Abstract
NF-κB is a pivotal transcription factor that controls cell survival and proliferation in diverse physiological processes. The activity of NF-κB is tightly controlled through its cytoplasmic sequestration by specific inhibitors, IκBs. Various cellular stimuli induce the activation of an IκB kinase (IKK), which phosphorylates IκBs and triggers their proteasomal degradation, causing nuclear translocation of activated NF-κB. Under normal conditions, the activation of NF-κB occurs transiently, thus ensuring rapid but temporary induction of target genes. Deregulated NF-κB activation contributes to the development of various diseases, including cancers and immunological disorders. Accumulated studies demonstrate that the NF-κB signaling pathway is a target of several human oncogenic viruses, including the human T-cell leukemia virus type 1 (HTLV1), the Kaposi sarcoma-associated herpesvirus (KSHV), and the Epstein bar virus (EBV). These viruses encode specific oncoproteins that target different signaling components of the NF-κB pathway, leading to persistent activation of NF-κB. This chapter will discuss the molecular mechanisms by which NF-κB is activated by the viral oncoproteins.
1. Introduction
The transcription factor NF-κB mediates inducible expression of a large number of genes involved in immune and inflammatory responses, cell proliferation and survival, and many other biological processes (Hayden and Ghosh 2008; Vallabhapurapu and Karin 2009). NF-κB represents a family of structurally related DNA-binding proteins, which in mammals includes RelA/p65, RelB, c-Rel, NF-κB1/p50, and NF-κB2/p52. The different NF-κB members can function as various homodimers and heterodimers that transactivate target genes bearing a κB enhancer sequence. Additionally, NF-κB also regulates gene expression via an epigenetic mechanism (Dong et al. 2008). The primary mechanism of NF-κB regulation involves its cytoplasmic sequestration by the inhibitory κB (IκB) family of proteins, including IκBα and homologous ankyrin repeat-containing proteins (Baldwin 1996). NF-κB1 and NF-κB2 are produced as precursor proteins, p105 and p100, which contain a C-terminal IκB-homologous portion and function as atypical IκB molecules (Beinke and Ley 2004). The canonical pathway of NF-κB activation involves rapid phosphorylation and degradation of the prototypical IκB member, IκBα, and concomitant nuclear translocation of p50-containing NF-κB dimeric complexes (Karin and Ben-Neriah 2000). This NF-κB signaling pathway is induced by diverse cellular stimuli and mediates pleotropic biological functions. NF-κB activation in specific cell types, such as B lymphocytes and lymphoid stromal cells, also involves a noncanonical pathway that is dependent on site-specific phosphorylation and processing of the NF-κB2 precursor protein p100 (Xiao et al. 2001b). This atypical pathway is specifically elicited by a subset of immune receptors that belong to the TNF receptor superfamily and mediates nuclear accumulation of p52/RelB dimer (Claudio et al. 2002; Coope et al. 2002; Dejardin et al. 2002; Kayagaki et al. 2002; Novack et al. 2003).
A central step in NF-κB signaling is activation of the IκB kinase (IKK). The IKK that mediates canonical NF-κB signaling pathway is composed of two catalytic subunits, IKKα and IKKβ, and a regulatory subunit, NEMO (also known as IKKγ and FIP-3) (Karin and Ben-Neriah 2000). Activation of the noncanonical NF-κB does not require IKKβ or NEMO but relies on IKKα as well as its upstream kinase NIK (Senftleben et al. 2001; Xiao et al. 2001b). Consistently, IKKα efficiently phosphorylates the C-terminal region of p100 (Senftleben et al. 2001). Under physiological conditions, NF-κB activation occurs transiently when cells receive a stimulus. This is due to the negative regulation of NF-κB signaling at multiple levels. However, the NF-κB pathway is constitutively activated in various cancer cells (Sun and Xiao 2003). The deregulated NF-κB activation may involve genetic mutations of regulatory factors or persistent stimulation of NF-κB signaling by pathogens (Sun and Xiao 2003). In particular, the NF-κB signaling pathway is a major cellular target of several human oncogenic viruses, including the human T-cell leukemia virus type 1 (HTLV1), the Kaposi sarcoma-associated herpesvirus (KSHV), and the Epstein bar virus (EBV). These viruses encode specific proteins that deregulate the NF-κB signaling pathway. In this review, we will discuss the molecular mechanisms by which the viral oncoproteins induce persistent NF-κB activation.
2. NF-κB activation by HTLV1
2.1 HTLV1 and adult T-cell leukemia
HTLV1 belongs to the Delta retrovirus genera, which also includes the HTLV1-related virus HTLV2, the simian T-cell leukemia virus type 1, and the bovine leukemia virus (Burmeister 2001). HTLV1 is the etiologic agent of adult T-cell leukemia (ATL), an acute malignancy of CD4+ T cells (Poiesz et al. 1980; Takatsuki 2005; Yoshida et al. 1982). This pathogen is endemic in certain areas of the world, including Southern Japan, Caribbean islands, South America, and sub-Saharan Africa (Verdonck et al. 2007). HTLV1 transmission occurs primarily via sexual contact, blood transfusion, and breast-feeding. An estimated 20 million people worldwide are infected with HTLV1, among which about 4% (6% male and 2% female) are expected to develop ATL (Taylor and Matsuoka 2005).
The major clinical features of ATL include the presence of CD4+CD25+ leukemic T cells with flower-shaped nuclei in peripheral blood, skin lesion with infiltrated leukemic T cells, hypercalcemia, and lymphadenopathy (Yasunaga and Matsuoka 2007). The development of ATL in HTLV1-infected individuals follows a long clinical latency (20-30 years), and the disease progress can be divided into four stages: asymptomatic, pre-ATL, chronic/smouldering ATL, and acute ATL (Yasunaga and Matsuoka 2007; Yoshida 2001). The low frequency and long clinical latency of ATL are a consequence of the oncogenic mechanism of HTLV1. Unlike the acute transforming retroviruses of animals (Burmeister 2001), HTLV1 lacks a typical oncogene of cellular origin and induces T-cell transformation through a so-called “transactivating” mechanism that involves aberrant induction of cellular genes regulating T-cell growth and survival (Matsuoka 2003). HTLV1 infection stimulates T cells to express the T-cell growth factor interleukin-2 (IL-2) (Maruyama et al. 1987; Siekevitz et al. 1987) and the α subunit of its high affinity receptor complex (CD25 or IL-2Rα) (Cross et al. 1987; Inoue et al. 1986; Siekevitz et al. 1987). In addition, HTLV1 induces the abnormal expression of various other cytokines, apoptosis inhibitors, cell cycle regulators, and proto-oncogenes (de La Fuente et al. 2000; Harhaj et al. 1999; Koga et al. 2004; Mori et al. 2002; Pise-Masison et al. 2002; Sasaki et al. 2005; Sinha-Datta et al. 2004; Tsukasaki et al. 2004). It is generally believed that induction of ATL by HTLV1 involves an early-phase of polyclonal T-cell proliferation and acquisition of anti-apoptotic ability, followed by T-cell immortalization characterized by indefinite proliferation in the presence of the T-cell growth factor IL-2. Over time, genetic and epigenetic abnormalities accumulate in the immortalized T cells, which promote the generation of a transformed T-cell clone that grows independently of IL-2 and contributes to leukemogenesis (Matsuoka and Jeang 2007; Yoshida 2001).
2.2 Tax as a primary oncogenic mediator of HTLV1
In addition to the structural genes common to all retroviruses, the HTLV1 genome contains a region termed pX, which encodes two regulatory proteins, Tax and Rex, as well as a number of accessory proteins (Matsuoka and Jeang 2007). Several of these pX-encoded proteins display signaling functions, with the most notable one being the 40-kD Tax protein. Tax serves as the transactivator of HTLV1-encoded genes and, thus, is required for viral replication (Franchini et al. 2003; Jeang 2001; Yoshida 2001). Moreover, Tax is largely responsible for the induction of cellular gene expression by HTLV1 (Ng et al. 2001; Sun and Ballard 1999). Strong evidence suggests that Tax is the primary oncogenic mediator of HTLV1 (Franchini et al. 2003). Studies using an HTLV1 molecular clone demonstrate that Tax is essential for the in vitro induction of T-cell immortalization by HTLV1 (Robek and Ratner 1999). Furthermore, expression of Tax in the absence of other HTLV1 gene products is sufficient to immortalize human CD4+ cord blood T cells and transform fibroblasts in cell culture (Grassmann et al. 1992; Grassmann et al. 1989; Pozzatti et al. 1990; Tanaka et al. 1990). The in vivo oncogenic potential of Tax has been firmly demonstrated using animal models (Lairmore et al. 2005). Transgenic expression of Tax using HTLV1 long-terminal repeat (LTR) or cellular promoters induces different types of tumors in mice (Lairmore et al. 2005). In particular interest are transgenic mice that express Tax under the control of the T cell-specific Lck distal promoter. At old ages, a proportion of these mice spontaneously develop pre-T cell leukemia and mature T-cell leukemia (Hasegawa et al. 2006; Ohsugi et al. 2007). As seen with ATL patients, the clinical latency of the Tax-transgenic mice is extremely long, which is probably why T-cell leukemia was not detected in some other studies using similar mouse models (Lairmore et al. 2005). In some cases, the severe inflammation of the Tax transgenic mice causes early lethality, which precludes the examination of tumorigenesis at old ages (Kwon et al. 2005). Notwithstanding, these studies establish Tax as a primary oncogenic mediator of HTLV1.
2.3 Persistent activation of NF-κB by Tax
Despite its potent gene induction function, Tax is not an intrinsic transcription factor due to its lack of a DNA-binding domain. Instead, Tax induces target gene expression by modulation of cellular transcription factors, most notably members of the CREB/ATF and NF-κB families (Sun and Ballard 1999). Tax directly interacts with CREB/ATF to form a transcription factor complex that activates the transcription of HTLV1-encoded genes through binding to Tax-responsive enhancer elements within the viral LTR (Suzuki et al. 1993; Zhao and Giam 1991; Zhao and Giam 1992). On the other hand, Tax stimulates the nuclear translocation of NF-κB, which is largely responsible for the induction of various cellular genes (Sun and Ballard 1999). In contrast to its tight regulation in normal T cells, NF-κB is constitutively activated in HTLV1-transformed and Tax-expressing cells. NF-κB activation is also a hallmark of tumor cells isolated from Tax-transgenic mice (Lairmore et al. 2005). Inhibition of NF-κB by antisense oligonucleotides inhibits the growth of Tax-transformed cells and causes tumor regression in Tax-transgenic mice (Kitajima et al. 1992). Studies using an infectious HTLV1 molecular clone further demonstrate that the NF-κB-activating function of Tax is required for HTLV1-induced immortalization of human T cells (Robek and Ratner 1999).
A hallmark of Tax-stimulated NF-κB activation is the involvement of both canonical and noncanonical pathways (Sun and Yamaoka 2005). Under normal conditions, stimulation of T cells by mitogens or the T-cell receptor (TCR) signal does not lead to strong activation of the noncanonical NF-κB (Xiao et al. 2001a). Remarkably, Tax expression in both T cells and nonlymphoid cells results in potent induction of noncanonical NF-κB signaling, characterized by processing of p100 and activation of p52-containing NF-κB dimers (Xiao et al. 2001a). Recent evidence suggests that Tax, but not the HTLV2-encoded Tax2 protein, activates the noncanonical NF-κB pathway, although both Tax and Tax2 activate the canonical NF-κB pathway (Higuchi et al. 2007). This finding has important implications, since HTLV1, but not HTLV2, is etiologically linked to human malignancies (Feuer and Green 2005). At least in vitro, the ability of Tax to activate noncanonical NF-κB is required for Tax-mediated induction of T-cell transformation, as determined by the conversion of a T-cell line from IL-2 dependent to IL-2 independent growth (Higuchi et al. 2007). As will be discussed in a following section, activation of the noncanonical NF-κB pathway is also a major feature in freshly isolated ATL cells.
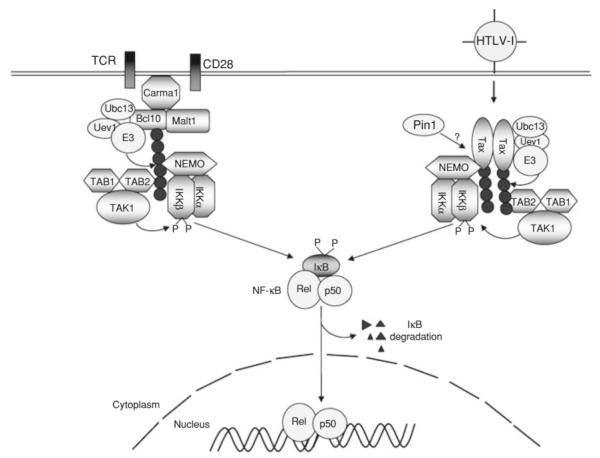
2.4 Targeting IKK signaling components by Tax
Activation of NF-κB by TCR and the CD28 costimulatory molecule involves the transient assembly of a signaling complex, composed of the scaffold protein Carma1, the adaptor protein Bcl10, the paracaspase Malt1, a yet-to-be characterized E3 ubiquitin ligase, and the lysine 63 (K63)-specific ubiquitin-conjugating enzyme (E2) dimer Ubc13/Uev1 (Fig. 1). K63-linked ubiquitin chains facilitate the recruitment and activation of IKK and its activating kinase, Tak1. Both NEMO and the Tak1-binding protein 2 (TAB2) contain a ubiquitin-association (UBA) domain, which mediates the ubiquitin-dependent IKK/Tak1 recruitment (Skaug et al. 2009). Due to the presence of multiple negative regulators, IKK activation by TCR and other immune receptors occurs transiently (Coornaert et al. 2009; Sun 2009; Sun and Ley 2008). However, IKK is constitutively activated in HTLV1-infected T cells and Tax-transfected cells (Chu et al. 1998; Geleziunas et al. 1998; Sun and Ballard 1999; Uhlik et al. 1998; Yin et al. 1998). Strong evidence suggests that Tax-mediated persistent activation of IKK involves stable assembly of a Tax/IKK signaling complex (Fig. 1). The assembly of this virus-specific signaling complex requires physical interaction between Tax and the IKK regulatory subunit, NEMO (Chu et al. 1999; Harhaj and Sun 1999; Jin et al. 1999). NEMO has two homologous leucine zipper (LZ) domains that are required for interaction with Tax (Xiao et al. 2000). Although Tax does not contain a typical LZ domain, it has a leucine-rich repeat region that is critical for interaction with NEMO (Xiao et al. 2000). Point mutations of the leucines or a conserved upstream motif of this region of Tax abolishes its binding to NEMO. Studies using Tax mutants and NEMO-Tax chimera proteins clearly demonstrate an adaptor function of NEMO in the recruitment of Tax to the IKK catalytic subunits (Xiao et al. 2000; Xiao and Sun 2000). Together, these studies establish the Tax/IKK physical association as a mechanism by which Tax persistently activates NF-κB.
Fig. 1.
Canonical NF-κB activation by TCR/CD28 and HTLV1 Tax. Canonical NF-κB activation by the T-cell receptor (TCR) and CD28 costimulatory molecule involves transient assembly of an intermediate signaling complex composed of Carma1, Bcl10, and Malt1. This so-called CBM complex is also associated with the ubiquitin-conjugating enzyme (E2) Ubc13/Uev1 and a yet-to-be characterized E3 ubiquitin ligase. Within this signaling complex, Bcl10 and probably also Malt1 are conjugated with K63-linked ubiquitin chains that function as a platform to recruit the IKK and Tak1 complexes for their activation. Tax forms a stable complex with IKK and Tak1 and thereby persistently activates these kinases and NF-κB. This viral pathway involves K63 type of ubiquitination of Tax, although how ubiquitination regulates the Tax-specific NF-κB signaling is less clear. Ubiquitination, and possibly Pin1-mediated isomerization, of Tax may facilitate the binding of Tax to NEMO. It is also possible that Tax ubiquitination facilitates its binding by the Tak1 complex via the ubiquitin-association function of Tab2.
The Tax-NEMO binding appears to occur directly, since it has also been detected by yeast two-hybrid assays (Jin et al. 1999). However, optimal Tax-NEMO interaction may require additional cellular factors or involve post-translational modifications of Tax or NEMO (Fig. 1). As will be discussed in a following section, ubiquitination of Tax has been suggested to promote Tax/NEMO binding (Nasr et al. 2006). A recent study reveals that the stable association of Tax with NEMO also requires a peptidylproline cis-transisomerase, Pin1 (Peloponese et al. 2009). Pin1 is known to isomerize phosphorylated serine/threonin-proline bonds in target proteins, an action that may modulate the activity of signaling molecules by causing their conformational changes (Lu and Zhou 2007). Pin1 is over expressed in Tax-expressing and HTLV1-transformed T cell lines and is required for Tax-mediated NF-κB activation (Peloponese et al. 2009). Since Pin1 binds to Tax, it is possible that Pin1 promotes Tax-NEMO association through isomerization of Tax (Fig. 1). However, it is currently unclear whether the enzymatic activity of Pin1 is essential for promoting the Tax-NEMO interaction.
How the Tax-IKK physical association leads to IKK activation is incompletely understood. One possible mechanism is Tax-stimulated oligomerization of NEMO, which may facilitate the catalytic activation of IKK catalytic subunits (Huang et al. 2002). However, the IKK oligomerization is unlikely sufficient for triggering its catalytic activity, since upstream kinases are also required for Tax-stimulated IKK activation (Wu and Sun 2007; Yin et al. 1998). In particular, Tax physically associates with and stimulates the catalytic activity of the IKK-activating kinase Tak1 (Wu and Sun 2007) (Fig. 1). Tak1 is constitutively activated in both Tax-transfected and HTLV1-infected cells (Wu and Sun 2007; Yu et al. 2008). Tax is physically assembled into the Tak1/IKK complex in HTLV1-infected T cells. In transfected cells, Tax not only activates Tak1 but also induces its association with the IKK complex (Wu and Sun 2007). Tak1 activation by cellular signals involves the Tak1-associated ubiquitin-binding protein TAB2 (Adhikari et al. 2007). Since TAB2 is also involved in Tax-mediated activation of Tak1 and NF-κB (Yu et al. 2008), it suggests the possible involvement of ubiquitination in the association between Tax and the Tak1 complex (Fig. 1). It is also likely that the Tax-mediated persistent IKK activation involves Tax-stimulated assembly of Tak1/IKK complex (Fig. 1).
IKK activation by antigen receptors involves its recruitment into lipid raft and the central region of the immunological synapse (Hara et al. 2004; Khoshnan et al. 2000; Su et al. 2002). The lipid-raft relocalization of IKK is mediated by the scaffold protein Carma1 (Hara et al. 2004), which constitutively associates with lipid raft and recruits downstream signaling factors upon phosphorylation by PKCtheta (Gaide et al. 2002; Matsumoto et al. 2005; Sommer et al. 2005). Interestingly, Tax possesses lipid raft-associating function, and in HTLV1-transformed T cell lines, the Tax/IKK complex is constitutively present in the Golgi-associated lipid raft microdomains (Huang et al. 2009). It would be important to examine whether the Tax-mediated lipid raft recruitment of IKK is independent of Carma1.
2.5. Tax-specific mechanism of noncanonical NF-κB activation
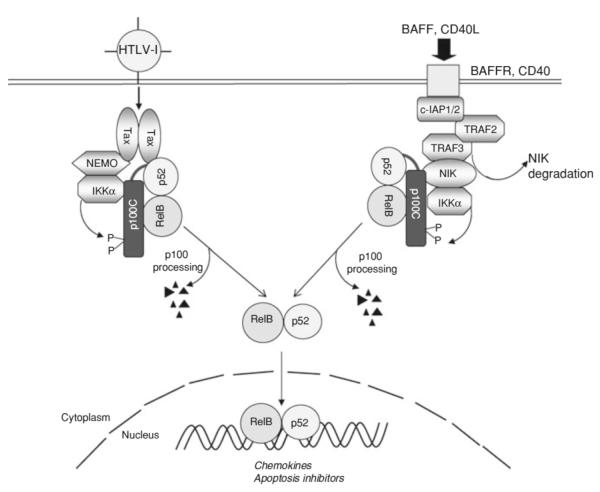
Under physiological conditions, active processing of p100 (the central step of noncanonical NF-κB signaling) occurs in B cells but not in T cells. This is because of the selective response of the noncanonical NF-κB signaling pathway to specific signals, including those delivered by CD40 and BAFF on B cells (Pomerantz and Baltimore 2002) (Fig. 2). The active processing of p100 in HTLV1-transformed T cells is mediated by a Tax-specific signaling mechanism that has both similarities and major differences from the cellular mechanism (Xiao et al. 2001a) (Fig. 2). As seen with cellular signals, Tax-stimulated p100 processing requires its C-terminal phosphorylation by IKKα. However, the Tax-specific pathway does not seem to require NIK, which is a central component of the cellular pathway (Xiao et al. 2001a; Xiao et al. 2001b). Furthermore, although NEMO is dispensable for the induction of p100 processing by cellular signals, this IKK regulatory subunit is essential for the Tax-specific noncanonical NF-κB pathway (Senftleben et al. 2001; Xiao et al. 2001a). A major function of NEMO in the Tax-specific pathway is to serve as an adaptor in the assembly of the Tax/IKKα signaling complex (Fig. 2). Another unique feature of Tax-stimulated p100 processing is the requirement of Tax/p100 physical interaction (Fig. 2). Tax forms a stable complex with p100 in both HTLV1-transformed T cells and Tax-transfected cells (Béraud et al. 1994), and this molecular interaction requires two N-terminal alpha helices of p100 and is essential for Tax-stimulated p100 processing (Xiao et al. 2001a). The Tax/p100 interaction may allow Tax to recruit p100 to IKKα for phoshorylation. In both transfected cells and HTLV1-transformed T cells, Tax is present in a complex that contains p100, IKKα, and NEMO. Like Tax, NIK physically interacts with both IKKα and p100 and induces IKKα/p100 complex formation (Xiao et al. 2004). However, unlike Tax, NIK binds to IKKα independently of NEMO, which explains why the Tax-specific pathway, but not the cellular pathway, requires NEMO (Fig. 2). Another function of NIK is to stimulate the catalytic activity of IKKα. Since NIK seems to be dispensable for Tax-stimulated p100 processing (Xiao et al. 2001a), it is unclear whether Tax-mediated activation of IKKα involves a different upstream kinase.
