Abstract
Objectives
To investigate the role of bone morphogenetic protein (BMP) signaling in acute pancreatitis (AP) by administration of noggin, an endogenous BMP antagonist, in a cerulein-induced AP model.
Methods
AP was induced by 9 hourly intraperitoneal injections of cerulein (50 µg/kg). Control mice received PBS injections. In a separate group, noggin (0.5 mg/kg) was given intraperitoneally at 1 hour before, and 2, 4, and 6 hours after AP induction. The mice were euthanized at one hour after completion of AP induction. The blood samples and the pancreas were harvested for analysis. Isolated pancreatic acini from normal mice and AR42J cells were treated with BMP2 and cerulein. AR42J cells were also treated with noggin. Phosphorylation of Smad1/5/8 was measured.
Results
BMP signaling was upregulated in AP mouse pancreas. BMP2 and cerulein induced phosphorylation of Smad1/5/8 in the acinar cells in vitro, which was blocked by noggin. Noggin administration in vivo attenuated AP induction, decreased vacuole formation in acinar cells, blocked LC3-II levels and partially restored Beclin-1 and Lamp-2 levels.
Conclusions
BMP signaling appears to promote AP induction and autophagy, as suggested by our study showing that noggin ameliorates AP and partially restores autophagic homeostasis.
Keywords: acute pancreatitis, cerulein, noggin, autophagy
INTRODUCTION
It is becoming increasingly clear that signaling pathways important in prenatal development continue to have vital roles in adult life. These pathways direct differentiation, determine cell fate, maintain stem cell niches, coordinate appropriate cellular responses to injury, and influence many disease processes, including inflammation.1 The bone morphogenetic protein (BMP) signaling pathway is an example of such a pathway. BMPs are a major subgroup of the transforming growth factor (TGF)-β superfamily and were initially identified as vital bone inductive factors.2 BMPs play important roles during embryonic development and postnatal homeostasis of various organs and tissues, by controlling cellular differentiation, proliferation, and apoptosis.2 In the canonical BMP signaling pathway, BMPs bind to BMP receptors type I (BMPR1) and type II (BMPR2) on the cell surface and subsequently activate intracellular mediators, receptor-activated Smads (R-Smads), Smad1, Smad5 and Smad8, which form a complex with Smad4 and translocate to the nucleus to regulate transcription of target genes. Noggin is an endogenous BMP antagonist that acts by sequestering BMPs and preventing their interactions with cell surface receptors.3–4 The crystal structure of noggin-BMP7 complex demonstrates that noggin blocks the molecular interfaces of the binding epitopes for both type I and type II receptors to inhibit BMP signaling.3–4 Thus, the primary physiological role of noggin is to antagonize the action of BMPs directly. The function of noggin is essential in skeletal and joint development. Homozygous mice for noggin-null mutant have excess cartilage and fail to initiate joint formation, while heterozygous mice appear normal.5 However, heterozygous mutations in the human noggin locus result in apical joint fusions, indicating functional haploinsufficiency.6–7 Overexpression of noggin in mature osteoblasts in mice results in dramatic decreases in bone mineral density and bone formation rates.8 Noggin also plays essential roles in other tissues. Noggin creates a niche for adult neurogenesis by antagonizing BMP signaling.9 Transgenic expression of noggin leads to the formation of ectopic crypts in mouse intestine.10
Several lines of evidence demonstrate that BMP signaling has proinflammatory properties. Elevated BMP2 protein and phosphorylation of intracellular mediators Smad1/5/8 have been shown in bronchial epithelial cells during airway inflammation.11 BMP2 is up-regulated in atherosclerotic arteries.12 The proinflammatory cytokine tumor necrosis factor-α and H2O2 increase endothelial expression of BMP2, but not BMP4. The increased levels of BMP2 induce endothelial dysfunction, oxidative stress, and endothelial activation.13 However, the role of BMP signaling in pancreatitis is largely unknown.
Acute pancreatitis (AP) is a necrotizing inflammatory condition of the pancreas. Accumulation of large vacuoles in acinar cells is a prominent and early feature of acute pancreatitis.14–17 Experimental evidence has defined these vacuoles as autophagic in origin.15–16, 18–19 Autophagy is a lysosomal degradation pathway that is essential for survival, differentiation, development, and homeostasis.20 Autophagy principally serves an adaptive role to protect organisms against diverse pathologies, including infections, cancer, neurodegeneration, aging, and heart disease. However, in certain experimental disease settings, the prosurvival functions of autophagy may be deleterious.20 In the early stage of acute pancreatitis, autophagy exerts devastating effects in pancreatic acinar cells by activation of trypsinogen to trypsin within pancreatic acinar cells. Mice deficient in Atg5, a key autophagy protein, show reduced signs of pancreatitis, suggesting that inhibition of autophagy is protective.18 However, more recent findings suggest that impaired autophagy enhances the disease. Mareninova et al.19 reported that acute pancreatitis causes inhibition of autophagy processing/maturation and lysosomal degradation, thereby suggesting a dysregulation of autophagic ‘flux’ responsible for the progression of AP. Further studies have also shown that the lyososomal protein, lysosomal-associated membrane protein 2 (Lamp-2), was depleted in experimental and in human pancreatitis, which was correlated with massive accumulation of autophagosomes.21
In this study, we evaluated whether the BMP signaling pathway plays a role in cerulein-induced acute pancreatitis, and found that BMP signaling was activated in the mouse pancreas after cerulein treatment. Furthermore, we administered an endogenous BMP antagonist, noggin, in vivo to cerulein-treated mice and found that noggin attenuated the manifestations of acute pancreatitis, reduced autophagic vacuoles and altered autophagic protein levels. Thus, we provide evidence that noggin attenuates acute pancreatitis likely by partially restoring autophagic homeostasis, suggesting that BMP signaling may play a promoting role in acute pancreatitis and in impaired autophagy in the disease.
MATERIALS AND METHODS
Materials
Cerulein (CR), the decapeptide analog of the potent pancreatic secretagogue cholecystokinin, was purchased from Bachem Americas, Inc. (Torrance, CA); the Phadebas Amylase Test from Magle Life Sciences (Cambridge, MA); collagenase type IV from Worthington Biochemical; soybean trypsin inhibitor, and Lamp-2 antibody from Sigma-Aldrich (St. Louis, MO); phosphorylated Smad1/5/8 (pSmad1/5/8) and Beclin-1 antibodies from Cell Signaling Technology, Inc. (Danvers, MA); LC3 antibody from Novus Biologicals, LLC (Littleton, CO); COX-2 antibody from Cayman Chemical Co. (Ann Arbor, MI); recombinant BMP2 and noggin proteins, BMP2 antibody from R&D Systems, Inc. (Minneapolis, MN); IL-6 Elisa kit from BD Biosciences; horseradish peroxidase-conjugated goat antibodies from Bio-Rad Laboratories (Hercules, CA); the enhanced chemiluminescence (Luminol substrate PicoWest) system for Western blotting from Pierce Biotechnology Inc. (Rockford, IL).
