Abstract
In rat arterial chemoreceptors, background potassium channels play an important role in maintaining resting membrane potential and promoting depolarization and excitation in response to hypoxia or acidosis. It has been suggested that these channels are a heterodimer of TASK-1 and TASK-3 based on their similarity to heterologously expressed TASK-1/3 fusion proteins. In this study, we sought to confirm the identity of these channels through germline ablation of Task-1 (Kcnk3) and Task-3 (Kcnk9) in mice. Background K-channels were abundant in carotid body type-1 cells from wild-type mice and comparable to those previously described in rat type-1 cells with a main conductance state of 33 pS. This channel was absent from both Task-1−/− and Task-3−/− cells. In its place we observed a larger (38 pS) K+-channel in Task-1−/− cells and a smaller (18 pS) K+-channel in Task-3−/− cells. None of these channels were observed in Task-1−/−/Task-3−/− double knock-out mice. We therefore conclude that the predominant background K-channel in wild-type mice is a TASK-1/TASK-3 heterodimer, whereas that in Task-1−/− mice is TASK-3 and, conversely, that in Task-3−/− mice is TASK-1. All three forms of TASK channel in type-1 cells were inhibited by hypoxia, cyanide and the uncoupler FCCP, but the greatest sensitivity was seen in TASK-1 and TASK-1/TASK-3 channels. In summary, the background K-channel in type-1 cells is predominantly a TASK-1/TASK-3 heterodimer. Although both TASK-1 and TASK-3 are able to couple to the oxygen and metabolism sensing pathways present in type-1 cells, channels containing TASK-1 appear to be more sensitive.
Key points
TASK-like background potassium channels play a key role in the sensing of hypoxic, metabolic and acidic stimuli in arterial chemoreceptor cells.
In this study, we investigated the roles of TASK-1 and TASK-3 in forming these channels by using gene deletion in mice.
Deletion of Task-1 (Kcnk3) and/or Task-3 (Kcnk9) disrupted the main form of background K-channel activity.
In single knock-out mice, we observed, instead of the wild-type channel, the homomeric forms of TASK-1 in Task-3−/− and TASK-3 in Task-1−/−.
All forms of TASK were inhibited by hypoxia, cyanide and the uncoupler FCCP.
We conclude that the main form of background K-channel in chemoreceptor cells is a TASK-1/TASK-3 heterodimer and that both TASK-1 and TASK-3 subunits can couple to both oxygen and metabolic signalling pathways in these cells.
Introduction
TASK-like potassium channels are thought to play a central role in mediating the excitatory response of arterial chemoreceptors to hypoxia, acidosis and hypercapnia (Buckler et al. 2000). Their presence in carotid body chemoreceptor cells was first suggested based on biophysical and pharmacological similarities between cloned TASK channels in heterologous expression systems and a native oxygen- and acid-sensitive background potassium current found in rat carotid body type-1 cells (Buckler, 1997; Buckler et al. 2000). The channels responsible for mediating this background current (originally termed ‘KB-channels’) are very abundant in the type-1 cell membrane and share a number of characteristics with TASK channels, including minimal voltage sensitivity, acid sensitivity, resistance to the classical K-channel inhibitors TEA and 4-AP, and the ability to be activated by halothane. It was originally suggested that KB-channels might be comprised of TASK-1, and TASK-1 mRNA was shown to be present in type-1 cells (Buckler et al. 2000). Further, more detailed, biophysical studies of KB-channels, together with the cloning and characterization of another closely related member of the TASK channel family, TASK-3 (Chapman et al. 2000; Kim et al. 2000; Rajan et al. 2000), revealed some subtle differences between KB-channels and TASK channels, principally relating to the magnesium sensitivity of single-channel conductance. These differences led us to speculate that the native channel might be a heteromer of TASK-1 and TASK-3 (Williams & Buckler, 2004) as TASK-3 was also reported to be expressed in type-1 cells (Yamamoto et al. 2002).
TASK channels belong to the tandem-p-domain K-channel (K2P) family, which possesses two pore-forming domains, each of which is sandwiched between two membrane-spanning domains in a tandem repeat (Goldstein et al. 1996; Lesage et al. 1996a). The functional form of K2P channels is believed to be dimeric (Lesage et al. 1996b; Brohawn et al. 2012; Miller & Long, 2012). The first suggestions of heterodimerization among some members of this family of channels were based on the pharmacological properties of whole cell currents produced in heterologous expression systems containing both TASK-1 and TASK-3 (Czirjak & Enyedi, 2002). Single-channel recordings of heteromultimeric channels formed in heterologous expression systems have never been reported, but fusion protein constructs (TASK-1–TASK-3 and TASK-3–TASK-1) expressed in heterologous systems generate TASK-like currents (Czirjak & Enyedi, 2002; Kang et al. 2004) and display single-channel properties which more closely resemble the predominant form of native KB-channel activity in type-1 cells than either TASK-1 or TASK-3 alone (Kim et al. 2009). Thus, the current hypothesis is that the background K-channels in type-1 cells are predominantly TASK-1/TASK-3 heterodimers and include a small number of homomeric TASK-1 and TASK-3.
Defining the structure of native channels in the carotid body is important in a number of respects, but first and foremost investigations into the regulation of these channels by natural stimuli will ultimately depend upon the identification of regulatory motifs that couple to the relevant sensory transduction pathway. Before this can be achieved, it is necessary to confirm the channel's identity. For example, recent investigations into the mechanisms of oxygen sensing in these cells have focused upon a role for metabolism in which mitochondrial ATP formation may be linked to the control of channel activity via AMP kinase (Evans et al. 2005; Wyatt & Evans, 2007). Interestingly, however, it has been suggested that only TASK-3 is regulated by AMP kinase and that TASK-1 is not (Dallas et al. 2009).
In this study, we therefore sought to: (i) investigate the role of Task-1 (Kcnk3) and Task-3 (Kcnk9) genes in the formation of background K-channels in type-1 cells, and (ii) test the sensitivity of the different forms of channel possible (TASK-1, TASK-3 and TASK-1/TASK-3) to modulation by hypoxia and inhibitors of mitochondrial metabolism.
Methods
Animals
The Task-1−/− and Task-3−/− mice used in these studies have been described in detail elsewhere (Aller et al. 2005; Brickley et al. 2007). For both Task-1−/− and Task-3−/−, the first coding exons are disrupted and no mutant allele is transcribed. Task-1−/−/Task-3−/− double knock-out animals were produced by crossing the two single knock-out lines (Trapp et al. 2008). Although Task-1−/− and Task-3−/− have been described as mostly of the C57BL/6 strain, we identified animals with wild-type alleles produced during our Task-1−/− and Task-3−/− crossing programme, which were subsequently mated to generate the control (wild-type) line used in these experiments. Genotypes were confirmed by PCR of genomic DNA extracted from ear biopsies. All procedures involving animals were approved by the University of Oxford's Local Ethical Review Process and were conducted in accordance with project and personal licences issued under the UK Animals (Scientific Procedures) Act 1986.
Type-1 cell isolation
Carotid bodies were excised from young mice (aged 4–8 weeks) under terminal anaesthesia (2–4% halothane) in accordance with project and personal licences issued under the UK Animals (Scientific Procedures) Act 1986. Type-1 cells were isolated using enzymatic dissociation with 0.25 mg ml−1 trypsin (Sigma-Aldrich Ltd, Gillingham, Dorset, UK) and 1.75 mg ml−1 collagenase P (Roche Diagnostics Ltd, Burgess Hill, West Sussex, UK) in Hank's balanced salt solution (HBSS) (pre-equilibrated with 5% CO2, 11% O2 air) for 25 min at 37°C. Tissue was subsequently transferred to enzyme-free culture medium and triturated through fire-polished pipettes. This cell suspension was plated out onto coverslips coated with a 20:1 mixture of poly-L-lysine and laminin (Sigma-Aldrich Ltd), which were incubated at 37°C for 2 h before additional culture medium was added. Culture medium comprised Ham's F-12 supplemented with 10% heat-inactivated fetal bovine serum, 2 mm l-glutamine and 4 μg ml−1 insulin. Cell isolates were used within 8 h.
Electrophysiology
Cell-attached patch clamp recordings were performed using an Axopatch 200B (Molecular Devices LLC, Sunnyvale, CA, US). Pipettes were fabricated from borosilicate glass capillaries (Harvard Apparatus Ltd, Kent, UK) and were sylgard-coated and fire-polished immediately before use. Cell-attached filling solution contained 140 mm KCl, 1 mm MgCl2, 1 mm EGTA, 10 mm Hepes, 10 mm tetraethylammonium and 5 mm 4-aminopyridine. The pH of the filling solution was adjusted to 7.4 at 37°C. All single-channel recordings were carried out in high K+ (100 mm K+), nominally Ca2+-free solutions (detailed below).
Single-channel recordings were filtered at 2–5 kHz and current was recorded and digitalized at 20 kHz. Voltage clamp control, data acquisition and analysis were performed using Spike2 4.21 (Cambridge Electronic Design, Cambridge, UK). Single-channel activity was defined by nPopen. Main conductance states for channel activity were established in an all-points histogram and used to set a 50% open threshold for nPopen definition analysis. Current levels which exceeded 150% of the main conductance state were classed as multiple openings.
Measurement of [Ca2+]i
Intracellular calcium measurements were performed using Indo-1 and a microspectrofluorimeter based on a Nikon Diaphot 200 (Nikon Corp., Tokyo, Japan) equipped with a xenon lamp to provide an excitation light source and cooled (−20°C) photomultiplier tubes (PMT; Thorn EMI, London, UK) to detect emitted fluorescence. Indo-1 was loaded into cells by incubation with 2–5 μm of the acetoxymethyl ester derivative in culture medium for 1 h at room temperature. Indo-1 was excited at 340 nm and fluorescence intensity measured at 405 ± 16 nm and 495 ± 10 nm. The fluorescence emission ratio (405:495) for Indo-1 was calibrated as previously described (Buckler & Vaughan Jones, 1993).
Solutions
Standard HCO3− buffered Tyrode solution contained 117 mm NaCl, 4.5 mm KCl, 2.5 mm CaCl2, 1 mm MgCl2, 23 mm NaHCO3 and 11 mm glucose. High K+ Tyrode contained 100 mm KCl, 21.5 mm NaCl, 1 mm MgCl2, 23 mm NaHCO3 and 11 mm glucose. Normoxic solutions were equilibrated with 5% CO2 and 95% air; hypoxic solutions were equilibrated with 5% CO2 and 95% N2 (Po2= 2 Torr). Both standard and high K+ Tyrode were pH 7.4 at 37°C.
Solutions containing NaCN were bubbled for > 15 min with 5% CO2 95% air prior to the addition of the compound. NaCN was added immediately before use and solutions were subsequently maintained under an atmosphere of 5% CO2 95% air to reduce loss of volatile HCN from solutions. Tyrodes containing NaCN were replaced with fresh solutions after approximately 1 h of experimentation.
