Abstract
The major histocompatibility complex (MHC) glycoprotein family, also referred to as human leukocyte antigens, present endogenous and exogenous antigens to T lymphocytes for recognition and response. These molecules play a central role in enabling the immune system to distinguish self from non-self, which is the basis for protective immunity against pathogenic infections and disease while at the same time representing a serious obstacle for tissue transplantation. All known MHC family members, like the majority of secreted, cell surface, and other immune-related molecules, carry asparagine (N)-linked glycans. The immune system has evolved increasing complexity in higher-order organisms along with a more complex pattern of protein glycosylation, a relationship that may contribute to immune function beyond the early protein quality control events in the endoplasmic reticulum that are commonly known. The broad MHC family maintains peptide sequence motifs for glycosylation at sites that are highly conserved across evolution, suggesting importance, yet functional roles for these glycans remain largely elusive. In this review, we will summarize what is known about MHC glycosylation and provide new insight for additional functional roles for this glycoprotein modification in mediating immune responses.
Keywords: Glycosylation, MHC, HLA, N-Glycan, Antigen presentation, Class I, Class II, Non-classical class I
Introduction
The major histocompatibility complex (MHC) is a ≈3.6-Mb cluster of genes in the mammalian genome that encodes integral components of the immune system. The MHC was first discovered in the mouse in the 1930s, referred to as the histocompatibility 2 (H2) complex, and has since been characterized in humans, also referred to as the human leukocyte antigen (HLA) complex, in addition to numerous other species [1]. MHC molecules play a well-established role in T cell development in the thymus and in maintaining immune homeostasis [2, 3] in addition to their central role in mediating the cellular and humoral immune responses that combat disease and pathogenic infection.
Members of the MHC family include the classical, highly polymorphic class I (MHCIa) and class II (MHCII) glycoprotein molecules, which act as support structures for the presentation of antigens to T cells. MHCIa molecules are expressed by almost all nucleated cells and present antigens derived from the cytoplasm to CD8+ T cells. MHCII molecules are mainly expressed by professional antigen presenting cells such as dendritic cells, macrophages, B cells, and thymic epithelial cells. MHCII molecules utilize an endocytic pathway for presentation of exogenous antigens to CD4+ T cells. In mice, the classical MHCIa molecules are encoded by the K and D regions, whereas the MHCII molecules are encoded by the IA and IE regions. Conversely, the human HLA complex encodes MHCIa molecules in the A, B, and C regions and MHCII molecules in the DP, DQ, and DR regions. The human HLA-DQ and HLA-DR molecules most closely resemble the mouse H2-A and H2-E molecules, respectively [1]. Like the majority of secreted, cell surface, and immune-related molecules, these proteins contain post-translational glycan modifications that are initiated in the lumen of the endoplasmic reticulum (ER). Although O-glycan structures have been shown to play important roles on immune-associated molecules [4], the prominent glycans found on MHC molecules are N-linked. In fact, essentially all of the MHC family maintain Asn-X-Thr/Ser (where X is any amino acid except proline) acceptor sites for N-glycosylation that are highly conserved across species, suggesting a functional role for these structures.
In addition to these classical MHCIa and MHCII molecules, more recent characterization of non-classical MHC class I (MHCIb) molecules have demonstrated their important role in the cellular immune response [5]. In general, the MHCIb molecules are less polymorphic and have more diverse functional roles than the class Ia molecules [6]. In this review, we will focus on the role of post-translational glycosylation in MHCIa and MHCII function, with only a limited discussion of the MHCIb glycoproteins.
Glycosylation is a non-template driven enzymatic process orchestrated by the collective action of glycosidases and glycosyltransferases, primarily but not exclusively in the Golgi apparatus (Fig. 1). The N-linked acceptor site is recognized by the oligosaccharyltransferase (OST) enzyme that removes the common Glc3Man9GlcNAc2 moiety from the dolichol phosphate lipid precursor and covalently attaches it to Asn residues within the consensus sequence while the nascent polypeptide remains in the ER. After addition of this common precursor, the N-glycans are further processed and modified. The three categorizations of N-glycans in the secretory pathway are high mannose, hybrid, and complex, which reflect the structure of the glycans as they are progressively modified. Important to this pathway are the Mgat genes and the acetylglucosaminyltransferase enzymes they encode. UDP-GlcNAc:α3-D-mannoside β1,2-N-acetylglucosaminyltransferase I (GlcNAcT-I; encoded by Mgat1) adds β1,2-linked GlcNAc to the non-reducing Man-α1,3Manβ1,4-terminus of Man5GlcNAc2, an essential step in the conversion from high mannose to hybrid N-glycan [7]. The hybrid N-glycan is then trimmed to a trimannosyl core by α-mannosidase enzymes, which creates a substrate for UDP-GlcNAc:α6-D-mannoside β1,2-N-acetylglucosaminyltransferase II (GlcNAcT-II; encoded by Mgat2). The activity of GlcNAcT-II transitions the N-glycan from hybrid to complex-type structures, the branching patterns of which can vary depending on the activity of additional GlcNAc transferases (GlcNAcT III–VIII, encoded in part by Mgat3–5). Branch chains can then be further extended through the activity of numerous fucosyl, galactosyl, and sialyl transferases [8]. Taken together, complex N-glycans can have extensive bi-, tri-, or tetra-antennary branches containing multiple galactose, fucose, and sialic acid linkages not found on the hybrid or high mannose precursors. In general, the N-glycans found in mammals are referred to as complex due to the more complex array of non-mannose linkages and inclusion of terminal sialic acid residues, as opposed to the high mannose N-glycans found on lower-order organisms like yeast and fungi [4].
Fig. 1.
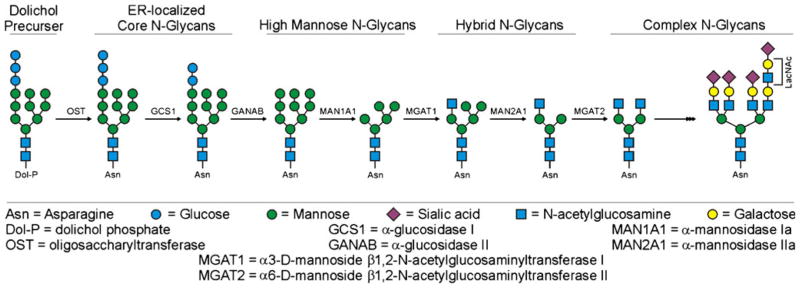
Simplified schematic of the N-glycosylation pathway in mammalian cells. Shown are the different classes of N-glycans found in mammals and some, but not all, of the key enzymes that create these structures for the readers easy reference. While this figure depicts events in a particular order, it must also be noted that the pathway is more flexible than shown and some of the enzymes can act in different order. For example, the MGAT1/MAN2A1 reactions can occur in reverse order from what is shown
Roles for protein N-glycosylation
Protein glycosylation can play a variety of roles in the context of different immune-associated molecules. These potential roles include a check point for proper protein folding and molecular trafficking to the cell surface [9, 10], for protein stability and protection from proteolysis [11] and to facilitate appropriate geometric spacing of cell surface molecules to prevent unwanted molecular cis-interactions and to optimize cell–cell adhesion and communication [12, 13].
Addition of N-glycans in the ER can play an important role in proper protein folding and trafficking. MHCIa molecules are a well-studied example of the role of N-glycans and protein folding. Blocking MHCIa N-glycosylation, such as through acceptor site mutation, results in significant increases in intracellular misfolded protein along with decreases in cell surface expression [14]. Similar to other glycoproteins, early glucose trimming of the newly attached Glc3Man9GlcNAc2 N-glycan core facilitates proper interactions with the lectin chaperones calnexin and calreticulin, which are involved in the chaperone-assisted protein folding and assembly pathway. Additional lectins can also contribute to this protein quality control pathway and transport from ER to Golgi [15]. Following correct protein folding and molecular assembly (i.e., β2-microglobulin and peptide antigen binding in the case of MHCIa), the N-glycan is further modified as it moves through the Golgi. The N-glycans can themselves facilitate glycoprotein trafficking through the Golgi for surface expression or secretion. For example, Brennan et al. recently demonstrated that the conserved C terminus N-glycan of perforin, a soluble cytotoxic molecule employed by the immune system, is necessary for the rapid and efficient delivery of the glycoprotein from the Golgi to secretory granules [16].
The potential protein folding and trafficking role of the conserved N-glycans on MHCII is much less clear. In fact, it appears that the N-glycosylated invariant chain (Ii) chaperone, which associates with the MHCII heterodimer in the ER, is the essential mediator of proper protein folding and trafficking from the ER to the Golgi and subsequently to endocytic compartments where MHCII binds to antigens for cell surface presentation [17, 18]. This is supported by observations where surface MHCII concentrations were unaltered for N-mutants and upon treatment with tunicamycin to inhibit the ER-localized OST [19]. Although the N-glycans found on Ii may play a partial role in MHCII trafficking [20], a signal sequence found in its cytoplasmic domain appears to be the critical factor in the transport of MHCII [21].
The polarized nature of typical epithelial cells, such as those found in the small intestine and lung, requires selective glycoprotein trafficking from the Golgi to either the apical or basolateral surfaces. Secreted and surface-bound glycoproteins designed for apical expression can use lectin accessory molecules to direct trafficking, indicated by the unrestricted localization when the glycans are removed [22]. Two such molecules identified to have apical sorting functions are the mannose-binding lectin VIP36 and the galactose-binding lectin galectin-3. Although most glycoproteins relegated to basolateral surface expression are also N-glycosylated, such as the MHC molecules, it appears that their sorting relies more exclusively on protein signal sequences rather than carbohydrate–lectin interactions [22, 23].
The role of N-glycans in glycoprotein stability can be mediated in multiple ways. N-Glycosylation can provide structural rigidity and/or maintain binding accessibility to residues both immediately surrounding an acceptor site as well as more distant domains, collectively increasing the overall stability of a protein [24] while maintaining the folded confirmation of the glycoprotein [25]. N-Glycans also can provide protection to the protein backbone from proteolysis. This protection is believed to occur either via direct steric hindrance of the approach of proteases or indirectly through changes in the protein structure to alter enzyme recognition site accessibility. It remains unclear if the N-glycans found on MHC molecules protect the protein structure from enzymatic degradation; however, the N-glycans of the chaperone Ii are important for preventing premature enzymatic proteolysis and dissociation from MHCII prior to its transport from the secretory pathway to endocytic vesicles [26]. The controlled proteolysis of Ii that occurs in endocytic vesicles by resident cathepsins [27] allows MHCII to bind antigenic peptides that are generated from proteolytic processing of exogenous antigens.
At the cell surface, protein glycosylation is thought to act as a molecular spacer to correctly orient signaling and adhesion molecules to facilitate proper cell-to-cell communications. Rudd and colleagues proposed that the N-glycans of MHC molecules could act as lateral spacers for cell surface molecules to prevent close protein–protein interactions [13]. This model is based largely on molecular packing patterns of MHC and other immune-related surface glycoproteins within crystals for crystallographic analysis, and it suggests that glycosylated MHC and T cell receptor (TCR) molecules would be spaced apart at the cell surface by the extended N-glycans, thereby constraining lateral association of glycoproteins. Their analysis also found that the maximum width of a MHC glycoprotein is similar to that of its cognate TCR ligand on T cells, which suggests that the N-glycans would limit the geometry and interaction of any TCR-MHC clusters on the cell surface [13].