Animals
All animal experiments were performed according to the protocols approved by the Animal Welfare Committee of the University of Texas Health Science Center at Houston. Adult (8–10 weeks old) male C57BL/6 mice were purchased from Harlan Laboratories, Inc. (Indianapolis, IN). The animals were housed in a climate-controlled room with an ambient temperature of 23°C and a 12:12-hour light-dark cycle. Animals were fed standard laboratory chow, given water ad libitum, and randomly assigned to control or experimental groups.
AP induction and noggin administration
For induction of AP, C57BL/6 mice were given 9 hourly intraperitoneal injections of a supramaximally stimulating concentration of cerulein (50 µg/kg). The control mice received equal volume and frequency of PBS injections. Seven to nine mice were assigned to each group. At one hour after the last cerulein injection (completion of AP induction), the mice were euthanized, and the blood and the pancreas were harvested. In a separate experiment, noggin (0.5 mg/kg) or PBS was given to the mice at 1 hour before, 2, 4 and 6 hours after AP induction. Seven mice per group were assigned to noggin or PBS group. The mice were euthanized one hour after completion of AP induction, and the blood and the pancreas were harvested. Serum was obtained for measurement of amylase and IL-6 levels. The pancreas samples were fixed in 10% formalin for morphological studies, or frozen in liquid nitrogen and stored at -80°C for protein extraction.
Morphological examination
Paraffin-embedded pancreas samples were sectioned (5 µm), stained with hematoxylin & eosin (H&E), and examined by an experienced pathologist who was blinded of the sample identity. Histopathological changes of AP was evaluated according to the criteria proposed by Schmidt et al22 with minor modifications and scored from 0 to 4 as absence to the most severe injury on the extent of edema, inflammation, acinar necrosis, and vacuole formation.
Transmission electron microscopy
Fresh pancreatic tissue samples were fixed in a mixture of 2% formaldehyde and 2% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) overnight, postfixed in 1% OsO4 in the same buffer, en bloc stained with 2% aqueous uranyl acetate, dehydrated in ethanol and embedded in Poly/Bed 812 (Polysciences, Warrington, PA). Ultrathin sections were cut on Leica EM UC7 ultramicrotome, stained with lead citrate and examined in a Philips CM 100 electron microscope at 60 kV.
Biochemical assays
Serum amylase levels were determined using the Phadebeas Amylase Test and IL-6 levels were determined using IL-6 Elisa kit as described by the manufacturer.
Cells and treatments
The rat pancreatic acinar cell line, AR42J, was obtained from the American Type Culture Collection (ATCC). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Mediatech, Inc., Manassas, VA) supplemented with 10% fetal bovine serum (FBS, Invitrogen Inc., Carlsbad, CA). The primary pancreatic acini were freshly prepared from normal untreated C57BL/6 (male, 8–10 weeks old) mouse pancreas by collagenase digestion as described.23 The isolated acini were seeded in tissue culture dishes coated with 0.01% laminin (Invitrogen Inc.), and cultured in DMEM supplemented with 10% FBS, 1% penicillin/streptomycin (Invitrogen Inc.). The cells were cultured at 37°C in a humidified incubator containing 5% CO2. The cells were treated with BMP2, cerulein, or noggin at different concentrations for the specified time periods indicated in the respective figures and then cell lysates were prepared. The vehicle (0.1 % BSA, 4 mM HCl) used for dilution of BMP2 and noggin was added to separate groups of cells as a control.
Western blotting analysis
Protein samples were prepared from cell lysates as previously described.24 The pancreatic tissue samples stored at −80°C were thawed and homogenized in 1 X ice-cold lysis buffer (Cell Signaling Technology Inc.), and then protein lysates were prepared. Western blotting analysis was performed using specific antibodies.
Statistical analysis
Data are expressed as mean ± SEM. A p value < 0.05 is considered significant. Differences between two groups were analyzed using Student’s t-test. The in vitro experiments were repeated at least twice and similar results were obtained.
BMP signaling is activated in AP
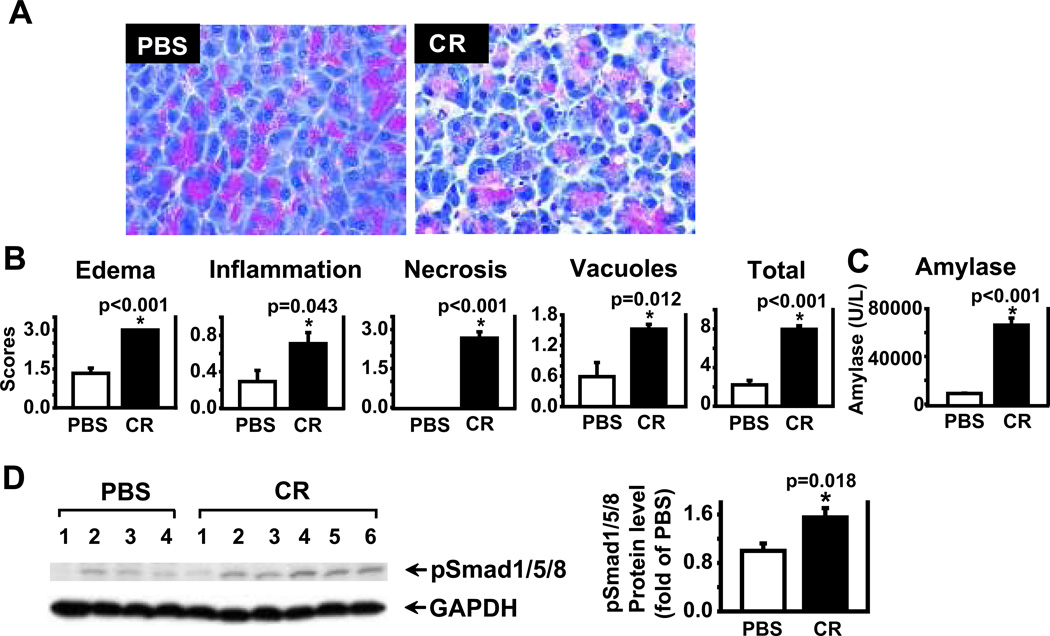
To begin to investigate the role of BMP signaling in AP, C57BL/6 mice were given cerulein (9 hourly intraperitoneal injections) for the induction of AP, a well-established rodent AP model.25–26 Mice pancreata were harvested at 1 hour after the completion of AP induction for histopathological evaluation as described.22 As expected, significant edema, inflammation, acinar cell necrosis, and vacuole formation were observed in the pancreatic sections with H&E staining in cerulein-treated mice, compared to PBS control mice (Fig. 1A). The histopathological scores of edema, inflammation, acinar necrosis, vacuole formation, and the total scores including all these changes were shown as a quantitative measurement of pancreatic injury (Fig. 1B). Serum amylase levels were elevated in cerulein-treated mice, compared to PBS control (Fig. 1C). To assess the levels of BMP signaling, protein lysates were prepared from the mouse pancreata at one hour after completion of AP induction and Western blotting analysis was performed. As shown in Figure 1D, phosphorylation of Smad1/5/8 (pSmad1/5/8) was significantly higher in cerulein-treated mice, compared to PBS control (p=0.018). However, BMP2 protein was undetectable (data not shown). These findings indicate that BMP signaling is activated in cerulein-induced AP, and support a role for BMP signaling in development of acute pancreatitis.
