Abstract
T cells constitute a crucial arm of the adaptive immune system and their optimal function is required for a healthy immune response. After the initial step of T cell-receptor (TCR) triggering by antigenic peptide complexes on antigen presenting cell (APC), the T cell exhibits extensive cytoskeletal remodeling. This cytoskeletal remodeling leads to formation of an “immunological synapse” [1] characterized by regulated clustering, segregation and movement of receptors at the interface. Synapse formation regulates T cell activation and response to antigenic peptides and proceeds via feedback between actin cytoskeleton and TCR signaling. Actin polymerization participates in various events during the synapse formation, maturation, and eventually its disassembly. There is increasing knowledge about the actin effectors that couple TCR activation to actin rearrangements [2, 3], and how defects in these effectors translate into impairment of T cell activation. In this review we aim to summarize and integrate parts of what is currently known about this feedback process. In addition, in light of recent advancements in our understanding of TCR triggering and translocation at the synapse, we speculate on the organizational and functional diversity of microfilament architecture in the T cell.
1. Introduction
Recognition of antigenic peptides bound to major histocompatibility complex (pMHC) molecules by the TCR is the key step in T cell activation. While as few as 10 pMHC are capable of actuating a T cell response [4], this is only possible due to the concerted action of accessory interactions that mediate adhesion and supplemental signals known collectively as co-stimulation. The T cell must first find the antigenic pMHC-bearing cells through a process of active migration in lymphoid tissues at speeds up to several cell diameters per minute. This process of pMHC sampling relies heavily on lamellipodial dynamics of the T cell. Upon locating antigenic pMHC the T cell must transform rapidly from loosely adherent and highly motile to a tightly adherent and arrested cell in a matter of seconds. This rapid change demands determinative cytoskeletal alterations, achieved via major actin and microtubule remodeling. Once activated, TCR signaling induces actin polymerization, which then feeds back for optimal TCR triggering and T cell-APC contact expansion, generating a stable interface or immunological synapse. Actin dynamics has been envisaged to participate extensively from the very first step of TCR triggering to the completion of a successful activation cycle, serving as highly versatile machinery. Initial TCR ligation sets up robust signaling cascades to achieve actin polymerization, rearrangement and dynamics. Diverse sets of molecules including TCR-associated kinases, GTPases and adaptor molecules orchestrate this process. The role and regulation of filamentous actin (F-actin) at the T cell synapse has been a focus of extensive investigation for over a decade. Ectopic expression studies, genetic lesions affecting F-actin integrity, as well as advent of better visualization tools have greatly enhanced our knowledge of molecular regulation of actin polymerization at the synapse. There are many reviews summarizing the role of actin effectors in T cell development, differentiation and activation [3, 5–9], as well as several recent insights into the signaling modules that could selectively regulate the actin architectures. In this review we will discuss the diversity of TCR-responsive molecular regulators and potential role in specific steps of T cell activation. Although we are far from understanding the precise spatio-temporal events occurring between TCR activation and establishment of mature synaptic actin cytoskeleton, recent findings provide significant clues towards a better understanding of the structural and functional heterogeneity within F-actin at the synapse.
2. Tools to study actin in T cell activation
2.1. Perturbation tools
Cell-permeable pharmacological reagents can be used to perturb F-actin and its formation. Latrunculin A binds G-actin monomers and prevents polymerization and cytochalasin D (cytoD) results in an increase in ADP-bound G-actin through acceleration of ATP hydrolysis in actin dimers. Both of the drugs lead to F-actin depolymerization by reducing the amount of ATP-bound G-actin that can be added to filaments after an initial increase in F-actin. In addition, Jasplakinolide causes stabilization of existing filaments. As discussed in the later sections, initial insights into the role of F-actin in T cell activation were deduced using these actin-targeting drugs. Since the mechanism of action of these inhibitors on F-actin is well characterized, these were also employed to discern between actin depolymerization vs. polymerization regulating TCR microcluster movement at the synapse [10–13]. The small molecule inhibitor of actin motor myosin activity, blebbistatin, has been utilized to study myosin-mediated actin rearrangements upon T cell activation [14]. Another class of inhibitors reported recently, Arp2/3 and formin inhibitors [15, 16], target these specific actin nucleation factors selectively. Although not yet utilized to investigate actin dynamics in T cells, these are powerful tools to dissect the molecular machinery responsible for actin polymerization at the synapse. Further understanding of the molecular regulation of actin polymerization was deduced using genetic tools. These studies involved the analysis of T cells derived from gene targeted mice, siRNA-mediated silencing, or ectopic expression of dominant negative proteins. In addition, human T cells derived from patients with immunodeficiencies such as Wiskott-Aldrich syndrome (WAS) [17] and a subset of common variable immunodeficiency [18], that have defects in the actin-regulatory proteins WASP and Vav1, respectively, have provided significant clues into the regulation of actin polymerization at the synapse, and its consequence in T cell activation (see section 4).
2.2. Visualization tools
Much of the studies investigating F-actin at the synapse in fixed T cells have been carried out using fluorescently labeled fungal toxin phalloidin. Phalloidin labels F-actin in fixed cells, enhancing the contrast of microfilaments detection even in the presence of large amounts of G-actin. Actin has been monitored in live Jurkat T cells following transfection with DNA constructs encoding β–actin tagged with green fluorescent protein (GFP) [19]. However, the actin-GFP system can lack contrast due to the equivalent detection of F-actin and G-actin. Two F-actin reporters that can be used in live cells have been established as useful tools. A 17 amino acid peptide derived from actin crosslinking protein ABP120, referred to as LifeAct, binds to F-actin selectively and with sufficiently low affinity that it appears not to affect F-actin while allowing visualization with excellent contrast. LifeAct has been utilized to visualize F-actin in live T cells [20]. The actin-targeting domain (residues 9–52) of the enzyme inositol trisphosphate 3-kinase, referred to as F-tractin, also binds F-actin with low affinity [10, 21], enabling visualization of the native F-actin in live cells when fused to a monomeric fluorescent protein. In addition to reporters, the development of advanced microscopy methodology (reviewed in [22]), analysis and tracking tools, and use of reconstituted systems (as discussed in 2.3) have greatly enhanced the knowledge of actin distribution and dynamics at the T cell synapse.
2.3. T cell activation systems
A variety of T cell activation tools have been used in studies of F-actin at the synapse. For a quantitative estimate of actin polymerization at the synapse, in initial studies T cell-APC conjugates were fixed and labeled with phalloidin and analyzed using flow cytometry or confocal microscopy. However, in this setup the details of F-actin distribution in the plane of synapse and underlying qualitative features were missing. To overcome these limitations and visualize F-actin in greater detail, a range of reconstituted planar TCR activation systems have been developed. These include glass coverslips coated with anti-CD3 antibody [23] and supported lipid bilayers (SLB) reconstituted with an adhesion molecule like ICAM-1 plus antigenic pMHC or with anti-CD3 antibody [1]. Lipid anchored molecules in SLB are laterally mobile, which enables the translocation of ligated protein clusters and the formation of supramolecular synaptic patterns. Apart from providing a planar synapse orientation for better visualization, these systems also allow the assessment of the effect of individual T cell surface receptors in isolation and the control of antigen density for TCR triggering. SLB interspersed with nano-scale chromium barriers enables creation of geometrically repatterned immunological synapses [24]. When T cells are incubated with these surfaces, the flow of the pMHC-TCR complex is discontinuous within the cells and is blocked at the diffusion barriers [25]. This system has been utilized to study the coupling between F-actin, in the cytosol, and TCR, at the T cell surface.
Total internal reflection fluorescent (TIRF) microscopy is routinely utilized to study membrane proximal behavior of F-actin at the synapse at cell interfaces with solid phase antibodies or SLB [26, 27]. More recently, higher resolution (superresolution) microscopy techniques such as photoactivation localization microscopy (PALM) have started being exploited to study the effects of F-actin manipulation in the TCR signalosome at the synapse [28, 29]. Apart from planar surfaces, reconstituted non-planar surfaces have also been developed to study mechanical forces resulting from actin dynamics in response to TCR ligation. One such methodology utilizes biomembrane force probe technique coupled with optical microscopy. In this approach, a bead coated with anti-CD3 antibody is brought in contact with the T cell, and the T cell cytoskeletal and mechanical responses following contact are analyzed by optical microscopy and mechanical transduction via the force probe [30]. In this system it was shown that a pure anti-CD3 antibody signal generates a thick F-actin rich protrusion that can extend several microns. Other non-planar reconstituted activation systems involve biomimetic droplet systems [31], artificial antigen presenting cells generated by 293 T cells ectopically overexpressing TCR-associated machinery [32] and red blood cells labeled with pMHC and used to study early events following TCR engagement [33]. Although the latter systems have not yet been utilized for the visualization of actin dynamics in T cells, these possess immense potential for understanding the molecular and biophysical properties of actin cytoskeleton in a 3D activation environment. Similarly, development of tools to study actin dynamics in T cells in vivo will strengthen our knowledge of how cellular environment can influence actin cytoskeleton in T cells.
3. Actin in T cell activation
Initial clues to the role of actin cytoskeleton in regulation of T cell activation were provided by the use of the pharmacological reagent cytoD and the study of its effect on Ca2+ mobilization and interleukin-2 (IL-2) production [34]. CytoD-treated T cells elicited mixed results when instigated with TCR-activation stimuli, revealing complex actin dynamics in the process of T cell activation. CytoD-treated T cell-APC conjugates exhibited reduced Ca2+ influx [34], and upon incubation with soluble anti-CD3/CD28 antibodies showed diminished IL-2 reported activity [35]. The role of F-actin integrity in Ca2+ signaling was further corroborated by reports utilizing SLB that also revealed the concomitant disappearance of small TCR clusters (see section 3.1.3)[36]. In contrast, a few reports illustrated a negative regulatory role of F-actin in Ca2+ signaling and IL-2 production [37, 38]. While the assay conditions could affect the results to an extent (continuous cytoD treatment in culture in Holsinger et al., vs. only pretreatment in Rivaz et al. studies), both studies suggest that actin dynamics plays a pleiotropic and pliable role in T cell activation. Later studies characterizing genetic lesions affecting T cell activation revealed diversity of actin effectors important for cytoskeletal homeostasis and optimal T cell function. It also became increasingly clear that actin dynamics plays an integral part at various stages of T cell synapse, as discussed in section 3.1.
3.1. Actin in various stages of synapse lifetime
3.1.1. Resting phase (steady state) of T cells
Prevention of non-specific T cell activation in the resting state is potentially as important as the mechanism that triggers it, to avert an unwanted signaling response. One crucial step in T cell signaling is the recruitment and phosphorylation of linker for the activation of T cells (LAT) at TCR clusters, which in turn is essential for phospholipase Cγ (PLCγ) activation and increase of intracellular Ca2+ levels, known to be critical for T cell activation [39]. There are different views on how TCR and LAT can come together. 1) TCR-CD3 complexes and LAT reside within distinct protein islands in the T cell membrane and these fuse together after the TCR recognizes the pMHC complex [40]; 2) LAT is localized to sub-synaptic vesicles (SSVs) that upon TCR triggering dock with TCR clusters enabling LAT phosphorylation [41, 42]. 3) LAT and TCR are segregated in resting cells and able to intermix in the membrane following TCR interaction with pMHC [29]. In all models the cortical actin cytoskeleton is envisioned to have a role in steady state segregation and ligation-induced proximity of TCR and LAT [40, 43]. Interestingly, F-actin inhibits B cell antigen receptor signals and disruption of F-actin results in B cell activation [44].
3.1.2. Pre-Synapse
T cells are highly motile cells that are constantly in transit through the tissues, which allows them to scan the APCs for cognate pMHC.
