Abstract
Significant advances in cellular reprogramming technologies and hematopoietic differentiation from human pluripotent stem cells (hPSCs) have already enabled the routine production of multiple lineages of blood cells in vitro and opened novel opportunities to study hematopoietic development, model genetic blood diseases, and manufacture immunologically matched cells for transfusion and cancer immunotherapy. However, the generation of hematopoietic cells with robust and sustained multilineage engraftment has not been achieved. Here, we highlight the recent advances in understanding the molecular and cellular pathways leading to blood development from hPSCs and discuss potential approaches that can be taken to facilitate the development of technologies for de novo production of hematopoietic stem cells.
Introduction
Pluripotent stem cells (PSCs) are defined as cells capable of self-renewal and differentiation into derivatives of all 3 germ layers. The first successful derivation of human PSCs (hPSCs), embryonic stem cells (hESCs), by James Thomson in 19981 dramatically elevated the interest in PSC biology because many viewed hESCs as a novel unlimited source of human cells for cell replacement therapies, drug screening, and developmental studies. In 2006, advances in understanding of the core transcriptional regulatory circuitry in mouse and human ESCs led to another crucial discovery by Shinya Yamanaka,2 who identified the set of reprogramming factors capable of inducing ESC-like cells (induced PSCs [iPSCs]) from mouse somatic fibroblasts. One year later, iPSCs were obtained from human somatic cells.3-5 Human iPSCs (hiPSCs) offer a novel tool to study and treat diseases because they capture the entire genome of a particular patient and provide an inexhaustible supply of immunologically compatible cells for experimentation and transplantation. Although initially iPSCs were generated from fibroblasts using retroviral vectors, multiple strategies for generating transgene-free iPSCs from fibroblasts and other cell types, including blood, have been developed within a short period (reviewed by Hussein and Nagy6 and Gonzalez et al7). With the iPSC field progressing very rapidly, the next challenge will be to demonstrate the functional usefulness of iPSC-derived cells in preclinical models of various human diseases and eventually move this technology into the clinic.
Hematopoietic stem cell (HSC) transplantation has become the standard of care for otherwise incurable blood cancers and deadly genetic diseases. The expansion of HSC donor registries, along with the development of alternative sources for HSC transplantation, including cord blood and haploidentical donors, and the use of novel conditioning regimens have significantly improved access to transplantation for patients with hematologic diseases.8,9 However, transplant engraftment failure, graft-versus-host disease, and delayed reconstitution still remain significant causes of morbidity and mortality after bone marrow transplantation8,9 leaving ∼50% of patients with a permanent disability or without a cure.10 Because iPSCs can be expanded indefinitely ex vivo and potentially differentiated into hematopoietic cells with blood-reconstituting capability,11,12 they open a unique opportunity to improve the outcomes of bone marrow transplantation by providing a supply of unlimited number of immunologically matched HSCs.13,14 Patients with monogenic hematologic and immune diseases would benefit the most from a iPSC-based bone marrow transplantation procedure. Currently, a lack of methodology for efficient expansion and genetic modifications of somatic HSCs and the risk for insertional mutagenesis with viral vectors remain the major limitations for HSC-based gene therapy.15 As shown in Figure 1, autologous iPSC lines can be generated from patients with genetic defects, precisely corrected with the wild-type gene by homologous recombination and then used to produce healthy hematopoietic cells for transplantation without the risk for graft-versus-host disease. The successful treatment of sickle cell anemia in a mouse model using gene-corrected iPSCs provided proof-of-principle that the clinical application of iPSCs to treat geneticblood diseases is feasible.16 In the setting of leukemia, iPSCs can be used to produce immunologically matched HSCs as well as T cells targeted to leukemia antigens and antigen-loaded dendritic cells to induce an anti-leukemia immune response.17,18 In addition, autologous panmyeloid progenitors can be generated form iPSCs19 for the management of cytopenias in patients with delayed engraftment.
Figure 1.
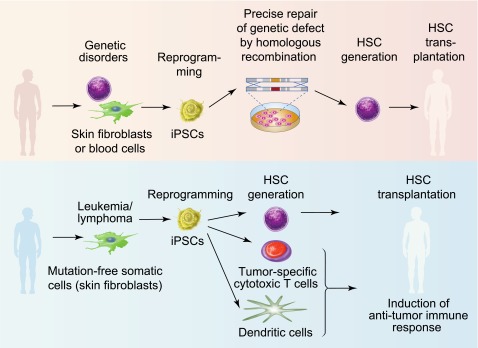
Therapeutic potential of hPSCs for blood diseases. iPSCs can be potentially used to treat patients with monogenic genetic blood diseases such as sickle cell anemia, β-thalassemia, Fanconi anemia, or SCID (upper panel). Autologous skin or blood cells from these patients can be reprogrammed into iPSCs. The defective gene in iPSCs can be repaired using homologous recombination. De novo generation of HSCs from gene-corrected iPSCs would provide immunologically matched cells for bone marrow transplantation. For cancer therapy, autologous iPSCs could be generated from skin fibroblasts or other somatic cells lacking leukemia mutation and used to generate HSCs for bone marrow transplantation as well as immune cells to induce an anti-leukemia immune response (lower panel). Professional illustration by Paulette Dennis.
In recent years, major progress has been made in developing systems for hematopoietic differentiation and producing major types of blood cells from hPSCs (reviewed by Kaufman14). However, the generation of hematopoietic cells with robust long-term reconstitution potential from hPSCs remains a significant challenge. The identification of sequential progenitors and molecular mechanisms leading to formation of various blood lineages from hPSCs is critical in overcoming this limitation. In this review, I focus on recent progress made in understanding cellular and molecular pathways leading to hematopoietic specification from hPSCs and discuss key approaches that could be undertaken to induce the formation of engraftable blood cells from hPSCs.
Translating embryonic hematopoiesis to PSC differentiation
Mesodermal development and HSC specification in the embryo
The knowledge gained from studies of embryogenesis and mouse ESC differentiation provided major insights into key pathways that regulate the sequential commitment of PSCs to blood cells and laid the foundation for the development of hematopoietic differentiation protocols for hPSCs. During embryogenesis, gastrulation is the first critical step in specification of pluripotent embryonic cells into blood. The beginning of gastrulation is marked by formation of primitive streak (PS). Epiblast cells ingress into the PS to give rise to the mesoderm and definitive endoderm.20 Although the entire PS expresses the T gene (also known as Brachyury),21 the subset of cells with hematoendothelial potential within the PS can be identified by the expression of inert domain-containing receptor (KDR) (Flk1 or vascular endothelial growth factor [VEGF] receptor 2).22,23 KDR+ cells migrate into the yolk sac, where they form vascular plexus and blood islands. Nodal, bone morphogenetic protein 4 (BMP4), WNT3, and fibroblast growth factor 2 (FGF2) are the most critical factors required for PS and mesoderm induction.24-27 Importantly, FGF2 upregulates expression of KDR on mesodermal precursors and makes them sensitive to VEGF.28 At this stage, the interaction of VEGF-A produced by the visceral endoderm with KDR becomes essential for the normal development of endothelial and blood lineages.23,29 Manipulation of these pathways in mouse ESC cultures helped to optimize mesodermal differentiation and provided additional knowledge regarding molecular regulation of hematopoietic mesoderm (reviewed by Keller30 and Murry and Keller31).
In the mouse embryo, the first blood progenitors are formed in the yolk sac where they can be identified using hematopoietic colony-forming assays as early as embryonic day 7.25 (E7.25). The yolk sac initially generates primitive hematopoietic cells, including nucleated red blood cells expressing embryonic hemoglobin, macrophages, and megakaryocytes.32,33 The second wave of yolk sac hematopoiesis, termed definitive erythromyeloid hematopoiesis, emerges when distinct blood islands can be recognized morphologically at E8.25.32,34 This wave is associated with an expansion of erythroid precursors expressing adult β-globins and unilineage and multilineage myeloid precursors.32 The first HSCs capable of reconstituting the entire hematopoietic system of wild-type adult animals are observed in the aorta-gonado-mesonephros (AGM) region, vitelline and umbilical arteries and placenta by E10.5-11.35-40 At E11.5, it is estimated that the AGM region contains ∼1 HSC.41 Emerging HSCs undergo expansion in the fetal liver and subsequently migrate to bone marrow, which becomes the predominant site of hematopoiesis in postnatal life.42
Hemogenic specification: concept of hemangioblast and hemogenic endothelium
During embryogenesis, endothelial and hematopoietic cells develop in parallel. The close spatial and temporal relationships between these 2 lineages were first noted by early embryologists in the late 19th century.43 In 1917, Florence Sabin postulated the existence of a common precursor for blood and endothelial cells based on her observation of blood development within the yolk sac of chicken embryos.44 This common precursor was later named hemangioblast by Murray, who defined it as aggregates of yolk sac mesenchyme (mesoderm) from which endothelial and hematopoietic cells develop.45 Although the term hemangioblast initially designated the mesodermal precursor, the modern literature applies it very broadly to describe any type of cell that can produce both endothelial and blood cells after culture in specific conditions in vitro. It became clear, however, that cells with hemangioblastic activity represent a very heterogeneous group of progenitors, which include cells at the mesodermal stage of development in the yolk sac and cells with typical endothelial characteristics in the AGM region.46-48
Within the AGM, hematopoietic cells were found to bud off from the endothelium lining the wall of the aorta.49 Recent studies in mice have provided direct evidence that this process represents the formation of definitive blood cells and HSCs from a unique population of endothelial cells on the ventral wall of the dorsal aorta, defined as hemogenic endothelium (HE), through an endothelial-hematopoietic transition.50-52 Dynamic tracing and imaging studies conducted in vivo have demonstrated that endothelial-hematopoietic transition represents a continuous process in which endothelial cells gradually acquire hematopoietic morphology and phenotype.50,53,54 Although the concept of HE was initially developed based on AGM studies, it became clear that endothelium in other embryonic and extraembryonic sites possess hemogenic potential. Among these sites are the vitelline and umbilical arteries,55 placenta,38 head vasculature,56 endocardium,57 and nascent yolk sac capillaries.58
Hematopoietic development from hPSCs
In general, the approaches for hESC differentiation into blood cells are similar to those employed for mouse ESCs. The first successful differentiation of hESCs was achieved by Kaufman et al using coculture with the S17 mouse stromal cell line.59 Since then, several embryoid body formation and 2D culture protocols, including serum- and feeder-free, have been developed for hematopoietic differentiation of hESCs.60-66 After successful reprogramming of human somatic cells to pluripotency, hESC protocols have also been applied to differentiate hiPSCs. It has been shown that the patterns of hematopoietic differentiation from hESCs and iPSCs are very similar.66-69 Many factors that are important for hematopoietic specification of mouse PSCs appear to play critical roles in induction of mesoderm and hematopoietic commitment in the hPSCs as well. As expected from murine studies, BMP4, WNT, FGF2, and VEGF have been shown to promote hematopoiesis from hESCs (Figure 2A).60,62,65,70-74 Hematopoietic cytokines are important components of hPSC differentiation systems and are required for amplification of emerging hematopoietic colony-forming cells (CFCs) and specification toward lymphoid lineages. The protocols for hematopoietic differentiation of hPSCs are extensively reviewed by Kardel and Eaves.75 Overall, hESC studies have revealed many similarities in hematopoietic differentiation with mouse ESCs, including requirements for intrinsic and extrinsic signaling and hierarchical organization of hematopoietic precursors. However, several differences have also been noted and will be discussed below.