Figure 1. BMP signaling pathway is activated in CR-induced acute pancreatitis (AP).
AP was induced in C57BL/6 mice by 9 hourly cerulein (CR) injections (50 µg/kg). Control groups received PBS. The mouse blood samples and pancreas were harvested at 1 hour after the completion of CR injections . A. Representative H&E-stained sections of the pancreas in PBS control mice and in mice receiving CR. B. H&E-stained sections of the pancreas were scored. The scores of edema, inflammation, acinar necrosis, vacuole formation, and total are presented. C. Serum amylase levels were measured from PBS- and CR-treated mice. D. Western blot analysis was performed using antibodies against phospho-Smad1/5/8 (pSmad1/5/8). GAPDH served as a protein loading control. A representative image was shown. Protein signals were quantified and normalized against GAPDH. Data are presented as mean ± SEM. n= 7–9 mice/group. * p < 0.05 compared to PBS control.
Noggin inhibits BMP2- and cerulein-induced pSmad1/5/8 signaling in vitro
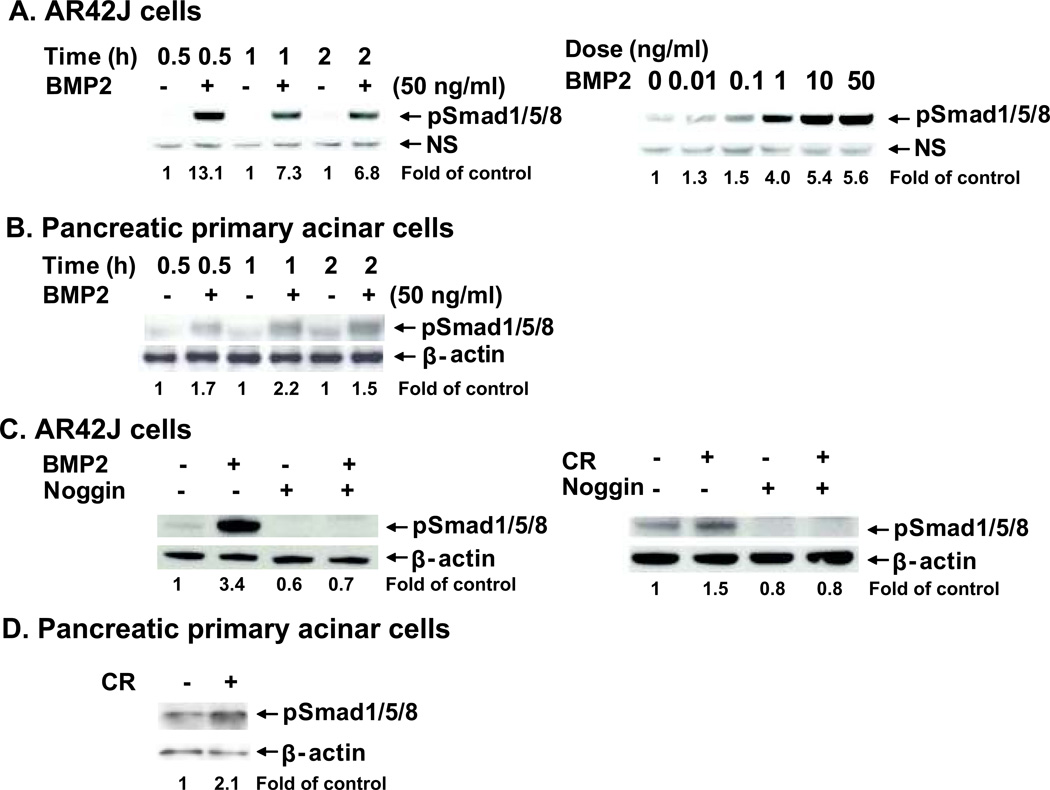
To specifically evaluate BMP signaling in pancreatic acinar cells in vitro, the rat pancreatic acinar cell line, AR42J, and primary acinar cells isolated from normal untreated C57BL/6 mice were used to test their responses to BMP2 and cerulein. The levels of pSmad1/5/8 were measured as an indication of BMP signaling response. A time-course evaluation of BMP2-mediated signaling revealed that pSmad1/5/8 reached peak levels at 0.5 hour; these levels slightly dropped, but persisted up to 2 hours after BMP2 stimulation. A dose-response study performed in AR42J cells showed that pSmad1/5/8 reached peak levels at a BMP2 dose of 50 ng/ml (Fig. 2A). A time-course study performed in primary acinar cells showed that pSmad1/5/8 reached peak levels at 1 hour after BMP2 stimulation (Fig. 2B). Pretreatment of noggin in AR42J cells blocked BMP2-induced pSmad1/5/8 signaling. Interestingly, cerulein treatment increased pSmad1/5/8 levels, although the induction was less brisk when compared with BMP2 treatment. Noggin pretreatment blocked cerulein-induced pSmad1/5/8 (Fig. 2C). Of note, noggin also abolished the basal levels of pSmad1/5/8 in vehicle control groups. Additionally, cerulein treatment also increased pSmad1/5/8 levels in primary acinar cells (Fig. 2D). However, BMP2 levels were undetectable (data not shown). These indicate that pancreatic acinar cells respond to BMP2 and cerulein stimulation, and noggin blocks the response to BMP2 and cerulein stimulation.