Both T cell and APC are covered with glycocalyx – a layer consisting of large glycoproteins [45]–which poses a repulsive barrier between these two cells. To counteract this effect the integrin-based adhesion (between lymphocyte function associated antigen-1 -LFA-1- on the T cell, and ICAM-I on the APC) brings the two cells’ membranes within 40 nm of each other. T cell and APC need to be at a distance of 15nm so that TCR can bind the pMHC complex, the integrin-based adhesion provides a start point for the formation of actin polymerization-based protrusions (invasive pseudopodia) of the T cell that reach the target APC, thus reducing the distance between the opposing membranes. This allows the optimal sampling of the APC surface pMHC complexes. Once the TCR recognizes and binds to a matching pMHC complex a sustained 15 nm contact is established. Recently, using the artificial APC and memory/effector T cell system, antigen-sampling micron-scale “invadosome-like protrusions” (ILP) have been visualized at high resolution [46].
The recognition of pMHC triggers signaling downstream of TCR and three pieces of evidence highlight the central role of actin cytoskeleton in TCR triggering: 1) pharmacological agents that disrupt F-actin completely abrogate TCR triggering [34]. 2) The leading edge of a motile T cell is similar to a lamellipodium and is the most sensitive part of a polarized T cell to TCR triggering [47]. 3) Formation of actin “cloud” correlates with costimulatory role of LFA-1 and CD8 molecules [48]. The mechanism of TCR triggering is a longstanding question as the structure of the TCR in a complex with the CD3 subunits has been elusive. Several models have been put forward to explain TCR triggering that evoke active participation of actin cytoskeleton [49]. Recent studies provide an emerging picture for explaining the role of microfilament dynamics in TCR triggering. On the T cell surface the TCR is present in apparent clusters of 5–20 molecules in “confinement zones” or “protein islands” likely to be held within the actin meshwork and/or anchored to cortical F-actin [40]. This promotes rapid re-binding of pMHC and thus allows for kinetic proofreading and imparts “gain” on the response by triggering multiple TCRs [34]. The receptor deformation model posits that the mechanical pulling and/or shear force causes conformational changes in the TCR [50]. This model is proposed to explain the known sensitivity, specificity, and versatility of the TCR signaling response [49]. While the details of the conformational changes are not established, evidence for the basic tenet is mounting. As the pMHC-TCR interaction occurs between two active cells, mechanical force is an inevitable consequence. For example, F-actin driven translocation exerts a force of the TCR-pMHC interaction that could accelerate the off-rate [19, 36, 51]. Supporting evidence that TCR is mechano-sensitive is discussed in section 5.
TCR triggering leads to rapid nucleation of F-actin that is coupled to the adaptors downstream of TCR (discussed in section 4). This may promote coalescence of TCRs into “microclusters”, which are also critically dependent on an intact actin cytoskeleton [26, 36]. As discussed later (section 4), the actin cytoskeleton has an additional supportive role in promoting continued signaling after the initial triggering.
Continued TCR signaling delivers what has been historically referred as a “stop” signal, wherein the motile T cell becomes sessile to engage the APC for a longer duration [52]. It is important to note that “deceleration” rather than stopping is a better description of the phenomenon associated with antigen recognition in vivo [53, 54]. Accumulated evidence points to multiple mechanisms acting in concert to ensure global reorganization of the cytoskeleton and “deceleration” of the T cell. One such pathway is Ca2+ influx upon TCR triggering, although the mechanistic details are not fully understood [54, 55]. Ca2+-dependent myosin heavy chain phosphorylation that prevents bipolar filament assembly has been proposed as an effector pathway [56]. Other Ca2+-modulated actin-binding proteins may function to globally reduce the cortical tension and collapse the uropod structure. Similarly, concomitant reduction in RhoA activity and increase in Rac1 activity upon TCR signaling suppresses the polarized state of the motile T cell [57]. Overall F-actin architecture generated by TCR microclusters, perpendicular to the synaptic surface may provide anchorage to the opposing APC membrane [46]. Furthermore, integrin activation via PKCs and other inside-out integrin effectors are also likely to be important for stable interaction between the T cell and APC [58, 59].
3.1.3. Synapse
During the phase of initial antigen sampling, the productive initial encounter of T cell with APC leads to the formation of a stable conjugate or synapse. Synapse formation in the T cell is characterized by micron-scale organization and segregation of a variety of cell surface receptors including TCR and the integrin molecule LFA-1. Once formed in the periphery of the synapse, TCR microclusters translocate centripetally and fuse into the central Supra Molecular Activation Cluster (cSMAC) zone. LFA-1 clusters on the other hand are organized around the cSMAC to form a highly contractile zone, the peripheral SMAC (pSMAC). Peripheral TCR microclusters in the pSMAC are the sites of active signaling and these continue to signal until their delivery into the cSMAC. With the aid of pharmacological and genetic manipulations, the role of F-actin has been investigated in different stages of synapse formation and maturation.
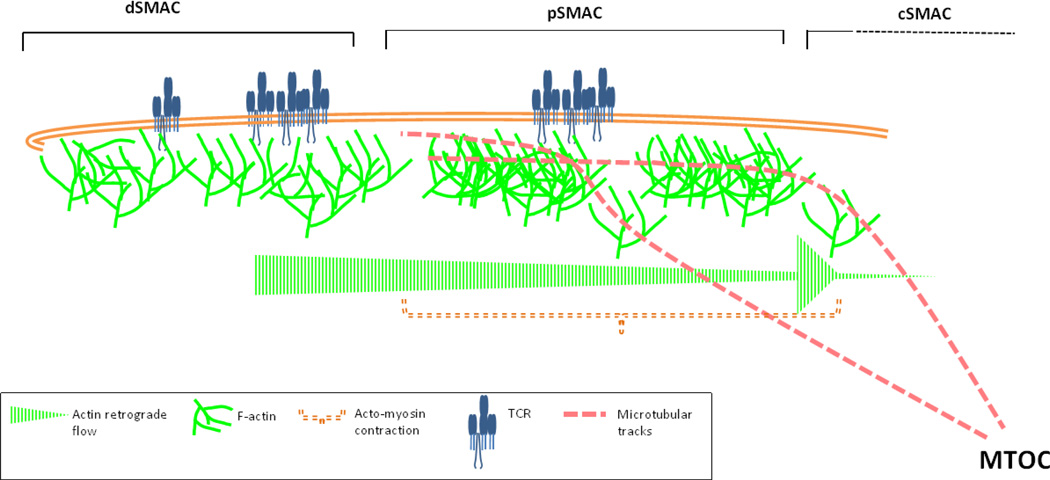
Synapse formation on a SLB can be broadly classified into two phases, first of spreading where microclusters continue to form, and second of lammellipodia contraction and TCR microclusters migration towards the cSMAC. The actin organization and dynamics are likely to be different in these two phases. In the spreading phase there is rapid increase in actin polymerization in an expanding ring following the lamellipodium-like leading edge. At the end of this first phase, synaptic actin is organized into three distinct zones: peripheral actin-rich distal SMAC (dSMAC), middle actomyosin-rich pSMAC and a central F-actin-depleted cSMAC. The second phase involves actin retrograde flow towards the cSMAC, and actomyosin-driven pSMAC contraction (Figure 1). Using Jurkat T cells, recent studies have highlighted actin and acto-myosin dynamics in different subsynaptic zones, and their role in microcluster translocation to the cSMAC. The first report to suggest coupling of actin with the TCR microcluster originated in a study by Kaizuka et al. where Jurkat T cells expressing actin-GFP were tracked live. Albeit slower, microcluster velocities correlated with actin speckles and the difference in actin-microcluster velocities was proposed to arise from microcluster “slippage” from underlying actin centripetal flow, during the course of movement [19]. This idea was further forwarded by DeMond et al. using SLB with variably angled chrome barriers to obstruct TCR-pMHC translocation, but not the flow of actin, to the synapse [25]. When TCR microclusters encountered angled barriers, they continued to move towards the synapse center by sliding along the barrier at an angle to the F-actin flow. This indicated a “frictional coupling” of microclusters with F-actin flow. At the moment it is not clear how microclusters are coupled to F-actin and mechanistically how F-actin dynamics influences microcluster translocation. One hypothesis is that actin polymerization in the dSMAC exerts pushing force on the microcluster and the associated signalosome cluster, which thus transits into the pSMAC area. Myosin-mediated actin “arc” contraction in the pSMAC area appears to be an additional driver of microcluster movement across the synapse. Inhibition of myosinII activity using blebbistatin or myosinII depletion through myosinII siRNA results in impaired delivery of the microclusters to the cSMAC [10, 14, 60]; in addition, Yi et al. have shown, using Jurkat T cells activated on reconstituted lipid bilayer, that myosinII perturbation using blebbistatin results in reduced microcluster velocity. However there are studies where myosin depletion or inhibition does not affect microcluster velocity per se, but affects the directionality of microcluster movement [60]. This could explain the defective cSMAC formation in blebbistatin-treated T cells reported in Ilani et al. [14]. In some reports blebbistatin has been ineffectual in affecting F-actin flow at the synapse, which could arise due to differences in assay conditions or pharmacological properties of the inhibitor; simultaneous treatment with actin and myosin inhibitors results in a more potent disruption of microcluster velocity than either of them used alone, suggesting that both actin polymerization and myosin activity coordinate microcluster movement at the synapse [10, 11]. A recent study characterized actin-dependent microcluster movement in the dynamic synapse in motile T cells, and found that F-actin depolymerization provides force for microcluster translocation in unstable synapse and that myosin-based contraction is dispensable for the process [12]. Actin dynamics is unanimously considered critical for TCR microcluster mobility. More recently participation of microtubular framework was also investigated and has yielded interesting results [13]. The Saito group demonstrated association of overexpressed negative-end microtubule motor with microclusters, and showed that inhibition of this motor in addition to actin depolymerization led to reduced microcluster motility. Thus both intact actin and microtubular cytoskeleton are required for microcluster transport at the synapse (Figure 1). However, at the moment, the cross-talk mechanisms between F-actin dynamics and microtubule machinery at the synapse are not clear, and further experiments will be required to clarify the molecular links and functional coupling between the two and with TCR microclusters.
Figure 1.
Actin polymerization and remodeling at the synapse is critical for TCR microcluster movement to the cSMAC. The mature radially symmetrical synapse exhibits three spatially distinct zones of actin organization. The outermost ring of rapid polymerization zone - dSMAC-involves feedback between TCR triggering and actin dynamics. Actin polymerization aids in TCR signaling and microcluster formation via an unknown mechanism, and TCR triggering in turn potentiates actin polymerization. Actin polymerization then drives movement of TCR microclusters from the dSMAC, towards the pSMAC, and is the onset site of fast actin centripetal flow (0.1µm/sec). The pSMAC is the middle zone that undergoes contractions guided by actomyosin network, and actin retrograde flow. Actin flow occurs at slower speed in the pSMAC (0.04µm/sec), and is achieved majorly via actin polymerization and possibly with contribution from myosinII activity. The contractile network is visible as F-actin “arcs” in Jurkat T cell system (shown as interrupted network in pSMAC). TCR microclusters exhibit kinetic coupling with actin flow all across the synapse interface; sometimes translocating slower than the flow due to occasional slippage, and sometimes exhibiting similar translocation rate as F-actin speckles. At the inner boundary of the pSMAC, there is rapid decline and collapse of F-actin flow, generating a sharp boundary of the F-actin-rich pSMAC and F-actin-poor cSMAC. Actin polymerization appears to be a major driving force for microcluster movement, however the myosinII activity and actomyosin contraction provides directional persistence to reduce meandering and efficient delivery of microclusters to the cSMAC. In addition, TCR microclusters are capable of interacting with microtubular motor protein dynein, thus utilizing microtubular tracks for navigating the synaptic interface, and eventual delivery to the cSMAC.
