Abstract
Scaffold-assisted signaling cascades guide cellular decision-making. In budding yeast, one such signal transduction pathway called the mitotic exit network (MEN) governs the transition from mitosis to the G1 phase of the cell cycle. The MEN is conserved and in metazoans is known as the Hippo tumor-suppressor pathway. We found that signaling through the MEN kinase cascade was mediated by an unusual two-step process. The MEN kinase Cdc15 first phosphorylated the scaffold Nud1. This created a phospho-docking site on Nud1, to which the effector kinase complex Dbf2-Mob1 bound through a phosphoserine-threonine binding domain, in order to be activated by Cdc15. This mechanism of pathway activation has implications for signal transmission through other kinase cascades and might represent a general principle in scaffold-assisted signaling.
Signaling complex assembly must be tightly controlled in both space and time. The use of protein scaffolds and phosphorylation-dependent protein-protein interactions is central to this spatiotemporal control. The mitotic exit network (MEN) is a conserved guanosine triphosphatase (GTPase) signaling cascade that governs exit from mitosis (1, 2). During anaphase, MEN signaling complexes assemble at spindle pole bodies (SPBs) (3, 4) to trigger exit from mitosis and to couple this cell cycle transition with nuclear position (5). The Ras-like GTPase Tem1 and the Polo protein kinase Cdc5 coordinately recruit the Hippo-like kinase Cdc15 to SPBs (3). Once localized to SPBs, Cdc15 is activated to phosphorylate the kinase Dbf2 and its coactivator Mob1 (6). Phosphorylation activates Dbf2-Mob1, which then promotes the release of the MEN effector protein phosphatase Cdc14 from the nucleolus, resulting in exit from mitosis (2).
Scaffold proteins serve as assembly platforms for kinase cascades and may function as signaling insulators (7). Our results show that, rather than functioning as a passive platform onto which MEN components assemble, the SPB-resident MEN scaffold Nud1 is a dynamic participant in MEN signal transmission. Nud1 is a phospho-protein and its phosphorylation increases during mitosis (fig. S1, A to C, and table S1) (8–10). We generated a NUD1 allele in which the 38 high-confidence mitotic phosphorylation sites and 4 lower-confidence sites were mutated to alanine (henceforth nud1-42A). The nud1-42A protein was stable and localized to SPBs, but phosphorylation during mitosis was reduced (fig. S1, B to D).
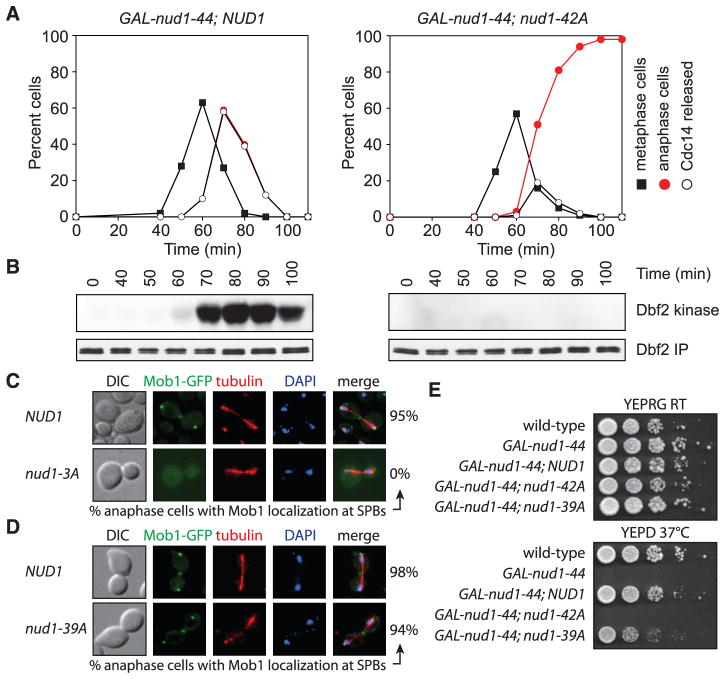
To examine the effects of the nud1-42A allele on MEN activity, we introduced the allele into a strain expressing the temperature-sensitive nud1-44 allele under the control of the galactose-inducible and glucose-repressible GAL1-10 promoter. GAL-nud1-44 nud1-42A cells, like MEN loss-of-function mutants, arrested in late anaphase with inactive Dbf2-Mob1 and nucleolar-restricted Cdc14 under conditions in which nud1-44 is inactive (Fig. 1, A and B, and fig. S1, E and F). Thus, nud1-42A cells are defective in MEN signaling.