Fig. 2.
Tax-specific noncanonical NF-κB pathway. Noncanonical NF-κB signaling pathway is stimulated in B cells by the BAFFR and CD40 signals. This pathway is negatively regulated by TRAF3, which recruits the E3 ubiquitin ligase c-IAP1 or c-IAP2 via TRAF2 and induces ubiquitin-dependent degradation of NIK. The receptor signals induce degradation of TRAF3 and TRAF2, leading to accumulation of NIK and NIK/IKKα-mediated p100 C-termimal phosphorylation. The phosphorylated p100 is then processed through the ubiquitin/proteasome pathway and produce the mature NF-κB2 p52 as a dimer with RelB. Under normal conditions, active processing of p100 does not occur in T cells. However, in HTLV1 infected T cells, Tax initiates an active noncanonical NF-κB pathway by bridging p100 and IKKα. In contrast to the cellular pathway, which is independent of NEMO, the viral pathway requires NEMO, which may function as an adaptor for Tax/IKKα association.
2.6 Ubiquitination in Tax-mediated IKK/NF-κB activation
Ubiquitination has emerged as a central mechanism that mediates activation of the NF-κB signaling pathway (Chen 2005; Sun and Ley 2008). Polyubiquitin chains are formed through linkage of the carboxy-terminal glycine residue of one ubiquitin to an internal lysine (K) residue of another ubiquitin, with K48- and K63-linked polyubiquitin chains been the most extensively studied. Whereas lysine 48 (K48)-linked polyubiquitin chains mediate protein degradation by 26S proteasome(Chau et al. 1989), K63-linked polyubiquitin chains are involved in assembly of signal transduction complexes(Chen 2005; Sun and Ley 2008). Activation of IKK is associated with K63-linked ubiquitination of NEMO as well as specific upstream adaptors, such as TRAF6 and RIP1 (Chen et al. 2006; Sun et al. 2004; Zhou et al. 2004). Ubiquitinated adaptors serve as a platform that recruits IKK and the IKK-activating kinase, Tak1, for their activation (Ea et al. 2006; Li et al. 2006; Wu et al. 2006) (Fig. 1).
Strong evidence suggests that Tax-mediated IKK activation also involves ubiquitination. Tax undergoes polyubiquitination in both transfected cells and HTLV1-transformed T cells (Lamsoul et al. 2005; Nasr et al. 2006). The ubiquitination of Tax appears to be required for its association with the IKK complex and induction of NF-κB nuclear translocation. Tax also undergoes sumoylation, which regulates the colocalization of Tax with p300 and NF-κB RelA in nuclear bodies and full NF-κB transcriptional activation (Lamsoul et al. 2005; Nasr et al. 2006). The ubiquitin chains conjugated on Tax are predominantly K63-linked, and consistently, the Tax ubiquitination is largely dependent on the K63-specific ubiquitin-conjugating enzyme Ubc13 (Shembade et al. 2007b) (Fig. 1). Ubiquitination of Tax seems to be important for Tax-NEMO physical interaction (Nasr et al. 2006). NEMO interaction with cellular activators, such as RIP1 and TRAF6, is also dependent on K63-linked ubiquitination (Ea et al. 2006; Li et al. 2006; Wu et al. 2006). NEMO has a UBA motif that facilitates NEMO binding to ubiquitinated RIP1. However, the ubiquitin-binding function of NEMO is dispensable for Tax-mediated activation of NF-κB (Wu and Sun 2007). Thus, it remains unclear how Tax ubiquitination promotes its association with NEMO and mediates activation of NF-κB.
Another potential function of Tax ubiquitination is to target this viral protein, as well as its associated IKK complex, to specific cellular compartments. In both HTLV1-infected and Tax-expressing T-cell lines, Tax and IKK are colocalized in perinuclear “hot spots” associated with the Golgi (Harhaj et al. 2007). This signaling event, which is induced by Tax, requires Tax-NEMO interaction and correlates with the NF-κB-activation activity of Tax. Tax ubiquitination appears to be important for relocalization of IKK into the Golgi-signaling complex, since a Tax mutant lacking its major ubiquitination-acceptor sites is defective in this function (Harhaj et al. 2007). Another study suggests that the Tax/IKK complex is present in the lipid raft microdomains of the Golgi, suggesting a lipid raft-dependent mechanism of IKK activation (Huang et al. 2009). It has also been suggested that Tax ubiquitination may promote the localization of Tax to the centrosome (Kfoury et al. 2008), a microtubule structure that regulates not only mitosis but also multiple other cellular functions, including signal transduction (Doxsey et al. 2005). The K63-ubiquitinated Tax seems to colocalize with NEMO in the centrosome, suggesting the possibility that Tax activation of IKK occurs at centrosome (Kfoury et al. 2008).
How Tax ubiquitination is regulated is still poorly understood, but a potential mechanism is suggested by the recent finding that ubiquitinated Tax is bound by NEMO-related protein (NRP) (Journo et al. 2009). NRP was originally identified as an apoptosis-regulatory protein, FIP-2, that binds to the adenoviral protein E3-14.7K (Li et al. 1998). More recently, NRP was shown to be mutated in patients with open-angle glaucoma, and therefore it was also named Optineurin (Rezaie et al. 2002). Like NEMO, NRP contains a UBA motif, which is required for interaction with ubiquitinated Tax (Journo et al. 2009). NRP appears to stabilize the polyubiquitination chains of Tax and, thereby, promote Tax-stimulated NF-kB signaling (Journo et al. 2009). NRP also interacts with TAX1BP1, and these two proteins cooperate to enhance Tax ubiquitination and Tax-mediated NF-κB activation. This latter finding is unexpected, since TAX1BP1 is known to promote deubiquitination and negatively regulate NF-κB activation by cellular stimuli (Shembade et al. 2007a). Tax1BP1 functions as an adaptor of A20 and is required for A20-mediated deubiquitination of important NF-κB signaling components, RIP1 and TRAF6 (Shembade et al. 2007a). Tax1BP1 also recruits the K48-specific ubiquitin ligase Itch to A20, thereby mediating the degradation of RIP1 and termination of TNFa-stimulated NF-κB activation (Shembade et al. 2008). It is likely that Tax may dysregulate the function of Tax1BP1 and prevents its participation in the A20 ubiquitin editing function. This idea is supported by the finding that Tax disrupts the complex assembly between Tax1BP1, A20, and Itch (Shembade et al. 2008). Since Tax1BP1 binds to Tax, it is possible that Tax1BP1 promotes Tax ubiquitination by recruiting an E3 ubiquitin ligase to Tax.
In addition to inducing polyubiquitination of NEMO, Tax induces monoubiquitination of the IKK catalytic subunit IKKβ (Carter et al. 2003). The IKKβ monoubiquitination, which can also be stimulated by cellular signals, is triggered by its phosphorylation at Ser-177/Ser-181 within the T loop (Carter et al. 2005). The T-loop phosphorylation of IKKβ recruits a ubiquitin ligase Ro52 (also called TRIM21), which catalyzes monoubiquitination of IKKβ in cooperation with the ubiquitin-conjugating enzyme UbcH5b (Wada et al. 2009). Fusion of IKKβ with ubiquitin inhibits IKKβ function, suggesting a negative role of monoubiquitination in IKKβ regulation (Wada et al. 2009). Consistently, Ro52 overexpression suppresses IKKβ-mediated NF-κB activation, and Ro52-deficient embryonic fibroblasts display hyper activation of NF-κB in response to toll-like receptor stimulation (Yoshimi et al. 2009). It will be important to examine whether Ro52 knockout or knockdown attenuates Tax-stimulated IKKβ monoubiquitination and promotes Tax-mediated IKK activation.
2.7 Tax-independent activation of NF-κB in ATL cells
Although Tax is essential for HTLV1-induced T-cell transformation, this viral gene product may not be required for the late-stage of HTLV1 leukemogenesis. Freshly isolated ATL cells often lack detectable expression of viral gene products, including Tax, which is thought to be due to the antiviral immune surveillance (Horie 2007; Sun and Yamaoka 2005). Interestingly, the Tax-negative ATL cells still display constitutive NF-κB activity, thus emphasizing a role for NF-κB in regulating both the initiation and maintenance of HTLV1-induced leukemogenesis (Hironaka et al. 2004). It is currently unclear how the Tax-independent NF-κB activation is mediated in ATL cells, but it appears to involve constitutive activation of IKK (Hironaka et al. 2004). Expression of a dominant-negative IKKα, but not dominant-negative IKKβ or NEMO, inhibits the constitutive NF-κB activity in ATL cells. Consistently, the ATL cells display elevated levels of p52, a product of the non-canonical NF-κB pathway mediated by IKKα (Hironaka et al. 2004). A more recent study further reveals that the IKKα-activating kinase, NIK (NF-κB inducing kinase), is overexpressed in ATL cells derived from a large proportion of patients (Saitoh et al. 2008). Deregulated expression of NIK may contribute to the Tax-independent activation of NF-κB in at least some of the ATL cells. Of note, Tax-dependent activation of the noncanonical NF-κB seems to be independent of NIK but is dependent on NEMO (Xiao et al. 2001a). This is because Tax physically targets IKKα via the adaptor function of NEMO. Thus, NIK is an attractive therapeutic target for treating Tax-negative ATLs, although inhibition of NIK may not be sufficient for blocking NF-κB in Tax-positive ATL cells.
Since NIK overexpression was not detected in some of the ATL cells, additional mechanism is obviously involved in the Tax-independent NF-κB activation. It would be important to examine whether the rest of the ATL cells have overexpression or activation of other IKK-activating kinases, such as MEKK1, MEKK3, Tak1, and Cot (also known as Tpl2). All of these kinases are potent IKK activators, although only some of them display non-redundant physiological IKK-regulatory functions (Sun and Xiao 2003). In fact, both Cot and Tak1 are constitutively activated in Tax-positive HTLV1-transformed T-cell lines (Babu et al. 2006; Wu and Sun 2007), although their expression level and activity in freshly isolated ATL cells have not been analyzed.
Given the complex nature of the HTLV1 genome, the involvement of a yet-to-be characterized viral gene product in Tax-independent NF-κB activation is also a possibility. Recent studies have led to the identification of a novel HTLV1 gene product, HTLV1 basic leucine zipper (HBZ), which is encoded by an antisense mRNA transcribed from the 3′LTR (Matsuoka and Green 2009; Satou et al. 2006). Unlike other viral gene products, HBZ is consistently expressed in HTLV1-transformed T-cell lines and freshly isolated ATL cells, implying an important role in leukemogenesis (Matsuoka and Green 2009). HBZ expression enhances the proliferation capacity of HTLV1-infected T cells in vitro and sensitizes T-cell activation in transgenic mice (Arnold et al. 2008; Satou et al. 2006). Surprisingly, despite its growth-stimulatory function, HBZ inhibits Tax-stimulated canonical, although not noncanonical, NF-kB activation under overexpression conditions (Zhao et al. 2009). HBZ acts through promoting the ubiquitination and subsequent degradation of RelA. It will be interesting to examine whether RNAi suppression of HBZ expression in ATL cells affects the Tax-independent activation of NF-κB, particularly the noncanonical pathway.
2.8 NF-κB in HTLV1-stimulated T-cell transformation
A major pathological characteristic of HTLV1-transformed T cells is their high resistance to apoptosis induction by different mechanisms (Taylor and Nicot 2008). It is generally believed that apoptosis inhibition contributes to both HTLV1-mediated T-cell transformation and the resistance of the ATL cells to conventional chemotherapies. The HTLV1-infected and Tax-expressing cells overexpress various anti-apoptotic genes, such as c-IAP1, c-IAP2, c-FLIP, Bcl-XL, and survivin (Harhaj et al. 1999; Okamoto et al. 2006; Sanda et al. 2006; Tsukahara et al. 1999; Wäldele et al. 2006). Since these survival genes are typical targets of the NF-κB signaling pathway, it is likely that the constitutive NF-κB activation is responsible for the anti-apoptotic phenotype of HTLV1-transformed T cells. This idea has indeed been confirmed by a number of studies (Portis et al. 2001; Taylor and Nicot 2008; Tsukahara et al. 1999; Wäldele et al. 2006). Tax-mediated apoptosis inhibition also involves activation of PI3 kinase (PI3K) and its downstream survival kinase AKT (Jeong et al. 2005b; Liu et al. 2001). AKT promotes cell survival by activating NF-κB, which in turn induces expression of apoptosis inhibitors (Jeong et al. 2005b). Recent evidence suggests that AKT and NF-κB may be mutually regulated. AKT activation by Tax involves NF-κB, which promotes the activation of PI3K and AKT through suppressing the expression of PI3K-inhibitory phosphatases, PTEN and SHIP-1 (Fukuda et al. 2009).
Another potential mechanism by which NF-κB promotes HTLV1-mediated T-cell transformation is inhibition of the tumor-suppressor p53. In HLTV1-transformed T cells, p53 is functionally inactive despite its competent expression and lack of structural alterations (Cereseto et al. 1996; Gartenhaus and Wang 1995; Pise-Masison et al. 1998; Reid et al. 1993; Takemoto et al. 2000). The p53 inactivation is mediated by Tax and involves activation of NF-κB (Pise-Masison and Brady 2005). Tax induces phosphorylation of the NF-κB component RelA at serine-536, which promotes the physical interaction between RelA and p53 and, thereby, inactivation of the transactivation function of p53 (Jeong et al. 2005a). The Tax-mediated RelA phosphorylation and p53 inactivation involve both IKKβ and AKT, although the precise underlying mechanism has not been clearly defined (Jeong et al. 2005a; Jeong et al. 2005b). These findings suggest the intriguing possibility that IKK inhibitors may function as a double sword in ATL therapy, since they may both block NF-κB activation and restore the function of p53.
Tumorigenesis is often promoted by chronic inflammation, a pathologic process that is largely dependent on NF-κB (Karin and Greten 2005). Inflammation is a typical feature of HTLV1-infected patients and Tax transgenic mice (Kwon et al. 2005; Peloponese et al. 2006). Whether inflammation contributes to development of ATL is unclear. Nevertheless, a recent study suggests that Tax-induced inflammation precedes the onset of lymphoma formation in mice (Rauch et al. 2009). As seen with tumorigenesis, Tax-induced inflammation in mice is dependent on NF-κB activation (Kwon et al. 2005). Therefore, it is likely that NF-κB promotes HTLV1-mediated T-cell malignancy via different mechanisms, including inhibition of apoptosis, repression of tumor suppressors, and induction of inflammation.
3. NF-κB Activation by KSHV
3.1 Diseases associated with KSHV infection
KSHV, also called human herpesvirus 8 (HHV-8), is found invariably in Kaposi’s sarcoma ( Chang et al. 1994; Boshoff and Weiss 1998) and in several lymphoproliferative disorders, that include primary effusion lymphoma (PEL) (Cesarman et al. 1995a), multicentric Castleman’s disease (MCD) (Soulier et al. 1995) and MCD-associated plasmablastic lymphoma (Dupin et al. 2000).
3.1.1 Kaposi’s sarcoma (KS)
Compelling evidence indicates that KSHV is an etiologic agent for KS (Chang et al. 1994). Over 22 cohort and 80 case control epidemiologic studies have confirmed the association between KSHV and KS (Bouvard et al. 2009). KS is the most common cancer in HIV-infected individuals, and it is currently one of the most common malignancies in many subequatorial African countries, where endemic KS had been relatively common even before the epidemic of HIV/AIDS (Parkin 2006; Sinfield et al. 2007). Although the incidence of AIDS-KS in the Western world has declined since the widespread implementation of highly active antiretroviral treatment (HAART), it remains increased as compared to the pre-AIDS era (Eltom et al. 2002).
Four clinical-epidemiological forms of KS have been described, which are: 1) sporadic or European; 2) endemic or African; 3) epidemic or AIDS-realted; 4) iatrogenic (associated with therapeutic immunosuppresion as in transplant recipients). These four forms have indistinguishable histologic features. KS is composed of a variable mixture of irregularly shaped, round capillary and slit-like vascular spaces that are lined by endothelial cells, and spindle-shaped cells accompanied by an inflammatory cell infiltrate that always includes macrophages and lymphocytes. The spindle cells are of lymphatic endothelial origin, and considered to be the tumor cells. These cells sometimes line vascular spaces, and sometimes form sheets. A variable proportion of these spindle cells contain KSHV, which can be detected by immunohistochemistry using monoclonal antibodies to viral latency associated nuclear antigen (LANA)(Katano et al. 1999; Kellam et al. 1999; Parravicini et al. 2000).
3.1.2 Primary effusion lymphomas (PEL)
Primary effusion lymphomas are a rare subset of malignant lymphomas with distinctive and unusual clinicopathologic features, including their presentation as lymphomatous effusions in body cavities, therefore being initially called body-cavity-based lymphomas (Cesarman et al. 1995a; Nador et al. 1996). While more common in HIV-positive males, PELs also occur in HIV-negative men and women (Nador et al. 1995; Said et al. 1996). These lymphomas contain KSHV, and the presence of this virus has become a diagnostic criterion for PEL. In addition, over 90% are also coinfected with EBV. The presence of KSHV in this subset of lymphomas allowed the development of cell lines that have been used as a tool for its propagation, characterization of the viral life cycle, and for serologic assays (Arvanitakis et al. 1996; Boshoff et al. 1998; Cesarman et al. 1995b; Renne et al. 1996). Purified virus from PEL cell lines has been used to demonstrate its ability to infect B-cells (Mesri et al. 1996; Rappocciolo et al. 2008) and endothelial cells (Cannon et al. 2000; Flore et al. 1998; Moses et al. 1999).
Some non-Hodgkin’s lymphomas without an effusion component have also been found to contain KSHV. They usually present as solid extranodal lymphomas and are diagnosed as diffuse large cell, immunoblastic, or anaplastic large cell lymphomas, in which the presence of KSHV can be demonstrated in practically all the tumor cells by immunohistochemistry, and confirmed by molecular techniques (Carbone et al. 2005; Chadburn et al. 2004; Deloose et al. 2005; Engels et al. 2003). These lymphomas appear to fall in the spectrum of PEL, as they usually lack expression of B cell antigens and immunoglobulin, they have a similar morphology, and they are frequently co-infected with EBV.
3.1.3 Multicentric Castleman’s disease
Castleman’s disease is a poorly understood atypical lymphoproliferative disorder, usually described as a polyclonal, non-neoplastic condition. Two distinct histopathologic subtypes had been reported before the identification of KSHV: the hyaline vascular type, by far more common, and the plasma cell type. However, mixed types exist, and it is not always possible to distinguish these. Clinically, Castleman’s disease can be localized, or the patient may have multiple enlarged lymph nodes, therefore called “multicentric” MCD. Approximately 90% of patients with MCD have the plasma cell type morphology. These patients have a variety of constitutional symptoms and frequently develop malignancies, most commonly KS and NHL, consistent with an association with KSHV infection (Soulier et al. 1995). In fact, the presence of a single lymph node containing KS and Castleman’s disease is not uncommon in HIV-positive patients. Notably, MCD, also called multicentric angiofollicular hyperplasia, is characterized by a vascular proliferation, which is reminiscent of KS.
Since the identification of KSHV in MCD, the understanding of the histology of this disease has changed. KSHV has been reported in MCD with both hyaline vascular and plasma cell morphology (Larroche et al. 2002), but it appears that the majority of cases described in the literature more closely resemble the plasma cell type of MCD. One study reported that the KSHV-positive cases showed the highest intensity of angiosclerosis and germinal center and perifollicular vascular proliferation, while plasmacytosis is less pronounced than in the KSHV-negative cases of the plasma cell type (Suda et al. 2001). We now believe that the KSHV-positive cases represent a distinct morphologic variant, resembling more the plasma cell type, but in addition showing the presence of larger cells in the mantle zones, which are approximately twice the size of mantle zone lymphocytes, and characterized by a moderate amount of amphophilic cytoplasm and a large vesicular nucleus containing prominent nucleoli. These cells have been called plasmablasts, although they frequently have immunoblastic features (Dupin et al. 2000). These cells can be numerous, coalesce and form microlymphomas or frank plasmablastic lymphomas, and they contain KSHV which can be detected by immunohistochemistry for KSHV LANA (Dupin et al. 1999). These KSHV-infected plasmablasts are B cells that for some unknown reason are monotypic but polyclonal, almost invariably expressing IgMλ (Du et al. 2001). One study showed that KSHV-positive endothelial cells can also be found in MCD lymph nodes, in both HIV-positive and –negative patients (Brousset et al. 2001). In addition antibodies to vIL-6 are useful, as this viral protein is also frequently expressed in MCD in scattered plamablasts surrounding the lymphoid follicles (Cannon et al. 1999; Parravicini et al. 1997; Staskus et al. 1999), and expression of this viral cytokine may confer a worse prognosis (Menke et al. 2002). Lytic antigens are also expressed more frequently in KSHV-infected cells in MCD that in other disorders associated with this virus, suggesting that lytic viral replication may be a feature of MCD (Katano et al. 2000).
