Abstract
Simvastatin and pravastatin are inhibitors of 3-hydroxy-3-methylglutaryl CoA reductase, and are used as antihypercholesterolemia drugs. Simvastatin, but not pravastatin, binds to the inserted domain of leukocyte function antigen (LFA)-1 and inhibits the function of LFA-1, including adhesion and costimulation of lymphocytes. Epstein-Barr virus (EBV)-transformed lymphoblastoid cell lines (LCLs) express high levels of LFA-1 on their surface and grow in tight clumps. Here we show that simvastatin (2 μM) inhibits clump formation and induces apoptosis of EBV-transformed LCLs. The apoptosis-inducing effect of simvastatin depends on binding to the inserted domain of LFA-1. Simvastatin, but not pravastatin, dissociates EBV latent membrane protein 1 from lipid rafts of LCLs, resulting in down-regulation of nuclear factor κB activity and induction of apoptosis. Analysis of multiple EBV-positive and -negative cell lines indicated that both LFA-1 and EBV latent membrane protein 1 expression were required for simvastatin's effects. Administration of simvastatin to severe combined immunodeficiency mice followed by inoculation with LCLs resulted in delayed development of EBV lymphomas and prolonged survival of animals. To our knowledge, this is the first report in which a drug that targets LFA-1 has been used to treat B cell lymphoma. These data suggest that simvastatin may have promise for treatment or prevention of EBV-associated lymphomas that occur in immunocompromised persons.
Infection of primary B cells with Epstein-Barr virus (EBV) results in transformation with growth of the cells in tight clumps and immortalization of the cells. These immortalized B cells have an immunoblastic morphology and express each of the EBV nuclear antigens (EBNAs) and latent membrane proteins (LMPs) (1, 2). EBNA-2 is a transactivator that up-regulates expression of cellular genes and LMPs. LMP-1 is an oncoprotein that constitutively activates nuclear factor κB (NF-κB) to induce B cell proliferation (3). LMP-1 also induces expression of adhesion molecules leukocyte function antigen (LFA)-1, LFA-3, and intercellular adhesion molecule 1 (ICAM-1) on the surface of EBV-transformed B cells (4, 5). The high level expression of adhesion molecules contributes to clumping of EBV-infected B cells in vitro (6).
EBV-associated immunoblastic lymphomas occur in immunocompromised patients such as those with AIDS or transplant recipients (7, 8). Because these EBV-associated immunoblastic lymphomas express each of the EBNAs and LMPs (8) that induce proliferation of B cells, the virus is thought to be directly responsible for the pathogenesis of these tumors (3). LMP-1 in EBV-associated lymphoma cells binds to tumor necrosis factor receptor-associated factors, and the tumors show activation of NF-κB (9). Many immunocompromised patients with EBV-associated immunoblastic lymphoma have tumors at extranodal sites such as the brain, lung, or gastrointestinal tract. The high-level expression of LFA-1 and other cellular adhesion molecules in these tumors may contribute to their extranodal location (10). The prognosis of these lymphomas is often poor for patients with irreversible immunosuppression, and treatment options are limited.
Simvastatin is a member of the statin family of drugs that inhibit 3-hydroxy-3-methylglutaryl CoA reductase (11). Statins lower plasma cholesterol levels, resulting in reduction of the risk of cardiovascular disease (12). Weitz-Schmidt et al. (13) demonstrated that certain statins, including simvastatin and lovastatin, bind to the I (inserted) domain of LFA-1 and inhibit its function (13). In contrast, other statins such as pravastatin do not bind to LFA-1. LFA-1 is expressed on the surface of various leukocytes and plays an important role in cell adhesion and costimulation of T cells. The I domain of LFA-1 is the binding site for ICAM-1, a ligand of LFA-1 (14, 15). The binding of simvastatin or lovastatin to the LFA-1 I domain induces a conformational change in LFA-1, resulting in inhibition of the interaction of LFA-1 with ICAM-1 (13). As a result of their binding to LFA-1, these statins inhibit the costimulatory activity of LFA-1 and suppress the inflammatory response in a murine model of peritonitis (13).
Here, we investigate the ability of simvastatin to inhibit EBV-positive B cell proliferation. Because simvastatin binds to and inhibits the function of LFA-1, we postulated that the drug would inhibit the growth of these cells both in vitro and in vivo. Inoculation of EBV-transformed lymphoblastoid cell lines (LCLs) into severe combined immunodeficiency (SCID) mice results in the formation of EBV-associated immunoblastic lymphomas that contain EBV genomes and express EBNAs, LMPs, and adhesion molecules including LFA-1 (16, 17). In the present study, we treated EBV-transformed LCLs in vitro with simvastatin, and administered the drug and inoculated SCID mice with LCLs to assess development of B cell lymphomas.
Materials and Methods
Cell Culture and Viability Assay. Three EBV-transformed LCLs, 12A1, 6B10, and 295H, EBV-positive Burkitt lymphoma cell lines [P3HR-1 (18), Akata (19), Mutu-1 (20), and Mutu-3 (20)], a human herpesvirus-8-positive EBV-negative primary effusion lymphoma cell line (BCBL-1) (21), an EBV-negative Burkitt lymphoma cell line (BJAB) (22), and EBV-negative T cell lines [Jurkat (23) and II-23 (24) cells; obtained from Carl Ware, La Jolla Institute for Allergy and Immunology, San Diego] were tested. For cell proliferation and viability assays, 2 × 104 cells per ml were cultured in 12- or 24-well plates for 5-7 days. Cell viability was assessed with XTT (Cell Proliferation Kit II, Roche Molecular Biochemicals), trypan blue, or propidium iodide (PI) staining. For PI staining, cells were washed with PBS, PI (5 μg/ml) was added, cells were washed, and fluorescent intensity was assessed with flow cytometry. Percent cell death was determined by the ratio of PI-positive cells to all gated cells.
Reagents and Antibodies. Simvastatin and pravastatin (Calbiochem) were converted to their open acid forms before use in vitro (25). Soluble ICAM-1 was purchased from R & D Systems. LFA-1 antibodies TS1/22 (American Type Culture Collection) (26) and G25.2 (BD Pharmingen) were used as primary antibodies, and fluorescein isothiocyanate (FITC)-conjugated F(ab′)2 fragment of goat anti-mouse Ig (Caltag, Burlingame, CA) was used as the secondary antibody for immunofluorescence and flow cytometry. TS1/22 antibody was obtained from hybridoma cells.
Lipid Raft Studies. Detergent extraction and flotation assay for lipid rafts were performed as described (27). Immunoblotting was performed by using anti-LMP-1 monoclonal antibody (S-12, BD Pharmingen), anti-CD71 monoclonal antibody (Zymed), and anti-Lyn monoclonal antibody (Santa Cruz Biotechnology).
Nuclear Extraction and Electrophoretic Mobility-Shift Assays. Nuclear extracts were prepared from 1 × 107 cells as described (28). Activation of NF-κB was determined by using 5 μg of nuclear extract in the gel-shift assay system (Promega) according to the manufacturer's instructions.
Apoptosis Assays. DNA fragmentation by apoptosis was detected by PI staining as described (29). Terminal deoxytransferase-mediated dUTP nick end labeling (TUNEL) assays were performed by using the in situ cell death detection kit (Roche Molecular Biochemicals) according to the manufacturer's instructions. DNA ladder formation was performed by extracting DNA from cells with the genomic DNA purification kit (Gentra Systems), and electrophoresis was performed.
Animal Experiments. Simvastatin tablets (ZOCOR, Merck) were mixed with mouse food at a ratio of 160 mg of simvastatin per 65 g of powdered food. Untreated animals received powdered food without simvastatin. LCLs (0.25 × 106, 1 × 106, or 4 × 106) were inoculated i.p. into 8-week-old SCID mice. Simvastatin was given either 3 days before (pretreatment group) or 7 days after (treatment group) inoculation of cells and continued until 4-6 weeks after inoculation. Thereafter, food without simvastatin was given to all mice, because of the side effects of prolonged high-dose simvastatin. All dead mice were autopsied and examined for the presence of lymphomas.
Results
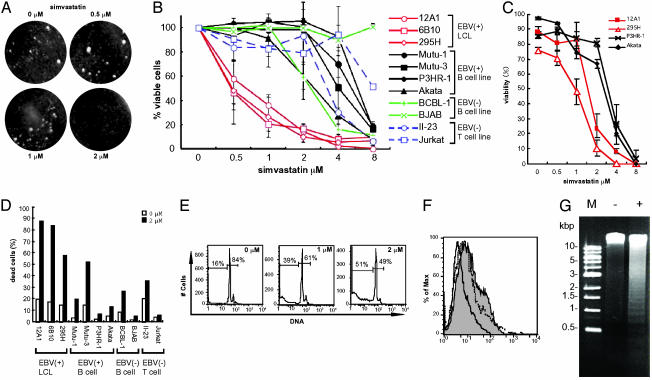
Simvastatin Inhibits Clump Formation and Induces Apoptosis of LCLs. LCLs and Burkitt lymphoma cells that express each of the EBNAs, LMPs, and LFA-1 grow in tight clumps. Binding of anti-LFA-1 antibody TS1/22 to the I domain of LFA-1 inhibits clumping of phorbol myristate acetate-stimulated LCLs after 18 h (6). To determine whether simvastatin affects clumping of unstimulated LCLs, we treated an LCL (12A1) with various concentrations of the drug. Five days after the addition of simvastatin, LCL clumps broke apart in wells treated with ≥2 μM simvastatin, whereas clumps remained in wells treated with ≤1 μM simvastatin (Fig. 1A). Dissociation of clumps was also observed in other LCLs (6B10 and 295H) and in Mutu-3 cells (data not shown). Cell viability assays were performed on various cell lines expressing different levels of LFA-1, ICAM-1, and LMP-1 (Table 1 and Fig. 5, which is published as supporting information on the PNAS web site). XTT cell proliferation assays showed that simvastatin decreased the number of viable cells in a dose-dependent manner (Fig. 1B). A loss in viability of LCLs was induced by 0.5 μM simvastatin, whereas ≥2 μM simvastatin was required to reduce viability of other cells. Trypan blue staining showed that LCLs 12A1 and 295H had >50% reduction in viability with 2 μM simvastatin, whereas Akata and P3HR-1 cells required higher concentrations of the drug (4 μM) to achieve >50% reduction in viability (Fig. 1C). The number of dead cells began to increase 5 days after addition of simvastatin at the time when clumps started to dissociate. PI staining followed by flow cytometry was also used to determine cell viability with 2 μM simvastatin. Cells expressing LMP-1 such as LCLs (12A1, 6B10, and 295H) and Mutu-3 showed low (<50%) viability after culture in 2 μM simvastatin for 7 days (Fig. 1D). Thus, incubation of cells with 2 μM simvastatin for 5 days inhibits clump formation and induces death in cells expressing LMP-1 (Table 1).