Figure 2. Pancreatic acinar cells are responsive to BMP2 and CR stimulation, noggin blocks BMP2- and CR-induced phosphorylation of Smad1/5/8 (pSmad1/5/8) in vitro.
A. A time course and a dose curve of BMP2 responses in AR42J cells. The cells were treated with BMP2 (50 ng/ml) or vehicle and harvested at 0.5, 1, and 2 hours for the time course. The cells were treated with BMP2 at different doses (0. 0.01, 0.1, 1, 10, and 50 ng/ml) and harvested at 0.5 hour for the dose curve. B. A time course of BMP2 responses in primary pancreatic acinar cells. The cells were isolated, cultured and treated with BMP2 (50 ng/ml) or vehicle and harvested at 0.5, 1, and 2 hours. C. AR42J cells were pretreated with noggin (1 mg/ml) for 1 hour followed by BMP2 (10 ng/ml) or cerulein (CR, 100 nM) treatment and harvested at 0.5 hour. Western blotting was performed using anti-pSmad1/5/8 antibody. pSmad1/5/8 levels were normalized against NS (non-specific bands) or β-actin and quantified. D. Primary pancreatic acinar cells were isolated, cultured and treated with cerulein (100 nM) or vehicle and harvested at 0.5 hour. The quantification data are expressed as relative fold changes in the cells treated with BMP2, noggin, or CR compared to the vehicle control.
Noggin attenuates AP induction in vivo
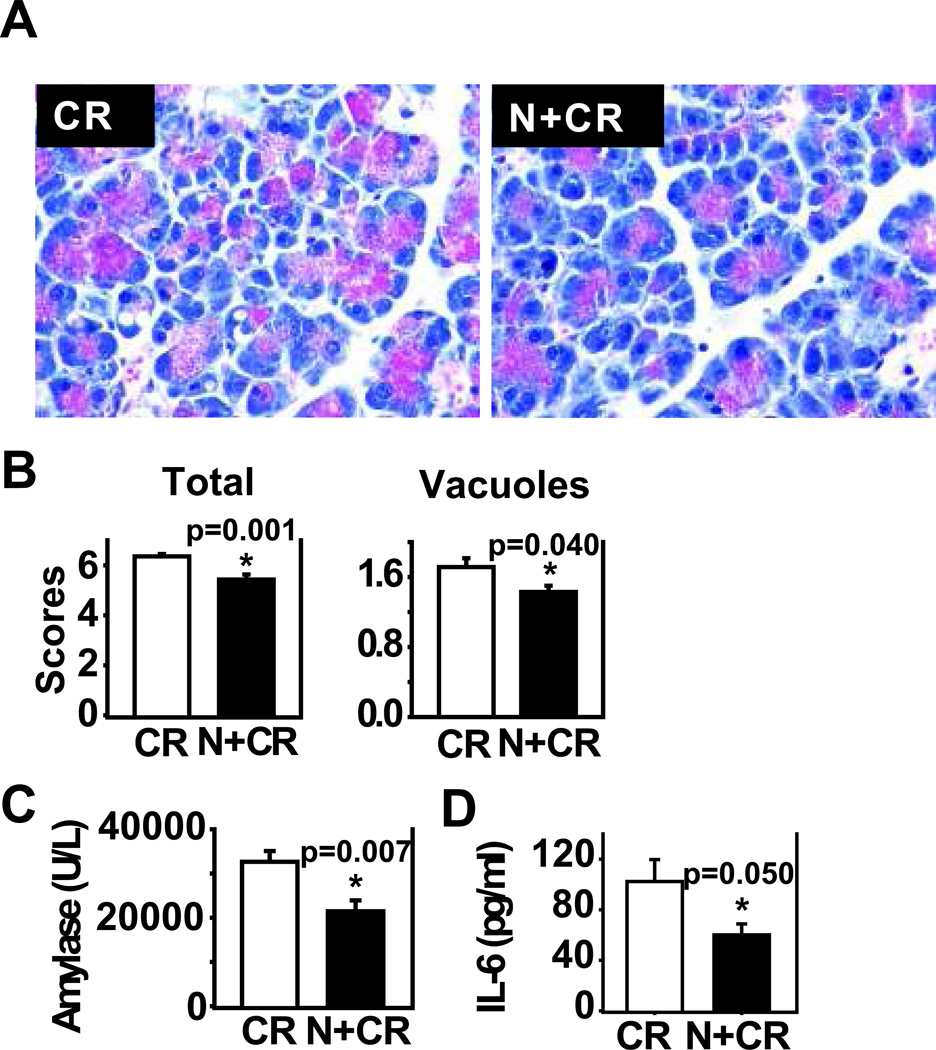
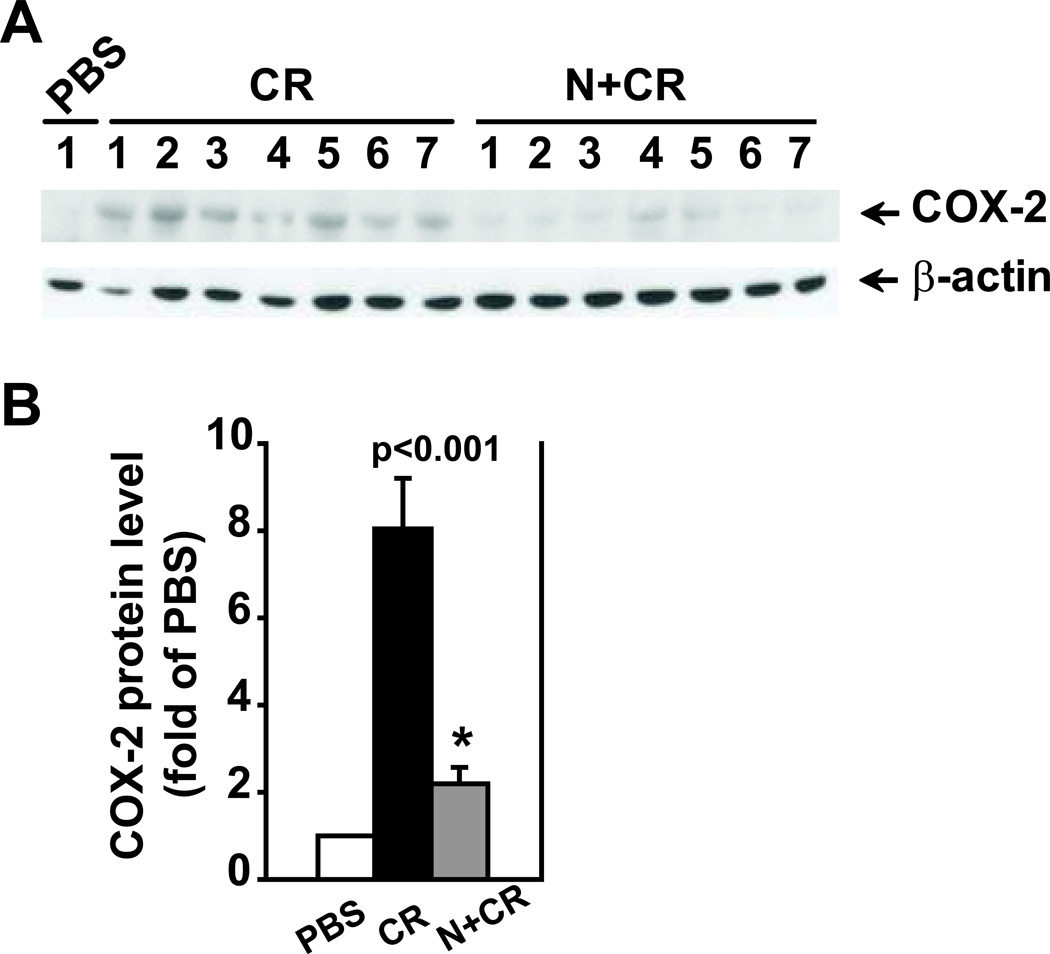
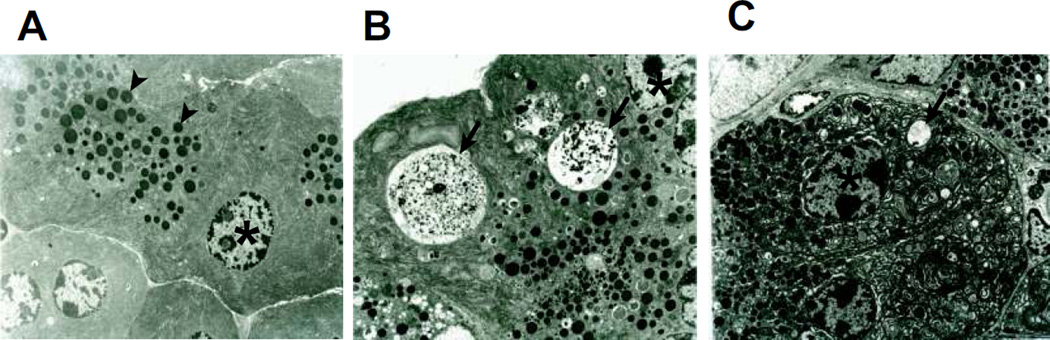
To further investigate the role of BMP signaling in cerulein-induced AP, we administered noggin in vivo to cerulein-treated mice and observed its overall effects on AP pathology. Prior to investigating the effects of noggin on acute pancreatitis, we performed pilot studies to examine if noggin administration in control mice (PBS alone, n=2 mice/group) had any effects compared to the mice without noggin administration. We observed no differences in total histological score or vacuolization; there was also no difference in amylase activity. These findings were observed in C57BL/6 mice, the same strain used in this study (Supplemental Fig. 1), as well as in Swiss Webster mice (n=2 mice/group, data not shown). These data from different strains of mice indicate that noggin administration in vivo had no effects on normal pancreas histology and amylase activity. Subsequently, we focused on the effects of noggin on acute pancreatitis by administering noggin to cerulein-treated mice. PBS was used as the vehicle control of cerulein and noggin. Compared to the mice receiving with cerulein injections, the mice with noggin and cerulein injections exhibited a reduced total histopathological score (p=0.001), serum amylase levels (p=0.007) and IL-6 levels (p=0.05, Fig. 3), and COX-2 protein levels (Fig. 4. P<0.001). Interestingly, noggin administration decreased vacuole formation (p=0.040, Fig. 3), but did not significantly affect