Abstract
The dog is recognized as a highly predictive model for pre-clinical research. Its size, life span, physiology and genetics more closely match human parameters than do those of the mouse model. Investigations of the genetic basis of disease and of new regenerative treatments have frequently taken advantage of canine models. However, full utility of this model hasn’t been realized because of the lack of easy transgenesis. Blastocyst-mediated transgenic technology developed in mice has been very slow to translate to larger animals, and somatic cell nuclear transfer remains technically challenging, expensive, and low yield. Spermatogonial stem cell (SSC) transplantation, which does not involve manipulation of ova or blastocysts, has proven to be an effective alternative approach for generating transgenic offspring in rodents, and in some large animals. Our recent demonstration that canine testis cells can engraft in a host testis, and generate donor-derived sperm, suggests that SSC transplantation may offer a similar avenue to transgenesis in the canine model. Here, we explore the potential of SSC transplantation in dogs as a means of generating canine transgenic models for pre-clinical models of genetic diseases. Specifically, we 1) established markers for identification and tracking canine spermatogonial cells; 2) established methods for enrichment and genetic manipulation of these cells; 3) described their behavior in culture; and 4) demonstrated engraftment of genetically manipulated SSC, and production of transgenic sperm. These findings help set the stage for generation of transgenic canine models via SSC transplantation.
Introduction
Spermatogonial stem cells (SSC) are the stem cells in testis that generate spermatozoa throughout the adult life of the male. As with all true stem cells, SSC can undergo both self-renewal, and differentiation divisions, thereby maintaining a static population of stem cells, while generating a constant supply of spermatozoa. In mice, these cells can be isolated and expanded indefinitely without genetic drift or loss of stem cell potential (Shinohara et al. 2000a, Shinohara & Brinster 2000, Shinohara et al. 2000b, Kanatsu-Shinohara et al. 2003, Nagano et al. 2003, Kubota et al. 2004, Hamra et al. 2005, Kanatsu-Shinohara et al. 2005, Oatley & Brinster 2006, Hamra et al. 2008, Oatley & Brinster 2008, Oatley et al. 2010). They can be genetically manipulated efficiently by transduction and transfection (Kanatsu-Shinohara et al. 2004, Hamra et al. 2005, Kanatsu-Shinohara et al. 2006, Kanatsu-Shinohara & Shinohara 2007, Takehashi et al. 2010, Kanatsu-Shinohara et al. 2011). SSC provide an alternative approach for the generation of transgenic mice. When transplanted into the testes of sterile male mice, SSC efficiently repopulate the seminiferous tissue, and re-initiate spermatogenesis. The offspring of these males carry the genetic properties of the donor cells (Kanatsu-Shinohara et al. 2004, Kanatsu-Shinohara et al. 2006, Kanatsu-Shinohara & Shinohara 2007, Takehashi et al. 2010, Kanatsu-Shinohara et al. 2011). Mouse SSC can also be converted into pluripotent cells, without genetic manipulation (Guan et al. 2006, Conrad et al. 2008, Izadyar et al. 2008, Golestaneh et al. 2009b, Mizrak et al. 2010). These germ line-derived pluripotent cells (gPS) acquire an expression profile similar to embryonic stem cells (ESC; (Silva et al. 2009)), and are functionally indistinguishable from ESC: They form complex teratomas, differentiate into three germ layers in culture, and contribute to all the tissues of mice generated from chimeric blastocysts (Takehashi et al. 2007, Conrad et al. 2008, Izadyar et al. 2008). Thus, at least in the mouse, SSCs provide an unusually versatile source of material for stem cell and developmental research, including transgenic animal technology, and stem cell-based cell therapy.
It would be extremely valuable to translate this technology to large animals for modeling human diseases. The SSC could be used directly to generate transgenic animal models for preclinical research, and the gPS would serve to bypass both the ethical concerns regarding ESC and the potential of genetic anomalies created in the multi-gene insertion approaches to generating “induced pluripotent stem” (iPS) cells. Other approaches to transgenic models in large animals have been very difficult and inefficient. While multiple lines of canine (Hayes et al. 2008, Wilcox et al. 2009), and other large animal (Kumar De et al. 2011, Vassiliev et al. 2011, Kim et al. 2012) embryonic stem cells (ESCs) have been reported, all non-rodent lines tend to show genetic drift, and loss of pluripotency over time (Yang et al. 2010, Gerwe et al. 2011). In addition, demonstration of germ-line transmission and generation of transgenic large-animal models from ESC have been largely unsuccessful. Genetically chimeric pigs have been produced recently (West et al. 2010) by implanting iPS into early embryos, but this approach has not yet succeeded in other large animals. Several transgenic dogs (Hong et al. 2009, Hong et al. 2011), and other large-animal models (An et al. 2012, Giraldo et al. 2012, Jung et al. 2012) have been generated through somatic nuclear transfer but, so far, this approach has been extremely labor, cost and animal intensive.
Several authors have reported isolation and short-term culture of SPG from large animals (Kim et al. 2006, Rodriguez-Sosa et al. 2006, Goel et al. 2007, Hermann et al. 2007, Aponte et al. 2008) and humans (Wu et al. 2009a), as well as conversion of these cells into pluripotent gPS cells (Golestaneh et al. 2009b). SSC transplantation, and subsequent donor sperm production has now been reported in pigs, sheep, bulls, goats, monkeys and dogs (Izadyar et al. 2003, Kim et al. 2008, Herrid et al. 2011, Jahnukainen et al. 2011, Hermann et al. 2012, Zeng et al. 2012, Zeng et al. 2013). More importantly, SSC transplants in both sheep (Herrid et al. 2009) and goats (Honaramooz et al. 2003) have led to the birth of donor-derived offspring through normal mating. So this technology is clearly applicable to large-animals.
In this light, the canine has major potential as a pre-clinical transgenic model for two major reasons. First, research with the canine model has proven to be highly translatable to the clinical setting. This model better reflects the size, life span, physiology, and genetics of humans than does the mouse model (Tsai et al. 2007). The canine model is also much more cost-effective than primate models. It is a primary preclinical model for hematopoietic stem cell transplantation ((Lupu & Storb 2007)), gene therapy (Nowend et al. 2011, Okazuka et al. 2011) and cancer research (Gordon & Khanna 2010, Rowell et al. 2011). The dog displays a large repertoire of naturally occurring genetic diseases with human counterparts (Athanasiou et al. 1995, Lingaas et al. 2003, Acland et al. 2005) and where data are available, the same genes are involved in both species (Tsai et al. 2007). Second, the ability of canine SSC to engraft and reinitiate spermatogenesis in a recipient testis has been demonstrated (Kim et al. 2008). So far, canine SSC activity has only been shown in freshly prepared, un-fractionated testis cells. It remains to be demonstrated that canine SSC can retain engraftment potential after purification, genetic manipulation, culture, and cryopreservation.
Here, we describe, for the first time, the identification, isolation, culture, and genetic manipulation of canine SSC. We demonstrate engraftment and generation of mature transgenic sperm by canine SSC transplanted into the testes of recipient males. A true SSC can only be distinguished from committed and differentiating spermatogonia by its ability to engraft. Therefore, the term, “SSC”, will be reserved henceforth for true stem cells, while the more general term “spermatogonia” (SPG) will be used when that distinction is not verifiable.
Materials and Methods
Reagents
Hanks’ balanced salt solution (HBSS: cat #14175), penicillin/streptomycin (15140- 122), B27-vitamin A supplement (12587-010), trypsin-EDTA (25200-056), Superscript II reverse transcriptase (18064-014), all Alexa-tagged secondary antibodies (Supplemental Table I), Dispase (17105-041), dispase (17105-041), mouse LIF (LIF2005), and heat-inactivated Fetal Bovine Serum (FBS) (10082147), were all from Invitrogen (Eugene, OR). Biosprint 96 blood DNA isolation kits (940057), and RNAeasy RNA isolation mini kits (74104), were from Qiagen (Valencia, CA). Type 4 collagenase (LS004212) and hyaluronidase (LS005475) were from Worthington (Lakewood, NJ). Primary antibodies were purchased as indicated in Supplementary Table I. DME/F12 medium (SH30023.01) and buffered formalin (SF98-4) were from Fisher Scientific (Waltham, MA). Primer synthesis was done by Operon (Huntsville, AI). Trypsin (T4799), DNase I (D4513-1VL), laminin (L2020), gelatin (G1890-100G), L-Glutamine (G6392- 10L), BSA (A9576-50ml), donkey serum (D9663-10ML), and human FGF2 (F0291-25UG) were from Sigma (St. Louis, MO). Advantage 2 polymerase mix (639202) was from Clontech (Mountain View, CA). Human EGF (354052) was from BD Biosciences. Human GDNF (212-GD- 050) was from Roche Biochemicals(Indianapolis IN). Rat soluble GFRA1 (560-GR-100), human BDNF (284-BD-025), human NT3 (267-N3-025), human NT4 (268-N4-025), human Pleiotropin (252-PL-250), and human WNT3A (5036-WIN-010) were all from R&D Systems (Minneapolis, MN). Target retrieval solution (S1699) was from DAKO (Carpinteria, CA). Falcon Tissue culture flasks and plates were from BD Biosciences (San Jose, CA).
Canine testis tissues
Testes were obtained from the Seattle Spay and Neuter Clinic, immediately following routine neutering for unrelated purposes. Tissue was collected from a variety of breeds in the age range of 3 to 12 months. Testes were transported in sterile Hanks Buffered Saline on ice, and used within 2 hours of castration.
Isolation and culture of SPG
Testes from 3–5 month old (pre-pubertal) dogs were washed twice in fresh, sterile HBSS. The testis parenchyma, containing the testicular cords, was dissected away from the tunica vaginalis and tunica albuginea, epididymis, and other non-seminiferous tissue, washed twice in HBSS, minced to < 1 mm pieces, and digested for 30 minutes at 37°C in 30 ml DME/F12 medium containing 1 mg/ml type 4 collagenase and 33 μg/ml DNase I. Tissue was dispersed into single cells and small lengths of cords by vigorous pipetting, then filtered through sterile gauze. The filtrate was washed twice in HBSS by centrifugation at 200 × g for 5 minutes. The washed cells and cord segments were plated in 225 cm2 culture flasks at an estimated 5×107 cells per flask in DMEM/F12 medium containing 10% FBS, 50 units/ml penicillin, and 50 μg/ml streptomycin. At 3 days culture, dead cells were removed by replacing the medium with HBSS. Loose cells were then dislodged from the tissue culture layer by 15–20 vigorous horizontal raps of the flask against a styrofoam block. The loose cells were pooled, and selected for non-adhesion to gelatin by 2 rounds of plating for 2 hours on a gelatin-coated 10 cm tissue culture dish and re-collection of all non-adherent cells. The remaining loose cells were either used directly for analysis, or cryo-preserved under liquid nitrogen in 70% DME/F12, 20% FBS, 10% DMSO. Cells were cultured in either of two basic media: A) Complete DMEM/F12: DME/F12 with 10% FBS and Pen/Strep or B) SG: a serum-free medium optimized for rat SSC culture (Wu et al. 2009b). Additional modifications to the culture conditions, including substratum, feeder cells, serum level, and growth factors, are specified in Supplementary Table II. Mouse Embryonic Feeder Cells (MEFs) were expanded from 14 day mouse embryos, mitotically inactivated by γ irradiation (3000 Rad), and plated at 25,000 cells/cm2 1 day prior to addition of SPG. Testis fibroblast feeder cells were adherent cells from 3 month old canine testis, expanded for at least 3 passages and at least 10 days of constant culture, then irradiated at 3000 Rad and plated at 25,000 cells/cm2 1 day prior to addition of SPG. Cultures were maintained at 37°C in a humidified tissue culture incubator with 5% CO2, and media were changed every 48 hours.
Lentiviral constructs
Self-inactivating lentivral reporter construct, PL-SIN-EF1a-EGFP (Hotta et al. 2009), driven by the widely expressed mouse EF1α promoter, was purchased through Addgene. A second self-inactivating lentiviral construct carrying a widely expressed PGK- mCherry reporter (pRCH-PCh-W), was obtained from the Viral Vector Production Core at Fred Hutchinson Cancer Research Center. Packaging vector, psPAX2 (plasmid 12260) and vsv-G envelope vector, pMD2.G (plasmid 12259) were from the Trono lab and purchased from Addgene (http://www.addgene.org/Didier_Trono).
Lentiviral Transduction of SPG
PL-SIN-EF1a-EGFP and pRCH-PCh-W were packaged in HEK293 cells as VSVG-pseudotyped lentiviral particles according to Addgene protocol E.2 (http://www.addgene.org), filtered through a .45 μM filter (Millipore, Billerica, MA), concentrated by centrifugation at 13,000g for 15 hours, and stored in aliquots at −80°C. Infectious particle concentrations were determined by titered infection of HEK293 cells (15 hours transduction in the presence of 4 μg/ml polybreen on 70% confluent cells), followed by determination of percentage of reporter-expressing cells at 48 hours post-infection. Final titers were 1–2×107 infectious units/ml. Transduction of freshly isolated SPG (“loose cells) was performed by incubating 0.5–2×106 cells with virus (moi = 5–15) in 1 ml transduction medium (DMEM/F12/10%FBS/4 μg/ml polybreen) for 15 hours in a tissue culture incubator. Cells were then washed twice with HBSS, and plated in SG medium. Expression of reporter genes was monitored by flow cytometry and by epifluorescence microscopy.
Histology and Immunohistochemistry
Seminiferous tissue was cut into 1 cm cubes, fixed for 72 hours in buffered formalin, embedded in paraffin and sectioned at 1.5 μm thickness. Sections were deparaffinized in 3 washes of xylene, rehydrated through an ethanol series (100%,100%, 85%, 70%, water), and washed twice in TBST (50 mM Tris, 150 mM NaCl, 0.1% Tween 20, pH 7.6) all for 10 minute intervals. Antigen retrieval was performed by a 20 minute incubation at 95°C in a pre-warmed target retrieval buffer (DAKO Carpinteria, CA), followed by 3 washes in HBSS. Treated sections were incubated for 30 minutes in blocking buffer (TBST + 5% BSA and 5% Donkey Serum), then for 15 hours at 4°C in blocking buffer plus primary antibody (see Supplementary Table I for antibodies and concentrations). Sections were washed 5 × 3 minutes in TBST, incubated 2 hours at RT in a 1000 X dilution of an appropriate Alexa Fluor-tagged donkey secondary antibody (Supplemental Table I) in TBST, washed similarly, and observed by epifluorescent microscopy. Cultured cells were fixed on the plate for 20 minutes in 10% buffered formalin, and probed as described for post-antigen retrieval sections. Dolichos biflorus agglutinin (DBA) mediated localization of α-D-N-acetyl-galactosamine was performed as described (Izadyar et al. 2002).
Microscopy
Phase and Epifluorescent microscopy were done on an Eclipse Ti-U inverted microscope equipped with several phase lenses, a TI-FL Epi-fl epi-illumination system, and digital DS-Qi1Mc monochrome camera (Nikon). Images were recorded and pseudo-colored using NIS-Elements D3.2 software from Nikon and color-merged with Photoshop software (Adobe).
Flow cytometry analysis
For dual reporter expression studies, cells were washed twice with HBSS, dissociated with Trypsin-EDTA for 5 minutes at room temperature, washed twice with HBSS and observed by flow cytometry on a FACS Calibur flow cytometer (BD Biosciences). For comparison of GFP reporter expression to VASA immunolocalization, cells were washed twice with HBSS, dissociated with Trypsin-EDTA for 5 minutes at room temperature, fixed for 5 minutes in 10% buffered formalin, washed 3 times in HBSS, blocked 10 minutes in blocking buffer, incubated at RT for 1 hour in blocking buffer containing anti VASA antibody (Supplemental Table I) at 100 X dilution, re-washed and incubated with donkey anti rabbit-Alexa 594 secondary antibody (Supplemental Table I), rewashed, and observed by flow cytometry as above. All washes were performed by re-suspension in HBSS and centrifugation at 200 × g for 5 minutes.
RTPCR analysis of gene expression
Three separate experiments were performed using dissociated testicular cells from three different pre-pubertal dogs: a 16-week old Black Labrador, and two 10-weeks old German Shepherd littermates. For each testis sample, RNA was extracted from intact seminiferous tissue at day 0, from the total cultured cells at 3 days, from the “loose” and adherent cell populations isolated at day 3, and from duplicate loose and adherent populations after 30 days in culture. RNA from testis tissue and cultured cells was extracted using an RNeasy mini kit (Qiagen) according to manufacturer’s instructions, and quantitated by UV spectral analysis using a Nanodrop™ 1000 spectrophotometer (ThermoFisher Scientific Inc). Two μg RNA was reverse transcribed using oligo dT18 primers and Superscript II Reverse Transcriptase (Invitrogen) according to manufacturer’s instructions. Prior to expression analysis, cDNAs were normalized to beta Actin (ACTB) by adjusting the cDNA concentrations until they gave similar signals by ACTB RTPCR. The adjusted cDNAs were used for all subsequent expression analysis. Primers for amplification of each specific mRNA target (Supplemental Table III) were designed to amplify a region spanning one or more introns of significant size, thus eliminating false positive signals from contaminating genomic DNA. Each primer pair was verified by cloning and sequencing the product amplified from testis cDNA. Primers were designed with a Tm of 72–74°C to accommodate a uniform two-step cycle of 20 sec at 94°C and 2 minutes at 68°C. Amplifications were performed as 20 μl reactions containing 2 μl normalized cDNA, primers at 200 nM, dNTPs at 200 μM and Advantage 2 polymerase mix and buffer. Products were separated by agarose, gel electrophoresis and detected by ethidium bromide staining.
Transplantation of SSC
Several preparations of donor SPG (Supplementary Table IV) were prepared and transduced with lentivirus, as described above, washed in serum-free and growth factor-free SG medium, and cryopreserved in SG / 20% BSA / 10% DMSO. On the day of transplantation, each cell preparation was thawed, washed 3 times in DME/F12, re-suspended in 1 ml DME/F12, and held at 4°C until use (1–3 hours). Transplantation of SPG and all animal care and handling was done at Cornell University, and all related protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Cornell University. Three 5- months-old hounds (Marshall BioResources, North Rose, NY, USA) had their testes subjected to focal external beam radiation to deplete their endogenous germ cells as described (Kim et al. 2008), except that the irradiation regimen was reduced from 3 consecutive days of 3 Gy/day, 8 weeks prior to transplant, to a single 3 Gy dose on the day of transplant. The smaller dose was designed to lower the apparently excessive toxicity to the testis tissues observed in our previous study, which had resulted in complete loss of libido, and almost no recovery of testis size, histological evidence of spermatogenesis, or sperm count. One ml of medium containing 2–5×105 SPG was injected in retrograde fashion into the rete testis under the guidance of ultrasound scanning (Aloka 633, Colormetrics Medical Systems Inc., Wallingford, CT, USA) as previously described (Kim et al. 2008).
VNTR analysis of donor/recipient sperm production
DNA was purified by silica binding from ejaculated sperm, using a Qiagen Biosprint magnetic bead DNA isolation kit, according to the manufacturer’s instructions. Donor and recipient DNA standards were prepared respectively from testis tissue used to generate each SSC preparation, and blood from pre-transplant recipients. The contribution of recipient and donor sperm cells was quantified by fluorescent VNTR (Variable Number Tandem Repeat) analysis of microsatellite loci, as described (Scharf et al. 1995, Hilgendorf et al. 2005) and modified (Graves et al. 2007). Micro-satellite FH2001, was used to distinguish the DNA of recipients 795941 and 795917 from their respective donor cells. Microsatellites FH2611 and FH2199 were used to distinguish Recipient 795933 from its donor cells. The primers used for these VNTRs are listed in Supplemental Table V. In all cases standard curves showed a threshold of unequivocal detection of donor DNA at 1–2% of total.
PCR detection of transgenic cells in sperm
Sperm DNA was analyzed for the presence of PL-SIN-EF1a-EGFP sequence by PCR. Three distinct regions within the construct were amplified in separate reactions as a means of corroborating low signal reactions (see Supplementary Table V for primer sequences and Fig. 7b for target positions). Amplification was in 20 μl reactions containing 100 ng DNA, 200 nM primers, 200 μM dNTPs, and 1X Advantage 2 polymerase mix and buffer. Reactions were run for 35–40 cycles under the 2-step conditions used for RTPCR. As standards for semi-quantitative estimation of vector copy #, reactions were run simultaneously on a titration series of 0, 20, 60, 200, and 2000 copies of linearized PL-SIN-EF1a-EGFP plasmid (based on a plasmid size of 7601 base pairs, and a calculated mass of 7.78 atograms per molecule) in 100 ng canine DNA (estimated at 40,000 haploid genomes, based on a haploid canine genome size of 2.5 × 109 base pairs or 2.56 pg (http://www.genome.ucsc.edu/).
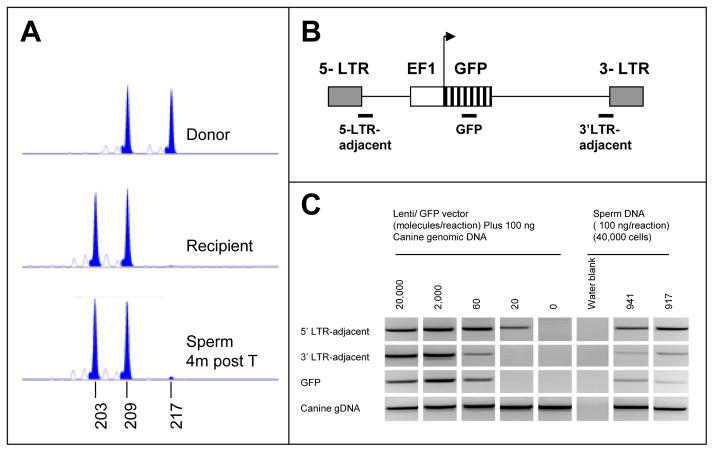
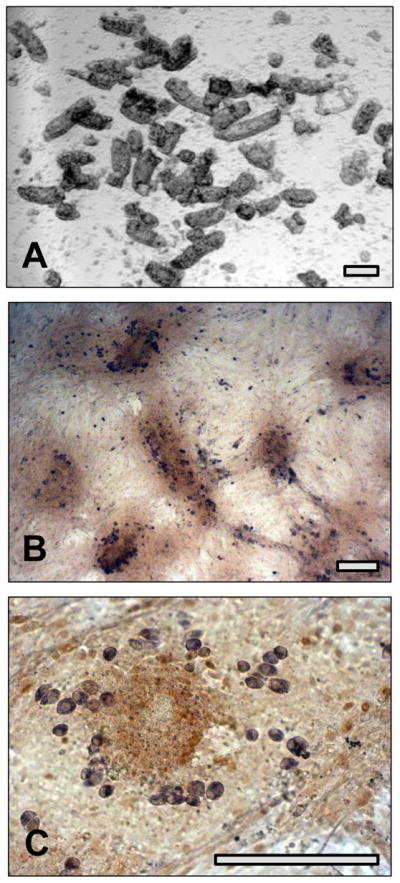
Figure 7. Engraftment of transgenic SSC.
(A) At 4 months post-transplant, a sperm sample from recipient 795933, was tested for donor sperm contribution by VNTR. Microsatellite FM2611 was amplified from donor (A) and recipient (B) DNA as well as from post-transplant sperm. The donor-specific allele (217 base pair product) was also evident in the sperm DNA sample at a level of about 1%, as calculated by comparison of the areas under the donor and recipient-specific (203 base pair) peaks. (B) Diagram of EF1-GFP vector, showing three regions amplified for corroborated detection of vector. (C) PCR of EF1-GFP vector from ejaculated sperm of two transplanted dogs. Sperm was taken 14 months after each dog (941 and 917) had been transplanted with SPG that had been transduced with the EF1-GFP virus. 100 ng sperm DNA, representing approximately 40,000 haploid cells, were analyzed in three separate PCR reactions representing three regions of the vector. Control reactions contained 100 ng normal dog DNA plus a tittered number of copies of the vector. A fourth PCR of the canine Myc gene served as an internal control for genomic DNA. Primers are listed in Supplemental Table III.
Results
Establishment of molecular markers for canine SPG
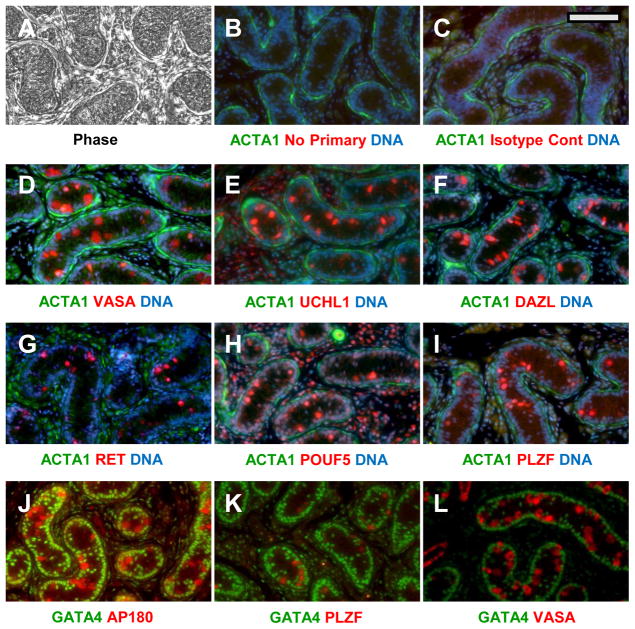
In order to identify and track canine SPG in culture, we screened a battery of commercially available antibodies to established murine and human SPG markers for specificity in canine pre-pubertal (4 months) testis. At this stage, the testicular cords present a simple architecture compared to mature testis ((Nayernia et al. 2004); see Fig. 1A). Only two cell types, the gonocytes/SPG and Sertoli cells, are packed in the dense cord-like structures, bound by a basement membrane. Both cell types are attached to the basement membrane at this age, but the Sertoli cell nuclei align along the periphery while the nuclei of the SPG are more centrally located (Nayernia et al. 2004). All SPG are SSC at this stage, since spermatogenesis has not yet started. Antibodies against reported SPG and SSC- specific target antigens; VASA (Noce et al. 2001), UCHL1 (Goel et al. 2007), DAZL (Anderson et al. 2007), PLZF (Jijiwa et al. 2008), AP180 (von Kopylow et al. 2010), POUF5 (Anderson et al. 2007) and RET (Anderson et al. 2007, Tyagi et al. 2009) all marked the interior SSC of canine pre-pubertal testis (Fig. 1). In contrast, ACTA1 was enriched outside of the cords in peri-tubular myoid and Leydig cells (Fig. 1B–I). GATA4, known as a Sertoli cell marker in mice (Ketola et al. 2002) and pigs (McCoard et al. 2001), was confined to the peripheral Sertoli nuclei of the cords (Fig. 1J–L). The VASA antibody was the most reliable probe for SSC, yielding a consistent, strong signal in all experiments. Dual-label staining demonstrated co-localization of VASA, UCHL1, DAZL, PLZF, and AP180 in the same set of interior cells (Supplemental Fig. 1). Unfortunately we were unable to demonstrate SSC-specific labeling by antibodies against established murine SSC surface antigens (data not shown), such as GFRA1, B1 and A6 integrins, and GPR125, each of which could be useful for immunofluorescent sorting of mouse SSC (Shinohara et al. 1999, Bjarnadottir et al. 2004, Grisanti et al. 2009). Localization of cRet, another SPG surface antigen in mice ((Tyagi et al. 2009)), was sporadic in pre-pubertal canine testis, and appeared to be nuclear rather than cell-surface (Fig. 1G). The reported localization of α-D-N-acetyl-galactosamine on the surface of porcine (Goel et al. 2007) and bovine (Izadyar et al. 2002) SSC also could not be confirmed in the dog using a similar dolichos biflorus agglutinin (DBA) binding assay.
Figure 1. Localization of SPG antigens in pre-pubertal canine testis.
(A) Phase image of tissue after deparaffinization. (B–L) Duel label probes in which putative SPG antigens (red) and somatic cell antigens (green) were marked. Putative SPG antigens (red) were probed with either rabbit (C–I) or goat (J–L) primary antibodies as indicated, followed by the appropriate Alexa-594 conjugated donkey secondary antibody. Somatic cells (green) were labeled either with mixed mouse antibodies against αActin and vimentin, (B–I) or GATA4 (J–L) followed by an anti-mouse Alexa 488 conjugate. The specific antibodies and dilutions used are shown in Supplemental Table I. Controls for background labeling were done identically except that they included either no primary antibody (B) or a rabbit isotype control (C). Specific antibodies and dilutions are listed in Supplemental Table I. Nuclei were counterstained blue with Hoechst 33342 in some sections (B–I). Bar = 100 um.
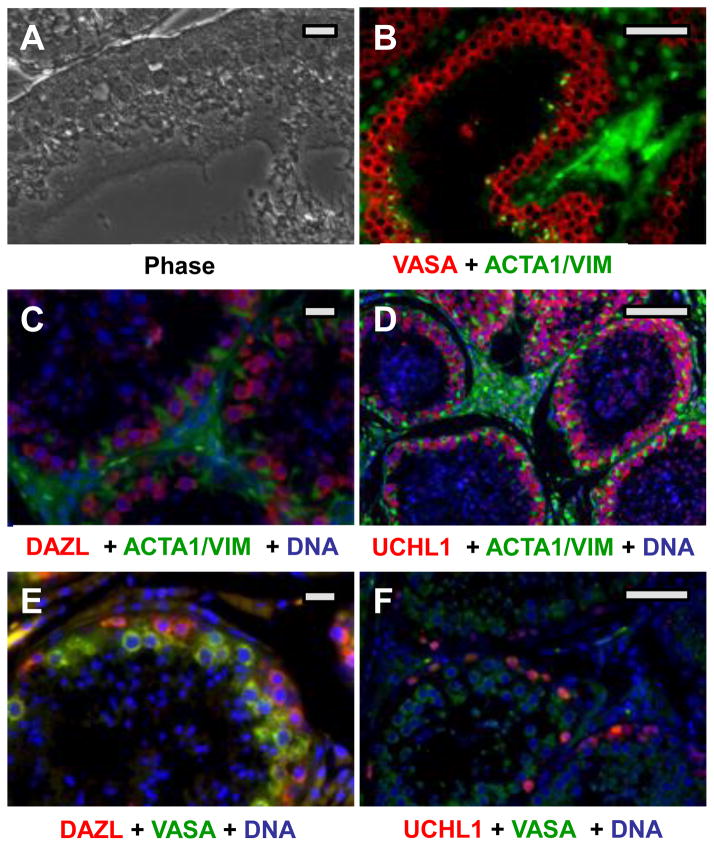
The same probes were used to localize SPG in mature testis (Fig. 2). As the testis matures, a central lumen forms in the cords, creating seminiferous tubules. The SSCs are confined at the basement membrane by Sertoli cells. Successive advancing stages of male germ cell development (spermatogonia, spermatocytes, spermatids, and spermatozoa) advance spatially toward the lumen, whereas Sertoli cell processes interdigitate among the male germ cells, supporting the developmental process ((Nayernia et al. 2004); see Fig. 2A). Most of the SPG/SSC markers seen in pre-pubertal testis were more difficult to detect in mature testis. All immunofluorescence signals were lower in adults. However, VASA, UCHL1 and DAZL were all clearly expressed in cells located either at the basement membranes of tubules, or in layers near these peripheral cells (Fig. 2B–F), consistent with SPGs and early stages of spermatogenesis. As detected by immunohistochemistry, VASA expression appeared to be higher in differentiating SPGs and spermatocytes than in the most peripheral cells, including the SSC (see Supplemental Figure 4). This pattern is similar to that seen in Human and Rhesus testis (Castrillon et al. 2000, Hermann et al. 2007). Thus VASA may be a poor marker for adult canine SSC, and a clear marker has not yet emerged. As indicated in Figure 2E–F, DAZL and UCHL1, may detect earlier stages than VASA, but the identity of the positive cells is not yet clear.
Figure 2. Localization of SPG antigens in adult canine testis.
(A) Phase image of tissue after deparaffinization. (B–D) Duel label probes in which putative SPG antigens (red) and somatic cell antigens (green) were marked. Putative SPG antigens (red) were probed with either rabbit (B) or goat (C,D) primary antibodies as indicated, followed by the appropriate Alexa-594 conjugated donkey secondary antibody. Somatic cells (green) were labeled with mixed mouse antibodies against alpha actin and vimentin, followed by an anti-mouse Alexa 488 conjugate. (E,F) Duel label probes for two SPG antigens. Note that the UCHL1 staining intensity in F is much lower than in D, showing only the most strongly positive cells. Bars = 150 um.