Fig. 1.
Simvastatin inhibits clump formation and induces apoptosis in LCLs. (A) Cell clumping of LCLs was observed after simvastatin was added at 0-2 μM for 5 days. (B) XTT cell proliferation assay was performed after addition of simvastatin (0-8 μM) to various cells lines for 7 days. Error bars indicate standard deviations for four independent experiments. (C) Cell viability was assayed by trypan blue staining after cell lines were cultured with 0-8 μM simvastatin for 7 days. Error bars indicate standard deviations for three separate experiments. (D) Percentage of dead cells in the absence (open bars) or presence (filled bars) of 2 μM simvastatin for 7 days as determined by PI staining and flow cytometry. PI-positive cells were counted as dead cells. (E) PI staining. Cells were treated with or without simvastatin for 5 days. Cell populations in sub-G0-G1 and G0-G1-S-M phase are indicated. (F) TUNEL assays were performed for cells treated with 2 μM simvastatin for 5 days (gray area with solid line), no simvastatin for 5 days (white area with solid line), or serum starvation for 72 h (white area with dotted line). (G) DNA ladder formation for cells cultured with (+) or without (-) 2 μM simvastatin for 5 days.
Table 1. Protein expression, activation of NF-κB, and cell death induced by simvastatin in cell lines.
| Cell line | Description | Clumping formation | LFA-1* | ICAM-1* | EBV† | LMP-1‡ | NF-κB‡ | Cell death by simvastatin§ |
|---|---|---|---|---|---|---|---|---|
| 12A1 | LCL | ++ | ++ | ++ | + | +++ | ++ | + |
| 6B10 | LCL | ++ | ++ | ++ | + | +++ | +++ | + |
| 295H | LCL | + | + | ++ | + | +++ | +++ | + |
| Mutu-1 | B cell line (BL) | − | − | − | + | − | − | − |
| Mutu-3 | B cell line (BL) | + | + | ++ | + | + | + | + |
| P3HR-1 | B cell line (BL) | − | ++ | ++ | + | − | + | − |
| Akata | B cell line (BL) | − | − | − | + | − | + | − |
| BCBL-1 | HHV-8-positive cell | − | − | + | − | − | − | − |
| BJAB | B cell line (BL) | − | + | ++ | − | − | − | − |
| II-23 | T cell line | − | ++ | + | − | − | + | − |
| Jurkat | T cell line | − | − | + | − | − | − | − |
Expression levels of LFA-1 and ICAM-1 were determined with flow cytometry (Fig. 5).
Expression level of LMP-1 and constitutive activation of NF-κB were determined by immunoblot and gel shift assay, respectively (Fig. 5).
Cell death induced by 2 μM simvastatin is defined as >50% cell death by PI staining (Fig. 1D). BL, Burkitt lymphoma; HHV-8, human herpesvirus 8.
Some statins induce apoptosis of certain tumor cells in vitro (30). PI staining showed that treatment of LCLs with simvastatin for 5 days induced a dose-dependent increase in fragmented DNA that was smaller than G0-G1 DNA (2n), indicative of apoptosis (29) (Fig. 1E). TUNEL assay confirmed that simvastatin induced DNA fragmentation in LCLs (Fig. 1F). Gel electrophoresis showed that simvastatin induced DNA fragment in LCLs resulting in formation of a DNA ladder (Fig. 1G). Thus, simvastatin induces apoptosis in LCLs.
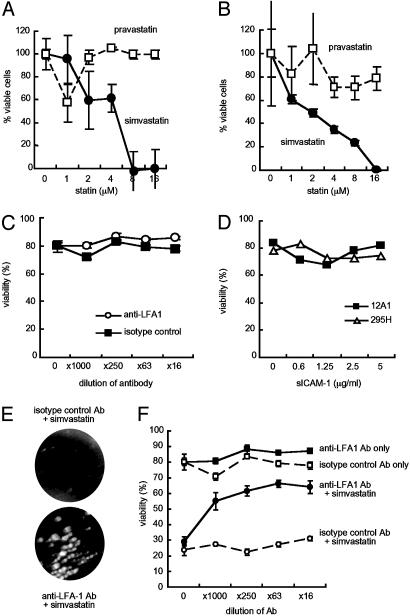
The Apoptosis-Inducing Effect Is Specific for Simvastatin and Depends on Binding to the I Domain of LFA-1. To determine whether loss of cell viability of LCLs occurs with a statin with different binding properties than simvastatin, LCLs were incubated with pravastatin, which does not bind to LFA-1 (13). Pravastatin had little effect on viability of LCL 12A1 or 6B10 (Fig. 2 A and B). Anti-LFA-1 monoclonal antibody TS1/22 and soluble ICAM-1 (sICAM-1) bind to the I domain of LFA-1 (26, 31), which is the site on LFA-1 targeted by simvastatin (13). Anti-LFA-1 TS1/22 antibody bound to LFA-1 on the cell surface at a 1:100 dilution by flow cytometry (Fig. 5); however, the antibody did not affect viability of 12A1 cells at concentrations up to 1:16 (Fig. 2C). sICAM-1 used at concentrations (5 μg/ml) that block rhinovirus infection (31) did not affect viability of LCLs 12A1 and 295H (Fig. 2D). Thus, antibody or another ligand that binds to the I domain of LFA-1 does not induce death of LCLs.
