Abstract
While some prior work suggests that medial prefrontal cortex (MFC) regions mediate freely chosen actions, other work suggests that the lateral frontal pole (LFP) is responsible for control of abstract, internal goals. The present study uses fMRI to determine whether the voluntary selection of a task in pursuit of an overall goal relies on MFC regions or the LFP. To do so, we used a modified voluntary task switching (VTS) paradigm, in which participants choose an individual task to perform on each trial (i.e., a subgoal), under instructions to perform the tasks equally often and in a random order (i.e. the overall goal). In conjunction, we examined patterns of activation in the face of irrelevant, but task-related external stimuli that might nonetheless influence task selection. While there was some evidence that the MFC was involved in voluntary task selection, we found that the LFP and anterior insula (AI) were crucial to task selection in the pursuit of an overall goal. In addition, activation of the LFP and AI increased in the face of environmental stimuli that might serve as an interfering or conflicting external bias on voluntary task choice. These findings suggest that the LFP supports task selection according to abstract, internal goals, and leaves open the possibility that MFC may guide action selection in situations lacking in such top-down biases. As such, the current study represents a critical step towards understanding the neural underpinnings of how tasks are selected voluntarily to enable an overarching goal.
Keywords: executive function, cognitive flexibility, goal maintenance, volition, fMRI
Introduction
The ability to flexibly adapt one's behavior to changing goals and environmental information is a hallmark of the human executive functions. Difficulties in flexibility, such as those exhibited after frontal lobe damage, lead to habitual behavior associated with deficits in planning, multitasking, and adapting to change (Duncan, 1986). Such a lack of cognitive flexibility often occurs because environmental stimuli trigger action patterns and responses, rather than actions occurring on the basis of internal goals (e.g., Lhermitte's environmental dependency syndrome; Besnard et al., 2011). While stimulus-guided behavior may be adaptive in routine situations, such as cooking a well-known recipe or performing a menial task, relying on stimuli to guide behavior is not desirable in novel situations or when environmental stimuli afford multiple, competing tasks (Miller and J. D. Cohen, 2001). Thus, executive functions are crucial when internal goals must be maintained in the face of irrelevant external information.
A common experimental paradigm used to examine the ability to flexibly control is the task switching paradigm. Typically in these paradigms, participants are shown target stimuli upon which two separate operations may be performed (e.g., determining item color or determining item shape). In most, a cue indicates which of the tasks should be performed on each trial (e.g., color identification) (for a review of different task switching paradigms, see Monsell, 2003), which we refer to as explicit task switching paradigms. Executive control processes are thought to be required in a task switch so that the cognitive system can be reconfigured to engage the new task set (Meiran, 1996; Rogers & Monsell, 1995; see Kiesel et al., 2010 for a review). In these explicit task-switching paradigms the choice of task is always indicated by external information (i.e., the cue), rather than being under the control of the participant. Hence, these paradigms are limited in the information they can provide, as they do not provide any insights on how individuals decide which task set to invoke at any given time. This limitation becomes clearer when one considers that in the real world, participants must often rely on both internal goals and external stimuli to select the appropriate task(s) (Ouellette and Wood, 1998).
In consideration of these limitations, Arrington & Logan (2004) introduced the voluntary task switching (VTS) paradigm. Participants voluntarily choose which of two (or three) tasks to perform on each trial, under instructions to choose the tasks equally often and in a random order. According to Arrington & Logan, the requirement to select a task in the absence of an external cue should necessarily involve cognitive control, more specifically to choose the goal (i.e., which task to perform). In line with the suggestion that voluntary task switching engages more efficient control than explicit task switching, switch costs have been shown to be reduced under voluntary compared to explicit conditions (Arrington and Logan, 2005; Orr and Weissman, 2011). This reduced switch cost is thought to arise because cognitive control mechanisms had to be engaged to select the appropriate task under the voluntary condition, and hence are already available for task-switching as well.
Yet in real life the selection of a task does not occur in a vacuum, and indeed research has shown that either present or past information from the environment, which we will refer to as external biases, can influence voluntary task selection. For instance, participants are biased to repeat a task when the specific experimental stimuli repeat (Mayr and Bell, 2006), presumably because the stimulus-task association from the previous trial is automatically retrieved when the stimulus is shown again (e.g., Hommel, 2004). These stimulus-task associations develop quickly and can be long lasting, biasing participants to choose the task that was first associated with a stimulus even after many subsequent trials (Arrington et al., 2010; Demanet et al., 2013; Demanet et al., 2010).
In order to investigate external biases on task choice independent from biases engendered by specific stimuli, we created a paradigm where task choice and responses to task stimuli are separated in time (Orr and Weissman, 2011). At the beginning of each trial a cue is presented that instructs the participant to indicate which one of two tasks they will perform on the upcoming target stimulus. Distracter items are presented concurrently with the cue and are used in the paradigm to represent environmental influences on task performance. Some of the distractors are associated with one of the two tasks, while others are not. Not surprisingly, we found an influence of environmental factors, such that participants are biased to choose the task that is associated with the distracters as opposed to the task not associated with the distracters. Moreover, succumbing to the bias provided by the distracter stimuli (as compared to not) negatively influenced task performance, as indicated by increased switch costs. This finding suggests that internal task selection that is not biased by external information is associated with more efficient cognitive control, presumably because during these trials, participants are better able to represent the overall task goal of selecting tasks randomly.
Work on intentional control has suggested that the MFC is critical for voluntarily choosing between different response alternatives (see Brass & Haggard, 2008 for a review and model). While most of this work has focused on choosing between simple response alternatives, there is evidence that the MFC is also involved in voluntarily choosing between different task options. Forstmann and colleagues (2006) presented participants with a cue indicating which task to perform, or a cue indicating from which of 2 or 3 tasks participants could freely choose to perform, with the restriction that participants could not repeat the same task from trial to trial. In this study, greater activation for the voluntary task choice trials compared to the explicit trials was observed in the MFC and the inferior parietal lobule (IPL). The authors suggested that the MFC was involved in resolving uncertainty between the different task alternatives arising from the lack of a top-down goal to guide behavior. More recently, Demanet and colleagues (2013) compared explicit task switching to VTS and found that the MFC was more active on voluntary task choice trials then on explicit trials. While they found other regions (e.g., DLPFC, frontal pole, anterior insula) that were also active, they concluded that the MFC was critical in voluntarily choosing between different task options.
However, other work has suggested a role for the lateral portion of the frontal pole (LFP) in guiding voluntary task choices. The LFP has been shown to be activated in a variety of situations where the task structure is ill-defined or requires maintaining abstract intentions or goals (for reviews, see Badre, 2008; Burgess, Dumontheil, & Gilbert, 2007; Gilbert, Gonen-Yaacovi, Benoit, Volle, & Burgess, 2010; Pollmann, 2012). In other words, the LFP is crucial when optimal behavior is not obvious or should be guided by internally maintained goals, rather than by concrete stimulus-response associations. Such is the case in the VTS paradigm where participants voluntarily choose tasks in order to best approximate a random sequence. This abstract goal must be maintained and incorporated with the recent history of task choices held in memory (Arrington and Logan, 2005; Mayr and Bell, 2006; Vandierendonck et al., 2012). On explicit trials, however, participants’ task choices are determined by the cue stimulus, requiring retrieval of the stored task rule in order to perform the appropriate task. Such ‘stimulus-dependent’ processing has been associated with more posterior portions of PFC and medial, rather than lateral, fronto-polar regions (Burgess et al., 2007, 2005).
Soon and colleagues have recently used fMRI to decode brain activity that predicts an upcoming choice, even before conscious awareness of the choice to be made (Soon et al., 2013, 2008). They found that the frontal pole was the first region whose activity predicted an upcoming voluntary choice between different responses or tasks, followed by the precuneus/posterior cingulate and the pre-SMA/SMA. Indeed, activity in the pre-SMA/SMA peaked after the decision had reached conscious awareness. Brass and colleagues have suggested that the pre-SMA/SMA is involved in the decision of when to make a response whereas the decision of what response to make involves the dorsal anterior cingulate cortex (dACC) (Brass and Haggard, 2008; Brass et al., 2013). However, the results of Soon and colleagues suggest that such the frontal pole may be involved in critical, early aspects of the task choice decision.
Given these discordant findings in the literature, the current study had two main goals. The first goal was to further elucidate the neural mechanisms underlying task selection when they are internally guided as compared to being dictated by the experimental paradigm. We hypothesize that the FP will be involved in representing the abstract goal to choose the tasks randomly, and will show greater activation for voluntary task choice trials than for explicit task trials. In line with previous studies (e.g., Demanet et al., 2013; Forstmann et al., 2006), we predicted that the MFC will also be recruited more on voluntary than explicit trials in order to help resolve conflict or uncertainty during task choices. The second goal was to examine the neural mechanisms involved in making internally guided choices when there are task-relevant environmental cues. Orr & Weissman (2011) suggested that overcoming external biases in the environment when making a task choice requires participants to represent abstract goals to a greater degree than in the absence of environmental biases. Therefore, we predicted that regions of the frontal pole would become even more engaged for voluntary task choices based on internal goals rather than based on directed cues when environmental information was discordant with those task goals.
Method
Participants
Thirty-one right-handed healthy individuals from the Boulder, CO area (mean age = 21.6, SD = 3.8; 13 males) participated in the experiment. All participants had normal or corrected-to-normal vision and had no history of psychiatric or neurological disorder. Informed consent was obtained from each participant prior to participation, and all study procedures were approved by the University of Colorado Boulder Institutional Review Board. One participant's data was excluded for excessive movement (>3 mm) and two participants’ data were excluded due to task-related issues (malfunctioning buttons and/or inability to follow task instructions to perform the tasks equally often and in a random order). Thus, data from 28 participants were included in the final data analyses.
Stimuli
Participants performed a voluntary task-switching version of the numerical Stroop task, which involves comparing a pair of digits in terms of their numerical or physical size (Henik and Tzelgov, 1982). On each trial, participants voluntarily chose to perform one of these two tasks or were explicitly instructed by a cue as to which of these two tasks to perform (see Fig. 1). Voluntary and explicit trials were presented in a random order throughout the experiment. Stimuli were created and presented using E-Prime 2 (Psychological Software Tools, Inc., Pittsburgh, PA).