Results
Standard recording conditions
Cell-attached patch recordings were performed on isolated type-1 cells perfused with 100 mm K+ HCO3− buffered Tyrode and a pipette solution containing 140 mm K+, plus 10 mm TEA and 5 mm 4-AP (see Methods for full composition). Pipette potential (Vp) was set at +80 mV for most recordings (unless otherwise indicated). These recording conditions were chosen so that: (i) any actions of hypoxia or metabolic inhibitors upon background K current would have minimal effect on cell membrane potential (estimated to be equivalent to EK=−9 mV) so that a constant potential across the cell-attached patch of approximately −89 mV could be maintained under all conditions, and (ii) voltage-activated and Ca2+-activated K+-channels in the patch would be largely inhibited by the inclusion of TEA and 4-AP and the negative patch potential.
Background K+-channel activity in wild-type mouse type-1 cells
Recordings from the type-1 cells of wild-type animals conducted under the conditions described above routinely displayed brief flickery inward currents. Analysis of these recordings revealed one main conductance level with a mean ± standard error of the mean (s.e.m.) amplitude of 2.51 ± 0.04 pA (n= 25). Other less frequent lower and higher conductance levels were also observed in most patches consistent with unresolved partial openings of channels and the presence of multiple channels (Fig. 1A and B). Despite the appearance of variable conductance levels, the majority of patches showed only a single peak when analysed by all-points histograms (Fig. 1D). Occasionally, however, a second peak or shoulder of lower amplitude was also resolved, at approximately 1.3 pA (Fig. 1C).
Figure 1. Background channel activity in cell-attached wild-type patches.
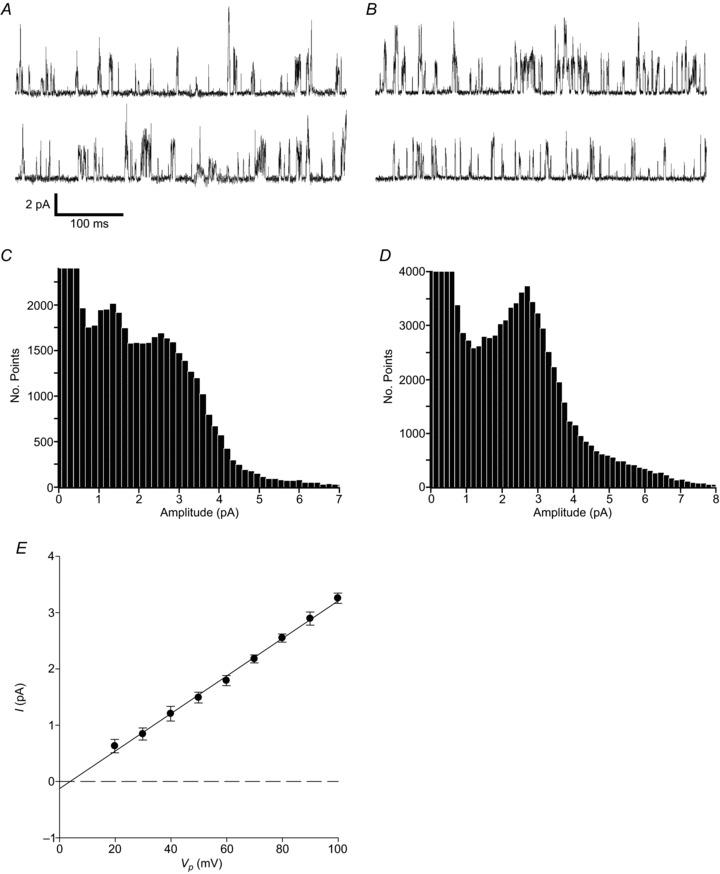
A, B, cell-attached recordings from different type-1 cells, each with two contiguous sections of 500 ms in length. Upward deflections represent inward currents (open channel events with current flow into the cell). Cells were superfused with 100 mm K+ Tyrode, with 140 mm K+ in the pipette solution. Pipette potential (Vp) was +80 mV. C, D, all-points histograms (150 fA bin widths) generated from 10 s segments of cell-attached recordings under control conditions from the same cells as in A and B. E, mean single-channel current–voltage relationship (I–V) for background channel activity (n= 15). Mean data from inward currents were fitted by least squares linear regression and yielded a mean ±s.e.m. slope conductance of 33.4 ± 0.7 pS (r2= 0.997).
Channels were active instantaneously and did not run down or inactivate over time. The most frequently observed channels were characterized by flickery kinetics with a mean ±s.e.m.nPopen of 0.20 ± 0.04 (n= 30) (the main conductance state as determined from all-points histograms was used to set a 50% threshold level for the determination of nPopen). The current–voltage (I–V) relationship of the main conductance level for inward currents was linear between 20 mV and 100 mV Vp, with a mean ±s.e.m. slope conductance of 33.4 ± 0.7 pS (n= 15) (Fig. 1E). The generally noisy nature of data recorded at negative pipette potentials precluded the sensible estimation of slope conductance of outward currents, but the reversal potential estimated by linear regression was at a Vp of approximately 4 mV. This is consistent with a potassium conductance.
The channels described above display properties similar to those of the background TASK-like potassium channels previously described in rat type-1 cells (see Discussion). It has been proposed that these background channels are probably formed from a TASK-1/TASK-3 heterodimer in rat type-1 cells with the possible co-presence of a small number of TASK-1 and TASK-3 homodimers (Kim et al. 2009). We sought to test this hypothesis using germline ablation of Task-1 and Task-3 genes.
Channel activity in Task-1−/− and Task-3−/− mouse type-1 cells
Cell-attached recordings from both Task-1−/− and Task-3−/− type-1 cells were performed under the same conditions as those used to study background K+-channel activity in wild-type cells (see above).
Most cell-attached recordings in type-1 cells from Task-1−/− mice also displayed spontaneous channel activity that did not run down over time (Fig. 2A). Channel openings had a flickery appearance similar to that seen in channels from wild-type cells, but all-points histograms resolved only a single, well-defined peak with a mean ±s.e.m. amplitude of 3.31 ± 0.07 pA (n= 29) (Fig. 2B), which was significantly larger (P < 0.0001, unpaired two-tailed Student's t test) than that seen in wild-type cells. Channel activity was, however, significantly lower than in patches from wild-type cells, with a mean ±s.e.m.nPopen of only 0.008 ± 0.002 (n= 21; P < 0.001, unpaired two-tailed t test). Single-channel currents in Task-1−/− cells showed inward rectification with mean slope conductance values calculated from the I–V relationship of 37.6 ± 0.8 pS for inward currents (n= 13) (Fig. 2C). The estimated reversal potential for single-channel currents was at Vp=−6.4 mV, consistent with a potassium channel.
Figure 2. Background channel activity in cell-attached Task-1−/− and Task-3−/− patches.
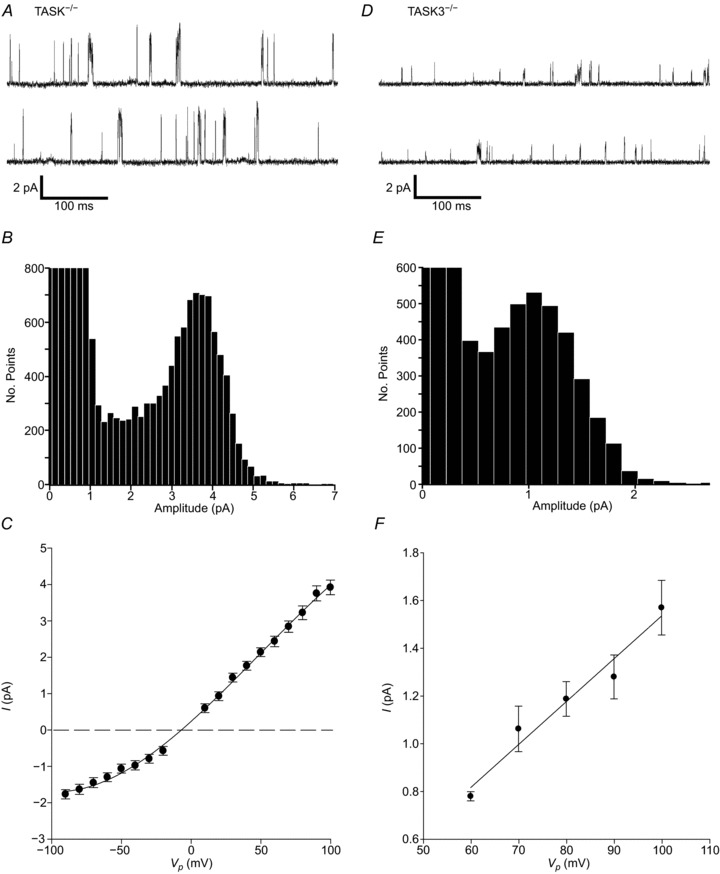
A, D, cell-attached recording of background channel activity from Task-1−/− and Task-3−/− type-1 cells respectively, each showing two contiguous 500 ms sections of recording. Recording conditions were as described for wild-type, type-1 cells. B, E, all-points histograms generated from 20 s and 40 s segments of cell-attached recordings for Task-1−/− and Task-3−/− type-1 cells, respectively. Bin widths were 150 fA. C, mean current–voltage (I–V) relationship for background channel activity in Task-1−/− type-1 cells (n= 13 for most points). Data for inward and outward currents were fitted independently by least squares linear regression and yielded a mean ±s.e.m. slope conductance of 37.6 ± 0.8 pS (r2= 0.997) for the inward current. F, mean single-channel I–V relationship for background channel activity in Task-3−/− type-1 cells (n= 5 for most points). Mean data for inward currents were fitted by least squares linear regression, which yielded a mean ±s.e.m. slope conductance of 18 ± 0.21 pS (r2= 0.96).
Background channel activity was also evident in cell-attached patches from Task-3−/− type-1 cells (Fig. 2D), but was of significantly lower amplitude than either wild-type or Task-1−/− channel currents (P < 0.0001 and P < 0.0001, respectively, unpaired two-tailed t test). Currents recorded at +80 mV Vp had a mean ±s.e.m. amplitude of 1.24 ± 0.05 pA (n= 19) for the principle peak and some other much less frequent higher conductance levels were also present, as determined by the all-points histogram (Fig. 2E). Channel kinetics were also flickery, with a relatively low mean ±s.e.m.nPopen of 0.047 ± 0.032 (n= 17), which was significantly lower than that in the wild-type (P < 0.02). Task-3−/− channels were instantaneously active and activity did not run down with time. The I–V relationship of inward currents between 60 mV and 100 mV Vp yielded a mean ±s.e.m. slope conductance of 18 ± 0.21 pS (n= 5). An unfavourable signal:noise ratio at more negative pipette potentials precluded an estimation of outward conductance. Current reversal was estimated by linear regression to be at Vp=7.1 mV.
