Abstract
Spatiotemporal regulation of the activity of a vast array of intracellular proteins and signaling pathways by reactive oxygen species (ROS) governs normal cardiovascular function. However, data from experimental and animal studies strongly support that dysregulated redox signaling, resulting from hyper-activation of various cellular oxidases or mitochondrial dysfunction, is integral to the pathogenesis and progression of cardiovascular disease (CVD). In this review, we address how redox signaling modulates the protein function, the various sources of increased oxidative stress in CVD, and the labyrinth of redox-sensitive molecular mechanisms involved in the development of atherosclerosis, hypertension, cardiac hypertrophy and heart failure, and ischemia–reperfusion injury. Advances in redox biology and pharmacology for inhibiting ROS production in specific cell types and subcellular organelles combined with the development of nanotechnology-based new in vivo imaging systems and targeted drug delivery mechanisms may enable fine-tuning of redox signaling for the treatment and prevention of CVD.
Keywords: Reactive oxygen species, NADPH oxidases, Mitochondria, Endothelial dysfunction, Atherosclerosis, Free radicals
Introduction
Reactive oxygen species (ROS)1 are formed from incomplete reduction of oxygen during normal respiration in all aerobic organisms. ROS are highly reactive and include free radicals containing one or more unpaired electrons, such as superoxide (O2•−) and hydroxyl radical (•OH), and nonradicals such as hydrogen peroxide (H2O2). It is estimated that between 0.2 and 2.0% of molecular oxygen consumed by the mitochondria in vitro may be converted to O2•− by the electron transport chain, but the amount of O2•− produced in vivo may be far less [1,2]. In addition to mitochondrial respiration, O2•− is generated by NADPH oxidases (Nox’s), uncoupled nitric oxide synthase, xanthine oxidase, lipoxygenases, myeloperoxidase, and cytochrome P450 isozymes. Because ROS production is inherent to normal physiology, cells have evolved both enzymatic and nonenzymatic antioxidant defense mechanisms to scavenge ROS and to maintain redox balance. A shift in redox homeostasis to an imbalance between ROS generation and endogenous antioxidant mechanisms results in oxidative stress, which has been implicated in the pathogenesis of various diseases including those of the cardiovascular system.
Reactive oxygen species in the cardiovascular system
A vast array of data from cell culture studies and experimental animal models as well as human studies supports the role of oxidative stress in the development of cardiovascular diseases such as atherosclerosis, hypertension, cardiac hypertrophy, heart failure, and ischemia-reperfusion injury [3–12]. Upregulation of oxidative stress markers has been shown to predict cardiovascular diseases [13–16]. However, data from a majority of randomized clinical trials and meta-analysis studies failed to show any preventive effect of antioxidant vitamins on the pathogenesis of cardiovascular diseases or mortality [17–20]. The ineffectiveness of antioxidants highlights the complexity of redox reactions in biological systems including vascular cells and the limitations of our current approaches to modulating the redox signaling to effect positive outcomes against cardiovascular diseases.
Oxidative stress causes cellular damage by free radical-induced oxidation of lipids, proteins, and DNA Molecular oxygen, itself a radical, is sparingly reactive, as its two unpaired electrons are in different molecular orbitals and have parallel spins. One-electron reduction of oxygen produces O2•−, which is membrane-impermeative and has a short half-life in an aqueous environment. Superoxide is rapidly dismutated to H2O2 by the action of superoxide dismutase (SOD) enzymes. As H2O2 is more stable than O2•− and membrane-permeative, it is important in cellular redox homeostasis and signaling. Reaction of H2O2 with transition metal ions such as Fe2+ generates •OH, a highly reactive and damaging ROS (Fenton reaction). Catalase, glutathione peroxidase, and per-oxiredoxins reduce H2O2 to water. Nitric oxide (•NO) is another important ROS in the cardiovascular system generated by the endothelial nitric oxide synthase (eNOS) and inducible NOS (iNOS) enzymes. When both are produced in the cells, O2•− reacts with •NO at a much faster rate than with SOD, generating peroxynitrite (ONOO−), a potent oxidizing radical in vascular cells. Myeloperoxidase secreted by neutrophils and monocytes can amplify the oxidative potential of H2O2 at physiological chloride concentrations by generating hypochlorous acid (HOCl), which is a strong oxidant that causes chlorination of tyrosine and oxidation of lysine, cysteine, and methionine (discussed later) [21–24].
Redox signaling
The term “redox signaling” describes a process in which physiological levels of ROS/reactive nitrogen species (RNS) induce modifications to proteins that are discrete, site-specific, and reversible [25]. Data accumulated over the past 2 decades provide evidence that ROS modulate the activity of a vast array of intracellular proteins and signaling pathways and this redox signaling is spatially and temporally regulated to generate specific effects. Even in the case of phagocytosis, wherein the microbicidal action has hitherto been attributed to the direct action of ROS generated by the concerted action of NADPH oxidase and myeloperoxidase, it is now revealed that the destruction of the invading pathogen is achieved by stimulation of cellular signaling pathways involved in the activation of proteases consequent to the ROS-induced increase in anionic charge and its compensation by the surge of K+ ions [26].
The reversible modification of the sulfur-containing amino acids methionine and cysteine serves as a posttranslational mechanism for the regulation of protein functions. ROS-induced oxidation of the sulfur atom in methionine yields methionine sulfoxide, which can be reduced back to methionine by methionine sulfoxide reductase (Msr) in a thioredoxin-dependent reaction. Methionine oxidation together with tyrosine chlorination of apolipoprotein A-1 (apoA-1) caused by ROS impairs the ABCA1 (ATP-binding cassette transporter A1)-dependent cholesterol efflux activity of apoA-1, which might enhance foam cell formation and atherogenesis [27]. Methionine oxidation is completely reversed by Msr, suggesting that in vivo modulation of this enzyme might prevent the loss of ABCA1 activity of apoA-1 under oxidative stress conditions and attenuate atherosclerosis. Angiotensin II (AngII)-induced oxidation of methionine 281/282 activates CaMKII (calcium/calmodulin-dependent protein kinase II), causing myocardial apoptosis in vitro and in vivo [28]. Further supporting the regulatory role of methionine oxidation in cardiac remodeling, AngII-stimulated CaMKII oxidation, cardiomyocyte apoptosis, and cardiac dysfunction are enhanced in MsrA−/− mice. In addition to increased oxidative stress, MsrA−/− mice have a decreased life span [29].
The main mechanisms by which ROS generate specific cellular effects, however, are the posttranslational covalent modification of cysteine thiols within the active and allosteric sites of proteins, oxidation of iron-sulfur cluster-containing proteins, S-glutathi-onylation (disulfide link between protein thiol and glutathione), and S-nitrosylation/S-nitrosation (•NO reacts with a thiol radical or nitrosonium ion reacts with protein thiolate to form protein-S-nitrosothiols) [30,31]. Redox signaling can induce acute effects, such as when the target proteins are ion channels and contractile proteins, or long-term effects, when the target is a protein kinase or a redox-sensitive transcription factor [30].
Modulation of protein function via alteration of cysteine thiols by H2O2 influences a wide variety of signal transduction cascades and diverse biological processes. H2O2, at low-micromolar concentrations, oxidizes catalytic cysteine residues in proteins first to generate sulfenic acid (SOH) and then disulfides (SS) [32,33]. SS formation can occur between two adjacent cysteines (intrapro-tein), between two proteins (interprotein), or as a mixed disulfide formed between a protein thiol and glutathione (S-glutathionyla-tion). Protein thiols and disulfides can undergo further oxidation by H2O2 to generate sulfinic (SO2−) and sulfonic (SO3−) acids. In addition, cysteine thiols can undergo •NO-dependent electrophilic and oxidative modification (S-nitrosylation) to generate protein-S-nitrosothiol (SNO), which with further oxidation can form SOH, SS, SO2−, and SO3− [34]. Oxidized or nitrosylated cysteine thiols in the cells are reduced back to cysteine by several enzymatic and nonenzymatic systems. For example, sulfenic acids, protein disulfides, and protein-S-nitrosothiols are reduced by thioredoxin, and thioredoxin reductase and S-glutathionylated protein cysteines by glutaredoxin [34,35]. Cysteine sulfinic acids, formed by the hyperoxidation of active-site Cys residues in typical 2-Cys peroxiredoxins, can be reduced by the enzyme sulfiredoxin. However, they might be irreversibly oxidized to cause damage to most proteins [36–38]. Sulfonic acids are an example of irreversible protein modification and a marker of cumulative oxidative stress [39]. Theoretically, the posttranslational modifications of cysteines could be impeded by antioxidant enzymes as they can remove ROS before protein modification occurs. Peroxiredoxins, by virtue of their ubiquitous presence, abundance, and high rate constants, reduce H2O2 and other hydroperoxides far more efficiently than any other thiol-containing proteins, impeding cysteine modifications [40]. The cysteine sulfinic acid generated in this reaction in the case of glutathione-dependent peroxiredoxins rapidly forms disulfide with glutathione, which is then recycled to the reduced state by glutaredoxin or ascorbic acid. In thioredoxin-dependent peroxiredoxins, the sulfenic acid rapidly reacts with proximal thiols to form a homo-intermolecular disulfide, which is recycled to the reduced state by thioredoxin [41–44]. The progression from reversible S-nitrosylation to SOH, SS, SO2−, and irreversible SO3− represents a graded transition of cellular signaling from adaptation and maintenance of cellular redox state in the face of nitrosative and oxidative stress to toxicity [34].
•NO also modulates protein function by targeting cysteine thiols in peptides and proteins, and S-nitrosylation is a principal mechanism by which •NO regulates signaling cascades across a multitude of protein classes [34]. The basis for S-nitrosylation specificity is not in the primary sequence of the target proteins, as high-throughput proteomic approaches failed to identify a linear Cys-flanking motif that predicts stable trans-nitrosylation of cysteines across various protein classes [45]. The proximity of a protein Cys to NOS may be a determinant of S-nitrosylation [46,47], whereas the electrostatic environment, hydrophobicity, and contiguity and orientation of aromatic amino acid chains arising from the tertiary protein structure and protein-protein interactions also regulate S-nitrosylation and denitrosylation [34,48]. Redox modification of active-site thiols is a principal mechanism for dynamic posttranslational regulation of all major protein classes, including phosphatases, kinases, transcription factors, ion channels and transporters, cytoskeletal and structural proteins, GTPases, metabolic and antioxidant enzymes, and respiratory proteins [31,34].
Phosphatases
Protein tyrosine phosphorylation is a key regulatory mechanism in signal transduction, affecting many cellular functions. Sundaresan et al. [49] demonstrated a correlation between the magnitude and duration of an increase in H2O2 levels and the protein tyrosine phosphorylation in VSMCs treated with various growth factors. They hypothesized that increased protein tyrosine phosphorylation was due to the transient inactivation of PTPs. Several subsequent papers provided the evidence for redox regulation of PTPs by growth factor-induced ROS. Lee et al. [50] elucidated reversible inactivation of PTP1B in A431 cells treated with epidermal growth factor (EGF), and later PTP1B inactivation was attributed to oxidation by H2O2, as inhibition of its accumulation prevented protein tyrosine phosphorylation [51]. Reversible inactivation of Src-homology 2 domain-containing PTP (SHP2) in VSMCs treated with platelet-derived growth factor (PDGF) requires association with the PDGF receptor and is necessary for the receptor activation [52]. Treatment of VSMCs with antioxidants increased growth factor-induced activity of SHP2 and several other PTPs, further confirming their redox regulation [53,54]. It was demonstrated that rapid inactivation of PTP family members by low-micromolar concentrations of H2O2 as a result of the oxidation of the essential catalytic cysteine residue to a Cys-SOH intermediate renders the PTPs inactive against phosphorylated substrates [29]. Unlike most cysteines in cellular proteins, which have a pKa > 8.0 at physiological pH, the catalytic cysteine residues of PTPs have low pKa ( > 6.0) and are present in thiolate form at physiological pH, making them extremely reactive [55,56]. The thiolate nucleophile attacks the electrophilic phosphorus atom of the substrate, removing the phosphate group and forming an enzyme thiol-phosphate intermediate [57]. However, because of their strong nucleophilic character, the thiolate anions are susceptible to oxidation by H2O2.
The Cys-SOH intermediate formed during PTP1B oxidation is rapidly converted into a sulfenyl-amide species by covalent linking of a sulfur atom of the catalytic cysteine with the main chain nitrogen of an adjacent residue [58,59]. This results in large conformational changes in the catalytic site, which inhibit substrate binding as well as protecting it from irreversible oxidation to sulfonic acid and allowing redox regulation of the enzyme by promoting its reversible reduction by thiols. Glutathionylation of the Cys-SOH also protects PTP1B from irreversible oxidation [60]. Other PTPs are protected from irreversible oxidation by the formation of a disulfide either between two vicinal cysteines in the catalytic site, as reported for LMW-PTP [61,62], or between the catalytic cysteine and a nearby backdoor cysteine, as observed in Cdc25 [63], RPTPα [64], and PTEN [65]. In the case of the SHPs, rereduction of Cys-SOH is dependent on the formation of an intramolecular disulfide between two conserved backdoor cysteines [66]. Meng et al. [54] demonstrated that reversible oxidative inactivation of SHP2 is necessary for PDGF-induced mitogenic signaling in fibroblasts. Induction of endogenous •NO and exposure to •NO donors inhibited the activity of several cellular PTPs, including those in endothelial cells [67–69]. However, S-nitrosylation of the catalytic cysteine in PTP1B protected it from ROS-induced irreversible oxidation [70].
Protein kinases
In addition to indirect regulation resulting from the concomitant inhibition of PTPs, both receptor (RTK) and nonreceptor tyrosine kinases also undergo oxidation-dependent activation. Examples of RTKs that undergo direct oxidation include insulin, EGF and PDGF receptors, and Ret kinase [71]. Schmid et al. [72] reported that increased kinase activity and insulin responsiveness of the insulin receptor (IR) may require “redox priming” and results from a decrease in IR β-chain sulfhydryl groups due to oxidation. In fact, 3-D models of the IR showed that conversion of any of the four cysteine residues (1056, 1138, 1234, and 1245) into sulfenic acid produces conformational changes, bringing Tyr1158 into close contact with Asp1083, which renders the catalytic site at Asp1132 and Tyr1162 accessible and facilitates its autophosphorylation in the activation loop [73].
Among the nonreceptor tyrosine kinases, Src is regulated by many stimuli that generate ROS, including hypoxia/reoxygenation, stretch, integrins, growth factors, and vasoactive agonists such as AngII and thrombin [74]. Hypoxia-induced mitochondrial ROS production activates Src in VSMCs, resulting in increased hypoxia-inducible factor 1α (HIF1α) expression [75]. Antioxidant-inhibitable Src activation was observed in endothelial cells subjected to cyclic strain or H2O2 treatment [76]. Integrin-stimulated Src activation was biphasic, with an early activation phase driven mainly by Tyr527 dephosphorylation mediated by PTPa and a subsequent Tyr418 autophosphorylation. The late phase involves oxidation of Cys245 and Cys487 by H2O2, resulting in the hyper-phosphorylation of Tyr418 and further activation of the kinase [77]. Src is also activated in a Tyr527-independent manner by nitrosylation and via generation of an intermolecular S-S bond, resulting in aggregation of adjacent Src molecules and Tyr416 autophosphorylation [78].
Serine/threonine kinases, such as protein kinase C (PKC), undergo redox regulation by direct oxidation of cysteine residues. All 12 PKC isozymes contain cysteine residues in the regulatory as well as catalytic domains and the stimulation or inhibition of the enzyme depends on which of the cysteines undergo redox modification [79,80]. Low levels of ROS oxidize cysteine residues in the regulatory region, promoting the release of zinc and forming intramolecular disulfide bonds, which causes Ca2+, diacylglycerol, or other lipid-independent activation of PKC by the dissociation of autoinhibitory pseudo-substrate [79–82]. In contrast, oxidation of catalytic domain cysteines inhibits PKC activity [80]. On the other hand, high-glucose-induced activation of PKC increased ROS production and cyclooxygenase 2 expression and reduced •NO availability and altered prostanoid expression, causing endothelial dysfunction [83]. ROS-induced PKC activity also regulates VCAM-1-dependent lymphocyte transendothelial migration [84]. Cyclic AMP-dependent (PKA) and cyclic guanosine monophosphate (cGMP)-dependent (PKG) protein kinases are also redox-sensitive and undergo cyclic nucleotide-independent activation by forming an interprotein disulfide linking two subunits in cells on exposure to H2O2 [85,86]. PKG activation represents one mechanism by which H2O2 can act as a vasorelaxant in the cardiovascular system [76]. In addition, activities of protein kinases such as Akt [87] and JNK1 [88] are regulated by S-nitrosylation of cysteine residues, resulting in their inactivation. Independent of classic regulation by guanine nucleotide exchange factors and GTPase-activating proteins, oxidizing agents also regulate the activity of GTPases. Lander et al. [89] showed for the first time that S-nitrosylation of Cys118 enhanced the activity of Ras by promoting the exchange of GDP for GTP. Adachi et al. [90] demonstrated that S-glutathionylation of Cys118 regulates AngII-induced hypertrophic signaling in VSMCs. More recently, Burridge’s group [91] showed that oxidation of Cys16 and Cys20 in the phosphoryl binding group activates RhoA and induces stress fiber formation in fibroblasts exposed to oxidants, suggesting that redox regulation of GTPases is a widespread signaling mechanism.
Transcription factors
Redox regulation of transcription factors such as NF-κB, nuclear factor E2-related factor-2 (Nrf2), AP-1, p53, and HIF plays an important role in vascular homeostasis and pathogenesis [31,92]. NF-κB regulates gene expression in immunity, stress responses, and inflammation, including in endothelial cells and cardiac myocytes [93]. Inhibitors of NF-κB (IκB) bind the inactive NF-κB p50–p65 heterodimer, the prototype of NF-κB family, and sequester it in the cytoplasm under basal conditions. Under oxidative stress conditions, activation of IκB via phosphorylation of Ser32 and Ser36 residues by inhibitory κB kinases (IKKs) targets IκB for ubiquitination and proteasomal degradation, allowing NF-κB to translocate to the nucleus and modulate gene expression [94,95]. Redox regulation of NF-κB is complex, as Cys62 of the p50 subunit is oxidized in the cytoplasm, and its reduction, by thioredoxin or possibly by Ref-1, is essential for its DNA binding in the nucleus [96–98]. The IKK complex contains catalytic IKKa and IKKp sub-units and a noncatalytic IKKγ subunit. The Cys178 and Cys179 in the kinase domains of the IKKα and IKKβ, respectively, regulate enzyme activity by promoting phosphorylation of activation-loop serines and interaction with ATP [99–101]. These cysteine residues also mediate redox regulation of NF-κB activity, as direct binding of electrophilic compounds to them inhibits enzyme activity and this inhibition was reversed by reducing agents. S-glutathionylation of Cys179 in the IKKβ also regulates reversible inhibition of NF-κB by endogenous H2O2 [102]. S-nitrosylation of the Cys179 in IKKβ inhibits the enzyme activity and NF-κB stimulation, a mechanism by which •NO exerts its anti-inflammatory efforts [103]. In addition, S-nitrosylation of Cys62 in the p50 subunit inhibits NF-κB-dependent DNA binding, promoter activity, and gene transcription [104,105].
The consensus DNA cis elements to which NF-κB dimers bind are known as “ κB sites” (5’-GGGRNWYYCC-3’, where R is A or G, N is any nucleotide, W is A or T, and Y is C or T) and are present in the promoter/enhancer regions of many target genes that regulate a diverse array of functions, including inflammation, proliferation, angiogenesis, matrix degradation, and pro- as well as antiapoptosis [93,106,107]. In cardiomyocytes, functional NF-κB signaling pathways are essential for protection against apoptosis induced by cytokines and acute myocardial ischemia [108,109]. However, chronic NF-κB activation under pathophysiological settings such as heart failure exacerbates cardiac remodeling by stimulating proinflammatory and profibrotic genes and inducing myocytes apoptosis [110]. The endothelial NF-κB signal transduction system is primed for activation in regions of disturbed flow and its activity is increased by exposure to stimuli that enhance atherosclerosis [111]. Further support for NF-κB in atherogenesis is evident from the reports that its activation regulates cytokine-induced expression of the cellular adhesion molecules vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) in endothelial cells [112,113]. NF-κB is activated by H2O2 in endothelial cells [114], whereas its activity is inhibited in H2O2-treated epithelial cells [92], which suggests that redox regulation of NF-κB and its attendant effects on cellular outcomes are determined by the duration and cellular context [31,93].
Nrf2 is another redox-sensitive transcription factor that helps maintain cellular redox homeostasis by upregulating antioxidant and phase II detoxifying enzymes under oxidative and electrophilic stress conditions [115]. The gene upregulation is achieved by the interaction of Nrf2 with electrophile and antioxidant (ARE) response elements and the upregulated genes include heme oxygenase-1 (HO-1), the catalytic subunit of glutamate-cysteine ligase, glutathione S-transferase, and NAD(P)H:quinine oxidore-ductase 1. Nrf2 activation and induction of downstream antioxidant genes confers protection against oxidative stress in cardiomyocytes and VSMCs and inhibits vascular inflammation [116,117]. Activation of Nrf2-dependent antioxidant gene expression by advanced glycation end products may protect the endothelium against chronic oxidative stress in diabetes [118]. Furthermore, atheroprotective laminar flow activates, whereas proatherogenic oscillatory flow inhibits, Nrf2 activity in human endothelial cells, underlying the importance of Nrf2-regulated gene expression in vascular homeostasis [119,120]. Under redox conditions where there may be a limited availability of tetrahy-drobiopterin (BH4), the eNOS cofactor, Nrf2 activation maintains endothelial homeostasis by downregulating eNOS levels via increased HO-1 activity and thus maintaining stoichiometric balance between BH4 and eNOS [121].
Nrf2 is sequestered in the cytoplasm under basal conditions by a cysteine-rich protein, Kelch-like ECH-associated protein 1 (Keap1), which binds to the Neh2 domain of Nrf2 and targets it for ubiquitin-dependent proteasomal degradation [122,123]. Two cysteine residues in Keap1, Cys273 and Cys288, are necessary for the ubiquitination of Nrf2. Electrophiles and oxidants disrupt the Keap1–Nrf2 complex, perhaps by the oxidation of Cys273 and Cys288, leading to stabilization and enhanced nuclear localization of Nrf2 and increased transcription of ARE-containing genes [124]. Furthermore, Cys151 in Keap1 is required for inhibition of Nrf2 degradation during oxidative stress, perhaps by inducing confor-mational changes. Fourquet et al. [125] reported that intermole-cular disulfide formation between Cys152 residues by ROS and RNS results in Keap1 inactivation and Nrf2 stabilization. The same is observed with simultaneous inactivation of the thioredoxin and glutathione pathways.
AP-1 regulates gene expression in cells in response to a broad spectrum of environmental stimuli, including oxidative stress. It is a dimer consisting of members of the Jun and Fos families, which complex through a leucine zipper domain into homo (Jun/Jun) or heterodimers (Jun/Fos) [126,127]. Dimerization juxtaposes the conserved basic regions of constituent proteins, forming a bipartite DNA-binding domain. Classic regulation of the activity of AP-1, either by an increase in the transcription of the Fos and Jun genes or by phosphorylation of the Fos and Jun proteins, often occurs downstream of redox-sensitive protein kinase activation [128,129]. AP-1 activity is also regulated in a redox-sensitive manner, as a conserved cysteine residue in the DNA-binding domains of the Fos and Jun proteins is susceptible to oxidation resulting in the loss of DNA binding [130]. These data are supported by the loss of redox regulation observed when the conserved cysteine is substituted by a serine residue [131]. The c-Jun binding domain contains one cysteine residue (Cys269) in the basic region that directly binds DNA and another (Cys320) close to the leucine zipper domain [132]. A decrease in the ratio of reduced/oxidized glutathione under oxidative stress conditions induces S-glutathiolation of Cys269 and the formation of an intermolecular disulfide bridge between Cys320 residues, with the former enabling reversible redox regulation of c-Jun DNA binding. In addition, reversible S-nitrosoglutathione-dependent S-glutathiolation of Cys269 may regulate c-Jun DNA binding [133]. We and others have demonstrated that AP-1 regulates vasoactive agonist-induced expression of adhesion molecules such as CD44 in VSMCs in a redox-sensitive manner [134,135]. In isolated hearts, an increase in AP-1 activity correlates with the duration of ischemia and reperfusion, whereas in adapted myocardium AP-1 activity is at the basal level, which indicates that AP-1 stimulates oxidative stress-induced apopto[136].
Several lines of evidence suggest that DNA binding or tran-scriptional activity of p53 is highly prone sis to oxidative inactivation. For example, DNA binding of p53 to its cognate sequence in vitro requires reductants such as 2-mercaptoethanol or dithiothreitol in the binding buffers and is sensitive to H2O2 and other oxidants such as diamide [137]. In addition, pharmacological oxidizing and reducing agents modulate gene transactivation by p53 in human cells [138]. More recently, Velu et al. [139] demonstrated that S-glutathionylation, in addition to other posttranslational modifications such as site-specific phosphorylation, ubiquitination, and 182 as the sites of sumoylation [140], governs the activity of p53 under stress conditions. Whereas most of the posttranslational modifications of p53 after genotoxic stress enhance its transcriptional competency to induce cell cycle checkpoints, S-glutathionylation is a negative and defensive regulatory mechanism under acute stress. Even though mass spectrometry identified cysteines 124, 141, and 182 as the sites of glutathionylation, cysteine 141 is the most reactive one on the surface of the p53 [139]. In addition, molecular modeling studies showed cysteines 124 and 141 at the dimer interface of p53, and glutathionylation of either residue interferes with protein dimerization and inhibits p53-DNA association. Inhibition of DNA binding and disruption of tetramerization under mild oxidizing conditions are correlated with the formation of a disulfide bond in p53 [141]. Reduction of disulfide bonds by thioredoxin and Ref-1 reactivates oxidized p53 and stimulates p53-mediated transactivation [142,143]. Interestingly, redox regulation of p53 in turn modulates cellular redox status. Sablina et al. [144] reported that low levels of p53 in unstressed or physiologically stressed cells upregulate several genes with antioxidant products, resulting in a decrease in intracellular ROS levels. In contrast, downregulation of p53 causes oxidative DNA damage and mutagenesis, which are prevented by an antioxidant supplement.
The role of endogenous p53 in atherosclerosis is controversial. p53 levels, cell proliferation, and apoptosis are predominant in human plaque areas with chronic inflammation [145]. An increase in macrophage p53 levels is associated with the enlargement of necrotic core, plaque rupture, and transient ischemic attacks in patients with carotid atherosclerosis [146]. Adenoviral overexpres-sion of p53 increased VSMC apoptosis and induced plaque rupture in preexisting atherosclerotic lesions [147]. However, Mercer et al. [148] demonstrated that endogenous p53 reduces atherosclerosis by protecting VSMCs and stromal cells from death and promoting apoptosis in macrophages. These data are supported by the observation of Boesten et al. [149] that macrophage-specific deletion of p53 enhances plaque vulnerability by increasing the lesion macrophage area and necrotic core formation.
Physiological roles of ROS
ROS regulate many physiological functions in the cardiovascular system under normal conditions. For example, •NO mediates endothelium-dependent vasomotor tone and flow responses in many vascular beds [150,151]. It is also suggested that •NO regulates endothelium-dependent microvascular and epicardial vasodilation under metabolic stimulation [152]. The functions of •NO include inhibition of platelet aggregation, disaggregation of aggregated platelets, and inhibition of platelet as well as leukocyte adhesion to the vascular endothelium [150,153,154]. Superoxide affects vascular tone by inactivating •NO [155] as well as by dismutating to H2O2 [30]. Several studies suggest that H2O2 is the endothelium-derived hyperpolarizing factor (EDHF) that regulates vasorelaxation in murine and human mesenteric arteries and flow-induced dilation in human coronary arterioles [156–159]. H2O2 and other hydroperoxides stimulate the activity of cycloox-ygenase, also known as prostaglandin endoperoxide H synthase, to produce the vasodilator prostacyclin and other prostanoids [160–162]. This effect is termed the “peroxide tone” and is evident at very low concentrations of peroxides (2–20 nM). It is suggested that eNOS regulates the EDHF-like activity of H2O2 and that •NO and H2O2 compensate for each other to cause endothelium-dependent relaxation [156]. SOD may play a critical role in endothelium-dependent relaxation by prolonging the half-life of •NO and by converting the vasoconstrictor O2•− to H2O2. In this context, it is worth noting that prolonged SOD2 deficiency results in decreased agonist-induced aortic relaxation and impaired aortic compliance in mice [163]. Both •NO and H2O2 regulate vasomotor tone by activating the enzyme soluble guanylate cyclase (sGC) [164–166]. •NO activates sGC by directly binding to the ferrous (Fe2 +) core of the heme prosthetic group, effecting a conformational change [167]. The product cGMP causes vasodilation by relaxing VSMCs, in part, by lowering Ca2+ by decreasing its influx, increasing efflux, promoting sequestration in the endoplasmic reticulum, and attenuating mobilization [167,168].
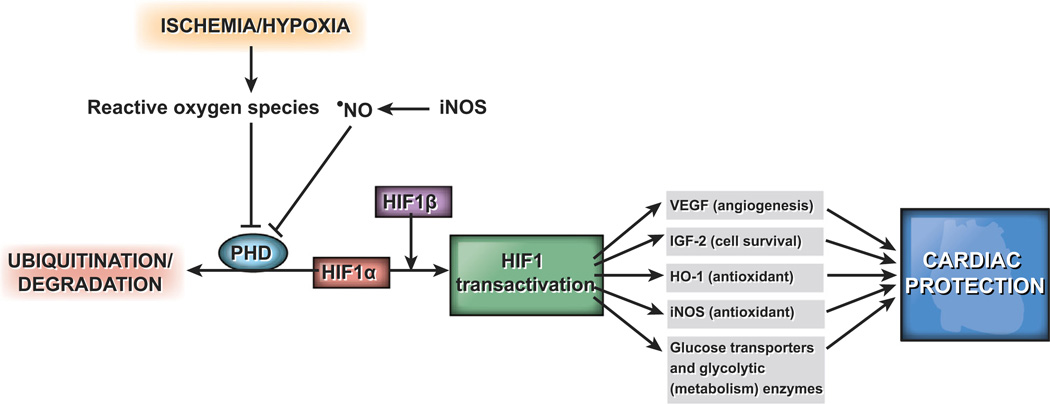
Oxygen homeostasis at the tissue level is vital for development, growth, and survival, and hence, cells have evolved a number of mechanisms to sense and respond to low oxygen levels. In humans, glomus type I chemoreceptor cells of the carotid body, located at the bifurcation of the carotid artery, release neurotrans-mitters in response to hypoxia and increase alveolar ventilation [169]. The neuroepithelial bodies of the intrapulmonary airways regulate hypoxic pulmonary vasoconstriction, optimizing ventilation–perfusion matching [170]. In contrast, vasodilation occurs in response to hypoxia in systemic vascular beds, such as coronary and cerebral circulations, to maintain O2 delivery [171]. In addition, physiological adaptation to hypoxia includes activation of transcription factor HIF1 and its downstream targets. HIF1 is a basic helix-loop-helix/PAS heterodimer, with an O2-sensitive HIF1α subunit and a constitutive HIF1β (ARNT) subunit (Fig. 1). Hydroxylation of proline residues 402 and 564 by prolyl hydro-xylases in normoxia enables HIF1α to interact with the von Hippel-Lindau tumor suppressor protein, which has ubiquitin ligase activity, and undergo degradation [172–175]. Hypoxia decreases hydroxylation of HIF1α and stabilizes it by not allowing interaction with the von Hippel-Lindau tumor suppressor protein. In cardiovascular cells, HIF1 induces the expression of genes involved in angiogenesis and vascular remodeling, energy metabolism, erythropoiesis, vasomotor reactivity, and vascular tone [176].
Fig. 1.
HIF transactivation confers cardiac protection during ischemia. Abbreviations used: HIF, hypoxia-inducible factor; VEGF, vascular endothelial growth factor; IGF-2, insulin-like growth factor-2; HO-1, heme oxygenase-1; iNOS, inducible nitric oxide synthase; •NO, nitric oxide.
The mechanisms by which cells detect a decrease in O2 levels to cause activation of HIF1 are still emerging but considerable evidence supports the role of increased mitochondrial ROS, particularly at complex III, in the induction of HIF1 under hypoxia [177–179]. Evidence in support of this notion includes increased ROS levels, as determined using fluorescent probes and ESR spectroscopy, and decreased reduced glutathione and cysteine levels [180–182]. Inactivation of Rieske iron–sulfur protein in mitochondrial complex III abrogated hypoxic stabilization of HIF1 [183]. Mansfield et al. [184] demonstrated impaired hypoxic HIF1 stabilization in murine embryonic cells lacking cytochrome c and therefore mitochondrial activity, further supporting the necessity of mitochondrial ROS in this process.