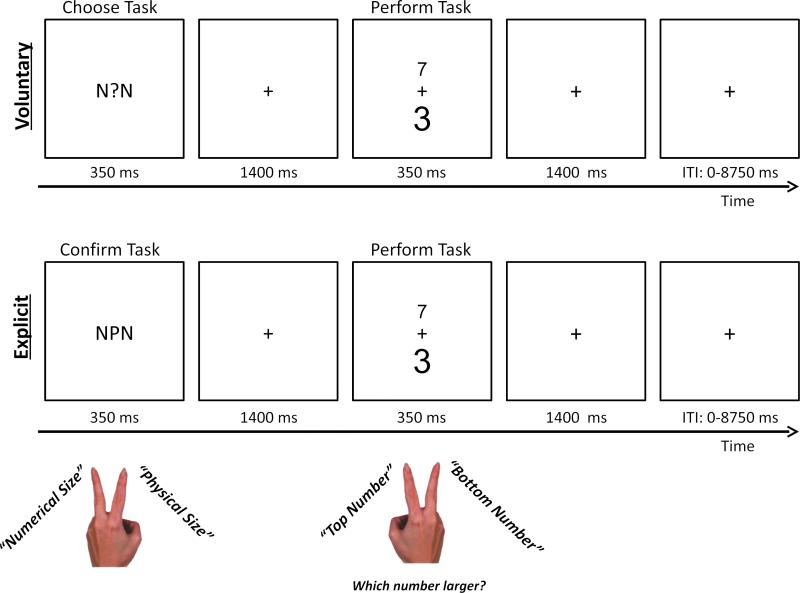
Figure 1.
Examples of typical voluntary task choice and explicit task trials. Participants performed a task switching version of the numerical Stroop task, which involved comparing two digits with respect to their numerical size or with respect to their physical size. Each voluntary task choice trial began with the presentation of a central question mark (“?”), which indicated that participants should voluntarily choose which task to perform in the current trial. Participants were instructed to indicate their task choice by pressing a button with the middle or index finger of their left hand. Each explicit task trial began with the presentation of a central cue letter, which indicated that participants should choose to perform either the numerical or the physical size comparison task. Participants were instructed to confirm their task choice by pressing a button with the middle or index finger of their left hand. In both voluntary and explicit task trials, the central cue was flanked by two identical distracter letters that were associated with the numerical size comparison task (i.e., two “Ns”), the physical size comparison task (i.e., two “Ps”), or neither task (i.e., two “Os”). The cue and flanking distracter letters were presented for 350 ms. After a delay of 1400 ms, the imperative task stimuli (i.e., two digits) appeared. One was numerically larger (e.g., “7”) while the other was numerically smaller (e.g., “3”). Further, one was presented in a larger font (e.g., “3”) while the other was presented in a smaller font (e.g., “7”). Depending on the task, participants indicated which of the two digits (top or bottom) was numerically larger or physically larger. The target stimuli remained on the screen for 350 ms and the trial ended after a delay of 1400 ms. The next trial began after a jittered inter-trial interval ranging from 0 to 8750 ms, in units of 1750 ms.
At the start of each trial, a task cue was presented for 350 ms. For voluntary trials, the cue was a question mark that prompted participants to voluntarily choose the next task. In explicit task trials, the cue was either an ‘N’ or a ‘P’ that instructed participants to perform either the numerical or the physical size comparison task, respectively. The cue was replaced by a central fixation cross, which remained on the screen for 1400 ms. Participants were instructed to indicate the task they were intending to perform before the end of this interval (i.e., they had to respond within 1750 ms of cue onset), by pressing a button with either their left index or left middle finger. We required a confirmatory response on explicit trials in order to equate the response demands on voluntary and explicit trials and to ensure participants were paying attention to the cue. On 1.6% of explicit trials, participants did not indicate the correct task and these trials were coded as errors, which were not included in data analyses. The task choice mapping was counterbalanced across participants. Instructions for how to choose the task on voluntary trials were modeled on the task instructions published by Arrington & Logan (2004), which emphasize choosing the tasks equally often and in a random order. The instructions suggest thinking of each task choice as a mental coin flip, with one side of a coin representing the numerical size comparison task, and the other side representing the physical size comparison task.
To introduce a possible external bias (i.e. environmental influence) on participants’ task choices, the central cue on each trial was flanked by two identical distracter letters (Fig. 1), which remained on screen for the duration of the cue stimulus. The distracter letters could be ‘Ns’, ‘Ps’, or ‘Os’. As N and P sometimes served as explicit task cues, we predicted that these letters would serve as environmental influences and introduce a bias to choose the numerical or physical size comparison task, respectively. As O was never an explicit task cue, the O distracter letters served as neutral distracters. The instructions stated that the distracter letters were irrelevant and that their task was to focus on the center cue stimulus.
After the task choice period, the target stimuli were presented. Two digits appeared 51 pixels above and below fixation. One digit was numerically larger (7, 8, or 9) and the other was numerically smaller (1, 2, or 3). Further, one digit was physically larger (size 32 Calibri Bold Font) while the other was physically smaller (size 18 Calibri Bold Font). The numerical and physical size of the two digits was crossed, so that there was an equal number of trials where the numerically larger digit was also physically larger (i.e., compatible targets) and trials where the numerically larger digit was physically smaller (i.e. incompatible targets). The digits remained on the screen for 350 ms and were then replaced by a fixation cross. Participants had 1750 ms from the onset of the digits to indicate the spatial position (top or bottom) of the digit that was larger at the relevant dimension (i.e., numerical size or physical size) by pressing a button with their right middle finger (the digit on top) or their right index finger (the digit on the bottom). Thus, the cue and target periods each lasted for 1750 ms for a total trial length of 3500 ms. The next trial began after a jittered inter-trial interval (ITI), which ranged from 0 to 5 TR's (i.e., 0-8750 ms), following a pseudo-logarithmic distribution favoring short ITIs.
Procedure
The experiment consisted of four parts: three practice sessions and then the actual experiment. First, participants practiced the numerical and physical size comparison tasks separately. Each practice block (one for each task) consisted of 15 trials, and the order in which the tasks were practiced was counterbalanced across participants. Second, participants practiced voluntarily choosing to perform either the numerical or the physical size comparison task when prompted by a question mark. Specifically, they performed a single block of 36 voluntary task choice trials in the absence of distracter letters. This practice emphasized trying to choose the tasks equally often and in a random order. Third, to prepare for the actual experiment, participants practiced randomly alternating between voluntary and explicit trials. They performed a single block of 48 trials in which voluntary and explicit task trials appeared in a random order. As in the actual experiment, each cue was flanked by two identical distracter letters.
At the end of each of the voluntary task choice and mixed voluntary-explicit practice blocks above, participants were told the proportion of trials in which they (a) performed each task and (b) switched tasks. If either proportion was less than 40% or greater than 60%, they performed that particular practice block again. If accuracy in any of the practice blocks fell below 80% the block was repeated. Each type of practice block (i.e., voluntary and mixed voluntary-explicit) was practiced no more than 2 times for each participant. The practice blocks were performed in a training room down the hall from the scanner on a laptop computer. Participants were then situated in the scanner. Foam pillows were placed around their head in order to minimize head movement. For the scanner session participants performed 7 blocks of 72 trials similar to those they had performed in the third mixed voluntary-explicit practice session. No feedback on choice proportions was presented during the scan runs. Stimuli were projected at a resolution of 1280×1024 on a screen placed at the back of the scanner bore, which participants viewed via a mirror system mounted on the head coil. Participants responded with button boxes held in their left and right hands.
fMRI Data Acquisition
Functional and structural MRI was performed on a 3-T Siemens Magnetom TIM Trio scanner (Siemens AG, Munich, Germany) with a 12-channel head coil. Whole-brain structural images were collected with a high-resolution 3D magnetization prepared rapid gradient echo (MPRAGE) sequence (1 mm3 isotropic voxels, 192 slices; 256 mm field of vision; repetition time = 2530 ms; echo times: 1.64, 3.5, 5.36, 7.22, 9.08 ms; inversion time: 1200 ms; flip angle: 7°; GRAPPA parallel image factor: 2). Functional BOLD (blood oxygenation level dependent) images were collected using gradient echo T2*-weighted echoplanar imaging (EPI); (repetition time = 1750 ms; echo time: 25 ms; 240 mm field of vision; 64 × 64 matrix; 31 axial slices, 3 mm slice thickness, 1 mm slice gap, 3.4 × 3.4mm voxels; flip angle = 67°). Slices were oriented obliquely along the AC-PC line. The first four volumes from each run were discarded to allow for magnetic field equilibration.
fMRI Data Processing
Image processing and data analysis were implemented using the FSL package (Analysis group, FMRIB, Oxford, UK, http://www.fmrib.ox.ac.uk/fsl/). Standard pre-processing was applied: MCFLIRT – linear slice-time correction/motion correction, BET – brain extraction, time-series prewhitening, high pass filter (0.01 Hz), and registration and spatial normalization to the Montreal Neurological Institute (MNI) 152-T1 2-mm template. Individual's functional images were first registered to their high-resolution MPRAGE scans via a 6 parameter linear registration, and the MPRAGE images were in turn registered to the MNI template via a 12 parameter linear registration. These registrations were combined in order to align the functional images to the template. Functional images were resampled in to standard space with 2-mm isotropic voxels and were smoothed with a Gaussian kernel of 8-mm full-width at half-maximum.
Lower-level statistics were implemented in FEAT. Using multiple regression analysis, statistical maps representing the association between the observed time series (e.g., BOLD signal) and one or a linear combination of regressors for each subject were constructed. Regressors in the main analysis were constructed from all 12 combinations of the following factors: agency (voluntary, explicit), task alternation (repeat, switch), and distracter-task congruency (congruent, incongruent, neutral). For each regressor, a double-gamma HRF was convolved with an event vector starting at the cue stimulus onset with a duration of 3500 ms, which consisted of the entire cue and target period. Head motion parameters (6 linear parameters: X,Y, Z, roll, pitch, yaw) were included as confound regressors. Errors were also included as a confound regressor. Contrasts of interest were formulated as linear combinations of the 12 main regressors. Lower-level models were passed to group-level analyses which employed non-parametric permutation methods through FSL's randomise function (Nichols and Holmes, 2002). One-sample t-tests for each contrast of interest were performed using the Threshold-Free Cluster Enhancement (TFCE) method, which detects clusters of contiguous voxels without first setting an arbitrary statistical cut-off (e.g., Z > 2.58), and controls the family-wise error (FWE) rate at p < .05 (Smith and Nichols, 2009). Each contrast underwent 5000 permutations. Randomise produces corrected 1-p maps, which we used to mask t-score maps for all figures and tables. Figures of statistical maps were created using Caret software version 5.65 (Van Essen et al., 2001; http://brainvis.wustl.edu/wiki/index.php/Caret) on the Conte69 atlas (Van Essen et al., 2012). Region-of-interest analyses were conducted on percent signal change data as calculated by FSL's featquery tool.