Channel activity in Task-1−/−/Task-3−/− (double knock-out) type-1 cells
Background channel activity was infrequently observed in cell-attached patches in type-1 cells from Task-1−/−/Task-3−/− double knock-out animals. In 20 patches recorded, a large conductance channel with flickery kinetics was recorded on four occasions (Fig. 3A). Single-channel currents at +80 mV Vp had a mean ±s.e.m. amplitude of 7.5 ± 0.8 pA (n= 3), as determined by an all-points histogram (Fig. 3B). The I–V relationship for inward currents between 20 mV and 100 mV Vp gave a mean ±s.e.m. slope conductance of 108 ± 4.8 pS (n= 2). In one recording channel, activity appeared to be inhibited by hypoxia; however, this was not replicable in the other three patches tested.
Figure 3. Background channel activity in cell-attached Task-1−/−/Task-3−/− patches.
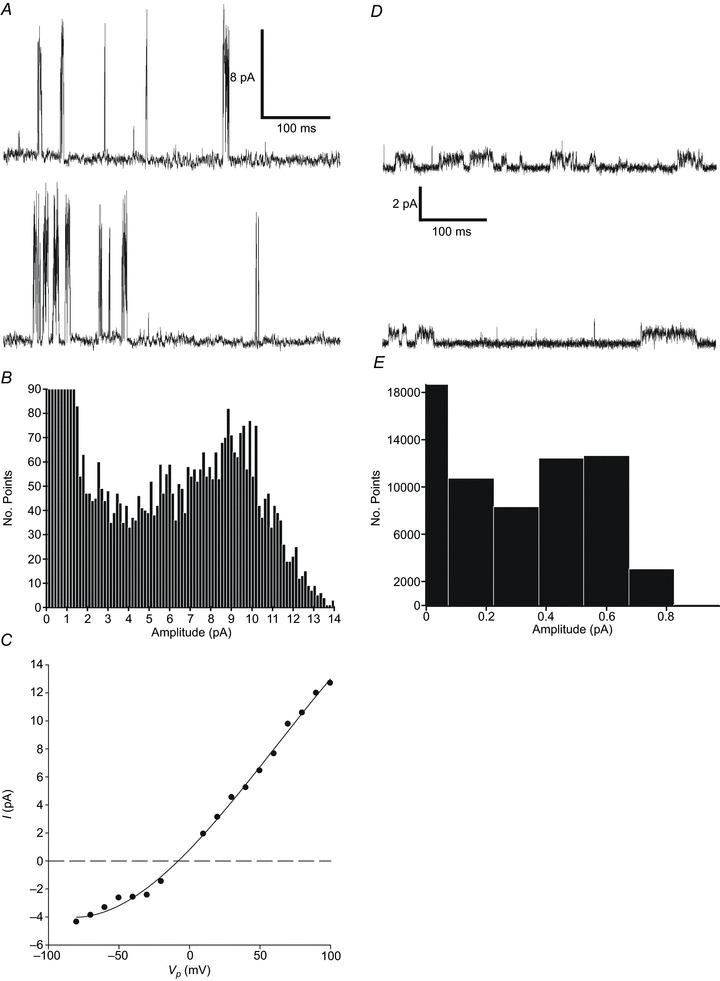
A, cell-attached recording of a large conductance channel in a Task-1−/−/Task-3−/− type-1 cell. Two contiguous 500 ms sections of recording are shown. Recording conditions were as described in Fig. 1. B, all-points histogram generated from a 20 s segment of cell-attached recording under control conditions, with 150 fA bins. C, single-channel current–voltage (I–V) relationship for a large conductance channel present in a Task-1−/−/Task-3−/− null type-1 cell (n= 1). Data for inward currents (n= 2) were fitted by least squares regression and yielded a mean ±s.e.m. slope conductance of 108 ± 4.8 pS (r2= 0.987). D, cell-attached recording of single-channel activity of a small conductance channel from a Task-1−/−/Task-3−/− type-1 cell. E, all-points histogram generated from a 10 s segment of cell-attached recording under control conditions, with 150 fA bins. Calculation of single-channel conductance at 80 mV yielded a value of 7.5 pS.
Another channel of far smaller conductance was also observed in three patches. This channel was characterized by long, stable, open states (Fig. 3D). Currents recorded at +80 mV Vp were approximately 0.6 pA in amplitude as determined by an all-points histogram (Fig. 3E). Because of the low frequency of occurrence of channels in Task-1−/−/Task-3−/− type-1 cell patches, they were not studied further.
Comparison of K+-channels in wild-type, Task-1−/− and Task-3−/− type-1 cells
Mean nPopen values for channel activity in wild-type and Task knock-out animals were compared using an unpaired t test. Channel activity in wild-type animals was much higher, by roughly 24-fold, than that seen in Task-1−/− (P < 0.001) and 4-fold greater than that observed in Task-3−/− (P < 0.02). There was no significant difference in channel activity between Task-1−/− and Task-3−/− (P= 0.19) (Fig. 4A). The frequency of observation of TASK-like channel activity also differed between subject groups. Of patches formed (> 10 GΩ resistance), 82% of wild-type (n= 45; total patches: 55), 55% of Task-1−/− (n= 44; total patches: 80) and 68% of Task-3−/− (n= 32; total patches: 47) patches contained TASK-like channel activity (Fig. 4B).
Figure 4. Task-1−/− and Task-3−/− single-channel summary.
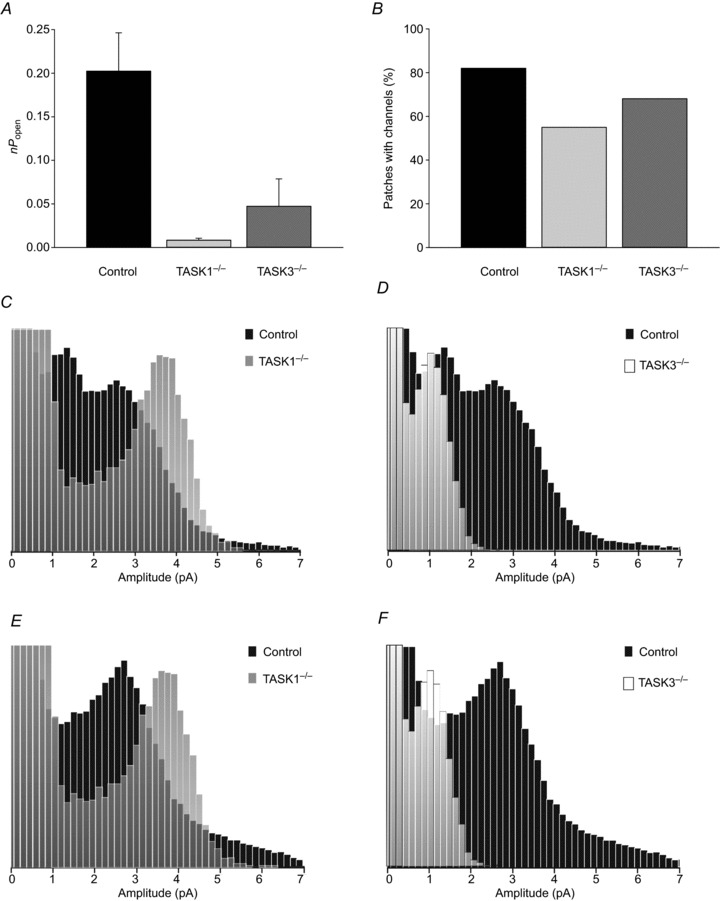
A, nPopen values for channel activity calculated under normoxic conditions at 80 mV Vp for control (n= 30), Task-1−/− (n= 21) and Task-3−/− (n= 17) type-1 cells. B, numbers of channel-containing patches as a percentage of total patches for control (n= 45), Task-1−/− (n= 44), and Task-3−/− (n= 32) cells. C, D, all-points histogram from a control type-1 cell with Task-1−/− and Task-3−/− superimposed, respectively. E, F, alternative all-points histogram from a control type-1 cell with Task-1−/− and Task-3−/−, respectively. Note all histograms in this figure have been arbitrarily scaled such that peaks in the different histograms are of similar size.
The superimposition of scaled all-points histograms derived from control and knock-out type-1 cells generally resolved a clear separation of different amplitude peaks for the different genotypes. The superimposition of both single- and two-peaked wild-type all-points histograms with a Task-1−/− histogram placed the Task-1−/− peak clearly to the right of the wild-type peaks (Fig. 4C and E). Performing the same action with a Task-3−/− histogram saw the mutant peak align closely with the leftward peak in wild-type ‘double peak’ histograms at around 1.0–1.5 pA amplitude (Fig. 4D) and to the left of the principle peak in single-peaked histograms (Fig. 4E).
These data support the following conclusions: (i) the predominant form of background K+-channel present in wild-type cells is a TASK-1/TASK-3 heteromultimer; (ii) the predominant form of background K+-channel present in Task-1−/− cells is a TASK-3 homomultimer, and (iii) the predominant form of background K+-channel present in Task-3−/− cells is a TASK-1 homomultimer (see Discussion).
Modulation of background channel activity
The oxygen sensitivity of background channel activity in rat type-1 cells has been well described (Buckler et al. 2000; Kim et al. 2009). It has also been demonstrated that rat type-1 cell background channels are strongly inhibited by metabolic poisons affecting oxidative phosphorylation (Williams & Buckler, 2004; Varas et al. 2007; Buckler, 2012). Little is known about the oxygen and metabolic sensitivity of wild-type TASK-1/TASK-3 channels in mouse type-1 cells or, indeed, of the sensitivities of the homomeric channels TASK-1 and TASK-3. The sensitivity of background/TASK K+-channel activity to hypoxia and inhibitors of oxidative phosphorylation were therefore investigated.
Effects of hypoxia
Acute exposure to hypoxia was achieved using 100 mm K+ HCO3− buffered Tyrode, pre-equilibrated with 5% CO2 95% N2. Exposure of isolated control mouse type-1 cells to hypoxia caused a rapid and reversible inhibition of single-channel activity, manifested as a general reduction in apparent noise when viewed on a long time base (Fig. 5A). Close examination of single-channel activity showed a decrease in multiple channel openings and a decreased frequency of single openings (Fig. 5B). All-points histograms revealed that hypoxia caused a decrease in frequency of all open channel current amplitudes, corresponding to a near uniform inhibition of background K+-channel activity at all conductance levels (Fig. 5C). The main conductance state determined from all-points histograms was used independently to set 50% threshold levels for the determination of nPopen under normoxic and hypoxic conditions. In concordance with previous studies in rat type-1 cells, nPopen under hypoxia fell to a mean ±s.e.m. of 48.7 ± 4.3% of the normoxic control level (n= 24; P < 0.001, single-sample one-tailed t test) (Fig. 5D).
Figure 5. Oxygen sensitivity of TASK channels in type-1 cells.