cerulein-induced pancreatic edema, inflammation and acinar necrosis (data not shown). Furthermore, the hisopathalogical findings on vacuole formation were confirmed by electron microscopy studies that revealed marked differences in pancreatic acinar morphology between cerulein alone and noggin plus cerulein groups. As shown in Figure 5, the pancreas tissue section from a control mouse with PBS injections alone (a baseline control) exhibited normal, healthy pancreatic acinar cells with compact endoplasmic reticulum cisternae (Fig. 5A). The mouse undergoing cerulein-induced AP demonstrated extensive formation of autophagic vacuoles containing partially degraded intracellular cargo, along with endoplasmic reticulum dilatation and marked cell swelling (Fig. 5B). The mouse receiving noggin and cerulein showed a decrease in both size and number of autophagic vacuoles (Fig. 5C). These indicate that noggin administration attenuates AP induction and reduces autophagic vacuole formation.
Figure 3. Noggin administration in vivo attenuates severity of CR-induced AP.
C57BL/6 mice were given noggin (N, 0.5 mg/kg) or PBS intraperitoneally at 1 hour before, 2, 4, and 6 hours after the first CR injection. AP was induced by 9 hourly CR injections (50 µg/kg). The mouse blood samples and the pancreas were harvested at 1 hour after the completion of CR injections. A. Representative pancreatic images of H&E stained sections from CR and N+CR groups. B. Total histological scores (comprises edema, inflammation, acinar necrosis, and vacuole formation) and vacuole formation are presented. C. Serum amylase levels. D. Serum IL-6 levels. Data are presented as mean ± SEM. n=7 mice/group. * p<0.05 compared to CR group.
Figure 4. Noggin decreases COX-2 protein levels.
Pancreatic tissue samples were obtained from the groups described in Figure 3. The pancreas from the mice treated with PBS alone was included as a baseline control. Protein lysates were prepared for Western blotting analysis using specific antibodies against COX-2. β-actin served as a protein loading control. A. Western blotting images. B. Quantification of the corresponding Western blots. The protein levels were normalized against β-actin and quantified. The data are expressed as relative fold changes to PBS group. n=6–7 mice in CR or N+CR group. * p<0.05 compared N+CR to CR.
Figure 5. Noggin reduces formation of autophagic vacuoles in CR-induced AP demonstrated by transmission electron microscopy (TEM).
Fresh pancreatic tissue samples were obtained from one mouse from each group described in Figure 3 and prepared for TEM studies. The pancreas from the mouse treated with PBS alone was included as a baseline control. Representative images were acquired from A. PBS control, B. CR, C. N+CR. Arrow head: zymogene granule. Asterisk: nucleus. Arrow: autophagic vacuole.
