Abstract
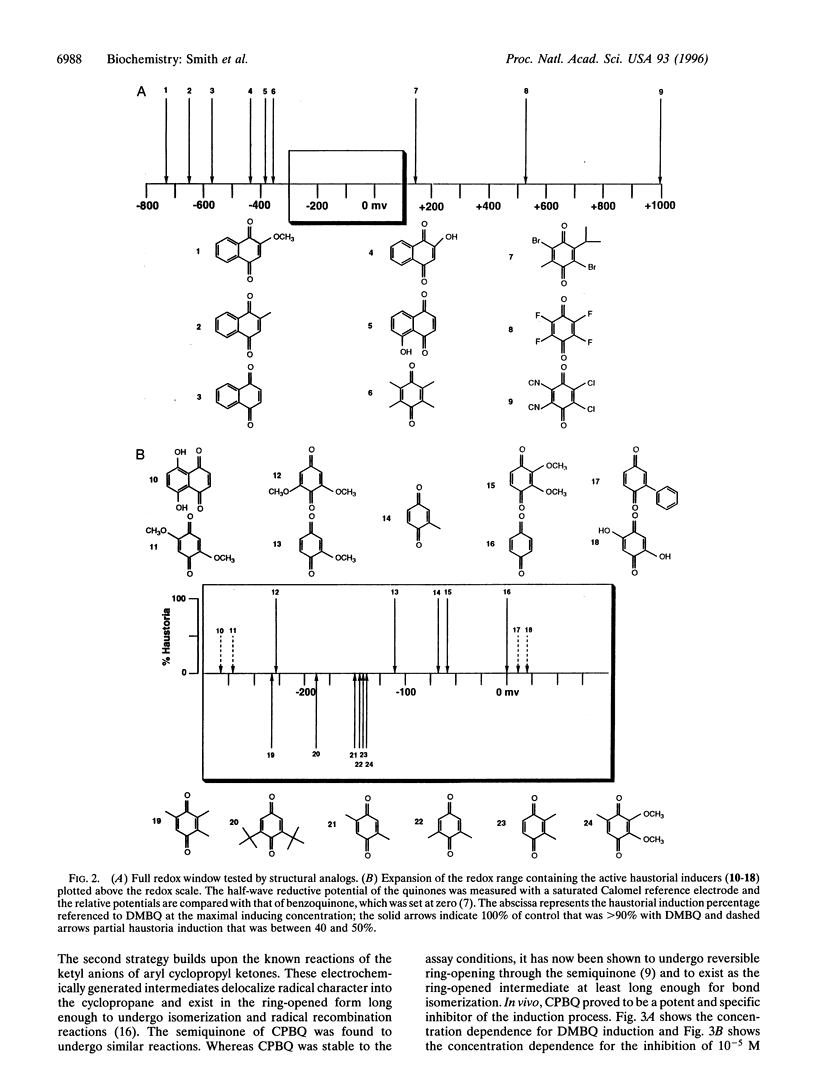
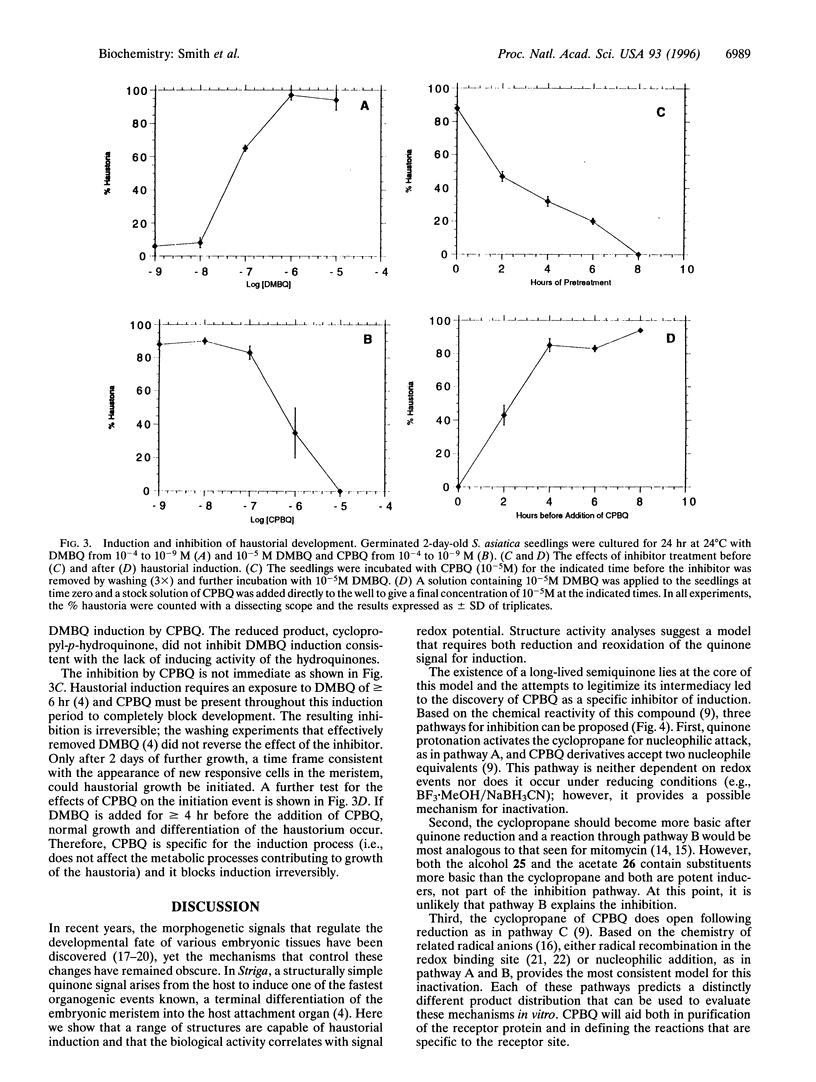
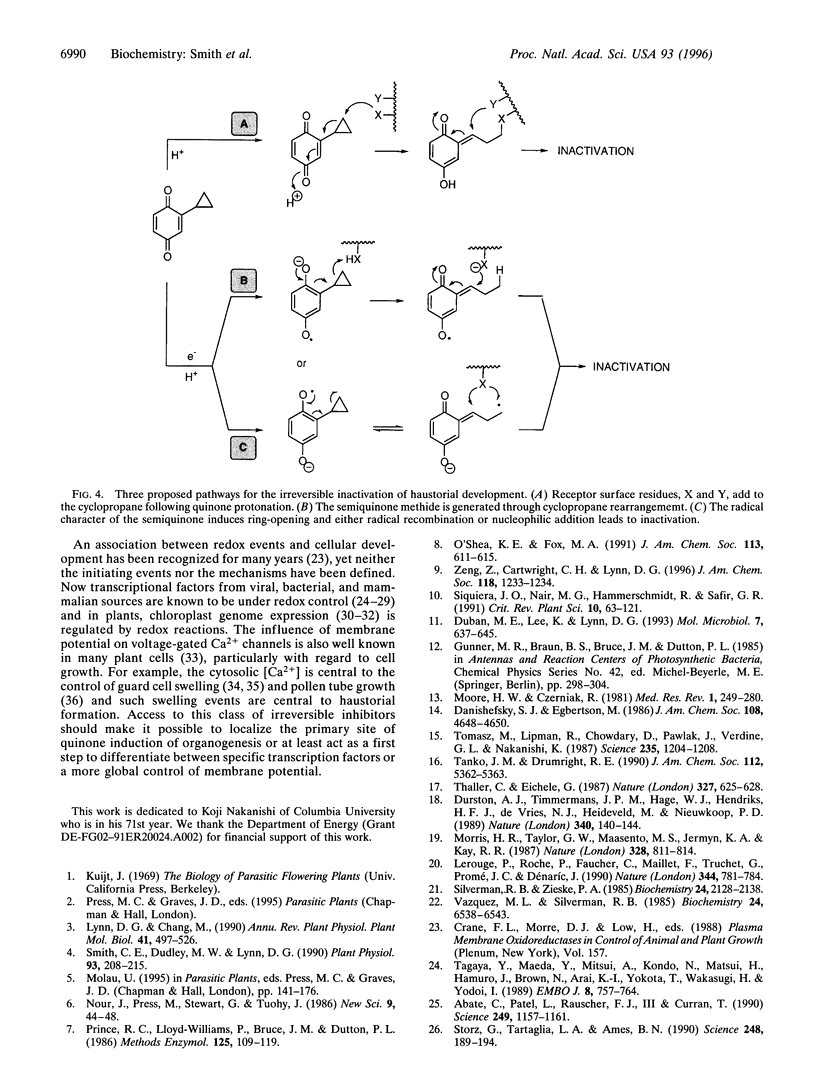
Parasitic strategies are widely distributed in the plant kingdom and frequently involve coupling parasite organogenesis with cues from the host. In Striga asiatica, for example, the cues that initiate the development of the host attachment organ, the haustorium, originate in the host and trigger the transition from vegetative to parasitic mode in the root meristem. This system therefore offers a unique opportunity to study the signals and mechanisms that control plant cell morphogenesis. Here we establish that the biological activity of structural analogs of the natural inducer displays a marked dependence on redox potential and suggest the existence of a semiquinone intermediate. Building on chemistry that exploits the energetics of such an intermediate, cyclopropyl-p-benzoquinone (CPBQ) is shown to be a specific inhibitor of haustorial development. These data are consistent with a model where haustorial development is initiated by the completion of a redox circuit.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abate C., Patel L., Rauscher F. J., 3rd, Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science. 1990 Sep 7;249(4973):1157–1161. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- Allen J. F. Redox control of transcription: sensors, response regulators, activators and repressors. FEBS Lett. 1993 Oct 18;332(3):203–207. doi: 10.1016/0014-5793(93)80631-4. [DOI] [PubMed] [Google Scholar]