Isolation of canine SSC
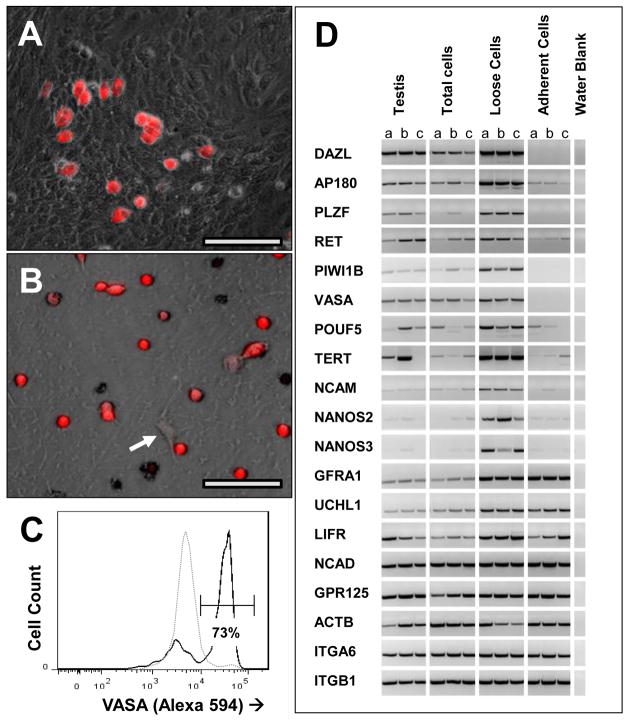
Pre-pubertal testes (3–6 months) were used as the source of SSC, taking advantage of the lower cellular complexity of the tissue at this stage. Spermatogenesis has not yet begun, and SSC are the only germ-lineage cells in the testes. In contrast to the mouse, a standard two-step digestion of canine pre-pubertal testis tissue with collagenase, followed by collagenase plus trypsin, and/or hyaluronidase (Hamra et al. 2008) did not generate a usable single-cell suspension. Considerable cell lysis was observed within the cords before the basement membrane dissociated. Thus we chose a gentler collagenase-only digestion, and plated incompletely digested pieces of seminiferous cords (Fig. 3A). By 3 days, most cells had migrated out of the cord structures onto the plate surface. Most of the VASA+ cells were rounded, loosely adherent cells that resided on top of a super-confluent layer of fibroblast-like cells (Fig. 3B,C and 4A). These VASA+ cells could be dislodged by vigorous agitation of the flask, and harvested with the medium. The “loose” cells were then further enriched by depleting collagen-adhesive cells (Hamra et al. 2008), with 2 rounds of short-term culture on gelatin-coated plates. The final preparation typically contained about 0.5–1 × 106 of 70% pure SPG, as judged by VASA immunohistochemistry (Fig. 4B) and by flow cytometry (Fig. 4C). Prior to enrichment, less than 3% of cells were VASA-positive, indicating an enrichment of about 23-fold. Cryo-preservation of the enriched cells, followed by thawing and washing, resulted in about 70% viability, based on trypan blue exclusion. RTPCR analysis of several SPG markers confirmed the enrichment of SPGs (Fig. 4D). Canine homologue mRNAs of the well-established mouse SPG markers, DAZL, AP180, PLZF, cRet, Piwi1 (Lee et al. 2006), VASA, POUF5, NCAM (Li et al. 1998, Kubota et al. 2004) Telomerase (TERT:(Riou et al. 2005)) and NANOS2 and 3 (Suzuki et al. 2009) all were enriched in the “loose cell” prep and depleted from the residual adherent cells, as compared to total testis cells. These data strongly support the hypothesis that the “loose cells” were canine SSC. Putative SPG marker RNAs; GFRA1, GPR125, ITGA6 and ITGB1 did not enrich with the canine SPGs, consistent with the lack of SPG-specific localization of the respective antigens by immunohistochemistry in canine testis (above). In addition UCHL1 mRNA was not enriched in the SPG pool, consistent with a post-transcriptional regulation in germ cells, or perhaps up-regulation of the gene in cultured adherent cells as reported previously (Luo et al. 2006).
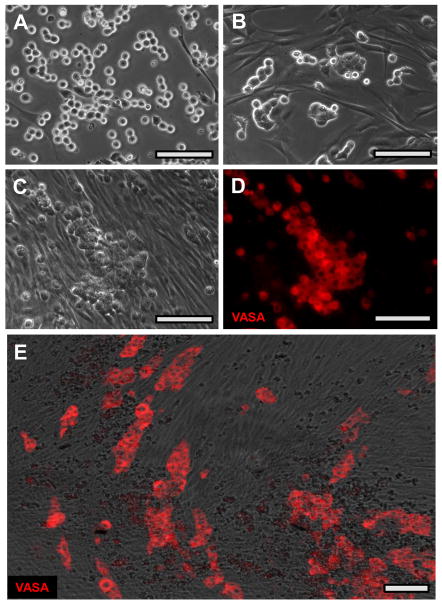
Figure 3. Emergence of SPG as a “loose cell” population in cultures of total testis cells.
(A) Bright field image of partially digested seminiferous tubules used to initiate culture. (B) Immunohistochemistry for VASA (purple) and GATA4 after 3 days culture of total testis cells. Note remnants of tubule pieces (dark brown) are firmly attached to substratum. (C) Higher power image of culture shown in (B). The VASA-positive cells are rounded and loosely scattered on top of the dense fibroblast-like lawn. Bars = 100 um.
Figure 4. Enrichment of SPG by differential adhesion.
(A) Composite phase/ fluorescent image of 3 day testis cell culture before harvest of SPG. VASA-positive cells are shown in red. (B) Final preparation of SPG after harvest of “loose” cells from 3-day culture, and 2 rounds of differential adhesion to gelatin. The isolated cells were allowed to adhere to a laminin surface, and were then probed for VASA by immunofluorescence. The arrow indicates a VASA-negative fibroblast. (C) FLOW data showing VASA immunofluoresence before and after enrichment of SPG by differential adhesion. Dotted line: unfractionated testis cells, collected at day 3 of culture, prior to removal of “loose” cells. Cells (which had fully emerged from tubule structures by this time) were re- suspended by trypsin. Solid line: enriched SPG (loose cells), after 2 rounds of depletion of adherent cells from duplicate 3-day culture. Both cell populations were prepared for FACS analysis of VASA expression as described in Methods. (D) Co-enrichment of SPG -specific transcripts with the “loose” cell population. Candidate SPG -enriched transcripts were amplified by RTPCR from ACTB-normalized cDNAs extracted at three days of culture from total testis cells, enriched SPG (loose cells) and remaining adherent cells. ACTB RTPCR was included as an internal control. Each panel of 3 lanes represents three RTPCRs of 3 separate cell preparations derived from the same set of three donor testis cell suspensions. Lane a is from a 16 week old Black Lab. Lanes b and c are from 10 week old German Sheppard littermates. Primers are listed in Supplemental Table III. Bars = 50 um.
Gene transfer into canine SPG
To test the efficiency of gene transfer by lentivirus, freshly enriched SPG were transduced with lentiviral vectors containing either an EF1-GFP or a CMV-mCherry reporter, and analyzed by flow cytometry for reporter expression. When cells were transduced with both vectors, at a multiplicity of infection (MOI) of 5 each, and analyzed at 48 hours (Fig. 5A–B) about 32% of the cells expressed at least one reporter and 22% expressed both. Since fibroblasts tend to rapidly out-grow the SPG in these cultures, a second experiment focused on reporter expression within the VASA+ population. Cells were transduced at an MOI of 20 with the EF1-GFP reporter vector, then fixed, permeabilized and labeled with the VASA antibody at 96 hours for flow cytometry (Fig. 5C). The SPG population (retaining VASA expression) showed a transduction efficiency of 8.8% under the conditions used here, based on GFP expression. Thus it is possible to insert and express transgenes in these cells by standard lentiviral transduction at a significant level. Our data might underestimate the true transduction efficiency of SPG if a portion of the cells left the VASA+ pool (differentiated or died) during the incubation period.
Figure 5. Gene transfer and expression in SPG by lentiviral transduction.
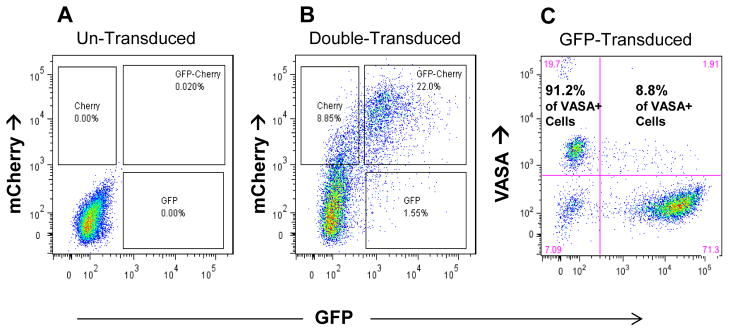
(A,B) Co- transduction with EF1-GFP and CMV-mCherry at an moi of 5 each. (A) FLOW diagram showing untreated control cells. (B) Same FLOW parameters showing expression of both vectors 4 days after a 15 hr transduction. (C) Expression of EFI-GFP in VASA-positive cells. Freshly isolated SPGs were transduced at moi of 20 for 15 hr and cultured for 4 days. Cells were fixed, permeabilized, probed for VASA using an Alexa-594 secondary antibody conjugate, and analyzed by FLOW cytometry. Two dimensional dot plot of VASA and GFP gated on total live cells. Large print indicates percent distribution of GFP-expressing cells within VASA+ population.
Behavior of canine SPG in culture
Conditions for in vitro survival and expansion of rodent SSC have been extensively studied, suggesting appropriate conditions to try for canine SPG culture. The essential factor for self-renewal-type expansion of rodent SSC in culture is Glial cell Derived Neurotrophic Factor (GDNF (Oatley et al. 2006)). However, EGF and βFGF and LIF enhance the proliferative effect of GDNF (Kanatsu-Shinohara et al. 2003). The inositol phosphate-anchored GDNF co-receptor, GFRα1, which can be added effectively as a soluble agent, is essential in some strains of mice, suggesting it is a limiting factor in those strains (Kubota et al. 2004). Additional signaling factors, including BDNF (Pyle et al. 2006), FGF9 (DiNapoli et al. 2006), NT3 (Pyle et al. 2006), NT4 (Pyle et al. 2006), Pleiotropin (Soh et al. 2007), and Wnt3a (Golestaneh et al. 2009a) have been implicated as having facilitating roles in survival and/or self-renewal; either in SSC directly, or in the closely related ESC. Serum levels and feeder cells have both had mixed effects on SSC survival in the literature (Hamra et al. 2005). Matrix components, particularly laminin and collagen, are predicted to affect morphology, mobility, and regulation of SSC, based on the localization of these materials at the SSC niche, high levels of B1 and A6 integrins on SSCs and an affinity for laminin (Shinohara et al. 1999).
In an attempt to establish an optimal culture environment for canine SSC, the enriched cells were cultured under a variety of conditions (Supplemental Table I). Base conditions were either a serum free medium (SG), optimized for murine SSC culture (Wu et al. 2009b), or DME/F12 with 10% FBS. Additional variables included feeder cells (canine, murine, none), substratum (laminin, gelatin, none), altered serum content (10%, 1%, none), and a variety of growth factors implicated in the mouse literature as promoters of SSC self-renewal. As an initial criterion for successful SPG culture, we recorded presence or absence of VASA-positive cells after one month in culture. As seen in Supplemental Table I, all culture conditions that included the established required components for rodent SSC self-renewal (SG medium with GDNF, FGF2, EGF, soluble GFRA1, and LIF (Wu et al. 2009b)) maintained significant numbers of VASA-positive cells for at least a month (e.g. Fig. 6E). The individual components of this cocktail were not tested separately. Addition of feeder cells, matrix, or any of the other signaling molecules did not noticeably alter the number, staining intensity, morphology, or association of VASA-positive cells, as determined by microscopic observation over a 1-month period. Therefore all subsequent studies used culture condition #12 (SG medium with GDNF, FGF2, EGF, soluble GFRA1, LIF, and a laminin substratum).
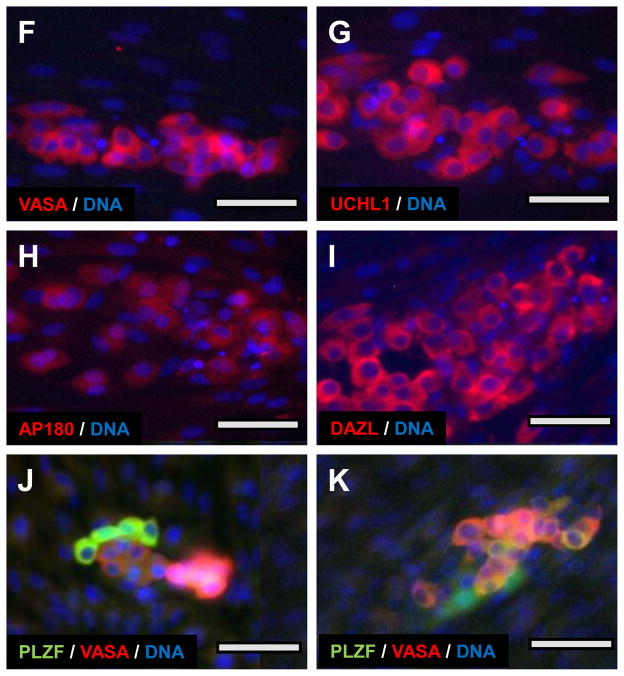
Figure 6. Behavior of canine SPG in culture.
