Abstract
Solar UV (UV)-B-radiation exerts both beneficial and adverse effects on human health. On the one hand, it is the most important environmental risk factor for the development of non-melanoma skin cancer [NMSC; most importantly basal (BCC) and squamous (SCC) cell carcinomas], that represent the most common malignancies in Caucasian populations. On the other hand, the human body’s requirements of vitamin D are mainly achieved by UV-B-induced cutaneous photosynthesis. This dilemma represents a serious problem in many populations, for an association of vitamin D-deficiency and multiple independent diseases including various types of cancer has been convincingly demonstrated. In line with these findings, epidemiologic and laboratory investigations now indicate that vitamin D and its metabolites have a risk reducing effect for NMSC. Potential mechanisms of action include inhibition of the hedgehog signaling pathway (BCC) and modulation of p53-mediated DNA damage response (SCC). As a consequence of these new findings it can be concluded that UV-B-radiation exerts both beneficial and adverse effects on risk and prognosis of NMSC. It can be assumed that many independent factors, including frequency and dose of UV-B exposure, skin area exposed, and individual factors (such as skin type and genetic determinants of the skin`s vitamin D status and of signaling pathways that are involved in the tumorigenesis of NMSC) determine whether UV-B exposure promotes or inhibits tumorigenesis of NMSC. Moreover, these findings may help to explain many of the differential effects of UV-B radiation on risk of NMSC, including variation in the dose-dependent risk for development of SCC in situ (actinic keratosis, AK), invasive SCC, and BCC. In this review, we analyze the relevance of the vitamin D endocrine system (VDES) for tumorigenesis, prevention, and treatment of NMSC and give an overview of present concepts and future perspectives.
Keywords: vitamin D endocrine system (VDES), tumorigenesis, prevention, and treatment of non-melanoma skin cancer (NMSC), vitamin D, solar UV radiation, skin cancer, basal cell carcinoma, squamous cell carcinoma
Introduction
The important function of the skin as the site of photosynthesis of vitamin D3 is well known.1-4 The presence of the vitamin D receptor (VDR) in most cell types of the skin, including keratinocytes, hair follicle cells, melanocytes, fibroblasts and others identifies the human skin as a target of biologically active vitamin D compounds and strongly indicates a major relevance of vitamin D for skin physiology including signaling pathways that are of relevance for tumorigenesis of non-melanoma skin cancer [NMSC; most importantly basal (BCC) and squamous (SCC) cell carcinomas].1–8. It has been demonstrated that 1,25-dihydroxyvitamin D3 [1,25(OH)2D3, calcitriol] the biologically active natural metabolite of vitamin D, has great impact on keratinocyte growth and differentiation and consequently is, together with calcipotriol and other analogs, successfully used for the treatment of the hyperproliferative skin disorder psoriasis.9,10
Solar and artificial UV (UV)-B-radiation (280–320 nm) exerts both beneficial and adverse effects on human health.1-6 On the one hand, UV-B-radiation is the most important environmental risk factor for the development of NMSC, that represent the most common malignancies in Caucasian populations.1-6 On the other hand, the human body`s requirements of vitamin D are mainly achieved by UV-B-induced cutaneous photosynthesis.1-8 This dilemma represents a serious problem in many populations, for an association of vitamin D-deficiency and multiple independent diseases including various types of cancer has been convincingly demonstrated.1-6,11,12 In line with these findings, clinical, epidemiologic, animal and in vitro investigations now indicate that vitamin D and its metabolites have a risk reducing effect for NMSC.5-8 Potential mechanisms of action include inhibition of the hedgehog signaling pathway (BCC) and modulation of p53-mediated DNA damage response (SCC). The functional integrity of the VDES can be affected at different levels. Relevant parameters that can be analyzed include vitamin D status (most importantly 25(OH)D serum concentration), expression and single nucleotide polymorphism (SNP) analysis of VDR, enzymes involved in the VDES (CYP2R1, CYP27A1, CYP27B1, CYP24A1, and vitamin D binding protein (DBP, GC). In this review, we analyze the relevance of the vitamin D endocrine system (VDES) for tumorigenesis, prevention, and treatment of NMSC and give an overview of present concepts and future perspectives.
Epidemiology and Photocarcinogenesis of Basal Cell Carcinoma (BCC) and Cutaneous Squamous Cell Carcinoma (SCC): The Two Most Predominant Types of Non-Melanoma Skin Cancer (NMSC)
Epidemiology of basal cell carcinoma (BCC) and cutaneous squamous cell carcinoma (SCC)
Cutaneous squamous cell carcinoma (SCC) and basal cell carcinoma (BCC) represent the two types of non-melanoma skin cancer (NMSC) with the highest incidence and prevalence rates worldwide.2,5,6,13 While the incidence of skin cancer has dramatically increased during the last decades, it is now accepted that the reasons for this development are multifactoral.2,5,6,13 It has been speculated that besides the age pyramid and other factors, cultural changes that result in increased UV-exposure, may be of particular importance.2,5,6,13 BCCs and SCCs show a locally aggressive and invasive growth pattern, but in comparison with SCCs (metastatic potential in about 5% of all cases), BCCs only very rarely metastasize (0.003–0.1%). Actinic keratoses (AK) are precursors of SCC and are now classified as SCC in situ. Epidemiological studies have convincingly shown that living in parts of the world with increased erythemal UV or high average annual bright sun results in increased risks of SCC and BCC, with the greatest increased risk for SCC.5,14 These investigations are in line with studies of personal exposure, demonstrating that higher levels of occupational and total UV exposure increase the risk for NMSC, with greater correlation for SCC than for BCC. “Intermittent” sun exposure, such as high exposure only at weekends or holidays tends to be associated to some extend with increased risk of BCC.5,14 Sunburn at any age increases the risk of BCCs and SCCs, with greater correlation for BCC than for SCC.5,14,15 The age at which high UV exposures occur may also be of importance, since there is epidemiological evidence showing that the risks of all major skin cancers are reduced by half in people who migrate to a high solar UV environment, like Australia after the age of 10 y, as compared with people who live since birth in a high solar UV environment.5,16,17 Pale skin increases the risk of SCC and BCC. SCCs and BCCs tend to occur in constantly sun-exposed skin areas like the face, ears, neck and back of the hands, with greater association for SCC than for BCC. Standard therapy for both skin cancers is surgical excision. Due to a high percentage of local and systemic recurrence, dermatologists have been looking for chemopreventive and/or chemotherapeutic agents for years. Interestingly, it has been shown that vitamin D compounds have chemopreventive effects at least for BCCs. The combination of retinoids and calctriol has been reported to be effective in the chemotherapy of cutaneous malignancies, including BCCs and SCCs.18
Vitamin D status in BCC and SCC
Vitamin D can be absorbed from the diet or synthesized from 7-dehydrocholesterol (7-DHC) in the skin by the action of sunlight (UV-B).1,3,4 It is metabolized in the liver by CYP2R1 or CYP27A1 to 25-hydroxyvitamin D [25(OH)D] and then in the kidney or in other tissues by CYP27B1 to the biologically active metabolite 1,25-dihydroxyvitamin D [1,25(OH)2D].1,3,4 In the blood, vitamin D metabolites are mostly bound to a specific transport protein, the vitamin D binding protein (VDBP, GC).1,3,4 1,25(OH)2D is metabolized in target cells at least in part by 1,25(OH)2D-24-hydroxylase (CYP24A1), resulting in a specific C-24 oxidation pathway to yield the biliary excretory product calcitroic acid.1,3,4 It is well accepted that the serum 25(OH)D concentration represents the best parameter to determine a person`s vitamin D status.1,3,4 Individual factors that predispose for a person`s vitamin D status, such as skin type and UV exposure, have been identified.1,3,4 Vitamin D deficiency is common in many populations1,3,4 and low serum 25(OH)D concentrations are associated with an increased incidence and an unfavorable outcome of multiple diseases, such as various types of cancer, infectious, cardio-vascular, and autoimmune diseases.1-4 At present, epidemiologic studies do not show a clear relationship between serum 25(OH)D concentration and risk of NMSC.7,8 However, the interpretation of these epidemiologic studies is difficult due to many limitations that include low case numbers and potentially confounding factors such as UV-B radiation. In many of these investigations, that are difficult to compare due to differences in the study design (including use of different parameters to determine vitamin D status, e.g., analysis of 25(OH)D and/or 1,25(OH)2D serum concentrations; use of different assays to measure 25(OH)D serum concentration; different location/latitude of study populations) the positive relationship of UV exposure with both vitamin D status and NMSC risk makes it difficult to interprete the findings. A nested case-control study (Osteoporotic fractures in men, MrOS) in ambulatory elderly men with (n = 178) or without (n = 930) NMSC showed that individuals with the highest baseline serum 25(OH)D concentrations (> 30 ng/ml) had 47% lower odds ratios for NMSC (95% confidence interval, 0.3–0.93; p = 0.026), compared with those with the lowest 25(OH)D concentrations.19 The authors concluded that high 25(OH)D levels may be associated with a reduced risk for NMSC, and that a diagnosis of NMSC does not indicate an adequate vitamin D status. However, some epidemiologic studies do not support the hypothesis that an adequate vitamin D status reduces the risk of NMSC. A prospective investigation evaluated the association between baseline plasma 25(OH)D levels and the risk of incident SCC and BCC among 4,641 women from the Nurses' Health Study (NHS) and the NHS II with 510 incident BCC cases and 75 incident SCC cases.20 In that study, plasma 25(OH)D levels were positively associated with risk of BCC after adjusting for age at blood draw, season of blood draw, lab batch, hair color, burning tendency, the number of sunburns, and UV B flux of residence at blood collection. Women in the highest quartile of 25(OH)D had more than 2-fold increased risk of BCC compared with women in the lowest quartile (OR = 2.07, 95% CI = 1.52–2.80, P for trend < 0.0001). The authors also found a significantly positive association between plasma 25(OH)D levels and SCC risk after adjusting for the same covariates (OR, highest vs. lowest quartile = 3.77, 95% CI = 1.70–8.36, P for trend = 0.0002). In this prospective study of women, plasma 25(OH)D levels were positively associated with NMSC risk. The authors concluded that, considering that most circulating vitamin D is due to sun exposure, the positive association between plasma 25(OH)D and NMSC is confounded by sun exposure and that their data suggest that one-time measurement of plasma vitamin D levels may reasonably reflect long-term sun exposure and predict the risk of NMSC.
Results of a case control study (Kaiser Permanente Northern California population) indicate that higher prediagnostic 25(OH)D levels may be associated with a small increased risk of BCC.21 Prospective cohort studies in women published in 199222 and in men published in 200023 using dietary questionaires found no association between intake of vitamin D and risk of BCC. A prospective investigation analyzing white individuals of a Heath maintenance organization cohort who sought low-bone-density or osteoporosis related advice reported that higher 25(OH)D serum concentrations (> 15 ng/ml) are associated with an increased risk of NMSC, although these findings were statistically not significant.24 Vitamin D-binding protein (VDBP) single nucleotide polymorphisms (SNP) affect 25(OH)D levels and thereby may influence skin carcinogenesis.25 One study tested the association between two functional VDBP SNPs and the susceptibility to (multiple) BCCs.25 Of the 7983 participants, 5790 (72.5%) and 5823 (72.9%) participants were genotyped for rs7041 and rs4588, respectively, and three haplotypes (Gc1s, Gc2 and Gc1f) were analyzed. Two hundred and 33 persons developed a BCC of whom 122 (52.4%) developed multiple BCCs during a mean follow-up of 11.6 y. In that study, the VDBP genotype was not associated with (multiple) BCC development using Cox proportional hazards and Andersen-Gill analyses, respectively. Stratifying age groups demonstrated that in the youngest age-group, the A/T variant of rs7041 was associated with BCC development [adjusted hazard ratio (HR) = 1.88 (95%CI 1.10–3.20)], while homozygote Gc1s carriers had a significantly lower BCC risk [adjusted HR = 0.53 (95%CI 0.31–0.91)].25 The authors concluded that in their study, the VDBP polymorphisms were not associated with susceptibility to (multiple) BCCs, but that age-gene interactions were observed.25
Some pilot studies indicate that patients with basal cell nevus syndrome (BCNS; Golz-Gorlin syndrome) or xeroderma pigmentosum, that are prone to develop BCCs and/or SCCs due to mutations in genes of the hedgehog signaling pathway or in DNA repair genes, and who therefore have to protect themselves consequently from UV radiation have relatively low serum concentrations of 25(OH)D.26,27 In a retrospective cohort study (41 ambulatory patients with BCNS who participated in a 2-y chemoprevention clinical trial vs. 360 population-based controls), 23 patients with BCNS (56%) were vitamin D deficient (defined as a 25[OH]D level of ≤ 20 ng/mL).27 Patients with BCNS had mean 25(OH)D levels below those of the general population (-3 ng/mL; p = 0.02) and were 3 times more likely to be vitamin D deficient (56% vs 18%; p < 0.001). It can be concluded that patients with BCNS are at increased risk for vitamin D deficiency, depending on their adherence to photoprotection.27
Expression of vitamin D receptor (VDR) in BCC and SCC
Effects of 1,25(OH)2D3 on target cells are at least in part mediated via its corresponding intranuclear receptor (VDR), which belongs to the superfamily of transacting transcriptional regulatory factors, including the steroid and thyroid hormone receptors as well as the retinoid-X receptors (RXRs) and retinoic acid receptors (RARs).3,4,28 Different classes of vitamin D response elements (VDRE) have been characterized in target genes that are activated either by VDR-homodimers or by heterodimers of VDR and RXRs.3,4,28 It was demonstrated that ligands of RXRs (e.g., 9-cis retinoic acid) can enhance the transcriptional activity in 1,25(OH)2D3-mediated nuclear signaling pathways. Thus, there are different vitamin D signaling pathways that are determined by the VDR, its dimerization-partner, corresponding ligands, and the nature of the VDRE.28 Increasing evidence now indicates that the VDR protects against the development of NMSC.29 It has been shown that mice lacking the VDR are sensitive to epidermal tumor formation induced by the carcinogen DMBA or following UV radiation (UVR).29-31 The epidermis of VDR null mice shows hyperproliferation of keratinocyte cell layers, and distortion of hair follicles, structures from which these carcinogen-induced skin tumors may develop.29-31
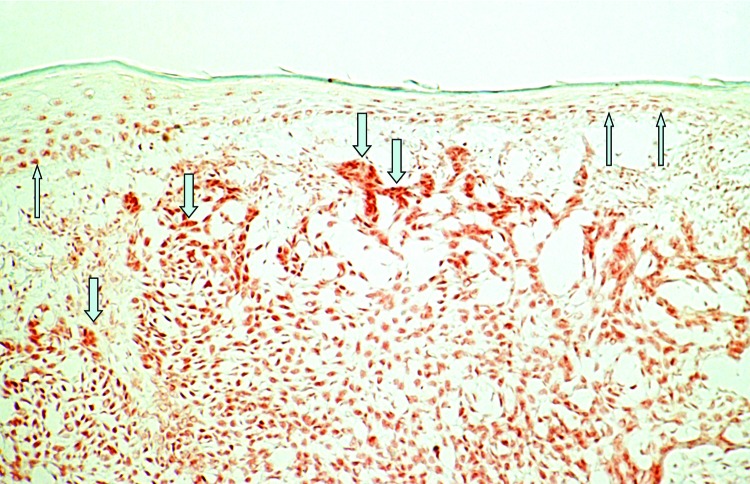
Strong VDR immunoreactivity and mRNA expression has been reported both in BCCs32-34 (Fig. 1) and SCCs.34,35 An in vitro investigation using real-Time RT-PCR technology showed a statistically highly significantly increased ratio of VDR/GAPDH gene expression in BCCs (median: 16.54) and SCCs (median, 37.00) as compared with normal human skin (median, 0.00021 for the BCC study and 0.000006 for the SCC study; both p < 0.005).33-35 Immunohistochemical in vitro investigations demonstrate that almost every tumor cell of BCCs and SCCs reveals nuclear immunoreactivity for VDR.32-35 In these studies, VDR staining intensity was markedly stronger in both skin tumor types as compared with adjacent epidermis or to distant unaffected epidermis of the same sections. In BCCs, VDR immunoreactivity was pronounced in the palisaded array of peripheral tumor cells.32 There was no visual difference comparing VDR staining pattern in the different variants of BCCs (nodular type, superficial type, fibrosing type) or of SCCs (poorly, moderately, well differentiated).32-35 Analyzing immunohistochemically the expression of nuclear VDR cofactors in BCCs, strong staining for RXR-α has been reported while in contrast, RXR-β and RXR-γ were not or only weakly detectable in BCCs.34 Analysis of the VDR heterodimerization partners suggests that selective vitamin D analogs activating exclusively the predominant VDR-RXR-α heterodimer may be most effective in the treatment of BCC with little risk of side effects.
Figure 1. Immunohistochemical analysis of vitamin D receptor (VDR) expression in a basal cell carcinoma (BCC). Please note strong nuclear staining that is increased in tumor cells (↓) as compared with unaffected overlying epidermis (↑) of human skin (labeled streptavidin-biotin technique using mAb 9A7γ).
When the immunohistochemical staining of VDR was analyzed in BCC and SCC for correlation with the proliferation marker Ki-67, no visual correlation of labeling patterns was found.32-35 Heterogeneous Ki-67 immunoreactivity with no visual differences between central and peripheral areas was found in most SCC specimens (11 of 15), although some SCCs revealed pronounced labeling for Ki-67 antigen in peripheral tumor cells (4 of 15). Confocal laser scanning microscopy confirmed these results, showing that double-stained sections for VDR and Ki-67 revealed no visual correlation of labeling patterns in both tumor (Fig. 2). Double-stained sections for VDR and the differentiation markers transglutaminase K or cytokeratin 10 also revealed no visual correlation of labeling patterns. When the immunohistochemical staining of VDR was analyzed for correlation with apoptotic cells, no visual correlation of labeling patterns was found. In that study, all BCC and SCC specimens revealed single scattered terminal UTP nucleotide end-labeled apoptotic cells with considerable variation in their number. Distribution of apoptotic cells within the tumors was heterogeneous. There were markedly fewer apoptotic cells than VDR-positive cells. In summary, comparison of staining patterns revealed no evidence for strong correlation of VDR expression and apoptosis and/or cell proliferation/differentiation in BCC or SCC.32-35
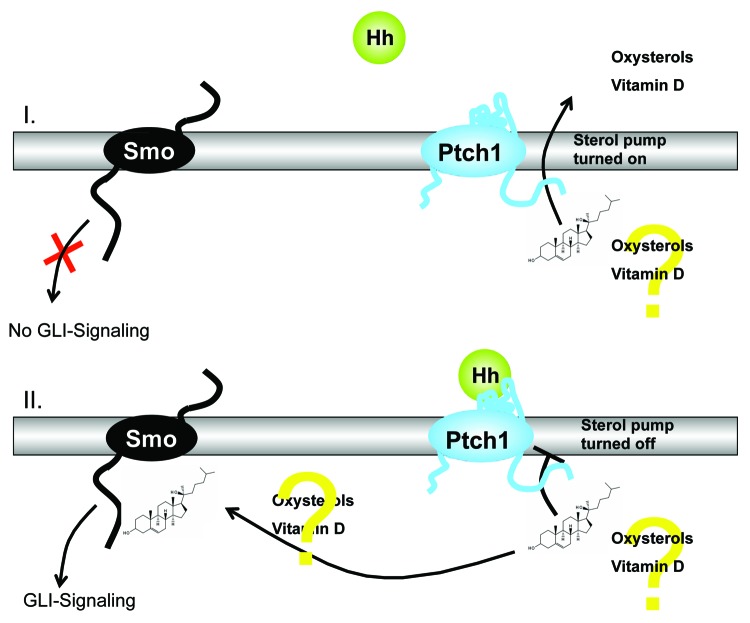
Figure 2. Schematic illustration of the theory suggesting that Ptch regulates Smo by removing oxysterols. Activation of the Hedgehog (Hh)-signaling pathway due to deficiency in the Hh receptor Patched1 (Ptch) is the crucial molecular defect that causes the formation of BCCs in human skin. Ptch1 possesses a sterol sensing domain (SSD), which is important for suppression of the activity of Smoothened (Smo), the signal transduction partner of Ptch. A current theory suggests that Ptch regulates Smo by removing oxysterols from Smo. Ptch acts like a sterol pump and removes oxysterols that have been created by 7-dehydrocholesterol reductase. Upon binding of a Hh protein or a mutation in the SSD of Ptch the pump is turned off allowing oxysterols to accumulate around Smo. This accumulation of sterols allows Smo to become active via GLI signaling or to remain on the cell membrane for a longer period of time.
Vitamin D receptor (VDR) polymorphisms (SNPs) in BCC and SCC
As outlined above, vitamin D deficiency is associated with various types of cancer. Functional polymorphisms (most importantly single nucleotide polymorphisms, SNPs) along the 105 kilobyte VDR gene have important implications for mediating actions of 1,25(OH)2D3.36,37 The VDR gene encompasses two promoter regions, eight protein-coding exons namely 2–9 and six untranslated exons (1a-1f).36,37 Many VDR polymorphisms have been discovered which are located in the promoter, in and around exons 2–9 and in the 3`UTR region.36,37 Most of the VDR polymorphisms, that may represent restriction fragment lengths polymorphisms (RFLP), have an unknown functional effect.36,37 In some cases, it has been indicated that they may be linked to truly functional polymorphisms elsewhere in the VDR gene (or in a nearby gene).36,37 Consequently, it has been shown that VDR polymorphisms are associated with various malignancies, including cancers of the breast, colon, and prostate.36 There is a significant increase in breast cancer risk for carriers of Fok1 ff compared with FF genotype, and a significant decrease of prostate cancer risk for Bsm1 Bb in comparison with bb carriers.36 Little is known about VDR polymorphisms and the occurrence of skin cancer. In a study analyzing SCC, the BB genotype of VDR was significantly associated with increased cancer risk (OR = 1.51).38 Moreover, an interaction between the Bsm1 polymorphism and total vitamin D intake was observed in SCC patients with > 2-fold higher risk seen in women with the BB genotype and high vitamin D intake (OR = 2.38, p interaction = 0.08).38 Another study suggested that the Taq1 polymorphism TT was associated with an increased number of BCCs that developed per year, particulary in combination with skin type I and male sex.39 In 2009, a review and meta-analysis of 67 independent studies on the association between the two most studied VDR polymorphisms (FokI and BsmI) and cancer risk was reported. When comparing FokI ff with FF carriers, a significant increase in skin cancer risk [SOR; 95% confidence intervals (CIs): 1.30; 1.04–1.61].40 Analyzing the BsmI genotypes in Caucasian populations, both Bb and BB carriers had a significant reduced risk of skin cancer. In conclusion, this meta-analysis strongly supports the concept that VDR FokI and BsmI polymorphisms modulate the risk of NMSC. In a pilot study in the German population, we have analyzed the presence of several VDR polymorphisms (Apa1, Taq1, Bgl1) in BCC and cutaneous SCC as compared with healthy controls.41 Variations were observed in the distribution of VDR polymorphisms in the tumor groups compared with the healthy controls (Apa1 Aa genotype: 56.1% in BCC, 51.1% in SCC, 45.1% in healthy controls; Taq1 Tt genotype: 58.6% in BCC, 50.0% in SCC, 48.0% in healthy controls; Bgl1 Bb genotype: 54.5% in BCC, 50.0% in SCC, 43.1%).41 Interestingly, there were associations indicating that Apa1 and Taq1 genotypes may be of importance for photocarcinogenesis of BCCs, but not of SCCs.41 These data indicate that VDR polymorphisms are of importance for the tumorigenesis of BCC and SCC, and may contribute to the differential role of UV-B radiation for the development of these malignancies.
Expression of CYP27A1, CYP27B1 and CYP24A1 in BCC and SCC
The formation of 1,25(OH)2D3, the biologically most active natural ligand of the VDR, is mediated through several main enzymes that facilitate hydroxylations of vitamin D at position 25 in the liver (CYP2R1, CYP27A1) and of resulting 25(OH)D3 at position 1 in the kidneys (CYP27B1).3,5,6 These hydroxylases belong to a class of cytochrome P450 mixed function monooxidases. Extrarenal activity of CYP27B1 has been reported in various cell types, including macrophages, keratinocytes, as well as prostate and colon cancer cells.3,5,6 It has been found that modulation of these enzymes influences the proliferation and differentiation status of 1,25(OH)2D3-sensitive cells, benign or neoplastic.3,5,6 Using array-competitive genomic hybridization (CGH), amplification of CYP24A1 was detected as a likely target oncogene of the amplification unit 20q13.2 in breast cancer cell lines and tumors.42 It has been speculated that increased expression of CYP24A1 due to gene amplification may abrogate vitamin D3-mediated growth control. Moreover, amplification of the CYP27B1 gene has been demonstrated in glioblastomas.43
In in vitro investigations, real-time PCR analysis showed mRNA ratios of CYP27B1/GAPDH (median: 0.739 in BCCs and 2.02 in SSCs) and CYP24A1/GAPDH (median, 0.0058 in BCCs and 0.382 in SCCs) gene expression in BCCs and SCCs, significantly increased as compared with normal human skin (median, 0.0008; 0.0000004, respectively).33-35 The ratio of CYP27A1/GAPDH was only in SCCs significantly increased to normal skin (median SCC, 33.00 vs NS, 0.00004; p < 0.02), whereas in BCCs ratio of CYP27A1/GAPDH were not significantly altered (median BCCs, 0.17 vs NS, 0.0166; p = 0.62).33-35
It is not known whether increased expression of the CYP27A1, CYP27B1 or CYP24A1 mRNA in NMSC is a result of gene amplification or of other mechanisms such as transcriptional regulation.33-35 If the increased expression of CYP27B1 results in an increased production of biologically active 1,25(OH)2D3, the accumulation of this metabolite should inhibit growth, invasion and metastasis of NMSC.33-35 It can be speculated whether increased expression of CYP27A1 and CYP27B1 in NMSC may represent a physiological feed-back loop coupled to the increased proliferative activity in these tumors. However, one has to keep in mind that 1,25(OH)2D3 may be rapidly metabolized via CYP24A1, whose expression is increased in BCCs and SCCs as well.33-35 It is not known whether increased expression of CYP27A1, CYP27B1 and CYP24A1 genes results in increased or reduced levels of 1,25(OH)2D3 in NMSC.33-35 Therefore, the question of cellular and systemic consequences of the increased expression of CYP27A1, CYP27B1 and CYP24A1 in skin tumors remains to be clarified. Nevertheless, it can be speculated whether precursors of biologically active 1,25(OH)2D3 or inhibitors of CYP24A1 may be of benefit for the prevention or treatment of BCCs and SCCs.33-35
Carcinogenesis of Basal Cell Carcinoma (BCC) and Cutaneous Squamous Cell Carcinoma (SCC): Convincing Evidence for Suppression of Skin Carcinogenesis by the Vitamin D Endocrine System
In mouse skin, the abnormal activation of two interacting pathways critical for epidermal and hair follicle function, β-catenin and hedgehog (Hh), leads to epidermal tumors.29-31 The canonical hedgehog signaling pathway represents a key regulator of development in humans and in animals and is present in every bilaterian.5,6,30 The pathway is called after its polypeptide ligand Hedgehog (Hh), an intercellular signaling molecule discovered in fruit flies of the genus Drosophila.5,6,30 Hh is one of Drosophila's segment polarity gene products, involved in creating the molecular and structural basis of the fly body plan.5,6,30 The molecule remains important during many stages of embryogenesis and metamorphosis. Sonic hedgehog (SHH) is the best investigated ligand of the vertebrate pathway.5,6,30 It is now accepted that activation of the Hh-signaling pathway due to deficiency in the Hh receptor Patched1 (Ptch) is the crucial molecular defect that causes the formation of BCCs in human skin.5,6,30 Ptch1 possesses a sterol sensing domain (SSD), which has been shown to be important for suppression of the activity of Smoothened (Smo), the signal transduction partner of Ptch.5,6,30 A current theory suggests that Ptch regulates Smo by removing oxysterols from Smo. Ptch acts like a sterol pump and removes oxysterols that have been created by 7-dehydrocholesterol reductase.5,6,30 Upon binding of a Hh protein or a mutation in the SSD of Ptch the pump is turned off allowing oxysterols to accumulate around Smo.5,6,30 This accumulation of sterols allows Smo to become active via GLI signaling or to stay on the cell membrane for a longer period of time.5,6,30 This hypothesis is supported by the presence of a number of small molecule agonists and antagonists of the pathway that act on Smo.5,6,30 The binding of SHH relieves Smo inhibition, leading to activation of the GLI transcription factors: the activators Gli1 and Gli2 and the repressor Gli3.5,6,30 It has been shown that all elements of the Hh signaling pathway are elevated in the epidermis and utricles of VDR null mice, and that 1,25(OH)2D3 blocks expression of these elements in normal mouse skin.29 In addition the transcriptional activity of β-catenin is increased in keratinocytes lacking the VDR.31 Using primary cultured human keratinocytes, it was demonstrated that 1,25(OH)2D3 suppresses cyclin D1 and Gli1 which are regulated by β-catenin/TCF signaling and have a critical role in epidermal carcinogenesis.31 Blockage of VDR by siRNA resulted in hyperproliferation of keratinocytes, and increased expression of cyclin D1 and Gli1.31 Moreover, it was demonstrated that 1,25(OH)2D3/VDR directly regulates transcriptional activity of β-catenin/TCF signaling using the -catenin reporter TopGlow.31 Using K14 driven tamoxifen-induced cre recombinase to delete both VDR and β-catenin in keratinocytes of mice following the first hair follicle cycle, it was found that ablation of epidermal specific β-catenin cannot rescue VDR null mice from UVB-induced skin tumor formation.31 Moreover, convincing evidence indicates the Ptch-dependent secretion of a vitamin D3-related compound, which acts as an endogenous inhibitor of Hh signaling by blocking the activity of Smo, the signal transduction partner of Ptch.30 It has been suggested that this substance is lacking in Ptch-deficient tumor cells, which in turn may result in activation of Hh-signaling.30 It has been demonstrated that the application of 1,25(OH)2D3 inhibits proliferation and growth of BCC in Ptch mutant mice in vitro and in vivo.30 This effect is associated with activation of VDR and induction of BCC differentiation.30 In addition, it was shown that 1,25(OH)2D3 inhibits Hh signaling at the level of Smo in a VDR-independent manner.30 The concomitant antiproliferative effects of 1,25(OH)2D3 on BCC growth were shown to be stronger than those of the Hh-specific inhibitor cyclopamine, even though the latter more efficiently inhibits Hh signaling.30 In conclusion, there is convincing evidence that exogenous supply of 1,25(OH)2D3 controls the activity of 3 independent pathways, Hh, β-catenin/TCF and VDR signaling, which are relevant for tumorigenesis and treatment of BCC. These data strongly support the concept that vitamin D compounds may represent promising options for prevention and treatment of BCC and that the VDR acts as a tumor suppressor in skin.
For SCC development, UV-induced DNA damage is the most important environmental risk factor.5,6 UV-R often causes gene mutations that may lead to cellular transformation and malignancy.5,6,44-48 DNA damage also initiates and promotes mechanisms that suppress immune surveillance responsible for detecting and eliminating transformed cells.5,6,49,50 UV exposure causes different types of DNA lesions that are produced either photochemically and directly or indirectly by UV activation of various photoreceptors that are able to alter the cellular redox equilibrium, thereby generating reactive oxygen species (ROS).5 ROS induced by UV radiation are able to cause oxidative damage to DNA, and lipid peroxidation.5 Moreover, it has been shown that excess levels of nitric oxide (NO) are induced by UV-mediated upregulation of nitric oxide synthase,5,51-53 and also by UV-A (320–400 nm) mediated decomposition of NO stores in nitrosothiols and nitrite.5,54,55 Pathophysiologically elevated levels of NO and ROS have been demonstrated to combine to form genotoxic NO derivatives such as peroxynitrite that cause oxidative and nitrosative modifications to the sugar-phosphate scaffold and bases of DNA.5
It is generally acknowledged that mutagenic effects of UV radiation represent a hallmark in the carcinogenesis of SCC, and that promutagenic pyrimidine dimers are the major forms of DNA damage produced directly by UV.5,6,56 The predominant type of pyrimidine dimer detected after UV exposure in human skin is the thymine-thymine dimer, a cys-syn cyclobutane pyrimidine dimer (CPD), while thymine-cytosine, cytosine-cytosine bipyrimidines, and 6–4 photoproducts are less common.5,6,57-60 CPDs are generated by the perturbation of the 5–6 double bonds in two adjacent pyrimidines, followed by abnormal covalent binding that connects the 2 pyrimidines by a stable ring configuration forming a bipyrimidine product.5,6,61,62 It is well accepted that CPD production requires the wavelengths of UV-B (290–320 nm).5,6 However there is some evidence for generation of thymine dimer by UV-A wavelengths below 330 nm.5,6,60,63-66 Wavelengths of UV-A are less energetic but considerably more abundant (20-fold higher) than UV-B in sunlight, and can penetrate to deeper skin levels.5,67-69 The shorter, more highly energetic UV-C wavelengths below 290 nm can also induce CPDs.5 However, they are completely absorbed by the stratosphere and are therefore not present at the earth`s surface.5 They may only become hazardous in the future if stratospheric ozone levels should be depleted.5 CPDs can also be produced chemically in isolated DNA, probably be via a triplet energy transfer mechanism.5,70,71 This mechanism may explain the production of CPDs in skin cells by a nitric oxide donor in the absence of UV,5,72 and a decrease in UV-induced CPDs in skin cells treated with inhibitors of nitric oxide synthase.5,73
Another important promutagenic photolesion detected in human skin is 8-hydroxy-2’deoxyguanosine, which is produced indirectly by oxidation of the base guanine by peroxynitrite.5,74,75 Peroxynitrite is also able to cause nitrosative damage to form 8-nitroguanine,5,76-79 which is converted to a promutagenic abasic site within a few hours. DNA strand breaks have also been shown to be induced by nitrosation of primary amines by another NO derivative, nitrous anhydride.5,80
Photolesions resistant to DNA repair are able to cause deleterious gene mutations that form either by deletion, base mispairing, or substitutions during DNA replication when adenine is inserted as the default base.5 Mutations that affect cellular function can promote skin carcinogenesis.5,6 Sequence alterations such as C to T transitions are associated with bipyrimidine sites, and correlate with mutations found in the p53 tumor suppressor gene in various types of tumors including skin cancers and their precursors.5,6,81-84 G to T transversions are associated with 8-hydroxy-2’deoxyguanosine5,85 and occur in isolated DNA exposed to peroxynitrite.5,86
UV-Induced DNA Damage Response: Modulation by Vitamin D Signaling
In order to protect genome integrity, cells respond to DNA damage by inducing signal transduction pathways that cause cell cycle arrest before the affected cells can replicate.5 This enables either DNA-repair or the elimination of severely damaged cells by apoptosis.5,6,87,88
Apoptosis, representing a mode of programmed cell death, is induced following UV-B-irradiation when cellular damage is too severe to be repaired.5,6,89-93 It has convincingly been shown that the biologically active vitamin D metabolite 1,25(OH)2D protects human skin cells from UV-induced cell death and apoptosis.5,6,89-93 In these studies, cytoprotective effects of 1,25(OH)2D on UV-B-irradiated keratinocytes were seen morphologically and using a colorimetric cell survival assay.5,6,89-93 Moreover, using an ELISA that detects DNA-fragmentation, it was shown that pretreatment with 1,25(OH)2D suppresses UV-B-induced apoptosis by 55–70%.5,6,89-93 This suppression requires pharmacological concentrations of 1,25(OH)2D and a preincubation period of several hours.5,6,89-93 In addition, it was demonstrated that pretreatment with 1,25(OH)2D also inhibits mitochondrial cytochrome C release, a hallmark event of UV-B-induced apoptosis.5,6,89-93
Furthermore, it was demonstrated that 1,25(OH)2D reduces two important mediators of the UV-response, namely, c-Jun-NH2-terminal kinase (JNK) activation and interleukin-6 (IL-6) production.5,6,89-93 As shown by western blotting, pretreatment of keratinocytes with 1,25(OH)2D [REMOVED INCLUDEPICTURE FIELD]diminishes UV-B-stimulated JNK activation by more than 30%. Furthermore, 1,25(OH)2D treatment [REMOVED INCLUDEPICTURE FIELD]reduces the UV-B-induced IL-6 mRNA expression and protein secretion by 75–90%. Analyzing the cleavage of PARP further supported these observations. Pretreatment of keratinocytes with 1,25(OH)2D inhibits efficiently, but not completely, this UV-B-induced PARP-cleavage.5,6,89-93
Metallothionein (MT)-induction may be relevant for the photoprotective effects of 1,25(OH)2D. MT acts as a radical scavenger in oxygen-mediated UV-B-injury.5,6,89-93 MTs are a class of small cysteine-rich proteins that bind and exchange heavy metal ions but also have clear scavenging properties for ROS.5,6,89-93 Part of the UVB-induced damage to cells occurs through the formation of ROS and antioxidative agents such as MT have been demonstrated to be photoprotective.5,6,89-93 In these studies, MT mRNA expression was shown to be clearly induced by 1,25(OH)2D.6
The anti-apoptotic effect of 1,25(OH)2D in keratinocytes was confirmed, using cisplatin and doxorubicin as apoptotic triggers.6,89-93 In that study, it was demonstrated that 1,25(OH)2D activated two independent survival pathways in keratinocytes: the MEK/extracellular signal regulated kinase (ERK) and the phosphatidylinositol 3-kinase (PI-3K)/Akt pathway.6,89-93 Activation of ERK and Akt by 1,25(OH)2D was transient, required a minimal dose of 10−9 mol/L and could be blocked by actinomycin D and cycloheximide.6 Moreover, inhibition of Akt or ERK activity with a PI-3K inhibitor (LY294002) or MEK inhibitors (PD98059, UO126) respectively, partially or totally suppressed the anti-apoptotic capacity of 1,25(OH)2D.6 Finally, 1,25(OH)2D modulates the expression of different apoptosis regulators belonging to the Bcl-2 family.6 It has been shown that 1,25(OH)2D treatment increases levels of the anti-apoptotic protein Bcl-2 and decreases levels of the pro-apoptotic proteins Bax and Bad in a time- and dose-dependent way.6,89-93 The authors of these investigations concluded that 1,25(OH)2D protects keratinocytes against apoptosis by activating the MEK/ERK and the PI-3K/Akt survival pathways and by increasing the Bcl-2 to Bax and Bad ratio.6,89-93
Moreover, it has been demonstrated that 1,25(OH)2D protects primary human keratinocytes against the UV-B-induced generation of CPDs.5,6,89-95 In some studies, this protection required pharmacologic doses of 1,25(OH)2D and an incubation period of at least 8 h before UV-B-irradiation.5,6 CPDs are primarily eleminated by the nucleotide excision repair (NER) pathway that has a relatively long half-life of 7–12 h.5,6,94-97 Individuals with the inherited disorder xeroderma pigmentosum bear a defect in one of the key enzymes of NER pathway and are highly prone to UV-induced skin carcinogenesis.5,6,98,99 Oxidative DNA damage is repaired by the more rapid alternate base excision repair (BER) pathway.5,6,100-104 However, the repair enzyme human 8-oxoguanine-DNA glycolase 1 is less abundant in the basal layers than the upper layers of the human epidermis, which indicates that repair of oxidative damage in the dividing keratinocytes of the epidermis is less efficient.5,104
The tumor suppressor protein p53, representing a key regulator of the DNA damage response as mentioned above, is activated by DNA damage.5,6,66,75,105,106 Physiological doses of UV-A and UV-B can induce inactivating mutations in the p53 gene. Mutations in the tumor suppressor p53 in engineered human skin were found to be predominantly UV-A finger print mutations induced by oxidative damage located in the basal layer of the epidermis.5,75 A positive association between mutations in the tumor suppressor p53 gene in UV-damaged cells in mouse and human skin before skin tumors appear, provides evidence for involvement in skin carcinogenesis.5,75 Activation of p53 is achieved by post-translational phosphorylations and acetylations on multiple sites.5 These modifications enhance p53 accumulation by inhibiting degradation by negative regulators including MDM2, and/or by increasing its transcription.5 These mechanisms result in nuclear accumulation of p53 that reaches maximum levels 12 h after UV radiation.5,107 p53 regulates the transcription of multiple genes that control cell growth,5,93 nucleotide excision repair5,6,108,109 and base excision repair5,102 pathways, as well as pro- or anti-apoptotic pathways.5,6,110 p53 mediates the gene transcription of GADD45, that assists DNA repair by binding to DNA, increasing accessibility to repair enzymes.5,111 DNA repair has been shown to be blocked in cells transfected with a dominant negative p53 construct,5,112 and early onset tumor formation is increased in homozygote p53 knockout mice.5,113 The gene for the DNA strand sensor protein kinase (ATM) acts by phosphorylating p53 at serine 15 and is inactivated in patients with the genetic disorder ataxia telangiectasia.5 These patients suffer from genome instability, immunodeficiency and cancer.5,114-116 Inactivation of the p53 phosphorylation site at serine 392 represents a mutational hotspot of the p53 gene, resulting from UV-induced DNA damage.5 A knock-in mutation in mice that blocks the phosphorylation of serine 398 (the murine equivalent of human serine 392) has been demonstrated to promote photocarcinogenesis in mice.5,117 UV-R has been shown to increase p53 expression in human skin cells and concurrent treatment with 1,25(OH)2D further enhanced this effect several fold, at 3 h and 6 h after UV-R.5,92 Combined with previously reported lower nitrite levels in the presence of 1,25(OH)2D, it has been speculated that this increased p53 expression may favor DNA repair over apoptosis.5,92,93 Additionally, it has convincingly been shown that topical application of 1,25(OH)2D or its analog QW suppressed solar simulated UV (SSUVR)-induced pyrimidine dimers in the epidermis of irradiated hairless Skh:HR1 mice, measured 24 h after irradiation.5,92,93 Furthermore, UV-induced immunosuppression in the mice can be markedly reduced by topical application of either 1,25(OH)2D or QW.5,92,93 Taken these data together, a protective effect of vitamin D compounds against UV-B-induced photodamage was convincingly shown in vitro and in vivo.5,6 It is tempting to speculate that the UV-B-induced cutaneous production of vitamin D may represent an evolutionary highly conserved feed-back mechanism that protects the skin from the hazardous effects of solar UV-radiation.6
UV-Induced Immune Suppression
Cutaneous immune responses that would normally detect and prevent the development of tumors in skin are suppressed by low doses of UV-R.5 This was demonstrated by the progressive growth of tumors transplanted into irradiated mice, while the tumors were rejected in unirradiated mice.5,118 Pyrimidine dimers are important mediators of photoimmune suppression.5,49,50 This was first demonstrated in the opossum where pyrimidine dimers are normally repaired in the presence of visible light by photolyase, an endogenous photoreactivating enzyme, which is present in most living organisms, but lost in mammals.5 A reduction in pyrimidine dimers correlated with a reduction in photoimmune suppression in the opossum after treatment with visible light immediately after UV irradiation.5,49 Photommune suppression was also reduced in irradiated mice after reduction of CPDs by application of encapsulated T4 endonuclease, the specific repair enzyme for pyrimidine dimers.5,50
There are other mediators of photoimmune suppression which include the release of pro-inflammatory cytokines that inhibit the antigen presenting function of Langerhan’s cells, resulting in decreased T cell differentiation and activation and the suppression of T-cell-mediated responses.5,119,120 Cis-urocanic acid formed by UV isomerisation of the photoreceptor trans-urocanic acid located in the outermost layers of skin also inhibits the antigen propensity of Langerhan’s cells.5,121,122 Depletion of Langerhan’s cells in the skin by DNA damage and oxidative stress reduces their antigen-presenting propensity.5,123 Free radicals generated by UV contribute to immune suppression by releasing platelet-activating factor (PAF) from epidermal cells.5 The peroxidation of lipids by peroxynitrite and PAF is implicated in prostaglandin and cytokine production and release, which in turn modulate regulatory T cells (Tregs) suppressing immune responses at distant sites.5,6,119,124 Tregs are involved in immune homeostasis by maintaining the balance between immunosuppression and autoimmunity, and reside in skin as well as skin draining lymph nodes5,120 and therefore could also be subjected to DNA damage and oxidative stress.
Antioxidant treatment has been shown to abolish immune suppression mediated by the lipid peroxidation pathway in irradiated mice.5,125 UV activation of Src, located on the inner surface of the keratinocyte plasma membrane, triggers signaling cascades that activate the transcription factors AP-1 and NF-κB that regulate immune regulatory cytokines, which is also blocked by antioxidant treatment.5,119,126,127 Activation of an alternate complement pathway has also been implicated in inflammatory and immune modulating activities.5,128,129
Both UVB and UVA components of sunlight are immunosuppressive in mice and humans,5,6,130-133 while certain wavelengths of UVA have been shown to have a protective effect against UVB-induced immunosuppression in mice.5,134 However there is some conflicting data from studies in mice and humans regarding the particular wavelengths of UVA and their immunomodulatory effects.5,133-135
Vitamin D compounds exert potent effects on the immune system and modulate the UV-induced immune response.5,6 The cytokine IL-6 represents an important mediator of the sunburn reaction, of UV-B-dependent immune suppression, and has been implicated in the tumorigenesis of BCC.5,6,89-93 UV-B-irradiation strongly induces IL-6 mRNA and release of IL-6 protein by human keratinocytes.5,6,89-93 In cultured human keratinocytes, 1,25(OH)2D treatment [REMOVED INCLUDEPICTURE FIELD]reduces the UV-B-induced IL-6 mRNA expression and protein secretion by 75–90%.6 Moreover, vitamin D compounds exert potent effects on the UV-induced immune response via many other mechanisms that include modulation of expression and function of regulatory T-cells.3,6
Summary
The data presented in this review provide convincing evidence that the VDES is of high importance for prevention (Tables 1 and 2) and treatment of NMSC. Besides a sufficient vitamin D status, the molecular basis that underlies these preventive effects of vitamin D compounds is the expression and functional integrity of the VDR and other key components of the VDES. While some of the complex interactions of the VDES with other signaling pathways that contribute to skin carcinogenesis (e.g., hedgehog signaling) have been identified, future laboratory investigations will address unanswered questions and will increase our knowledge about the impact of the VDES on skin carcinogenesis. Most importantly, additional well-designed observational and interventional studies are needed to define the efficacy and safety of vitamin D compounds in the prevention and treatment of NMSC.
Table 1. The role of the VDES for BCC prevention.
| BCC risk | evidence | |||
|---|---|---|---|---|
| convincing | presumable | possible | insufficient | |
| 25(OH)D serum concentration | ||||
| Clinical studies | ↓ (results difficult to interprete due to UV radiation as confounder) | |||
| Animal studies | ↓ | |||
| In vitro investigations | ↓ (via regulation of hedgehog signaling) | |||
| VDR polymorphisms (SNPs) | ||||
| Clinical studies | ↓ or ↑ | |||
| Animal studies | Ø (lack of data) | |||
| In vitro investigations | Ø (lack of data) | |||
| Polymorphisms (SNPs) in other VDES-related genes (CYP27A1, CYP27B1, CYP24A1, CYP2R1, GC) | ||||
| Clinical studies | ↓ or ↑ (GC) | Ø (lack of data) | ||
| Animal studies | Ø (lack of data) | |||
| In vitro investigations | Ø (lack of data) | |||
↓ Reduction of BCC risk resulting from substitution or treatment with vitamin D compounds (e.g., in interventional studies), from increasing 25(OH)D-serum concentration, from association with SNPs (in observational studies), or from in vitro investigations; ↑ Increase of BCC risk resulting from substitution or treatment with vitamin D compounds (e.g., in interventional studies), from increasing 25(OH)D-serum concentration, or from association with SNPs (in observational studies); ○ no association; Ø insufficient evidence.
Table 2. The role of the VDES for SCC prevention.
| SCC risk | evidence | |||
|---|---|---|---|---|
| convincing | presumable | possible | insufficient | |
| 25(OH)D serum concentration | ||||
| Clinical studies | ↓ (results difficult to interprete due to UV radiation as confounder) | |||
| Animal studies | ↓ | |||
| In vitro investigation | ↓ (via regulation of apoptosis, reduction of CPDs following UVR) | |||
| VDR polymorphisms (SNPs) | ||||
| Clinical studies | ↓ or ↑ | |||
| Animal studies | Ø (lack of data) | |||
| In vitro investigations | Ø (lack of data) | |||
| Polymorphisms (SNPs) in other VDES-related genes (CYP27A1, CYP27B1, CYP24A1, CYP2R1, GC) | ||||
| Clinical studies | Ø (lack of data) | |||
| Animal studies | Ø (lack of data) | |||
| In vitro investigations | Ø (lack of data) | |||
↓ Reduction of SCC risk resulting from substitution or treatment with vitamin D compounds (e.g., in interventional studies), from increasing 25(OH)D-serum concentration (in observational studies), from association with SNPs (in observational studies), or from in vitro investigations; ↑ Increase of SCC risk resulting from substitution or treatment with vitamin D compounds (e.g., in interventional studies), from increasing 25(OH)D-serum concentration (in observational studies), from association with SNPs (in observational studies), or from in vitro investigations; ○ no association; Ø insufficient evidence.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/dermatoendocrinology/article/24156
References
- 1.Holick MF. The photobiology of vitamin D and its consequences for humans. Ann N Y Acad Sci. 1985;453:1–13. doi: 10.1111/j.1749-6632.1985.tb11793.x. [DOI] [PubMed] [Google Scholar]
- 2.Reichrath J. The challenge resulting from positive and negative effects of sunlight: how much solar UV exposure is appropriate to balance between risks of vitamin D deficiency and skin cancer? Prog Biophys Mol Biol. 2006;92:9–16. doi: 10.1016/j.pbiomolbio.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 4.Lehmann B, Querings K, Reichrath J. Vitamin D and skin: new aspects for dermatology. Exp Dermatol. 2004;13(Suppl 4):11–5. doi: 10.1111/j.1600-0625.2004.00257.x. [DOI] [PubMed] [Google Scholar]
- 5.Mason RS, Reichrath J. Sunlight Vitamin D and Skin Cancer. Anticancer Agents Med Chem. 2013;13:83–97. doi: 10.2174/187152013804487272. [DOI] [PubMed] [Google Scholar]
- 6.Reichrath J, Reichrath S. Hope and challenge: the importance of ultraviolet (UV) radiation for cutaneous vitamin D synthesis and skin cancer. Scand J Clin Lab Invest Suppl. 2012;243:112–9. doi: 10.3109/00365513.2012.682876. [DOI] [PubMed] [Google Scholar]
- 7.Tang JY, Fu T, Lau C, Oh DH, Bikle DD, Asgari MM. Vitamin D in cutaneous carcinogenesis Part I. J Am Acad Dermatol. 2012;67(5)803.e1-803.e12. 10.1016/j.jaad.2012.05.044 [DOI] [PMC free article] [PubMed]
- 8.Tang JY, Fu T, Lau C, Oh DH, Bikle DD, Asgari MM. Vitamin D in cutaneous carcinogenesis Part II. J Am Acad Dermatol. 2012;67(5)817.e1-817.e11. 10.1016/j.jaad.2012.07.022 [DOI] [PMC free article] [PubMed]
- 9.Holick MF, Reichrath J. Clinical Utility of 1,25-Dihydroxyvitamin D3 and its Analogues for the Treatment of Psoriasis. In: M.F. Holick (editor). Vitamin D: Physiology, Molecular Biologic and Clinical Aspects, 2nd Edition. The Humana Press Inc., Totowa, New York, 2010, 1043-1060. [Google Scholar]
- 10.Reichrath J, Müller SM, Kerber A, Baum HP, