Abstract
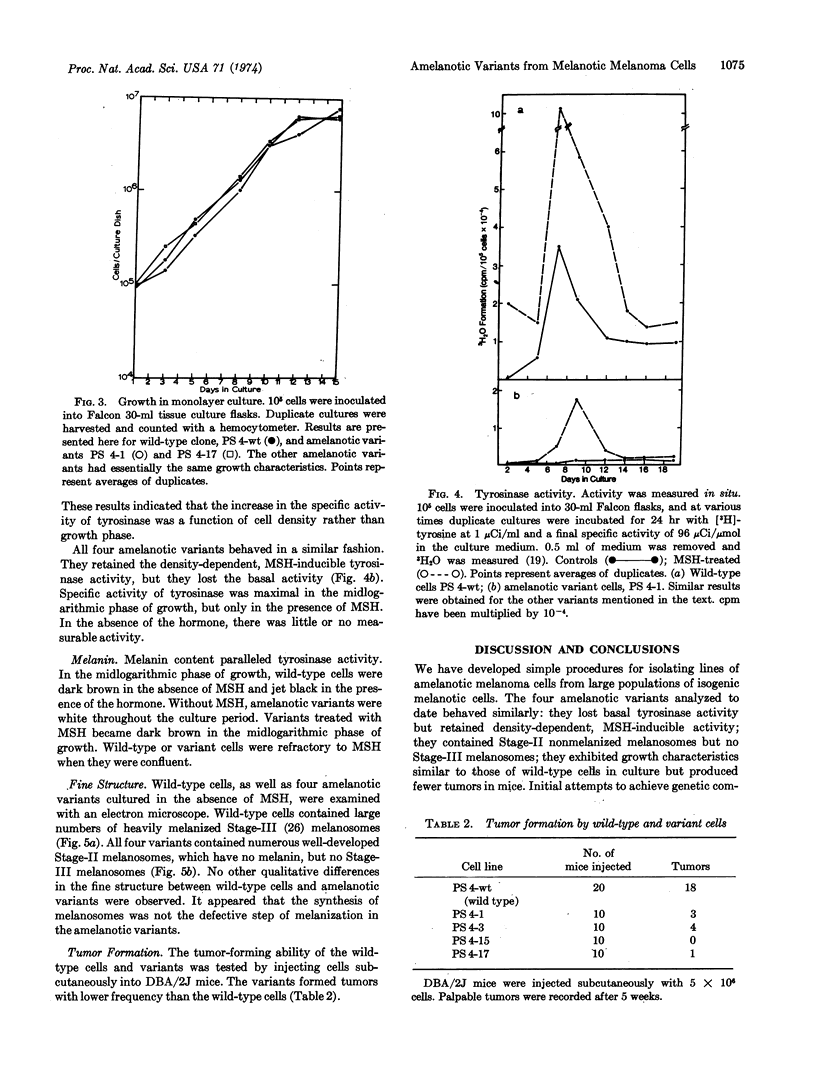
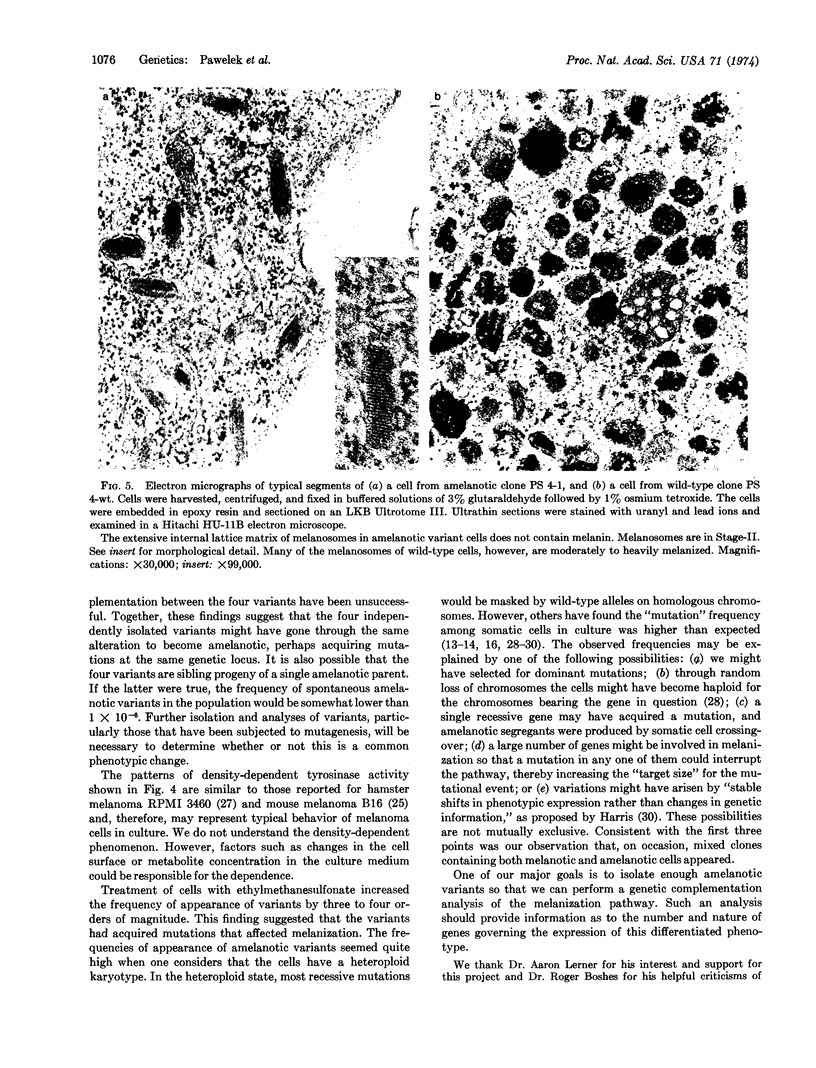
Melanoma cells express a phenotype that is easy to recognize: the synthesis of melanin. We used this marker to isolate clones of amelanotic variants from large populations of wild-type melanotic clones. Cloudman mouse melanoma (S91, clone M-3, CCL 53.1) was chosen as the parental line because the cells are highly pigmented, grow well as clones in soft agar, and fuse readily with Sendai virus. Subclones (107) of this line were screened without prior mutagenesis, and nine amelanotic variants were isolated. The mutagen ethylmethanesulfonate increased the frequency of variants by three to four orders of magnitude. Wild-type cells had both basal and melanocyte stimulating hormone-inducible tyrosinase activities. The four amelanotic variants that we have examined to date all behaved similarly: they lost basal tyrosinase (EC 1.14.18.1; monophenol monooxygenase) activity but retained melanocyte stimulating hormone-inducible activity; they contained Stage-II melanosomes but no melanized melanosomes; they exhibited growth characteristics similar to those of wild-type cells in culture but produced fewer tumors in mice.
Keywords: amelanotic melanoma variants, cell culture, mutagenesis, melanocyte stimulating hormone, tyrosinase
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chu M. Y., Fischer G. A. The incorporation of 3H-cytosine arabinoside and its effect on murine leukemic cells (L5178Y). Biochem Pharmacol. 1968 May;17(5):753–767. doi: 10.1016/0006-2952(68)90012-9. [DOI] [PubMed] [Google Scholar]
- Coffino P., Scharff M. D. Rate of somatic mutation in immunoglobulin production by mouse myeloma cells. Proc Natl Acad Sci U S A. 1971 Jan;68(1):219–223. doi: 10.1073/pnas.68.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R., Ephrussi B., Yamamoto K. Regulation of melanin synthesis in mammalian cells, as studied by somatic hybridization. I. Evidence for negative control. J Cell Physiol. 1968 Oct;72(2):115–127. doi: 10.1002/jcp.1040720206. [DOI] [PubMed] [Google Scholar]
- Davis F. M., Adelberg E. A. Use of somatic cell hybrids for analysis of the differentiated state. Bacteriol Rev. 1973 Jun;37(2):197–214. doi: 10.1128/br.37.2.197-214.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M. Mutation rates in cells at different ploidy levels. J Cell Physiol. 1971 Oct;78(2):177–184. doi: 10.1002/jcp.1040780204. [DOI] [PubMed] [Google Scholar]
