Abstract
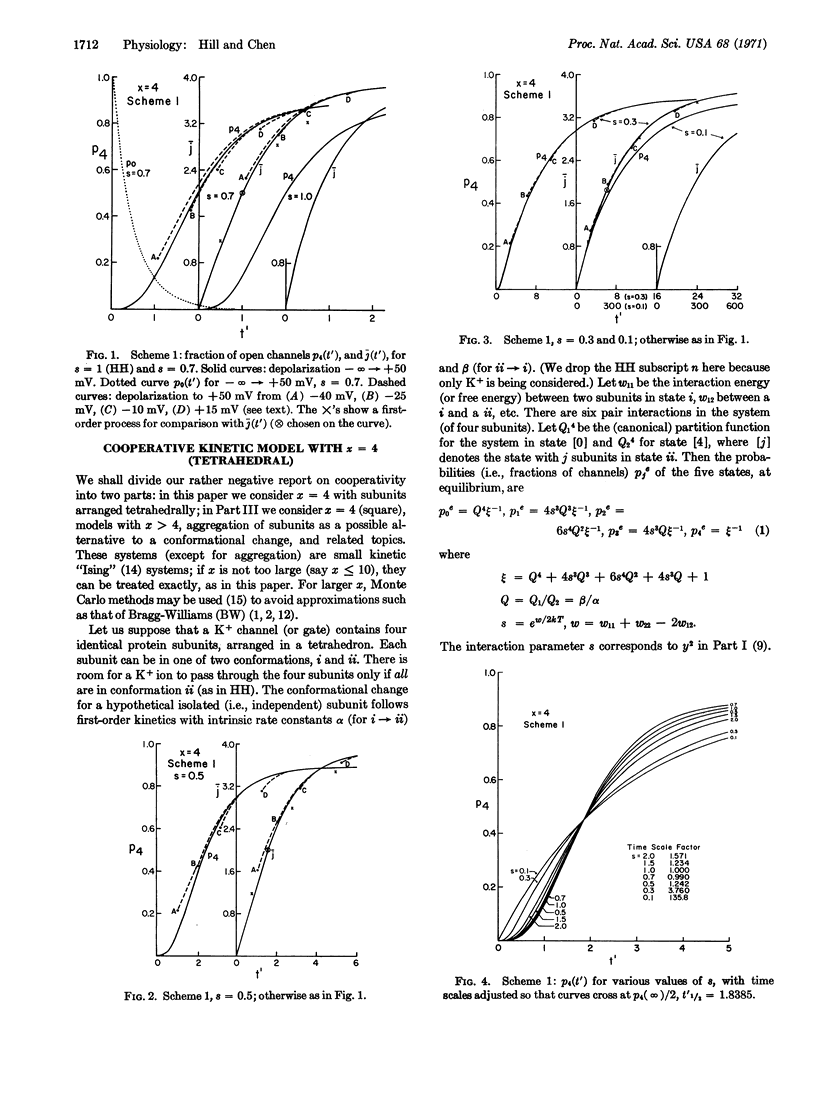
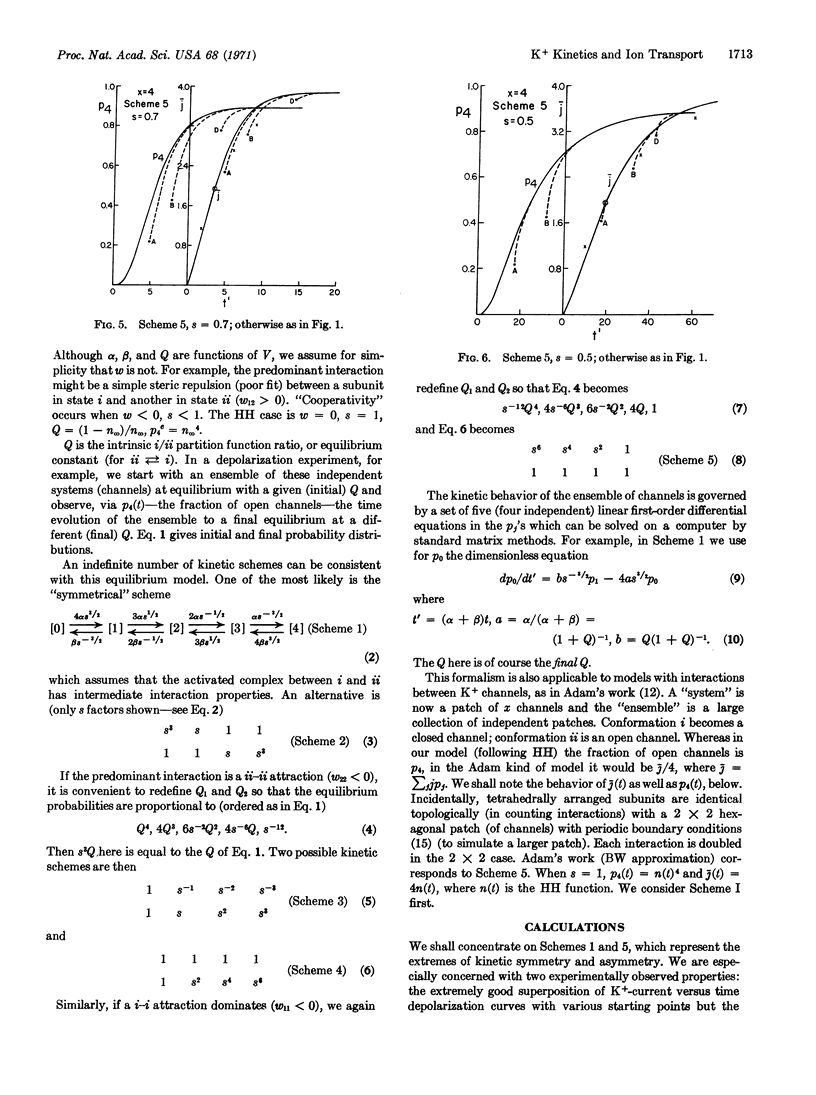
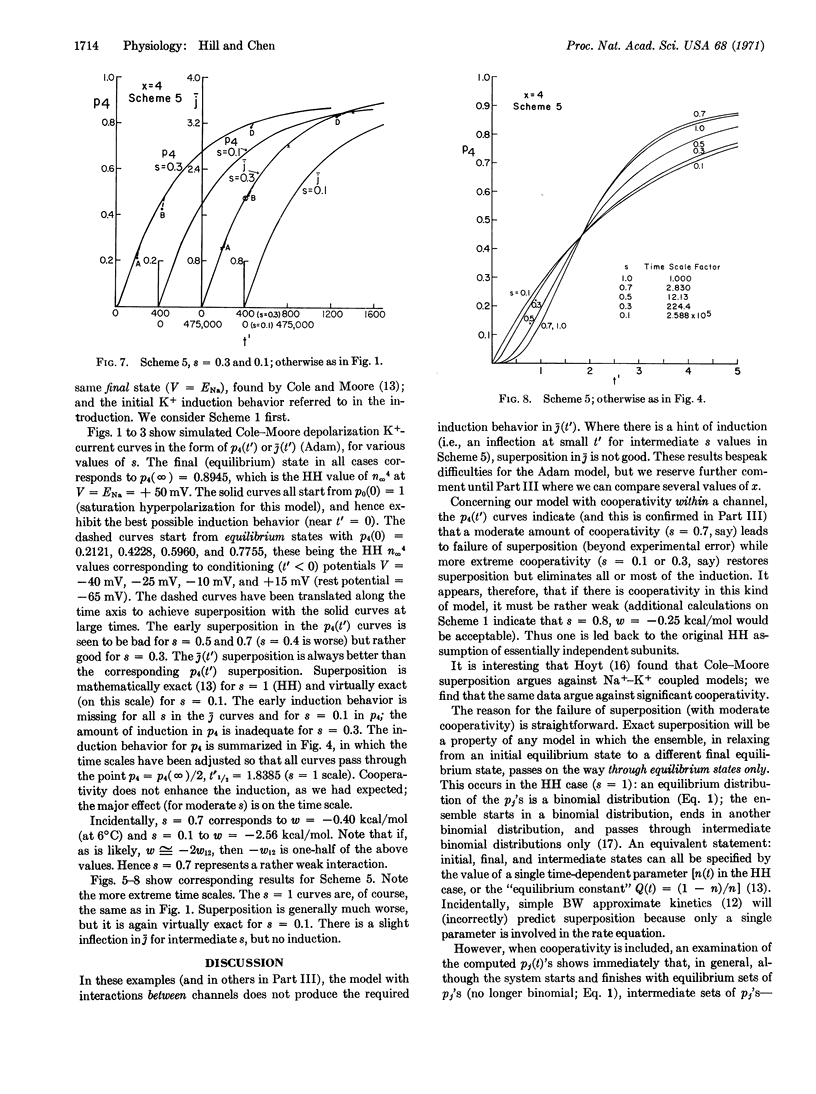
We use a tetrahedral model of four interacting protein subunits to represent the K+ channel or gate in the squid nerve membrane. The kinetic predictions, with varying degrees of cooperativity, are compared with experimental observations, especially those of Hodgkin and Huxley (J. Physiol. 117, 500, 1952) and of Cole and Moore (Biophys. J. 1, 1, 1960). The tentative conclusion reached is that if there is any cooperativity present it must be rather weak. There is no indication here that cooperativity improves the Hodgkin-Huxley assumption of independent “subunits”. Other related models will be discussed in Part III. We also find evidence against the suggestion that there is cooperativity between K+ channels arranged in patches of a two-dimensional lattice.
Keywords: Hodgkin and Huxley, Cole and Moore
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELMAN W. J., Jr, DYRO F. M., SENFT J. LONG DURATION RESPONSES OBTAINED FROM INTERNALLY PERFUSED AXONS. J Gen Physiol. 1965 May;48:SUPPL–SUPPL:9. doi: 10.1085/jgp.48.5.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M. Time course of TEA(+)-induced anomalous rectification in squid giant axons. J Gen Physiol. 1966 Nov;50(2):491–503. doi: 10.1085/jgp.50.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLE K. S., MOORE J. W. Potassium ion current in the squid giant axon: dynamic characteristic. Biophys J. 1960 Sep;1:1–14. doi: 10.1016/s0006-3495(60)86871-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The components of membrane conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):473–496. doi: 10.1113/jphysiol.1952.sp004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L., Chen Y. Cooperative effects in models of steady-state transport across membranes. 3. Simulation of potassium ion transport in nerve. Proc Natl Acad Sci U S A. 1970 Jul;66(3):607–614. doi: 10.1073/pnas.66.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L., Chen Y. Cooperative effects in models of steady-state transport across membranes. I. Proc Natl Acad Sci U S A. 1970 Apr;65(4):1069–1076. doi: 10.1073/pnas.65.4.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt R. C. Independence of the sodium and potassium conductance channels. A kinetic argument. Biophys J. 1971 Jan;11(1):110–122. doi: 10.1016/S0006-3495(71)86199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. W., Narahashi T., Shaw T. I. An upper limit to the number of sodium channels in nerve membrane? J Physiol. 1967 Jan;188(1):99–105. doi: 10.1113/jphysiol.1967.sp008126. [DOI] [PMC free article] [PubMed] [Google Scholar]