Results
Behavioral Results
Task Choice Proportions
Participants complied with task instructions, choosing to perform the numerical and physical size comparison tasks relatively equally often [numerical: 48.4%, physical: 51.6%; t(27) < 1.95, n.s.]. Participants in the VTS paradigm typically show a reference for repeating rather than switching tasks (Arrington and Logan, 2005; Mayr and Bell, 2006), though no such repeat bias was observed here as the repeat proportion on voluntary trials did not differ from 50% (explicit: 50.1%, 95% CI: 49.0-51.4%; voluntary: 50.1%, 95% CI: 46.8-54.6%; explicit vs. voluntary: F(1,27) = 0.08, n.s.) Following Orr & Weissman (2011), we assessed whether participants’ task choices were biased by the distracter letters. To this end we calculated a task choice index (TCI) by subtracting the proportion of voluntary task choice trials where participants chose the numerical size comparison task by the proportion of trials where participants chose the physical size comparison task. A positive TCI indicates a bias to choose the numerical size comparison task, and a negative TCI indicates a bias to choose the physical size comparison task (see Inline Supplementary Figure 1). TCI data were submitted to a repeated-measures ANOVA with the factors of agency of the previous trial (voluntary, explicit), task alternation (repeat, switch), and distracter letter identity (N, P, O). For the mean TCI data, see Inline Supplementary Table 1. In line with Orr & Weissman (2011), there was a main effect of distracter identity (F(1,27) = 8.1, p < .001). The TCI was significantly more positive when the distracter letters were N's compared to the neutral O's (6.7% vs. −5.5%, F(1,27) = 6.6, p = .01), and significantly more negative when the distracter letters were P's compared to the neutral O's (−14.9% vs. −5.5%, F(1,27) = 5.3, p < .05). These results demonstrate that participants’ task choices were biased by the distracter letters. No other effects were significant.
Reaction Time and Accuracy Data
Task choice reaction time data (i.e., the time it took participants to respond to the cue to indicate the current task) were submitted to a repeated-measures ANOVA with the factors agency (voluntary, explicit), task alternation (repeat, switch), and task choice congruency (congruent, incongruent, neutral). For the mean performance data, see Inline Supplementary Table 2. Violations of the sphericity assumption were adjusted with the Greenhouse-Geisser method. The main effect of agency was significant (F(1,27) = 6.4, p < .05), as participants were faster to voluntarily choose the next task (767 ms) than they were to confirm processing of the explicit task cues (800 ms). In line with previous findings (Arrington & Logan, 2005; Orr et al., 2012; Orr & Weissman, 2011), participants were faster to choose to repeat tasks (778 ms) than to switch tasks (788 ms; F(1,27) = 4.9, p < .05). Further, there was a main effect of choice congruency (F(1,27) = 7.7, p = .001). A post-hoc pairwise Newman-Keuls test revealed that incongruent task choices (796 ms) were made more slowly than congruent (776 ms; p < .005) and neutral task choices (778 ms; p < .005). There was no difference between congruent and neutral task choices. Finally, there was a significant interaction of agency and cue congruency (F(2,54) = 5.3, p < .01). Further analyses revealed a larger difference between incongruent and neutral task choices on voluntary compared to explicit trials (F(1,27) = 5.3, p < .05), and no significant difference between incongruent and congruent task choices on voluntary compared to explicit trials (F(1,27) = 0.52, n.s.). In other words, voluntary choices were made especially quickly when the distractors were neutral, possibly because they were irrelevant to task choice.
Reaction time data for actual task performance (i.e., the time it took participants to respond to the target digits) were submitted to a repeated-measures ANOVA with the factors agency (voluntary, explicit), task alternation (repeat, switch) and task choice congruency (congruent, incongruent, and neutral). For mean task performance data, see Inline Supplementary Table 3. Violations of the sphericity assumption were adjusted with the Greenhouse-Geisser method. As expected, the main effect of task alternation was significant (F(1,27) = 24.7, p < .001), as participants were faster to perform a task repeat than a task switch (744 ms vs. 776 ms). No other effects were significant.
Accuracy data were submitted to a repeated-measures ANOVA with the factors agency (voluntary, explicit), task alternation (repeat, switch), and task choice congruency (congruent, incongruent, neutral), and the mean data are presented in Inline Supplementary Table 3. Violations of the sphericity assumption were adjusted with the Greenhouse-Geisser method. As expected, there was a main effect of task alternation (F(1,27) = 23.5, p < .001), as participants were more accurate on task repeat trials (95.3%) than on switch trials (93.3%). No other effects were significant.
fMRI Results
Voluntary Task Choice vs. Explicit Task
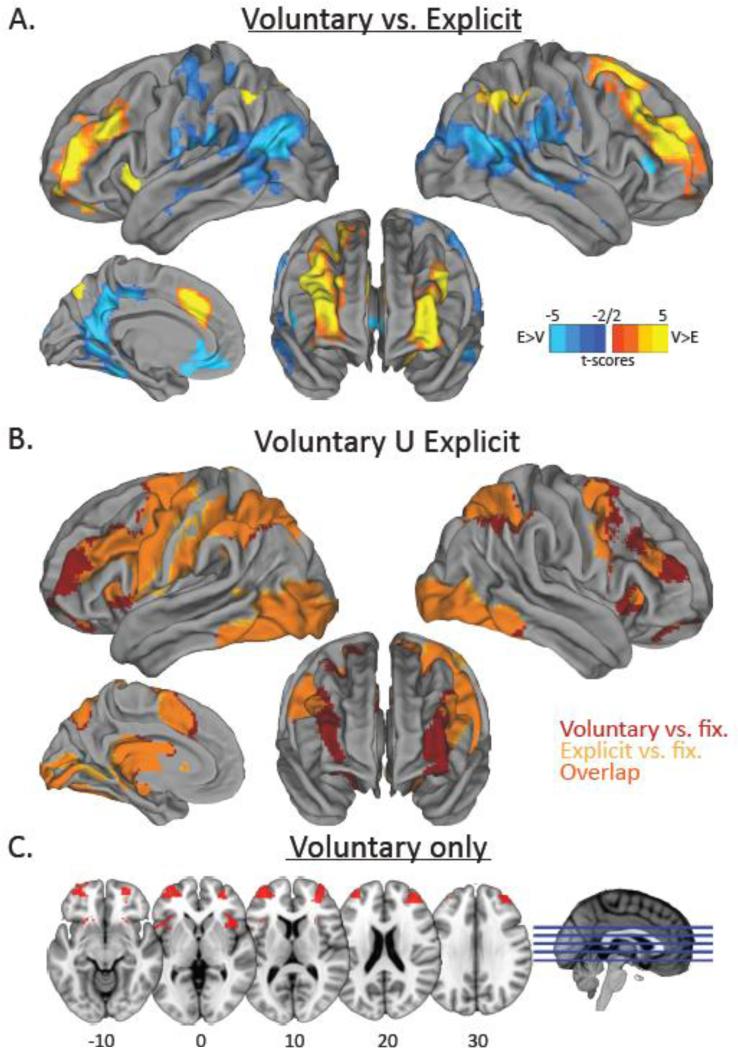
We were first interested in the overall differences between the regions involved in voluntary task choices as compared to explicit tasks. As mentioned above, Forstmann et al. (2006) suggested that the MFC is particularly involved in voluntary selection. In the Voluntary > Explicit contrast we observed a robust activation across the frontoparietal control network (DLPFC, IPL), the cingulo-opercular network (CON; consisting of MFC, AI/ frontal operculum, and LFP; Dosenbach et al., 2008), and cerebellar Crus I (see Table 1; Fig. 2a). Conversely, the Explicit > Voluntary contrast revealed increased activation across the default mode network, as well as the right inferior frontal gyrus (IFG) triangularis, and left primary somatosensory cortex. In order to assess whether the above findings suggest a failure for explicit trials to activate control regions or simply reflect greater recruitment of control regions for voluntary than explicit trials, we examined the conjunction of the Voluntary > baseline and Explicit > baseline contrasts (e.g., similar to the approach of Nichols, Brett, Andersson, Wager, & Poline, 2005). As shown in Fig. 2b, voluntary and explicit trials both activated the frontoparietal control network as well as MFC, cerebellum, thalamus, and caudate. Furthermore, we examined whether the regions that showed greater activation for explicit versus voluntary task choice trials were less deactivated on explicit trials compared to voluntary trials. Analyses of the percent signal change extracted from 5-mm radius spheres around the peak cluster activations revealed that explicit trials showed less deactivation than voluntary trials in the default mode network (explicit: −0.13%, voluntary: −0.26%; t(27) = 6.97, p < .001), primary somatosensory cortex (explicit: −0.004%, voluntary: −0.09%; t(27) = 3.24, p < .005), as well as right IFG triangularis (explicit: −0.16%, voluntary: −0.25%, t(27) = 6.63, p < .001). Thus, both voluntary and explicit trial recruited cognitive control regions, but these regions were activated more strongly (and default mode regions were more deactivated) during voluntary task choices than during explicit tasks.
Table 1.
Significant clusters of activation in the comparison of voluntary and explicit choice trials. Values presented are for the peak of activation in each cluster. For large clusters, the cluster name or global peak is bolded and local maxima are indented. Coordinate are in MNI space.
| Voluntary > Explicit | ||||||
|---|---|---|---|---|---|---|
| Region | # of voxels | Brodmann Area | t-score | x | y | z |
| Right Anterior Prefrontal Cluster | 8168 | -- | -- | -- | -- | -- |
| Right Anterior DLPFC | -- | BA10 | 8.91 | 34 | 42 | 18 |
| Right Mid-DLPFC | -- | BA9 | 7.59 | 44 | 34 | 34 |
| Dorsal ACC | -- | BA32 | 7.32 | 0 | 24 | 38 |
| Right Superior Frontal Cortex | -- | BA8 | 5.62 | 28 | 12 | 50 |
| Right Dorsal Premotor Cortex | -- | BA6 | 5.17 | 16 | 12 | 66 |
| Right Lateral Frontal Pole | -- | BA10 | 5.03 | 30 | 60 | 10 |
| Right Inferior Frontal Pole | -- | BA11 | 4.26 | 30 | 50 | −12 |
| Left Anterior Prefrontal Cluster | 3500 | -- | -- | -- | -- | -- |
| Left Mid-DLPFC | -- | BA9/46 | 6.91 | −44 | 32 | 32 |
| Left Inferior Inferior Frontal Pole | -- | BA11 | 6.37 | −20 | 40 | −20 |
| Left Lateral Frontal Pole | -- | BA10 | 5.52 | −34 | 60 | −8 |
| Left Anterior DLPFC | -- | BA9 | 4.2 | −28 | 46 | 38 |
| Right Inferior Parietal Lobule | 1481 | BA40 | 7.55 | 48 | −46 | 48 |
| Right Angular Gyrus | -- | BA7 | 5.51 | 32 | −62 | 46 |
| Left Inferior Parietal Lobule | 586 | BA40 | 6.56 | −44 | −52 | 48 |
| Left Precuneus | 401 | BA7 | 6.31 | −8 | −72 | 50 |
| Left Insula | 397 | BA13 | 7.47 | −34 | 16 | 2 |
| Left Cerebellar Crus I | 152 | n/a | 7.17 | −34 | −56 | −36 |
|
Explicit > Voluntary
| ||||||
| Visual and Parietal Cluster | 24812 | -- | -- | -- | -- | -- |
| Posterior Cingulate | -- | BA23 | 7.96 | −6 | −58 | 12 |
| Left Tempero-occipital Cortex | -- | BA39 | 6.67 | −50 | −66 | 16 |
| Right Tempero-occipital Cortex | -- | BA39 | 6.34 | 46 | −56 | 18 |
| Left Fusiform Gyrus | -- | BA37 | 5.66 | −34 | −38 | −20 |
| Left Dorsal Posterior Cingulate | -- | BA31 | 5.43 | −14 | −28 | 42 |
| Left Tempero-parietal Junction | -- | BA42 | 5.11 | −66 | −34 | 20 |
| Right Inferior Parietal Lobule | -- | BA40 | 4.97 | 66 | −28 | 24 |
| Left Middle Temporal Gyrus | -- | BA20 | 4.9 | −52 | −14 | −16 |
| Right Cuneus | -- | BA19 | 4.72 | 18 | −96 | 18 |
| Left Somatosensory Cortex | -- | BA3 | 4.57 | −62 | −18 | 36 |
| Right Parahippocampal Gyrus | -- | BA36 | 4.3 | 30 | −34 | −16 |
| Subgenual ACC | 1050 | BA25 | 6.26 | −4 | 18 | −18 |
| Ventral Medial Prefrontal Cortex | -- | BA11 | 5.55 | 0 | 38 | −14 |
| Right IFG triangularis | 59 | BA45 | 8.91 | 54 | 32 | 6 |
Figure 2.
