Abstract
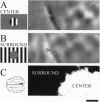
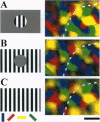
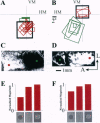
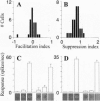
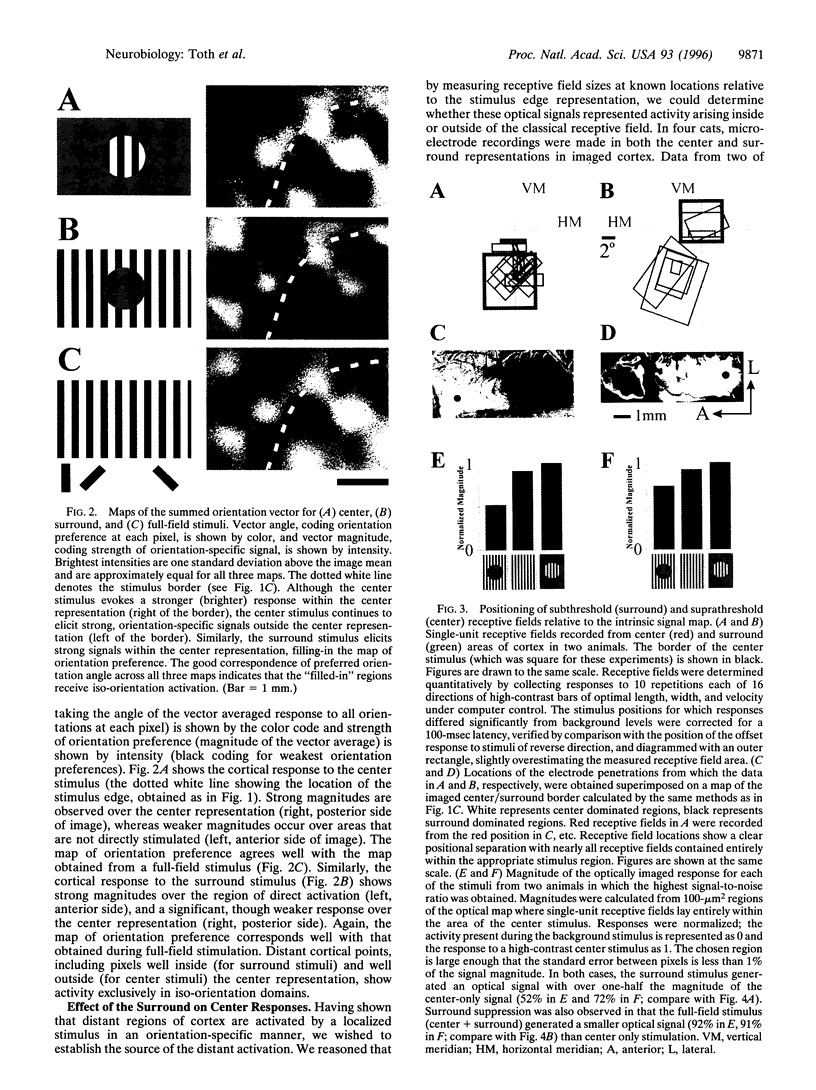
Neurons in primary visual cortex (area 17) respond vigorously to oriented stimuli within their receptive fields; however, stimuli presented outside the suprathreshold receptive field can also influence their responses. Here we describe a fundamental feature of the spatial interaction between suprathreshold center and subthreshold surround. By optical imaging of intrinsic signals in area 17 in response to a stimulus border, we show that a given stimulus generates activity primarily in iso-orientation domains, which extend for several millimeters across the cortical surface in a manner consistent with the architecture of long-range horizontal connections in area 17. By mapping the receptive fields of single neurons and imaging responses from the same cortex to stimuli that include or exclude the aggregate suprathreshold receptive field, we show that intrinsic signals strongly reveal the subthreshold surround contribution. Optical imaging and single-unit recording both demonstrate that the relative contrast of center and surround stimuli regulates whether surround interactions are facilitative or suppressive: the same surround stimulus facilitates responses when center contrast is low, but suppresses responses when center contrast is high. Such spatial interactions in area 17 are ideally suited to contribute to phenomena commonly regarded as part of "higher-level" visual processing, such as perceptual "popout" and "filling-in."