Fig. 1. Dbf2-Mob1 recruitment to SPBs and MEN activation requires Nud1 phosphorylation.
(A and B) Dbf2 kinase activity and cell cycle progression in GAL-nud1-44 NUD1 (A29878) and GAL-nud1-44 nud1-42A (A29881) cells. Cells were arrested in G1 with α-factor and released under conditions in which nud1-44 is inactive (12). (C) Mob1 localization in anaphase NUD1 (A29453) and nud1-3A (A31169) cells. (D) Mob1 localization in anaphase NUD1 (A24631) and nud1-39A (A31477) cells. DAPI, 4′,6-diamidino-2-phenylindole; DIC, differential interference contrast. (E) Growth of 10-fold serial dilutions of A2587, A29248, A29685, A29500, and A32295 cells on YEP plates containing either galactose and raffinose (YEPRG) or glucose (YEPD) (12).
Localization of the MEN components Tem1, Cdc15, Dbf2, and Mob1 to SPBs is essential for Dbf2-Mob1 activation and requires NUD1 (3, 4, 11). Localization of Nud1, Bfa1 [a Tem1 GTPase-activating protein (GAP) complex component], Tem1, and Cdc15 was normal in nud1-42A cells (12) (fig. S2, A to D), but Mob1 and Dbf2 were absent from SPBs (fig. S2, E and F). nud1-42A cells also harbored mispositioned anaphase spindles and detached astral microtubules (fig. S1F). Thus, the nud1-42A allele is defective in recruitment of Dbf2-Mob1 to SPBs and astral microtubule anchorage (11).
Further analyses (12) (fig. S3, A to C) revealed that Nud1 T78 was especially critical for MEN signaling, with two additional residues, S53 and S63, contributing to this function. A NUD1 allele carrying alanine substitutions of S53, S63, and T78 (nud1-3A; table S1) caused an anaphase arrest when overexpressed in the presence of wild-type NUD1 (fig. S3C) and failed to restore viability to cells expressing the GAL-nud1-44 allele grown under restrictive conditions (fig. S3D). The anaphase delay caused by a NUD1 allele that included the S53A and S63A mutations but not T78A was minor (fig. S3C). Replacing S53, S63, and T78 with residues that mimic phosphorylation (Asp or Glu) disrupted Nud1 function (12), precluding us from examining the consequences of constitutive phosphorylation of these residues. S53, S63, and T78 are conserved across fungal orthologs (fig. S4). Thus, these residues may have similarly important roles in other fungal species.
Localization of Mob1 to SPBs was disrupted in nud1-3A cells as it was in nud1-42A cells (Fig. 1C), but astral microtubule organization was not affected (fig. S5A). In contrast, a NUD1 allele in which all mitotic phosphorylation sites were mutated to alanine with the exception of S53, S63, and T78 (nud1-39A allele; table S1) facilitated normal Mob1 localization and restored viability to cells expressing the GAL-nud1-44 allele under restrictive conditions (Fig. 1, D and E). nud1-39A cells did exhibit a 2-min delay in exit from mitosis (fig. S5B), suggesting that other phosphorylation sites in Nud1 play a very minor role in MEN activation. nud1-39A cells also harbored mispositioned anaphase spindles and detached astral microtubules (fig. S5, A, C, and D). The spindle positioning and astral microtubule anchoring defect of nud1-39A cells was suppressed by forcing localization of the astral microtubule anchor Spc72 to SPBs (fig. S5E), suggesting that nud1-39A cells are defective in Spc72 recruitment. Indeed, Spc72 localization to SPBs was impaired in nud1-39A cells (fig. S5F). These results distinguish the two Nud1 functions: MEN activation and astral microtubule anchorage.