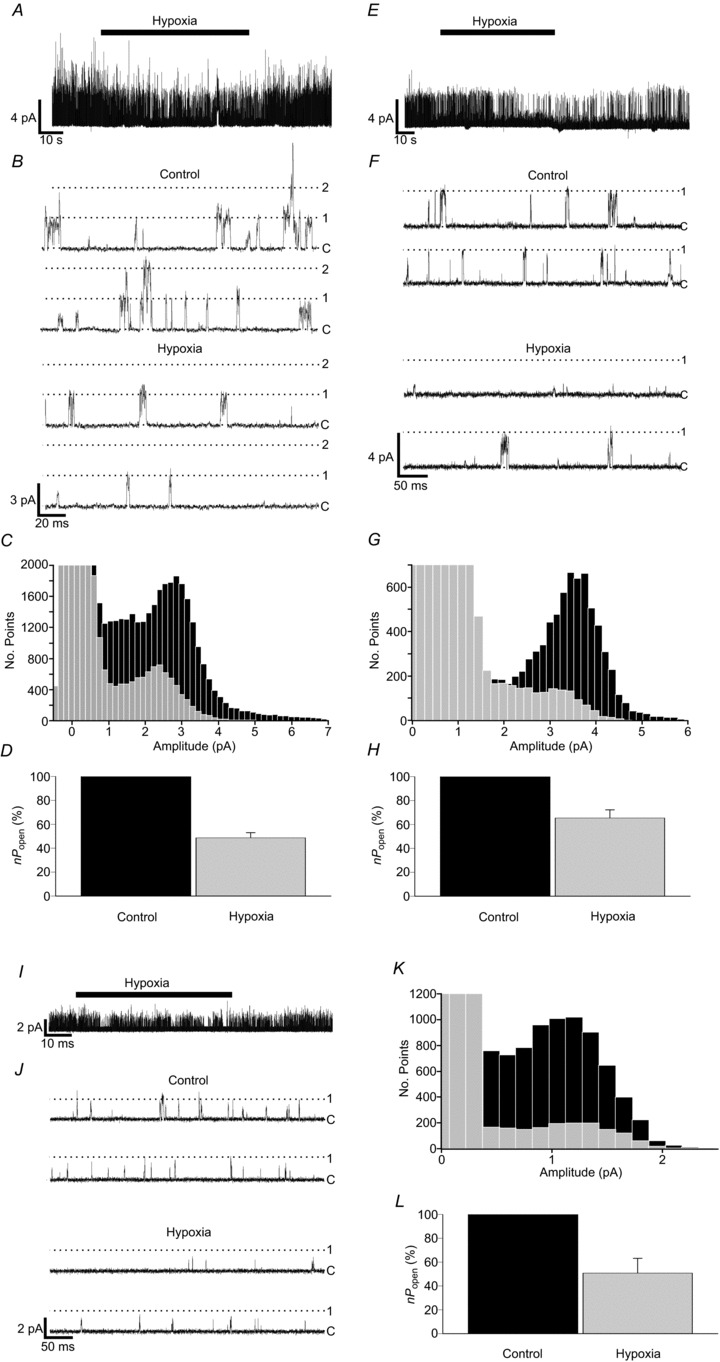
A, B, representative single-channel recording showing reversible inhibition of background channel activity by hypoxia in control type-1 cells. Cells were bathed in 100 mm K+, nominally Ca2+-free Tyrode solution. Hypoxia was induced by gassing Tyrode solutions with 5% CO2 and 95% N2. Pipette solution contained 140 mm K+, with a pipette potential of +80 mV. C, superimposition of all-points histograms derived from recordings made under control and hypoxic conditions. D, comparison of background channel activity under control and hypoxic conditions (n= 24 patches). E, F, single-channel recording from a Task-1−/− type-1 cell showing the reversibility of inhibition of channel activity by hypoxia. G, superimposed histograms constructed from Task-1−/− data obtained under control and hypoxic conditions. H, relative channel activity for Task-1−/− under control and hypoxic conditions (n= 16 patches). I, J, Task-3−/− single-channel activity inhibition by hypoxia. K, superimposition of all-points histograms created from Task-3−/− type-1 cell control and hypoxia recordings. L, comparison of relative channel activity in Task-3−/− cells under control and hypoxic conditions (n= 9 patches).
Examination of Task-1−/− type-1 cell patches revealed similar hypoxia-induced effects. Hypoxia brought about a rapid and reversible inhibition of TASK-3 K+-channel activity (Fig. 5E and F), which was reflected by a lower frequency of current amplitudes corresponding to the main open level in all-points histograms (Fig. 5G). Under hypoxia nPopen fell significantly to 65.4 ± 6.8% of the normoxic control level (n= 16; P < 0.001, single-sample one-tailed t test) (Fig. 5H).
TASK-1 K+-channel activity in Task-3−/− type-1 cells was similarly rapidly and reversibly inhibited by hypoxia (Fig. 5I and J). Under hypoxic conditions nPopen was reduced to 50.8 ± 12.3% of the normoxic value (n= 9; P < 0.005, single-sample one-tailed t test) (Fig. 5L). All-points histograms showed a decrease in frequency of single-channel current at all levels (Fig. 5K).
Effects of CN
The effects of cyanide on background channel activity were analogous to those of hypoxia as described above. Cyanide was added to high K+ Tyrode as NaCN to a concentration of 2 mm. Superfusion of wild-type type-1 cells with NaCN Tyrode caused a rapid inhibition of single-channel activity, which quickly recovered on return to standard Tyrode (Fig. 6A and B). These visual impressions were confirmed by the construction of all-points histograms, which showed a decrease in open-channel current across the whole range of amplitudes, corresponding to a near uniform inhibition of background K+-channel activity at all conductance levels during NaCN exposure (Fig. 6C). Calculated nPopen values under NaCN exposure were 27.2 ± 8.6% of the control value (n= 14; P < 0.001, single-sample one-tailed t test) (Fig. 6D).
Figure 6. Cyanide sensitivity of TASK channels in type-1 cells.
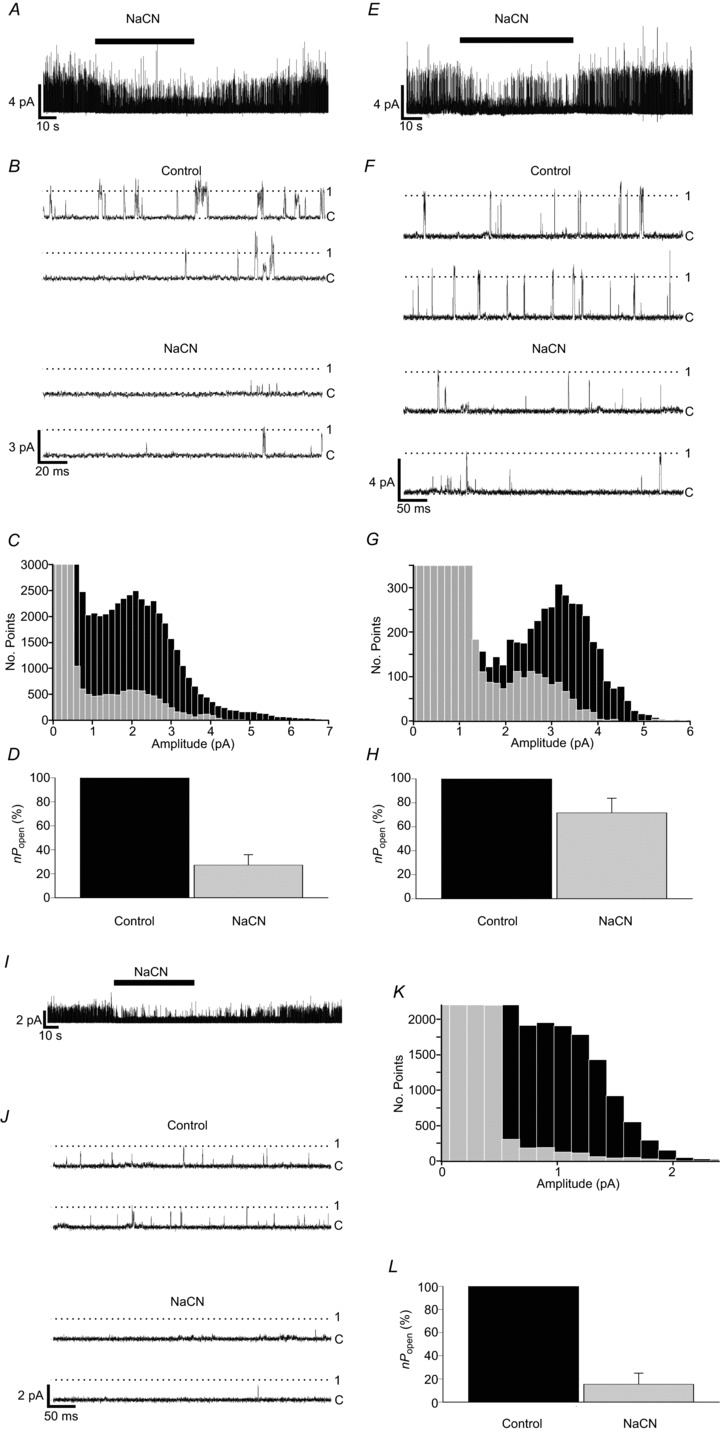
A, B, representative single-channel recording showing the reversible inhibition of background channel activity by 2 mm NaCN in control type-1 cells. Recording conditions were as in Fig. 5. C, superimposition of all-points histograms derived from recordings made during superfusion with control and cyanide Tyrode solutions. D, comparison of relative background channel activity in cell-attached patches under control and cyanide exposure (n= 14 patches). E, F, single-channel recording from a Task-1−/− type-1 cell showing the effects of cyanide. G, superimposed histograms constructed from Task-1−/− data obtained during control and cyanide Tyrode superfusion. H, relative channel activity for Task-1−/− during control and cyanide exposure (n= 6 patches). I, J, Task-3−/− single-channel activity inhibition by cyanide. K, Superimposition of all-points histograms created from Task-3−/− type-1 cell control and cyanide recordings. L, comparison of relative channel activity in Task-3−/− cells during cyanide exposure (n= 6 patches).
Exposure of Task-1−/− type-1 cells to NaCN caused a rapid and reversible inhibition of single-channel activity (Fig. 6E and F). Correspondingly, all-points histograms plotted from these data showed a decrease in frequency of current amplitudes corresponding to the main open state of TASK-3 (Fig. 6G). The effects of NaCN exposure on activity as determined by nPopen calculation appeared to be smaller than those observed in wild-type cells, with a fall in nPopen to 71.6 ± 12.1% of the control value (n= 6; P < 0.05, single-sample one-tailed t test) (Fig. 6H).
Background K+-channel activity in Task-3−/− cells also appeared to be strongly inhibited by NaCN. In these type-1 cells, exposure to 2 mm NaCN caused a rapid and reversible decline in single-channel activity (Fig. 6I and J), with a corresponding decrease in the frequency of current amplitudes corresponding to TASK-1 open events as defined by all-points histograms (Fig. 6K). Using a 50% threshold derived from the main (TASK-1) conductance level, nPopen was calculated to fall to just 15.4 ± 9.6% of the control value (n= 6; P < 0.001, single-sample one-tailed t test) (Fig. 6L).
