Significance
The modulation of ionic current flowing through an individual pore can be used for the stochastic sensing of a wide variety of analytes. Here we have produced protein pores by inserting peptide sequences that remain attached in an unconstrained conformation after limited proteolysis. The engineered pores were used for the label-free, single-molecule discrimination of Pim protein kinases. Further, we found unusually high, electrostatically enhanced, association rates for the Pim kinases and a consensus substrate, which suggests an additional element of control between cell-signaling pathways.
Keywords: substrate binding kinetics, single-molecule sensor, Coulombic interaction, phosphorylation
Abstract
In stochastic sensing, the association and dissociation of analyte molecules is observed as the modulation of an ionic current flowing through a single engineered protein pore, enabling the label-free determination of rate and equilibrium constants with respect to a specific binding site. We engineered sensors based on the staphylococcal α-hemolysin pore to allow the single-molecule detection and characterization of protein kinase–peptide interactions. We enhanced this approach by using site-specific proteolysis to generate pores bearing a single peptide sensor element attached by an N-terminal peptide bond to the trans mouth of the pore. Kinetics and affinities for the Pim protein kinases (Pim-1, Pim-2, and Pim-3) and cAMP-dependent protein kinase were measured and found to be independent of membrane potential and in good agreement with previously reported data. Kinase binding exhibited a distinct current noise behavior that forms a basis for analyte discrimination. Finally, we observed unusually high association rate constants for the interaction of Pim kinases with their consensus substrate Pimtide (∼107 to 108 M–1⋅s–1), the result of electrostatic enhancement, and propose a cellular role for this phenomenon.
Stochastic sensing is a powerful single-molecule approach for the detection of a wide range of analytes (1–3). Sensing is achieved by the modulation of ionic current flowing under an applied potential through an individual protein pore, such as the heptameric α-hemolysin (αHL) pore, reconstituted in an artificial lipid bilayer. The versatility and specificity of stochastic sensing have been enhanced by introducing sensing elements (analyte binding sites) into the αHL pore by protein engineering, chemical modification, or the use of adapter molecules (1, 4, 5). Through such approaches, the stochastic detection of ions (1, 6), small molecules (1, 7, 8), reactive molecules (9, 10), and protein–ligand interactions has been achieved (1, 11–14). The stochastic detection of proteins has also been achieved with functionalized solid-state nanopores (15). Polymer molecules passing through a protein pore can be detected by ionic current modulation (1, 16–19), which has provided a basis for polynucleotide sequencing (20–25). Further, this approach can be applied to the analysis of heterogeneous populations of nucleic acids for determination of purity, phosphorylation state, and chemical integrity (26). The threading of polynucleotides has also been used to study DNA–protein complexes (27). Finally, recent studies of protein translocation suggest that nanopore proteomics may be possible (19, 28).
Efforts have been made to apply stochastic sensing to protein kinases (11, 14). Protein kinases comprise one of the largest gene families in eukaryotes, with 518 kinase domains identified in the human genome (29). As most cellular and physiological processes are mediated or modulated by protein kinases, aberrant kinase activity can have transformative effects, leading, for example, to cancers. Protein kinases are hence under intense investigation as therapeutic targets. The stochastic sensing of protein kinases might, in addition to allowing the study of the fundamental kinetics of substrate interactions or the detection of kinases in different activation states, be extended to the screening of inhibitors. The label-free, single-molecule nature of the technique provides potential advantages over existing methods that largely rely on radiometric or fluorescence detection.
Sensors for the catalytic subunit of cAMP-dependent protein kinase (PKA) were previously engineered either by chemically attaching to the trans mouth of the αHL pore a peptide comprising residues 5–24 of the heat-stable protein inhibitor of PKA (14) or by genetically fusing this sequence within a single trans loop of the β-barrel domain (11). However, chemical modification requires time-consuming synthesis and does not achieve full modification (in the case cited: ∼70%). There again, fusion of the sensor element within the trans loop conformationally constrains the peptide, which may alter binding kinetics in contrast with a linear peptide (11).
In the present work, we aimed to (i) design a stochastic sensor for the Pim family of protein kinases (30) and (ii) investigate alternative engineering strategies that might circumvent the limitations of previous methods. The Pim kinase family comprises three serine/threonine protein kinases that are constitutively active monomers not requiring phosphorylation of the activation loop for activity. They lack regulatory domains and so are regulated at the transcriptional and translational level, for example by cytokine signaling through the Janus kinase/signal transducers and activators of transcription (JAK/STAT) pathway (31, 32). They have roles in survival signaling and cell proliferation, and their up-regulation can contribute to the progression of leukemias and tumors (33–37). Pim kinases possess an unusual ATP-binding pocket, widened by an insertion in the hinge region and the presence of a proline residue, which lacks one of two typically conserved residues that form backbone hydrogen bonds to the adenine ring of ATP (38–42). Their unusual ATP-binding sites and roles in cancer have prompted the investigation of potential Pim-selective inhibitors for cancer therapy (30, 43).
We first constructed a trans loop-constrained, genetically encoded sensor for Pim kinases, analogous to that previously made for the detection of PKA (11). We chose the Pim kinase consensus substrate Pimtide (ARKRRRHPS*GPPTA) as the sensor element, which had been identified by a peptide library screen and been shown to bind all three Pim kinases with high affinity (38, 44). Because Pimtide is a substrate for Pim kinases rather than an inhibitory peptide, it also offers the prospect of extending stochastic sensing to the measurement of catalytic activity.
We then enhanced this approach by using site-specific, posttranslational cleavage of the loop to liberate one end of the sensor peptide. We also made an analogous sensor for PKA. These proteolytically cleaved sensors allowed us to study the kinetics of kinase–substrate interactions independent of membrane potential. We also found differing noise characteristics for each kinase when bound, which could form a basis for analyte discrimination. Further, we found that the association rates of the Pim kinase family are electrostatically enhanced, and propose a possible role for this phenomenon in vivo. We hence demonstrated the utility of proteolytically cleaved αHL pores for the study of protein kinase substrate interactions.
Results
Initial Pore Engineering and Optimization.
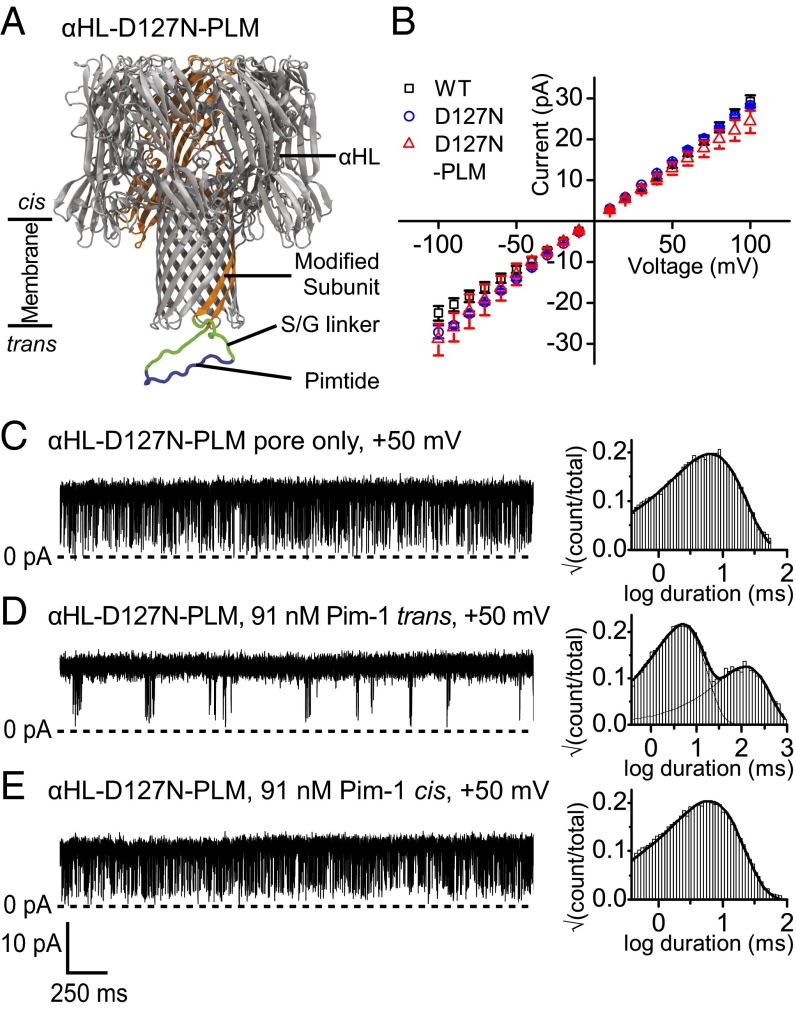
We first sought to engineer a genetically encoded sensor for the Pim kinase family by using a previously described strategy (11). In this approach, the Pimtide sensor element flanked by linker sequences of eight Ser/Gly residues on either side is fused into the trans mouth loop of one subunit of a heteroheptameric αHL pore (Fig. 1A). The heteromeric pore (αHL–PLM–D8)1(αHL–WT)6, [hereafter αHL–PLM, where PLM (Pimtide loop mutant) refers to the fused sensor element], was produced by in vitro transcription and translation (IVTT) and assembly on rabbit red blood cell membranes (Materials and Methods). However, when αHL–PLM was reconstituted into planar lipid bilayers, no alteration of its electrical behavior was observed upon the addition of Pim-1 to the trans chamber; a conductance state that reflected a single open pore continued to be observed at both positive and negative applied potentials (Fig. S1A).
Fig. 1.
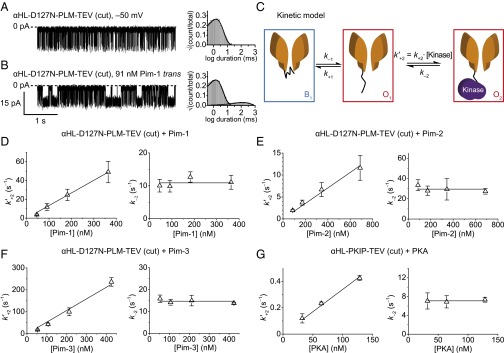
Design and characterization of an engineered αHL pore for Pim kinase detection with a trans loop fusion sensor element. (A) Illustration of the αHL–D127N–PLM pore indicating the position of the Pimtide sensor element (blue) flanked by serine/glycine linkers (green) and genetically fused into the trans loop of one subunit (orange) of αHL–D127N. (B) Current–voltage relationships for αHL–WT (□) and αHL–D127N (○) homoheptamers and the αHL–D127N–PLM (△) heteroheptamer. Error bars represent SDs (n = 3). Representative current recordings and dwell-time histograms for the open current level with an applied potential of +50 mV are shown for (C) the αHL–D127N–PLM pore only, (D) the αHL–D127N–PLM pore with 91 nM Pim-1 present in the trans chamber, and (E) the αHL–D127N–PLM pore with 91 nM Pim-1 present in the cis chamber. Histograms were fitted with one- or two-component probability density functions. All measurements were performed in 15 mM Mops, pH 6.8, 300 mM KCl, 5 mM DTT, and the filter corner frequency was 2 kHz.
