Abstract
Interpretation of quantitative trait locus (QTL) studies of agronomic traits is limited by lack of knowledge of biochemical pathways leading to trait expression. To more fully elucidate the biological significance of detected QTL, we chose a trait that is the product of a well-characterized pathway, namely the concentration of maysin, a C-glycosyl flavone, in silks of maize, Zea mays L. Maysin is a host-plant resistance factor against the corn earworm, Helicoverpa zea (Boddie). We determined silk maysin concentrations and restriction fragment length polymorphism genotypes at flavonoid pathway loci or linked markers for 285 F2 plants derived from the cross of lines GT114 and GT119. Single-factor analysis of variance indicated that the p1 region on chromosome 1 accounted for 58.0% of the phenotypic variance and showed additive gene action. The p1 locus is a transcription activator for portions of the flavonoid pathway. A second QTL, represented by marker umc 105a near the brown pericarp1 locus on chromosome 9, accounted for 10.8% of the variance. Gene action of this region was dominant for low maysin, but was only expressed in the presence of a functional p1 allele. The model explaining the greatest proportion of phenotypic variance (75.9%) included p1, umc105a, umc166b (chromosome 1), r1 (chromosome 10), and two epistatic interaction terms, p1 x umc105a and p1 x r1. Our results provide evidence that regulatory loci have a central role and that there is a complex interplay among different branches of the flavonoid pathway in the expression of this trait.
Full text
PDF
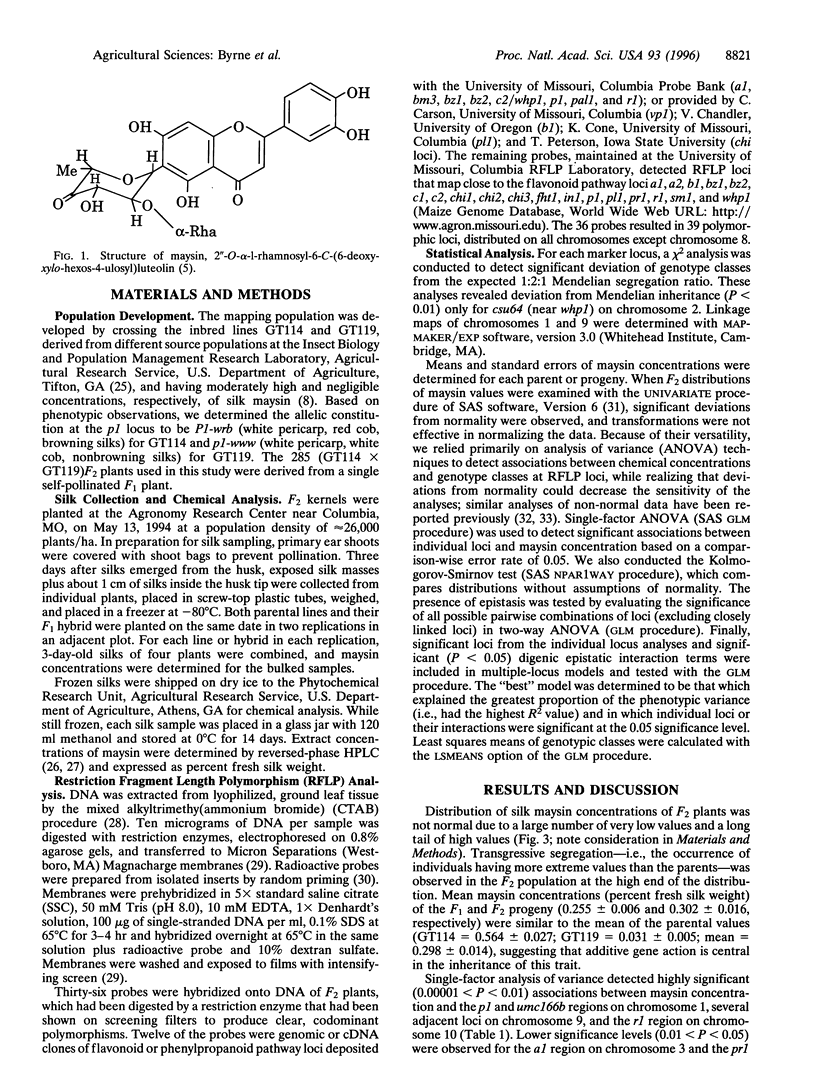
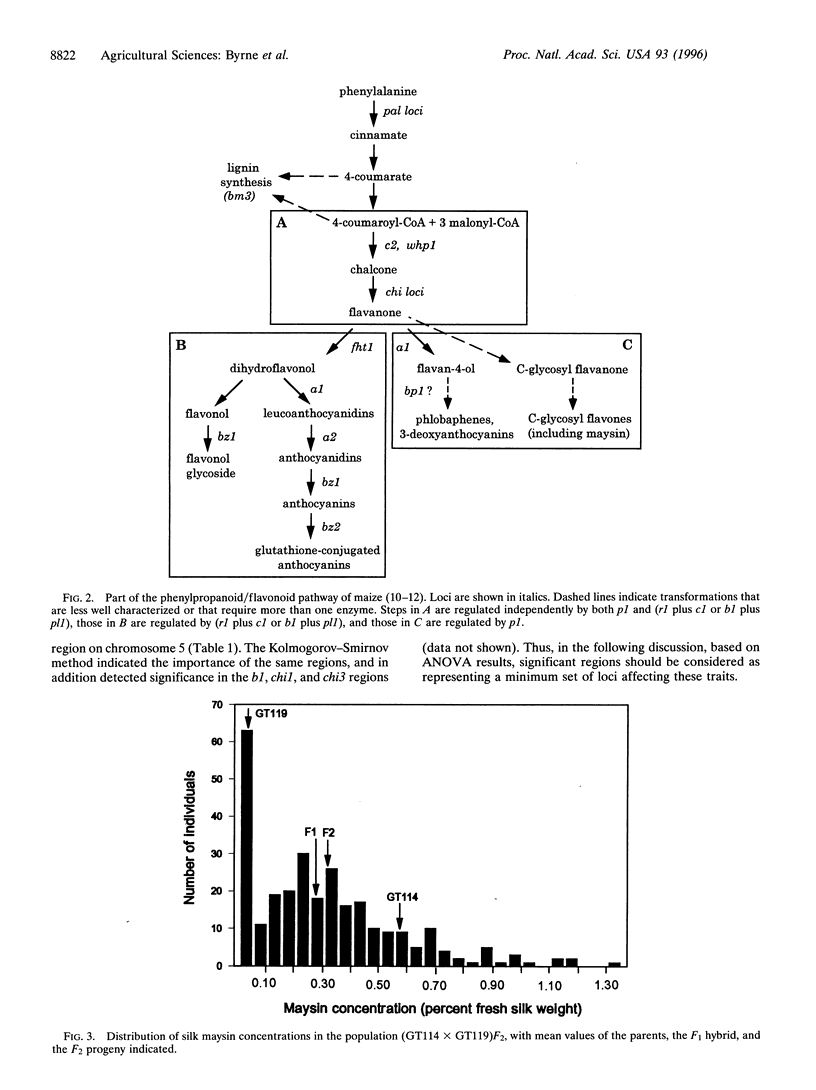
