Review of the alternative ways HIV-1 Env proteins can adapt to permit macrophage infection, and the role of macrophages as persistent viral reservoirs.
Keywords: CD4, CCR5, CXCR4, reservoir
Abstract
Myeloid cells residing in the CNS and lymphoid tissues are targets for productive HIV-1 replication, and their infection contributes to the pathological manifestations of HIV-1 infection. The Envs can adopt altered configurations to overcome entry restrictions in macrophages via a more efficient and/or altered mechanism of engagement with cellular receptors. This review highlights evidence supporting an important role for macrophages in HIV-1 pathogenesis and persistence, which need to be considered for strategies aimed at achieving a functional or sterilizing cure. We also highlight that the molecular mechanisms underlying HIV-1 tropism for macrophages are complex, involving enhanced and/or altered interactions with CD4, CCR5, and/or CXCR4, and that the nature of these interactions may depend on the anatomical location of the virus.
Introduction
HIV-1 infection of humans results in progressive destruction of CD4+ T cells, as well as chronic immune activation that causes immune exhaustion and depletion of uninfected immune cells. Together, these factors predispose the infected individual to opportunistic infections and cancers defining AIDS, as well as inflammatory manifestations leading to organ dysfunction [1]. Despite the great successes of cART, treatment is required for life, as virus persists in long-lived, latently infected resting memory CD4+ T cells in blood and lymphoid tissues (reviewed in refs. [2–7]). Long-lived persistent infection can also be established in other cells, including naive T cells, monocyte/macrophages, and astrocytes. Whereas macrophages have a principal role in the neuropathogenesis of HIV-1 and SIV infection [3, 7] and have been shown to be involved in the systemic pathogenesis of SIV [8] and SHIV infection [9, 10], the role for macrophages in the systemic pathogenesis of HIV-1 infection and as a barrier to eradication is less clear. Contributing to this uncertainty is that the virus–cell interactions permitting efficient HIV-1 entry into macrophages in vitro, which define M-tropic phenotypes, are incompletely understood.
The importance of macrophages in HIV-1 infection has been reviewed extensively recently [11]. In this review, we highlight evidence that M-tropic HIV-1 variants contribute to HIV-1 pathogenicity in the CNS and peripheral organs. We also underscore that the tropism of HIV-1 for macrophages is complex and is dependent on several divergent mechanisms that depend on altered interactions between the viral Envs and CD4 and/or either of the HIV-1 coreceptors CCR5 or CXCR4. This review aims to clarify issues in the field regarding the role of M-tropism and M-tropic viral strains in HIV-1 pathogenesis and to highlight the knowns and unknowns about myeloid cells as HIV-1 reservoirs.
MACROPHAGE LINEAGE CELLS AND HIV-1 INFECTION IN VIVO
Wheras it is long established that actively turning-over CD4+ T cells in lymphoid tissues are the principal source of plasma viremia during productive infection [12–14], and that latently infected resting CD4+ memory T cells are a principal reservoir in the blood and lymphoid tissue during suppressive cART [15], the role of myeloid lineage cells in productive or latent infection has been less clear.
Several studies have identified viral DNA in circulating monocytes, albeit at lower levels than in T cells [16, 17], or have described host cell proteins incorporated into virions, suggesting monocyte/macrophage origin [18]. However, monocytes are generally resistant to productive infection in vitro [19], and monocytes remain in circulation for only a short time before entering tissues and differentiating into macrophages. In blood, the inflammatory CD16+ monocytes appear to be preferentially infected compared with classical CD14+ monocytes [20]. However, infected monocytes do participate in viral dissemination into tissues, especially virus transport into the CNS [7, 21, 22].
In contrast to circulating monocytes, tissue macrophages persist for prolonged periods of time, and in vitro, macrophages support robust virus production and are relatively resistant to viral killing. In the absence of cART, tissue macrophages have been clearly identified as a major source of virus production at sites of opportunistic coinfections [23, 24]. Whether macrophages contribute substantially to plasma viremia during typical chronic-phase infection is less certain. Early studies showed that the large, rapid drop in plasma viremia upon initiating cART, ascribed to short-lived CD4+ T cells, was followed by a slower decay phase with a longer half-life, which was thought to be a result of long-lived, infected macrophages and DCs [13]. However, more recent studies have shown a marked decrease or loss of the second-order decay phase following cART that contains the integrase inhibitor raltegravir, which argues against macrophages as a key source of this virus and with mathematical modeling, suggests that T cells containing unintegrated virus may contribute to this second phase of decay [25, 26].
Important insights into the mechanisms regulating macrophage infection have been provided by simian models of infection. In both humans infected with HIV-1 and rhesus macaques infected with SIV, high-level plasma viremia persists even during late-stage disease when virtually all circulating and tissue CD4+ T cells have been lost. With the use of a highly pathogenic SHIV strain, a previous study showed by immunohistochemistry that in infected macaques with end-stage disease, >95% of infected cells were tissue macrophages [9]. More recently, a CD4+ T cell-depleted model of SIV infection revealed the potential for extensive macrophage infection in vivo. Here, the investigators artificially depleted CD4+ T cells in macaques prior to SIVmac infection [8]. Whereas peak viral loads during acute infection were similar in CD4+ T cell-depleted and control animals, there was no postpeak viral-load decline in CD4+ T cell-depleted animals as is seen normally. This phase of high-level viremia was characterized by extensive tissue macrophage infection. Thus, tissue macrophages outside of the CNS have the capacity to serve as sites of high-level virus production, at least under particular circumstances. Analysis of these models suggests that macrophage infection develops in settings where there is a paucity of CD4+ T cells that selects for viruses that can use/infect alternative cellular targets and a permissive immune environment that allows the emergence of M-tropic variants. The highly activated nature and rapid turnover of myeloid cells in this setting may also contribute to permissiveness to infection [27] (reviewed in ref. [28]). First, in the highly pathogenic SHIV infection and the antibody-mediated CD4+ T cell-depletion model, macrophage infection does not occur until after CD4+ T cells are depleted [8, 9, 29]. Second, in the experimentally CD4+ T cell-depleted model, Env-specific antibody-binding titers are reduced dramatically, and Envs, derived from the plasma of these animals, are highly sensitive to neutralization by mAb that are normally non-neutralizing against T cell-tropic Envs and by heterologous SIV+ plasma (but not by autologous plasma from these animals that lack CD4+ T cells) [30]. An additional factor contributing to immune control of macrophage infection may be direct antiviral activity of CD4+ T cells that is uniquely active in suppressing virus in macrophages [31, 32]. Of note, macaque infection with highly M-tropic SHIV in the absence of immune suppression generally does not cause disease and results instead in rapid disappearance of plasma viremia and induction of neutralizing antibodies [33].
Some uncertainty remains about extrapolating the results of SIV infection of nonhuman primates to HIV-1 infection in humans with respect to macrophage infection. High-level replication of SIV strains in macaque myeloid cells may be facilitated by the presence of Vpx that can antagonize cellular restrictions to RT [34, 35], shown recently to be conferred by SAMHD1 [36, 37]. In contrast, HIV-1 does not express Vpx and therefore, cannot overcome the restriction to replication in myeloid cells and resting CD4+ T cells that express SAMHD1 (reviewed in ref. [38]). On the other hand, robust HIV-1 replication occurs in brain macrophages and microglia (discussed further below), indicating that myeloid cells are not refractory to productive infection.
These experimental settings where M-tropic variants dominate, as a result of limited CD4+ T cell targets and an immunologically permissive environment, may mimic the macrophage-dependent and immunologically privileged CNS (discussed further below), as well as late-stage disease following extensive CD4+ T cell depletion.
In contrast to systemic infection, productive infection within the brain is an almost-exclusively myeloid-dependent process. Parenchymal microglia (which are the resident macrophage lineage cells of the brain) are long-lived, relatively fixed cells of the CNS, whereas perivascular macrophages turn over slowly through replenishment by blood monocytes. Both microglia and perivascular macrophages are infected by HIV-1, whereas CD4+ T cells do not contribute substantively to infection in the brain [39–41]. Whereas myeloid cells are also responsible for brain infection in rhesus macaques infected with SIV, the pattern is somewhat different in that infection is largely restricted to perivascular macrophages [42]. Neurological damage in HIV-1-associated neurological disorders results from soluble factors released by infected and activated/uninfected macrophages and microglia, including small molecule excitotoxic transmitters, cytokines and chemokines, and viral proteins [41].
ARE MACROPHAGE-LINEAGE CELLS A HIV-1 RESERVOIR IN PATIENTS ON cART?
CNS infection and neurological complications can persist despite effective cART, but it remains unclear if this is a direct result of viral reservoirs in the CNS or altered immune regulation and persistent immune activation. Given the paucity of studies of tissue from the CNS from patients on suppressive cART, this question has been difficult to answer. Interestingly, only a minority of patients on cART has persistent, detectable HIV-1 RNA in CSF [43], and those with detectable HIV-1 RNA in CSF were more likely to be taking a regimen of cART with poorer CNS penetration [43]. These data raise the possibility that with an effective ART regimen with optimal CNS penetration, persistence of HIV-1 in brain may be rare.
HIV-1 infection of macrophages has been demonstrated in liver within Kupffer cells, in lung within alveolar macrophages, and in splenic macrophages [44–47]. However, there are no studies that have systematically examined virus persistence in macrophages at these sites in patients on cART. The use of stringent controls to ensure that there is no T cell contamination of the clinical samples is critical when detecting low levels of infection by PCR, and there are now elegant approaches to do this by quantifying TCR mRNA in the sorted cell populations [16, 48, 49]. Although early studies demonstrated HIV-1 DNA and infectious virus in circulating monocytes [17, 50], more recent work has challenged whether circulating monocytes are truly a persistent reservoir in patients on cART [48].
Improved cART regimens that can effectively control SIV replication now allow for some of these invasive tissue studies to be performed in macaque models [51, 52]. Following effective control of RT-SHIV with cART, there was significant residual HIV-1 DNA and RNA in lymphoid tissue from LN, spleen, and the GI tract [51]; however, specific sorting of T cells and macrophages was not performed in this study. Similarly, the role of the CNS as a reservoir on cART can now be evaluated systematically using unique neuropathic strains of SIV [53].