Fig. 2.
Apoptosis-inducing effect of simvastatin depends on binding to the I domain of LFA-1. (A and B) XTT cell proliferation assay was performed for LCLs 12A1 (A) and 6B10 (B) in the presence of simvastatin or pravastatin after 5 days. (C) Cell viability was assayed by trypan blue staining for LCL 12A1 in the presence of anti-LFA-1 antibody TS1/22, which recognizes the I domain of LFA-1, or an isotype control antibody for 7 days. (D) Cell viability was measured by trypan blue staining of LCLs 12A1 and 295H cultured with soluble ICAM-1 (sICAM-1) for 7 days. (E) LCL 12A1 was cultured with anti-LFA-1 (TS1/22) or isotype control antibody for 1 h, simvastatin (2 μM) or no compound was added, and the cells were cultured for 7 days. Cell clumping is reduced with isotype control antibody and simvastatin (Upper) but not with anti-LFA-1 antibody and simvastatin (Lower). (F) Cell viability was assayed by trypan blue staining of LCLs cultured with an anti-LFA-1 or isotype control antibody. Error bars indicate standard deviations of three separate experiments.
Antibody to LFA-1 (TS1/22) has been shown to induce signal transduction in lymphocytes (32). Therefore, pretreatment of LCLs with the antibody might inhibit the effects of simvastatin. Pretreatment of cells with anti-LFA-1 antibody TS1/22 blocked the ability of simvastatin to dissociate clumps of LCLs (Fig. 2E) and to reduce viability of LCLs (Fig. 2F).
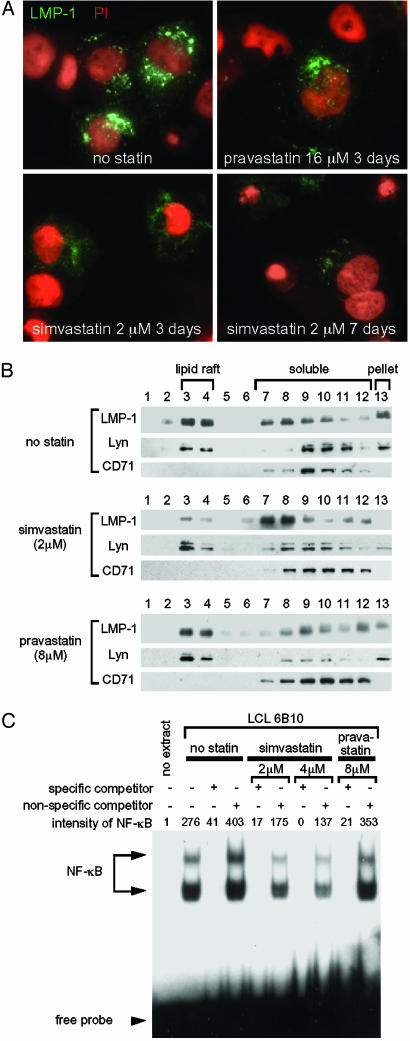
Simvastatin, but Not Pravastatin, Dissociates LMP-1 from Lipid Rafts and Reduces Activation of NF-κB. In vitro assays indicate that cholesterol depletion by high doses of statins disrupts lipid rafts and alters the localization and function of proteins in lipid rafts on the cell membrane (33-35). EBV LMP-1 localizes in lipid rafts and constitutively activates NF-κB via tumor necrosis factor receptor-associated factors (3, 27, 36). Therefore, we postulated that simvastatin may affect localization of LMP-1 in lipid rafts and impair signal transduction by LMP-1. To determine whether simvastatin alters localization of LMP-1, we treated LCLs with simvastatin and performed immunofluorescence assays for the viral protein. LMP-1 localized to large punctate structures in LCLs in the absence of simvastatin (Fig. 3A). After treatment with simvastatin for 3 or 7 days, LMP-1 showed a fine granular pattern in most of the cells that was much fainter and more diffuse than in untreated cells. In contrast, LMP-1 maintained its large punctate structures in cells treated with pravastatin. To further examine the effect of statins on LMP-1 localization, cell extracts were fractionated by using centrifugation and floatation in sucrose gradients (27). Lyn localizes to lipid rafts, whereas CD71 is in the soluble fraction (27). Immunoblotting showed that lipid raft fractions (fractions 3 and 4) contained the highest concentrations of LMP-1 in untreated or pravastatin-treated LCLs. At 2 μM simvastatin, the highest concentrations of LMP-1 shifted to soluble fractions (fractions 7-12), but Lyn remained in lipid rafts (fractions 3 and 4) (Fig. 3B). Quantitative analysis showed that 47% of LMP-1 was localized in lipid rafts of untreated cells; after treatment with simvastatin for 3 days, only 7% of LMP-1 was located in rafts. These data indicate that simvastatin, but not pravastatin, alters the localization of LMP-1 in the cell and dissociates LMP-1 from lipid rafts.
Fig. 3.