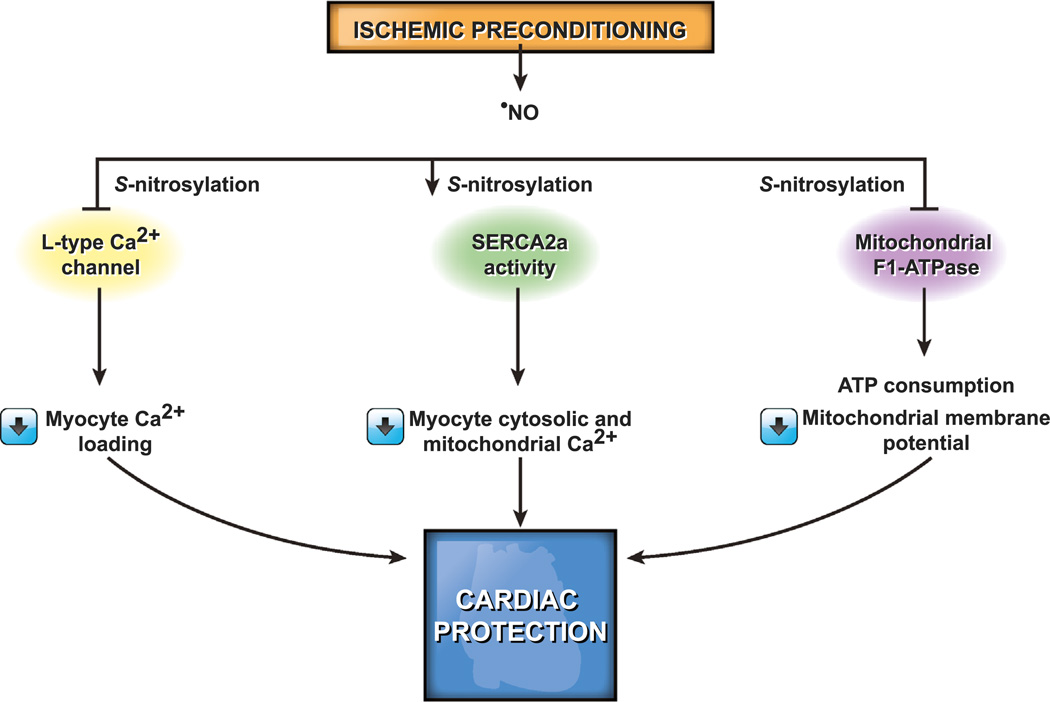
Low concentrations of ROS generated during ischemic preconditioning, in which one or more short periods of ischemia are separated by short periods of reperfusion, confer cardiac protection by reducing necrosis and the severity of arrhythmias and improving functional recovery when challenged with a longer period of ischemia [185–187]. Exogenous ROS mimic the protective effect of ischemic preconditioning [188] and the protective effect of preconditioning is decreased in the presence of antioxidants [189,190], suggesting that ROS generation is an innate physiologic adaptive process against potentially lethal ischemic injury. Ischemic preconditioning activates a number of signaling pathways, which converge on the mitochondria, resulting in activation of the mitoKATP channel and inhibition of the mitochondrial permeability transition pore [191]. More recently it was shown that ischemic preconditioning preserves mitochondrial efficiency by decreasing H+ leak and ROS production during ischemia-reperfusion [192].
ROS play a critical role in the activation of mechanotrans-duction signaling pathways that regulate the physiology and pathophysiology of heart function. A physiologic stretch of cardi-omyocytes, as happens in diastole, instantaneously increases ROS production via activation of Nox2 in a microtubule-dependent manner, a process termed X-ROS signaling [193]. Nox2 activation occurs in sarcolemmal and transverse tubule membranes and the resultant increase in local ROS levels sensitizes the nearby ryanodine receptors (RyR2) in the sarcoplasmic reticulum by oxidation. This triggers a burst of Ca2+ sparks, which causes muscle contraction and normalization of X-ROS signaling [193,194]. When the Ca2 + concentration drops to basal level, the muscle relaxes, completing the cycle [195]. Thus, the release and recapture of Ca2+ by the sarcoplasmic reticulum in each contraction-relaxation cycle underlies the heartbeat and is regulated by X-ROS signaling under normal physiological conditions [194]. Exogenous H2O2 reversibly increases a Ca2+ spark rate similar to that in a physiological stretch, and myocytes lacking Nox2 expression do not show stretch-dependent increase in ROS levels, further supporting the role of ROS in cardiac contraction and relaxation [193]. However, hyperactive X-ROS signaling may cause cardiomyopathy through aberrant Ca2+ release from sarcoplasmic reticulum. Various redox modifications, such as S-nitrosylation, S-glutathionylation, and disulfide crosslinking, dysregulate RyR2 and cause abnormal Ca2+ in several disease states [196–199]. In addition to the ryanodine receptors, the release and recapture of Ca2+ by the sarcoplasmic reticulum is regulated by sarco/endoplasmic reticulum ATPase (SERCA) and several accessory proteins, including phospholamban and calsequestrin [199]. SERCA, which transports cytosolic Ca2+ into the lumen of the sarcoplasmic reticulum in an ATP-dependent manner, is also regulated by redox mechanisms via the oxidation of cysteines or nitration of tyrosines. SERCA is activated by low (physiologic) and inactivated by high pathological levels of ROS because of irreversible oxidative modifications [200–203]. Activation of SERCA by NO decreases the intracellular Ca2+ concentration, relaxing cardiac, skeletal, and vascular smooth muscle. S-glutathionylation of Cys674 in SERCA2b—the major SERCA iso-form in vascular smooth muscle—by ONOO− increases its activity in vascular smooth muscle during normal endothelium-mediated relaxation [200]. The irreversible oxidation of this residue in atherosclerosis impairs NO-induced vasorelaxation. Similarly, the positive inotropic effects of HNO•− in normal and failing hearts involves increased Ca2+ into the sarcoplasmic reticulum and is mediated by reversible S-glutathionylation of Cys674 in SERCA2a —the major SERCA isoform in cardiac muscle [199,204,205].
Insulin sensitivity plays a vital role in cardiovascular health, and chronic oxidative stress is implicated in the development of insulin resistance, a state of diminished response to endogenous insulin [206]. Strong experimental evidence from human and animal models shows that increased mitochondrial ROS generation induces insulin resistance [206–208] and this can be rapidly reversed with mitochondrial uncouplers, electron transport chain (ETC) inhibitors, or mitochondrial superoxide dismutase (SOD2) mimetics or by overexpression of SOD2 [208]. The mechanisms by which mitochondrial ROS might contribute to insulin resistance include activation of JNK [209,210] and apoptosis signal-regulating kinase 1 [211]. In contrast to the role of increased oxidative stress in insulin resistance, recent evidence indicates that ROS also promote insulin sensitivity. Loh et al. [212] reported that mice deficient in the cytosolic and mitochondrial antioxidant enzyme glutathione peroxidase 1 (GPx-1) were protected from high-fat diet-induced insulin resistance. The protection resulted from ROS-induced increase in PI3K/Akt signaling and consequent AS160 phosphorylation and glucose uptake in the muscle, but not from altered insulin receptor and insulin receptor substrate 1 activation. Consistent with this idea, pharmacologic depletion of glutathione in C57BL/6J mice enhanced insulin sensitivity [213]. Interestingly, mice are also protected from diet-induced obesity in both these models. These data suggest that ROS have a physiological role in insulin sensitivity, and ROS levels, difference in sensitivity of tissues to ROS, and pathophysiological background are the major determinants of impaired versus enhanced insulin sensitivity [214,215].
Sources of ROS
Mitochondria
Mitochondria are the major source of ROS in mammals under physiological conditions [1,216] and increased mitochondrial ROS production underlies cardiovascular and many other diseases [4,217–224]. The mitochondrial ETC is the main source of ROS production in mitochondria [225]. Electrons from NADH and FADH2 generated in the Krebs cycle are transferred through the ETC to reduce molecular oxygen to water, a process that involves four one-electron reduction reactions. Complex IV (cytochrome c oxidase), the terminal component of the ETC, retains all the partially reduced intermediates until full reduction of oxygen is achieved. However, other complexes may leak electrons, generating O2•− by the partial reduction of oxygen.
Complex I and III are the main sources of O2•− production in mitochondria [225], with the former being the predominant source in vivo [226,227]. Whereas O2•− from complex I is released into the matrix, complex III-derived O2•− is released into both the mitochondrial matrix and the intermembranous space [228,229]. Superoxide production from complex I occurs in vivo when NADH levels are high, from damage to the respiratory chain, slow respiration, or ischemia [2]. This may occur by the mitochondrial loss of cytochrome c, as happens in the failing human heart [230,231], and probably by the inhibition of cytochrome c oxidase by enhanced formation of •NO [232]. ROS production also depends on the metabolic state of mitochondria, with more O2•− production in State 4 respiration (low oxygen consumption, electron flow, and ATP synthesis, limiting ADP level; high NADH/NAD+ ratio) than in State 3 respiration (high electron flow, fast ATP synthesis, partial depolarization, and decreased NADH/NAD+ ratio) [233]. It has been suggested that an increase in pathophysiological ROS levels would occur at the extremes of overall intracellular and intramitochondrial redox potential, which in turn depends on redox couples involved in ROS generation (NADH/NAD+) and ROS scavenging (NADPH/NADP+) [234]. An increase in ROS generation occurs when mitochondrial redox potential is significantly reduced, as happens in hypoxia, or significantly oxidized, as may happen during heart failure. In the latter case, the increase in ROS levels results from a depletion of antioxidant capacity as a consequence of the decrease in NADPH levels. Recently, it was shown that elevated [Na+] in cardiomyocytes of failing hearts reduced mitochondrial Ca2+ by accelerating Ca2+ efflux and decreased NADPH levels resulting in increased mitochondrial ROS formation [235]. A decrease in Ca2+ during increased workload attenuates the mitochondrial antioxidant capacity by decreasing the activity of Krebs cycle dehydrogenases.
In addition to the inner membrane, ROS are also produced at other sites in mitochondria [236]. For example, the p66Shc protein, partially localized in the mitochondrial intermembrane space, forms a molecular complex with cytochrome c and subtracts electrons, resulting in a reduction of oxygen and formation of H2O2 [237]. Data from experimental animal models suggest that activation of p66Shc plays a role in cardiovascular pathophysiology. Mice deficient in p66Shc were protected against AngII-induced myocardial damage [238] and diabetic cardiomyopathy [239] and early atherogenesis induced by a high-fat diet [240].
Monoamine oxidase, existing in two isoforms (MAO A and MAO B), is a mitochondrial outer-membrane-bound flavoprotein and is another important source of mitochondrial ROS that catalyzes the deamination of neurotransmitters and biogenic amines [241]. H2O2 generated during degradation of serotonin by MAO A induced receptor-independent apoptosis in isolated cardiac myocytes, and MAO inhibitors significantly decreased in vivo myocardial injury during ischemia–reperfusion [242]. Increased MAO A activity coupled with high intramyocardial norepinephrine levels plays an important role in the evolution of maladaptive hypertrophy into cardiac failure [243].
NADPH oxidases
Nox’s, a family of enzymes with the sole function of producing ROS, are implicated in the pathophysiology of many cardiovascular diseases [244]. The phagocyte (neutrophils and macrophages) oxidase, the first characterized NADPH oxidase, is a multicompo-nent complex that catalyzes the formation of O2•− during phagocytosis [245]. In the resting cell, the phagocyte NADPH oxidase has a membrane-bound catalytic core of the enzyme, flavocytochrome b558, and the cytosolic regulatory subunits p47phox, p40phox, p67phox, and small G-protein Rac1 or Rac2. The flavocytochrome b558 is a heterodimer consisting of a large glycoprotein, gp91phox (Nox2), and a small protein, p22phox, and the close association of these two proteins stabilizes the flavocytochrome [246].
Upon cell stimulation, the regulatory subunits translocate to the membrane and assemble with the flavocytochrome b558 to cause activation of the enzyme. In the resting neutrophils, p47phox, p67phox, and p40phox exist as a complex stabilized by SH3 domain interactions [30,247], whereas Rac is tethered to RhoGDI, a RhoGDP-dissociation inhibitor [248]. However, binding to the flavocytochrome is prevented because p47phox exists in an autoinhibited conformation in which its tandem SH3 domains are masked owing to intramolecular interaction with the C-terminal segment. During activation, multiple serine residues in the C-terminus of p47phox are phosphorylated, liberating the N-terminal SH3 domain for interaction with the proline-rich region of p22phox and translocation to the membrane [249–252]. This allows the proline-rich activation domain in p67phox to bind with an activation sequence in the C-terminus of Nox2 to initiate electron transfer, thus activating the enzyme [30,253].
The existence of similar, albeit lower ROS-generating, oxidases in nonphagocytic cells has been identified in the past decade, with the identification of Nox1, the first homolog of Nox2 [254]. Unlike the phagocyte oxidase, the nonphagocyte oxidases are active during normal metabolism and generate low levels of ROS even in the absence of extrinsic stimulation; however, their ROS generation is increased in response to agonist stimulation. In total, the Nox family comprises seven members, each with a distinct catalytic isoform: Nox’s 1–5 and Duox1 and Duox2 [246,248]. The predicted structure of Nox’s 1–4 consists of an N-terminal transmembrane region with six α-helical domains containing four conserved histidines, two each in the third and fifth domain spanning two asymmetrical hemes. The cytoplasmic C-terminus dehydrogenase domain contains conserved binding sites for FAD and NADPH. Nox5 is distinct from Nox’s 1–4 by the presence of a calmodulin-like EF domain with four Ca2+-binding sites in the long N-terminus, which enables rapid enzyme activation in response to elevated cytosolic Ca2+ levels [255,256]. The Duox proteins are further different from Nox5 in containing an N-terminal perox-idase-like domain that is connected to the EF domain by an additional transmembrane domain [257–259].
The expression of Nox catalytic subunits varies among different cell types of the cardiovascular system, with more than one subunit expression in the cell types [30] (Table 1). Nox1 is mainly expressed in VSMCs [10,254,260,261], although endothelial cell [10,262] and fibroblast [10] expression was also observed. Nox2 is present in endothelial cells [10,263,264,265,266], fibroblasts [267], cardiomyocytes [268,269], and VSMCs in human resistance arteries [270]. Nox4 expression is fairly abundant in VSMCs [10,271,272,273], endothelial cells [10,264], fibroblasts [10,274], and cardiomyocytes [275,276]. Nox5 is present in human VSMCs [277] and endothelial cells [278], whereas it is absent in rodents [279]. Nox3 and Duox2 expression was not reported in cardiovascular cells, but Kalinina et al. [280] observed Duox1 expression in the human aortic VSMCs.
Table 1.
Cardiovascular cell-specific expression of Nox isoforms.
| Cell type | Nox isoform | Tissue | Ref. |
|---|---|---|---|
| VSMCs | Nox1 | Mouse, rat aorta | [261,254,260] |
| VSMCs | Nox1 | Human coronary artery | [10] |
| VSMCs | Nox2 | Human resistance artery | [270] |
| VSMCs | Nox4 | Mouse, rat aorta | [271,272] |
| VSMCs | Nox4 | Human aorta | [273] |
| VSMCs | Nox4 | Human coronary artery | [10] |
| VSMCs | Nox5 | Human aorta | [277] |
| VSMCs | Duox1 | Human aorta | [280] |
| Endothelial cells | Nox1 | Human coronary artery | [10] |
| Endothelial cells | Nox1 | Human umbilical vein | [262] |
| Endothelial cells | Nox2 | Mouse, rat aorta | [263,264] |
| Endothelial cells | Nox2 | Human coronary artery | [10] |
| Endothelial cells | Nox2 | Human umbilical vein | [265,266] |
| Endothelial cells | Nox4 | Rat aorta | [264] |
| Endothelial cells | Nox4 | Human coronary artery | [10] |
| Endothelial cells | Nox4 | Human heart | [10] |
| Fibroblasts | Nox1 | Human heart | [10] |
| Fibroblasts | Nox2 | Human coronary artery | [10] |
| Fibroblasts | Nox2 | Human heart | [10] |
| Cardiomyocytes | Nox2 | Mouse, rat heart | [265,269] |
| Cardiomyocytes | Nox4 | Mouse heart | [275] |
Like Nox2, binding with p22phox is essential for the activity of Nox1 and Nox4. For Nox1, the cytosolic regulatory subunits are NoxO1 and NoxA1, the homologs of p47phox and p67phox, respectively, as well as Rac1. However, the subunit expression and NADPH oxidase composition may vary depending on the vascular beds and species. We and others have recently shown that Nox1 interacts with p47phox and NoxA1 in mouse VSMCs [11,281]. Nox4 does not require interaction with cytosolic regulatory subunits for activity and hence is constitutively active, with regulation mainly dependent on expression levels. Nox5, Duox1, and Duox2 activities are regulated by Ca2+ and do not require any subunit for activation [246].
Activated NADPH oxidases (Nox1 and Nox2) generate O2•− by transferring two electrons from NADPH in the cytosol to FAD and then to the two heme groups, with the second heme group reducing two successive molecules of molecular oxygen on the other side of the membrane [246,282]. Because the transfer of electrons across the plasma membrane generates depolarization, electroneutrality is ensured by the conduction of protons, which are generated from the NADPH hydrolysis in the cytosol, through a channel in the oxidase [283–285]. In contrast, Nox4 predominantly produces H2O2, which has been attributed to Cys226, Cys270, and a highly conserved His222 residue in the third extracytosolic loop [286]. The histidine could serve as a source of protons for the spontaneous dismutation of O2•− forming H2O2. ROS production from NADPH oxidases could be either extracellular or intracellular depending on the biological membranes in which the enzyme is expressed, which include plasma membrane, endosome, phagosome, caveolae, endoplasmic reticulum, mitochondria, and nucleus. Nox1, Nox2, Nox4, and Nox5 can be located either at the plasma membrane or within the cell and hence can generate extracellular or intracellular ROS [287].
Xanthine oxidase
Xanthine oxidase (XO) has been identified as a major source of O2•− in atherosclerosis [288,289] and congested heart failure [290,291]. XO and xanthine dehydrogenase (XDH) are interconvertible isozymes of the enzyme xanthine oxidoreductase (XOR) and catalyze the final two steps of the purine (adenosine) degradation pathway, reducing hypoxanthine and xanthine to uric acid. However, XDH preferentially reduces NAD+, whereas XO reduces only molecular O2, producing O2•− and H2O2. XDH is the predominant form in well-oxygenated tissue [292], which is converted to XO by reversible sulfhydryl oxidation or by irreversible proteolytic modification [293] under pathophysiological conditions such as ischemia-hypoxia [294,295]. Both forms of the enzymes act as NADH oxidases generating ROS, with the oxidation induced by XDH higher than that observed with XO [296,297], which may play an important role in cellular injury under conditions of increased NADH concentration, as happens in ischemia [298].
XOR has wide tissue distribution, but its plasma levels, low in healthy mammals, increase significantly under pathophysiological conditions [299]. Circulating XO binds to the vascular endothelial cells because of its affinity with the positively charged glycosami-noglycans on the cell surface [300,301], generating ROS and decreasing the bioavailability of •NO to cause endothelial dysfunction and impair vasorelaxation [289]. This is supported by the data, which show an inverse relationship between endothelium-bound XO activity and endothelium-dependent vasodilation in patients with CAD [302]. Increased functional XOR levels were observed in monocytes/macrophages in drug- and coronary artery ligation-induced heart failure in rats [303]. However, this increase was not observed in hypertrophic ventricles, suggesting its potential role in the progression from cardiac hypertrophy to heart failure. Supporting this notion, a significant increase in endothelium-bound XO activity was observed in patients with chronic heart failure [304].
Nitric oxide synthases
The NOS family of enzymes generates •NO from the conversion of L-arginine to L-citrulline. NOSs are homodimeric oxidoreduc-tases in which the heme-containing oxygenase domain is linked via a calmodulin-binding linker peptide to a NADPH-cytochrome P450 reductase-like diflavin domain [305]. Upon activation, the FAD of the flavoprotein domain transfers electrons from NADPH to FMN, which reduces heme iron and results in O2 activation followed by oxidation of the guanidino N atom of L-arginine, forming •NO and citrulline. BH4, a cofactor and critical determinant of the enzyme activity, binds close to the heme active site at the interface of the two monomers, stabilizing the dimer [306,307].
Three NOS isoforms are present in the cardiovascular system, of which neuronal (nNOS) and eNOS are constitutive, with activity regulated at a posttranslational level [308]. The iNOS isoform is produced in response to proinflammatory agonists such as cytokines and is regulated mostly at the transcriptional level [309].
Under normal conditions, eNOS exerts antiatherogenic effects in the vascular wall, including inhibition of cell growth [310,311], leukocyte adhesion [153], and platelet aggregation [312]. Increased coronary atherosclerosis observed in eNOS-deficient apoE−/− mice on a Western-type diet [313,314] was attributed to increased inflammation and leukocyte-endothelial interaction [315]. •NO derived from eNOS regulates VSMC tone and blood pressure as evidenced by systemic hypertension in eNOS-knockout mice [316,317] and hypotension in eNOS-transgenic mice [318]. In the heart, eNOS is expressed in the endocardium and cardiomyocytes and eNOS−/− mice exhibit attenuated left-ventricular function and increased mortality after myocardial infarction and during chronic pressure overload [319,320]. However, when eNOS activity becomes “uncoupled,” as happens in pathophysiological conditions, increased O2•− generation occurs because the transfer of electrons from NADPH through the flavins to molecular oxygen continues [307] (discussed later in regard to endothelial dysfunction).
In contrast, a rapid and large increase in •NO generation by upregulation of iNOS expression and activity was linked to cardiovascular pathology. Wild-type mice with iNOS deficiency had increased myocardial contractility and decreased mortality after myocardial infarction [321] and were protected from systolic overload-induced myocardial dysfunction [322], whereas apoE−/− /iNOS−/− mice had a significantly reduced atherosclerotic lesion area [323–325]. However, the role of iNOS in cardiovascular pathology was questioned as its overexpression in the mouse myocardium had no effect on viability and left-ventricular function [326]. It is likely that the high flux of •NO from iNOS has pathological effects only under oxidative stress conditions, particularly with increased O2•− levels [327].
In addition to neurons [328], nNOS is expressed in skeletal muscle [329], kidney [330], endothelial cells and SMCs [331], and cardiomyocytes [332]. Recent evidence indicates that nNOS has a protective function against atherosclerosis and in the heart. Kuhlencordt et al. [333] reported increased atherosclerotic plaque formation and decreased survival in nNOS-deficient apoE−/− mice. The physical proximity of nNOS to XOR in sarcoplasmic reticulum of the cardiomyocytes regulates the activity of the latter and nNOS deficiency decreases myocardial excitation coupling via increased activity of XOR [334].
Lipoxygenases
Lipoxygenases (LOXs), non-heme iron-containing dioxygenases that oxidize polyunsaturated fatty acids released from the cell membrane under inflammatory conditions to hydroperoxy fattyacid derivatives, are another important source of ROS production in the vascular wall [335,336]. Humans have six ALOX genes (LOX genes are named “ALOX” by convention, for arachidonic acid lipoxygenase), whereas mice have seven functional genes [337] and the LOX enzymes are named for the numbered carbon atom of the polyunsaturated fatty acid that gets oxidized (e.g., 5-LOX). Of the LOXs, 5-LOX and 12/15-LOX (also known as leukocyte-type LOX and 15-LOX1; referred to as 12/15-LOX as they can form similar products from common substrates) are important for cardiovascular function and atherosclerosis because of their expression in the vascular wall and inflammatory cells [337]. Mice express only 12-LOX and not 15-LOX [338].
5-LOX catalyzes the transformation of free arachidonic acid to leukotriene A4, which on hydrolysis yields leukotriene B4 (LTB4), a potent chemoattractant and leukocyte activator [339]. The conjugation of leukotriene A4 with glutathione by the action of leukotriene C4 synthase yields cysteinyl leukotrienes, which are associated with vasoconstriction. 5-LOX and LTB4 are highly expressed in human atherosclerotic plaques and LTB4 is involved in SMC recruitment [340,341]—antagonism of its receptor decreases monocytic foam cells in mice [342].
12/15-LOX catalyzes the oxidation of arachidonic acid to yield 12- and 15-hydroperoxyeicosatetraenoic acids (12- and 15-HPETEs), which are rapidly reduced by cellular peroxidases to the corresponding hydroxides, 12-HETE and 15-HETE, respectively [336]. This enzyme also oxidizes α-linoleic acid, another polyunsaturated ω-3 fatty acid, generating 13-hydroperoxyoctadecadienoic acid (13-HPODE), which is reduced to 13-HODE. In addition to free fatty acids, 12/15-LOX oxidizes polyunsaturated acyl chains in phospholipids and cholesteryl esters, key lipid components of LDL [343,344]. In macrophages, LDL oxidation requires binding LDL particles to the low-density lipoprotein receptor-related protein [345] and translocating 12/15-LOX from the cytosol to the plasma membrane [346]. Another line of evidence for the role of 12/15-LOX in LDL oxidation comes from the 12/15-LOX, apoE double-knockout mice on a high-fat diet, which have less atherosclerosis, significantly lower titers of autoantibodies against oxidized LDL in plasma, and lower isoprostane levels in urine compared with apoE−/− mice [347]. Protection against atherosclerosis in the 12/15-LOX, apoE double-knockout was attributed in part to decreased adhesion of monocytes to endothelial cells [348,349], an initiating event in atherogenesis, resulting from decreased activation of RhoA and NF-κB [350,351]. As a corollary to this, the overexpression of human 15-LOX in the vascular endothelium of LDL receptor-deficient mice enhanced early atherosclerosis [352]. However, some reports suggest that 12/15-LOX products may also exert an antiatherogenic effect because of their anti-inflammatory and vasodilatory properties [336].
12/15-LOX is markedly upregulated in heart failure, and transgenic mice with cardiomyocyte-specific overexpression of the enzyme develop systolic dysfunction, aging-associated cardiac fibrosis, and increased macrophage infiltration and MCP-1 expression [353]. Supporting this, 12/15-LOX-deficient mice had significantly reduced cardiac MCP-1 expression, macrophage infiltration, and reduced systolic dysfunction during chronic pressure overload.
Myeloperoxidase
Myeloperoxidase (MPO) generates several oxidants that initiate lipid peroxidation and induce modification of amino acid residues in proteins, including nitration, chlorination, and carbamylation [31]. Brennan et al. [354] demonstrated a significant decrease in 3-nitrotyrosine levels in MPO-deficient mice in response to inflammation. In addition, MPO-deficient mice had a significant reduction in the levels of F2-isoprostanes, HPETEs, and HPODEs in an acute model of inflammation, supporting a major role for MPO in in vivo lipid peroxidation [355]. Immunofluorescent staining revealed the presence of MPO in the neutrophils in intermediate and advanced atherosclerotic lesions of LDLR−/− mice [356]. MPO induces protein carbamylation in the presence of H2O2 at sites of inflammation and in atherosclerotic plaques by the oxidation of thiocyanate, an anion abundant in blood whose levels are elevated in smokers [357]. The product, cyanate, then covalently modifies lysine residues in proteins and lipoproteins, forming homocitrul-line (e-carbamyllysine). LDL-homocitrulline stimulates foam cell formation, VSMC proliferation, and endothelial apoptosis. In addition, the blood levels of protein-homocitrulline correlated with increased cardiovascular risk in case-control studies. An increase in LDL carbamylation in human atherosclerotic lesions by MPO causes cholesterol accumulation and lipid-droplet formation in macrophages through enhanced binding to the LDL receptor SR-A1. Similarly, an increase in HDL carbamylation by MPO induces cholesterol accumulation in macrophages through enhanced binding to the scavenger receptor SR-B1 [358]. More recently, Shao et al. [359] demonstrated that chlorination of Tyr192 in apoA-I of HDL in human plasma and atherosclerotic tissue generates dysfunctional HDL.
The proinflammatory and proatherogenic actions of MPO may include promotion of leukocyte recruitment at sites of inflammation by its positive surface charge [360]. MPO-deficient mice had reduced neutrophil infiltration in inflamed tissues, and an infusion of MPO into the circulation caused neutrophil adhesion even in uninflamed blood vessels, supporting the notion that neutrophil recruitment is mediated by the strong positive charge of MPO. However, it is suggested that MPO augments adhesion molecule-mediated interaction only between endothelial cells and neutrophils. In addition, a significant increase in systemic MPO levels was associated with coronary plaque erosion in patients with acute coronary syndrome [361]. Together, these data provide evidence that oxidative stress mediated by MPO could increase atherogenicity.
Substantial evidence also suggests that MPO converts nitrite, a major end product of •NO metabolism, into RNS, most probably nitrogen dioxide (•NO2), in a H2O2-dependent reaction [362–365]. The •NO2 generated by the MPO-H2O2-nitrite system catalyzes the nitration of tyrosine residues and oxidation of tryptophan residues and promotes lipid peroxidation of LDL [363,366]. However, MPO-generated •NO2 is not a major product, particularly in human leukocytes, and its significantly greater amounts in murine compared with human phagocytes might be due to higher local nitrite concentrations in the mice [367–370].
Endothelial dysfunction
Robert Furchgott [371] first demonstrated that relaxation of blood vessels by acetylcholine requires the presence of endothelial cells, whereas Rubanyi et al. [372] observed that this process is mediated by the release of a vasoactive substance, termed endothelium-derived relaxing factor (EDRF). The EDRF diffuses to the VSMCs and induces the production of cGMP by stimulating sGC [373]. cGMP activates PKG, causing a decline in cytosolic Ca2+, which in turn suppresses the activity of myosin light chain (MLC) kinase, resulting in increased levels of dephosphorylated MLC and relaxation of SMCs [374]. Activation of MLC phosphatase by PKG also causes vasodilation by increasing dephosphorylated MLC levels. The EDRF, which is rapidly degraded by O2•−, was identified as •NO [375,376], synthesized by NOS, mostly eNOS in the vasculature [377,378].
In addition to the regulation of vasodilation, endothelium modulates inflammation, SMC growth, platelet aggregation, and coagulation, and the dysregulation of these processes is termed “endothelial dysfunction,” which is evident as impaired vasorelaxation in response to endothelium-dependent vasodilators such as acetylcholine [379]. The healthy endothelium responds to vasoactive agonists released from the aggregating platelets by the activation of eNOS and increased production of •NO [380,381]. With endothelial injury, the aggregating platelets come into contact with VSMCs, causing contraction by releasing thromboxane A2 and serotonin [382]. The endothelium-dependent response to aggregating platelets is highly active in the coronary and cerebral circulations.
Endothelial dysfunction is an initial event in the development of atherosclerosis and ischemic heart disease [383,384] and an independent predictor of CVD [385–388]. Schächinger et al. [389] demonstrated that coronary endothelial vasodilator dysfunction is a prognostic indicator of cardiovascular events, including cardiovascular death, unstable angina, myocardial infarction, and ischemic stroke. In a 30-day follow-up of nonemergent vascular surgery, patients in the upper tertile of brachial artery flow-mediated dilation ( 4 8.1%) had significantly fewer adverse cardiovascular events than patients with low flow-mediated dilation [384]. Similarly, endothelial dysfunction in the forearm has been shown to predict adverse cardiovascular events in subjects with no apparent heart disease [390] as well as in patients with peripheral arterial disease [391,392]. Increased ROS production and impaired endothelium-dependent and -independent vasodilator responses resulting from eNOS uncoupling in platelets were observed in patients with congestive cardiac failure [393].
The mechanisms underlying endothelial dysfunction are multifactorial [394,395], with oxidative stress playing a major role. The kinetics of the reaction of O2•− with •NO are three times faster than the reaction rate of O2•− with SOD. Thus, it is likely that some O2•− always reacts with •NO within the cells and extracellular space, but endogenous antioxidant defenses minimize this interaction [396]. However, in pathophysiological conditions such as in hypercholesterolemic rabbits, impaired endothelium-dependent vascular relaxation results from the interaction of •NO with O2•−, because polyethylene-glycolated SOD markedly improved endothelium-dependent vascular relaxation in these but not in normocholesterolemic animals [397]. Hypercholesterolemia also enhances oxidative stress by upregulating the expression of the AT1 receptor, genetic disruption of which improved endothelial function and inhibited diet-induced atherosclerosis in apoE−/− mice [398]. Likewise, oxidized LDL but not native LDL inhibits endothelium-dependent relaxation in isolated vessels [399], whereas antioxidant vitamins enhance endothelium-dependent vasodilation in both the coronary and the forearm circulation in subjects with CVD [400,401]. Emphasizing the role of oxidative stress in endothelial dysfunction, deletion of various SOD isoforms impaired •NO-mediated arterial relaxation [402–404].
Another mechanism by which oxidative stress causes endothelial dysfunction is via the uncoupling of the eNOS. Deficiency of either eNOS substrate L-arginine or eNOS cofactor BH4 induces eNOS uncoupling to produce O2•− and H2O2. Increased O2•− production in the aortas of spontaneously hypertensive stroke-prone rats was reduced by treatment with NG-nitro-L-arginine methyl ester (L-NAME), an inhibitor of NOS, or removal of endothelium, indicating that the tissue and enzymatic sources of this increased O2•− are the endothelium and eNOS, respectively [405]. Activation of arginase I, which degrades L-arginine, and eNOS uncoupling were reported in diabetes, pulmonary hypertension, ischemia-reperfusion, atherosclerosis, and aging [406–410]. Arginase inhibition restores NOS coupling and reverses endothelial dysfunction [411].
Decreased BH4 availability, resulting in eNOS uncoupling, and increased oxidative stress were observed in diabetes [412,413]. Intra-arterial infusion of BH4 improved endothelium-dependent vasodilation in chronic smokers, supporting the notion that BH4 depletion contributes to eNOS dysfunction [414]. BH4 supplementation reversed left-ventricular hypertrophy (LVH), fibrosis, and cardiomyocyte dysfunction induced by pressure overload, highlighting the importance of cardiac myocyte eNOS uncoupling in hypertrophic heart disease [415,416]. Further support for the regulatory role of BH4 concentrations as a critical determinant in eNOS uncoupling was evident in apoE−/− mice with endothelial-specific overexpression of eNOS [417]. These mice had enhanced endothelium- and eNOS-dependent O2•−production and increased atherosclerosis compared with apoE−/− mice. BH4 supplementation attenuated both endothelial ROS and atherosclerosis. Consistent with these data, endothelial-specific overexpression of GTP-cyclohydrolase 1, the rate-limiting enzyme for BH4 synthesis, in apoE−/−/eNOS-transgenic mice partially restored eNOS coupling [418] and decreased ROS levels and atherosclerosis [419,420]. In vivo depletion of BH4 could also occur from oxidative modification by peroxynitrite [421]. Zou et al. [422] reported that the main mechanism of peroxynitrite-induced eNOS uncoupling is the release of Zn from a zinc-thiolate cluster, as this process is 10–100 times more sensitive than BH4 oxidation.
Serum asymmetrical dimethylarginine (ADMA), an endogenous L-arginine analog, is inversely correlated with flow-mediated dilation in the brachial artery of subjects, independent of risk factors for atherosclerosis [423]. ADMA inhibits eNOS activity by competitive inhibition of endothelial cell arginine uptake. Furthermore, the activities of S-adenosylmethionine-dependent protein arginine N-methyltransferases and dimethylarginine dimethylaminohydrolase, enzymes involved in ADMA synthesis and degradation, respectively, are redox sensitive, with the former enhanced and the latter decreased under oxidative stress conditions [424,425].
O2•− required for eNOS uncoupling is generated by several sources. Administration of oxypurinol, an inhibitor of XO, improved impaired vasodilation in hypercholesterolemic [426] and CHF patients [427,428], implicating XO-derived O2•−production in eNOS uncoupling. However, Dworakowski et al. [429] reported that increased activity of Nox, particularly of Nox4, contributes to increased O2•− production and vascular endothelial dysfunction in CHF patients. Impaired acetylcholine-induced relaxation of spontaneously hypertensive aged rat aortas was significantly improved by VAS2870, a pan-Nox inhibitor, by inhibiting the ectopically expressed Nox1 in endothelial cells [430]. Similarly, Nox1 overexpression in VSMCs impaired endothelium-dependent relaxation in response to AngII infusion, which was reversed by BH4 supplementation [431].