HIV-1 ENTRY PATHWAYS
The HIV-1 Env is organized into trimers on virions and consists of the gp120 surface and gp41 transmembrane subunits. HIV-1 attachment to cells involves the interaction of the gp120 Env glycoproteins with cellular CD4 and a secondary coreceptor, which is usually one of the chemokine receptors CCR5 or CXCR4 (reviewed in ref. [1]). HIV-1 may also incorporate cellular adhesion molecules and/or interact with such molecules on the cell surface to maximize the efficiency of receptor engagement (reviewed in refs. [54, 55]). The initial binding of gp120 to CD4 occurs with high-affinity [56] and triggers a conformational change in gp120 that exposes the coreceptor binding site [57, 58]. Current models of gp120 binding to coreceptor suggest that the gp120 V3 loop tip interacts principally with the ECL2 region, whereas the V3 loop stem and gp120 bridging sheet interact with the coreceptor N-terminus [59–62]. The V3 loop of gp120 is the primary (but not exclusive) determinant of coreceptor specificity [63, 64]. The interaction of CD4-bound gp120 with coreceptor induces further conformational changes to expose a fusion peptide within the N-terminus of the viral gp41 transmembrane Env glycoprotein, which facilitates fusion between the viral and cellular membranes and release of the viral core into the cell.
In addition to CCR5 and CXCR4, certain other chemokine receptors, such as CCR1, CCR2b, CCR3, CCR8, CX3CR1, CXCR6, formylpeptide receptor 1, GPR1, GPR15, apelin receptor, and D6, can act as alternative lentiviral coreceptors and mediate entry of certain HIV-1, HIV-2, and SIV strains into transfected cell lines [65–68]. Whereas alternative coreceptors do not appear to have a broad role in mediating the entry of HIV-1 into primary cells, a recent report described acute HIV-1 infection with a variant that could not use CCR5 or CXCR4 and used only GPR15 efficiently [69], which raises the possibility for a larger potential role than currently recognized. Wheres SIV does not normally use CXCR4, its alternative coreceptor use is typically much more robust and widespread than for HIV-1. Recent studies identified a novel CCR5-null mutation in sooty mangabeys that abrogated CCR5 expression but did not prevent infection and evidence that nonpathogenic SIV infection in this natural host in vivo is mediated through CXCR6, GPR15, and GPR1 [70, 71]. Similarly, earlier studies showed that rcm usually lack functional CCR5 and that CCR2b was commonly used by SIVrcm strains [72]. These reports indicate a role for alternative entry pathways in SIV natural host infection, suggesting a potentially distinct range of target cells in these hosts that may contribute to lack of pathogenesis.
HIV-1 CORECEPTOR SPECIFICITY, M-TROPISM, AND DISEASE PROGRESSION
The coreceptor specificity of HIV-1 strains is a principal determinant of viral transmission and disease progression and is also the central factor determining whether CCR5 antagonists may be appropriate for treatment regimens (reviewed in ref. [1]). Observations that individuals homozygous for the CCR5Δ32 allele are largely protected from HIV-1 infection [73, 74] and biological studies of recently transmitted HIV-1 strains [75, 76] indicate that R5 viruses are transmitted from person to person and are dominant in the early and chronic phases of HIV-1 infection. However, in up to 40–50% of individuals infected with HIV-1 subtype B, progression to late stages of infection is associated with evolution of viral coreceptor specificity, with emergence of viral variants able to use X4 or R5X4 viruses [77, 78]. As CXCR4 is more widely expressed on CD4+ T cell subsets than CCR5, including naive T cells, which may be critical for immune cell homeostasis (reviewed in ref. [1]), and as most R5X4 viruses have preferential CXCR4 use for HIV-1 entry into CD4+ T cells [79, 80], the broadened cell tropism of X4 and R5X4 viruses contributes to more rapid disease progression in subjects who experience broadened coreceptor use. Nonetheless, most individuals progress to late stages of disease, while exclusively harboring R5 viruses [81], including people infected with HIV-1 subtype C, which evolves to use CXCR4 less frequently [82, 83] (reviewed in ref. [84]). Whether disease progression and CD4+ T cell loss in the presence of only R5 strains reflect mainly cumulative damage of ongoing replication or indicate the emergence of variants with increased pathogenicity is an important question. However, recent studies have shown that compared with R5 viruses isolated from subjects with chronic infection, R5 viruses isolated from subjects with AIDS tend to have increased fitness, increased exposure of the CD4 binding domain in gp120, increased ability to use low levels of CD4 and CCR5 for entry, reduced sensitivity to inhibition by CCR5 antagonists, and an increased ability to cause apoptosis of CD4+ T cells (reviewed in ref. [1]). Chronic R5 virus may also exhibit a more flexible recognition of CCR5 compared with transmitted/founder viruses [85, 86]. Importantly, our previous studies and those of others have shown that the tropism of R5 HIV-1 strains for macrophages can be enhanced in pediatric subjects and adults who progress to late stages of infection, while exclusively harboring R5 viruses [87–89]. Together, these studies suggest that late-stage R5 viruses may exhibit conformational alterations in gp120 that augment the interaction with CD4 and/or CCR5 and modulation of M-tropism, which may reflect enhancement of pathogenicity.
THE CORECEPTOR SPECIFICITY OF HIV-1 INFLUENCES BUT DOES NOT DETERMINE CELLULAR TROPISM
The pathways used by HIV-1 for entry influence the viral tropism for CD4+ cell types (reviewed in refs. [81, 90]). M-tropic viruses can infect macrophages and primary CD4+ T cells and usually use CCR5 for entry [91–93], whereas T cell line-tropic viruses can infect primary CD4+ T cells and T cell lines via CXCR4 and typically infect macrophages poorly [94]. Dual-tropic (R5X4) viruses can potentially infect macrophages, primary CD4+ T cells, and T cell lines [95, 96].
There is, however, an important distinction between HIV-1 coreceptor specificity and cellular tropism [1]. Whereas most M-tropic viruses use CCR5 for cellular entry, not all R5 viruses are M-tropic [1, 97, 98]. In fact, recent studies suggest that most R5 viruses isolated from blood and lymphoid tissues that are capable of replicating in CD4+ T cells are non-M-tropic [86, 98–101], (with the principal bottleneck to macrophage infection at the level of HIV-1 entry [102]), although M-tropic R5 viruses can be detected in the blood of some subjects with advanced infection [87–89]. On the other hand, most R5 viruses isolated from the CNS are highly M-tropic [99, 102–109], suggesting that the CNS selects for viral variants that have characteristics favorable for efficient entry into perivascular macrophages and microglia or facilitates their evolution via a unique microenvironment that may involve reduced immune surveillance and low concentrations of anti-HIV-1-neutralizing antibodies.
Adding an even greater level of complexity to the mechanisms underlying M-tropism of HIV-1, a subset of highly M-tropic HIV-1 strains enters macrophages efficiently via CXCR4 [102, 110–113]. Furthermore, the coreceptor preference of R5X4 viruses for macrophages and primary CD4+ T cells can vary dramatically depending on the primary cell type and the viral strain [79, 80, 110, 111, 114–116]. These studies illustrate that the viral determinants underlying M-tropism of HIV-1 are significantly more complex than the coreceptor specificity of the HIV-1 Env glycoproteins per se.
Thus, “coreceptor specificity” and “cell tropism” are inter-related but distinct features of a HIV-1 isolate. Despite a significant body of recent literature describing the complexity of Env–receptor interactions underlying cellular tropism of HIV-1 (reviewed in refs. [1, 80, 90, 98]), the coreceptor specificity of HIV-1, for example, which is inferred from phenotypic tests, such as Trofile [117], and from gp120 V3 loop prediction algorithms, such as Geno-2-Pheno [118], is still frequently but incorrectly used synonymously with cellular tropism. For example, R5 strains are often collectively grouped as M-tropic viruses [1]. The frequent reference to the Trofile assay and genotypic prediction methods as “tropism” tests, rather than coreceptor specificity tests (for example, refs. [119, 120]), reflects a lack of precision in recognizing the distinctions between coreceptor use and target cell tropism. On the other hand, coreceptor specificity per se is relevant to the clinical question, for example, of whether a CCR5 antagonist may be appropriate in a given patient.
M-TROPIC HIV-1 STRAINS DERIVED FROM THE CNS FREQUENTLY EXHIBIT REDUCED DEPENDENCE ON CELL-SURFACE CD4 LEVELS
As non-M-tropic R5 HIV-1 variants enter macrophages inefficiently [102] and as macrophages express lower levels of CD4 on the cell surface compared with CD4+ T cells [121–123], several studies have investigated whether M-tropic R5 viruses have adopted mechanisms to overcome entry restrictions imposed by limiting CD4 levels. Studies using HIV-1 entry assays into cells expressing limiting CD4 levels have shown that M-tropic R5 viruses derived from the CNS frequently exhibit reduced CD4 dependence and have undergone conformational alterations within gp120 that promote the stabilization and/or exposure of the CD4 binding domain [99, 100, 103–109, 124–127]. These alterations are often strain-specific and include changes in the V1, V2, C2, V3, C3, and V4 regions of gp120 (reviewed in ref. [98]). One particular amino acid variant that occurs frequently in M-tropic R5 viruses derived from brain is Asn283 within the C2 region of gp120 [104]. Here, biophysical analysis showed that Asn283 reduces CD4 dependence and permits efficient macrophage entry via the formation of an additional hydrogen bond between gp120 and Gln40 of CD4, which increases gp120-CD4 affinity by decreasing the gp120-CD4 dissociation rate. Studies of single-molecule bond-force spectroscopy and receptor mobility in living cells have demonstrated that gp120-CD4 binding is relatively short-lived and weak compared with gp120-CD4 complex binding to CCR5 and that CCR5 is more mobile in the cell membrane than CD4 [128, 129]. Therefore, enhanced CD4 binding may not only increase the capacity of gp120 to scavenge low levels of CD4 on the macrophage surface, but also, it could potentially permit the Env-CD4 complex to more readily colocalize with CCR5, thus indirectly increasing the efficiency of CCR5 use. Whereas there is abundant evidence for the former possibility [98], the latter possibility is supported by some studies [87, 102, 130, 131] but not others [100] that have demonstrated reduced CCR5 dependence by certain M-tropic viruses derived from brain and blood.