3.1.4. Post-synapse
To complete a full activation cycle, T cells must disengage the synapse, in order to move on. Late-stage synapse, or asymmetrical synapse of a motile T cell, is generally referred to as “kinapse” [61]. The process of synapse-kinapse transition is still poorly understood. While various actin effectors, such as WASp, regulate synapse stability [53], the mechanism of cytoskeletal modulation leading to kinapse onset is unknown. Intra-vital imaging studies have clearly demonstrated that T cells in the lymph node exhibit a range of morphologies, motility behavior and contact duration when interacting with APCs [62]. Recent in vitro studies have reported naive murine T cells forming mobile junctions on lipid bilayers presenting pMHC and ICAM1 [53, 63]. This suggests that cell-intrinsic factors can control the stability of the synapse. Interestingly, motile naive T cells can also transition back to form stable synapse in a cell-autonomous manner. Further, PKCθ null T cells form more stable synapses than control cells, whereas T cells lacking WASP have defects in re-establishing symmetry [53]. The observation that myosin II is a central player in multiple model systems used to study symmetry breaking, may be applicable in naive T cells. Furthermore, it is possible that PKCθ impacts the myosin regulatory chain phosphorylation and thus promotes the transition to the 'kinapse' state. WASP may support “repair” of the lamellar actin network through actin nucleation and thus promote resymmetrization. It is intuitive to think that all these molecular signals impinge upon the actin cytoskeleton to determine the stability of T cells’ contact with APCs. However, in the absence of systematic studies, the mechanism of synapse stabilization by T cell cytoskeleton in the native milieu is rather unclear.
4. TCR to actin trigger
TCR engagement at the T cell surface triggers multiple signaling pathways that regulate actin organization and rearrangements at different scales. The actin polymerization at the synapse is a result of complex molecular interactions, and a diverse set of TCR-downstream molecular machineries participate in actin polymerization. Once the T cell is ligated with the cognate receptor, the immunoreceptor tyrosine-based motifs (ITAM) in the cytosolic tail of the TCR-associated CD3 complex undergo phosphorylation, which then serve as docking site for the Syk-kinase zeta chain-associated protein of 70kDa (ZAP70) [64]. CD3-recruited ZAP70 then leads to phosphorylation and activation of two key adaptor molecules that associate with a variety of molecules in TCR-associated signaling complexes. First, ZAP70 phosphorylates LAT, which in turn associates with and phosphorylates the SH2 domain-containing leukocyte protein of 76kDa (SLP76). Phosphorylated LAT also directly interacts with PLCγ1, a major regulator of Ca2+ influx in response to TCR engagement. SLP76 acts as scaffold for a plethora of actin effectors including Rho GTPases nucleotide exchange factor, Vav1, adaptor molecule Nck and nucleation promoting factors (NPFs) [2]. Rho GTPases can also bind and activate NPFs, which in turn activate the actin nucleation factors - the key drivers of actin polymerization (Figure 2). While genetic manipulation of actin polymerization effectors yields significant consequences on T cell activation, F-actin severing proteins are also reported to be crucial for T cell function [65]. Thus the actin dynamics at the T cell synapse is tightly regulated to achieve optimal T cell function. Below are the various components of actin-regulatory machinery downstream of TCR, broadly categorized according to their role in actin remodeling in the T cell.
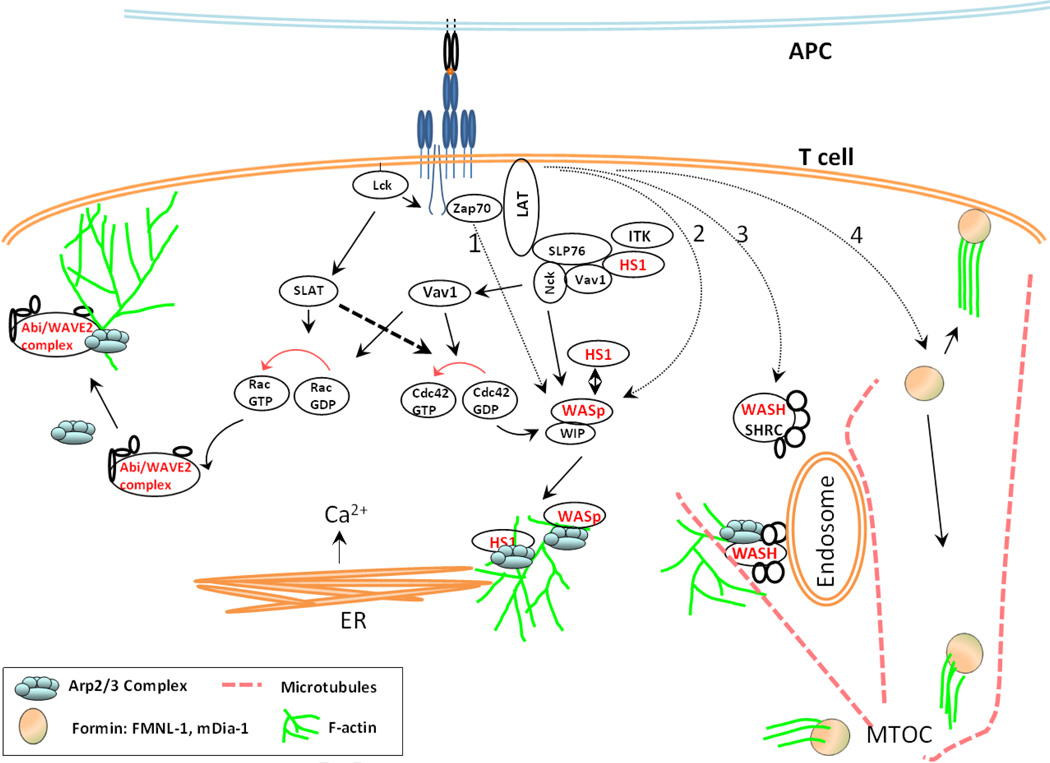
Figure 2.
Schematic view of integration of TCR signal transduction with diverse components of the actin polymerization machinery. TCR ligation with the pMHC complex on APC leads to Lck-mediated phosphorylation of cytosolic ITAM motifs in the CD3 zeta chain, which then serve as docking sites for ZAP70 kinase and it is accompanied by ZAP70 phosphorylation. From this initial step, TCR signaling diversifies to trigger a variety of signaling modules that yield actin polymerization. ZAP70 can activate NPF WASp via WIP-CrkL pathway (1), or via recruitment of Nck and thus of WASp. ZAP70-mediated recruitment of LAT and SLP76 complex, which then recruits Vav1, can activate Rho GTPase Cdc42, which in turn can release WASp auto-inhibition. Alternatively, WASp can be activated by PIP2-dependent-, Fyn-dependent or PST-PIP pathway (2). Activated WASp then recruits Arp2/3 complex and initiates actin polymerization. HS1, another NPF with cortactin activity, is recruited in an ITK-dependent manner and interacts with WASp, or can independently bind Arp2/3 and stabilize F-actin. WASP and HS1 activation in response to TCR engagement promotes release of Ca2+ from intracellular stores. In addition to Vav1, Lck-dependent activation of another GEF - SLAT - causes Rac GTPase activation, which in turn causes Abi/WAVE complex-dependent Arp2/3 recruitment to generate lamellipodial spreading and Ca2+ entry from the extracellular milieu. SLAT also activates Cdc42. Activation of WASH (3), a WASp-family NPF, causes the binding of WASH regulatory complex (SHRC) with endosomes, Arp2/3 complex recruitment and subsequent actin polymerization at the endosomes. SHRC also interacts with microtubules, and regulates endocytic recycling of cell surface receptors. Apart from the actin nucleating factor Arp2/3, the formin family proteins respond to TCR activation, and act to elongate the actin filaments linearly. These proteins localize to filopodia, as well as the perinuclear centrosomal area, where they regulate MTOC polarization to the synapse. (NPFs in the signaling cascades shown in red).
4.1. Actin polymerization effectors
4.1.1. Rho GTPases and GEFs
Rho family GTPases involved in TCR signaling include primarily Rac1, Rac2, Cdc42 and RhoH. Knock-out models for all four proteins show defects in T cell development, activation and function [66]. Rac and Cdc42 GTPases bind to and activate effectors such as NPFs when GTP-loaded (section 4.1.2). Among the other effectors of Rac and Cdc42, pak1 has been shown to be an important player, which impacts on the actin cytoskeleton in multiple ways and also leads to activation of MAPKs. While it is known that RhoH does not possess intrinsic GTPase activity and suppresses the activity of other Rho family GTPases, mechanistic details of how RhoH functions in TCR signaling have been controversial. One group has shown that ZAP70 activation is intact in RhoH-deficient thymocytes [67] whereas another group has shown that ITAM-like sequences of RhoH are important for ZAP70 recruitment and activation [68]. GTPases of the Rho family are activated by GTPase exchange factors (GEFs) that catalyze the exchange of GDP to GTP. Multiple GEFs have been shown to participate in TCR signaling. These include Vav1-3, Dock2, Dock8 and SLAT (also known as IBP or Def6). Knock-out models for these GEFs show multiple defects associated with T cell development, activation and function [66].
Vav1-deficient T cells have profound defects in TCR signaling, including Ca2+ influx, cytoskeletal rearrangements, activation of Erk, Akt, and the transcription factors NFAT and NF-κB [66]. Vav1 is recruited to phosphorylated SLP76 via its SH2 domain and is phosphorylated by ZAP70 (Figure 2). This phosphorylation relieves the auto-inhibitory conformation leading to GEF activity towards Rac. The C-terminal proline-rich region and SH3 domains of Vav1 are involved in interactions with Itk, PLCγ1 and Nck [69, 70]. This suggests that Vav1 may stabilize the SLP76-containing signalosome components through multiple mutual interactions. Consistent with this idea, expression of GEF-deficient Vav1 in Vav1-null cells rescues many of the TCR signaling defects [71]. Similar results were obtained from knock-in mice with GEF-deficiency [72]. Thus, the scaffolding function of Vav1 is necessary for Ca2+ influx and activation of Erk, whereas GEF activity is essential for Rac activity, F-actin polymerization and conjugation with APCs. SLAT exhibits GEF activity towards Cdc42 and, to a lesser extent, towards Rac. SLAT is recruited to the immunological synapse through tyrosine phosphorylation of its ITAM-like sequence by Lck and recognition of phosphatydilinositol 3–4–5 triphosphate (PIP3) by its PH domain [73]. SLAT-deficient T cells have defects in Ca2+ influx, activation of Cdc42, Rac, Erk, and NFAT during TCR signaling [74]. These cells also have defective actin polymerization at the synapse [75].
Dock2 belongs to the Dock-A family of GEFs and shows hematopoietic lineage-restricted expression. Dock2 functions together with one of the ELMO family members as a bipartite Rac GEF. Membrane recruitment of Dock2 is mediated by binding of DHR1 domain to PIP3 and also by Elmo [76]. While much of the TCR signaling is intact in Dock2-deficient T cells, they exhibit impaired Rac activity [77]. Also, Dock2-deficient T cells have reduction in clustering of TCR, accumulation of PKCθ and lipid rafts at the synapse and antigen-induced proliferation. Dock8 belongs to the Dock-C family of GEFs and acts on Cdc42 [76]. Inactivating mutations in Dock8 leads to immunodeficiency syndrome both in humans and mice. Dock8-deficient T cells from patients with this syndrome have reduced anti-CD3/CD28 antibody-induced proliferation [78]. Dock8-deficient murine CD8 T cells show defects in actin polymerization and LFA-1 polarization at the synapse.
4.1.2. Actin nucleation factors
Actin filament formation from the monomeric actin subunits (G-actin) is a kinetically unfavorable process and requires the aid of a set of proteins called nucleation factors. Nucleation factors bind G-actin subunits and F-actin simultaneously, therefore promoting filament nucleation and branching. Actin related proteins (Arp2/3) complex and the formin protein family represent two well-characterized nucleation factors in T cells. The Arp2/3 complex consists of a multimeric assembly of seven subunits and requires NPF-dependent recruitment to F-actin and activation. The Arp2 and Arp3 subunits of the complex are proposed to mimic the growing end of an actin filement. Once bound to F-actin, the Arp2/3 complex is presented with an actin monomer bound to NPF, causing daughter filament elongation at 70° to the mother filament.