Figure 2.
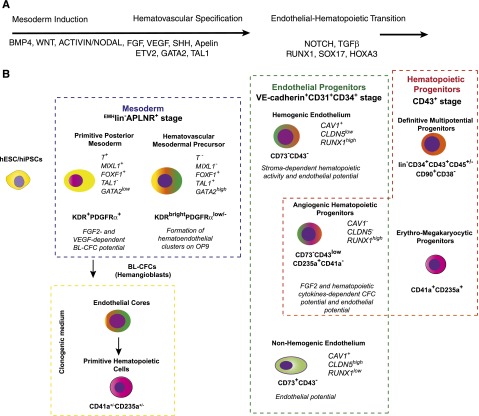
A model of hematopoietic development from hPSCs. (A) The most critical factors involved in specification of hematovascular precursors from PSCs and regulation of blood formation from HE. (B) Stages of hematopoietic development from hPSCs. Mesodermal stage of development is defined as expression of the mesodermal markers, APLNR and KDR.62,77,80 The lack of expression of typical endothelial (CD31, VE-cadherin), endothelial/mesenchymal (CD73, CD105), and hematopoietic (CD43, CD45) markers, ie, EMHlin− phenotype, separates mesoderm from lineage-committed cells.86,90 The most primitive mesodermal precursors with hematopoietic potential arise in coculture with OP9 or an embryoid body system on day 3 of differentiation. These cells have features of a posterior PS, coexpress KDR, APLNR, and PDGFRα and capable of forming BL (hemangioblast) colonies in the presence of FGF2 and VEGF.62,77,80,86 The formation of BL colonies in clonogenic medium proceeds through VE-cadherin+ endothelial intermediates, which generate primitive hematopoietic cells with erythroid, megakaryocytic, and macrophage potentials.77,86 Progressive mesodermal commitment to endothelial and hematopoietic cells is associated with downregulation of PDGFRα71,86 and PS genes, and upregulation of KDR, TAL1, and GATA2 genes associated with angiohematopoietic development leading to formation of EMHlin−KDRbrightAPLNR+PDGFRαlow/− hematovascular mesodermal precursors (HVMPs).86 HVMPs lack BL-CFC potential, but are highly enriched in cells that form hematoendothelial clusters on OP9.86 The endothelial stage of development was defined as expression of the typical endothelial markers VE-cadherin, CD31, and CD34 and the absence of the panhematopoietic marker CD43 (supplemental Table 1; see the Blood Web site).66,86,90,101,102 Within the VE-cadherin+CD43− population, HE cells (ie, cells lacking hematopoietic CFC potential but capable of forming blood cells after culture with stromal cells) were discriminated from non-HE cells based on lack of CD73 expression.86,102 The first hematopoietic progenitors emerging from the VE-cadherin+ population express CD235a, low levels of CD43, and lack CD41a expression. These cells have a unique potential to form hematopoietic colonies in the presence of FGF2 and hematopoietic cytokines, but also retain endothelial potential and therefore were designated as angiogenic hematopoietic progenitors (AHPs).86 Advanced hematopoietic development is associated with upregulation of CD43 expression; segregation of all hematopoietic CFCs to the CD43+ fraction66,100,128; and establishment of distinct subsets of CD43+ hematopoietic cells, including CD41a+CD235a+ erythro-megakaryocytic progenitors100,115,117 and lin−CD34+CD43+CD45+/− multipotent myelolymphoid progenitors.66,100,128 Progressive acquisition of the angiogenic and hematopoietic program by differentiated cells is emphasized by green and red colors, respectively.
Mesoderm induction from hPSCs
Understanding the mechanisms regulating induction and specification of mesoderm to hematovascular progenitors is essential in tracing the development of pre-HSCs and in defining factors required for their specification. Similar to mouse ESCs, the early stages of mesodermal development from hESCs can be monitored by expression of KDR (FLK1) and platelet-derived growth factor receptor alpha (PDGFRα).62,70,71,76,77 However, in contrast to mouse ESCs, low levels of KDR can be detected in undifferentiated hESCs.77-79 The successful targeting of green fluorescent protein (GFP) reporter to the locus of MIXL1, a gene transiently expressed in the PS, enabled the more accurate identification and isolation of the mesodermal populations at the PS stage in hESC cultures.76 Molecular profiling of MIXL1-GFP cells or wild-type hESCs at early stages of differentiation revealed apelin receptor (APLNR) as a novel marker of the PS population in hPSC cultures.77,80 APLNR was found to be expressed in cells with features of posterior mesoderm and anterior mesendoderm.77,80 In contrast to KDR, undifferentiated hESCs do not express APLNR, and because APLNR expression is homogeneous, positive and negative populations can be clearly separated by flow cytometry.
Using the MIXL1-GFP cell line, Davis et al76 demonstrated the essential role of BMP4 in induction of PS mesoderm from hESCs. The formation of mesoderm is also dependent on Activin A and FGF signaling.81-83 Inhibition of these pathways using small molecules completely blocks mesoderm development from hESCs.70 Several studies have documented the important role of canonical WNT signaling in blood development from hESC,71,72 which can be at least partially attributed to the enhancement of mesoderm formation after WNT signaling activation. Canonical WNT/β-catenin signaling is required for establishing nascent PS/mesendoderm from hESCs, whereas specification of nascent PS to anterior and posterior PS is regulated by the balance between BMP and Activin/Nodal signaling.84 Furthermore, activation of canonical WNT signaling promotes the development of more mature hematovascular mesoderm expressing a high level of KDR (KDRbright mesoderm) and hematoendothelial progenitors from hESCs.71,72 Vijayaragavan et al reported that mesoderm formation from hESCs is also affected by noncanonical WNT signaling.85
Hemangioblastic potential of hPSC-derived mesodermal precursors
Similar to mouse ESCs, the onset of hematopoiesis in hESC and hiPSC cultures is marked by the emergence of blast CFCs (BL-CFCs).62,77,80,86 Because BL-CFCs consist of vascular and hematopoietic progenitors, they are commonly referred as hemangioblasts. BL-CFCs are detected at the mesodermal stage of development, before the appearance of hematopoietic progenitors that form colonies in response to hematopoietic cytokines. Development of BL colonies from mesodermal cells requires FGF2 and VEGF but not hematopoietic cytokines.62,77 In defined serum-free clonogenic medium, FGF2 alone is sufficient to induce BL colonies from APLNR+ mesodermal cells.77,86 The formation of BL-CFCs is also promoted by the addition of apelin peptides to differentiation cultures or clonogenic medium.80,86 Similar to findings in mouse systems,87 human hemangioblasts (BL-CFCs) generate hematopoietic colonies through endothelial intermediates.77,86 Using time-lapse studies, we demonstrated that development of BL colonies in clonogenic cultures proceed through a core stage at which highly motile mesodermal cells undergo several divisions, upregulate expression of KDR and other endothelial genes (including CDH5, PECAM, and ESAM), and form immotile tight aggregates composed of ∼30 VE-cadherin+ epithelioid cells (cores). The core stage of differentiation is readily identifiable after 3 days of culture of mesodermal cells in semisolid clonogenic medium. Subsequently, VE-cadherin+ cells undergo endothelial-hematopoietic transition leading to the formation of CD235a+/−/CD41+/− cells with erythroblast morphology. The hematopoietic potential of BL-CFCs is mostly restricted to primitive erythroid and megakaryocytic cells, and macrophages.62,86 Interestingly, the development of BL colonies in culture closely recapitulates events leading to blood formation in vivo. In chicken embryo, FGF produced by endodermal cells induces the aggregation of migrating PS cells adjacent to the endoderm, upregulation of KDR, and formation of angioblasts and hemangioblasts.28,88 In differentiating hPSC cultures (Figure 2A), the BL-CFCs with hemangioblastic activity are highly enriched within the KDR+ and APLNR+PDGFRα+ nascent mesodermal population expressing MIXL1 and other PS genes.62,77,80,86 However, the proportion of BL-CFCs within isolated KDR+ or APLNR+ cells remains low at 1.5% to 4%.62,77,80
Stepwise specification toward hematopoietic and endothelial lineages in mouse ESC cultures proceeds through conversion of KDR+PDGFRα+ primitive mesodermal cells into KDR+PDGFRα− cells with properties of lateral plate mesoderm.89 To define the mesodermal subsets of differentiating hPSCs, we analyzed the kinetics of expression of APLNR, PDGFRα, and KDR mesodermal markers in hPSCs differentiated on the OP9 bone marrow stromal cell line.77 Because these markers could also be found on differentiated cells at postmesodermal stages, we demarcated mesodermal stage of development as EMHlin−, ie, the stage at which cells lack the expression of endothelial (CD31, VE-cadherin), endothelial/mesenchymal (CD73, CD105), and hematopoietic (CD43, CD45) lineage markers.86,90 On the basis of these analyses, we identified 2 distinct phases of mesodermal development. EMHlin−APLNR+PDGFRα+ cells, the most primitive mesodermal cells, emerge at days 2 to 3 of differentiation in OP9 coculture (Figure 2). These cells are reminiscent of the primitive posterior mesoderm in the embryo and express genes associated with PS (T, MIXL1, EOMES) and lateral plate/extraembryonic mesoderm (FOXF1, WNT5a, BMP4). As discussed above, the day 3 EMHlin−APLNR+PDGFRα+ cells have the potential to form BL colonies in response to FGF2.77,86 The next step of more advanced mesodermal commitment is associated with the emergence of HVMPs, which can be detected based on high expression of KDR and low to no expression of PDGFRα in EMHlin−APLNR+ cells, ie, EMHlin−KDRbrightAPLNR+PDGFRalow/− phenotype. Similar to day 3 posterior mesoderm cells, HVMPs express lateral plate/extraembryonic mesoderm genes. However, they upregulate expression of TAL1, HHEX, LMO2, GATA2, and ETV2 genes associated with angiohematopoietic development, and downregulate expression of PS genes (Figure 2). HVMPs lack BL-CFC potential but are highly enriched in bipotential cells that can form hematoendothelial clusters when cocultured on OP9 and produce the entire spectrum of myeloid progenitors.86 It has been shown that KDR+PDGFRαlow mesodermal population with angiohematopoietic potential is also present in hESC cultures differentiated using the embryoid body method, and that generation of such cells is significantly elevated by the addition of BMP4 and WNT3a.71 Collectively, these results indicate that at least 2 distinct mesodermal subsets are formed during hESC specification to endothelial and hematopoietic lineages. Both of these populations (posterior mesoderm cells and HVMPs) have endothelial and hematopoietic potentials when cultured in vitro (ie, possess hemangioblastic activity). Primitive hematopoietic potential can be detected within immature posterior mesoderm cells, whereas more mature HVMPs generate blood cells with definitive features.