More recently, it has become clear that galectins, a family of animal lectins that share a common carbohydrate recognition domain, are a missing link in the “spacer” model. Most galectin family members are multivalent molecules that bind derivations of the common disaccharide N-acetyllactosamine (Galβ1,4GlcNAc; LacNAc) found on branched N-glycans and thus link together cell surface glycoproteins into an ordered lattice-like conformation [28, 29]. The selective glycan binding preferences of individual galectins can differ substantially, which has been suggested to be the basis of the functional differences in their biological activity. Variations in glycan binding are mainly associated with the extent of N-glycan branching, the multiplicity of LacNAc residues, and/or the modification of terminal residues (i.e., sialylation or fucosylation) [28]. Illustrating the role of galectin–glycan interactions at the cell surface, it was found that the N-glycosylated TCR on the surface of naïve T cells forms a lattice that blocks spontaneous oligomerization in order to prevent unwanted signaling events. Conversely, upon T cell activation, the N-glycosylation pattern changes notably (e.g., four- to eightfold increase in branching), which alters galectin binding domains and the lattice structure, thus influencing T cell activity [12]. Although not described, galectins may also act to organize MHC molecules on the surface of antigen presenting cells. Differences in N-glycan structure and sialylation between cell types [30, 31] may indicate differences in galectin-associated molecular organization at the cell surface and thus influence T cell responses. Additionally, the differences in number of N-glycans between human (1 glycan) and mouse (two to three glycans) MHCIa molecules compared to MHCII molecules (three glycans) may reflect a specialized organizational lattice designed for optimal CD8+ T cell or CD4+ T cell stimulation, respectively.
MHCIa
MHCIa molecules are composed of a heavy chain glycoprotein that associates with the non-glycosylated β2-microglobulin protein, forming the heterodimer that is responsible for antigenic peptide presentation at the cell surface. The MHCIa alleles have a single highly conserved acceptor site for N-glycosylation at approximately Asn86 on the heavy chain subunit (Figs. 2 and 3). After the core Glc3Man9GlcNAc2 structure is added to Asn86, it is quickly processed by glucosidases I and II to generate monogluco-sylated Glc1Man9GlcNAc2, which enables interaction with calnexin and calreticulin. Along with additional accessory molecules, these chaperones function in the ER to ensure proper folding of the heavy chain, association with β2-microglobulin, and loading of antigenic peptide to the binding groove [32]. It is important to note that for MHCIa, antigen loading occurs in the ER prior to the majority of N-glycan modifications in the Golgi, whereas antigen loading for MHCII occurs after exiting the secretory pathway when it has fully complex and mature N-glycans. This temporal and spatial difference in MHC antigen loading appears to delineate an additional functional role for complex N-glycans in MHCII antigen binding and presentation [33].
Fig. 2.
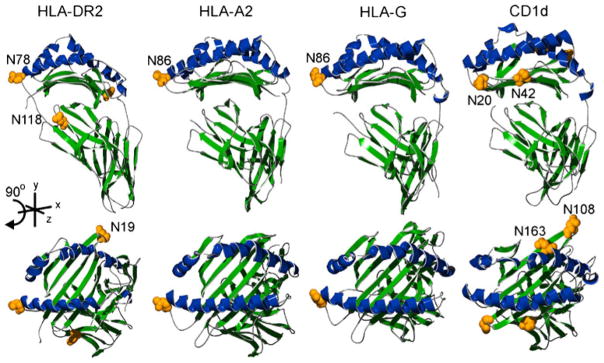
Structural comparison of human MHC molecules. Images were created using previously defined crystal structures of HLA-DR2 (pdb| 1BX2), HLA-A2 (pdb|3D25), HLA-G (pdb|1YDP), and CD1d (pdb|1ZT4). Alpha helices of the antigen binding groove are shaded blue and the rest of the molecule in green. The upper panel shows lateral views with the corresponding top view of each molecule found directly below. Putative N-glycan acceptor sites are shaded yellow and shown as space-filled residues
Fig. 3.
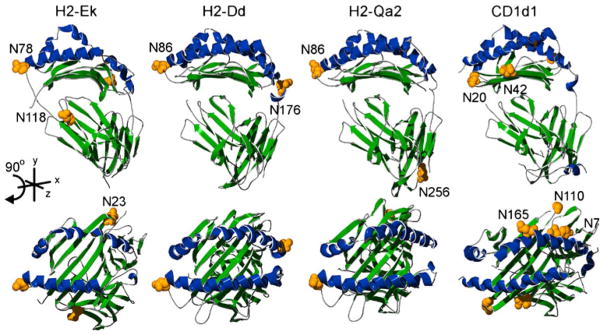
Structural comparison of mouse MHC molecules. Images were created using previously defined crystal structures of mouse H2-Ek (pdb|3QIB), H2-Dd (pdb|3E6F), Qa2 (pdb|1K8D), and CD1d1 (pdb| 3HE6). Alpha helices of the antigen binding groove are shaded blue and the rest of the molecule in green. The upper panel shows lateral views with the corresponding top view of each molecule found directly below. Putative N-glycan acceptor sites are shaded yellow and shown as space-filled residues. The N7 and N110 designations on CD1d1 show the approximate locations of the asparagine residues due to missing coordinate data in the available PDB structure file
Although it is well established that the precursor high mannose N-glycan plays a critical role in proper folding and assembly of the MHCIa, much less is known about the role of the mature complex N-glycan found on MHCIa as it exits the secretory pathway for cell surface localization. Computer modeling of oligosaccharide structures in the context of mouse MHCIa crystallographic information indicates that the glycans control crystal packing [13]. This suggests that, similar to the TCR [12], at the cell surface, the class Ia N-glycans could physically space the molecules from adjacent surface receptors and control the extent of clustering. Additionally, a study by Mandal and Mukhopadhyay which modeled MHCIa glycoprotein–glycan dynamics indicated that the type and length of branching found on complex N-glycans might modify the local protein conformation, which could potentially influence ligand binding sites on the molecule and T cell recognition specificity [34].
Unlike the structural heterogeneity of N-glycans typically associated with individual glycosylation sites of mammalian glycoproteins [35], Barber et al. found that across multiple allotypes of HLA-A, HLA-B, or HLA-C isolated from EBV-transformed human B cell lines that there was surprisingly limited structural diversity in the MHCIa-associated Asn86 N-glycan [36]. HLA-A and HLA-B were limited to one of two disialylated complex N-glycans, SA2Gal3Man3Glc-NAc5Fuc1 (triantennary) or SA2Gal2Man3GlcNAc4Fuc1 (bi-antennary), where the bi-antennary N-glycan predominated. Either of these two structures could be found on HLA-C in addition to two alternative structures that lack sialylation and galactose linkages (i.e., Man3GlcNAc4Fuc1 or Man3GlcNAc4). Although not found on HLA-A or HLA-B, these alternative structures composed a significant fraction of N-glycans found on HLA-C. To confirm that the structures found on the B cell lines were relevant to physiological states rather than artifacts of the cell transformation process [37] or in vitro cell culture conditions [38], HLA glycoproteins from peripheral blood lymphocytes (PBL) of healthy individuals were directly isolated for N-glycan analysis. In pooled HLA preparations, the four N-glycan structures appeared to be present in addition to a monosialylated structure. The major N-glycan found on PBLs was identified as the same bi-antennary N-glycan on the HLA allotypes of the B cell lines [36]. Analysis of mouse B cell and T cell lymphoma lines showed that bi-antennary N-glycans also predominate among mouse MHCIa molecules [39, 40]. Comparable to human MHCIa, the conserved Asn86 site of mouse MHCIa exhibits similar branching patterns and similar size distribution; however, a second mouse-conserved site (Asn176) appears to have a more heterogeneous N-glycosylation pattern indicated by the disparate proportion of triantennary structures between allotypes [39], suggesting that the mechanism(s) constraining Asn86 site N-glycan modifications may be more characteristic of the site rather than the molecule. While the branched core structure of the Asn86 site N-glycan appears to be relatively homogeneous within lymphocyte lineages (i.e., B cells and PBLs), the degree of terminal sialylation may be more variable between tissues. Although the branching pattern was not determined and the study was limited to the mouse system, a comparison of MHCIa allotypes isolated from macrophages, lymphocytes, and hepatocytes found an increasing degree of N-glycan sialylation, respectively [41]. The biological role for the relative homogeneity of MHCIa N-glycosylation pattern in the context of varying sialylation usage remains unclear. Due to the broad expression pattern of MHCIa across most nucleated cells, a similar N-glycan structure at a highly conserved site may play a role in providing consistent geometric spacing and orientation of the MHC at the cell surface that could be modified in a cell dependent fashion based on sialylation [28], rather than conferring additional information directly to CD8+ T cells [13]. The absence of N-glycans or changes in MHCIa sialylation do not appear to affect the allospecific or peptide-specific cytotoxic T lymphocyte (CTL) recognition of MHCIa molecules [42].
It is interesting to speculate that the alternative N-glycans found on HLA-C may correlate with a functional role separate from those of HLA-A and HLA-B. Unlike HLA-A and HLA-B, HLA-C is expressed at the cell surface at much lower levels, and it appears to bind a more restricted repertoire of peptides, which suggests a reduced role in antigen presentation to CD8+ T cells. Rather, HLA-C appears to play a more prominent role in natural killer (NK) cell receptor interactions. Human NK cells express a family of killer cell immunoglobulin-like receptors (KIR), similar to the mouse Ly49 receptors, which can confer cell activating or inhibitory signals upon ligation. The inhibitory p58 KIRs (CD158a and CD158b) can discriminate between two groups of HLA-C allotypes depending on the presence of a lysine or asparagine at position 80 of the heavy chain and upon ligation provide a dominant inhibitory signal to prevent NK cell lysis. The relative proximity of the Asn86 N-glycan to this recognition site was thought to possibly influence CD158 interactions. By mutating the Asn86 acceptor site, Baba et al. found that HLA-C molecules lacking N-glycosylation had decreased binding to CD158a. The decreased binding by HLA-C mutants was also reflected functionally by decreases in CD158a-mediated inhibition of NK cell lysis. Interestingly, the presence of an immature high-mannose N-glycan on HLA-C rather than the complex-type did not appear to affect CD158a binding [43]. This suggests that the physical presence of the Asn86 N-glycan on HLA-C may be more important than its specific structure or electrostatic charge provided by terminal sialic acids and that the potential functional role for the alternative N-glycan structures characterized by Barber et al. is not related to CD158a binding. However, the N-glycan may be contributing indirectly to CD158a binding by influencing the confirmation of the protein backbone, which is a reported role for N-glycans in other settings [24], or the core structure of the N-glycan is sufficient to mediate interactions with CD158a.
In addition to the highly conserved Asn86 site, some non-human species have additional acceptor sites for N-glycans on the MHCIa heavy chain. For example, all mouse allotypes of MHCIa have a second conserved heavy chain N-glycosylation site at Asn176 and less frequently at Asn256 [39]. It remains unclear what functional relevance the Asn176 N-glycan on MHCIa plays in mouse immune responses. Interestingly, introduction of the second Asn176 N-glycosylation site to human MHCIa molecules could only slightly rescue its cell surface expression when the highly conserved Asn86 site was absent [14]. This indicates that the Asn176 likely plays an uncharacterized but separate, non-redundant role from the Asn86 N-glycan and that MHCIa chaperone interactions and subsequent molecular transport through the secretory system appear to rely on both the Asn86 N-glycan and the underlying protein structure of the heavy chain.
One possible explanation for the additional Asn176 N-glycan on murine MHCIa may involve mediating innate immune responses through interactions with Ly-49 receptors [44]. Ly-49 glycoproteins are members of the C-type lectin-like, NK cell family of receptors that transmit activating or inhibitory signals upon ligation. The Ly-49 receptors have a single lectin-like extracellular domain indicating carbohydrate binding activity, although its exact role in receptor function is not completely clear. Interestingly, coinciding with the absence of multiple N-glycans on human MHCIa, functional Ly-49 receptors are not found in humans, suggesting that the additional N-glycans on mouse MHCIa may represent intraspecies immune co-evolution with NK cells to specifically protect against especially virulent mouse pathogens. Conversely, pathogens may have co-evolved with mouse MHCIa to exploit potential immune evasion mechanisms. For example, there is evidence that the mouse cytomegalovirus (CMV)-encoded m154 glycoprotein, a structural mimic of MHCIa, evolved to functionally “hide” CMV-infected cells from immunodetection by binding an inhibitory member of the Ly-49 family [45]. Similar to MHCIa, the N-glycosylation of m154 may have a role in interactions with Ly-49 molecules [46] in that N-glycans on m154 appeared to play an important role in its extraction and physical transfer (trogocytosis) to adjacent Ly-49 expressing cells [46]. Trogocytosis is a phenomenon by which NK cells and other lymphocytes can acquire cognate ligands, such as MHCIa molecules, through specific receptor binding [47, 48]. Considering the m154 mimicry of MHCIa, an unexplored potential role for the complex N-glycans found on MHCIa may involve the trogocytosis process.