As noted above, immune pressures are believed to be one important factor that normally restrains the emergence of M-tropic variants capable of using CCR5 in the presence of exceedingly low CD4 levels. It is long recognized that the requirement for CD4 binding prior to CCR5 engagement renders Env more resistant to neutralization, in part, by occluding conserved structures involved in CCR5 interactions [132–134] and, in part, by maintaining the Env protein in a more structurally constrained state that is less likely to assume a conformation that will engage CCR5 or be structurally altered and neutralized by antibodies [135]. In SIV-infected rhesus macaques, antibodies develop that do not normally neutralize virus but can neutralize if virus is first pretreated with soluble CD4. In CD4+ T cell-depleted macaques infected with SIV, in which extensive macrophage infection develops, variants emerge that are capable of using CCR5 with little or no CD4, and these variants are highly sensitive to neutralization by plasma from normal SIV-infected macaques even in the absence of soluble CD4 triggering [30]. The CD4+ T cell-depleted animals do not produce these antibodies, however, which likely enables emergence of these variants that do not require high levels of CD4 prior to CCR5 binding.
M-TROPIC HIV-1 STRAINS DERIVED FROM BLOOD CAN EXHIBIT REDUCED CCR5 DEPENDENCE
In addition to a possible indirect enhancement of CCR5 use that may be secondary to increased gp120-CD4 affinity, several recent studies of blood-derived M-tropic viruses have shown that these viruses can exhibit an increased interaction between gp120 and CCR5 [110, 131] and that reducing the affinity of gp120 for CCR5 can attenuate CCR5-mediated M-tropism [136].
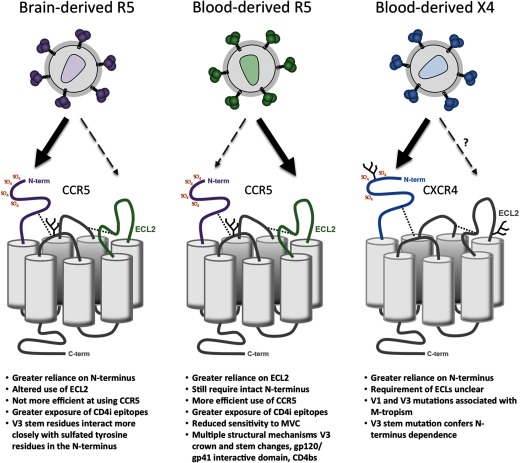
Recent characterization of the properties of a panel of gp120 proteins cloned from primary R5 HIV-1 isolates derived from blood showed correlations between their infectivity levels for macrophages and their ability to enter cells expressing very low levels of CCR5 [131]. Here, the M-tropic gp120s had greater exposure of CD4-induced epitopes that overlap the coreceptor binding domain, suggesting greater CCR5 binding-site exposure [131]; were less sensitive to inhibition by the CCR5 antagonist MVC, suggesting a more efficient interaction with CCR5 [131]; and exhibited reduced dependence on elements within the CCR5 N-terminus and increased dependence on elements within the CCR5 ECL2 region, suggesting an altered mechanism of gp120–CCR5 engagement [110, 131] (see Fig. 1). Thus, the results from these studies suggest that an enhanced and altered interaction between gp120 and CCR5 favoring the CCR5 ECL2 region may contribute to M-tropism of blood-derived HIV-1 strains. Other studies showed that increasing the expression levels of CCR5 on macrophages could lead to restoration of efficient entry by certain non-M-tropic blood-derived viruses but that CCR5 overexpression has no effect on the entry of highly M-tropic viruses derived from the blood of subjects with advanced disease, suggesting a threshold level of CCR5 that is necessary for efficient macrophage entry of blood-derived viruses [87].
Figure 1. Alternative mechanisms of coreceptor engagement by M-tropic HIV-1 Envs.
The models shown, which illustrate potential differences in Env–coreceptor engagement contributing to M-tropism of HIV-1, are inferred from recent studies by Salimi et al. [109], Sterjovski et al. [131], and Cashin et al. [110]. Increased reliance on a particular coreceptor region is shown by thick black arrows. SO4, Sulfate; N-term, N-terminus; CD4i, CD4-inducible; CD4bs, CD4 binding site.
The relationship between the efficiency of gp120–CCR5 engagement and magnitude of M-tropism of blood-derived viruses can also be inferred from recent studies of HIV-1 resistance to the CCR5 antagonist MVC [136]. Here, studies of a MVC-resistant strain of HIV-1 that was generated in vitro showed that use of MVC-occupied CCR5 by this virus was less efficient than that of unoccupied CCR5 but that the ability to interact with CD4 was unaffected by MVC. Whereas the MVC-resistant variant could efficiently enter PBMC in the presence of MVC, its M-tropic properties were abolished in the presence of MVC as a result of a less-efficient interaction with CCR5. Similar findings have been observed using primary MVC-resistant viruses isolated from plasma of subjects who developed resistance during Phase III clinical trials of MVC [137, 138]. Consistent with these observations, recent studies of HIV-1 resistance to another CCR5 antagonist—APL—demonstrated tropism alterations for CD4+ T cell subsets by an APL-resistant HIV-1 strain as a result of a less-efficient interaction with CCR5, characterized by a tropism shift toward effector memory T cells and relative sparing of central memory T cells [139].
Together, these studies indicate that a reduction in the efficiency of the interaction between gp120 and CCR5 can attenuate HIV-1 entry of blood-derived HIV-1 strains into primary CD4+ cells that have limiting CCR5 levels, including macrophages. Whereas reduced CD4 dependence appears to be the dominant pathway to efficient macrophage entry by brain-derived viruses, M-tropic viruses isolated from blood appear to have distinctive gp120 conformations that alter CCR5 engagement, in addition to altered interactions with CD4, to enter macrophages efficiently.
ALTERED MECHANISMS OF Env–CCR5 ENGAGEMENT CONTRIBUTE TO M-TROPISM OF BRAIN- AND BLOOD-DERIVED HIV-1 STRAINS
Although there is an abundance of evidence to suggest that reduced CD4 dependence is the major pathway to efficient macrophage entry by CNS-derived M-tropic HIV-1 strains, altered gp120–CCR5 interactions have been noted in some [130] but not all studies of brain-derived R5 HIV-1 Envs [100]. A recent study showed that highly M-tropic HIV-1 Envs originating from brain-derived isolates exhibit an altered mechanism of gp120–CCR5 engagement that occurs in tandem with a highly efficient interaction with CD4 [109]. This altered mechanism of gp120–CCR5 engagement was associated with increased exposure of CD4-induced epitopes and could be characterized by an increased dependence of gp120 on the CCR5 N-terminus and an altered recognition of the CCR5 ECL1 and ECL2 regions (see Fig. 1) [109]. Interestingly, quantitative HIV-1 receptor profiling using the 293-Affinofile system [140, 141] showed that this altered mechanism of gp120 engagement with CCR5 was not more efficient than that of corresponding LN-derived R5 Envs that were non-M-tropic and that did not exhibit increased dependence on the CCR5 N-terminus. These phenotypes of M-tropic R5 Envs derived from the brain, namely the increased dependence on the CCR5 N-terminus and lack of increased CCR5 affinity, contrast sharply with the phenotypes of M-tropic R5 Envs derived from blood that are characterized by reduced dependence on the CCR5 N-terminus and a more efficient interaction with CCR5 (see Fig. 1) [110, 131]. Together, these studies suggest a dichotomous mechanism of altered gp120–CCR5 engagement by M-tropic Envs, depending on whether they were isolated from brain or from blood—the former involving increased dependence on the CCR5 N-terminus that does not increase the efficiency of the Env–CCR5 interaction and the latter involving reduced dependence on the CCR5 N-terminus that increases Env–CCR5 affinity. Thus, the R5 M-tropic phenotype, or “color”, as termed by Swanstrom and colleagues [98], appears to be complex and may be comprised of at least two distinct M-tropic subphenotypes.
EVIDENCE THAT TISSUE MACROPHAGES MAY HARBOR M-TROPIC X4
As detailed in the preceding sections, not only is HIV-1 M-tropism limited to just a subset of R5 HIV-1 isolates that have unique phenotypic characteristics, but also, certain X4 and R5X4 HIV-1 strains can enter and replicate in macrophages very efficiently via CXCR4 [80, 96, 102, 110, 111, 113, 116, 142–144], adding a further layer of complexity to the gp120–receptor interactions that govern HIV-1 M-tropism. The fact that subtype B HIV-1 strains, harbored by 40–50% of infected subjects, are CXCR4-using at late stages of infection [77, 78], when CD4+ T cells are often ostensibly depleted, despite the maintenance of high plasma HIV-1 RNA levels, suggests that nonlymphocytic cells are likely to be the major factories of CXCR4-using HIV-1 during immunodeficiency. Indeed, studies of SHIV-infected macaques have shown that tissue macrophages residing in LN, spleen, GI tract, liver, and kidney sustain persistently high plasma viral loads of X4 when virtually all CD4+ T cells have been depleted from the body [9], and the strictly T cell tropic viral inoculum used for these studies (SHIVDH12R) underwent adaptive alterations in gp120 in vivo to facilitate efficient CXCR4-mediated macrophage infection ex vivo [10]. Further studies showed that X4 M-tropic HIV-1 viruses are more likely to arise in infected subjects with low CD4+ T cell counts, providing further evidence that macrophages may be targeted by X4 and contribute to HIV-1 pathogenesis in humans [142].
AN ALTERED MECHANISM OF Env–CXCR4 ENGAGEMENT CONTRIBUTES TO M-TROPISM OF X4 HIV-1 STRAINS
Several studies have been undertaken to better understand the nature of the interactions between gp120 and CXCR4 that permit efficient CXCR4-mediated HIV-1 entry and replication in macrophages. Although the CXCR4 N-terminus and ECL2 regions have been shown to be important for recognition of X4 HIV-1 viruses [62, 145], the coreceptor requirements for efficient CXCR4-mediated HIV-1 entry into macrophages are unclear. However, recent studies showed that efficient CXCR4-mediated macrophage entry of primary blood- and lymphoid tissue-derived viruses was associated with increased dependence on the CXCR4 N-terminus (Fig. 1) [110], which involved the presence of Ile326 within the V3 loop [110, 142]. These results illustrate a mechanism of gp120–coreceptor engagement by blood/lymphoid tissue-derived M-tropic X4 viruses that is related to that which occurs between gp120 and CCR5 of brain-derived M-tropic R5 viruses (i.e., increased dependence on the CCR5 N-terminus) [109] but that differs sharply to the mechanism of gp120–CCR5 engagement displayed by blood-derived M-tropic R5 viruses (i.e., reduced dependence on the CCR5 N-terminus) [110, 131]. These findings further illustrate the complex and tissue-specific nature of the alternative gp120–coreceptor interactions that contribute to efficient macrophage entry of HIV-1 via CXCR4 or CCR5.
