Abstract
Alpha crystallins are small heat shock proteins essential to normal ocular lens function. They also help maintain homeostasis in many non-ocular vertebrate tissues and their expression levels change in multiple diseases of the nervous and cardiovascular system and during cancer. The specific roles that α-crystallins may play in eye development are unclear. Studies with knockout mice suggested that only one of the two mammalian α-crystallins is required for normal early lens development. However, studies in two fish species suggested that reduction of αA-crystallin alone could inhibit normal fiber cell differentiation, cause cataract and contribute to lens degeneration. In this study we used synthetic antisense morpholino oligomers to suppress the expression of zebrafish αA-crystallin to directly test the hypothesis that, unlike mammals, the zebrafish requires αA-crystallin for normal early lens development. Despite the reduction of zebrafish αA-crystallin protein to undetectable levels by western analysis through 4 days of development we found no changes in fiber cell differentiation, lens morphology or transparency. In contrast, suppression of AQP0a expression, previously shown to cause lens cataract, produced irregularly shaped lenses, delay in fiber cell differentiation and lens opacities detectable by confocal microscopy. The normal development observed in αA-crystallin deficient zebrafish embryos may reflect similarly non-essential roles for this protein in the early stages of both zebrafish and mammalian lens development. This finding has ramifications for a growing number of researchers taking advantage of the zebrafish's transparent external embryos to study vertebrate eye development. Our demonstration that lens cataracts can be visualized in three-dimensions by confocal microscopy in a living zebrafish provides a new tool for studying the causes, development and prevention of lens opacities.
Keywords: alpha crystalline, ocular lens, small heat shock protein, lens development, cataract, morpholino, zebrafish
1. Introduction
Alpha crystallins are an evolutionarily related group of small heat shock proteins (sHsps) that play a role in producing and maintaining the transparency and refractive power of the vertebrate ocular lens (Horwitz, 2003; Andley, 2009). The two mammalian α-crystallins, αA- and αB-crystallin, are also expressed in tissues outside the lens where they may help maintain cellular homeostasis by inhibiting the aggregation of stress-denatured proteins (Bhat and Nagineni, 1989; Dubin et al., 1991; Horwitz, 1992; Srinivasan et al., 1992). Investigations into the extralenticular roles of αB-crystallin have identified changes in expression levels linked to multiple neurological disorders (Renkawek et al., 1999; Clark and Muchowski, 2000; Ousman et al., 2007) and cancer (Stegh et al., 2008), and a mutation in αB-crystallin that causes an inherited cardiac myopathy (Vicart et al., 1998). Recent studies have detailed a range of extralenticular roles for αA-crystallin as well, including the protection of retinal cells against diabetes (Kim et al., 2012) and autoimmune uveitis (Rao et al., 2008; 2012), and a potential role in retinoblastoma tumor growth (Kase et al., 2009). Alpha A-crystallin has also been shown to be an inhibitor of pancreatic carcinogenesis (Deng et al., 2010). Both αA- and αB-crystallin have been found to regulate apoptosis, but by different mechanisms, supporting the view that these two proteins can play divergent functions even within the same tissue (Morozov and Wawrousek, 2006; Ninkovic et al., 2012; Hu et al., 2012), This conclusion is further supported by evidence that the two mammalian α-crystallins can be found within distinct subcellular regions (Gangalum et al., 2012).
Relatively little experimental data exist demonstrating how α-crystallins may regulate or contribute to lens development. Boyle et al. (2000) showed that α-crystallins play a role in the differentiation of lens epithelial cells into elongated and denucleated fiber cells. Some of the best data on the function of α-crystallins in lens development come from a series of gene knockout studies performed in mice. Mice lacking a functional gene for αA-crystallin underwent normal fiber cell differentiation, but lenses were smaller than wildtypes and developed opacification by 7 weeks due in part to the aggregation of insoluble αB-crystallin (Brady et al., 1997). Disruption of the mouse αB-crystallin gene produced no noticeable effect on the lens (Brady et al., 2001), but the combined knockout of both αA- and αB-crystallin led to abnormal fiber cell differentiation, lack of posterior sutures, ectopic fiber cell nuclei and other gross morphological differences (Boyle et al., 2003). A follow up study with the double knockout mouse identified elevated levels of caspase activity regionally correlated with secondary fiber cell disintegration, suggesting that α-crystallins may help regulate the apoptosis-like differentiation of fiber cells (Morozov and Wawrousek, 2006).
The primary use of mammals, particularly rodents, to study α-crystallin function has limited our understanding of its roles in early lens development due to the inaccessibility of in utero early stage mammalian lenses and the associated costs for harvesting this tissue. Multiple researchers are now using the externally developing, transparent zebrafish embryo as a model for investigating lens development and function as this species contains similar lens crystallin components and patterns in lens development to mammals (Posner et al., 2008; Greiling and Clark, 2012; Wages et al., 2013). The zebrafish genome contains three α-crystallin genes compared to the two found in mammals. The zebrafish expresses two αB-crystallins resulting from the genome duplication that occurred at the base of teleost fish radiation (Smith et al., 2006) but only one, the ubiquitously expressed αBb-crystallin, is found prior to 5 dpf (Elicker and Hutson, 2007). The single zebrafish αA-crystallin is more evolutionarily conserved with its mammalian ortholog in structure, protective anti-protein aggregation properties and expression pattern (Runkle et al., 2002; Dahlman et al., 2005). The lens-preferred expression pattern of mammalian αA-crystallin is regulated by the transcription factors Pax6, c-Maf and CREB through the activity of chromatin remodeling enzymes (Yang and Cvekl, 2005; Yang et al., 2006). It is yet to be shown if the mechanisms of αA-crystallin transcriptional regulation are also conserved between zebrafish and mammals.
In contrast to the parallel gene knockout study in mice, two recent studies suggest that reduced amounts of αA-crystallin can lead to abnormalities in the early stages of fish lens development. Goishi et al. (2006) found that lenses of the zebrafish mutants clochem39 and cloches5 expressed reduced amounts of αA-crystallin, developed cataracts by 2.5 days post fertilization and contained aggregated γ-crystallin. Injection of αA-crystallin mRNA to increase levels of expressed protein prevented γ-crystallin aggregation and cataract, leading to the conclusion that reduced αA-crystallin caused this cataract. Strickler et al. (2007) found that the blind cave fish, a close relative of the zebrafish, downregulated αA-crystallin levels prior to its normal, regulated lens degeneration. The authors concluded that reduced αA-crystallin levels might initiate and/or maintain the degeneration of the cavefish lens through altered regulation of apoptosis.
The discrepancy between the lack of abnormal fiber cell differentiation in the αA-crystallin knockout mouse and the more severe effects correlated with reduced αA-crystallin expression in two fish species led us to directly test the hypothesis that αA-crystallin is needed for normal early development of the zebrafish lens. In this present study we suppressed the translation of αA-crystallin in zebrafish embryos using synthetic antisense morpholino oligomers and assessed the resulting effects on lens structure and transparency. While western analysis confirmed an efficient reduction in αA-crystallin to undetectable levels through 4 days post fertilization (dpf), we found no resulting changes in fiber cell differentiation, lens structure or lens transparency. A parallel analysis of zebrafish embryos injected with a morpholino against the water channel AQP0a, the lack of which is already known to cause cataract, confirmed that our methods were capable of detecting abnormalities in lens development. We also demonstrate, for the first time, that confocal microscopy can be used to produce three-dimensional images of cataract in the zebrafish lens. These results resolve a discrepancy in the literature by showing that αA-crystallin is not needed for normal early lens development in the zebrafish. This resolution has ramifications for a growing number of researchers utilizing the zebrafish as a model to understand lens function and development.
2. Materials and methods
2.1. Fish Maintenance and Breeding
ZDR strain zebrafish were housed in 10 liter aquaria on a recirculating filtering system maintained at 28°C with a 14:10 hour light and dark cycle. Fish were fed twice each day with either commercial flake food or live Artemia brine shrimp. Two males and two females were placed in one liter breeding tanks the afternoon prior to morning egg collections. All animal procedures were approved by Ashland University's Animal Care Committee.
2.2. Morpholino design, injection and verification of αA-crystallin translation blocking
Two morpholinos (MOs) designed by Gene Tools (Philomath, OR) were used in a 50:50 mixture to block the translation of αA-crystallin. One MO was designed to anneal to the start codon (5′ GTGTTGGATCGCAATATCCATAATG 3′) and another to the upstream 5′ untranslated region (5′ AGACCTGGTAACTCCTTACTGTAAC 3′). Control injections used a standard control MO (5′ CCTCTTACCTCAGTTACAATTTATA 3′; Gene Tools) to assess general toxicity from MO injection. An MO against AQP0a was also used as a positive control to induce lens cataract (5′ AACTCCCACATGGCTGCAAAAAGTC 3′; provided by Daniel M. Clemens, UC Irvine). Various concentrations of MOs were diluted in Danieau solution (58 mM NaCl, 0.7 mM KCl, 0.4 mM MgSO4, 0.6 mM Ca(NO3)2, 5.0 mM HEPES, pH 7.6) with 0.4% filtered phenol red as a tracer prior to injection into the yolks of 1-4 cell stage zebrafish embryos. Injections were performed with a Harvard Apparatus PL-90 picoinjector (Holliston, MA) using needles prepared with a Sutter P97 Micropipette Puller (Novato, CA). Injection pressures were adjusted to inject 1 nl of MO solution with each 20 msec pulse. Concentrations of the MO were typically adjusted to deliver the needed dose in a 1 nl injection. A range of concentrations for each MO was tested to find doses that did not cause whole body abnormalities in embryo development. Because MOs can produce toxicity resulting from binding to non-specific mRNA targets we attempted to use the lowest dose of αA-crystallin MOs that would still provide effective inhibition of translation. We found that a 9 ng dose of αA-crystallin MOs produced some abnormalities in embryos. Both 4.5 ng and 6 ng doses did not produce abnormal embryos, but did provide equal suppression of αA-crystallin expression. We, therefore, chose a 4.5 ng dose of the 50:50 αA-crystallin MOs mixture for all experiments reported in this study. We used the same dose for our control MO, which similarly caused abnormal embryo development at 9 ng but not at 4.5 or 6 ng. We injected 2 ng of AQP0a MO based on a previously published method (Froger et al., 2010). To summarize, the experiments reported in this study used the following doses: αA-crystallin MO: 4.5 ng; control MO: 4.5 ng; AQP0a MO: 2 ng.
The inhibition of αA-crystallin translation was assessed by western analysis. Zebrafish embryos from 3-5 days post fertilization were anesthetized on ice and decapitated anterior to the remaining yolk sac. Heads from 30-60 embryos were homogenized in 0.02 M NaPO4 (pH 7.4)/0.01 M EDTA/0.5% Triton X-100, centrifuged for 10 minutes at 13,000 g, and the protein concentration of the supernatant was quantified by Bradford assay (Biorad, Hercules, CA). Sixty to ninety micrograms of homogenized head protein was separated by SDS-PAGE on 12% gels (Pierce, Rockford, IL), transferred to PVDF membranes and probed with an antibody to zebrafish αA-crystallin (provided by Hyde lab, University of Notre Dame). Transfer membranes were blocked in 1% dehydrated non-fat milk in PBS/0.05% Tween-20 (PBST), probed with a 1:5000 dilution of αA-crystallin antibody in PBST, washed in PBST 3 times for 5 minutes each, probed with a 1:10,000 dilution of a goat anti-rabbit horseradish peroxidase bound secondary antibody (Sigma, St. Louis, MO) in PBST, washed in PBST 3 times for 5 minutes each, and then detected with SuperSignal West Pico Chemiluminescent Substrate (Pierce). Membranes were imaged using a Kodak 440 CF Imagestation (Kodak, Rochester, NY) and band intensities were quantified by densitometry using Kodak 1D Image Analysis Software (Kodak). Reported band intensities for αA-crystallin were normalized to the intensity of the βB1-crystallin loading control.
2.3. Assessment of potential lens phenotypes
Uninjected and MO injected embryos were assessed for differences in eye growth, examined histologically to identify abnormalities in fiber cell differentiation, and imaged by confocal microscopy to detect light scattering in the lens. Uninjected embryos and those injected with αA-crystallin or control MOs were anesthetized in ice water at 4 dpf and observed under a dissecting microscope to measure standard length (snout to base of caudal fin elements) and eye diameter (at its widest point). The software package Prism (www.graphpad.com) was used to compare measurements between the three groups for statistically significant differences.
