Abstract
The isolation and characterization of Escherichia coli O157:H7 strains from 22 out of 174 fecal samples from petting zoo animals representing twenty-two different species (camel, lion, goats, zebra, bear, baboon monkey, Siberian monkey, deer, elk, llama, pony, horses, fox, kangaroo, wolf, porcupine, chickens, tiger, ostrich, hyena, dogs, and wildcats) were investigated. One petting Al-Zawraa zoological society of Baghdad was investigated for E. coli O157:H7 over a 16-month period that spanned two summer and two autumn seasons. Variation in the occurrence of E. coli O157:H7-positive petting zoo animals was observed, with animals being culture positive only in the summer months but not in the spring, autumn, or winter. E. coli O157:H7 isolates were distinguished by agglutination with E. coli O157:H7 latex reagent (Oxoid), identified among the isolates, which showed that multiple E. coli strains were isolated from one petting zoo animal, in which a single animal simultaneously shed multiple E. coli strains; E. coli O157:H7 was isolated only by selective enrichment culture of 2 g of petting zoo animal feces. In contrast, strains other than O157:H7 were cultured from feces of petting zoo animals without enrichment.
1. Introduction
Since it was first identified in the early 1980s [1], the enterohemorrhagic Escherichia coli (EHEC) strains are a subset of Shiga toxin-producing E. coli strains that have been associated with animals and human diseases. In humans including self-limited watery diarrhea, hemorrhagic colitis, and the hemolytic-uremic syndrome (HUS) [2], this syndrome happened in 2–7% of people with E. coli 0157:H7 infection causing bloody diarrhea [3], in many areas of the world [4–8]. Among the EHEC serotypes, O157:H7, which expresses somatic (O) antigen 157 and flagellar (H) antigen 7, causes serious morbidity and large disease outbreaks, making this bacterium one of the most important food-borne and waterborne pathogens worldwide [9–11]. In 1995, an outbreak of E. coli O157:H7 infections in people was traced to jerky made from deer meat [12].
The vulnerable sectors of the community (children and the elderly) are at the most risk of developing severe infection, making it a very emotive issue in public health and across the food and agricultural industries [13–17].
Cattle appear to be major reservoir for verotoxin-producing E. coli O157 [18–22], although it has also been found in sheep, goats, heifer, birds, deer, geese, turkey, seabirds, dogs, cat, gull, chicken, pig, monkey, reptiles, llama, and horses, as well as on flies [23–30]. However, the extent to which these animal species play a role in the epidemiology of O157 infection remains to be established. Although most infections of O157 in humans have been linked to exposure to a food vehicle or water, person-to-person transmission of O157 and transmission by direct contact with animals or animal manure have also been reported [31].
Petting zoo visits are popular leisure activities and also have become an important feature of education for young children. Such visits are highly beneficial to children in helping them to learn about aspects of animal husbandry and farm produce. Close contact with the animals is often encouraged,such as petting and feeding animals, especially to the main group of visitors, young children, of acquiring severe zoonotic infections during visits to petting zoos. Several outbreak recorded of Escherichia coli O157 infections occurred among agricultural fair, festival, and petting zoo visitors in farm visits occurred in many country in Pennsylvania, Washington [32], Canada [33], and North Wales [34]. During 2003 to2004the capacity of STEC 0157 to persist and multiplicative in the farm environment (animal feces, straw, soil, water) [35] and their natural occurrence in several wild animal species from which interspecies transmission to domestic animals may occur [36], preventing the introduction of the infection, routine testing of brought-in replacement animals, culling infected animals, and closing infected petting zoos, all do not appear to be feasible or effective control measures.
Consistent with this, Pritchard et al. [37] found no obvious value in preentry of bacteriological testing of animals during a longitudinal study on a farm open to the public. Escherichia coli O157:H7 has been detected in the feces of white-tailed deer (Odocoileus virginianus), but the extent of direct or indirect zoonotic risk of this source of E. coli O157:H7 has yet to be determined [38].