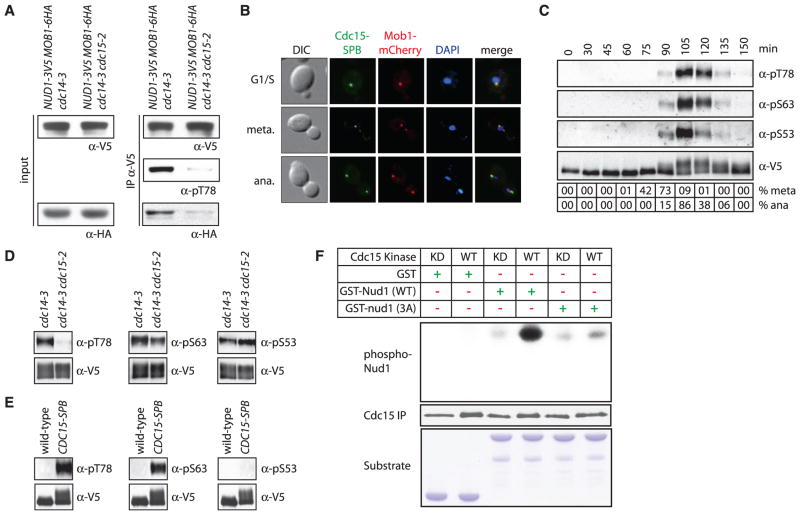
Next, we sought to identify the kinase(s) that phosphorylates Nud1 S53, S63, and T78. Cdc15 is a likely candidate because it, like Nud1 phosphorylation, is essential for Dbf2-Mob1 SPB localization (fig. S6) (13). Mob1 coimmunoprecipitated with Nud1 in anaphase-arrested cells in which CDC15 is active (cdc14-3 cells) but not in cells in which CDC15 is inactive (cdc14-3 cdc15-2 cells; Fig. 2A). Cdc15 kinase activity was also sufficient for localization of Dbf2-Mob1 to SPBs. Constitutive targeting of Cdc15 to SPBs by fusing CDC15 to the SPB (CDC15-SPB) (3) led to recruitment of Dbf2-Mob1 to SPBs soon after expression of CDC15-SPB and induced premature phosphorylation of Nud1 (Fig. 2B and fig. S7). To directly test whether Cdc15 phosphorylates Nud1 in vivo, we raised phospho-specific antibodies to S53, S63, and T78 (fig. S8). The antibodies bound to wild-type Nud1 only in anaphase cells (Fig. 2C). T78 phosphorylation depended on CDC15, S63 phosphorylation was partially dependent, and S53 phosphorylation was not (Fig. 2D). Consistent with this, premature targeting of Cdc15 to SPBs was sufficient to cause phosphorylation of Nud1 T78 and S63, but not S53 (Fig. 2E). In vitro, Cdc15 phosphorylated a recombinant N-terminal fragment of Nud1 (amino acids 1 to 150) but not the nud1-3A protein (Fig. 2F). Thus, Cdc15 phosphorylates Nud1 on residues T78 and S63 during anaphase to promote binding of Dbf2-Mob1 to Nud1.
Fig. 2. Phosphorylation of Nud1 by Cdc15 in anaphase promotes Mob1 recruitment to SPBs.
(A) Nud1-3V5 was immunoprecipitated from anaphase-arrested A31602 and A31661 cells, and bound Mob1 (IPα-HA), Nud1 (IPα-V5), Nud1 phosphorylated at T78 (IPα-pT78), total Mob1 (input α-HA), and total Nud1 (input α-V5) were examined. (B) Localization of Cdc15 and Mob1 in G1/S, metaphase, and anaphase MET3-CDC15-SPB MOB1-mCherry (A28499) cells grown in medium lacking methionine to induce expression of CDC15-SPB. (C) Phosphorylation of Nud1 T78 (α-pT78), S63 (α-pS63), S53 (α-pS53), and total Nud1 during a synchronized cell cycle (A24513). Percent meta-phase (% meta) and anaphase (% ana) cells are shown. (D) Phosphorylation of Nud1 as in Fig. 2C in anaphase-arrested NUD1-3V5 cdc14-3 (A29851) and NUD1-3V5 cdc14-3 cdc15-2 (A31661) cells. (E) Phosphorylation of Nud1 as in Fig. 2C in S-phase–arrested NUD1-3V5 (A24513) and NUD1-3V5 MET3-CDC15-SPB (A31422) cells (12). CDC15-SPB expression was induced as in Fig. 2B. (F) Phosphorylated Nud1, immunoprecipitated Cdc15 [Cdc15 IP; wild type (WT): A24957; kinase-dead (KD) Cdc15: A30371], and GST fusion protein substrate added to the kinase reaction (Substrate) are shown.