3.1.4 Plasmablastic lymphomas associated with MCD
Plasmablastic lymphomas associated with multicentric Castleman’s disease have also been described in HIV positive patients (Dupin et al. 2000). While these plasmablastic lymphomas are KSHV positive, they differ from PEL in a number of ways. Plasmablastic lymphomas are EBV negative, do not contain mutations in the Ig genes, and are thought to arise from naïve IgM lambda-expressing B cells rather than terminally differentiated B cells (Du et al. 2001). In addition, KSHV has been documented in germinotropic lymphoproliferative disorders in HIV-negative patients (Du et al. 2002), suggesting that this virus is present in a heterogenous but distinct group of lymphoproliferative diseases, and may be more common than initially thought.
3.3 KSHV latent and lytic infection
The majority of cells in PEL and KS lesions are latently infected by KSHV. Latency allows the virus to remain in the infected cell, ensuring that the cell survives and is not recognized as infected by the host immune system. Upon initial infection, KSHV produces viral proteins that inhibit innate antiviral responses, and subsequently during latency it produces a protein (LANA) that ensures maintenance of viral DNA in the form of extrachromosomal circles, called episomes, in dividing cells. It also produces proteins during latency that promote proliferation and survival of the infected cells, thereby having the potential of promoting tumorigenesis. PEL cell lines have been used to classify KSHV viral gene expression. Upon stimulation with butyrate or phorbol esters, PEL cells are induced to express lytic viral genes (Miller et al. 1997; Renne et al. 1996). Early lytic genes include those coding for viral proteins required for DNA replication or viral gene expression, whereas late lytic genes are those coding for viral structural proteins, like envelope and capsid proteins, required for assembly of viral particles (virions). The genes expressed during latency, and therefore constitutively in most PEL cells and KS spindle cells, include LANA, viral cyclin (vCYC), viral FLIP (FLICE inhibitor protein, vFLIP) (Dittmer et al. 1998; Dupin et al. 1999; Rainbow et al. 1997; Reed et al. 1998; Sturzl et al. 1999), as well as the viral-encoded miRNAs (Cai et al. 2005; Pfeffer et al. 2005; Samols et al. 2005). LANA, vCYC and vFLIP are expressed from the same promoter and at least two alternatively spliced mRNAs (Nakamura et al. 2003; Sarid et al. 1999). Certain viral genes could have a latent expression pattern in PEL but not in KS, like vIL-6 and IRF-3 (LANA2)(Cannon et al. 1999; Rivas et al. 2001). Another series of transcripts, called kaposins (or T0.7), are expressed in latently infected PEL cells, and of the potential proteins in this locus, Kaposin B has been reported to be expressed in some, but not all latently infected PELs (Sadler et al. 1999). Apart from vCYC and vFLIP, most of the cellular orthologues encoded by KSHV and pirated from the host genome, are only expressed during lytic reactivation.
3.4 NF-κB in KSHV infection and reactivation
There is an essential interrelationship between the KSHV life cycle and the NF-κB pathway, where the virus can affect NF-κB activity and in turn NF-κB signaling can affect viral latency, both directly by binding to viral promoters and indirectly though cellular gene expression.
3.3.1 NF-κB during KSHV binding and entry
NF-κB activation has been found to occur as early as five minutes after KSHV infection, and binding of the virus to the receptors in the cell surface can itself play a role in this activation. KSHV also contains a viral tegument protein (encoded by ORF75) that has been shown to activate NF-κB (Konrad et al. 2009). This tegument protein may contribute to NF-κB activation immediately after infection and independently of new protein synthesis, but the exact mechanism remains to be elucidated. The NF-κB activation induced immediately after infection stimulates the expression of viral genes, including a cluster of latent genes that are controlled from a single latent promoter, and include LANA, vCYC and vFLIP, as well as many cellular genes that play a role in the establishment of latency (Sadagopan et al. 2007).
3.3.2 NF-κB during KSHV latency
NF-κB activity is essential for the survival of latently KSHV infected PEL cells; selective inhibition of this pathway, results in downregulation of a very specific set of antiapoptotic genes, apoptosis of cells in culture, and tumor responses in mice (Keller et al. 2006; Keller et al. 2000). vFLIP has been identified as the major latent activator of NF-κB in KSHV-infected cells (Chaudhary et al. 1999a; Guasparri et al. 2004). Recently, a comprehensive screening for potential KSHV modulators of the NF-κB pathway performed by Konrad et al (2009) confirmed vFLIP as the most important activator, followed by the product of viral ORF75, which as mentioned above is a tegument protein (Konrad et al. 2009). KSHV vFLIP and the ORF75 product seem to cooperate for NF-κB activation in vitro, but it is unclear whether they can be expressed concomitantly in naturally occurring infection.
In contrast, vIRF3 (LANA2), encoded by ORF K10.5, was reported to inhibit NF-κB by binding to IKKβ (Seo et al. 2004). This protein is expressed during latency in PEL cells, but not in KS. Binding of vIRF3 to IKKβ was demonstrated by coimmunoprecipitation in transfected 293T cells, but could not be shown in a naturally infected PEL cell lines, so that its role as an NF-κB inhibitor in natural infection is unclear. Nevertheless, a screening for potential KSHV modulators confirmed the inhibitory potential of vIRF3 in 293T cells (Konrad et al. 2009).
3.3.3 NF-κB in KSHV lytic reactivation
Viral lytic reactivation requires NF-κB downregulation (Brown et al. 2003). The major protein involved in the switch from latency to lytic reactivation in KSHV is RTA, encoded by ORF50. vFLIP inhibits the expression of lytic genes through a NF-κB-mediated suppression of the AP-1 pathway, which has a detrimental effect on KSHV RTA activity (Seo et al. 2004). It has been shown that cellular components of the NF-κB cascade are per se negative regulators of KSHV RTA in almost all viral promoters, another mechanism for maintenance of viral latency. This effect seems to be related to the availability of RBP-Jk, a cellular transcriptional regulator. The RBP-Jk binding core sequence is relatively common in the KSHV genome, and even more frequent among the KSHV RTA-responsive promoters. Activation of NF-κB prevents RBP-Jk from anchoring KSHV RTA to the lytic viral promoters, and thereby suppresses their expression (Izumiya et al. 2009). While NF-κB inhibits expression of lytic viral proteins, the lytic viral proteins encoded by ORFs K1, K9, and K14 have been reported to inhibit NF-κB in transfected 293T cells. The K1 protein was shown to suppress both vFLIP and ORF75-mediated NF-κB activation in a dose-dependent fashion (Konrad et al. 2009). Intriguingly, it was previously reported that K1 expression in transfected BC-3 cells actually induces NF-κB-dependent promoter activity in luciferase reporter assays (Samaniego et al. 2001). The same group also reported increased NF-κB activity in B lymphocytes from K1-transgenic mice compared to non-transgenic animals (Prakash et al. 2002). The apparent conflicting information in these studies may be attributable to the distinct experimental methods and models used.
Apparently paradoxically, once lytic reactivation takes place, some lytic viral proteins may actually upregulate NF-κB and counterbalance the expression of other viral lytic genes. One possible explanation for this phenomenon is that a new increase in NF-κB activity might be required to induce expression of anti-apoptotic genes during the lytic cycle, perhaps necessary to increase cell viability long enough to accomplish maximum virion production and release. The KSHV G-protein coupled receptor (vGPCR; KSHV ORF74), for instance, is a lytic protein that can potently activate the NF-κB pathway in PEL (Cannon and Cesarman 2004) and in endothelial cells (Martin et al. 2008). While vGPCR is a lytic gene, it has been shown to inhibit the KSHV lytic cycle consistent with a negative regulatory role of NF-kB in lytic viral gene expression (Cannon et al. 2006).
An additional role for NF-κB has also been shown during the post-entry replicative steps of KSHV and during virion maturation (Sgarbanti et al. 2004). The abolishment of NF-κB activation with a super-repressor IkBα2NΔ4 in a KSHV-infected PEL cell line treated with an inducer of lytic replication causes a striking negative effect in the production of KSHV viral particles. Although viral particles derived from IκBα2NΔ4-transfected PEL cells entered endothelial cells, activation of NF-κB was found to be impaired, leading to defective viral gene expression and impaired establishment of infection.
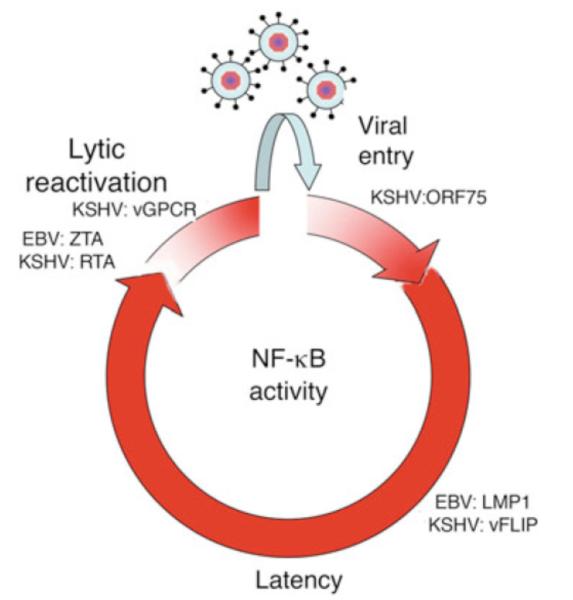
We believe that the dynamics of NF-κB infection and viral replication are tightly co-regulated, and this control is important for establishment of latency, viral replication as evasion of immune responses. A model of NF-κB activity in the context of the KSHV life cycle is illustrated in Figure 3.
Fig 3.
Model for the NF-κB activity kinetics throughout the course KSHV or EBV infection. Viral entry can trigger NF-κB activation, which can be caused by receptor binding and activity of the tegument protein encoded in ORF75 or KSHV. NF-κB induces expression of latent genes, such as EBV LMP1 and KSHV vFLIP, that in turn contribute to constitutive activation of the NF-κB pathway in the latently infected cells. Viral latency persists for a variable period of time, until unknown triggers downregulate NF-κB signaling and/or perturb the latent/lytic phase homeostasis. Consequently, a proportional increment in the expression of the EBV ZTA, EBV RTA and KSHV RTA major viral lytic activators occurs, which in turn further downregulates NF-κB, thereby propagating the lytic cascade. Once a biological threshold is reached, the latent-lytic switch is completed and viral replication occurs. Later in the lytic cascade, the expression of some viral lytic genes, such as KSHV vGPCR, may contribute to a new wave of NF-κB activation, which may have a role in extending the cell lifespan sufficiently to allow release of new viral particles until cytopathic effects of viral infection cause cell death.
3.4 Mechanism of NF-κB activation by vFLIP
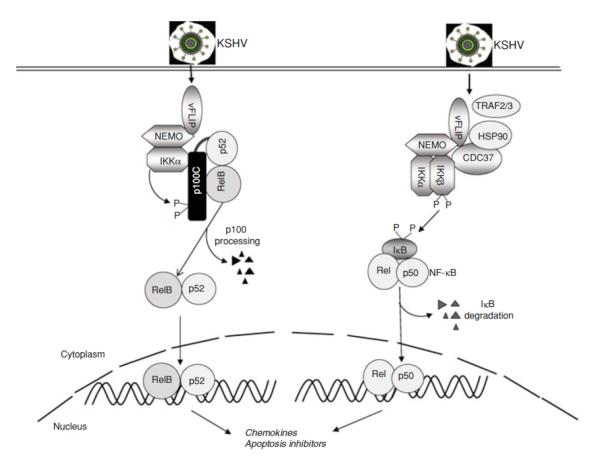
KSHV vFLIP is part of a group of viral and cellular proteins that are inhibitors of death receptor-induced apoptosis. FLIP proteins contain two death effector domains (DED) and have been shown to inhibit DED-DED interactions between FADD and procaspases 8 and 10. vFLIP is essential for the survival of PEL cells (Guasparri et al. 2004; Godfrey et al. 2005). vFLIP appears to inhibit cell death by several mechanisms. It may be able to do it directly, like the cellular FLIP proteins, by competing with caspase 8 for binding to death receptors, specifically by interacting with the FADD component of the death-inducing signaling complex (DISC) via one of two conserved DED domains (Djerbi et al. 1999). vFLIP can also suppress autophagy, which was shown to be due to prevention of Atg3 binding and processing of LC3 by vFLIP (Lee et al. 2009). In addition, by activating both cannonical and non-cannonical NF-κB pathways, expression of a full anti-apoptotic program is induced by vFLIP (Chaudhary et al. 1999b; Chugh et al. 2005; Guasparri et al. 2004; Keller et al. 2006; Keller et al. 2000; Liu et al. 2002; Matta and Chaudhary 2004) (Figure 4).
Fig 4.
NF-κB activation by KSHV vFLIP. The vFLIP protein encoded by KSHV can induce both the canonical (right) and noncanonical (left) NF-κB pathways. Direct binding of vFLIP to NEMO results in activation of IKKα and IKKβ, which in turn lead to cleavage of p100 and phosphorylation of IκB to induce nuclear translocation of RelB/p52 and Rel (p65)/p50 complexes, respectively.
The mechanism by which vFLIP induces NF-κB is incompletely understood, but we have acquired significant insights based on our understanding of the NF-κB pathway and of the interaction of vFLIP with specific cellular proteins in this pathway. vFLIP is present in the IKK complex or signalosome, the kinases IKKα and IKKβ, and the regulatory subunit NEMO. This complex also includes Hsp90 and cdc37 (Chen et al. 2002a. vFLIP binds directly to NEMO (Field et al. 2003; Guasparri et al. 2006; Liu et al. 2002). TRAF2 and 3 can also be found in this complex and appear to be involved in signaling in some experimental conditions (Chaudhary et al. 1999b; Guasparri et al. 2006), but not others (Matta et al. 2007). RIP and NIK are not in the vFLIP-containing complexes, and appear to be dispensable for NF-κB activation (Chaudhary et al. 1999b).
The structure of vFLIP binding to NEMO has been solved (Bagneris et al. 2008). The region of NEMO recognized by vFLIP was mapped to the HLX2 alpha helical domain, which forms a nonstandard intermolecular coiled coil composed of two IKKγ monomers in a parallel arrangement. A model for vFLIP activation of NEMO was proposed based on the crystal structure where inactive NEMO is in a helical bundle conformation but transitions to an open conformation upon an intermolecular coiled-coil formation catalyzed by vFLIP. This configuration, stabilized by vFLIP, was proposed to induce recruitment of the IKKβ and/or IKKα kinases for phosphorylation and subsequent phosphorylation of the IκBs. While inactivation of this complex in normal cells involves the recruitment of phosphatases or other proteins that restore the resting conformation of NEMO, these may be prevented from engaging with NEMO due to the presence of vFLIP, leading to constitutive activation of NF-κB.
3.5 Role of NF-κB in KSHV-mediated oncogenesis
The relevance of the NF-κB pathway in KSHV-mediated lymphomagenesis has been provided by studies using a pharmacological inhibitor called Bay11-7082 (Keller et al. 2006). These studies have shown that elimination of NF-κB activity induces apoptosis of PEL cell lines in vitro and in mice, indicating that this is an essential pathway that is induced by viral oncoproteins. Confirmation that vFLIP plays a central role in activating NF-κB, which in turn is essential for the survival of infected lymphoma cells, comes from experiments using RNA interference, where elimination of vFLIP results in decrease of NF-κB and apoptosis of PEL cells and inhibition of tumor growth in mice (Guasparri et al. 2004; Godfrey et al. 2005).
4. NF-κB Activation by EBV
4.1 EBV infection in healthy humans and EBV life cycle
EBV infection is practically ubiquitous in healthy adults, so it has been challenging to establish the exact role of this virus in lymphomagenesis. Nevertheless, extensive epidemiologic and experimental data support the notion that EBV is an oncogenic virus, which is supported by the well established fact that EBV can infect and transform normal human B-cells in vitro, resulting in their "immortalization" and leading to continuously growing lymphoblastoid cell lines (LCLs) (Rickinson and Kieff 1996).
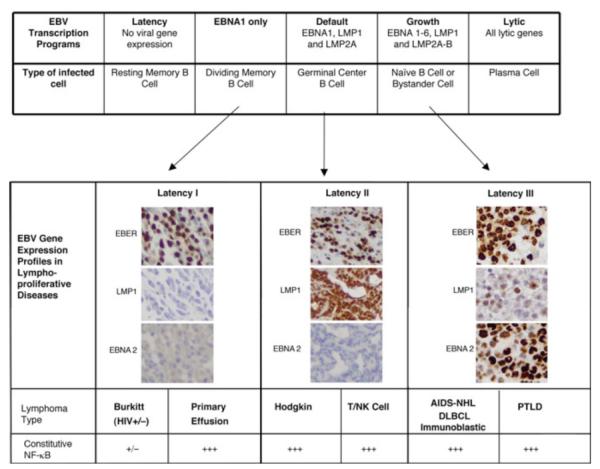
EBV establishes a lifelong infection in the vast majority of people without causing any disease. Careful analysis of expression patterns in different tissues from EBV-infected immunocompetent individuals led to the description of five different transcription programs that are used to establish and maintain EBV infection (Figure 5); reviewed by (Thorley-Lawson 2001; Thorley-Lawson and Gross 2004). Most of the same transcriptional programs are recapitulated in lymphomas and lymphoproliferative diseases.
Fig 5.
Patterns of EBV latent gene expression in healthy individuals and in malignant lymphomas. The patterns of EBV gene expression infection described in different B cell subsets are shown in the upper table. Corresponding expression profiles in malignant lymphomas have been designated Latencies I, II, and III, and are shown in the lower table. EBER in situ hybridization is used to detect the presence of EBV, immunohistochemical positivity for LMP1 denotes latency II or III, and EBNA 2 protein expression together with LMP1 is indicative of latency III. Those lymphomas expressing LMP1 have constitutive NF-κB activity, while in other lymphomas this activity may be present but more variable and sometimes induced by exogenous signals, such as that induced by CD40, BAFF and APRIL or by cellular genetic alterations, such as inactivating mutations of A20 or CARD11. Primary effusion lymphomas, while not expressing LMP1, also contain KSHV which induces NF-κB through expression of vFLIP.
When EBV first infects a naïve B cell in a healthy individual, a transient “growth program” is established, where EBV expresses EBNA 1-6, as well as LMP1, LMP2A and LMP2B. These proteins force the infected cells to become proliferating B cell blasts, probably allowing EBV infection to be propagated. In vitro generated LCLs express this growth program indefinitely. However, since many of these proteins are antigenic, this state is only transient in immunocompetent individuals. As soon as an immune response is established, most of the cells with this program are eliminated, or otherwise switch to a “default program” of EBV expression, where only EBNA1, LMP1 and LMP2A are expressed. This stage is also temporary because LMP1 and LMP2A mimick CD40 and antigen receptor signaling respectively, thereby inducing the B cells in peripheral lymphoid organs to behave like germinal center B cells and differentiate into resting memory B cells (Babcock et al. 2000; Laichalk et al. 2002). These infected cells in turn switch to a “latency program” where no viral genes are expressed, allowing lifetime persistence of EBV. Dividing peripheral blood infected memory B cells express only EBNA1, which is not immunogenic but allows the EBV episome to segregate and be propagated in dividing cells. Differentiation of memory B cells into plasma cells induces lytic replication, mediated by expression of the plasma cell transcription factor XBP-1, which induces expression of EBV ZTA initiating the lytic cascade (further described in section 4.5) (Bhende et al. 2007; Laichalk and Thorley-Lawson 2005; Sun and Thorley-Lawson 2007). Differentiation of B cells into plasma cells frequently occurs in lymphoid tissues near mucosal surfaces, notably in the Weldeyer’s ring, leading to viral shedding in saliva.
4.2 Diseases caused by EBV infection
Three different patterns of EBV expression have been described in infected cells in lymphoproliferative disorders: Latency I, II or III, illustrated in Figure 5 (Kieff 1996; Rickinson and Kieff 1996) . In Latency I, EBNA1 is the major viral protein produced. In the other extreme is Latency III, which corresponds to the “growth program” and involves the unrestricted expression of all 9 latent genes including six EBV-encoded nuclear antigens (EBNA1-6) (Kieff 1996) and three latent membrane proteins (LMP1, LMP2A, and LMP2B). Latency II corresponds to the “default program”, and consists of expression of EBNA1 and varying amounts of the three LMP proteins.
Because EBNA proteins are immunogenic, with the exception of EBNA1, an important feature of Latency III is the recognition and elimination of the EBV-infected cells by the immune system. Therefore, lymphomas with unrestricted EBV latency are generally only encountered in immunodeficient individuals. In contrast, most lymphomas in immunocompetent hosts will have Latency I or II, because down-regulation of the immunogenic EBNA proteins is an important mechanism of immune evasion by EBV (Rickinson and Kieff 1996).
Acute infection with EBV is frequently asymptomatic, but in some instances leads to infectious mononucleosis. Age of infection, viral dosage upon original infection and immune responses are though to determine whether primary infection is symptomatic in some individuals but not others. While infectious mononucleosis is usually self-limited, in rare instances chronic active EBV can develop. In addition, in the context of congenital or acquired immunodeficiency, a variety of lymphoproliferative disorders may develop. EBV is well known to be associated with the following malignancies:
4.2.1. Burkitt’s lymphoma (BL)
Epstein-Barr virus is invariably present in African (endemic) BL, but is found only in a minority of sporadic cases. Most of our understanding of EBV gene expression was originally derived from the study of BL cell lines; however, in vivo expression has also been examined in endemic BL tissue biopsies (Tao et al. 1998). EBV-positive BLs have EBNA1, and usually LMP2A, transcripts, in the absence of lytic transcripts or other latent transcripts. A subset of BL has a deletion of EBNA2, and in these cases there is expression of EBNA3A-C (Kelly et al. 2002; Kelly et al. 2005).