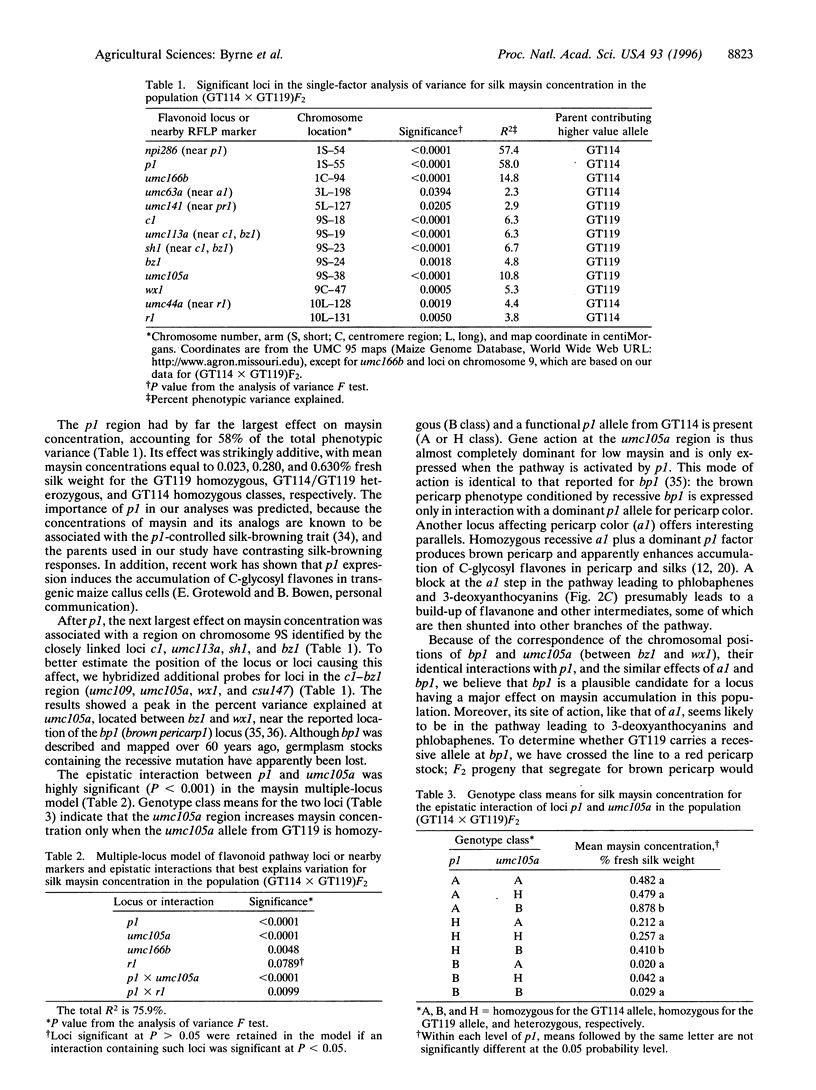


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Deboo G. B., Albertsen M. C., Taylor L. P. Flavanone 3-hydroxylase transcripts and flavonol accumulation are temporally coordinate in maize anthers. Plant J. 1995 May;7(5):703–713. doi: 10.1046/j.1365-313x.1995.07050703.x. [DOI] [PubMed] [Google Scholar]
- Doebley J., Stec A., Wendel J., Edwards M. Genetic and morphological analysis of a maize-teosinte F2 population: implications for the origin of maize. Proc Natl Acad Sci U S A. 1990 Dec 15;87(24):9888–9892. doi: 10.1073/pnas.87.24.9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooner H. K., Robbins T. P., Jorgensen R. A. Genetic and developmental control of anthocyanin biosynthesis. Annu Rev Genet. 1991;25:173–199. doi: 10.1146/annurev.ge.25.120191.001133. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gardiner J. M., Coe E. H., Melia-Hancock S., Hoisington D. A., Chao S. Development of a core RFLP map in maize using an immortalized F2 population. Genetics. 1993 Jul;134(3):917–930. doi: 10.1093/genetics/134.3.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotewold E., Drummond B. J., Bowen B., Peterson T. The myb-homologous P gene controls phlobaphene pigmentation in maize floral organs by directly activating a flavonoid biosynthetic gene subset. Cell. 1994 Feb 11;76(3):543–553. doi: 10.1016/0092-8674(94)90117-1. [DOI] [PubMed] [Google Scholar]
- Grotewold E., Peterson T. Isolation and characterization of a maize gene encoding chalcone flavonone isomerase. Mol Gen Genet. 1994 Jan;242(1):1–8. doi: 10.1007/BF00277341. [DOI] [PubMed] [Google Scholar]