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albus K. A quantitative study of the projection area of the central and the paracentral visual field in area 17 of the cat. I. The precision of the topography. Exp Brain Res. 1975 Dec 22;24(2):159–179. doi: 10.1007/BF00234061. [DOI] [PubMed] [Google Scholar]
- Bergen J. R., Julesz B. Parallel versus serial processing in rapid pattern discrimination. Nature. 1983 Jun 23;303(5919):696–698. doi: 10.1038/303696a0. [DOI] [PubMed] [Google Scholar]
- Born R. T., Tootell R. B. Single-unit and 2-deoxyglucose studies of side inhibition in macaque striate cortex. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7071–7075. doi: 10.1073/pnas.88.16.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A., Gilbert C. D. Long-range horizontal connections and their role in cortical reorganization revealed by optical recording of cat primary visual cortex. Nature. 1995 Jun 29;375(6534):780–784. doi: 10.1038/375780a0. [DOI] [PubMed] [Google Scholar]
- De Weerd P., Gattass R., Desimone R., Ungerleider L. G. Responses of cells in monkey visual cortex during perceptual filling-in of an artificial scotoma. Nature. 1995 Oct 26;377(6551):731–734. doi: 10.1038/377731a0. [DOI] [PubMed] [Google Scholar]
- DeAngelis G. C., Anzai A., Ohzawa I., Freeman R. D. Receptive field structure in the visual cortex: does selective stimulation induce plasticity? Proc Natl Acad Sci U S A. 1995 Oct 10;92(21):9682–9686. doi: 10.1073/pnas.92.21.9682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferster D. Orientation selectivity of synaptic potentials in neurons of cat primary visual cortex. J Neurosci. 1986 May;6(5):1284–1301. doi: 10.1523/JNEUROSCI.06-05-01284.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries W., Albus K., Creutzfeldt O. D. Effects of interacting visual patterns on single cell responses in cats striate cortex. Vision Res. 1977;17(9):1001–1008. doi: 10.1016/0042-6989(77)90002-5. [DOI] [PubMed] [Google Scholar]
- Frostig R. D., Lieke E. E., Ts'o D. Y., Grinvald A. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6082–6086. doi: 10.1073/pnas.87.16.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. Clustered intrinsic connections in cat visual cortex. J Neurosci. 1983 May;3(5):1116–1133. doi: 10.1523/JNEUROSCI.03-05-01116.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. Columnar specificity of intrinsic horizontal and corticocortical connections in cat visual cortex. J Neurosci. 1989 Jul;9(7):2432–2442. doi: 10.1523/JNEUROSCI.09-07-02432.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. Morphology and intracortical projections of functionally characterised neurones in the cat visual cortex. Nature. 1979 Jul 12;280(5718):120–125. doi: 10.1038/280120a0. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. Receptive field dynamics in adult primary visual cortex. Nature. 1992 Mar 12;356(6365):150–152. doi: 10.1038/356150a0. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. The influence of contextual stimuli on the orientation selectivity of cells in primary visual cortex of the cat. Vision Res. 1990;30(11):1689–1701. doi: 10.1016/0042-6989(90)90153-c. [DOI] [PubMed] [Google Scholar]
- Grinvald A., Lieke E. E., Frostig R. D., Hildesheim R. Cortical point-spread function and long-range lateral interactions revealed by real-time optical imaging of macaque monkey primary visual cortex. J Neurosci. 1994 May;14(5 Pt 1):2545–2568. doi: 10.1523/JNEUROSCI.14-05-02545.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinvald A., Lieke E., Frostig R. D., Gilbert C. D., Wiesel T. N. Functional architecture of cortex revealed by optical imaging of intrinsic signals. 1986 Nov 27-Dec 3Nature. 324(6095):361–364. doi: 10.1038/324361a0. [DOI] [PubMed] [Google Scholar]
- Gulyás B., Orban G. A., Duysens J., Maes H. The suppressive influence of moving textured backgrounds on responses of cat striate neurons to moving bars. J Neurophysiol. 1987 Jun;57(6):1767–1791. doi: 10.1152/jn.1987.57.6.1767. [DOI] [PubMed] [Google Scholar]