Although vascular relaxation in large vessels mediated by endothelium-derived •NO bioactivity is mainly dependent on O2•− concentrations, endothelium-dependent relaxation in small resistance vessels is mediated by H2O2 [31]. Matoba et al. [432] reported that endothelium-dependent relaxation and hyperpolar-ization of VSMCs in the small mesenteric arteries of mice in response to acetylcholine was inhibited by catalase and first proposed that H2O2 is the EDHF. H2O2-dependent vascular relaxation was reported in subjects with CVD. Miura et al. [159,433] reported that H2O2 has a more prominent role in flow-induced dilation of coronary arterioles in subjects with CAD compared with those without CAD. Also, under conditions of BH4 depletion, H2O2 mediates endothelium-dependent relaxation in coronary arteries [434]. Likewise, in mice deficient in GTP cyclohydrolase H2O2 produced as a result of eNOS dysfunction mediates aortic relaxation in response to acetyl choline [435]. However, the regulatory function of H2O2 may vary depending on the vascular bed, species, age, and pathophysiological conditions. For example, the vascular cell-specific overexpression of catalase decreased blood pressure in mice, indicating the vasoconstrictive function of H2O2 [436]. Both genetic and pharmacological evidence suggests that H2O2 also impairs endothelium-dependent vasorelaxation because polyethylene glycol-catalase or transgenic overexpression of GPx-1 protected mice against AngII-induced endothelial dysfunction of carotid arteries [437].
Atherosclerosis
Endothelial dysfunction and activation in the presence of atherosclerosis risk factors such as hypercholesterolemia and hypertension induce the expression of the cell adhesion molecules VCAM-1, ICAM-1, E-selectin, and P-selectin [438,439]. Activated endothelium also permits increased permeability to macromole-cules such as LDL The induction of cell adhesion molecules enables the adherence of circulating monocytes and T lymphocytes to the endothelium and the subsequent transmigration into the subendothelial space. The activation of inflammatory cells is associated with the stimulation of oxidant enzymes such as NADPH oxidases and MPO, generation of ROS, and oxidation of phospholipids and protein in LDL, resulting in the accumulation of oxidized LDL (oxLDL), an important effector of atherogenesis [440,441]. ROS generated in the vascular wall cells as well as in inflammatory cells by the activation of oxidant enzymes stimulates redox signaling pathways that could affect atherogenesis at multiple levels [442].
It was shown recently that oxLDL increases O2•− generation in human aortic endothelial cells by phosphorylating p66Shc at Ser36 [443]. This effect of oxLDL is dependent on the binding of the lipoprotein to its LOX-1 receptor, followed by the sequential activation of protein kinase Cp2 and c-Jun N-terminal kinase. Genetic deletion of p66Shc decreased oxidative stress, lipid peroxidation, and atherosclerosis in apoE−/− mice, indicating the important role of systemic oxidative stress in atherosclerosis [240,444]. Interestingly, the PKCp–JNK pathway is a critical effector of oxLDL-mediated induction of MMP2 expression and activity, and deletion of PKCp2 or JNK2 significantly decreases oxidative stress and atherosclerosis in apoE−/− mice [445–447]. Suppression of p66Shc expression inhibited oxLDL-induced p47phox expression as well as ROS production, indicating that NADPH oxidase is a major source of oxLDL-induced ROS in vascular cells [448]. Increases in p66Shc mRNA levels were reported in patients with high plasma LDL levels [433] and in angiographically confirmed CAD patients [449], implicating oxidative stress in atherogenesis.
In addition to oxidizing LDL, oxidative stress also affects cardiovascular health by inhibiting the cholesterol efflux function of HDL. Myeloperoxidase-induced chlorination of apoA-I, the major protein component of HDL, impairs the ability of apoA-I to promote cholesterol efflux through ABCA1, the macrophage ATP-binding cassette transporter [450,451]. The lecithin-cholesterol acyltransferase (LCAT) binding site on apoA-I is the preferred target for oxidative modification in atheroma, which diminished LCAT activity, resulting in a dysfunctional form of HDL [452–454]. This, in turn, could increase ROS production and inflammation, as Peshavariya et al. [455] showed that reconstituted HDL (apoA-I complexed with 1-palmitoyl-2-linoleoyl phosphatidylcholine in a molar ratio of 1:100) inhibits leukocyte NADPH oxidase activity, probably by disrupting the assembly of the enzyme subunits at lipid rafts. It is suggested that MPO catalyzes oxidation of HDL and converts it into a proinflammatory molecule [456].
Further supporting the proatherogenic role of MPO, Sugiyama et al. [457,458] reported the accumulation of a subset of MPO-containing macrophages in the subendothelial space at sites of coronary plaque erosion or rupture and suggested that MPO-positive macrophage-derived HOCl promotes acute coronary syndrome by stimulating endothelial cell death and tissue factor expression. Additional evidence for the role of MPO in the pathophysiology of atherosclerosis is evident in population-based studies of initially healthy men and women in whom high levels of circulating MPO were predictors of future risk of CHD [16,459,460].
Evidence accumulated over the past decade has shown increased expression of NADPH oxidase subunits and increased ROS levels in human atherosclerotic lesions, indicating the clinical relevance of redox signaling and oxidative stress in atherosclerosis. Sorescu et al. [10] reported increased Nox2 and p22phox expression along with increased O2•− generation in the shoulder region of atherosclerotic plaques. Azumi et al. [461,462] observed increased p22phox expression and ROS generation in atherosclerotic coronary arteries and in the coronary plaques of unstable angina patients. Simultaneous intravascular ultrasound and immunohistochemical analysis indicated that NADPH oxidase-derived ROS are involved in the coronary arterial remodeling associated with plaque vulnerability [463].
Evidence for the contribution of NADPH oxidase-derived ROS to atherosclerosis was also found in experimental mouse models that are deficient in various subunits of NADPH oxidase (Table 2). We demonstrated decreased aortic ROS levels in mice that are deficient in p47phox and decreased aortic atherosclerotic lesion area in apoE−/−/p47phox−/− compared with apoE−/− mice [3,464]. A decrease in the atherosclerotic lesion area and attenuated neoin-timal hyperplasia in response to arterial injury in apoE−/− -/p47phox−/− compared with apoE−/− mice is associated with decreased expression of adhesion molecule CD44 in aortic/arterial cross sections [464]. An allogeneic, sex-mismatched bone marrow transplantation study demonstrated that the atheroprotective effect of p47phox deletion resulted from the inhibition of NADPH oxidase in the vascular wall cells as well as in bone marrow-derived monocytes/macrophages [465]. Absence of a functional NADPH oxidase in neointimal SMCs caused attenuated activation of redox-sensitive mitogenic proteins, including Janus kinase 2, and decreased neointima formation in apoE−/−/p47phox−/− mice. Interestingly, Fenyo et al. [466] reported that the Janus kinase 2 inhibitor tyrphostin AG490 decreased the expression of Nox1, Nox2, and Nox4 subunits and NADPH oxidase activity and reduced the atherosclerotic lesion size in apoE−/− mice that were fed a high-fat diet. Our data on the effect of p47phox deficiency in atherogenesis in apoE−/− mice are supported by recent evidence of decreased aortic ROS production, increased •NO bioavailability, and significantly reduced atherosclerotic lesion size in apoE−/−/ Nox2y/- compared with apoE−/− mice fed a high-fat diet [263]. Similarly, reduced neointima formation in response to arterial injury and decreased leukocyte accumulation was observed in Nox2y/- mice [467]. Recent data also indicate that Nox1 activation contributes to atherosclerosis. The aortic atherosclerotic lesion area and the macrophage content in the aortic sinus area were significantly decreased in apoE−/−/Nox1y/- compared with apoE−/− mice that were fed a high-fat diet [468]. Nox1 is also involved in the response to vascular injury as attenuated wire injury-induced femoral artery neointima formation and decreased VSMC proliferation and migration were observed in Nox1y/− compared with wild-type mice [469]. Mechanistic studies revealed that Nox1 regulates VSMC migration by modulating the actin cytoskeleton via its effects on cofilin (a regulator of actin depolymerization), mDia1 (a RhoA adapter protein), and PAK1 (a serine/threonine kinase that promotes cytoskeletal reorganization). We demonstrated that adenovirus-mediated overexpression of the Nox activator NoxA1 increases neointimal hyperplasia in injured mouse carotid arteries and that NoxA1 expression is increased in aortas and atherosclerotic lesions of apoE−/− mice and in human carotid atherosclerotic lesions [11]. Furthermore, we showed that an inhibitor of Nox1 and Nox4, GKT136901, decreases ROS generation and expression of CD44 and its principal ligand, hyaluronan, in atherosclerotic lesions [134].
Table 2.
Experimental mouse models support the role of oxidative stress in atherosclerosis.
| Effector | Genetic model/pharmacologic agent | Phenotype | Ref. |
|---|---|---|---|
| p66Shc | ApoE−/−/p66Shc−/− | Decreased oxidative stress, lipid peroxidation and atherosclerosis | [444] |
| PKCβ | ApoE−/−/PKCβ−/− | Decreased MMP expression and activity; decreased atherosclerosis | [445] |
| JNK2 | ApoE−/−/JNK2−/− | Decreased foam cell formation and atherosclerosis | [446] |
| p47phox (Nox1/2 NADPH oxidase activity) | ApoE−/−/p47phox−/− | Decreased vascular ROS levels and atherosclerosis | [3,464] |
| Nox1/2/4 expression/activity | AG490 | Decreased atherosclerosis in apoE−/− mice | [466] |
| Nox2 | ApoE−/−/Nox2y/− | Decreased vascular ROS levels; increased NO bioavailability and decreased atherosclerosis | [263] |
| Nox1 | ApoE−/−/Nox1y/− | Decreased vascular ROS levels and atherosclerosis | [468] |
| Nox1/4 | GKT136901 | Decreased vascular ROS levels and atherosclerosis | [134] |
| Catalase; SOD1 and catalase | ApoE−/−/hCatTg0/+; apoE−/−/hSOD1Tg0/ +/hCatTg0/+ | Decreased lipid peroxidation and atherosclerosis | [471] |
| SOD2 | ApoE−/−/SOD2+/− | Increased mitochondrial ROS levels and mitochondrial DNA damage; increasedatherosclerosis | [4] |
| GPx | ApoE−/−/GPx−/− | Increased vascular ROS levels and atherosclerosis | [476] |
| eNOS | ApoE−/−/eNOS−/− | Accelerated atherosclerosis, aortic aneurysm, and ischemic heart disease | [314] |
| PON1 | PON1−/−; apoE−/−/PON1−/− | Increased atherosclerosis | [483,484] |
| PON2 | PON2-deficient apoE−/− | Increased mitochondrial oxidative stress | [486] |
| PON3 | hPON3Tg0/+; apoE−/−/hPON3Tg0/+ | Decreased atherosclerosis | [487] |
Endogenous antioxidant systems protect against atherogenesis, by virtue of their ability to scavenge ROS, facilitate endothelium-dependent vasorelaxation, inhibit inflammatory cell adhesion to endothelium, and alter vascular cellular responses, such as VSMC and endothelial cell apoptosis, VSMC proliferation, hypertrophy, and migration [470]. The protective role of SOD isoforms against atherogenesis has been elucidated using genetically modified mouse models. Overexpression of SOD1 and catalase or over-expression of catalase alone decreased plasma and aortic F2-isoprostane levels and retarded atherosclerosis in apoE−/− mice [471]. We showed that increased mitochondrial oxidant generation, resulting from SOD2 deficiency, induced mitochondrial DNA damage and accelerated atherosclerosis in young apoE−/− mice [4]. Interestingly, mitochondrial DNA damage not only correlated with the prevalence of aortic atherosclerosis in humans and apoE−/−mice, but also preceded atherogenesis in young apoE−/− mice. We also demonstrated that SOD1 and SOD2 deficiency results in SMC hyperplasia and hypertrophy, albeit through preferential activation of extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinases in SOD1+/− SMCs and the Janus kinase/signal transducer and activator of transcriptase pathway in SOD2+/− SMCs [472]. It was shown that recombinant SOD3 decreases LDL oxidation by endothelial cells [473,474]. Supporting the role of SOD3 in atheroprotection, Wang et al. [475] reported that low levels of plasma SOD3 are independently associated with a history of myocardial infarction in patients with angiographically documented CAD.
Further highlighting the importance of antioxidant defenses in atherosclerosis, Torzewski et al. [476] reported increased aortic ROS levels, decreased •NO levels, and increased atherosclerosis in apoE−/− mice that were deficient in GPx-1. Congruent with this, overexpression of GPx-4 reduced aortic F2-isoprostane levels and decreased atherosclerosis and delayed lesion progression in apoE−/−mice [477]. Human studies also indicate that GPx-1 is important in protection against atherosclerosis. For instance, GPx-1 activity is either decreased or absent in human carotid atherosclerotic lesions and its absence is associated with lesion severity [478]. In a prospective study of 636 patients with CAD, low GPx-1 activity in erythrocytes was associated with an increased risk of cardiovascular events independent of traditional risk factors or atherosclerosis [479]. In fact, a meta-analysis of 42 case-control and 3 prospective studies revealed that higher activities of circulating GPx, SOD, and catalase confer protection against CHD [480].
Paraoxonase, which exists in three isoforms (PON1, PON2, and PON3), is another antioxidant enzyme that has atheroprotective effects. PON1 is associated with HDL and was shown to block LDL as well as HDL oxidation in vitro ([481,482]. Congruent with this, PON1-knockout mice had higher levels of oxidized phospholipids compared with wild-type mice, and HDL from these mice was unable to block LDL oxidation in vitro [483]. Furthermore, PON1 deficiency increased aortic atherosclerosis in wild-type and apoE−/−mice [483,484]. PON2 was shown to attenuate macrophage triglyceride accumulation and foam cell formation via the inhibition of redox-sensitive microsomal diacylglycerol acyltransferase 1 [485]. Devarajan et al. [486] reported that PON2-deficient apoE−/− mice develop enhanced mitochondrial oxidative stress and exacerbated atherosclerosis on both chow and Western diet. An increase in atheroprotection was attributed to decreased apoptosis of vascular wall cells and macrophages, resulting from the interaction of PON2 with mitochondrial coenzyme Q10. Correspondingly, PON3-transgenic mice had significantly smaller atherosclerotic lesions on both B6 and apoE−/− backgrounds when fed an atherogenic diet [487]. Likewise, adenoviral overexpression of PON3 not only significantly decreased the atherosclerotic lesion area but also lowered the serum lipid hydroperoxide levels and enhanced the ability of cholesterol-loaded macrophages to efflux cholesterol [488]. Highlighting the clinical relevance of PON1 against systemic oxidative stress and atherosclerosis, Bhattacharyya et al. [489] reported that patients in the highest PON1 activity quartile had a lower incidence of cardiac events compared to those in the lowest quartile. Together, cell culture data, experimental mouse models (Table 2), and human studies support a contributory role for oxidative stress and redox-sensitive signaling in atherogenesis.
Hypertension
In 1991, Nakazono et al. [490] indicated a role for ROS in the etiology of hypertension when they observed a significant decrease in the blood pressure of spontaneously hypertensive rats, an animal model of hypertension, after the administration of heparin-bound SOD1. Increased oxidative stress is also associated with many other experimental models of hypertension, including spontaneously hypertensive stroke-prone rats and surgically induced, hormone-induced, and diet-induced hypertension [491]. In addition to the vasculature, the mechanisms of ROS-induced hypertension involve other organ systems such as the heart, kidneys, and central nervous system.
Supporting the role of NADPH oxidase in hypertension, an increase in the activity of this enzyme was observed in VSMCs and endothelial cells upon stimulation with AngII [492,493], as well as in various animal models of hypertension, such as those induced by AngII [494,495] and deoxycorticosterone acetate (DOCA-salt) [496]; in renovascular hypertension [497]; and in spontaneously hypertensive rats [498]. The causative role of various Nox homologs or Nox subunits in hypertension is elucidated using genetically altered mice. Genetic deletion of Nox1 in mice resulted in the loss of sustained blood pressure increase induced by AngII infusion [499,500], and L-NAME abolished the effect of Nox1 deletion on the pressor response to AngII. However, endothelium-dependent vascular relaxation was preserved in AngII-infused Nox1y/- aortas [499], suggesting that Nox1-derived ROS are important in hypertension. Supporting these data, overexpression of Nox1 in VSMCs increased medial O2•−production in response to AngII infusion, which resulted in eNOS uncoupling, decreased •NO bioavailability, impaired vasorelaxation, and an increase in systolic blood pressure [431,500].
Nox2 expression is increased in several organ systems in hypertension and particularly in resistance arteries, the site of blood pressure control [270]. Pagano’s group [501] reported that infusion of Nox2ds-tat, a competitive inhibitor of the interaction of Nox2 with p47phox, significantly decreased AngII-induced increase in ROS production and systolic blood pressure, supporting the role of Nox2-derived oxidative stress in hypertension. A decreased hypertensive response to AngII infusion in p47phox-deficient mice [502] and an increased response in mice with SMC-specific overexpression of p22phox [503] were also indicative of the role of Nox2 activation in hypertension, although these subunits also affect the activity of other Nox homologs. Increased Nox2 activity also contributes to renovascular hypertension by decreasing •NO bioavailability [504]. Zimmerman et al. [505] reported that hypertensive response to chronic systemic infusion of AngII is correlated with a marked increase in O2•− production in the subfornical organ of the brain, a region lying outside the blood-brain barrier (BBB). Studies using adenoviral vectors expressing small interfering RNA demonstrated that both Nox2 and Nox4 enzymes are required for the full vasopressor effects of AngII in this region [506]. Nox4 in the kidney may also mediate hypertension or hypertension-induced renal injury as it is upregulated in several animal models of hypertension [442]. BH4 supplementation decreased vascular ROS production, increased •NO bioavailability, and attenuated DOCA-salt-induced hypertension, indicating that eNOS uncoupling could result in hypertension [496]. Recent evidence indicates a cross talk between Nox and mitochondrial sources of ROS, and administration of a mitochondria-targeted antioxidant, mitoTEMPO, attenuated AngII and DOCA-salt-induced hypertension in mice [7,507]. MitoTEMPO also decreased mitochondrial and total cellular O2•−, reduced cellular NADPH oxidase activity, and restored bioavailability of •NO. Overexpression of SOD2 attenuated AngII-induced hypertension and vascular oxidative stress, further supporting the role of mitochondrial oxidative stress in hypertension [7]. Together, the various experimental animal models of hypertension support a causal role for oxidative stress in blood pressure regulation.
Cardiac hypertrophy and heart failure
Oxidative stress and redox signaling are important contributing factors for LVH, which occurs initially as an adaptive response to environmental stress to augment pump function and to reduce wall stress [508]. The heart undergoes pathologic cardiac hypertrophy in response to a long-term increase in workload, most often as a consequence of hypertension (pressure overload) or ischemic heart disease, resulting eventually in chronic heart failure [30]. Many of the molecular mechanisms of cardiac hypertrophy are redox sensitive and the transition from pathological hypertrophy to heart failure involves a decrease in contractility, ventricular remodeling and dilatation, myocardial fibrosis, and myocyte apoptosis.
Low-amplitude cyclic stretch induces hypertrophy in isolated cardiomyocytes via increased ROS production and activation of ERK1/2, whereas high amplitude causes apoptosis via activation of JNK, indicating how mechanical strain contributes to cardiac hypertrophy and heart failure [509]. Many stimuli of pathological cardiac hypertrophy act by phosphorylating class II histone dea-cetylases (HDACs), master negative regulators of cardiac hypertrophy [510] (Fig. 2). Phosphorylated class II HDACs dissociate from transcription factors such as MEF-2, NFAT, and CAMTA2, which then promote hypertrophy, whereas dissociated HDACs translocate from the nucleus to the cytoplasm. However, Sadoshima’s group [511] has shown that thioredoxin 1-sensitive oxidative modification of class II HDACs is a potent mechanism for their translocation from the nucleus and induction of hypertrophy. Thioredoxin 1 forms a multiprotein complex with HDAC4 and DnaJb5, a heat shock protein, in cardiomyocytes. Treatment of cardiomyocytes with ROS-generating G-protein-coupled receptor agonists such as phenylephrine stimulates oxidation of cysteine residues in DnaJb5 (Cys274, Cys276) and HDAC4 (Cys667, Cys 669), forming intramolecular disulfide bonds that enable HDAC4 to translocate to cytoplasm, resulting in hypertrophy. Thioredoxin regulates the nucleocytoplasmic shuttling of HDAC4, independent of its phos-phorylation status, by reducing Cys667 and Cys669, which inhibits hypertrophy [511,512]. Further supporting the role of ROS in hypertrophy, antioxidant treatment inhibited pressure overload-induced LVH in mice [513] and prevented the transition from compensatory hypertrophy to heart failure in guinea pigs [514]. Congruent with this, overexpression of antioxidant enzymes GPx and heme oxygenase-1 protected against pathologic left-ventricular remodeling and dysfunction in mice [515,516]. Furthermore, myocardial dysfunction and the severity of heart failure in patients are correlated with increased oxidative stress [30].
Fig. 2.
Redox signaling pathways regulate cardiac hypertrophy. Nox2 NADPH oxidase stimulation in response to G-protein-coupled receptor agonists or mechanical stretch activates the Ask1–NF-κB pathway, inducing cardiac hypertrophy. HDAC4 suppresses the activity of prohypertrophic transcription factors. Phosphorylation or oxidation of HDAC4 during oxidative stress conditions results in its export to the cytosol, leading to hypertrophy [511]. Abbreviations used: AngII, angiotensin II; Ask1, apoptosis signaling kinase 1; CaMKII, calcium/calmodulin-dependent kinase II; CAMTA2, calmodulin-binding transcription activator 2; CRM1, chromosomal region maintenance-1; Dnajb5, DnaJ homolog subfamily B member 5; GPCR, G-protein-coupled receptor; GRK, G-protein-coupled receptor kinase; HDAC, histone deacetylase; HDAC-P, phosphorylated HDAC; Imp, importin α; JNK, c-Jun N-terminal kinase; MEF-2, myocyte enhancer factor-2; NFAT, nuclear factor of activated T cells; NF-κB, nuclear factor-κB; ox, oxidized; red, reduced; TBP-2, thioredoxin binding protein-2; Trx1, thioredoxin 1.
Cardiac hypertrophy induced in response to chronic infusion of a subpressor dose of AngII was inhibited in Nox2y/− mice [268]. Inhibition of Nox2 enzyme activity by cardiomyocyte-specific deletion of Rac1 also decreased AngII-induced myocardial oxidative stress, activation of the redox-sensitive Ask1-NF-κB signaling pathway, and cardiac hypertrophy, further supporting the role of this enzyme in LVH [517] (Fig. 2). Nox2 enzyme activity and expression are increased in pressure overload-induced cardiac hypertrophy [518–520] as well as in the myocardium of failing human hearts [5,521,522]. However, Nox2 is not essential for pressure overload-induced LVH although Nox2y/− mice had less cardiac interstitial fibrosis and less contractile dysfunction compared with wild-type mice when subjected to chronic pressure overload [523]. Cardiac hypertrophy, cardiomyocyte apoptosis, and interstitial fibrosis were substantially reduced in p47phox−/− mice after myocardial infarction, indicating that Nox2 activation is involved in cardiac remodeling after myocardial infarction [524].
Nox4 expression is enhanced in cardiomyocytes treated with hypertrophic stimulants such as AngII and phenylephrine and in response to pressure overload [525,526]. Mice with cardiac-specific deletion of Nox4 had less mitochondrial O2•−, interstitial fibrosis, and cardiomyocyte apoptosis along with attenuated cardiac hypertrophy and better cardiac function in response to cardiac pressure overload compared with the wild type [527]. Correspondingly, cardiac-specific overexpression of Nox4 exacerbated cardiac dysfunction, fibrosis, and apoptosis in response to pressure overload, but did not cause cardiac hypertrophy. Also, Nox4 overexpression in vitro in cardiomyocytes induced apoptotic cell death but not hypertrophy [525], which suggests that the primary effect of an increase in Nox4 expression in a failing heart is cardiomyocyte apoptosis rather than hypertrophy. However, Shah’s group [526] reported exaggerated contractile dysfunction, hypertrophy, and cardiac dilatation during exposure to chronic pressure overload in global Nox4-knockout mice, whereas Nox4-transgenic mice with cardiomyocyte-specific overexpression were protected. The protective effect of Nox4 overexpression was attributed to preservation of myocardial capillary density during pressure overload. This view is supported by the recent findings of Brandes and colleagues [528] that ischemia-induced angiogenesis was impaired in Nox4−/− mice in a femoral artery ligation model. The reason for the discrepancy in the reported Nox4 function is unclear but may be related to the total as well as cell-type-specific concentration of H2O2 and the differences in vasoactive effectors produced in various model systems.
It has been suggested that ROS generation by XO contributes to impaired myocardial energy metabolism in heart failure [290,291]. Evidence for this was obtained using transgenic mice containing a troponin I truncation, a model of progressive dilated cardiomyo-pathy in which chronic XO inhibition with allopurinol delayed heart failure progression by preventing myofibrillar protein oxidation and improving cardiac muscle force generation [529]. The improvement in contractile function with the suppression of XO activity was corroborated in mice with coronary artery ligation, using echocardiography and MRI [530,531]. In a rather small study of nine patients with idiopathic dilated cardiomyopathy, allopur-inol significantly improved myocardial efficiency [291]. However, in a prospective OPT-CHF study (the efficacy and safety study of oxypurinol in patients with symptomatic heart failure), oxypur-inol, the active metabolite of allopurinol, failed to show improvement in the primary composite end point of heart failure clinical status and mortality [532].
Ischemia–reperfusion injury
The levels of ROS generation during ischemia are low, but cardiac ischemia–reperfusion injury occurs upon the restoration of blood flow to the ischemic myocardium, resulting from a large burst of ROS generation [191]. ROS generated during early reperfu-sion cause extensive damage to cardiomyocytes, resulting in the loss of cell viability. It was shown that heterozygous deficiency of GPx1 impaired myocardial recovery after ischemia–reperfusion injury [533]. Global knockdown of GPx1 in mice resulted in impaired cardiac recovery after ischemia–reperfusion injury [534]. GPx-1−/− mice had increased mitochondrial ROS production, increased oxidative mitochondrial DNA damage, and decreased expression of mitochondrial proteins including complex I, resulting in a decrease in NADH and ATP generation [534]. These results suggest that restoring homeostatic redox signaling and cardiac energy bioavailability is important for recovery from ischemia– reperfusion injury [535]. Thioredoxin 1 (Trx1; cytosolic isoform) and Trx2 (mitochondrial isoform) are key regulators of the cellular redox state, whose function is regulated by thioredoxin-interacting protein, an endogenous inhibitor. Yoshioka et al. [536] recently demonstrated improved left-ventricular function after ischemia–reperfusion injury in global as well as cardiomyocyte-specific thioredoxin-interacting protein-knockout mice compared with the wild type (Fig. 3). Protection against injury in these knockout mice was associated with a significant increase in Trx2 activity and decrease in myocardial ROS production. Furthermore, thioredoxin-interacting protein deficiency repressed mitochondrial respiration and enhanced anaerobic glycolysis, increasing cellular ATP levels.
Fig. 3.
Thioredoxin-interacting protein (Txnip) regulates cardiac ischemia–reperfusion injury. In wild-type mice, Txnip translocates to mitochondria during myocardial ischemia–reperfusion and induces mitochondrial dysfunction by inhibiting thioredoxin 2 (Trx2) activity and increasing ROS levels. Txnip deficiency protects against cardiac ischemia–reperfusion injury by allowing efficient scavenging of mitochondrial ROS by Trx2 and maintaining energy homeostasis through enhanced anaerobic metabolism [536].
HIF confers protection during and/or after ischemia via activation of downstream effector genes that increase glycolytic capacity, antioxidant defense, angiogenesis, and cell survival signaling [199] (Fig. 1). Cardiomyocyte-specific deletion of HIF1α in mice decreased contractility, vascularization, and high-energy phosphate content via altered gene expression, supporting a central role for HIF1α in coordinating energy availability and utilization in the heart [537]. As a corollary to this, cardiomyocyte-specific overexpression of HIF1α in mice attenuated infarct size and improved cardiac function 4 weeks after myocardial infarction, in association with an increase in capillary density as well as vascular endothelial growth factor and iNOS expression in the peri-infarct regions [538]. Similarly, activation of HIF1a by silencing HIF1a–prolyl 4-hydroxylase in the heart using small interfering RNA significantly increased iNOS mRNA expression and decreased infarct size and cardiac dysfunction after ischemia-reperfusion [539]. Furthermore, this protection was lost in iNOS−/− mice, indicating the critical role played by iNOS-dependent pathways against reperfusion injury. Highlighting the importance of •NO in protection against ischemia-reperfusion injury, Sun et al. [540] showed that ischemic preconditioning of rat hearts causes nitrosylation of proteins involved in Ca2+ handling and energetics (Fig. 4). For example, ischemic preconditioning causes S-nitrosylation and a decrease in the activity of the mitochondrial F1-ATPase, reducing the rate of decline in ATP levels. Further supporting the role of S-nitrosylation in protection against ischemia, perfusion of hearts with S-nitrosoglutathione improved left-ventricular function.
Fig. 4.
Preconditioning protects against myocardial ischemia–reperfusion injury by the S-nitrosylation of proteins regulating intracellular Ca2+ levels and mitochondrial energetics [adapted from 540]. Abbreviation used: SERCA, sarcoplasmic/endoplasmic reticulum Ca2+-ATPase.
ROS generation and redox signaling during the early preconditioning period may be involved in the eventual cardioprotective effect afforded by some ischemic preconditioning stimuli [189,199]. Diazoxide-induced cardioprotection against ischemia-reperfusion injury is mediated by ROS-induced activation of mitochondrial ATP-sensitive potassium channel, and antioxidants block the protection afforded by diazoxide as well as ischemic preconditioning [190,541]. The ROS generated during early preconditioning can be from mitochondria (slightly swollen with increased respiration) [542] or from Nox2 [543] and may act via stimulation of PKC.
Cerebral ischemia resulting from the occlusion of vessels in the brain tissue in the vast majority of strokes causes brain damage. Recanalization of the occluded vessels and restoration of the blood flow is the most effective treatment for the stroke. As in the heart, reperfusion results in increased ROS generation and oxidative stress, which significantly limit the benefits of the stroke therapies [544,545]. Intrinsic high metabolic activity, very high concentrations of the neurotransmitter-excitotoxin glutamate, and limited antioxidant defense mechanisms make the brain very susceptible to oxidative damage [546–548].
During reperfusion in the brain microvasculature, ROS break down the BBB, consisting of endothelial cells, pericytes, the surrounding basement membrane, and attached astrocyte endfeet, which can result in cerebral edema and/or brain hemorrhage, neurovascular injury, and neuronal death [544,549]. More recently, it was posited that the cell-cell and cell-matrix interaction between the BBB and neuronal and glial cells constituting the “neurovascular unit” integrates the brain function in response to various insults, including ischemia-reperfusion injury [548]. For example, sublethal levels of oxidative stress downregulate the production of cerebral endothelium-produced brain-derived neurotrophic factor, indicating the importance of cerebral endothelium as a critical source of homeostatic support for neurons and cell-cell interaction in coordinating protection against ischemia-reperfusion injury.
Mitochondria-derived ROS generation has hitherto been considered the major source of oxidative stress in the ischemia-reperfusion-induced BBB opening. Mitochondria are abundant in the brain [550] and dephosphorylation of oxidative phosphorylation complexes induced by ischemia causes hyperactive electron transport and hyperpolarization of mitochondrial membrane potential upon reperfusion, resulting in excess ROS production [551]. However, recent data suggest that Nox2 activity plays a dominant role in O2•− generation during N-methyl-D-aspartate receptor stimulation in ischemia in neurovascular cells and is the molecular mechanism underlying excitotoxicity-induced neuronal death [552,553]. Further affirming the important role of Nox2 in excitotoxicity, ROS generation and cell death were blocked in neurons lacking p47phox or with Nox inhibition [553]. Interestingly, Nox2 expression and activity observed in the brain after ischemia were markedly decreased in SOD1-transgenic mice, whereas they were significantly increased in SOD1−/− mice, indicating that Nox expression is redox sensitive [554].
Microglia, the resident macrophages of the brain that express Nox1 and Nox2, are activated very early in ischemia and their activation precedes macrophage infiltration [555,556]. However, Nox1 does not have any neuroprotective effect in severe cerebral ischemia [557,558]. Microglial Nox-derived O2•− enhanced astrocyte and cerebral endothelial cell death in a cell culture model of ischemia, whereas inhibition of microglia activation in mice markedly reduced BBB dysfunction and infarct volume in experimental stroke [559]. Additionally, activated astrocytes can produce large amounts of ROS and RNS via the stimulation of NADPH oxidase and iNOS [560,561], although this has not been shown under ischemia–reperfusion conditions. Infiltration of highly active Nox2-dependent NADPH oxidase-containing inflammatory cells such as neutrophils occurs for 3 days followed by that of blood-derived monocytes/macrophages, which peaks between 3 and 7 days after brain ischemia–reperfusion [555,562].
Nox4 protein is also expressed in neurons and cerebrovascular endothelial cells [558,563], with very high expression in basilar compared with systemic arteries [564]. Nox4 expression in mouse neurons is increased within 24 h after ischemia, peaks between days 7 and 15, and declines but remains high at day 30 [563]. Nox4 expression is also induced in the neurons and cerebrovascular endothelial cells of stroke patients [558]. Interestingly, Nox4 deletion had an overwhelmingly protective effect against cerebral infarction, BBB leakage, and neuronal apoptosis in mice subjected to both transient and permanent cerebral ischemia [558]. More recently, Hara and colleagues [565] have shown that Nox4 associates with Toll-like receptor 4 (TLR4; an essential component of innate immunity) in the brain tissue of mice and humans after ischemia–reperfusion. Deletion or pharmacological inhibition of TLR4 reduced Nox4 expression and suppressed ROS/RNS generation and neuronal apoptosis, indicating that activation of the TLR4–Nox4 signaling pathway is a potential pathophysiological mechanism in ischemic injury.