Embryos were prepared for histological examination using the method of Vihtelic et al. (2001). After anesthetization and fixation in 4% PFA/5% sucrose/PBS for at least 12 hours at 4°C, embryos were washed in 5% sucrose/PBS three times for 15 minutes each. Embryos were then embedded in 1.5% agarose/5% sucrose/PBS (cooled to 55-60°C) in a 24-well plate. The solidified agarose was trimmed so that each embryo was contained in a rectangular block, which was then incubated in 30% sucrose/PBS overnight at 4°C. The next day the agarose blocks were incubated in a 1:1 mixture of CRYO-OCT embedding compound (Tissue-Tek) and 30% sucrose/PBS for eight hours, transferred to 100% CRYO-OCT and incubated at 4°C overnight, after which they were stored at 4°C in CRYO-OCT until used. Blocks were frozen onto the chuck of a Leica CM1850UV cryostat in a drop of CRYO-OCT with the embryo oriented so that the head pointed away from the chuck. A series of 10-micrometer transverse sections were collected through the head of the fish until the entire lens was sampled. Sections were either stained with hematoxylin and eosin using a standard protocol or with DAPI using Prolong Antifade (Invitrogen). At least three embryos were examined for each timepoint, with the exception of the 77 hour DAPI stained timepoint, for which 2 embryos were examined.
For confocal imaging, 3 dpf live embryos were anesthetized with tricaine and mounted in low melting agarose (0.75%) in dishes with cover slip bottoms, with one eye perpendicular to the plane of the coverslip. Confocal z-series were collected at 1 uM increments using a 20x oil lens on a Leica SP5 confocal microscope in reflectance mode. Image series were then rendered as three-dimensional surface projections using Imaris image analysis software (Bitplane).
3. Results
3.1. Zebrafish αA-crystallin translation was inhibited to undetectable levels through 4 days post fertilization
Two synthetic antisense morpholino oligomers (MOs) were injected into the yolks of 1-4 cell stage zebrafish embryos to block the translation of αA-crystallin mRNA. Western analysis of homogenized embryonic heads showed that αA-crystallin protein was reduced to visually undetectable levels through 4 days post fertilization (dpf) (Fig. 1A). Densitometric analysis showed a 93% and 97% decrease in αA-crystallin band intensity at 3 and 4 dpf, respectively, compared to uninjected embryos. Embryos injected with a control MO that does not anneal to any zebrafish mRNA sequences also showed reductions in αA-crystallin levels at 3 dpf (55% decrease) and 4 dfp (32 % decrease). This reduction in control MO injected embryos disappeared by 5 dpf and may be due to developmental delay from general toxicity of the control MO. Western analysis also showed that αA-crystallin levels in uninjected embryos increased 2.5 fold between 3 and 5 dpf. Western analysis with an antibody against zebrafish βB1-crystallin showed that the inhibitory effect of the αA MO was gene specific and that βB1-crystallin levels remained relatively constant between days 3 and 5 (Fig. 1B).
Fig. 1.
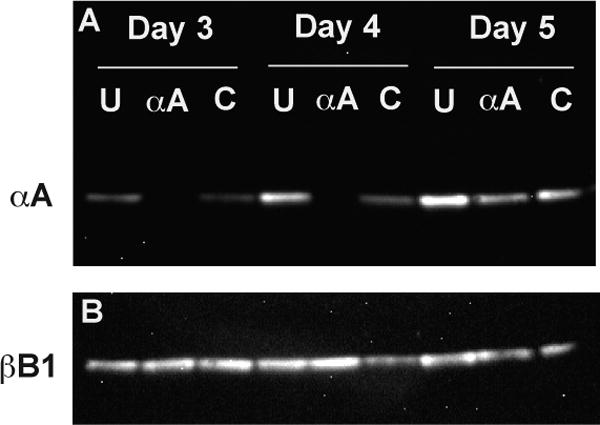
Alpha A-crystallin was undetectable by western analysis in MO knockdown embryos through four days post fertilization (dpf). Separate groups of 1-4 cell stage embryos were either left uninjected (U), or injected with morpholinos against αA-crystallin (αA) or a control morpholino (C) that does not recognize any zebrafish mRNA. Protein from the heads of embryos at three, four and five dpf were probed with antibodies against αA- and βB1-crystallin and detected with a chemiluminescent secondary antibody.
3.2. Knockdown of αA-crystallin does not affect lens development
The potential effects of blocking αA-crystallin protein production were assessed by gross anatomical measurements, histology and confocal microscopy. We found no difference in eye diameter relative to body length between the αA-crystallin and control MO injected embryos (Fig. 2). However, the relative eye diameter of both MO injected treatments did differ from the uninjected embryos (ANOVA, Tukey's post test, p = 0.009). While the MO doses used in this study did not produce any gross defects in embryonic development, the eye diameter/body length data suggest that the use of any MO, even a control with no known target in the zebrafish, can produce background toxicity that slows eye growth more than that of body length. There were no statistically significant differences in body length alone (ANOVA, p = 0.61) or eye diameter alone (ANOVA, p = 0.08) between the three treatments.
Fig. 2.
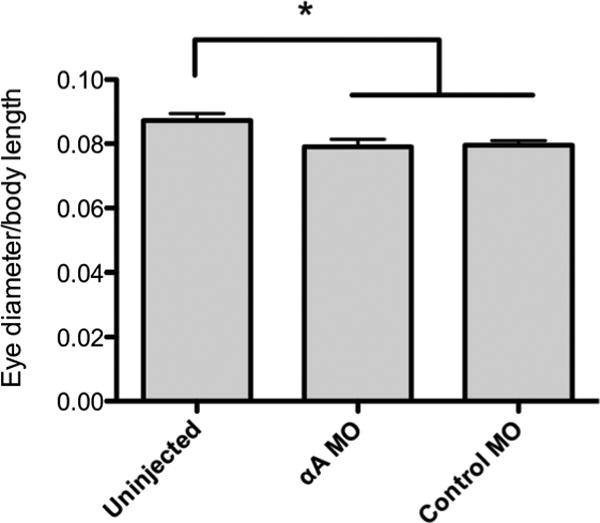
Knockdown of αA-crystallin did not affect eye size. Both αA-crystallin and control MO injected fish had smaller eyes as a ratio to body length compared to uninjected fish, but there was no significant difference between the αA-crystallin MO and control MO treated groups. Error bars indicate standard error of the mean (n=15). Asterisk indicates statistical significance at p < 0.05 (ANOVA with Tukey's multiple comparison test).