Enriched SPG from a 4 month old black lab were cultured under condition #12 (Supplemental Table II. (A–C) Phase images of SPG on day 1, 3, and 8 of culture, respectively. (D) Fluorescent image of same field as (C) probed for VASA. (E) Low power composite phase/fluorescence image of culture at 30 day showing clusters of tightly associated VASA-positive cells arranged on top of a confluent layer of fibroblast-like cells. (F–I) Localization of VASA (F), UCHL1 (G), AP180 (H) and DAZL (I) at two weeks. (J–K) Co-localization of PLZF (green) and VASA (red) within SPG clusters. Nuclei in F–K are counterstained (Blue) with Hoechst 33342. Bars = 50 um (A–E) or 25 uM F–K).
In early cultures, most of the cells adhered to substratum, but maintained a relatively rounded morphology compared to the elongated fibroblast-like cells in the culture (Fig. 6A and B). The rounded cells were often arrayed as connected chains, reminiscent of the syncytial chains of type A-aligned spermatogonia in vivo (Hermann et al. 2009). By 8 days, fibroblasts had proliferated to form a confluent layer, and the less adherent SPG-derived cells had formed irregular flattened clumps on top of the fibroblasts (Fig. 6C). These clumps retain VASA expression for many weeks, as shown in Fig. 6D and E, and remain distinct from the rapidly expanding somatic cell population (Supplemental Figure 2). Similar clumps of cells with similar morphology expressed several SPG markers in culture, including DAZL, UCHL1 and AP180 as well as VASA. All localized to similar clumps of cells with similar rounded morphology and round nuclei (Fig 6F–I). The level of expression of a given marker varied significantly among members of the same clump (Fig 6D), and the relative expression of different SPG markers varied among closely associated cells in the clumps (Fig 6J–K). This might reflect varying stages of spermatogonial differentiation, as is the case in mature testis (see Fig. 2E). In fact, over 2 weeks of culture, cells expressing DAZL, UCHL1 or AP180 became relatively rare, while VASA-positive cells remained numerous (data not shown). The germ-line-specific mRNA expression profiles seen at the time of isolation, was lost by 30 days (Supplemental Figure 3), consistent with the rapid expansion of somatic cells, and the probable loss of SPG over this period.
Although the marker-positive canine cells persisted for extended periods in culture, we have not yet been able to establish proliferating (non-differentiating) lines using available human and mouse growth factors. In one experiment a set of replicate plates from a single SPG preparation was cultured without passage for 30 days. Plates were fixed and stained for VASA at intervals and the VASA+ cells manually counted. There was no detectable loss or expansion of VASA+ cells over this experiment. So far, any attempt to passage SPG -clumps or entire populations leads to rapid loss of expression of the SPG markers.
Transplantation of genetically manipulated SPG and production of transgenic sperm
The only rigorous way to verify that the isolated “loose” cells were SSC, and that these cells retained true SSC characteristics after genetic manipulation, is to demonstrate engraftment and donor spermatogenesis after transplantation into a host testis. To this end, 6 separate SPG preparations were transplanted into 6 recipient testes of 3 hounds. Four of the transplants, used cells that had been previously transduced with a retroviral reporter (see Supplemental Table IV). Recipient hounds were prepared by focal irradiation of the testes, and 2–5×105 SPG were injected into the seminiferous tubules of each testis via retrograde injection into the rete testis. Due to the low recovery of spermatogenesis, and complete lack of libido observed in previous transplants (Kim et al. 2008), the irradiation dose in this study was reduced from 3 days of 3 Gy/day to a single dose of 3 Gy.
Post-procedure, the recipient dogs showed normal sexual maturation, including normal sized testes, normal libido and interest in female urine. They also trained easily for manual ejaculation, allowing for periodic collections of sperm. Upon castration at 14 months post-transplant, the testis histology showed normal spermatogenic activity throughout the seminiferous tissue (Supplemental Figure 4). Epidydimal sperm content, determined at the time of castration, was in the normal range, varying from 1.1–4.6×108 cells in the two dogs that received transgenic SPG (Supplemental Table IV).
Sperm samples were analyzed for donor DNA contribution at 4 months by VNTR. In replicate reactions, each animal showed donor signals at or near the threshold of detection (1% donor) by VNTR assay (e.g. Fig. 7A). Standard curves for donor chimerism indicated no reliable quantitative information below 1%, due to random noise. Similar equivocal VNTR evidence for donor sperm was obtained through 15 months post-transplant (data not shown). Thus, donor contribution in these dogs remained at or below 1%, and could not be accurately quantified by this method.
As a more sensitive alternative assay, sperm DNA was analyzed for the presence of the reporter vector, PL-SIN-EF1a-EGFP, by PCR in the two animals that received transgenic SPGs. As shown in Fig. 7B, 3 separate PCR reactions, amplifying 3 regions of the vector, were performed in parallel for internal verification. Both dogs yielded vector-positive sperm (Fig. 7C) by this assay, while sperm from the non-transduced dog was negative. Based on the included vector standards, the copy number was between 20 and 60 copies per 40,000 haploid cells. If we assume a vector copy number of 40 per 40,000 cells, an SPG transduction efficiency of 9% (shown above) and a vector copy/cell value of 1 (predicted from the low transduction efficiency), then we can estimate that about 0.1% of the total sperm were transgenic, and that about 1.0% were of donor origin. This calculated figure was consistent with our VNTR results, showing positive signals at the 1% detection threshold.
Discussion
Our long-term goal is to develop a robust strategy for generating transgenic canine models. Traditional approaches to transgenics have focused on introducing transgenes either directly into oocytes and zygotes or indirectly into early embryos via replacement of inner cell mass cells with ES or cloned cells. These approaches all require the ability to harvest significant numbers of oocytes or embryos from the female reproductive tract, manipulate them in vitro without damage, and implant them efficiently into a surrogate mother. The difficulties in performing these tasks, the low yields and high costs of traditional cloning, and the absence of clear cut, genetically stable ES cells in most species, combined to make development of transgenesis in large animal models a very expensive and time consuming endeavor. The development of SSC transplantation in rodents, as an alternative to embryo-mediated gene transfer, opened a new opportunity to explore this relatively less invasive, more efficient and lower cost approach in canines and other larger animal models. Successful SSC transplantations in several large animals (Izadyar et al. 2003, Kim et al. 2008, Herrid et al. 2011, Jahnukainen et al. 2011, Hermann et al. 2012, Zeng et al. 2012), led us believe this approach could be applied to the dog model.
Previously, we demonstrated that canine SSC can be transplanted successfully, that they can re-establish regions of normal spermatogenesis, and that they can generate mature donor-derived sperm in a host testis (Kim et al. 2008). In that study, unfractionated seminiferous tubule cells were transplanted to a donor testis without enrichment, culture, or genetic manipulation. In order to exploit SSC transplantation as a viable approach to canine transgenesis, the efficiencies of SSC collection, gene transfer, engraftment, and donor sperm production will have to be optimized. Ideally, this may require clonal expansion of lines of transgenic SSC, to achieve sufficient cell numbers and genetic homogeneity to guarantee a high level of transgenic sperm production, and thus a practical ratio of transgenic to wild-type offspring. Here, we extended the previous findings by developing and assessing methods to isolate, culture, and genetically manipulate canine SPG. We then tested whether or not engraftment potential is maintained after these manipulations.
Based on the rich data available on SPG markers in rodents and other animals, we were able to identify similar antigens and mRNAs that uniquely identified SPG and their progeny in canine testis. Several of these markers, including VASA, PLZF, DAZL, UCHLI and AP180, were detectable, using commercially available antibodies, in prepubertal gonocytes, adult spermatogonia, and cultured SPG. These antibodies facilitated tracking SPG through isolation and culture. Interestingly, five cell surface molecules, (GFRA1, GPR125, ITGA6, ITGB1 and α-D-N-acetyl-galactosamine), which have been useful for flow cytometry-based enrichment of SPG from other species, were either undetectable or non-specific for canine SPG. In the absence of a useful surface marker, we exploited a differential matrix affinity method for enrichment of canine SPG from pre-pubertal testis to 70% purity. The identity of this population was confirmed by the co-enrichment of mRNAs for 11 well-established SPG -specific genes. Lentiviral transduction of the enriched SPG yielded about 9% reporter-positive cells. Thus it is now possible to prepare and cryopreserve a population of 0.5–1 × 106 70% pure canine SPG with 9% lentiviral marking from an average sized pre-pubertal dog.
When cultured under the optimal conditions established for mice, the enriched canine SPG persisted as VASA+ cells for several weeks. Under these conditions, some cells remain positive for several markers of SPG, including VASA, DAZL, UCHL1, AP180, and PLZF, suggesting that they may retain their SSC phenotype in culture. However, most of the VASA-positive cells lost expression of other primitive markers, suggesting loss of the SSC phenotype. Furthermore, there was no evidence of expansion of germ-line cells. The standard methods used by mouse SSC investigators of serially passaging clumps of rounded cells led to loss of SPG markers, as did trypsin-mediated or manual passage of the cells. Extensive testing of culture conditions did not reveal any advantage conferred by addition of feeder cells, matrix components, or other growth factors reported to enhance self-renewal divisions of mouse SSC. Thus, while canine SPG can be maintained in culture for weeks, conditions for expansion remain to be established. For the most part, these results parallel those reported from other large animal spermatogonial cell cultures. Short term expansions of germ-line cells with SPG markers, reported for human (He et al. 2010), pig (Kuijk et al. 2009), and bovine (Aponte et al. 2008) suggest that large-animal SSC lines may soon be possible. However, in no case, has a stable line of SSC been reported from a large animal.
We have demonstrated, for the first time in dogs that transplantation of transgenic SPG can lead to engraftment in a host testis and transmit the transgene to mature spermatozoa. This extends the findings in our previous study (Kim et al. 2008), establishing engraftment of un-fractionated, un-manipulated canine testis cells. In addition to the changes in donor cell manipulation this study significantly changed the preconditioning regimen for host testes. In the first study a fractionated dose of 9 Gy, 8 weeks prior to transplantation was used to eliminate endogenous stem cells. While this regimen was consistent with those used successfully in other large animals (Kim et al. 2006, Herrid et al. 2009, Jahnukainen et al. 2011, Zeng et al. 2012), it may have delivered too high a dose for the dog, since testis size, sperm count and libido did not recover and sperm had to be recovered from the epididymis. Therefore, we reduced the irradiation dose to 3 Gy in the present study. The lower radiation dose used resulted in complete recovery of libido, testis size, spermatogenesis, and sperm count. However, most of the recovery was undoubtedly from endogenous SSC that escaped the preconditioning, possibly inhibiting donor engraftment. The typical interval of several weeks between irradiation and transplant is based on the logic that tissue damage and inflammation caused by irradiation will interfere with homing of stem cells to their niche (Herrid et al. 2011). However, there is not a compelling body of empirical data to confirm this. Arguably, disruption of the tissue might actually facilitate entry of the SSC to their niche by disturbing the sertoli cell tight junctions that block entry to that niche (Takashima et al. 2011). Hematopoietic stem cell homing to bone marrow is actually enhanced, not blocked, by local irradiation 24 hr prior to infusion of the cells (Bastianutto et al. 2007). Thus we used a single dose of 3Gy, immediately prior to transplant. The optimal preconditioning regimen probably lies between those of our two studies.