Simvastatin alters the localization of LMP-1, displaces LMP-1 from lipid rafts, and inhibits NF-κB activation in LCLs. (A) Immunofluorescence of LMP-1 in LCL 6B10 in the absence or presence of simvastatin or pravastatin. Cells were fixed, permeabilized, and incubated with anti-LMP-1 antibody (CS1-4, DakoCytomation, Carpinteria, CA), followed by FITC-conjugated secondary antibody and PI. LMP-1 is green (FITC) and nuclei are red (PI). (B) Immunoblotting of LCL fractions. LCLs were cultured in the absence or presence of simvastatin or pravastatin for 3 days, and cell lysates were fractionated by sucrose gradient ultracentrifugation. Equal aliquots of each fraction beginning at the top of the centrifuge tube were probed with antibodies to LMP-1, Lyn (a tyrosine kinase that localizes in lipid rafts), and CD71 (a transferrin receptor that does not localize in rafts). (C) Electrophoretic mobility-shift assays for NF-κB. LCL 6B10 was cultured in the absence or presence of simvastatin or pravastatin for 3 days, and nuclear extracts were used in electrophoretic mobility-shift assays with a radiolabeled NF-κB probe. Specific or nonspecific nonradioactive competitor oligonucleotides were added to some assays. The intensity of NF-κB-specific bands was measured by using a phosphorimager.
Each of the LCLs used in the present study showed constitutive activation of NF-κB (Fig. 5). Electrophoretic mobility-shift assays showed that treatment of LCLs with simvastatin (2 μM or 4 μM) for 3 days reduced the level of activated NF-κB, whereas pravastatin (8 μM) did not reduce NF-κB (Fig. 3C). Cell viability at 3 days ranged from 80-90% in cells treated with either statin or in untreated cells. Because inhibition of NF-κB induces apoptosis of LCLs (28), our results suggest that reduction of NF-κB by simvastatin results in induction of apoptosis in LCLs.
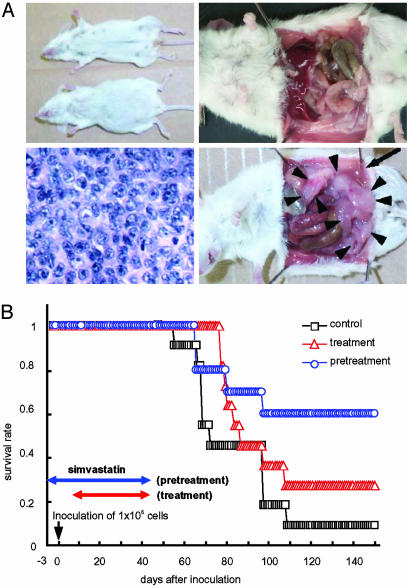
Simvastatin Delays the Onset of EBV Lymphomas and Prolongs Survival in SCID Mice Inoculated with EBV-Transformed LCLs. Because simvastatin induced apoptosis of LCLs, we tested the effect of the drug on SCID mice with EBV lymphomas. Simvastatin (250 mg/kg/day) was given orally to SCID mice beginning 3 days before i.p. inoculation with EBV-transformed LCLs. Control animals did not receive simvastatin. Four or 6 weeks later, simvastatin was discontinued because of side effects from the drug (Table 2) and mice were followed for development of tumors. Seven weeks after inoculation with LCLs, 80% of mice in the control group that received 0.25 × 106 cells developed ascites, whereas none of the animals treated with simvastatin that received the same number of cells had ascites (Fig. 4A). Nine weeks after inoculation, >70% of the control mice were dead of lymphoma, whereas all mice treated with simvastatin that received the same number of cells were alive, although a few had ascites. Mice pretreated with simvastatin that received different doses of LCLs all survived significantly longer compared to mice that did not receive the drug (Table 2). At autopsy all of these control mice had immunoblastic lymphomas (Fig. 4A). Flow cytometry showed that expression of LFA-1 in tumor cell ascites was reduced in mice treated with simvastatin compared with mice that did not receive the drug (data not shown). In a separate series of experiments using LCL 6B10, we compared mice that received simvastatin 3 days before inoculation with LCLs (pretreatment group) with mice that received the drug 7 days after inoculation (treatment group). Mice in the pretreatment group survived significantly longer (P < 0.04) compared with animals in the control group; however, the difference in survival was not significant for animals in the treatment group versus the control group (P = 0.2135) (Table 2 and Fig. 4B).
Table 2. SCID mouse experiments with simvastatin.
| LCL | Group | No. of cells | No. of mice* | Time of therapy† | Simvastatin days (mg/kg/day) | 50% survival, days | Day of death, range in days | p (logrank)‡ | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 12A1 | 1 | 0.25 × 106 | 6 | Pretreatment | −3 to +28 (250) | 81 | 70 to >105 | ] | 0.0496 | |
| 2 | 0.25 × 106 | 10 | No treatment | −(0) | 56 | 45-105 | ||||
| 3 | 1 × 106 | 6 | Pretreatment | −3 to +28 (250) | 56 | 53 to >105 | ] | 0.0350 | ||
| 4 | 1 × 106 | 12 | No treatment | −(0) | 53 | 48-56 | ||||
| 5 | 4 × 106 | 10 | Pretreatment | −3 to +28 (250) | 56 | 49-57 | ] | <0.0001 | ||
| 6 | 4 × 106 | 12 | No treatment | −(0) | 46 | 43-50 | ||||
| 6B10 | 7 | 1 × 106 | 10 | Pretreatment | −3 to +44 (250) | >100 | 64 to >100 | ]
|
0.0375 | |
| 8 | 1 × 106 | 11 | Treatment | +7 to +44 (250) | 85 | 76 to >100 | ]
|
0.2135 | ||
| 9 | 1 × 106 | 11 | No treatment | −(0) | 71 | 54 to >100 |
Number of mice analyzed. At 4 weeks, six animals receiving 0.25 × 106 of LCL 12AI and simvastatin, six animals receiving 1 × 106 of 12A1 and simvastatin, and two animals receiving 4 × 106 12A1 and simvastatin died or were sacrificed because of simvastatin side effects. These included severe weight loss, hunched body, or red swollen eyes. Autopsies showed no evidence of ascites or lymphomas in these mice and they were not included in the table and were excluded from the analysis.