Conclusions and future perspectives
In conclusion, evidence from experimental and animal studies supports a decisive role for redox signaling in cardiovascular home-ostasis and disease. As mentioned earlier, translation of this knowledge into human therapy for CVD has not been particularly successful as demonstrated by the disappointing data from large antioxidant clinical trials [20,566]. Evolving consensus suggests that targeting the source of ROS using specific inhibitors might be more effective in treating CVD than the use of antioxidants [567]. The NADPH oxidases are perhaps the best example of this strategy, as direct inhibitors of various Nox catalytic subunits are in development [568]. The complexity of the regulation of these enzymes may also lend itself to therapeutic manipulation in a cell- and tissue-specific manner, with fewer off-target effects. Other potential means to modulate redox signaling and treat CVD include targeting uncoupled NOS and mitochondrial ROS and augmenting endogenous antiox-idant gene regulators such as Nrf2 and antioxidant systems such as thioredoxin and peroxiredoxins [569]. Because of the intricate nature of redox biology and the need to target ROS in specific organ systems, advances in the clinical management of CVD may depend on progress in other fields such as gene therapy, systems biology, and nanotechnology. A good example in this regard is the recent successful and safe completion of SERCA2 (a protein inactivated by high levels of ROS) gene therapy for the treatment of heart failure [570]. Advances in nanotechnology may help in the detection of activated/dysfunctional endothelium by imaging the inflammatory markers [571–573]. Furthermore, nanotechnology-based drug delivery mechanisms targeted to specific cell types/organs (cardiomyo-cytes/heart) and organelles (mitochondria) may help transform the treatment of CVD and other diseases of oxidative stress.
Acknowledgment
This work was supported by National Institutes of Health Grants HL111664, AG024282, and TR000083.
Abbreviations
- AngII
angiotensin II
- AP-1
activator protein-1
- apoA-1
apolipoprotein A-1
- Ask1
apoptosis signaling kinase-1
- CAD
coronary artery disease
- CAMTA
calmodulin-binding transcription activator
- CHD
coronary heart disease
- CVD
cardiovascular disease
- CHF
congestive heart failure
- EDHF
endothelium-derived hyperpolarizing factor
- EGF
epidermal growth factor
- eNOS
endothelial nitric oxide synthase
- ETC
electron transport chain
- iNOS
inducible nitric oxide synthase
- GPx
glutathione peroxidase
- HDAC
histone deacetylase
- HDL
high-density lipoprotein
- HIF
hypoxia-inducible factor
- JNK
c-Jun N-terminal kinase
- Keap1
Kelch-like ECH-associated protein 1
- L-NAME
N G-nitro-l-arginine methyl ester
- LDL
low-density lipoprotein
- LOX
lipoxygenase
- LVH
left-ventricular hypertrophy
- MPO
myeloperoxidase
- NF-κB
nuclear factor-κB
- Nox
NADPH oxidase
- NOS
nitric oxide synthase
- nNOS
neuronal nitric oxide synthase
- Nrf2
nuclear factor E2-related factor 2
- PDGF
platelet-derived growth factor
- PKC
protein kinase C
- PKG
protein kinase G
- PON
paraoxonase
- PTP
protein tyrosine phosphatase
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- SHP2
Src homology phosphatase 2
- VCAM
vascular cell adhesion molecule
- VSMC
vascular smooth muscle cell
- XO
xanthine oxidase
References
- 1.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Murphy MP. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barry-Lane PA, Patterson C, van der Merwe M, Hu Z, Holland SM, Yeh ET, Runge MS. p47phox is required for atherosclerotic lesion progression in ApoE(−/−) mice. J. Clin. Invest. 2001;108:1513–1522. doi: 10.1172/JCI11927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballinger SW, Patterson C, Knight-Lozano CA, Burow DL, Conklin CA, Hu Z, Reuf J, Horaist C, Lebovitz R, Hunter GC, McIntyre K, Runge MS. Mitochondrial integrity and function in atherogenesis. Circulation. 2002;106:544–549. doi: 10.1161/01.cir.0000023921.93743.89. [DOI] [PubMed] [Google Scholar]
- 5.Heymes C, Bendall JK, Ratajczak P, Cave AC, Samuel JL, Hasenfuss G, Shah AM. Increased myocardial NADPH oxidase activity in human heart failure. J. Am. Coll. Cardiol. 2003;41 doi: 10.1016/s0735-1097(03)00471-6. [DOI] [PubMed] [Google Scholar]
- 6.Dai DF, Johnson SC, Villarin JJ, Chin MT, Nieves-Cintrón M, Chen T, Marcinek DJ, Dorn GW, 2nd, Kang YJ, Prolla TA, Santana LF, Rabinovitch PS. Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and Galphaq overexpression-induced heart failure. Circ. Res. 2011;108:837–846. doi: 10.1161/CIRCRESAHA.110.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, Dikalov SI. Therapeutic targeting of mitochon-drial superoxide in hypertension. Circ. Res. 2010;107:106–116. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hidaka T, Nakagawa K, Goto C, Soga J, Fujii Y, Hata T, Idei N, Fujimura N, Chayama K, Kihara Y, Higashi Y. Pioglitazone improves endothelium-dependent vasodilation in hypertensive patients with impaired glucose tolerance in part through a decrease in oxidative stress. Atherosclerosis. 2010;210:521–524. doi: 10.1016/j.atherosclerosis.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 9.Minuz P, Patrignani P, Gaino S, Degan M, Menapace L, Tommasoli R, Seta F, Capone ML, Tacconelli S, Palatresi S, Bencini C, Del Vecchio C, Mansueto G, Arosio E, Santonastaso CL, Lechi A, Morganti A, Patrono C. Increased oxidative stress and platelet activation in patients with hypertension and renovascular disease> . Circulation. 2002;106:2800–2805. doi: 10.1161/01.cir.0000039528.49161.e9. [DOI] [PubMed] [Google Scholar]
- 10.Sorescu D, Weiss D, Lassègue B, Clempus RE, Szöcs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor WR, Griendling KK. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation. 2002;105:1429–1435. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- 11.Niu XL, Madamanchi NR, Vendrov AE, Tchivilev I, Rojas M, Madamanchi C, Brandes RP, Krause KH, Humphries J, Smith A, Burnand KG, Runge MS. Nox activator 1: a potential target for modulation of vascular reactive oxygen species in atherosclerotic arteries. Circulation. 2010;121:549–559. doi: 10.1161/CIRCULATIONAHA.109.908319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMurray J, Chopra M, Abdullah I, Smith WE, Dargie HJ. Evidence of oxidative stress in chronic heart failure in humans. Eur. Heart J. 1993;14:1493–1498. doi: 10.1093/eurheartj/14.11.1493. [DOI] [PubMed] [Google Scholar]
- 13.Keaney JF, Jr, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ. Framingham Study. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler. Thromb. Vasc. Biol. 2003;23:434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 14.Schwedhelm E, Bartling A, Lenzen H, Tsikas D, Maas R, Brümmer J, Gutzki FM, Berger J, Frölich JC, Böger RH. Urinary 8-iso-prostaglandin F2alpha as a risk marker in patients with coronary heart disease: a matched case-control study. Circulation. 2004;109:843–848. doi: 10.1161/01.CIR.0000116761.93647.30. [DOI] [PubMed] [Google Scholar]
- 15.Wu T, Rifai N, Willett WC, Rimm EB. Plasma fluorescent oxidation products: independent predictors of coronary heart disease in men. Am. J. Epidemiol. 2007;166:544–551. doi: 10.1093/aje/kwm120. [DOI] [PubMed] [Google Scholar]
- 16.Karakas M, Koenig W, Zierer A, Herder C, Rottbauer W, Baumert J, Meisinger C, Thorand B. Myeloperoxidase is associated with incident coronary heart disease independently of traditional risk factors: results from the MONICA/KORA Augsburg Study. J. Intern. Med. 2012;271:43–50. doi: 10.1111/j.1365-2796.2011.02397.x. [DOI] [PubMed] [Google Scholar]
- 17.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P, Vitamin E. supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N. Engl. J. Med. 2000;342:154–160. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 18.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Vitamin E in the primary prevention of cardiovascular disease and cancer. The Women's Health Study: a randomized controlled trial . JAMA. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 19.Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Glynn RJ, Gaziano JM. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians' Health Study II randomized controlled trial. JAMA. 2008;300:2123–2133. doi: 10.1001/jama.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet. 2003;361:2017–2023. doi: 10.1016/S0140-6736(03)13637-9. [DOI] [PubMed] [Google Scholar]
- 21.Schraufstatter IU, Browne K, Harris A, Hyslop PA, Jackson JH, Quehenberger O, Cochrane CG. Mechanisms of hypochlorite injury of target cells. J. Clin. Invest. 1990;85:554–562. doi: 10.1172/JCI114472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foote CS, Goyne TE, Lehrer RI. Assessment of chlorination by human neutrophils. Nature. 1983;301:715–716. doi: 10.1038/301715a0. [DOI] [PubMed] [Google Scholar]
- 23.Herdener M, Heigold S, Saran M, Bauer G. Target cell-derived superoxide anions cause efficiency and selectivity of intercellular induction of apoptosis. Free Radic. Biol. Med. 2000;29:1260–1271. doi: 10.1016/s0891-5849(00)00422-6. [DOI] [PubMed] [Google Scholar]
- 24.Hazell LJ, Stacker R. Oxidation of low-density lipoprotein with hypochlorite causes transformation of the lipoprotein into a high-uptake form for macrophages. Biochem. J. 1993;290:165–172. doi: 10.1042/bj2900165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foster DB, Van Eyk JE, Marbán E, O'Rourke B. Redox signaling and protein phosphorylation in mitochondria: progress and prospects. J. Bioe-nerg. Biomembr. 2009;41:159–168. doi: 10.1007/s10863-009-9217-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reeves EP, Lu H, Jacobs HL, Messina CG, Bolsover S, Gabella G, Potma EO, Warley A, Roes J, Segal AW. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 2002;416:291–297. doi: 10.1038/416291a. [DOI] [PubMed] [Google Scholar]
- 27.Shao B, Oda MN, Bergt C, Fu X, Green PS, Brot N, Oram JE, Heinecke JW. Myeloperoxidase impairs ABCA1-dependent cholesterol efflux through methionine oxidation and site-specific tyrosine chlorination of apolipoprotein A-I J. Biol. Chem. 2006;281:9001–9004. doi: 10.1074/jbc.C600011200. [DOI] [PubMed] [Google Scholar]
- 28.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O'Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moskovitz J, Bar-Noy S, Williams WM, Requena J, Berlett BS, Stadtman ER. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc. Natl. Acad. Sci. USA. 2001;98:12920–12925. doi: 10.1073/pnas.231472998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cave AC, Brewer AC, Narayanapanicker A, Ray R, Grieve DJ, Walker S, Shah AM. NADPH oxidases in cardiovascular health and disease. Antioxid. Redox Signaling. 2006;8:691–728. doi: 10.1089/ars.2006.8.691. [DOI] [PubMed] [Google Scholar]
- 31.Thomas SR, Witting PK, Drummond GR. Redox control of endothelial function and dysfunction: molecular mechanisms and therapeutic opportunities. Antioxid. Redox Signaling. 2008;10:1713–1765. doi: 10.1089/ars.2008.2027. [DOI] [PubMed] [Google Scholar]
- 32.Claiborne A, Miller H, Parsonage D, Ross RP. Protein-sulfenic acid stabilization and function in enzyme catalysis and gene regulation. FASEB J. 1993;7:1483–1490. doi: 10.1096/fasebj.7.15.8262333. [DOI] [PubMed] [Google Scholar]
- 33.Denu JM, Tanner KG. Specific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide: evidence for a sulfenic acid intermediate and implications for redox regulation. Biochemistry. 1998;37:5633–5642. doi: 10.1021/bi973035t. [DOI] [PubMed] [Google Scholar]
- 34.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat. Rev. Mol. Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 35.Benhar M, Forrester MT, Hess DT, Stamler JS. Regulated protein denitrosylation by cytosolic and mitochondrial thioredoxins. Science. 2008;320:1050–1054. doi: 10.1126/science.1158265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woo HA, Jeong W, Chang TS, Park KJ, Park SJ, Yang JS, Rhee SG. Reduction of cysteine sulfinic acid by sulfiredoxin is specific to 2-Cys peroxiredoxins. J. Biol. Chem. 2005;280:3125–3128. doi: 10.1074/jbc.C400496200. [DOI] [PubMed] [Google Scholar]
- 37.Jönsson TJ, Johnson LC, Lowther WT. Structure of the sulphiredoxin-peroxiredoxin complex reveals an essential repair embrace. Nature. 2008;451:98–101. doi: 10.1038/nature06415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klomsiri C, Karplus PA, Poole LB. Cysteine-based redox switches in enzymes. Antioxid. Redox Signaling. 2011;14:1065–1077. doi: 10.1089/ars.2010.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim JC, Choi HI, Park YS, Nam HW, Woo HA, Kwon KS, Kim YS, Rhee SG, Kim K, Chae HZ. Irreversible oxidation of the active-site cysteine of peroxiredoxin to cysteine sulfonic acid for enhanced molecular chaperone activity. J. Biol. Chem. 2008;283:28873–28880. doi: 10.1074/jbc.M804087200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winterbourn CC, Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic. Biol. Med. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 41.Claiborne A, Yeh JI, Mallett TC, Luba J, Crane EJ, 3rd, Charrier V, Parsonage D. Protein-sulfenic acids: diverse roles for an unlikely player in enzyme catalysis and redox regulation. Biochemistry. 1999;38:15407–15416. doi: 10.1021/bi992025k. [DOI] [PubMed] [Google Scholar]
- 42.Rhee SG, Chae HZ, Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic. Biol. Med. 2005;38:1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 43.Monteiro G, Horta BB, Pimenta DC, Augusto O, Netto LE. Reduction of 1-Cys peroxiredoxins by ascorbate changes the thiol-specific antioxidant paradigm, revealing another function of vitamin C. Proc. Natl. Acad. Sci. USA. 2007;104:4886–891. doi: 10.1073/pnas.0700481104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shao D, Oka S, Brady CD, Haendeler J, Eaton P, Sadoshima J. Redox modification of cell signaling in the cardiovascular system. J. Mol. Cell. Cardiol. 2012;52:550–558. doi: 10.1016/j.yjmcc.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hao G, Derakhshan B, Shi L, Campagne E, Gross SS. SNOSID a proteomic method for identification of cysteine S-nitrosylation sites in complex protein mixtures. Proc. Natl. Acad. Sci. USA. 2006;103:1012–1017. doi: 10.1073/pnas.0508412103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fang M, Jaffrey SR, Sawa A, Ye K, Luo X, Snyder SH. Dexrasl: a G protein specifically coupled to neuronal nitric oxide synthase via CAPON. Neuron. 2000;28:183–193. doi: 10.1016/s0896-6273(00)00095-7. [DOI] [PubMed] [Google Scholar]
- 47.Choi YB, Tenneti L, Le DA, Ortiz J, Bai G, Chen HS, Lipton S. A Molecular basis of NMDA receptor-coupled ion channel modulation by S-nitrosylation. Nat. Neurosci. 2000;3:15–21. doi: 10.1038/71090. [DOI] [PubMed] [Google Scholar]
- 48.Nedospasov A, Rafikov R, Beda N, Nudler E. An autocatalytic mechanism of protein nitrosylation. Proc. Natl. Acad. Sci. USA. 2000;97:13543–13548. doi: 10.1073/pnas.250398197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H202 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 50.Lee SR, Kwon KS, Kim SR, Rhee SG. Reversible inactivation of protein-tyrosine phosphatase IB in A431 cells stimulated with epidermal growth factor. Biol. Chem. 1998;273:15366–15372. doi: 10.1074/jbc.273.25.15366. [DOI] [PubMed] [Google Scholar]
- 51.Bae YS, Kang SW, Seo MS, Baines IC, Tekle E, Chock PB, Rhee SG. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide: role in EGF receptor-mediated tyrosine phosphorylation. J. Biol. Chem. 1997;272:217–221. [PubMed] [Google Scholar]
- 52.Kappert K, Sparwel J, Sandin A, Seiler A, Siebolts U, Leppanen O, Rosenkranz S, Ostman A. Antioxidants relieve phosphatase inhibition and reduce PDGF signaling in cultured VSMCs and in restenosis. Arterioscler. Thromb. Vasc. Biol. 2006;26:2644–2651. doi: 10.1161/01.ATV.0000246777.30819.85. [DOI] [PubMed] [Google Scholar]
- 53.Tabet E, Schiffrin EL, Callera GE, He Y, Yao G, Ostman A, Kappert K, Tonks NK, Touyz RM. Redox-sensitive signaling by angiotensin II involves oxidative inactivation and blunted phosphorylation of protein tyrosine phosphatase SHP-2 in vascular smooth muscle cells from SHR. Circ. Res. 2008;103:149–158. doi: 10.1161/CIRCRESAHA.108.178608. [DOI] [PubMed] [Google Scholar]
- 54.Meng TC, Fukada T, Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol. Cell. 2002;9:387–399. doi: 10.1016/s1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- 55.Xu D, Rovira II, Finkel T. Oxidants painting the cysteine chapel: redox regulation of PTPs. Dev. Cell. 2002;3:251–252. doi: 10.1016/s1534-5807(02)00132-6. [DOI] [PubMed] [Google Scholar]
- 56.Tonks N. K Redox redux: revisiting PTPs and the control of cell signaling. Cell. 2005;121:667–670. doi: 10.1016/j.cell.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 57.Denu JM, Lohse DL, Vijayalakshmi J, Saper MA, Dixon JE. Visualization of intermediate transition-state structures in protein-tyrosine phosphatase catalysis. Proc. Natl. Acad. Sci. USA. 1996;93:2493–2498. doi: 10.1073/pnas.93.6.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salmeen A, Andersen JN, Myers MP, Meng TC, Hinks JA, Tonks NK, Barford D. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature. 2003;423:769–773. doi: 10.1038/nature01680. [DOI] [PubMed] [Google Scholar]
- 59.van Montfort RL, Congreve M, Tisi D, Carr R, Jhoti H. Oxidation state of the active-site cysteine in protein tyrosine phosphatase 1B. Nature. 2003;423:773–777. doi: 10.1038/nature01681. [DOI] [PubMed] [Google Scholar]
- 60.Barrett WC, DeGnore JP, König S, Fales HM, Keng YE, Zhang ZY, Yim MB, Chock PB. Regulation of PTP1B via glutathionylation of the active site cysteine 215. Biochemistry. 1999;38:6699–6705. doi: 10.1021/bi990240v. [DOI] [PubMed] [Google Scholar]
- 61.Caselli A, Marzocchini R, Camici G, Manao G, Moneti G, Pieraccini G, Ramponi G. The inactivation mechanism of low molecular weight phosphotyrosine-protein phosphatase by H202. J. Biol. Chem. 1998;273:32554–32560. doi: 10.1074/jbc.273.49.32554. [DOI] [PubMed] [Google Scholar]
- 62.Chiarugi P, Fiaschi T, Taddei ML, Talini D, Giannoni E, Raugei G, Ramponi G. Two vicinal cysteines confer a peculiar redox regulation to low molecular weight protein tyrosine phosphatase in response to platelet-derived growth factor receptor stimulation. J. Biol. Chem. 2001;276:33478–33487. doi: 10.1074/jbc.M102302200. [DOI] [PubMed] [Google Scholar]
- 63.Savitsky PA, Finkel T. Redox regulation of Cdc25C. J. Biol. Chem. 2002;277:20535–20540. doi: 10.1074/jbc.M201589200. [DOI] [PubMed] [Google Scholar]
- 64.Blanchetot C, Tertoolen LG, den Hertog J. Regulation of receptor protein-tyrosine phosphatase alpha by oxidative stress. EMBO J. 2002;21:493–503. doi: 10.1093/emboj/21.4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leslie NR, Bennett D, Lindsay YE, Stewart H, Gray A, Downes CP. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J. 2003;22:5501–5510. doi: 10.1093/emboj/cdg513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen C, Willard D, Rudolph J. Redox regulation of SH2-domain-containing protein tyrosine phosphatases by two backdoor cysteines. Biochemistry. 2009;48:1399–1409. doi: 10.1021/bi801973z. [DOI] [PubMed] [Google Scholar]
- 67.Caselli A, Chiarugi P, Camici G, Manao G, Ramponi G. In vivo inactivation of phosphotyrosine protein phosphatases by nitric oxide. FEBS Lett. 1995;374:249–252. doi: 10.1016/0014-5793(95)01120-4. [DOI] [PubMed] [Google Scholar]
- 68.Callsen D, Sandau KB, Brüne B. Nitric oxide and superoxide inhibit platelet-derived growth factor receptor phosphotyrosine phosphatases. Free Radic. Biol. Med. 1999;26:1544–1553. doi: 10.1016/s0891-5849(99)00015-5. [DOI] [PubMed] [Google Scholar]
- 69.Hsu ME, Meng TC. Enhancement of insulin responsiveness by nitric oxide-mediated inactivation of protein-tyrosine phosphatases. J. Biol. Chem. 2010;285:7919–7928. doi: 10.1074/jbc.M109.057513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen YY, Chu HM, Pan KT, Teng CH, Wang DL, Wang AH, Khoo KH, Meng TC. Cysteine S-nitrosylation protects protein-tyrosine phosphatase 1B against oxidation-induced permanent inactivation. J. Biol. Chem. 2008;283:35265–35272. doi: 10.1074/jbc.M805287200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chiarugi P. PTPs versus PTKs: the redox side of the coin. Free Radic. Res. 2005;39:353–364. doi: 10.1080/10715760400027987. [DOI] [PubMed] [Google Scholar]
- 72.Schmid E, El Benna J, Gaiter D, Klein G, Dröge W. Redox priming of the insulin receptor beta-chain associated with altered tyrosine kinase activity and insulin responsiveness in the absence of tyrosine autophosphorylation. FASEB J. 1998;12:863–870. doi: 10.1096/fasebj.12.10.863. [DOI] [PubMed] [Google Scholar]
- 73.Schmid E, Hotz-Wagenblatt A, Hacj V, Dröge W. Phosphorylation of the insulin receptor kinase by phosphocreatine in combination with hydrogen peroxide: the structural basis of redox priming. FASEB J. 1999;13:1491–1500. doi: 10.1096/fasebj.13.12.1491. [DOI] [PubMed] [Google Scholar]
- 74.Knock GA, Ward JPT. Redox regulation of protein kinases as a modulator of vascular function. Antioxid. Redox Signaling. 2011;15:1531–1547. doi: 10.1089/ars.2010.3614. [DOI] [PubMed] [Google Scholar]
- 75.Sato H, Sato M, Kanai H, Uchiyama T, Iso T, Ohyama Y, Sakamoto H, Tamura J, Nagai R, Kurabayashi M. Mitochondrial reactive oxygen species and c-Src play a critical role in hypoxic response in vascular smooth muscle cells. Cardiovasc. Res. 2005;67:714–722. doi: 10.1016/j.cardiores.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 76.Cheng JJ, Chao YJ, Wang D. L Cyclic strain activates redox-sensitive proline-rich tyrosine kinase 2 (PYK2) in endothelial cells. J. Biol. Chem. 2002;277:48152–8157. doi: 10.1074/jbc.M110937200. [DOI] [PubMed] [Google Scholar]
- 77.Giannoni E, Buricchi F, Raugei G, Ramponi G, Chiarugi P. Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Mol. Cell. Biol. 2005;25:6391–6403. doi: 10.1128/MCB.25.15.6391-6403.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Akhand AA, Pu M, Senga T, Kato M, Suzuki H, Miyata T, Hamaguchi M, Nakashima I. Nitric oxide controls src kinase activity through a sulfhydryl group modification-mediated Tyr-527-independent and Tyr-416-linked mechanism. J. Biol. Chem. 1999;274:25821–25826. doi: 10.1074/jbc.274.36.25821. [DOI] [PubMed] [Google Scholar]
- 79.Knapp LT, Klann E. Superoxide-induced stimulation of protein kinase C via thiol modification and modulation of zinc content. J. Biol. Chem. 2000;275:24136–24145. doi: 10.1074/jbc.M002043200. [DOI] [PubMed] [Google Scholar]
- 80.Gopalakrishna R, Jaken S. Protein kinase C signaling and oxidative stress. Free Radio. Biol. Med. 2000;28:1349–1361. doi: 10.1016/s0891-5849(00)00221-5. [DOI] [PubMed] [Google Scholar]
- 81.Gopalakrishna R, Anderson WB. Ca2+- phospholipid-independent activation of protein kinase C by selective oxidative modification of the regulatory domain. Proc. Natl. Acad. Sci. USA. 1989;86:6758–6762. doi: 10.1073/pnas.86.17.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gopalakrishna R, Gundimeda U, Schiffman JE, McNeill TH. A direct redox regulation of protein kinase C isoenzymes mediates oxidant-induced neuritogenesis in PC12 cells. J. Biol. Chem. 2008;283:14430–14444. doi: 10.1074/jbc.M801519200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cosentino E, Eto M, De Paolis P, van der Loo B, Bachschmid M, Ullrich V, Kouroedov A, Delli Gatti C, Joch H, Volpe M, Lüscher T. E High glucose causes upregulation of cyclooxygenase-2 and alters prostanoid profile in human endothelial cells: role of protein kinase C and reactive oxygen species. Circulation. 2003;107:1017–1023. doi: 10.1161/01.cir.0000051367.92927.07. [DOI] [PubMed] [Google Scholar]
- 84.Abdala-Valencia H, Cook-Mills JM. VCAM-1 signals activate endothelial cell protein kinase C± via oxidation. J. Immunol. 2006;177:6379–6387. doi: 10.4049/jimmunol.177.9.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brennan JP, Bardswell SC, Burgoyne JR, Fuller W, Schroder E, Wait R, Begum S, Kentish JC, Eaton P. Oxidant-induced activation of type 1 protein kinase A is mediated by RI subunit interprotein disulfide bond formation. J. Biol. Chem. 2006;281:21827–21836. doi: 10.1074/jbc.M603952200. [DOI] [PubMed] [Google Scholar]
- 86.Burgoyne JR, Madhani M, Cuello E, Charles RL, Brennan JP, Schroder E, Browning DD, Eaton P. Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science. 2007;317:1393–1397. doi: 10.1126/science.1144318. [DOI] [PubMed] [Google Scholar]
- 87.Yasukawa T, Tokunaga E, Ota H, Sugita H, Martyn JA, Kaneki M. S-nitrosylation-dependent inactivation of Akt/protein kinase B in insulin resistance. J. Biol. Chem. 2005;280:7511–7518. doi: 10.1074/jbc.M411871200. [DOI] [PubMed] [Google Scholar]
- 88.Park HS, Huh SH, Kim MS, Lee SH, Choi EJ. Nitric oxide negatively regulates c-Jun N-terminal kinase/stress-activated protein kinase by means of S-nitrosylation. Proc. Natl. Acad. Sci USA. 2000;97:14382–14387. doi: 10.1073/pnas.97.26.14382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lander HM, Milbank AJ, Tauras JM, Hajjar DP, Hempstead BL, Schwartz GD, Kraemer RT, Mirza UA, Chait BT, Burk SC, Quilliam LA. Redox regulation of cell signalling. Nature. 1996;381:380–381. doi: 10.1038/381380a0. [DOI] [PubMed] [Google Scholar]
- 90.Adachi T, Pimentel DR, Heibeck T, Hou X, Lee YJ, Jiang B, Ido Y, Cohen RA. S-glutathiolation of Ras mediates redox-sensitive signaling by angiotensin II in vascular smooth muscle cells. J. Biol. Chem. 2004;279:29857–29862. doi: 10.1074/jbc.M313320200. [DOI] [PubMed] [Google Scholar]
- 91.Adachi T, Wittchen ES, Campbell SL, Burridge K. Direct activation of RhoA by reactive oxygen species requires a redox-sensitive motif. PLoS One. 2009:e8045. doi: 10.1371/journal.pone.0008045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cheng X, Siow RC, Mann GE. Impaired redox signaling and antioxidant gene expression in endothelial cells in diabetes: a role for mitochondria and the nuclear factor-E2-related factor 2-Kelch-like ECH-associated protein 1 defense pathway. Antioxid. Redox Signaling. 2011;14:469–187. doi: 10.1089/ars.2010.3283. [DOI] [PubMed] [Google Scholar]
- 93.Gordon JW, Shaw J, Kirshenbaum LA. A Multiple facets of NF-κB in the heart: to be or not to NF-κB. Circ. Res. 2011;108:1122–1132. doi: 10.1161/CIRCRESAHA.110.226928. [DOI] [PubMed] [Google Scholar]
- 94.Traenckner EB, Pahl HL, Henkel T, Schmidt KN, Wilk S, Baeuerle PA. Phosphorylation of human I kappa B-alpha on serines 32 and 36 controls I kappa B-alpha proteolysis and NF-kappa B activation in response to diverse stimuli. EMBO J. 1995;14:2876–2883. doi: 10.1002/j.1460-2075.1995.tb07287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 96.Matthews JR, Wakasugi N, Virelizier JL, Yodoi J, Hay RT. Thioredoxin regulates the DNA binding activity of NF-kappa B by reduction of a disulphide bond involving cysteine 62. Nucleic Acids Res. 1992;20:3821–3830. doi: 10.1093/nar/20.15.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hirota K, Murata M, Sachi Y, Nakamura H, Takeuchi J, Mori K, Yodoi J. Distinct roles of thioredoxin in the cytoplasm and in the nucleus: a two-step mechanism of redox regulation of transcription factor NF-kappaB. J. Biol. Chem. 1999;274:27891–27897. doi: 10.1074/jbc.274.39.27891. [DOI] [PubMed] [Google Scholar]
- 98.Nishi T, Shimizu N, Hiramoto M, Sato I, Yamaguchi Y, Hasegawa M, Aizawa S, Tanaka H, Kataoka K, Watanabe H, Handa H. Spatial redox regulation of a critical cysteine residue of NF-kappa B in vivo. J. Biol. Chem. 2002;277:44548–4556. doi: 10.1074/jbc.M202970200. [DOI] [PubMed] [Google Scholar]
- 99.Byun MS, Choi J, Jue DM. Cysteine-179 of IkappaB kinase beta plays a critical role in enzyme activation by promoting phosphorylation of activation loop serines. Exp. Mol. Med. 2006;38:546–552. doi: 10.1038/emm.2006.64. [DOI] [PubMed] [Google Scholar]
- 100.Park MH, Song HS, Kim KH, Son DJ, Lee SH, Yoon DY, Kim Y, Park IY, Song S, Hwang BY, Jung JK, Hong JT. Cobrotoxin inhibits NF-kappa B activation and target gene expression through reaction with NF-kappa B signal molecules. Biochemistry. 2005;44:8326–8336. doi: 10.1021/bi050156h. [DOI] [PubMed] [Google Scholar]
- 101.Kapahi P, Takahashi T, Natoli G, Adams SR, Chen Y, Tsien RY, Karin M. Inhibition of NF-kappa B activation by arsenite through reaction with a critical cysteine in the activation loop of Ikappa B kinase. J. Biol. Chem. 2000;275:36062–36066. doi: 10.1074/jbc.M007204200. [DOI] [PubMed] [Google Scholar]
- 102.Reynaert NL, van der Vliet A, Guala AS, McGovern T, Hristova M, Pantano C, Heintz NH, Heim J, Ho YS, Matthews DE, Wouters EE, Janssen-Heininger YM. Dynamic redox control of NF-kappaB through glutaredoxin-regulated S-glutathionylation of inhibitory kappaB kinase beta. Froc. Natl. Acad. Sci. USA. 2006;103:13086–13091. doi: 10.1073/pnas.0603290103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reynaert NL, Ckless K, Korn SH, Vos N, Guala AS, Wouters EE, van der Vliet A, Janssen-Heininger YM. Nitric oxide represses inhibitory kappaB kinase through S-nitrosylation. Froc. Natl. Acad. Sci. USA. 2004;101:8945–8950. doi: 10.1073/pnas.0400588101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Matthews JR, Botting CH, Panico M, Morris HR, Hay RT. Inhibition of NF-kappaB DNA binding by nitric oxide. Nucleic Acids Res. 1996;24:2236–2242. doi: 10.1093/nar/24.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marshall HE, Stamler JS. Inhibition of NF-kappa B by S-nitrosylation. Biochemistry. 2001;40:1688–1693. doi: 10.1021/bi002239y. [DOI] [PubMed] [Google Scholar]
- 106.Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 107.Dhingra R, Shaw JA, Aviv Y, Kirshenbaum LA. Dichotomous actions of NF-kappaB signaling pathways in heart. J. Cardiovasc. Transl. Res. 2010;3:344–354. doi: 10.1007/s12265-010-9195-5. [DOI] [PubMed] [Google Scholar]
- 108.Mustapha S, Kirshner A, De Moissac D, Kirshenbaum LA. A direct requirement of nuclear factor-kappa B for suppression of apoptosis in ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H939–H945. doi: 10.1152/ajpheart.2000.279.3.H939. [DOI] [PubMed] [Google Scholar]
- 109.Misra A, Haudek SB, Knuefermann P, Vallejo JG, Chen ZJ, Michael LH, Sivasubramanian N, Olson EN, Entman ML, Mann DL. Nuclear factor-kappaB protects the adult cardiac myocyte against ischemia-induced apoptosis in a murine model of acute myocardial infarction. Circulation. 2003;108:3075–3078. doi: 10.1161/01.CIR.0000108929.93074.0B. [DOI] [PubMed] [Google Scholar]