- Hirsch J. A., Gilbert C. D. Long-term changes in synaptic strength along specific intrinsic pathways in the cat visual cortex. J Physiol. 1993 Feb;461:247–262. doi: 10.1113/jphysiol.1993.sp019512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Uniformity of monkey striate cortex: a parallel relationship between field size, scatter, and magnification factor. J Comp Neurol. 1974 Dec 1;158(3):295–305. doi: 10.1002/cne.901580305. [DOI] [PubMed] [Google Scholar]
- Humphrey A. L., Sur M., Uhlrich D. J., Sherman S. M. Projection patterns of individual X- and Y-cell axons from the lateral geniculate nucleus to cortical area 17 in the cat. J Comp Neurol. 1985 Mar 8;233(2):159–189. doi: 10.1002/cne.902330203. [DOI] [PubMed] [Google Scholar]
- Kisvárday Z. F., Martin K. A., Freund T. F., Maglóczky Z., Whitteridge D., Somogyi P. Synaptic targets of HRP-filled layer III pyramidal cells in the cat striate cortex. Exp Brain Res. 1986;64(3):541–552. doi: 10.1007/BF00340492. [DOI] [PubMed] [Google Scholar]
- Knierim J. J., van Essen D. C. Neuronal responses to static texture patterns in area V1 of the alert macaque monkey. J Neurophysiol. 1992 Apr;67(4):961–980. doi: 10.1152/jn.1992.67.4.961. [DOI] [PubMed] [Google Scholar]
- Lund J. S., Yoshioka T., Levitt J. B. Comparison of intrinsic connectivity in different areas of macaque monkey cerebral cortex. Cereb Cortex. 1993 Mar-Apr;3(2):148–162. doi: 10.1093/cercor/3.2.148. [DOI] [PubMed] [Google Scholar]
- Martin K. A., Whitteridge D. Form, function and intracortical projections of spiny neurones in the striate visual cortex of the cat. J Physiol. 1984 Aug;353:463–504. doi: 10.1113/jphysiol.1984.sp015347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire B. A., Gilbert C. D., Rivlin P. K., Wiesel T. N. Targets of horizontal connections in macaque primary visual cortex. J Comp Neurol. 1991 Mar 15;305(3):370–392. doi: 10.1002/cne.903050303. [DOI] [PubMed] [Google Scholar]
- Nelson S., Toth L., Sheth B., Sur M. Orientation selectivity of cortical neurons during intracellular blockade of inhibition. Science. 1994 Aug 5;265(5173):774–777. doi: 10.1126/science.8047882. [DOI] [PubMed] [Google Scholar]
- Pettet M. W., Gilbert C. D. Dynamic changes in receptive-field size in cat primary visual cortex. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8366–8370. doi: 10.1073/pnas.89.17.8366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran V. S., Gregory R. L. Perceptual filling in of artificially induced scotomas in human vision. Nature. 1991 Apr 25;350(6320):699–702. doi: 10.1038/350699a0. [DOI] [PubMed] [Google Scholar]
- Ratzlaff E. H., Grinvald A. A tandem-lens epifluorescence macroscope: hundred-fold brightness advantage for wide-field imaging. J Neurosci Methods. 1991 Feb;36(2-3):127–137. doi: 10.1016/0165-0270(91)90038-2. [DOI] [PubMed] [Google Scholar]
- Sillito A. M., Grieve K. L., Jones H. E., Cudeiro J., Davis J. Visual cortical mechanisms detecting focal orientation discontinuities. Nature. 1995 Nov 30;378(6556):492–496. doi: 10.1038/378492a0. [DOI] [PubMed] [Google Scholar]
- Stemmler M., Usher M., Niebur E. Lateral interactions in primary visual cortex: a model bridging physiology and psychophysics. Science. 1995 Sep 29;269(5232):1877–1880. doi: 10.1126/science.7569930. [DOI] [PubMed] [Google Scholar]
- Tootell R. B., Switkes E., Silverman M. S., Hamilton S. L. Functional anatomy of macaque striate cortex. II. Retinotopic organization. J Neurosci. 1988 May;8(5):1531–1568. doi: 10.1523/JNEUROSCI.08-05-01531.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman A. M., Gelade G. A feature-integration theory of attention. Cogn Psychol. 1980 Jan;12(1):97–136. doi: 10.1016/0010-0285(80)90005-5. [DOI] [PubMed] [Google Scholar]
- Tusa R. J., Palmer L. A., Rosenquist A. C. The retinotopic organization of area 17 (striate cortex) in the cat. J Comp Neurol. 1978 Jan 15;177(2):213–235. doi: 10.1002/cne.901770204. [DOI] [PubMed] [Google Scholar]