Effects of FCCP
When exposed to 1 μm FCCP (carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone), single-channel activity in wild-type cells rapidly decreased. Figure 7A shows an example of a multi-channel patch; note that FCCP caused a reduction in multiple and single opening events (Fig. 7A and B) as evidenced by the decline in frequency at all levels of channel current as shown in the all-points histogram (Fig. 7C). The main peak in the all-points histogram was used to set a 50% threshold from which nPopen was determined. FCCP decreased nPopen to just 13.4 ± 4.2% of the control value (n= 13; P < 0.001, single-sample one-tailed t test) (Fig. 7D). Background K+-channel activity rapidly returned to control levels once the toxin was washed off. Task-1−/− type-1 cell background channel activity responded similarly, with a rapid reversible inhibition of channel activity by FCCP exposure (Fig. 7E and F). All-points histograms revealed a substantial decline in the frequency of current amplitudes corresponding to single TASK-3 channel activity (Fig. 7G). Examination of relative channel activity showed a reduction in nPopen during FCCP exposure to 57.3 ± 9.3% of the control value (n= 14; P < 0.001, single-sample one-tailed t test) (Fig. 7H).
Figure 7. FCCP sensitivity of TASK-like channels in type-1 cells.
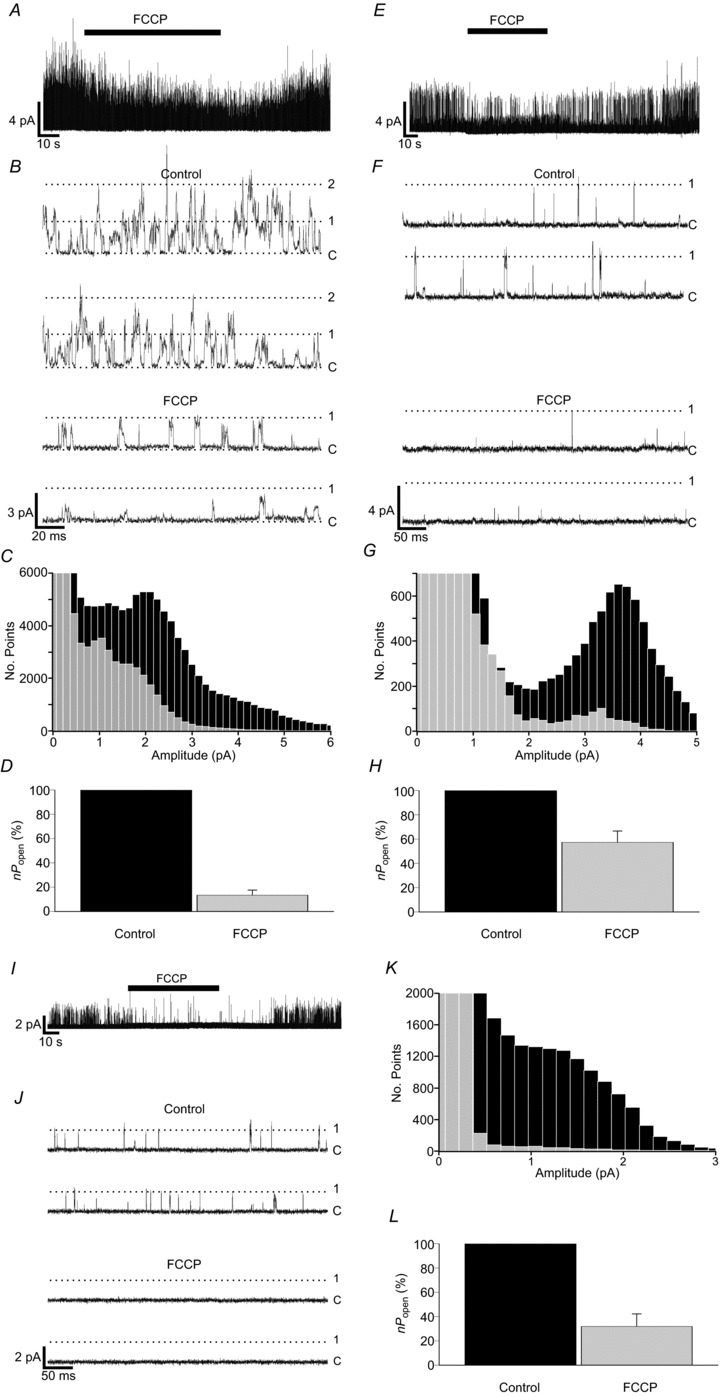
A, B, representative single-channel recording showing the reversibility of inhibition of background channel activity by 1 μm FCCP in control type-1 cells. Recording conditions were as for hypoxia and cyanide experiments. C, superimposition of all-points histograms derived from recordings made during superfusion with control and FCCP Tyrode solutions. D, comparison of relative background channel activity in cell-attached patches during control and FCCP exposure (n= 13 patches). E, F, single-channel recording from a Task-1−/− type-1 cell showing the reversibility of inhibition of channel activity by FCCP. G, superimposed histograms constructed from Task-1−/− data obtained during control and FCCP Tyrode superfusion. H, relative channel activity for Task-1−/− during control and FCCP exposure (n= 14 patches). I, J, Task-3−/− single-channel activity inhibition by FCCP. K, superimposition of all-points histograms created from Task-3−/− type-1 cell control and FCCP recordings. L, comparison of relative channel activity in Task-3−/− cells during FCCP exposure (n= 8 patches).
Application of 1 μm FCCP to Task-3−/− type-1 cells caused an almost complete and reversible inhibition of single-channel activity (Fig. 7I and J). Histograms plotted from data recorded during FCCP exposure had little resolvable peak relative to controls (Fig. 7K), but the decline in the frequencies of current amplitudes relative to the control value was consistent with the inhibition of a TASK-1 channel. Quantification of this response to FCCP by calculation of nPopen revealed that activity was reduced to 31.9 ± 10.3% of the control value (n= 8; P < 0.001, paired one-tailed t test) (Fig. 7L).
Ca2+-signalling in TASK knock-out cells
Having established that the oxygen-sensitive background K+ currents are mediated by TASK-1/TASK-3 channels, we sought to observe the effects of genetic deletion of these channels on calcium signalling in isolated mouse type-1 cells. Baseline levels of calcium were measured in bicarbonate buffered Tyrode equilibrated with 20% oxygen, 5% CO2 at pH 7.4 and 36°C in type-1 cells isolated from all four genotypes used in this study. Mean ±s.e.m. baseline [Ca2+]i in wild-type cells was 112 ± 10 nm (n= 9). Similar levels were observed in Task-1−/− cells ([Ca2+]i= 134 ± 30 nm; n= 11), Task-3−/− cells ([Ca2+]i= 139 ± 36 nm; n= 4) and Task-1−/−/Task-3−/− cells ([Ca2+]i= 125 ± 14 nm; n= 6). None of the values obtained for baseline [Ca2+]i in any of the knock-out genotypes were significantly different from that in wild-type cells.
Type-1 cells were also exposed to hypoxia (95% N2, 5% CO2), which induced a brisk rise in [Ca2+]i in wild-type cells (Δ[Ca2+]i 247 ± 54 nm; n= 9), Task-1−/− cells (Δ[Ca2+]i= 272 ± 44 nm; n= 11), Task-3−/− cells (Δ[Ca2+]i= 226 ± 95 nm; n= 4) and Task-1−/−/Task-3−/− cells (Δ[Ca2+]i= 263 ± 64 nm; n= 6). In all instances the hypoxia-induced rise in [Ca2+]i was substantially inhibited in calcium-free medium by 90 ± 6.5% (n= 4; P < 0.001) in control cells, 92 ± 2.7% (n= 11; P < 0.001) in Task-1−/− cells, 96 ± 2.0% (n= 4; P < 0.001) in Task-3−/− cells and 87 ± 4.7% (n= 3; P < 0.002, single-sample one-tailed t test) in Task-1−/−/Task-3−/− cells (Fig. 8).
Figure 8. [Ca2+]i signalling in wild-type and TASK knock-out type-1 cells.
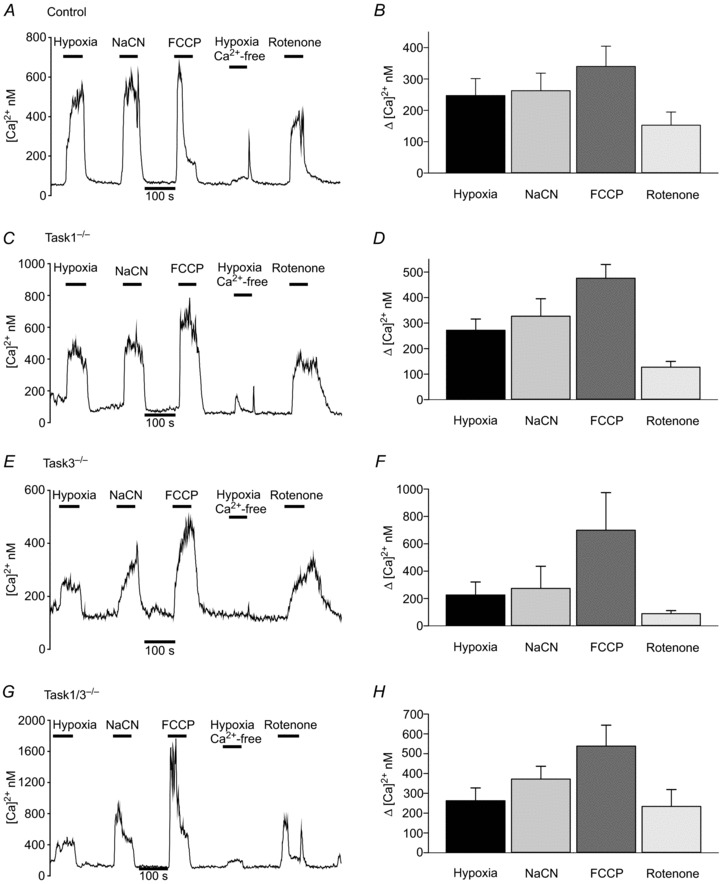
Individual recordings of intracellular [Ca2+]i and summary data showing the effects of hypoxia, hypoxia in a Ca2+-free Tyrode, NaCN (2 mm), FCCP (1 μm) and rotenone (1 μm) in type-1 cells from A, B wild-type mice, C, D Task-1−/− mice, E, F Task-3−/− mice, and G, H Task-1−/−/Task-3−/− mice. Summary data are means ± standard error of the mean.
We further tested cells from all four genotypes for sensitivity to a variety of metabolic/mitochondrial inhibitors including cyanide (2 mM), rotenone and FCCP (both 1 μm). All three agents elicited a robust and statistically significant rise in [Ca2+]i in type-1 cells from mice of all four genotypes (P < 0.05, assessed using a single-sample one-tailed t test on normalized data) (Fig. 8). There were no significant effects of genotype on responses to hypoxia, cyanide, rotenone and FCCP as assessed by two-way ANOVA.
Discussion
The aims of this study were to characterize background K+-channels of the mouse type-1 cell, to establish the roles of Task-1 and Task-3 in forming these channels, and to investigate the hypoxia and metabolic sensitivity of any TASK channels present.