Time of therapy. Animals received simvastatin 3 days before (pretreatment) or 7 days after (treatment) inoculation with LCLs.
Significance. P values indicated are for mice treated with simvastatin versus mice not treated with the drug for each cell number.
Fig. 4.
Simvastatin prolongs survival of SCID mice inoculated with LCLs. (A) Appearance of mice at 45 days after inoculation. The mouse that did not receive simvastatin (Upper Left, lower mouse) has ascites, but the simvastatin-treated mouse (upper mouse) has no ascites. The simvastatin-treated mouse (Upper Right) has no ascites or solid tumors, whereas the untreated mouse (Lower Right) has ascites (arrows) and solid tumors (arrowheads). Immunoblastic lymphoma is present in the mouse not treated with simvastatin (Lower Left). (B) Kaplan-Meier survival curves of SCID mice that were inoculated with 1.0 × 106 6B10 LCLs. Mice were pretreated with simvastatin beginning 3 days before inoculation with cells (blue circles), treated with drug beginning 7 days after inoculation with cells (red triangles), or not treated with drug (squares).
Discussion
We have shown that simvastatin induces cell death of EBV-transformed LCLs in vitro and prolongs survival of SCID mice with EBV lymphomas. This effect was seen with simvastatin, which binds to LFA-1, but not with pravastatin, which does not bind to LFA-1. Simvastatin dissociated LMP-1 from lipid rafts, inhibited NF-κB activation, and induced apoptosis in LCLs. Expression of LMP-1 and LFA-1 and constitutive NF-κB activation were critical for simvastatin-induced death of LCLs (Table 1). To our knowledge, this is the first report in which a drug that targets LFA-1 has been used to treat B cell lymphoma.
Apoptosis of LCLs occurred after 5-7 days of treatment with simvastatin. During the first 2 days of treatment there was no change in the morphology or viability of LCLs. However, 3 days after addition of simvastatin, LCL clumps became smaller, but the cells were still viable. At 5 days most clumps had dissociated and apoptosis was detected; at day 7 >80% of the cells were dead. We postulate that the relatively long time required for simvastatin to induce cell death is likely due to initiation of several processes in the cell. First, simvastatin blocks the binding of ICAM-1 to the I domain of LFA-1. LFA-1 binding to ICAM-1 on T cells induces cell proliferation (37), and because simvastatin inhibits LFA-1 binding to its receptor, the proliferation signal from LFA-1 is impaired by simvastatin. Second, simvastatin displaces EBV LMP-1 from lipid rafts and prevents signaling by the viral protein. LMP-1 recruits tumor necrosis factor receptor-associated factor 3 to lipid rafts and localization of LMP-1 to rafts activates NF-κB (36). Three days after treatment of LCLs with simvastatin, LMP-1 was dissociated from rafts and activation of NK-κB was inhibited in nuclear extracts from the cells. Because constitutive activation of NF-κB is required for growth of LCLs (28), simvastatin may induce apoptosis by turning off activation of NF-κB in these cells. This hypothesis is consistent with our observations that 2 μM simvastatin induced >50% death only in cells that expressed LMP-1 in which NF-κB was constitutively activated (Table 1). Although LFA-1 also localizes in lipid rafts of stimulated T cells (38) and simvastatin might have an effect on LFA-1, we did not observe a reduction in LFA-1 levels after treatment of LCLs with simvastatin in vitro (unpublished data). We did, however, note down-regulation of LFA-1 in EBV-transformed B cells in mice after treatment for 4-6 weeks. It is uncertain whether long-term simvastatin treatment in vivo resulted in a direct down-regulation of LFA-1 expression, or whether lower (trough) levels of simvastatin in serum may have allowed for selection and expansion of rare EBV-transformed B cells with low LFA-1 expression on their surface.
Simvastatin prolonged survival in SCID mice inoculated with EBV-transformed LCLs. Statins have previously been reported to have antitumor effects; however, these effects occurred at higher levels of drug (5-400 μM) (39-41) than were used in our study. In addition, other studies of statins showed that the effects were not specific for individual cell types and were seen with a wide variety of tumors both in vitro and in animal models (39-44). Clinical trials of lovastatin have shown partial responses in some patients with cancer (30). Simvastatin had an adjuvant effect in some, but not all, studies when given with other antitumor drugs (40, 41). The plasma levels of statins achieved in humans in clinical trials (2.32 ± 1.27 μM at peak concentration) (45) were comparable to the concentration of simvastatin (2 μM) that induced apoptosis of EBV-transformed LCLs in vitro and did not result in severe drug toxicity in the patients (30). Although a high dose of simvastatin was used in mice in this study (250 mg/kg/day), administration of simvastatin to mice at 100-400 mg/kg/day resulted in mean plasma drug levels about four to eight times higher than the mean human plasma drug level after an 80-mg oral dose, which is the maximum dose for cholesterol-lowering effects (46). Mice metabolize simvastatin much more rapidly than humans and therefore lower doses might be effective in humans.