We hypothesized that the sensor loop may have been trapped in a conformation that did not favor binding due to electrostatic interactions between the highly basic Pimtide and the αHL trans mouth charged residues Asp127, Asp129, and Lys131. We therefore screened a number of charge neutralization mutants at these positions. One of these, D127N, when present in the heteromeric pore (αHL–D127N–PLM–D8)1(αHL–D127N)6 (hereafter αHL–D127N–PLM), enabled a change in the electrical behavior of the pore upon the addition of Pim-1 to the trans chamber (Fig. 1 C and D). In the absence of Pim-1, and unlike αHL–PLM, αHL–D127N–PLM exhibited rapid transitions to a partially closed state of mean duration 0.41 ± 0.03 ms, with a mean interevent interval 7 ± 1 ms (both n = 3; Fig. S1B). The addition of Pim-1 to the trans chamber caused intermittent abolition of these closures, such that two distinct mean interevent intervals were now apparent (Fig. 1D). In the presence of 91 nM Pim-1, the mean duration of the closures was 0.46 ± 0.03 ms, and the mean interevent intervals were 4.7 ± 0.2 and 100 ± 10 ms (all values n = 3; Fig. S1C).
The two-state gating behavior exhibited by the pore at positive potentials in the absence of Pim-1 is similar to that seen for other pores modified with conformationally flexible attachments (11, 45, 46), and we ascribe this behavior to transient blockade of the pore by the modified trans loop. When the kinase binds to the loop, the motion is restrained, and the blockades are abolished. The addition of Pim-1 to the cis compartment did not produce a change in pore behavior (Fig. 1E). Current–voltage relationships for the open states of (αHL–D127N)7 and αHL–D127N–PLM show modest changes in rectification with respect to (αHL–WT)7, presumably because of alterations in charge distribution near the trans mouth arising from the modifications (Fig. 1B).
Site-Specific Proteolytic Cleavage Yields Unconstrained Peptide Sensor Elements.
We subsequently sought to produce pores bearing a sensor peptide attached by a single terminus, as these would better resemble the synthetic, linear peptides often used in other methods for measuring kinase substrate interactions (38, 47, 48). This had previously been achieved by forming an αHL pore with one subunit chemically coupled through a cysteine residue at the trans mouth to a peptide sensor element by using an S-pyridyl-functionalized tetra(ethylene glycol) linker (14). We sought a similar outcome through a genetically encoded procedure, thus avoiding the need for peptide synthesis, functionalization, and subunit modification. Genetically encoded sensors would also be amenable to the construction of combinatorial peptide libraries.
We introduced into the αHL–D127N–PLM–D8 polypeptide a tobacco etch virus (TEV) protease cleavage site (ENLYFQG) at the C-terminal end of the Pimtide sequence and preceding the Ser/Gly linker, so that posttranslational, site-specific proteolytic cleavage would liberate one end of the sensor peptide (Fig. 2 A and B). The cleaved subunit would then be mixed with αHL–D127N monomer, oligomerized, and the desired heteroheptamer purified by SDS/PAGE (Fig. 2C, Fig. S2, and SI Materials and Methods).
Fig. 2.
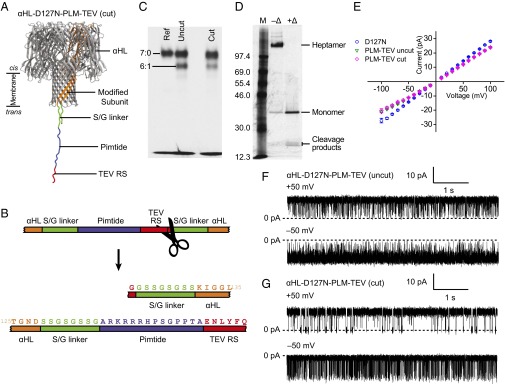
Design and characterization of a TEV protease-cleavable αHL trans loop fusion pore for Pim kinase detection. (A) Illustration of the protease-cleaved form of αHL–D127N–PLM–TEV indicating the positions of the Pimtide sensor element (blue), the serine/glycine linkers (green), and the TEV protease recognition site (“TEV RS,” red). (B) Scheme for the TEV protease-cleavable trans loop fusion in αHL–D127N–PLM–TEV. (C) Preparative SDS/PAGE showing a reference homoheptameric αHL marker (“Ref” lane) and lanes containing mixtures of (αHL–D127N)x(αHL–D127N–PLM–TEV)y where the αHL–D127N–PLM–TEV subunits were cleaved before assembly (cut) or uncleaved (uncut) by TEV protease. (D) Confirmation of the incorporation of the proteolytically cleaved αHL–D127N–PLM–TEV subunit into (αHL–D127N)6(αHL–D127N–PLM–TEV)1. Gel-purified (αHL–D127N)6(αHL–D127N–PLM–TEV)1 was electrophoresed with (+Δ) and without (–Δ) heating before loading. (E) Current–voltage relationships for the αHL–D127N (○) homoheptamer and the uncut (▽) and cut (◇) forms of the αHL–D127N–PLM–TEV heteroheptamer. Error bars represent SDs (n = 3). Representative current traces for the uncut (F) and cut (G) forms of the αHL–D127N–PLM–TEV pore at applied potentials of ±50 mV. All measurements were performed in 15 mM Mops, pH 6.8, 300 mM KCl, 5 mM DTT, and the filter corner frequency was 2 kHz.
Heteroheptamers, (αHL–D127N–PLM–TEV–D8)1(αHL–D127N)6 (hereafter αHL–D127N–PLM–TEV), were successfully formed with both cleaved (“cut”) and uncleaved (“uncut”) sensor subunits (Fig. 2C). Gel-purified, cut αHL–D127N–PLM–TEV was denatured by heating at 95 °C for 20 min and analyzed by SDS/PAGE alongside nondenatured, cut αHL–D127N–PLM–TEV (Fig. 2D). In the heated sample, two bands were seen at around half the apparent molecular mass of the monomer band, consistent with the expected sizes of the cleavage products of the sensor subunit, confirming that the cleaved sensor subunits had been incorporated into the heteroheptamers.
Single-Channel Characterization of Pores.
We then characterized the electrical properties of both cut and uncut αHL–D127N–PLM–TEV. The open state current–voltage relationships were indistinguishable and both exhibited altered rectification compared with αHL-D127N homoheptamer, which may be due to the presence of additional charged residues near the trans mouth (Fig. 2E). In the absence of Pim kinase, uncut αHL–D127N–PLM–TEV displayed large amplitude blockades at both +50 and –50 mV (Fig. 2F). At +50 mV, the blockade duration histogram was best fit with a two-component probability density function, giving mean blockade durations of 0.49 ± 0.03 and 3.7 ± 0.9 ms (n = 3; Fig. S3A). The mean interevent interval was 32 ± 5 ms (n = 3; Fig. S3A). Analysis at –50 mV was complicated by a large number of poorly resolved events, so the mean durations may possess substantial systematic error. The blockade duration histogram was best fit with a two-component probability density function, giving mean blockade durations of 0.49 ± 0.09 and 3 ± 1 ms, and the mean interevent interval was 100 ± 60 ms (all n = 3; Fig. S3A).
Protease-cleaved αHL–D127N–PLM–TEV also exhibited blockades in the absence of Pim kinase at both positive and negative applied potentials, but with altered kinetics. At +50 mV, the mean interevent interval was 50 ± 10 ms (n = 3; Fig. S3B). The blockade duration histogram was best fit with a three-component probability density function, giving mean durations 0.22 ± 0.05, 2.2 ± 0.9, and 18 ± 7 ms (all n = 3; Fig. S3B). At –50 mV, the mean interevent interval was 1.4 ± 0.2 ms, and the mean blockade duration was 8 ± 2 ms (both n = 6; Fig. S3B).
We also produced a sensor similar to αHL–D127N–PLM–TEV but with the TEV protease cleavage site placed at the N-terminal side of the Pimtide sequence. However, when reconstituted into planar lipid bilayers, the protease-cleaved pore gave a very noisy current signal without defined conductance states and so was not pursued further. From here on, the properties of the protease-cleaved form of αHL–D127N–PLM–TEV are further discussed.
Single-Molecule Detection and Kinetic Analysis of Pim Kinase Binding.
The addition of 91 nM Pim-1 to the trans chamber under an applied potential of –50 mV altered the αHL–D127N–PLM–TEV pore’s pattern of blockades (Fig. 3 A and B). Specifically, an additional population of longer events appeared in the duration histogram for the open current level (interevent intervals; Fig. 3B). In the presence of 91 nM Pim-1, the mean interevent interval for the additional population was 100 ± 20 ms (n = 3; Fig. S4A). The mean blockade and interevent interval durations were unchanged, within error, and were 8 ± 2 and 1.5 ± 0.2 ms, respectively (n = 3; Fig. S4A). We ascribe the long-duration intervals at the open current level to the binding of Pim-1 to the sensor peptide, which prevents the peptide from blocking the pore. Pim-1 that had been denatured by heating did not produce a change in pore behavior (Fig. S4B).
Fig. 3.