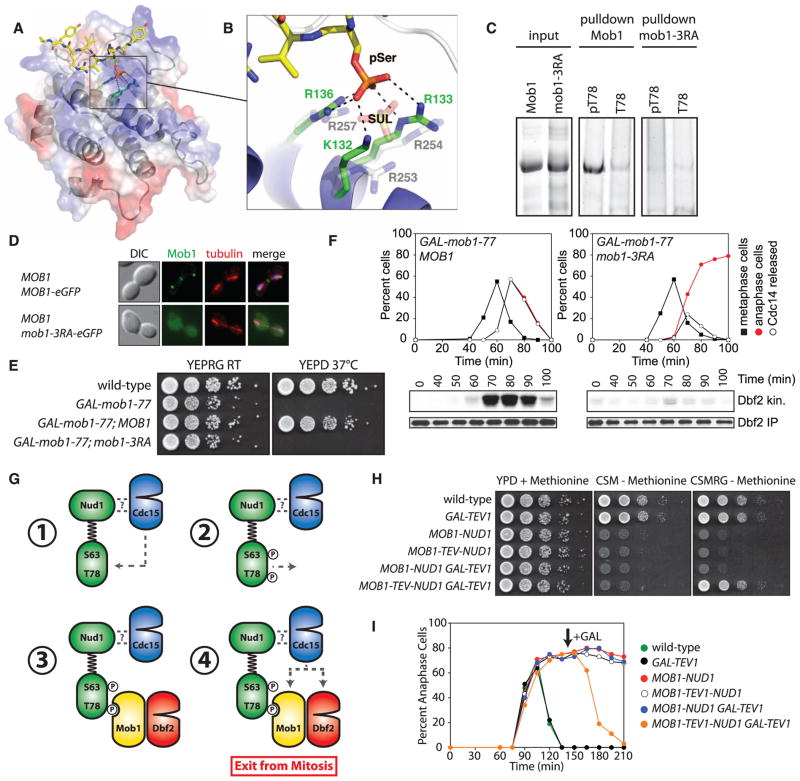
Mob1 bound to SPBs in the absence of Dbf2 (Fig. 3A), but Dbf2 did not bind to SPBs in the absence of Mob1 (Fig. 3B). This observation, together with the finding that Mob1 and Nud1 form a complex in a CDC15-dependent manner (Fig. 2A), suggests that Mob1 binds Nud1 in a phosphorylation-dependent manner. To test this, we assayed the interaction of recombinant Mob1 with Nud1 phosphopeptides in vitro. Glutathione S-transferase (GST)–Mob1 (amino acids 79 to 314) that contains the conserved Mob1 core but not the N terminus unique to yeast (14) preferentially bound to phosphorylated T78 Nud1 peptides (Fig. 3C). Oriented peptide library screening (12) revealed that Mob1 displayed a preference for Y or F in the −2 position and preferred aliphatic, hydrophobic, or R residues in the +1 to +4 positions. The Nud1 protein sequence surrounding T78 shows features consistent with these results (fig. S9, A to C). We measured binding of GST-Mob1 to filter-bound peptides predicted to be optimal ligands on the basis of the peptide library screening. Mob1 bound to these optimal peptides in a phosphorylation-specific manner (Fig. 3D). The binding of Mob1 to the optimal ligand was stronger than that to the Nud1 pT78 peptide (dissociation constant Kd of 174 nM and 2.4 μM, respectively; fig. S9, D and E). These values are similar to those observed for the interaction of FHA phosphopeptide binding domains with their optimal and physiologic peptides (15, 16). Thus, Mob1 directly binds Nud1 T78 in a phosphorylation-dependent manner.
Fig. 3. Phosphorylation-dependent binding of Mob1 to Nud1.