Results from comparison of voluntary and explicit trials. (a) Activations in hot colors represent regions that were significantly more active for voluntary task choice trials compared to explicit task trials. Activations in cold colors represent regions that were significantly more active for explicit task trials compared to voluntary task choice trials. (b) Regions shown in red were significantly active for voluntary task choice trials (vs. baseline), regions shown in yellow were significantly active for explicit trials (vs. baseline), and regions shown in orange were active for both voluntary and explicit trials relative to baseline. (c) Activations shown in red represent regions that were significantly active for voluntary task choice trials (vs. baseline), but not for explicit trials (vs. baseline).
Next, we examined which regions were activated during voluntary task choices that were not activated during explicit tasks. According to previous work, voluntary task choices rely critically on the MFC (Demanet et al., 2013; Forstmann et al., 2006). However, in our data this region was active for both voluntary trials as well as explicit trials, albeit more strongly for the former. To identify the regions that are uniquely activated during voluntary task choice trials (but not during explicit trials), we masked the Voluntary > baseline contrast map with the Voluntary > baseline and Explicit > baseline conjunction map. As shown in Fig. 2c, this procedure revealed activations in bilateral LFP and bilateral AI. Thus, these regions appear to be uniquely activated by voluntary task choices. This conclusion is further tested in an ROI analysis presented below.
Task Switching Effects
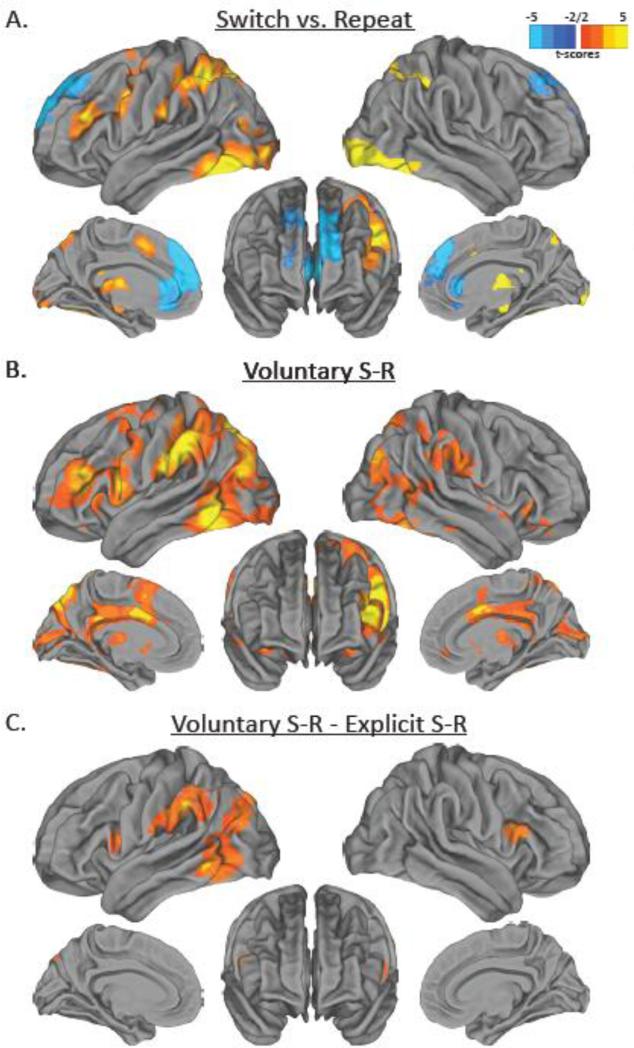
We then turned to examining the regions involved in task switching, and asked whether or not these regions differed between voluntary and explicit trials. Peak activations are detailed in Tables 2-4. First, and in line with most imaging studies of task switching (for a review, see Ruge et al., 2011), the Switch > Repeat contrast revealed that, collapsed across both voluntary and explicit trials, switching tasks activated regions of the left-lateralized frontoparietal control network, MFC, cerebellum, thalamus, and putamen (see Fig. 3a). The Repeat > Switch contrast revealed activation in the anterior medial prefrontal cortex.
Table 2.
Report of significant clusters of activation in the comparison of repeat and switch trials. Values presented are for the peak of activation in each cluster. For large clusters, the cluster name is bolded and local maxima are indented. Coordinate are in MNI space.
| Switch > Repeat | ||||||
|---|---|---|---|---|---|---|
| Region | # of voxels | Brodmann Area | t-score | x | y | z |
| Cerebellum, Visual, and Parietal Cluster | 24722 | -- | -- | -- | -- | -- |
| Right Cerebellum Lobule VI | -- | n/a | 12.5 | 32 | −50 | −30 |
| Left Cerebellum Lobule VI | -- | n/a | 11.4 | −38 | −46 | −34 |
| Left Fusiform Gyrus | -- | BA37 | 9.50 | −42 | −62 | −20 |
| Left Inferior Parietal Lobule | -- | BA40 | 9.45 | −40 | −42 | 40 |
| Right Fusiform Gyrus | -- | BA19 | 7.62 | 42 | −76 | −16 |
| Left Cuneus | -- | BA18 | 7.59 | −36 | −94 | −4 |
| Right Cuneus | -- | BA18 | 6.33 | 18 | −102 | −4 |
| Right Inferior Parietal Lobule | -- | BA40 | 6.31 | 32 | −48 | 38 |
| Right Superior Parietal Lobule | -- | BA7 | 5.55 | 20 | −70 | 56 |
| Right Tempero-occipital Cortex | -- | BA37 | 5.47 | 54 | −54 | −24 |
| Right Lateral Occipital Cortex | -- | BA19 | 5.16 | 32 | −66 | 26 |
| Left Superior Parietal Lobule | -- | BA7 | 5.00 | −32 | −48 | 68 |
| Subcortical and Prefrontal Cluster | 11403 | -- | -- | -- | -- | -- |
| Left Thalamus | -- | n/a | 9.70 | −10 | −20 | 10 |
| Left Dorsal Premotor Cortex | -- | BA6 | 8.92 | −40 | 2 | 34 |
| Left Pallidum | -- | n/a | 8.82 | −22 | 6 | 0 |
| Right Pallidum | -- | n/a | 7.97 | 16 | −4 | 0 |
| Left Mid-DLPFC | -- | BA46 | 6.52 | −44 | 32 | 22 |
| Dorsal ACC | -- | BA32 | 5.61 | −6 | 8 | 48 |
| Left Lateral Premotor Cortex | -- | BA9 | 5.41 | −58 | 8 | 26 |
| Repeat > Switch | ||||||
| Medial Prefrontal Cortex Cluster | 6029 | -- | -- | -- | -- | -- |
| Dorsal Medial Prefrontal Cortex | -- | BA8 | 7.55 | −4 | 44 | 42 |
| Rostral ACC | -- | BA32 | 6.55 | −8 | 44 | 12 |
| Right Superior Frontal Cortex | -- | BA9 | 5.2 | 22 | 52 | 44 |
| Ventral Medial Prefrontal Cortex | -- | BA11 | 5.12 | −8 | 30 | −12 |
| Medial Frontal Pole | -- | BA10 | 4.69 | −2 | 66 | 32 |
| Left Superior Frontal Cortex | -- | BA8 | 4.68 | −32 | 30 | 54 |
Table 4.
Report of significant clusters of activation in the comparison of the switch-repeat difference in voluntary and explicit choice trials. Values presented are for the peak of activation in each cluster. For large clusters, the global peak is bolded and local maxima are indented. Coordinates are in MNI space.
| Voluntary Switch-Repeat > Explicit Switch-Repeat | ||||||
|---|---|---|---|---|---|---|
| Region | # of voxels | Brodmann Area | t-score | x | y | z |
| Left Occipital/Parietal Cluster | 5053 | -- | -- | -- | -- | -- |
| Left Tempero-occipital Cortex | -- | BA19 | 3.89 | −48 | −60 | −8 |
| Left Lateral Occipital Cortex | -- | BA39 | 3.66 | −44 | −76 | 24 |
| Left Inferior Parietal Lobule | -- | BA40 | 4.01 | −60 | −36 | 32 |
| Left Lateral Occipital Cortex | -- | BA7 | 3.01 | −12 | −74 | 56 |
| Left Inferior Frontal Gyrus | 402 | BA44 | 3.68 | −50 | 4 | 14 |
| Right Inferior Frontal Gyrus | 310 | BA44 | 3.56 | 54 | 16 | 18 |
Figure 3.
Results from comparison of task switch and task repeat trials. (a) Activations in hot colors represent regions that were significantly more active for task switch trials compared to task repeat trials. Activations in cold colors represent regions that were significantly more active for task repeat trials compared to task switch trials. (b) Activations in hot colors represent regions that were significantly more active for voluntary task switch trials compared to voluntary task repeat trials. (c) Activations in hot colors represent regions that were significantly more active for voluntary task switch minus task repeat trials compared to explicit task switch minus task repeat trials.
Next we examined the Switch > Repeat contrast separately for voluntary and explicit trials. For voluntary trials, the Switch > Repeat contrast revealed activations in largely the same regions as the overall Switch > Repeat contrast, with perhaps less activation in the right hemisphere (see Fig. 3b). For explicit trials, the Switch > Repeat contrast did not yield any significant differences. Confirming this impression, the contrast of Voluntary Switch-Repeat > Explicit Switch-Repeat resulted in activation in bilateral inferior frontal junction (IFJ), left IPL, left occipitotemporal cortex, and left lateral temporo-occipital cortex (see Fig. 3c). There were no regions activated in the Repeat > Switch contrast for either voluntary or explicit trials. Given the large difference in neural activation for the switch effect between voluntary and explicit trials, it is somewhat surprising that there were no differences in behavioral switch costs as previous studies have found decreased switch costs for voluntary compared to explicit trials (Arrington & Logan, 2005; Orr & Weissman, 2011). However the engagement of these neural regions may have helped to reduce such costs.