Translocation of c-myc into one of the immunoglobulin loci is considered by some to be a prerequisite for classification of a lymphoma as BL or atypical BL. The most common translocation is a t(8;14), involving the c-myc and immunoglobulin heavy chain genes, but in 10% of the cases it can involve c-myc and one of the light chain genes. It is thought that this translocation leads to deregulated expression of the c-myc gene. Mutations of the c-myc locus also occur in Burkitt lymphoma, and these may also lead to abnormal expression. Gene expression profiling experiments have concluded that there are cases with a typical and unique BL signature, while other cases are more heterogenous (Dave et al. 2006; Hummel et al. 2006). The latter tend to have more complex karyotypes than the former, where usually translocations involving c-myc are the only detectable cytogenetic abnormality.
4.2.2. Post-transplantation lymphoproliferative disorder
Post-transplantation lymphoproliferative disorders (PTLDs) develop in the setting of iatrogenic immunosuppression following solid organ transplantation or allogeneic bone marrow transplantation. The incidence of these lesions varies based on the type of organ transplanted as well as on the type and amount of immunosuppression employed. As with other immunodeficiency-related lymphoproliferative disorders, the development of PTLDs is highly associated with EBV infection. The relative incidence of these lesions is higher in patients who are EBV negative at the time of transplantation and become infected when already immunosuppressed. Most PTLDs exhibit type III latency, with the exception of the monomorphic lesions, which often exhibit the latency type I pattern of EBV gene expression, and also often carry cellular genetic alterations in oncogenes and tumor suppressor genes and thereby may be less dependent on EBV (Knowles et al. 1995).
4.2.3 AIDS-related non-Hodgkin’s lymphomas
The incidence of non-Hodgkin’s lymphomas (NHL) in HIV positive individuals is estimated to be between 4 and 10%, but the incidence of at least some subsets has decreased with combination antiretroviral therapy. The incidence of Hodgkin’s lymphoma is somewhat increased in HIV-infected individuals, but it is not considered to be an AIDS-defining condition. The pathogenesis of NHL in the context AIDS is complex and thought to be related to disrupted immune surveillance, chronic antigenic stimulation, genetic alterations, cytokine dysregulation and herpes virus infection (Carbone 2003; Carbone et al. 1998; Knowles 2001). Although HIV-related lymphomas are almost always of B cell origin, they are morphologically diverse. Several subtypes are similar to lymphomas occurring in immunocompetent patients, while others preferentially develop in the context of AIDS. HIV-related lymphomas can be classified by morphology (as in the WHO classification), and/or by primary site of presentation (i.e. systemic, primary central nervous system, body cavity)(Carbone et al. 2009; Knowles 2001; Raphaël et al. 2008).
Lymphomas also occurring in immunocompetent patients
HIV-related BL includes cases exhibiting the features of classical BL (described above), those showing plasmacytoid differentiation and those exhibiting features of atypical Burkitt/Burkitt-like lymphoma. In terms of EBV infection, AIDS-related BL resembles sporadic BL, with around 30% of cases being positive. Diffuse large B-cell lymphoma (DLBCL) can be divided into centroblastic (CB) and immunoblastic (IB) categories. While these morphologically and immunophenotypically resemble lesions found in immunocompetent individuals, the frequent association with EBV is almost exclusive of immunodefficient patients. The IB type is more frequently associated with EBV infection, and patients with these lymphomas are usually significantly immunosuppressed with low CD4 counts (median <100 ×106/L) and approximately one-third have been previously diagnosed with an AIDS-defining illness. This degree of immune dysfunction allows EBV to be the driving proliferative force, with expression of the oncogenic but also immunogenic LMP and EBNA proteins. In addition, while DLBCL express adhesion molecules that are important for immune recognition, BLs do not. These observations suggest that defective EBV immunity is involved in the pathogenesis of DLBCLs (Kersten et al. 1998).
Lymphomas occurring primarily in HIV-positive patients
Primary central nervous system lymphomas (PCNSL) differ from systemic DLBCLs, with the majority of cases exhibiting IB morphology and EBV-positivity. According to one study, PCNSL can be divided into two categories-those with immunoblastic features, which express LMP-1 in conjunction with BCL-2 but no BCL-6 expression, and those with a large, noncleaved cell morphology, which do not express LMP-1 or BCL-2, but express BCL-6 (Larocca et al. 1998). Another type of lymphoma described in the context of HIV infection is the plasmablastic lymphoma of the oral cavity. It has features similar to IB lymphomas, but is less heterogeneous and polymorphic. A majority of these are associated with EBV infection. Polymorphic B-cell lymphomas (PTLD-like) are extremely rare lesions but morphologically resemble polymorphic PTLDs. The last category of lymphoma predominantly occurring in HIV-positive patients is primary effusion lymphoma. The latter, while positive for EBV, also contains KSHV, and was discussed in the previous section.
4.2.4 T/natural killer cell lymphomas
The angiocentric (nasal and nasal-type) T/natural killer (T/NK)-cell lymphomas are always associated with EBV infection (Jaffe et al. 1999). These have a high prevalence in Asia, but cases from other countries have also shown an association with EBV (Elenitoba-Johnson et al. 1998). Studies on cell lines indicate that T/NK cell lymphomas have a Latency II (Kanegane et al. 1998; Tsuchiyama et al. 1998). Although EBV has also been reported to be present in peripheral T-cell lymphomas, it has been shown to be preferentially localized in B-cells rather than the neoplastic T cells (Ho et al. 1998).
4.2.5 Hodgkin’s lymphoma (HL)
EBV is present in approximately 40% of cases of HL in Western countries and more frequently in developing countries and in younger patients (Harris 1998). HL results from a monoclonal expansion of B-cells containing somatic hypermutations of the immunoglobulin genes. These mutations may be “crippling”, resulting in lack of antigen-receptor expression. Therefore, the Hodgkin’s-Reed-Sternberg cells are derived from germinal B-cells destined to undergo apoptosis, but they are postulated to be protected by some transforming event, such as EBV (Küppers and Rajewsky 1998). In HL, EBV establishes Latency II within HRS cells, with expression of LMP-1 and LMP2, which are subdominant targets for CTL recognition.
4.2.6 Nasopharyngeal carcinoma (NPC)
This type of carcinoma is most common in Cantonese individuals, but also occurs in Arab, and Eskimo populations (Yu and Yuan 2002), and sporadically in the West. EBV is consistently associated with the non-keratinizing and undifferentiated subtypes of nasopharyngeal carcinomas. Biopsies show lesions composed of large neoplastic epithelial cells disposed in a syncytium-like array, admixed with a prominent inflammatory component consisting of normal appearing lymphocytes. This Histologic appearance gave rise to the misnomer or lymphoepithelioma. Well-differentiated carcinomas of the nasopharynx are often EBV associated as well (Pathmanathan et al. 1995). Nasopharyngeal carcinomas express EBV EBNA1, LMP2 and sometimes LMP1, classified as type II latency. Viral DNA is present in plasma in patients with nasopharyngeal carcinoma and has emerged as a highly reliable guide to determining prognosis and monitoring therapy (Lechowicz et al. 2002; Lin et al. 2004; Lo et al. 2000). This DNA is not encapsidated but is fragmented DNA released from tumor cells undergoing apoptosis (Chan et al. 2002). High levels of viral DNA in plasma at the start of therapy has emerged as an important prognostic factor, persistence of high levels in the face of therapy is a marker of relapse or progression (Chan et al. 2002).
4.3 NF-κB activation during EBV binding and entry
To infect naïve B lymphocytes, the major envelope glycoprotein of EBV, gp350/220, binds CD21, which is the complement receptor type 2 (CR2), on the cell surface. Binding to CD21 triggers NF-κB activation, which has been demonstrated by inhibition with a soluble gp105 fragment of EBV gp350/220 protein and with anti-CR2 monoclonal antibody OKB7 (Sugano et al. 1997). This binding results in IκBα phosphorylation by protein kinase C (PKC), followed by its degradation and nuclear accumulation of p50 and RelA hetero- or homodimers. As NF-κB is activated, the viral DNA enters the cell nucleus and the transcription of latent viral products can take place, a consequence of triggering NF-κB responsive elements in the EBV Wp promoter (Sugano et al. 1997). Therefore, the EBV binding initiates NF-κB activation, required for successful cell infection. In turn, the NF-κB activity also upregulates the expression of the CD21 molecule (Sugano et al. 1997), which may provide a positive feedback loop to enhance the cell susceptibility to EBV entry.
4.4 NF-κB during latent EBV infection
EBV LMP1 protein is required for the establishment of viral latency, although it appears to be expressed only transiently during primary EBV infection in humans. It is found in EBV-immortalized lymphoblastoid cell lines (LCL) in vitro, as well as in the newly-infected B lymphocytes during latent infection. LMP1 strongly activates the NF-κB pathway, and in turn it is itself upregulated by NF-κB (Johansson et al. 2009). This creates an amplification loop that keeps steady high levels of NF-κB during EBV latency. EBV LMP1 is important for latency maintenance, and can suppress lytic reactivation by both NF-κB-dependent and NF-κB-independent pathways (Prince et al. 2003). LMP2 has also been shown to affect NF-κB in EBV-associated lymphomas through indirect mechanisms (Guasparri et al. 2008), as well as in transgenic mice (Swanson-Mungerson et al. 2006).
Paradoxically, the EBNA1 protein was recently demonstrated to downregulate NF-κB in NPC cells by inhibiting the phosphorylation of IKKα/β (Valentine et al. 2010). EBNA1 is expressed in all EBV+ dividing cells, so it may be postulated that EBV has developed a mechanism to downregulate this transcription factor during infection in vivo in order to avoid immune recognition. It is possible that NF-κB is only transiently upregulated during latency but for long-term viral infection, this activity may remain low. However, as this downregulation of NF-κB by EBNA1 has only been reported in epithelial cells, it may be cell-specific and not occurring in latently infected memory B cell reservoirs.
4.5 NF-κB in lytic reactivation of EBV reactivation
EBV encodes an immediate early protein, called ZTA, BZLF1, bZIP or ZEBRA, which is important for the switch to lytic replication. Expression of ZTA is inhibited by NF-κB, specifically by the RelA protein (Figure 3). In turn, while ZTA induces the nuclear translocation of RelA, it inhibits its transcriptional activity (Morrison and Kenney 2004). Thereby, NF-κB is important to maintain latency, and cellular changes that lead to a reduction of NF-κB can result in expression of ZTA. Conversely, induction of expression of ZTA, for example by induction of XBP-1 during B cell differentiation to plasma cells (Bhende et al. 2007; Laichalk and Thorley-Lawson 2005; Sun and Thorley-Lawson 2007), leads to a reduction of NF-κB which in turn allows higher levels of ZTA expression thereby propagating the lytic cascade.
NF-κB may also play an important role during lytic viral replication by affecting the surrounding non-infected inflammatory cells. The release of the EBV dUTPase, a non-structural viral protein produced during the lytic phase, into the microenvironment of peripheral blood mononuclear cells (PBMC) and monocyte-derived macrophages was shown to activate NF-κB in these cells through the Toll-like receptor (TLR) 2. The EBV dUTPase-driven NF-κB activation ultimately modulates the local immune response as a result of increase in the secretion of cytokines, notably IL-6 and IL-10 (Ariza et al. 2009).
4.6 Mechanism of NF-κB activation by the latent viral proteins LMP1 and LMP2
LMP1 is the most important EBV product that causes constitutive NF-κB activity in the majority of EBV-associated malignancies, and it is capable of transforming both in vitro (Wang et al. 1985) and in vivo (Homig-Holzel et al. 2008; Kulwichit et al. 1998; Panagopoulos et al. 2004). EBV LMP1 mimics a constitutively activated ligand-independent receptor of the TNF receptor family. It binds TRAFs and recruits TRADD, ultimately inducing the expression of NF-κB-regulated genes that promote cell survival and proliferation (Cahir McFarland et al. 1999; Gires et al. 1997; Kieser 2008). Although two cytoplasmic carboxy-terminal regions in the LMP1 protein – namely the C-terminal-activating region 1 and 2 (CTAR1 and CTAR2) – are able to activate the NF-κB cascade, CTAR1 is enough for cell transformation due to its unique features in inducing multiple signaling pathways (Mainou et al. 2007). LMP1 associates with TRAF-1 and TRAF-3 in vivo in EBV-associated lymphomas, eventually leading to the downstream activation of NF-κB (Liebowitz 1998). While LMP1 binds several TRAFs, the ones that are essential for NF-κB activation depend on the experimental system used. TRAF-3 (Xie et al. 2004) and TRAF-6 (Luftig et al. 2003) have been found to be involved in LMP1 signaling in murine knockout systems, while in these, TRAF-2 has been shown to be dispensable. In contrast, TRAF2 is essential for NF-κB signaling in EBV-infected lymphoma cell lines (Guasparri et al. 2008). Knock-down of TRAF-2 by RNA interference, but not of the other TRAFs, results in downregulation of NF-κB and apoptotic cell death.
LMP2A is a functional homolog of the B-cell receptor (BCR), although it also inhibits antigen-induced activation of the BCR signal transduction cascade which is thought to prevent BCR induced lytic replication of EBV in LCLs (Miller et al. 1994). LMP2A is not essential for generation of LCLs (Rochford et al. 1997; Speck et al. 1999), but at least according to one study, it does contribute to the efficiency of B cell immortalization (Brielmeier et al. 1996) and has been found to have several effects on B cells. Experiments with transgenic mice expressing LMP2A targeting expression to the B-cell lineage showed that LMP2A sends survival signals and allows B cells to bypass developmental checkpoints and escape the bone marrow to colonize peripheral lymphoid organs (Caldwell et al. 2000; Caldwell et al. 1998; Merchant et al. 2000). These functions are mediated by an ITAM motif, through which LMP2A activates PI3K>Akt->mTOR signaling in B lymphocytes by activating members of the Src family of tyrosine kinases, and also associates with the BCR signaling effector Syk kinase, a mechanism through which it induces epithelial cell migration (Fruehling and Longnecker 1997; Fukuda and Longnecker 2007; Lu et al. 2006; Moody et al. 2005; Swart et al. 2000). LMP2A can also engage the JNK mitogen-activated protein (MAP) kinase and beta-catenin signaling pathways (Chen et al. 2002b; Morrison et al. 2003). While transfected LMP2 has not been reported to activate NF-κB, two recent studies have shown activation of NF-κB by LMP2A by evaluating the role of this viral protein in conjunction with known BCR signals in double transgenic mice expressing LMP2A and BCR restricted to hen egg lysozyme (HEL) or ribonucleoprotein Smith (Sm) (Swanson-Mungerson et al. 2005; Wang et al. 2006). Both of these studies found that B cells expressing LMP2A in vivo display constitutive NF-κB activity, as compared to LMP2A-negative control B cells. LMP2A signaling appears not to affect B cell proliferation in vivo (Rochford et al. 1997), but rather provides survival signals in BCR-negative B cells (Caldwell et al. 2000). Although LMP2A cannot activate NF-κB on its own, its ability augment signaling from LMP1 by increasing its half-life has been reported (Dawson et al. 2001). In contrast, a different study found that LMP2A inhibits NF-κB activity in carcinoma cell lines infected in vitro with wild type recombinant EBV, as compared with virus in which LMP2A was deleted (Stewart et al. 2004). In LCLs and EBV infected lymphoma cell lines, LMP2A was found to be essential for NF-κB signaling, as knock-down of this viral protein resulted in suppression of NF-κB (Guasparri et al. 2008). However, in contrast to the previous study by Dawson et al. (Dawson et al. 2001), this effect was not mediated by LMP1 stabilization, but rather by the transcriptional control of TRAF-2 mediated by LMP2A-induced signaling (Guasparri et al. 2008). In the absence of LMP2A, TRAF-2 transcription is downregulated and LMP1 signaling is impaired.
4.7 Role of NF-κB in EBV-mediated oncogenesis
The relevance of the NF-κB pathway in EBV-mediated lymphomagenesis has been supported by studies in which inhibition of NF-κB using an IκB phosphorylation-deficient mutant which sequesters NF-κB in the cytoplasm (Cahir-McFarland et al. 2000; Feuillard et al. 2000) or a pharmacological IKK inhibitor called Bay11-7082 (Cahir-McFarland et al. 2004; Keller et al. 2006). These studies have shown that elimination of NF-κB activity induces apoptosis of EBV-infected lymphoma cell lines in vitro and in mice, indicating that this is an essential pathway that is induced by viral oncoproteins. Confirmation that LMP1 plays a central role in activating NF-κB, which in turn is essential for the survival of infected lymphoma cells, comes from experiments using RNA interference, where elimination of LMP1 or LMP2A results in decrease of NF-κB and apoptosis of EBV-infected lymphoma cells (Guasparri et al. 2008).
5. Concluding Remarks
NF-κB is a common cellular target of HTLV1, EBV, and KSHV oncogenic viruses that are etiologically associated with human lymphoid malignancies. Despite their tremendous differences in genomic structure and life cycles, these pathogens share the ability to persistently activate NF-κB via specific viral proteins. HTLV1 Tax protein physically interacts with IKK and activates IKK independently of upstream signaling molecules. Similar to HTLV1 Tax, the KSHV vFLIP protein directly binds NEMO, thereby activating the IKK complex independently of upstream signaling molecules. In contrast, the EBV LMP1 protein interacts with and activates TRAFs, thereby mediating TRAF-dependent NF-κB activation. A common feature of these viral NF-κB inducers is the induction of both canonical and noncanonical NF-κB pathways. Persistent activation of the NF-κB pathways by these human pathogens plays a central role in their induction of host cell transformation. Thus, NF-κB represents an attractive therapeutic target in the treatment of the lymphoid malignancies associated with infection by these viruses.
Acknowledgements
Work performed in the authors’ laboratories is supported by the National Institutes of Health Grants (AI064639, AI057555, and GM084459 to SCS; CA068939 and CA103646 to EC) and the Starr Cancer Consortium (Award to EC).
