Abstract
Several studies have demonstrated enhanced auditory processing in the blind, suggesting that they compensate their visual impairment in part with greater sensitivity of the other senses. However, several physiological studies show that early visual deprivation can impact negatively on auditory spatial localization. Here we report for the first time severely impaired auditory localization in the congenitally blind: thresholds for spatially bisecting three consecutive, spatially-distributed sound sources were seriously compromised, on average 4.2-fold typical thresholds, and half performing at random. In agreement with previous studies, these subjects showed no deficits on simpler auditory spatial tasks or with auditory temporal bisection, suggesting that the encoding of Euclidean auditory relationships is specifically compromised in the congenitally blind. It points to the importance of visual experience in the construction and calibration of auditory spatial maps, with implications for rehabilitation strategies for the congenitally blind.
Keywords: congenital blindness, auditory localization, visual deprivation cross-sensory calibration
Introduction
It is generally assumed that the blind enjoy enhanced sensitivity of non-visual senses, which they rely on to a greater extent than sighted people. Much physiological work supports this intuitive view. The visual cortex is highly plastic, particularly in young animals, and retains a good deal of plasticity even in adulthood (Merabet and Pascual-Leone, 2010). This plasticity allows the visual cortex in the congenitally blind to become colonized to some extent by the auditory and somatosensory systems (Sadato et al., 1996; Weeks et al., 2000). Even a few days of binocular deprivation is sufficient to reveal colonization of primary visual cortex by touch (Merabet et al., 2008). There is also psychophysical evidence that the congenitally blind have enhanced tactile discrimination (Goldreich and Kanics, 2003), auditory pitch discrimination (Gougoux et al., 2004), sound localization (Lessard et al., 1998; Roder et al., 1999), and are properly able to form spatial topographical maps (Tinti et al., 2006; Fortin et al., 2008). Interestingly, the enhancement is not uniform, but depends somewhat on condition. For example, simple localization of peripheral, but not central stimuli exceeds that of controls (Roder et al., 1999), and is similar for the localization along the horizontal, but poorer for the vertical meridian (Zwiers et al., 2001). This is consistent with anatomical evidence showing that the peripheral but not central visual field has strong auditory projections (Falchier et al., 2002), possibly facilitating colonization.
On the other hand, it is well known that auditory spatial maps can be modified by vision, suggesting that vision may be important for spatial auditory localization. Owls reared with distorting prisms show systematic and persistent biases in auditory localization (Knudsen and Knudsen, 1985). Comparable (but transitory) effects have also been demonstrated in humans, after relatively short periods of adaptation to systematically non-aligned auditory and visual stimuli (Recanzone, 1998; Zwiers et al., 2003). Total visual deprivation in young ferrets has been shown to cause disordered development of auditory spatial maps (King and Carlile, 1993). If similar effects were to occur in humans, we may expect congenitally blind humans, who have been visually deprived since birth, to exhibit specific deficits in localization of auditory sound sources. However, no such deficits have been reported; on the contrary, several studies have reported enhanced auditory skills in congenitally blind humans, mentioned above (Lessard et al., 1998; Roder et al., 1999; Doucet et al., 2005). Similar results have also been reported in visually deprived ferrets (King and Parsons, 1999).
A possible reason why no auditory spatial deficits have been reported to date is that the auditory tasks have not taxed a metric representation of auditory space. Most studies test pitch or timbre discrimination (Gougoux et al., 2004; Doucet et al., 2005), or localization of single sounds in space (Lessard et al., 1998; Roder et al., 1999), which do not require estimation and comparison of positions in space. For this reason we studied the ability of non-sighted humans to judge the relative position of a sound source in a sequence of three spatially separated sounds. We have previously used this bisection task to study typical development of representation of auditory space (Gori et al., 2012a), and shown that children as young as 6 years of age can manage it well, with thresholds only slightly higher than adults.
The choice of the use of this task was strongly motivated by theory and empirical studies suggesting that cross-sensory calibration may be essential for normal sensory development. Our work shows that lack of early vision impacts on haptic orientation judgements (Gori et al., 2010), and lack of early haptic perception impacts on visual size judgement (Gori et al., 2012b). As much experimental evidence suggests that vision is fundamental for space perception (for review see King, 2009; Gori et al., 2012a) we predicted that congenitally blind subjects should show an impairment in a task requiring auditory spatial representation, namely auditory space bisection. We show here that congenitally blind individuals have severe difficulties with the spatial bisection task, yielding thresholds ranging from four times typical thresholds to a total inability to perform the task. On the other hand, thresholds for simple pointing, minimal angle acuity and temporal bisection were similar to control subjects. We suggest this highlights the importance of visual spatial representations in establishing and calibrating auditory spatial representations.