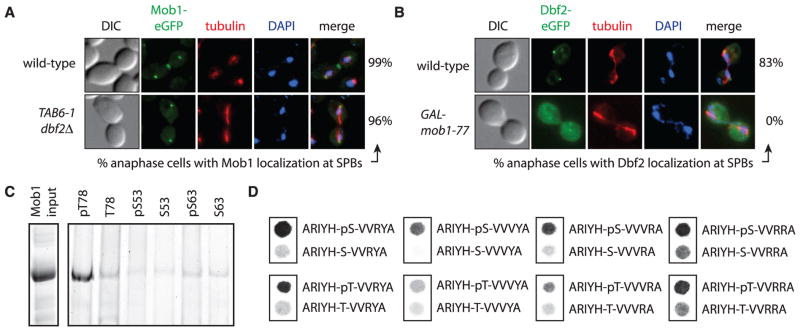
(A) Mob1 localization in anaphase MOB1-eGFP (A24631) and MOB1-eGFP dbf2Δ TAB6-1 (A32656) cells. TAB6-1 is a dominant active CDC14 allele that was necessary to keep dbf2Δ cells alive (20). (B) Dbf2 localization in anaphase DBF2-eGFP (A29921) and DBF2-eGFP GAL-mob1-77 (A32654) cells. eGFP, enhanced green fluorescent protein. (C) Bead-bound phosphorylated (pT78, pS53, or pS63) or nonphosphorylated (T78, S53, or S63) Nud1 peptides were incubated with GST-Mob1 and input and eluates analyzed. (D) Binding of GST-Mob1 to an array of peptide spots. Abbreviations for the amino acid residues are as follows: A, Ala; H, His; I, Ile; R, Arg; S, Ser; T, Thr; V, Val; and Y, Tyr.
We attempted to crystallize human (hMob1) and yeast Mob1 as complexes with the Nud1 pT78 and optimal phosphopeptides. Of these combinations, hMob1 bound to the peptide TVARIYHpSVVRYAPS yielded crystals of sufficient quality for structure determination and refinement at a resolution of 2.1 Å (table S4). The phosphopeptide binds across a shallow pocket on the Mob1 surface (Fig. 4A and fig. S10A). The structural basis for selection of aromatic residues in the pSer/pThr −2 position (fig. S9, A and B) is explained by the packing of the phenolic side chain of Tyr −2 against the aliphatic portion of K84. In yeast Mob1, this lysine is substituted by a proline, which would also make favorable van der Waals interactions. Discrimination for small-to medium-sized hydrophobic residues at the +1 and +2 positions is also explained by their interactions with a largely conserved hydrophobic depression on the Mob1 surface.
Fig. 4. Mob1 binding to phospho-Nud1 through a conserved basic pocket.
(A) Structure of the hMob1-phosphopeptide complex. The peptide is shown as yellow sticks and hMob1 is shown in ribbon representation overlaid with a transparent electrostatic potential surface. Surface electrostatic potential between −10 kT (red) and +10 kT (blue) is shown. (B) View of the basic pocket of Mob1. pSer is coordinated by hMob1 K132, R133, and R136, and the SO42− ion by yeast Mob1 R253, R254, and R257. (C) Binding of phosphorylated (pT78) or nonphosphorylated (T78) Nud1 peptides to GST-Mob1 or GST-mob1-3RA as in Fig. 3C. (D) Mob1 localization in anaphase A31500 and A31504 cells. (E) Growth of A2587, A32452, A32586, and A32589 cells on YEPRG and YEPD plates as in Fig. 1E. (F) Dbf2 kinase activity and cell cycle progression in MOB1 GAL-mob1-77 (A32818) and mob1-3RA GAL-mob1-77 (A32823) cells grown as in Fig. 1A. (G) Model for Dbf2-Mob1 activation. (H) Growth of A2587, A5588, A32336, A33289, A33551, and A33285 cells on YEPD+methioinine, CSM-methionine+glucose, or CSM-methionine+raffinose and galactose (CSMRG) plates as in Fig. 1E. (I) Anaphase kinetics in A1411, A33291, A32668, A33549, A33287, and A33283 cells. Cells were arrested in G1 with α-factor in YEPRaff+ methionine medium and released into CSM-methionine+raffinose medium to induce expression of the Mob1-Nud1 fusion. Galactose was added (arrow) to induce expression of GAL-TEV1.