Abbreviations
- IKK
IκB kinase
- HTLV1
human T-cell leukemia virus type 1
- KSHV
Kaposi sarcoma-associated herpesvirus
- EBV
Epstein bar virus
- IκB
inhibitory κB
- ATL
adult T-cell leukemia
- IL-2
interleukin-2
- LTR
long-terminal repeat
- TCR
T-cell receptor
- K63
lysine 63
- UBA
ubiquitin-association
- TAB2
Tak1-binding protein 2
- LZ
leucine zipper
- NRP
NEMO-related protein
- NIK
NF-κB inducing kinase
- HBZ
HTLV1 basic leucine zipper
- PI3K
PI3 kinase
- MCD
multicentric Castleman’s disease
- KS
Kaposi’s sarcoma
- HAART
highly active antiretroviral treatment
- LANA
latency associated nuclear antigen
- PEL
primary effusion lymphomas
- vCYC
viral cyclin
- vFLIP
viral FLICE inhibitor protein
- GPCR
G-protein coupled receptor
- TPA
tetradecanoyl phorbol acetate
- DED
death effector domains
- DISC
death-inducing signaling complex
- LCL
lymphoblastoid cell line
- EBNA
EBV-encoded nuclear antigen
- LMP
latent membrane protein
- BL
Burkitt lymphoma
- PTLD
post-transplantation lymphoproliferative disorders
- NHL
non-Hodgkin lymphoma
- DLBCL
diffuse large B-cell lymphoma
- CB
centroblastic
- IB
immunoblastic
- PCNSL
primary central nervous system lymphoma
- T/NK
T/natural killer
- HL
Hodgkin lymphoma
- NPC
nasopharyngeal carcinoma
- CR2
complement receptor type 2
- PKC
protein kinase C
- LCL
lymphoblastoid cell lines
- PBMC
peripheral blood mononuclear cells
- TLR
Toll-like receptor
- CTAR
C-terminal-activating region
- BCR
B-cell receptor
- MAP
mitogen-activated protein
- HEL
hen egg lysozyme
- Sm
ribonucleoprotein Smith
References
- Adhikari A, Xu M, Chen ZJ. Ubiquitin-mediated activation of TAK1 and IKK. Oncogene. 2007;26:3214–3226. doi: 10.1038/sj.onc.1210413. [DOI] [PubMed] [Google Scholar]
- Ariza ME, Glaser R, Kaumaya PT, Jones C, Williams MV. The EBV-encoded dUTPase activates NF-kappa B through the TLR2 and MyD88-dependent signaling pathway. J Immunol. 2009;182:851–9. doi: 10.4049/jimmunol.182.2.851. [DOI] [PubMed] [Google Scholar]
- Arnold J, Zimmerman B, Li M, Lairmore MD, Green PL. Human T-cell leukemia virus type-1 antisense-encoded gene, Hbz, promotes T-lymphocyte proliferation. Blood. 2008;112:3788–3797. doi: 10.1182/blood-2008-04-154286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitakis L, Mesri EA, Nador R, Said JW, Asch AS, Knowles DM, Cesarman E. Establishment and characterization of a primary effusion (body cavity-based) lymphoma cell line (BC-3) harboring Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) in the absence of Epstin-Barr virus. Blood. 1996;88:2648–2654. [PubMed] [Google Scholar]
- Babcock GJ, Hochberg D, Thorley-Lawson AD. The expression pattern of Epstein-Barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B cell. Immunity. 2000;13:497–506. doi: 10.1016/s1074-7613(00)00049-2. [DOI] [PubMed] [Google Scholar]
- Babu G, Waterfield M, Chang M, Wu X, Sun S-C. Deregulated activation of oncoprotein kinase Tpl2/Cot in HTLV-I-transformed T cells. J. Biol. Chem. 2006;281:14041–14047. doi: 10.1074/jbc.M512375200. [DOI] [PubMed] [Google Scholar]
- Bagneris C, Ageichik AV, Cronin N, Wallace B, Collins M, Boshoff C, Waksman G, Barrett T. Crystal structure of a vFlip-IKKgamma complex: insights into viral activation of the IKK signalosome. Mol Cell. 2008;30:620–31. doi: 10.1016/j.molcel.2008.04.029. [DOI] [PubMed] [Google Scholar]
- Baldwin AS., Jr. The NF-κB and IκB proteins: new discoveries and insights. Annu. Rev. Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Beinke S, Ley SC. Functions of NF-kappaB1 and NF-kappaB2 in immune cell biology. Biochem. J. 2004;382:393–409. doi: 10.1042/BJ20040544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béraud C, Sun S-C, Ganchi PA, Ballard DW, Greene WC. Human T-cell leukemia virus type I Tax associates with and is negatively regulated by the NF-κB2 p100 gene product: implications for viral latency. Mol. Cell Biol. 1994;14:1374–1382. doi: 10.1128/mcb.14.2.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhende PM, Dickerson SJ, Sun X, Feng WH, Kenney SC. X-box-binding protein 1 activates lytic Epstein-Barr virus gene expression in combination with protein kinase D. J Virol. 2007;81:7363–70. doi: 10.1128/JVI.00154-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshoff C, Gao SJ, Healy LE, Matthews S, Thomas AJ, Coignet L, Warnke RA, Strauchen JA, Matutes E, Kamel OW, Moore PS, Weiss RA, Chang Y. Establishing a KSHV+ cell line (BCP-1) from peripheral blood and characterizing its growth in Nod/SCID mice. Blood. 1998;91:1671–1679. [PubMed] [Google Scholar]
- Boshoff C, Weiss RA. Kaposi’s sarcoma-associated herpesvirus. Adv Cancer Res. 1998;75:57–86. doi: 10.1016/s0065-230x(08)60739-3. [DOI] [PubMed] [Google Scholar]
- Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V. A review of human carcinogens--Part B: biological agents. Lancet Oncol. 2009;10:321–2. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- Brielmeier M, Mautner J, Laux G, Hammerschmidt W. The latent membrane protein 2 gene of Epstein-Barr virus is important for efficient B cell immortalization. J Gen Virol. 1996;77(Pt 11):2807–18. doi: 10.1099/0022-1317-77-11-2807. [DOI] [PubMed] [Google Scholar]
- Brousset P, Cesarman E, Meggetto F, Lamant L, Delsol G. Colocalization of the viral interleukin-6 with latent nuclear antigen-1 of human herpesvirus-8 in endothelial spindle cells of Kaposi’s sarcoma and lymphoid cells of multicentric Castleman’s disease. Hum Pathol. 2001;32:95–100. doi: 10.1053/hupa.2001.21131. [DOI] [PubMed] [Google Scholar]
- Brown HJ, Song MJ, Deng H, Wu TT, Cheng G, Sun R. NF-kappaB inhibits gammaherpesvirus lytic replication. J Virol. 2003;77:8532–40. doi: 10.1128/JVI.77.15.8532-8540.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister T. Oncogenic retroviruses in animals and humans. Rev. Med. Virol. 2001;11:369–380. doi: 10.1002/rmv.331. [DOI] [PubMed] [Google Scholar]
- Cahir McFarland ED, Izumi KM, Mosialos G. Epstein-barr virus transformation: involvement of latent membrane protein 1-mediated activation of NF-kappaB. Oncogene. 1999;18:6959–64. doi: 10.1038/sj.onc.1203217. [DOI] [PubMed] [Google Scholar]
- Cahir-McFarland ED, Carter K, Rosenwald A, Giltnane JM, Henrickson SE, Staudt LM, Kieff E. Role of NF-kappa B in cell survival and transcription of latent membrane protein 1-expressing or Epstein-Barr virus latency III-infected cells. J Virol. 2004;78:4108–19. doi: 10.1128/JVI.78.8.4108-4119.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahir-McFarland ED, Davidson DM, Schauer SL, Duong J, Kieff E. NF-kappa B inhibition causes spontaneous apoptosis in Epstein-Barr virus-transformed lymphoblastoid cells. Proc Natl Acad Sci U S A. 2000;97:6055–60. doi: 10.1073/pnas.100119497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Lu S, Zhang Z, Gonzalez CM, Damania B, Cullen BR. Kaposi’s sarcoma-associated herpesvirus expresses an array of viral microRNAs in latently infected cells. Proc Natl Acad Sci U S A. 2005;102:5570–5. doi: 10.1073/pnas.0408192102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell RG, Brown RC, Longnecker R. Epstein-Barr virus LMP2A-induced B-cell survival in two unique classes of EmuLMP2A transgenic mice. J Virol. 2000;74:1101–13. doi: 10.1128/jvi.74.3.1101-1113.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell RG, Wilson JB, Anderson SJ, Longnecker R. Epstein-Barr virus LMP2A drives B cell development and survival in the absence of normal B cell receptor signals. Immunity. 1998;9:405–11. doi: 10.1016/s1074-7613(00)80623-8. [DOI] [PubMed] [Google Scholar]
- Cannon JS, Ciufo D, Hawkins AL, Griffin CA, Borowitz MJ, Hayward GS, Ambinder RF. A new primary effusion lymphoma-derived cell line yields a highly infectious Kaposi’s sarcoma herpesvirus-containing supernatant. J Virol. 2000;74:10187–93. doi: 10.1128/jvi.74.21.10187-10193.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon JS, Nicholas J, Orenstein JM, Mann RB, Murray PG, Browning PJ, DiGiuseppe JA, Cesarman E, Hayward GS, Ambinder RF. Heterogeneity of viral IL-6 expression in HHV-8-associated diseases. Journal of Infectious Diseases. 1999;180:824–828. doi: 10.1086/314956. [DOI] [PubMed] [Google Scholar]
- Cannon M, Cesarman E, Boshoff C. KSHV G protein-coupled receptor inhibits lytic gene transcription in primary-effusion lymphoma cells via p21-mediated inhibition of Cdk2. Blood. 2006;107:277–84. doi: 10.1182/blood-2005-06-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon ML, Cesarman E. The KSHV G protein-coupled receptor signals via multiple pathways to induce transcription factor activation in primary effusion lymphoma cells. Oncogene. 2004;23:514–23. doi: 10.1038/sj.onc.1207021. [DOI] [PubMed] [Google Scholar]
- Carbone A. Emerging pathways in the development of AIDS-related lymphomas. Lancet Oncol. 2003;4:22–9. doi: 10.1016/s1470-2045(03)00957-4. [DOI] [PubMed] [Google Scholar]
- Carbone A, Cesarman E, Spina M, Gloghini A, Schulz TF. HIV-associated lymphomas and gamma-herpesviruses. Blood. 2009;113:1213–24. doi: 10.1182/blood-2008-09-180315. [DOI] [PubMed] [Google Scholar]
- Carbone A, Gaidano G, Gloghini A, Larocca LM, Capello D, Canzonieri V, Antinori A, Tirelli U, Falini B, Dalla-Favera R. Differential expression of BCL-6, CD138/syndecan-1, and Epstein-Barr virus-encoded latent membrane protein-1 identifies distinct histogenetic subsets of acquired immunodeficiency syndrome-related non-Hodgkin’s lymphomas. Blood. 1998;91:747–755. [PubMed] [Google Scholar]
- Carbone A, Gloghini A, Vaccher E, Cerri M, Gaidano G, Dalla-Favera R, Tirelli U. Kaposi’s sarcoma-associated herpesvirus/human herpesvirus type 8-positive solid lymphomas: a tissue-based variant of primary effusion lymphoma. J Mol Diagn. 2005;7:17–27. doi: 10.1016/S1525-1578(10)60004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RS, Pennington KN, Arrate P, Oltz EM, Ballard DW. Site-specific monoubiquitination of IkappaB kinase IKKbeta regulates its phosphorylation and persistent activation. J. Biol. Chem. 2005;280:43272–43279. doi: 10.1074/jbc.M508656200. [DOI] [PubMed] [Google Scholar]
- Carter RS, Pennington KN, Ungurait BJ, Arrate P, Ballard DW. Signal-induced ubiquitination of I kappaB Kinase-beta. J. Biol. Chem. 2003;278:48903–48906. doi: 10.1074/jbc.M310686200. [DOI] [PubMed] [Google Scholar]
- Cereseto A, Diella F, Mulloy JC, Cara A, Michieli P, Grassmann R, Franchini G, Klotman ME. p53 functional impairment and high p21waf1/cip1 expression in human T-cell lymphotropic/leukemia virus type I-transformed T cells. Blood. 1996;88:1551–1560. [PubMed] [Google Scholar]
- Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi’s Sarcoma-associated Herpesvirus-like DNA sequences in AIDS-related body cavity-based lymphomas. N Eng J Med. 1995a;332:1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- Cesarman E, Moore PS, Rao P, Inghirami G, Knowles DM, Chang Y. In vitro establishment and characterization of two acquired immunodeficiency syndrome-related lymphoma cell lines (BC-1 and BC-2) containing Kaposi’s sarcoma-associated herpesvirus-like (KSHV) DNA sequences. Blood. 1995b;86:2708–2714. [PubMed] [Google Scholar]
- Chadburn A, Hyjek E, Mathew S, Cesarman E, Said J, Knowles DM. KSHV-positive solid lymphomas represent an extra-cavitary variant of primary effusion lymphoma. Am J Surg Pathol. 2004;28:1401–16. doi: 10.1097/01.pas.0000138177.10829.5c. [DOI] [PubMed] [Google Scholar]
- Chan AT, Lo YM, Zee B, Chan LY, Ma BB, Leung SF, Mo F, Lai M, Ho S, Huang DP, Johnson PJ. Plasma Epstein-Barr virus DNA and residual disease after radiotherapy for undifferentiated nasopharyngeal carcinoma. J Natl Cancer Inst. 2002;94:1614–9. doi: 10.1093/jnci/94.21.1614. [DOI] [PubMed] [Google Scholar]
- Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- Chau V, Tobias JW, Bachmair A, Marriott D, Ecker DJ, Gonda DK, Varshavsky A. A multiubiquitin chain is confined to specific lysine in a targeted short-lived protein. Science. 1989;243:1576–1583. doi: 10.1126/science.2538923. [DOI] [PubMed] [Google Scholar]
- Chaudhary PM, Jasmin A, Eby MT, Hood L. Modulation of the NF-kappa B pathway by virally encoded death effector domains-containing proteins. Oncogene. 1999a;18:5738–46. doi: 10.1038/sj.onc.1202976. [DOI] [PubMed] [Google Scholar]
- Chaudhary PM, Jasmin A, Eby MT, Hood L. Modulation of the NF-kappa B pathway by virally encoded death effector domains-containing proteins. Oncogene. 1999b;14:5738–5746. doi: 10.1038/sj.onc.1202976. [DOI] [PubMed] [Google Scholar]
- Chen G, Cao P, Goeddel DV. TNF-induced recruitment and activation of the IKK complex require Cdc37 and Hsp90. Mol Cell. 2002a;9:401–10. doi: 10.1016/s1097-2765(02)00450-1. [DOI] [PubMed] [Google Scholar]
- Chen SY, Lu J, Shih YC, Tsai CH. Epstein-Barr virus latent membrane protein 2A regulates c-Jun protein through extracellular signal-regulated kinase. J. Virol. 2002b;76:9556–9561. doi: 10.1128/JVI.76.18.9556-9561.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZJ. Ubiquitin signalling in the NF-kappaB pathway. Nat. Cell Biol. 2005;7:758–765. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZJ, Bhoj V, Seth RB. Ubiquitin, TAK1 and IKK: is there a connection? Cell Death Differ. 2006;13:687–692. doi: 10.1038/sj.cdd.4401869. [DOI] [PubMed] [Google Scholar]
- Chu Z-L, DiDonato JA, Hawiger J, Ballard DW. The Tax oncoprotein of human T-cell leukemia virus type 1 associates with and persistently activates IκB kinases containing IKKα and IKKβ. J. Biol. Chem. 1998;273:15891–15894. doi: 10.1074/jbc.273.26.15891. [DOI] [PubMed] [Google Scholar]
- Chu Z-L, Shin Y-A, Yang J-M, DiDonato JA, Ballard DW. IKKγ mediates the interaction of cellular IκB kinases with the Tax transforming protein of human T cell leukemia virus type 1. J. Biol. Chem. 1999;274:15297–15300. doi: 10.1074/jbc.274.22.15297. [DOI] [PubMed] [Google Scholar]
- Chugh P, Matta H, Schamus S, Zachariah S, Kumar A, Richardson JA, Smith AL, Chaudhary PM. Constitutive NF-{kappa}B activation, normal Fas-induced apoptosis, and increased incidence of lymphoma in human herpes virus 8 K13 transgenic mice. Proc Natl Acad Sci U S A. 2005 doi: 10.1073/pnas.0408577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claudio E, Brown K, Park S, Wang H, Siebenlist U. BAFF-induced NEMO-independent processing of NF-kappaB2 in maturing B cells. Nat. Immunol. 2002;3:958–965. doi: 10.1038/ni842. [DOI] [PubMed] [Google Scholar]
- Coope HJ, Atkinson PG, Huhse B, Belich M, Janzen J, Holman MJ, Klaus GG, Johnston LH, Ley SC. CD40 regulates the processing of NF-kappaB2 p100 to p52. EMBO J. 2002;15:5375–5385. doi: 10.1093/emboj/cdf542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coornaert B, Carpentier I, Beyaert R. A20: central gatekeeper in inflammation and immunity. J. Biol. Chem. 2009;284:8217–8221. doi: 10.1074/jbc.R800032200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross SL, Feinberg MB, Wolf JB, Holbrook NJ, Wong-Staal F, Leonard WJ. Regulation of the human interleukin-2 receptor a chain promoter: activation of a non functional promoter by the transactivator gene of HTLV-1. Cell. 1987;49:47–56. doi: 10.1016/0092-8674(87)90754-9. [DOI] [PubMed] [Google Scholar]
- Dave SS, Fu K, Wright GW, Lam LT, Kluin P, Boerma EJ, Greiner TC, Weisenburger DD, Rosenwald A, Ott G, Muller-Hermelink HK, Gascoyne RD, Delabie J, Rimsza LM, Braziel RM, Grogan TM, Campo E, Jaffe ES, Dave BJ, Sanger W, Bast M, Vose JM, Armitage JO, Connors JM, Smeland EB, Kvaloy S, Holte H, Fisher RI, Miller TP, Montserrat E, Wilson WH, Bahl M, Zhao H, Yang L, Powell J, Simon R, Chan WC, Staudt LM. Molecular diagnosis of Burkitt’s lymphoma. N Engl J Med. 2006;354:2431–42. doi: 10.1056/NEJMoa055759. [DOI] [PubMed] [Google Scholar]
- Dawson CW, George JH, Blake SM, Longnecker R, Young LS. The Epstein-Barr virus encoded latent membrane protein 2A augments signaling from latent membrane protein 1. Virology. 2001;289:192–207. doi: 10.1006/viro.2001.1142. [DOI] [PubMed] [Google Scholar]
- de La Fuente C, Deng L, Santiago F, Arce L, Wang L, Kashanchi F. Gene expression array of HTLV type 1-infected T cells: Up-regulation of transcription factors and cell cycle genes. AIDS. Res. Hum. Retroviruses. 2000;16:1695–1700. doi: 10.1089/08892220050193164. [DOI] [PubMed] [Google Scholar]
- Dejardin E, Droin NM, Delhase M, Haas E, Cao Y, Makris C, Li ZW, Karin M, Ware CF, Green DR. The Lymphotoxin-beta Receptor Induces Different Patterns of Gene Expression via Two NF-kappaB Pathways. Immunity. 2002;17:525–535. doi: 10.1016/s1074-7613(02)00423-5. [DOI] [PubMed] [Google Scholar]
- Deloose ST, Smit LA, Pals FT, Kersten MJ, van Noesel CJ, Pals ST. High incidence of Kaposi sarcoma-associated herpesvirus infection in HIV-related solid immunoblastic/plasmablastic diffuse large B-cell lymphoma. Leukemia. 2005;19:851–5. doi: 10.1038/sj.leu.2403709. [DOI] [PubMed] [Google Scholar]
- Dittmer D, Lagunoff M, Renne R, Staskus K, Haase A, Ganem D. A cluster of latently expressed genes in Kaposi’s sarcoma-associated herpesvirus. J Virol. 1998;72:8309–15. doi: 10.1128/jvi.72.10.8309-8315.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djerbi M, Screpanti V, Catrina AI, Bogen B, Biberfeld P, Grandien A. The inhibitor of death receptor signaling, FLICE-inhibitory protein defines a new class of tumor progression factors [see comments] J Exp Med. 1999;190:1025–32. doi: 10.1084/jem.190.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Zhong H, Hayden MS, Ghosh G. Repression of gene expression by unphosphorylated NF-κB p65 through epigenetic mechanisms. Genes Devel. 2008;22:1159–1173. doi: 10.1101/gad.1657408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxsey S, McCollum D, Theurkauf W. Centrosomes in cellular regulation. Annu. Rev. Cell. Dev. Biol. 2005;21:411–434. doi: 10.1146/annurev.cellbio.21.122303.120418. [DOI] [PubMed] [Google Scholar]
- Du MQ, Diss TC, Liu H, Ye H, Hamoudi RA, Cabecadas J, Dong HY, Harris NL, Chan JK, Rees JW, Dogan A, Isaacson PG. KSHV- and EBV-associated germinotropic lymphoproliferative disorder. Blood. 2002;100:3415–8. doi: 10.1182/blood-2002-02-0487. [DOI] [PubMed] [Google Scholar]
- Du MQ, Liu H, Diss TC, Ye H, Hamoudi RA, Dupin N, Meignin V, Oksenhendler E, Boshoff C, Isaacson PG. Kaposi sarcoma-associated herpesvirus infects monotypic (IgM lambda) but polyclonal naive B cells in Castleman disease and associated lymphoproliferative disorders. Blood. 2001;97:2130–6. doi: 10.1182/blood.v97.7.2130. [DOI] [PubMed] [Google Scholar]
- Dupin N, Diss TL, Kellam P, Tulliez M, Du MQ, Sicard D, Weiss RA, Isaacson PG, Boshoff C. HHV-8 is associated with a plasmablastic variant of Castleman disease that is linked to HHV-8-positive plasmablastic lymphoma. Blood. 2000;95:1406–12. [PubMed] [Google Scholar]
- Dupin N, Fisher C, Kellam P, Ariad S, Tulliez M, Franck N, van Marck E, Salmon D, Gorin I, Escande JP, Weiss RA, Alitalo K, Boshoff C. Distribution of human herpesvirus-8 latently infected cells in Kaposi’s sarcoma, multicentric Castleman’s disease, and primary effusion lymphoma. Proc Natl Acad Sci U S A. 1999;96:4546–51. doi: 10.1073/pnas.96.8.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ea CK, Deng L, Xia ZP, Pineda G, Chen ZJ. Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol. Cell. 2006;22:245–257. doi: 10.1016/j.molcel.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Elenitoba-Johnson KS, Zarate-Osorno A, Meneses A, Krenacs L, Kingma DW, Raffeld M, Jaffe ES. Cytotoxic granular protein expression, Epstein-Barr virus strain type, and latent membrane protein-1 oncogene deletions in nasal T-lymphocyte/natural killer cell lymphomas from Mexico. Mod. Pathol. 1998;11:754–761. [PubMed] [Google Scholar]