By our calculation, the enriched and genetically modified SSC used here engrafted almost as well as did the un-enriched, untreated canine cells of our previous study. Based on histology, only a 5–15% recovery of spermatogenesis was observed in the first study, after 9 Gy of total irradiation. This was supported by about a 10% of normal sperm count. Thus, the observed 20% donor sperm represented about 2% of normal sperm production. In contrast, after a 3 Gy dose in the present study, both histology and sperm count were normal, indicating a 100% recovery of spermatogenesis. Thus, the 1% donor sperm generated in these animals represented 1% of normal total germ cell activity and sperm production. Since a large fraction of endogenous SSC survived the present 3 Gy preconditioning, it is also likely that these cells inhibited homing and engraftment of donor cells, either by physical exclusion, or by homeostatic signaling mechanisms that regulate self-renewal (Caires et al. 2010, Caires et al. 2012). From this comparison, we conclude that the level of donor engraftment in these two studies was similar, in spite of differences in preconditioning of the recipient tissue or isolation and manipulation of the donor cells. Similar studies with other large animals have yielded variable levels of donor sperm production (Honaramooz et al. 2003, Izadyar et al. 2003, Herrid et al. 2009, Herrid et al. 2011, Jahnukainen et al. 2011, Hermann et al. 2012, Zeng et al. 2012, Zeng et al. 2013), ranging from less than 1% to as high as 30% in sheep (Herrid et al. 2009).
The relatively low donor sperm levels observed here must be improved before canine SSC transplantation can be exploited as a practical approach for transgenesis. Optimization of the preconditioning regimen may yield a large improvement. Another area worthy of improvement may be immune-compatibility. In this study, no attempt was made to match host and donor. The blood-testis barrier, which protects developing germ cells in the tubule lumen from immune surveillance, may not fully protect SSC. These cells reside outside the barrier formed by Sertoli cell tight junctions. However, there is also some degree of immune privilege outside the barrier, since immunologically unmatched SSC transplants in bull, (Izadyar et al. 2003), goat (Honaramooz et al. 2003), pig (Mikkola et al. 2006), as well as dog (Kim et al. 2008), have all led to engraftment. Of note, unrelated SSC transplants in bulls yield significantly lower levels of engraftment than autologous transplants (Izadyar et al. 2003), suggesting that work on this point might help improve efficiency. The importance of donor/host interactions for canine SSC transplants remains to be determined.
Finally, establishment of culture conditions for true clonal expansion of canine SSC, such as is routine in mice, will also be extremely useful. A uniform clone of transgenic SSC would yield a >10 fold increase in the percent of transgenic cells over a primary transduction efficiency of 9%. It would also facilitate unlimited expansion: a 100 fold increase in the number of total SSC injected, would not exceed the cell numbers injected in our first study. It would also facilitate selection confirmation and expansion of low frequency events, such as homologous recombination, prior to transplantation. All of these factors will greatly affect the level of transgenic sperm production, and hence, the efficiency of generating transgenic offspring. A major obstacle to expansion of canine SSC may be species-specific qualities of self-renewal regulation. First, the available non-canine growth factors used in this study may not be fully compatible with the canine receptors. Second, canine SSC may utilize one or more alternative signaling pathways not observed in mice. With these caveats in mind, we are presently exploring both the production of canine growth factors, particularly GDNF, and genetic manipulation of canine SSC to bypass the requirement for GDNF (Lee et al. 2007, Lee et al. 2009).
Supplementary Material
Acknowledgments
Funding
This work was supported in part by Pilot Grant 20003812 from the Northwest Genome Engineering Consortium (NIH grant 1-UL1-RR024921-01) and by NIH Challenge Grant: NHBLI- 1-RC1-HL100270-01. We acknowledge the services of the Core Center of Excellence in Hematology (funded by NIH grant 3P30DK056465-11S2) for provision of canine mRNAs and Reporter constructs, as well as design of RTPCR primers and isolation of DNA and RNA samples.
We thank Dr. Margaret McEntee and her staff at the Jane M. Turrel Radiation Therapy Suite at Cornell’s College of Veterinary Medicine for their generous assistance with recipient preparation. We thank Deborah Higginbotham, Gretchen Johnson, Emily Meyer, Darlene John for technical assistance. We thank Bonnie Larson and Helen Crawford for assistance with the preparation and editing of the manuscript.
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the review.
Disclaimer: This is not the definitive version of record of this article. This manuscript has been accepted for publication in Reproduction, but the version presented here has not yet been copy edited, formatted or proofed. Consequently, Bioscientifica accepts no responsibility for any errors or omissions it may contain. The definitive version is now freely available at doi: 10.1530/REP-13-0086 (2013).
References
- Acland GM, Aguirre GD, Bennett J, Aleman TS, Cideciyan AV, Bennicelli J, Dejneka NS, Pearce-Kelling SE, Maguire AM, Palczewski K, Hauswirth WW, Jacobson SG. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol Ther. 2005;12:1072–1082. doi: 10.1016/j.ymthe.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An LY, Yuan YG, Yu BL, Yang TJ, Cheng Y. Generation of human lactoferrin transgenic cloned goats using donor cells with dual markers and a modified selection procedure. Theriogenology. 2012;78:1303–1311. doi: 10.1016/j.theriogenology.2012.05.027. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Fulton N, Cowan G, Coutts S, Saunders PT. Conserved and divergent patterns of expression of DAZL, VASA and OCT4 in the germ cells of the human fetal ovary and testis. BMC Dev Biol. 2007;7:136. doi: 10.1186/1471-213X-7-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aponte PM, Soda T, Teerds KJ, Mizrak SC, van de Kant HJ, de Rooij DG. Propagation of bovine spermatogonial stem cells in vitro. Reproduction. 2008;136:543–557. doi: 10.1530/REP-07-0419. [DOI] [PubMed] [Google Scholar]
- Athanasiou KA, Agarwal A, Muffoletto A, Dzida FJ, Constantinides G, Clem M. Biomechanical properties of hip cartilage in experimental animal models. Clin Orthop Relat Res. 1995:254–266. [PubMed] [Google Scholar]
- Bastianutto C, Mian A, Symes J, Mocanu J, Alajez N, Sleep G, Shi W, Keating A, Crump M, Gospodarowicz M, Medin J, Minden M, Liu FF. Local radiotherapy induces homing of hematopoietic stem cells to the irradiated bone marrow. Cancer Res. 2007;67:10112–10116. doi: 10.1158/0008-5472.CAN-07-2192. [DOI] [PubMed] [Google Scholar]
- Bjarnadottir TK, Fredriksson R, Hoglund PJ, Gloriam DE, Lagerstrom MC, Schioth HB. The human and mouse repertoire of the adhesion family of G-protein-coupled receptors. Genomics. 2004;84:23–33. doi: 10.1016/j.ygeno.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Caires K, Broady J, McLean D. Maintaining the male germline: regulation of spermatogonial stem cells. J Endocrinol. 2010;205:133–145. doi: 10.1677/JOE-09-0275. [DOI] [PubMed] [Google Scholar]
- Caires KC, de Avila JM, Cupp AS, McLean DJ. VEGFA family isoforms regulate spermatogonial stem cell homeostasis in vivo. Endocrinology. 2012;153:887–900. doi: 10.1210/en.2011-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillon DH, Quade BJ, Wang TY, Quigley C, Crum CP. The human VASA gene is specifically expressed in the germ cell lineage. Proc Natl Acad Sci U S A. 2000;97:9585–9590. doi: 10.1073/pnas.160274797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad S, Renninger M, Hennenlotter J, Wiesner T, Just L, Bonin M, Aicher W, Buhring HJ, Mattheus U, Mack A, Wagner HJ, Minger S, Matzkies M, Reppel M, Hescheler J, Sievert KD, Stenzl A, Skutella T. Generation of pluripotent stem cells from adult human testis. Nature. 2008;456:344–349. doi: 10.1038/nature07404. [DOI] [PubMed] [Google Scholar]
- DiNapoli L, Batchvarov J, Capel B. FGF9 promotes survival of germ cells in the fetal testis. Development. 2006;133:1519–1527. doi: 10.1242/dev.02303. [DOI] [PubMed] [Google Scholar]
- Gerwe BA, Angel PM, West FD, Hasneen K, Young A, Orlando R, Stice SL. Membrane proteomic signatures of karyotypically normal and abnormal human embryonic stem cell lines and derivatives. Proteomics. 2011;11:2515–2527. doi: 10.1002/pmic.201000032. [DOI] [PubMed] [Google Scholar]
- Giraldo AM, Ball S, Bondioli KR. Production of transgenic and knockout pigs by somatic cell nuclear transfer. Methods Mol Biol. 2012;885:105–123. doi: 10.1007/978-1-61779-845-0_8. [DOI] [PubMed] [Google Scholar]
- Goel S, Sugimoto M, Minami N, Yamada M, Kume S, Imai H. Identification, isolation, and in vitro culture of porcine gonocytes. Biol Reprod. 2007;77:127–137. doi: 10.1095/biolreprod.106.056879. [DOI] [PubMed] [Google Scholar]
- Golestaneh N, Beauchamp E, Fallen S, Kokkinaki M, Uren A, Dym M. Wnt signaling promotes proliferation and stemness regulation of spermatogonial stem/progenitor cells. Reproduction. 2009a;138:151–162. doi: 10.1530/REP-08-0510. [DOI] [PubMed] [Google Scholar]
- Golestaneh N, Kokkinaki M, Pant D, Jiang J, DeStefano D, Fernandez-Bueno C, Rone JD, Haddad BR, Gallicano GI, Dym M. Pluripotent stem cells derived from adult human testes. Stem Cells Dev. 2009b;18:1115–1126. doi: 10.1089/scd.2008.0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon IK, Khanna C. Modeling opportunities in comparative oncology for drug development. ILAR J. 2010;51:214–220. doi: 10.1093/ilar.51.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves SS, Hogan W, Kuhr CS, Diaconescu R, Harkey MA, Georges GE, Sale GE, Zellmer E, Baran SW, Jochum C, Stone B, Storb R. Stable trichimerism after marrow grafting from 2 DLA-identical canine donors and nonmyeloablative conditioning. Blood. 2007;110:418–423. doi: 10.1182/blood-2007-02-071282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisanti L, Falciatori I, Grasso M, Dovere L, Fera S, Muciaccia B, Fuso A, Berno V, Boitani C, Stefanini M, Vicini E. Identification of spermatogonial stem cell subsets by morphological analysis and prospective isolation. Stem Cells. 2009;27:3043–3052. doi: 10.1002/stem.206. [DOI] [PubMed] [Google Scholar]
- Guan K, Nayernia K, Maier LS, Wagner S, Dressel R, Lee JH, Nolte J, Wolf F, Li M, Engel W, Hasenfuss G. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440:1199–1203. doi: 10.1038/nature04697. [DOI] [PubMed] [Google Scholar]