- Buchanan B. B. Regulation of CO2 assimilation in oxygenic photosynthesis: the ferredoxin/thioredoxin system. Perspective on its discovery, present status, and future development. Arch Biochem Biophys. 1991 Jul;288(1):1–9. doi: 10.1016/0003-9861(91)90157-e. [DOI] [PubMed] [Google Scholar]
- Danon A., Mayfield S. P. Light-regulated translation of chloroplast messenger RNAs through redox potential. Science. 1994 Dec 9;266(5191):1717–1719. doi: 10.1126/science.7992056. [DOI] [PubMed] [Google Scholar]
- Duban M. E., Lee K., Lynn D. G. Strategies in pathogenesis: mechanistic specificity in the detection of generic signals. Mol Microbiol. 1993 Mar;7(5):637–645. doi: 10.1111/j.1365-2958.1993.tb01155.x. [DOI] [PubMed] [Google Scholar]
- Durston A. J., Timmermans J. P., Hage W. J., Hendriks H. F., de Vries N. J., Heideveld M., Nieuwkoop P. D. Retinoic acid causes an anteroposterior transformation in the developing central nervous system. Nature. 1989 Jul 13;340(6229):140–144. doi: 10.1038/340140a0. [DOI] [PubMed] [Google Scholar]
- Kinoshita T., Nishimura M., Shimazaki Ki. Cytosolic Concentration of Ca2+ Regulates the Plasma Membrane H+-ATPase in Guard Cells of Fava Bean. Plant Cell. 1995 Aug;7(8):1333–1342. doi: 10.1105/tpc.7.8.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerouge P., Roche P., Faucher C., Maillet F., Truchet G., Promé J. C., Dénarié J. Symbiotic host-specificity of Rhizobium meliloti is determined by a sulphated and acylated glucosamine oligosaccharide signal. Nature. 1990 Apr 19;344(6268):781–784. doi: 10.1038/344781a0. [DOI] [PubMed] [Google Scholar]