EFFECT OF STEM-CELL TRANSPLANTATION ON INFECTED MONOCYTE/MACROPHAGES: IMPLICATIONS FOR HIV-1 CURE
The recent reports of HIV-1 cure following stem-cell transplantation with a CCR5Δ32 homozygous donor (the Berlin patient [146]) and with a CCR5 WT donor (the Boston patients [147]) raise some new and important insights regarding the turnover of monocyte/macrophages and their role as a long-term reservoir on cART. Macrophages are largely resistant to radiation and chemotherapy; however, in the Berlin patient, macrophages from liver, brain, and GI tract were all of donor origin, i.e., CCR5Δ32 homozygous within 12, 17, and 24 months, respectively, post-transplant [148]. These data demonstrate that long-lived tissue macrophages do indeed turn over and can be replaced following transplantation and therefore, that this reservoir too was eliminated post-transplantation.
The situation in the Boston patients is somewhat different, as the donor cells expressed WT CCR5 but were “protected” from infection as the transplant was performed in the presence of continuous cART [147]. The Boston patients have only recently stopped antiviral therapy and showed no evidence of viral rebound in the periphery [149], but further studies of viral load in multiple tissue sites and cell subsets, including monocyte/macrophages, are awaited. Similar to the Berlin patient, it is possible that there has been replacement of tissue macrophages with donor cells.
CONCLUDING REMARKS
This review describes the role of tissue macrophages in HIV-1 pathogenesis and persistence, both within and outside of the CNS, and provides a foundation for understanding the complex and divergent mechanisms that HIV-1 can exploit to enter macrophages efficiently, which may depend on the tissue of origin of the virus. The role of macrophages in HIV-1 persistence on cART remains controversial, and further work is required to understand their contribution to the viral reservoir, mechanisms of persistence in the presence of cART, and response to interventions currently being developed to purge virus from latently infected T cells.
ACKNOWLEDGMENTS
P.R.G. is the recipient of an Australian Research Council Future Fellowship (FT2) and is supported by grants from the Australian National Health and Medical Research Council (#1006534 and #1022066) and funds from the Victorian Operational Infrastructure Support Program received by the Burnet Institute. R.G.C. was supported by U.S. National Institutes of Health grants MH061139 and AI091516 and received assistance from the Penn Center for AIDS Research (CFAR; AI045008). N.F. was supported by U.S. National Institutes of Health Grant T32-AI007632.
We thank Jasminka Sterjovski for assistance with preparing the figure.
Footnotes
- APL
- aplaviroc
- ART
- antiretroviral therapy
- cART
- combined antiretroviral therapy
- ECL1/2
- first/second coreceptor extracellular loop
- Env
- HIV-1 envelope glycoprotein
- GI
- gastrointestinal
- GPR
- GPCR
- M-tropic
- macrophage tropic
- MVC
- maraviroc
- R5 virus
- CCR5-using virus
- R5X4 virus
- virus that can use CCR5 and CXCR4
- rcm
- red-capped mangabeys
- SAMHD1
- sterile α motif domain and HD domain-containing protein 1
- SHIV
- chimeric human SIV
- X4 virus
- CXCR4-using virus
AUTHORSHIP
P.R.G., N.F., S.R.L., and R.G.C. wrote the manuscript and have read and approved the final version.
DISCLOSURES
The authors have no competing interests to declare.
REFERENCES
- 1. Gorry P. R., Ancuta P. (2011) Coreceptors and HIV-1 pathogenesis. Curr. HIV/AIDS Rep. 8, 45–53 [DOI] [PubMed] [Google Scholar]
- 2. Churchill M. J., Wesselingh S. L., Cowley D., Pardo C. A., McArthur J. C., Brew B. J., Gorry P. R. (2009) Extensive astrocyte infection is prominent in human immunodeficiency virus-associated dementia. Ann. Neurol. 66, 253–258 [DOI] [PubMed] [Google Scholar]
- 3. Gonzalez-Scarano F., Martin-Garcia J. (2005) The neuropathogenesis of AIDS. Nat. Rev. Immunol. 5, 69–81 [DOI] [PubMed] [Google Scholar]
- 4. Palmer S., Josefsson L., Coffin J. M. (2011) HIV reservoirs and the possibility of a cure for HIV infection. J. Int. Med. 270, 550–560 [DOI] [PubMed] [Google Scholar]
- 5. Trono D., Van Lint C., Rouzioux C., Verdin E., Barre-Sinoussi F., Chun T. W., Chomont N. (2010) HIV persistence and the prospect of long-term drug-free remissions for HIV-infected individuals. Science 329, 174–180 [DOI] [PubMed] [Google Scholar]
- 6. Crowe S. M. (2006) Macrophages and residual HIV infection. Curr. Opin. HIV AIDS 1, 129–133 [DOI] [PubMed] [Google Scholar]
- 7. Dunfee R., Thomas E., Gorry P. R., Wang J., Ancuta P., Gabuzda D. (2006) Mechanisms of HIV-1 neurotropism. Curr. HIV Res. 4, 267–278 [DOI] [PubMed] [Google Scholar]
- 8. Ortiz A. M., Klatt N. R., Li B., Yi Y., Tabb B., Hao X. P., Sternberg L., Lawson B., Carnathan P. M., Cramer E. M., Engram J. C., Little D. M., Ryzhova E., Gonzalez-Scarano F., Paiardini M., Ansari A. A., Ratcliffe S., Else J. G., Brenchley J. M., Collman R. G., Estes J. D., Derdeyn C. A., Silvestri G. (2011) Depletion of CD4(+) T cells abrogates post-peak decline of viremia in SIV-infected rhesus macaques. J. Clin. Invest. 121, 4433–4445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Igarashi T., Brown C. R., Endo Y., Buckler-White A., Plishka R., Bischofberger N., Hirsch V., Martin M. A. (2001) Macrophage are the principal reservoir and sustain high virus loads in rhesus macaques after the depletion of CD4+ T cells by a highly pathogenic simian immunodeficiency virus/HIV type 1 chimera (SHIV): implications for HIV-1 infections of humans. Proc. Natl. Acad Sci. USA 98, 658–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Igarashi T., Imamichi H., Brown C. R., Hirsch V. M., Martin M. A. (2003) The emergence and characterization of macrophage-tropic SIV/HIV chimeric viruses (SHIVs) present in CD4+ T cell-depleted rhesus monkeys. J. Leukoc. Biol. 74, 772–780 [DOI] [PubMed] [Google Scholar]
- 11. Koppensteiner H., Brack-Werner R., Schindler M. (2012) Macrophages and their relevance in human immunodeficiency virus type I infection. Retrovirology 9, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ho D. D., Neumann A. U., Perelson A. S., Chen W., Leonard J. M., Markowitz M. (1995) Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 373, 123–126 [DOI] [PubMed] [Google Scholar]
- 13. Perelson A. S., Neumann A. U., Markowitz M., Leonard J. M., Ho D. D. (1996) HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science 271, 1582–1586 [DOI] [PubMed] [Google Scholar]
- 14. Wei X., Ghosh S. K., Taylor M. E., Johnson V. A., Emini E. A., Deutsch P., Lifson J. D., Bonhoeffer S., Nowak M. A., Hahn B. H., et al. (1995) Viral dynamics in human immunodeficiency virus type 1 infection. Nature 373, 117–122 [DOI] [PubMed] [Google Scholar]
- 15. Finzi D., Hermankova M., Pierson T., Carruth L. M., Buck C., Chaisson R. E., Quinn T. C., Chadwick K., Margolick J., Brookmeyer R., Gallant J., Markowitz M., Ho D. D., Richman D. D., Siliciano R. F. (1997) Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278, 1295–1300 [DOI] [PubMed] [Google Scholar]
- 16. Sonza S., Mutimer H. P., Oelrichs R., Jardine D., Harvey K., Dunne A., Purcell D. F., Birch C., Crowe S. M. (2001) Monocytes harbour replication-competent, non-latent HIV-1 in patients on highly active antiretroviral therapy. AIDS 15, 17–22 [DOI] [PubMed] [Google Scholar]
- 17. Zhu T., Muthui D., Holte S., Nickle D., Feng F., Brodie S., Hwangbo Y., Mullins J. I., Corey L. (2002) Evidence for human immunodeficiency virus type 1 replication in vivo in CD14(+) monocytes and its potential role as a source of virus in patients on highly active antiretroviral therapy. J. Virol. 76, 707–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lawn S. D., Roberts B. D., Griffin G. E., Folks T. M., Butera S. T. (2000) Cellular compartments of human immunodeficiency virus type 1 replication in vivo: determination by presence of virion-associated host proteins and impact of opportunistic infection. J. Virol. 74, 139–145 [PMC free article] [PubMed] [Google Scholar]
- 19. Triques K., Stevenson M. (2004) Characterization of restrictions to human immunodeficiency virus type 1 infection of monocytes. J. Virol. 78, 5523–5527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ellery P. J., Tippett E., Chiu Y. L., Paukovics G., Cameron P. U., Solomon A., Lewin S. R., Gorry P. R., Jaworowski A., Greene W. C., Sonza S., Crowe S. M. (2007) The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J. Immunol. 178, 6581–6589 [DOI] [PubMed] [Google Scholar]
- 21. Buckner C. M., Calderon T. M., Willams D. W., Belbin T. J., Berman J. W. (2011) Characterization of monocyte maturation/differentiation that facilitates their transmigration across the blood-brain barrier and infection by HIV: implications for NeuroAIDS. Cell. Immunol. 267, 109–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nottet H. S., Persidsky Y., Sasseville V. G., Nukuna A. N., Bock P., Zhai Q. H., Sharer L. R., McComb R. D., Swindells S., Soderland C., Gendelman H. E. (1996) Mechanisms for the transendothelial migration of HIV-1-infected monocytes into brain. J. Immunol. 156, 1284–1295 [PubMed] [Google Scholar]
- 23. Lawn S. D., Pisell T. L., Hirsch C. S., Wu M., Butera S. T., Toossi Z. (2001) Anatomically compartmentalized human immunodeficiency virus replication in HLA-DR+ cells and CD14+ macrophages at the site of pleural tuberculosis coinfection. J. Infect. Dis. 184, 1127–1133 [DOI] [PubMed] [Google Scholar]
- 24. Orenstein J. M., Fox C., Wahl S. M. (1997) Macrophages as a source of HIV during opportunistic infections. Science 276, 1857–1861 [DOI] [PubMed] [Google Scholar]