Stochastic detection of protein kinases using TEV protease-cleaved αHL trans loop fusion pores. Representative current traces of the αHL–D127N–PLM–TEV (cut) pore under an applied potential of –50 mV before (A) and after (B) the addition of 91 nM Pim-1 to the trans chamber are shown together with representative dwell-time histograms for the open-pore current level. (C) Kinetic model for the observed current signal from proteolytically cleaved sensors in the presence of a protein kinase. The state B1 corresponds to the blocked current level of the pore, which is assumed to be due to obstruction of the conductive channel by the peptide attached at the trans mouth. State O1 corresponds to the open pore, where the pore is not bound to a kinase, and state O2 corresponds to the open pore, where kinase is bound to the pore through the attached peptide. Note: an additional blocked state B2 connected to O2 was included for Pim-3 to account for an additional population of blockades that occur when Pim-3 is bound to the pore (Materials and Methods). Plots of the concentration dependence of the pseudo-first-order kinase association rate constants 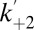 and first-order kinase dissociation rate constants k–2 are shown for the interaction of the αHL–D127N–PLM–TEV (cut) pore with (D) Pim-1, (E) Pim-2, (F) Pim-3, and (G) for the interaction of the αHL–PKIP–TEV (cut) pore with cAMP-dependent protein kinase (PKA). Error bars represent SDs (Pim kinases: n = 4; PKA: n = 3). Association rate constant plots were fitted to the equation y = mx, where the gradient, m, gave the final value for the biomolecular association rate constant, and dissociation rate constant plots were fitted to the equation y = c, where the intercept, c, gave the final value for the dissociation rate constant (Table 1). Measurements were performed in 15 mM Mops, pH 6.8, 300 mM KCl for PKA and in 15 mM Mops, pH 6.8, 300 mM KCl, 5 mM DTT for the Pim kinases. The applied potential was –50 mV, and the filter corner frequency was 2 kHz.
and first-order kinase dissociation rate constants k–2 are shown for the interaction of the αHL–D127N–PLM–TEV (cut) pore with (D) Pim-1, (E) Pim-2, (F) Pim-3, and (G) for the interaction of the αHL–PKIP–TEV (cut) pore with cAMP-dependent protein kinase (PKA). Error bars represent SDs (Pim kinases: n = 4; PKA: n = 3). Association rate constant plots were fitted to the equation y = mx, where the gradient, m, gave the final value for the biomolecular association rate constant, and dissociation rate constant plots were fitted to the equation y = c, where the intercept, c, gave the final value for the dissociation rate constant (Table 1). Measurements were performed in 15 mM Mops, pH 6.8, 300 mM KCl for PKA and in 15 mM Mops, pH 6.8, 300 mM KCl, 5 mM DTT for the Pim kinases. The applied potential was –50 mV, and the filter corner frequency was 2 kHz.
We studied the kinetics of kinase association and dissociation with a model in which it is assumed that the kinase binds only when the peptide is not blocking the pore, i.e., from the open state only (Fig. 3C). We measured the Pim-1 concentration dependence of the dissociation rate constant, k–2, and the observed pseudo-first-order association rate constant, 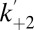 . k–2 was found to be independent of Pim-1 concentration, consistent with unimolecular dissociation, and
. k–2 was found to be independent of Pim-1 concentration, consistent with unimolecular dissociation, and 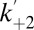 was found to be linearly dependent on Pim-1 concentration, consistent with bimolecular association of Pim-1. We also applied this analysis to measurements of the interactions of Pim-2 and Pim-3 with αHL–D127N–PLM–TEV (Fig. 3 E and F and Fig. S5 A and B). Pim-3 required a modified kinetic model due to additional resolved “noise” events in the kinase-bound state (below and Materials and Methods). From these analyses, we determined the dissociation rate constants, k–2, and bimolecular association rate constants, k+2, and hence the equilibrium dissociation constants, Kd = k–2/k+2, for all three Pim kinase family members (Table 1).
was found to be linearly dependent on Pim-1 concentration, consistent with bimolecular association of Pim-1. We also applied this analysis to measurements of the interactions of Pim-2 and Pim-3 with αHL–D127N–PLM–TEV (Fig. 3 E and F and Fig. S5 A and B). Pim-3 required a modified kinetic model due to additional resolved “noise” events in the kinase-bound state (below and Materials and Methods). From these analyses, we determined the dissociation rate constants, k–2, and bimolecular association rate constants, k+2, and hence the equilibrium dissociation constants, Kd = k–2/k+2, for all three Pim kinase family members (Table 1).
Table 1.
Association and dissociation rate constants and equilibrium dissociation constants for several protein kinase-peptide interactions
| Source | Pore | Kinase | k+2 (M–1⋅s–1) | k–2 (s–1) | Kd (μM) |
| This work | αHL–D127N–PLM–TEV (cut) | Pim-1 | (1.34 ± 0.03) × 108 | 10.9 ± 0.6 | 0.081 ± 0.005 |
| Pim-2 | (1.76 ± 0.08) × 107 | 30 ± 1 | 1.7 ± 0.1 | ||
| Pim-3 | (5.3 ± 0.3) × 108 | 14.7 ± 0.5 | 0.028 ± 0.002 | ||
| αHL–PKIP–TEV (cut) | PKA | (3.4 ± 0.1) × 106 | 7.1 ± 0.1 | 2.09 ± 0.07 | |
| Method | Kinase | k+2 | k–2 | Kd | |
| From the literature | ITC* | Pim-1 | — | — | 0.058 |
| ITC* | Pim-2 | — | — | 0.64 | |
| ITC* | Pim-3 | — | — | 0.039 | |
| Gel filtration† | PKA | — | — | 2.3 | |
| SPR‡ | PKA (+MgATP) | 1.5 × 106 | 7.6 × 10−4 | 0.0005 | |
| Stochastic sensing (14) | PKA | (2.5 ± 0.5) × 106 | 0.19 ± 0.05 | 0.08 ± 0.01 | |
| Stochastic sensing (11) | PKA | (1.5 ± 0.1) × 106 | 0.2 ± 0.1 | 0.13 ± 0.01 |
Constants in this work were determined under an applied potential of –50 mV, in 15 mM Mops, pH 6.8, 300 mM KCl, and 5 mM DTT, except for PKA, where the buffer did not contain DTT. Values are mean ± SE, determined from curve fitting as shown in Fig. 3, where the fitted means were derived from n = 4 for the three Pim kinases, and n = 3 for PKA. αHL, α-hemolysin; ITC, isothermal titration calorimetry; PKIP, protein kinase inhibitor peptide (residues 5–24); PLM, Pimtide loop mutant; SPR, surface plasmon resonance; TEV, tobacco etch virus.
Pim kinase affinities for Pimtide peptide (30).
Interaction of full-length heat-stable protein kinase inhibitor (PKI) with PKA catalytic subunit (48).
Interaction of GST–PKI fusion with PKA catalytic subunit in the presence of 1 mM ATP and 10 mM MgCl2 (47).
We applied the same kinetic analysis to the interaction of Pim-1 with our original loop–fusion sensor αHL–D127N–PLM and determined rate constants k+2 = (1.90 ± 0.09) × 108 M–1⋅s–1 and k–2 = 6.8 ± 0.6 s–1 and the equilibrium dissociation constant Kd = 36 ± 4 nM (all mean ± SE). Differences in rate constants were also observed when comparing previous chemically modified and loop-constrained sensors for PKA (11, 14), but these differences and those in the present work are too small to be attributed with confidence to conformational constraints.
Stochastic Detection of cAMP-Dependent Protein Kinase.
To test the general applicability of the approach, we constructed an analogous sensor for the catalytic subunit of cAMP-dependent protein kinase (PKA) by placing a TEV protease cleavage site after residues 5–24 of the heat-stable protein kinase inhibitor (PKIP5–24) (49) in the αHL–PKIP pore used previously for the stochastic detection of PKA (11). We also introduced a lysine immediately after PKIP5–24 to maintain the net neutral charge of the sensor peptide (an additional negative charge is introduced by the TEV protease cleavage site).
The TEV–protease cleaved form of (αHL–PKIP–TEV)1(αHL–WT)6 (hereafter αHL–PKIP–TEV) exhibited similar blockade behavior at ±50 mV in the absence of PKA compared with the Pimtide-based sensor in the absence of Pim kinase, but with altered kinetics. This reinforces the conclusion that the blockades observed in the absence of kinase arise from motion of the sensor peptide element and not the vestigial Ser/Gly linker that remains on the adjacent strand after proteolytic cleavage (Fig. S5C). At +50 mV the duration histogram for the blocked current level was best fit by a two-component probability density function, giving mean blockade durations of 0.25 ± 0.07 and 2.8 ± 0.7 ms; the mean interevent interval was 120 ± 20 ms (all n = 3; Fig. S5C). At –50 mV, the mean interevent interval was 4.5 ± 0.3 ms, and the mean blockade duration was 2.5 ± 0.4 ms (both n = 3; Fig. S5C).
At –50 mV and in the presence of 128 nM PKA catalytic subunit, the mean blockade duration was 2.6 ± 0.4 ms, the mean interevent interval duration was 5.0 ± 0.2 ms, and the mean event duration due to kinase binding was 140 ± 10 ms (all n = 3; Fig. S5C). The rate constants and equilibrium dissociation constant for the association of PKA with αHL–PKIP–TEV were determined as for the Pim kinases (Fig. 3G and Table 1).
To investigate substrate selectivity between PKA and Pim-1, we looked for signs of interaction between Pim-1 and αHL–PKIP–TEV and between PKA and αHL–D127N–PLM–TEV. For both αHL–PKIP–TEV in the presence of 182 nM Pim-1 and for αHL–D127N–PLM–TEV in the presence of 128 nM PKA, binding was not observed (Fig. S6).
Voltage Independence of Kinase Binding.
As the kinetics of association and dissociation were measured under an applied transmembrane potential, it was important to examine the voltage dependence of the rate constants and equilibrium constants. For Pim-1 interacting with αHL–D127N–PLM–TEV (Fig. S7 A–C), there was no statistically significant voltage dependence from –20 to –80 mV for k+2, k–2, or Kd [t(26) = –0.55, P = 0.59; t(26) = –1.66, P = 0.11; t(26) = 0.18, P = 0.86; respectively, where the null hypothesis was that the slope of the linear regression was zero]. This is an improvement over previous kinase stochastic sensors, which did exhibit voltage-dependent interactions (14). At higher negative potentials, we found that the αHL–D127N–PLM–TEV pore closed with increasing frequency. The closures were reversed if the polarity of the applied potential was alternated, but they prohibited experiments at potentials more negative than –80 mV.
Discrimination of Pim Kinase Family Members.