Formation of HE in hPSC cultures
The evidence that HSCs originate from HE with definitive hematopoietic potential underscores the need to identify HE progenitors in hPSC cultures.91 In embryos, HE can be reliably identified based on morphology and anatomical location.49 However, identification of HE in hESC differentiation cultures faces significant challenges because of the considerable overlap in expression of surface markers by endothelial and hematopoietic cells. One of the most specific markers for endothelial cells, VE-cadherin,92-94 is commonly used to isolate endothelial cells from mouse embryo and ESC cultures.95-97 In mouse systems, VE-cadherin staining is typically combined with blood cell–specific antibodies CD41a (early marker of embryonic hematopoietic progenitors) and CD45 (panhematopoietic marker) to separate endothelial and hematopoietic phases of development and identify a transient population of HE emerging around the onset of blood cell generation.87,97,98 Initially, Wang et al99 demonstrated that hESC-derived VE-cadherin+ cells expressing CD31 (PECAM) and KDR (FLK1) but lacking CD45 have hematopoietic and endothelial potentials. Recently, major progress has been made in further dissection of this population to enrich toward HE activity and separate HE more precisely from already established blood cells and non-HE (supplemental Table 1). Identification of CD43 as a marker that covers the entire population of hematopoietic cells, including CD45− CFCs, in hPSC cultures,67,100 made it possible to accurately segregate the endothelial stage from the hematopoietic stage. CD31+CD43− or CD34+CD43− cells isolated from differentiated hESC cells entirely lacked hematopoietic CFC potential but had the capacity to generate blood and endothelial cells after culture with OP9 stromal cells (ie, displayed HE properties).100,101 Recently, Rafii et al102 engineered hESCs to express fluorescent reporters under the VE-cadherin and CD41a promoter (Pr). This system makes it possible to directly observe the formation of round CD41aPr+ blood cells from VE-cadherinPr+CD41aPr− epithelioid precursors. Round cells developed from VE-cadherinPr+ cells expressed CD43 and possessed broad hematopoietic CFC potential. Although CD43 enables the precise separation of VE-cadherin+ blood cells from endothelium, further narrowing down the HE phenotype requires the identification of markers that distinguish HE from non-HE. By analyzing the kinetics of VE-cadherin and other endothelial marker expression in hPSC coculture with OP9, we identified 2 distinct populations of cells within VE-cadherin+CD43− endothelial cells on the basis of expression of CD73 (5′-nucleotidase; Figure 2B).86 Both VE-cadherin+CD43−CD73+ and CD73− populations expressed KDR, CD31, ESAM, CD34, and other typical endothelial molecules and were capable of generating endothelial cells but lacked hematopoietic colony-forming activity (formation of blood colonies in semisolid medium supplemented with hematopoietic cytokines). However, only VE-cadherin+CD43−CD73− cells displayed HE properties (ie, the ability to generate blood cells after secondary coculture with OP9). In contrast to non-HE, HE expressed high levels of RUNX1, which is known to mark HE in the embryo,103 and was capable of generating the entire spectrum of myeloid progenitors, including erythroid cells expressing adult β-hemoglobin. The segregation of HE from non-HE on the basis of expression of CD73 was also shown using VE-cadherinPr/CD41Pr dual reporter transgenic hESCs.102 Kennedy et al66 found that cells with HE phenotype possessed T lymphoid potential and demonstrated the critical role of Activin/Nodal signaling in establishing a lymphoid program in HE. It has also been found, that, in parallel with the acquisition of endothelial markers, differentiated hESCs begin to express angiotensin-converting enzyme (CD143). When CD143+CD43−CD45−CD41a− cells were isolated and analyzed, they showed hemangioblastic properties.104 Angiotensin-converting enzyme activity was found to be important for regulation of hematopoietic vs endothelial differentiation of isolated CD143+ cells. Inhibition of angiotensin II type 1 receptor enhanced hematopoietic potential of CD143+ cells, whereas inhibition of angiotensin II type 2 receptor skewed their differentiation toward endothelial cells.104
The mechanisms regulating HE development and endothelial-hematopoietic transition remain largely unknown. Mouse studies have identified Runx152 as a positive regulator and HoxA3105 as a negative regulator of blood formation from endothelium, and showed the pivotal role of Sox17 in HE expansion106 and Hes-mediated Notch signaling in HSC formation from HE in the AGM region.107 The critical role of SOX17 in regulation of endothelial-hematopoietic transition has been also demonstrated in hPSCs.101 Conditional expression of SOX17 in hESC-derived CD34+CD43− cells promoted the formation of a unique population of VE-cadherin+CD43+CD45− cells with HE properties while knockdown of its expression inhibited blood formation, indicating that SOX17 is essential for induction and maintenance of the hemogenic program in endothelial cells. This conclusion was also supported by findings that overexpressed SOX17 binds to promoters and regulatory regions of a large number of hematopoietic and endothelial genes.101 Lee et al108 found that formation of hematopoietic progenitors from hPSC-derived CD31+CD45− cells can be enhanced by HES1-mediated activation of NOTCH signaling using Jag1. The developmental progression from HE to hematopoietic progenitors depends on transforming growth factor beta (TGFβ) signaling. In an embryoid body differentiation system, treatment of hESCs at the CD31+CD43− stage of development with TGFβ1 suppressed formation of CD43+ cells, whereas TGFβ inhibitors exerted the opposite effect.74
Specification and diversification of hematopoietic lineages from hPSCs
In hPSC differentiation cultures, the cells that are already committed to a hematopoietic fate and capable of forming various colonies of blood cells in response to hematopoietic cytokines arise within the CD34+ population before expression of a CD45 typical panhematopoietic marker.59,61,78,109 Although CD34 specifically identifies hematopoietic progenitors and stem cells within the somatic hematopoietic compartment, in hPSC cultures, CD34 is expressed by a variety of cell types, including endothelial and mesenchymal stem cells.100,110 Thus, the use of CD34 alone may not be sufficient to select for a pure population of hematopoietic progenitors from hPSCs. By analyzing the kinetics of expression of specific hematopoietic markers in hESCs differentiated on OP9, Vodyanik et al100 identified leukosialin (CD43) as a marker that reliably separates CD43+ colony-forming hematopoietic progenitors from CD43−CD31+ endothelial cells and CD43−CD31− cells with mesenchymal characteristics within the CD34+ population generated from hESCs in coculture with OP9. Similarly, selection of CD43+ cells from hiPSCs differentiated on OP9 or in embryoid bodies made it possible to separate hematopoietic CFCs from nonhematopoietic cells.66,67 Recent time-lapse video recordings of endothelial-hematopoietic transition demonstrated that the gain of CD43 expression tightly correlated with the transformation of epithelioid endothelial cells into round blood cells.86 Because CD43 provides a repulsive barrier around the cell,111 acquisition of CD43 expression may have functional significance for budding and separation of blood cells from the endothelium.
In hPSC cultures differentiated on OP9, the first CD43+ cells were detected within emerging VE-cadherin+ cells as early as day 4 of differentiation.86,100 These cells were CD41a− and expressed the erythroid marker, CD235a (glycophorin A), and low levels of CD43, which was best detected using highly sensitive fluorochromes. The emerging VE-cadherin+CD43lowCD235a+CD41a− cells had primarily hematopoietic characteristics and colony-forming potential and lacked the expression of CLDN5 and CAV1, typical endothelial genes; however, they retained the capacity to grow endothelial cells (Figure 2B). Because of these properties, VE-cadherin+CD43lowCD235a+CD41a− cells were defined as AHPs.86,90 Although AHPs expressed the erythroid cell marker CD235a, they had a unique potential to form a broad spectrum of hematopoietic colonies in serum-free medium supplemented with hematopoietic cytokines and FGF2.86 Gross morphologic examinations of some of these colonies resemble hemangioblast/BL colonies formed by BL-CFCs. However, in contrast to BL colonies, colonies generated by AHPs do not develop through an endothelial core stage. Whether AHPs represent a unique transition stage between HE and blood or a distinct wave of hematopoietic progenitors remains to be determined.
In mouse embryo and ESC cultures, CD41a (GPIIb) expression marks the initiation of hematopoiesis and precedes the expression of the erythroid marker, TER119.112-114 However, in hPSC cultures, the first CD41a+ cells were detected within the already established CD43+CD235a+ population.86 Thus, the vast majority of CD41a+ cells coexpress CD235a. Although early CD41a+CD235a+ cells express high levels of VE-cadherin, they are completely devoid of endothelial potential.86 Analysis of the colony-forming capacity of CD41a+CD235a+ cells revealed that they are highly enriched in erythro-megakaryocytic progenitors,100,115 but also show some myeloid potential in serum-free clonogenic medium.86 hESC- and hiPSCs-derived CD235a+CD41a+ progenitors with erythro-megakaryocytic potential can be expanded and differentiated into erythroid or megakaryocytic cells.116,117 The expansion and fate switch of CD235a+CD41a+ cells is regulated by the aryl hydrocarbon receptor.117 Multiple studies have demonstrated the feasibility of high-scale production of red blood cells and platelets in bulk hPSC cultures (reviewed by Chang et al118 and Takayama and Eto119). However, erythroid cells generated in these cultures have primitive and fetal-like characteristics.120,121 They express mostly embryonic and fetal hemoglobins with low levels of adult hemoglobin and do not enucleate efficiently. Although several studies have demonstrated that hPSC-derived platelets are capable of initiating thrombus formation, the hemostatic properties of these cells remain to be evaluated in animal models of thrombocytopenia.