Silencing Arp2 or Arp3 proteins in Jurkat T cells results in their failure to spread on anti-CD3 antibody-coated glass coverslips. F-actin accumulation is also impaired in the Tcell-APC conjugate interface of Arp2/3-depleted T cells, suggesting that Arp2/3 is critical for actin polymerization at the synapse [79]. In Arp2/3-deficient cells filopodial extensions still form, indicating that actin-nucleating factor(s), in addition to Arp2/3 complex, are likely to participate in actin polymerization at the synapse. Formins are the other class of nucleating factors in T cells that bind to barbed end of actin filaments in a dimeric state and prevent access of the capping proteins. Formin-bound filaments grow processively, further aided by the binding of profiling to the formin. Jurkat T cells depleted of the predominant formin family member Formin-like protein 1 (FMNL-1) exhibit normal F-actin accumulation at the synapse. In addition, filopodia in Arp2/3-deficient cells formed independently of FMNL-1 as well, suggesting the involvement of additional nucleation factors. While FMNL-1 and another formin, mammalian diaphanous (mDia-1), colocalize with the microtubule-organizing center (MTOC) and Jurkat T cells deficient for FMNL-1 or mDia-1 fail to polarize MTOC towards the synapse, Arp2/3-deficient cells show normal MTOC orientation. Recently, FMNL-1 silencing increased overall cellular F-actin in Jurkat T cells and fragmented the Golgi complex [80]. T cells derived from mDia-1 knockout mice show defective proliferative responses when activated; it is unclear if these defects are a manifestation of impaired centrosomal reorientation [81]. The identity of the nucleation factor driving filopodia formation in Jurkat cells in the absence of Arp2/3 is unclear [79]. It is notable that a challenge of interpreting the loss-of-function experiments with actin nucleators and their activators may arise from redundancy in the polymerization pathways. It is likely that different actin nucleators compete for G-actin and suppression of one pathway may result in compensatory increase in the other by making more G-actin available to the remaining pathways.
4.1.3. Actin nucleation promoting factors (NPF)
NPFs are the modular scaffold proteins that activate nucleating factors, as well as plethora of upstream signaling proteins, both diversifying actin polymerization responses and integrating TCR-signaling with actin polymerization. The WASp family of proteins includes the major NPFs in T cells. These include neuronal Wiskott-Aldrich syndrome protein (N-WASp), its hematopoietic cell-specific homolog WASp, WASp family-verprolin homology proteins (WAVE/SCAR) and WASp and SCAR homolog protein (WASH). The WASp family is characterized by the presence of a C-terminal verprolin homology cofilin homology acidic (VCA) domain and proline rich region (PRR)[7]. The VCA domain binds to actin and Arp2/3, and the PRR-domain of NPFs interacts with various SH3 domain-containing signaling proteins. N-terminal domains are variable amongst WASp family members and dictate subcellular localization and functional specificity of these proteins.
The N-terminal portion of the WASp and N-WASp proteins contains a WH1 domain (EVH1) and GTPase binding domain (GBD). Under resting conditions, these proteins exist in auto-inhibited state, where the GBD domain masks the VCA domain. Upon RhoGTPase Cdc42 binding at the GBD domain, there is a conformational change that relieves auto-inhibition and makes the VCA domain available to interact with Arp2/3 and actin. Besides the VCA masking mechanism, there are additional mechanisms that promote WASp activation. For example, phosphorylation of WASp on the Tyr291 (human)/Tyr293 (mouse) residue by protein tyrosine kinase appears to regulate WASp activity, by enhancing sensitivity to Cdc42. Overexpression of non-phosphorylatable Tyr293F mutant of WASp in WASp knockout mice induces similar immune dysregulation as in WASp knockout mice [82, 83]. In addition, WASp can be activated in a Cdc42-independent manner in T cells [84, 85]. This regulation is proposed to occur via phosphatydilinositol 4–5 biphosphate (PIP2)-mediated recruitment, via LAT/SLP76-mediated recruitment of NCK and thus WASP, via interaction with Fyn, via ZAP70-CrkL-WIP-WASP pathway and PST-PIP pathway [27, 83, 86, 87]. The WH1 domain of WASp also plays a regulatory role in WASp activation, via binding to the WASp interacting protein (WIP). In immune cells, WASp exists in a constitutive complex with WIP, which serves as chaperone for WASp. In the absence of WIP, WASp protein cannot be detected.
WASp deficiency manifests as Wiskott-Aldrich syndrome (WAS) in humans, and a large number of missense mutations in WAS patients are found in the WIP binding region of WASp. WAS is characterized by immunodeficiency, thrombocytopenia and eczema. WAS patients show a spectrum of immunological defects including recurrent infections and autoimmunity. T cells from WAS patients show cytoskeletal defects, aberrant morphology and impaired proliferation in response to TCR activation [88–90]. In mice lacking WASp, T cells exhibit defective proliferation in response to antigenic stimulus. T cells from WASp-/- mice have unstable synapses and reduced Ca2+ influx and IL2-production [53, 91–93]. While WASp is required for T cell activation and IL-2 production, it has a redundant role with N-WASp in T cell development. T cells develop normally in WASp-/- mice, however in WASp N-WASp double knockout mice T cell development is impaired [94]. In addition, there have been variable reports regarding the role of WASp in actin organization at the synapse in the mouse system [91, 92, 95]. Whether the defects in T cell activation in WASp-deficient cells arise from its role in actin polymerization remains to be established.
Owing to the variable WASp knockout phenotype for actin polymerization at the synapse, another WASp-family protein - WAVE2 - was investigated for its role in actin remodeling at the synapse. WAVE2 exists in a protein complex, associating with five proteins: Abi1 and2, HSPC300, HEM1 and PIR121. This is an obligate complex as shRNA-mediated depletion of any of its members results in loss of the other binding partners [96]. In resting conditions the Abi/WAVE2 complex is thought to be constitutively assembled [97]. The WAVE2 complex is activated by allosteric changes induced by simultaneous binding of Rac1-GTP and acidic phospholipids [98]. How TCR signaling provides these signals for WAVE2 activation is unclear. In Jurkat T cells activated on anti-CD3 antibody-coated glass coverslips and expressing eYFP-Abi1 to mark the WAVE2 complex, Abi1 localized to the lamellipodia and was enriched on the tip, suggesting WAVE2-dependent actin polymerization contributes to lamellipodial growth [97]. WAVE2 is required for lammellipodial spreading, for proper conjugate formation and actin accumulation at the synapse in the T cell-APC conjugates [96]. Silencing WAVE2 results in diminished Ca2+ mobilization in T cells, however, this was found to occur downstream of IP3-mediated Ca2+ release from the endoplasmic reticulum. Treatment of WAVE2-depleted cells with thapsigargin, an inhibitor of endoplasmic reticulum Ca2+-ATPases, thus triggering the Ca2+ release from intracellular stores, did not rescue Ca2+defects. The defect was specific to the Ca2+ entry from extracellular medium [96]. This result implicated WAVE2 in the regulation of CRAC. It is possible that WAVE2-driven actin polymerization in the lammellipodia influences the interaction of STIM-1/2 in the endoplasmic reticulum with the Orai channels in the plasma membrane, a proposed mechanism for Ca2+-induced Ca2+entry from extracellular source [99]. TCR-mediated activation of WAVE2 is also implicated inside-out signaling for activation of integrins via the interaction of WAVE2 with vinculin and talin [100].
WASH is the most recent NPF to be identified in T cells. Like the WAVE2 protein, it exists in a multi-protein complex, termed as WASH regulatory complex (SHRC). The assembly complex includes family with sequence similarity 21, member C (FAM21C), Strumpellin, Strumpellin and WASH interacting protein (SWIP), and Coiled-coil domain-containing protein 53 (CCDC53). As in the case of WAVE2 complex; SHRC stabilizes the levels of individual SHRC components and regulates WASH function [101]. WASH localizes at the plasmamembrane and intracellular vesicles, and regulates endosome-associated Arp2/3 activity [102]. Although T cells derived from WASH knockout (WASHout) mice show normal T cell signaling and activation, T cell proliferation is compromised. These cells also show impaired trafficking of TCR and other key T cell surface proteins including GLUT1, targeting them for lysosomal degradation [103]. Although to what extent these sorting defects arise via NPF activity of WASH has not been addressed yet, these results pose an interesting possibility that WASH regulates T cell endomembrane trafficking via maintenance of actin dynamics at endosomes. Long–term dysregulation of receptor homeostasis and T cell proliferation results from defects in this F-actin pool. Future experiments should yield more information on the mechanism of how endosomal actin affects T cell response, and how WASH integrates the two.
4.2. Other regulatory proteins
4.2.1. Hematopoietic cell-specific protein, HS1
HS1 is the hematopoietic cell-specific form of cortactin. Capable of interacting with both Arp2/3 complex and F-actin, HS1 promotes stable branching. HS1 also has low NPF behaviour; however the significance of this low activity in T cells is still unclear. HS1 is rapidly phosphorylated in response to antigenic stimuli in T cell, and interacts with a variety of proteins including Lck, ITK, Vav1 and WASp. Consistent with its F-actin stabilizing role, HS1-deficient T cells show unstable lamellipodia when activated on anti-CD3 antibody-coated glass coverslips. Loss of HS1 also results in impaired Ca2+ influx and T cell proliferation [104, 105]. Although the exact role of HS1 in T cell activation is not clear, existing data indicates that it regulates PLCγ1 dynamics at the synapse. TCR engagement induces ZAP70-dependent tyrosine phosphorylation and ITK-dependent recruitment of HS1 at the synapse. Phospho-HS1 interacts with PLCγ1 and regulates its association with F-actin [106]. In addition, phospho-Tyr on HS1 can serve as docking sites for Vav1 and is critical for Vav1 stabilization and thus for actin accumulation at the synapse [105]. This can be further assisted by HS1-WASp interaction [107]. Considering that HS1 is capable of interacting with a variety of SH2-domain-containing TCR-downstream signaling proteins, it may employ multiple signaling routes to regulate actin polymerization at the synapse.
4.2.2. Endocytic proteins
The well-characterized endocytosis regulators from other cell types appear to play an important role in the regulation of actin cytoskeleton in T cells. Loss of endosome scission protein dynamin2 (dyn2) in T cells results in defective actin accumulation at the synapse. The dyn2 PRD domain was found to interact with Vav1, and dyn2-depleted cells exhibit defects in Ca2+ elevation and PLCγ1 phosphorylation. The Ca2+ influx defects in dyn2-silenced cells were independent of LAT complex [108]. Similarly, endocytic coat protein clathrin is recruited to the synapse, localizes with TCR and affects actin accumulation at the synapse [109]. Interestingly, clathrin depletion leads to reduction in dyn2 and Arp2/3 recruitment at the synapse, suggesting that endocytosis of TCR at the synapse may accompany actin polymerization. In addition, the previously characterized role of dynamin in actin polymerization may in part accompany TCR endocytosis.
In addition to actin polymerization effectors, actin depolymerization proteins are also required to maintain actin dynamics at the synapse. Treatment of Jurkat T cells with F-actin stabilizing drugs causes accumulation of F-actin at the distinct subsynaptic boundaries of dSMAC and pSMAC [10], suggesting an actin depolymerization hotspot between dSMAC and pSMAC, and between pSMAC and cSMAC. While this could be due to myosinII-mediated contraction and thus bundle disassembly, F-actin severing protein cofilin has also been implicated in maintaining F-actin dynamics at synapse. Cofilin is recruited to the T cell-B cell synapse, outside of the cSMAC. Inhibition of cofilin using cell-permeable inhibitory cofilin-homolog peptides results in reduced synapse formation, activation-induced T cell proliferation and Interferon-γ production [65]. Considering that cofilin can competitively inhibit myosinII binding with F-actin, it will be interesting to investigate which of these proteins contributes to the generation of the F-actin-poor cSMAC zone in T cells [110]. Another component of the actin polymerization inhibition machinery is coronin-1. A hemoatopoetic cell-specific protein, coronin-1 can bind to the Arp2/3 complex and inhibit its actin nucleation activity. Coronin-1 homotrimerizes, and binds actin and plasma membrane simultaneously creating a molecular link between extracellular signals and actin cytoskeleton [111]. Interestingly, although coronin-deficient mouse T cells show more accumulation of F-actin and actin polymerization molecules such as WASP and Arp2/3 at the synapse, actin dynamics and synapse stability are compromised [112]. Consequently, these cells show aberrant development, differentiation and antigen-induced distal signaling. These studies have illustrated a tight and complex regulation of actin dynamics and its role in T cell activation, where F-actin polymerization or its stability alone are not sufficient to drive optimal T cell responses.