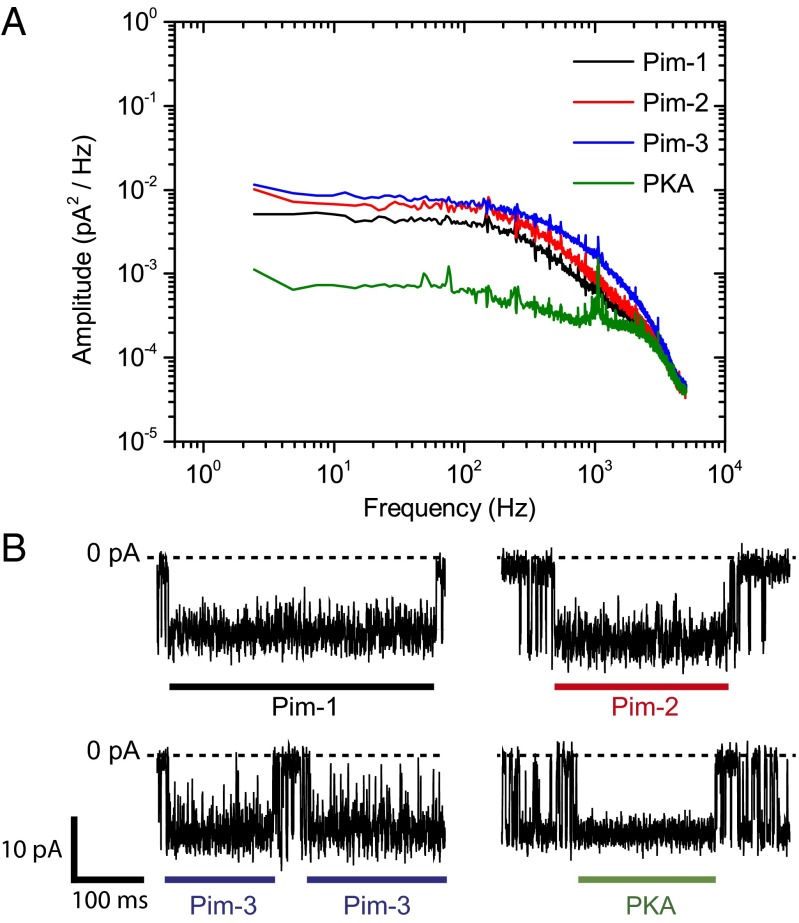
Stochastic sensing offers the potential for analyte discrimination based on blockade amplitude and/or the mean duration of binding. In the present work, binding was not associated with current blockade. We did, however, observe differences with respect to the noise in the kinase-bound conductance states; current traces corresponding to the kinase-bound state of the Pim kinases showed additional current noise compared with those for PKA-bound levels with αHL–PKIP–TEV, and the magnitude of the additional noise varied between the three Pim kinases [Fig. 4 A and B; current–trace SDs: 1.78 pA (Pim-1), 2.07 pA (Pim-2), 2.45 pA (Pim-3), and 1.19 pA (PKA)]. In the case of Pim-3, the noise was sufficiently resolved that it was necessary to include an additional state in the kinetic model for the determination of rate constants (Materials and Methods). Exploiting these noise differences enables an alternative means for analyte discrimination.
Fig. 4.
Power spectra of the kinase-bound states for four different protein kinases. (A) Power spectral densities of events corresponding to Pim-1, Pim-2, or Pim-3 bound to the αHL–D127N–PLM–TEV pore and the catalytic subunit of cAMP-dependent protein kinase (PKA) bound to the αHL–PKIP–TEV pore. For clarity, coincident spikes present in all four spectra at odd harmonics of the power-line frequency (50 Hz) have been removed by notch filtering. The unfiltered spectra are shown in Fig. S8. (B) Representative traces showing individual events that correspond to the kinase-bound states as described above, where the thick bars indicate the duration of binding. A downward deflection corresponds to a negative current. Measurements were performed in 15 mM Mops, pH 6.8, 300 mM KCl for PKA and with the addition of 5 mM DTT for the Pim kinases. The applied potential was –50 mV, and the filter corner frequency was 2 kHz.
Association Rate of Pim-1 for Pimtide is Electrostatically Enhanced.
The association rate constants determined for the Pim kinases are significantly higher than that determined for PKA, particularly in the cases of Pim-1 and Pim-3, which both have association rate constants around two orders of magnitude higher than PKA. Orientation requirements usually limit protein–protein association rate constants to 105–106 M–1⋅s–1 (50). However, it has been shown that rate constants as high as 109 M–1⋅s–1 can be achieved through long-range electrostatic interactions between binding partners, which accelerate the formation of a loosely associated “transient complex” preceding the formation of the native complex (51). Such electrostatic enhancement can be modulated by varying the ionic strength of the solution. A significant decrease in an electrostatically enhanced association rate constant (k+2) occurs with increasing ionic strength, and a linear relationship is observed when log k+2 is plotted against 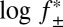 , where
, where 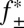 is the mean activity coefficient of the electrolyte given by the extended Debye–Hückel equation (52):
is the mean activity coefficient of the electrolyte given by the extended Debye–Hückel equation (52):
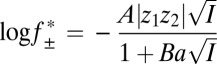 |
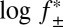 is related to the electrostatic potential between the two binding partners and log k+2 to the activation energy for association (52).
is related to the electrostatic potential between the two binding partners and log k+2 to the activation energy for association (52).
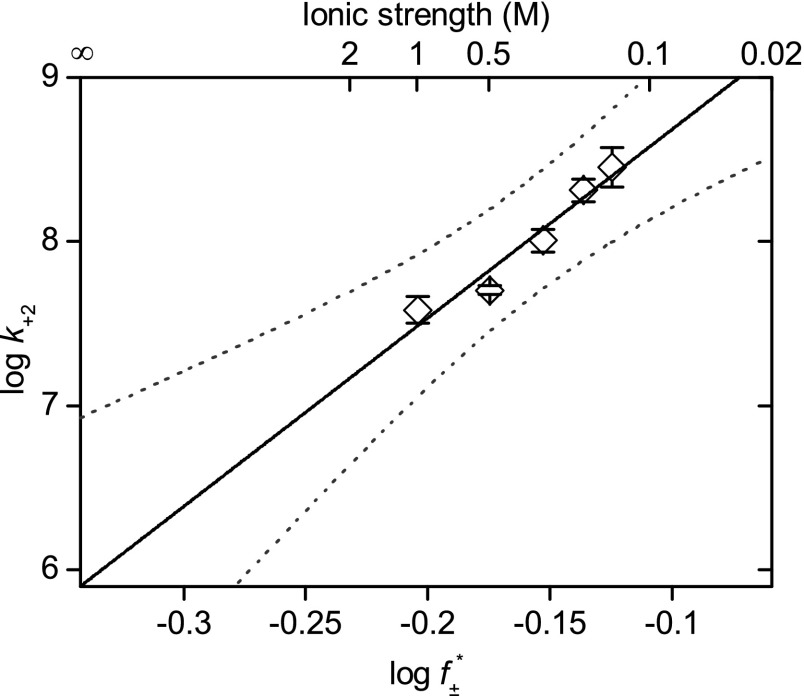
We measured the association rate constant for Pim-1 with αHL–D127N–PLM–TEV at ionic strengths of 150–1,000 mM. We observed a strong linear dependence for 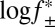 versus log k+2 (R2 = 0.97), which suggests that the association of Pim-1 with its substrate is electrostatically enhanced (Fig. 5). Extrapolation to infinite ionic strength allows a rough approximation of the basal association rate constant in the absence of electrostatic forces. The value for Pim-1, ∼8 × 105 M–1⋅s–1, is similar in magnitude to other basal association rate constants estimated for electrostatically enhanced protein–protein interactions (51). We calculated electrostatic potential maps for Pim-1 and Pim-2 using Adaptive Poisson-Boltzmann Solver (APBS) (53) and visualized them in Visual Molecular Dynamics (VMD) (54) (Fig. S9 A and B). The maps clearly illustrate strong negative electrostatic potentials across the peptide-binding pockets that would form long-range electrostatic interactions with the highly positively charged Pimtide substrate, consistent with the phenomenon described above. In contrast, PKA does not have this feature (Fig. S9C).
versus log k+2 (R2 = 0.97), which suggests that the association of Pim-1 with its substrate is electrostatically enhanced (Fig. 5). Extrapolation to infinite ionic strength allows a rough approximation of the basal association rate constant in the absence of electrostatic forces. The value for Pim-1, ∼8 × 105 M–1⋅s–1, is similar in magnitude to other basal association rate constants estimated for electrostatically enhanced protein–protein interactions (51). We calculated electrostatic potential maps for Pim-1 and Pim-2 using Adaptive Poisson-Boltzmann Solver (APBS) (53) and visualized them in Visual Molecular Dynamics (VMD) (54) (Fig. S9 A and B). The maps clearly illustrate strong negative electrostatic potentials across the peptide-binding pockets that would form long-range electrostatic interactions with the highly positively charged Pimtide substrate, consistent with the phenomenon described above. In contrast, PKA does not have this feature (Fig. S9C).
Fig. 5.
Association of Pim-1 with the αHL–PLM–TEV pore at different ionic strengths. Log k+2 (where k+2 is the second-order association rate constant) was plotted against 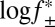 (where
(where 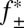 is the mean activity coefficient, calculated from the extended Debye–Hückel equation). At the limit [KCl] → ∞,
is the mean activity coefficient, calculated from the extended Debye–Hückel equation). At the limit [KCl] → ∞, 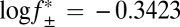 , and k+2
(basal) ∼8 × 105 M–1⋅s–1. Measurements were performed in 15 mM Mops, pH 6.8, with the ionic strength adjusted with KCl. The ionic strength contribution from the Mops was ∼4 mM. The applied potential was –50 mV, and the filter corner frequency was 2 kHz. Error bars represent SDs (n = 5). Dotted lines indicate the 95% prediction interval for the linear regression.
, and k+2
(basal) ∼8 × 105 M–1⋅s–1. Measurements were performed in 15 mM Mops, pH 6.8, with the ionic strength adjusted with KCl. The ionic strength contribution from the Mops was ∼4 mM. The applied potential was –50 mV, and the filter corner frequency was 2 kHz. Error bars represent SDs (n = 5). Dotted lines indicate the 95% prediction interval for the linear regression.
Discussion
Stochastic sensors for the catalytic subunit of cAMP-dependent protein kinase (PKA) have previously been described (11, 14). These either relied upon chemical attachment of a peptide sensor element to αHL (14) or the fusion of a peptide sensor element within the trans loop of αHL (11). Here we have described an engineering strategy that combines advantages from each approach while eliminating several weaknesses. We thereby extended the scope of stochastic sensing of protein kinases and used the approach to gain insight into the kinetics of substrate interactions for the Pim kinase family.
Proteolytically Cleaved trans Loops in αHL as Stochastic Sensors.