- 110.Hamid T, Guo SZ, Kingery JR, Xiang X, Dawn B, Prabhu SD. Cardiomyocyte NF-κB p65 promotes adverse remodelling, apoptosis, and endoplasmic reticulum stress in heart failure. Cardiovasc. Res. 2011;89:129–138. doi: 10.1093/cvr/cvq274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hajra L, Evans AI, Chen M, Hyduk SJ, Collins T, Cybulsky MI. The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc. Natl. Acad. Sci. USA. 2000;97:9052–9057. doi: 10.1073/pnas.97.16.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Marui N, Offermann MK, Swerlick R, Kunsch C, Rosen CA, Ahmad M, Alexander RW, Medford RM. Vascular cell adhesion molecule-1 (VCAM-1) gene transcription and expression are regulated through an antioxidant-sensitive mechanism in human vascular endothelial cells. J. Clin. Invest. 1993;92:1866–1874. doi: 10.1172/JCI116778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen XL, Zhang Q, Zhao R, Ding X, Tummala PE, Medford RM. Rac1 and superoxide are required for the expression of cell adhesion molecules induced by tumor necrosis factor-alpha in endothelial cells. J. Pharmacol. Exp. Ther. 2003;305:573–580. doi: 10.1124/jpet.102.047894. [DOI] [PubMed] [Google Scholar]
- 114.Csiszar A, Smith KE, Roller A, Kaley G, Edwards JG, Ungvari Z. Regulation of bone morphogenetic protein-2 expression in endothelial cells: role of nuclear factor-kappaB activation by tumor necrosis factor-alpha, H202, and high intravascular pressure. Circulation. 2005;111:2364–2372. doi: 10.1161/01.CIR.0000164201.40634.1D. [DOI] [PubMed] [Google Scholar]
- 115.Motohashi H, Yamamoto M. Nrf2-Keapl defines a physiologically important stress response mechanism. Trends Mol. Med. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 116.Dreger H, Westphal K, Weller A, Baumann G, Stangl V, Meiners S, Stangl K. Nrf2-dependent upregulation of antioxidative enzymes: a novel pathway for proteasome inhibitor-mediated cardioprotection. Cardiovasc. Res. 2009;83:354–361. doi: 10.1093/cvr/cvp107. [DOI] [PubMed] [Google Scholar]
- 117.Levonen AL, Inkala M, Heikura T, Jauhiainen S, Jyrkkanen HK, Kansanen E, Määttä K, Romppanen E, Turunen P, Rutanen J, Yla-Herttuala S. Nrf2 gene transfer induces antioxidant enzymes and suppresses smooth muscle cell growth in vitro and reduces oxidative stress in rabbit aorta in vivo. Arterioscler. Thromb. Vasc. Biol. 2007;27:741–747. doi: 10.1161/01.ATV.0000258868.80079.4d. [DOI] [PubMed] [Google Scholar]
- 118.He M, Siow RC, Sugden D, Gao L, Cheng X, Mann GE. Induction of HO-1 and redox signaling in endothelial cells by advanced glycation end products: a role for Nrf2 in vascular protection in diabetes. Nutr. Metab. Cardiovasc. Dis. 2011;21:277–285. doi: 10.1016/j.numecd.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 119.Hosoya T, Maruyama A, Kang MI, Kawatani Y, Shibata T, Uchida K, Warabi E, Noguchi N, Itoh K, Yamamoto M. Differential responses of the Nrf2-Keapl system to laminar and oscillatory shear stresses in endothelial cells. J. Biol. Chem. 2005;280:27244–27250. doi: 10.1074/jbc.M502551200. [DOI] [PubMed] [Google Scholar]
- 120.Dai G, Vaughn S, Zhang Y, Wang ET, Garcia-Cardena G, Gimbrone MA., Jr Biomechanical forces in atherosclerosis-resistant vascular regions regulate endothelial redox balance via phosphoinositol 3-kinase/Akt-depen-dent activation of Nrf2. Circ. Res. 2007;101:723–733. doi: 10.1161/CIRCRESAHA.107.152942. [DOI] [PubMed] [Google Scholar]
- 121.Heiss EH, Schachner D, Werner ER, Dirsch VM. Active NF-E2-related factor (Nrf2) contributes to keep endothelial NO synthase (eNOS) in the coupled state: role of reactive oxygen species (ROS), eNOS, and heme oxygenase (HO-1) levels. J. Biol. Chem. 2009;284:31579–31586. doi: 10.1074/jbc.M109.009175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keapl represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Itoh K, Wakabayashi N, Katoh Y, Ishii T, O'Connor T, Yamamoto M. Keap1 regulates both cytoplasmic-nuclear shuttling and degradation of Nrf2 in response to electrophiles. Genes Cells. 2003;8:379–391. doi: 10.1046/j.1365-2443.2003.00640.x. [DOI] [PubMed] [Google Scholar]
- 124.Zhang DD, Hannink M. Distinct cysteine residues in Keapl are required for Keapl-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol. Cell. Biol. 2003;23:8137–8151. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fourquet S, Guerois R, Biard D, Toledano MB. Activation of NRF2 by nitrosative agents and H202 involves KEAP1 disulfide formation. J. Biol. Chem. 2010;285:8463–8471. doi: 10.1074/jbc.M109.051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gentz R, Rauscher EJ, 3rd, Abate C, Curran T. Parallel association of Fos and Jun leucine zippers juxtaposes DNA binding domains. Science. 1989;243:1695–1699. doi: 10.1126/science.2494702. [DOI] [PubMed] [Google Scholar]
- 127.Turner R, Tjian R. Leucine repeats and an adjacent DNA binding domain mediate the formation of functional cFos-cJun heterodimers. Science. 1989;243:1689–1694. doi: 10.1126/science.2494701. [DOI] [PubMed] [Google Scholar]
- 128.Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K, Noda T, Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen K, Thomas SR, Keaney JF., Jr Beyond LDL oxidation: ROS in vascular signal transduction. Free Radic. Biol. Med. 2003;35:117–132. doi: 10.1016/s0891-5849(03)00239-9. [DOI] [PubMed] [Google Scholar]
- 130.Abate C, Patel L, Rauscher FJ, 3rd, Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science. 1990;249:1157–1161. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- 131.Okuno H, Akahori A, Sato H, Xanthoudakis S, Curran T, Iba H. Escape from redox regulation enhances the transforming activity of Fos. Oncogene. 1993;8:695–701. [PubMed] [Google Scholar]
- 132.Klatt P, Molina EP, De Lacoba MG, Padilla CA, Martinez-Galesteo E, Barcena JA, Lamas S. Redox regulation of c-Jun DNA binding by reversible S-glutathiolation. FASEB J. 1999;13:1481–1490. doi: 10.1096/fasebj.13.12.1481. [DOI] [PubMed] [Google Scholar]
- 133.Klatt P, Molina EP, Lamas S. Nitric oxide inhibits c-Jun DNA binding by specifically targeted S-glutathionylation. J. Biol. Chem. 1999;274:15857–15864. doi: 10.1074/jbc.274.22.15857. [DOI] [PubMed] [Google Scholar]
- 134.Vendrov AE, Madamanchi NR, Niu XL, Molnar KC, Runge M, Szyndralewiez C, Page P, Runge MS. NADPH oxidases regulate CD44 and hyaluronic acid expression in thrombin-treated vascular smooth muscle cells and in atherosclerosis J. Biol. Chem. 2010;285:26545–26557. doi: 10.1074/jbc.M110.143917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Foster LC, Wiesel P, Huggins GS, Pañares RM, Chin MT, Pellacani A, Perrella MA. Role of activating protein-1 and high mobility group-I (Y) protein in the induction of CD44 gene expression by interleukin-lbeta in vascular smooth muscle cells. FASEBJ. 2000;14:368–378. doi: 10.1096/fasebj.14.2.368. [DOI] [PubMed] [Google Scholar]
- 136.Maulik N, Sasaki H, Addya S, Das DK. Regulation of cardiomyocyte apoptosis by redox-sensitive transcription factors. FEBS Lett. 2000;485:7–12. doi: 10.1016/s0014-5793(00)02174-8. [DOI] [PubMed] [Google Scholar]
- 137.Hainaut P, Milner J. Redox modulation of p53 conformation and sequence-specific DNA binding in vitro. Cancer Res. 1993;53:4469–4473. [PubMed] [Google Scholar]
- 138.Verhaegh GW, Richard MJ, Hainaut P. Regulation of p53 by metal ions and by antioxidants: dithiocarbamate down-regulates p53 DNA-binding activity by increasing the intracellular level of copper. Mol. Cell. Biol. 1997;17:5699–5706. doi: 10.1128/mcb.17.10.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Velu CS, Niture SK, Doneanu CE, Pattabiraman N, Srivenugopal KS. Human p53 is inhibited by glutathionylation of cysteines present in the proximal DNA-binding domain during oxidative stress. Biochemistry. 2007;46:7765–7780. doi: 10.1021/bi700425y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bode AM, Dong Z. Post-translational modification of p53 in tumorigenesis. Nat. Rev. Cancer. 2004;4:793–805. doi: 10.1038/nrc1455. [DOI] [PubMed] [Google Scholar]
- 141.Sun XZ, Vinci C, Makmura L, Han S, Tran D, Nguyen J, Hamann M, Grazziani S, Sheppard S, Gutova M, Zhou F, Thomas J, Momand J. Formation of disulfide bond in p53 correlates with inhibition of DNA binding and tetramerization. Antioxid. Redox Signaling. 2003;5:655–665. doi: 10.1089/152308603770310338. [DOI] [PubMed] [Google Scholar]
- 142.Ueno M, Masutani H, Arai RJ, Yamauchi A, Hirota K, Sakai T, Inamoto T, Yamaoka Y, Yodoi J, Nikaido T. Thioredoxin-dependent redox regulation of p53-mediated p21 activation. J. Biol. Chem. 1999;274:35809–35815. doi: 10.1074/jbc.274.50.35809. [DOI] [PubMed] [Google Scholar]
- 143.Seemann S, Hainaut P. Roles of thioredoxin reductase 1 and APE/Ref-1 in the control of basal p53 stability and activity. Oncogene. 2005;24:3853–3863. doi: 10.1038/sj.onc.1208549. [DOI] [PubMed] [Google Scholar]
- 144.Sablina AA, Budanov AV, Ilyinskaya GV, Agapova LS, Kravchenko JE, Chumakov PM. The antioxidant function of the p53 tumor suppressor. Nat. Med. 2005;11:1306–1313. doi: 10.1038/nm1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ihling C, Haendeler J, Menzel G, Hess RD, Fraedrich G, Schaefer HE, Zeiher AM. Co-expression of p53 and MDM2 in human atherosclerosis: implications for the regulation of cellularity of atherosclerotic lesions. J. Pathol. 1998;185:303–312. doi: 10.1002/(SICI)1096-9896(199807)185:3<303::AID-PATH106>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 146.Yuan XM, Osman E, Miah S, Zadeh SN, Xu L, Forssell C, Li W. p53 expression in human carotid atheroma is significantly related to plaque instability and clinical manifestations. Atherosclerosis. 2010;210:392–399. doi: 10.1016/j.atherosclerosis.2009.11.048. [DOI] [PubMed] [Google Scholar]
- 147.von der Thusen JH, van Vlijmen BJ, Hoeben RC, Kockx MM, Havekes LM, van Berkel TJ, Biessen EA. Induction of atherosclerotic plaque rupture in apolipoprotein E−/− mice after adenovirus-mediated transfer of p53. Circulation. 2002;105:2064–2070. doi: 10.1161/01.cir.0000015502.97828.93. [DOI] [PubMed] [Google Scholar]
- 148.Mercer J, Figg N, Stoneman V, Braganza D, Bennett MR. Endogenous p53 protects vascular smooth muscle cells from apoptosis and reduces atherosclerosis in ApoE knockout mice. Circ. Res. 2005;96:667–674. doi: 10.1161/01.RES.0000161069.15577.ca. [DOI] [PubMed] [Google Scholar]
- 149.Boesten LS, Zadelaar AS, van Nieuwkoop A, Hu L, Teunisse AF, Jochemsen AG, Evers B, van de Water B, Gijbels MJ, van Vlijmen BJ, Havekes LM, de Winther MP. Macrophage p53 controls macrophage death in atherosclerotic lesions of apolipoprotein E deficient mice. Atherosclerosis. 2009;207:399–04. doi: 10.1016/j.atherosclerosis.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 150.Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 151.Moncada S, Palmer RM, Higgs EA. The discovery of nitric oxide as the endogenous nitrovasodilator. Hypertension. 1988;12:365–372. doi: 10.1161/01.hyp.12.4.365. [DOI] [PubMed] [Google Scholar]
- 152.Quyyumi AA, Dakak N, Andrews NP, Gilligan DM, Panza JA, Cannon RO., 3rd Contribution of nitric oxide to metabolic coronary vasodilation in the human heart. Circulation. 1995;92:320–326. doi: 10.1161/01.cir.92.3.320. [DOI] [PubMed] [Google Scholar]
- 153.Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. proc. Natl. Acad. Sci. USA. 1991;88:4651–655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ahluwalia A, Foster P, Scotland RS, McLean PG, Mathur A, Perretti M, Moncada S, Hobbs AJ. Antiinflammatory activity of soluble guanylate cyclase: cGMP-dependent down-regulation of P-selectin expression and leukocyte recruitment. Froc. Natl. Acad. Sci. USA. 2004;101:1386–1391. doi: 10.1073/pnas.0304264101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cooke CL, Davidge ST. Endothelial-dependent vasodilation is reduced in mesenteric arteries from superoxide dismutase knockout mice. Cardiovasc Res. 2003;60:635–642. doi: 10.1016/j.cardiores.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 156.Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kanaide H, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J. Clin. Invest. 2000;106:1521–1530. doi: 10.1172/JCI10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Matoba T, Shimokawa H, Kubota H, Morikawa K, Fujiki T, Kunihiro I, Mukai Y, Hirakawa Y, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in human mesenteric arteries. Biochem. Biophys. Res. Commun. 2002;290:909–913. doi: 10.1006/bbrc.2001.6278. [DOI] [PubMed] [Google Scholar]
- 158.Morikawa K, Shimokawa R, Matoba T, Kubota H, Akaike T, Talukder MA, Hatanaka M, Fujiki T, Maeda H, Takahashi S, Takeshita A. Pivotal role of Cu,Zn-superoxide dismutase in endothelium-dependent hyperpolarization. J. Clin. Invest. 2003;112:1871–8799. doi: 10.1172/JCI19351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Miura H, Bosnjak JJ, Ning G, Saito T, Miura M, Gutterman DD. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ. Res. 2003;92:e31–40. doi: 10.1161/01.res.0000054200.44505.ab. [DOI] [PubMed] [Google Scholar]
- 160.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu. Rev. Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 161.Kulmacz RJ, Wang LH. Comparison of hydroperoxide initiator requirements for the cyclooxygenase activities of prostaglandin H synthase-1 and −2. J. Biol. Chem. 2005;270:24019–24023. doi: 10.1074/jbc.270.41.24019. [DOI] [PubMed] [Google Scholar]
- 162.Schildknecht S, Bachschmid M, Ullrich V. Peroxynitrite provides the peroxide tone for PGHS-2-dependent prostacyclin synthesis in vascular smooth muscle cells. FASEB J. 2005;19:1169–1171. doi: 10.1096/fj.04-3465fje. [DOI] [PubMed] [Google Scholar]
- 163.Zhou RH, Vendrov AE, Tchivilev I, Niu XL, Molnar KC, Rojas M, Carter JD, Tong H, Stouffer GA, Madamanchi NR, Runge MS. Mitochondrial oxidative stress in aortic stiffening with age: the role of smooth muscle cell function. Arterioscler. Thromb. Vasc. Biol. 2012;32:745–755. doi: 10.1161/ATVBAHA.111.243121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ignarro LJ, Kadowitz PJ. The pharmacological and physiological role of cyclic GMP in vascular smooth muscle relaxation. Annu. Rev. Pharmacol. Toxicol. 1985;25:171–191. doi: 10.1146/annurev.pa.25.040185.001131. [DOI] [PubMed] [Google Scholar]
- 165.Burke TM, Wolin MS. Hydrogen peroxide elicits pulmonary arterial relaxation and guanylate cyclase activation. Am. J. Physiol. Heart Circ. Physiol. 1987;252:H721–H732. doi: 10.1152/ajpheart.1987.252.4.H721. [DOI] [PubMed] [Google Scholar]
- 166.Burke-Wolin TM, Wolin MS. H202 and cGMP may function as an 02 sensor in the pulmonary artery. J. Appl. Physiol. 1989;66:167–170. doi: 10.1152/jappl.1989.66.1.167. [DOI] [PubMed] [Google Scholar]
- 167.Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, Chepenik KP, Waldman SA. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol. Rev. 2000;52:375–14. [PubMed] [Google Scholar]
- 168.Kazerounian S, Pitari GM, Ruiz-Stewart I, Schulz S, Waldman SA. Nitric oxide activation of soluble guanylyl cyclase reveals high and low affinity sites that mediate allosteric inhibition by calcium. Biochemistry. 2002;41:3396–3404. doi: 10.1021/bi0110894. [DOI] [PubMed] [Google Scholar]
- 169.Bunn HF, Poyton RO. Oxygen sensing and molecular adaptation to hypoxia. Physiol. Rev. 1996;76:839–885. doi: 10.1152/physrev.1996.76.3.839. [DOI] [PubMed] [Google Scholar]
- 170.Van Lommel A. Pulmonary neuroendocrine cells (PNEC) and neuroepithelial bodies (NEB): chemoreceptors and regulators of lung development. Paediatr. Respir. Rev. 2001;2:171–176. doi: 10.1053/prrv.2000.0126. [DOI] [PubMed] [Google Scholar]
- 171.Wadsworth RM. Vasoconstrictor and vasodilator effects of hypoxia. Trends Pharmacol. Sci. 1994;15:47–53. doi: 10.1016/0165-6147(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 172.Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 173.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’ Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJC. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 174.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for 02 sensing. Science. 2001;292:464–68. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 175.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim AV, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by 02-regulated prolyl hydroxylation. Science. 2001;292:468–72. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 176.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J. Clin. Invest. 2005;115:500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc. Natl. Acad. Sci. USA. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-lalpha during hypoxia: a mechanism of 02 sensing. J. Biol. Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 179.Agani FH, Pichiule P, Chavez JC, LaManna JC. The role of mitochondria in the regulation of hypoxia-inducible factor 1 expression during hypoxia. J. Biol. Chem. 2000;275:35863–35867. doi: 10.1074/jbc.M005643200. [DOI] [PubMed] [Google Scholar]
- 180.Waypa GB, Schumacker PT. 02 sensing in hypoxic pulmonary vasoconstriction: the mitochondrial door reopens. Respir. Physiol. Neurobiol. 2002;132:81–91. doi: 10.1016/s1569-9048(02)00051-4. [DOI] [PubMed] [Google Scholar]
- 181.Giaccia AJ, Simon MC, Johnson R. The biology of hypoxia: the role of oxygen sensing in development, normal function, and disease. Genes Dev. 2004;18:2183–2194. doi: 10.1101/gad.1243304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1:401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 183.Brunelle JK, Bell EL, Quesada NM, Vercauteren K, Tiranti V, Zeviani M, Scarpulla RC, Chandel NS. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 2005;1:409–14. doi: 10.1016/j.cmet.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 184.Mansfield KD, Guzy RD, Pan Y, Young RM, Cash TP, Schumacker PT, Simon MC. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation. Cell Metab. 2005;1:393–399. doi: 10.1016/j.cmet.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Murry CE, Richard VJ, Reimer KA, Jennings RB. Ischemic preconditioning slows energy metabolism and delays ultra structural damage during a sustained ischemic episode. Circ. Res. 1990;66:913–931. doi: 10.1161/01.res.66.4.913. [DOI] [PubMed] [Google Scholar]
- 186.Liu Y, Downey JM. Ischemic preconditioning protects against infarction in rat heart. Am. J. Physiol. 1992;263:H1107–H1112. doi: 10.1152/ajpheart.1992.263.4.H1107. [DOI] [PubMed] [Google Scholar]
- 187.Lasley RD, Anderson GM, Mentzer RM. Ischemic and hypoxic preconditioning enhance postischemic recovery of function in the rat heart. Cardiovasc. Res. 1993;27:565–570. doi: 10.1093/cvr/27.4.565. [DOI] [PubMed] [Google Scholar]
- 188.Tritto I, D'Andrea D, Eramo N, Scognamiglio A, De Simone C, Violante A, Esposito A, Chiariello M, Ambrosio G. Oxygen radicals can induce preconditioning in rabbit hearts. Circ. Res. 1997;80:743–748. doi: 10.1161/01.res.80.5.743. [DOI] [PubMed] [Google Scholar]
- 189.Chen W, Gabel S, Steenbergen C, Murphy E. A redox-based mechanism for cardioprotection induced by ischemic preconditioning in perfused rat heart. Circ. Res. 1995;77:424–29. doi: 10.1161/01.res.77.2.424. [DOI] [PubMed] [Google Scholar]
- 190.Forbes RA, Steenbergen C, Murphy E. Diazoxide-induced cardioprotection requires signaling through a redox-sensitive mechanism. Circ. Res. 2001;88:802–809. doi: 10.1161/hh0801.089342. [DOI] [PubMed] [Google Scholar]
- 191.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol. Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Quarrie R, Cramer BM, Lee DS, Steinbaugh GE, Erdahl W, Pfeiffer DR, Zweier JL, Crestanello JA. Ischemic preconditioning decreases mitochondria] proton leak and reactive oxygen species production in the postischemic heart. J. Surg. Res. 2011;165:5–14. doi: 10.1016/j.jss.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Prosser BL, Ward CW, Lederer WJ. X-ROS signaling: rapid mechano-chemo transduction in heart. Science. 2011;333:1440–1445. doi: 10.1126/science.1202768. [DOI] [PubMed] [Google Scholar]
- 194.Hidalgo C, Donoso P. Cell signaling: getting to the heart of mechanotrans-duction. Science. 2011;333:1388–1390. doi: 10.1126/science.1212183. [DOI] [PubMed] [Google Scholar]
- 195.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 196.Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- 197.Sánchez G, Pedrozo Z, Domenech RJ, Hidalgo C, Donoso P. Tachycardia increases NADPH oxidase activity and RyR2 S-glutathionylation in ventricular muscle. J. Mol. Cell. Cardiol. 2005;39:982–991. doi: 10.1016/j.yjmcc.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 198.Hidalgo C, Sanchez G, Barrientos G, Aracena-Parks PA. transverse tubule NADPH oxidase activity stimulates calcium release from isolated triads via ryanodine receptor type 1 S-glutathionylation. J. Biol. Chem. 2006;281:26473–26482. doi: 10.1074/jbc.M600451200. [DOI] [PubMed] [Google Scholar]
- 199.Santos CX, Anilkumar N, Zhang M, Brewer AC, Shah AM. Redox signaling in cardiac myocytes. Free Radic. Biol. Med. 2011;50:777–793. doi: 10.1016/j.freeradbiomed.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Adachi T, Weisbrod RM, Pimentel D, Ying J, Sharov VS, Schoneich C, Cohen RA. S-glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide: a mechanism targeted by oxidants in vascular disease. Nat. Med. 2004;10:1200–1207. doi: 10.1038/nm1119. [DOI] [PubMed] [Google Scholar]
- 201.Ishi T, Sunami O, Saitoh N, Nishio H, Takeuchi T, Hata F. Inhibition of skeletal muscle sarcoplasmic reticulum Ca2+-ATPase by nitric oxide. FEBS Lett. 1998;440:218–222. doi: 10.1016/s0014-5793(98)01460-4. [DOI] [PubMed] [Google Scholar]
- 202.Viner RI, Williams TD, Schoneich C. Nitric oxide-dependent modification of the sarcoplasmic reticulum Ca-ATPase: localization of cysteine target sites. Free Radic. Biol. Med. 2000;29:489–96. doi: 10.1016/s0891-5849(00)00325-7. [DOI] [PubMed] [Google Scholar]
- 203.Schoneich C, Sharov VS. Mass spectrometry of protein modifications by reactive oxygen and nitrogen species. Free Radic. Biol. Med. 2006;41:1507–1520. doi: 10.1016/j.freeradbiomed.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 204.Tocchetti CG, Wang W, Froehlich JP, Huke S, Aon MA, Wilson GM, Di Benedetto G, O'Rourke B, Gao WD, Wink DA, Toscano JP, Zaccolo M, Bers DM, Valdivia HH, Cheng H, Kass DA, Paolocci N. Nitroxyl improves cellular heart function by directly enhancing cardiac sarcoplasmic reticulum Ca2+ cycling. Circ. Res. 2007;100:96–104. doi: 10.1161/01.RES.0000253904.53601.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lancel S, Zhang J, Evangelista A, Trucillo MP, Tong X, Siwik DA, Cohen RA, Colucci WS. Nitroxyl activates SERCA in cardiac myocytes via glutathiolation of cysteine 674. Circ. Res. 2009;104:720–723. doi: 10.1161/CIRCRESAHA.108.188441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 207.Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, 3rd, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H202 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Invest. 2009;119:573–581. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hoehn KL, Salmon AB, Hohnen-Behrens C, Turner N, Hoy AJ, Maghzal GJ, Stacker R, Van Remmen H, Kraegen EW, Cooney GJ, Richardson AR, James DE. Insulin resistance is a cellular antioxidant defense mechanism. Froc. Natl. Acad. Sci. USA. 2009;106:17787–17792. doi: 10.1073/pnas.0902380106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 210.Sabio G, Kennedy NJ, Cavanagh-Kyros J, Jung DY, Ko HJ, Ong H, Barrett T, Kim JK, Davis RJ. Role of muscle c-Jun NH2-terminal kinase 1 in obesity-induced insulin resistance. Mol. Cell. Biol. 2010;30:106–115. doi: 10.1128/MCB.01162-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Liu H, Nishitoh H, Ichijo H, Kyriakis JM. Activation of apoptosis signal-regulating kinase 1 (ASK1) by tumor necrosis factor receptor-associated factor 2 requires prior dissociation of the ASK1 inhibitor thioredoxin. Mol. Cell. Biol. 2010;20:2198–2208. doi: 10.1128/mcb.20.6.2198-2208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Loh K, Deng H, Fukushima A, Cai X, Boivin B, Galic S, Bruce C, Shields BJ, Skiba B, Ooms LM, Stepto N, Wu B, Mitchell CA, Tonks NK, Watt MJ, Febbraio MA, Crack PJ, Andrikopoulos S, Tiganis T. Reactive oxygen species enhance insulin sensitivity. Cell Metab. 2009;10:260–272. doi: 10.1016/j.cmet.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Findeisen HM, Gizard F, Zhao Y, Qing H, Jones KL, Cohn D, Heywood EB, Bruemmer D. Glutathione depletion prevents diet-induced obesity and enhances insulin sensitivity. Obesity. 2011;19:2429–2432. doi: 10.1038/oby.2011.298. [DOI] [PubMed] [Google Scholar]
- 214.Tiganis T. Reactive oxygen species and insulin resistance: the good, the bad and the ugly. Trends Pharmacol. Sci. 2011;32:82–89. doi: 10.1016/j.tips.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 215.Iwakami S, Misu H, Takeda T, Sugimori M, Matsugo S, Kaneko S, Takamura T. Concentration-dependent dual effects of hydrogen peroxide on insulin signal transduction in H4IIEC hepatocytes. PLoS One. 2011:e27401. doi: 10.1371/journal.pone.0027401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide: general properties and effect of hyperbaric oxygen. Biochem. J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Corral-Debrinski M, Stepien G, Shoffner JM, Lott MT, Kanter K, Wallace DC. Hypoxemia is associated with mitochondrial DNA damage and gene induction: implications for cardiac disease. JAMA. 1991;266:1812–1816. [PubMed] [Google Scholar]
- 218.Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, Chan PH, Wallace DC, Epstein CJ. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat. Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 219.Wallace DC. Mitochondrial diseases in man and mouse. Science. 1999;283:1482–1488. doi: 10.1126/science.283.5407.1482. [DOI] [PubMed] [Google Scholar]
- 220.Ide T, Tsutsui H, Kinugawa S, Utsumi H, Kang D, Hattori N, Uchida K, Arimura K, Egashira K, Takeshita A. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ. Res. 1999;85:357–363. doi: 10.1161/01.res.85.4.357. [DOI] [PubMed] [Google Scholar]
- 221.Nakamura R, Egashira K, Machida Y, Hayashidani S, Takeya M, Utsumi H, Tsutsui H, Takeshita A. Probucol attenuates left ventricular dysfunction and remodeling in tachycardia-induced heart failure: roles of oxidative stress and inflammation. Circulation. 2002;106:362–367. doi: 10.1161/01.cir.0000021430.04195.51. [DOI] [PubMed] [Google Scholar]
- 222.Lin PH, Lee SH, Su CP, Wei YH. Oxidative damage to mitochondria] DNA in atrial muscle of patients with atrial fibrillation. Free Radic. Biol. Med. 2003;35:1310–1318. doi: 10.1016/j.freeradbiomed.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 223.Wu SB, Ma YS, Chen YC, Wei YH. Mitochondrial, DNA mutation-elicited oxidative stress, oxidative damage, and altered gene expression in cultured cells of patients with MERRF syndrome. Mol. Neurobiol. 2010;41:256–266. doi: 10.1007/s12035-010-8123-7. [DOI] [PubMed] [Google Scholar]
- 224.Coskun P, Wyrembak J, Schriner S, Chen HW, Marciniack C, Laferla F, Wallace DC. A mitochondrial etiology of Alzheimer and Parkinson disease. Biochim. Biophys. Acta. 2012;1820:553–564. doi: 10.1016/j.bbagen.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Turrens J. F Mitochondrial formation of reactive oxygen species. J. Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Barja G, Herrero A. Localization at complex I and mechanism of the higher free radical production of brain nonsynaptic mitochondria in the short-lived rat than in the longevous pigeon. J. Bioenerg. Biomembr. 1998;30:235–243. doi: 10.1023/a:1020592719405. [DOI] [PubMed] [Google Scholar]
- 227.Hirst J, King MS, Pryde KR. The production of reactive oxygen species by complex I. Biochem. Soc. Trans. 2008;36:976–980. doi: 10.1042/BST0360976. [DOI] [PubMed] [Google Scholar]
- 228.Han D, Canali R, Rettori D, Kaplowitz N. Effect of glutathione depletion on sites and topology of superoxide and hydrogen peroxide production in mitochondria. Mol. Pharmacol. 2003;64:1136–1144. doi: 10.1124/mol.64.5.1136. [DOI] [PubMed] [Google Scholar]
- 229.Camello-Almaraz C, Gomez-Pinilla PJ, Pozo MJ, Camello P. Mitochondrial reactive oxygen species and Ca2+ signaling. Am. J. Physiol. Cell Physiol. 2006;291:C1082–C1088. doi: 10.1152/ajpcell.00217.2006. [DOI] [PubMed] [Google Scholar]
- 230.Scheubel RJ, Bartling B, Simm A, Silber RE, Drogaris K, Darmer D, Holtz J. Apoptotic pathway activation from mitochondria and death receptors without caspase-3 cleavage in failing human myocardium: fragile balance of myocyte survival? J. Am. Coll. Cardiol. 2002;39:481–88. doi: 10.1016/s0735-1097(01)01769-7. [DOI] [PubMed] [Google Scholar]
- 231.Scheubel RJ, Tostlebe M, Simm A, Rohrbach S, Prondzinsky R, Gellerich FN, Silber RE, Holtz J. Dysfunction of mitochondrial respiratory chain complex I in human failing myocardium is not due to disturbed mitochondrial gene expression. J. Am. Coll. Cardiol. 2002;40:2174–2181. doi: 10.1016/s0735-1097(02)02600-1. [DOI] [PubMed] [Google Scholar]
- 232.Forfia PR, Hintze TH, Wolin MS, Kaley G. Role of nitric oxide in the control of mitochondrial function. Adv. Exp. Med. Biol. 1999;471:381–388. doi: 10.1007/978-1-4615-4717-4_46. [DOI] [PubMed] [Google Scholar]
- 233.Kokoszka JE, Coskun P, Esposito LA, Wallace DC. Increased mitochondrial oxidative stress in the Sod2 (+/−) mouse results in the age-related decline of mitochondrial function culminating in increased apoptosis. Froc Natl. Acad. Sci. USA. 2001;98:2278–2283. doi: 10.1073/pnas.051627098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Aon MA, Cortassa S, O'Rourke B. Redox-optimized, ROS balance: a unifying hypothesis. Biochim. Biophys. Acta. 2010;1797:865–877. doi: 10.1016/j.bbabio.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kohlhaas M, Liu T, Knopp A, Zeller T, Ong MF, Böhm M, O'Rourke B, Maack C. Elevated cytosolic Na+ increases mitochondrial formation of reactive oxygen species in failing cardiac myocytes. Circulation. 2010;121:1606–1613. doi: 10.1161/CIRCULATIONAHA.109.914911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Di Lisa F, Kaludercic N, Carpi A, Menabò R, Giorgio M. Mitochondria] pathways for ROS formation and myocardial injury: the relevance of p66 (Shc) and monoamine oxidase. Basic Res. Cardiol. 2009;104:131–139. doi: 10.1007/s00395-009-0008-4. [DOI] [PubMed] [Google Scholar]
- 237.Giorgio M, Migliaccio E, Orsini E, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M, Pinton P, Rizzuto R, Bernardi P, Paolucci E, Pelicci PG. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 238.Graiani G, Lagrasta C, Migliaccio E, Spillmann E, Meloni M, Madeddu P, Quaini E, Padura IM, Lanfrancone L, Pelicci P, Emanueli C. Genetic deletion of the p66Shc adaptor protein protects from angiotensin H-induced myocardial damage. Hypertension. 2005;46:433–140. doi: 10.1161/01.HYP.0000174986.73346.ba. [DOI] [PubMed] [Google Scholar]