The progenitors with broad myelolymphoid potential and lin−CD34+CD43+CD45− phenotype can be detected in hPSC cultures shortly after emergence of CD235a+CD41a+ cells. Acquisition of CD45 expression by lin− cells is associated with progressive myeloid commitment.100 The lin−CD34+CD43+CD45+/− hematopoietic progenitors are capable of forming the entire spectrum of hematopoietic colonies in serum-containing CFC medium. The CFC potentials of these cells was comparable or even higher than that of phenotypically similar cord blood cells.122 The hESC- and hiPSC-derived lin−CD34+CD43+CD45+/− cells display many phenotypical features of HSCs, including CD90 and c-kit expression and the lack of CD38 and CD45RA expression.67,100 These cells have high aldehyde dehydrogenase activity, the ability to efflux rhodamine-123, and LTC-IC potential.78,100,123 The lin−CD34+CD43+CD45+ cells could be expanded with granulocyte-macrophage colony-stimulating factor and differentiated into myelomonocytic cells including neutrophils, eosinophils, macrophages, dendritic and Langerhans cells, and osteoclasts.19 CD34+ and CD43+ cells generated from hESCs or hiPSCs also possess natural killer and B lymphoid potentials.78,100,124-126 Natural killer cells generated from hPSCs have cytolytic function and can be generated in large numbers using defined conditions.125,126 However, B lymphoid cultures produced very low numbers of CD19+CD10+ cells. Although these cells exhibited multiple genomic D-JH rearrangements, they did not express IgM or CD5, indicating that their development failed to progress beyond the pre–B-cell stage.124 The T-cell potential of hESCs was initially demonstrated using in vivo studies, which found that hESC-derived CD34+ cells transplanted into human thymus/fetal liver grafts in SCID-hu mice generated T cells, including CD4+CD8+ T-cell precursors.127 Later, T cells were generated from hESCs and iPSCs in vitro using OP9 cells expressing the NOTCH ligands δ-like 1 (DLL1) or 4 (DLL4).18,66,128,129 By analyzing the T-cell potential of CD43+ populations expressing different levels of CD34 and CD43, Timmermans et al found that T cells were derived exclusively from the CD34highCD43low population of hematopoietic progenitors.128 T-cell potential could also be detected when NOTCH signaling is activated in CD34+CD43−CD45− cells expressing endothelial markers.66 Collectively, these studies indicate that cells with panmyeloid and lymphoid potentials (ie, definitive hematopoietic cells) can be generated from hPSCs.
Hematopoietic engraftment potential of hPSC-derived cells
Following the initial studies demonstrating the presence of CD34+ cells with hematopoietic colony-forming potential in hESC cultures, there was significant interest in evaluating the hematopoietic repopulation potential of these cells. Several reports have detected human hematopoietic cells in the bone marrow of immunocompromised mice and sheep many months after intravenous or intrafemoral injection of an entire population of differentiated hESCs or hESC-derived CD34+ cells.130-134 However, bone marrow engraftment observed in these studies was low (within 0.1%-2%) and was mostly restricted to the myeloid lineage. Similarly low levels of engraftment were reported after intrafemoral transplantation of differentiated hiPSCs.135 It was suggested that hPSC-derived cells have limited migratory potential and seem unable to complete maturation within the adult bone marrow environment.131,135 To find out whether the neonatal environment provides a better support for hematopoietic engraftment of differentiating hESCs, Tian et al transplanted CD34+ cells obtained from luciferase-expressing hESCs into newborn NSG mice.136 Although the authors detected luciferase+ cells several months after transplantation, they found that these cells were mostly endothelial and not hematopoietic. Recently, 2 studies have demonstrated that hematopoietic differentiation could be achieved in vivo during teratoma formation from hiPSCs.137,138 The generation of blood cells within teratomas was enhanced by injecting hiPSCs together with OP9 stromal cells ectopically expressing DLL1 and WNT3a. When CD34+CD45+ cells were isolated from these teratomas and transplanted into NSG mice, the pattern of hematopoietic engraftment was very similar to that observed after transplantation of in vitro differentiated hPSCs (ie, very limited 0.1%-2% hematopoietic chimerism in the bone marrow with predominant myeloid engraftment).137 In another study, hematopoietic differentiation within teratomas was amplified by administration of human SCF and TPO via an micro-osmotic pump. However, only ∼4% NOD/SCID and ∼30% of NOD/SCID/JAK3null mice showed a low level of hematopoietic chimerism after transplantation of 600 CD34+CD45+ teratoma-derived cells.138 Although these studies demonstrated the feasibility of generating hematopoietic cells with limited engraftment potential from hPSCs, they also indicate that current differentiation conditions do not reproduce the complexity of embryonic hematopoietic development that leads to HSC specification and expansion.
Defining conditions that allow engineering HSCs
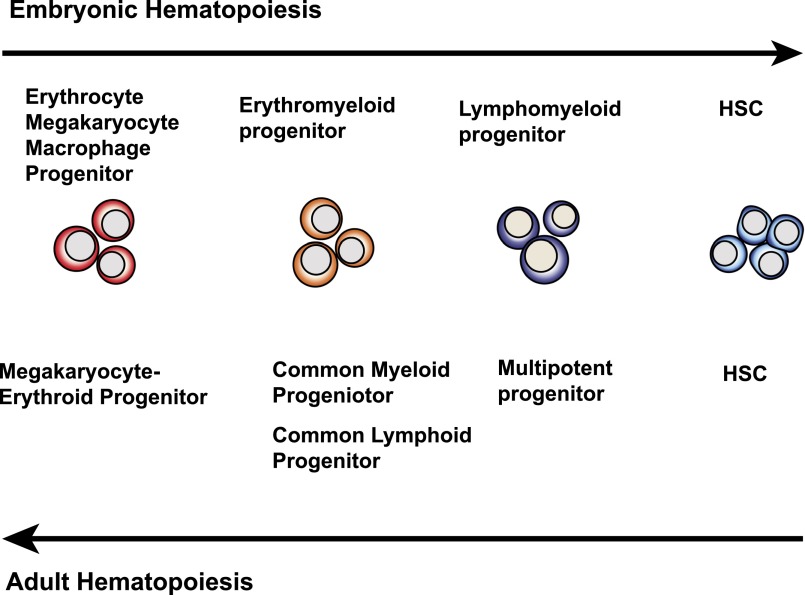
Why do in vitro hPSC cultures fail to yield cells with robust hematopoietic engraftment potential? Because HSC specification in the AGM region of an embryo is a very rare event (1 HSC per AGM at E11.5)41 followed by HSC expansion in the fetal liver, one can argue that hPSC differentiation cultures produce rare HSCs but fail to expand them. However, this situation is probably unlikely. During embryogenesis, the hematopoietic hierarchy appears in reverse sequence as compared with an adult one,139 ie, the formation of progenitors with more restricted hematopoietic potential precedes emergence of HSCs (Figure 3). In mice, panmyeloid progenitors and T and B lymphoid precursors were first identified in the yolk sac before HSC potential could be detected in the embryo proper.139-141 In addition, clusters of multipotential blood cells are formed at many sites within the aorta; however, HSCs are restricted to the ventral wall of the dorsal aorta.55,142 This indicates that finely tuned local signaling is required for multipotential blood cells to acquire repopulation potential. Therefore, it is more likely that multipotential blood cells generated in hPSC cultures represent a pre-HSC stage of development and that blood cells arising in hPSC cultures simply fail to complete the HSC specification after transition from the endothelial to the hematopoietic stage.
Figure 3.
Schematic diagram demonstrating the opposite sequence of blood cell development between embryos and adults. In embryos, cells with restricted hematopoietic potential appear before HSC specification. In adults, hematopoiesis proceeds through gradual maturation of HSCs, leading to formation of progenitors with more restricted potential.
Currently, 2 major models exist to explain the relationship between adult and embryonic hematopoietic hierarchies.139 According to the first model, the embryonic and adult hierarchies arise from a common hematopoietic ancestor that gives rise to both primitive yolk sac and definitive embryonic hematopoiesis. The other model suggests that primitive and definitive hierarchies arise independently. Studies in Xenopus found that separation of adult and embryonic hematopoiesis occurs as early as the 32-cell blastomere stage.143 Independent origins of yolk and definitive embryonic hematopoiesis are also supported by most of the mouse studies.35-37 Nevertheless, the contribution of the yolk sac precursors to definitive adult hematopoiesis cannot be entirely excluded.144,145 Regardless of which model more accurately predicts the in vivo process, the most critical question in relationship to hPSC differentiation studies is whether the more primitive yolk sac precursors can acquire adult HSC properties after exposure to particular signaling events. Studies by Yoder et al strongly support the hypothesis that local environment can induce HSC potential in yolk sac cells.146,147 In contrast to AGM, yolk sac organ cultures are unable to initiate and support HSC development ex vivo or repopulate adult recipients.41 However, CD34+c-kit+ or CD34+c-kit+CD41+ cells isolated from the yolk sac of E9-9.5 embryos are capable of reconstituting multilineage long-term hematopoiesis when transplanted into busulfan-conditioned neonates.112,146,147 Similarly, hematopoietic progenitors with multilineage potential that engraft in neonates can be isolated from the para-aortic splanchnopleura (the AGM primordium) of an E9 mouse embryo, before definitive HSC potential can be detected in an adult recipient repopulation assay.146 Thus, embryonic cells with detectable myeloid and lymphoid potentials in vitro, but lacking adult repopulation potential, can mature into HSCs in the neonatal environment. On the basis of these studies, induction of the equivalent of yolk sac/para-aortic splanchnopleura multilineage newborn-repopulating cells in hPSC cultures is considered a critical step toward establishing protocols for generating HSCs ex vivo.30 By using T-cell potential as a major criterion for the identification of such cells in hPSC cultures, Kennedy et al found that progenitors with T lymphoid potential reside within the CD34+CD43−CD45− population of cells expressing CD34 and other endothelial markers.66 Importantly, the production of these T lymphoid progenitors could be enhanced by manipulation of Activin/Nodal signaling pathways during the first 4 days of hPSC differentiation,66 indicating that the definitive hematopoietic program can be enhanced during the mesodermal stage. Further engraftment studies will be required to determine whether hPSC-derived cells with lymphoid potential can repopulate newborn mice, similar to cells produced in vivo during embryogenesis.