By designing αHL trans loop sensor element fusions that can be site-specifically cleaved by TEV protease, we were able to produce stochastic sensors for the Pim kinase family and for PKA that do not require chemical modification and which bear a sensor peptide attached through a peptide bond to a single terminus. The procedure is fast, comprising only a simple additional step within the existing method for producing heteromeric αHL pores. As the sensor element is genetically encoded, it should be possible to construct combinatorial sensor libraries by this approach. Pores bearing peptides attached to the trans mouth could also have other applications. For example, peptides containing genetically encoded affinity tags could be used to immobilize pores on a solid substrate, or to attach them within a solid-state nanopore for the formation of hybrid nanopore devices (55).
Stochastic Detection of Protein Kinases.
We determined the association and dissociation rate constants, and hence the equilibrium dissociation constants, for the interaction of all three Pim kinases with their consensus substrate peptide Pimtide by using the sensor αHL–D127N–PLM–TEV (cut) and for PKA with the inhibitory peptide PKIP5–24 by using the sensor αHL–PKIP–TEV (cut) (Table 1). Our findings with Pim-1 show that measured constants were independent of transmembrane potential, allowing direct comparison with values determined in bulk solution, in contrast with previous studies (14). Kinase binding occurs without a reduction in pore current, unlike previously described sensors (11, 14). This suggests that binding and dissociation occur sufficiently far from the pore and lipid bilayer to not be influenced by ionic flow through the pore or the transmembrane electric field.
Equilibrium dissociation constants, but not rate constants, for Pim kinases with Pimtide have previously been determined by isothermal titration calorimetry (ITC) (38). Values determined here correspond well with those from ITC (Table 1), given somewhat different determination conditions (ITC: 50 mM Hepes, pH 7.5, 150 mM NaCl, 1 mM DTT, at 10 °C; this work: 15 mM Mops, pH 6.8, 300 mM KCl, 5 mM DTT, at 21 °C), and the presence of the TEV recognition site adjacent to Pimtide in the sensor peptide. Pim-3 bound strongest (this work: 28 nM; ITC: 39 nM), with Pim-1 binding slightly more weakly (this work: 81 nM; ITC: 58 nM), and Pim-2 binding much more weakly (this work: 1.7 μM; ITC: 640 nM).
For the PKA–PKIP5–24 interaction, the measured association rate constant (3.4 × 106 M–1⋅s–1) is consistent with previous values from stochastic detection (2.5 × 106 M–1⋅s–1, 1.5 × 106 M–1⋅s–1) (11, 14) and a value determined by surface plasmon resonance (SPR) (1.5 × 106 M–1⋅s–1) for a GST–PKI fusion with a PKA catalytic subunit (but in the presence of MgATP, and where PKI is the full-length heat-stable protein kinase inhibitor) (56). However, the dissociation rate constant differs from those previously determined by stochastic sensing (7.1 s–1 in the present work versus 0.19 and 0.2 s–1) (11, 14). The affinities determined in the previous studies also differed substantially from that determined by analytical gel filtration (80 and 130 nM versus 2.3 μM) (57), whereas the Kd value determined here (2.09 μM) corresponds well, suggesting that our determination of the dissociation rate constant is reliable. The origin of the discrepancies in previous stochastic sensing studies is unclear.
An intriguing property of the sensors is that the electrical noise in the kinase-bound states depends on the identity of the kinase (Fig. 4). This phenomenon might be exploited as a means to enhance analyte identification. Inspection of the 3D structures of Pim-1, Pim-2, and PKA (Protein Data Bank ID codes 2BIL, 2IWI, and 2CPK, respectively) suggests these differences may be due to interactions between unstructured regions of the Pim kinase C termini and the pore, although it is debatable whether dynamic behavior can be inferred from static structures. Pim-1 and Pim-2 have C termini orientated on the side of the kinases that is expected to face the pore in the bound state, with the last 7 and 24 residues, respectively, unresolved in the structures. In contrast, PKA has a longer C-terminal tail that wraps around the surface of the enzyme, contains the turn and hydrophobic motifs common to AGC family kinases, and terminates on the opposite side of the kinase, away from the pore and with no unresolved terminal residues (58). Noise differences between Pim family members may arise from differences of length or charge distribution in their unstructured C termini.
Comparison with Other Methods.
Stochastic detection has several advantages over traditional methods for determining rate and equilibrium constants. Our method does not use spectroscopic phenomena, is label-free, and may be more amenable to parallelization/high throughput than stopped-flow spectroscopy. However, the engineering strategy may limit the scope of interactions studied, as one binding partner must be encoded within the αHL trans loop.
SPR experiments must be performed carefully to avoid rebinding and mass transport effects. Reliable determination of very high association rate constants (>106 M–1⋅s–1, depending on analyte size) and dissociation rate constants outside the range 10−5 to 1 s–1 is difficult (59). In contrast, our method uses a single sensor molecule under steady-state conditions and so does not suffer these limitations. Notably, we were able to determine very rapid association rate constants for Pim kinases (∼107–108 M–1⋅s–1).
Although our method determines equilibrium constants indirectly from the association and dissociation rate constants, the simultaneous determination of rate and equilibrium constants is itself advantageous. If droplet interface bilayers are used, where compartment volumes are typically 200 nL or less (60), analyte consumption in stochastic sensing can be very low (a few ng in 200 nL droplets) compared with ITC (61). Our method might also be coupled with high-throughput single-channel recording methods (62–65). Finally, it is possible to estimate the entropic and enthalpic contributions to the change in free energy upon binding by measuring the temperature dependence of the equilibrium dissociation constant according to the van’t Hoff equation (66–68).
Electrostatic Enhancement of Pim-1–Pimtide Association.
The measured association rate constants for all three Pim kinases with Pimtide were one to two orders of magnitude higher than that of PKA for PKIP5–24 and were near the top of the range typically measured for protein–protein interactions (51). Studies on other very fast protein–protein interactions have concluded that the dominant factor that enhances association rates is the presence of long-range electrostatic interactions between binding partners (51). We observed a strong decrease in the Pim-1–Pimtide association rate constant with increasing ionic strength, as seen for other protein–protein interactions with electrostatically enhanced association rates (52).
Pimtide is a highly optimized Pim kinase substrate obtained from a peptide library screen, whereas known natural substrates bind with lower affinity. Some do, however, possess high net charge similar to Pimtide [e.g., p21tide: RKRRQTSMTD, PAP-1tide: KKRKHKASKSS (38)]. Compared with Pim-1, PKA does not possess the same extensive distribution of negative electrostatic potential across its binding pocket (Fig. S9), and PKIP5–24 has low net charge. Pim kinases may therefore be distinctly capable of potentially rapid electrostatically enhanced substrate association rates.
We propose that substrate association rates and their electrostatic modulation may be important when considering signaling pathways at the systems level where several proteins or pathways compete. For instance, the transient expression of a kinase with higher association rate for a particular shared substrate than another kinase could lead to the faster kinase outcompeting the slower for that substrate, driving the slower kinase toward a “second-preference” substrate, and hence effecting a shift between signaling pathways. However, very few kinase substrate-binding rate constants have so far been determined. Our approach could enable a detailed investigation of the contribution of electrostatic enhancement to the overall control of signaling through the study of binding kinetics for a range of protein kinases and natural substrates.
In conclusion, we have developed a versatile, label-free method for measuring the kinetics and affinities of kinase–substrate interactions and have applied it to the interaction of Pim kinases with the consensus substrate Pimtide and the interaction of PKA with the inhibitory peptide PKIP5–24. We thus identified electrostatic enhancement in Pim-1–Pimtide association, a phenomenon that may be important in the cellular context.
We envisage several extensions of the method. First, it may be possible to specifically screen for type II kinase inhibitors, as their stabilization of the inactive “DFG-out” kinase conformation (where DFG refers to a conserved motif in the activation loop) should interfere with substrate binding. Type I inhibitors would be expected to occupy the ATP-binding pocket but not significantly interfere with substrate binding. Second, it should be possible to study phosphorylation of the Pimtide sensor element when MgATP is present. In addition, it would be desirable to establish turnover of the substrate by the addition of a suitable protein phosphatase. Finally, it should be possible to generate a pseudosubstrate sensor element by mutation of the Pimtide phosphoacceptor serine residue and hence study the interaction of Pim kinases with the sensor in the presence of nucleotides, but in the absence of phosphorylation. Previous measurements with PKA have shown a synergistic effect between MgATP and peptide binding (11, 14, 57), and it may be possible to measure the modulation of this effect by ATP-competitive (type I) inhibitors.
Materials and Methods
All chemicals, reagents, and oligonucleotides were from Sigma Aldrich unless otherwise indicated.
Pore Engineering.
pT7–αHL was described previously (69). pT7–αHL–PLM–D8 was prepared from pT7–αHL–RL4–D8 (11) by cassette mutagenesis. A DNA cassette encoding the Pimtide sequence, flanking glycine/serine linkers, and AgeI and StuI sites at the 5′ and 3′ ends, respectively, was prepared by assembly PCR using the following oligonucleotides: 5′-GCGCGCACCGGTGATGATAG-3′, 5′-GCCGCTACTGCCGCTTCCGCTGCTATCATCACCGGTGCGC-3′, 5′-AGCGGCAGTAGCGGCGCACGTAAACGTCGTCGTCATCCGA-3′, 5′-GCTACCCGCGGTCGGTGGGCCGCTCGGATGACGACGACGT-3′, 5′-CCGACCGCGGGTAGCTCCGGGAGCGGCTCTAGCAAAATTG-3′, and 5′-CCCGGGAGGCCTCCAATTTTGCTAGAGCCGCTC-3′. The cassette was digested with AgeI and StuI (New England Biolabs) and ligated into pT7–αHL–RL4–D8 that had also been digested with AgeI and StuI.
pT7–αHL–D127N was generated from pT7–αHL by in vivo homologous recombination (70, 71). Two PCR reactions were performed using pT7–αHL as the template. For the first reaction, the template was linearized by NdeI (New England Biolabs) before PCR using the forward mutagenic primer: 5′-GTTACTGGTAATGATACAGGAAAAATTGGC-3′ and the reverse nonmutagenic primer (SC47): 5′-CAGAAGTGGTCCTGCAACTTTAT-3′. The second PCR used template linearized by HindIII; the reverse mutagenic primer was: 5′-GCCAATTTTTCCTGTATCATTACCAGTAAC-3′, and the forward nonmutagenic primer (SC46) was 5′-ATAAAGTTGCAGGACCACTTCTG-3′. PCR was performed with Phusion Flash HF Master Mix (New England Biolabs) using the cycling conditions of the manufacturer. Equal volumes of each PCR (typically 5 μL) were then mixed and transformed into chemically competent XL-10 Gold cells (Novagen), which were subsequently spread onto LB (Lysogeny Broth) carbenicillin plates and incubated at 37 °C overnight. Plasmid DNA was isolated from several colonies taken from the plates. Successful constructs were confirmed by DNA sequencing.