The morbidity and the mortality associated with outbreaks of gastrointestinal illnesses caused by STEC have highlighted the threat they pose to public health. Therefore, monitoring the presence of E. coli in animals will assure prompt diagnosis and identify the source of infection that may assist in risk management.
Epidemiological investigation of O157 strain in animal populations has focused mainly on the bovine reservoir and recently in horses, so the prevalence in other animals is not well known in Iraq. The aim of this study was to determine the prevalence of E. coli O157 in fecal samples collected from zoo animals in the Al-Zawraa zoological society of Baghdad in Iraq.
2. Material and Methods
2.1. Animals
One hundred seventy-four fecal samples were collected from animals kept in Al_Zawraa zoological society of Baghdad of different breeds, ages, and sexes which were included in the study during the period from June 2010 to June 2013. Twenty-two animal species, representing, camel, lion, goats, zebra, bear, baboon monkey, Siberian monkey, deer, elk, llama, pony, horses, fox, kangaroo, wolf, porcupine, chickens, tiger, ostrich, hyena, dogs, and wildcats.
2.2. Sample Inoculation
Three g of each fecal sample was mixed with normal saline and centrifuged. The supernatant was discarded and the deposit was inoculated with 5 mL of buffered peptone water (Oxoid).
2.3. Selective Enrichment and Isolation of E. coli O157:H7
One loopful from cultured broth was placed in plate with Cefixime Tellurite-Sorbitol MacConkey (CT-SMAC) agar. The plate was streaked for isolation and incubated overnight. After incubation, all nonsorbitol-fermenting (grey/white) colonies were plated on both blood agar and Levine Eosin Methylene blue agar and incubated overnight at 37°C.
2.4. Agglutination Test
Individual isolates colonies of nonsorbitol-fermenting colonies on CT-SMAC medium were tested for the presence of the O157 and the H7 antigens by agglutination with E. coli O157 and H7 latex reagent (Oxoid).
3. Results
E. coli O157:H7 was isolated from twenty-two out of 174 fecal samples collected in Al_Zawraa zoological societies of Baghdad which are summarized in Table 1. E. coli O157:H7 was isolated from 30% bear, 22.2% deer, 28.6% pony, 16.7% horses, 20% zebra, 66.7% ostrich, 12.5% hyena, 16.7% llama, 25% goat, and 28.6% jaguar. The percentage of EHEC E. coli isolate and characteristic of E. coli O157:H7 are shown in Table 2.
Table 1.
Number of positive E. coli O157:H7 animals from fecal samples collected and date of each collection for different animal species in Al-Zawraa zoological society of Baghdad.
| Animal spp. | Number of samples | Month of sample | No. of positive animals | Percentage of positive animals for E. coli O157:H7 |
|---|---|---|---|---|
| Bear | 10 | June | 3 | 30% |
| Deer | 9 | June | 2 | 22.2% |
| Pony | 7 | June | 2 | 28.6% |
| Lion | 22 | April | 3 | 13.6% |
| Elk | 8 | April | 1 | 12.5% |
| Dog | 8 | September | Nil | 0% |
| Horse | 12 | April | 2 | 16.7% |
| Wildcat | 5 | September | Nil | 0% |
| Zebra | 5 | September | 1 | 20% |
| Siberia monkey | 9 | February | Nil | 0% |
| Ostrich | 3 | June | 2 | 66.7% |
| Baboon monkey | 9 | February | Nil | 0% |
| Hyena | 8 | April | 1 | 12.5% |
| Kangaroo | 1 | April | Nil | 0% |
| Wolf | 5 | February | Nil | 0% |
| Camel | 9 | June | Nil | 0% |
| Fox | 6 | June | Nil | 0% |
| Porcupine | 5 | February | Nil | 0% |
| Llama | 6 | June | 1 | 16.7% |
| Goat | 8 | June | 2 | 25% |
| Jaguar | 7 | April | 2 | 28.6% |
| Chicken | 6 | February | Nil | 0% |
Table 2.