- 239.Rota M, LeCapitaine N, Hosoda T, Boni A, De Angelis A, Padin-Iruegas ME, Esposito G, Vitale S, Urbanek K, Casarsa C, Giorgio M, Lüscher TE, Pelicci PG, Anversa P, Leri A, Kajstura J. Diabetes promotes cardiac stem cell aging and heart failure, which are prevented by deletion of the p66shc gene. Circ. Res. 2006;99:42–52. doi: 10.1161/01.RES.0000231289.63468.08. [DOI] [PubMed] [Google Scholar]
- 240.Napoli C, Martin-Padura I, de Nigris E, Giorgio M, Mansueto G, Somma P, Condorelli M, Sica G, De Rosa G, Pelicci P. Deletion of the p66Shc longevity gene reduces systemic and tissue oxidative stress, vascular cell apoptosis, and early atherogenesis in mice fed a high-fat diet. Proc. Natl. Acad. Sci. USA. 2003;100:2112–2116. doi: 10.1073/pnas.0336359100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Edmondson DE, Mattevi A, Binda C, Li M, Hubálek E. Structure and mechanism of monoamine oxidase. Curr. Med. Chem. 2004;11:1983–1993. doi: 10.2174/0929867043364784. [DOI] [PubMed] [Google Scholar]
- 242.Bianchi P, Kunduzova O, Masini E, Cambon C, Bani D, Raimondi L, Seguelas MH, Nistri S, Colucci W, Leducq N, Parini A. Oxidative stress by monoamine oxidase mediates receptor-independent cardiomyocyte apoptosis by serotonin and postischemic myocardial injury. Circulation. 2005;112:3297–3305. doi: 10.1161/CIRCULATIONAHA.104.528133. [DOI] [PubMed] [Google Scholar]
- 243.Kaludercic N, Takimoto E, Nagayama T, Feng N, Lai EW, Bedja D, Chen K, Gabrielson KL, Blakely RD, Shih JC, Pacak K, Kass DA, Di Lisa E, Paolocci N. Monoamine oxidase A-mediated enhanced catabolism of norepinephrine contributes to adverse remodeling and pump failure in hearts with pressure overload. Circ. Res. 2010;106:193–202. doi: 10.1161/CIRCRESAHA.109.198366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Selemidis S, Sobey CG, Wingler K, Schmidt HH, Drummond G. R NADPH oxidases in the vasculature: molecular features, roles in disease and pharmacological inhibition. Pharmacol. Ther. 2008;120:254–291. doi: 10.1016/j.pharmthera.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 245.Vignais PV. The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell Mol. Life Sci. 2002;59:1428–1459. doi: 10.1007/s00018-002-8520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 247.Lapouge K, Smith SJ, Groemping Y, Rittinger K. Architecture of the p40-p47-p67phox complex in the resting state of the NADPH oxidase: a central role for p67phox. J. Biol. Chem. 2002;277:10121–10128. doi: 10.1074/jbc.M112065200. [DOI] [PubMed] [Google Scholar]
- 248.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 249.Huang J, Kleinberg ME. Activation of the phagocyte NADPH oxidase protein p47(phox): phosphorylation controls SH3 domain-dependent binding to p22(phox) J. Biol. Chem. 1999;274:19731–19737. doi: 10.1074/jbc.274.28.19731. [DOI] [PubMed] [Google Scholar]
- 250.Leto TL, Adams AG, de Mendez I. Assembly of the phagocyte NADPH oxidase: binding of Src homology 3 domains to proline-rich targets. Proc. Natl. Acad. Sci. USA. 1994;91:10650–10654. doi: 10.1073/pnas.91.22.10650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.DeLeo ER, Nauseef WM, Jesaitis AJ, Burritt JB, Clark RA, Quinn MT. A domain of p47phox that interacts with human neutrophil flavocytochrome b558. J. Biol. Chem. 1995;270:26246–26251. doi: 10.1074/jbc.270.44.26246. [DOI] [PubMed] [Google Scholar]
- 252.Inanami O, Johnson J, McAdara JK, Benna JE, Faust LR, Newburger PE, Babior BM. Activation of the leukocyte NADPH oxidase by phorbol ester requires the phosphorylation of p47PHOX on serine 303 or 304. J. Biol. Chem. 1998;273:9539–9543. doi: 10.1074/jbc.273.16.9539. [DOI] [PubMed] [Google Scholar]
- 253.Nisimoto Y, Motalebi S, Han CH, Lambeth JD. The p67(phox) activation domain regulates electron flow from NADPH to flavin in flavocytochrome b (558) J. Biol. Chem. 1999;274:22999–23005. doi: 10.1074/jbc.274.33.22999. [DOI] [PubMed] [Google Scholar]
- 254.Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, Lambeth JD. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 255.Bánfi B, Molnár G, Maturana A, Steger K, Hegedûs B, Demaurex N, Krause KH. A Ca2+-activated NADPH oxidase in testis, spleen, and lymph nodes. J. Biol. Chem. 2001;276:37594–37601. doi: 10.1074/jbc.M103034200. [DOI] [PubMed] [Google Scholar]
- 256.Bánfi B, Tirone E, Durussel I, Knisz J, Moskwa P, Molnár GZ, Krause KH, Cox JA. Mechanism of Ca2+ activation of the NADPH oxidase 5 (NOX5) J. Biol. Chem. 2004;279:18583–18591. doi: 10.1074/jbc.M310268200. [DOI] [PubMed] [Google Scholar]
- 257.Dupuy C, Ohayon R, Valent A, Noël-Hudson MS, Dème D. Virion, A Purification of a novel flavoprotein involved in the thyroid NADPH oxidase: cloning of the porcine and human cDNAs. J. Biol. Chem. 1999;274:37265–37269. doi: 10.1074/jbc.274.52.37265. [DOI] [PubMed] [Google Scholar]
- 258.De Deken X, Wang D, Many MC, Costagliola S, Libert F, Vassart G, Dumont JE, Miot F. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J. Biol. Chem. 2000;275:23227–23233. doi: 10.1074/jbc.M000916200. [DOI] [PubMed] [Google Scholar]
- 259.Edens WA, Sharling L, Cheng G, Shapira R, Kinkade JM, Lee T, Edens HA, Tang X, Sullards C, Flaherty DB, Benian GM, Lambeth JD. Tyrosine cross-linking of extracellular matrix is catalyzed by Duox, a multi-domain oxidase/peroxidase with homology to the phagocyte oxidase subunit gp91phox. J. Cell Biol. 2001;154:879–892. doi: 10.1083/jcb.200103132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Lassegue B, Sorescu D, Szocs K, Yin Q, Akers M, Zhang Y, Grant SL, Lambeth JD, Griendling KK. Novel gp91phox homologues in vascular smooth muscle cells: Nox1 mediates angiotensin H-induced superoxide formation and redox-sensitive signaling pathways. Circ. Res. 2001;88:888–894. doi: 10.1161/hh0901.090299. [DOI] [PubMed] [Google Scholar]
- 261.Grote K, Flach I, Luchtefeld M, Akin E, Holland SM, Drexler H, Schieffer B. Mechanical stretch enhances mRNA expression and proenzyme release of matrix metalloproteinase-2 (MMP-2) via NAD(P)H oxidase-derived reactive oxygen species. Circ. Res. 2003;92:e80–e86. doi: 10.1161/01.RES.0000077044.60138.7C. [DOI] [PubMed] [Google Scholar]
- 262.Hwang J, Kleinhenz DJ, Lassegue B, Griendling KK, Dikalov S, Hart CM. Peroxisome proliferator-activated receptor-a ligands regulate endothelial membrane superoxide production. Am J. Physiol. Cell Physiol. 2005;288:C899–C905. doi: 10.1152/ajpcell.00474.2004. [DOI] [PubMed] [Google Scholar]
- 263.Judkins CP, Diep H, Broughton BR, Mast AE, Hooker EU, Miller AA, Selemidis S, Dusting GJ, Sobey CG, Drummond GR. Direct evidence of a role for Nox2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in ApoE−/− mice. Am. J. Physiol. Heart Circ. Physiol. 2010;298:H24–H32. doi: 10.1152/ajpheart.00799.2009. [DOI] [PubMed] [Google Scholar]
- 264.Ago T, Kitazono T, Ooboshi H, Iyama T, Han YH, Takada J, Wakisaka M, Ibayashi S, Utsumi H, Iida M. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation. 2004;109:227–233. doi: 10.1161/01.CIR.0000105680.92873.70. [DOI] [PubMed] [Google Scholar]
- 265.Jones SA, O’Donnell VB, Wood JD, Broughton JP, Hughes EJ, Jones OT. Expression of phagocyte NADPH oxidase components in human endothelial cells. Am. J. Physiol. 1996;271:H1626–H1634. doi: 10.1152/ajpheart.1996.271.4.H1626. [DOI] [PubMed] [Google Scholar]
- 266.Gorlach A, Brandes RP, Nguyen K, Amidi M, Dehghani E, Busse R. A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ Res. 2000;87:26–32. doi: 10.1161/01.res.87.1.26. [DOI] [PubMed] [Google Scholar]
- 267.Wang HD, Xu S, Johns DG, Du Y, Quinn MT, Cayatte AJ, Cohen RA. Role of NADPH oxidase in the vascular hypertrophic and oxidative stress response to angiotensin II in mice. Circ. Res. 2001;88:947–953. doi: 10.1161/hh0901.089987. [DOI] [PubMed] [Google Scholar]
- 268.Bendall JK, Cave AC, Heymes C, Gall N, Shah AM. Pivotal role of a gp91phox-containing NADPH oxidase in angiotensin H-induced cardiac hypertrophy in mice. Circulation. 2002;105:293–296. doi: 10.1161/hc0302.103712. [DOI] [PubMed] [Google Scholar]
- 269.Xiao L, Pimentel DR, Wang J, Singh K, Colucci WS, Sawyer DB. Role of reactive oxygen species and NAD(P)H oxidase in alpha 1-adrenoceptor signaling in adult rat cardiac myocytes. Am. J. Physiol. Cell Physiol. 2002;282:C926–C934. doi: 10.1152/ajpcell.00254.2001. [DOI] [PubMed] [Google Scholar]
- 270.Touyz RM, Chen X, Tabet E, Yao G, He G, Quinn MT, Pagano PJ, Schiffrin E. L Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ. Res. 2002;90:1205–1213. doi: 10.1161/01.res.0000020404.01971.2f. [DOI] [PubMed] [Google Scholar]
- 271.Ellmark SH, Dusting GJ, Fui MN, Guzzo-Pernell N, Drummond G. R The contribution of Nox4 to NADPH oxidase activity in mouse vascular smooth muscle. Cardiovasc. Res. 2005;65:495–504. doi: 10.1016/j.cardiores.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 272.Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, Schmidt HH, Lassegue B, Griendling KK. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler. Thromb. Vasc. Biol. 2007;27:42–48. doi: 10.1161/01.ATV.0000251500.94478.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Pedruzzi E, Guichard C, Ollivier V, Driss E, Fay M, Prunet C, Marie JC, Pouzet C, Samadi M, Elbim C, O’Dowd Y, Bens M, Vandewalle A, Gougerot-Pocidalo MA, Lizard G, Ogier-Denis E. NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol. Cell. Biol. 2004;24:10703–10717. doi: 10.1128/MCB.24.24.10703-10717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Colston JT, de la Rosa SD, Strader JR, Anderson MA, Freeman G. L H202 activates Nox4 through PLA2-dependent arachidonic acid production in adult cardiac fibroblasts. FEBS Lett. 2005;579:2533–2540. doi: 10.1016/j.febslet.2005.03.057. [DOI] [PubMed] [Google Scholar]
- 275.Peng T, Lu X, Feng Q. Pivotal role of gp91phox-containing NADH oxidase in lipopolysaccharide-induced tumor necrosis factor-α expression and myocardial depression. Circulation. 2005;111:1637–1644. doi: 10.1161/01.CIR.0000160366.50210.E9. [DOI] [PubMed] [Google Scholar]
- 276.Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ. Res. 2010;106:1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Jay DB, Papaharalambus CA, Seidel-Rogol B, Dikalova AE, Lassegue B, Griendling KK. Nox5 mediates PDGF-induced proliferation in human aortic smooth muscle cells. Free Radic. Biol. Med. 2008;45:329–335. doi: 10.1016/j.freeradbiomed.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.BelAiba RS, Djordjevic T, Petry A, Diemer K, Bonello S, Banfi B, Hess J, Pogrebniak A, Bickel C, Gorlach A. NOX5 variants are functionally active in endothelial cells. Free Radic. Biol. Med. 2007;42:446–459. doi: 10.1016/j.freeradbiomed.2006.10.054. [DOI] [PubMed] [Google Scholar]
- 279.Bedard K, Jaquet V, Krause KH. NOX5: from basic biology to signaling and disease. Free Radic. Biol. Med. 2012;52:725–734. doi: 10.1016/j.freeradbiomed.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 280.Kalinina N, Agrotis A, Tararak E, Antropova Y, Kanellakis P, Ilyinskaya O, Quinn MT, Smirnov V, Bobik A. Cytochrome b558-dependent NAD(P) H oxidase-phox units in smooth muscle and macrophages of atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2002;22:2037–2043. doi: 10.1161/01.atv.0000040222.02255.0f. [DOI] [PubMed] [Google Scholar]
- 281.Ambasta RK, Schreiber JG, Janiszewski M, Busse R, Brandes RP. Noxal is a central component of the smooth muscle NADPH oxidase in mice. Free Radic. Biol. Med. 2006;41:193–201. doi: 10.1016/j.freeradbiomed.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 282.Lassègue B, Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R277–R297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 283.Maturana A, Arnaudeau S, Ryser S, Banfi B, Hossle JP, Schlegel W, Krause KH, Demaurex N. Heme histidine ligands within gp91phox modulate proton conduction by the phagocyte NADPH oxidase. J. Biol. Chem. 2001;276:30277–30284. doi: 10.1074/jbc.M010438200. [DOI] [PubMed] [Google Scholar]
- 284.Kimball RA, Saier MH. Voltage-gated H+ channels associated with human phagocyte superoxide-generating NADPH oxidases: sequence comparisons, structural predictions, and phylogenetic analyses. Mol. Membr. Biol. 2002;19:137–147. doi: 10.1080/09687680210127887. [DOI] [PubMed] [Google Scholar]
- 285.DeCoursey TE, Morgan D, Cherny VV. The gp91phox component of NADPH oxidase is not a voltage-gated proton channel. J. Gen. Physiol. 2002;120:773–779. doi: 10.1085/jgp.20028704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Takac I, Schroder K, Zhang L, Lardy B, Anilkumar N, Lambeth JD, Shah AM, Morel E, Brandes RP. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J. Biol. Chem. 2011;286:13304–13313. doi: 10.1074/jbc.M110.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Lassegue B, Griendling KK. NADPH oxidases: functions and pathologies in the vasculature. Arteriosder. Thromb. Vase. Biol. 2010;30:653–661. doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Ohara Y, Peterson TE, Harrison DG. Hypercholesterolemia increases endothelial superoxide anion production. J. Clin. Invest. 1993;91:2546–2551. doi: 10.1172/JCI116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.White CR, Darley-Usmar V, Berrington WR, McAdams M, Gore JZ, Thompson JA, Parks DA, Tarpey MM, Freeman BA. Circulating plasma xanthine oxidase contributes to vascular dysfunction in hypercholesterole-mic rabbits. Proc. Natl. Acad. Sci. USA. 1996;93:8745–8749. doi: 10.1073/pnas.93.16.8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Ekelund UE, Harrison RW, Shokek O, Thakkar RN, Tunin RS, Senzaki H, Kass DA, Marban E, Hare JM. Intravenous allopurinol decreases myocardial oxygen consumption and increases mechanical efficiency in dogs with pacing-induced heart failure. Circ. Res. 1999;85:437–145. doi: 10.1161/01.res.85.5.437. [DOI] [PubMed] [Google Scholar]
- 291.Cappola TP, Kass DA, Nelson GS, Berger RD, Rosas GO, Kobeissi ZA, Marban E, Hare JM. Allopurinol improves myocardial efficiency in patients with idiopathic dilated cardiomyopathy. Circulation. 2001;104:2407–2411. doi: 10.1161/hc4501.098928. [DOI] [PubMed] [Google Scholar]
- 292.Sarnesto A, Linder N, Raivio KO. Organ distribution and molecular forms of human xanthine dehydrogenase/xanthine oxidase protein. Lab Invest. 1996;74:48–56. [PubMed] [Google Scholar]
- 293.Berry CE, Hare JM. Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J. Physiol. 2004;555:589–606. doi: 10.1113/jphysiol.2003.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Stirpe E, Delia Corte E. The regulation of rat liver xanthine oxidase: conversion in vitro of the enzyme activity from dehydrogenase (type D) to oxidase (type 0) J. Biol. Chem. 1969;244:3855–3863. [PubMed] [Google Scholar]
- 295.Nishino T, Okamoto K, Eger BT, Pai EF, Nishino T. Mammalian xanthine oxidoreductase—mechanism of transition from xanthine dehydrogenase to xanthine oxidase. FEBS J. 2008;275:3278–3289. doi: 10.1111/j.1742-4658.2008.06489.x. [DOI] [PubMed] [Google Scholar]
- 296.Sanders SA, Eisenthal R, Harrison R. NADH oxidase activity of human xanthine oxidoreductase: generation of superoxide anion. Eur. J. Biochem. 1997;245:541–548. doi: 10.1111/j.1432-1033.1997.00541.x. [DOI] [PubMed] [Google Scholar]
- 297.Maia L, Vala A, Mira L. NADH oxidase activity of rat liver xanthine dehydrogenase and xanthine oxidase-contribution for damage mechanisms. Free Radic. Res. 2005;39:979–986. doi: 10.1080/10715760500210962. [DOI] [PubMed] [Google Scholar]
- 298.Stoner JD, Angelos MG, Clanton TL. Myocardial contractile function during postischemic low-flow reperfusion: critical thresholds of NADH and 02 delivery. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H375–H380. doi: 10.1152/ajpheart.00436.2003. [DOI] [PubMed] [Google Scholar]
- 299.Martin HM, Hancock JT, Salisbury V, Harrison R. Role of xanthine oxidoreductase as an antimicrobial agent. Infect. Immun. 2004;72:4933–1939. doi: 10.1128/IAI.72.9.4933-4939.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Adachi T, Fukushima T, Usami Y, Hirano K. Binding of human xanthine oxidase to sulphated glycosaminoglycans on the endothelial cell surface. Biochem. J. 1993;289:523–527. doi: 10.1042/bj2890523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Radi R, Rubbo H, Bush K, Freeman BA. Xanthine oxidase binding to glycosaminoglycans: kinetics and superoxide dismutase interactions of immobilised xanthine oxidase-heparin complexes. Arch. Biochem. Biophys. 1997;339:125–135. doi: 10.1006/abbi.1996.9844. [DOI] [PubMed] [Google Scholar]
- 302.Spiekermann S, Landmesser U, Dikalov S, Bredt M, Gamez G, Tatge H, Reepschlager N, Hornig B, Drexler H, Harrison D. Electron spin resonance characterization of vascular xanthine and NAD(P)H oxidase activity in patients with coronary artery disease: relation to endothelium-dependent vasodilation. Circulation. 2003;107:1383–1389. doi: 10.1161/01.cir.0000056762.69302.46. [DOI] [PubMed] [Google Scholar]
- 303.de Jong JW, Schoemaker RG, de Jonge R, Bernocchi P, Keijzer E, Harrison R, Sharma HS, Ceconi C. Enhanced expression and activity of xanthine oxidoreductase in the failing heart. J. Mol. Cell. Cardiol. 2000;32:2083–2089. doi: 10.1006/jmcc.2000.1240. [DOI] [PubMed] [Google Scholar]
- 304.Landmesser U, Spiekermann S, Dikalov S, Tatge H, Wilke R, Kohler C, Harrison DG, Hornig B, Drexler H. Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure: role of xanthine-oxidase and extracellular superoxide dismutase. Circulation. 2002;106:3073–3078. doi: 10.1161/01.cir.0000041431.57222.af. [DOI] [PubMed] [Google Scholar]
- 305.Marietta M. Nitric oxide synthase: aspects concerning structure and catalysis. Cell. 1994;78:927–930. doi: 10.1016/0092-8674(94)90268-2. [DOI] [PubMed] [Google Scholar]
- 306.Raman CS, Li H, Martasek P, Krai V, Masters BS, Poulos TL. Crystal structure of constitutive endothelial nitric oxide synthase: a paradigm for pterin function involving a novel metal center. Cell. 1998;95:939–950. doi: 10.1016/s0092-8674(00)81718-3. [DOI] [PubMed] [Google Scholar]
- 307.Channon KM. Tetrahydrobiopterin: regulator of endothelial nitric oxide synthase in vascular disease. Trends Cardiovasc. Med. 2004;14:323–327. doi: 10.1016/j.tcm.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 308.Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochem. J. 1994;298:249–258. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Nathan C. Natural resistance and nitric oxide. Cell. 1995;82:873–876. doi: 10.1016/0092-8674(95)90019-5. [DOI] [PubMed] [Google Scholar]
- 310.Garg UC, Hassid A. Nitric oxide-generating vasodilators 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis proliferation of cultured rat vascular smooth muscle cells. Clin. Invest. 1989;83:1774–1777. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.D'Souza EM, Sparks RL, Chen H, Kadowitz PJ, Jeter JR., Jr Mechanism of eNOS gene transfer inhibition of vascular smooth muscle cell proliferation. Am. J. Physiol. Cell Physiol. 2003;284:C191–C199. doi: 10.1152/ajpcell.00179.2002. [DOI] [PubMed] [Google Scholar]
- 312.Radomski MW, Palmer RM, Moncada S. Proc. Natl. Acad. Sci. USA. An L-arginine/nitric oxide pathway present in human platelets regulates aggregation. 1990;87:5193–5197. doi: 10.1073/pnas.87.13.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Knowles JW, Reddick RL, Jennette JC, Shesely EG, Smithies O, Maeda N. Enhanced atherosclerosis and kidney dysfunction in eNOS(−/−) apoE(−/−) mice are ameliorated by enalapril treatment. J. Clin. Invest. 2008;105:451–58. doi: 10.1172/JCI8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Kuhlencordt PJ, Gyurko R, Han E, Scherrer-Crosbie M, Aretz TH, Hajjar R, Picard MH, Huang PL. Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/ endothelial nitric oxide synthase double-knockout mice. Circulation. 2001;104:448–54. doi: 10.1161/hc2901.091399. [DOI] [PubMed] [Google Scholar]
- 315.Ponnuswamy P, Schrottle A, Ostermeier E, Griiner S, Huang PL, Ertl G, Hoffmann U, Nieswandt B, Kuhlencordt PJ. eNOS protects from atherosclerosis despite relevant superoxide production by the enzyme in apoE mice. PLoS One. 2012:e30193. doi: 10.1371/journal.pone.0030193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Huang PL, Huang ZH, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 317.Kojda G, Laursen JB, Ramasamy S, Kent JD, Kurz S, Burchfield J, Shesely EG, Harrison DG. Protein expression, vascular reactivity and soluble guanylate cyclase activity in mice lacking the endothelial cell nitric oxide synthase: contributions of NOS isoforms to blood pressure and heart rate control. Cardiovasc. Res. 1999;42:206–213. doi: 10.1016/s0008-6363(98)00315-0. [DOI] [PubMed] [Google Scholar]
- 318.Ohashi Y, Kawashima S, Hirata K, Yamashita T, Ishida T, Inoue N, Sakoda T, Kurihara H, Yazaki Y, Yokoyama M. Hypotension and reduced nitric oxide-elicited vasorelaxation in transgenic mice overexpressing endothelial nitric oxide synthase. J Clin. Invest. 1998;102:2061–2071. doi: 10.1172/JCI4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Scherrer-Crosbie M, Ullrich R, Bloch KD, Nakajima H, Nasseri B, Aretz HT, Lindsey ML, Vancon AC, Huang PL, Lee RT, Zapol WM, Picard MH. Endothelial nitric oxide synthase limits left ventricular remodeling after myocardial infarction in mice. Circulation. 2001;104:1286–1291. doi: 10.1161/hc3601.094298. [DOI] [PubMed] [Google Scholar]
- 320.Ichinose E, Bloch KD, Wu JC, Hataishi R, Aretz HT, Picard MH, Scherrer-Crosbie M. Pressure overload-induced LV hypertrophy and dysfunction in mice are exacerbated by congenital NOS3 deficiency. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H1070–H1075. doi: 10.1152/ajpheart.00940.2003. [DOI] [PubMed] [Google Scholar]
- 321.Feng Q, Lu X, Jones DL, Shen J, Arnold JM. Increased inducible nitric oxide synthase expression contributes to myocardial dysfunction and higher mortality after myocardial infarction in mice. Circulation. 2001;104:700–704. doi: 10.1161/hc3201.092284. [DOI] [PubMed] [Google Scholar]
- 322.Zhang P, Xu X, Hu X, van Deel ED, Zhu G, Chen Y. Inducible nitric oxide synthase deficiency protects the heart from systolic overload-induced ventricular hypertrophy and congestive heart failure. Circ. Res. 2007;100:1089–1098. doi: 10.1161/01.RES.0000264081.78659.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Detmers PA, Hernandez M, Mudgett J, Hassing H, Burton C, Mundt S, Chun S, Fletcher D, Card DJ, Lisnock J, Weikel R, Bergstrom JD, Shevell DE, Hermanowski-Vosatka A, Sparrow CP, Chao YS, Rader DJ, Wright SD, Pure E. Deficiency in inducible nitric oxide synthase results in reduced atherosclerosis in apolipoprotein E-deficient mice. J. Immunol. 2000;165:3430–3435. doi: 10.4049/jimmunol.165.6.3430. [DOI] [PubMed] [Google Scholar]
- 324.Kuhlencordt PJ, Chen J, Han E, Astern J, Huang P. L Genetic deficiency of inducible nitric oxide synthase reduces atherosclerosis and lowers plasma lipid peroxides in apolipoprotein E-knockout mice. Circulation. 2001;103:3099–3104. doi: 10.1161/01.cir.103.25.3099. [DOI] [PubMed] [Google Scholar]
- 325.Miyoshi T, Li Y, Shih DM, Wang X, Laubach VE, Matsumoto AH, Helm GA, Lusis AJ, Shi W. Deficiency of inducible NO synthase reduces advanced but not early atherosclerosis in apolipoprotein E-deficient mice. Life Sci. 2006;79:525–531. doi: 10.1016/j.lfs.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 326.Heger J, Godecke A, Flogel U, Merx MW, Molojavyi A, Kuhn-Velten WN, Schrader J. Cardiac-specific overexpression of inducible nitric oxide synthase does not result in severe cardiac dysfunction. Circ. Res. 2002;90:93–99. doi: 10.1161/hh0102.102757. [DOI] [PubMed] [Google Scholar]
- 327.Loscalzo J. Adverse effects of supplemental L-arginine in atherosclerosis: consequences of methylation stress in a complex catabolism? Arteriosder Thromb. Vasc. Biol. 2003;23:3–5. doi: 10.1161/01.atv.0000040860.71626.9d. [DOI] [PubMed] [Google Scholar]
- 328.Bredt DS, Glatt CE, Hwang PM, Fotuhi M, Dawson TM, Snyder SH. Nitric oxide synthase protein and mRNA are discretely localized in neuronal populations of the mammalian CNS together with NADPH diaphorase. Neuron. 1991;7:615–624. doi: 10.1016/0896-6273(91)90374-9. [DOI] [PubMed] [Google Scholar]
- 329.Mungrue IN, Bredt DS. nNOS at a glance: implications for brain and brawn J. Cell Sci. 2004;117:2627–2629. doi: 10.1242/jcs.01187. [DOI] [PubMed] [Google Scholar]
- 330.Tojo A, Kimoto M, Wilcox CS. Renal expression of constitutive NOS and DDAH: separate effects of salt intake and angiotensin. Kidney Int. 2000;58:2075–2083. doi: 10.1111/j.1523-1755.2000.00380.x. [DOI] [PubMed] [Google Scholar]
- 331.Papapetropoulos A, Rudic RD, Sessa WC. Molecular control of nitric oxide synthases in the cardiovascular system. Cardiovasc. Res. 1999;43:509–520. doi: 10.1016/s0008-6363(99)00161-3. [DOI] [PubMed] [Google Scholar]
- 332.Burger DE, Lu X, Lei M, Xiang EL, Hammoud L, Jiang M, Wang H, Jones DL, Sims SM, Feng Q. Neuronal nitric oxide synthase protects against myocardial infarction-induced ventricular arrhythmia and mortality in mice. Circulation. 2009;120:1345–1354. doi: 10.1161/CIRCULATIONAHA.108.846402. [DOI] [PubMed] [Google Scholar]
- 333.Kuhlencordt PJ, Hotten S, Schodel J, Rutzel S, Hu K, Widder J, Marx A, Huang PL, Ertl G. Atheroprotective effects of neuronal nitric oxide synthase in apolipoprotein E knockout mice. Arteriosder. Thromb. Vase. Biol. 2006;26:1539–1544. doi: 10.1161/01.ATV.0000223143.88128.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Khan SA, Lee K, Minhas KM, Gonzalez DR, Raju SV, Tejani AD, Li D, Berkowitz DE, Hare JM. Neuronal nitric oxide synthase negatively regulates xanthine oxidoreductase inhibition of cardiac excitation-contraction coupling. Proc. Natl. Acad. Sci. USA. 2004;101:15944–15948. doi: 10.1073/pnas.0404136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Droge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 336.Dobrian AD, Lieb DC, Cole BK, Taylor-Fishwick DA, Chakrabarti SK, Nadler JL. Functional and pathological roles of the 12- and 15-lipoxygenases. Prog. Lipid Res. 2011;50:115–131. doi: 10.1016/j.plipres.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Funk CD, Chen XS, Johnson EN, Zhao L. Lipoxygenase genes and their targeted disruption. Prostaglandins Other Lipid Mediat. 2002;68-69:303–312. doi: 10.1016/s0090-6980(02)00036-9. [DOI] [PubMed] [Google Scholar]
- 338.Chen XS, Kurre U, Jenkins NA, Copeland NG, Funk CD. CDNA cloning, expression, mutagenesis of C-terminal isoleucine, genomic structure, and chromosomal localizations of murine 12-lipoxygenases. J. Biol. Chem. 1994;269:13979–13987. [PubMed] [Google Scholar]
- 339.Bäck M. Leukotriene signaling in atherosclerosis and ischemia. Cardiovasc. Drugs Ther. 2009;23:41–48. doi: 10.1007/s10557-008-6140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Bäck M, Bu DX, Bränström R, Sheikine Y, Yan ZQ, Hansson GK. Leukotriene B4 signaling through NF-kappaB-dependent BLT1 receptors on vascular smooth muscle cells in atherosclerosis and intimal hyperplasia. Proc. Natl. Acad. Sci. USA. 2005;102:17501–17506. doi: 10.1073/pnas.0505845102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Heller EA, Liu E, Tager AM, Sinha S, Roberts JD, Koehn SL, Libby P, Aikawa ER, Chen JQ, Huang P, Freeman MW, Moore KJ, Luster AD, Gerszten RE. Inhibition of atherogenesis in BLT1-deficient mice reveals a role for LTB4 BLT1 in smooth muscle cell recruitment. Circulation. 2005;112:578–586. doi: 10.1161/CIRCULATIONAHA.105.545616. [DOI] [PubMed] [Google Scholar]
- 342.Aiello RJ, Bourassa PA, Lindsey S, Weng W, Freeman A, Showell HJ. Leukotriene B4 receptor antagonism reduces monocytic foam cells in mice. Arterioscler. Thromb. Vasc. Biol. 2002;22:443–449. doi: 10.1161/hq0302.105593. [DOI] [PubMed] [Google Scholar]
- 343.Belkner J, Stender H, Kühn H. The rabbit 15-lipoxygenase preferentially oxygenates LDL cholesterol esters, and this reaction does not require vitamin E. J. Biol. Chem. 1998;273:23225–23232. doi: 10.1074/jbc.273.36.23225. [DOI] [PubMed] [Google Scholar]
- 344.Harkewicz R, Hartvigsen K, Almazan F, Dennis EA, Witztum JL, Miller YI. Cholesteryl ester hydroperoxides are biologically active components of minimally oxidized low density lipoprotein. J. Biol. Chem. 2008;283:10241–10251. doi: 10.1074/jbc.M709006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Xu W, Takahashi Y, Sakashita T, Iwasaki T, Hattori H, Yoshimoto T. Low density lipoprotein receptor-related protein is required for macrophage-mediated oxidation of low density lipoprotein by 12/15-lipoxygenase. J. Biol. Chem. 2001;276:36454–36459. doi: 10.1074/jbc.M105093200. [DOI] [PubMed] [Google Scholar]
- 346.Zhu H, Takahashi Y, Xu W, Kawajiri H, Murakami T, Yamamoto M, Iseki S, Iwasaki T, Hattori H, Yoshimoto T. Low density lipoprotein receptor-related protein-mediated membrane translocation of 12/15-lipox-ygenase is required for oxidation of low density lipoprotein by macrophages. J. Biol. Chem. 2003;278:13350–13355. doi: 10.1074/jbc.M212104200. [DOI] [PubMed] [Google Scholar]
- 347.Cyrus T, Praticò D, Zhao L, Witztum JL, Rader DJ, Rokach J, FitzGerald GA, Funk CD. Absence of 12/15-lipoxygenase expression decreases lipid peroxidation and atherogenesis in apolipoprotein E-deficient mice. Circulation. 2001;103:2277–2282. doi: 10.1161/01.cir.103.18.2277. [DOI] [PubMed] [Google Scholar]
- 348.Patricia Kim MK, Harper JA, Shih CM, Berliner PT, Natarajan JA, Nadler R, Hedrick JLCC. Lipoxygenase products increase monocyte adhesion to human aortic endothelial cells. Arterioscler. Thromb. Vasc. Biol. 1999;19:2615–2622. doi: 10.1161/01.atv.19.11.2615. [DOI] [PubMed] [Google Scholar]
- 349.Reilly KB, Srinivasan S, Hatley ME, Patricia MK, Lannigan J, Bolick DT, Vandenhoff G, Pei H, Natarajan R, Nadler JL, Hedrick CC. 12/15-Lipoxygenase activity mediates inflammatory monocyte/endothelial interactions and atherosclerosis in vivo. J. Biol. Chem. 2004;279:9440–9450. doi: 10.1074/jbc.M303857200. [DOI] [PubMed] [Google Scholar]
- 350.Bolick DT, Orr AW, Whetzel A, Srinivasan S, Hatley ME, Schwartz MA, Hedrick CC. 12/15-Lipoxygenase regulates intercellular adhesion molecule-1 expression and monocyte adhesion to endothelium through activation of RhoA and nuclear factor-kappaB. Arterioscler. Thromb. Vasc. Biol. 2005;25:2301–2307. doi: 10.1161/01.ATV.0000186181.19909.a6. [DOI] [PubMed] [Google Scholar]
- 351.Bolick DT, Srinivasan S, Whetzel A, Fuller LC, Hedrick CC. 12/15 Lipoxygenase mediates monocyte adhesion to aortic endothelium in apoli-poprotein E-deficient mice through activation of RhoA and NFκB. Arterioscler. Thromb. Vasc. Biol. 2006;26:1260–1266. doi: 10.1161/01.ATV.0000217909.09198.d6. [DOI] [PubMed] [Google Scholar]
- 352.Harats D, Shaish A, George J, Mulkins M, Kurihara H, Levkovitz H, Sigal E. Overexpression of 15-lipoxygenase in vascular endothelium accelerates early atherosclerosis in LDL receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2000;20:2100–2105. doi: 10.1161/01.atv.20.9.2100. [DOI] [PubMed] [Google Scholar]
- 353.Kayama Y, Minamino T, Toko H, Sakamoto M, Shimizu I, Takahashi H, Okada S, Tateno K, Moriya J, Yokoyama M, Nojima A, Yoshimura M, Egashira K, Aburatani H, Komuro I. Cardiac 12/15 lipoxygenase-induced inflammation is involved in heart failure. J. Exp. Med. 2009;206:1565–1574. doi: 10.1084/jem.20082596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Brennan ML, Wu W, Fu X, Shen Z, Song W, Frost H, Vadseth C, Narine L, Lenkiewicz E, Borchers MT, Lusis AJ, Lee JJ, Lee NA, Abu-Soud HM, Ischiropoulos H, Hazen SL. A tale of two controversies: defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. J. Biol. Chem. 2002;277:17415–17427. doi: 10.1074/jbc.M112400200. [DOI] [PubMed] [Google Scholar]
- 355.Zhang R, Brennan ML, Shen Z, MacPherson JC, Schmitt D, Molenda CE, Hazen SL. Myeloperoxidase functions as a major enzymatic catalyst for initiation of lipid peroxidation at sites of inflammation. J. Biol. Chem. 2002;277:46116–46122. doi: 10.1074/jbc.M209124200. [DOI] [PubMed] [Google Scholar]
- 356.van Leeuwen M, Gijbels MJ, Duijvestijn A, Smook M, van de Gaar MJ, Heeringa P, de Winther MP, Tervaert JW. Accumulation of myeloperoxidase-positive neutrophils in atherosclerotic lesions in LDLR-/mice. Arterioscler. Thromb. Vasc. Biol. 2008;28:84–89. doi: 10.1161/ATVBAHA.107.154807. [DOI] [PubMed] [Google Scholar]
- 357.Wang Z, Nicholls SJ, Rodriguez ER, Kummu O, Horkko S, Barnard J, Reynolds WF, Topol EJ, DiDonato JA, Hazen SL. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat. Med. 2007;13:1176–1184. doi: 10.1038/nm1637. [DOI] [PubMed] [Google Scholar]
- 358.Holzer M, Gauster M, Pfeifer T, Wadsack C, Fauler G, Stiegler P, Koefeler H, Beubler E, Schuligoi R, Heinemann A, Marsche G. Protein carbamylation renders high-density lipoprotein dysfunctional. Antioxid. Redox Signaling. 2011;14:2337–2346. doi: 10.1089/ars.2010.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Shao B, Pennathur S, Heinecke JW. Myeloperoxidase targets apolipo-protein A-I, the major high density lipoprotein protein, for site-specific oxidation in human atherosclerotic lesions. J. Biol. Chem. 2012;287:6375–6386. doi: 10.1074/jbc.M111.337345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Klinke A, Nussbaum C, Kubala L, Friedrichs K, Rudolph TK, Rudolph V, Paust HJ, Schröder C, Benten D, Lau D, Szocs K, Furtmüller PG, Heeringa P, Sydow K, Duchstein HJ, Ehmke H, Schumacher U, Meinertz T, Sperandio M, Baldus S. Myeloperoxidase attracts neutrophils by physical forces. Blood. 2011;117:1350–1358. doi: 10.1182/blood-2010-05-284513. [DOI] [PubMed] [Google Scholar]
- 361.Ferrante G, Nakano M, Prati F, Niccoli G, Mallus MT, Ramazzotti V, Montone RA, Kolodgie FD, Virmani R, Crea F. High levels of systemic myeloperoxidase are associated with coronary plaque erosion in patients with acute coronary syndromes: a clinicopathological study. Circulation. 2010;122:2505–2513. doi: 10.1161/CIRCULATIONAHA.110.955302. [DOI] [PubMed] [Google Scholar]
- 362.Eiserich JP, Hristova M, Cross CE, Jones AD, Freeman BA, Halliwell B, van der, Vliet A. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- 363.Podrez EA, Schmitt D, Hoff HF, Hazen SL. Myeloperoxidase-generated reactive nitrogen species convert LDL into an atherogenic form in vitro. J. Clin. Invest. 1999;103:1547–1560. doi: 10.1172/JCI5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Podrez EA, Febbraio M, Sheibani N, Schmitt D, Silverstein RL, Hajjar DP, Cohen PA, Frazier WA, Hoff HF, Hazen SL. Macrophage scavenger receptor CD36 is the major receptor for LDL modified by monocyte-generated reactive nitrogen species. J. Clin. Invest. 2000;105:1095–1108. doi: 10.1172/JCI8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Zhang R, Shen Z, Nauseef WM, Hazen SL. Defects in leukocyte-mediated initiation of lipid peroxidation in plasma as studied in myeloperoxidase-deficient subjects: systematic identification of multiple endogenous diffusible substrates for myeloperoxidase in plasma. Blood. 2002;99:1802–1810. [PubMed] [Google Scholar]
- 366.Byun J, Mueller DM, Fabjan JS, Heinecke JW. Nitrogen dioxide radical generated by the myeloperoxidase-hydrogen peroxide-nitrite system promotes lipid peroxidation of low density lipoprotein. FEBS Lett. 1999;455:243–246. doi: 10.1016/s0014-5793(99)00893-5. [DOI] [PubMed] [Google Scholar]
- 367.Jiang Q, Hurst JK. Relative chlorinating, nitrating, and oxidizing capabilities of neutrophils determined with phagocytosable probes. J. Biol. Chem. 1997;272:32767–32772. doi: 10.1074/jbc.272.52.32767. [DOI] [PubMed] [Google Scholar]
- 368.Nauseef WM. The proper study of mankind. J. Clin. Invest. 2001;107:401–403. doi: 10.1172/JCI11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 370.Nathan C, Shiloh MU. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. USA. 2000;97:8841–8848. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 372.Rubanyi GM, Lorenz RR, Vanhoutte PM. Bioassay of endothelium-derived relaxing factor(s): inactivation by catecholamines. Am. J. Physiol. 1985;249:H95–H101. doi: 10.1152/ajpheart.1985.249.1.H95. [DOI] [PubMed] [Google Scholar]
- 373.Ignarro LJ, Harbison RG, Wood KS, Kadowitz PJ. Activation of purified soluble guanylate cyclase by endothelium-derived relaxing factor from intrapulmonary artery and vein: stimulation by acetylcholine, bradykinin and arachidonic acid. J. Pharmacol. Exp. Ther. 1986;237:893–900. [PubMed] [Google Scholar]
- 374.Morgado M, Cairrão E, Santos-Silva AJ, Verde I. Cyclic nucleotide-dependent relaxation pathways in vascular smooth muscle. Cell Mol. Life Sci. 2012;69:247–266. doi: 10.1007/s00018-011-0815-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Rubanyi GM, Vanhoutte PM. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am. J. Physiol. 1986;250:H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- 376.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 377.Palmer RM, Moncada S. A novel citrulline-forming enzyme implicated in the formation of nitric oxide by vascular endothelial cells. Biochem. Biophys. Res. Commun. 1989;158:348–352. doi: 10.1016/s0006-291x(89)80219-0. [DOI] [PubMed] [Google Scholar]
- 378.Förstermann U, Münzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 379.Schulz E, Gori T, Münzel T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens. Res. 2011;34:665–673. doi: 10.1038/hr.2011.39. [DOI] [PubMed] [Google Scholar]
- 380.Shimokawa H, Vanhoutte PM. Angiographic demonstration of hyper-constriction induced by serotonin and aggregating platelets in porcine coronary arteries with regenerated endothelium. J. Am. Coll. Cardiol. 1991;17:1197–1202. doi: 10.1016/0735-1097(91)90854-3. [DOI] [PubMed] [Google Scholar]
- 381.Motley Eguchi ED, Patterson K, Palmer MM, Suzuki PD, Eguchi HS. Mechanism of endothelial nitric oxide synthase phosphorylation and activation by thrombin. Hypertension. 2007;49:577–583. doi: 10.1161/01.HYP.0000255954.80025.34. [DOI] [PubMed] [Google Scholar]
- 382.Vanhoutte PM, Shimokawa H, Tang EH, Feletou M. Endothelial dysfunction and vascular disease. Acta Physiol. (Oxford) 2009;196:193–222. doi: 10.1111/j.1748-1716.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- 383.Vanhoutte PM. Endothelial dysfunction and atherosclerosis. Eur. Heart J. 1997;18:E19–29. doi: 10.1016/s0195-668x(97)90005-1. [DOI] [PubMed] [Google Scholar]
- 384.Vanhoutte PM. Endothelial control of vasomotor function: from health to coronary disease. Circ. J. 2003;67:572–575. doi: 10.1253/circj.67.572. [DOI] [PubMed] [Google Scholar]
- 385.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Münzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 386.Gokce N, Keaney JE, Jr, Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risks tratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation. 2002;105:1567–1572. doi: 10.1161/01.cir.0000012543.55874.47. [DOI] [PubMed] [Google Scholar]
- 387.Behrendt D, Ganz P. Endothelial function: from vascular biology to clinical applications. Am. J. Cardiol. 2002;90:40L–48L. doi: 10.1016/s0002-9149(02)02963-6. [DOI] [PubMed] [Google Scholar]
- 388.Fischer D, Rossa S, Landmesser U, Spiekermann S, Engberding N, Hornig B, Drexler H. Endothelial dysfunction in patients with chronic heart failure is independently associated with increased incidence of hospitaliza-tion, cardiac transplantation, or death. Eur. Heart J. 2005;26:65–69. doi: 10.1093/eurheartj/ehi001. [DOI] [PubMed] [Google Scholar]
- 389.Schächinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 390.Shechter M, Issachar A, Marai I, Koren-Morag N, Freinark D, Shahar Y, Shechter A, Feinberg MS. Long-term association of brachial artery flow-mediated vasodilation and cardiovascular events in middle-aged subjects with no apparent heart disease. Int. J. Cardiol. 2009;134:52–58. doi: 10.1016/j.ijcard.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 391.Brevetti G, Silvestro A, Schiano V, Chiariello M. Endothelial dysfunction and cardiovascular risk prediction in peripheral arterial disease: additive value of flow-mediated dilation to ankle-brachial pressure index. Circulation. 2003;108:2093–2098. doi: 10.1161/01.CIR.0000095273.92468.D9. [DOI] [PubMed] [Google Scholar]
- 392.Perrone-Filardi P, Cuocolo A, Brevetti G, Silvestro A, Storto G, Delle-grottaglie S, Corrado L, Cafiero M, Camerino R, Polimeno M, Zarrilli A, Caiazzo G, Maglione A, Petretta A, Chiariello M. Relation of brachial artery flow-mediated vasodilation to significant coronary artery disease in patients with peripheral arterial disease. Am. J. Cardiol. 2005;96:1337–1341. doi: 10.1016/j.amjcard.2005.06.084. [DOI] [PubMed] [Google Scholar]
- 393.Dixon LJ, Morgan DR, Hughes SM, McGrath LT, El-Sherbeeny NA, Plumb RD, Devine A, Leahey W, Johnston GD, McVeigh GE. Functional consequences of endothelial nitric oxide synthase uncoupling in congestive cardiac failure. Circulation. 2003;107:1725–1728. doi: 10.1161/01.CIR.0000066283.13253.78. [DOI] [PubMed] [Google Scholar]
- 394.Kelm M, Preik M, Hafner DJ, Strauer BE. Evidence for a multifactorial process involved in the impaired flow response to nitric oxide in hypertensive patients with endothelial dysfunction? Hypertension. 1996;27:346–353. doi: 10.1161/01.hyp.27.3.346. [DOI] [PubMed] [Google Scholar]
- 395.Cooke JP. Does ADMA cause endothelial dysfunction. Arterioscler. Thromb. Vasc. Biol. 2000;20:2032–2037. doi: 10.1161/01.atv.20.9.2032. [DOI] [PubMed] [Google Scholar]
- 396.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ. Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 397.Mugge A, Elwell JH, Peterson TE, Hofmeyer TG, Heistad DD, Harrison DG. Chronic treatment with polyethylene-glycolated superoxide dismutase partially restores endothelium-dependent vascular relaxations in cholesterol-fed rabbits. Circ. Res. 1991;69:1293–1300. doi: 10.1161/01.res.69.5.1293. [DOI] [PubMed] [Google Scholar]
- 398.Wassmann S, Czech T, van Eickels M, Fleming I, Böhm M, Nickenig G. Inhibition of diet-induced atherosclerosis and endothelial dysfunction in apolipoprotein E/angiotensin II type 1A receptor double-knockout mice. Circulation. 2004;110:3062–3067. doi: 10.1161/01.CIR.0000137970.47771.AF. [DOI] [PubMed] [Google Scholar]
- 399.Tanner FC, Noll G, Boulanger CM, Luscher TF. Oxidized low density lipoproteins inhibit relaxations of porcine coronary arteries: role of scavenger receptor and endothelium-derived nitric oxide. Circulation. 1991;83:2012–2020. doi: 10.1161/01.cir.83.6.2012. [DOI] [PubMed] [Google Scholar]
- 400.Ting HH, Timimi FK, Boles KS, Creager SJ, Ganz P, Creager MA. Vitamin C improves endothelium-dependent vasodilation in patients with non-insulin-dependent diabetes mellitus. J. Clin. Invest. 1996;97:22–28. doi: 10.1172/JCI118394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Solzbach U, Hornig B, Jeserich M, Just H. Vitamin C improves endothelial dysfunction of epicardial coronary arteries in hypertensive patients. Circulation. 1997;96:1513–1519. doi: 10.1161/01.cir.96.5.1513. [DOI] [PubMed] [Google Scholar]
- 402.Lynch SM, Frei B, Morrow JD, Roberts LJ, 2nd, Xu A, Jackson T, Reyna R, Klevay LM, Vita JA, Keaney JF., Jr Vascular superoxide dismutase deficiency impairs endothelial vasodilator function through direct inactivation of nitric oxide and increased lipid peroxidation. Arterioscler. Thromb. Vasc. Biol. 1997;17:2975–2981. doi: 10.1161/01.atv.17.11.2975. [DOI] [PubMed] [Google Scholar]
- 403.Ohashi M, Runge MS, Faraci FM, Heistad DD. MnSOD deficiency increases endothelial dysfunction in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2006;26:2331–2336. doi: 10.1161/01.ATV.0000238347.77590.c9. [DOI] [PubMed] [Google Scholar]
- 404.Jung O, Marklund SL, Geiger H, Pedrazzini T, Busse R, Brandes RP. Extracellular superoxide dismutase is a major determinant of nitric oxide bioavailability: in vivo and ex vivo evidence from ecSOD-deficient mice. Circ. Res. 2003;93:622–629. doi: 10.1161/01.RES.0000092140.81594.A8. [DOI] [PubMed] [Google Scholar]
- 405.Kerr S, Brosnan MJ, McIntyre M, Reid JL, Dominiczak AF, Hamilton CA. Superoxide anion production is increased in a model of genetic hypertension: role of the endothelium. Hypertension. 1999;33:1353–1358. doi: 10.1161/01.hyp.33.6.1353. [DOI] [PubMed] [Google Scholar]
- 406.Romero MJ, Platt DH, Tawfik HE, Labazi M, El-Remessy AB, Bartoli M, Caldwell RB, Caldwell RW. Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circ. Res. 2008;102:95–102. doi: 10.1161/CIRCRESAHA.107.155028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Zhang C, Hein TW, Wang W, Miller MW, Fossum TW, McDonald MM, Humphrey JD, Kuo L. Upregulation of vascular arginase in hypertension decreases nitric oxide-mediated dilation of coronary arterioles. Hypertension. 2004;44:935–943. doi: 10.1161/01.HYP.0000146907.82869.f2. [DOI] [PubMed] [Google Scholar]
- 408.Hein TW, Zhang C, Wang W, Chang CI, Thengchaisri N, Kuo L. Ischemia-reperfusion selectively impairs nitric oxide-mediated dilation in coronary arterioles: counteracting role of arginase. FASEB J. 2003;17:2328–2330. doi: 10.1096/fj.03-0115fje. [DOI] [PubMed] [Google Scholar]
- 409.Ryoo S, Gupta G, Benjo A, Lim HK, Camara A, Sikka G, Lim HK, Sohi D, Santhanam L, Soucy K, Tuday E, Baraban E, Ilies M, Gerstenblith G, Nyhan D, Shoukas A, Christianson DW, Alp NJ, Champion HC, Huso D, Berkowitz DE. Endothelial arginase II: a novel target for the treatment of atherosclerosis. Circ. Res. 2008;102:923–932. doi: 10.1161/CIRCRESAHA.107.169573. [DOI] [PubMed] [Google Scholar]
- 410.White AR, Ryoo S, Li D, Champion HC, Steppan J, Wang D, Nyhan D, Shoukas AA, Hare JM, Berkowitz DE. Knockdown of arginase I restores NO signaling in the vasculature of old rats. Hypertension. 2006;47:245–251. doi: 10.1161/01.HYP.0000198543.34502.d7. [DOI] [PubMed] [Google Scholar]
- 411.Kim JH, Bugaj LJ, Oh YJ, Bivalacqua TJ, Ryoo S, Soucy KG, Santhanam L, Webb A, Camara A, Sikka G, Nyhan D, Shoukas AA, Ilies M, Christianson DW, Champion HC, Berkowitz DE. Arginase inhibition restores NOS coupling and reverses endothelial dysfunction vascular stiffness in old rats. J. Appl. Physiol. 2009;107:1249–1257. doi: 10.1152/japplphysiol.91393.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Shinozaki K, Kashiwagi A, Nishio Y, Okamura T, Yoshida Y, Masada M, Toda N, Kikkawa R. Abnormal biopterin metabolism is a major cause of impaired endothelium-dependent relaxation through nitric oxide/O2-imbalance in insulin-resistant rat aorta. Diabetes. 1999;48:2437–2445. doi: 10.2337/diabetes.48.12.2437. [DOI] [PubMed] [Google Scholar]
- 413.Meininger CJ, Marinos RS, Hatakeyama K, Martinez-Zaguilan R, Rojas JD, Kelly KA, Wu G. Impaired nitric oxide production in coronary endothelial cells of the spontaneously diabetic BB rat is due to tetrahydro-biopterin deficiency. Biochem. J. 2000;349:353–356. doi: 10.1042/0264-6021:3490353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Heitzer T, Brockhoff C, Mayer B, Warnholtz A, Mollnau H, Henne S, Meinertz T, Münzel T. Tetrahydrobiopterin improves endothelium-dependent vasodilation in chronic smokers: evidence for a dysfunctional nitric oxide synthase. Circ. Res. 2000;86:E36–E41. doi: 10.1161/01.res.86.2.e36. [DOI] [PubMed] [Google Scholar]
- 415.Takimoto E, Champion HC, Li M, Ren S, Rodriguez ER, Tavazzi B, Lazzarino G, Paolocci N, Gabrielson KL, Wang Y, Kass DA. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J. Clin. Invest. 2005;115:1221–1231. doi: 10.1172/JCI21968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Moens AL, Takimoto E, Tocchetti CG, Chakir K, Bedja D, Cormaci G, Ketner EA, Majmudar M, Gabrielson K, Halushka MK, Mitchell JB, Biswal S, Channon KM, Wolin MS, Alp NJ, Paolocci N, Champion HC, Kass DA. Reversal of cardiac hypertrophy and fibrosis from pressure overload by tetrahydrobiopterin: efficacy of recoupling nitric oxide synthase as a therapeutic strategy. Circulation. 2008;117:2626–2636. [Google Scholar]
- 417.Ozaki M, Kawashima S, Yamashita T, Hirase T, Namiki M, Inoue N, Hirata K, Yasui H, Sakurai H, Yoshida Y, Masada M, Yokoyama M. Overexpression of endothelial nitric oxide synthase accelerates atherosclerotic lesion formation in apoE-deficient mice. J. Clin. Invest. 2002;110:331–340. doi: 10.1172/JCI15215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Bendall JK, Alp NJ, Warrick N, Cai S, Adlam D, Rockett K, Yokoyama M, Kawashima S, Channon KM. Stoichiometric relationships between endothelial tetrahydrobiopterin, endothelial NO synthase (eNOS) activity, and eNOS coupling in vivo: insights from transgenic mice with endothelial-targeted GTP cyclohydrolase 1 and eNOS overexpression. Circ. Res. 2005;97:864–871. doi: 10.1161/01.RES.0000187447.03525.72. [DOI] [PubMed] [Google Scholar]
- 419.Alp NJ, McAteer MA, Khoo J, Choudhury RP, Channon KM. Increased endothelial tetrahydrobiopterin synthesis by targeted transgenic GTP-cyclohydrolase I overexpression reduces endothelial dysfunction and atherosclerosis in ApoE-knockout mice. Arterioscler. Thromb. Vasc. Biol. 2004;24:445–450. doi: 10.1161/01.ATV.0000115637.48689.77. [DOI] [PubMed] [Google Scholar]
- 420.Takaya T, Hirata K, Yamashita T, Shinohara M, Sasaki N, Inoue N, Yada T, Goto M, Fukatsu A, Hayashi T, Alp NJ, Channon KM, Yokoyama M, Kawashima S. A specific role for eNOS-derived reactive oxygen species in atherosclerosis progression Arterioscler. Thromb. Vasc. Biol. 2007;27:1632–1637. doi: 10.1161/ATVBAHA.107.142182. [DOI] [PubMed] [Google Scholar]
- 421.Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T, Harrison DG. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103:1282–1288. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- 422.Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J. Clin. Invest. 2002;109:817–826. doi: 10.1172/JCI14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Juonala M, Viikari JS, Alfthan G, Marniemi J, Kähönen M, Taittonen L, Laitinen T, Raitakari OT. Brachial artery flow-mediated dilation and asymmetrical dimethylarginine in the cardiovascular risk in young Finns study. Circulation. 2007;116:1367–1373. doi: 10.1161/CIRCULATIONAHA.107.690016. [DOI] [PubMed] [Google Scholar]
- 424.Boger RH, Sydow K, Borlak J, Thum T, Lenzen H, Schubert B, Tsikas D, Bode-Boger SM. LDL cholesterol upregulates synthesis of asymmetrical dimethylarginine in human endothelial cells: involvement of S-adenosylmethionine-dependent methyltransferases. Circ. Res. 2000;87:99–105. doi: 10.1161/01.res.87.2.99. [DOI] [PubMed] [Google Scholar]
- 425.Tran CT, Leiper JM, Vallance P. The DDAH/ADMA/NOS pathway. Atherosclerosis. Suppl. 2003;4:33–40. doi: 10.1016/s1567-5688(03)00032-1. [DOI] [PubMed] [Google Scholar]
- 426.Cardillo C, Kilcoyne CM, Cannon RO, 3rd, Quyyumi AA, Panza JA. Xanthine oxidase inhibition with oxypurinol improves endothelial vasodilator function in hypercholesterolemic but not in hypertensive patients. Hypertension. 1997;30:57–63. doi: 10.1161/01.hyp.30.1.57. [DOI] [PubMed] [Google Scholar]
- 427.Farquharson CA, Butler R, Hill A, Belch JJ, Struthers AD. Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation. 2002;106:221–226. doi: 10.1161/01.cir.0000022140.61460.1d. [DOI] [PubMed] [Google Scholar]
- 428.Doehner W, Schoene N, Rauchhaus M, Leyva-Leon F, Pavitt DV, Reaveley DA, Schuler G, Coats AJ, Anker SD, Hambrecht R. Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: results from 2 placebo-controlled studies. Circulation. 2002;105:2619–2624. doi: 10.1161/01.cir.0000017502.58595.ed. [DOI] [PubMed] [Google Scholar]
- 429.Dworakowski R, Walker S, Momin A, Desai J, El-Gamel A, Wendler O, Kearney MT, Shah AM. Reduced nicotinamide adenine dinucleotide phosphate oxidase-derived superoxide and vascular endothelial dysfunction in human heart failure. J. Am Coll Cardiol. 2008;51:1349–1356. doi: 10.1016/j.jacc.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 430.Wind S, Beuerlein K, Armitage ME, Taye A, Kumar AH, Janowitz D, Neff C, Shah AM, Wingler K, Schmidt HH. Oxidative stress and endothelial dysfunction in aortas of aged spontaneously hypertensive rats by NOX1/2 is reversed by NADPH oxidase inhibition. Hypertension. 2010;56:490–497. doi: 10.1161/HYPERTENSIONAHA.109.149187. [DOI] [PubMed] [Google Scholar]
- 431.Dikalova AE, Gόgora MC, Harrison DG, Lambeth JD, Dikalov S, Griendling KK. Upregulation of Nox1 in vascular smooth muscle leads to impaired endothelium-dependent relaxation via eNOS uncoupling. Am. J. Physiol. Heart. Circ. Physiol. 2010;299:H673–H679. doi: 10.1152/ajpheart.00242.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kanaide H, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J. Clin. Invest. 2000;106:1521–1530. doi: 10.1172/JCI10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Miura H, Wachtel RE, Liu Y, Loberiza FR, Jr, Saito T, Miura M, Gutterman DD. Flow-induced dilation of human coronary arterioles: important role of Ca2+-activated K+ channels. Circulation. 2001;103:1992–1998. doi: 10.1161/01.cir.103.15.1992. [DOI] [PubMed] [Google Scholar]
- 434.Cosentino F, Katusić ZS. Tetrahydrobiopterin and dysfunction of endothe-lial nitric oxide synthase in coronary arteries. Circulation. 1995;91:139–144. doi: 10.1161/01.cir.91.1.139. [DOI] [PubMed] [Google Scholar]
- 435.Cosentino F, Barker JE, Brand MP, Heales SJ, Werner ER, Tippins JR, West N, Channon KM, Volpe M, Lüscher TF. Reactive oxygen species mediate endothelium-dependent relaxations in tetrahydrobiopterin-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2001;21:496–502. doi: 10.1161/01.atv.21.4.496. [DOI] [PubMed] [Google Scholar]
- 436.Suvorava T, Lauer N, Kumpf S, Jacob R, Meyer W, Kojda G. Endogenous vascular hydrogen peroxide regulates arteriolar tension in vivo. Circulation. 2005;112:2487–2495. doi: 10.1161/CIRCULATIONAHA.105.543157. [DOI] [PubMed] [Google Scholar]
- 437.Chrissobolis S, Didion SP, Kinzenbaw DA, Schrader LI, Dayal S, Lentz SR, Faraci FM. Glutathione peroxidase-1 plays a major role in protecting against angiotensin II-induced vascular dysfunction. Hypertension. 2008;51:872–877. doi: 10.1161/HYPERTENSIONAHA.107.103572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Madamanchi NR, Vendrov A, Runge MS. Oxidative stress vascular disease. Arterioscler. Thromb. Vasc. Biol. 2005;25:29–38. doi: 10.1161/01.ATV.0000150649.39934.13. [DOI] [PubMed] [Google Scholar]
- 439.Hulsmans M, Holvoet P. The vicious circle between oxidative stress and inflammation in atherosclerosis. J. Cell. Mol. Med. 2010;14:70–78. doi: 10.1111/j.1582-4934.2009.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Witztum JL, Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J. Clin. Invest. 1991;88:1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Stocker R, Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol. Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 442.Lassève B, San Martín A, Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ. Res. 2012;110:1364–1390. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Shi Y, Cosentino F, Camici GG, Akhmedov A, Vanhoutte PM, Tanner FC, Lüscher TF. Oxidized low-density lipoprotein activates p66Shc via lectin-like oxidized low-density lipoprotein receptor-1 and protein kinase C-beta c-Jun N-terminal kinase in human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2011;31:2090–2097. doi: 10.1161/ATVBAHA.111.229260. [DOI] [PubMed] [Google Scholar]
- 444.Martin-Padura I, de Nigris F, Migliaccio E, Mansueto G, Minardi S, Rienzo M, Lerman LO, Stendardo M, Giorgio M, De Rosa G, Pelicci PG, Napoli C. p66Shc deletion confers vascular protection in advanced atherosclerosis in hypercholesterolemic apolipoprotein E knockout mice. Endothelium. 2008;15:276–287. doi: 10.1080/10623320802487791. [DOI] [PubMed] [Google Scholar]
- 445.Harja E, Chang JS, Lu Y, Leitges M, Zou YS, Schmidt AM, Yan SF. Mice deficient in PKCβ and apolipoprotein E display decreased atherosclerosis. FASEB J. 2009;23:1081–1091. doi: 10.1096/fj.08-120345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Ricci R, Sumara G, Sumara I, Rozenberg I, Kurrer M, Akhmedov A, Hersberger M, Erikssoson U, Eberli FR, Becher B, Boren J, Chen M, Cybulsky MI, Moore KJ, Freeman MW, Wagner EF, Matter CM, Luscher TF. Requirement of JNK2 for scavenger receptor A-mediated foam cell formation in atherogenesis. Science. 2004;306:1558–1561. doi: 10.1126/science.1101909. [DOI] [PubMed] [Google Scholar]
- 447.Osto E, Matter CM, Kouroedov A, Malinski T, Bachschmid M, Camici GG, Kilic U, Stallmach T, Boren J, Iliceto S, Luscher TF, Cosentino F. c-Jun N-terminal kinase 2 deficiency protects against hypercholesterolemia-induced endothelial dysfunction and oxidative stress. Circulation. 2008;118:2073–2080. doi: 10.1161/CIRCULATIONAHA.108.765032. [DOI] [PubMed] [Google Scholar]
- 448.Bosutti A, Grassi G, Zanetti M, Aleksova A, Zecchin M, Sinagra G, Biolo G, Guarnieri G. Relation between the plasma levels of LDL-cholesterol and the expression of the early marker of inflammation long pentraxin PTX3 and the stress response gene p66ShcA in pacemaker-implanted patients. Clin. Exp. Med. 2007;7:16–23. doi: 10.1007/s10238-007-0118-y. [DOI] [PubMed] [Google Scholar]
- 449.Noda Y, Yamagishi S, Matsui T, Ueda S, Ueda S, Jinnouchi Y, Hirai Y, Imaizumi T. The p66shc gene expression in peripheral blood monocytes is increased in patients with coronary artery disease. Clin. Cardiol. 2010;33:548–552. doi: 10.1002/clc.20761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Bergt C, Pennathur S, Fu X, Byun J, O'Brien K, McDonald TO, Singh P, Anantharamaiah GM, Chait A, Brunzell J, Geary RL, Oram JF, Heinecke JW. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc. Natl. Acad. Sci. USA. 2004;101:13032–13037. doi: 10.1073/pnas.0405292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Shao B, Oda MN, Bergt C, Fu X, Green PS, Brot N, Oram JF, Heinecke JW. Myeloperoxidase impairs ABCA1-dependent cholesterol efflux through methionine oxidation and site-specific tyrosine chlorination of apolipoprotein A-I. J. Biol. Chem. 2006;281:9001–9004. doi: 10.1074/jbc.C600011200. [DOI] [PubMed] [Google Scholar]
- 452.Wu Z, Wagner MA, Zheng L, Parks JS, Shy JM, 3rd, Smith JD, Gogonea V, Hazen SL. The refined structure of nascent HDL reveals a key functional domain for particle maturation and dysfunction. Nat. Struct. Mol. Biol. 2007;14:861–868. doi: 10.1038/nsmb1284. [DOI] [PubMed] [Google Scholar]
- 453.Shao B, Cavigiolio G, Brot N, Oda MN, Heinecke JW. Methionine oxidation impairs reverse cholesterol transport by apolipoprotein A-I. Proc. Natl. Acad. Sci. USA. 2008;105:12224–12229. doi: 10.1073/pnas.0802025105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Navab M, Reddy ST, Van Lenten BJ. Fogelman AMHDL and cardiovascular disease: atherogenic and atheroprotective mechanisms. Nat. Rev. Cardiol. 2011;8:222–232. doi: 10.1038/nrcardio.2010.222. [DOI] [PubMed] [Google Scholar]
- 455.Peshavariya H, Dusting GJ, Di Bartolo B, Rye KA, Barter PJ, Jiang F. Reconstituted high-density lipoprotein suppresses leukocyte NADPH oxidase activation by disrupting lipid rafts Free. Radic. Res. 2009;43:772–782. doi: 10.1080/10715760903045304. [DOI] [PubMed] [Google Scholar]
- 456.Undurti A, Huang Y, Lupica JA, Smith JD, DiDonato JA, Hazen SL. Modification of high density lipoprotein by myeloperoxidase generates a pro-inflammatory particle. J. Biol. Chem. 2009;284:30825–30835. doi: 10.1074/jbc.M109.047605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Sugiyama S, Okada Y, Sukhova GK, Virmani R, Heinecke JW, Libby P. Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am. J. Pathol. 2001;158:879–891. doi: 10.1016/S0002-9440(10)64036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Sugiyama S, Kugiyama K, Aikawa M, Makamura S, Ogawa H, Libby P. Hypochlorous acid a macrophage product induces endothelial apoptosis tissue factor expression: involvement of myeloperoxidase-mediated oxidant in plaque erosion and thrombogenesis. Arterioscler. Thromb. Vasc. Biol. 2004;24:1309–1314. doi: 10.1161/01.ATV.0000131784.50633.4f. [DOI] [PubMed] [Google Scholar]
- 459.Meuwese MC, Stroes ES, Hazen SL, van Miert JN, Kuivenhoven JA, Schaub RG, Wareham NJ, Luben R, Kastelein JJ, Khaw KT, Boekholdt SM. Serum myeloperoxidase levels are associated with the future risk of coronary artery disease in apparently healthy individuals: the EPIC-Norfolk Prospective Population Study. J. Am. Coll. Cardiol. 2007;50:159–165. doi: 10.1016/j.jacc.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 460.Rana JS, Arsenault BJ, Despres JP, Côté M, Talmud PJ, Ninio E, Wouter Jukema J, Wareham NJ, Kastelein JJ, Khaw KT, Boekholdt SM. Inflammatory biomarkers, physical activity, waist circumference, and risk of future coronary heart disease in healthy men and women. Eur. Heart. J. 2011;32:336–344. doi: 10.1093/eurheartj/ehp010. [DOI] [PubMed] [Google Scholar]
- 461.Azumi H, Inoue N, Takeshita S, Rikitake Y, Kawashima S, Hayashi Y, Itoh H, Yokoyama M. Expression of NADH/NADPH oxidase p22phox in human coronary arteries. Circulation. 1999;100:1494–1498. doi: 10.1161/01.cir.100.14.1494. [DOI] [PubMed] [Google Scholar]
- 462.Azumi H, Inoue N, Ohashi Y, Terashima M, Mori T, Fujita H, Awano K, Kobayashi K, Maeda K, Hata K, Shinke T, Kobayashi S, Hirata K, Kawashima S, Itabe H, Hayashi Y, Imajoh-Ohmi S, Itoh H, Yokoyama M. Superoxide generation in directional coronary atherectomy specimens of patients with angina pectoris: important role of NAD(P)H oxidase. Arter-ioscler. Thromb. Vasc. Biol. 2002;22:1838–1844. doi: 10.1161/01.atv.0000037101.40667.62. [DOI] [PubMed] [Google Scholar]