MHCIb
The MHCIb subfamily includes both glycosylated [49, 50] and non-glycosylated [6] molecules expressed at the cell surface. Unlike the well-defined function of the “classical” MHCIa and MHCII molecules in antigen presentation to αβ TCR-expressing CD8+ T cells and CD4+ T cells, respectively, the “non-classical” MHCIb categorization includes a more functionally diverse group of molecules. Within species, the MHCIb molecules are structurally related to MHCIa molecules, including associations with β2-microglobulin; however, they lack the high degree of polymorphism seen in the classical MHCIa and MHCII molecules [5]. Also, it is well established that some MHCIb molecules can act as innate immune receptors (i.e., NK cell receptors). MHCIb molecules include HLA-E, HLA-F, HLA-G, and HLA-H (HLE) in humans and transcripts from the H2-M, H2-Q, and H2-T regions in mice.
HLA-E and its functional homologue in mice, Qa-1b, are relatively non-polymorphic MHCIb molecules that bind and present nonameric leader peptides derived from the signal sequences of the MHCIa heavy chains, in addition to various pathogen-associated and antigen presentation defect-associated peptides [51]. Similar to their related intraspecies MHCIa molecules (e.g., HLA-A and H2-D; Figs. 2 and 3), HLA-E and Qa-1b maintain the conserved one and two acceptor sites for N-glycosylation, respectively. This suggests that the role for N-glycans on these molecules is likely similar to that of the classical class Ia molecules.
Based on available heavy chain sequence information, the potential N-glycosylation pattern for the mouse Q9 (Qa-2 isoform) and H2-M3 molecules more closely resembles the human MHCIa and Ib molecules in that only the highly conserved acceptor site found across species (Asn86) is present. Although little is known about the role of N-glycosylation of Q9 and H2-M3, it is reasonable to speculate that it is at least involved in a common activity to facilitate early events in protein folding and quality control.
In addition to HLA-E, humans express three additional β2-microglobulin-associated MHCIb molecules (HLA-G, HLA-F, HLA-H). Like the MHCIa molecules, HLA-G and HLA-F, both carry a single N-glycan at the same highly conserved acceptor site. In the mouse, the non-classical Qa-2 molecule is thought to be a functional homolog to HLA-G [52]. Unlike HLA-G, Qa-2 has an additional potential N-glycan acceptor site at Asn256 of the heavy chain (Fig. 3). This Asn256 site is also found in some mouse MHCIa allotypes (H2-Db, Dk, Kd) but not others (H2-Dd, Kb, Kk) as described above [14, 39], although a possible functional role remains unclear. HLA-G plays an important role in immune tolerance through direct binding with inhibitory receptors immunoglobulin-like transcript-2 and immunoglobulin-like transcript-4 and the inhibitory KIR 2DL4 [53]. The expression pattern of HLA-G includes seven different protein isoforms, generated by alternative splicing of the primary mRNA transcript. HLA-G1, HLA-G2, HLA-G3, and HLA-G4 are membrane-bound isoforms whereas HLA-G5, HLA-G6, and HLA-G7 are secreted [53]. Of the membrane-bound forms, HLA-G1 (Fig. 2) is more structurally similar to MHCIa molecules and is expressed at the cell surface with a mature complex N-glycan. However, HLA-G2, HLA-3, and HLA-4 are truncated versions of the heavy chain that do not associate with β2-microglobulin and are expressed with an immature high mannose/hybrid N-glycan [53, 54]. Although a well-defined functional role for HLA-F has yet to be established, these molecules also have a propensity to be expressed at the cell surface with high mannose/hybrid N-glycans [55]. Similar to observations with the MHCI-like lipid-presenting molecule CD1d, the absence of β2-microglobulin association in the ER may play a role in relegating these glycoproteins to an alternative pathway that results in the high mannose/hybrid glycosylation pattern [55, 56].
HLA-H is now more commonly referred to in humans and mice as HFE after it was found to be mutated in most cases of hereditary hemochromatosis [57]. Although HFE appears to play a prominent role in iron metabolism, it has also been characterized as possibly having immune-associated activities. Hepatic iron overload in TCR delta chain knockout mice provides indirect evidence that suggests that HFE is recognized by γδ T cells [58]. All three of the putative N-glycosylation acceptor sites of human HFE appear to be occupied with N-glycans. One of these sites, Asn110, is in a comparable structural location to the highly conserved site of MHCIa molecules [59], which suggests that it may contribute a similar role in early protein folding and quality control events in the ER. However, the presence of the additional two N-glycans appears to provide a similar function if the Asn110 N-glycan is absent [60]. It remains unknown whether changes in the N-glycan branching pattern at the cell surface may influence cognate ligand interactions.
Although CD1 molecules are expressed by a genetic locus distinct from the MHC locus, these important glycoproteins can also be characterized as “non-classical” MHC molecules. These lipid and glycolipid antigen presenting molecules are represented by four isoforms in humans (a, b, c, and d) and one in mice (CD1d). The overall structure and domain organization of CD1 is similar to MHCIa, with a heavy chain non-covalently associated with β2-microglobulin; however, more significant differences are found in the antigen-binding grooves and number of N-glycan acceptor sites. In the case of CD1d, the bound lipid antigens are recognized by T cells expressing a CD1d-restricted invariant α chain and semi-invariant β chain TCR. The human and mouse CD1d molecules have four [61] and five [62] occupied N-glycan acceptor sites, respectively (Figs. 2 and 3). Studies have shown that CD1d N-glycans associate with the lectin chaperones calnexin and calreticulin [61], although calreticulin appears to be particularly critical for CD1d folding and assembly [63]. Selective removal of the Asn42 site can affect the stability of the heavy chain and β2-microglobulin heterodimer but does not appear to negatively affect surface expression [64]. Although there are conflicting reports about the roles of the other CD1d N-glycans, a common finding is that the complete absence of CD1d N-glycosylation eliminates cell surface expression of both mouse and human molecules; however, the lack of any individual N-glycan is not sufficient to replicate this phenotype [64, 65]. This suggests that the N-glycans may serve multiple and partially redundant roles in early chaperone-mediated folding and trafficking events in the ER. At the cell surface, the presence of CD1d N-glycans does not appear to directly affect T cell recognition of CD1d bound lipid antigens [64, 66]; rather, these complex N-glycans are proposed to serve an indirect role by providing stability and proper organization and spacing to sustain TCR interactions, similar to that proposed for MHCIa [13, 65].
MHCII
MHCII molecules are heterodimers composed of non-covalently associated alpha and beta chain glycoproteins. MHCII has two highly conserved acceptor sites for N-glycosylation at approximately Asn78 on the alpha chain and at Asn19 on the beta chain (Fig. 4). Although not as highly conserved across evolution, within many species, including humans and mice, there is a conserved second acceptor site on MHCII alpha chains at Asn118, which is more frequently found on DQ-related rather than DR-related MHCII molecules (Fig. 4). Interestingly, the Rhesus and cynomolgus macaque monkey species that are commonly used in biomedical research appear to lack the DR alpha Asn118 site (Fig. 4; cynomolgus macaque not shown), a distinction that is not found in different ape species or in humans (Fig. 4; chimpanzee, gorilla, and gibbon not shown). On the other hand, some species of pig (i.e., Sus scrofa) have the second Asn118 site in addition to a third potential N-glycosylation site on their DR alpha chain, as compared to the more evolutionarily distant DR-related alpha chain in chickens [67] that maintains only the single highly conserved Asn78 site. The variable presence of acceptor sites on DR-related rather than DQ-related MHCII molecules may correlate with separate functional roles of the MHCII allotypes and indicate different immune-related selective pressures, beyond ER protein quality control events, as the evolutionary branches diverged. For example, the Asn118 site of mouse DR-related MHCII (H2-E) appears to influence the adaptive immune response through T cell repertoire selection (see below) [68].
Fig. 4.

MHCII N-glycan acceptor sites are conserved across species. Peptide sequence alignments of MHCII DR-related alpha and beta chains (top) and DQ-related alpha and beta chains (bottom). The consensus N-X-T/S acceptor sites (where X is any amino acid except proline) are in bold text with the glycan-linked asparagine residue highlighted in red
Like MHCIa, the MHCII heterodimer is formed and folded in the ER in the context of linked immature core N-glycans; however, a significant difference is that the MHCII antigen binding groove is bound with the Ii chaperone rather than antigenic molecules [17, 69, 70]. The evolutionary conservation of acceptor sites, as seen with MHCIa, suggests an important functional role for the N-glycans; however, unlike MHCIa, a common role for MHCII N-glycosylation has been elusive despite the striking structural similarity of the Asn78 site to the analogous Asn86 site in MHCIa molecules (Figs. 2 and 3). In stark contrast to MHCIa, the MHCII N-glycans expressed on different antigen presenting cell populations are heterogeneous in branching pattern and sialylation usage [30]. This is even true within a particular cell type and in homogeneous cell populations where the MHCIa N-glycans are relatively homogeneous [36, 39]. For example, the analysis of a homogenous B cell lymphoma line (AKTB-1b) found that the N-glycans linked at specific acceptor sites on MHCII contain microheterogeneity, a phenomenon that is thought to be influenced by context of the acceptor site within the peptide backbone [39, 71]. Due to the numerous diverse roles of N-glycans described earlier, it is interesting to speculate that changes in the N-glycosylation pattern of MHCII may reflect a cellular mechanism to diversify the function of a limited selection of MHCII proteins and thus may contribute to “fine tuning” the outward CD4+ T cell response to antigens in a cell type-specific context.
Computer modeling of a crystallized human MHCII molecule indicates that both alpha and beta chain N-glycans likely protrude outward from the MHC surface [72]. Interestingly, Asn78 is located at the edge of the antigen binding groove suggesting it may influence TCR interactions, whereas Asn118 is located closer to the cell surface [68] (Figs. 2 and 3). Ishikawa and colleagues published a study in 1995 using transgenic mice with mutated MHCII (H2-E) alpha chain acceptor sites that suggested a role for Asn118, but not Asn78, in T cell thymic selection based on differential Vβ TCR usage [68]. It remains unclear whether the effect on thymic selection was due to a direct role of the N-glycans in antigen presentation or receptor binding, or to an indirect role through structural misalignment of the binding surface, though the position of Asn118 distal to the TCR binding sites supports a model in which it plays an indirect role in receptor association. An earlier study by Wei and colleagues also examined roles for N-glycans at the alpha chain Asn78 in addition to the beta chain Asn19 acceptor sites using vector-expressed mouse class II (H2-A) in B lymphoma cells lines [73]. Like Asn78, beta chain Asn19 is located proximal to the antigen-binding groove [13]. Loss of N-glycans at Asn78 and Asn19 appeared to have mixed influences on the stimulation of different peptide-specific T hybridoma cell lines, which is in contrast to studies showing no effect of the N-glycans on peptide antigen binding, presentation, and specific T cell responses [33, 74, 75]. Furthermore, removal of the beta chain Asn19 site resulted in the loss of an alpha chain serologic epitope, which suggests that an N-glycan at this site may influence the structure of MHCII molecules [73]. In both studies, the role for N-glycans could not be attributed to changes in the relative surface expression of cell surface MHCII, but these results must be considered in the context of the experimental systems utilized. Transformed antigen presenting cell lines and the responding T hybridomas, due to their malignant nature, can have significant differences in N-glycosylation patterns than that of their primary cell counterparts [76, 77]. In fact, aberrant glycosylation patterns are hallmarks of many cancer cell glycoproteins [78, 79] that are targets in ongoing cancer vaccination efforts [80–82].
Unlike MHCIa, a role for MHCII N-glycans in mediating ER-localized chaperone interactions has not been established. In fact, neither blockade of N-glycosylation with tunicamycin [19] nor expression of MHCII lacking N-glycosylation acceptor sites [68, 73] significantly reduces the cell surface concentration or localization of MHCII molecules, demonstrating independence from the calnexin/ calreticulin pathway. Instead, the invariant chain (Ii) trimeric complex makes contact with alpha and beta chain heterodimers within the antigen binding groove [83] and along a conserved region of the beta chain [84]. These two regions lack N-glycans, demonstrating reliance upon Ii-mediated protein–protein interactions for appropriate trafficking of MHCII through the secretory pathway.
MHCII N-glycosylation: a new functional role
While the argument in support of the N-glycans serving to induce proper spacing of the MHCII on the cell surface for T cell recognition has precedent for other membrane glycoproteins [12, 85], the biological application of that hypothesis for MHCII has not been demonstrated and remains a model based solely on the packing of MHCII molecules in crystal lattices for structure determination [13]. Likewise, the model in which N-glycans protect MHCII from proteolytic cleavage within vesicles and at the cell surface does not hold up experimentally in that cell surface concentrations of MHCII are not significantly affected by the inhibition of MHCII N-glycosylation [33, 68].
We recently found that complex N-glycans on MHCII molecules have a direct functional role in antigen presentation to CD4+ T cells [33]. While studying MHCII antigen presentation of a new class of zwitterionic polysaccharide T cell-dependent antigens (e.g., type I Streptococcus pneumoniae capsular polysaccharide, Sp1; Bacteroides fragilis polysaccharide A, PSA) we found that, unlike peptide antigens, these carbohydrate antigens (glycoantigens) failed to significantly associate with bacterially expressed human MHCII molecules. This was a surprising finding since the glycoantigens, like peptide antigens, bind with nanomolar affinity to the same MHCII molecule expressed by mammalian cells [86]. It is well-known that bacteria, along with other lower order organisms such as yeast and fungi, lack the machinery to produce complex-type N-glycans. Further examination found that it was in fact the lack of mammalian complex N-glycosylation that precluded antigen binding and presentation.
The function of complex N-glycans on MHCII was defined in part using the glucosidase inhibitor castanosper-mine and the mannosidase inhibitor kifunensine. By blocking these enzymes in the N-glycan synthesis pathway (Fig. 1), the MHCII glycoproteins will still have an N-glycan structure at their respective acceptor sites but will be mostly limited to high mannose and hybrid N-glycans (structural intermediates in the secretory pathway) rather than the normal complex-type branching of the native N-glycan structures. Similar to previous studies [74], we found that the absence of complex N-glycans did not affect cell surface localization of MHCII or its function in presenting selected peptide antigens. Furthermore, enzymatic removal of terminal sialic acids with neuraminidase showed that sialylation of complex N-glycans on MHCII did not appear to be responsible for antigen binding. Likewise, glycoantigens failed to associate with oligosaccharides in the absence of the MHCII protein backbone; thus, it appears that both the structure of the complex N-glycans and the MHCII backbone are both important for glycoantigen presentation since neither structure alone could recapitulate antigen binding [33].
MHCII binding of glycoantigen and peptide antigen is competitive and dependent on HLA-DM expression [86, 87], suggesting that the glycoantigens interact within the peptide binding groove in some way. However, the processed 3–10-kDa polysaccharide that is presented by MHCII [86, 88] is much larger than the 13–18 amino acid peptide that is typically found associated with the peptide binding groove. Moreover, we have demonstrated that the glycoantigen helical conformation [89, 90] is also required for stable association with MHCII [91], yet the size of this helix is too large to fit into the peptide groove in any classical fashion.
While it is presently unknown how complex N-glycans on MHCII contribute to glycoantigen binding and presentation, there are at least two explanations. First, it is possible that the change from complex to high mannose/hybrid N-glycans causes structural alterations in MHCII that inhibit proper glycoantigen binding. This model, however, suffers from the observation that peptide binding is unchanged under these conditions, suggesting that any change in structure does not significantly affect the peptide binding groove. Likewise, the lack of complex N-glycans does not appear to adversely affect the action of HLA-DM in catalyzing the exchange of the invariant chain peptide with antigenic peptides in antigen presenting cells, further arguing against structural changes. Nevertheless, it remains possible that the change to more primitive high mannose/hybrid N-glycans causes conformational differences. If this model is correct, it would mean that any crystal structure of an MHCII molecule lacking mammalian complex N-glycans is lacking functionally significant differences in protein structure.
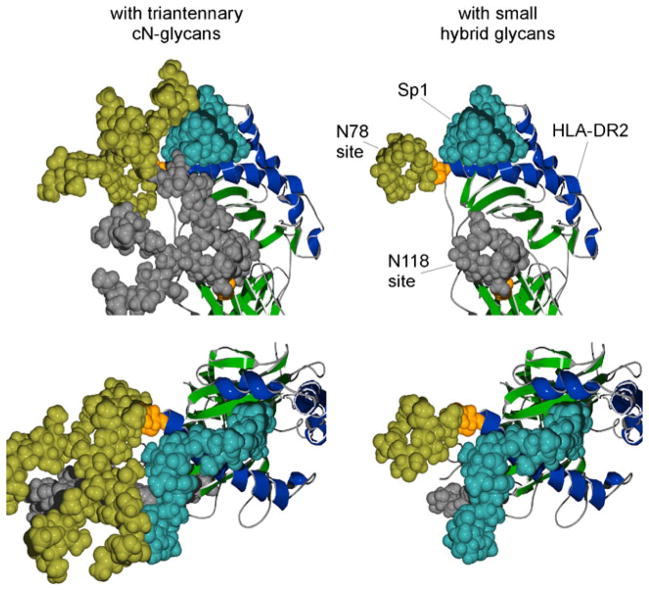
The second possible explanation is that the complex N-glycans directly interact with the glycoantigen during presentation. We believe that this possibility is more likely correct, although this model would be controversial in that it requires direct carbohydrate–carbohydrate interactions to be critical during presentation of glycoantigens by MHCII. However, direct participation in the binding event fits with all of the data reported thus far. Based on molecular modeling of MHCII glycoproteins [13], it is likely that the antigen binding platform could be significantly extended by glycan structures protruding from the protein backbone. Since complex N-glycans are physically large structures that are comparable in size to an immunoglobulin domain (i.e., 30×10× 10 Å)[13], it is conceivable that binding of the relatively large glycoantigens to the MHCII glycoprotein is stabilized by the increased surface area provided by the complex N-glycan(s) beyond that of the characterized peptide binding groove. In fact, the N-glycans found at Asn78 and Asn19 are almost exclusively highly branched complex oligosaccharides [39], which are not only critical for glycoantigen binding [33] but would also offer the greatest additional binding surface area. Furthermore, the helix formed by the glycoantigen [89, 90] is characterized as having a major groove of a size (5×10×10 Å) appropriate for engulfing a protein alpha helix [90] in a similar fashion as some DNA-binding proteins [92]. Taken together, these data suggest a mode of binding between glycoantigens and glycosylated MHCII where the glycoantigen makes contacts within the peptide binding groove as well as with the complex N-glycans while straddling one or both of the alpha helices on MHCII lining the groove, as modeled in Figs. 5 and 6. Indeed, when the N-glycans are digitally reduced to hybrid structures, the potential contacts with the glycoantigen are lost in the model (Fig. 6). Although merely a hypothesis, this structural model is consistent with all of the data reported to date and may provide a guide to unraveling the true mode of glycoantigen binding to MHCII and the role complex N-glycans are playing.
Fig. 5.
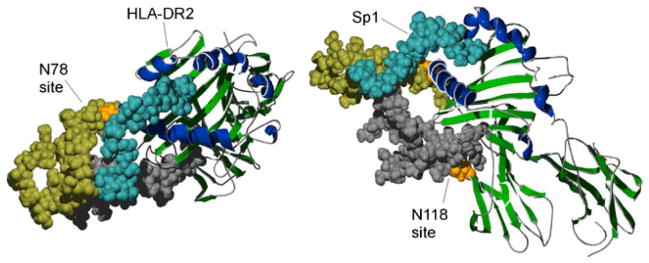
The predicted association of glycoantigen to the N-glycosylated MHCII molecule. This model is based on recent evidence showing that glycoantigen binding is dependent on both the MHCII peptide backbone and the presence of complex N-glycans [33]. Furthermore, glycoantigen binding is competitive with traditional peptide antigens suggesting its association with the antigen binding groove [86]. Alpha helices of the antigen binding groove are shaded dark blue and the rest of the molecule in green. Using space filling, the HLA-DR2 alpha chain N-glycan acceptor sites N78 and N118 are shown in orange and the attached complex N-glycan structures are shown in yellow and gray, respectively. The Sp1 glycoantigen is shown in light blue (coordinates provided by Julia Wang [89]). The left and right panels are showing top and lateral views of the same complex. Images were created using previous structural analysis of HLA-DR2 [122] and Sp1 (from S. pneumoniae) [89]. The glycosylated HLA-DR2 model was created using the GLYCAM web tool developed by Dr. Robert Woods [123]
Fig. 6.
Structural model of Sp1 glycoantigen bound to human HLA-DR2 in the presence or absence of complex N-glycans. Images were created using previously defined crystal structures of HLA-DR2 [122] with modeled N-glycan structures (GLYCAM web tool [123]). The upper and lower images are showing different views of Sp1 (coordinates provided by Julia Wang [89]) associated with HLA-DR2 in the presence of attached triantennary complex N-glycans (left) or hybrid N-glycans (right). Alpha helices of the antigen binding groove are shaded blue and the rest of the molecule in green. The alpha chain N-glycan acceptor sites N78 and N118 are shown in orange as space-filled residues with the attached N-glycan structures shown in yellow and gray, respectively. The predicted association of a glycoantigen (Sp1 from S. pneumoniae; blue) is shown
Context-dependent control of MHC N-glycosylation
The repertoire of N-glycans produced by each cell is a reflection of the type of glycosyltransferase and glycosidase enzymes expressed, chosen from a collection in excess of 200 in the mammalian genome [4]. The wide range of combinatorial enzymatic activities expressed at any given time greatly increases the complexity of the possible oligosaccharide structures (i.e., complex N-glycans) and potentially introduces context-dependent functions for a given glycoprotein. In fact, individual glycoproteins can be found with a range of N-glycosylation patterns, although the underlying amino acid sequence remains unchanged. For example, the N-glycans found on the human erythrocyte CD59 glycoprotein can be extremely heterogeneous, with more than 120 glycosylation variants identified [93]. It is possible, if not likely, that alterations in complex N-glycan structure found on cell surface-expressed glycoproteins and secreted glycoproteins may transmit functional information beyond that of the protein backbone itself.
The idea that post-translational protein modifications can transmit functional information is not a new one. Protein phosphorylation is a well-known and widely studied mechanism by which cells relay intracellular and intercellular information. In the immune system, the MHC glycoproteins, due to their antigen presentation capabilities, are critical for information exchange with T cells. An early observation by Cullen et al. showed that MHCII molecules were differentially glycosylated when expressed by different antigen presenting cell types found in the spleen [30]. Immunoprecipitated MHCII molecules isolated from B cells or the adherent cell population, known to contain mainly macrophages and dendritic cells, were analyzed by various electrophoretic methods to show that the B cell alpha chains appeared to contain a larger quantity of sialylation, whereas the beta chains appeared to have a similar pattern between the antigen presenting cell types. Neuraminidase removal of sialic acid residues resulted in a B cell alpha chain glycosylation pattern that was comparable to the adherent cells [30]. A more recent study by Barrera et al. also suggested that different cell types may differentially N-glycosylate MHCII molecules. Human MHCII molecules immunoprecipitated from a gastric epithelial cell line (KATO III) and a B cells line (JESTHOM) had different electrophoretic mobilities when analyzed by two-dimensional electrophoresis. The apparent differences in MHCII molecular weight was attributed to differences in the glycosylation pattern between the two cell types [31], and although it was not directly examined, the differences between MHCII expressed by the gastric epithelial cells and B cells appeared to result from changes not limited to variations in sialylation.