pT7–αHL–D127N–PLM–D8 was generated from pT7–αHL–PLM–D8 by in vivo homologous recombination. The forward mutagenic primer was 5′-GTCACCGGTAATGATAGCAGCGGAAG-3′, and the reverse mutagenic primer was 5′-GCTTCCGCTGCTATCATTACCGGTGAC-3′. The nonmutagenic primers were SC47 and SC46 as above.
pT7–αHL–D127N–PLM–TEV–D8 was generated from pT7–αHL–D127N–PLM–D8 by in vivo homologous recombination. The forward mutagenic primer was 5′-GAAAATCTGTATTTTCAAGGGGGTAGCTCCGGGAGCGGC-3′, and the reverse mutagenic primer was 5′-CCCTTGAAAATACAGATTTTCCGCGGTCGGTGGGCCGCT-3′. The nonmutagenic primers were SC47 and SC46 as above.
pT7–αHL–PKIP–TEV–D8 was generated from pT7–αHL–PKIP–D8 (11) by in vivo homologous recombination. The forward mutagenic primer was 5′-AAAGAAAATCTGTATTTTCAAGGGGGCAGCAGCGGCAGCGGC-3′, and the reverse mutagenic primer was 5′-CCCTTGAAAATACAGATTTTCTTTATCATGAATCGCATTGCGACG-3′. The nonmutagenic primers were SC47 and SC46 as above.
Pore Synthesis.
Engineered αHL pores were produced using the S30 T7 High-Yield Protein Expression System (Promega), a coupled IVTT system. A standard reaction comprises: 500 ng plasmid DNA, 10 μL S30 Premix Plus (supplied with kit), 9 μL S30 extract (supplied with kit), 1 μL l-[35S]methionine (1,175 Ci⋅mmol–1, 10 mCi⋅mL–1, MP Biomedicals), and sufficient nuclease-free water to achieve a total reaction volume of 25 μL. To suppress endogenous expression, the S30 extract was pretreated with rifampicin (1 μg⋅mL–1 final) before addition to the IVTT reaction. Reaction mixtures were incubated at 37 °C with shaking at 1,200 rpm in a Thermomixer Comfort (Eppendorf) for 1 h. Mutant αHL homoheptamers were produced in 25-μL reactions supplemented with 3 μL of rabbit erythrocyte membranes [∼1 mg (protein)⋅mL–1]. After incubation, reactions were centrifuged for 10 min at 25,000 × g. The supernatant was removed and the pellet resuspended in 200 μL MBSA buffer (10 mM Mops, pH 7.4, 150 mM NaCl, 1 mg⋅mL–1 BSA). The suspension was then centrifuged and washed once more before being centrifuged a final time. The resulting pellet was resuspended in 50 μL of 1X Laemmli sample buffer [62.5 mM Tris⋅HCl, pH 6.8, 2.3% (wt/vol) SDS, 5% (vol/vol) β-mercaptoethanol, 10% (wt/vol) glycerol] and then electrophoresed in a 5% SDS/PAGE gel at 80 V overnight (∼18–20 h).
To produce heteroheptamers, 100-μL-scale reactions were set up with the two different plasmids present in a 6:2 ratio, where the higher-concentration plasmid is the αHL mutant that does not contain the sensing element, and the lower-concentration plasmid encodes αHL bearing the sensor element and a C-terminal D8 tag for the separation of oligomers of different stoichiometry by gel mobility shift (11, 72). The procedure was then the same as for homoheptamers.
To extract homo- and heteroheptameric pores after electrophoresis, the gel was dried on Whatman 3M filter paper under vacuum for 3–4 h at room temperature. The dried gel was autoradiographed using Kodak BioMax MR film. With the aid of an aligned autoradiograph, the desired bands were excised from the gel with a scalpel. Homoheptamers appear as a single band. Heteroheptamers appear as a ladder of bands due to the varying stoichiometries of incorporated D8-tagged sensor subunits, which produce a downward gel shift. Each excised gel slice was rehydrated in 500 μL TE buffer (10 mM Tris⋅HCl, pH 8.0, 1 mM EDTA) for 1 h at room temperature. The backing filter paper was then removed, and the rehydrated gel was macerated with a pestle. The resulting mixture was filtered through a 0.2-μm cellulose acetate filter (Rainin Microfilterfuge tube). The filtrate was aliquoted in 10-μL portions and stored at –80 °C.
To produce TEV protease-cleaved heteroheptamer pores, two IVTT reactions were first prepared: a 75-μL reaction containing DNA encoding the nonsensor subunit and a 25-μL reaction containing DNA encoding the protease-cleavable sensor subunit. After incubation, as described above, translation was stopped in both reactions by the addition of chloramphenicol to a final concentration of 34 μg⋅mL–1. AcTEV protease (10 units, Invitrogen) was added to the sensor subunit reaction, which was then incubated at 4 °C for 4 h. The two tubes were then mixed, followed by the addition of rabbit erythrocyte membranes (5 μL, 1 mg⋅mL–1). The resulting mixture was incubated at room temperature for 1 h before recovery of the membranes by centrifugation for 10 min at 25,000 × g. The membrane pellet was then washed twice with MBSA before resuspension in Laemmli sample buffer and SDS/PAGE purification of the assembled αHL pores as described above.
Expression and Purification of Kinases.
The catalytic subunit of murine cAMP-dependent protein kinase (α isoform) was purchased from New England Biolabs and was supplied at a concentration of 0.13 mg⋅mL–1.
Pim kinases were expressed and purified from Escherichia coli by affinity and size-exclusion chromatography (SI Materials and Methods). Pim kinase concentrations were obtained from their absorbances at 280 nm as determined with a NanoDrop 1000 (Thermo Scientific), using the following molecular weights (Mr) and extinction coefficients (ε): Pim-1 Mr = 35,546 Da, ε = 48,930 M–1⋅cm–1; Pim-2 Mr = 34,277 Da, ε = 47,440 M–1⋅cm–1; Pim-3 Mr = 36,830 Da, ε = 47,440 M–1⋅cm–1. All measurements were made in quadruplicate.
Single-Channel Recording.
Single-channel recording was performed by using the method of Montal and Mueller (73). A 25-μm-thick Teflon film containing an aperture 60 μm in diameter separated two Delrin chambers. Before bilayer formation, the aperture was pretreated with 1 μL of 1% (vol/vol) hexadecane in pentane on each side. Each chamber was then filled with 1 mL of buffer solution, which comprised 15 mM Mops, pH 6.8, 300 mM KCl, and 5 mM DTT, except where PKA was used, in which case DTT was omitted. Mops-based buffers were titrated to the desired pH with 1 M KOH; 1,2-diphytanoyl-sn-glycero-3-phosphocholine (DPhPC) in pentane (5 μL, 10 mg⋅mL–1) was then dropped onto the buffer surface of each chamber. After 5 min, lipid bilayers were folded across the Teflon aperture by the repeated raising and lowering of the buffer level above and below the aperture in each chamber by pipetting. Measurements were made at 21 ± 1 °C. Electrical currents were measured with two Ag/AgCl electrodes, each contained within salt bridges formed of 3 M KCl in 3% (wt/vol) low-melt agarose. The electrodes were connected to the headstage of a patch-clamp amplifier (Axopatch 200B, Molecular Devices) operating in voltage-clamp mode. The cis chamber was defined as the chamber connected to the grounded electrode and to which αHL protein was added. The trans chamber was connected to the working electrode. Current signals were filtered with a low-pass Bessel filter (80 dB/decade) with a corner frequency of 2 kHz. Signals were digitized by using a Digidata 1320A digitizer (Molecular Devices), connected to a computer running the pCLAMP 9.2 software suite (Molecular Devices). Current signals were sampled at a frequency of 10 kHz. To obtain the insertion of a single engineered αHL pore, 0.1–0.5 μL of protein (typically ∼1 ng⋅μL–1) was added to the cis chamber. A potential of +150 mV was then applied with stirring of the cis chamber until pore insertion was observed as a step increase in current.
Single-Channel Current Analysis.
Single-channel data were analyzed with QuB 2.0 software (www.qub.buffalo.edu). Current traces were idealized by using the segmental k-means algorithm (74) of QuB, according to the kinetic models described (Fig. 3C). Dwell time analysis and rate constant estimation were performed by using the maximum interval likelihood algorithm of QuB (75), with retrospective application of a dead time of 300 μs for approximate correction of missed events due to low-pass filtering. In a few cases, where significant numbers of events were near the limit of time resolution, a shorter dead time of 200 μs was used to minimize errors due to missed events and improve histogram fitting. Additionally, a higher dead time of 1 ms was used for voltage dependence data obtained at –20 and –30 mV to minimize the effect of short, false events caused by difficulties in idealizing traces where the open pore current is very small.
Kinetic analysis of the interaction of Pim-3 with αHL–D127N–PLM–TEV required a modification of the model shown in Fig. 3C, because the noise (seen for all Pim kinase-bound states) was sufficiently resolved in this case to form transitions to an additional blocked level. Hence, we added a blocked state, B2, which was connected to the kinase-bound open state O2. The overall kinetic model was of the form: B1 ⇔ O1 ⇔ O2 ⇔ B2, where states other than B2 are the same as in Fig. 3C.
Duration histograms were made from logarithmically binned durations with square-root y ordinates (76). OriginPro 8.5.1 (OriginLab) was used for the plotting, fitting, and presentation of data. Fitting was performed without error weighting. Numerical data are reported as mean ± SD, unless otherwise indicated. All statistical tests were performed with an alpha value of 0.05. Significance testing (Student's t test) for the voltage dependence of kinase binding was performed on constituent independent data points rather than the means that are shown in Fig. S7 A–C. t test values are reported with degrees of freedom in parentheses.
For spectral analysis, several hundred events corresponding to the kinase-bound state were manually extracted from a single recording by using Clampfit 10.3 (Molecular Devices). The extracted events were concatenated to form a single continuous trace. The baseline was then adjusted by subtracting the mean current value of the concatenated trace. Power spectra were calculated in Clampfit using segment lengths of 4,096 samples (spectral resolution 2.44 Hz), to which were applied Hamming window functions and which were averaged together with 50% window overlap. The resulting power spectral densities for all four protein kinases were then plotted in OriginPro.
Ionic-Strength Dependence of Pim-1 Association.
Experiments were performed in 15 mM Mops, pH 6.8, with the ionic strength adjusted with KCl at 20 ± 1 °C. The ionic strength contribution from the Mops was ∼4 mM. The Pim-1 concentration used in all cases was 182 nM. 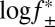 was calculated according to the extended Debye–Hückel equation (77, 78):
was calculated according to the extended Debye–Hückel equation (77, 78):
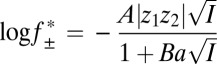 |
where I is the ionic strength of the solution, z1 and z2 are the charges of the anion and cation, A and B are constants dependent on the temperature and dielectric constant of the solution (78), and a is the ion size parameter. Values of 0.5046 dm3/2⋅mol–1/2 and 3.276 × 108 dm1/2⋅mol–1/2 were used for A and B, respectively (79). A value of 4.5 Å was used for a (note: the units of a are dm in the above equation). Schreiber et al. (52) used a value of 5.6 Å for a, which is somewhat larger than literature values for the hydrated diameters of Na+ and Cl–, but which gave the best fit to their data. To be consistent, we calculated our value of a for KCl by scaling the NaCl value by a factor of 0.8 to account for the smaller hydrated diameter of K+ vs. Na+ (80). A justification for the use of the extended Debye–Hückel equation at the high electrolyte concentrations used here is given in Schreiber et al. (52) and should hold for KCl as well as for NaCl.
Visualization of Electrostatic Potentials.
Electrostatic potentials were calculated with APBS (53) and visualized in VMD (54) (SI Materials and Methods).
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health and Oxford Nanopore Technologies. L.H. was supported in part by a Biotechnology and Biological Sciences Research Council doctoral training grant. S.K. and L.T.A. are supported by the Structural Genomics Consortium, a registered charity (1097737) that receives funds from AbbVie, Boehringer Ingelheim, the Canada Foundation for Innovation, the Canadian Institutes for Health Research, Genome Canada, GlaxoSmithKline, Janssen, Lilly Canada, the Novartis Research Foundation, the Ontario Ministry of Economic Development and Innovation, Pfizer, Takeda, and the Wellcome Trust.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1312739110/-/DCSupplemental.
References
- 1.Bayley H, Cremer PS. Stochastic sensors inspired by biology. Nature. 2001;413(6852):226–230. doi: 10.1038/35093038. [DOI] [PubMed] [Google Scholar]
- 2.Howorka S, Siwy Z. Nanopore analytics: Sensing of single molecules. Chem Soc Rev. 2009;38(8):2360–2384. doi: 10.1039/b813796j. [DOI] [PubMed] [Google Scholar]
- 3.Majd S, et al. Applications of biological pores in nanomedicine, sensing, and nanoelectronics. Curr Opin Biotechnol. 2010;21(4):439–476. doi: 10.1016/j.copbio.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Astier Y, Braha O, Bayley H. Toward single molecule DNA sequencing: direct identification of ribonucleoside and deoxyribonucleoside 5′-monophosphates by using an engineered protein nanopore equipped with a molecular adapter. J Am Chem Soc. 2006;128(5):1705–1710. doi: 10.1021/ja057123+. [DOI] [PubMed] [Google Scholar]
- 5.Wu H-C, Astier Y, Maglia G, Mikhailova E, Bayley H. Protein nanopores with covalently attached molecular adapters. J Am Chem Soc. 2007;129(51):16142–16148. doi: 10.1021/ja0761840. [DOI] [PubMed] [Google Scholar]
- 6.Hammerstein AF, Shin S-H, Bayley H. Single-molecule kinetics of two-step divalent cation chelation. Angew Chem Int Ed Engl. 2010;49(30):5085–5090. doi: 10.1002/anie.200906601. [DOI] [PubMed] [Google Scholar]
- 7.Cheley S, Gu LQ, Bayley H. Stochastic sensing of nanomolar inositol 1,4,5-trisphosphate with an engineered pore. Chem Biol. 2002;9(7):829–838. doi: 10.1016/s1074-5521(02)00172-2. [DOI] [PubMed] [Google Scholar]
- 8.Guan X, Gu LQ, Cheley S, Braha O, Bayley H. Stochastic sensing of TNT with a genetically engineered pore. ChemBioChem. 2005;6(10):1875–1881. doi: 10.1002/cbic.200500064. [DOI] [PubMed] [Google Scholar]
- 9.Shin S-H, Luchian T, Cheley S, Braha O, Bayley H. Kinetics of a reversible covalent-bond-forming reaction observed at the single-molecule level. Angew Chem Int Ed Engl. 2002;41(19):3707–3709, 3523. doi: 10.1002/1521-3773(20021004)41:19<3707::AID-ANIE3707>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 10.Wu H-C, Bayley H. Single-molecule detection of nitrogen mustards by covalent reaction within a protein nanopore. J Am Chem Soc. 2008;130(21):6813–6819. doi: 10.1021/ja8004607. [DOI] [PubMed] [Google Scholar]
- 11.Cheley S, Xie H, Bayley H. A genetically encoded pore for the stochastic detection of a protein kinase. ChemBioChem. 2006;7(12):1923–1927. doi: 10.1002/cbic.200600274. [DOI] [PubMed] [Google Scholar]
- 12.Howorka S, Nam J, Bayley H, Kahne D. Stochastic detection of monovalent and bivalent protein-ligand interactions. Angew Chem Int Ed Engl. 2004;43(7):842–846. doi: 10.1002/anie.200352614. [DOI] [PubMed] [Google Scholar]
- 13.Rotem D, Jayasinghe L, Salichou M, Bayley H. Protein detection by nanopores equipped with aptamers. J Am Chem Soc. 2012;134(5):2781–2787. doi: 10.1021/ja2105653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie H, Braha O, Gu L-Q, Cheley S, Bayley H. Single-molecule observation of the catalytic subunit of cAMP-dependent protein kinase binding to an inhibitor peptide. Chem Biol. 2005;12(1):109–120. doi: 10.1016/j.chembiol.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Wei R, Gatterdam V, Wieneke R, Tampé R, Rant U. Stochastic sensing of proteins with receptor-modified solid-state nanopores. Nat Nanotechnol. 2012;7(4):257–263. doi: 10.1038/nnano.2012.24. [DOI] [PubMed] [Google Scholar]
- 16.Bezrukov SM, Vodyanoy I, Parsegian VA. Counting polymers moving through a single ion channel. Nature. 1994;370(6487):279–281. doi: 10.1038/370279a0. [DOI] [PubMed] [Google Scholar]
- 17.Movileanu L, Schmittschmitt JP, Scholtz JM, Bayley H. Interactions of peptides with a protein pore. Biophys J. 2005;89(2):1030–1045. doi: 10.1529/biophysj.104.057406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robertson JWF, et al. Single-molecule mass spectrometry in solution using a solitary nanopore. Proc Natl Acad Sci USA. 2007;104(20):8207–8211. doi: 10.1073/pnas.0611085104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez-Larrea D, Bayley H. Multistep protein unfolding during nanopore translocation. Nat Nanotechnol. 2013;8(4):288–295. doi: 10.1038/nnano.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasianowicz JJ, Brandin E, Branton D, Deamer DW. Characterization of individual polynucleotide molecules using a membrane channel. Proc Natl Acad Sci USA. 1996;93(24):13770–13773. doi: 10.1073/pnas.93.24.13770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cherf GM, et al. Automated forward and reverse ratcheting of DNA in a nanopore at 5-Å precision. Nat Biotechnol. 2012;30(4):344–348. doi: 10.1038/nbt.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayden EC. Nanopore genome sequencer makes its debut. Nature. 2012 doi: 10.1038/nature.2012.10051. [DOI] [Google Scholar]
- 23.Manrao EA, et al. Reading DNA at single-nucleotide resolution with a mutant MspA nanopore and phi29 DNA polymerase. Nat Biotechnol. 2012;30(4):349–353. doi: 10.1038/nbt.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pennisi E. Genome sequencing. Search for pore-fection. Science. 2012;336(6081):534–537. doi: 10.1126/science.336.6081.534. [DOI] [PubMed] [Google Scholar]
- 25.Stoddart D, Heron AJ, Mikhailova E, Maglia G, Bayley H. Single-nucleotide discrimination in immobilized DNA oligonucleotides with a biological nanopore. Proc Natl Acad Sci USA. 2009;106(19):7702–7707. doi: 10.1073/pnas.0901054106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H, Dunning JE, Huang AP, Nyamwanda JA, Branton D. DNA heterogeneity and phosphorylation unveiled by single-molecule electrophoresis. Proc Natl Acad Sci USA. 2004;101(37):13472–13477. doi: 10.1073/pnas.0405568101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hornblower B, et al. Single-molecule analysis of DNA-protein complexes using nanopores. Nat Methods. 2007;4(4):315–317. doi: 10.1038/nmeth1021. [DOI] [PubMed] [Google Scholar]
- 28.Nivala J, Marks DB, Akeson M. Unfoldase-mediated protein translocation through an α-hemolysin nanopore. Nat Biotechnol. 2013;31(3):247–250. doi: 10.1038/nbt.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298(5600):1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 30.Blanco-Aparicio C, Carnero A. Pim kinases in cancer: Diagnostic, prognostic and treatment opportunities. Biochem Pharmacol. 2013;85(5):629–643. doi: 10.1016/j.bcp.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 31.Allen JD, Verhoeven E, Domen J, van der Valk M, Berns A. Pim-2 transgene induces lymphoid tumors, exhibiting potent synergy with c-myc. Oncogene. 1997;15(10):1133–1141. doi: 10.1038/sj.onc.1201288. [DOI] [PubMed] [Google Scholar]
- 32.Bachmann M, Möröy T. The serine/threonine kinase Pim-1. Int J Biochem Cell Biol. 2005;37(4):726–730. doi: 10.1016/j.biocel.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 33.Amson R, et al. The human protooncogene product p33pim is expressed during fetal hematopoiesis and in diverse leukemias. Proc Natl Acad Sci USA. 1989;86(22):8857–8861. doi: 10.1073/pnas.86.22.8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen AM, et al. Increased expression of the hPim-2 gene in human chronic lymphocytic leukemia and non-Hodgkin lymphoma. Leuk Lymphoma. 2004;45(5):951–955. doi: 10.1080/10428190310001641251. [DOI] [PubMed] [Google Scholar]