- 25. Murray J. M., Emery S., Kelleher A. D., Law M., Chen J., Hazuda D. J., Nguyen B. Y., Teppler H., Cooper D. A. (2007) Antiretroviral therapy with the integrase inhibitor raltegravir alters decay kinetics of HIV, significantly reducing the second phase. AIDS 21, 2315–2321 [DOI] [PubMed] [Google Scholar]
- 26. Spivak A. M., Rabi S. A., McMahon M. A., Shan L., Sedaghat A. R., Wilke C. O., Siliciano R. F. (2011) Short communication: dynamic constraints on the second phase compartment of HIV-infected cells. AIDS Res. Hum. Retroviruses 27, 759–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hasegawa A., Liu H., Ling B., Borda J. T., Alvarez X., Sugimoto C., Vinet-Oliphant H., Kim W. K., Williams K. C., Ribeiro R. M., Lackner A. A., Veazey R. S., Kuroda M. J. (2009) The level of monocyte turnover predicts disease progression in the macaque model of AIDS. Blood 114, 2917–2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mir K. D., Mavigner M., Silvestri G. (2012) The myeloid cytokine network in AIDS pathogenesis. Cytokine Growth Factor Rev. 23, 223–231 [DOI] [PubMed] [Google Scholar]
- 29. Igarashi T., Brown C. R., Byrum R. A., Nishimura Y., Endo Y., Plishka R. J., Buckler C., Buckler-White A., Miller G., Hirsch V. M., Martin M. A. (2002) Rapid and irreversible CD4+ T-cell depletion induced by the highly pathogenic simian/human immunodeficiency virus SHIV(DH12R) is systemic and synchronous. J. Virol. 76, 379–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Francella N., Gwyn S. E., Yi Y., Li B., Ziao P., Elliott S. T., Oritz A. M., Hoxie J. A., Paiardini M., Silvestri G., Derdeyn C. A., Collman R. G. (2013) CD4+ T cells support production of SIV Env antibodies that enforce CD4-dependent entry and shape tropism in vivo. J. Virol. 87, 9719–9732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sacha J. B., Giraldo-Vela J. P., Buechler M. B., Martins M. A., Maness N. J., Chung C., Wallace L. T., Leon E. J., Friedrich T. C., Wilson N. A., Hiraoka A., Watkins D. I. (2009) Gag- and Nef-specific CD4+ T cells recognize and inhibit SIV replication in infected macrophages early after infection. Proc. Natl. Acad. Sci. USA 106, 9791–9796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vojnov L., Martins M. A., Bean A. T., Veloso de Santana M. G., Sacha J. B., Wilson N. A., Bonaldo M. C., Galler R., Stevenson M., Watkins D. I. (2012) The majority of freshly sorted simian immunodeficiency virus (SIV)-specific CD8(+) T cells cannot suppress viral replication in SIV-infected macrophages. J. Virol. 86, 4682–4687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Igarashi T., Donau O. K., Imamichi H., Nishimura Y., Theodore T. S., Iyengar R., Erb C., Buckler-White A., Buckler C. E., Martin M. A. (2007) Although macrophage-tropic simian/human immunodeficiency viruses can exhibit a range of pathogenic phenotypes, a majority of isolates induce no clinical disease in immunocompetent macaques. J. Virol. 81, 10669–10679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kaushik R., Zhu X., Stranska R., Wu Y., Stevenson M. (2009) A cellular restriction dictates the permissivity of nondividing monocytes/macrophages to lentivirus and gammaretrovirus infection. Cell Host Microb. 6, 68–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sharova N., Wu Y., Zhu X., Stranska R., Kaushik R., Sharkey M., Stevenson M. (2008) Primate lentiviral Vpx commandeers DDB1 to counteract a macrophage restriction. PLoS Pathog. 4, e1000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hrecka K., Hao C., Gierszewska M., Swanson S. K., Kesik-Brodacka M., Srivastava S., Florens L., Washburn M. P., Skowronski J. (2011) Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature 474, 658–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Laguette N., Sobhian B., Casartelli N., Ringeard M., Chable-Bessia C., Segeral E., Yatim A., Emiliani S., Schwartz O., Benkirane M. (2011) SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 474, 654–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Laguette N., Benkirane M. (2012) How SAMHD1 changes our view of viral restriction. Trends Immunol. 33, 26–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cosenza M. A., Zhao M. L., Si Q., Lee S. C. (2002) Human brain parenchymal microglia express CD14 and CD45 and are productively infected by HIV-1 in HIV-1 encephalitis. Brain Pathol. 12, 442–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fischer-Smith T., Croul S., Sverstiuk A. E., Capini C., L'Heureux D., Regulier E. G., Richardson M. W., Amini S., Morgello S., Khalili K., Rappaport J. (2001) CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J. Neurovirol. 7, 528–541 [DOI] [PubMed] [Google Scholar]
- 41. Yadav A., Collman R. G. (2009) CNS inflammation and macrophage/microglial biology associated with HIV-1 infection. J. Neuroimmune Pharmacol. 4, 430–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Williams K. C., Corey S., Westmoreland S. V., Pauley D., Knight H., deBakker C., Alvarez X., Lackner A. A. (2001) Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J. Exp. Med. 193, 905–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cusini A., Vernazza P. L., Yerly S., Decosterd L. A., Ledergerber B., Fux C. A., Rohrbach J., Widmer N., Hirschel B., Gaudenz R., Cavassini M., Klimkait T., Zenger F., Gutmann C., Opravil M., Gunthard H. F. (2013) Higher CNS penetration-effectiveness of long-term combination antiretroviral therapy is associated with better HIV-1 viral suppression in cerebrospinal fluid. J. Acquir. Immune Defic. Syndr. 62, 28–35 [DOI] [PubMed] [Google Scholar]
- 44. Hufert F. T., Schmitz J., Schreiber M., Schmitz H., Racz P., von Laer D. D. (1993) Human Kupffer cells infected with HIV-1 in vivo. J. Acquir. Immune Defic. Syndr. 6, 772–777 [PubMed] [Google Scholar]
- 45. Clarke J. R., Krishnan V., Bennett J., Mitchell D., Jeffries D. J. (1990) Detection of HIV-1 in human lung macrophages using the polymerase chain reaction. AIDS 4, 1133–1136 [DOI] [PubMed] [Google Scholar]
- 46. Lewin S. R., Kirihara J., Sonza S., Irving L., Mills J., Crowe S. M. (1998) HIV-1 DNA and mRNA concentrations are similar in peripheral blood monocytes and alveolar macrophages in HIV-1-infected individuals. AIDS 12, 719–727 [DOI] [PubMed] [Google Scholar]
- 47. Pearce T. E., Nowakowski M., Eden E., Huang Z. B., Steiner P., Shahabuddin M., Potash M. J., Volsky D. J. (1993) Uniform detection of HIV-1 in alveolar macrophages of pediatric but not adult AIDS patients. J. Leukoc. Biol. 53, 722–726 [DOI] [PubMed] [Google Scholar]
- 48. Josefsson L., Eriksson S., Sinclair E., Ho T., Killian M., Epling L., Tan A., Lemey P., Faria N. R., Shao W., Hunt P., Somsouk M., Douek D., Bacchetti P., Loeb L., Custer J., Poole L., Deeks S., Hecht F. M., Palmer S. (2012) Characterization of persistent HIV-1 in a broad spectrum of CD4+ T cells isolated from peripheral blood and gut associated lymphoud tissue from patients on loing-term supressive therapy. THAA0105. Program and Abstracts of the 14th International AIDS Conference, Washington, DC (abs.) [Google Scholar]
- 49. Aleixo L. F., Goodenow M. M., Sleasman J. W. (1995) Molecular analysis of highly enriched populations of T-cell-depleted monocytes. Clin. Diagn. Lab. Immunol. 2, 733–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lambotte O., Taoufik Y., de Goer M. G., Wallon C., Goujard C., Delfraissy J. F. (2000) Detection of infectious HIV in circulating monocytes from patients on prolonged highly active antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 23, 114–119 [DOI] [PubMed] [Google Scholar]
- 51. North T. W., Higgins J., Deere J. D., Hayes T. L., Villalobos A., Adamson L., Shacklett B. L., Schinazi R. F., Luciw P. A. (2010) Viral sanctuaries during highly active antiretroviral therapy in a nonhuman primate model for AIDS. J. Virol. 84, 2913–2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Deere J. D., Schinazi R. F., North T. W. (2011) Simian immunodeficiency virus macaque models of HIV latency. Curr. Opin. HIV AIDS 6, 57–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Clements J. E., Gama L., Graham D. R., Mankowski J. L., Zink M. C. (2011) A simian immunodeficiency virus macaque model of highly active antiretroviral treatment: viral latency in the periphery and the central nervous system. Curr. Opin. HIV AIDS 6, 37–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cantin R., Methot S., Tremblay M. J. (2005) Plunder and stowaways: incorporation of cellular proteins by enveloped viruses. J. Virol. 79, 6577–6587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tsegaye T. S., Pohlmann S. (2010) The multiple facets of HIV attachment to dendritic cell lectins. Cell. Microbiol. 12, 1553–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. (1984) The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature 312, 763–767 [DOI] [PubMed] [Google Scholar]
- 57. Doms R. W. (2000) Beyond receptor expression: the influence of receptor conformation, density, and affinity in HIV-1 infection. Virology 276, 229–237 [DOI] [PubMed] [Google Scholar]
- 58. Doms R. W., Trono D. (2000) The plasma membrane as a combat zone in the HIV battlefield. Genes Dev. 14, 2677–2688 [DOI] [PubMed] [Google Scholar]
- 59. Cormier E. G., Dragic T. (2002) The crown and stem of the V3 loop play distinct roles in human immunodeficiency virus type 1 envelope glycoprotein interactions with the CCR5 coreceptor. J. Virol. 76, 8953–8957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Huang C. C., Tang M., Zhang M. Y., Majeed S., Montabana E., Stanfield R. L., Dimitrov D. S., Korber B., Sodroski J., Wilson I. A., Wyatt R., Kwong P. D. (2005) Structure of a V3-containing HIV-1 gp120 core. Science 310, 1025–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Farzan M., Mirzabekov T., Kolchinsky P., Wyatt R., Cayabyab M., Gerard N. P., Gerard C., Sodroski J., Choe H. (1999) Tyrosine sulfation of the amino terminus of CCR5 facilitates HIV-1 entry. Cell 96, 667–676 [DOI] [PubMed] [Google Scholar]
- 62. Brelot A., Heveker N., Adema K., Hosie M. J., Willett B., Alizon M. (1999) Effect of mutations in the second extracellular loop of CXCR4 on its utilization by human and feline immunodeficiency viruses. J. Virol. 73, 2576–2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hoffman T. L., Doms R. W. (1999) HIV-1 envelope determinants for cell tropism and chemokine receptor use. Mol. Membr. Biol. 16, 57–65 [DOI] [PubMed] [Google Scholar]
- 64. Cardozo T., Kimura T., Philpott S., Weiser B., Burger H., Zolla-Pazner S. (2007) Structural basis for coreceptor selectivity by the HIV type 1 V3 loop. AIDS Res. Hum. Retroviruses 23, 415–426 [DOI] [PubMed] [Google Scholar]
- 65. Farzan M., Choe H., Martin K., Marcon L., Hofmann W., Karlsson G., Sun Y., Barrett P., Marchand N., Sullivan N., Gerard N., Gerard C., Sodroski J. (1997) Two orphan seven-transmembrane segment receptors which are expressed in CD4-positive cells support simian immunodeficiency virus infection. J. Exp. Med. 186, 405–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gorry P. R., Dunfee R. L., Mefford M. E., Kunstman K., Morgan T., Moore J. P., Mascola J. R., Agopian K., Holm G. H., Mehle A., Taylor J., Farzan M., Wang H., Ellery P., Willey S. J., Clapham P. R., Wolinsky S. M., Crowe S. M., Gabuzda D. (2007) Changes in the V3 region of gp120 contribute to unusually broad coreceptor usage of an HIV-1 isolate from a CCR5 Δ32 heterozygote. Virology 362, 163–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. McKnight A., Dittmar M. T., Moniz-Periera J., Ariyoshi K., Reeves J. D., Hibbitts S., Whitby D., Aarons E., Proudfoot A. E., Whittle H., Clapham P. R. (1998) A broad range of chemokine receptors are used by primary isolates of human immunodeficiency virus type 2 as coreceptors with CD4. J. Virol. 72, 4065–4071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Reeves J. D., Hibbitts S., Simmons G., McKnight A., Azevedo-Pereira J. M., Moniz-Pereira J., Clapham P. R. (1999) Primary human immunodeficiency virus type 2 (HIV-2) isolates infect CD4-negative cells via CCR5 and CXCR4: comparison with HIV-1 and simian immunodeficiency virus and relevance to cell tropism in vivo. J. Virol. 73, 7795–7804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jiang C., Parrish N. F., Wilen C. B., Li H., Chen Y., Pavlicek J. W., Berg A., Lu X., Song H., Tilton J. C., Pfaff J. M., Henning E. A., Decker J. M., Moody M. A., Drinker M. S., Schutte R., Freel S., Tomaras G. D., Nedellec R., Mosier D. E., Haynes B. F., Shaw G. M., Hahn B. H., Doms R. W., Gao F. (2011) Primary infection by a human immunodeficiency virus with atypical coreceptor tropism. J. Virol. 85, 10669–10681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Riddick N. E., Hermann E. A., Loftin L. M., Elliott S. T., Wey W. C., Cervasi B., Taafe J., Engram J. C., Li B., Else J. G., Li Y., Hahn B. H., Derdeyn C. A., Sodora D. L., Apetrei C., Paiardini M., Silvestri G., Collman R. G. (2010) A novel CCR5 mutation common in sooty mangabeys reveals SIVsmm infection of CCR5-null natural hosts and efficient alternative coreceptor usage in vivo. PLoS Pathog. 6, e1001064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Elliott S. T., Riddick N. E., Francella N., Paiardini M., Vanderford T. H., Li B., Apetrei C., Sodora D. L., Derdeyn C. A., Silvestri G., Collman R. G. (2012) Cloning and analysis of sooty mangabey alternative coreceptors that support simian immunodeficiency virus SIVsmm entry independently of CCR5. J. Virol. 86, 898–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chen Z., Kwon D., Jin Z., Monard S., Telfer P., Jones M. S., Lu C. Y., Aguilar R. F., Ho D. D., Marx P. A. (1998) Natural infection of a homozygous Δ24 CCR5 red-capped mangabey with an R2b-tropic simian immunodeficiency virus. J. Exp. Med. 188, 2057–2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Michael N. L., Chang G., Louie L. G., Mascola J. R., Dondero D., Birx D. L., Sheppard H. W. (1997) The role of viral phenotype and CCR-5 gene defects in HIV-1 transmission and disease progression. Nat. Med. 3, 338–340 [DOI] [PubMed] [Google Scholar]
- 74. Michael N. L., Louie L. G., Sheppard H. W. (1997) CCR5-Δ 32 gene deletion in HIV-1 infected patients. Lancet 350, 741–742 [DOI] [PubMed] [Google Scholar]
- 75. Salazar-Gonzalez J. F., Salazar M. G., Keele B. F., Learn G. H., Giorgi E. E., Li H., Decker J. M., Wang S., Baalwa J., Kraus M. H., Parrish N. F., Shaw K. S., Guffey M. B., Bar K. J., Davis K. L., Ochsenbauer-Jambor C., Kappes J. C., Saag M. S., Cohen M. S., Mulenga J., Derdeyn C. A., Allen S., Hunter E., Markowitz M., Hraber P., Perelson A. S., Bhattacharya T., Haynes B. F., Korber B. T., Hahn B. H., Shaw G. M. (2009) Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J. Exp. Med. 206, 1273–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Isaacman-Beck J., Hermann E. A., Yi Y., Ratcliffe S. J., Mulenga J., Allen S., Hunter E., Derdeyn C. A., Collman R. G. (2009) Heterosexual transmission of human immunodeficiency virus type 1 subtype C: macrophage tropism, alternative coreceptor use, and the molecular anatomy of CCR5 utilization. J. Virol. 83, 8208–8220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bjorndal A., Deng H., Jansson M., Fiore J. R., Colognesi C., Karlsson A., Albert J., Scarlatti G., Littman D. R., Fenyo E. M. (1997) Coreceptor usage of primary human immunodeficiency virus type 1 isolates varies according to biological phenotype. J. Virol. 71, 7478–7487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Connor R. I., Sheridan K. E., Ceradini D., Choe S., Landau N. R. (1997) Change in coreceptor use coreceptor use correlates with disease progression in HIV-1–infected individuals. J. Exp. Med. 185, 621–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yi Y., Shaheen F., Collman R. G. (2005) Preferential use of CXCR4 by R5X4 human immunodeficiency virus type 1 isolates for infection of primary lymphocytes. J. Virol. 79, 1480–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Loftin L.M., Kienzle M., Yi Y., Collman R. G. (2011) R5X4 HIV-1 coreceptor use in primary target cells: implications for coreceptor entry blocking strategies. J. Translat. Med. 9 (Suppl. 1), S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gorry P., Sterjovski J., Churchill M., Witlox K., Gray L., Cunningham A., Wesselingh S. (2004) The role of viral coreceptors and macrophage tropism in human immunodeficiency virus type 1 disease progression. Sexual Health 1, 23–35 [DOI] [PubMed] [Google Scholar]
- 82. Jakobsen M. R., Cashin K., Roche M., Sterjovski J., Ellett A., Borm K., Flynn J., Erikstrup C., Gouillou M., Gray L. R., Saksena N., Wang B., Purcell D. F. J., Kallestrup P., Zinyama-Gutsire R., Gomo E., Ullum H., Ostergaard L., Lee B., Ramsland P. A., Churchill M. J., Gorry P. R. (2013) Longitudinal analysis of CCR5 and CXCR4 usage alterations in a cohort of antiretroviral therapy naive subjects with progressive HIV-1 subtype C infection. PLoS One 8, e65950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cashin K., Jakobsen M. R., Sterjovski J., Roche M., Ellett A., Flynn J. K., Borm K., Gouillou M., Churchill M. J., Gorry P. R. (2013) Linkages between HIV-1 specificity for CCR5 or CXCR4 and in vitro usage of alternative coreceptors during progressive HIV-1 subtype C infection. Retrovirology 10, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Jakobsen M. R., Ellett A., Churchill M. J., Gorry P. R. (2010) Viral tropism, fitness and pathogenicity of HIV-1 subtype C. Future Virol. 5, 219–231 [Google Scholar]
- 85. Parker Z. F., Iyer S. S., Wilen C. B., Parrish N. F., Chikere K. C., Lee F. H., Didigu C. A., Berro R., Klasse P. J., Lee B., Moore J. P., Shaw G. M., Hahn B. H., Doms R. W. (2013) Transmitted/founder and chronic HIV-1 envelope proteins are distinguished by differential utilization of CCR5. J. Virol. 87, 2401–2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ping L. H., Joseph S. B., Anderson J. A., Abrahams M. R., Salazar-Gonzalez J. F., Kincer L. P., Treurnicht F. K., Arney L., Ojeda S., Zhang M., Keys J., Potter E. L., Chu H., Moore P., Salazar M. G., Iyer S., Jabara C., Kirchherr J., Mapanje C., Ngandu N., Seoighe C., Hoffman I., Gao F., Tang Y., Labranche C., Lee B., Saville A., Vermeulen M., Fiscus S., Morris L., Karim S. A., Haynes B. F., Shaw G. M., Korber B. T., Hahn B. H., Cohen M. S., Montefiori D., Williamson C., Swanstrom R. (2013) Comparison of viral Env proteins from acute and chronic infections with subtype C human immunodeficiency virus type 1 identifies differences in glycosylation and CCR5 utilization and suggests a new strategy for immunogen design. J. Virol. 87, 7218–7233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gray L., Sterjovski J., Churchill M., Ellery P., Nasr N., Lewin S. R., Crowe S. M., Wesselingh S., Cunningham A. L., Gorry P. R. (2005) Uncoupling coreceptor usage of human immunodeficiency virus type 1 (HIV-1) from macrophage tropism reveals biological properties of CCR5-restricted HIV-1 isolates from patients with acquired immunodeficiency syndrome. Virology 337, 384–398 [DOI] [PubMed] [Google Scholar]
- 88. Li S., Juarez J., Alali M., Dwyer D., Collman R., Cunningham A., Naif H. M. (1999) Persistent CCR5 utilization and enhanced macrophage tropism by primary blood human immunodeficiency virus type 1 isolates from advanced stages of disease and comparison to tissue-derived isolates. J. Virol. 73, 9741–9755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tuttle D. L., Anders C. B., Aquino-De Jesus M. J., Poole P. P., Lamers S. L., Briggs D. R., Pomeroy S. M., Alexander L., Peden K. W., Andiman W. A., Sleasman J. W., Goodenow M. M. (2002) Increased replication of non-syncytium-inducing HIV type 1 isolates in monocyte-derived macrophages is linked to advanced disease in infected children. AIDS Res. Hum. Retroviruses 18, 353–362 [DOI] [PubMed] [Google Scholar]
