Abstract
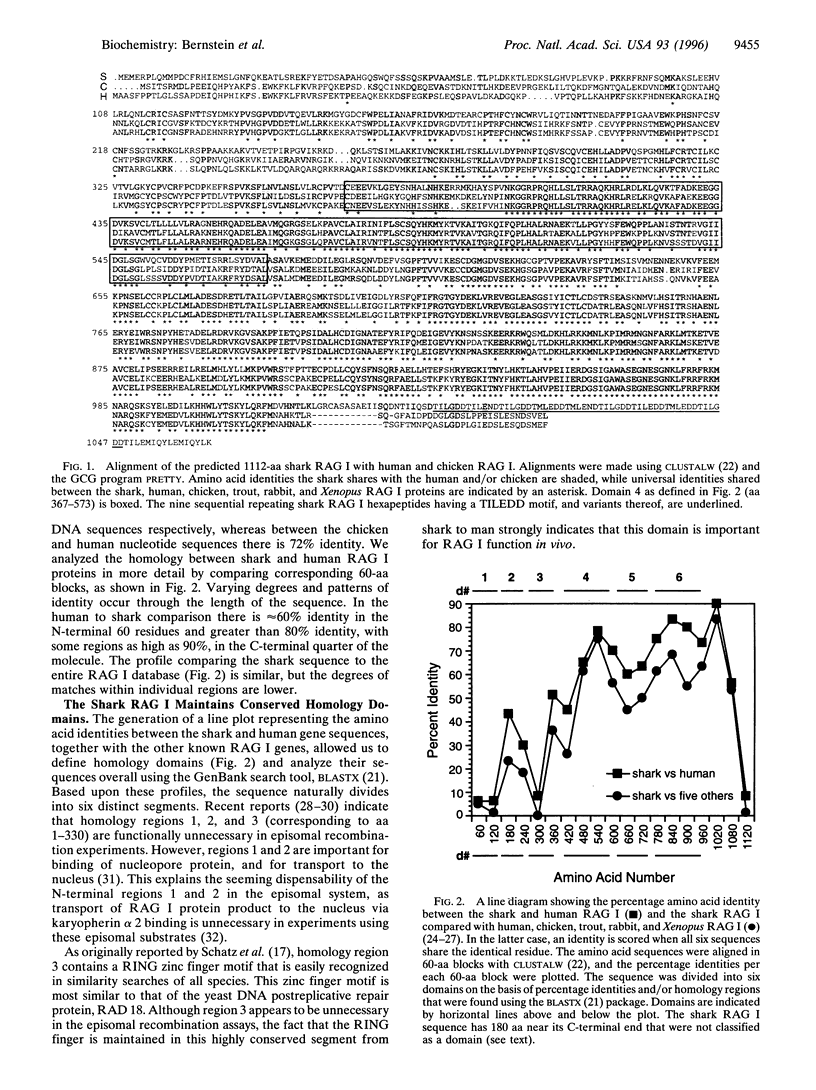
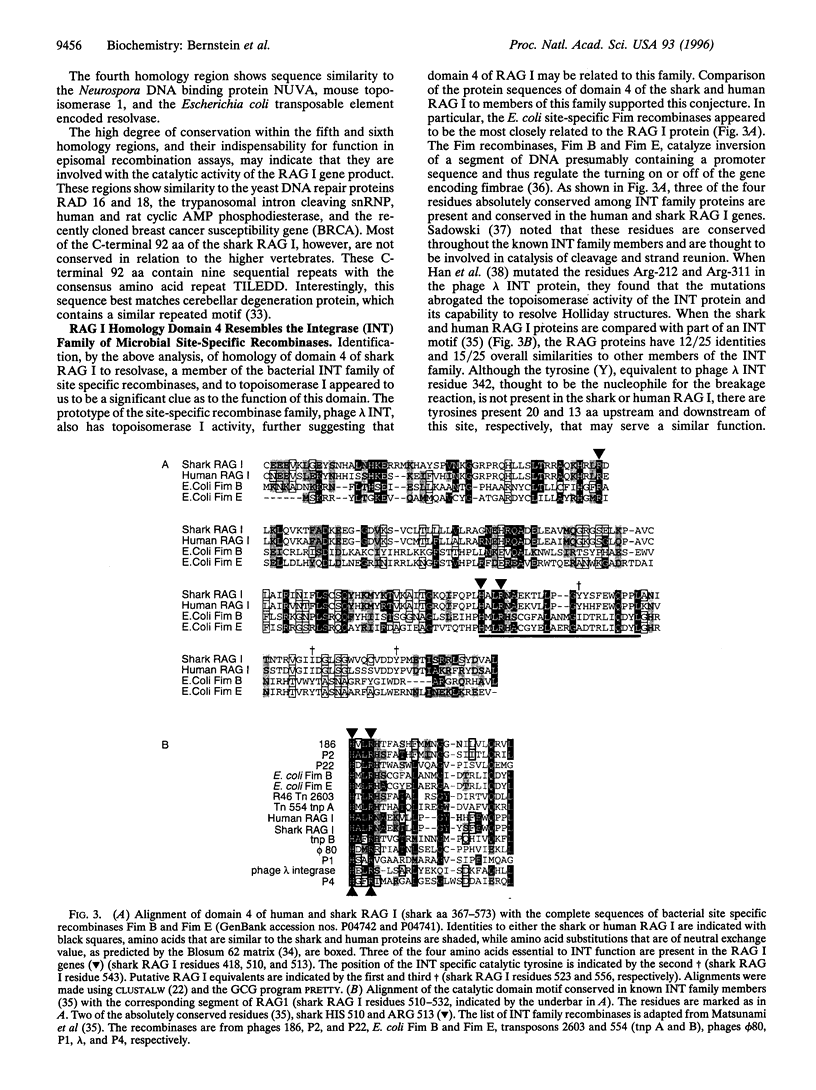
The rearrangement of antibody and T-cell receptor gene segments is indispensable to the vertebrate immune response. All extant jawed vertebrates can rearrange these gene segments. This ability is conferred by the recombination activating genes I and II (RAG I and RAG II). To elucidate their origin and function, the cDNA encoding RAG I from a member of the most ancient class of extant gnathostomes, the Carcharhine sharks, was characterized. Homology domains identified within shark RAG I prompted sequence comparison analyses that suggested similarity of the RAG I and II genes, respectively, to the integrase family genes and integration host factor genes of the bacterial site-specific recombination system. Thus, the apparent explosive evolution (or "big bang") of the ancestral immune system may have been initiated by a transfer of microbial site-specific recombinases.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Amemiya C. T., Ohta Y., Litman R. T., Rast J. P., Haire R. N., Litman G. W. VH gene organization in a relict species, the coelacanth Latimeria chalumnae: evolutionary implications. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6661–6665. doi: 10.1073/pnas.90.14.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein R. M., Schluter S. F., Lake D. F., Marchalonis J. J. Evolutionary conservation and molecular cloning of the recombinase activating gene 1. Biochem Biophys Res Commun. 1994 Nov 30;205(1):687–692. doi: 10.1006/bbrc.1994.2720. [DOI] [PubMed] [Google Scholar]
- Carlson L. M., Oettinger M. A., Schatz D. G., Masteller E. L., Hurley E. A., McCormack W. T., Baltimore D., Thompson C. B. Selective expression of RAG-2 in chicken B cells undergoing immunoglobulin gene conversion. Cell. 1991 Jan 11;64(1):201–208. doi: 10.1016/0092-8674(91)90221-j. [DOI] [PubMed] [Google Scholar]
- Chen J., Shinkai Y., Young F., Alt F. W. Probing immune functions in RAG-deficient mice. Curr Opin Immunol. 1994 Apr;6(2):313–319. doi: 10.1016/0952-7915(94)90107-4. [DOI] [PubMed] [Google Scholar]
- Cuomo C. A., Oettinger M. A. Analysis of regions of RAG-2 important for V(D)J recombination. Nucleic Acids Res. 1994 May 25;22(10):1810–1814. doi: 10.1093/nar/22.10.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dropcho E. J., Chen Y. T., Posner J. B., Old L. J. Cloning of a brain protein identified by autoantibodies from a patient with paraneoplastic cerebellar degeneration. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4552–4556. doi: 10.1073/pnas.84.13.4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D. I. Integration host factor: a protein for all reasons. Cell. 1988 Nov 18;55(4):545–554. doi: 10.1016/0092-8674(88)90213-9. [DOI] [PubMed] [Google Scholar]
