Significance
There are marked differences between the sexes in their immune response to infections and vaccination, with females often having significantly higher responses. However, the mechanisms underlying these differences are largely not understood. Using a systems immunology approach, we have identified a cluster of genes involved in lipid metabolism and likely modulated by testosterone that correlates with the higher antibody-neutralizing response to influenza vaccination observed in females. Moreover, males with the highest testosterone levels and expression of related gene signatures exhibited the lowest antibody responses to influenza vaccination. This study generates a number of hypotheses on the sex differences observed in the human immune system and their relationship to mechanisms involved in the antibody response to vaccination.
Keywords: aging, gender, immuno-endocrine, sexual dimorphism, immunosenescence
Abstract
Females have generally more robust immune responses than males for reasons that are not well-understood. Here we used a systems analysis to investigate these differences by analyzing the neutralizing antibody response to a trivalent inactivated seasonal influenza vaccine (TIV) and a large number of immune system components, including serum cytokines and chemokines, blood cell subset frequencies, genome-wide gene expression, and cellular responses to diverse in vitro stimuli, in 53 females and 34 males of different ages. We found elevated antibody responses to TIV and expression of inflammatory cytokines in the serum of females compared with males regardless of age. This inflammatory profile correlated with the levels of phosphorylated STAT3 proteins in monocytes but not with the serological response to the vaccine. In contrast, using a machine learning approach, we identified a cluster of genes involved in lipid biosynthesis and previously shown to be up-regulated by testosterone that correlated with poor virus-neutralizing activity in men. Moreover, men with elevated serum testosterone levels and associated gene signatures exhibited the lowest antibody responses to TIV. These results demonstrate a strong association between androgens and genes involved in lipid metabolism, suggesting that these could be important drivers of the differences in immune responses between males and females.
The variability in the biology of human populations poses significant challenges in understanding different disease outcomes and developing successful therapeutics. The sources of this variation are likely the consequence of genetics, epigenetics, and the history of antigenic exposure (1, 2). As therapies targeting immune function are developed to improve clinical outcomes in cancer, viral and bacterial infections, autoimmune diseases, and transplantation, identifying the sources of immunological variation and finding biomarkers for immune health and dysfunction are crucial for their success (3).
An important source of immunological variation is known to be the sex of the individual. Males experience a greater severity and prevalence of bacterial, viral, fungal, and parasitic infections than females, who also exhibit a more robust response to antigenic challenges such as infection and vaccination (4, 5). This stronger immune response in females could also explain why they more frequently develop immune-mediated pathologies during influenza infection, such as an overproduction of cytokines (cytokine storm) that contribute to an increase in capillary permeability and lung failure (6). Furthermore, females are at a higher risk for developing autoimmune diseases. In this later context, it is interesting to note that a recent study showed that females had, on average, 1.7 times the frequency of self-specific T cells as males (7). Despite the fact that initial observations relating the sex of the individual with the immune response were made many years ago (8), little is known about the mechanisms underlying these differences.
Some sex-specific variations in the immune response can be directly attributed to sex hormones (9). In humans, sex steroids can bind to intracellular receptors located in immune cells such as monocytes, B cells, and T cells and activate hormone-responsive genes, suggesting that they can directly affect sex-related differences in both innate and adaptive immune responses (10). Whereas estrogens are associated with inflammation and can stimulate proliferation and differentiation of lymphocytes and monocytes, androgens suppress the activity of immune cells by increasing the synthesis of anti-inflammatory cytokines (11, 12).
To date, no clear associations have been found between biological and clinical differences in the immune response between males and females in humans. In one study, results from public gene expression data (13) showed that many of the genes induced by a yellow fever vaccine were preferentially activated in females (14). However, whether these differences correlate with poor antibody outcomes remains to be determined.
In this study, we sought to determine whether we could identify biomarkers from peripheral blood that could explain the sex-related differences in the serological response to the trivalent inactivated seasonal influenza vaccine (TIV) in both young and older cohorts.
Young and older females had higher neutralizing antibodies than age-matched males, consistent with previous reports (15). Females also showed higher expression of inflammatory markers. However, none of these specific sex-related differences correlated with the observed disparities in the antibody response to TIV. Nevertheless, using a machine learning approach, we identified a set of genes previously shown to be regulated by testosterone and participating in lipid biosynthesis, whose expression was negatively associated with antibody responses to TIV in the male subjects in our study. Moreover, males with high levels of serum testosterone and expressing related gene signatures in blood cells showed the lowest neutralizing responses to TIV. These results suggest that testosterone might be immunosuppressive in vivo in humans, and indicate that its effect on an influenza vaccine and other immune responses could be due to the regulation of genes implicated in the metabolism of lipids.
Results
Elevated Levels of Neutralizing Antibodies upon Influenza Vaccination and Inflammatory Markers in Serum from Females Versus Males.
To study the differences in males’ versus females’ immune systems, we used data from a vaccination and systems immunology study conducted on 91 individuals (37 males and 54 females) of different ages (20–30 and 60–>89 y old) (Table 1) that we recently reported (16). We studied a variety of immune parameters from peripheral blood before vaccination, including cytokines, chemokines, and growth factors in serum, frequencies of diverse blood cell subsets, phosphorylation levels of signal transducer and activator of transcription (STAT) proteins in multiple cells stimulated with a variety of cytokines or unstimulated (96 conditions in total), and whole-blood gene expression. The gene expression data were reduced to 109 gene modules by cluster analysis and assignment of a set of transcription factors (regulatory program) to each gene module as described (16) (SI Materials and Methods). Four individuals were removed from the analysis: two outliers and two with incomplete datasets.
Table 1.