Internally and Externally Guided Voluntary Task Choice
The next set of analyses examined the brain regions involved in overcoming external (i.e., environmental) biases on task selection. To this end we tested for differences between incongruent and congruent task choices as a function of agency (voluntary, explicit) and task alternation (repeat, switch). A whole-brain search yielded no significant differences, which are likely accounted for by the conservative FWE-correction at the whole brain level. However, since we had hypothesized differences in the cingulo-opercular network, we conducted an ROI analysis using the factors of region (dACC, AI, LFP), agency (explicit, voluntary), task alternation (repeat, switch), and choice congruency (congruent, incongruent, neutral)1. For each level of the full factorial design of these factors, we extracted mean percent signal change from the peaks identified in the Voluntary > Explicit contrast (see Table 1), averaging over left and right hemisphere clusters. These peaks were located in the LFP (left: −34, 60, −8; right: 30, 60, 10), the left and right AI (±34, 16, 2), and from the MFC (0, 24, 38). Mean percent signal change data were entered in to a 4-way repeated measures ANOVA (see Table 5 for means).
Table 5.
Mean percent signal change data as a function of region, agency, task alternation, and choice congruency. In addition to mean values, standard error (SE), lower and upper bounds of 95% confidence intervals are also presented.
| Region | Agency | Alt | Choice Congruency | Mean | SE | −95% CI | +95% CI |
|---|---|---|---|---|---|---|---|
| dACC | Explicit | Repeat | Con | 0.10 | 0.05 | 0.00 | 0.19 |
| dACC | Explicit | Repeat | Inc | 0.10 | 0.04 | 0.02 | 0.18 |
| dACC | Explicit | Repeat | Neut | 0.10 | 0.05 | 0.01 | 0.20 |
| dACC | Explicit | Switch | Con | 0.13 | 0.05 | 0.03 | 0.22 |
| dACC | Explicit | Switch | Inc | 0.11 | 0.04 | 0.03 | 0.20 |
| dACC | Explicit | Switch | Neut | 0.12 | 0.04 | 0.04 | 0.19 |
| dACC | Voluntary | Repeat | Con | 0.21 | 0.05 | 0.12 | 0.30 |
| dACC | Voluntary | Repeat | Inc | 0.24 | 0.05 | 0.14 | 0.33 |
| dACC | Voluntary | Repeat | Neut | 0.21 | 0.04 | 0.12 | 0.30 |
| dACC | Voluntary | Switch | Con | 0.28 | 0.05 | 0.17 | 0.39 |
| dACC | Voluntary | Switch | Inc | 0.24 | 0.05 | 0.14 | 0.33 |
| dACC | Voluntary | Switch | Neut | 0.22 | 0.04 | 0.13 | 0.31 |
| AI | Explicit | Repeat | Con | 0.06 | 0.02 | 0.02 | 0.11 |
| AI | Explicit | Repeat | Inc | 0.09 | 0.03 | 0.03 | 0.14 |
| AI | Explicit | Repeat | Neut | 0.05 | 0.03 | 0.00 | 0.11 |
| AI | Explicit | Switch | Con | 0.07 | 0.03 | 0.02 | 0.13 |
| AI | Explicit | Switch | Inc | 0.08 | 0.02 | 0.03 | 0.13 |
| AI | Explicit | Switch | Neut | 0.07 | 0.02 | 0.02 | 0.12 |
| AI | Voluntary | Repeat | Con | 0.12 | 0.02 | 0.07 | 0.16 |
| AI | Voluntary | Repeat | Inc | 0.16 | 0.02 | 0.11 | 0.20 |
| AI | Voluntary | Repeat | Neut | 0.11 | 0.02 | 0.06 | 0.16 |
| AI | Voluntary | Switch | Con | 0.17 | 0.02 | 0.12 | 0.21 |
| AI | Voluntary | Switch | Inc | 0.13 | 0.02 | 0.08 | 0.17 |
| AI | Voluntary | Switch | Neut | 0.15 | 0.02 | 0.10 | 0.19 |
| LFP | Explicit | Repeat | Con | 0.02 | 0.05 | −0.08 | 0.12 |
| LFP | Explicit | Repeat | Inc | 0.01 | 0.07 | −0.12 | 0.15 |
| LFP | Explicit | Repeat | Neut | 0.01 | 0.05 | −0.09 | 0.11 |
| LFP | Explicit | Switch | Con | 0.00 | 0.05 | −0.10 | 0.10 |
| LFP | Explicit | Switch | Inc | 0.02 | 0.06 | −0.10 | 0.14 |
| LFP | Explicit | Switch | Neut | −0.05 | 0.04 | −0.13 | 0.04 |
| LFP | Voluntary | Repeat | Con | 0.04 | 0.05 | −0.06 | 0.13 |
| LFP | Voluntary | Repeat | Inc | 0.23 | 0.05 | 0.12 | 0.34 |
| LFP | Voluntary | Repeat | Neut | 0.06 | 0.05 | −0.05 | 0.17 |
| LFP | Voluntary | Switch | Con | 0.17 | 0.06 | 0.05 | 0.30 |
| LFP | Voluntary | Switch | Inc | 0.13 | 0.06 | 0.01 | 0.24 |
| LFP | Voluntary | Switch | Neut | 0.14 | 0.05 | 0.03 | 0.25 |
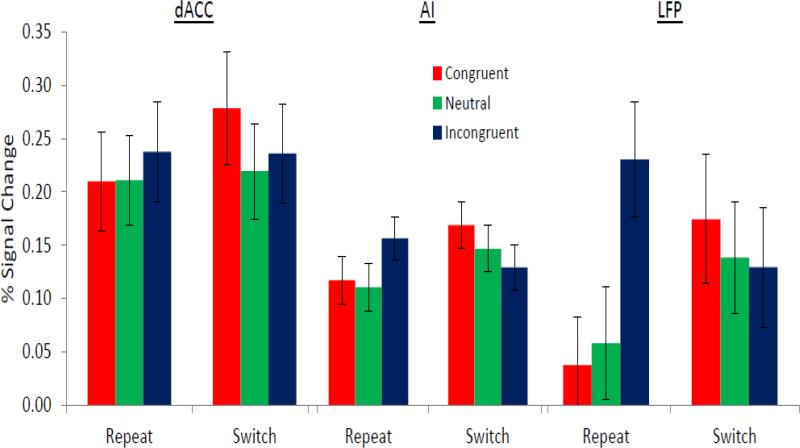
As would be expected from selecting these peaks from the contrast of Voluntary > Explicit, the simple effect of agency was significant in all three regions: dACC, F(1,27) = 41.6, p < .001); AI, F(1,27) = 36.1, p < .001); LFP, F(1,27) = 36.6, p < .001). Yet region interacted with agency (F(2,54) = 5.98, p < .01). There was a larger effect of agency in the dACC compared to the AI (F(1,27) = 13.3, p < .001) as well as in the LFP compared to the AI (F(1,27) = 9.4, p < .005). Confirming the exploratory conjunction analysis presented above, examining the 95% confidence intervals (see Table 5) revealed that the dACC was significantly activated in explicit trials compared to baseline, and activations in the LFP did not differ from baseline in explicit trials. Contrary to the whole-brain analysis, the AI did appear to show greater activity in explicit trials compared to baseline. These results confirm the uniqueness of LFP activation to voluntary trials.
Furthermore, there was a significant 4-way interaction of all of the factors (F(2,54) = 4.0, p < .05). To probe this effect further, we examined whether alternation interacted with congruency separately for each region and level of agency. For the dACC, task alternation did not interact with choice congruency for explicit (F(2,254) = 0.05, n.s.) or voluntary trials (F(2,54) = 1.2, n.s.). Hence, the activity of dACC appears to be mainly influenced by agency, although the dACC was still significantly active on explicit trials (see Fig. 4). For the AI, task alternation did not interact with choice congruency for explicit trials (F(2,54) = 0.33, n.s.), but there was an interaction for voluntary trials (F(2,54) = 4.2, p = .01). As shown in Fig. 4, for the AI in voluntary repeat trials, incongruent trials were numerically, but not significantly more active than congruent and neutrals trials, while the opposite pattern was observed in voluntary switch trials. Hence, the activity of the AI appears to be involved in overcoming a task choice bias from the distracters. For the FP, task alternation did not interact with choice congruency for explicit trial (F(2,54) = 0.47, n.s.), but there was an interaction for voluntary trials (F(2,54) = 5.1, p < .01). As shown in Fig. 4, for the LFP in voluntary repeat trials, incongruent trials were more strongly activated for incongruent compared to congruent (p < .001) and neutral trials (p < .001), while the latter two were equivalent (p = .72). In voluntary switch trials, however, there were no differences between the congruency conditions (all p's > .50), yet all levels of congruency were more active than baseline (all 95% CIs did not contain zero). Further, all levels of congruency in the switch conditions were more active than congruent repeat trials (all p's < .05). Thus, the FP was strongly involved in overcoming a task choice bias from the distracters.
Figure 4.
Mean percent signal change from peaks in the dACC, AI, and LFP as a function of task alternation and choice congruency. All values are from voluntary trials. Error bars represent standard error of the mean.
Individual Differences
We also examined whether individual differences amongst participants influenced the pattern of neural activation. In the current study, we observed that overall participants were faster to voluntarily choose the next task than they were to act on the explicit task cue. This finding suggests that participants (or at least some participants) may have used a “proactive” strategy to prepare a voluntary task choice ahead of time on each trial (as to be prepared should that condition appear). Such a strategy would yield a speed advantage when voluntarily choosing the next task and a disadvantage on a certain proportion of explicit trials where the cue indicated that the non-prepared task was to be performed. The degree to which participants utilize strategic, proactive control is known to show robust individual differences (Locke and Braver, 2008). Accordingly, the extent to which participants were faster to choose the next task on voluntary versus explicit trials varied greatly (M = 35 ms, range: −109 to 209 ms). Individual differences in the extent that people chose the task more quickly on voluntary vs. explicit trials may thus reflect differences in the utilization of proactive control.