The percentage of EHEC E. coli isolate and E. coli O157:H7 with its characteristic features.
| Animal spp. | Positive samples for EHEC | % of E. coli isolates | Positive sample for O157:H7 | Sorbitol fermentation | Positive for O157 antigen | Positive for H7 antigen |
|---|---|---|---|---|---|---|
| Bear | 5 | 50% | 3 | + | + | + |
| Deer | 3 | 33.3% | 2 | + | + | + |
| Pony | 1 | 14.3% | 2 | + | + | + |
| Lion | 1 | 4.55% | 3 | + | + | + |
| Elk | Nil | — | 1 | + | + | + |
| Dog | Nil | — | Nil | − | − | − |
| Horse | 1 | 8.3% | 2 | + | + | + |
| Wildcat | Nil | — | Nil | − | − | − |
| Zebra | Nil | — | 1 | + | + | + |
| Siberia monkey | Nil | — | Nil | − | − | − |
| Ostrich | 3 | 100% | 2 | + | + | + |
| Baboon monkey | Nil | — | Nil | − | − | − |
| Hyena | Nil | — | 1 | + | + | + |
| Kangaroo | Nil | — | Nil | − | − | − |
| Wolf | Nil | — | Nil | − | − | − |
| Camel | 1 | 11.1% | Nil | − | − | − |
| Fox | Nil | — | Nil | − | − | − |
| Porcupine | Nil | — | Nil | − | − | − |
| Llama | 1 | 16.7% | 1 | + | + | + |
| Goat | Nil | — | 2 | + | + | + |
| Jaguar | 2 | 28.6% | 2 | + | + | + |
| Chicken | Nil | — | Nil | − | − | − |
EHEC strains were isolated from 50% bear, 33.3% deer, 14.3% pony, 4.55% lion, 8.3% horse, 100% ostrich, 11.1% camel, 16.7% llama, and 28.6% jaguar.
The E. coli O157:H7 appears as sorbitol-nonfermented colonies on MacConkey agar (white-gray) as shown in Figure 1.
Figure 1.
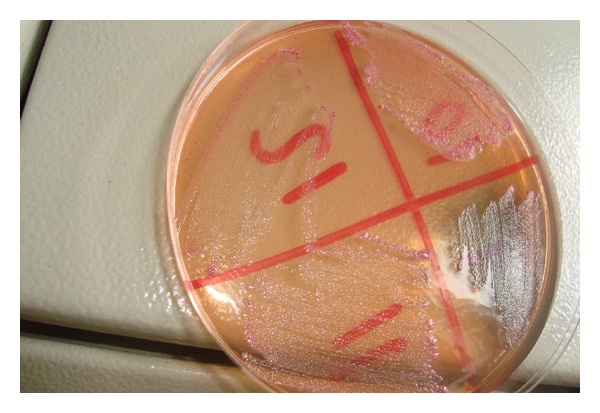
Sorbitol-nonfermented colonies of E. coli O157:H7 on CT-SMAC media.
Individual isolates colonies of non-sorbitol-fermenting colonies on CT-SMAC medium were agglutinated with E. coli O157 and H7 latex reagent (Oxoid) as shown in Figures 2 and 3.
Figure 2.
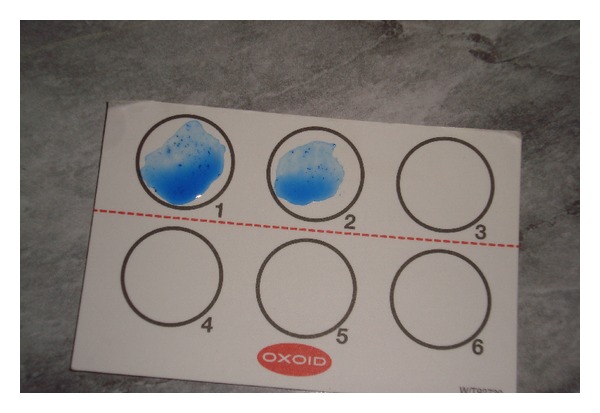
Agglutination of E. coli O157 and H7 latex reagent with isolates colonies of nonsorbitol-fermenting colonies on CT-SMAC medium.