- 90. Goodenow M. M., Collman R. G. (2006) HIV-1 coreceptor preference is distinct from target cell tropism: a dual-parameter nomenclature to define viral phenotypes. J. Leukoc. Biol. 80, 965–972 [DOI] [PubMed] [Google Scholar]
- 91. Alkhatib G., Combadiere C., Broder C. C., Feng Y., Kennedy P. E., Murphy P. M., Berger E. A. (1996) CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272, 1955–1958 [DOI] [PubMed] [Google Scholar]
- 92. Choe H., Farzan M., Sun Y., Sullivan N., Rollins B., Ponath P. D., Wu L., Mackay C. R., LaRosa G., Newman W., Gerard N., Gerard C., Sodroski J. (1996) The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell 85, 1135–1148 [DOI] [PubMed] [Google Scholar]
- 93. Dragic T., Litwin V., Allaway G. P., Martin S. R., Huang Y., Nagashima K. A., Cayanan C., Maddon P. J., Koup R. A., Moore J. P., Paxton W. A. (1996) HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381, 667–673 [DOI] [PubMed] [Google Scholar]
- 94. Feng Y., Broder C. C., Kennedy P. E., Berger E. A. (1996) HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science 272, 872–877 [DOI] [PubMed] [Google Scholar]
- 95. Collman R., Balliet J. W., Gregory S. A., Friedman H., Kolson D. L., Nathanson N., Srinivasan A. (1992) An infectious molecular clone of an unusual macrophage-tropic and highly cytopathic strain of human immunodeficiency virus type 1. J. Virol. 66, 7517–7521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Yi Y., Isaacs S. N., Williams D. A., Frank I., Schols D., De Clercq E., Kolson D. L., Collman R. G. (1999) Role of CXCR4 in cell-cell fusion and infection of monocyte-derived macrophages by primary human immunodeficiency virus type 1 (HIV-1) strains: two distinct mechanisms of HIV-1 dual tropism. J. Virol. 73, 7117–7125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Peters P. J., Duenas-Decamp M. J., Sullivan W. M., Clapham P. R. (2007) Variation of macrophage tropism among HIV-1 R5 envelopes in brain and other tissues. J. Neuroimmune Pharmacol. 2, 32–41 [DOI] [PubMed] [Google Scholar]
- 98. Arrildt K. T., Joseph S. B., Swanstrom R. (2012) The HIV-1 env protein: a coat of many colors. Curr. HIV/AIDS Rep. 9, 52–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Peters P. J., Bhattacharya J., Hibbitts S., Dittmar M. T., Simmons G., Bell J., Simmonds P., Clapham P. R. (2004) Biological analysis of human immunodeficiency virus type 1 R5 envelopes amplified from brain and lymph node tissues of AIDS patients with neuropathology reveals two distinct tropism phenotypes and identifies envelopes in the brain that confer an enhanced tropism and fusigenicity for macrophages. J. Virol. 78, 6915–6926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Peters P. J., Duenas-Decamp M. J., Sullivan W. M., Brown R., Ankghuambom C., Luzuriaga K., Robinson J., Burton D. R., Bell J., Simmonds P., Ball J., Clapham P. R. (2008) Variation in HIV-1 R5 macrophage-tropism correlates with sensitivity to reagents that block envelope: CD4 interactions but not with sensitivity to other entry inhibitors. Retrovirology 5, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Peters P. J., Sullivan W. M., Duenas-Decamp M. J., Bhattacharya J., Ankghuambom C., Brown R., Luzuriaga K., Bell J., Simmonds P., Ball J., Clapham P. R. (2006) Non-macrophage-tropic human immunodeficiency virus type 1 R5 envelopes predominate in blood, lymph nodes, and semen: implications for transmission and pathogenesis. J. Virol. 80, 6324–6332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Gorry P. R., Bristol G., Zack J. A., Ritola K., Swanstrom R., Birch C. J., Bell J. E., Bannert N., Crawford K., Wang H., Schols D., De Clercq E., Kunstman K., Wolinsky S. M., Gabuzda D. (2001) Macrophage tropism of human immunodeficiency virus type 1 isolates from brain and lymphoid tissues predicts neurotropism independent of coreceptor specificity. J. Virol. 75, 10073–10089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Dunfee R. L., Thomas E. R., Gabuzda D. (2009) Enhanced macrophage tropism of HIV in brain and lymphoid tissues is associated with sensitivity to the broadly neutralizing CD4 binding site antibody b12. Retrovirology 6, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Dunfee R. L., Thomas E. R., Gorry P. R., Wang J., Taylor J., Kunstman K., Wolinsky S. M., Gabuzda D. (2006) The HIV Env variant N283 enhances macrophage tropism and is associated with brain infection and dementia. Proc. Natl. Acad. Sci. USA 103, 15160–15165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Dunfee R. L., Thomas E. R., Wang J., Kunstman K., Wolinsky S. M., Gabuzda D. (2007) Loss of the N-linked glycosylation site at position 386 in the HIV envelope V4 region enhances macrophage tropism and is associated with dementia. Virology 367, 222–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Gonzalez-Perez M. P., O'Connell O. J., Lin R., Sullivan W. M., Bell J., Simmonds P., Clapham P. R. (2012) Independent evolution of macrophage-tropism and increased charge between HIV-1 R5 envelopes present in brain and immune tissue. Retrovirology 9, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Schnell G., Joseph S., Spudich S., Price R. W., Swanstrom R. (2011) HIV-1 replication in the central nervous system occurs in two distinct cell types. PLoS Pathog. 7, e1002286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Thomas E. R., Dunfee R. L., Stanton J., Bogdan D., Taylor J., Kunstman K., Bell J. E., Wolinsky S. M., Gabuzda D. (2007) Macrophage entry mediated by HIV Envs from brain and lymphoid tissues is determined by the capacity to use low CD4 levels and overall efficiency of fusion. Virology 360, 105–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Salimi H., Roche M., Webb N., Gray L. R., Chikere K., Sterjovski J., Ellett A., Wesselingh S. L., Ramsland P. A., Lee B., Churchill M., Gorry P. R. (2013) Macrophage-tropic HIV-1 variants from brain demonstrate alterations in the way gp120 engages both CD4 and CCR5. J. Leukoc. Biol. 93, 113–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Cashin K., Roche M., Sterjovski J., Ellett A., Gray L. R., Cunningham A. L., Ramsland P. A., Churchill M. J., Gorry P. R. (2011) Alternative coreceptor requirements for efficient CCR5- and CXCR4-mediated HIV-1 entry into macrophages. J. Virol. 85, 10699–10709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Gray L., Roche M., Churchill M. J., Sterjovski J., Ellett A., Poumbourios P., Sheffief S., Wang B., Saksena N., Purcell D. F., Wesselingh S., Cunningham A. L., Brew B. J., Gabuzda D., Gorry P. R. (2009) Tissue-specific sequence alterations in the human immunodeficiency virus type 1 envelope favoring CCR5 usage contribute to persistence of dual-tropic virus in the brain. J. Virol. 83, 5430–5441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ohagen A., Devitt A., Kunstman K. J., Gorry P. R., Rose P. P., Korber B., Taylor J., Levy R., Murphy R. L., Wolinsky S. M., Gabuzda D. (2003) Genetic and functional analysis of full-length human immunodeficiency virus type 1 env genes derived from brain and blood of patients with AIDS. J. Virol. 77, 12336–12345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Yi Y., Chen W., Frank I., Cutilli J., Singh A., Starr-Spires L., Sulcove J., Kolson D. L., Collman R. G. (2003) An unusual syncytia-inducing human immunodeficiency virus type 1 primary isolate from the central nervous system that is restricted to CXCR4, replicates efficiently in macrophages, and induces neuronal apoptosis. J. Neurovirol. 9, 432–441 [DOI] [PubMed] [Google Scholar]
- 114. Gray L., Churchill M. J., Keane N., Sterjovski J., Ellett A. M., Purcell D. F., Poumbourios P., Kol C., Wang B., Saksena N., Wesselingh S. L., Price P., French M., Gabuzda D., Gorry P. R. (2006) Genetic and functional analysis of R5X4 human immunodeficiency virus type 1 envelope glycoprotiens derived from two individuals homozygous for the CCR5delta32 allele. J. Virol. 80, 3684–3691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Loftin L. M., Kienzle M. F., Yi Y., Lee B., Lee F. H., Gray L., Gorry P. R., Collman R. G. (2010) Constrained use of CCR5 on CD4+ lymphocytes by R5X4 HIV-1: efficiency of Env-CCR5 interactions and low CCR5 expression determine a range of restricted CCR5-mediated entry. Virology 402, 135–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Yi Y., Rana S., Turner J. D., Gaddis N., Collman R. G. (1998) CXCR-4 is expressed by primary macrophages and supports CCR5-independent infection by dual-tropic but not T-tropic isolates of human immunodeficiency virus type 1. J. Virol. 72, 772–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Whitcomb J. M., Huang W., Fransen S., Limoli K., Toma J., Wrin T., Chappey C., Kiss L. D., Paxinos E. E., Petropoulos C. J. (2007) Development and characterization of a novel single-cycle recombinant-virus assay to determine human immunodeficiency virus type 1 coreceptor tropism. Antimicrob. Agents Chemother. 51, 566–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Swenson L. C., Moores A., Low A. J., Thielen A., Dong W., Woods C., Jensen M. A., Wynhoven B., Chan D., Glascock C., Harrigan P. R. (2010) Improved detection of CXCR4-using HIV by V3 genotyping: application of population-based and “deep” sequencing to plasma RNA and proviral DNA. J. Acquir. Immune Defic. Syndr. 54, 506–510 [DOI] [PubMed] [Google Scholar]
- 119. Vandekerckhove L. P., Wensing A. M., Kaiser R., Brun-Vezinet F., Clotet B., De Luca A., Dressler S., Garcia F., Geretti A. M., Klimkait T., Korn K., Masquelier B., Perno C. F., Schapiro J. M., Soriano V., Sonnerborg A., Vandamme A. M., Verhofstede C., Walter H., Zazzi M., Boucher C. A. (2011) European guidelines on the clinical management of HIV-1 tropism testing. Lancet Infect. Dis. 11, 394–407 [DOI] [PubMed] [Google Scholar]