In addition to regulating actin filament turnover, some actin effectors also regulate microfilament-microtubule interactions. Such proteins are vital in coordinating the organization and function of these cytoskeletal systems in various phases of the immunological synapse. As discussed in sections above, formins and WASH proteins fall under this category. Reviewed elsewhere recently [43], several other molecular bridges are being revealed, illustrating the importance of communication between the two types of cytoskeleton at the synapse.
5. Actin and TCR Mechanotransduction
Mechanical force has been implied in several biological processes including stem cell differentiation and self-renewal, mitosis, migration, among others (reviewed in [113]) and has also been proposed to play a role in T cell activation [114]. Actomyosin cytoskeleton is fundamental in mediating force transmission. It is well-positioned, spanning large distances within the cell and thus connects parts of the cell membrane as well as the cell membrane to the nucleus [115].
As mentioned above, in one of the first events of T cell activation, ITAM motifs of the TCR-associated CD3 chains are phosphorylated by Lck, enabling all subsequent events of the signaling cascade. In the inactive conformation, before TCR ligation, the TCR-CD3 complex cytoplasmic tails with ITAMs are thought to interact with the lipids in the inner leaflet of the plasma membrane leading to a reversible conformational change that may restrict access of the Lck to their tyrosine residues (safety model) [116–118]. Other studies however contradict this model and suggest that the dissociation of the TCR-CD3 cytoplasmic chains is not a cause but a consequence of the ITAM motifs’ phosphorylation [119]. It is therefore not yet well understood how the TCR ligation triggers the release of the CD3 ITAM motifs, among other possibilities, mechanical forces and/or local membrane environment have been suggested to play a role [120].
Several studies, by different groups, have indeed shown that applying mechanical forces to the TCR can activate T cells and that the TCR can act as a mechanosensor: lateral forces applied to TCR using non-stimulatory anti-CD3 monoclonal antibody were able to trigger cytoplasmic Ca2+ elevation [121] and axial forces applied by micromanipulation were able to rescue T cell stimulating defects associated with elongated pMHC [122]. It has also been established using a biomembrane force probe that T cells generate pushing and pulling forces when anti-CD3 antibody on beads engaged TCR complexes [30]. Furthermore, mouse [123] and human [124] T cells display correlations between substrate elastic modulus (stiffness) and T cell response. Mouse CD4+ T cells produced more IL-2 in response to polyacrylamide gels of increasing elastic modulus coated with anti-CD3 and anti-CD28 antibodies. Blebbistatin abolished the observed rigidity effect, which suggests that actomyosin cytoskeleton is involved in the T cell response to environmental mechanical rigidity. In the same study it was also shown that the mechanosensing response to the stiff substrate was mediated by the CD3 epsilon chain rather than CD28, under the conditions used [123]; the involvement of the TCR, rather than CD28, had also been suggested by Li et al.[122]. Consistent with the detection of increased level of IL-2 secretion in cells cultured on stiffer surfaces, clusters of phophorylated ZAP70 (pZAP70), phosphorylated Src family kinase proteins (Lck and Fyn) and phosphorylated proline rich tyrosine kinase-2 (pPYK2) were detected in the cell surface of these cells, whereas no (pZAP70 and pSFK) or only minor clusters (pPYK2) were observed in the cell surface of cells cultured on softer surfaces. Interestingly, under stiffer surfaces conditions, blebbistatin treatment did not alter the localization of pZAP70 and pSFK but had an effect on pPYK2 distribution, suggesting that PYK2 responds to cell contractility and contributes to the T cell mechanosensing mechanism [123]. It has been noted that correlations between cellular responses and substrate elastic modulus do not demonstrate a mechanical response, but may also be related to the chemistry of ligand presentation, which can also vary with gel and elastomer properties that alter elastic modulus [125].
Multiple factors may account for generation of force on the TCR in vivo. T cell migration and scanning of APC surfaces in search of a particular pMHC can lead to tensile mechanical force (as mentioned in 3.1.2). Once the TCR binds the pMHC, an extracellular mechanical torque can occur and result in structural changes within the TCR-pMHC complex and subsequent triggering of downstream signaling, though the same effect could not be observed when normal forces were applied [114, 121]. Within the synapse, the formation of microclusters and their actin-based movement can also generate force on the TCR. Methods to directly measure forces in TCR microclusters are on the horizon [126]. TCR clusters are formed in the periphery of the synapse where actin protrusions and myosin-dependent retraction occur to drive the movement of the TCR microclusters towards the center of the synapse, thus exposing the TCR to mechanical force [53, 127]. The fact that a deficiency in PYK2, which is closely related to mechanotransducer focal adhesion kinase (FAK), results in the loss of CD8+ effector cells suggests that PYK2 is critical for the CD8 T cell effector fate and supports mechanotransduction as a player in T cell signaling [128]. Mechanotranduction is becoming a field of great interest in the study of the immune cell activation and differentiation, much however remains to be understood regarding the actin cytoskeletal regulation that underlies the response of T cells to environmental mechanical forces.
6. Perspective
Since the discovery of actin polymerization as a critical regulator of immunological synapse formation and T cell activation, there have been as-yet increasing insights into the identity and interactions of actin effectors at the synapse. Recent studies have begun to unravel the diversity of functions that the actin machinery carries out for TCR signaling, however future experiments will be required to investigate the specific mechanism of actin participation in processes such as microcluster formation and molecular basis of their coupling with the retrograde actin flow. It is also unclear if actin remodeling is tuned depending upon the strength of TCR activating signal, and whether microcluster-F actin coupling can influence signal integration at single microcluster level. Careful examination of qualitative F-actin responses at high resolution, and characterization of subsynaptic F-actin organization, will aid in addressing these questions. Super-resolution studies of F-actin have been useful in the study of exocytosis in this regard, and will likely soon provide insight into mechanisms of TCR signaling [129]. In addition, mechanistic insights into the role and regulation of actin effectors will uncover the signaling pathways integrated to maintain F-actin at the synapse. For example, ubiquitination of actin regulators has recently emerged as a mechanism to regulate effector levels [130–132], whether this mechanism coincides with receptor ubiquitination in the T cells [133] remains to be investigated. In addition, T cells defective in a variety of actin regulators exhibit defective antigen-induced Ca2+ mobilization. Knowing how actin dynamics communicates with Ca2+ stores will be a key step in forwarding our understanding of the process of T cell activation and immune disorders where T cell activation is compromised. There is a need to expand the views on a more integrated picture of cytoskeletal regulation T cell behavior, by investigating ways of generation and disassembly of actin machinery, and its subcellular function.
Highlights.
Antigen-sampling and T cell receptor (TCR) activation relies heavily on the cortical actin dynamics.
Once triggered, TCR drives molecular programs leading to actin polymerization and rearrangement.
Polymerized actin (F-actin) in turn modulates various stages of T-cell immunological synapse and T cell activation.
Impaired TCR-microfilament crosstalk leads to defective T cell responses.
Acknowlegments
S.K. and V.M. are supported by the Cancer Research Institute postdoctoral fellowships. S.C. and M.L.D. are supported by the NIH Common Fund Nanomedicine Development Center PN2EY016586.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 2.Burkhardt JK, Carrizosa E, Shaffer MH. The actin cytoskeleton in T cell activation. Annu Rev Immunol. 2008;26:233–259. doi: 10.1146/annurev.immunol.26.021607.090347. [DOI] [PubMed] [Google Scholar]
- 3.Reicher B, Barda-Saad M. Multiple pathways leading from the T-cell antigen receptor to the actin cytoskeleton network. FEBS Lett. 2010;584:4858–4864. doi: 10.1016/j.febslet.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Irvine DJ, Purbhoo MA, Krogsgaard M, Davis MM. Direct observation of ligand recognition by T cells. Nature. 2002;419:845–849. doi: 10.1038/nature01076. [DOI] [PubMed] [Google Scholar]
- 5.Thrasher AJ, Burns SO. WASP: a key immunological multitasker. Nature reviews. Immunology. 2010;10:182–192. doi: 10.1038/nri2724. [DOI] [PubMed] [Google Scholar]
- 6.Beemiller P, Krummel MF. Mediation of T-cell activation by actin meshworks. Cold Spring Harbor perspectives in biology. 2010;2:a002444. doi: 10.1101/cshperspect.a002444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, Dong B, Siminovitch KA. Contributions of Wiskott-Aldrich syndrome family cytoskeletal regulatory adapters to immune regulation. Immunological reviews. 2009;232:175–194. doi: 10.1111/j.1600-065X.2009.00846.x. [DOI] [PubMed] [Google Scholar]
- 8.Dustin ML. Cell adhesion molecules and actin cytoskeleton at immune synapses and kinapses. Curr Opin Cell Biol. 2007;19:529–533. doi: 10.1016/j.ceb.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Billadeau DD, Nolz JC, Gomez TS. Regulation of T-cell activation by the cytoskeleton. Nature reviews. Immunology. 2007;7:131–143. doi: 10.1038/nri2021. [DOI] [PubMed] [Google Scholar]
- 10.Yi J, Wu XS, Crites T, Hammer JA., 3rd Actin retrograde flow and actomyosin II arc contraction drive receptor cluster dynamics at the immunological synapse in Jurkat T cells. Molecular biology of the cell. 2012;23:834–852. doi: 10.1091/mbc.E11-08-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Babich A, Li S, O'Connor RS, Milone MC, Freedman BD, Burkhardt JK. F-actin polymerization and retrograde flow drive sustained PLCgamma1 signaling during T cell activation. J Cell Biol. 2012;197:775–787. doi: 10.1083/jcb.201201018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beemiller P, Jacobelli J, Krummel MF. Integration of the movement of signaling microclusters with cellular motility in immunological synapses. Nature immunology. 2012;13:787–795. doi: 10.1038/ni.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hashimoto-Tane A, Yokosuka T, Sakata-Sogawa K, Sakuma M, Ishihara C, Tokunaga M, Saito T. Dynein-driven transport of T cell receptor microclusters regulates immune synapse formation and T cell activation. Immunity. 2011;34:919–931. doi: 10.1016/j.immuni.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Ilani T, Vasiliver-Shamis G, Vardhana S, Bretscher A, Dustin ML. T cell antigen receptor signaling and immunological synapse stability require myosin IIA. Nature immunology. 2009;10:531–539. doi: 10.1038/ni.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rizvi SA, Neidt EM, Cui J, Feiger Z, Skau CT, Gardel ML, Kozmin SA, Kovar DR. Identification and characterization of a small molecule inhibitor of formin-mediated actin assembly. Chem Biol. 2009;16:1158–1168. doi: 10.1016/j.chembiol.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nolen BJ, Tomasevic N, Russell A, Pierce DW, Jia Z, McCormick CD, Hartman J, Sakowicz R, Pollard TD. Characterization of two classes of small molecule inhibitors of Arp2/3 complex. Nature. 2009;460:1031–1034. doi: 10.1038/nature08231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallego MD, Santamaria M, Pena J, Molina IJ. Defective actin reorganization and polymerization of Wiskott-Aldrich T cells in response to CD3-mediated stimulation. Blood. 1997;90:3089–3097. [PubMed] [Google Scholar]