Figure 3.
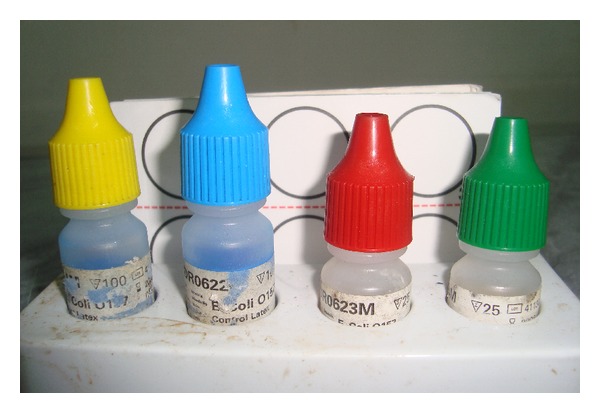
Agglutination kit (Oxoid) for diagnosis of E. coli O157:H7.
4. Discussion
This study includes the isolation of E. coli derived from twenty-two mammalian species from one zoo; this is the first report concerning the isolation of E. coli O157:H7 from petting zoo animals in Iraq.
Whereas ruminants are considered to be reservoir of E. coli O157:H7 infections, wild bird may play a key role in emergence by providing a “zoonotic pool” of the infectious agents mainly E. coli O157:H7; wild bird play an important role in dissemination of E. coli O157:H7; could be the main reservoir for E. coli O157:H7 especially gull that spreads E. coli O157:H7 to cattle and other animals [39, 40]. Other researcher [41] found that manure, rail and environmental of petting zoo animal that causes human infected cases with E. coli 0157 so that different isolated rate among animal species could be related with manure, animal food, water supply [11] contaminated with E. coli 0157 that may contaminated with feces of wild bird. The 100% carriage rate of E. coli O157:H7 in ostrich in this investigation is almost the same as that in cattle, which suggests that E. coli O157:H7 strains are probably widespread in ostrich populations. Because of the popularity of petting zoo, petting zoo animals with E. coli 0157:H7 have the potential to make large numbers of people ill.
Visitors had direct contact with animals that are potential sources of enteric pathogens. Visitors could eat and drink while interacting with animals, and, with the exception of strollers, there were no exclusions on items being brought into animal venues and visitors did not receive educational messages concerning disease risk and prevention measures.
There were insufficient hand washing signs and hand washing stations within the animal venues and midways.
Standards should outline the need for adequate hand washing facilities, appropriate disposal of manure, and proper cleaning of the environment, including rails and floors. Most of these animals did not carry E. coli 0157:H7 during the study period.
The seasonality of the incidence of E. coli O157:H7 in petting zoo animals, coupled with the increase in E. coli O157:H7 associated with food-borne illness during the summer months, suggests that environmental replication plays a key role in the epidemiology of infections [42, 43].