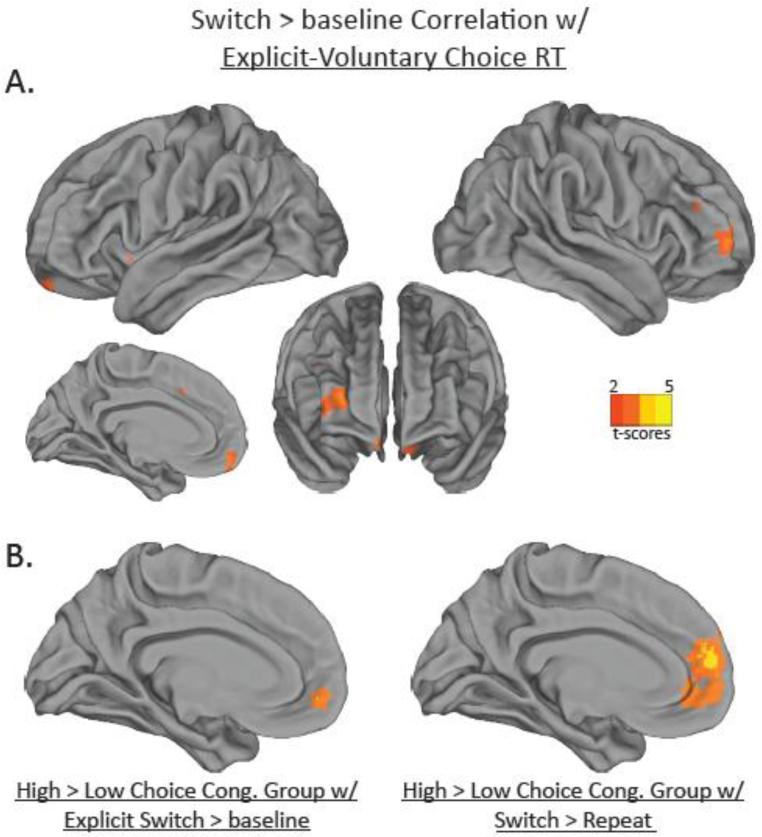
In order to examine the neural mechanisms that were associated with differences in proactive control, we included each participant's difference in choice reaction times for explicit and voluntary trials as a covariate in a GLM with the same contrasts used in the main GLM detailed above. The Switch > baseline contrast revealed activation in right LFP, as well as medial inferior LFP and dorsal ACC, as shown in Fig. 5a and Table 6. This LFP activation overlapped considerably with the LFP activation from the Voluntary > Explicit contrast. No other contrasts showed a significant correlation. In addition, we correlated the difference in explicit and voluntary choice RT with percent signal change for each of the ROIs investigated above, collapsed across conditions. Activity in the LFP was significantly correlated with the choice RT difference, such that greater activity in the LFP was associated with faster voluntary choice RTs compared to explicit choice RTs (r = .35, p < .05; see Inline Supplementary Figure 2). Neither the dACC (r = .27, n.s.) nor the AI (r = .15, n.s.) showed significant correlations. Our interpretation of these results is that participants who prepared a task early on (possibly using proactive control strategies) may have been better at representing abstract task goals and resolving decision conflict (possibly via recruitment of the MFC), which would be critical when switching to a new task.
Figure 5.
Results from individual difference analyses. (a) Activations in hot colors represent regions that were correlated with faster task choice reaction times on voluntary compared to explicit trials for the contrast of Switch vs. baseline. (b) Sagittal slices showing activations for regions that were more active in participants who made relatively more congruent task choices than in participants who made relatively fewer congruent task choices for the contrast of Explicit Switch vs. baseline (left) and for the contrast of Switch vs. Repeat (right).
Table 6.
The top panel details the correlation of the difference in Explicit and Voluntary choice RT with the Switch > baseline contrast. The bottom two panels details the clusters that were more active in the high choice congruency group than in the low choice congruency group. Values presented are for the peak of activation in each cluster. Coordinates are in MNI space.
| Explicit - Vo luntary Choice RT Correlations with Switch > baseline | ||||||
|---|---|---|---|---|---|---|
| Region | # of voxels | Brodmann Area | t-score | x | y | z |
| Right Lateral Frontal Pole | 475 | BA10 | 3.7 | 38 | 60 | 12 |
| Medial Frontal Pole | 287 | BA11 | 3.25 | −6 | 56 | −14 |
| Dorsal ACC | 111 | BA32 | 2.77 | −4 | 16 | 36 |
| High Choice Congruenc y Group > Low Choice Congruency Group with Explicit Switch | ||||||
|---|---|---|---|---|---|---|
| Region | # of voxels | Brodmann Area | t-score | x | y | z |
| Medial Frontal Pole | 1197 | BA10 | 4.59 | −12 | 54 | −8 |
| Right Frontal Pole | -- | BA10 | 4.43 | 16 | 68 | 2 |
| High Choice Congruency Group > Low Choice Congruency Group with Switch > Repeat | ||||||
|---|---|---|---|---|---|---|
| Region | # of voxels | Brodmann Area | t-score | x | y | z |
| Medial Frontal Pole | 1582 | BA10 | 4.35 | 0 | 52 | 0 |
| Superior Medial Frontal Pole | -- | BA10 | 3.21 | 0 | 68 | 30 |
| Inferior Medial Frontal Pole | -- | BA11 | 3.15 | 6 | 52 | −16 |
We then looked for individual differences in fMRI activations based on the voluntary task choice congruency proportion, i.e., how often participants chose the task associated with the distracter letters rather than the alternate task. First, we re-ran the main GLM described above with each participant's TCI now included as a covariate. However, this procedure did not yield any significant results. To increase power, we divided the 28 participants in to two groups based on their task choice congruency proportion (scatterplot shown in Supplementary Fig. 1). A high task choice congruency proportion would suggest that the participants had difficultly overcoming the bottom-up bias of the distracters. In order to avoid any confounds of different numbers of incongruent trials in the two groups, we randomly selected an equal number of incongruent trials for each level of agency and task alternation. The minimum number of trials in a given cell across participants was 18 after eliminating one participant who only had 5 trials in a cell. Therefore, 18 trials were randomly selected from each cell across the remaining 27 participants. We then performed a two-group comparison with the groups based on high and low task choice congruency (13 and 14 participants in the low and high task choice congruency groups, respectively), using the same lower-level design as described above. In two contrasts, Explicit Switch vs. baseline and Switch vs. Repeat, we found a significant group difference with greater activation in the anterior medial PFC for the high choice congruency group compared to the low choice congruency group (see Fig. 5b and Table 6). As a follow-up, we extracted the percent signal change from the medial frontal pole (0, 52, 0), and entered this data in to an ANOVA with the same factors as the ROI analysis presented above. Only the main effect of agency approached significance, with the medial frontal pole being less deactivated on voluntary trials explicit trials (−0.23) compared to (−0.26; F(1,27) = 3.3, p = .08). These findings suggest that the anterior medial PFC may have played a role in mediating the effect of the bottom-up bias of the distracters on voluntary task choice. As this region is thought to be a part of the default mode network, greater activation (or less deactivation) in the anterior medial PFC for the high vs. low task choice congruency group may have reflected decreased cognitive control, in line with Orr & Weissman (2011).
Discussion
In the current study, we examined the neural mechanisms underlying voluntary task selection in the pursuit of a long-term goal as compared to when task selection is explicitly cued. Further, we sought to contrast voluntary task selection in the face of external stimuli that might influence task choice from conditions in which such an influence was absent. In line with previous literature, we identified the dACC, LFP, and AI as having a role in voluntary task selection. Additionally, and contrary to the results of Demanet et al. (2013), we found that the LFP and AI were crucial for overcoming external biases on internal task selection. Below we discuss how the current findings fit with the extent literature on voluntary task switching. Further, we discuss the discrepancy between the current results and recent results positing a critical role of MFC in voluntary selection of task choice, and offer suggestions for future research to elucidate these differences.
Neural Mechanisms of Voluntary Task Selection
Our results indicated that there was unique activation of the LFP during voluntary as compared to explicit task selection. According to the gateway hypothesis model proposed by Burgess and colleagues, the LFP is important in guiding goal-directed behavior according to internal goals in the absence of any stimuli directly associated with the current task (e.g., Burgess et al., 2007). In the VTS paradigm, when a question mark is presented, participants must select the next task according to an abstract mental model of a random sequence (Arrington and Logan, 2005). On explicit trials, this mental model is not required, as the only current goal is to perform the instructed task. A recent model of task choice in the VTS paradigm suggests that participants generate short sequences which are maintained in working memory to guide task selection (Vandierendonck et al., 2012). Given that across a variety of tasks the LFP co-activates with the fronto-parietal control network and the other regions of the cingulo-opercular network (Gilbert et al., 2010), the LFP is in a position to represent overall task goals that can then be implemented by the more posterior control regions of the frontoparietal control network and the cingulo-opercular network. This suggestion is in line with recent models suggesting a rostro-caudal organization of abstraction in the prefrontal cortex (Badre, 2008; O'Reilly, 2010), and is supported by recent work demonstrating that activity in the frontal pole encodes voluntary actions earlier than more posterior PFC regions such as the pre-SMA/SMA (Soon et al., 2013, 2008). Therefore, we propose that the LFP guides voluntary task selection by maintaining the goal to respond randomly.
While we did find greater MFC activation for voluntary compared to explicit trials, the MFC was still reliably activated for explicit trials compared to baseline. Examination of Figure 3i in Forstmann et al. (2006) suggests that the MFC may have been active in both voluntary and explicit trials as well, but to a greater degree in voluntary trials. However, it should be noted that no baseline comparisons were presented for explicit or voluntary trials in that study so such a conclusion is tentative at best. Nevertheless, Demanet and colleagues (2013) found that the MFC was deactivated on explicit trials relative to baseline, and only showed a positive activation for unbiased voluntary task choices. Yet, when we examined the regions that were significant in voluntary trials, but not in explicit trials, we found activations only in LFP.
According to the ‘what, when, whether’ model of Brass & Haggard (2008), the MFC is involved in guiding the decision of ‘what’ response out of several alternatives to make. Specifically, it represents the weighing of different response alternatives. This possibility fits with general models of MFC function which posit that the MFC is involved in conflict monitoring (Botvinick et al., 2001), selecting information to guide responding (Banich, 2009), response outcome predictions (Alexander and Brown, 2011), or other performance monitoring functions (Holroyd and Yeung, 2012; Ridderinkhof et al., 2004). Brass and colleagues (2008, 2010, 2013) have proposed that within the MFC, the ACC selects the appropriate response (‘what’ response) which is signaled to the pre-SMA/SMA where the decision is made of when to make the response, and the dorsal fronto-median cortex decides whether or not to stop the response. However, other work has demonstrated that the decision of which action to perform is made early on in the frontal pole, which no involvement of the rostral cingulate zone (Soon et al., 2013, 2008). After the decision is made, the goal is maintained by the frontal pole until the appropriate time to produce the action, and the pre-SMA/SMA comes on-line just before the initiation of the response. Perhaps more generally, Dosenbach and colleagues (2006, 2008) have suggested that the frontal pole, AI, and dACC show similar patterns of sustained activation related to stable task-set control. However, these authors have not posited specific roles for these individual regions in task-set control. Thus, the exact role of MFC remains unclear, although our results suggest that it is not involved in abstract task choice.
External Biases on Task Choice
The current results demonstrated that overcoming a bias on task choice from the distracter letters relied on recruitment of the LFP and AI, the same regions that were involved in goal-directed task selection. This conclusion is supported by a number of pieces of evidence. First, in the ROI analysis, the AI and LFP showed a significant interaction of task alternation and choice congruency in voluntary trials, but not in explicit trials. Critically, both regions exhibited greater activation in voluntary incongruent repeat trials compared to either voluntary congruent or voluntary neutral repeat trials. Thus, these regions responded specifically to overcoming the bottom-up bias of the distracters. Activation for the LPF did not exceed baseline in voluntary congruent repeat and voluntary neutral repeat trials, and activation was equivalent, though greater than baseline, for all levels of choice congruency in voluntary switch trials. This pattern of activation does not appear to merely reflect decision conflict, as neutral trials were not associated with any decision conflict whereas there may have been unresolved conflict on congruent trials when participants were deciding to either go with or against the distracter letters. We suggest that these results suggest that succumbing to bottom-up biases of the distracters reflects a reduction of goal-directed control, specifically failing to maintain an abstract task goal of random task selection, in line with the conclusions of Orr & Weissman (2011).