Hematoxylin and eosin staining of uninjected and MO-injected embryos through 4 dpf, within the window of αA-crystallin knockdown, showed no abnormalities in lens development (Fig. 3A). At least three embryos were examined for each timepoint. By comparison, histology of aquaporin deficient AQP0a MO injected embryos showed a range of morphologies from normal to irregularities in lens shape (Fig. 3B). Typical irregularities were indentations in the equatorial regions of the lens (Fig. 3B arrows) and persistence of fiber cell nuclei. Three of the six AQP0a MO injected embryos examined showed some lens irregularity.
Fig. 3.
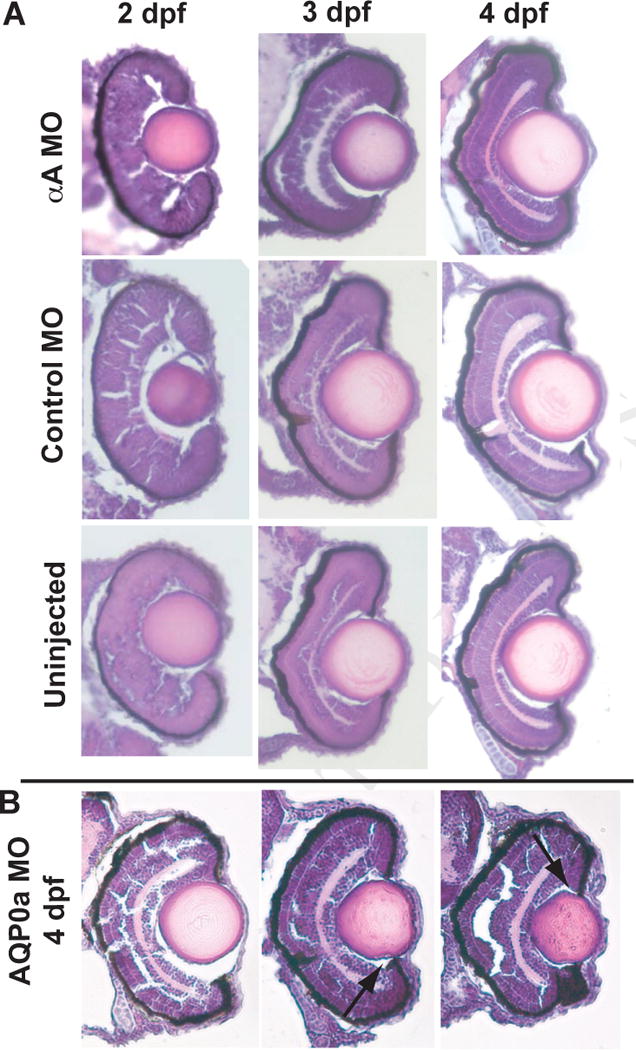
Knockdown of αA-crystallin produced no noticeable histological effects on lens development. A) Embryos on days two, three and four post fertilization were fixed, cryosectioned and stained with hematoxylin and eosin. These sections indicate no alterations in lens or retinal anatomy. Images are representative of at least three embryos examined for each timepoint, with none showing irregularities in lens development. B) Knockdown of AQP0a, which was already known to produce lens cataract by 3 dpf, produced a range of phenotypes including normal lenses and those with indentation (arrows) and persistent fiber cell nuclei. Three of six examined embryos showed some irregularities in lens development.
A previous study of the zebrafish cloche mutant, which contains reduced levels of αA-crystallin, observed retention of fiber cell nuclei due to halted fiber cell differentiation (Goishi et al., 2006). We used DAPI staining to determine if inhibition of αA-crystallin translation would replicate this phenotype. By 48 hours post fertilization (hpf) lenses from embryos in all treatments except the control MO injections showed a defined ring of epithelial cell nuclei (Fig. 4, thin arrows). Lenses of uninjected embryos had fiber cell nuclei restricted to a proliferating zone by 72 hpf, similar to that seen by Greiling et al. (2010) (Fig. 4, thick arrows). Alpha A-crystallin MO injected embryos reached a similar stage by 77 hpf, indicating that fiber cell denucleation was complete within the window of αA-crystallin knockdown (Fig. 1). All control MO injected embryos examined exhibited delayed fiber cell denucleation, with nuclei extending towards the cornea around the lens nucleus at 72 hpf (Fig. 4, asterisk). This result is likely due to a slight developmental delay caused by background toxicity of the control MO, which has no specific target in the zebrafish transcriptome. Interestingly, the AQP0a MO injected embryos seemed to develop normally at first, with denucleation proceeding similarly to uninjected fishes through 48 hpf, but with an apparent delay after this time. This timing in the delay of fiber cell denucleation differs from that of the control MO, suggesting that it may be due to AQP0a knockdown as opposed to nonspecific toxicity of the MO.
Fig. 4.
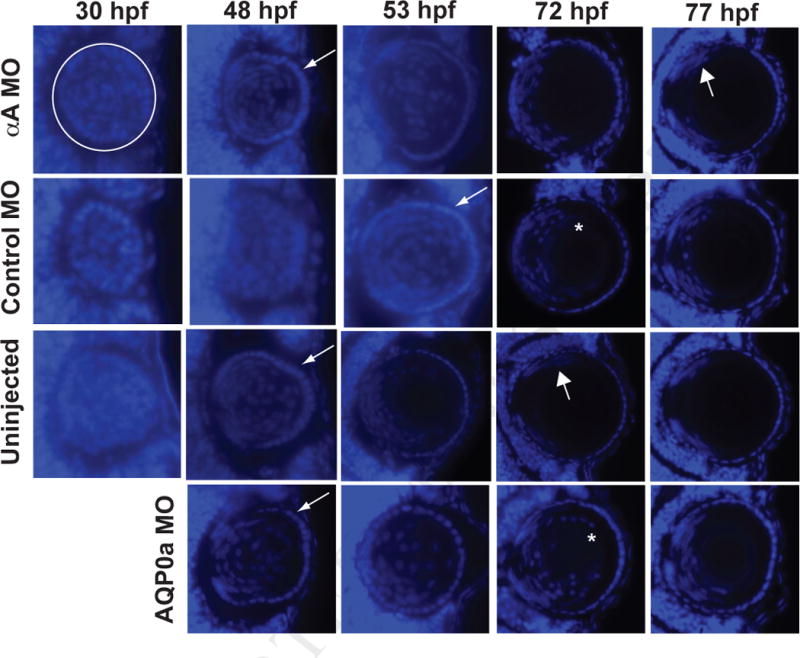
Knockdown of αA-crystallin did not inhibit fiber cell denucleation in comparison to control injected fish. Embryos were injected with either an MO that blocked the translation of αA-crystallin (αA MO), one that does not recognize any zebrafish mRNA sequence (Control MO) or one that blocks translation of a critical water channel (AQP0a MO). Other embryos were left uninjected. Embryos fixed at the specified times were cryosectioned and stained with DAPI to show cell nuclei. White circle in the upper left panel indicates the extent of the lens as a representative example with the cornea to the right. Thin arrows indicate first appearance of a noticeable lens epithelium as fiber cells become denucleated, thick arrows indicate first appearance of fiber cell nuclei restricted to a proliferative zone, and asterisks indicates residual fiber cell nuclei surrounding the lens nucleus. Times shown are hours post fertilization (hpf). At least three embryos were examined for all timepoints, except for the 77 hour timepoint, for which 2 embryos were examined.