To produce HSCs from hPSCs, it is also necessary to better understand the mechanisms regulating the formation of and specification of blood cells from HE cells. The acquisition of arterial identity through activation of NOTCH signaling and activation of hematopoietic programing by RUNX1 are the most critical factors for the establishment of HE cells in vivo (reviewed by Zape and Zovein148). However, it has become clear that HE cells are heterogeneous and not all HE cells can produce HSCs. Recent evidence indicates that at least 2 distinct populations of HE cells with erythromyeloid and HSC potentials exist in the mouse embryo, and that Ly6a (Sca1) specifically marks HSC-producing HE cells.149 It is already known that at least 2 distinct types of VE-cadherin+ cells with primitive and broad erythromyeloid hematopoietic potentials can be produced from hPSCs.66,86,91,102 However, the specification of cells with broad lymphomyeloid potential directly from HE cells remains to be shown using clonal studies. The recent identification of HE progenitors in hPSC cultures66,86 and the generation of genetically engineered cell lines to trace endothelial-hematopoietic transition102 have already provided a platform for assaying upstream factors required for HE formation with HSC potential and downstream factors that promote HSC expansion in the human system.
The reprogramming studies provided strong evidence that cellular identities are defined by gene regulatory networks controlled by few master regulatory factors. Identification of the master factors required for the specification of definitive/adult type HSCs from embryonic precursors would be one of the essential prerequisites to establishing a protocol for generating HSCs ex vivo. Mouse studies discovered HoxB4 or its upstream regulator Cdx4 as chief factors capable of inducing a self-renewal program in ESC-derived hematopoietic progenitors.12,150 The rationale for selecting HoxB4 to engineer HSCs from ESCs came from embryonic studies that demonstrated the lack of expression of several critical homeobox regulators of definitive HSCs, including HoxB4, in the yolk sac.151 Nevertheless, HoxB4-induced cells do not entirely mimic the function and phenotype of somatic HSCs and produce mostly myeloid engraftment. Recently, the Daley group152 performed comprehensive molecular profiling studies to compare in vitro–generated hematopoietic cells from mouse ESCs with cells from embryonic hematopoietic sites. These studies revealed that HoxB4-induced HSCs lack HoxA9 expression and a Notch signaling signature,152 which may explain their limited lymphoid potential. Although attempts have been made to induce HSCs from hPSCs using HOXB4, it was found that hESC-derived hematopoietic cells already express HOXB4, and its forced expression does not enhance engraftment of human cells.131,153 In a search for the intrinsic determinants required for HSC specification from hPSCs, several groups performed molecular profiling studies of ESC-derived and in vivo–produced human primitive hematopoietic cells. These studies revealed an apparent similarity between the transcriptomes of phenotypically identical fetal liver and hESC-derived primitive hematopoietic populations,122,154 although distinctive differences in the expression of genes regulating HSC self-renewal, homing, and chromatin remodeling were also noted.122 Interestingly, compared with the fetal liver primitive hematopoietic cells, hESC-derived hematopoietic progenitors showed much higher levels of expression of genes from the HOXB cluster, but significantly lower levels of genes from the HOXA cluster.122,154 Differences in genes involved in NOTCH signaling pathways,155 polycomb and trithorax complexes were also noted.122,156 Capacity to home to bone marrow is one of the critical features of HSCs. However, hESC-derived lin−CD34+CD43+CD45+/− hematopoietic progenitors express very low levels of the HSC-homing molecule CXCR4, which may indicate that these cells have yet to acquire competent homing capability.122 Overall, molecular profiling studies have identified several unique features of the transcriptome of human and mouse PSC-derived hematopoietic precursors. Insights gained from these studies can be further explored to define novel molecular targets capable of activating an adult-type HSC program during hPSC differentiation.
Concluding remarks
The de novo generation of HSCs remains a significant challenge. Achieving this goal requires collaborative efforts of developmental, stem cell, and molecular biologists. Embryonic studies have identified the essential role of Notch, Wnt, Hedgehog, and TGFβ/Smad signaling pathways in HSC development and the maintenance of an HSC program. Further understanding the molecular mechanisms and niche factors critical for specifying HE vs non-HE cells and HSCs vs non-HSC precursors from HE cells in the embryo will be an essential prerequisite to the development of optimal conditions for HSC production in vitro. The reconstruction of gene regulatory networks and identification of master regulatory factors that control HSC identity and drive HSC precursors to adopt HSC fate will ultimately enable conversion of hPSC-derived cells into adult-type HSCs (Figure 4). Recently, a hemogenic program was successfully induced in mouse and human fibroblasts by forced expression of Gata2, Gfi1b, cFos, and Etv6157 and OCT4, respectively.158 Although these reports demonstrated the feasibility of generating hematopoietic hierarchy for clinical application by direct transformation of somatic cells and bypassing the pluripotency stage, the next challenging step is to determine factors capable of inducing the hematopoietic self-renewal program in directly transformed somatic cells and achieve the scalability of the direct reprogramming process. Nevertheless, both approaches, PSC-based and direct reprogramming of somatic cells to HSCs, have to be pursued to better understand the genetic and epigenetic factors governing hematopoietic specification and the self-renewal program. No doubt, these studies will continue to provide fascinating insights into the fundamental questions of HSC biology, and eventually will lead to the development of novel stem cell therapies.
Figure 4.
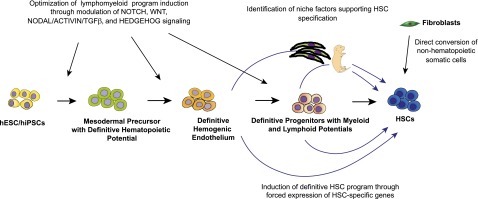
Potential approaches for de novo induction of HSCs.
Supplementary Material
Acknowledgments
I thank Gene Uenishi and Derek Theisen for proofreading the manuscript.
This work is supported by funds from the National Institutes of Health, National Heart, Lung, and Blood Institute (U01HL099773 and R01 HL116221) and the Charlotte Geyer Foundation. The author declares no competing financial interests.
Footnotes
The online version of this article contains a data supplement.
Authorship
Contribution: I.S. wrote the paper.
Conflict-of-interest disclosure: The author declares no competing financial interest relating to the topic of this article.
Correspondence: Igor I. Slukvin, Department of Pathology and Laboratory Medicine, University of Wisconsin, 1220 Capitol Court, Madison, WI 53715; e-mail: islukvin@wisc.edu.
References
- 1.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 5.Park IH, Zhao R, West JA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451(7175):141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 6.Hussein SM, Nagy AA. Progress made in the reprogramming field: new factors, new strategies and a new outlook. Curr Opin Genet Dev. 2012;22(5):435–443. doi: 10.1016/j.gde.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 7.González F, Boué S, Izpisúa Belmonte JC. Methods for making induced pluripotent stem cells: reprogramming à la carte. Nat Rev Genet. 2011;12(4):231–242. doi: 10.1038/nrg2937. [DOI] [PubMed] [Google Scholar]
- 8.Spitzer TR, Dey BR, Chen YB, Attar E, Ballen KK. The expanding frontier of hematopoietic cell transplantation. Cytometry B Clin Cytom. 2012;82(5):271–279. doi: 10.1002/cyto.b.21034. [DOI] [PubMed] [Google Scholar]
- 9.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354(17):1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 10.Daley GQ. The promise and perils of stem cell therapeutics. Cell Stem Cell. 2012;10(6):740–749. doi: 10.1016/j.stem.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sancho-Martinez I, Li M, Izpisua Belmonte JC. Disease correction the iPSC way: advances in iPSC-based therapy. Clin Pharmacol Ther. 2011;89(5):746–749. doi: 10.1038/clpt.2010.341. [DOI] [PubMed] [Google Scholar]
- 12.Kyba M, Perlingeiro RC, Daley GQ. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109(1):29–37. doi: 10.1016/s0092-8674(02)00680-3. [DOI] [PubMed] [Google Scholar]
- 13.Daley GQ. Hematology Am Soc Hematol Educ Program. 2007. Towards the generation of patient-specific pluripotent stem cells for combined gene and cell therapy of hematologic disorders. pp. 17–22. [DOI] [PubMed] [Google Scholar]
- 14.Kaufman DS. Toward clinical therapies using hematopoietic cells derived from human pluripotent stem cells. Blood. 2009;114(17):3513–3523. doi: 10.1182/blood-2009-03-191304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rivière I, Dunbar CE, Sadelain M. Hematopoietic stem cell engineering at a crossroads. Blood. 2012;119(5):1107–1116. doi: 10.1182/blood-2011-09-349993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanna J, Wernig M, Markoulaki S, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318(5858):1920–1923. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- 17.Iwamoto H, Ojima T, Hayata K, et al. Antitumor immune response of dendritic cells (DCs) expressing tumor-associated antigens derived from induced pluripotent stem cells: In comparison to bone marrow-derived DCs. Int J Cancer. 2013. Jul 3. doi: 10.1002/ijc.28367. [Epub ahead of print] [DOI] [PubMed]
- 18.Themeli M, Kloss CC, Ciriello G, et al. Generation of tumor-targeted human T lymphocytes from induced pluripotent stem cells for cancer therapy. Nature Biotechnol. 2013;31(10):928-933. [DOI] [PMC free article] [PubMed]
- 19.Choi KD, Vodyanik MA, Slukvin II. Generation of mature human myelomonocytic cells through expansion and differentiation of pluripotent stem cell-derived lin-CD34+CD43+CD45+ progenitors. J Clin Invest. 2009;119(9):2818–2829. doi: 10.1172/JCI38591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinder SJ, Tsang TE, Quinlan GA, Hadjantonakis AK, Nagy A, Tam PP. The orderly allocation of mesodermal cells to the extraembryonic structures and the anteroposterior axis during gastrulation of the mouse embryo. Development. 1999;126(21):4691–4701. doi: 10.1242/dev.126.21.4691. [DOI] [PubMed] [Google Scholar]