Subjects’ baseline characteristics
| Males | Females | P value | |
| Number of subjects | 37 | 54 | — |
| Age range (median), y | 20–>89 (63) | 20–>89 (68) | 0.14 |
| BMI range (median) | 19–36 (25) | 18–47 (24) | 0.61 |
| Cytomegalovirus (+), % | 61 | 57 | 0.73 |
| Epstein–Barr virus (+), % | 70 | 54 | 0.13 |
BMI, body mass index.
To determine the magnitude of the antibody response to TIV, we performed virus microneutralization assays. The seroconversion rate (percent of individuals with a fourfold or greater change in their post- versus prevaccination microneutralization titer) was computed for each group and strain in the vaccine (SI Materials and Methods). We conducted logistic regression analysis on each of the titer changes (corresponding to the H1N1, H3N2, and B strains) and included the age and sex variables in the model, because age was expected to modify vaccine responses. Females had a greater response than males to the H3N2 strain (P = 0.0027) and to a lesser extent to the B strain (P = 0.02). In contrast, despite a strong age effect (P = 0.0035), no differences according to sex were found for the H1N1 strain (Table 2).
Table 2.
Age and sex effects on microneutralization antibody titer responses to influenza vaccination
| Beta | SE | z value | P value | ||
| H1N1 | (Intercept) | −0.272 | 0.229 | −1.190 | 0.234 |
| Age | −0.690 | 0.236 | −2.919 | 0.004 | |
| Sex | −0.011 | 0.234 | −0.047 | 0.962 | |
| H3N2 | (Intercept) | −0.038 | 0.228 | −0.166 | 0.868 |
| Age | −0.190 | 0.236 | −0.804 | 0.421 | |
| Sex | −0.716 | 0.239 | −2.992 | 0.003 | |
| B | (Intercept) | −0.502 | 0.236 | −2.128 | 0.033 |
| Age | −0.583 | 0.246 | −2.367 | 0.018 | |
| Sex | −0.594 | 0.256 | −2.324 | 0.020 |
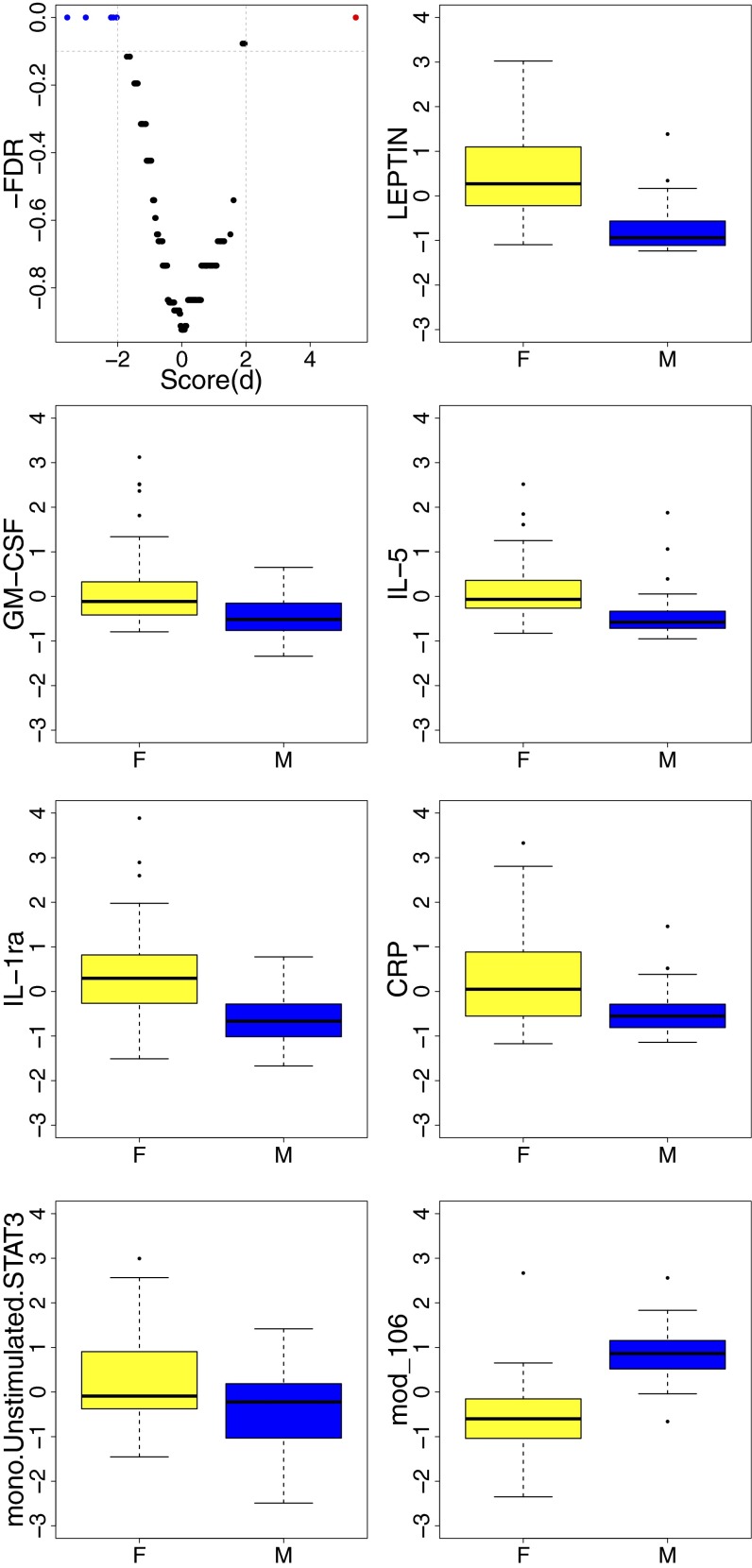
To determine the differences in the baseline’s immune measures in males versus females, we conducted differential expression analysis across a total of 278 parameters using significance analysis of microarrays (SAM) (17) and found significant differences in 7 parameters [false discovery rate (FDR) Q < 0.1], 6 of which were increased in females (Fig. 1). Strikingly, these included several known markers of inflammation, such as LEPTIN, interleukin (IL)-1 receptor agonist (RA), C-reactive protein (CRP), Granulocyte macrophage-colony stimulating factor (GM-CSF), and Interleukin IL-5, as well as the phosphorylation levels of STAT3 proteins in unstimulated monocytes (M-pSTAT3).