Second, in the individual differences analyses, the LFP was more active for participants who prepared a voluntary task choice more quickly than an explicit task, consistent with the notion that the LFP is involved in processing that relies on internally maintained information (Burgess et al., 2007). In contrast, the anterior medial PFC, including the medial frontal pole, was more active in participants who showed a large bias of the distracters compared to participants who showed a smaller bias. The medial frontal pole is thought to be involved in coordinating stimulus-dependent processing, that is processing that is tied to present stimuli. The anterior medial PFC has also been shown to be part of the default mode network, a set of regions that are more active at rest, and are anti-correlated with task-relevant brain regions (Fox et al., 2005; Raichle et al., 2001). Supporting this medial-lateral frontal pole dissociation, Gilbert and colleagues (2010) have demonstrated in a fMRI meta-analysis that the lateral frontal pole typically co-activates with control regions such as DLPFC and IPL whereas the medial frontal pole typically co-activates with default mode network regions. In our study, a specific analysis of the medial frontal pole activity suggested that this region was more deactivated on voluntary trials than on explicit trials. Taken together, these results suggest that participants who were better at maintaining an abstract task set showed more LFP activation, and less medial frontal pole activation. Additional work is needed to better understand the functional coupling of the medial and lateral frontal pole in voluntary task selection.
Although we have suggested that the distracters induced a bottom-up bias on voluntary task selection, it is possible that on some trials the distracters were treated as explicit cues. Thus, succumbing to bottom-up biases on voluntary trials may have reflected an explicit task choice, rather than a mere reduction of goal-directed control. However, there are several pieces of evidence that suggest that voluntary congruent trials were not simply treated like explicit trials. First, participants were faster to choose the task on voluntary neutral trials compared to either voluntary congruent or voluntary incongruent trials. This suggests that on voluntary congruent trials participants considered their task options to some extent, otherwise voluntary congruent choices would have been made the fastest. Furthermore, the mean percent signal change in all three ROIs was numerically larger for all voluntary conditions compared to the explicit conditions, which suggests that even voluntary congruent trials were qualitatively different than explicit trials. To directly assess whether voluntary congruent trials were treated like explicit trials, we contrasted mean percent signal change on voluntary congruent trials and explicit congruent trials. Indeed, in all three ROIs, mean percent signal change was significantly greater in voluntary congruent trials than in explicit congruent trials (dACC; F(1,27) = 31.6, p < .001; AI; F(1,27) = 25.4, p < .001; LFP; F(1,27) = 6.0, p < .05). Also, all three ROIs showed a similar pattern when comparing voluntary incongruent and explicit incongruent trials (all F's > 9.0), and voluntary neutral and explicit neutral trials (all F's > 10.0). Thus, at least in these ROIs, it does not appear that voluntary congruent trials, or any voluntary trials for that matter, were treated like explicit trials. Rather, we suggest that task choice on voluntary congruent trials reflected the priming of the signaled goal, which was selected via voluntary processes.
It is somewhat surprising that the MFC was not involved in overcoming an external bias. Regions within the MFC have been shown to be sensitive to conflicts at the task level (Desmet et al., 2011; Orr & Weissman, 2009), such as would be expected to occur when selection of the current task was discordant with that suggested by the distracter letters. Yet even in the explicit trials, where the cue directly conflicted with the distracters, there was no MFC activation in any of the contrasts comparing incongruent and congruent task choices in explicit trials. Rather, the current findings suggest that the AI may have been involved in resolving task-level conflict. Compared to the MFC, much less is known about the function of the AI. Nelson and colleagues have suggested that the AI is involved in abstract task-level attention (Nelson et al., 2010). Further, a recent study suggested that the AI is involved in evaluating voluntary action decisions (Brass and Haggard, 2010). Hence, the AI may have been involved in evaluating whether the current task choice (i.e., a congruent or incongruent task choice) was in line with the overall goal.
At first glance, the current findings appear to contradict previous findings by Forstmann and colleagues (2006) as well as Demanet and colleagues (2013). However, we would like to point out that the MFC was active across all three studies, and both the current paper and Demanet et al. implicated LFP, AI, and MFC as having a role in voluntary task selection. These three regions are all thought to be part of the cingulo-opercular network, proposed to implement stable, task-set control (Dosenbach et al., 2008). However, how these three regions interact is less clear.
On the one hand, the current design and the design used by Demanet and colleagues (2013) are quite similar. In both studies, participants alternated randomly between explicit and voluntary trials. And in both studies, participants were instructed on voluntary trials to choose the two tasks equally often and in a random or unpredictable order. The main difference was that Demanet and colleagues used a single-registrant design, where a single response indicated the task choice as well as the imperative task response. In contrast, the current study used a double-registrant design, where independent and temporally separate responses were made to indicate the task choice and the imperative task response. In the single-registrant design the external bias may have acted more strongly at the response level than at the goal level, while in the double-registrant design the external bias may have acted more strongly at the goal level than at the response level (cf., Logan & Gordon, 2001; Rubinstein et al., 2001). Indeed, Soon and colleagues (2008, 2013) have demonstrated that the frontal pole is the first region to predict a voluntary choice, followed by parietal and medial frontal structures, suggesting separable roles in time for these two brain regions. As the current design did not allow us to analyze the fMRI BOLD signal separately for the task choice and task performance periods, we were unable to test whether the activity of the LFP and AI reflected goal processing during earlier task selection followed by the ACC during target response selection. However, the double-registrant design would afford the inclusion of catch trials (i.e., task choice cues not followed by a target) that would allow for the separation of these time periods. This explanation is speculative and should be directly investigated.
We propose that the differences between the current results and those of Forstmann and colleagues derive from differences in the nature of the tasks employed. In Forstmann and colleagues (2006), as well as studies of voluntary action selection (e.g., Brass & Haggard, 2008; Nachev et al., 2005), participants are given explicit alternatives of the actions or tasks to perform. There is no overall internal goal to guide selection unlike in the VTS paradigm, where the instructions require participants to represent an overall internal goal of selecting tasks in a random order. The ambiguous nature of how tasks should be selected in studies other than the current one may introduce uncertainty (Volz et al., 2004) or conflict (Milham and Banich, 2005) between the possible action plans, requiring more involvement of the MFC to select an action. One possibility is that the MFC is called upon when there is no top-down bias from the LFP to drive task selection. When a top-down bias is present, there may still be a need to recruit the MFC to resolve conflicts during selection, but the MFC does not drive actual selection of the task.
Consistent with this suggestion, there is evidence supporting the idea that the frontal pole is involved in situations where the task rules are ill-defined. Burgess and colleagues have suggested that the frontal pole is involved in “ill-structured situations” where “one has to impose one's own structure (p. 229)” (Burgess et al., 2005). Indeed, randomness is an abstract concept and participants in the VTS paradigm need to formulate their own internal mental model of a random sequence (Arrington and Logan, 2005; Mayr and Bell, 2006; Vandierendonck et al., 2012). Even in free choice paradigms very similar to those used by Brass and colleagues, the frontal pole has been shown to guide action selection (Soon et al., 2008). Future experiments that compare activation in VTS and in free choice paradigms like that used by Forstmann and colleagues (2006) would provide crucial insight in to the neural mechanisms underlying voluntary task choices that are made under conditions of uncertainty or are made under the umbrella of long-term abstract goals.
Conclusions
The current study represents a timely examination of the neural mechanisms underlying goal-directed voluntary task selection. The current results demonstrate a role of the LFP and AI in guiding voluntary task selection in the absence of external cues to indicate what the task should be. Of note, these regions become even more strongly activated when external biases inconsistent with those choices are available. These findings, therefore, suggest a strong role for LFP and AI in self-directed behavior, an ability compromised in many psychiatric disorders and neurological syndromes. Together with the findings of Forstmann et al. (2006) and Demanet et al. (2013), the results suggest a dissociation of the mechanisms underlying voluntary task selection, with the LFP underlying task selection guided by internal goals, and the MFC underlying task selection guided by the weighing of different response alternatives. Future studies should test this dissociation by directly comparing voluntary task selection under free choice (e.g, Forstmann et al., 2006) and under internally represented goals as in the current study.
Supplementary Material
Highlights.
We investigated the neural mechanisms that underlie voluntary task selection.
Voluntary task choices were compared to explicit, cued task trials.
Voluntary task choices were associated with activation in frontal pole and insula.
Frontal pole was involved in overcoming distraction on choices.
Table 3.
Report of significant clusters of activation in the comparison of voluntary repeat and voluntary switch trials. Values presented are for the peak of activation in each cluster. For large clusters, the global peak is bolded and local maxima are indented. Coordinates are in MNI space.
| Voluntary Switch > Voluntary Repeat | ||||||
|---|---|---|---|---|---|---|
| Region | # of voxels | Brodmann Area | t-score | x | y | z |
| Occipital-Parietal Cluster | 11202 | -- | -- | -- | -- | -- |
| Left Tempero-occipital Cortex | -- | BA37 | 5.68 | −54 | −56 | −10 |
| Left Inferior Parietal Lobule | -- | BA40 | 5.41 | −58 | −34 | 34 |
| Left Lateral Occipital Cortex | -- | BA7 | 5.05 | −10 | −66 | 58 |
| Left Lateral Occipital Cortex | -- | BA19 | 4.5 | −28 | −72 | 36 |
| Right Lateral Occipital Cortex | -- | BA7 | 3.91 | 14 | −66 | 64 |
| Left Cerebellar Crus I/ Lobule VI | -- | n/a | 3.89 | −34 | −42 | 34 |
| Left Superior Parietal Lobule | -- | BA7 | 3.15 | −30 | −56 | 66 |
| Prefrontal Cortex Cluster | 8811 | -- | -- | -- | -- | -- |
| Left Inferior Frontal Gyrus | -- | BA44 | 5.18 | −50 | 6 | 14 |
| Left DLPFC | -- | BA46 | 5.14 | −46 | 32 | 14 |
| Left Dorsal Premotor Cortex | -- | BA6 | 4.53 | −52 | 0 | 38 |
| Dorsal ACC | -- | BA24 | 4.16 | −2 | 10 | 34 |
| Posterior Cingulate | -- | BA23 | 3.92 | −2 | −36 | 26 |
| Pre-Supplementary Motor Area | -- | BA6 | 3.82 | −2 | 10 | 54 |
| Anterior Insula/ Frontal Operculum | -- | BA13 | 3.67 | −28 | 26 | 4 |
| Left Thalamus | -- | n/a | 4.51 | −8 | −18 | 6 |
| Left Putamen | -- | n/a | 4.15 | −16 | 12 | −8 |
| Right Thalamus | -- | n/a | 3.98 | 12 | −6 | 10 |
| Right Thalamus | -- | n/a | 2.99 | 20 | −26 | 8 |
| Right Posterior Fusiform Cortex | 1118 | BA37 | 3.97 | 44 | −48 | −20 |
| Right Inferiror Parietal Lobule | 273 | BA40 | 3.5 | 60 | −36 | 32 |
| Right Cerebellar Lobule VI | 66 | n/a | 3.62 | 34 | −40 | −42 |
| Subgenual ACC | 13 | BA25 | 3.12 | −4 | 10 | −4 |
Acknowledgements
This work was supported by NIMH P50 079485 to M.T.B. and NIDA 1-F32-DA034412-01A1 to J.M.O. We thank two anonymous reviewers for critical comments on a previous version of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
While we were mostly interested in examining voluntary trials, we included explicit trials to examine whether these regions responded to choice congruency in general, or whether any possible effects of choice congruency were specific to voluntary trials. The peaks were identified in a contrast that is inherently biased against explicit trials, so the lack of any effects in explicit trials is not surprising.
