should be noted that high concentrations (0.5 and 1 mM) of DATS induced generalized protein thiol oxidation and aggregation (data not shown), which is also reflected by additional oxidized forms of PTEN (see additional band in Fig. 7A). Due to its lower initial polysulfide content, NaHS was significantly less efficient in oxidizing PTEN than the polysulfide-positive controls K2Sx and DATS. However, as previous studies have exposed cells to H2S donors for periods between 30 min and 48 h (28, 47), we considered the possibility that longer incubation times may lead to additional polysulfide formation during the incubation period. When cells were exposed to NaHS for 45 min, PTEN disulfide formation was indeed detectable at 100 μM and even occurred upon treatment with Na2S (from both Alfa Aesar and Sigma) at concentrations as low as 50 μM (Fig. 7B). Of note, 50 μM was also the lowest concentration of H2O2 leading to a detectable PTEN mobility shift under the given conditions (Fig. 7B).
FIG. 7.
Polysulfides modify PTEN in intact cells. (A) HEK293T cells were exposed to the specified concentrations of H2O2, K2Sx, NaHS (Cayman), Diallyl trisulfide (DATS), or DMSO for 15 min at 37°C, followed by NEM to block free thiols in intact cells. Cell lysates were subjected to nonreducing SDS-PAGE and immunoblotting to probe for PTEN. The NEM alkylation and immunoblot procedure was done for all panels of this figure. Cyanolysis of K2Sx and NaHS stock solutions was performed immediately after dissolution to quantitate S0 levels, which were used to calculate S0 concentrations in the respective cell treatments. S0 levels for DATS treatments equal the applied DATS concentration. Data are representative of two (DATS/DMSO) or seven (H2O2, K2Sx, NaHS) independent experiments. (B) HEK293T cells were exposed to the specified concentrations of H2O2, NaHS (Cayman), or Na2S (Alfa Aesar or Sigma) for 45 min at 37°C. Data are representative of three independent experiments. (C) TCEP at 0.5 mM (or buffer) was added to HEK293T cells just before exposure to the specified concentrations of K2Sx, H2O2, NaHS (Cayman), or Na2S (Sigma) for 45 min. Similarly, 2000 U/well bovine liver catalase (or buffer) was injected before treatment with these stimuli for 45 min (D). The proportion of oxidized PTEN was quantified by densitometry and is given below immunoblots (C, D). (C, D) are representative of two independent experiments. TCEP, tris(2-carboxyethyl)phosphine.
These results suggested de novo formation of polysulfides during the incubation period, in addition to the variable amounts of polysulfides that are introduced as pre-existing contaminants in sulfide salt solutions. We also considered the possibility that H2S treatment promotes the formation of oxidants such as H2O2, which would then be responsible for the observed oxidation of PTEN. To this end, we exposed HEK293T cells for 45 min to H2O2, K2Sx, NaHS, or Na2S in the presence or absence of tris(2-carboxyethyl)phosphine (TCEP) or catalase. TCEP is a cell-impermeable reductant that readily reduces sulfur–sulfur bonds, and is therefore expected to degrade polysulfides in the cell culture medium. Consistently, 0.5 mM TCEP largely prevented PTEN disulfide bond formation in response to K2Sx treatment (Fig. 7C). As TCEP also reacts with H2O2 (9), it similarly suppressed H2O2-induced PTEN oxidation (Fig. 7C). Importantly, however, TCEP completely abolished NaHS- and Na2S-induced PTEN disulfide bond formation (Fig. 7C), most likely through the reduction of polysulfides that formed in the culture medium during the incubation period. Since this experiment did not rule out the involvement of H2O2 that may have formed secondarily in the cellular supernatant during exposure to sulfide salts, we treated cells in the absence or presence of 2000 units of catalase. While catalase almost completely abolished H2O2-induced PTEN oxidation, it had only a relatively minor influence on K2Sx-, NaHS-, and Na2S-induced PTEN oxidation (Fig. 7D).
Taken together, these data suggest that polysulfides introduced to or formed in the cell culture supernatant enter cells and modify intracellular proteins. These observations imply that oxidative effects of H2S solutions in cell culture as reported in previous studies are more likely to be attributable to polysulfides than to H2S per se.
Discussion
Despite a wealth of recent publications suggesting new physiological roles for H2S, its molecular mechanism of action remains poorly defined. Several studies have reported S-sulfhydration of proteins in response to H2S, and have hypothesized this to be its major signaling mode (11, 20, 28, 29, 38, 47). However, an important question remains unanswered: how does H2S lead to an oxidative protein thiol modification, that is, S-sulfhydration? The results presented in this study strongly suggest that formation of polysulfides is the missing link in H2S-induced protein thiol oxidation.
We were able to show that the commonly used sulfide salts NaHS and Na2S form polysulfides in solution at physiological pH (7–8), as determined by UV/Vis spectroscopy, cyanolysis, and their oxidative effect on PTEN and roGFP2 in vitro (see Figs. 3 and 4). This finding was, however, not entirely unexpected, as the oxidation of H2S to polysulfides (and eventually elemental sulfur) at neutral or slightly alkaline pH and in the presence of molecular oxygen is a known phenomenon (2). Indeed, the difficulties of working with H2S have recently been discussed (13), and suggestions have been made as to how formation of polysulfides can be prevented. It is therefore somewhat surprising that polysulfide formation is not considered or discussed in the majority of studies investigating the biology of H2S.