Fig. 1.
Significant differences in baseline immune parameters between females and males. Expression of a total of 278 immune features and gene modules was compared between females (F) (n = 53) and males (M) (n = 34) of different age groups, including serum cytokines, chemokines, and growth factors; frequencies of over 15 blood cell subsets; phosphorylation events in multiple immune cells; and whole-genome gene expression using SAM. A cutoff of Q < 0.1 and absolute score(d) > 2 was considered significant (volcano plot; Top, Left). Inflammatory markers including LEPTIN, IL-1RA, and CRP and other serum proteins were elevated in females compared with males. mono.Unstimulated.STAT3, baseline levels of pSTAT3 in isolated monocytes. A single gene module (module 106) (Bottom, Right) was differentially expressed (and up-regulated in males). Module 106 is enriched for genes located on the Y chromosome (P < 10−9). Lower whisker represents the minimum value, lower hinge the first quartile, upper hinge the third quartile, and upper whisker the maximum value. Outliers are represented by circles.
One parameter (gene module 106) was up-regulated in males compared with females. A significant fraction of this gene module is composed of genes located on the Y chromosome (enrichment P < 10−9) (Table S1). Interestingly, genes participating in the activation of v-akt murine thymoma viral oncogene homolog (Akt) and phospholipase C (PLC) proteins such as mature T-cell proliferation 1 (MTCP1) and phosphatidylinositol-specific phospholipase C, X domain containing 1 (PLCXD1), respectively, clustered with Y chromosome-linked genes in module 106. The regulatory program derived for module 106 (Fig. S1) included genes previously shown to be differentially regulated in males versus females, such as CLOCK (18), ENY2 (19), and IRF1 and IRF7 (20).
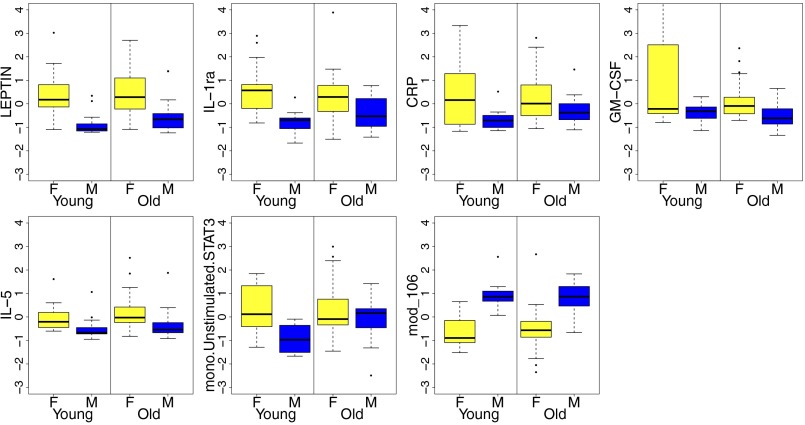
It has long been noted that inflammatory markers, especially IL-6, TNF-α, and IL-1β, among others, are increased in the elderly. Thus, we divided the individuals by age group (young, 20–30 y old; old, 60–>89 y old) and investigated whether these differences were also observed in our aging cohort. The serum levels of LEPTIN, IL1-RA, CRP, GM-CSF, and IL-5 were all higher in females regardless of age group (P < 0.05) (Fig. 2). However, the differences for CRP, IL1-RA, and LEPTIN were less pronounced in the older group due to an overall increase in the levels of these proteins in older compared with younger males (P = 0.007, by Fisher’s combined probability). Strikingly, M-pSTAT3 levels were significantly higher in females among young subjects (P = 0.002) but not in the elderly (P = 0.268), where both sexes had similar levels to those found in young females (Fig. 2). This suggested that other cytokines that signal through STAT3 (e.g., IL-6, IL-11, and LIF, among others) were elevated in both males and females in the older cohort. Because IL-6 is one of the hallmark cytokines of aging, we directly compared IL-6 levels in young versus older (without correction for multiple comparisons) and noticed elevated levels in elderly subjects (Fig. S2), consistent with multiple previous reports.
Fig. 2.
Differences in baseline immune parameters between females and males by age group. The individuals were first divided by age group (58 older and 29 young), and the significant differences identified between all females (yellow bars) and males (blue bars) using SAM (seven in total; Fig. 1) were used to investigate differences in expression by age group. With the exception of mono.Unstimulated.STAT3, all significant differences between all males and females identified previously were also observed in both age groups (P < 0.05). However, the differences in LEPTIN, IL-1RA, and CRP were less pronounced in older individuals due to an overall increase in the levels of these proteins in the serum of older males compared with young males (P < 0.05). F, females; M, males. mono.Unstimulated.STAT3, baseline levels of pSTAT3 in isolated monocytes; mod_106, module enriched for Y chromosome genes.