- 21.Wilkinson DG, Bhatt S, Herrmann BG. Expression pattern of the mouse T gene and its role in mesoderm formation. Nature. 1990;343(6259):657–659. doi: 10.1038/343657a0. [DOI] [PubMed] [Google Scholar]
- 22.Yamaguchi TP, Dumont DJ, Conlon RA, Breitman ML, Rossant J. flk-1, an flt-related receptor tyrosine kinase is an early marker for endothelial cell precursors. Development. 1993;118(2):489–498. doi: 10.1242/dev.118.2.489. [DOI] [PubMed] [Google Scholar]
- 23.Shalaby F, Ho J, Stanford WL, et al. A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. Cell. 1997;89(6):981–990. doi: 10.1016/s0092-8674(00)80283-4. [DOI] [PubMed] [Google Scholar]
- 24.Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9(17):2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- 25.Saxton TM, Pawson T. Morphogenetic movements at gastrulation require the SH2 tyrosine phosphatase Shp2. Proc Natl Acad Sci USA. 1999;96(7):3790–3795. doi: 10.1073/pnas.96.7.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22(4):361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- 27.Conlon FL, Lyons KM, Takaesu N, et al. A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development. 1994;120(7):1919–1928. doi: 10.1242/dev.120.7.1919. [DOI] [PubMed] [Google Scholar]
- 28.Flamme I, Breier G, Risau W. Vascular endothelial growth factor (VEGF) and VEGF receptor 2 (flk-1) are expressed during vasculogenesis and vascular differentiation in the quail embryo. Dev Biol. 1995;169(2):699–712. doi: 10.1006/dbio.1995.1180. [DOI] [PubMed] [Google Scholar]
- 29.Carmeliet P, Ferreira V, Breier G, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380(6573):435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 30.Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19(10):1129–1155. doi: 10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- 31.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132(4):661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Palis J, Robertson S, Kennedy M, Wall C, Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126(22):5073–5084. doi: 10.1242/dev.126.22.5073. [DOI] [PubMed] [Google Scholar]
- 33.Xu MJ, Matsuoka S, Yang FC, et al. Evidence for the presence of murine primitive megakaryocytopoiesis in the early yolk sac. Blood. 2001;97(7):2016–2022. doi: 10.1182/blood.v97.7.2016. [DOI] [PubMed] [Google Scholar]
- 34.Silver L, Palis J. Initiation of murine embryonic erythropoiesis: a spatial analysis. Blood. 1997;89(4):1154-1164. [PubMed]
- 35.de Bruijn MF, Ma X, Robin C, Ottersbach K, Sanchez MJ, Dzierzak E. Hematopoietic stem cells localize to the endothelial cell layer in the midgestation mouse aorta. Immunity. 2002;16(5):673–683. doi: 10.1016/s1074-7613(02)00313-8. [DOI] [PubMed] [Google Scholar]
- 36.Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86(6):897-906. [DOI] [PubMed]
- 37.Müller AM, Medvinsky A, Strouboulis J, Grosveld F, Dzierzak E. Development of hematopoietic stem cell activity in the mouse embryo. Immunity. 1994;1(4):291–301. doi: 10.1016/1074-7613(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 38.Gekas C, Dieterlen-Lièvre F, Orkin SH, Mikkola HK. The placenta is a niche for hematopoietic stem cells. Dev Cell. 2005;8(3):365–375. doi: 10.1016/j.devcel.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 39.Ottersbach K, Dzierzak E. The murine placenta contains hematopoietic stem cells within the vascular labyrinth region. Dev Cell. 2005;8(3):377–387. doi: 10.1016/j.devcel.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 40.de Bruijn MF, Speck NA, Peeters MC, Dzierzak E. Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. EMBO J. 2000;19(11):2465–2474. doi: 10.1093/emboj/19.11.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumaravelu P, Hook L, Morrison AM, et al. Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): role of the aorta-gonad-mesonephros (AGM) region and the yolk sac in colonisation of the mouse embryonic liver. Development. 2002;129(21):4891–4899. doi: 10.1242/dev.129.21.4891. [DOI] [PubMed] [Google Scholar]
- 42.Cumano A, Godin I. Ontogeny of the hematopoietic system. Annu Rev Immunol. 2007;25(25):745–785. doi: 10.1146/annurev.immunol.25.022106.141538. [DOI] [PubMed] [Google Scholar]
- 43.Maximow AA. Relation of blood cells to connective tissues and endothelium. Physiol Rev. 1924;4(4):533–563. [Google Scholar]
- 44.Sabin F. Origin and development of the primitive vessels of the chick and of the pig. Carnegie Inst Wash Publ Contribs Embryol. 1917;6:61–124. [Google Scholar]
- 45.Murray PDF. The development in vitro of the blood of the early chick embryo. Proc R Soc Lond, B. 1932;111(773):497–521. [Google Scholar]
- 46.Lacaud G, Gore L, Kennedy M, et al. Runx1 is essential for hematopoietic commitment at the hemangioblast stage of development in vitro. Blood. 2002;100(2):458–466. doi: 10.1182/blood-2001-12-0321. [DOI] [PubMed] [Google Scholar]
- 47.Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125(4):725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- 48.Huber TL, Kouskoff V, Fehling HJ, Palis J, Keller G. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature. 2004;432(7017):625–630. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- 49.Jaffredo T, Gautier R, Eichmann A, Dieterlen-Lièvre F. Intraaortic hemopoietic cells are derived from endothelial cells during ontogeny. Development. 1998;125(22):4575–4583. doi: 10.1242/dev.125.22.4575. [DOI] [PubMed] [Google Scholar]
- 50.Boisset JC, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E, Robin C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464(7285):116–120. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- 51.Zovein AC, Hofmann JJ, Lynch M, et al. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell. 2008;3(6):625–636. doi: 10.1016/j.stem.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009;457(7231):887–891. doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kissa K, Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010;464(7285):112–115. doi: 10.1038/nature08761. [DOI] [PubMed] [Google Scholar]
- 54.Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DY, Traver D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464(7285):108–111. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yokomizo T, Dzierzak E. Three-dimensional cartography of hematopoietic clusters in the vasculature of whole mouse embryos. Development. 2010;137(21):3651–3661. doi: 10.1242/dev.051094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Z, Lan Y, He W, et al. Mouse embryonic head as a site for hematopoietic stem cell development. Cell Stem Cell. 2012;11(5):663–675. doi: 10.1016/j.stem.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 57.Nakano H, Liu X, Arshi A, et al. Haemogenic endocardium contributes to transient definitive haematopoiesis. Nature Commun. 2013;4:1564. [DOI] [PMC free article] [PubMed]
- 58.Li W, Ferkowicz MJ, Johnson SA, Shelley WC, Yoder MC. Endothelial cells in the early murine yolk sac give rise to CD41-expressing hematopoietic cells. Stem Cells Dev. 2005;14(1):44–54. doi: 10.1089/scd.2005.14.44. [DOI] [PubMed] [Google Scholar]
- 59.Kaufman DS, Hanson ET, Lewis RL, Auerbach R, Thomson JA. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2001;98(19):10716–10721. doi: 10.1073/pnas.191362598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chadwick K, Wang L, Li L, et al. Cytokines and BMP-4 promote hematopoietic differentiation of human embryonic stem cells. Blood. 2003;102(3):906–915. doi: 10.1182/blood-2003-03-0832. [DOI] [PubMed] [Google Scholar]
- 61.Zambidis ET, Peault B, Park TS, Bunz F, Civin CI. Hematopoietic differentiation of human embryonic stem cells progresses through sequential hematoendothelial, primitive, and definitive stages resembling human yolk sac development. Blood. 2005;106(3):860–870. doi: 10.1182/blood-2004-11-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kennedy M, D’Souza SL, Lynch-Kattman M, Schwantz S, Keller G. Development of the hemangioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood. 2007;109(7):2679–2687. doi: 10.1182/blood-2006-09-047704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Salvagiotto G, Burton S, Daigh CA, Rajesh D, Slukvin II, Seay NJ. A defined, feeder-free, serum-free system to generate in vitro hematopoietic progenitors and differentiated blood cells from hESCs and hiPSCs. PLoS ONE. 2011;6(3):e17829. doi: 10.1371/journal.pone.0017829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Niwa A, Heike T, Umeda K, et al. A novel serum-free monolayer culture for orderly hematopoietic differentiation of human pluripotent cells via mesodermal progenitors. PLoS ONE. 2011;6(7):e22261. doi: 10.1371/journal.pone.0022261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pick M, Azzola L, Mossman A, Stanley EG, Elefanty AG. Differentiation of human embryonic stem cells in serum-free medium reveals distinct roles for bone morphogenetic protein 4, vascular endothelial growth factor, stem cell factor, and fibroblast growth factor 2 in hematopoiesis. Stem Cells. 2007;25(9):2206–2214. doi: 10.1634/stemcells.2006-0713. [DOI] [PubMed] [Google Scholar]
- 66.Kennedy M, Awong G, Sturgeon CM, et al. T lymphocyte potential marks the emergence of definitive hematopoietic progenitors in human pluripotent stem cell differentiation cultures. Cell Rep. 2012;2(6):1722-1735. [DOI] [PubMed]
- 67.Choi KD, Yu J, Smuga-Otto K, et al. Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells. 2009;27(3):559–567. doi: 10.1634/stemcells.2008-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Woods NB, Parker AS, Moraghebi R, et al. Brief report: efficient generation of hematopoietic precursors and progenitors from human pluripotent stem cell lines. Stem Cells. 2011;29(7):1158–1164. doi: 10.1002/stem.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Park TS, Zimmerlin L, Zambidis ET. Efficient and simultaneous generation of hematopoietic and vascular progenitors from human induced pluripotent stem cells. Cytometry A. 2013;83(1):114-126. [DOI] [PMC free article] [PubMed]
- 70.Zhang P, Li J, Tan Z, et al. Short-term BMP-4 treatment initiates mesoderm induction in human embryonic stem cells. Blood. 2008;111(4):1933–1941. doi: 10.1182/blood-2007-02-074120. [DOI] [PubMed] [Google Scholar]
- 71.Wang Y, Nakayama N. doi: 10.1016/j.scr.2009.06.001. WNT and BMP signaling are both required for hematopoietic cell development from human ES cells. Stem Cell Res. 2009;3(2-3):113-125. [DOI] [PubMed] [Google Scholar]
- 72.Woll PS, Morris JK, Painschab MS, et al. Wnt signaling promotes hematoendothelial cell development from human embryonic stem cells. Blood. 2008;111(1):122–131. doi: 10.1182/blood-2007-04-084186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cerdan C, Rouleau A, Bhatia M. VEGF-A165 augments erythropoietic development from human embryonic stem cells. Blood. 2004;103(7):2504–2512. doi: 10.1182/blood-2003-07-2563. [DOI] [PubMed] [Google Scholar]