Materials and methods
We measured auditory spatial discrimination in nine congenitally blind individuals with no vision residual (age: 33 ± 6 years, seven females and two males; see Supplementary Table 1 for clinical details) and 27 sighted individuals (age: 30 ± 2, 21 females and six males), all with normal hearing (assessed by audiometric test) and no cognitive impairments. The sighted subjects were blindfolded before entering the room, so they had no notion of the room or the speaker layout. Subjects sat 180 cm from the centre of a bank of 23 speakers, spanning ±25° of visual angle. A total of five tasks were measured, in two different sessions (randomized order within each session). In the first we measured spatial bisection and minimum angle. In the second (some months later, to obtain additional data) we measured pointing to a single sound source, temporal bisection and a slower version of the spatial bisection. For the spatial bisection task, three 75 ms stimuli were presented successively at 500 ms intervals, the first at −25°, the third at +25°, and the second at an intermediate speaker position determined by the QUEST adaptive algorithm (Watson and Pelli, 1983), which estimates the point of subjective equality after each response, and places the next trial near that estimate. To ensure that a wide range of positions was sampled, that estimate was jittered by a random amount, drawn from a Gaussian distribution of space constant 25°, and the nearest speaker to that estimate chosen. In practice, this meant that for the patient group, the whole range of positions was sampled, almost uniformly. Subjects reported verbally whether the second sound was closer to the left (first) or right (last) sound. To be certain of the generalization of our results, we used three different sound sources (with a random order of presentation), all 75 ms duration and 60 dB sound pressure level (SPL) intensity (measured at the subject position): 500 Hz sound (for which interaural time differences are more important for sound localization); 3000 Hz sound (for which interaural level differences are more important); and pink noise, ranging from 0 to 5 kHz (for which both are important) (see Supplementary material for individual psychometric functions of the three conditions). Each subject performed 60 trials for each condition.
For the task measuring minimal audible angle, two 75 ms stimuli of 500 Hz were presented successively with a 500 ms interval, one (randomly first or second) on the central speaker (0°), the other a certain distance left or right, following the QUEST algorithm. Subjects verbally reported whether the first or the second sound was more to the right. Each subject performed 60 trials. Data are plotted as a function of speaker distance, where positive means the probe stimulus to right.
For both tasks, the proportion of rightward responses was calculated for each speaker distance, and the data fit with a Gaussian error function. Figure 1 shows the results averaged over all subjects; individual results for the patients are reported in Supplementary Figs 1 and 2. The space constant (σ) of the fit was taken as the estimate of threshold for the space bisection task. As the precision in sound localization varied very little between the two stimuli and noise burst for both control and patient groups, we pooled the data for all sound types (separated psychometric functions are reported in Supplementary Figs 1 and 2).
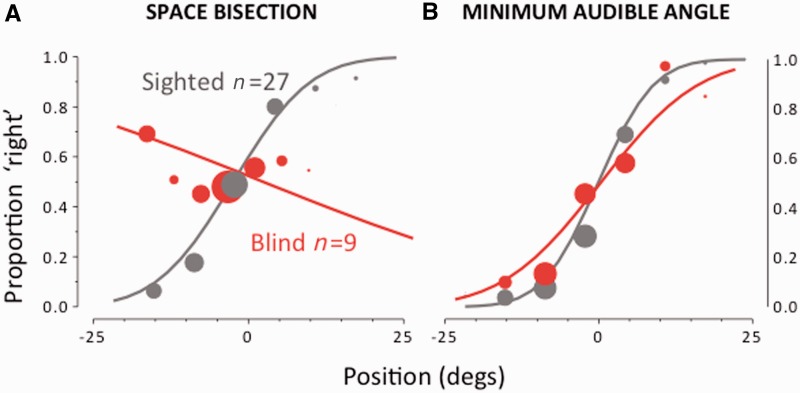
Figure 1.
Results of the bisection and minimal audible angle task. (A) Spatial bisection: proportion of trials (averaged over subjects) judged ‘closer to the right sound source’, plotted against speaker position. The area of the dots is the proportion of trials at that position, normalized by the total number of trials performed by all subjects in that group. Both sets of data are fit with the Gaussian error function. (B) Minimal audible angle: proportion of trials where the second of a two-sound sequence was reported to the right of the first, plotted against difference in speaker position. Again the fits are the Gaussian error function.