A major source of polysulfides appears to be the impurities in NaHS and Na2S crystals. Hughes et al. (13) recommend anhydrous Na2S from Alfa Aesar as the most stable preparation they could find. Consistently, we found that solutions of Alfa Aesar Na2S (with a stated purity of 96%) contain considerably lower levels of polysulfides than NaHS solutions. Nonetheless, we detected polysulfides immediately after dissolution of Alfa Aesar Na2S, and these solutions led to oxidation of PTEN in vitro (see Fig. 4A, C′–C′′). Similar results were obtained with Na2S from an alternative source (Sigma), which is specified as 100% pure, but still led to oxidation of PTEN in vitro (Supplementary Fig. S11). These results suggest that PTEN oxidation is mostly mediated by polysulfides formed during the assay rather than by preexisting impurities in the Na2S crystals. Consistently, the same degree of PTEN oxidation as observed with Sigma Na2S was found for the purest source of H2S, namely, the gas itself (see Fig. 5A, A′). Some researchers have suggested slow-releasing H2S donors, such as GYY4137, as the reagents of choice to best mimic the effects of endogenous H2S, due to their continuous generation of low levels of H2S (24). Apparently, even such low amounts of H2S lead to PTEN oxidation in vitro, which was prevented in the presence of cyanide and thus can be attributed to the formation of sulfane sulfur (see Fig. 5B, B′′′). Hughes et al. (13) recommended the deoxygenation of the solvent with N2 as well as supplementation with the metal chelator diethylene triamine pentaacetic acid (DTPA) to avoid HS− autoxidation. However, in our hands, these measures were largely ineffective in diminishing NaHS-, Na2S-, or GYY4137-induced PTEN oxidation (not shown). In any case, neither the complete elimination of oxygen nor the inclusion of extremely high concentrations of metal chelators is a practical or sensible measure in cell culture or animal studies. Indeed, all our results are consistent with a previous statement from John Toohey that “It is not possible to add HS− ion without simultaneously adding S0 (43).”
Polysulfide-mediated oxidation of PTEN most likely occurs via the addition of sulfane sulfur (e.g., S-sulfhydration) to the active site Cys-124 (and possibly to some extent other cysteines), followed by partial conversion to a disulfide bond between Cys-124 and the nearby Cys-71. Four lines of evidence support this conclusion: (1) the activity of NaHS-, but not H2O2-, treated PTEN-C71A is recovered by DTT, although intramolecular disulfide bond formation is not possible in this mutant. (2) PTEN-WT only partially shows a gel mobility shift indicative of the disulfide bonded state in response to NaHS, despite complete inactivation of the enzyme (see Fig. 6A, B). (3) H2S can be released from polysulfide-treated PTEN-WT with DTT (see Fig. 6C). The observed H2S:PTEN stoichiometry of 1.6±0.3 (mean±SEM) is consistent with the addition of two sulfur atoms to one or more cysteine residues of PTEN-WT that are partially lost due to disulfide or trisulfide formation. (4) Differential alkylation and mass spectrometry reveals NaHS-induced reversible oxidation of Cys-124 in the absence of Cys-71. Furthermore, our observations provide additional experimental support for the previously proposed hypothesis that sulfane sulfur addition/S-sulfhydration protects thiols from irreversible damage, that is, H2O2- induced overoxidation (20, 30) (see Fig. 6D).
In principle, S-sulfhydration could also arise as a result of H2S-mediated reduction of protein disulfides (31, 35). Our results, however, show that H2S, in the inevitable presence of polysulfides, is not capable of reducing oxidized roGFP2 or PTEN in vitro (see Fig. 3C and Supplementary Fig. S3). Also, in a physiological setting, H2S is expected to be a considerably weaker reductant than GSH, due to its lower abundance and higher redox potential (16). Hence, it appears rather unlikely that S-sulfhydration typically occurs as a consequence of H2S-mediated disulfide reduction.
In addition to recognizing that H2S solutions give rise to polysulfides at physiological pH and on biologically relevant time scales, we show that polysulfides readily enter cells, where they lead to oxidation of proteins such as PTEN (see Fig. 7A). Furthermore, polysulfides appear to form de novo during exposure of cells to the purest preparations of sulfide salts, as indicated by sensitive PTEN oxidation upon Na2S treatment for 45 min (see Fig. 7B), and the ability of TCEP, but not catalase, to completely abolish the NaHS- or Na2S-induced PTEN mobility shift (see Fig. 7C, D).
Presumably, the signaling consequences of polysulfide-mediated oxidation of intracellular PTEN are similar to those induced by H2O2, that is, increased flux through the PI3K-Akt pathway followed by enhanced cell growth and survival. In line with this hypothesis, exposure of cells to H2S has repeatedly been linked to increased Akt signaling, particularly in the context of cardioprotection, vasorelaxation, and angiogenesis (1, 4, 12, 33). The data presented here suggest that these previously reported observations, which have been attributed to H2S, might have in fact been mediated by polysulfide-induced oxidation of PTEN.