- Kao F. T., Johnson R. T., Puck T. T. Complementation analysis on virus-fused Chinese hamster cells with nutritional markers. Science. 1969 Apr 18;164(3877):312–314. doi: 10.1126/science.164.3877.312. [DOI] [PubMed] [Google Scholar]
- Kao F. T., Puck T. T. Genetics of somatic mammalian cells, VII. Induction and isolation of nutritional mutants in Chinese hamster cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1275–1281. doi: 10.1073/pnas.60.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao F. T., Puck T. T. Genetics of somatic mammalian cells. IV. Properties of Chinese hamster cell mutants with respect to the requirement for proline. Genetics. 1967 Mar;55(3):513–524. doi: 10.1093/genetics/55.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G., Bregula U., Wiener F., Harris H. The analysis of malignancy by cell fusion. I. Hybrids between tumour cells and L cell derivatives. J Cell Sci. 1971 May;8(3):659–672. doi: 10.1242/jcs.8.3.659. [DOI] [PubMed] [Google Scholar]
- Levisohn S. R., Thompson E. B. Tyrosine aminotransferase induction regulation variant in tissue culture. Nat New Biol. 1972 Jan 26;235(56):102–104. doi: 10.1038/newbio235102a0. [DOI] [PubMed] [Google Scholar]
- Littlefield J. W. The use of drug-resistant markers to study the hybridization of mouse fibroblasts. Exp Cell Res. 1966 Jan;41(1):190–196. doi: 10.1016/0014-4827(66)90558-1. [DOI] [PubMed] [Google Scholar]
- Loomis W. F., Jr, Wahrmann J. P., Luzzati D. Temperature-sensitive variants of an established myoblast line. Proc Natl Acad Sci U S A. 1973 Feb;70(2):425–429. doi: 10.1073/pnas.70.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiss H. K., Basilico C. Temperature sensitive mutants of BHK 21 cells. Nat New Biol. 1972 Sep 20;239(90):66–68. doi: 10.1038/newbio239066a0. [DOI] [PubMed] [Google Scholar]
- Mezger-Freed L. Puromycin resistance in haploid and heteroploid frog cells: gene or membrane determined? J Cell Biol. 1971 Dec;51(3):742–751. doi: 10.1083/jcb.51.3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migeon B. R., Childs B. Hybridization of mammalian somatic cells. Prog Med Genet. 1970;7:1–28. [PubMed] [Google Scholar]