- Malho R., Read N. D., Trewavas A. J., Pais M. S. Calcium Channel Activity during Pollen Tube Growth and Reorientation. Plant Cell. 1995 Aug;7(8):1173–1184. doi: 10.1105/tpc.7.8.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh M. R., Webb AAR., Taylor J. E., Hetherington A. M. Stimulus-Induced Oscillations in Guard Cell Cytosolic Free Calcium. Plant Cell. 1995 Aug;7(8):1207–1219. doi: 10.1105/tpc.7.8.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride A. A., Klausner R. D., Howley P. M. Conserved cysteine residue in the DNA-binding domain of the bovine papillomavirus type 1 E2 protein confers redox regulation of the DNA-binding activity in vitro. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7531–7535. doi: 10.1073/pnas.89.16.7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H. W., Czerniak R. Naturally occurring quinones as potential bioreductive alkylating agents. Med Res Rev. 1981 Fall;1(3):249–280. doi: 10.1002/med.2610010303. [DOI] [PubMed] [Google Scholar]
- Morris H. R., Taylor G. W., Masento M. S., Jermyn K. A., Kay R. R. Chemical structure of the morphogen differentiation inducing factor from Dictyostelium discoideum. 1987 Aug 27-Sep 2Nature. 328(6133):811–814. doi: 10.1038/328811a0. [DOI] [PubMed] [Google Scholar]
- Prince R. C., Lloyd-Williams P., Bruce J. M., Dutton P. L. Voltammetric measurements of quinones. Methods Enzymol. 1986;125:109–119. doi: 10.1016/s0076-6879(86)25010-7. [DOI] [PubMed] [Google Scholar]
- Silverman R. B., Zieske P. A. Mechanism of inactivation of monoamine oxidase by 1-phenylcyclopropylamine. Biochemistry. 1985 Apr 23;24(9):2128–2138. doi: 10.1021/bi00330a005. [DOI] [PubMed] [Google Scholar]