3.3. Confocal microscopy visualizes cataract in aquaporin deficient embryos but shows no cataract in αA-crystallin knockdown embryos
Confocal microscopy was used to detect light scattering in the lens and to produce three-dimensional visualizations of any lens cataract resulting from MO injections. None of the nine embryos examined after αA-crystallin MO injection produced detectable light scattering in the zebrafish lens (Fig. 5; Supp. Movie 1). Embryos were also injected with a control MO and an MO against the water channel AQP0a that is already known to induce cataract (Froger et al., 2010). At least seven embryos were examined for each condition. Approximately 60% of AQP0a MO injected embryos displayed cataract, which appeared as islands of light scattering within the lens (Fig. 5, white arrow). Our results with the AQP0a MO injected embryos not only showed that we were able to detect cataract when present, but that confocal imagery could provide three-dimensional imagery of the position and extent of lens cataract in live zebrafish (Supp. Movie 2).
Figure 5.
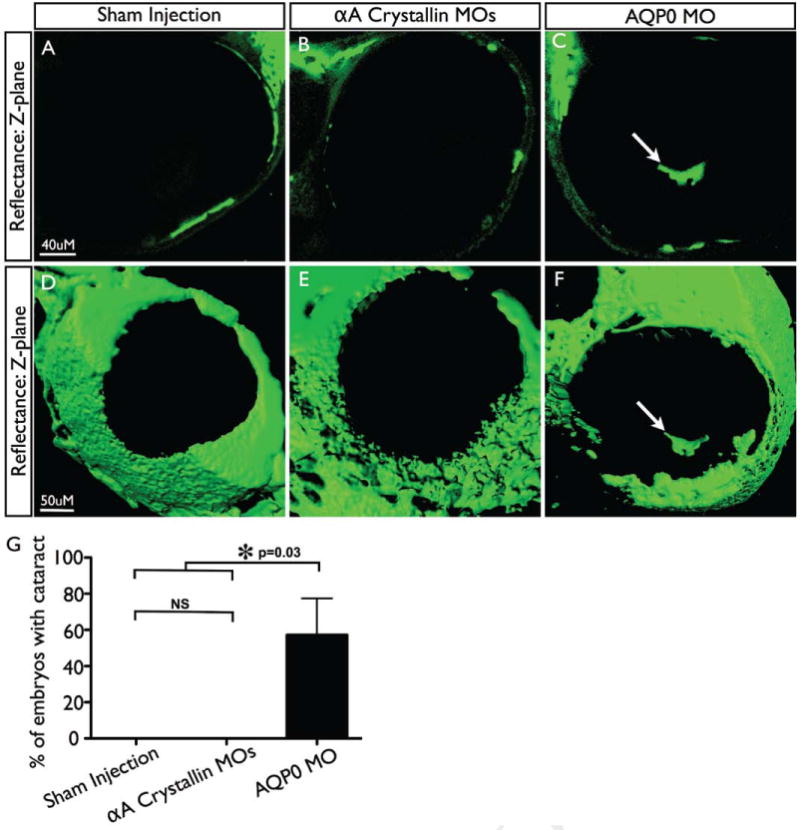
αA crystallin knockdown does not induce cataract formation. Representative z-plane (A-C) and 3D projections (D-F) of reflectance confocal image series taken of embryos injected with buffer only (A,D), αA crystallin MOs (B,E) or AQP0a MO (Positive control) (C,F). Arrows indicate cataracts, seen only in AQP0a injected MOs. (G) Percentage of embryos with cataracts (only one eye imaged per embryo), n≥7 for each condition. Error bars indicate standard deviation; two-tailed t-test used to measure statistical significance.
4. Discussion
Our data indicate that a significant reduction in αA-crystallin has no detectable effect on zebrafish lens development through 4 dpf. This result differs from past studies suggesting that loss of αA-crystallin in the fish lens can lead to γ-crystallin aggregation, inhibition of fiber cell differentiation, cataract (Goishi et al., 2006) and lens degeneration (Strickler et al., 2007) during this same developmental period. However, our results concur with analysis of the αA-crystallin knockout mouse (Brady et al., 1997), which showed no disruption in fiber cell differentiation and exhibited cataract only after seven weeks, far later than the comparable zebrafish developmental period investigated in this study. Conservation of αA-crystallin function in early lens development fits well with the observed similarities between mammal and zebrafish lens crystallin content (Posner et al., 2008; Greiling et al., 2009; Wages et al., 2013) and lens development (Greiling and Clark, 2009), further supporting the use of the zebrafish as a model species for studying α-crystallin function. The ability to manipulate protein expression in this model species along with novel techniques for genome editing promise to expand the use of the zebrafish for studies of lens function and cataract. Our confocal microscopy of AQP0a MO injected embryos also shows for the first time that the extent of cataract can be visualized in a live zebrafish.