References
- 1.Riley LW, Remis RS, Helgerson SD, et al. Hemmorhagic colitis associated with a rare Escherichia coli serotype. The New England Journal of Medicine. 1983;308(12):681–685. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- 2.Griffin PM, Tauxe RV. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiologic Reviews. 1991;13:60–98. doi: 10.1093/oxfordjournals.epirev.a036079. [DOI] [PubMed] [Google Scholar]
- 3.Easton L. Escherichia coli O157: occurrence, transmission and laboratory detection. British Journal of Biomedical Science. 1997;54(1):57–64. [PubMed] [Google Scholar]
- 4.Parry SM, Palmer SR. The public health significance of VTEC O157. Journal of Applied Microbiology Symposium Supplement. 2000;88(29):1–9. doi: 10.1111/j.1365-2672.2000.tb05326.x. [DOI] [PubMed] [Google Scholar]
- 5.Chapman PA, Wright DJ, Norman P. Verotoxin-producing Escherichia coli infections in Sheffield: cattle as a possible source. Epidemiology and Infection. 1989;102(3):439–445. doi: 10.1017/s0950268800030156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hancock DD, Besser TE, Kinsell ML, Tarr PI, Rice DH, Paros MG. The prevalence of Escherichia coli O157.H7 in dairy and beef cattle in Washington state. Epidemiology and Infection. 1994;113(2):199–207. doi: 10.1017/s0950268800051633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapman PA, Siddons CA, Cerdan Malo AT, Harkin MA. A 1-year study of Escherichia coli O157 in cattle, sheep, pigs and poultry. Epidemiology and Infection. 1997;119(2):245–250. doi: 10.1017/s0950268897007826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trevena WB, Hooper RS, Wray C, Willshaw GA, Cheasty T, Domingue G. Vero cytotoxin-producing Escherichia coli O157 associated with companion animals. The Veterinary Record. 1996;138(16, article 400) [PubMed] [Google Scholar]
- 9.Waterborne outbreak of gastroenteritis associated with a contaminated municipal water supply, Walkerton, Ontario, May-June 2000. Canada Communicable Disease Report. 2000;26(20):170–173. [PubMed] [Google Scholar]
- 10.Wetzel AN, LeJeune JT. Isolation of Escherichia coli O157:H7 strains that do not produce Shiga toxin from bovine, avian and environmental sources. Letters in Applied Microbiology. 2007;45(5):504–507. doi: 10.1111/j.1472-765X.2007.02228.x. [DOI] [PubMed] [Google Scholar]
- 11.Bonetta S, Borelli E, Bonetta S, Conio O, Palumbo F, Carraro E. Development of a PCR protocol for the detection of Escherichia coli O157:H7 and Salmonella spp. in surface water. Environmental Monitoring and Assessment. 2011;177(1–4):493–503. doi: 10.1007/s10661-010-1650-x. [DOI] [PubMed] [Google Scholar]
- 12.Keene WE, Sazie E, Kok J, et al. An outbreak of Escherichia coli O157:H7 infections traced to jerky made from deer meat. Journal of the American Medical Association. 1997;277(15):1229–1231. doi: 10.1001/jama.1997.03540390059036. [DOI] [PubMed] [Google Scholar]
- 13.Al-Jader L, Salmon RL, Walker AM, Williams HM, Willshaw GA, Cheasty T. Outbreak of Escherichia coli O157 in a nursery: lessons for prevention. Archives of Disease in Childhood. 1999;81(1):60–63. doi: 10.1136/adc.81.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heuvelink AE, Van Heerwaarden C, Van Oosterom R, Edink K, Van Duynhoven YT. Escherichia coli O157 on a farm for children. Tijdschrift voor Diergeneeskunde. 2000;125(24):761–762. [PubMed] [Google Scholar]
- 15.Pradel N, Livrelli V, De Champs C, et al. Prevalence and characterization of Shiga toxin-producing Escherichia coli isolated from cattle, food, and children during a one-year prospective study in France. Journal of Clinical Microbiology. 2000;38(3):1023–1031. doi: 10.1128/jcm.38.3.1023-1031.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Outbreaks of Escherichia coli O157:H7 infections among children associated with farm visits in Pennsylvania and Washington, 2000. Canada Communicable Disease Report. 2001;27:117–120. [PubMed] [Google Scholar]