Simvastatin, or other statins that block LFA-1 binding to ICAM-1, may have a role in the treatment of EBV-associated immunoblastic lymphomas that occur in immunocompromised persons. These tumor express high levels of LFA-1 (4) and LMP-1 (47) and have constitutive activation of NF-κB in vivo (9). Simvastatin might be used in combination with other agents for these patients. Simvastatin has not been reported to cause suppression of bone marrow function, and therefore it might not add to the hematopoietic toxicity that often occurs with other chemotherapeutic agents used to treat EBV-associated lymphomas. Recently we have found that lovastatin and atorvastatin also kill LCLs at similar concentrations as simvastatin (H.K. and J.I.C., unpublished results). Further studies are needed to assess the use of statins as adjunctive therapy for EBV-associated lymphomas in immunocompromised persons.
Supplementary Material
Abbreviations: EBV, Epstein-Barr virus; ICAM-1, intercellular adhesion molecule 1; I domain, inserted domain; LCL, lymphoblastoid cell line; LFA, leukocyte function antigen; LMP, latent membrane protein; PI, propidium iodide; SCID, severe combined immunodeficiency.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Kieff, E. & Rickinson, A. (2001) in Fields Virology, eds. Knipe, D. M. & Howley, P. M. (Lippincott Williams & Wilkins, Philadelphia), Vol. 2, pp. 2511-2573. [Google Scholar]
- 2.Rowe, M., Lear, A. L., Croom-Carter, D., Davies, A. H. & Rickinson, A. B. (1992) J. Virol. 66, 122-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mosialos, G., Birkenbach, M., Yalamanchili, R., VanArsdale, T., Ware, C. & Kieff, E. (1995) Cell 80, 389-399. [DOI] [PubMed] [Google Scholar]
- 4.Peng, M. & Lundgren, E. (1992) Oncogene 7, 1775-1782. [PubMed] [Google Scholar]
- 5.Wang, D., Liebowitz, D., Wang, F., Gregory, C., Rickinson, A., Larson, R., Springer, T. & Kieff, E. (1988) J. Virol. 62, 4173-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothlein, R. & Springer, T. A. (1986) J. Exp. Med. 163, 1132-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen, J. I. (2000) N. Engl. J. Med. 343, 481-492. [DOI] [PubMed] [Google Scholar]
- 8.Carbone, A. (2003) Lancet Oncol. 4, 22-29. [DOI] [PubMed] [Google Scholar]
- 9.Liebowitz, D. (1998) N. Engl. J. Med. 338, 1413-1421. [DOI] [PubMed] [Google Scholar]
- 10.Hamilton-Dutoit, S. J., Rea, D., Raphael, M., Sandvej, K., Delecluse, H. J., Gisselbrecht, C., Marelle, L., van Krieken, H. J. & Pallesen, G. (1993) Am. J. Pathol. 143, 1072-1085. [PMC free article] [PubMed] [Google Scholar]
- 11.Lennernas, H. & Fager, G. (1997) Clin. Pharmacokinet. 32, 403-425. [DOI] [PubMed] [Google Scholar]
- 12.Corsini, A., Maggi, F. M. & Catapano, A. L. (1995) Pharmacol. Res. 31, 9-27. [DOI] [PubMed] [Google Scholar]
- 13.Weitz-Schmidt, G., Welzenbach, K., Brinkmann, V., Kamata, T., Kallen, J., Bruns, C., Cottens, S., Takada, Y. & Hommel, U. (2001) Nat. Med. 7, 687-692. [DOI] [PubMed] [Google Scholar]
- 14.Randi, A. M. & Hogg, N. (1994) J. Biol. Chem. 269, 12395-12398. [PubMed] [Google Scholar]
- 15.Knorr, R. & Dustin, M. L. (1997) J. Exp. Med. 186, 719-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rowe, M., Young, L. S., Crocker, J., Stokes, H., Henderson, S. & Rickinson, A. B. (1991) J. Exp. Med. 173, 147-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Picchio, G. R., Cohen, J. I., Wyatt, E. R. & Mosier, D. E. (1993) Am. J. Pathol. 143, 342-349. [PMC free article] [PubMed] [Google Scholar]
- 18.Miller, G., Robinson, J., Heston, L. & Lipman, M. (1974) Proc. Natl. Acad. Sci. USA 71, 4006-4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takada, K., Horinouchi, K., Ono, Y., Aya, T., Osato, T., Takahashi, M. & Hayasaka, S. (1991) Virus Genes 5, 147-156. [DOI] [PubMed] [Google Scholar]
- 20.Gregory, C. D., Rowe, M. & Rickinson, A. B. (1990) J. Gen. Virol. 71, 1481-1495. [DOI] [PubMed] [Google Scholar]