The lack of a detectable lens phenotype in αA-crystallin deficient embryos suggests that lens abnormalities in the cloche mutant (Goishi et al., 2006) and blind cavefish (Strickler et al., 2007) result from factors other than lack of αA-crystallin. Goishi et al.'s (2006) finding that overexpression of αA-crystallin rescued the delayed fiber cell differentiation phenotype in the cloche mutant does seem to support a causal relationship between low αA-crystallin levels and altered lens development. However, it is also possible that the cloche mutant lens phenotype is induced by other genetic factors in this fish, and that phenotype rescue results from an abundance of an introduced stress protein. The lens degeneration phenotype observed in the blind cavefish (Strickler et al., 2007) is more extreme than that of the cloche mutant and is not seen in our αA-crystallin knockdown, supporting a hypothesis proposed by the authors that downregulation of αA-crystallin in the blind cavefish does not initiate lens degeneration but may contribute to sustained apoptosis following another induction mechanism. It is also possible that downregulation of αA-crystallin in the blind cavefish results from, but does not play a role in sustaining, lens degeneration.
While the roles that αA-crystallin may play in lens development are not well resolved, its interactions with the cytoskeleton and ability to regulate apoptosis suggest that it could regulate fiber cell differentiation. The ability of α-crystallin to induce fiber cell differentiation in cell culture provides some experimental evidence for this role (Boyle and Takemoto, 2000).
There are several possible explanations for the lack of an early developmental phenotype after αA-crystallin knockdown in the zebrafish lens. First, αA-crystallin's developmental role could be accomplished with exceptionally small amounts of protein. The MO approach used in this study is not a knockout approach, and while we were able to reduce αA-crystallin to undetectable levels by western analysis, there may still be small amounts remaining. Both the Goishi et al. (2006) and Strickler et al. (2007) studies also identified a reduction in αA-crystallin as opposed to a total loss of this protein, qualitatively similar to levels achieved in our study. It is possible that residual αA-crystallin after MO knockdown is sufficient to avoid phenotypes that would be caused by more efficient suppression of its translation. This possibility could only be excluded after a true αA-crystallin knockout zebrafish is produced. A second possible explanation for our results is that αA-crystallin may play no role in early development and, therefore, its loss would have no effect. A third possibility is that αA-crystallin serves functions that overlap with other proteins, such as αB-crystallin or other small heat shock protein expressed in the zebrafish lens like Hsp27 and MKBP (Elicker and Hutson, 2007; Marvin et al., 2008). Interestingly, knockdown of Hsp27, which is constitutively expressed in zebrafish cardiac and skeletal muscle, also produces no developmental abnormalities (Tucker et al., 2009). Redundancy between α-crystallins was suggested as an explanation for the lack of significant phenotypes in the individual knockout of mouse αA- and αB-crystallin (Boyle et al., 2003). Of the two zebrafish αB-crystallins only one, the ubiquitously expressed αBb-crystallin, is found prior to 5 dpf. Alpha Bb-crystallin mRNA is present by 2 dpf (Elicker and Hutson, 2007) and its protein product could replace the normal functions of αA-crystallin. Future experiments that overexpress native and altered versions of αA-crystallin and knockdown αB-crystallins could allow us to distinguish between these three possible explanations.
The amount of α-crystallin required for normal lens function is an interesting question in light of the lower levels found in the fish lens compared to mammals (Posner et al., 2008; Wages et al., 2013). While αA-crystallin is observable by two-dimensional gel electrophoresis by 4.5 dpf, αBb-crystallin is not apparent until between 6 weeks and 4 months (Wages et al., 2013). If αBb-crystallin replaces the functional role of missing αA-crystallin, the levels needed must be very low. The knockout of αA-crystallin in the mouse interestingly led to reduced lens and eye size (Brady et al., 1997), which we did not find in the knockdown zebrafish. However, Brady et al. (1997) only measured eye and lens size in adult mice while we analyzed embryos. Bhat (2004) has suggested that αA-crystallin may play a critical role in the initial development of lens transparency through some yet to be identified functions. Our data do not directly address this hypothesis due to the short time window of protein translation inhibition produced by our morpholinos. While we can conclude that suppression of αA-crystallin expression does not interfere with lens fiber cell differentiation or transparency through 4 dpf, we were not able to assess possible longer term effects of αA-crystallin reduction as seen in the knockout mouse.
This is the first study to use antisense morpholino oligomers to directly assess the function of a lens crystallin. While our results are not able to distinguish between the possibilities that αA-crystallin serves no function in early lens development, a function that does not manifest abnormalities when lacking until later in development, or a redundant function with another lens protein, our data do suggest that loss of αA-crystallin alone does not inhibit fiber cell differentiation or cause cataract in the early stages of zebrafish lens development. On the contrary, the zebrafish lens appears similar to that of the mouse, which undergoes normal fiber cell differentiation when lacking αA-crystallin. The recent development of methods for in vivo editing of the zebrafish genome, such as TALENS and the CRISPR-Cas system, will enhance the ability to manipulate lens crystallin expression and test hypotheses of crystallin function beyond early developmental stages (Chang et al., 2013; Hwang et al., 2013). We recently used evolutionary thermal adaptation of six fish αA-crystallins to identify specific amino acid changes that enhance anti-aggregation activity (Posner et al., 2012). Genome editing technologies and the confocal imaging of cataract demonstrated in this study will allow us to test the protective abilities of these and newly discovered modifications within the complex setting of a live lens.
Supplementary Material
Highlights.
Morpholinos can reduce αA-crystallin levels in the embryonic zebrafish lens
Loss of αA-crystallin does not alter early zebrafish lens development
Confocal microscopy can produce 3D images of zebrafish lens cataract
The role of αA-crystallin in lens development may be conserved in vertebrates
Our results are relevant to researchers using the zebrafish to study lens function
Acknowledgments
This work was funded by an AREA grant from the National Institutes of Health/National Eye Institute to M.P. (R15 EY13535). Two undergraduate student authors were provided summer room and board by Ashland University in support of this study. This study was initiated during a faculty sabbatical by M.P. supported by Ashland University and conducted in the Department of Biology at Case Western Reserve University. The AQP0a morpholino used in this study was provided by Daniel M. Clemens, UC Irvine and the crystallin antibodies were produced and provided by Thomas Vihtelic while at the University of Notre Dame. Neeley Meyers contributed to early experimental design for this study while an undergraduate student at Ashland University.
Footnotes
Appendix. Supplementary data.
Supplemental Movie 1. Three-dimensional confocal imagery of a representative zebrafish lens after inhibition of αA-crystallin translation showing lack of opacity.