Characteristics of the KB-channel in mouse type-1 cells
Cell-attached recordings from wild-type mouse type-1 cells revealed abundant single-channel activity at negative membrane potentials similar to that previously reported for KB-channels in rat type-1 cells (Williams & Buckler, 2004) (Varas et al. 2007; Buckler, 2012) and to TASK-1 and TASK-3 fusion dimers expressed in HeLa cells (Kim et al. 2009). Mouse KB-channels had flickery kinetics with short-lived open states and poorly defined or variable conductance levels, characteristics that are typical of rat KB activity. Most all-points amplitude histograms typically displayed a single but broad peak at around 2.5 pA (Vp= 80 mV), suggesting one main, if ill-defined, open state.
The KB-channel is predominantly a TASK-1/TASK-3 heteromultimer
We investigated the molecular identity of the background K+-channel in mice by using germline deletion of the two main candidate genes, Task-1−/− and Task-3−/−. The first obvious difference between recordings from wild-type type-1 cells and those from either Task-1−/− or Task-3−/− mice is that channel activity was much lower in both of the knock-out strains. This manifested both as a reduction in mean nPopen for patches with channels and in the number of patches with any TASK-like channel activity. The second obvious difference is in the amplitude of single-channel current. In the Task-1−/− mouse, the main peak observed in all-points histograms occurred at a significantly higher current amplitude than that seen in the wild-type mouse, whereas in the Task-3−/− mouse it occurred at a much lower amplitude than in the wild-type mouse. The differences in single-channel current recorded at a pipette potential of +80 mV were mirrored by differences in the slope conductance in wild-type (33 pS), Task-1−/− (38 pS) and Task-3−/− (18 pS) mice. Thus disruption of either Task-1 or Task-3 reduces background K+-channel activity substantially and fundamentally changes the biophysical properties of the remaining background K+-channels, eliminating the 33 pS channel that predominates in the wild-type cell. Deletion of both Task-1 and Task-3 eliminated almost all K-channel activity at negative membrane potentials. The main form of background K+-channel found in wild-type type-1 cells is therefore dependent upon functional copies of both Task-1 and Task-3 genes. These observations confirm that the predominant background K+-channel present in wild-type type-1 cells is a heteromer of TASK-1 and TASK-3.
TASK-1 and TASK-3 homomers in Task-3−/− and Task-1−/− type-1 cells
The biophysical properties of the background K+-channels remaining in single knock-out animals are comparable with those of TASK-1 and TASK-3 in heterologous expression systems [i.e. the main conductance state in Task-1−/− cells is similar to that reported for TASK-3, 36 pS (Han et al. 2002)] and the main conductance state in Task-3−/− cells is similar to that reported for TASK-1 [16 pS (Kim et al. 1998; Leonoudakis et al. 1998; Patel et al. 1999)]. Moreover, both of these channels were absent from Task-1−/−/Task-3−/− double knock-out type-1 cells. We therefore conclude that the principle form of background K+-channel activity in Task-1−/− type-1 cells is a TASK-3 homomer and that in Task-3−/− cells is a TASK-1 homomer.
Are homomeric forms of TASK-1 or TASK-3 also present in wild-type cells?
Kim et al. (2009) categorized three channel types in rat type-1 cells and attributed them to TASK-1, TASK-3 and TASK-1/TASK-3 heterodimers. An occasional feature (n= 6) of recordings from wild-type mouse type-1 cells was the appearance of a second peak in all-points histograms of conductance comparable with that in heterologously expressed TASK-1 and native TASK-1 channels in Task-3−/− mice. Although this may represent a sub-conductance state, the fact that its presence/activity relative to the main TASK-1/TASK-3 conductance state was highly variable between patches suggests it is more likely to reflect the presence of TASK-1 homomers in wild-type cells.
Although TASK-3 in Task-1−/− mice had a higher conductance than TASK-1/TASK-3 in wild-type cells,1 we did not resolve any higher conductance peaks in all-points histograms compatible with TASK-3 channels being present in wild-type cells. This does not prove that TASK-3 homomers are totally absent, as a low level of activity may well be masked by the variable conductance levels of TASK-1/TASK-3, but it suggests that TASK-3 channel activity is not a prominent feature of mouse type-1 cells.
A conspicuous feature of both Task-1−/− and Task-3−/− type-1 cells was a much reduced level of channel activity compared with that in wild-type cells (average wild-type channel activity is 3.6-fold greater than the sum of channel activity in both single mutants). This is greater than might be expected from a simple gene dosage effect and may reflect a number of potential differences between heteromeric and homomeric forms of TASK, such as: (i) TASK-1/TASK-3 heteromers may be more readily assembled and/or trafficked to the plasma membrane than homomers; (ii) TASK-1/TASK-3 may be less prone to turnover, and (iii) TASK-1/TASK-3 channels may be intrinsically more active than homomeric channels. In respect of (i), it is worthy of note that TASK-1 (K2P3) and TASK-3 (K2P9) can also form heteromeric assemblies with TWIK (K2P1), which is often SUMOylated and the resultant TASK-1/TWIK-1 and TASK-3/TWIK-1 heterodimeric channels silenced (Plant et al. 2012). Thus, if heterodimer formation between TASK-1 and TASK-3 were favoured over other forms, the number of functional channels might be significantly greater in the wild-type than in either of the single knock-out cells. Such factors may also help to explain the relatively minor role played by homomeric forms of these channels in wild-type cells (Kim et al. 2009).
Other channel types present in type-1 cells
Recordings from Task-1−/−/Task-3−/− type-1 cells revealed very little in the way of channel activity at negative membrane potentials of any description. This is in agreement with a substantive increase in input resistance for Task-1−/−/Task-3−/− mouse type-1 cells (Ortega-Saenz et al. 2010). However, Task-1−/−/Task-3−/− type-1 cells were not completely devoid of channel activity. The most conspicuous of the channels observed was a large conductance (108 pS) channel of low activity, which had characteristics similar to those of TREK/TRAAK channels in terms of conductance and flickery kinetics (Patel et al. 1998; Honore et al. 2002). TREK-1 mRNA and TRAAK immunoreactivity have both been found in type-1 cells (Yamamoto et al. 2002; Yamamoto & Taniguchi, 2006). This large conductance channel was also seen in seven patches from Task-1−/− mice and eight patches from Task-3−/− mice. We have not as yet observed this channel in type-1 cell patches from wild-type mice.
Another channel of lower conductance (approximately 7.5 pS) with long and very stable open states was also identified. This channel was found in all mouse genotypes studied and in rat type-1 cells (Buckler K.J., unpublished observations, 2013) and may be the same as an 8 pS channel briefly described by Kim et al. (2009). In a number of patches from knock-out genotypes, we also observed a small voltage-activated channel of approximately 1.4 pA at 80 mV Vp. Activity in this channel was low at negative membrane potentials, but increased by 13-fold upon depolarization (Fig. S1, online).
We did not observe any channel activity that could be ascribed to KATP channels, which have recently been reported in rat type-1 cells (Kim et al. 2011). However, we have previously seen such channels in rat type-1 cells in excised patches under certain conditions, albeit very rarely (K. J. Buckler, unpublished observations, 2001).
It should be noted that both TEA and 4-AP were included in our pipette-filling solution to help isolate TASK from other K+-channels. As a consequence these studies cannot be considered to represent a comprehensive survey of all the channels present in the membranes of type-1 cells. They do, however, confirm a lack of any significant background K+-channel activity other than that which can be attributed to channels derived from Task-1 and Task-3. We conclude therefore that, although other K+-channels are present in these cells, the TEA and 4-AP insensitive background K+ current seen in wild-type type-1 cells (Buckler, 1997) is primarily mediated by a TASK-1/TASK-3 heterodimer.
Effects of hypoxia and metabolic poisons on TASK channel activity
Hypoxia robustly inhibited background K+-channel activity in wild-type cells at all conductance levels (Fig. 5C), which is consistent with an inhibition of the predominant TASK-1/TASK-3 heterodimeric channel, as well as any homomeric TASK-1 channels present. These results confirm previous observations in rat type-1 cells (Buckler et al. 2000; Kim et al. 2009). The inhibitory effects of hypoxia on KB activity were recapitulated by the mitochondrial poisons NaCN (a complex IV inhibitor) and FCCP (an uncoupler), providing further evidence for the metabolic regulation of KB activity (Buckler & Vaughan-Jones, 1998; Williams & Buckler, 2004; Wyatt & Buckler, 2004; Varas et al. 2007). Similar patterns of channel inhibition were also seen with hypoxia, CN and FCCP for TASK-1, in Task-3−/− type-1 cells, and TASK-3, in Task-1−/− type-1 cells. Thus TASK-1, TASK-3 and TASK-1/TASK-3 channels are all regulated by both hypoxia and metabolic inhibition. This suggests that either TASK-1 and TASK-3 share a common regulatory motif or they can assemble/interact with a common regulatory subunit which then couples them to oxygen and metabolic sensing pathways.
Although the qualitative effects of hypoxia and metabolic inhibitors on background channel activity were consistent between genotypes, a two-way ANOVA was carried out to determine whether there were any quantitative differences between genotypes or between stimuli (hypoxia, NaCN and FCCP). This analysis indicated that there was no significant interaction between the effects of genotype and stimuli on channel activity [F(4, 101) = 1.997, P= 0.101]. Post hoc analysis using Tukey's honestly significant differences (HSD) test did, however, indicate that the effects of both NaCN and FCCP were greater than those of hypoxia (P < 0.02 for CN−vs. hypoxia and P < 0.01 for FCCP vs. hypoxia) and that channel activity in Task-1−/− mice was less sensitive to stimuli than that in Task-3−/− or wild-type mice. Thus TASK-3 would seem to be less sensitive to inhibition by hypoxia and metabolic inhibitors than either TASK-1 or the TASK-1/TASK-3 heterodimer.
It seems increasingly probable that both oxygen sensing and metabolic sensing are linked in type-1 cells (Mulligan & Lahiri, 1982; Wyatt & Buckler, 2004). Indeed, it is possible that they are one and the same as mitochondrial function in type-1 cells is extraordinarily oxygen-sensitive (Duchen & Biscoe, 1992a,b; Buckler & Turner, 2013). Two potential mechanisms that may couple TASK channels to metabolism have thus far been described. One hypothesis proposes that channel activity is inhibited through phosphorylation by an AMP-activated protein kinase (AMPK) (Wyatt et al. 2007). However, only TASK-3, not TASK-1, appears to be regulated by AMPK (Dallas et al. 2009). Therefore, this pathway alone cannot account for the regulation of TASK-1 by hypoxia and metabolic inhibitors in Task-3−/− mice. Consequently, should AMPK be an essential link in the hypoxia transduction pathway, the actions of AMPK upon TASK-1 would need to be indirect. A second hypothesis rests on the observation that the wild-type TASK-1/TASK-3 channel of rat type-1 cells can be activated in the excised patch by mm levels of ATP (Varas et al. 2007), suggesting that changes in cytosolic ATP may directly modulate channel function. This property (of ATP sensitivity) has not yet been observed in heterologously expressed TASK-1 or TASK-3 and thus is presumably dependent on some as yet unidentified cofactor or accessory subunit. There may therefore be more to these channels in the form of regulatory subunits yet to be discovered.