The authors declare no conflicts of interest.
References
- Alexander WH, Brown JW. Medial prefrontal cortex as an action-outcome predictor. Nat. Neurosci. 2011;14:1338–44. doi: 10.1038/nn.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrington CM, Logan GD. The cost of a voluntary task switch. Psychol. Sci. 2004;15:610–5. doi: 10.1111/j.0956-7976.2004.00728.x. [DOI] [PubMed] [Google Scholar]
- Arrington CM, Logan GD. Voluntary task switching: chasing the elusive homunculus. J. Exp. Psychol. Lean. 2005;31:683–702. doi: 10.1037/0278-7393.31.4.683. [DOI] [PubMed] [Google Scholar]
- Arrington CM, Weaver SM, Pauker RL. Stimulus-based priming of task choice during voluntary task switching. J. Exp. Psychol. Lean. 2010;36:1060–7. doi: 10.1037/a0019646. [DOI] [PubMed] [Google Scholar]
- Badre D. Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends Cogn. Sci. 2008;12:193–200. doi: 10.1016/j.tics.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Banich MT. Executive function: The search for an integrated account. Curr. Dir. Psychol. Sci. 2009;18:89–94. [Google Scholar]
- Besnard J, Allain P, Aubin G, Chauviré V, Etcharry-Bouyx F, Le Gall D. A contribution to the study of environmental dependency phenomena: the social hypothesis. Neuropsychologia. 2011;49:3279–94. doi: 10.1016/j.neuropsychologia.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol. Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Brass M, Haggard P. The what, when, whether model of intentional action. Neuroscientist. 2008;14:319–25. doi: 10.1177/1073858408317417. [DOI] [PubMed] [Google Scholar]
- Brass M, Haggard P. The hidden side of intentional action: the role of the anterior insular cortex. Brain Struct. Funct. 2010;214:603–610. doi: 10.1007/s00429-010-0269-6. [DOI] [PubMed] [Google Scholar]
- Brass M, Lynn MT, Demanet J, Rigoni D. Imaging volition: what the brain can tell us about the will. Exp. Brain Res. 2013 doi: 10.1007/s00221-013-3472-x. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Dumontheil I, Gilbert SJ. The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends Cogn. Sci. 2007;11:290–8. doi: 10.1016/j.tics.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Simons JS, Dumontheil I, Gilbert SJ. The gateway hypothesis of rostral prefrontal cortex (area 10) function, in: Measuring the Mind: Speed, Control, and Age. Oxford University Press; Oxford: 2005. pp. 217–248. [Google Scholar]
- Demanet J, De Baene W, Arrington CM, Brass M. Biasing free choices: The role of the rostral cingulate zone in intentional control. Neuroimage. 2013 doi: 10.1016/j.neuroimage.2013.01.052. [DOI] [PubMed] [Google Scholar]
- Demanet J, Verbruggen F, Liefooghe B, Vandierendonck A. Voluntary task switching under load: contribution of top-down and bottom-up factors in goal-directed behavior. Psychon. B. Rev. 2010;17:387–93. doi: 10.3758/PBR.17.3.387. [DOI] [PubMed] [Google Scholar]
- Desmet C, Fias W, Hartstra E, Brass M. Errors and conflict at the task level and the response level. J. Neurosci. 2011;31:1366–74. doi: 10.1523/JNEUROSCI.5371-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn. Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. Disorganisation of behavior after frontal lobe damage. Cognitive Neuropsychol. 1986;3:271–290. [Google Scholar]
- Forstmann BU, Brass M, Koch I, Von Cramon DY. Voluntary selection of task sets revealed by functional magnetic resonance imaging. J. Cognitive Neurosci. 2006;18:388–98. doi: 10.1162/089892906775990589. [DOI] [PubMed] [Google Scholar]
- Forstmann BU, Ridderinkhof KR, Kaiser J, Bledowski C. At your own peril: an ERP study of voluntary task set selection processes in the medial frontal cortex. Cogn. Affect. Behav. Neurosci. 2007;7:286–96. doi: 10.3758/cabn.7.4.286. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. P. Natl. Acad. Sci. USA. 2005;102:9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SJ, Gonen-Yaacovi G, Benoit RG, Volle E, Burgess PW. Distinct functional connectivity associated with lateral versus medial rostral prefrontal cortex: a meta-analysis. Neuroimage. 2010;53:1359–67. doi: 10.1016/j.neuroimage.2010.07.032. [DOI] [PubMed] [Google Scholar]
- Henik A, Tzelgov J. Is three greater than five: the relation between physical and semantic size in comparison tasks. Mem. Cognition. 1982;10:389–395. doi: 10.3758/bf03202431. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Yeung N. Motivation of extended behaviors by anterior cingulate cortex. Trends Cogn. Sci. 2012;16:122–8. doi: 10.1016/j.tics.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Hommel B. Event files: feature binding in and across perception and action. Trends Cogn. Sci. 2004;8:494–500. doi: 10.1016/j.tics.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Kiesel A, Steinhauser M, Wendt M, Falkenstein M, Jost K, Philipp AM, Koch I. Control and interference in task switching--a review. Psychol. Bull. 2010;136:849–74. doi: 10.1037/a0019842. [DOI] [PubMed] [Google Scholar]
- Locke HS, Braver TS. Motivational influences on cognitive control: Behavior, brain activation, and individual differences. Cogn. Affect. Behav. Neurosci. 2008;8:99–112. doi: 10.3758/cabn.8.1.99. [DOI] [PubMed] [Google Scholar]
- Logan GD, Gordon RD. Executive control of visual attention in dual-task situations. Psychol. Rev. 2001;108:393–434. doi: 10.1037/0033-295x.108.2.393. [DOI] [PubMed] [Google Scholar]
- Mayr U, Bell T. On how to be unpredictable: evidence from the voluntary task- switching paradigm. Psychol. Sci. 2006;17:774–80. doi: 10.1111/j.1467-9280.2006.01781.x. [DOI] [PubMed] [Google Scholar]
- Meiran N. Reconfiguration of processing mode prior to task performance. J. Exp. Psychol. Lean. 1996;22:1423–1442. [Google Scholar]
- Milham MP, Banich MT. Anterior cingulate cortex: an fMRI analysis of conflict specificity and functional differentiation. Hum. Brain Mapp. 2005;25:328–35. doi: 10.1002/hbm.20110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Nachev P, Rees G, Parton A, Kennard C, Husain M. Volition and Conflict in Human. Medial Frontal Cortex. 2005;15:122–128. doi: 10.1016/j.cub.2005.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Dosenbach NUF, Cohen AL, Wheeler ME, Schlaggar BL, Petersen SE. Role of the anterior insula in task-level control and focal attention. Brain Struct. Funct. 2010;214:669–80. doi: 10.1007/s00429-010-0260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TE, Brett M, Andersson J, Wager T, Poline J-B. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum. Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly RC. The What and How of prefrontal cortical organization. Trends Neurosci. 2010;33:355–61. doi: 10.1016/j.tins.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr JM, Carp J, Weissman DH. The influence of response conflict on voluntary task switching: a novel test of the conflict monitoring model. Psychol. Res. 2012;76:60–73. doi: 10.1007/s00426-011-0324-9. [DOI] [PubMed] [Google Scholar]
- Orr JM, Weissman DH. Anterior cingulate cortex makes 2 contributions to minimizing distraction. Cereb. Cortex. 2009;19:703–11. doi: 10.1093/cercor/bhn119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr JM, Weissman DH. Succumbing to bottom-up biases on task choice predicts increased switch costs in the voluntary task switching paradigm. Frontier Psychol. 2011;2:31. doi: 10.3389/fpsyg.2011.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette JA, Wood W. Habit and intention in everyday life: The multiple processes by which past behavior predicts future behavior. Psychol. Bull. 1998;124:54–74. [Google Scholar]
- Pollmann S. Anterior Prefrontal Contributions to Implicit Attention Control. Brain Sci. 2012;2:254–266. doi: 10.3390/brainsci2020254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. P. Natl. Acad. Sci. USA. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone E. a, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–7. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Monsell S. Costs of a predictable switch between simple cognitive tasks. J. Exp. Psychol. Gen. 1995;124:207–231. [Google Scholar]
- Rubinstein JS, Meyer DE, Evans JE. Executive control of cognitive processes in task switching. J. Exp. Psycho. Human. 2001;27:763–797. doi: 10.1037//0096-1523.27.4.763. [DOI] [PubMed] [Google Scholar]
- Ruge H, Jamadar S, Zimmermann U, Karayanidis F. The many faces of preparatory control in task switching: Reviewing a decade of fMRI research. Hum. Brain Mapp. 2011;00:1–24. doi: 10.1002/hbm.21420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Soon CS, Brass M, Heinze H-J, Haynes J-D. Unconscious determinants of free decisions in the human brain. Nat. Neurosci. 2008;11:543–545. doi: 10.1038/nn.2112. [DOI] [PubMed] [Google Scholar]
- Soon CS, He HH, Bode S, Haynes J-D. Predicting free choices for abstract intentions. P. Natl. Acad. Sci. USA. 2013;110:6217–6222. doi: 10.1073/pnas.1212218110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Drury HA, Dickson J, Harwell J, Hanlon D, Anderson CH. An integrated software suite for surface-based analyses of cerebral cortex. J. Am. Med. Inform. Assn. 2001;8:443–59. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Glasser MF, Dierker DL, Harwell, John, Coalson T. Parcellations and hemispheric asymmetries of human cerebral cortex analyzed on surface-based atlases. Cereb. Cortex. 2012;22:2241–2262. doi: 10.1093/cercor/bhr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandierendonck A, Demanet J, Liefooghe B, Verbruggen F. A chain-retrieval model for voluntary task switching. Cognitive Psychol. 2012;65:241–83. doi: 10.1016/j.cogpsych.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Volz KG, Schubotz RI, Von Cramon DY. Why am I unsure? Internal and external attributions of uncertainty dissociated by fMRI. Neuroimage. 2004;21:848–57. doi: 10.1016/j.neuroimage.2003.10.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.