In addition to low pKa thiols such as the PTEN active site cysteine, polysulfides also oxidize thiols of lower reactivity, as exemplified by roGFP2 and GSH (see Fig. 3A–B′′). Thus, polysulfides appear to be highly reactive and relatively unspecific in their reaction with thiols, and thus are expected to target numerous proteins not only in vitro, but also in cell lysates and intact cells. This may explain the large number of sulfhydrated proteins that have been identified in cell lysates exposed to H2S (28). Interestingly, substantial amounts of protein-bound sulfane sulfur have repeatedly been detected in cell culture, homogenized tissues, and plasma, by determination of H2S that is released upon addition of reducing agents (15, 40, 41). Notably, the amount of protein-bound sulfane sulfur increases upon exposure of tissue homogenates to exogenous H2S (15). While this pool of protein-bound sulfane sulfur has so far been viewed as a sulfide store that may release H2S in response to a physiological stimulus (18), it might actually represent the result of polysulfide-mediated protein thiol modification.
It is a long standing and unresolved question why relatively high concentrations of exogenously added H2S (μM–mM) are usually needed to observe various physiological responses, while physiological H2S concentrations are orders of magnitude lower (nM) (32). Our results may help to resolve this paradox by supporting the notion that H2S is not the actual signaling species, but that many physiological effects are mediated by and based on sulfane sulfur.
Whether, and how, polysulfides (or more generally sulfane sulfur) form endogenously, and whether they play a physiological role in vivo, remains to be addressed. It has been hypothesized that polysulfides form when H2S encounters intracellular oxidants such as H2O2, superoxide (O2.−), or hypochlorous acid (HOCl) (31, 35). Sulfane sulfur formation may also result from H2S-mediated reduction of cytochrome c or cytochrome c oxidase, which has been reported to occur at low H2S concentrations (5).
Alternatively, sulfane sulfur may be generated inside cells as the primary enzymatic product, with H2S being produced from sulfane sulfur as a secondary by-product. It has been suggested that cystathionine-γ-lyase (CSE) and cystathionine-β-synthase (CBS), commonly regarded as H2S generating enzymes, may actually produce sulfane sulfur species (43). Along these lines, the third H2S generating enzyme, 3-mercaptopyruvate sulfurtransferase (3MST), is known to become S-sulfhydrated upon reaction with its endogenous substrate, 3-mercaptopyruvate (generating pyruvate). Already, more than 50 years ago, it was found that in the absence of sulfane sulfur acceptors, 3MST can directly generate and release polysulfides (14). Only in the presence of dithiol reductants, like DTT, thioredoxin, or dihydrolipoic acid, does 3MST release H2S from the S-sulfhydrated catalytic cysteine (26). Notably, monothiol reductants such as cysteine and glutathione do not promote H2S release (26), most likely because the persulfide is transferred from 3MST to GSH or cysteine. The question arises whether proteins other than thioredoxin could also act as persulfide acceptors; this idea is supported by the observation that overexpression of 3MST in HEK293-F cells leads to an increase in protein-bound sulfane sulfur (41). Furthermore, the concept of persulfide transmission from one protein to the next is not new, but evolutionarily conserved and well known to occur during biosynthesis of vitamins and other cofactors, as well as RNA thionucleosides (27).
In conclusion, since it is chemically impossible that H2S by itself oxidizes protein thiols, an intermediate species must exist. Our data show that at least in vitro and in cell culture, these intermediate species are polysulfides, which very efficiently oxidize protein thiols and likely explain the numerous proteins that have been found to be S-sulfhydrated upon exposure to H2S in previous reports. To which extent and by which mechanisms polysulfides are endogenously produced in vivo remains to be further clarified. It is, however, plausible, if not likely, that polysulfides or, more generally, sulfane sulfur are the actual mediators of in vivo H2S signaling. In this case, H2S may only be released as the end product in the termination of polysulfide-based signaling, that is, as the product of polysulfide reduction. This scenario would also be compatible with the low concentrations (nM) of endogenously produced H2S.
In future studies, it will be important, but also difficult, to distinguish between effects induced by H2S per se and effects mediated by polysulfides. It seems that H2S cannot be studied without generating polysulfides and/or other sulfane sulfur species. However, after all, this may not necessarily only be seen as a disadvantage, if indeed polysulfides are the actual in vivo agents of H2S signaling.
Sulfane sulfur should be tested for its ability to induce H2S-sensitive signaling pathways [see (43) for suggested sulfane sulfur generating systems]. A very recent report highlights this necessity by showing that polysulfides induced the same effects on Ca+ influx in astrocytes that had previously been attributed to H2S (19).
Materials and Methods
Reagents
NaHS hydrate was from either Sigma-Aldrich (#161527) or Cayman (#10012555). Anhydrous Na2S was from either Alfa Aesar (#65122) or Sigma-Aldrich (#407410). NaHS and Na2S were stored desiccated under vacuum upon arrival. DiFMUP was obtained from Life Technologies, DTT from AppliChem, GSNO from Enzo Life Sciences, and the PTEN antibody from Cell Signaling Technology (#9552). SIN-1 chloride from Cayman was used as an ONOO− donor and dissolved in dimethylformamide. DATS from LKT Laboratories was diluted in DMSO before addition to cells or recombinant PTEN. TCEP was used as a neutral solution from Thermo Scientific. All other chemicals were from Sigma-Aldrich. GYY4137 was a kind gift from Matt Whiteman, University of Exeter, United Kingdom.
Preparation of H2S and polysulfide solutions
NaHS, Na2S, and GYY4137 were typically dissolved to 55–150 mM in 200 mM Tris-HCl, pH 8.0, which had been degassed for at least 2 h by applying vacuum and stirring vigorously. Stock solutions of NaHS, Na2S, and GYY4137 were diluted into the buffer required for each experiment and used within minutes after preparation. To prepare H2S(aq) solutions from H2S gas, ultrapure water was flushed with H2S gas for 20 min and kept in a brown glass bottle with minimum headspace and a double layer parafilm seal. H2S(aq) solutions were used within 2 h after preparation. K2Sx was typically dissolved to 16.5 mg/ml in 200 mM Tris-HCl, pH 9.2, and diluted into the buffer required for each experiment just before use. Determination of H2S and polysulfide levels regularly found ∼70 mM H2S and ∼75 mM S0 in these K2Sx stock solutions. Note, however, that the polysulfides in K2Sx tend to precipitate as elemental sulfur at pH<9.0, which is why polysulfide levels are likely to be lower than expected once K2Sx has been diluted into buffers of physiological pH.
Determination of H2S levels
To determine H2S concentrations, stock solutions were serially diluted into a 100 mM carbonate buffer, pH 9.6, which had been degassed with N2 for 40 min, and their absorbance measured at 230 nm using a Nanodrop 2000 spectrophotometer (Thermo Scientific). Extinction coefficients of H2S dissolved in Tris-HCl, pH 8.0 and 9.2, were determined to be 6511 and 6309 L mol−1 cm−1, respectively. The extinction coefficient of H2S dissolved in water was reported to be 7200 L mol−1 cm−1 (13), and was confirmed in our laboratory. The appropriate extinction coefficients were used for calculation of H2S levels in different buffers.
Cyanolysis
Determination of polysulfide levels by cyanolysis was done largely according to Wood (45). Briefly, 50 μl of K2Sx, NaHS, or Na2S stock solutions were diluted with 400 μl of ultrapure water. Buffers used for dissolution of the compounds were run in parallel and utilized for background correction. After addition of 50 μl of 0.5 M KCN (dissolved in ultrapure water), cyanolysis was allowed to proceed for 45 min at room temperature. Ten microliters of 37% formaldehyde was added, followed by 100 μl Goldstein's Reagent (61.88 mM ferric nitrate nonahydrate, 18.375% HNO3). Two hundred microliters of the samples were transferred in duplicate to clear 96-well plates, and blood-red [Fe(SCN)(H2O)5]2+ was detected by measuring absorbance at 460 nm using a microplate reader (FLUOstar Omega; BMG Labtech). Thiocyanate (SCN−) levels (representing S0 concentration) were quantified by use of a KSCN standard curve. In experiments, where the polysulfide content of “H2S donor” stock solutions was correlated with their oxidative effect on PTEN, stock solutions were prepared and diluted just before the start of the experiment, and then added to the PTEN activity assay or cells and immediately subjected to cyanolysis.
PTEN activity assay
Two consecutive tags, a streptavidin binding protein (SBP)-tag and a His6-tag, were added C-terminally to the WT PTEN construct by standard cloning procedures. For experiments in which PTEN cysteine mutants were required, mutagenesis of individual cysteines was performed using the QuikChange Site-Directed Mutagenesis kit (Stratagene). Recombinant PTEN-SBP-His was purified from Escherichia coli strain M15 (Qiagen) via consecutive hexahistidine and streptavidin affinity chromatography. The purified protein was dialyzed into 50 mM Tris-HCl, 150 mM NaCl, 2 mM EDTA, 2 mM DTT, pH 7.4, and supplemented with 10% glycerol for long-term storage at −80°C. For activity measurements, the PTEN assay buffer (100 mM Tris-HCl, 2 mM EDTA, 0.05% NP-40, pH 8.0) was degassed for a minimum of 2 h before use by applying vacuum and stirring vigorously. PTEN-SBP-His was prereduced with 20 mM DTT for 10 min, and passed through gel filtration columns (Thermo Scientific) pre-equilibrated with the PTEN assay buffer to remove DTT immediately before the start of the assay. In a total volume of 100 μl, 2 μg of PTEN-SBP-His (380 nM) was reacted with 200 μM of the artificial fluorogenic substrate DiFMUP at 37°C, and fluorescence of the product was measured every minute for 60 min at 355/460 nm (excitation/emission) using a microplate reader (FLUOstar Omega; BMG Labtech). Fluorescence signals were blank corrected using corresponding samples lacking the PTEN protein. Note that the C71S and C71A mutants of PTEN were found to show decreased reactivity compared to the WT protein (see Supplementary Fig. S7). PTEN-C71S and PTEN-C71A showed the same phenotype and were used interchangeably. Samples were run as triplicates unless otherwise noted.
roGFP2 redox assay
Purification of recombinant roGFP2-His under nonreducing conditions was performed as described previously (8). The roGFP2 assay buffer (100 mM potassium phosphate buffer, 5 mM EDTA, pH 7.0) was degassed for a minimum of 2 h before use by applying vacuum and stirring vigorously. Typically, roGFP2-His was prereduced with 20 mM DTT for 10 min before the experiment, and passed through gel filtration columns (Thermo Scientific) pre-equilibrated with the roGFP2 assay buffer to remove DTT immediately before the start of the assay. The redox state of roGFP2-His (1 μM, 100 μl) was determined at 37°C by measuring its fluorescence emission at 520 nm after excitation at 390 and 480 nm using a microplate reader (FLUOstar Omega; BMG Labtech). The degree of oxidation of roGFP2 was calculated as described previously and plotted against time (25).
Gel mobility shift of PTEN
For experiments involving recombinant PTEN, prereduced PTEN-SBP-His (380 nM) was exposed to 100 μM H2O2 or NaHS for the indicated times, followed by alkylation with 30 mM NEM either immediately or after reduction with 10 mM DTT. Samples were subjected to nonreducing sodium dodecyl sulfate (SDS) gel electrophoresis and immunoblotting with an anti-PTEN antibody following standard procedures. To determine the redox state of intracellular PTEN, 2×105 human embryonic kidney HEK293T cells were seeded into 24-well plates and grown over night to 95% confluency. H2O2, NaHS, or Na2S were dissolved to 120 mM in a 200 mM potassium phosphate buffer, pH 7.4, K2Sx was dissolved to 13.2 mg/ml in a 100 mM sodium carbonate buffer, pH 9.6, and DATS was diluted to 100 mM in DMSO. These solutions were diluted into a normal growth medium (the Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum and 100 U/ml penicillin plus 100 μg/ml streptomycin) and added to cells. After 15 or 45 min, the supernatant was replaced by 40 mM NEM in phosphate buffered saline (PBS) to alkylate free thiols in intact cells for 5 min on ice. Cells were lysed in 10 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, 40 mM NEM, protease inhibitor cocktail (Roche), for 20 min at 4°C. Cleared lysates were subjected to nonreducing SDS gel electrophoresis and immunoblotting for PTEN according to standard methods. In experiments investigating the modifying influence of TCEP or catalase, TCEP or bovine liver catalase (Sigma #C9322) were diluted/dissolved in PBS (pH 7.4) and added to cells for final concentrations of 0.5 mM TCEP or 2000 U/well catalase just before addition of H2O2/K2Sx/NaHS/Na2S. Immunoblots were quantified by densitometry using the ImageJ software (37) to determine the proportion of oxidized PTEN. To this end, the intensity of the lower (oxidized) PTEN band was divided by the sum of the intensities of both bands (representing the total PTEN pool).
Measurement of H2S release after reduction of polysulfide-treated PTEN
Approximately 10 μM recombinant PTEN (WT/C71A/C124S) was incubated with or without 1 mM DTT for 30 min in the PTEN assay buffer, pH 7.4 (±prereduction), followed by treatment with 2 mM polysulfides at room temperature (RT) for 30 min. Polysulfides were prepared by mixing 2 mM HOCl with 10 mM Na2S. Product analysis of this reaction mixture [as described in the supporting information of (31)] unambiguously revealed that HOCl is converted into polysulfide species under these conditions. The large excess of polysulfides was necessary to overwhelm the reducing capacity of DTT that was used for prereduction of PTEN. After polysulfide treatment, inorganic salts were removed by buffer exchange that was carried out by three consecutive concentration dilution steps using an amicon microconcentrator (30 kDa cutoff). This resulted in a>> 1000-fold dilution of the inorganic molecules and 1.4-fold concentration of a protein, with ∼100% protein recovery (as measured by the Lowry method). Protein samples were then split into two aliquots that were treated±1 mM DTT at RT for 30 min to liberate sulfide from persulfide species. Sulfide released from PTEN was measured by the MBB method as described previously (39). Briefly, after derivatization with MBB and HPLC separation the sulfide-dibimane product was measured by fluorescence detection.
Differential alkylation of recombinant PTEN and mass spectrometry
Prereduced recombinant PTEN-WT or -C71S (7.6 μM) was treated with or without 2 mM NaHS (Cayman) or H2O2 for 5 min at RT, before the reaction was stopped by addition of 50 mM NEM (15 min at RT). Samples were then split into two aliquots and subjected either to differential alkylation or directly to LTQ-OrbitrapVelos mass spectrometric analysis. For differential alkylation, the protein was denatured with 1% SDS to ensure complete alkylation of free thiols with NEM, followed by buffer exchange to remove NEM. Again in the presence of 1% SDS, previously oxidized thiols (including persulfides) were reduced by addition of 30 mM DTT for 30 min at RT. Newly reduced thiols were then alkylated by addition of 100 mM iodoacetamide, and samples were subjected to SDS-PAGE and Coomassie staining. PTEN bands were excised and double in-gel digested using AspN followed by trypsin. Peptides were subjected to reversed phase column chromatography and LTQ-OrbitrapVelos mass spectrometry as described previously (3). Post-translationally modified PTEN peptides were identified by searching all MS/MS spectra in dta format against the PTEN protein sequence using Sorcerer™-SEQUEST® (Sequest v. 2.7 rev. 11, Thermo Electron, including Scaffold_3_00_02; Proteome Software, Inc.) applying a stringent SEQUEST filter and the published parameters (3).
For semiquantitative analysis of the differentially alkylated Cys-124 peptide, NaHS- or H2O2-treated PTEN-WT or -C71S was analyzed by MALDI-TOF-TOF MS/MS. After AspN and trypsin double-digestion, peptides were manually spotted to MALDI-targets and the MALDI-TOF-TOF measurement performed on a Proteome-Analyzer 5800 (Applied Biosystems) as described previously (3).
Supplementary Material
Abbreviations Used
- 3MST
3-mercaptopyruvate sulfurtransferase
- C71S/C71A
cysteine 71-to-serine/alanine mutant
- CBS
cystathionine-β-synthase
- CN−
cyanide
- CSE
cystathionine-γ-lyase
- DATS
diallyl trisulfide
- DiFMUP
6,8-difluoro-4-methylumbelliferyl phosphate
- DTPA
diethylene triamine pentaacetic acid
- DTT
dithiothreitol
- E0
redox potential
- [Fe(SCN)(H2O)5]2+
iron (III) thiocyanate
- GSH
(reduced) glutathione
- GSNO
S-nitrosoglutathione
- GSSG
glutathione disulfide
- GYY4137
morpholin-4-ium 4-methoxyphenyl(morpholino) phosphinodithioate
- H2O2
hydrogen peroxide
- H2S
hydrogen sulfide
- HOCl
hypochlorous acid
- K2Sx
potassium (poly)sulfide
- KSCN
potassium thiocyanate
- Na2S
sodium sulfide
- NaHS
sodium hydrosulfide
- NEM
N-ethyl maleimide
- O2.−
superoxide radical
- ONOO−
peroxynitrite
- PI3K
phosphoinositide 3-kinase
- PTEN
phosphatase and tensin homolog
- roGFP2
reduction-oxidation-sensitive GFP2
- S0
sulfane sulfur
- TCEP
tris(2-carboxyethyl)phosphine
- WT
wild type
Acknowledgments
R.G. received a stipend from the German National Academic Foundation (Studienstiftung des deutschen Volkes). We thank Dr. Torsten Burkholz (University of Saarland, Germany) for help with the preparation of H2S(aq), Dr. Matt Whiteman (University of Exeter, United Kingdom) for kindly providing GYY4137, Dr. Nick Leslie (University of Dundee, United Kingdom) for advice regarding PTEN purification, and Attila Nagy for help with the MBB assay. H.A. was supported by grants from the Deutsche Forschungsgemeinschaft (AN 746/2-1 and AN 746/3-1). P.N. is supported by an FP7-PEOPLE-2010-RG Marie Curie International Reintegration Grant (grant No.: PIRG08-GA-2010-277006).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Cai WJ. Wang MJ. Moore PK. Jin HM. Yao T. Zhu YC. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc Res. 2007;76:29–40. doi: 10.1016/j.cardiores.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 2.Chen KY. Morris JC. Kinetics of oxidation of aqueous sulfide by O2. Environ Sci Technol. 1972;6:529. [Google Scholar]
- 3.Chi BK. Gronau K. Mader U. Hessling B. Becher D. Antelmann H. S-bacillithiolation protects against hypochlorite stress in Bacillus subtilis as revealed by transcriptomics and redox proteomics. Mol Cell Proteomics. 2011;10:M111.009506. doi: 10.1074/mcp.M111.009506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coletta C. Papapetropoulos A. Erdelyi K. Olah G. Modis K. Panopoulos P. Asimakopoulou A. Gero D. Sharina I. Martin E. Szabo C. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc Natl Acad Sci U S A. 2012;109:9161–9166. doi: 10.1073/pnas.1202916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collman JP. Ghosh S. Dey A. Decreau RA. Using a functional enzyme model to understand the chemistry behind hydrogen sulfide induced hibernation. Proc Natl Acad Sci U S A. 2009;106:22090–22095. doi: 10.1073/pnas.0904082106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Debiemme-Chouvy C. Wartelle C. Sauvage FX. First evidence of the oxidation and regeneration of polysulfides at a GaAs electrode, under anodic conditions. A study by in situ UV-visible spectroelectrochemistry. J Phys Chem B. 2004;108:18291–18296. [Google Scholar]
- 7.Denu JM. Dixon JE. Protein tyrosine phosphatases: mechanisms of catalysis and regulation. Curr Opin Chem Biol. 1998;2:633–641. doi: 10.1016/s1367-5931(98)80095-1. [DOI] [PubMed] [Google Scholar]
- 8.Gutscher M. Pauleau AL. Marty L. Brach T. Wabnitz GH. Samstag Y. Meyer AJ. Dick TP. Real-time imaging of the intracellular glutathione redox potential. Nat Methods. 2008;5:553–559. doi: 10.1038/nmeth.1212. [DOI] [PubMed] [Google Scholar]
- 9.Han J. Yen S. Han G. Han P. Quantitation of hydrogen peroxide using tris(2-carboxyethyl)phosphine. Anal Biochem. 1996;234:107–109. doi: 10.1006/abio.1996.0059. [DOI] [PubMed] [Google Scholar]
- 10.Hanson GT. Aggeler R. Oglesbee D. Cannon M. Capaldi RA. Tsien RY. Remington SJ. Investigating mitochondrial redox potential with redox-sensitive green fluorescent protein indicators. J Biol Chem. 2004;279:13044–13053. doi: 10.1074/jbc.M312846200. [DOI] [PubMed] [Google Scholar]
- 11.Hourihan JM. Kenna JG. Hayes JD. The gasotransmitter hydrogen sulfide induces Nrf2-target genes by inactivating the Keap1 ubiquitin ligase substrate adaptor through formation of a disulfide bond between Cys-226 and Cys-613. Antioxid Redox Signal. 2013;19:465–481. doi: 10.1089/ars.2012.4944. [DOI] [PubMed] [Google Scholar]
- 12.Hu Y. Chen X. Pan TT. Neo KL. Lee SW. Khin ES. Moore PK. Bian JS. Cardioprotection induced by hydrogen sulfide preconditioning involves activation of ERK and PI3K/Akt pathways. Pflugers Arch. 2008;455:607–616. doi: 10.1007/s00424-007-0321-4. [DOI] [PubMed] [Google Scholar]
- 13.Hughes MN. Centelles MN. Moore KP. Making and working with hydrogen sulfide: the chemistry and generation of hydrogen sulfide in vitro and its measurement in vivo: a review. Free Radic Biol Med. 2009;47:1346–1353. doi: 10.1016/j.freeradbiomed.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Hylin JW. Wood JL. Enzymatic formation of polysulfides from mercaptopyruvate. J Biol Chem. 1959;234:2141–2144. [PubMed] [Google Scholar]
- 15.Ishigami M. Hiraki K. Umemura K. Ogasawara Y. Ishii K. Kimura H. A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxid Redox Signal. 2009;11:205–214. doi: 10.1089/ars.2008.2132. [DOI] [PubMed] [Google Scholar]
- 16.Kabil O. Banerjee R. Redox biochemistry of hydrogen sulfide. J Biol Chem. 2010;285:21903–21907. doi: 10.1074/jbc.R110.128363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamyshny A. Goifman A. Gun J. Rizkov D. Lev O. Equilibrium distribution of polysulfide ions in aqueous solutions at 25 degrees C: a new approach for the study of polysulfides equilibria. Environ Sci Technol. 2004;38:6633–6644. doi: 10.1021/es049514e. [DOI] [PubMed] [Google Scholar]
- 18.Kimura H. Hydrogen sulfide: its production, release and functions. Amino Acids. 2011;41:113–121. doi: 10.1007/s00726-010-0510-x. [DOI] [PubMed] [Google Scholar]
- 19.Kimura Y. Mikami Y. Osumi K. Tsugane M. Oka JI. Kimura H. Polysulfides are possible H2S-derived signaling molecules in rat brain. FASEB J. 2013;27:2451–2457. doi: 10.1096/fj.12-226415. [DOI] [PubMed] [Google Scholar]
- 20.Krishnan N. Fu C. Pappin DJ. Tonks NK. H2S-Induced sulfhydration of the phosphatase PTP1B and its role in the endoplasmic reticulum stress response. Sci Signal. 2011;4:ra86. doi: 10.1126/scisignal.2002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwon J. Lee SR. Yang KS. Ahn Y. Kim YJ. Stadtman ER. Rhee SG. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc Natl Acad Sci U S A. 2004;101:16419–16424. doi: 10.1073/pnas.0407396101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SR. Yang KS. Kwon J. Lee C. Jeong W. Rhee SG. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- 23.Leslie NR. Bennett D. Lindsay YE. Stewart H. Gray A. Downes CP. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J. 2003;22:5501–5510. doi: 10.1093/emboj/cdg513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li L. Whiteman M. Guan YY. Neo KL. Cheng Y. Lee SW. Zhao Y. Baskar R. Tan CH. Moore PK. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation. 2008;117:2351–2360. doi: 10.1161/CIRCULATIONAHA.107.753467. [DOI] [PubMed] [Google Scholar]
- 25.Meyer AJ. Dick TP. Fluorescent protein-based redox probes. Antioxid Redox Signal. 2010;13:621–650. doi: 10.1089/ars.2009.2948. [DOI] [PubMed] [Google Scholar]
- 26.Mikami Y. Shibuya N. Kimura Y. Nagahara N. Ogasawara Y. Kimura H. Thioredoxin and dihydrolipoic acid are required for 3-mercaptopyruvate sulfurtransferase to produce hydrogen sulfide. Biochem J. 2011;439:479–485. doi: 10.1042/BJ20110841. [DOI] [PubMed] [Google Scholar]
- 27.Mueller EG. Trafficking in persulfides: delivering sulfur in biosynthetic pathways. Nat Chem Biol. 2006;2:185–194. doi: 10.1038/nchembio779. [DOI] [PubMed] [Google Scholar]