- 74.Wang C, Tang X, Sun X, et al. TGFβ inhibition enhances the generation of hematopoietic progenitors from human ES cell-derived hemogenic endothelial cells using a stepwise strategy. Cell Res. 2012;22(1):194–207. doi: 10.1038/cr.2011.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kardel MD, Eaves CJ. Modeling human hematopoietic cell development from pluripotent stem cells. Exp Hematol. 2012;40(8):601–611. doi: 10.1016/j.exphem.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 76.Davis RP, Ng ES, Costa M, et al. Targeting a GFP reporter gene to the MIXL1 locus of human embryonic stem cells identifies human primitive streak-like cells and enables isolation of primitive hematopoietic precursors. Blood. 2008;111(4):1876–1884. doi: 10.1182/blood-2007-06-093609. [DOI] [PubMed] [Google Scholar]
- 77.Vodyanik MA, Yu J, Zhang X, et al. A mesoderm-derived precursor for mesenchymal stem and endothelial cells. Cell Stem Cell. 2010;7(6):718–729. doi: 10.1016/j.stem.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vodyanik MA, Bork JA, Thomson JA, Slukvin II. Human embryonic stem cell-derived CD34+ cells: efficient production in the coculture with OP9 stromal cells and analysis of lymphohematopoietic potential. Blood. 2005;105(2):617–626. doi: 10.1182/blood-2004-04-1649. [DOI] [PubMed] [Google Scholar]
- 79.Yang L, Soonpaa MH, Adler ED, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453(7194):524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 80.Yu QC, Hirst CE, Costa M, et al. APELIN promotes hematopoiesis from human embryonic stem cells. Blood. 2012;119(26):6243–6254. doi: 10.1182/blood-2011-12-396093. [DOI] [PubMed] [Google Scholar]
- 81.D’Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23(12):1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- 82.Chng Z, Teo A, Pedersen RA, Vallier L. SIP1 mediates cell-fate decisions between neuroectoderm and mesendoderm in human pluripotent stem cells. Cell Stem Cell. 2010;6(1):59–70. doi: 10.1016/j.stem.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 83.Vallier L, Touboul T, Chng Z, et al. Early cell fate decisions of human embryonic stem cells and mouse epiblast stem cells are controlled by the same signalling pathways. PLoS ONE. 2009;4(6):e6082. doi: 10.1371/journal.pone.0006082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sumi T, Tsuneyoshi N, Nakatsuji N, Suemori H. Defining early lineage specification of human embryonic stem cells by the orchestrated balance of canonical Wnt/beta-catenin, Activin/Nodal and BMP signaling. Development. 2008;135(17):2969–2979. doi: 10.1242/dev.021121. [DOI] [PubMed] [Google Scholar]
- 85.Vijayaragavan K, Szabo E, Bossé M, Ramos-Mejia V, Moon RT, Bhatia M. Noncanonical Wnt signaling orchestrates early developmental events toward hematopoietic cell fate from human embryonic stem cells. Cell Stem Cell. 2009;4(3):248–262. doi: 10.1016/j.stem.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Choi KD, Vodyanik MA, Togarrati PP, et al. Identification of the hemogenic endothelial progenitor and its direct precursor in human pluripotent stem cell differentiation cultures. Cell Rep. 2012;2(3):553–567. doi: 10.1016/j.celrep.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lancrin C, Sroczynska P, Stephenson C, Allen T, Kouskoff V, Lacaud G. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature. 2009;457(7231):892–895. doi: 10.1038/nature07679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- 89.Sakurai H, Era T, Jakt LM, et al. In vitro modeling of paraxial and lateral mesoderm differentiation reveals early reversibility. Stem Cells. 2006;24(3):575–586. doi: 10.1634/stemcells.2005-0256. [DOI] [PubMed] [Google Scholar]
- 90.Slukvin II. Deciphering the hierarchy of angiohematopoietic progenitors from human pluripotent stem cells. Cell Cycle. 2013;12(5):720–727. doi: 10.4161/cc.23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sturgeon CM, Ditadi A, Clarke RL, Keller G. Defining the path to hematopoietic stem cells. Nat Biotechnol. 2013;31(5):416–418. doi: 10.1038/nbt.2571. [DOI] [PubMed] [Google Scholar]
- 92.Breier G, Breviario F, Caveda L, et al. Molecular cloning and expression of murine vascular endothelial-cadherin in early stage development of cardiovascular system. Blood. 1996;87(2):630–641. [PubMed] [Google Scholar]
- 93.Lampugnani MG, Resnati M, Raiteri M, et al. A novel endothelial-specific membrane protein is a marker of cell-cell contacts. J Cell Biol. 1992;118(6):1511–1522. doi: 10.1083/jcb.118.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vittet D, Buchou T, Schweitzer A, Dejana E, Huber P. Targeted null-mutation in the vascular endothelial-cadherin gene impairs the organization of vascular-like structures in embryoid bodies. Proc Natl Acad Sci USA. 1997;94(12):6273–6278. doi: 10.1073/pnas.94.12.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nishikawa SI, Nishikawa S, Hirashima M, Matsuyoshi N, Kodama H. Progressive lineage analysis by cell sorting and culture identifies FLK1+VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development. 1998;125(9):1747–1757. doi: 10.1242/dev.125.9.1747. [DOI] [PubMed] [Google Scholar]
- 96.Nishikawa SI, Nishikawa S, Kawamoto H, et al. In vitro generation of lymphohematopoietic cells from endothelial cells purified from murine embryos. Immunity. 1998;8(6):761–769. doi: 10.1016/s1074-7613(00)80581-6. [DOI] [PubMed] [Google Scholar]
- 97.Eilken HM, Nishikawa S, Schroeder T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature. 2009;457(7231):896–900. doi: 10.1038/nature07760. [DOI] [PubMed] [Google Scholar]
- 98.Hashimoto K, Fujimoto T, Shimoda Y, Huang X, Sakamoto H, Ogawa M. Distinct hemogenic potential of endothelial cells and CD41+ cells in mouse embryos. Dev Growth Differ. 2007;49(4):287–300. doi: 10.1111/j.1440-169X.2007.00925.x. [DOI] [PubMed] [Google Scholar]
- 99.Wang L, Li L, Shojaei F, et al. Endothelial and hematopoietic cell fate of human embryonic stem cells originates from primitive endothelium with hemangioblastic properties. Immunity. 2004;21(1):31–41. doi: 10.1016/j.immuni.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 100.Vodyanik MA, Thomson JA, Slukvin II. Leukosialin (CD43) defines hematopoietic progenitors in human embryonic stem cell differentiation cultures. Blood. 2006;108(6):2095–2105. doi: 10.1182/blood-2006-02-003327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nakajima-Takagi Y, Osawa M, Oshima M, et al. Role of SOX17 in hematopoietic development from human embryonic stem cells. Blood. 2013;121(3):447–458. doi: 10.1182/blood-2012-05-431403. [DOI] [PubMed] [Google Scholar]
- 102.Rafii S, Kloss CC, Butler JM, et al. Human ESC-derived hemogenic endothelial cells undergo distinct waves of endothelial to hematopoietic transition. Blood. 2013;121(5):770–780. doi: 10.1182/blood-2012-07-444208. [DOI] [PubMed] [Google Scholar]
- 103.North T, Gu TL, Stacy T, et al. Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development. 1999;126(11):2563–2575. doi: 10.1242/dev.126.11.2563. [DOI] [PubMed] [Google Scholar]
- 104.Zambidis ET, Park TS, Yu W, et al. Expression of angiotensin-converting enzyme (CD143) identifies and regulates primitive hemangioblasts derived from human pluripotent stem cells. Blood. 2008;112(9):3601–3614. doi: 10.1182/blood-2008-03-144766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Iacovino M, Chong D, Szatmari I, et al. HoxA3 is an apical regulator of haemogenic endothelium. Nat Cell Biol. 2011;13(1):72–78. doi: 10.1038/ncb2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Clarke RL, Yzaguirre AD, Yashiro-Ohtani Y, et al. The expression of Sox17 identifies and regulates haemogenic endothelium. Nat Cell Biol. 2013;15(5):502–510. doi: 10.1038/ncb2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guiu J, Shimizu R, D’Altri T, et al. Hes repressors are essential regulators of hematopoietic stem cell development downstream of Notch signaling. J Exp Med. 2013;210(1):71–84. doi: 10.1084/jem.20120993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee JB, Werbowetski-Ogilvie TE, Lee JH, et al. Notch-HES1 signaling axis controls hemato-endothelial fate decisions of human embryonic and induced pluripotent stem cells. Blood. doi: 10.1182/blood-2012-12-471649. 2013. 122(7):1162-1173. [DOI] [PubMed] [Google Scholar]
- 109.Ng ES, Davis RP, Azzola L, Stanley EG, Elefanty AG. Forced aggregation of defined numbers of human embryonic stem cells into embryoid bodies fosters robust, reproducible hematopoietic differentiation. Blood. 2005;106(5):1601–1603. doi: 10.1182/blood-2005-03-0987. [DOI] [PubMed] [Google Scholar]
- 110.Kopher RA, Penchev VR, Islam MS, Hill KL, Khosla S, Kaufman DS. Human embryonic stem cell-derived CD34+ cells function as MSC progenitor cells. Bone. 2010;47(4):718–728. doi: 10.1016/j.bone.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Manjunath N, Correa M, Ardman M, Ardman B. Negative regulation of T-cell adhesion and activation by CD43. Nature. 1995;377(6549):535–538. doi: 10.1038/377535a0. [DOI] [PubMed] [Google Scholar]
- 112.Ferkowicz MJ, Starr M, Xie X, et al. CD41 expression defines the onset of primitive and definitive hematopoiesis in the murine embryo. Development. 2003;130(18):4393–4403. doi: 10.1242/dev.00632. [DOI] [PubMed] [Google Scholar]
- 113.Mikkola HK, Fujiwara Y, Schlaeger TM, Traver D, Orkin SH. Expression of CD41 marks the initiation of definitive hematopoiesis in the mouse embryo. Blood. 2003;101(2):508–516. doi: 10.1182/blood-2002-06-1699. [DOI] [PubMed] [Google Scholar]
- 114.Otani T, Inoue T, Tsuji-Takayama K, et al. Progenitor analysis of primitive erythropoiesis generated from in vitro culture of embryonic stem cells. Exp Hematol. 2005;33(6):632–640. doi: 10.1016/j.exphem.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 115.Klimchenko O, Mori M, Distefano A, et al. A common bipotent progenitor generates the erythroid and megakaryocyte lineages in embryonic stem cell-derived primitive hematopoiesis. Blood. 2009;114(8):1506–1517. doi: 10.1182/blood-2008-09-178863. [DOI] [PubMed] [Google Scholar]
- 116.Dias J, Gumenyuk M, Kang H, et al. Generation of red blood cells from human induced pluripotent stem cells. Stem Cells Dev. 2011;20(9):1639–1647. doi: 10.1089/scd.2011.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Smith BW, Rozelle SS, Leung A, et al. The aryl hydrocarbon receptor directs hematopoietic progenitor cell expansion and differentiation. Blood. 2013;122(3):376–385. doi: 10.1182/blood-2012-11-466722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chang KH, Bonig H, Papayannopoulou T. Generation and characterization of erythroid cells from human embryonic stem cells and induced pluripotent stem cells: an overview. Stem Cells Int. 2011;2011:791604. [DOI] [PMC free article] [PubMed]