To identify associations between these sex-related features, we generated correlation matrices and conducted unsupervised clustering with or without IL-6. Interestingly, M-pSTAT3 clustered with CRP and GM-CSF (Fig. S3A), or with CRP, IL-6, and GM-CSF when IL-6 was incorporated in the clustering analysis (Fig. S3B), suggesting that the intracellular levels of phosphorylated STAT proteins in monocytes likely represent a functional readout corresponding to an inflammatory environment in vivo.
These results indicate that females have a stronger neutralizing response to influenza vaccination and an increased inflammatory serum profile, which correlates with the baseline levels of phosphorylated STAT3 proteins in peripheral monocytes.
Weaker Vaccine Responses in Males with High Expression of Genes Involved in Lipid Metabolism.
To identify features associated with the observed sex differences in vaccine responsiveness, we focused on the neutralizing activity to H3N2 because the largest differences between males and females were found for this strain. Also, this influenza strain is important in a public health context because it causes the highest rates of morbidity and mortality during the influenza season (21). An individual was considered a responder if they had the standard fourfold or greater change in their post- versus prevaccination microneutralization titer (seroconversion). For H3N2, 33 females and 10 males were responders and 20 females and 24 males were nonresponders. We first searched for possible confounding factors by investigating which features could substantially modify the observed sex effect. In brief, we performed forward stepwise logistic regression with sex as the initial predictor. In stepwise regression, each of the immune features was incorporated into the model iteratively and statistics were computed to account for modifications in the regression coefficient of sex (SI Materials and Methods). The iterations were stopped when the added feature did not modify the regression coefficient of sex to the standard threshold 20%. By this procedure, we identified two possible confounders: a gene module enriched for genes encoding for ribosomal proteins (module 042) (enrichment P < 10−6) and the acute-phase inflammatory marker CRP. Thus, we generated a first model (model 1), which included the variables of sex, module 042, and CRP. The resulting regression coefficient for sex in model 1, after adjusting for confounders, was −2.03 compared with −1.38 (sex variable alone) (Fig. S4).
We then searched for gene expression profiles that could explain the differences in vaccine responsiveness between males and females, namely features having different effects in males or females, while adjusting for confounding variables. To do so, we used the Interact package (SI Materials and Methods), which searches for significant interactions between predictors using permutation methods. A significant interaction (FDR Q < 0.1) was identified for a module enriched for genes participating in lipid biosynthesis (enrichment P < 0.001) (module 052) (Table S1). These genes included LTA4H, encoding for leukotriene A4 hydrolase, which converts leukotriene A4 (LTA4) to active LTB4; MIF (macrophage migration inhibitory factor), which plays a role in the anti-inflammatory effects of glucocorticoids (22); PDSS2 (decaprenyl-diphosphate synthase subunit 2), whose product synthesizes the prenyl side chain of coenzyme Q; and PEX5 (peroxisomal biogenesis factor 5), involved in fatty acid metabolism. The gene regulators derived for module 052 (Fig. S1B) included CLOCK (activator) and FBJ murine osteosarcoma viral oncogene homolog (FOS), Jun B proto-oncogene (JUNB), and Jun D proto-oncogene (JUND) (repressors), among others. Interestingly, the CLOCK gene is involved in the regulation of circadian rhythms, as well as in lipid metabolism (23).
We then generated a second model (model 2), which included the variables of sex and module 052, the interaction term (sex × module 052), and the covariates CRP and module 042. The resulting odds ratio (OR) estimate for vaccine response based on the expression of module 052 in model 2 was 0.39 [confidence interval (CI), 0.18–0.84] for males and 2.25 (CI, 1.08–4.67) for females (Fig. 3A). This indicates that the probability of being a high responder to TIV decreases significantly with an elevated expression of module 052 in males and with decreased expression of module 052 in females. To determine the extent to which module 052 and its interaction with sex contribute to the classification model, we computed a cross-validated area under the curve (cvAUC) for model 1 and model 2. The cvAUC was 0.712 for model 1, and 0.761 for model 2. Furthermore, direct comparison of the two models shows that model 1 is significantly better than model 2 (P = 0.0019, by likelihood ratio test).
Fig. 3.
Odds ratio for vaccine responses in males and females based on expression of module 052. Interaction analysis was conducted for sex and gene expression modules on the serological (microneutralization) responses to TIV (seroconversion to the H3N2 strain). A significant interaction was found between the variables sex and gene module 052. (A) Odds ratio for vaccine response given module 052 in females (red line) and males (blue line). (B) No significant interaction between sex and module 052 is observed for males with low levels of testosterone [Tlo (n = 17), brown line], although a significantly negative effect of module 052 is observed for males with high levels of testosterone [Thi (n = 17), blue line] (adjusted for confounders including age).
These results suggest that the observed sex differences in the neutralizing antibody responses to vaccination could be mediated by the expression of genes involved in lipid metabolism.
Blunted Vaccine Response in Males with High Levels of Testosterone and Elevated Expression of Genes Involved in Lipid Metabolism.