Consequences of TASK channel deletion
In rat carotid body type-1 cells, TASK-like potassium channels are highly abundant and are believed to be responsible for the majority of background potassium conductance. Our studies in mice demonstrate that the major form of potassium channel activity present in wild-type cells at negative membrane potentials is a TASK-1/TASK-3 heterodimer and that in Task-1−/−/Task-3−/− mice, background K-channel activity is very much reduced. This conclusion is supported by whole cell recordings that show a substantial decrease in resting membrane conductance in Task-1−/−/Task-3−/− mice (Ortega-Saenz et al. 2010). Given the fundamental importance of background K+ currents in setting resting membrane potential, the loss of TASK-1 and TASK-3 would be expected to result in a sustained depolarization of type-1 cells and a consequent increase in resting [Ca2+]i. Yet resting [Ca2+]i levels were similar in all genotypes. Ortega-Saenz et al. (2010) similarly reported that resting membrane potential was only slightly depolarized in type-1 cells from Task-1−/−/Task-3−/− mice. These data contrast strikingly with the effects of acute inhibition of background K currents by physiological stimuli, chemical stimuli or pharmacological inhibition with Ba2+, all of which cause much greater depolarization and a very marked increase in intracellular calcium (Buckler & Vaughan Jones, 1994b; Buckler & Vaughan-Jones, 1998; Buckler, 1999; Wyatt & Buckler, 2004). This indicates that in the setting of germline deletion of Task-1 and Task-3, the depolarizing effects of the loss of background K+-channel activity must be compensated for. As the resting membrane potential represents a balance between passive outward potassium current plus outward Na+-pump current against inward current generated by the passive influx of other cations (e.g. Na+ and Ca2+) and/or efflux of anions, any loss of background potassium current could be compensated for by changes in permeability to these other ions and/or electrogenic transporter activity. These other background currents are as yet poorly defined in the type-1 cell, but a substantial resting sodium current has been identified (Buckler, 1997; Carpenter & Peers, 2001). A further contributory factor in respect of the limited depolarization reported in type-1 cells from Task-1−/−/Task-3−/− mice may be the accompanying decrease in voltage-gated Ca2+ current density (Ortega-Saenz et al. 2010). Voltage-gated Ca2+ currents tend to become active at potentials positive to −50 mV with significant Ca2+ influx or Ca2+ current often evident at −40 mV (Urena et al. 1989; Buckler & Vaughan Jones, 1994a; Silva & Lewis, 1995). Voltage-gated Ca2+ current would therefore normally add to the depolarizing effects of background inward currents once membrane potential becomes positive to approximately −50 mV.
The idea that germline deletion of TASK-channels leads to compensatory changes in the expression and/or activity of other ion channels is supported not only by observed changes in voltage-gated Ca2+ and –K+ currents in Task-1−/−/Task-3−/− type-1 cells (Ortega-Saenz et al. 2010), but also by studies in other cells in which the knock-out of TASK channels has resulted in the upregulation of GABA(A) receptors in the brain (Linden et al. 2008). These observations make clear the difficulties inherent in attempts to determine the normal physiological role of any ion channel by gene deletion.
We also noted significant chemosensitivity, measured as an increase in [Ca2+]i, to both hypoxia and a range of metabolic inhibitors in all TASK knock-out cells. In Task-1−/− and Task-3−/− cells, this could be explained by the fact that the remaining homomeric forms of TASK-3 and TASK-1 retain sensitivity to both hypoxia and metabolic inhibitors. In the case of the double knock-out, however, the obvious implication is that there must be other currents present in the type-1 cells of Task-1−/−/Task-3−/− mice that are capable of being regulated by hypoxia and metabolic inhibitors. Large-conductance calcium-activated potassium channels (BKCa) have been reported to be oxygen- and cyanide-sensitive in rat type-1 cells (Peers, 1990; Peers & O’Donnell, 1990; Lopez Lopez et al. 1997), but paxilline, an inhibitor of BKCa, failed to mimic or alter the secretory responses to hypoxia in Task-1−/−/Task-3−/− mice (Ortega-Saenz et al. 2010). Voltage-activated potassium channels (KV) have been reported to be oxygen-sensitive in the mouse (Perez Garcia et al. 2004; Yamaguchi et al. 2004), but their activation threshold, particularly in the Task-1−/−/Task-3−/− type-1 cell, seems to be quite low (i.e. positive to −30 mV) (Perez Garcia et al. 2004; Ortega-Saenz et al. 2010). It would thus seem unlikely that oxygen-sensitive BKCa and/or Kv channels can account for hypoxic excitation of Task-1−/−/Task-3−/− type-1 cells. Consequently, our data suggest the presence of novel, as yet unidentified, oxygen- and metabolism-sensitive ionic currents in the type-1 cell. We did observe oxygen sensitivity in one patch from Task-1−/−/Task-3−/− cells in what appeared to be a larger conductance (TREK-like) channel and therefore other background K-channels may be oxygen-sensitive in these cells. Given the low level of background K-channel activity in Task-1−/−/Task-3−/− type-1 cells, however, any small oxygen-sensitive inward current, or possibly the inhibition of an outward pump current, may be sufficient to depolarize and excite these cells. Such currents could easily have been overlooked in previous studies, given the magnitude of the resting K-conductance in wild-type cells. We note from previous studies that the reversal potentials for oxygen-sensitive background currents in rat type-1 cells do not always coincide exactly with those of background K-channel inhibitors (see Fig. 2 in Buckler et al. 2000), which may indicate the presence of an oxygen-sensitive inward current. Task-1−/−/Task-3−/− mice may therefore provide a useful model in which to look for small, novel oxygen-sensitive currents in type-1 cells.
Conclusions
Despite the present considerations regarding the possible existence of other oxygen-sensitive currents in type-1 cells, it is clear from electrophysiological investigations that in normal cells inhibition of a background K current is a major contributor to membrane depolarization in response to both hypoxia and acidosis (Buckler, 1997; Buckler et al. 2000). Our data now confirm that this background K+ current in carotid body type-1 cells is predominantly carried via TASK-1/TASK-3 heterodimeric channels with a lesser contribution from TASK-1 homomeric channels. All three types of channel (TASK-1, TASK-3 and TASK-1/TASK-3) are sensitive to hypoxia and metabolic inhibitors, but the most sensitive seem to be those containing the TASK-1 subunit.
Acknowledgments
We would like to thank Professor William Wisden and Dr Stefan Trapp for the generous gifts of Task-1−/− and Task-3−/− mice. We would also like to thank Denise Jelfs for assistance and advice in maintaining the transgenic mouse lines used in this study.
Glossary
Abbreviations
- AMPK
adenosine monophosphate activated protein kinase
- EK
equilibrium potential for K+ ions
- FCCP
carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone
- GABA (A)
γ-aminobutyric acid ligand-gated ion channel. KB-channel, background K+ channel
- nPopen
estimated channel open probability
- Po2
partial pressure of oxygen
- TASK
TWIK-related acid-sensitive K+ channel
- TEA
tetraethylammonium
- TRAAK
TWIK-related arachidonic-acid-stimulated K+ channel
- TREK
TWIK-related K+-channel
- TWIK
tandem of p domains in a weak inward rectifying K+ channel
- Vp
pipette potential
- 4-AP
4-aminopyridine
Footnotes
Kim et al. (2009) did not observe an appreciable difference between the conductance of heterologously expressed TASK-3 and the TASK-1/3 fusion protein. This may be attributable to a genuine difference between naturally assembled TASK-1/TASK-3 heterodimers and TASK-1/3 fusion proteins, or it may reflect variations in ionic conditions, temperature or species.
Additional information
Competing interests
None declared.
Author contributions
All experiments were performed in the Department of Physiology, Anatomy and Genetics, University of Oxford. K.J.B. and P.J.T. conceived and designed the experiments. P.J.T. performed the experiments, collected data, and was primarily responsible for data analysis. K.J.B. and P.J.T. interpreted the data, K.J.B. drafted the manuscript and both authors participated in the critical review and revision of the manuscript for important intellectual content. Both authors have read and approved the final submitted version of this manuscript.
Funding
This work was funded by a British Heart Foundation project grant (PG/08/086/25849) and a Medical Research Council UK project grant (G101134).