- Oikawa A., Nakayasu M., Nohara M. Tyrosinase activities of cell-free extracts and living cells of cultured melanoma cells. Dev Biol. 1973 Jan;30(1):198–205. doi: 10.1016/0012-1606(73)90057-2. [DOI] [PubMed] [Google Scholar]
- Patel R. P., Okun M. R., Yee W. A., Wilgram G. F., Edelstein L. M. Inability of murine melanoma "tyrosinase" (dopa ozidase) to oxidize tyrosine in the presence or absence of dopa or dihydroxyfumarate cofactor. J Invest Dermatol. 1973 Aug;61(2):55–59. doi: 10.1111/1523-1747.ep12674766. [DOI] [PubMed] [Google Scholar]
- Pawelek J., Wong G., Sansone M., Morowitz J. Molecular biology of pigment cells. Molecular controls in mammalian pigmentation. Yale J Biol Med. 1973 Dec;46(5):430–443. [PMC free article] [PubMed] [Google Scholar]
- Pomerantz S. H. L-tyrosine-3,5-3H assay for tyrosinase development in skin of newborn hamsters. Science. 1969 May 16;164(3881):838–839. doi: 10.1126/science.164.3881.838. [DOI] [PubMed] [Google Scholar]
- Rao P. N., Johnson R. T. Mammalian cell fusion: studies on the regulation of DNA synthesis and mitosis. Nature. 1970 Jan 10;225(5228):159–164. doi: 10.1038/225159a0. [DOI] [PubMed] [Google Scholar]
- Ruddle F. H. Linkage analysis in man by somatic cell genetics. Nature. 1973 Mar 16;242(5394):165–169. doi: 10.1038/242165a0. [DOI] [PubMed] [Google Scholar]
- Sell E. K., Krooth R. S. Tabulation of somatic cell hybrids formed between lines of cultured cells. J Cell Physiol. 1972 Dec;80(3):453–461. doi: 10.1002/jcp.1040800316. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Mankovitz R., Baker R. M., Wright J. A., Till J. E., Siminovitch L., Whitmore G. F. Selective and nonselective isolation of temperature-sensitive mutants of mouse L-cells and their characterization. J Cell Physiol. 1971 Dec;78(3):431–440. doi: 10.1002/jcp.1040780312. [DOI] [PubMed] [Google Scholar]
- Wahrmann J. P., Luzzati D., Winand R. Changes in adenyl cyclase specific activity during differentiation on an established myogenic cell line. Biochem Biophys Res Commun. 1973 May 15;52(2):576–581. doi: 10.1016/0006-291x(73)90751-1. [DOI] [PubMed] [Google Scholar]
- Wong G., Pawelek J. Control of phenotypic expression of cultured melanoma cells by melanocyte stimulating hormones. Nat New Biol. 1973 Feb 14;241(111):213–215. doi: 10.1038/newbio241213a0. [DOI] [PubMed] [Google Scholar]