The structure explains the phospho-dependence of Mob1 binding through extensive interactions of the phosphoserine with three basic residues (K132, R133, and R136) that form part of a positively charged pocket located on the face opposite to that of the Dbf2-interacting surface. In yeast Mob1, three structurally equivalent arginine residues, R253, R254, and R257, form the conserved basic pocket and directly coordinate a sulfate ion derived from the crystallization mother liquor (Fig. 4B and fig. S10B) (14). Thus, the Mob1 protein family contains a distinct class of phosphoserine-threonine binding domains.
To test whether these arginine residues are important for Mob1 binding to Nud1, we assayed the interaction of GST-mob1-R253A, R254A, R257A (henceforth mob1-3RA) with phosphopeptides. The mob1-3RA protein was properly folded (fig. S11A), but failed to interact with Nud1 pT78 peptides (Fig. 4C). Thus, the basic pocket of Mob1 mediates phosphopeptide binding. Phosphopeptide binding was also essential for Mob1 function in vivo. The mob1-R→A proteins interacted with Dbf2 (fig. S11B) but did not localize to SPBs (Fig. 4D and fig. S11C) nor support viability (Fig. 4E and fig. S11D). Furthermore, these cells lacked Dbf2 kinase activity and failed to release Cdc14 from the nucleolus or to exit from mitosis (Fig. 4F). This cell cycle arrest was not suppressed by a dominant, constitutively active Dbf2 allele (DBF2-HyA; fig. S11E) (17). Thus, even if the defect in Dbf2 activation is corrected in mob1-3RA cells, the failure of mob1-3RA to bind phosphopeptides and presumably target Dbf2 to its relevant substrates results in an inability to exit from mitosis. Indeed, the phosphopeptide binding property of Mob1 may also be critical for targeting Dbf2-Mob1 to its substrates. Mob1 binds experimentally verified phosphorylation sites in the Dbf2-Mob1 substrate Hof1 (fig. S12) (18).
Our results show that MEN signaling requires an unusual two-step process (Fig. 4G). Rather than directly activating its downstream kinase Dbf2-Mob1, Cdc15 first creates phospho-docking sites on the MEN scaffold Nud1. Nud1 phosphorylation recruits Dbf2-Mob1 to SPBs followed by Cdc15-dependent activation of Dbf2-Mob1. As Cdc15 alone is sufficient to activate Dbf2-Mob1 in vitro (6), why do cells use a two-step mechanism for Dbf2-Mob1 activation? A two-step scaffold-assisted mechanism of activating downstream kinases may afford many advantages. In addition to allowing for greater regulatory power, this mechanism mitigates the “biphasic effect” in which scaffold proteins can exhibit concentration-dependent titration effects (19). Increasing concentrations of scaffold proteins initially favor interaction of partner proteins but, at higher concentrations, will sequester partner proteins into separate complexes. The dependency of terminal kinase binding on scaffold phosphorylation may ensure that terminal kinases only engage in complexes in which their upstream kinases are bound and active. Such a protective mechanism predicts that MEN signaling ought to be resistant to the overexpression of individual signaling components, and this is what we observe (fig. S13).
A two-step mechanism may also facilitate signal transmission and effector activation at distinct sites in the cell. Dbf2-Mob1 activated by Cdc15 at SPBs must phosphorylate Cdc14 in the nucleolus. This predicts that the Mob1-Nud1 interaction must be dynamic. In agreement, fusion of MOB1 to NUD1 (MOB1-NUD1) resulted in the constitutive localization of Dbf2 to SPBs (fig. S14, A and B) but, despite the proper localization pattern, did not restore viability to mob1-77 cells at restrictive conditions (fig. S14C). Indeed, expression of MOB1-NUD1 was lethal in wild-type cells because Cdc14 could not be released from the nucleolus (fig. S14, D to F). This lethality appears to result from failure of Dbf2-Mob1 to dissociate from SPBs because a Mob1-Nud1 fusion in which Mob1 could be proteolytically released from Nud1 restored proper MEN regulation (Fig. 4, H and I). Furthermore, lethality caused by expression of MOB1-NUD1 was not suppressed by expression of a dominant, constitutively active Dbf2-HyA allele (fig. S14G), indicating that even hyperactive Dbf2 that cannot dissociate from SPBs is not functional. During MEN signaling, dynamic interaction between Mob1 and Nud1 may be achieved by Cdc15 first generating a high-affinity scaffold docking site for Dbf2-Mob1. Phosphorylation of Dbf2-Mob1 by Cdc15 could then, in addition to activating the kinase, decrease affinity to the Nud1 scaffold, thereby promoting the dissociation of Dbf2-Mob1 from SPBs.
In this study, we have uncovered a distinct mechanism of scaffold-assisted activation of a kinase cascade. We propose that the two-step activation of terminal kinases may be a common feature of scaffold-assisted signaling that provides a potentially general means to avoid the biphasic effect and organize signaling pathways in which the site of pathway activation differs from the essential site of action.
Supplementary Material
Acknowledgments
We thank I. Cheeseman, F. Solomon, and the Amon lab for comments and E. Schiebel for reagents. Coordinates and structure factors for the hMob1-phosphopeptide complex have been deposited with the Protein Data Bank (PDBID: 4JIZ). Supported by the NIH (GM056800 to A.A.; CA112967 and ES015339 to M.B.Y; P41 RR011823 to J.R.Y. III and T. N. Davis, principal investigator; and GM51312 to M.W.); MRC UK (U117584228) to S.J.S; MRC centenary fellowship to L.S.; NIH F32 GM086038 to J.M.K.; and NSF Predoctoral Fellowship to J.M.R. A.A. is an investigator of the Howard Hughes Medical Institute.
Footnotes
References and Notes
- 1.Hergovich A, Hemmings BA. Semin Cell Dev Biol. 2012;23:794. doi: 10.1016/j.semcdb.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stegmeier F, Amon A. Annu Rev Genet. 2004;38:203. doi: 10.1146/annurev.genet.38.072902.093051. [DOI] [PubMed] [Google Scholar]
- 3.Rock JM, Amon A. Genes Dev. 2011;25:1943. doi: 10.1101/gad.17257711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valerio-Santiago M, Monje-Casas F. J Cell Biol. 2011;192:599. doi: 10.1083/jcb.201007044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caydasi AK, Pereira G. Exp Cell Res. 2012;318:1421. doi: 10.1016/j.yexcr.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 6.Mah AS, Jang J, Deshaies RJ. Proc Natl Acad Sci USA. 2001;98:7325. doi: 10.1073/pnas.141098998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Good MC, Zalatan JG, Lim WA. Science. 2011;332:680. doi: 10.1126/science.1198701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maekawa H, Priest C, Lechner J, Pereira G, Schiebel E. J Cell Biol. 2007;179:423. doi: 10.1083/jcb.200705197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park CJ, et al. Eukaryot Cell. 2008;7:444. doi: 10.1128/EC.00283-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keck JM, et al. Science. 2011;332:1557. doi: 10.1126/science.1205193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gruneberg U, Campbell K, Simpson C, Grindlay J, Schiebel E. EMBO J. 2000;19:6475. doi: 10.1093/emboj/19.23.6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Materials and methods are available as supplementary materials on Science Online.
- 13.König C, Maekawa H, Schiebel E. J Cell Biol. 2010;188:351. doi: 10.1083/jcb.200911128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mrkobrada S, Boucher L, Ceccarelli DF, Tyers M, Sicheri F. J Mol Biol. 2006;362:430. doi: 10.1016/j.jmb.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Durocher D, et al. Mol Cell. 2000;6:1169. doi: 10.1016/s1097-2765(00)00114-3. [DOI] [PubMed] [Google Scholar]
- 16.Ding Z, et al. Biochemistry. 2007;46:2684. doi: 10.1021/bi061763n. [DOI] [PubMed] [Google Scholar]
- 17.Geymonat M, Spanos A, de Bettignies G, Sedgwick SG. J Cell Biol. 2009;187:497. doi: 10.1083/jcb.200905114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elia AE, Cantley LC, Yaffe MB. Science. 2003;299:1228. doi: 10.1126/science.1079079. [DOI] [PubMed] [Google Scholar]
- 19.Levchenko A, Bruck J, Sternberg PW. Proc Natl Acad Sci USA. 2000;97:5818. doi: 10.1073/pnas.97.11.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shou W, et al. Mol Cell. 2001;8:45. doi: 10.1016/s1097-2765(01)00291-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.