- 17.Duffy G. Verocytoxigenic Escherichia coli in animal faeces, manures and slurries. Journal of Applied Microbiology Symposium Supplement. 2003;94(32):S94–S103. doi: 10.1046/j.1365-2672.94.s1.11.x. [DOI] [PubMed] [Google Scholar]
- 18.Blanco M, Blanco JE, Blanco J, et al. Prevalence and characteristics of Escherichia coli serotype O157:H7 and other verotoxin-producing E. Coli in healthy cattle. Epidemiology and Infection. 1996;117(2):251–257. doi: 10.1017/s0950268800001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blanco M, Blanco JE, Blanco J, et al. Distribution and characterization of faecal verotoxin-producing Escherichia coli (VTEC) isolated from healthy cattle. Veterinary Microbiology. 1997;54(3-4):309–319. doi: 10.1016/s0378-1135(96)01292-8. [DOI] [PubMed] [Google Scholar]
- 20.Jackson SG, Goodbrand RB, Johnson RP, et al. Escherichia coli O157:H7 diarrhoea associated with well water and infected cattle on an Ontario farm. Epidemiology and Infection. 1998;120(1):17–20. doi: 10.1017/s0950268897008479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnsen G, Wasteson Y, Heir E, Berget OI, Herikstad H. Escherichia coli O157:H7 in faeces from cattle, sheep and pigs in the southwest part of Norway during 1998 and 1999. International Journal of Food Microbiology. 2001;65(3):193–200. doi: 10.1016/s0168-1605(00)00518-3. [DOI] [PubMed] [Google Scholar]
- 22.Sheng H, Lim JY, Watkins MK, Minnich SA, Hovde CJ. Characterization of an Eschenchia coli O157:H7 O-antigen deletion mutant and effect of the deletion on bacterial persistence in the mouse intestine and colonization at the bovine terminal rectal mucosa. Applied and Environmental Microbiology. 2008;74(16):5015–5022. doi: 10.1128/AEM.00743-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kudva IT, Hatfield PG, Hovde CJ. Characterization of Escherichia coli O157:H7 and other shiga toxin- producing E. coli serotypes isolated from sheep. Journal of Clinical Microbiology. 1997;35(4):892–899. doi: 10.1128/jcm.35.4.892-899.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heuvelink AE, Van Heerwaarden C, Zwartkruis-Nahuis JTM, et al. Escherichia coli O157 infection associated with a petting zoo. Epidemiology and Infection. 2002;129(2):295–302. doi: 10.1017/s095026880200732x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Synge BA. Verocytotoxin-producing Escherichia coli: a veterinary view. Journal of Applied Microbiology Symposium Supplement. 2000;88(29):S31–S37. doi: 10.1111/j.1365-2672.2000.tb05330.x. [DOI] [PubMed] [Google Scholar]
- 26.Oswald E, Schmidt H, Morabito S, Karch H, Marchès O, Caprioli A. Typing of intimin genes in human and animal enterohemorrhagic and enteropathogenic Escherichia coli: characterization of a new intimin variant. Infection and Immunity. 2000;68(1):64–71. doi: 10.1128/iai.68.1.64-71.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Renter DG, Sargeant JM, Hygnstorm SE, Hoffman JD, Gillespie JR. Escherichia coli O157:H7 in free-ranging deer in Nebraska. Journal of Wildlife Diseases. 2001;37(4):755–760. doi: 10.7589/0090-3558-37.4.755. [DOI] [PubMed] [Google Scholar]
- 28.Lenahan M, O’Brien SB, Byrne C, et al. Molecular characterization of Irish E. coli O157:H7 isolates of human, bovine, ovine and porcine origin. Journal of Applied Microbiology. 2009;107(4):1340–1349. doi: 10.1111/j.1365-2672.2009.04320.x. [DOI] [PubMed] [Google Scholar]