- 119.Takayama N, Eto K. Pluripotent stem cells reveal the developmental biology of human megakaryocytes and provide a source of platelets for clinical application. Cell Mol Life Sci. 2012;69(20):3419–3428. doi: 10.1007/s00018-012-0995-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chang CJ, Mitra K, Koya M, et al. Production of embryonic and fetal-like red blood cells from human induced pluripotent stem cells. PLoS ONE. 2011;6(10):e25761. doi: 10.1371/journal.pone.0025761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kobari L, Yates F, Oudrhiri N, et al. Human induced pluripotent stem cells can reach complete terminal maturation: in vivo and in vitro evidence in the erythropoietic differentiation model. Haematologica. 2012;97(12):1795–1803. doi: 10.3324/haematol.2011.055566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Salvagiotto G, Zhao Y, Vodyanik M, et al. Molecular profiling reveals similarities and differences between primitive subsets of hematopoietic cells generated in vitro from human embryonic stem cells and in vivo during embryogenesis. Exp Hematol. 2008;36(10):1377–1389. doi: 10.1016/j.exphem.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ran D, Shia WJ, Lo MC, et al. RUNX1a enhances hematopoietic lineage commitment from human embryonic stem cells and inducible pluripotent stem cells. Blood. 2013;121(15):2882–2890. doi: 10.1182/blood-2012-08-451641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Carpenter L, Malladi R, Yang CT, et al. Human induced pluripotent stem cells are capable of B-cell lymphopoiesis. Blood. 2011;117(15):4008–4011. doi: 10.1182/blood-2010-08-299941. [DOI] [PubMed] [Google Scholar]
- 125.Woll PS, Martin CH, Miller JS, Kaufman DS. Human embryonic stem cell-derived NK cells acquire functional receptors and cytolytic activity. J Immunol. 2005;175(8):5095–5103. doi: 10.4049/jimmunol.175.8.5095. [DOI] [PubMed] [Google Scholar]
- 126.Knorr DA, Ni Z, Hermanson D, et al. Clinical-scale derivation of natural killer cells from human pluripotent stem cells for cancer therapy. Stem Cells Transl Med. 2013;2(4):274-283. [DOI] [PMC free article] [PubMed]
- 127.Galic Z, Kitchen SG, Kacena A, et al. T lineage differentiation from human embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103(31):11742-11747. Epub 12006 Jul 11714. [DOI] [PMC free article] [PubMed]
- 128.Timmermans F, Velghe I, Vanwalleghem L, et al. Generation of T cells from human embryonic stem cell-derived hematopoietic zones. J Immunol. 2009;182(11):6879–6888. doi: 10.4049/jimmunol.0803670. [DOI] [PubMed] [Google Scholar]
- 129.Vizcardo R, Masuda K, Yamada D, et al. Regeneration of human tumor antigen-specific T cells from iPSCs derived from mature CD8(+) T cells. Cell Stem Cell. 2013;12(1):31–36. doi: 10.1016/j.stem.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 130.Lu M, Kardel MD, O’Connor MD, Eaves CJ. Enhanced generation of hematopoietic cells from human hepatocarcinoma cell-stimulated human embryonic and induced pluripotent stem cells. Exp Hematol. 2009;37(8):924–936. doi: 10.1016/j.exphem.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 131.Wang L, Menendez P, Shojaei F, et al. Generation of hematopoietic repopulating cells from human embryonic stem cells independent of ectopic HOXB4 expression. J Exp Med. 2005;201(10):1603–1614. doi: 10.1084/jem.20041888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ledran MH, Krassowska A, Armstrong L, et al. Efficient hematopoietic differentiation of human embryonic stem cells on stromal cells derived from hematopoietic niches. Cell Stem Cell. 2008;3(1):85–98. doi: 10.1016/j.stem.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 133.Narayan AD, Chase JL, Lewis RL, et al. Human embryonic stem cell-derived hematopoietic cells are capable of engrafting primary as well as secondary fetal sheep recipients. Blood. 2006;107(5):2180–2183. doi: 10.1182/blood-2005-05-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tian X, Woll PS, Morris JK, Linehan JL, Kaufman DS. Hematopoietic engraftment of human embryonic stem cell-derived cells is regulated by recipient innate immunity. Stem Cells. 2006;24(5):1370–1380. doi: 10.1634/stemcells.2005-0340. [DOI] [PubMed] [Google Scholar]
- 135.Risueño RM, Sachlos E, Lee JH, et al. Inability of human induced pluripotent stem cell-hematopoietic derivatives to downregulate microRNAs in vivo reveals a block in xenograft hematopoietic regeneration. Stem Cells. 2012;30(2):131–139. doi: 10.1002/stem.1684. [DOI] [PubMed] [Google Scholar]
- 136.Tian X, Hexum MK, Penchev VR, Taylor RJ, Shultz LD, Kaufman DS. Bioluminescent imaging demonstrates that transplanted human embryonic stem cell-derived CD34(+) cells preferentially develop into endothelial cells. Stem Cells. 2009;27(11):2675–2685. doi: 10.1002/stem.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Amabile G, Welner RS, Nombela-Arrieta C, et al. In vivo generation of transplantable human hematopoietic cells from induced pluripotent stem cells. Blood. 2013;121(8):1255–1264. doi: 10.1182/blood-2012-06-434407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Suzuki N, Yamazaki S, Yamaguchi T, et al. Generation of engraftable hematopoietic stem cells from induced pluripotent stem cells by way of teratoma formation. Mol Ther. 2013;21(7):1424-1431. [DOI] [PMC free article] [PubMed]
- 139.Medvinsky A, Rybtsov S, Taoudi S. Embryonic origin of the adult hematopoietic system: advances and questions. Development. 2011;138(6):1017–1031. doi: 10.1242/dev.040998. [DOI] [PubMed] [Google Scholar]
- 140.Yoshimoto M, Porayette P, Glosson NL, et al. Autonomous murine T-cell progenitor production in the extra-embryonic yolk sac before HSC emergence. Blood. 2012;119(24):5706–5714. doi: 10.1182/blood-2011-12-397489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yoshimoto M, Montecino-Rodriguez E, Ferkowicz MJ, et al. Embryonic day 9 yolk sac and intra-embryonic hemogenic endothelium independently generate a B-1 and marginal zone progenitor lacking B-2 potential. Proc Natl Acad Sci USA. 2011;108(4):1468–1473. doi: 10.1073/pnas.1015841108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Taoudi S, Medvinsky A. Functional identification of the hematopoietic stem cell niche in the ventral domain of the embryonic dorsal aorta. Proc Natl Acad Sci USA. 2007;104(22):9399–9403. doi: 10.1073/pnas.0700984104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ciau-Uitz A, Walmsley M, Patient R. Distinct origins of adult and embryonic blood in Xenopus. Cell. 2000;102(6):787–796. doi: 10.1016/s0092-8674(00)00067-2. [DOI] [PubMed] [Google Scholar]
- 144.Samokhvalov IM, Samokhvalova NI, Nishikawa S. Cell tracing shows the contribution of the yolk sac to adult haematopoiesis. Nature. 2007;446(7139):1056–1061. doi: 10.1038/nature05725. [DOI] [PubMed] [Google Scholar]
- 145.Weissman IL, Papaioannou V, Grdner R. Fetal hematopoietic origins of the adult hematolymphoid cells. In: Clarkson B, Marks PA, Till JE, editors. Differentiation of Normal and Neoplastic Cells. New York: Cold Spring Harbor Laboratory Press; 1978. pp. 33–47. [Google Scholar]
- 146.Yoder MC, Hiatt K, Dutt P, Mukherjee P, Bodine DM, Orlic D. Characterization of definitive lymphohematopoietic stem cells in the day 9 murine yolk sac. Immunity. 1997;7(3):335–344. doi: 10.1016/s1074-7613(00)80355-6. [DOI] [PubMed] [Google Scholar]
- 147.Yoder MC, Hiatt K, Mukherjee P. In vivo repopulating hematopoietic stem cells are present in the murine yolk sac at day 9.0 postcoitus. Proc Natl Acad Sci USA. 1997;94(13):6776–6780. doi: 10.1073/pnas.94.13.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zape JP, Zovein AC. Hemogenic endothelium: origins, regulation, and implications for vascular biology. Semin Cell Dev Biol. 2011;22(9):1036–1047. doi: 10.1016/j.semcdb.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 149.Chen MJ, Li Y, De Obaldia ME, et al. Erythroid/myeloid progenitors and hematopoietic stem cells originate from distinct populations of endothelial cells. Cell Stem Cell. 2011;9(6):541–552. doi: 10.1016/j.stem.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang Y, Yates F, Naveiras O, Ernst P, Daley GQ. Embryonic stem cell-derived hematopoietic stem cells. Proc Natl Acad Sci USA. 2005;102(52):19081–19086. doi: 10.1073/pnas.0506127102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.McGrath KE, Palis J. Expression of homeobox genes, including an insulin promoting factor, in the murine yolk sac at the time of hematopoietic initiation. Mol Reprod Dev. 1997;48(2):145–153. doi: 10.1002/(SICI)1098-2795(199710)48:2<145::AID-MRD1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 152.McKinney-Freeman S, Cahan P, Li H, et al. The transcriptional landscape of hematopoietic stem cell ontogeny. Cell Stem Cell. 2012;11(5):701–714. doi: 10.1016/j.stem.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lee GS, Kim BS, Sheih JH, Moore M. Forced expression of HoxB4 enhances hematopoietic differentiation by human embryonic stem cells. Mol Cells. 2008;25(4):487–493. [PubMed] [Google Scholar]
- 154.Dou DR, Minasian A, Sierra M, et al. Inability to express HOXA cluster and BCL11A genes compromises self-renewal and multipotency of hESC-derived hematopoietic cells. In: Proceedings from American Society of Hematology 54th Annual Meeting; December 8-11, 2012; Atlanta, GA. Abstract 1190.
- 155.Shojaei F, Menendez P. Molecular profiling of candidate human hematopoietic stem cells derived from human embryonic stem cells. Exp Hematol. 2008;36(11):1436–1448. doi: 10.1016/j.exphem.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 156.Schnerch A, Lee JB, Graham M, Guezguez B, Bhatia M. Human embryonic stem cell-derived hematopoietic cells maintain core epigenetic machinery of the polycomb group/Trithorax Group complexes distinctly from functional adult hematopoietic stem cells. Stem Cells Dev. 2013;22(1):73–89. doi: 10.1089/scd.2012.0204. [DOI] [PubMed] [Google Scholar]
- 157.Pereira CF, Chang B, Qiu J, et al. Induction of a hemogenic program in mouse fibroblasts. Cell Stem Cell. 2013;13(2):205–218. doi: 10.1016/j.stem.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Szabo E, Rampalli S, Risueño RM, et al. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature. 2010;468(7323):521–526. doi: 10.1038/nature09591. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.