- Eltom MA, Jemal A, Mbulaiteye SM, Devesa SS, Biggar RJ. Trends in Kaposi’s sarcoma and non-Hodgkin’s lymphoma incidence in the United States from 1973 through 1998. J Natl Cancer Inst. 2002;94:1204–10. doi: 10.1093/jnci/94.16.1204. [DOI] [PubMed] [Google Scholar]
- Engels EA, Pittaluga S, Whitby D, Rabkin C, Aoki Y, Jaffe ES, Goedert JJ. Immunoblastic lymphoma in persons with AIDS-associated Kaposi’s sarcoma: a role for Kaposi’s sarcoma-associated herpesvirus. Mod Pathol. 2003;16:424–9. doi: 10.1097/01.MP.0000056629.62148.55. [DOI] [PubMed] [Google Scholar]
- Feuer G, Green PL. Comparative biology of human T-cell lymphotropic virus type 1 (HTLV-1) and HTLV-2. Oncogene. 2005;24:5996–6004. doi: 10.1038/sj.onc.1208971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuillard J, Schuhmacher M, Kohanna S, Asso-Bonnet M, Ledeur F, Joubert-Caron R, Bissieres P, Polack A, Bornkamm GW, Raphael M. Inducible loss of NF-kappaB activity is associated with apoptosis and Bcl-2 down-regulation in Epstein-Barr virus-transformed B lymphocytes. Blood. 2000;95:2068–75. [PubMed] [Google Scholar]
- Field N, Low W, Daniels M, Howell S, Daviet L, Boshoff C, Collins M. KSHV vFLIP binds to IKK-gamma to activate IKK. J. Cell Sci. 2003;116:3721–3728. doi: 10.1242/jcs.00691. [DOI] [PubMed] [Google Scholar]
- Flore O, Rafii S, Ely S, O’Leary JJ, Hyjek EM, Cesarman E. Transformation of primary human endothelial cells by Kaposi’s sarcoma-associated herpesvirus. Nature. 1998;394:588–592. doi: 10.1038/29093. [DOI] [PubMed] [Google Scholar]
- Franchini G, Nicot C, Johnson JM. Seizing of T cells by human T-cell leukemia/lymphoma virus type 1. Adv. Cancer Res. 2003;89:69–132. doi: 10.1016/s0065-230x(03)01003-0. [DOI] [PubMed] [Google Scholar]
- Fruehling S, Longnecker R. The immunoreceptor tyrosine-based activation motif of Epstein-Barr virus LMP2A is essential for blocking BCR-mediated signal transduction. Virology. 1997;235:241–51. doi: 10.1006/viro.1997.8690. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Longnecker R. Epstein-Barr Virus (EBV) Latent Membrane Protein 2A Mediates Transformation through Constitutive Activation of the Ras/PI3-K/Akt Pathway. J Virol. 2007 doi: 10.1128/JVI.00537-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda RI, Tsuchiya K, Suzuki K, Itoh K, Fujita J, Utsunomiya A, Tsuji T. Human T-cell leukemia virus type I tax down-regulates the expression of phosphatidylinositol 3,4,5-trisphosphate inositol phosphatases via the NF-kappaB pathway. J. Biol. Chem. 2009;284:2680–2689. doi: 10.1074/jbc.M806325200. [DOI] [PubMed] [Google Scholar]
- Gaide O, Favier B, Legler DF, Bonnet D, Brissoni B, Valitutti S, Bron C, Tschopp J, Thome M. CARMA1 is a critical lipid raft-associated regulator of TCR-induced NF-kappa B activation. Nat. Immunol. 2002;3:836–843. doi: 10.1038/ni830. [DOI] [PubMed] [Google Scholar]
- Gartenhaus RB, Wang P. Functional inactivation of wild-type p53 protein correlates with loss of IL-2 dependence in HTLV-I transformed human T lymphocytes. Leukemia. 1995;9:2082–2086. [PubMed] [Google Scholar]
- Geleziunas R, Ferrell S, Lin X, Mu Y, Cunningham ET, Jr., Grant M, Connelly MA, Hambor JE, Marcu KB, Greene WC. Human T-cell leukemia virus type 1 Tax induction of NF-κB involves activation of the IkB kinase α (IKKα) and IKKβ cellular kinases. Mol. Cell. Biol. 1998;18:5157–5165. doi: 10.1128/mcb.18.9.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gires O, Zimber-Strobl U, Gonnella R, Ueffing M, Marschall G, Zeidler R, Pich D, Hammerschmidt W. Latent membrane protein 1 of Epstein-Barr virus mimics a constitutively active receptor molecule. Embo J. 1997;16:6131–40. doi: 10.1093/emboj/16.20.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey A, Anderson J, Papanastasiou A, Takeuchi Y, Boshoff C. Inhibiting primary effusion lymphoma by lentiviral vectors encoding short hairpin RNA. Blood. 2005;105:2510–8. doi: 10.1182/blood-2004-08-3052. [DOI] [PubMed] [Google Scholar]
- Grassmann R, Berchtold S, Radant I, Alt M, Fleckenstein B, Sodroski JG, Haseltine WA, Ramstedt U. Role of human T-cell leukemia virus type I x region proteins in immortalization of primary human lymphocytes in culture. J. Virol. 1992;66:4570–4575. doi: 10.1128/jvi.66.7.4570-4575.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassmann R, Dengler C, Müller-Fleckenstein I, Fleckenstein B, McGuire K, Dokhelar M-C, Sodroski JG, Haseltine WA. Transformation to continuous growth of primary human T-lymphocytes by human T-cell leukemia virus type I X-region gene transduced by a herpesvirus saimiri vector. Proc. Natl. Acad. Sci. USA. 1989;86:3351–3355. doi: 10.1073/pnas.86.9.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guasparri I, Bubman D, Cesarman E. EBV LMP2A affects LMP1-mediated NF-kappaB signaling and survival of lymphoma cells by regulating TRAF2 expression. Blood. 2008;111:3813–20. doi: 10.1182/blood-2007-03-080309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guasparri I, Keller SA, Cesarman E. KSHV vFLIP is essential for the survival of infected lymphoma cells. J Exp Med. 2004;199:993–1003. doi: 10.1084/jem.20031467. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Guasparri I, Wu H, Cesarman E. The KSHV oncoprotein vFLIP contains a TRAF-interacting motif and requires TRAF2 and TRAF3 for signalling. EMBO Rep. 2006;7:114–9. doi: 10.1038/sj.embor.7400580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall PA, Donaghy M, Cotter FE, Stansfeld AG, Levison DA. An immunohistological and genotypic study of the plasma cell form of Castleman’s disease. Histopathology. 1989;14:333–46. doi: 10.1111/j.1365-2559.1989.tb02162.x. discussion 429-32. [DOI] [PubMed] [Google Scholar]
- Hara H, Bakal C, Wada T, Bouchard D, Rottapel R, Saito T, Penninger JM. The molecular adapter Carma1 controls entry of IkappaB kinase into the central immune synapse. J. Exp. Med. 2004;200:1167–1177. doi: 10.1084/jem.20032246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harhaj EW, Good L, Xiao G, Sun S-C. Gene expression profiles in HTLV-I-immortalized T cells: deregulated expression of genes involved in apoptosis regulation. Oncogene. 1999;18:1341–1349. doi: 10.1038/sj.onc.1202405. [DOI] [PubMed] [Google Scholar]
- Harhaj EW, Sun S-C. IKKg serves as a docking subunit of the IkB kinase and mediates interaction of IKK with the human T-cell leukemia virus Tax protein. J. Biol. Chem. 1999;274:22911–22914. doi: 10.1074/jbc.274.33.22911. [DOI] [PubMed] [Google Scholar]
- Harhaj NS, Sun SC, Harhaj EW. Activation of NF-kappa B by the human T cell leukemia virus type I (HTLV-I) tax oncoprotein is associated with ubiquitin-dependent relocalization of IKK. J. Biol. Chem. 2007;282:4185–4192. doi: 10.1074/jbc.M611031200. [DOI] [PubMed] [Google Scholar]
- Harris NL. The many faces of Hodgkin’s disease around the world: what have we learned from its pathology? Ann. Oncol. 1998;9:S45–56. doi: 10.1093/annonc/9.suppl_5.s45. [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Sawa H, Lewis MJ, Orba Y, Sheehy N, Yamamoto Y, Ichinohe T, Tsunetsugu-Yokota Y, Katano H, Takahashi H, Matsuda J, Sata T, Kurata T, Nagashima K, Hall WW. Thymus-derived leukemia-lymphoma in mice transgenic for the Tax gene of human T-lymphotropic virus type I. Nat. Med. 2006;12:466–472. doi: 10.1038/nm1389. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Tsubata C, Kondo R, Yoshida S, Takahashi M, Oie M, Tanaka Y, Mahieux R, Matsuoka M, Fujii M. Cooperation of NF-kappaB2/p100 activation and the PDZ domain binding motif signal in human T-cell leukemia virus type 1 (HTLV-1) Tax1 but not HTLV-2 Tax2 is crucial for interleukin-2-independent growth transformation of a T-cell line. J. Virol. 2007;81:11900–11907. doi: 10.1128/JVI.00532-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hironaka N, Mochida K, Mori N, Maeda M, Yamamoto N, Yamaoka S. Tax-independent constitutive IkappaB kinase activation in adult T-cell leukemia cells. Neoplasia. 2004;6:266–278. doi: 10.1593/neo.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho JW, Ho FC, Chan AC, Liang RH, Srivastava G. Frequent detection of Epstein-Barr virus-infected B cells in peripheral T-cell lymphomas. J. Pathol. 1998;185:79–85. doi: 10.1002/(SICI)1096-9896(199805)185:1<79::AID-PATH52>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Homig-Holzel C, Hojer C, Rastelli J, Casola S, Strobl LJ, Muller W, Quintanilla-Martinez L, Gewies A, Ruland J, Rajewsky K, Zimber-Strobl U. Constitutive CD40 signaling in B cells selectively activates the noncanonical NF-kappaB pathway and promotes lymphomagenesis. J Exp Med. 2008;205:1317–29. doi: 10.1084/jem.20080238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie R. NF-kappaB in pathogenesis and treatment of adult T-cell leukemia/lymphoma. Int Rev Immunol. 2007;26:269–281. doi: 10.1080/08830180701703364. [DOI] [PubMed] [Google Scholar]
- Huang GJ, Zhang ZQ, Jin DY. Stimulation of IKK-gamma oligomerization by the human T-cell leukemia virus oncoprotein Tax. FEBS Lett. 2002;531:494–498. doi: 10.1016/s0014-5793(02)03590-1. [DOI] [PubMed] [Google Scholar]
- Huang J, Ren T, Guan H, Jiang Y, Cheng H. HTLV-1 Tax is a critical lipid raft modulator that hijacks IkappaB kinases to the microdomains for persistent activation of NF-kappaB. J. Biol. Chem. 2009;284:6208–6217. doi: 10.1074/jbc.M806390200. [DOI] [PubMed] [Google Scholar]
- Hummel M, Bentink S, Berger H, Klapper W, Wessendorf S, Barth TF, Bernd HW, Cogliatti SB, Dierlamm J, Feller AC, Hansmann ML, Haralambieva E, Harder L, Hasenclever D, Kuhn M, Lenze D, Lichter P, Martin-Subero JI, Moller P, Muller-Hermelink HK, Ott G, Parwaresch RM, Pott C, Rosenwald A, Rosolowski M, Schwaenen C, Sturzenhofecker B, Szczepanowski M, Trautmann H, Wacker HH, Spang R, Loeffler M, Trumper L, Stein H, Siebert R. A biologic definition of Burkitt’s lymphoma from transcriptional and genomic profiling. N Engl J Med. 2006;354:2419–30. doi: 10.1056/NEJMoa055351. [DOI] [PubMed] [Google Scholar]
- Inoue J, Seiki M, Taniguchi T, Tsuru S, Yoshida M. Induction of interleukin-2 receptor gene expression by p40x encoded by human T-cell leukemia virus type I. EMBO J. 1986;5:2883–2888. doi: 10.1002/j.1460-2075.1986.tb04583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumiya Y, Izumiya C, Hsia D, Ellison TJ, Luciw PA, Kung HJ. NF-kappaB serves as a cellular sensor of Kaposi’s sarcoma-associated herpesvirus latency and negatively regulates K-Rta by antagonizing the RBP-Jkappa coactivator. J Virol. 2009;83:4435–46. doi: 10.1128/JVI.01999-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe ES, Krenacs L, Kumar S, Kingma DW, Raffeld M. Extranodal peripheral T-cell and NK-cell neoplasms. Am. J. Clin. Pathol. 1999;111:S46–55. [PubMed] [Google Scholar]
- Jeang KT. Functional activities of the human T-cell leukemia virus type I Tax oncoprotein: cellular signaling through NF-kappa B. Cytokine Growth Factor Rev. 2001;12:207–217. doi: 10.1016/s1359-6101(00)00028-9. [DOI] [PubMed] [Google Scholar]
- Jeong SJ, Pise-Masison CA, Radonovich MF, Park HU, Brady JN. A Novel NF-{kappa}B Pathway Involving IKK{beta} and p65/RelA Ser-536 Phosphorylation Results in p53 Inhibition in the Absence of NF-{kappa}B Transcriptional Activity. J. Biol. Chem. 2005a;280:10326–19332. doi: 10.1074/jbc.M412643200. [DOI] [PubMed] [Google Scholar]
- Jeong SJ, Pise-Masison CA, Radonovich MF, Park HU, Brady JN. Activated AKT regulates NF-kappaB activation, p53 inhibition and cell survival in HTLV-1-transformed cells. Oncogene. 2005b;24:6719–6728. doi: 10.1038/sj.onc.1208825. [DOI] [PubMed] [Google Scholar]
- Jin D-Y, Giordano V, Kibler KV, Nakano H, Jeang K-T. Role of adaptor function in oncoprotein-mediated activation of NF-κB: HTLV-I Tax interacts directly with IκB kinase γ. J. Biol. Chem. 1999;274:17402–17405. doi: 10.1074/jbc.274.25.17402. [DOI] [PubMed] [Google Scholar]
- Johansson P, Jansson A, Ruetschi U, Rymo L. Nuclear factor-kappaB binds to the Epstein-Barr Virus LMP1 promoter and upregulates its expression. J Virol. 2009;83:1393–401. doi: 10.1128/JVI.01637-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Journo C, Filipe J, About F, Chevalier SA, Afonso PV, Brady JN, Flynn D, Tangy F, Israël A, Vidalain PO, Mahieux R, Weil R. NRP/Optineurin Cooperates with TAX1BP1 to potentiate the activation of NF-kappaB by human T-lymphotropic virus type 1 tax protein. PLoS Pathog. 2009;5:e1000521. doi: 10.1371/journal.ppat.1000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanegane H, Yachie A, Miyawaki T, Tosato G. EBV-NK cells interactions and lymphoproliferative disorders. Leuk. Lymphoma. 1998;29:491–498. doi: 10.3109/10428199809050908. [DOI] [PubMed] [Google Scholar]
- Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu. Rev. Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- Katano H, Sato Y, Kurata T, Mori S, Sata T. High expression of HHV-8-encoded ORF73 protein in spindle-shaped cells of Kaposi’s sarcoma. Am J Pathol. 1999;155:47–52. doi: 10.1016/S0002-9440(10)65097-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katano H, Sato Y, Kurata T, Mori S, Sata T. Expression and localization of human herpesvirus 8-encoded proteins in primary effusion lymphoma, Kaposi’s sarcoma, and multicentric Castleman’s disease. Virology. 2000;269:335–44. doi: 10.1006/viro.2000.0196. [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Yan M, Seshasayee D, Wang H, Lee W, French DM, Grewal IS, Cochran AG, Gordon NC, Yin J, Starovasnik MA, Dixit VM. BAFF/BLyS Receptor 3 Binds the B Cell Survival Factor BAFF Ligand through a Discrete Surface Loop and Promotes Processing of NF-kappaB2. Immunity. 2002;17:515–524. doi: 10.1016/s1074-7613(02)00425-9. [DOI] [PubMed] [Google Scholar]
- Kellam P, Bourboulia D, Dupin N, Shotton C, Fisher C, Talbot S, Boshoff C, Weiss RA. Characterization of monoclonal antibodies raised against the latent nuclear antigen of human herpesvirus 8. J Virol. 1999;73:5149–55. doi: 10.1128/jvi.73.6.5149-5155.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller SA, Hernandez-Hopkins D, Vider J, Ponomarev V, Hyjek E, Schattner EJ, Cesarman E. NF-{kappa}B is essential for progression of KSHV- and EBV-infected lymphomas in vivo. Blood. 2006;107:3295–3302. doi: 10.1182/blood-2005-07-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller SA, Schattner EJ, Cesarman E. Inhibition of NF-κB induces apoptosis of KSHV-infected primary effusion lymphoma cells. Blood. 2000;96:2537–2542. [PubMed] [Google Scholar]
- Kelly G, Bell A, Rickinson A. Epstein-Barr virus-associated Burkitt lymphomagenesis selects for downregulation of the nuclear antigen EBNA2. Nat Med. 2002;8:1098–104. doi: 10.1038/nm758. [DOI] [PubMed] [Google Scholar]
- Kelly GL, Milner AE, Tierney RJ, Croom-Carter DS, Altmann M, Hammerschmidt W, Bell AI, Rickinson AB. Epstein-Barr virus nuclear antigen 2 (EBNA2) gene deletion is consistently linked with EBNA3A, -3B, and -3C expression in Burkitt’s lymphoma cells and with increased resistance to apoptosis. J Virol. 2005;79:10709–17. doi: 10.1128/JVI.79.16.10709-10717.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten MJ, Van Gorp J, Pals ST, Boon F, Van Oers MH. Expression of Epstein-Barr virus latent genes and adhesion molecules in AIDS-related non-Hodgkin’s lymphomas: correlation with histology and CD4-cell number. Leuk. Lymphoma. 1998;30:515–524. doi: 10.3109/10428199809057564. [DOI] [PubMed] [Google Scholar]
- Kfoury Y, Nasr R, Favre-Bonvin A, El-Sabban M, Renault N, Giron ML, Setterblad N, Hajj HE, Chiari E, Mikati AG, Hermine O, Saib A, de Thé H, Pique C, Bazarbachi A. Ubiquitylated Tax targets and binds the IKK signalosome at the centrosome. Oncogene. 2008;27:1665–1676. doi: 10.1038/sj.onc.1210804. [DOI] [PubMed] [Google Scholar]
- Khoshnan A, Bae D, Tindell CA, Nel AE. The physical association of protein kinase C theta with a lipid raft-associated inhibitor of kappa B factor kinase (IKK) complex plays a role in the activation of the NF-kappa B cascade by TCR and CD28. J. Immunol. 2000;165:6933–6940. doi: 10.4049/jimmunol.165.12.6933. [DOI] [PubMed] [Google Scholar]
- Kieff E. Epstein-Barr virus and its replication. In: Fields BN, Knipe DM, Howley PM, editors. Virology. Lippincott-Raven Publishers; Philadelphia: 1996. pp. 2343–2396. [Google Scholar]
- Kieser A. Pursuing different ‘TRADDes’: TRADD signaling induced by TNF-receptor 1 and the Epstein-Barr virus oncoprotein LMP1. Biol Chem. 2008;389:1261–71. doi: 10.1515/BC.2008.144. [DOI] [PubMed] [Google Scholar]
- Kitajima I, Shinohara T, Bilakovics J, Brown DA, Xu X, Nerenberg M. Ablation of transplanted HTLV-I Tax-transformed tumors in mice by antisense inhibition of NF-κB. Science. 1992;258:1792–1795. doi: 10.1126/science.1299224. [DOI] [PubMed] [Google Scholar]
- Knowles DM. Neoplastic Hematopathology. Williams and Wilkins; Baltimore: 2001. p. 1956. [Google Scholar]
- Knowles DM, Cesarman E, Chadburn A, Frizzera G, Chen J, Rose EA, Michler RE. Correlative morphologic and molecular genetic analysis demonstrates three distinct categories of posttransplantation lymphoproliferative disorders. Blood. 1995;85:552–565. [PubMed] [Google Scholar]
- Koga H, Imada K, Ueda M, Hishizawa M, Uchiyama T. Identification of differentially expressed molecules in adult T-cell leukemia cells proliferating in vivo. Cancer Sci. 2004;95:411–417. doi: 10.1111/j.1349-7006.2004.tb03224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad A, Wies E, Thurau M, Marquardt G, Naschberger E, Hentschel S, Jochmann R, Schulz TF, Erfle H, Brors B, Lausen B, Neipel F, Sturzl M. A systems biology approach to identify the combination effects of human herpesvirus 8 genes on NF-kappaB activation. J Virol. 2009;83:2563–74. doi: 10.1128/JVI.01512-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulwichit W, Edwards RH, Davenport EM, Baskar JF, Godfrey V, Raab-Traub N. Expression of the Epstein-Barr virus latent membrane protein 1 induces B cell lymphoma in transgenic mice. Proc Natl Acad Sci U S A. 1998;95:11963–8. doi: 10.1073/pnas.95.20.11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küppers R, Rajewsky K. The origin of Hodgkin and Reed/Sternberg cells in Hodgkin’s disease. Annu. Rev. Immunol. 1998;16:471–493. doi: 10.1146/annurev.immunol.16.1.471. [DOI] [PubMed] [Google Scholar]
- Kwon H, Ogle L, Benitez B, Bohuslav J, Montano M, Felsher DW, Greene WC. Lethal cutaneous disease in transgenic mice conditionally expressing type I human T cell leukemia virus Tax. J. BIol. Chem. 2005;280:35713–35722. doi: 10.1074/jbc.M504848200. [DOI] [PubMed] [Google Scholar]
- Laichalk LL, Hochberg D, Babcock GJ, Freeman RB, Thorley-Lawson DA. The dispersal of mucosal memory B cells: evidence from persistent EBV infection. Immunity. 2002;16:745–54. doi: 10.1016/s1074-7613(02)00318-7. [DOI] [PubMed] [Google Scholar]
- Laichalk LL, Thorley-Lawson DA. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein-Barr virus in vivo. J Virol. 2005;79:1296–307. doi: 10.1128/JVI.79.2.1296-1307.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lairmore MD, Silverman L, Ratner L. Animal models for human T-lymphotropic virus type 1 (HTLV-1) infection and transformation. Oncogene. 2005;24:6005–6015. doi: 10.1038/sj.onc.1208974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamsoul I, Lodewick J, Lebrun S, Brasseur R, Burny A, Gaynor RB, Bex F. Exclusive ubiquitination and sumoylation on overlapping lysine residues mediate NF-kappaB activation by the human T-cell leukemia virus tax oncoprotein. Mol. Cell. Biol. 2005;25:10391–10406. doi: 10.1128/MCB.25.23.10391-10406.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larocca LM, Capello D, Rinelli A, Nori S, Antinori A, Gloghini A, Cingolani A, Migliazza A, Saglio G, Cammilleri-Broet S, Raphael M, Carbone A, Gaidano G. The molecular and phenotypic profile of primary central nervous system lymphoma identifies distinct categories of the disease and is consistent with histogenetic derivation from germinal center-related B cells. Blood. 1998;92:1011–1099. [PubMed] [Google Scholar]