Our results showing that augmented expression of module 052 correlated with weaker vaccine responsiveness in males but not in females suggested that sex hormones could be involved in expression of this gene module. Indeed, results from chemical–gene interaction analysis (http://ctdbase.org) (24) show that expression of a significant fraction of genes in module 052 can be modulated by testosterone (P < 0.005, by hypergeometric test). Thus, we measured free (unbound, bioactive form) testosterone in the sera from the individuals in our study with the hypothesis that, in males, the observed effect of module 052 on vaccine response was dependent on the circulating levels of testosterone. We stratified the male subjects into testosterone high (Thi) or low (Tlo), if they were above or below the median for all of the male subjects (4.06 pg/mL; range, 0.58–24.78 pg/mL), and generated a third model (model 3) for vaccine-neutralizing response, in which male subjects were replaced by Thi (n = 17) and Tlo (n = 17). The median testosterone level in Thi subjects was 9.55 pg/mL (range, 4.25–24.78 pg/mL), and 2.34 pg/mL (range, 0.58–3.89 pg/mL) in Tlo subjects. The median age for Tlo and Thi males was 77 and 24 y, respectively. Thus, model 3 included the interaction terms module 052 × Thi and module 052 × Tlo, and was also adjusted for age, because of the effect of aging on testosterone levels. Strikingly, the interaction between testosterone levels and module 052 was significant only for the Thi group (P < 0.005) and not for Tlo males (P = 0.18), and the corresponding OR estimates for vaccine response, according to module 052, were 0.87 (CI, 0.28–2.69) for Tlo and 0.19 (CI, 0.04–0.80) for Thi males. We also tested testosterone levels as a continuous measure by replacing the interaction terms module 052 × Thi and module 052 × Tlo with module 052 × testosterone; the model was also adjusted by sex and age. Consistent with model 3, the interaction of module 052 and testosterone levels was significant (P = 0.012) (Fig. S5). This indicates that module 052 has a significant effect on vaccine response in males with high levels of testosterone but not in those with lower levels.
Together, these results show that in males with higher levels of testosterone and elevated expression of genes that participate in lipid metabolism, the antibody response to vaccination is severely down-regulated, whereas in those with low levels of testosterone, or in females, the contribution of module 052 is not detrimental and the responses to the vaccine remain intact.
Discussion
In this study, we have used a systems approach to the analysis of sex differences in the immune system in humans. These data reinforce and extend previous reports, and point toward a mechanistic hypothesis that may drive the sex disparities observed in responses to vaccination. Differences in vaccine responsiveness in males versus females have been reported for most commercially available vaccines including yellow fever, influenza, measles, mumps, rubella, and hepatitis, among others (5). As in these studies, we find stronger responses to influenza vaccination and significantly increased serum levels of proinflammatory molecules in females compared with males, specifically LEPTIN (25), IL-RA (26), and CRP (27). In addition, we find differences in GM-CSF and IL-5 and in the baseline pSTAT3 levels in monocytes, which correlate with serum CRP, IL-6, and GM-CSF. Consistent with this, LEPTIN and IL-6 activate STAT3 in monocytes, which results in the secretion of CRP and IL1-RA (28, 29). We also find that these sex differences in monocyte pSTAT3 are observed only in young subjects, possibly due to increased levels of other cytokines signaling through STAT3 in both sexes from the older cohort (e.g., IL-6). Therefore, the level of pSTAT3 in monocytes likely reflects the sum of diverse inflammatory stimuli targeting STAT3. Consistent with these observations, the phosphorylation levels of STAT3 and other STAT proteins have been found to be increased in asthma (30) and in other inflammatory conditions (31).
With respect to the influenza vaccine response, our results indicate that the natural variation in circulating free testosterone could drive many of the differences observed in the response to vaccines. In particular, males with elevated levels of serum testosterone and high expression of genes participating in lipid metabolism were significantly less likely to respond to TIV. These results are in agreement with previous findings showing an immunosuppressive role of testosterone in animals and in vitro (11, 32) with an increase in the synthesis of anti-inflammatory cytokines such as IL-10 (12). Consistently, men with androgen deficiencies have higher levels of inflammatory cytokines than healthy controls (33). However, we did not find an association between the proinflammatory cytokines that are differentially expressed in females and males and the response to vaccination. Rather, our data indicate that other molecules, such as those involved in lipid biosynthesis, are likely affected by testosterone and modulate the antibody response. In particular, our results suggest that testosterone could act by decreasing expression of transcription factors such as FOS, JUNB, and JUND that, in turn, repress the expression of gene module 052 (Fig. S1B). Consistent with this hypothesis, androgen receptor signaling antagonizes NF-κB and represses AP-1 (FOS/JUN), which mediates the production of proinflammatory and antiviral cytokines (34, 35).
A particularly interesting gene found in module 052 is LTA4H, one of the members of the epoxide hydrolase family. The product of this gene catalyzes the conversion of LTA4 (originating from arachidonic acid) to LTB4, a lipid mediator that has both proinflammatory (via surface receptors) and anti-inflammatory (via activation of peroxisome proliferator-activated receptors and decreased NF-κB expression) activities. Furthermore, LTB4 seems to participate in the differentiation of suppressor cells both from the myeloid (36) as well as from the lymphoid (37) compartments. More generally, several studies in both humans and mice have shown the ability of LTB4 precursors, such as omega-3 and -6 fatty acids, to suppress inflammatory responses (38, 39).
Other potentially relevant genes in module 052 are MIF, PDSS2, and PEX5. MIF participates in the synthesis of prostaglandin E2, a lipid compound that originates from arachidonic acid, binds to immune cells (T cells and dendritic cells), and suppresses inflammatory cytokine production (40, 41). A less clear association is observed for PDSS2, an enzyme that mediates isoprenoid biosynthesis and the incorporation of lipids in proteins. Last, PEX5 participates in the biogenesis of peroxisomes, which regulate various metabolic activities including the degradation of very long chain fatty acids. Peroxisomes have also been implicated in the innate immune system, with a significant reduction observed in inflammation, apparently related to the suppressive effect of TNF-α on peroxisome function (42).
Recent studies have also focused on the possibility that genes located on the X and Y chromosomes also affect the response to vaccination. Polymorphisms in genes on the X chromosome that encode for immunological proteins can influence immune responses to vaccines. For example, Toll-like receptor 7, located on the X chromosome, can escape X inactivation, resulting in higher expression in females than in males (43). Y chromosome genes have also been shown to affect sex-dependent susceptibility to autoimmune disease and possibly to other immune functions (44). However, our results indicate that the expression of genes on the Y chromosome might not be involved in the immune response to vaccines, because the sex-related gene module 106 was not associated with the differences in the response to TIV despite the observation that genes regulating important immune functions such as the activation of Akt and PLC proteins by MTCP1 and PLCXD1, respectively, are clustered together with Y chromosome genes in this module.