- 28.Mustafa AK. Gadalla MM. Sen N. Kim S. Mu W. Gazi SK. Barrow RK. Yang G. Wang R. Snyder SH. H2S signals through protein S-sulfhydration. Sci Signal. 2009;2:ra72. doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mustafa AK. Sikka G. Gazi SK. Steppan J. Jung SM. Bhunia AK. Barodka VM. Gazi FK. Barrow RK. Wang R. Amzel LM. Berkowitz DE. Snyder SH. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res. 2011;109:1259–1268. doi: 10.1161/CIRCRESAHA.111.240242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagahara N. Nirasawa T. Yoshii T. Niimura Y. Is novel signal transducer sulfur oxide involved in the redox cycle of persulfide at the catalytic site cysteine in a stable reaction intermediate of mercaptopyruvate sulfurtransferase? Antioxid Redox Signal. 2012;16:747–753. doi: 10.1089/ars.2011.4468. [DOI] [PubMed] [Google Scholar]
- 31.Nagy P. Winterbourn CC. Rapid reaction of hydrogen sulfide with the neutrophil oxidant hypochlorous acid to generate polysulfides. Chem Res Toxicol. 2010;23:1541–1543. doi: 10.1021/tx100266a. [DOI] [PubMed] [Google Scholar]
- 32.Olson KR. A practical look at the chemistry and biology of hydrogen sulfide. Antioxid Redox Signal. 2012;17:32–44. doi: 10.1089/ars.2011.4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papapetropoulos A. Pyriochou A. Altaany Z. Yang G. Marazioti A. Zhou Z. Jeschke MG. Branski LK. Herndon DN. Wang R. Szabo C. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci U S A. 2009;106:21972–21977. doi: 10.1073/pnas.0908047106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paul BD. Snyder SH. H(2)S signalling through protein sulfhydration and beyond. Nat Rev Mol Cell Biol. 2012;13:499–507. doi: 10.1038/nrm3391. [DOI] [PubMed] [Google Scholar]
- 35.Predmore BL. Lefer DJ. Gojon G. Hydrogen sulfide in biochemistry and medicine. Antioxid Redox Signal. 2012;17:119–140. doi: 10.1089/ars.2012.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramazzini B. De morbis artificum diatriba [diseases of workers]. 1713. Am J Public Health. 2001;91:1380–1382. doi: 10.2105/ajph.91.9.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneider CA. Rasband WS. Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sen N. Paul BD. Gadalla MM. Mustafa AK. Sen T. Xu R. Kim S. Snyder SH. Hydrogen sulfide-linked sulfhydration of NF-kappaB mediates its antiapoptotic actions. Mol Cell. 2012;45:13–24. doi: 10.1016/j.molcel.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen X. Pattillo CB. Pardue S. Bir SC. Wang R. Kevil CG. Measurement of plasma hydrogen sulfide in vivo and in vitro. Free Radic Biol Med. 2011;50:1021–1031. doi: 10.1016/j.freeradbiomed.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen X. Peter EA. Bir S. Wang R. Kevil CG. Analytical measurement of discrete hydrogen sulfide pools in biological specimens. Free Radic Biol Med. 2012;52:2276–2283. doi: 10.1016/j.freeradbiomed.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shibuya N. Tanaka M. Yoshida M. Ogasawara Y. Togawa T. Ishii K. Kimura H. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal. 2009;11:703–714. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- 42.Toohey JI. Sulphane sulphur in biological systems: a possible regulatory role. Biochem J. 1989;264:625–632. doi: 10.1042/bj2640625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toohey JI. Sulfur signaling: is the agent sulfide or sulfane? Anal Biochem. 2011;413:1–7. doi: 10.1016/j.ab.2011.01.044. [DOI] [PubMed] [Google Scholar]
- 44.Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev. 2012;92:791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 45.Wood JL. Sulfane sulfur. Methods Enzymol. 1987;143:25–29. doi: 10.1016/0076-6879(87)43009-7. [DOI] [PubMed] [Google Scholar]
- 46.Xiao Y. Yeong Chit Chia J. Gajewski JE. Sio Seng Lio D. Mulhern TD. Zhu HJ. Nandurkar H. Cheng HC. PTEN catalysis of phospholipid dephosphorylation reaction follows a two-step mechanism in which the conserved aspartate-92 does not function as the general acid—mechanistic analysis of a familial Cowden disease-associated PTEN mutation. Cell Signal. 2007;19:1434–1445. doi: 10.1016/j.cellsig.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 47.Yang G. Zhao K. Ju Y. Mani S. Cao Q. Puukila S. Khaper N. Wu L. Wang R. Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxid Redox Signal. 2013;18:1906–1919. doi: 10.1089/ars.2012.4645. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.