The differences in B cell alpha chain sialylation were interesting observations; however, a functional role of the increased MHCII sialic acid content has not been clearly identified. Neuraminidase treatment of isolated B cell MHCII does not appear to alter T cell recognition of presented peptide antigens [94]. Rather than playing a direct functional role, the increased sialic acid content on B cell alpha chains may reflect a global increase in sialic acid linkages on B cell glycoprotein N-glycans. For example, a study by Neel et al. provided evidence that the N-glycosylation pattern of isolated MHCII molecules closely resembled the glycosylation pattern of the total glycoprotein pool within each cell line, as compared to the major differences detected between cell lines [95]. MHCII N-glycan content was analyzed by lectin chromatography and compared between an antigen presenting cell (EBV-transformed human 430 B cell line) and that of the same serological class II molecule ectopically expressed in a mouse fibroblast cell line. While lectins can be useful in identifying the presence of N-glycans and differentiating general structural differences, more rigorous techniques, such as HPLC and mass spectrometry, are needed to define and compare protein N-glycans.
The use of cell lines has been common to previous studies characterizing the complex N-glycans found on MHC glycoproteins and other immune-related glycoproteins expressed by mammalian cells. Cell lines are attractive for use in these experiments since they have historically required very large numbers of homogeneous cells to yield the requisite amount of glycoprotein that is needed to then extract and analyze the glycans. However, as mentioned previously, the glycans found on these cell lines are likely quite different from their primary cell counterparts. Indeed, the majority of mammalian glycoproteins on primary cells carry complex N-glycans, yet glycoproteins from cell lines can express unusually large amounts of high-mannose N-glycans [95]. Going forward, it will be important to focus on the specific N-glycan structure(s) of MHC molecules on primary cells (i.e., non-transformed) or even cells in vivo (i.e., not cultured ex vivo) in order fully understand a physiological role of complex N-glycans on cell surface and secreted glycoproteins.
Unlike other MHCIa and Ib molecules, the non-classical human CD1d molecule has been shown to be expressed independent of β2-microglobulin, which appears to affect the N-glycosylation pattern of the heavy chain [56, 96]. Kim and colleagues showed that expression of CD1d in the β2-microglobulin-deficient FO-1 human melanoma cell line resulted in a N-glycosylation pattern with immature carbohydrate side chains that had not been further modified beyond the initial glycan transfer in the ER [56]. Interestingly, unlike the CD1d glycoprotein heterodimer with β2-microglobulin that is expressed on both the lateral and apical surface of the intestinal epithelium, a non-glycosylated, β2-microglobulin-independent form with apical surface restriction has been identified [96]. The functional role of these non-glycosylated CD1d molecules and its potential presence in additional tissues remains unknown, although it is clear that T cells are able to respond to CD1d-presented antigen from cells lacking β2-microglobulin [97–99]. In addition, CD1d-restricted T cell responses, independent of β2-microglobulin, are readily detectable in an airway hyperre-activity mouse model of asthma [100]. This indicates that β2-microglobulin is not necessary for all CD1d antigen presentation functions. However, it remains unclear how the N-glycosylation pattern and repertoire of antigens presented by β2-microglobulin -independent CD1d molecules might influence the observed in vivo T cell responses.
N-Glycosylation in health and disease
The N-glycan structures found in a homeostatic setting are fairly uniform in a cell-type and protein-specific manner; however, these structures can be altered significantly in inflammatory and disease settings. There are many potential factors that contribute to these changes, such as altered expression patterns of glycosidases and glycosyltransferases in the Golgi apparatus, altered availability of the carbohydrate building blocks, or increased expression of cellular proteins that may overwhelm the limits of the secretory pathway [79, 101].
Decreased cell surface expression of MHCIa due to changes in N-glycosylation has been proposed as one of the potential mechanisms by which viruses can evade immune detection in infected cells. Interference in MHCIa antigen presentation, by various means, is a common mechanism used by viruses to evade detection. Tardif and Siddiqui showed that human hepatic cells stably expressing hepatitis C virus (HCV) had significantly decreased surface expressed MHCIa, which was attributed to lower levels of properly folded heavy chain. The decrease in properly folded heavy chain correlated with a decrease in global protein glycosylation in addition to a decrease in folded heavy chain found in the Golgi apparatus [102]. Although no direct evidence was provided, it is likely that HCV was inhibiting the very early events in N-glycosylation, possibly at the initial Glc3Man9GlcNAc2 linkage by the OST or the subsequent glycan trimming by glucosidases I and II (see Fig. 1). The lack of these events would then limit the activity of the calnexin and calreticulin chaperones, which are important for proper heavy chain folding and assembly of MHCIa molecules [9, 10].
Changes in cellular glycosylation patterns are associated with conditions of chronic inflammation such as rheumatoid arthritis, cancer, and inflammatory bowel diseases. Inflammatory stimuli, including microbial products like LPS or various secreted cytokines such as IL1β, IL-6, and TNFα, can act directly on cells in the proximal environment or systemically via the circulatory system. Inflammatory stimuli can alter the glycan branching pattern and levels of sialylation of cell bound and secreted glycoproteins [101]. Although not characterized directly, changes in total cellular N-glycosylation patterns are also likely to be reflected on MHCII. It is known that inflammation stimuli can increase MHCII expression levels [103, 104]. While the increased expression of MHCII on antigen presenting cells (i.e., dendritic cells, macrophages, B cells) can be facilitated by numerous types of stimuli, increased expression on cells that do not constitutively express the molecule, such as epithelial cells, may require more selective cytokine stimulations [103]. Increased surface expression of MHCII coupled with changes in the N-glycosylation pattern has the potential to significantly alter the presentation of T cell antigens that are sensitive to changes in N-glycosylation.
In addition to cellular changes in glycosylation occurring secondarily to inflammatory stimuli responses, abnormal protein glycosylation can directly promote inflammatory conditions [105]. Originally generated for xenotransplantation research, it was found that mice expressing a human transgene of α1,2-fucosyltransferase (hFUT1) unexpectedly developed severe inflammatory colitis. Although these mice had profound disturbances in the glycosylation pattern at mucosal barrier surfaces, it was found that the severe colitis-associated condition was related to the abnormal glycosylation of cells in the immune system and IL-12 cytokine responses [106], indicating the importance of proper immune cell glycosylation in mediating and/or preventing intestinal inflammation. In the context of antigen presenting cells and the MHCII glycoprotein, which we have recently attributed a functional role for its complex N-glycan pattern as a direct mediator of proper glycoantigen binding and presentation, dysfunction of proper glycosylation may be selectively important for controlling intestinal inflammation. One of the most studied members of this family of T cell-dependent antigens is the capsular PSA glycoantigen expressed by the common gut commensal B. fragilis. Interestingly, the PSA has been shown to be a key bacterial factor that can maintain gastrointestinal immune balance. PSA administered to gnotobiotic animals (i.e., germ-free) appears to be sufficient to restore the Th1/Th2 T cell balance to that of normally colonized animals [107]. In fact, PSA can induce a regulatory immune response characterized by IL-10 production that shows remarkable efficacy in modulating T cell activity [108] and inflammation in animal models of inflammatory bowel disease [109]. This suggests that intestinal antigen presenting cells that encounter PSA are able to present the glycoantigen due to the complex N-glycans attached to the respective MHCII molecules. Presumably the glycoantigen presentation acts to stimulate a cohort of the CD4+ T cell repertoire to assist in controlling the inflammatory intestinal environment [109], yet if an underlying disease mechanism alters those N-glycans, it could result in loss of this anti-inflammatory T cell activity through inhibiting MHCII-dependent PSA presentation.
A recent study by Mkhikian and colleagues provided interesting evidence for a unifying molecular mechanism in multiple sclerosis (MS), a debilitating autoimmune and neurodegenerative disorder that is associated with risk factors of both genetic and environmental origin. The mechanistic theory suggests that both genetic variants (IL-7RA, IL-2RA, CTLA-4, and MGAT1) and environmental factors (vitamin D3/sunlight and metabolism) can converge to dys-regulate the common N-glycosylation pathway, which may significantly influence the pathologic potential of T cells [110]. Defects in the pathway are thought to have a causal role in MS, which is supported by the development of spontaneous inflammatory demyelination and neurodegeneration in Mgat1- and Mgat5-deficient mice [111, 112]. Furthermore, a recent genome-wide association study of 1,040 MS patients found that genetic variants of MGAT5, encoding the N-acetylglucosaminyltransferase V branching enzyme (GlcNAcT-V), were associated with disease severity. It should be noted that the greatest genetic risk on MS susceptibility is associated with the HLA locus [113], including different haplotypes of MHCII. Including T cells, select genetic variants that directly disrupt the N-glycosylation pathway (i.e., MGAT1, MGAT5) would likely also influence MHCII N-glycan patterns, which may also contribute to disease progression and/or severity. For example, changes in N-glycan branching could affect the ability of MHCII to present and stimulate glycoantigen responsive anti-inflammatory T cells [33]. In addition to controlling intestinal inflammation, the PSA glycoantigen has been shown to control neuroinflammation in experimental autoimmune encephalitis, an animal model of human MS [114, 115], thus providing a potential connection between the genetic susceptibility for MS with antigen presentation defects associated with changes in MHCII glycosylation.
Concluding remarks
The relationship between protein and glycan is a dynamic one. Attachment of the initial core/high-mannose N-glycan in the ER can act to stabilize the conformation of the protein structure both locally and at distant protein domains [11, 24]; however, later events in the Golgi that mediate complex N-glycan branching and branch extension may further modify local protein dynamics as well as the structure–function relationship [34]. Conversely, slight changes to the peptide outside the acceptor site sequence can affect the final glycan structure or site occupancy [116]. Thus, differences in the primary protein sequence that occur in nature, such as those associated with disease susceptibility, may adversely affect glycoprotein function in part by altering the glycosylation pattern. The MHC family is associated with hundreds of diseases including most autoimmune disorders. These disease-associated genetic variants may alter the peptide sequence in a way that changes the N-glycosylation pattern and thus could confer an altered function to the final glycoprotein product. Determining the extent of site-specific N-glycan microheterogeneity across the highly polymorphic MHC allotypes is increasingly more realistic as new analytical glycomic methods are developed [35, 117].
One of the intriguing facts of the MHC family of glycoproteins is that multiple sites for N-glycosylation have been evolutionarily conserved, yet it has been difficult to define a clear consensus for exactly what roles they play. The roles for some of these N-glycans, such as that on Asn78 of MHCIa, can at least partially be attributed to protein structure and quality control events in the ER. While this may be a more ancient role for MHC N-glycosylation that relates to genetic and three-dimensional structural analyses indicating a common evolutionary origin of the family [118, 119], it could be suggested that the roles for N-glycans became more diverse with the dual increases in complexity of the N-glycans themselves and the immune systems as a whole in higher-order organisms. For example, analysis of the MHCIa and MHCII crystal structure suggests that the highly conserved Asn86 site of MHCIa may have a similar counterpart in the Asn78 site of the MHCII alpha chain (Figs. 2 and 3). Both of the acceptor sites are positioned so that the attached N-glycan projects from the same distal end of their respective antigen binding grooves. However, while site occupancy on MHCIa Asn78 plays an important role in ER protein quality control events preceding cell surface expression, the MHCII N-glycan at Asn78 does not appear to significantly alter surface expression [68]. The divergent roles for N-glycans between MHC classes (i.e., II, Ia, Ib) may contribute to the respective functional differences of MHC molecules in innate and adaptive immunity. Supporting this is the fact that within MHC classes, there is a high degree of conservation of select N-glycosylation acceptor sites across species, indicating that they likely contribute critical functional roles and thus there are significant levels of evolutionary selective pressure to maintain the sites. However, with the possible exception of MHCIa molecules, it has been difficult to clearly define the functional roles of the MHC N-glycans. One possible explanation is that N-glycosylation may have multiple roles depending on variations in glycan structure in the context of the same acceptor site. For instance, while the presence of the precursor high-mannose N-glycan is important for MHCIa quality control and antigen loading in the ER, the MHCIa complex N-glycans found at the cell surface may serve an additional functional role such as mediating receptor–ligand interactions and/or molecular geometric spacing. Thus, simply eliminating these N-glycosylation acceptor sites may mask detection of secondary roles for the N-glycan structures in immune function.
To identify additional functional roles for N-glycosylation post-ER, recent studies have developed new methods to selectively regulate N-glycan branching and branch extension in the Golgi. For example, our recent study identifying a functional role for complex N-glycans in MHCII antigen presentation utilized controlled gene deletion of Mgat2, which encodes the Golgi-localized GlcNAcT-II enzyme, in order to focus on post-ER glycan functions [33]. Additionally, deletion of the Mgat5 gene encoding the GlcNAcT-V enzyme, a mediator of branch extension, identified a role for TCR associated complex N-glycans in mediating molecular cis-interactions at the cell surface via galectin–glycan interactions [120]. The galectin-mediated interactions could also play an important role in organizing other cell surface glycoproteins, such as MHC molecules, on the antigen presenting cell. In addition to the MHC glycoproteins, the majority of the other molecules found at the interface of the antigen presenting cell to T cell (i.e., the immune synapse) are also heavily glycosylated and may control cis-interactions with one another [13]. These genetic-based methods allow for a more clear understanding of how changes in cellular metabolism (e.g., during T cell stimulation) or cell lineage differentiation that contribute to significant changes in the protein N-glycosylation pattern [120, 121] relates to cell function.
Acknowledgments
This work was supported by the National Institutes of Health grants OD004225 and GM082916 to B.A. Cobb.
Footnotes
Conflict of interest: The authors declare that they have no conflict of interest.
References
- 1.Kumánovics A, Takada T, Lindahl KF. Genomic organization of the mammalian MHC. Annu Rev Immunol. 2003;21:629–657. doi: 10.1146/annurev.immunol.21.090501.080116. [DOI] [PubMed] [Google Scholar]
- 2.Koller BH, Marrack P, Kappler JW, Smithies O. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science. 1990;248(4960):1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- 3.Madsen L, Labrecque N, Engberg J, Dierich A, Svejgaard A, Benoist C, et al. Mice lacking all conventional MHC class II genes. Proc Natl Acad Sci U S A. 1999;96(18):10338–10343. doi: 10.1073/pnas.96.18.10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marth JD, Grewal PK. Mammalian glycosylation in immunity. Nat Rev Immunol. 2008;8(11):874–887. doi: 10.1038/nri2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodgers JR, Cook RG. MHC class Ib molecules bridge innate and acquired immunity. Nat Rev Immunol. 2005;5(6):459–471. doi: 10.1038/nri1635. [DOI] [PubMed] [Google Scholar]
- 6.Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol. 2004;22(1):817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 7.Schachter H. Mgat1-dependent N-glycans are essential for the normal development of both vertebrate and invertebrate metazoans. Semin Cell Dev Biol. 2010;21(6):609–615. doi: 10.1016/j.semcdb.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Lowe JB, Marth JD. A genetic approach to mammalian glycan function. Annu Rev Biochem. 2003;72:643–691. doi: 10.1146/annurev.biochem.72.121801.161809. [DOI] [PubMed] [Google Scholar]
- 9.Bergeron JJ, Zapun A, Ou WJ, Hemming R, Parlati F, Cameron PH, et al. The role of the lectin calnexin in conformation independent binding to N-linked glycoproteins and quality control. Adv Exp Med Biol. 1998;435:105–116. doi: 10.1007/978-1-4615-5383-0_11. [DOI] [PubMed] [Google Scholar]
- 10.Saito Y, Ihara Y, Leach MR, Cohen-Doyle MF, Williams DB. Calreticulin functions in vitro as a molecular chaperone for both glycosylated and non-glycosylated proteins. EMBO J. 1999;18 (23):6718–6729. doi: 10.1093/emboj/18.23.6718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solá RJ, Griebenow K. Effects of glycosylation on the stability of protein pharmaceuticals. J Pharm Sci. 2009;98(4):1223–1245. doi: 10.1002/jps.21504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grigorian A, Torossian S, Demetriou M. T-cell growth, cell surface organization, and the galectin-glycoprotein lattice. Immunol Rev. 2009;230(1):232–246. doi: 10.1111/j.1600-065X.2009.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudd PM, Wormald MR, Stanfield RL, Huang M, Mattsson N, Speir JA, et al. Roles for glycosylation of cell surface receptors involved in cellular immune recognition. J Mol Biol. 1999;293(2):351–366. doi: 10.1006/jmbi.1999.3104. [DOI] [PubMed] [Google Scholar]
- 14.Barbosa JA, Santos-Aguado J, Mentzer SJ, Strominger JL, Burakoff SJ, Biro PA. Site-directed mutagenesis of class I HLA genes. Role of glycosylation in surface expression and functional recognition. J Exp Med. 1987;166(5):1329–1350. doi: 10.1084/jem.166.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto K. Intracellular lectins involved in folding and transport in the endoplasmic reticulum. Biol Pharm Bull. 2009;32 (5):767–773. doi: 10.1248/bpb.32.767. [DOI] [PubMed] [Google Scholar]
- 16.Brennan AJ, Chia J, Browne KA, Ciccone A, Ellis S, Lopez JA, et al. Protection from endogenous perforin: glycans and the C terminus regulate exocytic trafficking in cytotoxic lymphocytes. Immunity. 2011;34(6):879–892. doi: 10.1016/j.immuni.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Elliott EA, Drake JR, Amigorena S, Elsemore J, Webster P, Mellman I, et al. The invariant chain is required for intra-cellular transport and function of major histocompatibility complex class II molecules. J Exp Med. 1994;179(2):681–694. doi: 10.1084/jem.179.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Germain RN, Rinker AG. Peptide binding inhibits protein aggregation of invariant-chain free class II dimers and promotes surface expression of occupied molecules. Nature. 1993;363(6431):725–728. doi: 10.1038/363725a0. [DOI] [PubMed] [Google Scholar]
- 19.Hart GW. The role of asparagine-linked oligosaccharides in cellular recognition by thymic lymphocytes. Effects of tunicamycin on the mixed lymphocyte reaction. J Biol Chem. 1982;257 (1):151–158. [PubMed] [Google Scholar]
- 20.Sevilla LM, Comstock SS, Swier K, Miller J. Endoplasmic reticulum-associated degradation-induced dissociation of class II invariant chain complexes containing a glycosylation-deficient form of p41. J Immunol. 2004;173(4):2586–2593. doi: 10.4049/jimmunol.173.4.2586. [DOI] [PubMed] [Google Scholar]
- 21.Bakke O, Dobberstein B. MHC class II-associated invariant chain contains a sorting signal for endosomal compartments. Cell. 1990;63(4):707–716. doi: 10.1016/0092-8674(90)90137-4. [DOI] [PubMed] [Google Scholar]
- 22.Vagin O, Kraut JA, Sachs G. Role of N-glycosylation in trafficking of apical membrane proteins in epithelia. Am J Physiol Renal Physiol. 2009;296(3):F459–F469. doi: 10.1152/ajprenal.90340.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bakke O, Nordeng TW. Intracellular traffic to compartments for MHC class II peptide loading: signals for endosomal and polarized sorting. Immunol Rev. 1999;172(1):171–187. doi: 10.1111/j.1600-065x.1999.tb01365.x. [DOI] [PubMed] [Google Scholar]
- 24.Imperiali B, O’Connor SE. Effect of N-linked glycosylation on glycopeptide and glycoprotein structure. Curr Opin Chem Biol. 1999;3(6):643–649. doi: 10.1016/s1367-5931(99)00021-6. [DOI] [PubMed] [Google Scholar]
- 25.Shental-Bechor D, Levy Y. Effect of glycosylation on protein folding: a close look at thermodynamic stabilization. Proc Natl Acad Sci U S A. 2008;105(24):8256–8261. doi: 10.1073/pnas.0801340105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neumann J, Schach N, Koch N. Glycosylation signals that separate the trimerization from the MHC class II-binding domain control intracellular degradation of invariant chain. J Biol Chem. 2001;276(16):13469–13475. doi: 10.1074/jbc.M010629200. [DOI] [PubMed] [Google Scholar]
- 27.Roche PA, Cresswell P. Proteolysis of the class II-associated invariant chain generates a peptide binding site in intracellular HLA-DR molecules. Proc Natl Acad Sci U S A. 1991;88 (8):3150–3154. doi: 10.1073/pnas.88.8.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rabinovich GA, Toscano MA. Turning ‘sweet’ on immunity: galectin–glycan interactions in immune tolerance and inflammation. Nat Rev Immunol. 2009;9(5):338–352. doi: 10.1038/nri2536. [DOI] [PubMed] [Google Scholar]
- 29.Garner OB, Baum LG. Galectin-glycan lattices regulate cell-surface glycoprotein organization and signalling. Biochem Soc Trans. 2008;36(Pt 6):1472–1477. doi: 10.1042/BST0361472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cullen SE, Kindle CS, Shreffler DC, Cowing C. Differential glycosylation of murine B cell and spleen adherent cell Ia antigens. J Immunol. 1981;127(4):1478–1484. [PubMed] [Google Scholar]
- 31.Barrera C, Espejo R, Reyes VE. Differential glycosylation of MHC class II molecules on gastric epithelial cells: implications in local immune responses. Hum Immunol. 2002;63(5):384–393. doi: 10.1016/s0198-8859(02)00386-5. [DOI] [PubMed] [Google Scholar]
- 32.Trombetta ES, Helenius A. Lectins as chaperones in glycoprotein folding. Curr Opin Struct Biol. 1998;8(5):587–592. doi: 10.1016/s0959-440x(98)80148-6. [DOI] [PubMed] [Google Scholar]
- 33.Ryan SO, Bonomo JA, Zhao F, Cobb BA. MHCII glycosylation modulates Bacteroides fragilis carbohydrate antigen presentation. J Exp Med. 2011;208(5):1041–1053. doi: 10.1084/jem.20100508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mandal TK, Mukhopadhyay C. Effect of glycosylation on structure and dynamics of MHC class I glycoprotein: a molecular dynamics study. Biopolymers. 2001;59(1):11–23. doi: 10.1002/1097-0282(200107)59:1<11::AID-BIP1001>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 35.An HJ, Froehlich JW, Lebrilla CB. Determination of glycosylation sites and site-specific heterogeneity in glycoproteins. Curr Opin Chem Biol. 2009;13(4):421–426. doi: 10.1016/j.cbpa.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barber LD, Patel TP, Percival L, Gumperz JE, Lanier LL, Phillips JH, et al. Unusual uniformity of the N-linked oligosaccharides of HLA-A, -B, and -C glycoproteins. J Immunol. 1996;156(9):3275–3284. [PubMed] [Google Scholar]
- 37.Wagner DD, Ivatt R, Destree AT, Hynes RO. Similarities and differences between the fibronectins of normal and transformed hamster cells. J Biol Chem. 1981;256(22):11708–11715. [PubMed] [Google Scholar]
- 38.Goochee CF, Monica T. Environmental effects on protein glycosylation. Nat Biotechnol. 1990;8(5):421–427. doi: 10.1038/nbt0590-421. [DOI] [PubMed] [Google Scholar]