- 29.DebRoy C, Roberts E. Screening petting zoo animals for the presence of potentially pathogenic Escherichia coli . Journal of Veterinary Diagnostic Investigation. 2006;18(6):597–600. doi: 10.1177/104063870601800614. [DOI] [PubMed] [Google Scholar]
- 30.Hamzah AM, Abed AL-Reda AMH, Khalaf JM. Prevalence of Escherichia coli O:157 and H:7 from horse feces in Baghdad, Iraq. Online Journal of Veterinary Research. 2013;17(2):96–99. [Google Scholar]
- 31.Swerdlow DL, Woodruff BA, Brady RC, et al. A waterborne outbreak in Missouri of Escherichia coli O157:H7 associated with bloody diarrhea and death. Annals of Internal Medicine. 1992;117(10):812–819. doi: 10.7326/0003-4819-117-10-812. [DOI] [PubMed] [Google Scholar]
- 32.Gage R, Crielly A, Baysinger M, et al. Outbreaks of Escherichia coli O157 : H7 infections among children associ-ated with farm visits—Pennsylvania and Washington. Morbidity and Mortality Weekly Report. 2001;50:293–297. [PubMed] [Google Scholar]
- 33.Helwig D. E. coli outbreak linked to fall fair. Canadian Medical Association Journal. 2000;162(article 245) [Google Scholar]
- 34.Payne CJI, Petrovic M, Roberts RJ, et al. Vero cytotoxin-producing Escherichia coli O157 gastroenteritis in farm visitors, North Wales. Emerging Infectious Diseases. 2003;9(5):526–530. doi: 10.3201/eid0905.020237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davies M, Engel J, Griffin D, et al. Outbreaks of Escherichia coli O157:H7 associated with petting zoos—North Carolina, Florida, and Arizona, 2004 and 2005. Morbidity and Mortality Weekly Report. 2005;54:1277–1280. [PubMed] [Google Scholar]
- 36.Hancock DD, Besser TE, Rice DH, Ebel ED, Herriott DE, Carpenter LV. Multiple sources of Escherichia coli O157 in feedlots and dairy farms in the Northwestern USA. Preventive Veterinary Medicine. 1998;35(1):11–19. doi: 10.1016/s0167-5877(98)00050-6. [DOI] [PubMed] [Google Scholar]
- 37.Pritchard GC, Willshaw GA, Bailey JR, Carson T, Cheasty T. Verocytotoxin-producing Escherichia coli O157 on a farm open to the public: outbreak investigation and longitudinal bacteriological study. Veterinary Record. 2000;147(10):259–264. doi: 10.1136/vr.147.10.259. [DOI] [PubMed] [Google Scholar]
- 38.Sargeant JM, Hafer DJ, Gillespie JR, Oberst RD, Flood SJA. Prevalence of Escherichia coli O157:H7 in white-tailed deer sharing rangeland with cattle. Journal of the American Veterinary Medical Association. 1999;215(6):792–794. [PubMed] [Google Scholar]
- 39.Wallace JS, Cheasty T, Jones K. Isolation of Vero cytotoxin-producing Escherichia coli O157 from wild birds. Journal of Applied Microbiology. 1997;82(3):399–404. doi: 10.1046/j.1365-2672.1997.00378.x. [DOI] [PubMed] [Google Scholar]
- 40.Foster G, Evans J, Knight HI, et al. Analysis of feces samples collected from a wild-bird garden feeding station in Scotland for the presence of verocytotoxin-producing Escherichia coli O157. Applied and Environmental Microbiology. 2006;72(3):2265–2267. doi: 10.1128/AEM.72.3.2265-2267.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warshawsky B, Gutmanis I, Henry B, et al. Outbreak of Escherichia coli 0157:H7 related to animal contact at a petting zoo. Canadian Journal of Infectious Diseases. 2002;13(3):175–181. doi: 10.1155/2002/873832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hancock DD, Besser TE, Rice DH, Herriott DE, Tarr PI. A longitudinal study of Escherichia coli O157 in fourteen cattle herds. Epidemiology and Infection. 1997;118(2):193–195. doi: 10.1017/s0950268896007212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stanford K, Bach SJ, Marx TH, et al. Monitoring Escherichia coli O157:H7 in inoculated and naturally colonized feedlot cattle and their environment. Journal of Food Protection. 2005;68(1):26–33. doi: 10.4315/0362-028x-68.1.26. [DOI] [PubMed] [Google Scholar]