- 18.Paccani SR, Boncristiano M, Patrussi L, Ulivieri C, Wack A, Valensin S, Hirst TR, Amedei A, Del Prete G, Telford JL, D'Elios MM, Baldari CT. Defective Vav expression and impaired F-actin reorganization in a subset of patients with common variable immunodeficiency characterized by T-cell defects. Blood. 2005;106:626–634. doi: 10.1182/blood-2004-05-2051. [DOI] [PubMed] [Google Scholar]
- 19.Kaizuka Y, Douglass AD, Varma R, Dustin ML, Vale RD. Mechanisms for segregating T cell receptor and adhesion molecules during immunological synapse formation in Jurkat T cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:20296–20301. doi: 10.1073/pnas.0710258105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riedl J, Crevenna AH, Kessenbrock K, Yu JH, Neukirchen D, Bista M, Bradke F, Jenne D, Holak TA, Werb Z, Sixt M, Wedlich-Soldner R. Lifeact: a versatile marker to visualize F-actin. Nat Methods. 2008;5:605–607. doi: 10.1038/nmeth.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson HW, Schell MJ. Neuronal IP3 3-kinase is an F-actin-bundling protein: role in dendritic targeting and regulation of spine morphology. Molecular biology of the cell. 2009;20:5166–5180. doi: 10.1091/mbc.E09-01-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dustin ML, Depoil D. New insights into the T cell synapse from single molecule techniques. Nature reviews. Immunology. 2011;11:672–684. doi: 10.1038/nri3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bunnell SC, Kapoor V, Trible RP, Zhang W, Samelson LE. Dynamic actin polymerization drives T cell receptor-induced spreading: a role for the signal transduction adaptor LAT. Immunity. 2001;14:315–329. doi: 10.1016/s1074-7613(01)00112-1. [DOI] [PubMed] [Google Scholar]
- 24.Mossman KD, Campi G, Groves JT, Dustin ML. Altered TCR signaling from geometrically repatterned immunological synapses. Science. 2005;310:1191–1193. doi: 10.1126/science.1119238. [DOI] [PubMed] [Google Scholar]
- 25.DeMond AL, Mossman KD, Starr T, Dustin ML, Groves JT. T cell receptor microcluster transport through molecular mazes reveals mechanism of translocation. Biophys J. 2008;94:3286–3292. doi: 10.1529/biophysj.107.119099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campi G, Varma R, Dustin ML. Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J Exp Med. 2005;202:1031–1036. doi: 10.1084/jem.20051182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barda-Saad M, Braiman A, Titerence R, Bunnell SC, Barr VA, Samelson LE. Dynamic molecular interactions linking the T cell antigen receptor to the actin cytoskeleton. Nature immunology. 2005;6:80–89. doi: 10.1038/ni1143. [DOI] [PubMed] [Google Scholar]
- 28.Hsu CJ, Baumgart T. Spatial association of signaling proteins and F-actin effects on cluster assembly analyzed via photoactivation localization microscopy in T cells. PloS one. 2011;6:e23586. doi: 10.1371/journal.pone.0023586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sherman E, Barr V, Manley S, Patterson G, Balagopalan L, Akpan I, Regan CK, Merrill RK, Sommers CL, Lippincott-Schwartz J, Samelson LE. Functional nanoscale organization of signaling molecules downstream of the T cell antigen receptor. Immunity. 2011;35:705–720. doi: 10.1016/j.immuni.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Husson J, Chemin K, Bohineust A, Hivroz C, Henry N. Force generation upon T cell receptor engagement. PloS one. 2011;6:e19680. doi: 10.1371/journal.pone.0019680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bourouina N, Husson J, Hivroz C, Henry N. Biomimetic droplets for artificial engagement of living cell surface receptors: the specific case of the T-cell. Langmuir. 2012;28:6106–6113. doi: 10.1021/la300398a. [DOI] [PubMed] [Google Scholar]
- 32.James JR, Vale RD. Biophysical mechanism of T-cell receptor triggering in a reconstituted system. Nature. 2012;487:64–69. doi: 10.1038/nature11220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang J, Zarnitsyna VI, Liu B, Edwards LJ, Jiang N, Evavold BD, Zhu C. The kinetics of two-dimensional TCR and pMHC interactions determine T-cell responsiveness. Nature. 2010;464:932–936. doi: 10.1038/nature08944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valitutti S, Dessing M, Aktories K, Gallati H, Lanzavecchia A. Sustained signaling leading to T cell activation results from prolonged T cell receptor occupancy. Role of T cell actin cytoskeleton. J Exp Med. 1995;181:577–584. doi: 10.1084/jem.181.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holsinger LJ, Graef IA, Swat W, Chi T, Bautista DM, Davidson L, Lewis RS, Alt FW, Crabtree GR. Defects in actin-cap formation in Vav-deficient mice implicate an actin requirement for lymphocyte signal transduction. Current biology : CB. 1998;8:563–572. doi: 10.1016/s0960-9822(98)70225-8. [DOI] [PubMed] [Google Scholar]
- 36.Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–127. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeBell KE, Conti A, Alava MA, Hoffman T, Bonvini E. Microfilament assembly modulates phospholipase C-mediated signal transduction by the TCR/CD3 in murine T helper lymphocytes. J Immunol. 1992;149:2271–2280. [PubMed] [Google Scholar]
- 38.Rivas FV, O'Keefe JP, Alegre ML, Gajewski TF. Actin cytoskeleton regulates calcium dynamics and NFAT nuclear duration. Mol Cell Biol. 2004;24:1628–1639. doi: 10.1128/MCB.24.4.1628-1639.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mingueneau M, Roncagalli R, Gregoire C, Kissenpfennig A, Miazek A, Archambaud C, Wang Y, Perrin P, Bertosio E, Sansoni A, Richelme S, Locksley RM, Aguado E, Malissen M, Malissen B. Loss of the LAT adaptor converts antigen-responsive T cells into pathogenic effectors that function independently of the T cell receptor. Immunity. 2009;31:197–208. doi: 10.1016/j.immuni.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 40.Lillemeier BF, Mortelmaier MA, Forstner MB, Huppa JB, Groves JT, Davis MM. TCR and Lat are expressed on separate protein islands on T cell membranes and concatenate during activation. Nature immunology. 2010;11:90–96. doi: 10.1038/ni.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Purbhoo MA, Liu H, Oddos S, Owen DM, Neil MA, Pageon SV, French PM, Rudd CE, Davis DM. Dynamics of subsynaptic vesicles and surface microclusters at the immunological synapse. Sci Signal. 2010;3:ra36. doi: 10.1126/scisignal.2000645. [DOI] [PubMed] [Google Scholar]
- 42.Williamson DJ, Owen DM, Rossy J, Magenau A, Wehrmann M, Gooding JJ, Gaus K. Pre-existing clusters of the adaptor Lat do not participate in early T cell signaling events. Nature immunology. 2011;12:655–662. doi: 10.1038/ni.2049. [DOI] [PubMed] [Google Scholar]
- 43.Martin-Cofreces NB, Alarcon B, Sanchez-Madrid F. Tubulin and actin interplay at the T cell and antigen-presenting cell interface. Front Immunol. 2011;2:24. doi: 10.3389/fimmu.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Treanor B, Depoil D, Bruckbauer A, Batista FD. Dynamic cortical actin remodeling by ERM proteins controls BCR microcluster organization and integrity. J Exp Med. 2011;208:1055–1068. doi: 10.1084/jem.20101125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cyster JG, Shotton DM, Williams AF. The dimensions of the T lymphocyte glycoprotein leukosialin and identification of linear protein epitopes that can be modified by glycosylation. The EMBO journal. 1991;10:893–902. doi: 10.1002/j.1460-2075.1991.tb08022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sage PT, Varghese LM, Martinelli R, Sciuto TE, Kamei M, Dvorak AM, Springer TA, Sharpe AH, Carman CV. Antigen recognition is facilitated by invadosome-like protrusions formed by memory/effector T cells. J Immunol. 2012;188:3686–3699. doi: 10.4049/jimmunol.1102594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei X, Tromberg BJ, Cahalan MD. Mapping the sensitivity of T cells with an optical trap: polarity and minimal number of receptors for Ca(2+) signaling. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:8471–8476. doi: 10.1073/pnas.96.15.8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suzuki J, Yamasaki S, Wu J, Koretzky GA, Saito T. The actin cloud induced by LFA-1-mediated outside-in signals lowers the threshold for T-cell activation. Blood. 2007;109:168–175. doi: 10.1182/blood-2005-12-020164. [DOI] [PubMed] [Google Scholar]
- 49.van der Merwe PA, Dushek O. Mechanisms for T cell receptor triggering. Nature reviews. Immunology. 2011;11:47–55. doi: 10.1038/nri2887. [DOI] [PubMed] [Google Scholar]
- 50.Ma Z, Sharp KA, Janmey PA, Finkel TH. Surface-anchored monomeric agonist pMHCs alone trigger TCR with high sensitivity. PLoS biology. 2008;6:e43. doi: 10.1371/journal.pbio.0060043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huppa JB, Axmann M, Mortelmaier MA, Lillemeier BF, Newell EW, Brameshuber M, Klein LO, Schutz GJ, Davis MM. TCR-peptide-MHC interactions in situ show accelerated kinetics and increased affinity. Nature. 2010;463:963–967. doi: 10.1038/nature08746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dustin ML, Bromley SK, Kan Z, Peterson DA, Unanue ER. Antigen receptor engagement delivers a stop signal to migrating T lymphocytes. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:3909–3913. doi: 10.1073/pnas.94.8.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sims TN, Soos TJ, Xenias HS, Dubin-Thaler B, Hofman JM, Waite JC, Cameron TO, Thomas VK, Varma R, Wiggins CH, Sheetz MP, Littman DR, Dustin ML. Opposing Effects of PKCtheta and WASp on Symmetry Breaking and Relocation of the Immunological Synapse. Cell. 2007;129:773–785. doi: 10.1016/j.cell.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 54.Skokos D, Shakhar G, Varma R, Waite JC, Cameron TO, Lindquist RL, Schwickert T, Nussenzweig MC, Dustin ML. Peptide-MHC potency governs dynamic interactions between T cells and dendritic cells in lymph nodes. Nature immunology. 2007;8:835–844. doi: 10.1038/ni1490. [DOI] [PubMed] [Google Scholar]
- 55.Bhakta NR, Oh DY, Lewis RS. Calcium oscillations regulate thymocyte motility during positive selection in the three-dimensional thymic environment. Nature immunology. 2005;6:143–151. doi: 10.1038/ni1161. [DOI] [PubMed] [Google Scholar]
- 56.Jacobelli J, Chmura SA, Buxton DB, Davis MM, Krummel MF. A single class II myosin modulates T cell motility and stopping, but not synapse formation. Nature immunology. 2004;5:531–538. doi: 10.1038/ni1065. [DOI] [PubMed] [Google Scholar]