Supplementary material
Fig. S1, online
References
- Aller MI, Veale EL, Linden AM, Sandu C, Schwaninger M, Evans LJ, Korpi ER, Mathie A, Wisden W, Brickley SG. Modifying the subunit composition of TASK channels alters the modulation of a leak conductance in cerebellar granule neurons. J Neurosci. 2005;25:11455–11467. doi: 10.1523/JNEUROSCI.3153-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Aller MI, Sandu C, Veale EL, Alder FG, Sambi H, Mathie A, Wisden W. TASK-3 two-pore domain potassium channels enable sustained high-frequency firing in cerebellar granule neurons. J Neurosci. 2007;27:9329–9340. doi: 10.1523/JNEUROSCI.1427-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brohawn SG, del Marmol J, MacKinnon R. Crystal structure of the human K2P TRAAK, a lipid- and mechano-sensitive K+ ion channel. Science. 2012;335:436–441. doi: 10.1126/science.1213808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ. A novel oxygen-sensitive potassium current in rat carotid body type I cells. J Physiol. 1997;498:649–662. doi: 10.1113/jphysiol.1997.sp021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ. Background leak K+-currents and oxygen sensing in carotid body type 1 cells. Respir Physiol. 1999;115:179–187. doi: 10.1016/s0034-5687(99)00015-8. [DOI] [PubMed] [Google Scholar]
- Buckler KJ. Effects of exogenous hydrogen sulphide on calcium signalling, background (TASK) K channel activity and mitochondrial function in chemoreceptor cells. Pflugers Arch. 2012;463:743–754. doi: 10.1007/s00424-012-1089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ, Turner PJ. Oxygen sensitivity of mitochondrial function in rat arterial chemoreceptor cells. J Physiol. 2013;591:3549–3563. doi: 10.1113/jphysiol.2013.257741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ, Vaughan-Jones RD. Effects of mitochondrial uncouplers on intracellular calcium, pH and membrane potential in rat carotid body type I cells. J Physiol. 1998;513:819–833. doi: 10.1111/j.1469-7793.1998.819ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ, Vaughan Jones RD. Effects of acidic stimuli on intracellular calcium in isolated type I cells of the neonatal rat carotid body. Pflugers Arch. 1993;425:22–27. doi: 10.1007/BF00374499. [DOI] [PubMed] [Google Scholar]
- Buckler KJ, Vaughan Jones RD. Effects of hypercapnia on membrane potential and intracellular calcium in rat carotid body type I cells. J Physiol. 1994a;478:157–171. doi: 10.1113/jphysiol.1994.sp020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ, Vaughan Jones RD. Effects of hypoxia on membrane potential and intracellular calcium in rat neonatal carotid body type I cells. J Physiol. 1994b;476:423–428. doi: 10.1113/jphysiol.1994.sp020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ, Williams BA, Honore E. An oxygen-, acid- and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells. J Physiol. 2000;525:135–142. doi: 10.1111/j.1469-7793.2000.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter E, Peers C. A standing Na+ conductance in rat carotid body type I cells. Neuroreport. 2001;12:1421–1425. doi: 10.1097/00001756-200105250-00025. [DOI] [PubMed] [Google Scholar]
- Chapman CG, Meadows HJ, Godden RJ, Campbell DA, Duckworth M, Kelsell RE, Murdock PR, Randall AD, Rennie GI, Gloger IS. Cloning, localization and functional expression of a novel human, cerebellum specific, two pore domain potassium channel. Brain Res Mol Brain Res. 2000;82:74–83. doi: 10.1016/s0169-328x(00)00183-2. [DOI] [PubMed] [Google Scholar]
- Czirjak G, Enyedi P. Formation of functional heterodimers between the TASK-1 and TASK-3 two-pore domain potassium channel subunits. J Biol Chem. 2002;277:5426–5432. doi: 10.1074/jbc.M107138200. [DOI] [PubMed] [Google Scholar]
- Dallas ML, Scragg JL, Wyatt CN, Ross F, Hardie DG, Evans AM, Peers C. Modulation of O2 sensitive K+ channels by AMP-activated protein kinase. Adv Exp Med Biol. 2009;648:57–63. doi: 10.1007/978-90-481-2259-2_6. [DOI] [PubMed] [Google Scholar]
- Duchen MR, Biscoe TJ. Mitochondrial function in type I cells isolated from rabbit arterial chemoreceptors. J Physiol. 1992a;450:13–31. doi: 10.1113/jphysiol.1992.sp019114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen MR, Biscoe TJ. Relative mitochondrial membrane potential and [Ca2+]i in type I cells isolated from the rabbit carotid body. J Physiol. 1992b;450:33–61. doi: 10.1113/jphysiol.1992.sp019115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e Silva MJ, Lewis DL. L- and N-type Ca2+ channels in adult rat carotid body chemoreceptor type I cells. J Physiol. 1995;489:689–699. doi: 10.1113/jphysiol.1995.sp021083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AM, Mustard KJ, Wyatt CN, Peers C, Dipp M, Kumar P, Kinnear NP, Hardie DG. Does AMP-activated protein kinase couple inhibition of mitochondrial oxidative phosphorylation by hypoxia to calcium signaling in O2-sensing cells. J Biol Chem. 2005;280:41504–41511. doi: 10.1074/jbc.M510040200. [DOI] [PubMed] [Google Scholar]
- Goldstein SA, Price LA, Rosenthal DN, Pausch MH. ORK1, a potassium-selective leak channel with two pore domains cloned from Drosophila melanogaster by expression in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1996;93:13256–13261. doi: 10.1073/pnas.93.23.13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Truell J, Gnatenco C, Kim D. Characterization of four types of background potassium channels in rat cerebellar granule neurons. J Physiol. 2002;542:431–444. doi: 10.1113/jphysiol.2002.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore E, Maingret F, Lazdunski M, Patel AJ. An intracellular proton sensor commands lipid- and mechano-gating of the K+ channel TREK-1. EMBO J. 2002;21:2968–2976. doi: 10.1093/emboj/cdf288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D, Han J, Talley EM, Bayliss DA, Kim D. Functional expression of TASK-1/TASK-3 heteromers in cerebellar granule cells. J Physiol. 2004;554:64–77. doi: 10.1113/jphysiol.2003.054387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Cavanaugh EJ, Kim I, Carroll JL. Heteromeric TASK-1/TASK-3 is the major oxygen-sensitive background K+ channel in rat carotid body glomus cells. J Physiol. 2009;587:2963–2975. doi: 10.1113/jphysiol.2009.171181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Fujita A, Horio Y, Kurachi Y. Cloning and functional expression of a novel cardiac two-pore background K+ channel (cTBAK-1) Circ Res. 1998;82:513–518. doi: 10.1161/01.res.82.4.513. [DOI] [PubMed] [Google Scholar]
- Kim D, Kim I, Papreck JR, Donnelly DF, Carroll JL. Characterization of the ATP-sensitive K+ channel in the rat carotid body glomus cells. Respir Physiol Neurobiol. 2011;177:247–255. doi: 10.1016/j.resp.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Bang H, Kim D. TASK-3, a new member of the tandem pore K+ channel family. J Biol Chem. 2000;275:9340–9347. doi: 10.1074/jbc.275.13.9340. [DOI] [PubMed] [Google Scholar]
- Leonoudakis D, Gray AT, Winegar BD, Kindler CH, Harada M, Taylor DM, Chavez RA, Forsayeth JR, Yost CS. An open rectifier potassium channel with two pore domains in tandem cloned from rat cerebellum. J Neurosci. 1998;18:868–877. doi: 10.1523/JNEUROSCI.18-03-00868.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage F, Guillemare E, Fink M, Duprat F, Lazdunski M, Romey G, Barhanin J. TWIK-1, a ubiquitous human weakly inward rectifying K+ channel with a novel structure. EMBO J. 1996a;15:1004–1011. [PMC free article] [PubMed] [Google Scholar]
- Lesage F, Reyes R, Fink M, Duprat F, Guillemare E, Lazdunski M. Dimerization of TWIK-1 K+ channel subunits via a disulfide bridge. EMBO J. 1996b;15:6400–6407. [PMC free article] [PubMed] [Google Scholar]
- Linden AM, Aller MI, Leppa E, Rosenberg PH, Wisden W, Korpi ER. K+ channel TASK-1 knockout mice show enhanced sensitivities to ataxic and hypnotic effects of GABA(A) receptor ligands. J Pharmacol Exp Ther. 2008;327:277–286. doi: 10.1124/jpet.108.142083. [DOI] [PubMed] [Google Scholar]
- Lopez Lopez JR, Gonzalez C, Perez Garcia MT. Properties of ionic currents from isolated adult rat carotid body chemoreceptor cells: effect of hypoxia. J Physiol. 1997;499:429–441. doi: 10.1113/jphysiol.1997.sp021939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AN, Long SB. Crystal structure of the human two-pore domain potassium channel K2P1. Science. 2012;335:432–436. doi: 10.1126/science.1213274. [DOI] [PubMed] [Google Scholar]
- Mulligan E, Lahiri S. Separation of carotid body chemoreceptor responses to O2 and CO2 by oligomycin and by antimycin A. Am J Physiol Cell Physiol. 1982;242:C200–C206. doi: 10.1152/ajpcell.1982.242.3.C200. [DOI] [PubMed] [Google Scholar]
- Ortega-Saenz P, Levitsky KL, Marcos-Almaraz MT, Bonilla-Henao V, Pascual A, Lopez-Barneo J. Carotid body chemosensory responses in mice deficient of TASK channels. J Gen Physiol. 2010;135:379–392. doi: 10.1085/jgp.200910302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AJ, Honore E, Lesage F, Fink M, Romey G, Lazdunski M. Inhalational anesthetics activate two-pore-domain background K+ channels. Nat Neurosci. 1999;2:422–426. doi: 10.1038/8084. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honore E, Maingret F, Lesage F, Fink M, Duprat F, Lazdunski M. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J. 1998;17:4283–4290. doi: 10.1093/emboj/17.15.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peers C. Hypoxic suppression of K+ currents in type I carotid body cells: selective effect on the Ca2+-activated K+ current. Neurosci Lett. 1990;119:253–256. doi: 10.1016/0304-3940(90)90846-2. [DOI] [PubMed] [Google Scholar]
- Peers C, O’Donnell J. Potassium currents recorded in type I carotid body cells from the neonatal rat and their modulation by chemoexcitatory agents. Brain Res. 1990;522:259–266. doi: 10.1016/0006-8993(90)91470-2. [DOI] [PubMed] [Google Scholar]
- Perez Garcia MT, Colinas O, Miguel Velado E, Moreno Dominguez A, Lopez Lopez JR. Characterization of the Kv channels of mouse carotid body chemoreceptor cells and their role in oxygen sensing. J Physiol. 2004;557:457–471. doi: 10.1113/jphysiol.2004.062281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant LD, Zuniga L, Araki D, Marks JD, Goldstein SA. SUMOylation silences heterodimeric TASK potassium channels containing K2P1 subunits in cerebellar granule neurons. Sci Signal. 2012;5:84. doi: 10.1126/scisignal.2003431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan S, Wischmeyer E, Xin Liu G, Preisig Muller R, Daut J, Karschin A, Derst C. TASK-3, a novel tandem pore domain acid-sensitive K+ channel. An extracellular histiding as pH sensor. J Biol Chem. 2000;275:16650–16657. doi: 10.1074/jbc.M000030200. [DOI] [PubMed] [Google Scholar]
- Trapp S, Aller MI, Wisden W, Gourine AV. A role for TASK-1 (KCNK3) channels in the chemosensory control of breathing. J Neurosci. 2008;28:8844–8850. doi: 10.1523/JNEUROSCI.1810-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urena J, Lopez Lopez J, Gonzalez C, Lopez Barneo J. Ionic currents in dispersed chemoreceptor cells of the mammalian carotid body. J Gen Physiol. 1989;93:979–999. doi: 10.1085/jgp.93.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varas R, Wyatt CN, Buckler KJ. Modulation of TASK-like background potassium channels in rat arterial chemoreceptor cells by intracellular ATP and other nucleotides. J Physiol. 2007;583:521–536. doi: 10.1113/jphysiol.2007.135657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BA, Buckler KJ. Biophysical properties and metabolic regulation of a TASK-like potassium channel in rat carotid body type 1 cells. Am J Physiol Lung Cell Mol Physiol. 2004;286:L221–L230. doi: 10.1152/ajplung.00010.2003. [DOI] [PubMed] [Google Scholar]
- Wyatt CN, Buckler KJ. The effect of mitochondrial inhibitors on membrane currents in isolated neonatal rat carotid body type I cells. J Physiol. 2004;556:175–191. doi: 10.1113/jphysiol.2003.058131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt CN, Evans AM. AMP-activated protein kinase and chemotransduction in the carotid body. Respir Physiol Neurobiol. 2007;157:22–29. doi: 10.1016/j.resp.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Wyatt CN, Mustard KJ, Pearson SA, Dallas ML, Atkinson L, Kumar P, Peers C, Hardie DG, Evans AM. AMP-activated protein kinase mediates carotid body excitation by hypoxia. J Biol Chem. 2007;282:8092–8098. doi: 10.1074/jbc.M608742200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Lande B, Kitajima T, Hori Y, Shirahata M. Patch clamp study of mouse glomus cells using a whole carotid body. Neurosci Lett. 2004;357:155–157. doi: 10.1016/j.neulet.2003.10.062. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Kummer W, Atoji Y, Suzuki Y. TASK-1, TASK-2, TASK-3 and TRAAK immunoreactivities in the rat carotid body. Brain Research. 2002;950:304–307. doi: 10.1016/s0006-8993(02)03181-5. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Taniguchi K. Expression of tandem P domain K+ channel, TREK-1, in the rat carotid body. J Histochem Cytochem. 2006;54:467–472. doi: 10.1369/jhc.5A6755.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