- Fuschiotti P., Harindranath N., Mage R. G., McCormack W. T., Dhanarajan P., Roux K. H. Recombination activating genes-1 and -2 of the rabbit: cloning and characterization of germline and expressed genes. Mol Immunol. 1993 Aug;30(11):1021–1032. doi: 10.1016/0161-5890(93)90127-w. [DOI] [PubMed] [Google Scholar]
- Greenhalgh P., Olesen C. E., Steiner L. A. Characterization and expression of recombination activating genes (RAG-1 and RAG-2) in Xenopus laevis. J Immunol. 1993 Sep 15;151(6):3100–3110. [PubMed] [Google Scholar]
- Greenhalgh P., Steiner L. A. Recombination activating gene 1 (Rag1) in zebrafish and shark. Immunogenetics. 1995;41(1):54–55. doi: 10.1007/BF00188438. [DOI] [PubMed] [Google Scholar]
- Han Y. W., Gumport R. I., Gardner J. F. Mapping the functional domains of bacteriophage lambda integrase protein. J Mol Biol. 1994 Jan 21;235(3):908–925. doi: 10.1006/jmbi.1994.1048. [DOI] [PubMed] [Google Scholar]
- Hansen J. D., Kaattari S. L. The recombination activation gene 1 (RAG1) of rainbow trout (Oncorhynchus mykiss): cloning, expression, and phylogenetic analysis. Immunogenetics. 1995;42(3):188–195. doi: 10.1007/BF00191224. [DOI] [PubMed] [Google Scholar]
- Henikoff S., Henikoff J. G. Amino acid substitution matrices from protein blocks. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10915–10919. doi: 10.1073/pnas.89.22.10915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds K. R., Litman G. W. Major reorganization of immunoglobulin VH segmental elements during vertebrate evolution. Nature. 1986 Apr 10;320(6062):546–549. doi: 10.1038/320546a0. [DOI] [PubMed] [Google Scholar]
- Hohman V. S., Schuchman D. B., Schluter S. F., Marchalonis J. J. Genomic clone for sandbar shark lambda light chain: generation of diversity in the absence of gene rearrangement. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):9882–9886. doi: 10.1073/pnas.90.21.9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallenbach S., Brinkmann T., Rougeon F. Rag-1: a topoisomerase? Int Immunol. 1993 Feb;5(2):231–232. doi: 10.1093/intimm/5.2.231. [DOI] [PubMed] [Google Scholar]
- Klein J. Are invertebrates capable of anticipatory immune responses? Scand J Immunol. 1989 May;29(5):499–505. doi: 10.1111/j.1365-3083.1989.tb01152.x. [DOI] [PubMed] [Google Scholar]
- Kokubu F., Litman R., Shamblott M. J., Hinds K., Litman G. W. Diverse organization of immunoglobulin VH gene loci in a primitive vertebrate. EMBO J. 1988 Nov;7(11):3413–3422. doi: 10.1002/j.1460-2075.1988.tb03215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landy A. Dynamic, structural, and regulatory aspects of lambda site-specific recombination. Annu Rev Biochem. 1989;58:913–949. doi: 10.1146/annurev.bi.58.070189.004405. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J., Schluter S. F. Development of an immune system. Ann N Y Acad Sci. 1994 Apr 15;712:1–12. doi: 10.1111/j.1749-6632.1994.tb33557.x. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J., Schluter S. F. On the relevance of invertebrate recognition and defence mechanisms to the emergence of the immune response of vertebrates. Scand J Immunol. 1990 Jul;32(1):13–20. doi: 10.1111/j.1365-3083.1990.tb02886.x. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J., Schluter S. F., Yang H. Y., Hohman V. S., McGee K., Yeaton L. Antigenic cross-reactions among immunoglobulin of diverse vertebrates (elasmobranchs to man) detected using xenoantisera. Comp Biochem Physiol Comp Physiol. 1992 Apr;101(4):675–687. doi: 10.1016/0300-9629(92)90343-o. [DOI] [PubMed] [Google Scholar]
- Matsunami N., Hamaguchi Y., Yamamoto Y., Kuze K., Kangawa K., Matsuo H., Kawaichi M., Honjo T. A protein binding to the J kappa recombination sequence of immunoglobulin genes contains a sequence related to the integrase motif. Nature. 1989 Dec 21;342(6252):934–937. doi: 10.1038/342934a0. [DOI] [PubMed] [Google Scholar]
- McBlane J. F., van Gent D. C., Ramsden D. A., Romeo C., Cuomo C. A., Gellert M., Oettinger M. A. Cleavage at a V(D)J recombination signal requires only RAG1 and RAG2 proteins and occurs in two steps. Cell. 1995 Nov 3;83(3):387–395. doi: 10.1016/0092-8674(95)90116-7. [DOI] [PubMed] [Google Scholar]
- McClain M. S., Blomfield I. C., Eisenstein B. I. Roles of fimB and fimE in site-specific DNA inversion associated with phase variation of type 1 fimbriae in Escherichia coli. J Bacteriol. 1991 Sep;173(17):5308–5314. doi: 10.1128/jb.173.17.5308-5314.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroianu J., Hijikata M., Blobel G., Radu A. Mammalian karyopherin alpha 1 beta and alpha 2 beta heterodimers: alpha 1 or alpha 2 subunit binds nuclear localization signal and beta subunit interacts with peptide repeat-containing nucleoporins. Proc Natl Acad Sci U S A. 1995 Jul 3;92(14):6532–6536. doi: 10.1073/pnas.92.14.6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash H. A., Robertson C. A. Heteroduplex substrates for bacteriophage lambda site-specific recombination: cleavage and strand transfer products. EMBO J. 1989 Nov;8(11):3523–3533. doi: 10.1002/j.1460-2075.1989.tb08518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oettinger M. A., Schatz D. G., Gorka C., Baltimore D. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science. 1990 Jun 22;248(4962):1517–1523. doi: 10.1126/science.2360047. [DOI] [PubMed] [Google Scholar]
- Rast J. P., Anderson M. K., Ota T., Litman R. T., Margittai M., Shamblott M. J., Litman G. W. Immunoglobulin light chain class multiplicity and alternative organizational forms in early vertebrate phylogeny. Immunogenetics. 1994;40(2):83–99. doi: 10.1007/BF00188170. [DOI] [PubMed] [Google Scholar]