- 57.Cernuda-Morollon E, Millan J, Shipman M, Marelli-Berg FM, Ridley AJ. Rac activation by the T-cell receptor inhibits T cell migration. PloS one. 2010;5:e12393. doi: 10.1371/journal.pone.0012393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Friedman RS, Jacobelli J, Krummel MF. Mechanisms of T cell motility and arrest: deciphering the relationship between intra- and extracellular determinants. Seminars in immunology. 2005;17:387–399. doi: 10.1016/j.smim.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 59.Mayya V, Dustin ML. Actin Cytoskeleton and the Dynamics of Immunological Synapse. In: Carlier MF, editor. Actin-based motility: cellular, molecular and physical aspects. Springer; 2010. [Google Scholar]
- 60.Kumari S, Vardhana S, Cammer M, Curado S, Santos L, Sheetz MP, Dustin ML. T Lymphocyte Myosin IIA is Required for Maturation of the Immunological Synapse. Front Immunol. 2012;3:230. doi: 10.3389/fimmu.2012.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dustin ML. Hunter to gatherer and back: immunological synapses and kinapses as variations on the theme of amoeboid locomotion. Annu Rev Cell Dev Biol. 2008;24:577–596. doi: 10.1146/annurev.cellbio.24.110707.175226. [DOI] [PubMed] [Google Scholar]
- 62.Azar GA, Lemaitre F, Robey EA, Bousso P. Subcellular dynamics of T cell immunological synapses and kinapses in lymph nodes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:3675–3680. doi: 10.1073/pnas.0905901107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Friedman RS, Beemiller P, Sorensen CM, Jacobelli J, Krummel MF. Real-time analysis of T cell receptors in naive cells in vitro and in vivo reveals flexibility in synapse and signaling dynamics. J Exp Med. 2010;207:2733–2749. doi: 10.1084/jem.20091201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang H, Kadlecek TA, Au-Yeung BB, Goodfellow HE, Hsu LY, Freedman TS, Weiss A. ZAP-70: an essential kinase in T-cell signaling. Cold Spring Harbor perspectives in biology. 2010;2:a002279. doi: 10.1101/cshperspect.a002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eibert SM, Lee KH, Pipkorn R, Sester U, Wabnitz GH, Giese T, Meuer SC, Samstag Y. Cofilin peptide homologs interfere with immunological synapse formation and T cell activation. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:1957–1962. doi: 10.1073/pnas.0308282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tybulewicz VL, Henderson RB. Rho family GTPases and their regulators in lymphocytes. Nature reviews. Immunology. 2009;9:630–644. doi: 10.1038/nri2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dorn T, Kuhn U, Bungartz G, Stiller S, Bauer M, Ellwart J, Peters T, Scharffetter-Kochanek K, Semmrich M, Laschinger M, Holzmann B, Klinkert WE, Straten PT, Kollgaard T, Sixt M, Brakebusch C. RhoH is important for positive thymocyte selection and T-cell receptor signaling. Blood. 2007;109:2346–2355. doi: 10.1182/blood-2006-04-019034. [DOI] [PubMed] [Google Scholar]
- 68.Gu Y, Chae HD, Siefring JE, Jasti AC, Hildeman DA, Williams DA. RhoH GTPase recruits and activates Zap70 required for T cell receptor signaling and thymocyte development. Nature immunology. 2006;7:1182–1190. doi: 10.1038/ni1396. [DOI] [PubMed] [Google Scholar]
- 69.Barda-Saad M, Shirasu N, Pauker MH, Hassan N, Perl O, Balbo A, Yamaguchi H, Houtman JC, Appella E, Schuck P, Samelson LE. Cooperative interactions at the SLP-76 complex are critical for actin polymerization. The EMBO journal. 2010;29:2315–2328. doi: 10.1038/emboj.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Braiman A, Barda-Saad M, Sommers CL, Samelson LE. Recruitment and activation of PLCgamma1 in T cells: a new insight into old domains. The EMBO journal. 2006;25:774–784. doi: 10.1038/sj.emboj.7600978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miletic AV, Graham DB, Sakata-Sogawa K, Hiroshima M, Hamann MJ, Cemerski S, Kloeppel T, Billadeau DD, Kanagawa O, Tokunaga M, Swat W. Vav links the T cell antigen receptor to the actin cytoskeleton and T cell activation independently of intrinsic Guanine nucleotide exchange activity. PloS one. 2009;4:e6599. doi: 10.1371/journal.pone.0006599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saveliev A, Vanes L, Ksionda O, Rapley J, Smerdon SJ, Rittinger K, Tybulewicz VL. Function of the nucleotide exchange activity of vav1 in T cell development and activation. Sci Signal. 2009;2:ra83. doi: 10.1126/scisignal.2000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Becart S, Altman A. SWAP-70-like adapter of T cells: a novel Lck-regulated guanine nucleotide exchange factor coordinating actin cytoskeleton reorganization and Ca2+ signaling in T cells. Immunological reviews. 2009;232:319–333. doi: 10.1111/j.1600-065X.2009.00839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Feau S, Schoenberger SP, Altman A, Becart S. SLAT Regulates CD8+ T Cell Clonal Expansion in a Cdc42-and NFAT1-Dependent Manner. J Immunol. 2013;190:174–183. doi: 10.4049/jimmunol.1201685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fanzo JC, Yang W, Jang SY, Gupta S, Chen Q, Siddiq A, Greenberg S, Pernis AB. Loss of IRF-4-binding protein leads to the spontaneous development of systemic autoimmunity. The Journal of clinical investigation. 2006;116:703–714. doi: 10.1172/JCI24096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Harada Y, Tanaka Y, Terasawa M, Pieczyk M, Habiro K, Katakai T, Hanawa-Suetsugu K, Kukimoto-Niino M, Nishizaki T, Shirouzu M, Duan X, Uruno T, Nishikimi A, Sanematsu F, Yokoyama S, Stein JV, Kinashi T, Fukui Y. DOCK8 is a Cdc42 activator critical for interstitial dendritic cell migration during immune responses. Blood. 2012;119:4451–4461. doi: 10.1182/blood-2012-01-407098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sanui T, Inayoshi A, Noda M, Iwata E, Oike M, Sasazuki T, Fukui Y. DOCK2 is essential for antigen-induced translocation of TCR and lipid rafts, but not PKC-theta and LFA-1, in T cells. Immunity. 2003;19:119–129. doi: 10.1016/s1074-7613(03)00169-9. [DOI] [PubMed] [Google Scholar]
- 78.Randall KL, Chan SS, Ma CS, Fung I, Mei Y, Yabas M, Tan A, Arkwright PD, Al Suwairi W, Lugo Reyes SO, Yamazaki-Nakashimada MA, Garcia-Cruz Mde L, Smart JM, Picard C, Okada S, Jouanguy E, Casanova JL, Lambe T, Cornall RJ, Russell S, Oliaro J, Tangye SG, Bertram EM, Goodnow CC. DOCK8 deficiency impairs CD8 T cell survival and function in humans and mice. J Exp Med. 2011;208:2305–2320. doi: 10.1084/jem.20110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gomez TS, Kumar K, Medeiros RB, Shimizu Y, Leibson PJ, Billadeau DD. Formins regulate the actin-related protein 2/3 complex-independent polarization of the centrosome to the immunological synapse. Immunity. 2007;26:177–190. doi: 10.1016/j.immuni.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Colon-Franco JM, Gomez TS, Billadeau DD. Dynamic remodeling of the actin cytoskeleton by FMNL1gamma is required for structural maintenance of the Golgi complex. J Cell Sci. 2011;124:3118–3126. doi: 10.1242/jcs.083725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eisenmann KM, West RA, Hildebrand D, Kitchen SM, Peng J, Sigler R, Zhang J, Siminovitch KA, Alberts AS. T cell responses in mammalian diaphanous-related formin mDia1 knock-out mice. The Journal of biological chemistry. 2007;282:25152–25158. doi: 10.1074/jbc.M703243200. [DOI] [PubMed] [Google Scholar]
- 82.Blundell MP, Bouma G, Metelo J, Worth A, Calle Y, Cowell LA, Westerberg LS, Moulding DA, Mirando S, Kinnon C, Cory GO, Jones GE, Snapper SB, Burns SO, Thrasher AJ. Phosphorylation of WASp is a key regulator of activity and stability in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15738–15743. doi: 10.1073/pnas.0904346106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Badour K, Zhang J, Shi F, Leng Y, Collins M, Siminovitch KA. Fyn and PTP-PEST-mediated regulation of Wiskott-Aldrich syndrome protein (WASp) tyrosine phosphorylation is required for coupling T cell antigen receptor engagement to WASp effector function and T cell activation. J Exp Med. 2004;199:99–112. doi: 10.1084/jem.20030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cannon JL, Labno CM, Bosco G, Seth A, McGavin MH, Siminovitch KA, Rosen MK, Burkhardt JK. Wasp recruitment to the T cell:APC contact site occurs independently of Cdc42 activation. Immunity. 2001;15:249–259. doi: 10.1016/s1074-7613(01)00178-9. [DOI] [PubMed] [Google Scholar]
- 85.Cory GO, Garg R, Cramer R, Ridley AJ. Phosphorylation of tyrosine 291 enhances the ability of WASp to stimulate actin polymerization and filopodium formation. Wiskott-Aldrich Syndrome protein. The Journal of biological chemistry. 2002;277:45115–45121. doi: 10.1074/jbc.M203346200. [DOI] [PubMed] [Google Scholar]
- 86.Sato M, Sawahata R, Takenouchi T, Kitani H. Identification of Fyn as the binding partner for the WASP N-terminal domain in T cells. Int Immunol. 2011;23:493–502. doi: 10.1093/intimm/dxr042. [DOI] [PubMed] [Google Scholar]
- 87.Sasahara Y, Rachid R, Byrne MJ, de la Fuente MA, Abraham RT, Ramesh N, Geha RS. Mechanism of recruitment of WASP to the immunological synapse and of its activation following TCR ligation. Mol Cell. 2002;10:1269–1281. doi: 10.1016/s1097-2765(02)00728-1. [DOI] [PubMed] [Google Scholar]
- 88.Cianferoni A, Massaad M, Feske S, de la Fuente MA, Gallego L, Ramesh N, Geha RS. Defective nuclear translocation of nuclear factor of activated T cells and extracellular signal-regulated kinase underlies deficient IL-2 gene expression in Wiskott-Aldrich syndrome. J Allergy Clin Immunol. 2005;116:1364–1371. doi: 10.1016/j.jaci.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 89.Calvez R, Lafouresse F, De Meester J, Galy A, Valitutti S, Dupre L. The Wiskott-Aldrich syndrome protein permits assembly of a focused immunological synapse enabling sustained T-cell receptor signaling. Haematologica. 2011;96:1415–1423. doi: 10.3324/haematol.2011.040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Molina IJ, Sancho J, Terhorst C, Rosen FS, Remold-O'Donnell E. T cells of patients with the Wiskott-Aldrich syndrome have a restricted defect in proliferative responses. J Immunol. 1993;151:4383–4390. [PubMed] [Google Scholar]
- 91.Cannon JL, Burkhardt JK. Differential roles for Wiskott-Aldrich syndrome protein in immune synapse formation and IL-2 production. J Immunol. 2004;173:1658–1662. doi: 10.4049/jimmunol.173.3.1658. [DOI] [PubMed] [Google Scholar]
- 92.Zhang J, Shehabeldin A, da Cruz LA, Butler J, Somani AK, McGavin M, Kozieradzki I, dos Santos AO, Nagy A, Grinstein S, Penninger JM, Siminovitch KA. Antigen receptor-induced activation and cytoskeletal rearrangement are impaired in Wiskott-Aldrich syndrome protein-deficient lymphocytes. J Exp Med. 1999;190:1329–1342. doi: 10.1084/jem.190.9.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Snapper SB, Rosen FS, Mizoguchi E, Cohen P, Khan W, Liu CH, Hagemann TL, Kwan SP, Ferrini R, Davidson L, Bhan AK, Alt FW. Wiskott-Aldrich syndrome protein-deficient mice reveal a role for WASP in T but not B cell activation. Immunity. 1998;9:81–91. doi: 10.1016/s1074-7613(00)80590-7. [DOI] [PubMed] [Google Scholar]
- 94.Cotta-de-Almeida V, Westerberg L, Maillard MH, Onaldi D, Wachtel H, Meelu P, Chung UI, Xavier R, Alt FW, Snapper SB. Wiskott Aldrich syndrome protein (WASP) and N-WASP are critical for T cell development. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:15424–15429. doi: 10.1073/pnas.0706881104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Krawczyk C, Oliveira-dos-Santos A, Sasaki T, Griffiths E, Ohashi PS, Snapper S, Alt F, Penninger JM. Vav1 controls integrin clustering and MHC/peptide-specific cell adhesion to antigen-presenting cells. Immunity. 2002;16:331–343. doi: 10.1016/s1074-7613(02)00291-1. [DOI] [PubMed] [Google Scholar]