In conclusion, our results are consistent with a large body of work in animals showing that testosterone is immunosuppressive in vivo and extend this to humans responding to a seasonal influenza vaccine and exhibiting typical variations in testosterone levels. We suggest that testosterone acts directly on immune cells by repressing transcription factors (such as FOS, JUN, and others) implicated in immune activation; these transcription factors would in turn repress the expression of genes involved in lipid metabolism with immunosuppressive activities, creating a negative feedback loop.
From an evolutionary perspective, the immunosuppressive effects of testosterone could be advantageous as a possible homeostatic mechanism to turn off the immune response. For instance, experiments with highly pathogenic viruses reconstructed from isolates from the 1918 influenza pandemic (which killed over 50 million people) show that infection with this strain in animal models results in an uncontrolled, deadly cytokine storm (45). Furthermore, suppression of this inflammatory response in infected mice ameliorates immunopathology and decreases mortality (6, 46). It has also been noted that testosterone treatment of castrated male mice made them less susceptible to LPS-induced shock (32). Because males of many species are more likely to experience trauma than females, this positive effect of testosterone may also help to balance out the consequences of reduced immunity to infection.
In summary, we have identified unique proinflammatory markers that are differentially expressed in females compared with males, as well as genes that participate in lipid metabolism that could be modulated by the levels of free testosterone in normal populations and correlate with the sex-related bias in the responsiveness to influenza vaccination.
Materials and Methods
Subjects, Specimens, and Vaccination Protocol.
With the exception of the neutralizing antibody response to the vaccine and the determination of testosterone measurements from serum, this study used baseline-level data from a previously published work conducted in 91 healthy donors who were enrolled in an influenza vaccine study at the Stanford-Lucile Packard Children's Hospital (LPCH) Vaccine Program during the 2008–2009 influenza season (16). Thus, only a brief description of the methods is included here. The protocol of the study was approved by the Institutional Review Board of the Research Compliance Office at Stanford University. Blood samples were obtained prevaccination and 28 ± 7 d after receiving a single dose of TIV Fluzone (Sanofi Pasteur). Whole blood was used for gene expression analysis as described (16). Peripheral blood mononuclear cells (PBMCs) were obtained by density gradient centrifugation (Ficoll-Paque) and frozen at −80 °C before transferring to liquid nitrogen. Serum was separated by centrifugation of clotted blood and stored at −80 °C before use. Whole blood, PBMCs, or serum from the first visit (baseline, day 0) was processed and used for determination of gene expression, leukocyte subset frequency, signaling responses to stimulation, serum cytokine and chemokine levels, testosterone levels, and CMV and EBV serostatus by ELISA (Calbiotech). Serum samples from day 0 and day ∼28 were used for virus microneutralization titer determination.
Virus Microneutralization Assay.
A standard plaque reduction virus microneutralization assay was performed. In brief, serum samples were heat-inactivated for 30 min at 56 °C, serially diluted (twofold) in virus diluent (DMEM, 1% BSA, antibiotics, and 25 mM Hepes), and mixed with 100 median tissue culture infective doses each of the H1N1, H3N2, and B strains (kind gift of George Kemble, MedImmune). Plates were incubated 1 h at 37 °C and 5% CO2, and 1.5 × 104 exponentially growing Madin–Darby canine kidney-London cells (kind gift of David Lewis, Stanford University) were added in 100 μL of virus diluent. Cell cultures were then incubated overnight at 37 °C and 5% CO2, and washed and fixed with ice-cold acetone for 10 min at room temperature. Fixative was discarded and plates were air-dried. After several washes with washing buffer (PBS, 0.1% Tween-20), wells were incubated for 1 h at room temperature with an anti-influenza A or B nucleoprotein mouse monoclonal antibody (KPL) at 1:4,000 in blocking buffer (PBS, 1% BSA, 0.1% Tween-20). After washing, a secondary antibody (goat anti-mouse IgG, HP-conjugated; KPL) was added at 1:2,000 in blocking buffer and incubated 1 h at room temperature before revealing with HRP substrate. Absorbance (OD) was read at 490 nm.
Whole-Blood Microarray Analysis of Gene Expression.
The procedures for RNA extraction, quantification, hybridization, and scanning were described previously (16). The original microarray probe-level data files can be accessed at the Gene Expression Omnibus repository under accession number GSE41080.
Leukocyte Subset Frequency Determination.
PBMCs were thawed, washed with FACS buffer (PBS supplemented with 2% FBS and 0.1% Na azide), and stained with three separate anti-human antibody mixtures containing (i) anti-CD3 AmCyan, CD4 Pacific Blue, CD8 allophycocyanin (APC) H7, and CD28 APC; (ii) CD3 AmCyan, CD4 Pacific Blue, CD8 APCH7, CD27 PE, and CD45RA PE-Cy5; and (iii) CD3 AmCyan, CD19 Alexa Fluor 700, CD56 PE, CD33 PE-Cy7, and TCR APC, all reagents from BD Biosciences. After incubation, cells were washed several times and data were collected using DIVA software on an LSR II instrument (BD Biosciences) and analyzed using FlowJo 8.8.6 (Tree Star).
Phosphorylation of Intracellular Proteins by Phosphoflow.
Cells were thawed with FACS buffer and stimulated with the indicated cytokines for 15 min in warm media (RPMI with 10% FBS). Cells were washed and fixed with paraformaldehyde and permeabilized with 95% ice-cold methanol. Different stimulus conditions were bar-coded using a 3 × 3 matrix with Pacific Orange and Alexa Fluor 750 (Invitrogen). Cell mixtures were stained with an antibody mixture as described previously (16). Data were collected using DIVA software and analyzed with FlowJo 8.8.6.
Serum Cytokine-Level Determination.
Cytokines were measured on a Luminex system. Fifty-plex kits were purchased from Millipore and used according to the manufacturer’s recommendations with some modifications.
Testosterone-Level Determination.
Serum levels of free testosterone were measured using the Free Testosterone ELISA Kit (Calbiotech) as recommended by the manufacturer.
Statistical Analysis.
Statistical procedures for gene module construction, interaction analysis, and modeling of vaccine responsiveness can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank the Human Immune Monitoring Core staff at Stanford, and the Stanford-LPCH Vaccine Program staff, Sally Mackey MS, Sue Swope RN, Cynthia Walsh RN, Kyrsten Spann, Thu Quan, and Michele Ugur, who enrolled subjects and obtained samples from the participants. This work has been supported by grants from the Ellison Medical Foundation (AG-SS-1788), Howard Hughes Medical Institute, and National Institutes of Health (NIH) (U19s AI057229 and AI090019) (to M.M.D.), and grants for the Stanford CTRU (NIH Contract M01 RR00070). D.F. was supported by a fellowship from the Stanford Center on Longevity. B.P.H. is supported by a grant from EHESP. R.T. is supported by a grant from the Vaccine Research Institute.
Footnotes
The authors declare no conflict of interest.
Data deposition: Probe-level expression data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE41080).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1321060111/-/DCSupplemental.
References
- 1.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8(4):253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knight JC. Genomic modulators of the immune response. Trends Genet. 2013;29(2):74–83. doi: 10.1016/j.tig.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis MM. A prescription for human immunology. Immunity. 2008;29(6):835–838. doi: 10.1016/j.immuni.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein SL. The effects of hormones on sex differences in infection: From genes to behavior. Neurosci Biobehav Rev. 2000;24(6):627–638. doi: 10.1016/s0149-7634(00)00027-0. [DOI] [PubMed] [Google Scholar]
- 5.Klein SL, Poland GA. Personalized vaccinology: One size and dose might not fit both sexes. Vaccine. 2013;31(23):2599–2600. doi: 10.1016/j.vaccine.2013.02.070. [DOI] [PubMed] [Google Scholar]
- 6.Robinson DP, Lorenzo ME, Jian W, Klein SL. Elevated 17β-estradiol protects females from influenza A virus pathogenesis by suppressing inflammatory responses. PLoS Pathog. 2011;7(7):e1002149. doi: 10.1371/journal.ppat.1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su LF, Kidd BA, Han A, Kotzin JJ, Davis MM. Virus-specific CD4(+) memory-phenotype T cells are abundant in unexposed adults. Immunity. 2013;38(2):373–383. doi: 10.1016/j.immuni.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grossman CJ. Interactions between the gonadal steroids and the immune system. Science. 1985;227(4684):257–261. doi: 10.1126/science.3871252. [DOI] [PubMed] [Google Scholar]
- 9.Sakiani S, Olsen NJ, Kovacs WJ. Gonadal steroids and humoral immunity. Nat Rev Endocrinol. 2013;9(1):56–62. doi: 10.1038/nrendo.2012.206. [DOI] [PubMed] [Google Scholar]
- 10.Pennell LM, Galligan CL, Fish EN. Sex affects immunity. J Autoimmun. 2012;38(2-3):J282–J291. doi: 10.1016/j.jaut.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Olsen NJ, Kovacs WJ. Gonadal steroids and immunity. Endocr Rev. 1996;17(4):369–384. doi: 10.1210/edrv-17-4-369. [DOI] [PubMed] [Google Scholar]
- 12.Liva SM, Voskuhl RR. Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. J Immunol. 2001;167(4):2060–2067. doi: 10.4049/jimmunol.167.4.2060. [DOI] [PubMed] [Google Scholar]
- 13.Gaucher D, et al. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J Exp Med. 2008;205(13):3119–3131. doi: 10.1084/jem.20082292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010;10(5):338–349. doi: 10.1016/S1473-3099(10)70049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook IF. Sexual dimorphism of humoral immunity with human vaccines. Vaccine. 2008;26(29-30):3551–3555. doi: 10.1016/j.vaccine.2008.04.054. [DOI] [PubMed] [Google Scholar]
- 16.Furman D, et al. Apoptosis and other immune biomarkers predict influenza vaccine responsiveness. Mol Syst Biol. 2013;9:659. doi: 10.1038/msb.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA. 2001;98(9):5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gómez-Abellán P, et al. Sexual dimorphism in clock genes expression in human adipose tissue. Obes Surg. 2012;22(1):105–112. doi: 10.1007/s11695-011-0539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao R, et al. In utero exposure to second-hand smoke aggravates adult responses to irritants: Adult second-hand smoke. Am J Respir Cell Mol Biol. 2012;47(6):843–851. doi: 10.1165/rcmb.2012-0241OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haslinger C, et al. Microarray gene expression profiling of B-cell chronic lymphocytic leukemia subgroups defined by genomic aberrations and VH mutation status. J Clin Oncol. 2004;22(19):3937–3949. doi: 10.1200/JCO.2004.12.133. [DOI] [PubMed] [Google Scholar]
- 21.Thompson WW, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 22.Al-Abed Y, et al. Thyroxine is a potential endogenous antagonist of macrophage migration inhibitory factor (MIF) activity. Proc Natl Acad Sci USA. 2011;108(20):8224–8227. doi: 10.1073/pnas.1017624108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turek FW, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308(5724):1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis AP, et al. The Comparative Toxicogenomics Database: Update 2013. Nucleic Acids Res. 2013;41(Database issue):D1104–D1114. doi: 10.1093/nar/gks994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hickey MS, et al. Gender differences in serum leptin levels in humans. Biochem Mol Med. 1996;59(1):1–6. doi: 10.1006/bmme.1996.0056. [DOI] [PubMed] [Google Scholar]
- 26.Lynch EA, Dinarello CA, Cannon JG. Gender differences in IL-1 alpha, IL-1 beta, and IL-1 receptor antagonist secretion from mononuclear cells and urinary excretion. J Immunol. 1994;153(1):300–306. [PubMed] [Google Scholar]
- 27.Lakoski SG, et al. Gender and C-reactive protein: Data from the Multiethnic Study of Atherosclerosis (MESA) cohort. Am Heart J. 2006;152(3):593–598. doi: 10.1016/j.ahj.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 28.Zhang D, Sun M, Samols D, Kushner I. STAT3 participates in transcriptional activation of the C-reactive protein gene by interleukin-6. J Biol Chem. 1996;271(16):9503–9509. doi: 10.1074/jbc.271.16.9503. [DOI] [PubMed] [Google Scholar]
- 29.Gabay C, Dreyer M, Pellegrinelli N, Chicheportiche R, Meier CA. Leptin directly induces the secretion of interleukin 1 receptor antagonist in human monocytes. J Clin Endocrinol Metab. 2001;86(2):783–791. doi: 10.1210/jcem.86.2.7245. [DOI] [PubMed] [Google Scholar]
- 30.Yang XO, et al. The signaling suppressor CIS controls proallergic T cell development and allergic airway inflammation. Nat Immunol. 2013;14(7):732–740. doi: 10.1038/ni.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Shea JJ, Holland SM, Staudt LM. JAKs and STATs in immunity, immunodeficiency, and cancer. N Engl J Med. 2013;368(2):161–170. doi: 10.1056/NEJMra1202117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rettew JA, Huet-Hudson YM, Marriott I. Testosterone reduces macrophage expression in the mouse of Toll-like receptor 4, a trigger for inflammation and innate immunity. Biol Reprod. 2008;78(3):432–437. doi: 10.1095/biolreprod.107.063545. [DOI] [PubMed] [Google Scholar]
- 33.Malkin CJ, et al. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89(7):3313–3318. doi: 10.1210/jc.2003-031069. [DOI] [PubMed] [Google Scholar]
- 34.Kallio PJ, Poukka H, Moilanen A, Jänne OA, Palvimo JJ. Androgen receptor-mediated transcriptional regulation in the absence of direct interaction with a specific DNA element. Mol Endocrinol. 1995;9(8):1017–1028. doi: 10.1210/mend.9.8.7476976. [DOI] [PubMed] [Google Scholar]
- 35.McKay LI, Cidlowski JA. Molecular control of immune/inflammatory responses: Interactions between nuclear factor-kappa B and steroid receptor-signaling pathways. Endocr Rev. 1999;20(4):435–459. doi: 10.1210/edrv.20.4.0375. [DOI] [PubMed] [Google Scholar]
- 36.Yokota Y, et al. Absence of LTB4/BLT1 axis facilitates generation of mouse GM-CSF-induced long-lasting antitumor immunologic memory by enhancing innate and adaptive immune systems. Blood. 2012;120(17):3444–3454. doi: 10.1182/blood-2011-10-383240. [DOI] [PubMed] [Google Scholar]
- 37.Juzan M, Hostein I, Gualde N. Role of thymus-eicosanoids in the immune response. Prostaglandins Leukot Essent Fatty Acids. 1992;46(4):247–255. doi: 10.1016/0952-3278(92)90030-m. [DOI] [PubMed] [Google Scholar]
- 38.Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr. 2002;21(6):495–505. doi: 10.1080/07315724.2002.10719248. [DOI] [PubMed] [Google Scholar]
- 39.Kanneganti TD, Dixit VD. Immunological complications of obesity. Nat Immunol. 2012;13(8):707–712. doi: 10.1038/ni.2343. [DOI] [PubMed] [Google Scholar]
- 40.Vassiliou E, Jing H, Ganea D. Prostaglandin E2 inhibits TNF production in murine bone marrow-derived dendritic cells. Cell Immunol. 2003;223(2):120–132. doi: 10.1016/s0008-8749(03)00158-8. [DOI] [PubMed] [Google Scholar]
- 41.Jing H, Vassiliou E, Ganea D. Prostaglandin E2 inhibits production of the inflammatory chemokines CCL3 and CCL4 in dendritic cells. J Leukoc Biol. 2003;74(5):868–879. doi: 10.1189/jlb.0303116. [DOI] [PubMed] [Google Scholar]
- 42.Schrader M, Fahimi HD. Peroxisomes and oxidative stress. Biochim Biophys Acta. 2006;1763(12):1755–1766. doi: 10.1016/j.bbamcr.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 43.Pisitkun P, et al. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312(5780):1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 44.Spach KM, et al. Cutting edge: The Y chromosome controls the age-dependent experimental allergic encephalomyelitis sexual dimorphism in SJL/J mice. J Immunol. 2009;182(4):1789–1793. doi: 10.4049/jimmunol.0803200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cillóniz C, et al. Lethal influenza virus infection in macaques is associated with early dysregulation of inflammatory related genes. PLoS Pathog. 2009;5(10):e1000604. doi: 10.1371/journal.ppat.1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Teijaro JR, et al. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell. 2011;146(6):980–991. doi: 10.1016/j.cell.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.