- 39.Swiedler SJ, Freed JH, Tarentino AL, Plummer TH, Jr, Hart GW. Oligosaccharide microheterogeneity of the murine major histocompatibility antigens. Reproducible site-specific patterns of sialylation and branching in asparagine-linked oligosaccharides. J Biol Chem. 1985;260(7):4046–4054. [PubMed] [Google Scholar]
- 40.Hayes BK, Esquivel F, Bennink JR, Yewdell JW, Varki A. Structure of the N-linked oligosaccharides of MHC class I molecules from cells deficient in the antigenic peptide transporter. Implications for the site of peptide association. J Immunol. 1995;155 (8):3780–3787. [PubMed] [Google Scholar]
- 41.Le AV, Doyle D. Differential regulation of mouse H-2 alloantigens. Biochemistry. 1982;21(23):5730–5738. doi: 10.1021/bi00266a001. [DOI] [PubMed] [Google Scholar]
- 42.Parham P. Functions for MHC class I carbohydrates inside and outside the cell. Trends Biochem Sci. 1996;21(11):427–433. doi: 10.1016/s0968-0004(96)10053-0. [DOI] [PubMed] [Google Scholar]
- 43.Baba E, Erskine R, Boyson JE, Cohen GB, Davis DM, Malik P, et al. N-linked carbohydrate on human leukocyte antigen-C and recognition by natural killer cell inhibitory receptors. Hum Immunol. 2000;61(12):1202–1218. doi: 10.1016/s0198-8859(00)00184-1. [DOI] [PubMed] [Google Scholar]
- 44.Parham P. NK cell receptors: of missing sugar and missing self. Curr Biol. 2000;10(5):R195–R197. doi: 10.1016/s0960-9822(00)00350-x. [DOI] [PubMed] [Google Scholar]
- 45.Abi-Rached L, Parham P. Natural selection drives recurrent formation of activating killer cell immunoglobulin-like receptor and Ly49 from inhibitory homologues. J Exp Med. 2005;201 (8):1319–1332. doi: 10.1084/jem.20042558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guseva NV, Fullenkamp CA, Naumann PW, Shey MR, Ballas ZK, Houtman JC, et al. Glycosylation contributes to variability in expression of murine cytomegalovirus m157 and enhances stability of interaction with the NK-cell receptor Ly49H. Eur J Immunol. 2010;40(9):2618–2631. doi: 10.1002/eji.200940134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahmed KA, Munegowda MA, Xie Y, Xiang J. Intercellular trogocytosis plays an important role in modulation of immune responses. Cell Mol Immunol. 2008;5(4):261–269. doi: 10.1038/cmi.2008.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caumartin J, LeMaoult J, Carosella ED. Intercellular exchanges of membrane patches (trogocytosis) highlight the next level of immune plasticity. Transpl Immunol. 2006;17(1):20–22. doi: 10.1016/j.trim.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 49.Wingren C, Crowley MP, Degano M, Chien Yh, Wilson IA. Crystal structure of a gammadelta T cell receptor ligand T22: a truncated MHC-like fold. Science. 2000;287(5451):310–314. doi: 10.1126/science.287.5451.310. [DOI] [PubMed] [Google Scholar]
- 50.McMaster M, Zhou Y, Shorter S, Kapasi K, Geraghty D, Lim KH, et al. HLA-G isoforms produced by placental cytotrophoblasts and found in amniotic fluid are due to unusual glycosylation. J Immunol. 1998;160(12):5922–5928. [PubMed] [Google Scholar]
- 51.van Hall T, Oliveira CC, Joosten SA, Ottenhoff THM. The other Janus face of Qa-1 and HLA-E: diverse peptide repertoires in times of stress. Microbes Infect. 2010;12(12–13):910–918. doi: 10.1016/j.micinf.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 52.Comiskey M, Goldstein CY, De Fazio SR, Mammolenti M, Newmark JA, Warner CM. Evidence that HLA-G is the functional homolog of mouse Qa-2, the ped gene product. Hum Immunol. 2003;64(11):999–1004. doi: 10.1016/j.humimm.2003.08.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Donadi EA, Castelli EC, Arnaiz-Villena A, Roger M, Rey D, Moreau P. Implications of the polymorphism of HLA-G on its function, regulation, evolution and disease association. Cell Mol Life Sci. 2011;68(3):369–395. doi: 10.1007/s00018-010-0580-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Riteau B, Rouas-Freiss N, Menier C, Paul P, Dausset J, Carosella ED. HLA-G2, -G3, and -G4 isoforms expressed as non-mature cell surface glycoproteins inhibit NK and antigen-specific CTL cytolysis. J Immunol. 2001;166(8):5018–5026. doi: 10.4049/jimmunol.166.8.5018. [DOI] [PubMed] [Google Scholar]
- 55.Lee N, Ishitani A, Geraghty DE. HLA-F is a surface marker on activated lymphocytes. Eur J Immunol. 2010;40(8):2308–2318. doi: 10.1002/eji.201040348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim HS, Garcia J, Exley M, Johnson KW, Balk SP, Blumberg RS. Biochemical characterization of CD1d expression in the absence of b2-microglobulin. J Biol Chem. 1999;274(14):9289–9295. doi: 10.1074/jbc.274.14.9289. [DOI] [PubMed] [Google Scholar]
- 57.Feder JN, Gnirke A, Thomas W, Tsuchihashi Z, Ruddy DA, Basava A, et al. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet. 1996;13 (4):399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- 58.Ehrlich R, Lemonnier FA. HFE—a novel nonclassical class I molecule that is involved in iron metabolism. Immunity. 2000;13(5):585–588. doi: 10.1016/s1074-7613(00)00058-3. [DOI] [PubMed] [Google Scholar]
- 59.Bennett MJ, Lebron JA, Bjorkman PJ. Crystal structure of the hereditary haemochromatosis protein HFE complexed with transferrin receptor. Nature. 2000;403(6765):46–53. doi: 10.1038/47417. [DOI] [PubMed] [Google Scholar]
- 60.Bhatt L, Murphy C, O’Driscoll LS, Carmo-Fonseca M, McCaffrey MW, Fleming JV. N-Glycosylation is important for the correct intracellular localization of HFE and its ability to decrease cell surface transferrin binding. FEBS J. 2010;277(15):3219–3234. doi: 10.1111/j.1742-4658.2010.07727.x. [DOI] [PubMed] [Google Scholar]
- 61.Kang SJ, Cresswell P. Calnexin, calreticulin, and ERp57 cooperate in disulfide bond formation in human CD1d heavy chain. J Biol Chem. 2002;277(47):44838–44844. doi: 10.1074/jbc.M207831200. [DOI] [PubMed] [Google Scholar]
- 62.De Silva AD, Park JJ, Matsuki N, Stanic AK, Brutkiewicz RR, Medof ME, et al. Lipid protein interactions: the assembly of CD1d1 with cellular phospholipids occurs in the endoplasmic reticulum. J Immunol. 2002;168(2):723–733. doi: 10.4049/jimmunol.168.2.723. [DOI] [PubMed] [Google Scholar]
- 63.Zhu Y, Zhang W, Veerapen N, Besra G, Cresswell P. Calreticulin controls the rate of assembly of CD1d molecules in the endoplasmic reticulum. J Biol Chem. 2010;285(49):38283–38292. doi: 10.1074/jbc.M110.170530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paduraru C, Spiridon L, Yuan W, Bricard G, Valencia X, Porcelli SA, et al. An N-linked glycan modulates the interaction between the CD1d heavy chain and b2-microglobulin. J Biol Chem. 2006;281(52):40369–40378. doi: 10.1074/jbc.M608518200. [DOI] [PubMed] [Google Scholar]
- 65.Sriram V, Willard CA, Liu J, Brutkiewicz RR. Importance of N-linked glycosylation in the functional expression of murine CD1d1. Immunology. 2008;123(2):272–281. doi: 10.1111/j.1365-2567.2007.02696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Karadimitris A, Gadola S, Altamirano M, Brown D, Woolfson A, Klenerman P, et al. Human CD1d-glycolipid tetramers generated by in vitro oxidative refolding chromatography. Proc Natl Acad Sci U S A. 2001;98(6):3294–3298. doi: 10.1073/pnas.051604498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Salomonsen J, Marston D, Avila D, Bumstead N, Johansson B, Juul-Madsen H, et al. The properties of the single chicken MHC classical class II a chain (B-LA) gene indicate an ancient origin for the DR/E-like isotype of class II molecules. Immunogenetics. 2003;55(9):605–614. doi: 10.1007/s00251-003-0620-7. [DOI] [PubMed] [Google Scholar]
- 68.Ishikawa S, Kowal C, Cole B, Thomson C, Diamond B. Replacement of N-glycosylation sites on the MHC class II E alpha chain. Effect on thymic selection and peripheral T cell activation. J Immunol. 1995;154(10):5023–5029. [PubMed] [Google Scholar]
- 69.Elliott WL, Stille CJ, Thomas LJ, Humphreys RE. An hypothesis on the binding of an amphipathic, alpha helical sequence in Ii to the desetope of class II antigens. J Immunol. 1987;138 (9):2949–2952. [PubMed] [Google Scholar]
- 70.Roche PA, Cresswell P. Invariant chain association with HLA-DR molecules inhibits immunogenic peptide binding. Nature. 1990;345(6276):615–618. doi: 10.1038/345615a0. [DOI] [PubMed] [Google Scholar]
- 71.Gorga JC, Horejsí V, Johnson DR, Raghupathy R, Strominger JL. Purification and characterization of class II histocompatibility antigens from a homozygous human B cell line. J Biol Chem. 1987;262(33):16087–16094. [PubMed] [Google Scholar]
- 72.Brown JH, Jardetzky TS, Gorga JC, Stern LJ, Urban RG, Strominger JL, et al. Three-dimensional structure of the human class II histocompatibility antigen HLA-DR1. Nature. 1993;364 (6432):33–39. doi: 10.1038/364033a0. [DOI] [PubMed] [Google Scholar]
- 73.Wei BY, Buerstedde JM, Bell M, Chase C, Nilson A, Browne A, et al. Functional effects of N-linked oligosaccharides located on the external domain of murine class II molecules. J Immunol. 1991;146(7):2358–2366. [PubMed] [Google Scholar]
- 74.Nag B, Passmore D, Kendrick T, Bhayani H, Sharma SD. N-linked oligosaccharides of murine major histocompatibility complex class II molecule. Role in antigenic peptide binding, T cell recognition, and clonal nonresponsiveness. J Biol Chem. 1992;267 (31):22624–22629. [PubMed] [Google Scholar]
- 75.Nag B, Wada HG, Arimilli S, Fok K, Passmore D, Sharma SD, et al. The role of N-linked oligosaccharides of MHC class II antigens in T cell stimulation. J Immunol Methods. 1994;172 (1):95–104. doi: 10.1016/0022-1759(94)90382-4. [DOI] [PubMed] [Google Scholar]
- 76.Fukuta K, Abe R, Yokomatsu T, Kono N, Nagatomi Y, Asanagi M, et al. Comparative study of the N-glycans of human monoclonal immunoglobulins M produced by hybridoma and parental cells. Arch Biochem Biophys. 2000;378(1):142–150. doi: 10.1006/abbi.2000.1806. [DOI] [PubMed] [Google Scholar]
- 77.Ilic V, Milosevic-Jovcic N, Petrovic S, Markovic D, Stefanovic G, Ristic T. Glycosylation of IgG B cell receptor (IgG BCR) in multiple myeloma: relationship between sialylation and the signal activity of IgG BCR. Glycoconj J. 2008;25(4):383–392. doi: 10.1007/s10719-007-9101-9. [DOI] [PubMed] [Google Scholar]
- 78.Li M, Song L, Qin X. Glycan changes: cancer metastasis and anti-cancer vaccines. J Biosci. 2010;34(4):665–673. doi: 10.1007/s12038-010-0073-8. [DOI] [PubMed] [Google Scholar]
- 79.Lis H, Sharon N. Protein glycosylation. Structural and functional aspects. Eur J Biochem. 1993;218(1):1–27. doi: 10.1111/j.1432-1033.1993.tb18347.x. [DOI] [PubMed] [Google Scholar]