- 120. Poveda E., Alcami J., Paredes R., Cordoba J., Gutierrez F., Llibre J. M., Delgado R., Pulido F., Iribarren J. A., Garcia Deltoro M., Hernandez Quero J., Moreno S., Garcia F. (2010) Genotypic determination of HIV tropism - clinical and methodological recommendations to guide the therapeutic use of CCR5 antagonists. AIDS Rev. 12, 135–148 [PubMed] [Google Scholar]
- 121. Lewin S. R., Sonza S., Irving L. B., McDonald C. F., Mills J., Crowe S. M. (1996) Surface CD4 is critical to in vitro HIV infection of human alveolar macrophages. AIDS Res. Hum. Retroviruses 12, 877–883 [DOI] [PubMed] [Google Scholar]
- 122. Wang J., Crawford K., Yuan M., Wang H., Gorry P. R., Gabuzda D. (2002) Regulation of CC chemokine receptor 5 and CD4 expression and human immunodeficiency virus type 1 replication in human macrophages and microglia by T helper type 2 cytokines. J. Infect. Dis. 185, 885–897 [DOI] [PubMed] [Google Scholar]
- 123. Lee B., Sharron M., Montaner L. J., Weissman D., Doms R. W. (1999) Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc. Natl. Acad. Sci. USA 96, 5215–5220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Duenas-Decamp M. J., Peters P. J., Burton D., Clapham P. R. (2009) Determinants flanking the CD4 binding loop modulate macrophage tropism of human immunodeficiency virus type 1 R5 envelopes. J. Virol. 83, 2575–2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Martin J., LaBranche C. C., Gonzalez-Scarano F. (2001) Differential CD4/CCR5 utilization, gp120 conformation, and neutralization sensitivity between envelopes from a microglia-adapted human immunodeficiency virus type 1 and its parental isolate. J. Virol. 75, 3568–3580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Martin-Garcia J., Cao W., Varela-Rohena A., Plassmeyer M. L., Gonzalez-Scarano F. (2006) HIV-1 tropism for the central nervous system: brain-derived envelope glycoproteins with lower CD4 dependence and reduced sensitivity to a fusion inhibitor. Virology 346, 169–179 [DOI] [PubMed] [Google Scholar]
- 127. Musich T., Peters P. J., Duenas-Decamp M. J., Gonzalez-Perez M. P., Robinson J., Zolla-Pazner S., Ball J. K., Luzuriaga K., Clapham P. R. (2011) A conserved determinant in the V1 loop of HIV-1 modulates the V3 loop to prime low CD4 use and macrophage infection. J. Virol. 85, 2397–2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Chang M. I., Panorchan P., Dobrowsky T. M., Tseng Y., Wirtz D. (2005) Single-molecule analysis of human immunodeficiency virus type 1 gp120-receptor interactions in living cells. J. Virol. 79, 14748–14755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Steffens C. M., Hope T. J. (2004) Mobility of the human immunodeficiency virus (HIV) receptor CD4 and coreceptor CCR5 in living cells: implications for HIV fusion and entry events. J. Virol. 78, 9573–9578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Gorry P. R., Taylor J., Holm G. H., Mehle A., Morgan T., Cayabyab M., Farzan M., Wang H., Bell J. E., Kunstman K., Moore J. P., Wolinsky S. M., Gabuzda D. (2002) Increased CCR5 affinity and reduced CCR5/CD4 dependence of a neurovirulent primary human immunodeficiency virus type 1 isolate. J. Virol. 76, 6277–6292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Sterjovski J., Roche M., Churchill M. J., Ellett A., Farrugia W., Gray L. R., Cowley D., Poumbourios P., Lee B., Wesselingh S., Cunningham A. L., Ramsland P. A., Gorry P. R. (2010) An altered and more efficient mechanism of CCR5 engagement contributes to macrophage tropism of CCR5-using HIV-1 envelopes. Virology 404, 269–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Hoffman T. L., LaBranche C. C., Zhang W., Canziani G., Robinson J., Chaiken I., Hoxie J. A., Doms R. W. (1999) Stable exposure of the coreceptor-binding site in a CD4-independent HIV-1 envelope protein. Proc. Natl. Acad. Sci. USA 96, 6359–6364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Kolchinsky P., Mirzabekov T., Farzan M., Kiprilov E., Cayabyab M., Mooney L. J., Choe H., Sodroski J. (1999) Adaptation of a CCR5-using, primary human immunodeficiency virus type 1 isolate for CD4-independent replication. J. Virol. 73, 8120–8126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Rizzuto C. D., Wyatt R., Hernandez-Ramos N., Sun Y., Kwong P. D., Hendrickson W. A., Sodroski J. (1998) A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science 280, 1949–1953 [DOI] [PubMed] [Google Scholar]
- 135. Haim H., Strack B., Kassa A., Madani N., Wang L., Courter J.R., Princiotto A., McGee K., Pacheco B., Seaman M.S., Smith A.B., III, Sodroski J. (2011) Contribution of intrinsic reactivity of the HIV-1 envelope glycoproteins to CD4-independent infection and global inhibitor sensitivity. PLoS Pathog. 7, e1002101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Roche M., Jakobsen M. R., Sterjovski J., Ellett A., Posta F., Lee B., Jubb B., Westby M., Lewin S. R., Ramsland P. A., Churchill M. J., Gorry P. R. (2011) HIV-1 escape from the CCR5 antagonist maraviroc associated with an altered and less efficient mechanism of gp120-CCR5 engagement that attenuates macrophage-tropism. J. Virol. 85, 4330–4342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Flynn J. K., Paukovics G., Moore M. S., Ellett A., Gray L. R., Duncan R., Salimi H., Jubb B., Westby M., Purcell D. F., Lewin S. R., Lee B., Churchill M. J., Gorry P. R., Roche M. (2013) The magnitude of HIV-1 resistance to the CCR5 antagonist maraviroc may impart a differential alteration in HIV-1 tropism for macrophages and T-cell subsets. Virology 442, 51–58 [DOI] [PubMed] [Google Scholar]
- 138. Roche M., Salimi H., Duncan R., Wilkinson B., Chikere K., Moore M. S., Webb N., Zappi H., Sterjovski J., Flynn J., Ellett A., Gray L. R., Lee B., Jubb B., Westby M., Ramsland P. A., Lewin S. R., Payne R., Churchill M. J., Gorry P. R. (2013) A common mechanism of clinical HIV-1 resistance to maraviroc despite divergent resistance levels and lack of common gp120 resistance mutations. Retrovirology 10, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Pfaff J. M., Wilen C. B., Harrison J. E., Demarest J. F., Lee B., Doms R. W., Tilton J. C. (2010) HIV-1 resistance to CCR5 antagonists associated with highly efficient use of CCR5 and altered tropism on primary CD4+ T cells. J. Virol. 84, 6505–6514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Johnston S. H., Lobriz M. A., Nguyen S., Lassen K., Delair S., Posta F., Bryson Y. J., Arts E. J., Chou T., Lee B. (2009) A quantitative affinity-profiling system that reveals distinct CD4/CCR5 usage patterns among human immunodeficiency virus type 1 and simian immunodeficiency virus strains. J. Virol. 83, 11016–11026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Chikere K., Chou T., Gorry P. R., Lee B. (2013) Affinofile profiling: how efficiency of CD4/CCR5 usage impacts the biological and pathogenic phenotype of HIV. Virology 435, 81–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Ghaffari G., Tuttle D. L., Briggs D., Burkhardt B. R., Bhatt D., Andiman W. A., Sleasman J. W., Goodenow M. M. (2005) Complex determinants in human immunodeficiency virus type 1 envelope gp120 mediate CXCR4-dependent infection of macrophages. J. Virol. 79, 13250–13261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Yi Y., Loftin L., Wang L., Ratcliffe S. J., Isaacman-Beck J., Collman R. G. (2008) Entry coreceptor use and fusion inhibitor T20 sensitivity of dual-tropic R5X4 HIV-1 in primary macrophage infection. J. Acquir. Immune Defic. Syndr. 47, 285–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Simmons G., Reeves J. D., McKnight A., Dejucq N., Hibbitts S., Power C. A., Aarons E., Schols D., De Clercq E., Proudfoot A. E., Clapham P. R. (1998) CXCR4 as a functional coreceptor for human immunodeficiency virus type 1 infection of primary macrophages. J. Virol. 72, 8453–8457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Labrosse B., Treboute C., Brelot A., Alizon M. (2001) Cooperation of the V1/V2 and V3 domains of human immunodeficiency virus type 1 gp120 for interaction with the CXCR4 receptor. J. Virol. 75, 5457–5464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Hutter G., Nowak D., Mossner M., Ganepola S., Mussig A., Allers K., Schneider T., Hofmann J., Kucherer C., Blau O., Blau I. W., Hofmann W. K., Thiel E. (2009) Long-term control of HIV by CCR5 Δ32/Δ32 stem-cell transplantation. N. Engl. J. Med. 360, 692–698 [DOI] [PubMed] [Google Scholar]
- 147. Henrich T. J., Hu Z., Li J. Z., Sciaranghella G., Busch M. P., Keating S. M., Gallien S., Lin N. H., Giguel F. F., Lavoie L., Ho V. T., Armand P., Soiffer R. J., Sagar M., Lacasce A. S., Kuritzkes D. R. (2013) Long-term reduction in peripheral blood HIV type 1 reservoirs following reduced-intensity conditioning allogeneic stem cell transplantation. J. Infect. Dis. 207, 1694–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Allers K., Hutter G., Hofmann J., Loddenkemper C., Rieger K., Thiel E., Schneider T. (2011) Evidence for the cure of HIV infection by CCR5Δ32/Δ32 stem cell transplantation. Blood 117, 2791–2799 [DOI] [PubMed] [Google Scholar]
- 149. Henrich T., Hanhauser E., Sirignano M., Davis B., Lee T-H., Keating S., Busch M., Marty F., LaCasce F., Armand P., Soiffer R., Altfeld M., Kuritzkes D. (2013) In depth investigation of peripheral and gut HIV-1 reservoirs, HIV-specific cellular immunity, and host microchimerism following allogeneic hematopoetic stem cell transplantation. WELBA05. Program and Abstracts of the 7th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention, Kuala Lumpur, Malaysia (abs.) [Google Scholar]