For the pointing task, a single sound (500 Hz stimulus) was played to one of 10 speakers positioned between −25° and +25°, in pseudo-random order. After the audio stimulation, subjects pointed to the sound direction with a hand-held laser (with the experimenter correcting pointing height for the first few trials). Pointing positions were then measured by the experimenter and registered. The computer calculated offline the error as the distance between the physical and the indicated position, and averaged over all trials to give an estimate of pointing accuracy. Seven congenitally blind individuals (five females and two males; Supplementary Table 1) and seven age- and gender-matched sighted individuals performed 40 trials of this task. The temporal bisection task was similar to the spatial bisection, except that all sounds were played on the central speaker, and subjects verbally reported whether the middle sound was temporally closer to the first or the last (the total duration remained 1 s, but the second stimulus varied in time, following the QUEST algorithm). Eight congenital blind individuals (six females and two males; Supplementary Table 1) and eight age- and gender-matched sighted individuals each performed 60 trials of this task. Again the results were fit by Gaussian error functions whose standard deviation estimated threshold. Most subjects completed all trials of the task when started but in few conditions if they were bored or complained we interrupted testing.
After the initial testing, showing that the patients performed poorly on the bisection task, we devised a slower version to see whether the longer intervals may facilitate discrimination. This test was similar to the initial space bisection version but the three 75 ms pink noise stimuli were presented successively at 1000 ms intervals. Each subject performed 60 trials. Six congenitally blind individuals (four females and two males; Supplementary Table 1) and six age- and gender-matched sighted individuals performed this task. The tests performed by each participant are reported in Supplementary Table 1.
For the statistical evaluation we used both the Wilcoxon Signed Ranks Test and the bootstrap sign-test (Efron, 1993), a technique that takes into account the error associated with each individual threshold as well as the between subject variance. One hundred thousand iterations were run. On each iteration, the data for each subject were independently sampled (with replacement), drawing N independent samples from the N data points for that subject on that condition, to yield an estimate of threshold for each subject (capping thresholds to 25°, half the total distance). The geometric mean of thresholds of the non-sighted subjects was compared with that for the controls, and the P-value taken as the proportion of iterations where the patient thresholds were lower than the controls. When 100 000 reiterations did not produce a single case where the thresholds for the blind were lower than the controls, we assume P < 10−5.
All participants gave informed consent before testing. The study was approved by the ethics committee of the local health service (Comitato Etico, ASL3, Genova).
Results
Figure 1A shows the results of the bisection task, plotting proportion of ‘closer to the third sound’ as a function of position of the second sound, averaged separately for the blind subjects (red symbols), and the typically sighted controls (grey symbols). For the sighted subjects, responses varied systematically with speaker position, well fit by a Gaussian error function of standard deviation (SD) 4.3° (the estimate of group threshold). However, the data for the congenitally blind group show no systematic variation with speaker position, effectively random responses, if anything in the wrong direction. Figure 1B shows results for the minimal audible angle task (Mills, 1958), where subjects judged whether the first or second of two successive sounds was more to the right. The results for this task were quite different: here the psychometric functions for blind and typically sighted were very similar, both of similar width (7.0° and 5.6°, respectively).
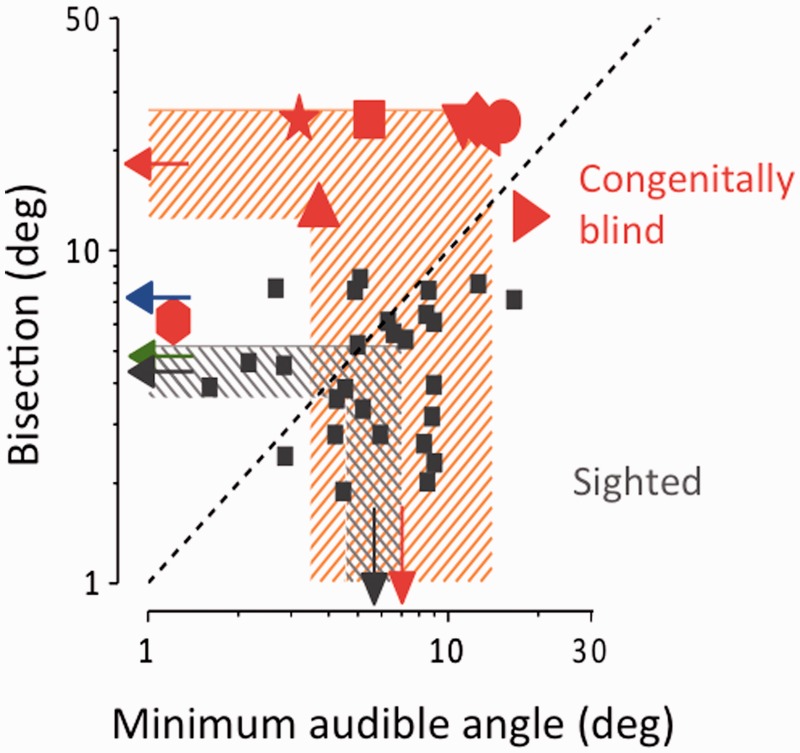
Figure 2 shows the consistency of the effects over subjects, plotting bisection thresholds against minimal audible angle. Although the minimal audible angle thresholds do not differ significantly between groups (t-test, P = 0.21), the difference in bisection thresholds is highly significant (Wilcoxon Signed Ranks Test, P < 0.01; bootstrap sign-test: P < 10−5); not one congenitally blind subject had a localization threshold falling within the 95% confidence range of the thresholds of the sighted subjects. Five blind subjects could not do the task at all, and were assigned an arbitrary threshold of 25° (half the total separation of the speakers). Perhaps thresholds would have been measurable on these subjects with a larger separation, but our equipment did not allow us to attempt this. The one subject (Subject 8) with bisection thresholds near those of the controls had particularly low thresholds for minimal audible angle (the lowest of all sighted and blind subjects), suggesting that she was in general very competent at these tasks, but she too was relatively worse at the bisection than the minimal audible angle task.
Figure 2.
Individual data, plotting bisection thresholds against minimal audible angle, calculated from the width of individual psychometric functions (Supplementary Figs 1 and 2). When no fit was plausible, they were assigned a value of 25° (50% the total spacing of the display). Arrows at the margin show the geometric means of each group and the shaded areas the 95% confidence intervals. The blue and green arrows show the average thresholds for 7- and 10-year-old children, respectively, taken from a previous study (Gori et al., 2012a). The dashed diagonal line is the equality line: whereas the thresholds of the sighted subjects are scattered around this line, all except one non-sighted subject is above it. Indeed, the only non-sighted subject with bisection thresholds falling within the control range (Subject 8) had a threshold for minimal audible angle threshold 6-fold lower than the mean of the controls, so her data point falls well above the bisection line.
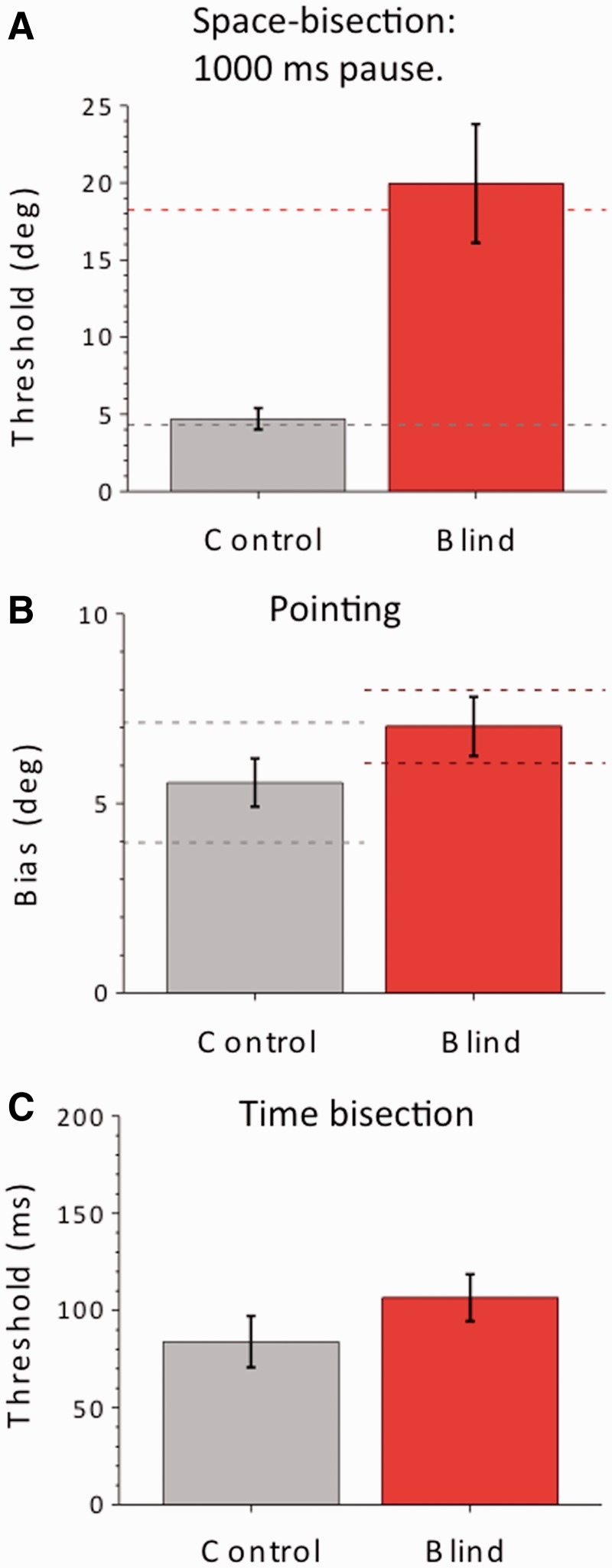
To understand better the specificity of the deficit, we performed three further tests on a subset of subjects who were available for further testing (Supplementary Table 1). Firstly, to test if the bisection task generalized over various conditions, we repeated the measurement (with a complex stimulus) with twice the temporal separation (1000 ms between sound-bursts). Figure 3A shows that also with the longer time duration, the thresholds remained 4-fold worse than the age-matched sighted controls (Wilcoxon Signed Ranks Test, P < 0.01; bootstrap sign-test: P < 10−5), showing that the choice of temporal separation is not crucial.
Figure 3.
Average thresholds in three additional tasks, for a subset of non-sighted patients and the same number of age- and gender-matched group of blindfolded sighted controls. The bars indicate ±1 standard error of the mean. (A) Space bisection with 1000 ms separation between the sounds. The impairment of the patient group remains highly significant (Bootstrap sign-test: P < 10−5). The test was performed in six non-sighted and six sighted individuals. Dashed red and grey lines refer to the average performance of subjects for the bisection task with the 500 ms separation. (B) Average error in laser-pointing to a single sound. The error measure (which comprises both accuracy, bars; and precision, dashed lines) is the average absolute distance between real and pointed values. The performance of the patient group is not significantly different from the sighted group (t-test, P = 0.07). The test was performed in seven non-sighted and seven sighted individuals. (C) Thresholds in the temporal bisection task, determined separately for each subject, then averaged. The performance of the patient group is not significantly different from the sighted group (t-test, P = 0.14). The test was performed in eight non-sighted and eight sighted individuals.
We then tested with a simpler spatial localization task, asking subjects to point with a laser to a single brief sound. Figure 3B shows the results, compared with the blindfolded age-matched sighted controls. The performance of the congenitally blind was well within the range of the controls (average bias of 7° for a range of 25°, compared with 5° for the controls: t-test, P > 0.05), consistent with the literature (Lessard et al., 1998). Precision (standard deviation of the responses around each subject’s mean) was also comparable between subject groups (1.6° ± 0.88 for the controls and 1.00 ± 0.15 for the visually impaired group, t-test P = 0.47). We then performed a temporal bisection task, which was similar to the spatial bisection, except that all sounds were played to the central speaker, and subjects reported whether the middle sound was temporally closer to the first or the last (the total duration remained 1 s, but the second stimulus varied in time, following the QUEST algorithm). Again, there was no statistical difference in the performance of the two groups (Fig. 3C, t-test, P > 0.05).
Discussion
In this study we report for the first time gross deficits in auditory spatial localization along the horizontal axis in the congenitally blind: for three of our subjects, thresholds for auditory localization were 3–5-fold worse than those of typical controls, and five of them could not do the task at all. This deficit is far larger than the perceptual enhancements that have been reported, and was evident only for the bisection task: minimal audible angle thresholds were well within the typical range, as were thresholds for simple pointing. What is special about bisection? A decision in a bisection task is not based on an instantaneous estimate but requires a representation of space that must remain in memory for the duration of the task (1 s), and therefore taxes heavily a topographical spatial map. One possibility is that the succession of sounds is interpreted differently by the blind, possibly more as apparent motion, and this interferes with the spatial representation. Blind subjects indeed seem to have lower auditory-motion thresholds than sighted subjects (Lewald, 2013). However, the fact that their thresholds were not improved by slowing the stimulus presentation to 1-s stimulus separation speaks against this suggestion.
We have previously used this identical task to measure thresholds in children as young as 6 years of age: they had no difficulty in understanding the task, and their thresholds were close to those of adults (Gori et al., 2012a). By 10 years of age, thresholds had reached adult levels (Fig. 2). Note also that the blind subjects had no difficulty with the temporal bisection, showing that the concept of bisection was not alien to them.
The spatial auditory deficit was specific for the spatial bisection task, which requires subjects to encode the position of three sounds, remember them over a period of 1 s and compare their remembered positions. That there was no deficit for the temporal bisection suggests that there was no deficit in memory per se. Nor was there a deficit in pointing to single targets. It seems that the subjects had a preserved topological representation of space, but an impaired Euclidian representation. Our paradigm did not involve jittering the positions of the two end speakers, so in principle, subjects could have performed the task by ignoring those and attending only to the central speaker. However, the poor thresholds for the blind suggest that they did not (or could not) use this strategy. With a limited group of sighted subjects, we measured bisection thresholds with the end positions jittered and found that it did not affect their results.
Although reduced auditory resolution may seem inconsistent with evidence of enhanced auditory performance and auditory colonization of visual cortex in the blind, it is consistent with the effects of visual deprivation on development of spatial maps in the superior colliculus of guinea pigs, ferrets and cats (Withington-Wray et al., 1990; King and Carlile, 1993; Wallace and Stein, 2007): the maps do develop, but are less well-ordered than in animals reared under normal lighting conditions. We do not know if the spatial bisection task in humans relies on the superior colliculus, rather than a cortical map; but if so, the effects of deprivation on development of the maps would be consistent with preserved topography, but impaired Euclidian representation.
There is good evidence that the visual system is fundamental in calibrating auditory localization: owls reared with distorting prisms show systematic and persistent biases in auditory localization (Knudsen and Knudsen, 1985); early visual deprivation of ferrets causes disordered development of superior collicular auditory spatial maps (King and Carlile, 1993); altered vision modifies the developing auditory map (King et al., 1988; Knudsen and Brainard, 1991; DeBello et al., 2001); and relatively brief periods of adaptation to spatially conflicting visual and auditory stimuli biases auditory localization in adults (Recanzone, 1998; Zwiers et al., 2003). Interestingly, in children <12 years of age, vision dominates over audition in spatial localization tasks along the horizontal axis, rather than integrating optimally, as in adults (Gori et al., 2012a), implying that in the developing child, calibration of the auditory system by the visual one is fundamental. These results point to the importance of vision in the formation of auditory spatial maps.
In previous studies we have highlighted the role of cross-sensory calibration in the developing child (Gori et al., 2008; Burr et al., 2011; Burr and Gori, 2011). We believe this to be a general property of sensory systems, particularly during development, when the sensory apparatus is still maturing. The idea, which goes back to Berkley’s (1709/1963) proposition that touch calibrates vision, is that the more ‘robust and accurate’ sense for a particular sensory task (not necessarily more precise) calibrates the other: a deficit in the more accurate ‘calibrating’ sense should also impact on the system it should calibrate. Following this prediction we have shown that congenitally blind subjects show severe but selective impairments in haptic discrimination tasks, for orientation but not size discriminations (Gori et al., 2010); and conversely, haptically impaired patients show poor visual size discrimination but not orientation discrimination (Gori et al., 2012b). The direction of the effects are consistent with the fact that in children <8 years of age, touch dominates vision in size judgements, and vision dominates touch in orientation judgements (Gori et al., 2008). Interestingly, in both cases the results were quite different with patients with acquired rather than congenital disabilities, suggesting that cross-sensory calibration at an early age is essential.
The present study provides strong evidence for cross-sensory calibration (Burr and Gori, 2011), suggesting that visual information is necessary for normal development of the auditory sense of space. Blind subjects were not uniformly bad at auditory tasks, but only in the particular bisection task, designed to tax a sophisticated, and well-calibrated spatial auditory map of Euclidean relationships. The simpler tasks tapping minimal audible angle and simple topographical representations may be achieved by less subtle mechanisms.
Besides the obvious theoretical relevance of the study, demonstrating impaired auditory localization and pointing to the role of cross-sensory interactions for normal development, the study could have repercussions for rehabilitation. Blind people rely strongly on auditory information to orient them in the environment. Sturdy spatial maps are clearly of paramount importance and their development in the absence of visual information has to be understood and recovered if impaired. It is possible that techniques could be devised where other senses, such as touch, can serve to calibrate the auditory spatial sense during development.
Supplementary Material
Acknowledgements
We thank all the participants for their kind willing contribution. Michele Richetti, Sara Sansalone and Stefania Repetto of Linear International SRL for their support with sound analysis and for the usage of their anechoic room. Laura Lucagrossi, and Alessandra Macchiavello of Istituto Chiossone for their help in subject recruitment and selection. Antonio Maviglia and Marco Jacono of IIT for the setup development and software support. Elisa Freddi for her help with subjects and testing.
Funding
Research supported by a European Research Council advanced grant “STANIB”, and the Italian ministry of research.
Supplementary material
Supplementary material is available at Brain online.
References
- Berkeley G. An essay towards a new theory of vision. Indianapolis: Bobbs-Merril; 1709/1963. [Google Scholar]
- Burr D, Binda P, Gori M. Combining information from different senses: dynamic adjustment of combination weights, and the development of cross-modal integration in children. In: Trommershauser J, Kording K, Landy MS, editors. Book of sensory cue integration. Oxford University Press; 2011. [Google Scholar]
- Burr D, Gori M. Multisensory integration develops late in humans. In: Murray MM, Wallace MT, editors. Frontiers in the neural bases of multisensory processes. Taylor & Francis Group; 2011. [PubMed] [Google Scholar]
- DeBello WM, Feldman DE, Knudsen EI. Adaptive axonal remodeling in the midbrain auditory space map. J Neurosci. 2001;21:3161–74. doi: 10.1523/JNEUROSCI.21-09-03161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet ME, Guillemot JP, Lassonde M, Gagné JP, Leclerc C, Lepore F. Blind subjects process auditory spectral cues more efficiently than sighted individuals. Exp Brain Res. 2005;160:194–202. doi: 10.1007/s00221-004-2000-4. [DOI] [PubMed] [Google Scholar]
- Efron BT, Tibshirani RJ. An introduction to the Bootstrap. New York, NY: Chapman & Hall; 1993. [Google Scholar]
- Falchier A, Clavagnier S, Barone P, Kennedy H. Anatomical evidence of multimodal integration in primate striate cortex. J Neurosci. 2002;22:5749–59. doi: 10.1523/JNEUROSCI.22-13-05749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin M, Voss P, Lord C, Lassonde M, Pruessner J, Saint-Amour D, et al. Wayfinding in the blind: larger hippocampal volume and supranormal spatial navigation. Brain. 2008;131:2995–3005. doi: 10.1093/brain/awn250. [DOI] [PubMed] [Google Scholar]
- Goldreich D, Kanics IM. Tactile acuity is enhanced in blindness. J Neurosci. 2003;23:3439–45. doi: 10.1523/JNEUROSCI.23-08-03439.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gori M, Del Viva M, Sandini G, Burr DC. Young children do not integrate visual and haptic form information. Curr Biol. 2008;18:694–8. doi: 10.1016/j.cub.2008.04.036. [DOI] [PubMed] [Google Scholar]
- Gori M, Sandini G, Martinoli C, Burr D. Poor haptic orientation discrimination in nonsighted children may reflect disruption of cross-sensory calibration. Curr Biol. 2010;20:223–5. doi: 10.1016/j.cub.2009.11.069. [DOI] [PubMed] [Google Scholar]
- Gori M, Sandini G, Burr D. Development of visuo-auditory integration in space and time. Front Integr Neurosci. 2012a;6:77. doi: 10.3389/fnint.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gori M, Tinelli F, Sandini G, Cioni G, Burr D. Impaired visual size-discrimination in children with movement disorders. Neuropsychologia. 2012b;50:1838–43. doi: 10.1016/j.neuropsychologia.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Gougoux F, Lepore F, Lassonde M, Voss P, Zatorre RJ, Belin P. Neuropsychology: pitch discrimination in the early blind. Nature. 2004;430:309. doi: 10.1038/430309a. [DOI] [PubMed] [Google Scholar]
- King AJ. Visual influences on auditory spatial learning. Philos Trans R Soc Lond B Biol Sci. 2009;364(1515):331–9. doi: 10.1098/rstb.2008.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AJ, Hutchings ME, Moore DR, Blakemore C. Developmental plasticity in the visual and auditory representations in the mammalian superior colliculus. Nature. 1988;332:73–6. doi: 10.1038/332073a0. [DOI] [PubMed] [Google Scholar]
- King AJ, Carlile S. Changes induced in the representation of auditory space in the superior colliculus by rearing ferrets with binocular eyelid suture. Exp Brain Res. 1993;94:444–55. doi: 10.1007/BF00230202. [DOI] [PubMed] [Google Scholar]
- King AJ, Parsons CH. Improved auditory spatial acuity in visually deprived ferrets. Eur J Neurosci. 1999;11:3945–56. doi: 10.1046/j.1460-9568.1999.00821.x. [DOI] [PubMed] [Google Scholar]
- Knudsen EI, Brainard MS. Visual instruction of the neural map of auditory space in the developing optic tectum. Science. 1991;253:85–7. doi: 10.1126/science.2063209. [DOI] [PubMed] [Google Scholar]
- Knudsen EI, Knudsen F. Vision guides the adjustment of auditory localization in young bran owls. Science. 1985;230:545–8. doi: 10.1126/science.4048948. [DOI] [PubMed] [Google Scholar]
- Lessard N, Paré M, Lepore F, Lassonde M. Early-blind human subjects localize sound sources better than sighted subjects. Nature. 1998;395:278–80. doi: 10.1038/26228. [DOI] [PubMed] [Google Scholar]
- Lewald J. Exceptional ability of blind humans to hear sound motion: implications for the emergence of auditory space. Neuropsychologia. 2013;51:181–6. doi: 10.1016/j.neuropsychologia.2012.11.017. [DOI] [PubMed] [Google Scholar]
- Merabet LB, Hamilton R, Schlaug G, Swisher JD, Kiriakopoulos ET, Pitskel NB, et al. Rapid and reversible recruitment of early visual cortex for touch. PLoS One. 2008;3:e3046. doi: 10.1371/journal.pone.0003046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merabet LB, Pascual-Leone A. Neural reorganization following sensory loss: the opportunity of change. Nat Rev Neurosci. 2010;11:44–52. doi: 10.1038/nrn2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills AW. On the minimum audible angle. J Acoust Soc Am. 1958;30:237–46. [Google Scholar]
- Recanzone GH. Rapidly induced auditory plasticity: the ventriloquism aftereffect. Proc Natl Acad Sci USA. 1998;95:869–75. doi: 10.1073/pnas.95.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder B, der-Sälejärvi W, Sterr A, Rösler F, Hillyard SA, Neville HJ. Improved auditory spatial tuning in blind humans. Nature. 1999;400:162–6. doi: 10.1038/22106. [DOI] [PubMed] [Google Scholar]
- Sadato N, Pascual-Leone A, Grafman J, Ibañez V, Deiber MP, Dold G, et al. Activation of the primary visual cortex by Braille reading in blind subjects. Nature. 1996;380:526–8. doi: 10.1038/380526a0. [DOI] [PubMed] [Google Scholar]
- Tinti C, Adenzato M, Tamietto M, Cornoldi C. Visual experience is not necessary for efficient survey spatial cognition: evidence from blindness. Q J Exp Psychol (Hove) 2006;59:1306–28. doi: 10.1080/17470210500214275. [DOI] [PubMed] [Google Scholar]
- Wallace MT, Stein BE. Early experience determines how the senses will interact. J Neurophysiol. 2007;97:921–6. doi: 10.1152/jn.00497.2006. [DOI] [PubMed] [Google Scholar]
- Watson AB, Pelli DG. QUEST: a Bayesian adaptive psychometric method. Percept Psychophys. 1983;33:113–20. doi: 10.3758/bf03202828. [DOI] [PubMed] [Google Scholar]
- Weeks R, Horwitz B, Aziz-Sultan A, Tian B, Wessinger CM, Cohen LG, et al. A positron emission tomographic study of auditory localization in the congenitally blind. J Neurosci. 2000;20:2664–72. doi: 10.1523/JNEUROSCI.20-07-02664.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withington-Wray DJ, Binns KE, Keating MJ. The maturation of the superior collicular map of auditory space in the guinea pig is disrupted by developmental visual deprivation. Eur J Neurosci. 1990;2:682–92. doi: 10.1111/j.1460-9568.1990.tb00458.x. [DOI] [PubMed] [Google Scholar]
- Zwiers MP, Van Opstal AJ, Cruysberg JR. A spatial hearing deficit in early-blind humans. J Neurosci. 2001;21 RC142:1–5. doi: 10.1523/JNEUROSCI.21-09-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwiers MP, Van Opstal AJ, Paige GD. Plasticity in human sound localization induced by compressed spatial vision. Nat Neurosci. 2003;6:175–81. doi: 10.1038/nn999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.