Supplemental Movie 2. Three-dimensional confocal imagery of a representative zebrafish lens after inhibition of AQP0a translation showing lens opacity.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andley UP. Effects of alpha-crystallin on lens cell function and cataract pathology. Curr Mol Med. 2009;9:887–892. doi: 10.2174/156652409789105598. [DOI] [PubMed] [Google Scholar]
- Bhat SP. Transparency and non-refractive functions of crystallins--a proposal. Exp Eye Res. 2004;79:809–816. doi: 10.1016/j.exer.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Bhat SP, Nagineni CN. alpha B subunit of lens-specific protein alpha-crystallin is present in other ocular and non-ocular tissues. Biochem Biophys Res Commun. 1989;158:319–325. doi: 10.1016/s0006-291x(89)80215-3. [DOI] [PubMed] [Google Scholar]
- Boyle DL, Takemoto LJ. A possible role for alpha-crystallins in lens epithelial cell differentiation. Mol Vis. 2000;6:63–71. [PubMed] [Google Scholar]
- Boyle DL, Takemoto LJ, Brady JP, Wawrousek EF. Morphological characterization of the Alpha A- and Alpha B-crystallin double knockout mouse lens. BMC Ophthalmology. 2003;3:3. doi: 10.1186/1471-2415-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady JP, Garland D, Duglas-Tabor Y, Robison WG, Groome A, Wawrousek EF. Targeted disruption of the mouse alpha A-crystallin gene induces cataract and cytoplasmic inclusion bodies containing the small heat shock protein alpha B-crystallin. Proc Natl Acad Sci USA. 1997;94:884–889. doi: 10.1073/pnas.94.3.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady JP, Garland DL, Green DE, Tamm ER, Giblin FJ, Wawrousek EF. AlphaB-crystallin in lens development and muscle integrity: a gene knockout approach. Invest Ophthalmol Vis Sci. 2001;42:2924–2934. [PubMed] [Google Scholar]
- Chang N, Sun C, Gao L, Zhu D, Xu X, Zhu X, Xiong JW, Xi JJ. Genome editing with RNA-guided Cas9 nuclease in Zebrafish embryos. Cell Res. 2013;23:465–472. doi: 10.1038/cr.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JI, Muchowski PJ. Small heat-shock proteins and their potential role in human disease. Curr Opin Struct Biol. 2000;10:52–59. doi: 10.1016/s0959-440x(99)00048-2. [DOI] [PubMed] [Google Scholar]
- Dahlman JM, Margot KL, Ding L, Horwitz J, Posner M. Zebrafish alpha-crystallins: protein structure and chaperone-like activity compared to their mammalian orthologs. Mol Vis. 2005;11:88–96. [PubMed] [Google Scholar]
- Deng M, Chen PC, Xie S, Zhao J, Gong L, Liu J, Zhang L, Sun S, Liu J, Ma H, et al. The small heat shock protein alphaA-crystallin is expressed in pancreas and acts as a negative regulator of carcinogenesis. Biochim Biophys Acta. 2010;1802:621–631. doi: 10.1016/j.bbadis.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Dubin RA, Gopal-Srivastava R, Wawrousek EF, Piatigorsky J. Expression of the murine alpha B-crystallin gene in lens and skeletal muscle: identification of a muscle-preferred enhancer. Mol Cell Biol. 1991;11:4340–4349. doi: 10.1128/mcb.11.9.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elicker KS, Hutson LD. Genome-wide analysis and expression profiling of the small heat shock proteins in zebrafish. Gene. 2007;403:60–69. doi: 10.1016/j.gene.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froger A, Clemens D, Kalman K, Németh-Cahalan KL, Schilling TF, Hall JE. Two distinct aquaporin 0s required for development and transparency of the zebrafish lens. Invest Ophthalmol Vis Sci. 2010;51:6582–6592. doi: 10.1167/iovs.10-5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangalum RK, Horwitz J, Kohan SA, Bhat SP. αA-crystallin and αB-crystallin reside in separate subcellular compartments in the developing ocular lens. Journal of Biological Chemistry. 2012;287:42407–42416. doi: 10.1074/jbc.M112.414854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goishi K, Shimizu A, Najarro G, Watanabe S, Rogers R, Zon LI, Klagsbrun M. AlphaA-crystallin expression prevents gamma-crystallin insolubility and cataract formation in the zebrafish cloche mutant lens. Development. 2006;133:2585–2593. doi: 10.1242/dev.02424. [DOI] [PubMed] [Google Scholar]
- Greiling TMS, Clark JI. Early lens development in the zebrafish: a three-dimensional time-lapse analysis. Dev Dyn. 2009;238:2254–2265. doi: 10.1002/dvdy.21997. [DOI] [PubMed] [Google Scholar]
- Greiling TMS, Clark JI. New Insights into the Mechanism of Lens Development Using Zebra Fish. Elsevier Inc.; 2012. [DOI] [PubMed] [Google Scholar]
- Greiling TMS, Aose M, Clark JI. Cell fate and differentiation of the developing ocular lens. Invest Ophthalmol Vis Sci. 2010;51:1540–1546. doi: 10.1167/iovs.09-4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiling TMS, Houck SA, Clark JI. The zebrafish lens proteome during development and aging. Mol Vis. 2009;15:2313–2325. [PMC free article] [PubMed] [Google Scholar]
- Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci USA. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz J. Alpha-crystallin. Exp Eye Res. 2003;76:145–153. doi: 10.1016/s0014-4835(02)00278-6. [DOI] [PubMed] [Google Scholar]
- Hu WF, Gong L, Cao Z, Ma H, Ji W, Deng M, Liu M, Hu XH, Chen P, Yan Q, et al. αA- and αB-Crystallins Interact with Caspase-3 and Bax to Guard Mouse Lens Development. Curr Mol Med. 2012;12:177–187. doi: 10.2174/156652412798889036. [DOI] [PubMed] [Google Scholar]