- Larroche C, Cacoub P, Soulier J, Oksenhendler E, Clauvel JP, Piette JC, Raphael M. Castleman’s disease and lymphoma: report of eight cases in HIV-negative patients and literature review. Am J Hematol. 2002;69:119–26. doi: 10.1002/ajh.10022. [DOI] [PubMed] [Google Scholar]
- Lechowicz MJ, Lin L, Ambinder RF. Epstein-Barr virus DNA in body fluids. Curr Opin Oncol. 2002;14:533–7. doi: 10.1097/00001622-200209000-00010. [DOI] [PubMed] [Google Scholar]
- Lee JS, Li Q, Lee JY, Lee SH, Jeong JH, Lee HR, Chang H, Zhou FC, Gao SJ, Liang C, Jung JU. FLIP-mediated autophagy regulation in cell death control. Nat Cell Biol. 2009;11:1355–62. doi: 10.1038/ncb1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Kobayashi M, Blonska M, You Y, Lin X. Ubiquitination of RIP is required for tumor necrosis factor alpha-induced NF-kappaB activation. J. Biol. Chem. 2006;281:13636–13643. doi: 10.1074/jbc.M600620200. [DOI] [PubMed] [Google Scholar]
- Li Y, Kang J, Horwitz M. Interaction of an adenovirus E3 14.7-kilodalton protein with a novel tumor necrosis factor alpha-inducible cellular protein containing leucine zipper domains. Mol. Cell. Biol. 1998;18:1601–1610. doi: 10.1128/mcb.18.3.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebowitz D. Epstein-Barr virus and a cellular signaling pathway in lymphomas from immunosuppressed patients. N Engl J Med. 1998;338:1413–21. doi: 10.1056/NEJM199805143382003. [DOI] [PubMed] [Google Scholar]
- Lin JC, Wang WY, Chen KY, Wei YH, Liang WM, Jan JS, Jiang RS. Quantification of plasma Epstein-Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N Engl J Med. 2004;350:2461–70. doi: 10.1056/NEJMoa032260. [DOI] [PubMed] [Google Scholar]
- Liu L, Eby MT, Rathore N, Sinha SK, Kumar A, Chaudhary PM. The human herpes Virus 8 encoded viral FLICE inhibitory protein physically associates with and persistently activates the IkappaB kinase complex. J Biol Chem. 2002;5:5. doi: 10.1074/jbc.M110480200. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang Y, Yamakuchi M, Masuda S, Tokioka T, Yamaoka S, Maruyama I, Kitajima I. Phosphoinositide-3 kinase-PKB/Akt pathway activation is involved in fibroblast Rat-1 transformation by human T-cell leukemia virus type I tax. Oncogene. 2001;20:2514–2526. doi: 10.1038/sj.onc.1204364. [DOI] [PubMed] [Google Scholar]
- Lo YM, Chan AT, Chan LY, Leung SF, Lam CW, Huang DP, Johnson PJ. Molecular prognostication of nasopharyngeal carcinoma by quantitative analysis of circulating Epstein-Barr virus DNA. Cancer Res. 2000;60:6878–81. [PubMed] [Google Scholar]
- Lu J, Lin WH, Chen SY, Longnecker R, Tsai SC, Chen CL, Tsai CH. Syk tyrosine kinase mediates Epstein-Barr virus latent membrane protein 2A-induced cell migration in epithelial cells. J Biol Chem. 2006;281:8806–14. doi: 10.1074/jbc.M507305200. [DOI] [PubMed] [Google Scholar]
- Lu KP, Zhou XZ. The prolyl isomerase PIN1: a pivotal new twist in phosphorylation signalling and disease. Nat. Rev. Mol. Cell. Biol. 2007;8:904–916. doi: 10.1038/nrm2261. [DOI] [PubMed] [Google Scholar]
- Luftig M, Prinarakis E, Yasui T, Tsichritzis T, Cahir-McFarland E, Inoue J, Nakano H, Mak TW, Yeh WC, Li X, Akira S, Suzuki N, Suzuki S, Mosialos G, Kieff E. Epstein-Barr virus latent membrane protein 1 activation of NF-kappaB through IRAK1 and TRAF6. Proc Natl Acad Sci U S A. 2003;100:15595–600. doi: 10.1073/pnas.2136756100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainou BA, Everly DN, Jr., Raab-Traub N. Unique signaling properties of CTAR1 in LMP1-mediated transformation. J Virol. 2007;81:9680–92. doi: 10.1128/JVI.01001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D, Galisteo R, Ji Y, Montaner S, Gutkind JS. An NF-kappaB gene expression signature contributes to Kaposi’s sarcoma virus vGPCR-induced direct and paracrine neoplasia. Oncogene. 2008;27:1844–52. doi: 10.1038/sj.onc.1210817. [DOI] [PubMed] [Google Scholar]
- Maruyama M, Shibuya H, Harada H, Hatakeyama M, Seiki M, Fujita T, Inoue JI, Yoshida M, Taniguchi T. Evidence for aberrant activation of the interleukine-2 autocrine loop by HTLV-1-encoded p40x and T3/Ti complex triggering. Cell. 1987;48:343–350. doi: 10.1016/0092-8674(87)90437-5. [DOI] [PubMed] [Google Scholar]
- Matsumoto R, Wang D, Blonska M, Li H, Kobayashi M, Pappu B, Chen Y, Wang D, Lin X. Phosphorylation of CARMA1 plays a critical role in T Cell receptor-mediated NF-kappaB activation. Immunity. 2005;23:575–585. doi: 10.1016/j.immuni.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Matsuoka M. Human T-cell leukemia virus type I and adult T-cell leukemia. Oncogene. 2003;22:5131–5140. doi: 10.1038/sj.onc.1206551. [DOI] [PubMed] [Google Scholar]
- Matsuoka M, Green PL. The HBZ gene, a key player in HTLV-1 pathogenesis. Retrovirology. 2009;6:71. doi: 10.1186/1742-4690-6-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka M, Jeang KT. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat. Rev. Cancer. 2007;7:270–280. doi: 10.1038/nrc2111. [DOI] [PubMed] [Google Scholar]
- Matta H, Chaudhary PM. Activation of alternative NF-kappa B pathway by human herpes virus 8-encoded Fas-associated death domain-like IL-1 beta-converting enzyme inhibitory protein (vFLIP) Proc Natl Acad Sci U S A. 2004;101:9399–404. doi: 10.1073/pnas.0308016101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta H, Mazzacurati L, Schamus S, Yang T, Sun Q, Chaudhary PM. Kaposi’s sarcoma-associated herpesvirus (KSHV) oncoprotein K13 bypasses TRAFs and directly interacts with the IkappaB kinase complex to selectively activate NF-kappaB without JNK activation. J Biol Chem. 2007;282:24858–65. doi: 10.1074/jbc.M700118200. [DOI] [PubMed] [Google Scholar]
- Menke DM, Chadbum A, Cesarman E, Green E, Berenson J, Said J, Tiemann M, Parwaresch R, Thome SD. Analysis of the human herpesvirus 8 (HHV-8) genome and HHV-8 vIL-6 expression in archival cases of castleman disease at low risk for HIV infection. Am J Clin Pathol. 2002;117:268–75. doi: 10.1309/7243-AV50-KJ28-V6J9. [DOI] [PubMed] [Google Scholar]
- Merchant M, Caldwell RG, Longnecker R. The LMP2A ITAM is essential for providing B cells with development and survival signals in vivo. J Virol. 2000;74:9115–24. doi: 10.1128/jvi.74.19.9115-9124.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesri EA, Cesarman E, Arvanitakis L, Rafii S, Moore MAS, Posnett DN, Knowles DM, Asch AS. Human herpesvirus-8/Kaposi’s sarcoma-associated herpesvirus is a new transmissible virus that infects B cells. Journal of Experimental Medicine. 1996;183:2385–2390. [Google Scholar]
- Miller CL, Lee JH, Kieff E, Longnecker R. An integral membrane protein (LMP2) blocks reactivation of Epstein-Barr virus from latency following surface immunoglobulin crosslinking. Proc Natl Acad Sci U S A. 1994;91:772–6. doi: 10.1073/pnas.91.2.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Heston L, Grogan E, Gradoville L, Rigsby M, Sun R, Shedd D, Kushnaryov VM, Grossberg S, Chang Y. Selective switch between latency and lytic replication of Kaposi’s sarcoma herpesvirus and Epstein-Barr virus in dually infected body cavity lymphoma cells. J Virol. 1997;71:314–324. doi: 10.1128/jvi.71.1.314-324.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody CA, Scott RS, Amirghahari N, Nathan CA, Young LS, Dawson CW, Sixbey JW. Modulation of the cell growth regulator mTOR by Epstein-Barr virus-encoded LMP2A. J Virol. 2005;79:5499–506. doi: 10.1128/JVI.79.9.5499-5506.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori N, Sato H, Hayashibara T, Senba M, Hayashi T, Yamada Y, Kamihira S, Ikeda S, Yamasaki Y, Morikawa S, Tomonaga M, Geleziunas R, Yamamoto N. Human T-cell leukemia virus type I Tax transactivates the matrix metalloproteinase-9 gene: potential role in mediating adult T-cell leukemia invasiveness. Blood. 2002;99:1341–1349. doi: 10.1182/blood.v99.4.1341. [DOI] [PubMed] [Google Scholar]
- Morrison JA, Klingelhutz AJ, Raab-Traub N. Epstein-Barr virus latent membrane protein 2A activates beta-catenin signaling in epithelial cells. J Virol. 2003;77:12276–84. doi: 10.1128/JVI.77.22.12276-12284.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison TE, Kenney SC. BZLF1, an Epstein-Barr virus immediate-early protein, induces p65 nuclear translocation while inhibiting p65 transcriptional function. Virology. 2004;328:219–32. doi: 10.1016/j.virol.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Moses AV, Fish KN, Ruhl R, Smith PP, Strussenberg JG, Zhu L, Chandran B, Nelson JA. Long-term infection and transformation of dermal microvascular endothelial cells by human herpesvirus 8. J Virol. 1999;73:6892–902. doi: 10.1128/jvi.73.8.6892-6902.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nador RG, Cesarman E, Chadburn A, Dawson DB, Ansari MQ, Said J, Knowles DM. Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi’s sarcoma-associated herpesvirus. Blood. 1996;88:645–656. [PubMed] [Google Scholar]
- Nador RG, Cesarman E, Knowles DM, Said JW. Herpes-like DNA sequences in a body-cavity-based lymphoma in an HIV-negative patient (Letter to the Editor) New England Journal of Medicine. 1995;333:943. doi: 10.1056/NEJM199510053331417. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Lu M, Gwack Y, Souvlis J, Zeichner SL, Jung JU. Global changes in Kaposi’s sarcoma-associated virus gene expression patterns following expression of a tetracycline-inducible Rta transactivator. J Virol. 2003;77:4205–20. doi: 10.1128/JVI.77.7.4205-4220.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasr R, Chiari E, El-Sabban M, Mahieux R, Kfoury Y, Abdulhay M, Yazbeck V, Hermine O, H. d T, Pique C, Bazarbachi A. Tax ubiquitylation and sumoylation control critical cytoplasmic and nuclear steps of NF-kappaB activation. Blood. 2006;107:4021–4029. doi: 10.1182/blood-2005-09-3572. [DOI] [PubMed] [Google Scholar]
- Ng PW, Iha H, Iwanaga Y, Bittner M, Chen Y, Jiang Y, Gooden G, Trent JM, Meltzer P, Jeang KT, Zeichner SL. Genome-wide expression changes induced by HTLV-1 Tax: evidence for MLK-3 mixed lineage kinase involvement in Tax-mediated NF-kappaB activation. Oncogene. 2001;20:4484–4496. doi: 10.1038/sj.onc.1204513. [DOI] [PubMed] [Google Scholar]
- Novack DV, Yin L, Hagen-Stapleton A, Schreiber RD, Goeddel DV, Ross FP, Teitelbaum SL. The IkappaB function of NF-kappaB2 p100 controls stimulated osteoclastogenesis. J Exp Med. 2003;198:771–81. doi: 10.1084/jem.20030116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsugi T, Kumasaka T, Okada S, Urano T. The Tax protein of HTLV-1 promotes oncogenesis in not only immature T cells but also mature T cells. Nat. Med. 2007;13:527–528. doi: 10.1038/nm0507-527. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Fujisawa J, Reth M, Yonehara S. Human T-cell leukemia virus type-I oncoprotein Tax inhibits Fas-mediated apoptosis by inducing cellular FLIP through activation of NF-kappaB. Genes Cells. 2006;11:177–191. doi: 10.1111/j.1365-2443.2006.00927.x. [DOI] [PubMed] [Google Scholar]
- Panagopoulos D, Victoratos P, Alexiou M, Kollias G, Mosialos G. Comparative analysis of signal transduction by CD40 and the Epstein-Barr virus oncoprotein LMP1 in vivo. J Virol. 2004;78:13253–61. doi: 10.1128/JVI.78.23.13253-13261.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–44. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- Parravicini C, Chandran B, Corbellino M, Berti E, Paulli M, Moore PS, Chang Y. Differential viral protein expression in Kaposi’s sarcoma-associated herpesvirus-infected diseases: Kaposi’s sarcoma, primary effusion lymphoma, and multicentric Castleman’s disease. Am J Pathol. 2000;156:743–9. doi: 10.1016/S0002-9440(10)64940-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parravicini C, Corbellino M, Paulli M, Magrini U, Lazzarino M, Moore PS, Chang Y. Expression of a virus-derived cytokine, KSHV vIL-6, in HIV-seronegative Castleman’s disease. Am. J. Pathol. 1997;151:1517–1522. [PMC free article] [PubMed] [Google Scholar]
- Pathmanathan R, Prasad U, Chandrika G, Sadler R, Flynn K, Raab-Traub N. Undifferentiated, nonkeratinizing, and squamous cell carcinoma of the nasopharynx. Variants of Epstein-Barr virus-infected neoplasia. Am J Pathol. 1995;146:1355–67. [PMC free article] [PubMed] [Google Scholar]
- Peloponese JJ, Yasunaga J, Kinjo T, Watashi K, Jeang KT. Peptidylproline cistrans-isomerase Pin1 interacts with human T-cell leukemia virus type 1 tax and modulates its activation of NF-kappaB. J. Virol. 2009;83:3238–3248. doi: 10.1128/JVI.01824-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peloponese JM, Yeung ML, Jeang KT. Modulation of nuclear factor-kappaB by human T cell leukemia virus type 1 Tax protein: implications for oncogenesis and inflammation. Immunol. Res. 2006;34:1–12. [PubMed] [Google Scholar]
- Pfeffer S, Sewer A, Lagos-Quintana M, Sheridan R, Sander C, Grasser FA, van Dyk LF, Ho CK, Shuman S, Chien M, Russo JJ, Ju J, Randall G, Lindenbach BD, Rice CM, Simon V, Ho DD, Zavolan M, Tuschl T. Identification of microRNAs of the herpesvirus family. Nat Methods. 2005;2:269–76. doi: 10.1038/nmeth746. [DOI] [PubMed] [Google Scholar]
- Pise-Masison CA, Brady JN. Setting the stage for transformation: HTLV-1 Tax inhibition of p53 function. Front. Biosci. 2005;10:919–930. doi: 10.2741/1586. [DOI] [PubMed] [Google Scholar]
- Pise-Masison CA, Choi KS, Radonovich M, Dittmer J, Kim SJ, Brady JN. Inhibition of p53 transactivation function by the human T-cell lymphotropic virus type 1 Tax protein. J. Virol. 1998;72:1165–1170. doi: 10.1128/jvi.72.2.1165-1170.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pise-Masison CA, Radonovich M, Mahieux R, Chatterjee P, Whiteford C, Duvall J, Guillerm C, Gessain A, Brady JN. Transcription profile of cells infected with human T-cell leukemia virus type I compared with activated lymphocytes. Cancer Res. 2002;62:3562–3571. [PubMed] [Google Scholar]
- Poiesz BF, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. Detection and isolation of a type C retrovirus particles from fresh cultured lymphocytes of a pateint with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz JL, Baltimore D. Two pathways to NF-kappaB. Mol. Cell. 2002;10:693–695. doi: 10.1016/s1097-2765(02)00697-4. [DOI] [PubMed] [Google Scholar]
- Portis T, Harding JC, Ratner L. The contribution of NF-kappa B activity to spontaneous proliferation and resistance to apoptosis in human T-cell leukemia virus type 1 Tax-induced tumors. Blood. 2001;98:1200–1208. doi: 10.1182/blood.v98.4.1200. [DOI] [PubMed] [Google Scholar]
- Pozzatti R, Vogel J, Jay G. The human T-lymphotropic virus type I tax gene can cooperate with the ras oncogene to induce neoplastic transformation of cells. Mol. Cell Biol. 1990;10:413–417. doi: 10.1128/mcb.10.1.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash O, Tang ZY, Peng X, Coleman R, Gill J, Farr G, Samaniego F. Tumorigenesis and aberrant signaling in transgenic mice expressing the human herpesvirus-8 K1 gene. J Natl Cancer Inst. 2002;94:926–35. doi: 10.1093/jnci/94.12.926. [DOI] [PubMed] [Google Scholar]
- Prince S, Keating S, Fielding C, Brennan P, Floettmann E, Rowe M. Latent membrane protein 1 inhibits Epstein-Barr virus lytic cycle induction and progress via different mechanisms. J Virol. 2003;77:5000–7. doi: 10.1128/JVI.77.8.5000-5007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainbow L, Platt GM, Simpson GR, Sarid R, Gao SJ, Stoiber H, Herrington CS, Moore PS, Schulz TF. The 222- to 234-kilodalton latent nuclear protein (LNA) of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) is encoded by orf73 and is a component of the latency-associated nuclear antigen. J Virol. 1997;71:5915–5921. doi: 10.1128/jvi.71.8.5915-5921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raphaël M, Said J, Borisch B, Cesarman E, Harris NL. Lymphomas associated with HIV infection. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; Lyon, France: 2008. pp. 340–342. [Google Scholar]
- Rappocciolo G, Hensler HR, Jais M, Reinhart TA, Pegu A, Jenkins FJ, Rinaldo CR. Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC-SIGN. J Virol. 2008;82:4793–806. doi: 10.1128/JVI.01587-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch D, Gross S, Harding J, Niewiesk S, Lairmore M, Piwnica-Worms D, Ratner L. Imaging spontaneous tumorigenesis: inflammation precedes development of peripheral NK tumors. Blood. 2009;113:1493–1500. doi: 10.1182/blood-2008-07-166462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JA, Nador RG, Spaulding D, Tani Y, Cesarman E, Knowles DM. Demonstration of Kaposi’s sarcoma-associated herpesvirus cyclin D homolog in cutaneous Kaposi’s sarcoma by colorimetric in situ hybridization using a catalyzed signal amplification system. Blood. 1998;91:3825–3832. [PubMed] [Google Scholar]
- Reid RL, Lindholm PF, Mireskandari A, Dittmer J, N. BJ. Stabilization of wild-type p53 in human T-lymphocytes transformed by HTLV-I. Oncogene. 1993;8:3029–3036. [PubMed] [Google Scholar]
- Renne R, Zhong W, Herndier B, McGrath M, Abbey N, Kedes D, Ganem D. Lytic growth of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat. Med. 1996;2:342–346. doi: 10.1038/nm0396-342. [DOI] [PubMed] [Google Scholar]
- Rezaie T, Child A, Hitchings R, Brice G, Miller L, Coca-Prados M, Héon E, Krupin T, Ritch R, Kreutzer D, Crick RP, Sarfarazi M. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science. 2002;295:1077–1079. doi: 10.1126/science.1066901. [DOI] [PubMed] [Google Scholar]
- Rickinson AB, Kieff E. Epstein-Barr virus. In: Fields BN, Knipe DM, Howley PM, editors. Virology. Lippincott-Raven Publishers; Philadelphia: 1996. pp. 2397–2446. [Google Scholar]
- Rivas C, Thlick AE, Parravicini C, Moore PS, Chang Y. Kaposi’s sarcoma-associated herpesvirus LANA2 is a B-cell-specific latent viral protein that inhibits p53. J Virol. 2001;75:429–38. doi: 10.1128/JVI.75.1.429-438.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robek MD, Ratner L. Immortalization of CD4(+) and CD8(+) T Lymphocytes by Human T-Cell Leukemia Virus Type 1 Tax Mutants Expressed in a Functional Molecular Clone. J. Virol. 1999;73:4856–4865. doi: 10.1128/jvi.73.6.4856-4865.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochford R, Miller CL, Cannon MJ, Izumi KM, Kieff E, Longnecker R. In vivo growth of Epstein-Barr virus transformed B cells with mutations in latent membrane protein 2 (LMP2) Arch Virol. 1997;142:707–20. doi: 10.1007/s007050050113. [DOI] [PubMed] [Google Scholar]
- Sadagopan S, Sharma-Walia N, Veettil MV, Raghu H, Sivakumar R, Bottero V, Chandran B. Kaposi’s sarcoma-associated herpesvirus induces sustained NF-kappaB activation during de novo infection of primary human dermal microvascular endothelial cells that is essential for viral gene expression. J Virol. 2007;81:3949–68. doi: 10.1128/JVI.02333-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler R, Wu L, Forghani B, Renne R, Zhong W, Herndier B, Ganem D. A complex translational program generates multiple novel proteins from the latently expressed kaposin (K12) locus of Kaposi’s sarcoma-associated herpesvirus. J Virol. 1999;73:5722–30. doi: 10.1128/jvi.73.7.5722-5730.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said JW, Tasaka T, Takeuchi S, Asou H, de Vos S, Cesarman E, Knowles DM, Koeffler HP. Primary effusion lymphoma in women: Report of two cases of Kaposi’s sarcoma-herpes virus-associated effusion-based lymphoma in human immunodeficiency virus-negative women. Blood. 1996;88:3124–3128. [PubMed] [Google Scholar]
- Saitoh Y, Yamamoto N, Dewan MZ, Sugimoto H, Martinez Bruyn VJ, Iwasaki Y, Matsubara K, Qi X, Saitoh T, Imoto I, Inazawa J, Utsunomiya A, Watanabe T, Masuda T, Yamamoto N, Yamaoka S. Overexpressed NF-kappaB-inducing kinase contributes to the tumorigenesis of adult T-cell leukemia and Hodgkin Reed-Sternberg cells. Blood. 2008;111:5118–5129. doi: 10.1182/blood-2007-09-110635. [DOI] [PubMed] [Google Scholar]
- Samaniego F, Pati S, Karp JE, Prakash O, Bose D. Human herpesvirus 8 K1-associated nuclear factor-kappa B-dependent promoter activity: role in Kaposi’s sarcoma inflammation? J Natl Cancer Inst Monogr. 2001:15–23. doi: 10.1093/oxfordjournals.jncimonographs.a024252. [DOI] [PubMed] [Google Scholar]
- Samols MA, Hu J, Skalsky RL, Renne R. Cloning and identification of a microRNA cluster within the latency-associated region of Kaposi’s sarcoma-associated herpesvirus. J Virol. 2005;79:9301–5. doi: 10.1128/JVI.79.14.9301-9305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanda T, Asamitsu K, Ogura H, Iida S, Utsunomiya A, Ueda R, Okamoto T. Induction of cell death in adult T-cell leukemia cells by a novel IkappaB kinase inhibitor. Leukemia. 2006;20:590–598. doi: 10.1038/sj.leu.2404129. [DOI] [PubMed] [Google Scholar]
- Sarid R, Wiezorek JS, Moore PS, Chang Y. Characterization and cell cycle regulation of the major Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) latent genes and their promoter. J Virol. 1999;73:1438–46. doi: 10.1128/jvi.73.2.1438-1446.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H, Nishikata I, Shiraga T, Akamatsu E, Fukami T, Hidaka T, Kubuki Y, Okayama A, Hamada K, Okabe H, Murakami Y, Tsubouchi H, Morishita K. Overexpression of a cell adhesion molecule, TSLC1, as a possible molecular marker for acute-type adult T-cell leukemia. Blood. 2005;105:1204–1213. doi: 10.1182/blood-2004-03-1222. [DOI] [PubMed] [Google Scholar]
- Satou Y, Yasunaga J, Yoshida M, Matsuoka M. HTLV-I basic leucine zipper factor gene mRNA supports proliferation of adult T cell leukemia cells. Proc. Natl. Acad. Sci. U S A. 2006;103:720–725. doi: 10.1073/pnas.0507631103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senftleben U, Cao Y, Xiao G, Kraehn G, Greten F, Chen Y, Hu Y, Fong A, Sun S-C, Karin M. Activation of IKKα of a second, evolutionary conserved, NF-κB signaling pathway. Science. 2001;293:1495–1499. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- Seo T, Park J, Lim C, Choe J. Inhibition of nuclear factor kappaB activity by viral interferon regulatory factor 3 of Kaposi’s sarcoma-associated herpesvirus. Oncogene. 2004;23:6146–55. doi: 10.1038/sj.onc.1207807. [DOI] [PubMed] [Google Scholar]
- Sgarbanti M, Arguello M, tenOever BR, Battistini A, Lin R, Hiscott J. A requirement for NF-kappaB induction in the production of replication-competent HHV-8 virions. Oncogene. 2004;23:5770–80. doi: 10.1038/sj.onc.1207707. [DOI] [PubMed] [Google Scholar]
- Shembade N, Harhaj NS, Liebl DJ, Harhaj EW. Essential role for TAX1BP1 in the termination of TNF-alpha-, IL-1- and LPS-mediated NF-kappaB and JNK signaling. EMBO J. 2007a;26:3910–3922. doi: 10.1038/sj.emboj.7601823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shembade N, Harhaj NS, Parvatiyar K, Copeland NG, Jenkins NA, Matesic LE, Harhaj EW. The E3 ligase Itch negatively regulates inflammatory signaling pathways by controlling the function of the ubiquitin-editing enzyme A20. Nat. Immunol. 2008;9:254–262. doi: 10.1038/ni1563. [DOI] [PubMed] [Google Scholar]
- Shembade N, Harhaj NS, Yamamoto M, Akira S, Harhaj EW. The human T-cell leukemia virus type 1 Tax oncoprotein requires the ubiquitin-conjugating enzyme Ubc13 for NF-kappaB activation. J. Virol. 2007b;81:13735–13742. doi: 10.1128/JVI.01790-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekevitz M, Feinberg MB, Holbrook N, Wong-Staal F, Greene WC. Activation of interleukin 2 and interleukin 2 receptor (Tac) promoter expression by the transactivator (tat) gene product of human T-cell leukemia virus type 1. Proc. Natl. Acad. Sci. USA. 1987;84:5389–5393. doi: 10.1073/pnas.84.15.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinfield RL, Molyneux EM, Banda K, Borgstein E, Broadhead R, Hesseling P, Newton R, Casabonne D, Mkandawire N, Nkume H, Hodgson T, Liomba G. Spectrum and presentation of pediatric malignancies in the HIV era: experience from Blantyre, Malawi, 1998-2003. Pediatr Blood Cancer. 2007;48:515–20. doi: 10.1002/pbc.20917. [DOI] [PubMed] [Google Scholar]
- Sinha-Datta U, Horikawa I, Michishita E, Datta A, Sigler-Nicot JC, Brown M, Kazanji M, Barrett JC, Nicot C. Transcriptional activation of hTERT through the NF-kappaB pathway in HTLV-I-transformed cells. Blood. 2004;104:2523–2531. doi: 10.1182/blood-2003-12-4251. [DOI] [PubMed] [Google Scholar]
- Skaug B, Jiang X, Chen ZJ. The role of ubiquitin in NF-kappaB regulatory pathways. Annu. Rev. Biochem. 2009;78:769–796. doi: 10.1146/annurev.biochem.78.070907.102750. [DOI] [PubMed] [Google Scholar]
- Sommer K, Guo B, Pomerantz JL, Bandaranayake AD, Moreno-Garcia ME, Ovechkina YL, Rawlings DJ. Phosphorylation of the CARMA1 linker controls NF-kappaB activation. Immunity. 2005;23:561–567. doi: 10.1016/j.immuni.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d’Agay MF, Clauvel J-P, Raphael M, Degos L, Sigaux F. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood. 1995;86:1275–1280. [PubMed] [Google Scholar]
- Speck P, Kline KA, Cheresh P, Longnecker R. Epstein-Barr virus lacking latent membrane protein 2 immortalizes B cells with efficiency indistinguishable from that of wild-type virus. J Gen Virol. 1999;80(Pt 8):2193–203. doi: 10.1099/0022-1317-80-8-2193. [DOI] [PubMed] [Google Scholar]
- Staskus KA, Sun R, Miller G, Racz P, Jaslowski A, Metroka C, Brett-Smith H, Haase AT. Cellular tropism and viral interleukin-6 expression distinguish human herpesvirus 8 involvement in Kaposi’s sarcoma, primary effusion lymphoma, and multicentric Castleman’s disease. J Virol. 1999;73:4181–7. doi: 10.1128/jvi.73.5.4181-4187.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart S, Dawson CW, Takada K, Curnow J, Moody CA, Sixbey JW, Young LS. Epstein-Barr virus-encoded LMP2A regulates viral and cellular gene expression by modulation of the NF-kappaB transcription factor pathway. Proc Natl Acad Sci U S A. 2004;101:15730–5. doi: 10.1073/pnas.0402135101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturzl M, Hohenadl C, Zietz C, Castanos-Velez E, Wunderlich A, Ascherl G, Biberfeld P, Monini P, Browning PJ, Ensoli B. Expression of K13/v-FLIP gene of human herpesvirus 8 and apoptosis in Kaposi’s sarcoma spindle cells. J Natl Cancer Inst. 1999;91:1725–33. doi: 10.1093/jnci/91.20.1725. [DOI] [PubMed] [Google Scholar]
- Su TT, Guo B, Kawakami Y, Sommer K, Chae K, Humphries LA, Kato RM, Kang S, Patrone L, Wall R, Teitell M, Leitges M, Kawakami T, Rawlings DJ. PKC-beta controls I kappa B kinase lipid raft recruitment and activation in response to BCR signaling. Nat. Immunol. 2002;3:780–786. doi: 10.1038/ni823. [DOI] [PubMed] [Google Scholar]
- Suda T, Katano H, Delsol G, Kakiuchi C, Nakamura T, Shiota M, Sata T, Higashihara M, Mori S. HHV-8 infection status of AIDS-unrelated and AIDS-associated multicentric Castleman’s disease. Pathol Int. 2001;51:671–9. doi: 10.1046/j.1440-1827.2001.01266.x. [DOI] [PubMed] [Google Scholar]
- Sugano N, Chen W, Roberts ML, Cooper NR. Epstein-Barr virus binding to CD21 activates the initial viral promoter via NF-kappaB induction. J Exp Med. 1997;186:731–7. doi: 10.1084/jem.186.5.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun CC, Thorley-Lawson DA. Plasma cell-specific transcription factor XBP-1s binds to and transactivates the Epstein-Barr virus BZLF1 promoter. J Virol. 2007;81:13566–77. doi: 10.1128/JVI.01055-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Deng L, Ea C-K, Xia Z-P, Chen ZJ. The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol. Cell. 2004;14:289–301. doi: 10.1016/s1097-2765(04)00236-9. [DOI] [PubMed] [Google Scholar]
- Sun S-C, Ballard DW. Persistent activation of NF-κB by the Tax transforming protein of HTLV-1: hijacking cellular IκB kinases. Oncogene. 1999;18:6948–6958. doi: 10.1038/sj.onc.1203220. [DOI] [PubMed] [Google Scholar]
- Sun S-C, Xiao G. Deregulation of NF-kB and its upstream kinases in cancer. Cancer and Metastasis Reviews. 2003;22:405–422. doi: 10.1023/a:1023733231406. [DOI] [PubMed] [Google Scholar]
- Sun SC. CYLD: a tumor suppressor deubiquitinase regulating NF-κB activation. Cell Death Differ. 2009 doi: 10.1038/cdd.2009.43. Epub ahead of print: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SC, Ley SC. New insights into NF-kappaB regulation and function. Trends Immunol. 2008;29:469–478. doi: 10.1016/j.it.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SC, Yamaoka S. Activation of NF-κB by HTLV-I and implications for cell transformation. Oncogene. 2005;24:5952–5964. doi: 10.1038/sj.onc.1208969. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Fujisawa JI, Toita M, Yoshida M. The trans-activator tax of human T-cell leukemia virus type I (HTLV-1) interacts with cAMP-responsive element (CRE) binding and CRE modulator proteins that bind to the 21-base-pair enhancer of HTLV-1. Proc. Natl. Acad. Sci. USA. 1993;90:610–614. doi: 10.1073/pnas.90.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson-Mungerson M, Bultema R, Longnecker R. Epstein-Barr virus LMP2A enhances B-cell responses in vivo and in vitro. J Virol. 2006;80:6764–70. doi: 10.1128/JVI.00433-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson-Mungerson MA, Caldwell RG, Bultema R, Longnecker R. Epstein-Barr virus LMP2A alters in vivo and in vitro models of B-cell anergy, but not deletion, in response to autoantigen. J Virol. 2005;79:7355–62. doi: 10.1128/JVI.79.12.7355-7362.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swart R, Ruf IK, Sample J, Longnecker R. Latent membrane protein 2A-mediated effects on the phosphatidylinositol 3-Kinase/Akt pathway. J Virol. 2000;74:10838–45. doi: 10.1128/jvi.74.22.10838-10845.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsuki K. Discovery of adult T-cell leukemia. Retrovirology. 2005;2:16. doi: 10.1186/1742-4690-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto S, Trovato R, Cereseto A, Nicot C, Kislyakova T, Casareto L, Waldmann T, Torelli G, Franchini G. p53 stabilization and functional impairment in the absence of genetic mutation or the alteration of the p14(ARF)-MDM2 loop in ex vivo and cultured adult T-cell leukemia/lymphoma cells. Blood. 2000;95:3939–3944. [PubMed] [Google Scholar]
- Tanaka A, Takahashi C, Yamaoka S, Nosaka T, Maki M, Hatanaka M. Oncogenic transformation by the tax gene of human T-cell leukemia virus type I in vitro. Proc. Natl. Acad. Sci. USA. 1990;87:1071–1075. doi: 10.1073/pnas.87.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Q, Robertson KD, Manns A, Hildesheim A, Ambinder RF. Epstein-Barr virus (EBV) in endemic Burkitt’s lymphoma: molecular analysis of primary tumor tissue. Blood. 1998;91:1373–1381. [PubMed] [Google Scholar]
- Taylor GP, Matsuoka M. Natural history of adult T-cell leukemia/lymphoma and approaches to therapy. Oncogene. 2005;24:6047–6057. doi: 10.1038/sj.onc.1208979. [DOI] [PubMed] [Google Scholar]
- Taylor JM, Nicot C. HTLV-1 and apoptosis: role in cellular transformation and recent advances in therapeutic approaches. Apoptosis. 2008;13:733–747. doi: 10.1007/s10495-008-0208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson DA. Epstein-Barr virus: exploiting the immune system. Nat Rev Immunol. 2001;1:75–82. doi: 10.1038/35095584. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med. 2004;350:1328–37. doi: 10.1056/NEJMra032015. [DOI] [PubMed] [Google Scholar]
- Tsuchiyama J, Yoshino T, Mori M, Kondoh E, Oka T, Akagi T, Hiraki A, Nakayama H, Shibuya A, Ma Y, Kawabata T, Okada S, Harada M. Characterization of a novel human natural killer-cell line (NK-YS) established from natural killer cell lymphoma/leukemia associated with Epstein-Barr virus infection. Blood. 1998;92:1374–1383. [PubMed] [Google Scholar]
- Tsukahara T, Kannagi M, Ohashi T, Kato H, Arai M, Nunez G, Iwanaga Y, Yamamoto N, Ohtani K, Nakamura M, Fujii M. Induction of Bcl-x(L) expression by human T-cell leukemia virus type 1 Tax through NF-kappaB in apoptosis-resistant T-cell transfectants with Tax. J. Virol. 1999;73:7981–7987. doi: 10.1128/jvi.73.10.7981-7987.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukasaki K, Tanosaki S, DeVos S, Hofmann WK, Wachsman W, Gombart AF, Krebs J, Jauch A, Bartram CR, Nagai K, Tomonaga M, Said JW, Koeffler HP. Identifying progression-associated genes in adult T-cell leukemia/lymphoma by using oligonucleotide microarrays. Int. J. Cancer. 2004;109:875–881. doi: 10.1002/ijc.20028. [DOI] [PubMed] [Google Scholar]
- Uhlik M, Good L, Xiao G, Harhaj EW, Zandi E, Karin M, Sun S-C. NF-kappaB-inducing kinase and IkappaB kinase participate in human T-cell leukemia virus I Tax-mediated NF-kappaB activation. J. Biol. Chem. 1998;273:21132–21136. doi: 10.1074/jbc.273.33.21132. [DOI] [PubMed] [Google Scholar]
- Valentine R, Dawson CW, Hu C, Shah KM, Owen TJ, Date KL, Maia SP, Shao J, Arrand JR, Young LS, O’Neil JD. Epstein-Barr virus-encoded EBNA1 inhibits the canonical NF-kappa-B pathway in carcinoma cells by inhibiting IKK phosphorylation. Mol. Cancer. 2010;9:1. doi: 10.1186/1476-4598-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu. Rev. Immunol. 2009;693:733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- Verdonck K, González E, Van Dooren S, Vandamme AM, Vanham G, Gotuzzo E. Human T-lymphotropic virus 1: recent knowledge about an ancient infection. Lancet Infect. Dis. 2007;7:266–281. doi: 10.1016/S1473-3099(07)70081-6. [DOI] [PubMed] [Google Scholar]
- Wada K, Niida M, Tanaka M, Kamitani T. Ro52-mediated monoubiquitination of IKK{beta} downregulates NF-{kappa}B signaling. J. Biochem. 2009;146:821–832. doi: 10.1093/jb/mvp127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wäldele K, Silbermann K, Schneider G, Ruckes T, Cullen BR, Grassmann R. Requirement of the human T-cell leukemia virus (HTLV-1) tax-stimulated HIAP-1 gene for the survival of transformed lymphocytes. Blood. 2006;107:4491–4499. doi: 10.1182/blood-2005-08-3138. [DOI] [PubMed] [Google Scholar]
- Wang D, Liebowitz D, Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985;43:831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- Wang H, Nicholas MW, Conway KL, Sen P, Diz R, Tisch RM, Clarke SH. EBV latent membrane protein 2A induces autoreactive B cell activation and TLR hypersensitivity. J Immunol. 2006;177:2793–802. doi: 10.4049/jimmunol.177.5.2793. [DOI] [PubMed] [Google Scholar]
- Wu CJ, Conze DB, Li T, Srinivasula SM, Ashwell JD. Sensing of Lys 63-linked polyubiquitination by NEMO is a key event in NF-kappaB activation. Nat. Cell Biol. 2006;8:398–406. doi: 10.1038/ncb1384. [DOI] [PubMed] [Google Scholar]
- Wu X, Sun SC. Retroviral oncoprotein Tax deregulates NF-kB by activating Tak1 and mediating Tak1-IKK physical association. EMBO Rep. 2007;8:510–515. doi: 10.1038/sj.embor.7400931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G, Cvijic ME, Fong A, Harhaj EW, Uhlik MT, Waterfield M, Sun SC. Retroviral oncoprotein Tax induces processing of NF-kappaB2/p100 in T cells: evidence for the involvement of IKKalpha. EMBO J. 2001a;20:6805–6815. doi: 10.1093/emboj/20.23.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G, Fong A, Sun SC. Induction of p100 processing by NF-kappaB-inducing kinase involves docking IkappaB kinase alpha (IKKalpha) to p100 and IKKalpha-mediated phosphorylation. J. Biol. Chem. 2004;279:30099–30105. doi: 10.1074/jbc.M401428200. [DOI] [PubMed] [Google Scholar]
- Xiao G, Harhaj EW, Sun S-C. Domain-specific interaction with IKKγ is an essential step in Tax-mediated activation of IKK. J. Biol. Chem. 2000;275:34060–34067. doi: 10.1074/jbc.M002970200. [DOI] [PubMed] [Google Scholar]
- Xiao G, Harhaj EW, Sun SC. NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol. Cell. 2001b;7:401–409. doi: 10.1016/s1097-2765(01)00187-3. [DOI] [PubMed] [Google Scholar]
- Xiao G, Sun SC. Activation of IKKalpha and IKKbeta through their fusion with HTLV-I tax protein. Oncogene. 2000;19:5198–5203. doi: 10.1038/sj.onc.1203894. [DOI] [PubMed] [Google Scholar]
- Xie P, Hostager BS, Bishop GA. Requirement for TRAF3 in signaling by LMP1 but not CD40 in B lymphocytes. J Exp Med. 2004;199:661–71. doi: 10.1084/jem.20031255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasunaga J, Matsuoka M. Human T-cell leukemia virus type I induces adult T-cell leukemia: from clinical aspects to molecular mechanisms. Cancer Control. 2007;14:133–140. doi: 10.1177/107327480701400206. [DOI] [PubMed] [Google Scholar]
- Yin M-J, Christerson LB, Yamamoto Y, Kwak Y-T, Xu S, Mercurio F, Barbose M, Cobb MH, Gaynor RB. HTLV-I Tax protein binds to MEKK1 to stimulate IκB kinase activity and NF-κB activation. Cell. 1998;93:875–884. doi: 10.1016/s0092-8674(00)81447-6. [DOI] [PubMed] [Google Scholar]
- Yoshida M. Multiple viral strategies of HTLV-1 for dysregulation of cell growth control. Annu. Rev. Immunol. 2001;19:475–496. doi: 10.1146/annurev.immunol.19.1.475. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Miyoshi I, Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc. Natl. Acad. Sci. USA. 1982;79:2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimi R, Chang TH, Wang H, Atsumi T, Morse H C r, Ozato K. Gene disruption study reveals a nonredundant role for TRIM21/Ro52 in NF-kappaB-dependent cytokine expression in fibroblasts. J. Immunol. 2009;182:7527–7538. doi: 10.4049/jimmunol.0804121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu MC, Yuan JM. Epidemiology of nasopharyngeal carcinoma. Semin Cancer Biol. 2002;12:421–9. doi: 10.1016/s1044579x02000858. [DOI] [PubMed] [Google Scholar]
- Yu Q, Minoda Y, Yoshida R, Yoshida H, Iha H, Kobayashi T, Yoshimura A, Takaesu G. HTLV-1 Tax-mediated TAK1 activation involves TAB2 adapter protein. Biochem. Biophys. Res. Commun. 2008;365:189–194. doi: 10.1016/j.bbrc.2007.10.172. [DOI] [PubMed] [Google Scholar]
- Zhao LJ, Giam CZ. Interaction of T-cell lymphotropic virus type I (HTLV-1) transcriptional activator Tax with cellular factors that bind specifically to the 21-base-pair repeats in the HTLV-1 enhancer. Proc. Natl. Acad. Sci. USA. 1991;88:11445–11449. doi: 10.1073/pnas.88.24.11445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao LJ, Giam CZ. Human T-cell lymphotropic virus type I (HTLV-1) transcriptional activator, Tax, enhances CREB binding to HTLV-1 21-base-pair repeats by protein-protein interaction. Proc. Natl. Acad. Sci. USA. 1992;89:7070–7474. doi: 10.1073/pnas.89.15.7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Yasunaga J, Satou Y, Nakao M, Takahashi M, Fujii M, Matsuoka M. Human T-cell leukemia virus type 1 bZIP factor selectively suppresses the classical pathway of NF-kappaB. Blood. 2009;113:2755–2764. doi: 10.1182/blood-2008-06-161729. [DOI] [PubMed] [Google Scholar]
- Zhou H, Wertz I, O’Rourke K, Ultsch M, Seshagiri S, Eby M, Xiao W, Dixit VM. Bcl10 activates the NF-kappaB pathway through ubiquitination of NEMO. Nature. 2004;427:167–171. doi: 10.1038/nature02273. [DOI] [PubMed] [Google Scholar]