- Hamra FK, Chapman KM, Nguyen DM, Williams-Stephens AA, Hammer RE, Garbers DL. Self renewal, expansion, and transfection of rat spermatogonial stem cells in culture. Proc Natl Acad Sci U S A. 2005;102:17430–17435. doi: 10.1073/pnas.0508780102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamra FK, Chapman KM, Wu Z, Garbers DL. Isolating highly pure rat spermatogonial stem cells in culture. Methods Mol Biol. 2008;450:163–179. doi: 10.1007/978-1-60327-214-8_12. [DOI] [PubMed] [Google Scholar]
- Hayes B, Fagerlie SR, Ramakrishnan A, Baran S, Harkey M, Graf L, Bar M, Bendoraite A, Tewari M, Torok-Storb B. Derivation, characterization, and in vitro differentiation of canine embryonic stem cells. Stem Cells. 2008;26:465–473. doi: 10.1634/stemcells.2007-0640. [DOI] [PubMed] [Google Scholar]
- He Z, Kokkinaki M, Jiang J, Dobrinski I, Dym M. Isolation, characterization, and culture of human spermatogonia. Biol Reprod. 2010;82:363–372. doi: 10.1095/biolreprod.109.078550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Sukhwani M, Lin CC, Sheng Y, Tomko J, Rodriguez M, Shuttleworth JJ, McFarland D, Hobbs RM, Pandolfi PP, Schatten GP, Orwig KE. Characterization, cryopreservation, and ablation of spermatogonial stem cells in adult rhesus macaques. Stem Cells. 2007;25:2330–2338. doi: 10.1634/stemcells.2007-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Sukhwani M, Simorangkir DR, Chu T, Plant TM, Orwig KE. Molecular dissection of the male germ cell lineage identifies putative spermatogonial stem cells in rhesus macaques. Hum Reprod. 2009;24:1704–1716. doi: 10.1093/humrep/dep073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Sukhwani M, Winkler F, Pascarella JN, Peters KA, Sheng Y, Valli H, Rodriguez M, Ezzelarab M, Dargo G, Peterson K, Masterson K, Ramsey C, Ward T, Lienesch M, Volk A, Cooper DK, Thomson AW, Kiss JE, Penedo MC, Schatten GP, Mitalipov S, Orwig KE. Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm. Cell Stem Cell. 2012;11:715–726. doi: 10.1016/j.stem.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrid M, Davey R, Stockwell S, Olejnik J, Schmoelzl S, Suchowerska N, Jackson M, Holland M, Hill JR. A shorter interval between irradiation of recipient testis and germ cell transplantation is detrimental to recovery of fertility in rams. Int J Androl. 2011;34:501–512. doi: 10.1111/j.1365-2605.2010.01113.x. [DOI] [PubMed] [Google Scholar]
- Herrid M, Olejnik J, Jackson M, Suchowerska N, Stockwell S, Davey R, Hutton K, Hope S, Hill JR. Irradiation enhances the efficiency of testicular germ cell transplantation in sheep. Biol Reprod. 2009;81:898–905. doi: 10.1095/biolreprod.109.078279. [DOI] [PubMed] [Google Scholar]
- Hilgendorf I, Weirich V, Zeng L, Koppitz E, Wegener R, Freund, Junghanss C. Canine haematopoietic chimerism analyses by semiquantitative fluorescence detection of variable number of tandem repeat polymorphism. Vet Res Commun. 2005;29:103–110. doi: 10.1023/b:verc.0000047486.01458.c5. [DOI] [PubMed] [Google Scholar]
- Honaramooz A, Behboodi E, Megee SO, Overton SA, Galantino-Homer H, Echelard Y, Dobrinski I. Fertility and germline transmission of donor haplotype following germ cell transplantation in immunocompetent goats. Biol Reprod. 2003;69:1260–1264. doi: 10.1095/biolreprod.103.018788. [DOI] [PubMed] [Google Scholar]
- Hong SG, Kim MK, Jang G, Oh HJ, Park JE, Kang JT, Koo OJ, Kim T, Kwon MS, Koo BC, Ra JC, Kim DY, Ko C, Lee BC. Generation of red fluorescent protein transgenic dogs. Genesis. 2009;47:314–322. doi: 10.1002/dvg.20504. [DOI] [PubMed] [Google Scholar]
- Hong SG, Oh HJ, Park JE, Kim MJ, Kim GA, Park EJ, Koo OJ, Kang SK, Jang G, Lee BC. Production of offspring from cloned transgenic RFP female dogs and stable generational transmission of the RFP gene. Genesis. 2011;49:835–840. doi: 10.1002/dvg.20772. [DOI] [PubMed] [Google Scholar]
- Hotta A, Cheung AY, Farra N, Vijayaragavan K, Seguin CA, Draper JS, Pasceri P, Maksakova IA, Mager DL, Rossant J, Bhatia M, Ellis J. Isolation of human iPS cells using EOS lentiviral vectors to select for pluripotency. Nat Methods. 2009;6:370–376. doi: 10.1038/nmeth.1325. [DOI] [PubMed] [Google Scholar]
- Izadyar F, Den Ouden K, Stout TA, Stout J, Coret J, Lankveld DP, Spoormakers TJ, Colenbrander B, Oldenbroek JK, Van der Ploeg KD, Woelders H, Kal HB, De Rooij DG. Autologous and homologous transplantation of bovine spermatogonial stem cells. Reproduction. 2003;126:765–774. [PubMed] [Google Scholar]
- Izadyar F, Pau F, Marh J, Slepko N, Wang T, Gonzalez R, Ramos T, Howerton K, Sayre C, Silva F. Generation of multipotent cell lines from a distinct population of male germ line stem cells. Reproduction. 2008;135:771–784. doi: 10.1530/REP-07-0479. [DOI] [PubMed] [Google Scholar]
- Izadyar F, Spierenberg GT, Creemers LB, den Ouden K, de Rooij DG. Isolation and purification of type A spermatogonia from the bovine testis. Reproduction. 2002;124:85–94. [PubMed] [Google Scholar]
- Jahnukainen K, Ehmcke J, Quader MA, Saiful Huq M, Epperly MW, Hergenrother S, Nurmio M, Schlatt S. Testicular recovery after irradiation differs in prepubertal and pubertal non-human primates, and can be enhanced by autologous germ cell transplantation. Hum Reprod. 2011;26:1945–1954. doi: 10.1093/humrep/der160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jijiwa M, Kawai K, Fukihara J, Nakamura A, Hasegawa M, Suzuki C, Sato T, Enomoto A, Asai N, Murakumo Y, Takahashi M. GDNF-mediated signaling via RET tyrosine 1062 is essential for maintenance of spermatogonial stem cells. Genes Cells. 2008;13:365–374. doi: 10.1111/j.1365-2443.2008.01171.x. [DOI] [PubMed] [Google Scholar]
- Jung EM, An BS, Kim YK, Hwang I, Lee JY, Shin TY, Hyun SH, Hwang WS, Jeung EB. Establishment of transgenic fibroblasts for producing recombinant human interferon-alpha and erythropoietin in bovine milk. Mol Med Report. 2012 doi: 10.3892/mmr.2012.1182. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Ikawa M, Takehashi M, Ogonuki N, Miki H, Inoue K, Kazuki Y, Lee J, Toyokuni S, Oshimura M, Ogura A, Shinohara T. Production of knockout mice by random or targeted mutagenesis in spermatogonial stem cells. Proc Natl Acad Sci U S A. 2006;103:8018–8023. doi: 10.1073/pnas.0601139103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Kato-Itoh M, Ikawa M, Takehashi M, Sanbo M, Morioka Y, Tanaka T, Morimoto H, Hirabayashi M, Shinohara T. Homologous Recombination in Rat Germline Stem Cells. Biol Reprod. 2011 doi: 10.1095/biolreprod.111.090837. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, Shinohara T. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69:612–616. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Ogonuki N, Iwano T, Lee J, Kazuki Y, Inoue K, Miki H, Takehashi M, Toyokuni S, Shinkai Y, Oshimura M, Ishino F, Ogura A, Shinohara T. Genetic and epigenetic properties of mouse male germline stem cells during long-term culture. Development. 2005;132:4155–4163. doi: 10.1242/dev.02004. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Shinohara T. Culture and genetic modification of mouse germline stem cells. Ann N Y Acad Sci. 2007;1120:59–71. doi: 10.1196/annals.1411.001. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Toyokuni S, Shinohara T. Transgenic mice produced by retroviral transduction of male germ line stem cells in vivo. Biol Reprod. 2004;71:1202–1207. doi: 10.1095/biolreprod.104.031294. [DOI] [PubMed] [Google Scholar]
- Ketola I, Anttonen M, Vaskivuo T, Tapanainen JS, Toppari J, Heikinheimo M. Developmental expression and spermatogenic stage specificity of transcription factors GATA-1 and GATA-4 and their cofactors FOG-1 and FOG-2 in the mouse testis. Eur J Endocrinol. 2002;147:397–406. doi: 10.1530/eje.0.1470397. [DOI] [PubMed] [Google Scholar]
- Kim EY, Noh EJ, Park HY, Park MJ, Noh EH, Lee JB, Jeong CJ, Lee DS, Riu KZ, Park SP. Establishment of bovine embryonic stem cell lines using a minimized feeder cell drop. Cell Reprogram. 2012;14:520–529. doi: 10.1089/cell.2012.0038. [DOI] [PubMed] [Google Scholar]
- Kim Y, Selvaraj V, Dobrinski I, Lee H, McEntee MC, Travis AJ. Recipient preparation and mixed germ cell isolation for spermatogonial stem cell transplantation in domestic cats. J Androl. 2006;27:248–256. doi: 10.2164/jandrol.05034. [DOI] [PubMed] [Google Scholar]
- Kim Y, Turner D, Nelson J, Dobrinski I, McEntee M, Travis AJ. Production of donor-derived sperm after spermatogonial stem cell transplantation in the dog. Reproduction. 2008;136:823–831. doi: 10.1530/REP-08-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci U S A. 2004;101:16489–16494. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijk EW, Colenbrander B, Roelen BA. The effects of growth factors on in vitro-cultured porcine testicular cells. Reproduction. 2009;138:721–731. doi: 10.1530/REP-09-0138. [DOI] [PubMed] [Google Scholar]
- Kumar De A, Malakar D, Akshey YS, Jena MK, Dutta R. Isolation and characterization of embryonic stem cell-like cells from in vitro produced goat (Capra hircus) embryos. Anim Biotechnol. 2011;22:181–196. doi: 10.1080/10495398.2011.622189. [DOI] [PubMed] [Google Scholar]
- Lee J, Kanatsu-Shinohara M, Inoue K, Ogonuki N, Miki H, Toyokuni S, Kimura T, Nakano T, Ogura A, Shinohara T. Akt mediates self-renewal division of mouse spermatogonial stem cells. Development. 2007;134:1853–1859. doi: 10.1242/dev.003004. [DOI] [PubMed] [Google Scholar]
- Lee J, Kanatsu-Shinohara M, Morimoto H, Kazuki Y, Takashima S, Oshimura M, Toyokuni S, Shinohara T. Genetic reconstruction of mouse spermatogonial stem cell self-renewal in vitro by Ras-cyclin D2 activation. Cell Stem Cell. 2009;5:76–86. doi: 10.1016/j.stem.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Lee JH, Schutte D, Wulf G, Fuzesi L, Radzun HJ, Schweyer S, Engel W, Nayernia K. Stem-cell protein Piwil2 is widely expressed in tumors and inhibits apoptosis through activation of Stat3/Bcl-XL pathway. Hum Mol Genet. 2006;15:201–211. doi: 10.1093/hmg/ddi430. [DOI] [PubMed] [Google Scholar]
- Li LH, Jester WF, Jr, Orth JM. Expression of 140-kDa neural cell adhesion molecule in developing testes in vivo and in long-term Sertoli cell-gonocyte cocultures. J Androl. 1998;19:365–373. [PubMed] [Google Scholar]
- Lingaas F, Comstock KE, Kirkness EF, Sorensen A, Aarskaug T, Hitte C, Nickerson ML, Moe L, Schmidt LS, Thomas R, Breen M, Galibert F, Zbar B, Ostrander EA. A mutation in the canine BHD gene is associated with hereditary multifocal renal cystadenocarcinoma and nodular dermatofibrosis in the German Shepherd dog. Hum Mol Genet. 2003;12:3043–3053. doi: 10.1093/hmg/ddg336. [DOI] [PubMed] [Google Scholar]