- 35.Dhanasekaran SM, et al. Delineation of prognostic biomarkers in prostate cancer. Nature. 2001;412(6849):822–826. doi: 10.1038/35090585. [DOI] [PubMed] [Google Scholar]
- 36.Ellwood-Yen K, et al. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell. 2003;4(3):223–238. doi: 10.1016/s1535-6108(03)00197-1. [DOI] [PubMed] [Google Scholar]
- 37.Fujii C, et al. Aberrant expression of serine/threonine kinase Pim-3 in hepatocellular carcinoma development and its role in the proliferation of human hepatoma cell lines. Int J Cancer. 2005;114(2):209–218. doi: 10.1002/ijc.20719. [DOI] [PubMed] [Google Scholar]
- 38.Bullock AN, Debreczeni J, Amos AL, Knapp S, Turk BE. Structure and substrate specificity of the Pim-1 kinase. J Biol Chem. 2005;280(50):41675–41682. doi: 10.1074/jbc.M510711200. [DOI] [PubMed] [Google Scholar]
- 39.Bullock AN, et al. Crystal structure of the PIM2 kinase in complex with an organoruthenium inhibitor. PLoS ONE. 2009;4(10):e7112. doi: 10.1371/journal.pone.0007112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacobs MD, et al. Pim-1 ligand-bound structures reveal the mechanism of serine/threonine kinase inhibition by LY294002. J Biol Chem. 2005;280(14):13728–13734. doi: 10.1074/jbc.M413155200. [DOI] [PubMed] [Google Scholar]
- 41.Kumar A, et al. Crystal structures of proto-oncogene kinase Pim1: a target of aberrant somatic hypermutations in diffuse large cell lymphoma. J Mol Biol. 2005;348(1):183–193. doi: 10.1016/j.jmb.2005.02.039. [DOI] [PubMed] [Google Scholar]
- 42.Qian KC, et al. Structural basis of constitutive activity and a unique nucleotide binding mode of human Pim-1 kinase. J Biol Chem. 2005;280(7):6130–6137. doi: 10.1074/jbc.M409123200. [DOI] [PubMed] [Google Scholar]
- 43.Drygin D, Haddach M, Pierre F, Ryckman DM. Potential use of selective and nonselective Pim kinase inhibitors for cancer therapy. J Med Chem. 2012;55(19):8199–8208. doi: 10.1021/jm3009234. [DOI] [PubMed] [Google Scholar]
- 44.Hutti JE, et al. A rapid method for determining protein kinase phosphorylation specificity. Nat Methods. 2004;1(1):27–29. doi: 10.1038/nmeth708. [DOI] [PubMed] [Google Scholar]
- 45.Howorka S, et al. A protein pore with a single polymer chain tethered within the lumen. J Am Chem Soc. 2000;122(11):2411–2416. [Google Scholar]
- 46.Movileanu L, Howorka S, Braha O, Bayley H. Detecting protein analytes that modulate transmembrane movement of a polymer chain within a single protein pore. Nat Biotechnol. 2000;18(10):1091–1095. doi: 10.1038/80295. [DOI] [PubMed] [Google Scholar]
- 47.Lew J, Coruh N, Tsigelny I, Garrod S, Taylor SS. Synergistic binding of nucleotides and inhibitors to cAMP-dependent protein kinase examined by acrylodan fluorescence spectroscopy. J Biol Chem. 1997;272(3):1507–1513. doi: 10.1074/jbc.272.3.1507. [DOI] [PubMed] [Google Scholar]
- 48.Rininsland F, et al. Metal ion-mediated polymer superquenching for highly sensitive detection of kinase and phosphatase activities. Proc Natl Acad Sci USA. 2004;101(43):15295–15300. doi: 10.1073/pnas.0406832101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng HC, et al. A potent synthetic peptide inhibitor of the cAMP-dependent protein kinase. J Biol Chem. 1986;261(3):989–992. [PubMed] [Google Scholar]
- 50.Northrup SH, Erickson HP. Kinetics of protein-protein association explained by Brownian dynamics computer simulation. Proc Natl Acad Sci USA. 1992;89(8):3338–3342. doi: 10.1073/pnas.89.8.3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schreiber G, Haran G, Zhou H-X. Fundamental aspects of protein-protein association kinetics. Chem Rev. 2009;109(3):839–860. doi: 10.1021/cr800373w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schreiber G, Fersht AR. Rapid, electrostatically assisted association of proteins. Nat Struct Biol. 1996;3(5):427–431. doi: 10.1038/nsb0596-427. [DOI] [PubMed] [Google Scholar]
- 53.Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proc Natl Acad Sci USA. 2001;98(18):10037–10041. doi: 10.1073/pnas.181342398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Humphrey W, Dalke A, Schulten K. VMD: Visual molecular dynamics. J Mol Graph. 1996;14(1):27–28. 33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 55.Hall AR, et al. Hybrid pore formation by directed insertion of α-haemolysin into solid-state nanopores. Nat Nanotechnol. 2010;5(12):874–877. doi: 10.1038/nnano.2010.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zimmermann B, Chiorini JA, Ma Y, Kotin RM, Herberg FW. PrKX is a novel catalytic subunit of the cAMP-dependent protein kinase regulated by the regulatory subunit type I. J Biol Chem. 1999;274(9):5370–5378. doi: 10.1074/jbc.274.9.5370. [DOI] [PubMed] [Google Scholar]
- 57.Herberg FW, Taylor SS. Physiological inhibitors of the catalytic subunit of cAMP-dependent protein kinase: Effect of MgATP on protein-protein interactions. Biochemistry. 1993;32(50):14015–14022. doi: 10.1021/bi00213a035. [DOI] [PubMed] [Google Scholar]
- 58.Knighton DR, et al. Crystal structure of the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science. 1991;253(5018):407–414. doi: 10.1126/science.1862342. [DOI] [PubMed] [Google Scholar]
- 59.van der Merwe PA. Surface plasmon resonance. In: Harding S, Chowdry B, editors. Protein-Ligand Interactions: Hydrodynamics and Calorimetry. Oxford, UK: Oxford Univ Press; 2001. pp. 137–170. [Google Scholar]
- 60.Bayley H, et al. Droplet interface bilayers. Mol Biosyst. 2008;4(12):1191–1208. doi: 10.1039/b808893d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pierce MM, Raman CS, Nall BT. Isothermal titration calorimetry of protein-protein interactions. Methods. 1999;19(2):213–221. doi: 10.1006/meth.1999.0852. [DOI] [PubMed] [Google Scholar]
- 62.Baaken G, Ankri N, Schuler A-K, Rühe J, Behrends JC. Nanopore-based single-molecule mass spectrometry on a lipid membrane microarray. ACS Nano. 2011;5(10):8080–8088. doi: 10.1021/nn202670z. [DOI] [PubMed] [Google Scholar]
- 63.Le Pioufle B, Suzuki H, Tabata KV, Noji H, Takeuchi S. Lipid bilayer microarray for parallel recording of transmembrane ion currents. Anal Chem. 2008;80(1):328–332. doi: 10.1021/ac7016635. [DOI] [PubMed] [Google Scholar]
- 64.Syeda R, Holden MA, Hwang WL, Bayley H. Screening blockers against a potassium channel with a droplet interface bilayer array. J Am Chem Soc. 2008;130(46):15543–15548. doi: 10.1021/ja804968g. [DOI] [PubMed] [Google Scholar]
- 65.Kawano R, et al. Automated parallel recordings of topologically identified single ion channels. Sci Rep. 2013;3:1995. doi: 10.1038/srep01995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Howorka S, Movileanu L, Braha O, Bayley H. Kinetics of duplex formation for individual DNA strands within a single protein nanopore. Proc Natl Acad Sci USA. 2001;98(23):12996–13001. doi: 10.1073/pnas.231434698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kang X-F, Gu L-Q, Cheley S, Bayley H. Single protein pores containing molecular adapters at high temperatures. Angew Chem Int Ed Engl. 2005;44(10):1495–1499. doi: 10.1002/anie.200461885. [DOI] [PubMed] [Google Scholar]
- 68.Nestorovich EM, Karginov VA, Berezhkovskii AM, Parsegian VA, Bezrukov SM. Kinetics and thermodynamics of binding reactions as exemplified by anthrax toxin channel blockage with a cationic cyclodextrin derivative. Proc Natl Acad Sci USA. 2012;109(45):18453–18458. doi: 10.1073/pnas.1208771109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Walker B, Krishnasastry M, Zorn L, Kasianowicz J, Bayley H. Functional expression of the alpha-hemolysin of Staphylococcus aureus in intact Escherichia coli and in cell lysates. Deletion of five C-terminal amino acids selectively impairs hemolytic activity. J Biol Chem. 1992;267(15):10902–10909. [PubMed] [Google Scholar]
- 70.Bubeck P, Winkler M, Bautsch W. Rapid cloning by homologous recombination in vivo. Nucleic Acids Res. 1993;21(15):3601–3602. doi: 10.1093/nar/21.15.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jones DH, Howard BH. A rapid method for recombination and site-specific mutagenesis by placing homologous ends on DNA using polymerase chain reaction. Biotechniques. 1991;10(1):62–66. [PubMed] [Google Scholar]
- 72.Howorka S, Cheley S, Bayley H. Sequence-specific detection of individual DNA strands using engineered nanopores. Nat Biotechnol. 2001;19(7):636–639. doi: 10.1038/90236. [DOI] [PubMed] [Google Scholar]
- 73.Montal M, Mueller P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc Natl Acad Sci USA. 1972;69(12):3561–3566. doi: 10.1073/pnas.69.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qin F. Restoration of single-channel currents using the segmental k-means method based on hidden Markov modeling. Biophys J. 2004;86(3):1488–1501. doi: 10.1016/S0006-3495(04)74217-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qin F, Auerbach A, Sachs F. Estimating single-channel kinetic parameters from idealized patch-clamp data containing missed events. Biophys J. 1996;70(1):264–280. doi: 10.1016/S0006-3495(96)79568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sigworth FJ, Sine SM. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J. 1987;52(6):1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Debye P, Hückel E. Zur Theorie der Elektrolyte. Phys Z. 1923;24:185–206. [Google Scholar]
- 78.Robinson RA, Stokes RH. Electrolyte Solutions. London: Butterworths; 1959. [Google Scholar]
- 79.Manov GG, Bates RG, Hamer WJ, Acree SF. Values of the constants in the Debye-Hückel equation for activity coefficients. J Am Chem Soc. 1943;65(9):1765–1767. [Google Scholar]
- 80.Kielland J. Individual activity coefficients of ions in aqueous solutions. J Am Chem Soc. 1937;59(9):1675–1678. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.