- Hwang WY, Fu Y, Reyon D, Maeder ML, Tsai SQ, Sander JD, Peterson RT, Yeh JRJ, Joung JK. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotechnol. 2013;31:227–229. doi: 10.1038/nbt.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kase S, Parikh JG, Rao NA. Expression of alpha-crystallin in retinoblastoma. Arch Ophthalmol. 2009;127:187–192. doi: 10.1001/archophthalmol.2008.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Park SY, Park J, Kim YS, Hwang EM, Park JY, Roh GS, Kim HJ, Kang SS, Cho GJ, et al. Reduction of experimental diabetic vascular leakage and pericyte apoptosis in mice by delivery of αA-crystallin with a recombinant adenovirus. Diabetologia. 2012;55:2835–2844. doi: 10.1007/s00125-012-2625-y. [DOI] [PubMed] [Google Scholar]
- Marvin M, O'Rourke D, Kurihara T, Juliano CE, Harrison KL, Hutson LD. Developmental expression patterns of the zebrafish small heat shock proteins. Dev Dyn. 2008;237:454–463. doi: 10.1002/dvdy.21414. [DOI] [PubMed] [Google Scholar]
- Morozov V, Wawrousek EF. Caspase-dependent secondary lens fiber cell disintegration in alphaA-/alphaB-crystallin double-knockout mice. Development. 2006;133:813–821. doi: 10.1242/dev.02262. [DOI] [PubMed] [Google Scholar]
- Ninkovic J, Pinto L, Petricca S, Lepier A, Sun J, Rieger MA, Schroeder T, Cvekl A, Favor J, Götz M. The transcription factor Pax6 regulates survival of dopaminergic olfactory bulb neurons via crystallin αA. Neuron. 2010;68:682–694. doi: 10.1016/j.neuron.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousman SS, Tomooka BH, van Noort JM, Wawrousek EF, O'Connor KC, Hafler DA, Sobel RA, Robinson WH, Steinman L. Protective and therapeutic role for alphaB-crystallin in autoimmune demyelination. Nature. 2007;448:474–479. doi: 10.1038/nature05935. [DOI] [PubMed] [Google Scholar]
- Posner M, Hawke M, Lacava C, Prince CJ, Bellanco NR, Corbin RW. A proteome map of the zebrafish (Danio rerio) lens reveals similarities between zebrafish and mammalian crystallin expression. Mol Vis. 2008;14:806–814. [PMC free article] [PubMed] [Google Scholar]
- Posner M, Kiss AJ, Skiba J, Drossman A, Dolinska MB, Hejtmancik JF, Sergeev YV. Functional Validation of Hydrophobic Adaptation to Physiological Temperature in the Small Heat Shock Protein αA-crystallin. PLoS ONE. 2012;7:e34438. doi: 10.1371/journal.pone.0034438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao NA, Saraswathy S, Pararajasegaram G, Bhat SP. Small Heat Shock Protein αA-Crystallin Prevents Photoreceptor Degeneration in Experimental Autoimmune Uveitis. PLoS ONE. 2012;7:e33582. doi: 10.1371/journal.pone.0033582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao NA, Saraswathy S, Wu GS, Katselis GS, Wawrousek EF, Bhat S. Elevated retina-specific expression of the small heat shock protein, alphaA-crystallin, is associated with photoreceptor protection in experimental uveitis. Invest Ophthalmol Vis Sci. 2008;49:1161–1171. doi: 10.1167/iovs.07-1259. [DOI] [PubMed] [Google Scholar]
- Renkawek K, Stege GJ, Bosman GJ. Dementia, gliosis and expression of the small heat shock proteins hsp27 and alpha B-crystallin in Parkinson's disease. Neuroreport. 1999;10:2273–2276. doi: 10.1097/00001756-199908020-00009. [DOI] [PubMed] [Google Scholar]
- Runkle S, Hill J, Kantorow M, Horwitz J, Posner M. Sequence and spatial expression of zebrafish (Danio rerio) alphaA-crystallin. Mol Vis. 2002;8:45–50. [PMC free article] [PubMed] [Google Scholar]
- Smith AA, Wyatt K, Vacha J, Vihtelic TS, Zigler JS, Wistow GJ, Posner M. Gene duplication and separation of functions in alphaB-crystallin from zebrafish (Danio rerio) Febs J. 2006;273:481–490. doi: 10.1111/j.1742-4658.2005.05080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan AN, Nagineni CN, Bhat SP. alpha A-crystallin is expressed in non-ocular tissues. J Biol Chem. 1992;267:23337–23341. [PubMed] [Google Scholar]
- Stegh AH, Kesari S, Mahoney JE, Jenq HT, Forloney KL, Protopopov A, Louis DN, Chin L, DePinho RA. Bcl2L12-mediated inhibition of effector caspase-3 and caspase-7 via distinct mechanisms in glioblastoma. Proceedings of the National Academy of Sciences. 2008;105:10703–10708. doi: 10.1073/pnas.0712034105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickler AG, Byerly MS, Jeffery WR. Lens gene expression analysis reveals downregulation of the anti-apoptotic chaperone alphaA-crystallin during cavefish eye degeneration. Dev Genes Evol. 2007;217:771–782. doi: 10.1007/s00427-007-0190-z. [DOI] [PubMed] [Google Scholar]
- Tucker NR, Ustyugov A, Bryantsev AL, Konkel ME, Shelden EA. Hsp27 is persistently expressed in zebrafish skeletal and cardiac muscle tissues but dispensable for their morphogenesis. Cell Stress Chaperones. 2009;14:521–533. doi: 10.1007/s12192-009-0105-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicart P, Caron A, Guicheney P, Li Z, Prévost MC, Faure A, Chateau D, Chapon F, Tomé F, Dupret JM, et al. A missense mutation in the alphaB-crystallin chaperone gene causes a desmin-related myopathy. Nat Genet. 1998;20:92–95. doi: 10.1038/1765. [DOI] [PubMed] [Google Scholar]
- Vihtelic TS, Yamamoto Y, Sweeney MT, Jeffery WR, Hyde DR. Arrested differentiation and epithelial cell degeneration in zebrafish lens mutants. Dev Dyn. 2001;222:625–636. doi: 10.1002/dvdy.1217. [DOI] [PubMed] [Google Scholar]
- Wages P, Horwitz J, Ding L, Corbin RW, Posner M. Changes in zebrafish (Danio rerio) lens crystallin content during development. Mol Vis. 2013;19:408–417. [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Cvekl A. Tissue-specific Regulation of the Mouse αA-crystallin Gene in Lens via Recruitment of Pax6 and c-Maf to its Promoter. J Mol Biol. 2005;351:453–469. doi: 10.1016/j.jmb.2005.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Stopka T, Golestaneh N, Wang Y, Wu K, Li A, Chauhan BK, Gao CY, Cveklova K, Duncan MK, et al. Regulation of alphaA-crystallin via Pax6, c-Maf, CREB and a broad domain of lens-specific chromatin. Embo J. 2006;25:2107–2118. doi: 10.1038/sj.emboj.7601114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