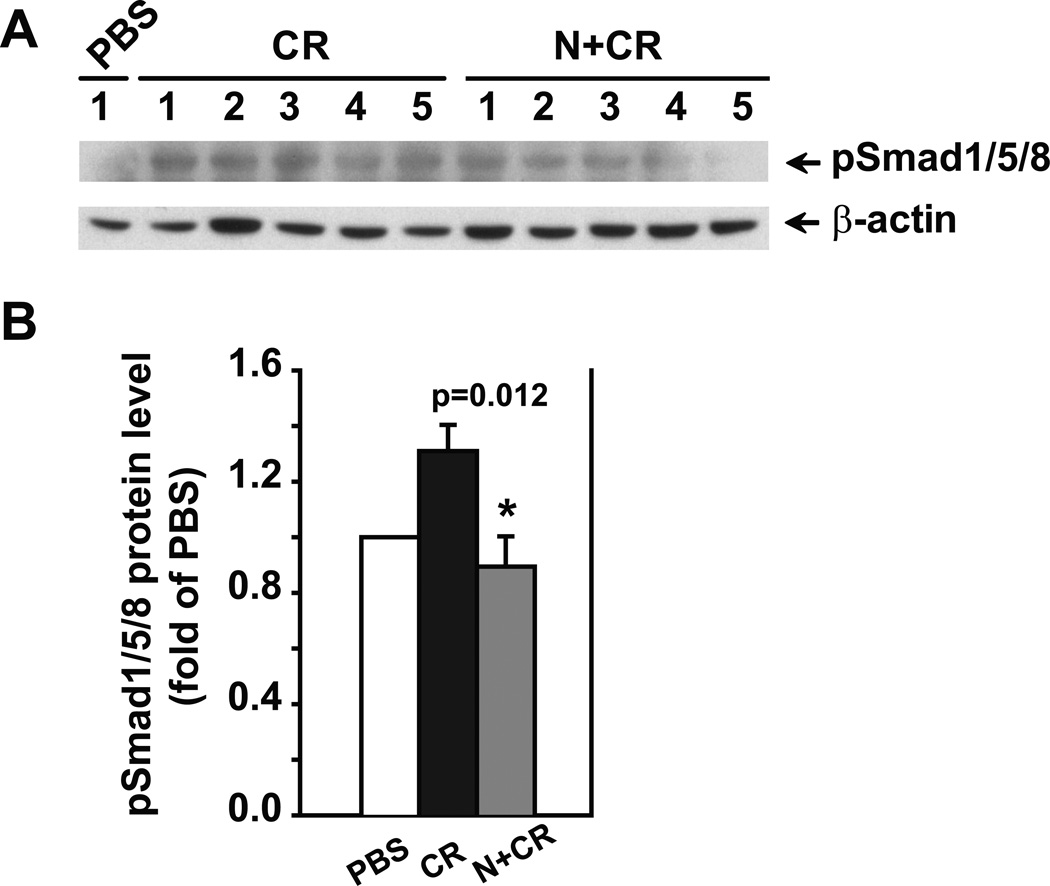
Furthermore, the effects of noggin administration in vivo on BMP signaling were evaluated by measuring pSmad1/5/8 levels. As shown in Figure 6, noggin decreased pSmad1/5/8 levels in cerulein-treated group compared to cerulein-treated group without noggin administration (p=0.012). These data suggest that noggin’s effects on pancreatitis are likely through targeting BMP signaling.
Figure 6. Noggin attenuates the activated BMP signaling in pancreatitis.
Pancreatic tissue samples were obtained from the groups described in Figure 3. The pancreas from the mice treated with PBS alone was included as a baseline control. Protein lysates were prepared for Western blotting analysis using specific antibodies against pSmad1/5/8. β-actin served as a protein loading control. A. Western blotting images. B. Quantification of the corresponding Western blots. The protein levels were normalized against β-actin and quantified. The data are expressed as relative fold changes to PBS group. n=6–7 mice in CR or N+CR group. * p<0.05 compared N+CR to CR.
Noggin regulates autophagic protein markers
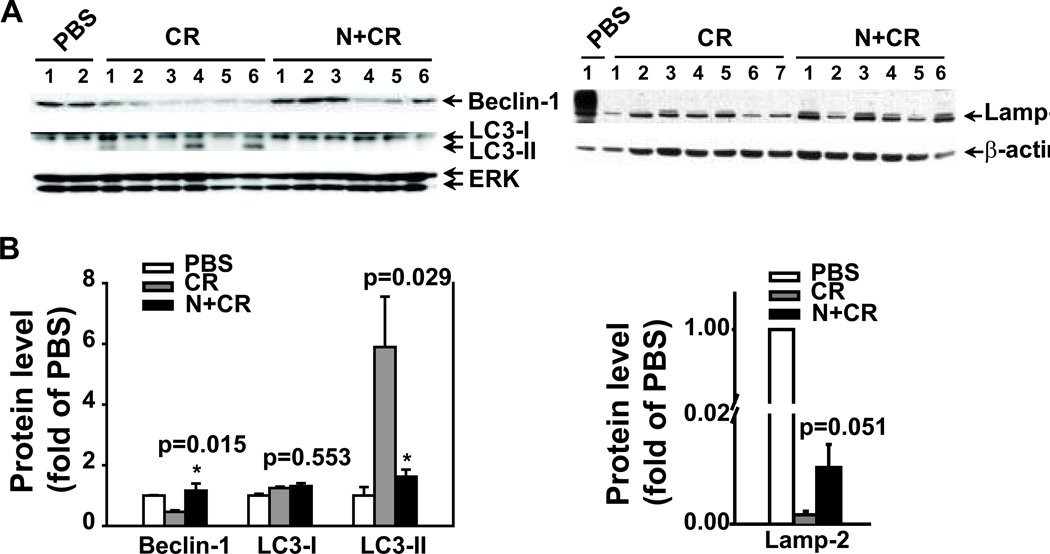
Based on the histopathalogical and electron microscopy findings, we focused on noggin regulation of autophagy in AP and further evaluated autophagic protein markers. Consistent with the previous reports,19, 21 cerulein-treated mouse pancreas had increased LC3-II levels and depleted Lamp-2 levels compared to PBS control (Fig. 7). In contract to the previous study,19, 21 Beclin-1 levels were decreased in the cerulein-treated mouse pancreas. Furthermore, noggin treatment blocked cerulein-induced LC3-II levels (p=0.029), partially restored cerulein-induced reduction in Beclin-1 levels (p=0.015) and depletion in Lamp-2 levels (p=0.051). These indicate that noggin partially restores the levels of autophagy protein markers, and suggest that this restoration may lead to the reduced autophagic vacuole formation in the pancreas during AP induction.
Figure 7. Noggin modulates levels of autophagy proteins in CR-induced AP.
Pancreatic tissue samples were obtained from the groups described in Figure 3. The pancreas from the mice treated with PBS alone was included as a baseline control. Protein lysates were prepared for Western blotting analysis using specific antibodies against Beclin-1, LC3 or Lamp-2. ERK or β-actin served as a protein loading control. A. Western blotting images. B. Quantification of the corresponding Western blots. The protein levels were normalized against ERK or β-actin and quantified. The data are expressed as relative fold changes to PBS group. n=6–7 mice in CR or N+CR group. * p<0.05 compared N+CR to CR.
DISCUSSION
The purpose of this study was to evaluate the role of BMP signaling in the pathogenesis of AP. We found that BMP signaling was activated in cerulein-induced AP as demonstrated by elevated levels of pSmad1/5/8. In vitro, pancreatic acinar cells responded to BMP2 and cerulein stimulation as shown by increased pSmad1/5/8 levels. Noggin, an endogenous BMP antagonist, blocked BMP2- and cerulein-induced pSmad1/5/8 in vitro. Further, noggin administration in vivo ameliorated AP and reduced autophagic vacuole formation, which was associated with a partial restoration in levels of three autophagy protein markers. Our findings led us to conclude that BMP signaling may promote AP and the impaired autophagy in the disease.
To investigate the role of BMP signaling in pancreatitis, we first evaluated BMP signaling molecules in the pancreas with cerulein-induced AP. We found that pSmad1/5/8 levels were elevated in the pancreas with cerulein-induced AP (Fig. 1D). Given our findings that an endogenous BMP antagonist, noggin, blocked the activation of BMP signaling to BMP2 and cerulein stimulation in pancreatic acinar cells in vitro (Fig. 2), we further determined whether noggin administered in vivo modulates AP induction. We found that noggin attenuated AP as shown by reduced histopathological scores, reduced serum amylase levels, and attenuated inflammatory molecules IL-6 (Fig. 3D) and COX-2 levels (Fig. 4). Of note, among the score criteria, vacuole formation was significantly reduced in the noggin and cerulein treatment group. Electron microscopy analysis demonstrated that the vacuoles are of autophagic origin, confirming the light microscopy findings that noggin administration in vivo attenuated vacuole formation as shown by a reduction in size and number of vacuoles (Fig. 5). We also observed that the values for serum amylase in pancreatitis differ greatly between Fig. 1 and Fig. 3. We reason that in Figure 3, additional fluid (PBS with or without noggin) was given to the animals, which may cause the injected cerulein to be absorbed at a slower rate than the animals in Figure 1. Therefore, this may decrease the effects of cerulein in both groups in Figure 3. Nonetheless, the proof-of-principle remains consistent – that is, noggin specifically decreases cerulein-induced amylase activity.
Noggin has been used as a tool to block BMP function because it is a relatively specific inhibitor of BMP activity 27. In our in vitro study, noggin blocked BMP2- and cerulein-induced activation of BMP signaling in AR42J cells (Fig. 2). In vivo, noggin treatment decreased pSmad1/5/8 levels (Fig. 6) in association with attenuation of acute pancreatitis. These data suggest that the effects of noggin both in vitro and in vivo are likely through targeting BMP signaling. However, other mechanisms contributing to the effects of noggin cannot be excluded at this point and deserve further investigation.
The observed decrease in the number of autophagic vacuoles with noggin could be caused by two opposite processes, autophagy inhibition or stimulation of autophagic flux. To resolve this, we chose to examine different stages of the autophagic process by studying markers that represent specific events in autophagy - early to late. We examined three autophagy-specific markers: Beclin-1, a marker of early phagophore formation or autophagy initiation; LC3-II, the categorical autophagy-specific marker and indicator of autophagosome maturation; and Lamp-2, a lysosomal membrane protein important for autolysosome formation. Our studies show that noggin treatment blocked cerulein-induced LC3-II levels (new Fig. 7, p=0.029), partially restored cerulein-induced reduction in Beclin-1 levels (p=0.015) and depletion in Lamp-2 levels (p=0.051). Our data suggest that noggin may restore or normalize autophagy initiation (as shown by Beclin-1 levels) and enhance autophagy completion or efficiency (as shown by Lamp-2 levels). Through these mechanisms, noggin may prompt an efficient clearance of autophagic vacuoles, resulting in reduced autophagic vacuole formation in the pancreas during acute pancreatitis. Therefore, noggin administration in our model favors stimulation of autophagic flux over autophagy inhibition.
In our study, cerulein-induced AP suppressed Beclin-1 level in the pancreas, which is different from the increased Beclin-1 level in AP rat pancreas as reported.19 Given that autophagy is a highly dynamic and complex process, it is possible that these differences may reflect different “snapshot looks” at autophagy at any one static point28 in different in vivo study settings, in addition to the different species used in the studies. Furthermore, the mechanisms and the consequences of Beclin-1 regulation in pancreatitis are not very clear and deserve further investigation.
Based on these findings, we postulate that in an AP disease state, BMP signaling inhibits Beclin-1 and promotes Lamp-2 depletion to inhibit autophagy completion in response to cytotoxic insults. Therefore, the acinar cells experience a backlog in autophagic vacuoles/machinery. This is evidenced by an increase in autophagic vacuoles by electron microscopy and LC3-II protein levels by Western blotting. In this scenario, autophagy process is dysfunctional, and no longer beneficial for the cell, but instead toxic and can lead to tissue damage. The administration of noggin appears to attenuate the impaired autophagy possibly caused by BMP signaling in AP, resulting in a decrease in LC3-II protein levels and a partial restoration in Beclin-1 and Lamp-2 protein levels. The partial restoration of dysfunctional autophagy process by noggin may be important in reducing the acinar cell injury associated with AP disease state. Therefore, targeting BMP signaling pathway may be a novel strategy to attenuate the impaired autophagy in AP.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank E. Figueroa and S. Schuenke in Department of Surgery, University of Texas Medical Branch for manuscript and figure preparation and submission.
Disclosure of funding received for this work: NIDDK (P01-DK035608), NSFC30871189 and NSFC81171841.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest to disclose.
REFERENCES
- 1.Lees C, Howie S, Sartor RB, et al. The hedgehog signalling pathway in the gastrointestinal tract: implications for development, homeostasis, and disease. Gastroenterology. 2005;129:1696–1710. doi: 10.1053/j.gastro.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Ye L, Lewis-Russell JM, Kyanaston HG, et al. Bone morphogenetic proteins and their receptor signaling in prostate cancer. Histol Histopathol. 2007;22:1129–1147. doi: 10.14670/HH-22.1129. [DOI] [PubMed] [Google Scholar]
- 3.Groppe J, Greenwald J, Wiater E, et al. Structural basis of BMP signalling inhibition by the cystine knot protein Noggin. Nature. 2002;420:636–642. doi: 10.1038/nature01245. [DOI] [PubMed] [Google Scholar]
- 4.Yanagita M. BMP antagonists: their roles in development and involvement in pathophysiology. Cytokine Growth Factor Rev. 2005;16:309–317. doi: 10.1016/j.cytogfr.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Brunet LJ, McMahon JA, McMahon AP, et al. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science. 1998;280:1455–1457. doi: 10.1126/science.280.5368.1455. [DOI] [PubMed] [Google Scholar]
- 6.Gong Y, Krakow D, Marcelino J, et al. Heterozygous mutations in the gene encoding noggin affect human joint morphogenesis. Nat Genet. 1999;21:302–304. doi: 10.1038/6821. [DOI] [PubMed] [Google Scholar]
- 7.Marcelino J, Sciortino CM, Romero MF, et al. Human disease-causing NOG missense mutations: effects on noggin secretion, dimer formation, and bone morphogenetic protein binding. Proc Natl Acad Sci U S A. 2001;98:11353–11358. doi: 10.1073/pnas.201367598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu XB, Li Y, Schneider A, et al. Impaired osteoblastic differentiation, reduced bone formation, and severe osteoporosis in noggin-overexpressing mice. J Clin Invest. 2003;112:924–934. doi: 10.1172/JCI15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim DA, Tramontin AD, Trevejo JM, et al. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- 10.Haramis AP, Begthel H, van den Born M, et al. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science. 2004;303:1684–1686. doi: 10.1126/science.1093587. [DOI] [PubMed] [Google Scholar]
- 11.Rosendahl A, Pardali E, Speletas M, et al. Activation of bone morphogenetic protein/Smad signaling in bronchial epithelial cells during airway inflammation. Am J Respir Cell Mol Biol. 2002;27:160–169. doi: 10.1165/ajrcmb.27.2.4779. [DOI] [PubMed] [Google Scholar]
- 12.Dhore CR, Cleutjens JP, Lutgens E, et al. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2001;21:1998–2003. doi: 10.1161/hq1201.100229. [DOI] [PubMed] [Google Scholar]
- 13.Csiszar A, Ahmad M, Smith KE, et al. Bone morphogenetic protein-2 induces proinflammatory endothelial phenotype. Am J Pathol. 2006;168:629–638. doi: 10.2353/ajpath.2006.050284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niederau C, Grendell JH. Intracellular vacuoles in experimental acute pancreatitis in rats and mice are an acidified compartment. J Clin Invest. 1988;81:229–236. doi: 10.1172/JCI113300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watanabe O, Baccino FM, Steer ML, et al. Supramaximal caerulein stimulation and ultrastructure of rat pancreatic acinar cell: early morphological changes during development of experimental pancreatitis. Am J Physiol. 1984;246:G457–G467. doi: 10.1152/ajpgi.1984.246.4.G457. [DOI] [PubMed] [Google Scholar]
- 16.Koike H, Steer ML, Meldolesi J. Pancreatic effects of ethionine: blockade of exocytosis and appearance of crinophagy and autophagy precede cellular necrosis. Am J Physiol. 1982;242:G297–G307. doi: 10.1152/ajpgi.1982.242.4.G297. [DOI] [PubMed] [Google Scholar]
- 17.Aho HJ, Nevalainen TJ, Havia VT, et al. Human acute pancreatitis: a light and electron microscopic study. Acta Pathol Microbiol Immunol Scand A. 1982;90:367–373. [PubMed] [Google Scholar]
- 18.Hashimoto D, Ohmuraya M, Hirota M, et al. Involvement of autophagy in trypsinogen activation within the pancreatic acinar cells. J Cell Biol. 2008;181:1065–1072. doi: 10.1083/jcb.200712156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mareninova OA, Hermann K, French SW, et al. Impaired autophagic flux mediates acinar cell vacuole formation and trypsinogen activation in rodent models of acute pancreatitis. J Clin Invest. 2009;119:3340–3355. doi: 10.1172/JCI38674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fortunato F, Burgers H, Bergmann F, et al. Impaired autolysosome formation correlates with Lamp-2 depletion: role of apoptosis, autophagy, and necrosis in pancreatitis. Gastroenterology. 2009;137:350. doi: 10.1053/j.gastro.2009.04.003. 360 e351-355. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt J, Lewandrowski K, Fernandez-del Castillo C, et al. Histopathologic correlates of serum amylase activity in acute experimental pancreatitis. Dig Dis Sci. 1992;37:1426–1433. doi: 10.1007/BF01296014. [DOI] [PubMed] [Google Scholar]
- 23.Han B, Ji B, Logsdon CD. CCK independently activates intracellular trypsinogen and NF-kappaB in rat pancreatic acinar cells. Am J Physiol Cell Physiol. 2001;280:C465–C472. doi: 10.1152/ajpcell.2001.280.3.C465. [DOI] [PubMed] [Google Scholar]
- 24.Cao Y, Gao X, Zhang W, et al. Dietary fiber enhances TGF-{beta} signaling and growth inhibition in the gut. Am J Physiol Gastrointest Liver Physiol. 2011;301:G156–G164. doi: 10.1152/ajpgi.00362.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niederau C, Ferrell LD, Grendell JH. Caerulein-induced acute necrotizing pancreatitis in mice: protective effects of proglumide, benzotript, and secretin. Gastroenterology. 1985;88:1192–1204. doi: 10.1016/s0016-5085(85)80079-2. [DOI] [PubMed] [Google Scholar]
- 26.Bhatia M, Saluja AK, Hofbauer B, et al. Role of substance P and the neurokinin 1 receptor in acute pancreatitis and pancreatitis-associated lung injury. Proc Natl Acad Sci U S A. 1998;95:4760–4765. doi: 10.1073/pnas.95.8.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Canalis E, Economides AN, Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr Rev. 2003;24:218–235. doi: 10.1210/er.2002-0023. [DOI] [PubMed] [Google Scholar]
- 28.Barth S, Glick D, Macleod KF. Autophagy: assays and artifacts. J Pathol. 2010:117–124. doi: 10.1002/path.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.