- Kacser H., Burns J. A. The molecular basis of dominance. Genetics. 1981 Mar-Apr;97(3-4):639–666. doi: 10.1093/genetics/97.3-4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knogge W., Schmelzer E., Weissenböck G. The role of chalcone synthase in the regulation of flavonoid biosynthesis in developing oat primary leaves. Arch Biochem Biophys. 1986 Nov 1;250(2):364–372. doi: 10.1016/0003-9861(86)90738-1. [DOI] [PubMed] [Google Scholar]
- Lander E. S., Botstein D. Mapping mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics. 1989 Jan;121(1):185–199. doi: 10.1093/genetics/121.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs K. A., Alfenito M. R., Lloyd A. M., Walbot V. A glutathione S-transferase involved in vacuolar transfer encoded by the maize gene Bronze-2. Nature. 1995 Jun 1;375(6530):397–400. doi: 10.1038/375397a0. [DOI] [PubMed] [Google Scholar]
- McCarty D. R., Carson C. B., Stinard P. S., Robertson D. S. Molecular Analysis of viviparous-1: An Abscisic Acid-Insensitive Mutant of Maize. Plant Cell. 1989 May;1(5):523–532. doi: 10.1105/tpc.1.5.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghai-Maroof M. A., Soliman K. M., Jorgensen R. A., Allard R. W. Ribosomal DNA spacer-length polymorphisms in barley: mendelian inheritance, chromosomal location, and population dynamics. Proc Natl Acad Sci U S A. 1984 Dec;81(24):8014–8018. doi: 10.1073/pnas.81.24.8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignols F., Rigau J., Torres M. A., Capellades M., Puigdomènech P. The brown midrib3 (bm3) mutation in maize occurs in the gene encoding caffeic acid O-methyltransferase. Plant Cell. 1995 Apr;7(4):407–416. doi: 10.1105/tpc.7.4.407. [DOI] [PMC free article] [PubMed] [Google Scholar]