- Smith C. E., Dudley M. W., Lynn D. G. Vegetative/Parasitic transition: control and plasticity in striga development. Plant Physiol. 1990 May;93(1):208–215. doi: 10.1104/pp.93.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal F. J., Roederer M., Herzenberg L. A., Herzenberg L. A. Intracellular thiols regulate activation of nuclear factor kappa B and transcription of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9943–9947. doi: 10.1073/pnas.87.24.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz G., Tartaglia L. A., Ames B. N. Transcriptional regulator of oxidative stress-inducible genes: direct activation by oxidation. Science. 1990 Apr 13;248(4952):189–194. doi: 10.1126/science.2183352. [DOI] [PubMed] [Google Scholar]
- Tagaya Y., Maeda Y., Mitsui A., Kondo N., Matsui H., Hamuro J., Brown N., Arai K., Yokota T., Wakasugi H. ATL-derived factor (ADF), an IL-2 receptor/Tac inducer homologous to thioredoxin; possible involvement of dithiol-reduction in the IL-2 receptor induction. EMBO J. 1989 Mar;8(3):757–764. doi: 10.1002/j.1460-2075.1989.tb03436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaller C., Eichele G. Identification and spatial distribution of retinoids in the developing chick limb bud. Nature. 1987 Jun 18;327(6123):625–628. doi: 10.1038/327625a0. [DOI] [PubMed] [Google Scholar]
- Tomasz M., Lipman R., Chowdary D., Pawlak J., Verdine G. L., Nakanishi K. Isolation and structure of a covalent cross-link adduct between mitomycin C and DNA. Science. 1987 Mar 6;235(4793):1204–1208. doi: 10.1126/science.3103215. [DOI] [PubMed] [Google Scholar]
- Vazquez M. L., Silverman R. B. Revised mechanism for inactivation of mitochondrial monoamine oxidase by N-cyclopropylbenzylamine. Biochemistry. 1985 Nov 5;24(23):6538–6543. doi: 10.1021/bi00344a035. [DOI] [PubMed] [Google Scholar]