- Rast J. P., Litman G. W. T-cell receptor gene homologs are present in the most primitive jawed vertebrates. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9248–9252. doi: 10.1073/pnas.91.20.9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadofsky M. J., Hesse J. E., Gellert M. Definition of a core region of RAG-2 that is functional in V(D)J recombination. Nucleic Acids Res. 1994 May 25;22(10):1805–1809. doi: 10.1093/nar/22.10.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadofsky M. J., Hesse J. E., McBlane J. F., Gellert M. Expression and V(D)J recombination activity of mutated RAG-1 proteins. Nucleic Acids Res. 1993 Dec 11;21(24):5644–5650. doi: 10.1093/nar/21.24.5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadofsky M. J., Hesse J. E., van Gent D. C., Gellert M. RAG-1 mutations that affect the target specificity of V(D)j recombination: a possible direct role of RAG-1 in site recognition. Genes Dev. 1995 Sep 1;9(17):2193–2199. doi: 10.1101/gad.9.17.2193. [DOI] [PubMed] [Google Scholar]
- Sadowski P. D. Site-specific genetic recombination: hops, flips, and flops. FASEB J. 1993 Jun;7(9):760–767. doi: 10.1096/fasebj.7.9.8392474. [DOI] [PubMed] [Google Scholar]
- Schatz D. G., Chun J. J. V(D)J recombination and the transgenic brain blues. New Biol. 1992 Mar;4(3):188–196. [PubMed] [Google Scholar]
- Schatz D. G., Oettinger M. A., Baltimore D. The V(D)J recombination activating gene, RAG-1. Cell. 1989 Dec 22;59(6):1035–1048. doi: 10.1016/0092-8674(89)90760-5. [DOI] [PubMed] [Google Scholar]
- Shamblott M. J., Litman G. W. Complete nucleotide sequence of primitive vertebrate immunoglobulin light chain genes. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4684–4688. doi: 10.1073/pnas.86.12.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver D. P., Spanopoulou E., Mulligan R. C., Baltimore D. Dispensable sequence motifs in the RAG-1 and RAG-2 genes for plasmid V(D)J recombination. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6100–6104. doi: 10.1073/pnas.90.13.6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. B. New insights into V(D)J recombination and its role in the evolution of the immune system. Immunity. 1995 Nov;3(5):531–539. doi: 10.1016/1074-7613(95)90124-8. [DOI] [PubMed] [Google Scholar]
- Thompson J. D., Higgins D. G., Gibson T. J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994 Nov 11;22(22):4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasue H., Iwami M., Koide Y., Ohtsubo E., Ishibashi M. The oncogenicity of avian adenoviruses. IV. Confirmatory evidence for recombination between viral and cellular DNA sequences and repetition of the recombinant in cells of a tumor line. Virology. 1989 Apr;169(2):447–451. doi: 10.1016/0042-6822(89)90170-0. [DOI] [PubMed] [Google Scholar]
- van Gent D. C., Mizuuchi K., Gellert M. Similarities between initiation of V(D)J recombination and retroviral integration. Science. 1996 Mar 15;271(5255):1592–1594. doi: 10.1126/science.271.5255.1592. [DOI] [PubMed] [Google Scholar]