- Luo J, Megee S, Rathi R, Dobrinski I. Protein gene product 9.5 is a spermatogonia-specific marker in the pig testis: application to enrichment and culture of porcine spermatogonia. Mol Reprod Dev. 2006;73:1531–1540. doi: 10.1002/mrd.20529. [DOI] [PubMed] [Google Scholar]
- Lupu M, Storb R. Five decades of progress in haematopoietic cell transplantation based on the preclinical canine model. Vet Comp Oncol. 2007;5:14–30. doi: 10.1111/j.1476-5829.2006.00114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoard SA, Lunstra DD, Wise TH, Ford JJ. Specific staining of Sertoli cell nuclei and evaluation of Sertoli cell number and proliferative activity in Meishan and White Composite boars during the neonatal period. Biol Reprod. 2001;64:689–695. doi: 10.1095/biolreprod64.2.689. [DOI] [PubMed] [Google Scholar]
- Mikkola M, Sironen A, Kopp C, Taponen J, Sukura A, Vilkki J, Katila T, Andersson M. Transplantation of normal boar testicular cells resulted in complete focal spermatogenesis in a boar affected by the immotile short-tail sperm defect. Reprod Domest Anim. 2006;41:124–128. doi: 10.1111/j.1439-0531.2006.00651.x. [DOI] [PubMed] [Google Scholar]
- Mizrak SC, Chikhovskaya JV, Sadri-Ardekani H, van Daalen S, Korver CM, Hovingh SE, Roepers-Gajadien HL, Raya A, Fluiter K, de Reijke TM, de la Rosette JJ, Knegt AC, Belmonte JC, van der Veen F, de Rooij DG, Repping S, van Pelt AM. Embryonic stem cell-like cells derived from adult human testis. Hum Reprod. 2010;25:158–167. doi: 10.1093/humrep/dep354. [DOI] [PubMed] [Google Scholar]
- Nagano M, Ryu BY, Brinster CJ, Avarbock MR, Brinster RL. Maintenance of mouse male germ line stem cells in vitro. Biol Reprod. 2003;68:2207–2214. doi: 10.1095/biolreprod.102.014050. [DOI] [PubMed] [Google Scholar]
- Nayernia K, Li M, Engel W. Spermatogonial stem cells. Methods Mol Biol. 2004;253:105–120. doi: 10.1385/1-59259-744-0:105. [DOI] [PubMed] [Google Scholar]
- Noce T, Okamoto-Ito S, Tsunekawa N. Vasa homolog genes in mammalian germ cell development. Cell Struct Funct. 2001;26:131–136. doi: 10.1247/csf.26.131. [DOI] [PubMed] [Google Scholar]
- Nowend KL, Starr-Moss AN, Murphy KE. The function of dog models in developing gene therapy strategies for human health. Mamm Genome. 2011;22:476–485. doi: 10.1007/s00335-011-9348-0. [DOI] [PubMed] [Google Scholar]
- Oatley JM, Avarbock MR, Telaranta AI, Fearon DT, Brinster RL. Identifying genes important for spermatogonial stem cell self-renewal and survival. Proc Natl Acad Sci U S A. 2006;103:9524–9529. doi: 10.1073/pnas.0603332103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatley JM, Brinster RL. Spermatogonial stem cells. Methods Enzymol. 2006;419:259–282. doi: 10.1016/S0076-6879(06)19011-4. [DOI] [PubMed] [Google Scholar]
- Oatley JM, Brinster RL. Regulation of spermatogonial stem cell self-renewal in mammals. Annu Rev Cell Dev Biol. 2008;24:263–286. doi: 10.1146/annurev.cellbio.24.110707.175355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatley JM, Kaucher AV, Avarbock MR, Brinster RL. Regulation of Mouse spermatogonial Stem Cell Differentiation by STAT3 Signaling. Biol Reprod. 2010 doi: 10.1095/biolreprod.109.083352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazuka K, Beard BC, Emery DW, Schwarzwaelder K, Spector MR, Sale GE, von Kalle C, Torok-Storb B, Kiem HP, Blau CA. Long-term regulation of genetically modified primary hematopoietic cells in dogs. Mol Ther. 2011;19:1287–1294. doi: 10.1038/mt.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyle AD, Lock LF, Donovan PJ. Neurotrophins mediate human embryonic stem cell survival. Nat Biotechnol. 2006;24:344–350. doi: 10.1038/nbt1189. [DOI] [PubMed] [Google Scholar]
- Riou L, Bastos H, Lassalle B, Coureuil M, Testart J, Boussin FD, Allemand I, Fouchet P. The telomerase activity of adult mouse testis resides in the spermatogonial alpha6-integrin-positive side population enriched in germinal stem cells. Endocrinology. 2005;146:3926–3932. doi: 10.1210/en.2005-0502. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Sosa JR, Dobson H, Hahnel A. Isolation and transplantation of spermatogonia in sheep. Theriogenology. 2006;66:2091–2103. doi: 10.1016/j.theriogenology.2006.03.039. [DOI] [PubMed] [Google Scholar]
- Rowell JL, McCarthy DO, Alvarez CE. Dog models of naturally occurring cancer. Trends Mol Med. 2011;17:380–388. doi: 10.1016/j.molmed.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf SJ, Smith AG, Hansen JA, McFarland C, Erlich HA. Quantitative determination of bone marrow transplant engraftment using fluorescent polymerase chain reaction primers for human identity markers. Blood. 1995;85:1954–1963. [PubMed] [Google Scholar]
- Shinohara T, Avarbock MR, Brinster RL. beta1- and alpha6-integrin are surface markers on mouse spermatogonial stem cells. Proc Natl Acad Sci U S A. 1999;96:5504–5509. doi: 10.1073/pnas.96.10.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara T, Avarbock MR, Brinster RL. Functional analysis of spermatogonial stem cells in Steel and cryptorchid infertile mouse models. Dev Biol. 2000a;220:401–411. doi: 10.1006/dbio.2000.9655. [DOI] [PubMed] [Google Scholar]
- Shinohara T, Brinster RL. Enrichment and transplantation of spermatogonial stem cells. Int J Androl. 2000;23(Suppl 2):89–91. doi: 10.1046/j.1365-2605.2000.00025.x. [DOI] [PubMed] [Google Scholar]
- Shinohara T, Orwig KE, Avarbock MR, Brinster RL. Spermatogonial stem cell enrichment by multiparameter selection of mouse testis cells. Proc Natl Acad Sci U S A. 2000b;97:8346–8351. doi: 10.1073/pnas.97.15.8346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva C, Wood JR, Salvador L, Zhang Z, Kostetskii I, Williams CJ, Strauss JF., 3rd Expression profile of male germ cell-associated genes in mouse embryonic stem cell cultures treated with all-trans retinoic acid and testosterone. Mol Reprod Dev. 2009;76:11–21. doi: 10.1002/mrd.20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh BS, Song CM, Vallier L, Li P, Choong C, Yeo BH, Lim EH, Pedersen RA, Yang HH, Rao M, Lim B. Pleiotrophin enhances clonal growth and long-term expansion of human embryonic stem cells. Stem Cells. 2007;25:3029–3037. doi: 10.1634/stemcells.2007-0372. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Sada A, Yoshida S, Saga Y. The heterogeneity of spermatogonia is revealed by their topology and expression of marker proteins including the germ cell-specific proteins Nanos2 and Nanos3. Dev Biol. 2009;336:222–231. doi: 10.1016/j.ydbio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Takashima S, Kanatsu-Shinohara M, Tanaka T, Takehashi M, Morimoto H, Shinohara T. Rac mediates mouse spermatogonial stem cell homing to germline niches by regulating transmigration through the blood-testis barrier. Cell Stem Cell. 2011;9:463–475. doi: 10.1016/j.stem.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Takehashi M, Kanatsu-Shinohara M, Miki H, Lee J, Kazuki Y, Inoue K, Ogonuki N, Toyokuni S, Oshimura M, Ogura A, Shinohara T. Production of knockout mice by gene targeting in multipotent germline stem cells. Dev Biol. 2007;312:344–352. doi: 10.1016/j.ydbio.2007.09.029. [DOI] [PubMed] [Google Scholar]
- Takehashi M, Kanatsu-Shinohara M, Shinohara T. Generation of genetically modified animals using spermatogonial stem cells. Dev Growth Differ. 2010 doi: 10.1111/j.1440-169X.2009.01167.x. [DOI] [PubMed] [Google Scholar]
- Tsai KL, Clark LA, Murphy KE. Understanding hereditary diseases using the dog and human as companion model systems. Mamm Genome. 2007;18:444–451. doi: 10.1007/s00335-007-9037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi G, Carnes K, Morrow C, Kostereva NV, Ekman GC, Meling DD, Hostetler C, Griswold M, Murphy KM, Hess RA, Hofmann MC, Cooke PS. Loss of Etv5 decreases proliferation and RET levels in neonatal mouse testicular germ cells and causes an abnormal first wave of spermatogenesis. Biol Reprod. 2009;81:258–266. doi: 10.1095/biolreprod.108.075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassiliev I, Vassilieva S, Truong KP, Beebe LF, McIlfatrick SM, Harrison SJ, Nottle MB. Isolation and in vitro characterization of putative porcine embryonic stem cells from cloned embryos treated with trichostatin A. Cell Reprogram. 2011;13:205–213. doi: 10.1089/cell.2010.0102. [DOI] [PubMed] [Google Scholar]
- von Kopylow K, Kirchhoff C, Jezek D, Schulze W, Feig C, Primig M, Steinkraus V, Spiess AN. Screening for biomarkers of spermatogonia within the human testis: a whole genome approach. Hum Reprod. 2010;25:1104–1112. doi: 10.1093/humrep/deq053. [DOI] [PubMed] [Google Scholar]
- West FD, Terlouw SL, Kwon DJ, Mumaw JL, Dhara SK, Hasneen K, Dobrinsky JR, Stice SL. Porcine induced pluripotent stem cells produce chimeric offspring. Stem Cells Dev. 2010;19:1211–1220. doi: 10.1089/scd.2009.0458. [DOI] [PubMed] [Google Scholar]
- Wilcox JT, Semple E, Gartley C, Brisson BA, Perrault SD, Villagomez DA, Tayade C, Becker S, Lanza R, Betts DH. Characterization of canine embryonic stem cell lines derived from different niche microenvironments. Stem Cells Dev. 2009;18:1167–1178. doi: 10.1089/scd.2008.0336. [DOI] [PubMed] [Google Scholar]
- Wu X, Schmidt JA, Avarbock MR, Tobias JW, Carlson CA, Kolon TF, Ginsberg JP, Brinster RL. Prepubertal human spermatogonia and mouse gonocytes share conserved gene expression of germline stem cell regulatory molecules. Proc Natl Acad Sci U S A. 2009a;106:21672–21677. doi: 10.1073/pnas.0912432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Falciatori I, Molyneux LA, Richardson TE, Chapman KM, Hamra FK. Spermatogonial culture medium: an effective and efficient nutrient mixture for culturing rat spermatogonial stem cells. Biol Reprod. 2009b;81:77–86. doi: 10.1095/biolreprod.108.072645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Lin G, Tan YQ, Deng LY, Yuan D, Lu GX. Differences between karyotypically normal and abnormal human embryonic stem cells. Cell Prolif. 2010;43:195–206. doi: 10.1111/j.1365-2184.2010.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W, Tang L, Bondareva A, Honaramooz A, Tanco V, Dores C, Megee S, Modelski M, Rodriguez-Sosa JR, Paczkowski M, Silva E, Wheeler M, Krisher RL, Dobrinski I. Viral transduction of male germline stem cells results in transgene transmission after germ cell transplantation in pigs. Biol Reprod. 2013;88:27. doi: 10.1095/biolreprod.112.104422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W, Tang L, Bondareva A, Luo J, Megee SO, Modelski M, Blash S, Melican DT, Destrempes MM, Overton SA, Gavin WG, Ayres S, Echelard Y, Dobrinski I. Non-viral transfection of goat germline stem cells by nucleofection results in production of transgenic sperm after germ cell transplantation. Mol Reprod Dev. 2012;79:255–261. doi: 10.1002/mrd.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.