- 96.Nolz JC, Gomez TS, Zhu P, Li S, Medeiros RB, Shimizu Y, Burkhardt JK, Freedman BD, Billadeau DD. The WAVE2 complex regulates actin cytoskeletal reorganization and CRAC-mediated calcium entry during T cell activation. Current biology : CB. 2006;16:24–34. doi: 10.1016/j.cub.2005.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zipfel PA, Bunnell SC, Witherow DS, Gu JJ, Chislock EM, Ring C, Pendergast AM. Role for the Abi/wave protein complex in T cell receptor-mediated proliferation and cytoskeletal remodeling. Current biology : CB. 2006;16:35–46. doi: 10.1016/j.cub.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 98.Lebensohn AM, Kirschner MW. Activation of the WAVE complex by coincident signals controls actin assembly. Mol Cell. 2009;36:512–524. doi: 10.1016/j.molcel.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Barr VA, Bernot KM, Srikanth S, Gwack Y, Balagopalan L, Regan CK, Helman DJ, Sommers CL, OhHora M, Rao A, Samelson LE. Dynamic movement of the calcium sensor STIM1 and the calcium channel Orai1 in activated T-cells: puncta and distal caps. Molecular biology of the cell. 2008;19:2802–2817. doi: 10.1091/mbc.E08-02-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nolz JC, Medeiros RB, Mitchell JS, Zhu P, Freedman BD, Shimizu Y, Billadeau DD. WAVE2 regulates high-affinity integrin binding by recruiting vinculin and talin to the immunological synapse. Mol Cell Biol. 2007;27:5986–6000. doi: 10.1128/MCB.00136-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jia D, Gomez TS, Metlagel Z, Umetani J, Otwinowski Z, Rosen MK, Billadeau DD. WASH and WAVE actin regulators of the Wiskott-Aldrich syndrome protein (WASP) family are controlled by analogous structurally related complexes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:10442–10447. doi: 10.1073/pnas.0913293107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gomez TS, Billadeau DD. A FAM21-containing WASH complex regulates retromer-dependent sorting. Dev Cell. 2009;17:699–711. doi: 10.1016/j.devcel.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gomez TS, Gorman JA, de Narvajas AA, Koenig AO, Billadeau DD. Trafficking defects in WASH-knockout fibroblasts originate from collapsed endosomal and lysosomal networks. Molecular biology of the cell. 2012;23:3215–3228. doi: 10.1091/mbc.E12-02-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Taniuchi I, Kitamura D, Maekawa Y, Fukuda T, Kishi H, Watanabe T. Antigen-receptor induced clonal expansion and deletion of lymphocytes are impaired in mice lacking HS1 protein, a substrate of the antigen-receptor-coupled tyrosine kinases. The EMBO journal. 1995;14:3664–3678. doi: 10.1002/j.1460-2075.1995.tb00036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gomez TS, McCarney SD, Carrizosa E, Labno CM, Comiskey EO, Nolz JC, Zhu P, Freedman BD, Clark MR, Rawlings DJ, Billadeau DD, Burkhardt JK. HS1 functions as an essential actin-regulatory adaptor protein at the immune synapse. Immunity. 2006;24:741–752. doi: 10.1016/j.immuni.2006.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Carrizosa E, Gomez TS, Labno CM, Klos Dehring DA, Liu X, Freedman BD, Billadeau DD, Burkhardt JK. Hematopoietic lineage cell-specific protein 1 is recruited to the immunological synapse by IL-2-inducible T cell kinase and regulates phospholipase Cgamma1 Microcluster dynamics during T cell spreading. J Immunol. 2009;183:7352–7361. doi: 10.4049/jimmunol.0900973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dehring DA, Clarke F, Ricart BG, Huang Y, Gomez TS, Williamson EK, Hammer DA, Billadeau DD, Argon Y, Burkhardt JK. Hematopoietic lineage cell-specific protein 1 functions in concert with the Wiskott-Aldrich syndrome protein to promote podosome array organization and chemotaxis in dendritic cells. J Immunol. 2011;186:4805–4818. doi: 10.4049/jimmunol.1003102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gomez TS, Hamann MJ, McCarney S, Savoy DN, Lubking CM, Heldebrant MP, Labno CM, McKean DJ, McNiven MA, Burkhardt JK, Billadeau DD. Dynamin 2 regulates T cell activation by controlling actin polymerization at the immunological synapse. Nature immunology. 2005;6:261–270. doi: 10.1038/ni1168. [DOI] [PubMed] [Google Scholar]
- 109.Calabia-Linares C, Robles-Valero J, de la Fuente H, Perez-Martinez M, Martin-Cofreces N, Alfonso-Perez M, Gutierrez-Vazquez C, Mittelbrunn M, Ibiza S, Urbano-Olmos FR, Aguado-Ballano C, Sanchez-Sorzano CO, Sanchez-Madrid F, Veiga E. Endosomal clathrin drives actin accumulation at the immunological synapse. J Cell Sci. 2011;124:820–830. doi: 10.1242/jcs.078832. [DOI] [PubMed] [Google Scholar]
- 110.Wiggan O, Shaw AE, DeLuca JG, Bamburg JR. ADF/cofilin regulates actomyosin assembly through competitive inhibition of myosin II binding to F-actin. Dev Cell. 2012;22:530–543. doi: 10.1016/j.devcel.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gatfield J, Albrecht I, Zanolari B, Steinmetz MO, Pieters J. Association of the leukocyte plasma membrane with the actin cytoskeleton through coiled coil-mediated trimeric coronin 1 molecules. Molecular biology of the cell. 2005;16:2786–2798. doi: 10.1091/mbc.E05-01-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mugnier B, Nal B, Verthuy C, Boyer C, Lam D, Chasson L, Nieoullon V, Chazal G, Guo XJ, He HT, Rueff-Juy D, Alcover A, Ferrier P. Coronin-1A links cytoskeleton dynamics to TCR alpha beta-induced cell signaling. PloS one. 2008;3:e3467. doi: 10.1371/journal.pone.0003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Eyckmans J, Boudou T, Yu X, Chen CS. A hitchhiker's guide to mechanobiology. Dev Cell. 2011;21:35–47. doi: 10.1016/j.devcel.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim ST, Shin Y, Brazin K, Mallis RJ, Sun ZY, Wagner G, Lang MJ, Reinherz EL. TCR Mechanobiology: Torques and Tunable Structures Linked to Early T Cell Signaling. Front Immunol. 2012;3:76. doi: 10.3389/fimmu.2012.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Khatau SB, Hale CM, Stewart-Hutchinson PJ, Patel MS, Stewart CL, Searson PC, Hodzic D, Wirtz D. A perinuclear actin cap regulates nuclear shape. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:19017–19022. doi: 10.1073/pnas.0908686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xu C, Gagnon E, Call ME, Schnell JR, Schwieters CD, Carman CV, Chou JJ, Wucherpfennig KW. Regulation of T cell receptor activation by dynamic membrane binding of the CD3epsilon cytoplasmic tyrosine-based motif. Cell. 2008;135:702–713. doi: 10.1016/j.cell.2008.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gagnon E, Schubert DA, Gordo S, Chu HH, Wucherpfennig KW. Local changes in lipid environment of TCR microclusters regulate membrane binding by the CD3{varepsilon} cytoplasmic domain. J Exp Med. 2012;209:2423–2439. doi: 10.1084/jem.20120790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shi X, Bi Y, Yang W, Guo X, Jiang Y, Wan C, Li L, Bai Y, Guo J, Wang Y, Chen X, Wu B, Sun H, Liu W, Wang J, Xu C. Ca2+ regulates T-cell receptor activation by modulating the charge property of lipids. Nature. 2013;493:111–115. doi: 10.1038/nature11699. [DOI] [PubMed] [Google Scholar]
- 119.Zhang H, Cordoba SP, Dushek O, van der Merwe PA. Basic residues in the T-cell receptor zeta cytoplasmic domain mediate membrane association and modulate signaling. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:19323–19328. doi: 10.1073/pnas.1108052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gaus K, Chklovskaia E, Fazekas de St Groth B, Jessup W, Harder T. Condensation of the plasma membrane at the site of T lymphocyte activation. J Cell Biol. 2005;171:121–131. doi: 10.1083/jcb.200505047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim ST, Takeuchi K, Sun ZY, Touma M, Castro CE, Fahmy A, Lang MJ, Wagner G, Reinherz EL. The alphabeta T cell receptor is an anisotropic mechanosensor. The Journal of biological chemistry. 2009;vol. 284:31028–31037. doi: 10.1074/jbc.M109.052712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li YC, Chen BM, Wu PC, Cheng TL, Kao LS, Tao MH, Lieber A, Roffler SR. Cutting Edge: mechanical forces acting on T cells immobilized via the TCR complex can trigger TCR signaling. J Immunol. 2010;184:5959–5963. doi: 10.4049/jimmunol.0900775. [DOI] [PubMed] [Google Scholar]
- 123.Judokusumo E, Tabdanov E, Kumari S, Dustin ML, Kam LC. Mechanosensing in T lymphocyte activation. Biophys J. 2012;102:L5–L7. doi: 10.1016/j.bpj.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.O'Connor RS, Hao X, Shen K, Bashour K, Akimova T, Hancock WW, Kam LC, Milone MC. Substrate rigidity regulates human T cell activation and proliferation. J Immunol. 2012;189:1330–1339. doi: 10.4049/jimmunol.1102757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Trappmann B, Gautrot JE, Connelly JT, Strange DG, Li Y, Oyen ML, Cohen Stuart MA, Boehm H, Li B, Vogel V, Spatz JP, Watt FM, Huck WT. Extracellular-matrix tethering regulates stem-cell fate. Nat Mater. 2012;11:642–649. doi: 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]
- 126.Stabley DR, Jurchenko C, Marshall SS, Salaita KS. Visualizing mechanical tension across membrane receptors with a fluorescent sensor. Nat Methods. 2012;9:64–67. doi: 10.1038/nmeth.1747. [DOI] [PubMed] [Google Scholar]
- 127.Dobereiner HG, Dubin-Thaler BJ, Hofman JM, Xenias HS, Sims TN, Giannone G, Dustin ML, Wiggins CH, Sheetz MP. Lateral membrane waves constitute a universal dynamic pattern of motile cells. Phys Rev Lett. 2006;97:038102. doi: 10.1103/PhysRevLett.97.038102. [DOI] [PubMed] [Google Scholar]
- 128.Beinke S, Phee H, Clingan JM, Schlessinger J, Matloubian M, Weiss A. Proline-rich tyrosine kinase-2 is critical for CD8 T-cell short-lived effector fate. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:16234–16239. doi: 10.1073/pnas.1011556107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Brown AC, Dobbie IM, Alakoskela JM, Davis I, Davis DM. Super-resolution imaging of remodeled synaptic actin reveals different synergies between NK cell receptors and integrins. Blood. 2012;120:3729–3740. doi: 10.1182/blood-2012-05-429977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ichikawa D, Mizuno M, Yamamura T, Miyake S. GRAIL (gene related to anergy in lymphocytes) regulates cytoskeletal reorganization through ubiquitination and degradation of Arp2/3 subunit 5 and coronin 1A. The Journal of biological chemistry. 2011;286:43465–43474. doi: 10.1074/jbc.M111.222711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Reicher B, Joseph N, David A, Pauker MH, Perl O, Barda-Saad M. Ubiquitylation-dependent negative regulation of WASp is essential for actin cytoskeleton dynamics. Mol Cell Biol. 2012;32:3153–3163. doi: 10.1128/MCB.00161-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Miura-Shimura Y, Duan L, Rao NL, Reddi AL, Shimura H, Rottapel R, Druker BJ, Tsygankov A, Band V, Band H. Cbl-mediated ubiquitinylation and negative regulation of Vav. The Journal of biological chemistry. 2003;278:38495–38504. doi: 10.1074/jbc.M305656200. [DOI] [PubMed] [Google Scholar]
- 133.Vardhana S, Choudhuri K, Varma R, Dustin ML. Essential role of ubiquitin and TSG101 protein in formation and function of the central supramolecular activation cluster. Immunity. 2010;32:531–540. doi: 10.1016/j.immuni.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]