- 463.Terashima M, Ohashi Y, Azumi H, Otsui K, Kaneda H, Awano K, Kobayashi S, Honjo T, Suzuki T, Maeda K, Yokoyama M, Inoue N. Impact of NAD(P)H oxidase-derived reactive oxygen species on coronary arterial remodeling: a comparative intravascular ultrasound and histochem-ical analysis of atherosclerotic lesions. Circ. Cardiovasc. Interv. 2009;2:196–204. doi: 10.1161/CIRCINTERVENTIONS.108.799502. [DOI] [PubMed] [Google Scholar]
- 464.Vendrov AE, Madamanchi NR, Hakim ZS, Rojas M, Runge MS. Thrombin and NAD(P)H oxidase-mediated regulation of CD44 and BMP4-Id pathway in VSMC, restenosis, and atherosclerosis. Circ. Res. 2006;98:1254–1263. doi: 10.1161/01.RES.0000221214.37803.79. [DOI] [PubMed] [Google Scholar]
- 465.Vendrov AE, Hakim ZS, Madamanchi NR, Rojas M, Madamanchi C, Runge MS. Atherosclerosis is attenuated by limiting superoxide generation in both macrophages and vessel wall cells. Arterioscler. Thromb. Vasc. Biol. 2007;27:2714–2721. doi: 10.1161/ATVBAHA.107.152629. [DOI] [PubMed] [Google Scholar]
- 466.Fenyo IM, Florea IC, Raicu M, Manea A. Tyrphostin AG490 reduces NADPH oxidase activity and expression in the aorta of hypercholesterolemic apolipoprotein E-deficient mice. Vasc. Pharmacol. 2011;54:100–106. doi: 10.1016/j.vph.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 467.Chen Z, Keaney JF, Jr, Schulz E, Levison B, Shan L, Sakuma M, Zhang X, Shi C, Hazen SL, Simon DI. Decreased neointimal formation in Nox2-deficient mice reveals a direct role for NADPH oxidase in the response to arterial injury. Proc. Natl. Acad Sci USA. 2004;101:13014–13019. doi: 10.1073/pnas.0405389101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Sheehan AL, Carrell S, Johnson B, Stanic B, Banfi B, Miller FJ., Jr Role for nox1 NADPH oxidase in atherosclerosis. Atherosclerosis. 2011;216:321–326. doi: 10.1016/j.atherosclerosis.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Lee MY, San Martin A, Mehta PK, Dikalova AE, Garrido AM, Datla SR, Lyons E, Krause KH, Banfi BLambeth JD, Lassègue B, Griendling KK. Mechanisms of vascular smooth muscle NADPH oxidase 1 (Nox1) contribution to injury-induced neointimal formation. Arterioscler. Thromb. Vasc. Biol. 2009;29:480–487. doi: 10.1161/ATVBAHA.108.181925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid. Redox. Signaling. 2011;15:1583–1606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Yang H, Roberts LJ, Shi MJ, Zhou LC, Ballard BR, Richardson A, Guo ZM. Retardation of atherosclerosis by overexpression of catalase or both Cu/Zn-superoxide dismutase catalase in mice lacking apolipopro-tein. E. Circ. Res. 2004;95:1075–1081. doi: 10.1161/01.RES.0000149564.49410.0d. [DOI] [PubMed] [Google Scholar]
- 472.Madamanchi NR, Moon SK, Hakim ZS, Clark S, Mehrizi A, Patterson C, Runge MS. Differential activation of mitogenic signaling pathways in aortic smooth muscle cells deficient in superoxide dismutase isoforms. Arterioscler. Thromb. Vasc. Biol. 2005;25:950–956. doi: 10.1161/01.ATV.0000161050.77646.68. [DOI] [PubMed] [Google Scholar]
- 473.Laukkanen MO, Lehtolainen P, Turunen P, Aittomaki S, Oikari P, Marklund SL, Yla-Herttuala S. Rabbit extracellular superoxide dismutase: expression and effect on LDL oxidation. Gene. 2000;254:173–179. doi: 10.1016/s0378-1119(00)00272-9. [DOI] [PubMed] [Google Scholar]
- 474.Takatsu H, Tasaki H, Kim HN, Ueda S, Tsutsui M, Yamashita K, Toyokawa T, Morimoto Y, Nakashima Y, Adachi T. Overexpression of EC-SOD suppresses endothelial cell-mediated LDL oxidation. Biochem. Biophys. Res. Commun. 2001;285:84–91. doi: 10.1006/bbrc.2001.5114. [DOI] [PubMed] [Google Scholar]
- 475.Wang XL, Adachi T, Sim AS, Wilcken DE. Plasma extracellular superoxide dismutase levels in an Australian population with coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 1998;18:1915–1921. doi: 10.1161/01.atv.18.12.1915. [DOI] [PubMed] [Google Scholar]
- 476.Torzewski M, Ochsenhirt V, Kleschyov AL, Oelze M, Daiber A, Li H, Rossmann H, Tsimikas S, Reifenberg K, Cheng F, Lehr HA, Blankenberg S, Förstermann U, Münzel T, Lackner KJ. Deficiency of glutathione peroxidase-1 accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2007;27:850–857. doi: 10.1161/01.ATV.0000258809.47285.07. [DOI] [PubMed] [Google Scholar]
- 477.Guo Z, Ran Q, Roberts LJ, 2nd, Zhou L, Richardson A, Sharan C, Wu D, Yang H. Suppression of atherogenesis by overexpression of glutathione peroxidase-4 in apolipoprotein E-deficient mice. Free. Radic. Biol Med. 2008;44:343–352. doi: 10.1016/j.freeradbiomed.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Lapenna D, de Gioia S, Ciofani G, Mezzetti A, Ucchino S, Calafiore AM, Napolitano AM, Di Ilio C, Cuccurullo F. Glutathione-related antiox-idant defenses in human atherosclerotic plaques. Circulation. 1998;97:1930–1934. doi: 10.1161/01.cir.97.19.1930. [DOI] [PubMed] [Google Scholar]
- 479.Blankenberg S, Rupprecht HJ, Bickel C, Torzewski M, Hafner G, Tiret L, Smieja M, Cambien F, Meyer J, Lackner KJ. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N. Engl. J. Med. 2003;349:1605–1613. doi: 10.1056/NEJMoa030535. [DOI] [PubMed] [Google Scholar]
- 480.Flores-Mateo G, Carrillo-Santisteve P, Elosua R, Guallar E, Marrugat J, Bleys J, Covas MI. Antioxidant enzyme activity coronary heart disease: meta-analyses of observational studies. Am. J. Epidemiol. 2009;170:135–147. doi: 10.1093/aje/kwp112. [DOI] [PubMed] [Google Scholar]
- 481.Aviram M, Rosenblat M, Bisgaier CL, Newton RS, Primo-Parmo SL, La Du BN. Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions: a possible peroxidative role for paraoxonase. J. Clin. Invest. 1998;101:1581–1590. doi: 10.1172/JCI1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Aviram M, Rosenblat M, Billecke S, Erogul J, Sorenson R, Bisgaier CL, Newton RS, La Du B. Human serum paraoxonase (PON 1) is inactivated by oxidized low density lipoprotein and preserved by antioxidants. Free. Radic. Biol. Med. 1999;26:892–904. doi: 10.1016/s0891-5849(98)00272-x. [DOI] [PubMed] [Google Scholar]
- 483.Shih DM, Gu L, Xia YR, Navab M, Li WF, Hama S, Castellani LW, Furlong CE, Costa LG, Fogelman AM, Lusis AJ. Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature. 1998;394:284–287. doi: 10.1038/28406. [DOI] [PubMed] [Google Scholar]
- 484.Shih DM, Xia YR, Wang XP, Miller E, Castellani LW, Subbana-gounder G, Cheroutre H, Faull KF, Berliner JA, Witztum JL, Lusis AJ. Combined serum paraoxonase knockout/apolipoprotein E knockout mice exhibit increased lipoprotein oxidation and atherosclerosis. J. Biol. Chem. 2000;275:17527–17535. doi: 10.1074/jbc.M910376199. [DOI] [PubMed] [Google Scholar]
- 485.Rosenblat M, Coleman R, Reddy ST, Aviram M. Paraoxonase 2 attenuates macrophage triglyceride accumulation via inhibition of diacylglycerol acyl-transferase 1. J. Lipid. Res. 2009;50:870–879. doi: 10.1194/jlr.M800550-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Devarajan A, Bourquard N, Hama S, Navab M, Grijalva VR, Morvardi S, Clarke CF, Vergnes L, Reue K, Teiber JF, Reddy ST. Paraoxonase 2 deficiency alters mitochondrial function and exacerbates the development of atherosclerosis. Antioxid. Redox. Signaling. 2011;14:341–351. doi: 10.1089/ars.2010.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Shih DM, Xia YR, Wang XP, Wang SS, Bourquard N, Fogelman AM, Lusis AJ, Reddy ST. Decreased obesity and atherosclerosis in human paraoxonase 3 transgenic mice. Circ. Res. 2007;100:1200–1207. doi: 10.1161/01.RES.0000264499.48737.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Ng CJ, Bourquard N, Hama SY, Shih D, Grijalva VR, Navab M, Fogelman AM, Reddy ST. Adenovirus-mediated expression of human paraoxonase 3 protects against the progression of atherosclerosis in apoli-poprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2007;27:1368–1374. doi: 10.1161/ATVBAHA.106.134189. [DOI] [PubMed] [Google Scholar]
- 489.Bhattacharyya T, Nicholls SJ, Topol EJ, Zhang R, Yang X, Schmitt D, Fu X, Shao M, Brennan DM, Ellis SG, Brennan ML, Allayee H, Lusis AJ, Hazen SL. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA. 2008;299:1265–1276. doi: 10.1001/jama.299.11.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Nakazono K, Watanabe N, Matsuno K, Sasaki J, Sato T, Inoue M. Does superoxide underlie the pathogenesis of hypertension? Proc. Natl. Acad. Sci. USA. 1991;88:10045–10048. doi: 10.1073/pnas.88.22.10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Montezano AC, Touyz RM. Oxidative stress, Noxs, and hypertension: experimental evidence and clinical controversies. Ann. Med. 2012;44(Suppl. 1):S2–S16. doi: 10.3109/07853890.2011.653393. [DOI] [PubMed] [Google Scholar]
- 492.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ. Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 493.Zhang H, Schmeisser A, Garlichs CD, Plötze K, Damme U, Mügge A, Daniel WG. Angiotensin II-induced superoxide anion generation in human vascular endothelial cells: role of membrane-bound NADH-/NADPH-oxi-dases. Cardiovasc. Res. 1999;44:215–222. doi: 10.1016/s0008-6363(99)00183-2. [DOI] [PubMed] [Google Scholar]
- 494.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation: contribution to alterations of vasomotor tone. J. Clin. Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Fukui T, Ishizaka N, Rajagopalan S, Laursen JB, Capers IVQ, Taylor WR, Harrison DG, de Leon H, Wilcox JN, Griendling KK. p22phox mRNA expression NADPH oxidase activity are increased in aortas from hypertensive rats. Circ. Res. 1997;80:45–51. doi: 10.1161/01.res.80.1.45. [DOI] [PubMed] [Google Scholar]
- 496.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J. Clin. Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Heitzer T, Wenzel U, Hink U, Krollner D, Skatchkov M, Stahl RA, MacHarzina R, Brasen JH, Meinertz T, Munzel T. Increased NAD(P)H oxidase-mediated superoxide production in renovascular hypertension: evidence for an involvement of protein kinase C. Kidney. Int. 1999;55:252–260. doi: 10.1046/j.1523-1755.1999.00229.x. [DOI] [PubMed] [Google Scholar]
- 498.Morawietz H, Weber M, Rueckschloss U, Lauer N, Hacker A, Kojda G. Upregulation of vascular NAD(P)H oxidase subunit gp91phox and impairment of the nitric oxide signal transduction pathway in hypertension. Biochem. Biophys. Res. Commun. 2001;285:1130–1135. doi: 10.1006/bbrc.2001.5312. [DOI] [PubMed] [Google Scholar]
- 499.Matsuno K, Yamada H, Iwata K, Jin D, Katsuyama M, Matsuki M, Takai S, Yamanishi K, Miyazaki M, Matsubara H, Yabe-Nishimura C. Nox1 is involved in angiotensin II-mediated hypertension: a study in Nox1-deficient mice. Circulation. 2005;112:2677–2685. doi: 10.1161/CIRCULATIONAHA.105.573709. [DOI] [PubMed] [Google Scholar]
- 500.Gavazzi G, Banfi B, Deffert C, Fiette L, Schappi M, Herrmann F, Krause KH. Decreased blood pressure in NOX1-deficient mice. FEBS Lett. 2006;580:497–504. doi: 10.1016/j.febslet.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 501.Rey FE, Cifuentes ME, Kiarash A, Quinn MT, Pagano PJ. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O2− and systolic blood pressure in mice. Circ. Res. 2001;89:408–414. doi: 10.1161/hh1701.096037. [DOI] [PubMed] [Google Scholar]
- 502.Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, Harrison DG. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension. 2002;40:511–515. doi: 10.1161/01.hyp.0000032100.23772.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Weber DS, Rocic P, Mellis AM, Laude K, Lyle AN, Harrison DG, Griendling KK. Angiotensin II-induced hypertrophy is potentiated in mice overexpressing p22phox in vascular smooth muscle. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H37–H42. doi: 10.1152/ajpheart.00638.2004. [DOI] [PubMed] [Google Scholar]
- 504.Jung O, Schreiber JG, Geiger H, Pedrazzini T, Busse R, Brandes RP. gp91phox-containing NADPH oxidase mediates endothelial dysfunction in renovascular hypertension. Circulation. 2004;109:1795–1801. doi: 10.1161/01.CIR.0000124223.00113.A4. [DOI] [PubMed] [Google Scholar]
- 505.Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ. Res. 2004;95:210–216. doi: 10.1161/01.RES.0000135483.12297.e4. [DOI] [PubMed] [Google Scholar]
- 506.Peterson JR, Burmeister MA, Tian X, Zhou Y, Guruju MR, Stupinski JA, Sharma RV, Davisson RL. Genetic silencing of Nox2 and Nox4 reveals differential roles of these NADPH oxidase homologues in the vasopressor and dipsogenic effects of brain angiotensin II. Hypertension. 2009;54:1106–1114. doi: 10.1161/HYPERTENSIONAHA.109.140087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ. Res. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 508.Claycomb WC. Cardiac muscle cell proliferation and cell differentiation in vivo and in vitro. Adv. Exp. Med. Biol. 1983;161:249–265. doi: 10.1007/978-1-4684-4472-8_14. [DOI] [PubMed] [Google Scholar]
- 509.Pimentel DR, Amin JK, Xiao L, Miller T, Viereck J, Oliver-Krasinski J, Baliga R, Wang J, Siwik DA, Singh K, Pagano P, Colucci WS, Sawyer DB. Reactive oxygen species mediate amplitude-dependent hypertrophic and apoptotic responses to mechanical stretch in cardiac myocytes. Circ. Res. 2001;89:453–460. doi: 10.1161/hh1701.096615. [DOI] [PubMed] [Google Scholar]
- 510.Oka S, Ago T, Kitazono T, Zablocki D, Sadoshima J. The role of redox modulation of class II histone deacetylases in mediating pathological cardiac hypertrophy. J. Mol. Med. 2009;87:785–791. doi: 10.1007/s00109-009-0471-2. [DOI] [PubMed] [Google Scholar]
- 511.Ago T, Liu T, Zhai P, Chen W, Li H, Molkentin JD, Vatner SF, Sadoshima J. A redox-dependent pathway for regulating class II HDACs and cardiac hypertrophy. Cell. 2008;133:978–993. doi: 10.1016/j.cell.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 512.Yamamoto M, Yang G, Hong C, Liu J, Holle E, Yu X, Wagner T, Vatner SF, Sadoshima J. Inhibition of endogenous thioredoxin in the heart increases oxidative stress and cardiac hypertrophy. J. Clin. Invest. 2003;112:1395–1406. doi: 10.1172/JCI17700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Date M, Morita T, Yamashita N, Nishida K, Yamaguchi O, Higuchi Y, Hirotani S, Matsumura Y, Hori M, Tada M, Otsu K. The antioxidant N-2-mercaptopropionyl glycine attenuates left ventricular hypertrophy in in vivo murine pressure-overload model. J. Am. Coll. Cardiol. 2002;39:907–912. doi: 10.1016/s0735-1097(01)01826-5. [DOI] [PubMed] [Google Scholar]
- 514.Dhalla AK, Hill MF, Singal PK. Role of oxidative stress in transition of hypertrophy to heart failure. J. Am. Coll. Cardiol. 1996;28:506–514. doi: 10.1016/0735-1097(96)00140-4. [DOI] [PubMed] [Google Scholar]
- 515.Shiomi T, Tsutsui H, Matsusaka H, Murakami K, Hayashidani S, Ikeuchi M, Wen J, Kubota T, Utsumi H, Takeshita A. Overexpression of glu-tathione peroxidase prevents left ventricular remodeling and failure after myocardial infarction in mice. Circulation. 2004;109:544–549. doi: 10.1161/01.CIR.0000109701.77059.E9. [DOI] [PubMed] [Google Scholar]
- 516.Wang G, Hamid T, Keith RJ, Zhou G, Partridge CR, Xiang X, Kingery JR, Lewis RK, Li Q, Rokosh DG, Ford R, Spinale FG, Riggs DW, Srivastava S, Bhatnagar A, Bolli R, Prabhu SD. Cardioprotective and antiapoptotic effects of heme oxygenase-1 in the failing heart. Circulation. 2010;121:1912–1925. doi: 10.1161/CIRCULATIONAHA.109.905471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Satoh M, Ogita H, Takeshita K, Mukai Y, Kwiatkowski DJ, Liao JK. Requirement of Rac1 in the development of cardiac hypertrophy. Proc. Natl. Acad. Sci USA. 2006;103:7432–7437. doi: 10.1073/pnas.0510444103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Li JM, Gall NP, Grieve DJ, Chen M, Shah AM. Activation of NADPH oxidase during progression of cardiac hypertrophy to failure. Hypertension. 2002;40:477–484. doi: 10.1161/01.hyp.0000032031.30374.32. [DOI] [PubMed] [Google Scholar]
- 519.Nadruz W, Jr, Lagosta VJ, Moreno H, Jr, Coelho OR, Franchini KG. Simvastatin prevents load-induced protein tyrosine nitration in overloaded hearts. Hypertension. 2004;43:1060–1066. doi: 10.1161/01.HYP.0000124252.43470.2c. [DOI] [PubMed] [Google Scholar]
- 520.Li HL, Huang Y, Zhang CN, Liu G, Wei YS, Wang AB, Liu YQ, Hui RT, Wei C, Williams GM, Liu DP, Liang CC. Epigallocatechin-3 gallate inhibits cardiac hypertrophy through blocking reactive oxidative species-dependent and -independent signal pathways. Free Radic. Biol. Med. 2006;40:1756–1775. doi: 10.1016/j.freeradbiomed.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 521.Maack C, Böhm M, Vlaskin L, Dabew E, Lorenz K, Schäfers HJ, Lohse MJ, Engelhardt S. Partial agonist activity of bucindolol is dependent on the activation state of the human beta1-adrenergic receptor. Circulation. 2003;108:348–353. doi: 10.1161/01.CIR.0000080325.94345.8B. [DOI] [PubMed] [Google Scholar]
- 522.Nediani C, Borchi E, Giordano C, Baruzzo S, Ponziani V, Sebastiani M, Nassi P, Mugelli A, d'Amati G, Cerbai E. NADPH oxidase-dependent redox signaling in human heart failure: relationship between the left and right ventricle. J. Mol. Cell. Cardiol. 2007;42:826–834. doi: 10.1016/j.yjmcc.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 523.Grieve DJ, Byrne JA, Siva A, Layland J, Johar S, Cave AC, Shah AM. Involvement of the nicotinamide adenosine dinucleotide phosphate oxidase isoform Nox2 in cardiac contractile dysfunction occurring in response to pressure overload. J. Am. Coll. Cardiol. 2006;47:817–826. doi: 10.1016/j.jacc.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 524.Doerries C, Grote K, Hilfiker-Kleiner D, Luchtefeld M, Schaefer A, Holland SM, Sorrentino S, Manes C, Schieffer B, Drexler H, Landmesser U. Critical role of the NAD(P)H oxidase subunit p47phox for left ventricular remodeling/dysfunction and survival after myocardial infarction. Circ. Res. 2007;100:894–903. doi: 10.1161/01.RES.0000261657.76299.ff. [DOI] [PubMed] [Google Scholar]
- 525.Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ. Res. 2010;106:1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Zhang M, Brewer AC, Schröder K, Santos CX, Grieve DJ, Wang M, Anilkumar N, Yu B, Dong X, Walker SJ, Brandes RP, Shah AM. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc. Natl. Acad. Sci. USA. 2010;107:18121–18126. doi: 10.1073/pnas.1009700107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc. Natl. Acad. Sci. USA. 2010;107:15565–15570. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Schröder K, Zhang M, Benkhoff S, Mieth A, Pliquett R, Kosowski J, Kruse C, Luedike P, Michaelis UR, Weissmann N, Dimmeler S, Shah AM, Brandes RP. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ. Res. 2012;110:1217–1225. doi: 10.1161/CIRCRESAHA.112.267054. [DOI] [PubMed] [Google Scholar]
- 529.Duncan JG, Ravi R, Stull LB, Murphy AM. Chronic xanthine oxidase inhibition prevents myofibrillar protein oxidation and preserves cardiac function in a transgenic mouse model of cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H1512–H1518. doi: 10.1152/ajpheart.00168.2005. [DOI] [PubMed] [Google Scholar]
- 530.Stull LB, Leppo MK, Szweda L, Gao WD, Marbán E. Chronic treatment with allopurinol boosts survival and cardiac contractility in murine post-ischemic cardiomyopathy. Circ. Res. 2004;9510:1005–1011. doi: 10.1161/01.RES.0000148635.73331.c5. [DOI] [PubMed] [Google Scholar]
- 531.Naumova AV, Chacko VP, Ouwerkerk R, Stull L, Marbán E, Weiss RG. Xanthine oxidase inhibitors improve energetics and function after infarction in failing mouse hearts. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H837–H843. doi: 10.1152/ajpheart.00831.2005. [DOI] [PubMed] [Google Scholar]
- 532.Hare JM, Mangal B, Brown J, Fisher C, Jr, Freudenberger R, Colucci WS, Mann DL, Liu P, Givertz MM, Schwarz RPOPT-CHF Investigators. Impact of oxypurinol in patients with symptomatic heart failure: results of the OPT-CHF study. J. Am. Coll. Cardiol. 2008;51:2301–2309. doi: 10.1016/j.jacc.2008.01.068. [DOI] [PubMed] [Google Scholar]
- 533.Forgione MA, Cap A, Liao R, Moldovan NI, Eberhardt RT, Lim CC, Jones J, Goldschmidt-Clermont PJ, Loscalzo J. Heterozygous cellular glutathione peroxidase deficiency in the mouse: abnormalities in vascular and cardiac function and structure. Circulation. 2002;106:1154–1158. doi: 10.1161/01.cir.0000026820.87824.6a. [DOI] [PubMed] [Google Scholar]
- 534.Thu VT, Kim HK, Ha SH, Yoo JY, Park WS, Kim N, Oh GT, Han J. Glutathione peroxidase 1 protects mitochondria against hypoxia/reoxygena-tion damage in mouse hearts. Pflugers Arch. 2010;460:55–68. doi: 10.1007/s00424-010-0811-7. [DOI] [PubMed] [Google Scholar]
- 535.Spindel ON, Berk BC. Redox redux: protecting the ischemic myocardium. J. Clin. Invest. 2012;122:30–32. doi: 10.1172/JCI61467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Yoshioka J, Chutkow WA, Lee S, Kim JB, Yan J, Tian R, Lindsey ML, Feener EP, Seidman CE, Seidman JG, Lee RT. Deletion of thioredoxin-interacting protein in mice impairs mitochondrial function but protects the myocardium from ischemia-reperfusion injury. J. Clin. Invest. 2012;122:267–279. doi: 10.1172/JCI44927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Huang Y, Hickey RP, Yeh JL, Liu D, Dadak A, Young LH, Johnson RS, Giordano FJ. Cardiac myocyte-specific HIF-1alpha deletion alters vascularization, energy availability, calcium flux, and contractility in the normoxic heart. FASEB J. 2004;18:1138–1140. doi: 10.1096/fj.04-1510fje. [DOI] [PubMed] [Google Scholar]
- 538.Kido M, Du L, Sullivan CC, Li X, Deutsch R, Jamieson SW, Thistlethwaite PA. Hypoxia-inducible factor 1-alpha reduces infarction and attenuates progression of cardiac dysfunction after myocardial infarction in the mouse. J. Am. Coll. Cardiol. 2005;46:2116–2124. doi: 10.1016/j.jacc.2005.08.045. [DOI] [PubMed] [Google Scholar]
- 539.Natarajan R, Salloum FN, Fisher BJ, Kukreja RC, Fowler AA., 3rd Hypoxia inducible factor-1 activation by prolyl 4-hydroxylase-2 gene silencing attenuates myocardial ischemia reperfusion injury. Circ. Res. 2006;98:133–140. doi: 10.1161/01.RES.0000197816.63513.27. [DOI] [PubMed] [Google Scholar]
- 540.Sun J, Morgan M, Shen RF, Steenbergen C, Murphy E. Preconditioning results in S-nitrosylation of proteins involved in regulation of mitochondrial energetics and calcium transport. Circ. Res. 2007;101:1155–1163. doi: 10.1161/CIRCRESAHA.107.155879. [DOI] [PubMed] [Google Scholar]
- 541.Pain T, Yang XM, Critz SD, Yue Y, Nakano A, Liu GS, Heusch G, Cohen MV, Downey JM. Opening of mitochondrial K(ATP) channels triggers the preconditioned state by generating free radicals. Circ. Res. 2000;87:460–466. doi: 10.1161/01.res.87.6.460. [DOI] [PubMed] [Google Scholar]
- 542.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, Olson EN, Sollott SJ. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J. Clin. Invest. 2004;113:1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Bell RM, Cave AC, Johar S, Hearse DJ, Shah AM, Shattock MJ. Pivotal role of NOX-2-containing NADPH oxidase in early ischemic preconditioning. FASEB J. 2005;14:2037–2039. doi: 10.1096/fj.04-2774fje. [DOI] [PubMed] [Google Scholar]
- 544.Kahles T, Brandes RP. NADPH oxidases as therapeutic targets in ischemic stroke. Cell. Mol. Life Sci. 2012;69:2345–2363. doi: 10.1007/s00018-012-1011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Chen H, Yoshioka H, Kim GS, Jung JE, Okami N, Sakata H, Maier CM, Narasimhan P, Goeders CE, Chan PH. Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid. Redox Signaling. 2011;14:1505–1517. doi: 10.1089/ars.2010.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Choi DW. Excitotoxic cell death. J. Neurobiol. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- 547.Adibhatla RM, Hatcher JF. Lipid oxidation and peroxidation in CNS health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signaling. 2010;12:125–169. doi: 10.1089/ars.2009.2668. [DOI] [PubMed] [Google Scholar]
- 548.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Jung JE, Kim GS, Chen H, Maier CM, Narasimhan P, Song YS, Niizuma K, Katsu M, Okami N, Yoshioka H, Sakata H, Goeders CE, Chan PH. Reperfusion and neurovascular dysfunction in stroke: from basic mechanisms to potential strategies for neuroprotection. Mol. Neurobiol. 2010;41:172–179. doi: 10.1007/s12035-010-8102-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Barksdale KA, Perez-Costas E, Gandy JC, Melendez-Ferro M, Roberts RC, Bijur GN. Mitochondrial viability in mouse and human postmortem brain. FASEB J. 2010;24:3590–35999. doi: 10.1096/fj.09-152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Sanderson TH, Reynolds CA, Kumar R, Przyklenk K, Hüttemann M. Molecular mechanisms of ischemia-reperfusion injury in brain: pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol. Neurobiol. 2013;47:9–23. doi: 10.1007/s12035-012-8344-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Brennan AM, Suh SW, Won SJ, Narasimhan P, Kauppinen TM, Lee H, Edling Y, Chan PH, Swanson RA. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat. Neurosci. 2009;12:857–863. doi: 10.1038/nn.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Girouard H, Park L, Anrather J, Zhou P, Iadecola C. Angiotensin II attenuates endothelium-dependent responses in the cerebral microcircula-tion through nox-2-derived radicals. Arterioscler. Thromb. Vasc. Biol. 2006;26:826–832. doi: 10.1161/01.ATV.0000205849.22807.6e. [DOI] [PubMed] [Google Scholar]
- 554.Chen H, Song YS, Chan PH. Inhibition of NADPH oxidase is neuro-protective after ischemia-reperfusion. J. Cereb. Blood Flow Metab. 2009;29:1262–1272. doi: 10.1038/jcbfm.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Schilling M, Besselmann M, Leonhard C, Mueller M, Ringelstein EB, Kiefer R. Microglial activation precedes and predominates over macrophage infiltration in transient focal cerebral ischemia: a study in green fluorescent protein transgenic bone marrow chimeric mice. Exp. Neurol. 2003;183:25–33. doi: 10.1016/s0014-4886(03)00082-7. [DOI] [PubMed] [Google Scholar]
- 556.Cheret C, Gervais A, Lelli A, Colin C, Amar L, Ravassard P, Mallet J, Cumano A, Krause KH, Mallat M. Neurotoxic activation of microglia is promoted by a Nox1-dependent NADPH oxidase. J. Neurosci. 2008;28:12039–12051. doi: 10.1523/JNEUROSCI.3568-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Kahles T, Kohnen A, Heumueller S, Rappert A, Bechmann I, Liebner S, Wittko IM, Neumann-Haefelin T, Steinmetz H, Schroeder K, Brandes RP. NADPH oxidase Nox1 contributes to ischemic injury in experimental stroke in mice. Neurobiol. Dis. 2010;40:185–192. doi: 10.1016/j.nbd.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 558.Kleinschnitz C, Grund H, Wingler K, Armitage ME, Jones E, Mittal M, Barit D, Schwarz T, Geis C, Kraft P, Barthel K, Schuhmann MK, Herrmann AM, Meuth SG, Stoll G, Meurer S, Schrewe A, Becker L, Gailus-Durner V, Fuchs H, Klopstock T, de Angelis MH, Jandeleit-Dahm K, Shah AM, Weissmann N, Schmidt HH. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol. 2010;8:e1000479. doi: 10.1371/journal.pbio.1000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Yenari MA, Xu L, Tang XN, Qiao Y, Giffard RG. Microglia potentiate damage to blood-brain barrier constituents: improvement by minocycline in vivo and in vitro. Stroke. 2006;37:1087–1093. doi: 10.1161/01.STR.0000206281.77178.ac. [DOI] [PubMed] [Google Scholar]
- 560.Shin CY, Choi JW, Ryu JR, Ryu JH, Kim W, Kim H, Ko KH. Immunostimulation of rat primary astrocytes decreases intracellular ATP level. Brain Res. 2001;902:198–204. doi: 10.1016/s0006-8993(01)02385-x. [DOI] [PubMed] [Google Scholar]
- 561.Choi JJ, Kim WK. Potentiated glucose deprivation-induced death of astrocytes after induction of iNOS. J. Neurosci. Res. 1998;54:870–875. doi: 10.1002/(SICI)1097-4547(19981215)54:6<870::AID-JNR15>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 562.Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J. Leukocyte Biol. 2010;87:779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Vallet P, Charnay Y, Steger K, Ogier-Denis E, Kovari E, Herrmann F, Michel JP, Szanto I. Neuronal expression of the NADPH oxidase NOX4, and its regulation in mouse experimental brain ischemia. Neuroscience. 2005;132:233–238. doi: 10.1016/j.neuroscience.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 564.Miller AA, Drummond GR, Mast AE, Schmidt HH, Sobey CG. Effect of gender on NADPH-oxidase activity, expression, and function in the cerebral circulation: role of estrogen. Stroke. 2007;38:2142–2149. doi: 10.1161/STROKEAHA.106.477406. [DOI] [PubMed] [Google Scholar]
- 565.Suzuki Y, Hattori K, Hamanaka J, Murase T, Egashira Y, Mishiro K, Ishiguro M, Tsuruma K, Hirose Y, Tanaka H, Yoshimura S, Shimazawa M, Inagaki N, Nagasawa H, Iwama T, Hara H. Pharmacological inhibition of TLR4-NOX4 signal protects against neuronal death in transient focal ischemia. Sci. Rep. 2012;2:896. doi: 10.1038/srep00896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Jialal I, Devaraj S. Antioxidants and atherosclerosis: don't throw out the baby with the bath water. Circulation. 2003;107:926–928. doi: 10.1161/01.cir.0000048966.26216.4c. [DOI] [PubMed] [Google Scholar]
- 567.Jaquet V, Scapozza L, Clark RA, Krause KH, Lambeth JD. Small-molecule NOX inhibitors: ROS-generating NADPH oxidases as therapeutic targets. Antioxid. Redox Signaling. 2009;11:2535–2552. doi: 10.1089/ars.2009.2585. [DOI] [PubMed] [Google Scholar]
- 568.Wingler K, Hermans JJ, Schiffers P, Moens A, Paul M, Schmidt HH. NOX1, 2, 4, 5: counting out oxidative stress. Br. J. Pharmacol. 2011;164:866–883. doi: 10.1111/j.1476-5381.2011.01249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Burgoyne JR, Mongue-Din H, Eaton P, Shah AM. Redox signaling in cardiac physiology and pathology. Circ. Res. 2012;111:1091–1106. doi: 10.1161/CIRCRESAHA.111.255216. [DOI] [PubMed] [Google Scholar]
- 570.Hajjar RJ. Potential of gene therapy as a treatment for heart failure. J. Clin. Invest. 2013;123:53–61. doi: 10.1172/JCI62837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Cyrus T, Wickline SA, Lanza GM. Nanotechnology in interventional cardiology. WIREs Nanomed. Nanobiotechnol. 2012;4:82–95. doi: 10.1002/wnan.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Thurn KT, Brown E, Wu A, Vogt S, Lai B, Maser J, Paunesku T, Woloschak GE. Nanoparticles for applications in cellular imaging. Nanoscale Res. Lett. 2007;2:430–441. doi: 10.1007/s11671-007-9081-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Li JM, Newburger PE, Gounis MJ, Dargon P, Zhang X, Messina LM. Local arterial nanoparticle delivery of siRNA for NOX2 knockdown to prevent restenosis in an atherosclerotic rat model. Gene Ther. 2010;17:1279–1287. doi: 10.1038/gt.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]