- 21.Renne, R., Zhong, W., Herndier, B., McGrath, M., Abbey, N., Kedes, D. & Ganem, D. (1996) Nat. Med. 2, 342-346. [DOI] [PubMed] [Google Scholar]
- 22.Menezes, J., Leibold, W., Klein, G. & Clements, G. (1975) Biomedicine 22, 276-284. [PubMed] [Google Scholar]
- 23.Schneider, U., Schwenk, H. U. & Bornkamm, G. (1977) Int. J. Cancer 19, 621-626. [DOI] [PubMed] [Google Scholar]
- 24.Ware, C. F., Green, L. M., Reade, J., Stern, M. L. & Berger, A. E. (1986) Lymphokine Res. 5, 313-324. [PubMed] [Google Scholar]
- 25.Liu, L., Moesner, P., Kovach, N. L., Bailey, R., Hamilton, A. D., Sebti, S. M. & Harlan, J. M. (1999) J. Biol. Chem. 274, 33334-33340. [DOI] [PubMed] [Google Scholar]
- 26.Huang, C. & Springer, T. A. (1995) J. Biol. Chem. 270, 19008-19016. [DOI] [PubMed] [Google Scholar]
- 27.Higuchi, M., Izumi, K. M. & Kieff, E. (2001) Proc. Natl. Acad. Sci. USA 98, 4675-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cahir-McFarland, E. D., Davidson, D. M., Schauer, S. L., Duong, J. & Kieff, E. (2000) Proc. Natl. Acad. Sci. USA 97, 6055-6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Darzynkiewicz, Z., Bruno, S., Del Bino, G., Gorczyca, W., Hotz, M. A., Lassota, P. & Traganos, F. (1992) Cytometry 13, 795-808. [DOI] [PubMed] [Google Scholar]
- 30.Wong, W. W., Dimitroulakos, J., Minden, M. D. & Penn, L. Z. (2002) Leukemia 16, 508-519. [DOI] [PubMed] [Google Scholar]
- 31.Marlin, S. D., Staunton, D. E., Springer, T. A., Stratowa, C., Sommergruber, W. & Merluzzi, V. J. (1990) Nature 344, 70-72. [DOI] [PubMed] [Google Scholar]
- 32.Perez, O. D., Mitchell, D., Jager, G. C., South, S., Murriel, C., McBride, J., Herzenberg, L. A., Kinoshita, S. & Nolan, G. P. (2003) Nat. Immunol. 4, 1083-1092. [DOI] [PubMed] [Google Scholar]
- 33.Gubina, E., Chen, T., Zhang, L., Lizzio, E. F. & Kozlowski, S. (2002) Blood 99, 2518-2525. [DOI] [PubMed] [Google Scholar]
- 34.Hansen, G. H., Niels-Christiansen, L. L., Thorsen, E., Immerdal, L. & Danielsen, E. M. (2000) J. Biol. Chem. 275, 5136-5142. [DOI] [PubMed] [Google Scholar]
- 35.Simons, K. & Toomre, D. (2000) Nat. Rev. Mol. Cell Biol. 1, 31-39. [DOI] [PubMed] [Google Scholar]
- 36.Kaykas, A., Worringer, K. & Sugden, B. (2001) EMBO J. 20, 2641-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Seventer, G. A., Shimizu, Y., Horgan, K. J. & Shaw, S. (1990) J. Immunol. 144, 4579-4586. [PubMed] [Google Scholar]
- 38.Leitinger, B. & Hogg, N. (2002) J. Cell Sci. 115, 963-972. [DOI] [PubMed] [Google Scholar]
- 39.Vitols, S., Angelin, B. & Juliusson, G. (1997) Lipids 32, 255-262. [DOI] [PubMed] [Google Scholar]
- 40.Soma, M. R., Baetta, R., De Renzis, M. R., Mazzini, G., Davegna, C., Magrassi, L., Butti, G., Pezzotta, S., Paoletti, R. & Fumagalli, R. (1995) Cancer Res. 55, 597-602. [PubMed] [Google Scholar]
- 41.Clutterbuck, R. D., Millar, B. C., Powles, R. L., Newman, A., Catovsky, D., Jarman, M. & Millar, J. L. (1998) Br. J. Haematol. 102, 522-527. [DOI] [PubMed] [Google Scholar]
- 42.Feleszko, W., Mlynarczuk, I. & Nowis, D. (2001) FEBS Lett. 503, 219-220. [DOI] [PubMed] [Google Scholar]
- 43.Matar, P., Rozados, V. R., Roggero, E. A. & Scharovsky, O. G. (1998) Cancer Biother. Radiopharm. 13, 387-393. [DOI] [PubMed] [Google Scholar]
- 44.Matar, P., Rozados, V. R., Binda, M. M., Roggero, E. A., Bonfil, R. D. & Scharovsky, O. G. (1999) Clin. Exp. Metastasis 17, 19-25. [DOI] [PubMed] [Google Scholar]
- 45.Thibault, A., Samid, D., Tompkins, A. C., Figg, W. D., Cooper, M. R., Hohl, R. J., Trepel, J., Liang, B., Patronas, N., Venzon, D. J., et al. (1996) Clin. Cancer Res. 2, 483-491. [PubMed] [Google Scholar]
- 46.Zocor-Simvastatin/Merck (2003) 57th Physician's Desk Reference (Medical Economics, Montvale, NJ), pp. 2126-2131.
- 47.Young, L., Alfieri, C., Hennessy, K., Evans, H., O'Hara, C., Anderson, K. C., Ritz, J., Shapiro, R. S., Rickinson, A., Kieff, E., et al. (1989) N. Engl. J. Med. 321, 1080-1085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.