- 80.Ragupathi G, Coltart DM, Williams LJ, Koide F, Kagan E, Allen J, et al. On the power of chemical synthesis: immunological evaluation of models for multiantigenic carbohydrate-based cancer vaccines. Proc Natl Acad Sci U S A. 2002;99(21):13699–13704. doi: 10.1073/pnas.202427599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ryan SO, Turner MS, Gariépy J, Finn OJ. Tumor antigen epitopes interpreted by the immune system as self or abnormal-self differentially affect cancer vaccine responses. Cancer Res. 2010;70 (14):5788–5796. doi: 10.1158/0008-5472.CAN-09-4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brooks CL, Schietinger A, Borisova SN, Kufer P, Okon M, Hirama T, et al. Antibody recognition of a unique tumor-specific glycopeptide antigen. Proc Natl Acad Sci U S A. 2010;107 (22):10056–10061. doi: 10.1073/pnas.0915176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Romagnoli P, Germain RN. The CLIP region of invariant chain plays a critical role in regulating major histocompatibility complex class II folding, transport, and peptide occupancy. J Exp Med. 1994;180(3):1107–1113. doi: 10.1084/jem.180.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Neumann J, Koch N. A novel domain on HLA-DRb chain regulates the chaperone role of the invariant chain. J Cell Sci. 2006;119 (20):4207–4214. doi: 10.1242/jcs.03177. [DOI] [PubMed] [Google Scholar]
- 85.Dennis JW, Lau KS, Demetriou M, Nabi IR. Adaptive regulation at the cell surface by N-glycosylation. Traffic. 2009;10 (11):1569–1578. doi: 10.1111/j.1600-0854.2009.00981.x. [DOI] [PubMed] [Google Scholar]
- 86.Cobb BA, Kasper DL. Characteristics of carbohydrate antigen binding to the presentation protein HLA-DR. Glycobiology. 2008;18(9):707–718. doi: 10.1093/glycob/cwn050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Velez CD, Lewis CJ, Kasper DL, Cobb BA. Type I Streptococcus pneumoniae carbohydrate utilizes a nitric oxide and MHC II-dependent pathway for antigen presentation. Immunology. 2009;127(1):73–82. doi: 10.1111/j.1365-2567.2008.02924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cobb BA, Wang Q, Tzianabos AO, Kasper DL. Polysaccharide processing and presentation by the MHCII pathway. Cell. 2004;117(5):677–687. doi: 10.016/j.cell.2004.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Choi YH, Roehrl MH, Kasper DL, Wang JY. A unique structural pattern shared by T-cell-activating and abscess-regulating zwitterionic polysaccharides. Biochemistry. 2002;41(51):15144–15151. doi: 10.1021/bi020491v. [DOI] [PubMed] [Google Scholar]
- 90.Wang Y, Kalka-Moll WM, Roehrl MH, Kasper DL. Structural basis of the abscess-modulating polysaccharide A2 from Bacteroides fragilis. Proc Natl Acad Sci U S A. 2000;97(25):13478–13483. doi: 10.1073/pnas.97.25.13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kreisman LS, Friedman JH, Neaga A, Cobb BA. Structure and function relations with a T-cell-activating polysaccharide antigen using circular dichroism. Glycobiology. 2007;17(1):46–55. doi: 10.1093/glycob/cwl056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang Y, Larsen CA, Stadler HS, Ames JB. Structural basis for sequence specific DNA binding and protein dimerization of HOXA13. PLoS One. 2011;6(8):e23069. doi: 10.1371/journal.pone.0023069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rudd PM, Morgan BP, Wormald MR, Harvey DJ, Van Den Berg CW, Davis SJ, et al. The glycosylation of the complement regulatory protein, human erythrocyte CD59. J Biol Chem. 1997;272 (11):7229–7244. doi: 10.1074/jbc.272.11.7229. [DOI] [PubMed] [Google Scholar]
- 94.Krieger J, Jenis DM, Chesnut RW, Grey HM. Studies on the capacity of intact cells and purified Ia from different B cell sources to function in antigen presentation to T cells. J Immunol. 1988;140(2):388–394. [PubMed] [Google Scholar]
- 95.Neel D, Merlu B, Turpin E, Rabourdin-Combe C, Mach B, Goussault Y, et al. Characterization of N-linked oligosaccharides of an HLA-DR molecule expressed in different cell lines. Biochem J. 1987;244(2):433–442. doi: 10.1042/bj2440433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Balk SP, Burke S, Polischuk JE, Frantz ME, Yang L, Porcelli S, et al. Beta 2-microglobulin-independent MHC class Ib molecule expressed by human intestinal epithelium. Science. 1994;265 (5169):259–262. doi: 10.1126/science.7517575. [DOI] [PubMed] [Google Scholar]
- 97.Amano M, Baumgarth N, Dick MD, Brossay L, Kronenberg M, Herzenberg LA, et al. CD1 expression defines subsets of follicular and marginal zone B cells in the spleen: b2-microglobulin-dependent and independent forms. J Immunol. 1998;161(4):1710–1717. [PubMed] [Google Scholar]
- 98.Cardell S, Tangri S, Chan S, Kronenberg M, Benoist C, Mathis D. CD1-restricted CD4+ T cells in major histocompatibility complex class II-deficient mice. J Exp Med. 1995;182(4):993–1004. doi: 10.1084/jem.182.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Murakami M, Paul WE. Age-dependent appearance of NK1.1+ T cells in the livers of b2-microglobulin knockout and SJL mice. J Immunol. 1998;160(6):2649–2654. [PubMed] [Google Scholar]
- 100.Koh YI, Kim HY, Meyer EH, Pichavant M, Akbari O, Yasumi T, et al. Activation of nonclassical CD1d-restricted NK T cells induces airway hyperreactivity in b2-microglobulin-deficient mice. J Immunol. 2008;181(7):4560–4569. doi: 10.4049/jimmunol.181.7.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Arnold JN, Saldova R, Hamid UM, Rudd PM. Evaluation of the serum N-linked glycome for the diagnosis of cancer and chronic inflammation. Proteomics. 2008;8(16):3284–3294. doi: 10.1002/pmic.200800163. [DOI] [PubMed] [Google Scholar]
- 102.Tardif KD, Siddiqui A. Cell surface expression of major histocompatibility complex class I molecules is reduced in hepatitis C virus subgenomic replicon-expressing cells. J Virol. 2003;77 (21):11644–11650. doi: 10.1128/JVI.77.21.11644-11650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hansen JJ, Holt L, Sartor RB. Gene expression patterns in experimental colitis in IL-10-deficient mice. Inflamm Bowel Dis. 2009;15(6):890–899. doi: 10.1002/ibd.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mayer L, Eisenhardt D, Salomon P, Bauer W, Plous R, Piccinini L. Expression of class II molecules on intestinal epithelial cells in humans. Differences between normal and inflammatory bowel disease. Gastroenterology. 1991;100(1):3–12. doi: 10.1016/0016-5085(91)90575-6. [DOI] [PubMed] [Google Scholar]
- 105.Lowe JB. Glycosylation, immunity, and autoimmunity. Cell. 2001;104(6):809–812. doi: 10.1016/s0092-8674(01)00277-x. [DOI] [PubMed] [Google Scholar]
- 106.Brown SJ, Miller AM, Cowan PJ, Slavin J, Connell WR, Moore GT, et al. Altered immune system glycosylation causes colitis in alpha1,2-fucosyltransferase transgenic mice. Inflamm Bowel Dis. 2004;10(5):546–556. doi: 10.1097/00054725-200409000-00008. [DOI] [PubMed] [Google Scholar]
- 107.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122(1):107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 108.Kreisman LS, Cobb BA. Glycoantigens induce human peripheral Tr1 cell differentiation with gut-homing specialization. J Biol Chem. 2011;286(11):8810–8818. doi: 10.1074/jbc.M110.206011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453 (7195):620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 110.Mkhikian H, Grigorian A, Li CF, Chen HL, Newton B, Zhou RW, et al. Genetics and the environment converge to dysregulate N-glycosylation in multiple sclerosis. Nat Commun. 2011;2:334. doi: 10.1038/ncomms1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ye Z, Marth JD. N-glycan branching requirement in neuronal and postnatal viability. Glycobiology. 2004;14(6):547–558. doi: 10.1093/glycob/cwh069. [DOI] [PubMed] [Google Scholar]
- 112.Lee SU, Grigorian A, Pawling J, Chen IJ, Gao G, Mozaffar T, et al. N-glycan processing deficiency promotes spontaneous inflammatory demyelination and neurodegeneration. J Biol Chem. 2007;282(46):33725–33734. doi: 10.1074/jbc.M704839200. [DOI] [PubMed] [Google Scholar]
- 113.McElroy JP, Oksenberg JR. Multiple sclerosis genetics 2010. Neurol Clin. 2011;29(2):219–231. doi: 10.1016/j.ncl.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 114.Ochoa-Repáraz J, Mielcarz DW, Ditrio LE, Burroughs AR, Begum-Haque S, Dasgupta S, et al. Central nervous system demyelinating disease protection by the human commensal Bacteroides fragilis depends on polysaccharide A expression. J Immunol. 2010;185(7):4101–4108. doi: 10.4049/jimmunol.1001443. [DOI] [PubMed] [Google Scholar]
- 115.Ochoa-Reparaz J, Mielcarz DW, Wang Y, Begum-Haque S, Dasgupta S, Kasper DL, et al. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 2010;3(5):487–495. doi: 10.1038/mi.2010.29. [DOI] [PubMed] [Google Scholar]
- 116.Jones J, Krag SS, Betenbaugh MJ. Controlling N-linked glycan site occupancy. Biochim Biophys Acta. 2005;1726(2):121–137. doi: 10.1016/j.bbagen.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 117.Tissot B, North SJ, Ceroni A, Pang PC, Panico M, Rosati F, et al. Glycoproteomics: past, present and future. FEBS Lett. 2009;583 (11):1728–1735. doi: 10.1016/j.febslet.2009.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Flajnik MF, Kasahara M. Comparative genomics of the MHC: glimpses into the evolution of the adaptive immune system. Immunity. 2001;15(3):351–362. doi: 10.1016/s1074-7613(01)00198-4. [DOI] [PubMed] [Google Scholar]
- 119.Madden DR. The three-dimensional structure of peptide–MHC complexes. Annu Rev Immunol. 1995;13:587–622. doi: 10.1146/annurev.iy.13.040195.003103. [DOI] [PubMed] [Google Scholar]
- 120.Demetriou M, Granovsky M, Quaggin S, Dennis JW. Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature. 2001;409(6821):733–739. doi: 10.1038/35055582. [DOI] [PubMed] [Google Scholar]
- 121.Bax M, García-Vallejo JJ, Jang-Lee J, North SJ, Gilmartin TJ, Hernández G, et al. Dendritic cell maturation results in pronounced changes in glycan expression affecting recognition by siglecs and galectins. J Immunol. 2007;179(12):8216–8224. doi: 10.4049/jimmunol.179.12.8216. [DOI] [PubMed] [Google Scholar]
- 122.Smith KJ, Pyrdol J, Gauthier L, Wiley DC, Wucherpfennig KW. Crystal structure of HLA-DR2 (DRA*0101, DRB1*1501) complexed with a peptide from human myelin basic protein. J Exp Med. 1998;188(8):1511–1520. doi: 10.1084/jem.188.8.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Woods Group. [Accessed 2 Aug 2011];GLYCAM web glycoprotein builder. 2011 http://glycam.ccrc.uga.edu/ccrc/gp/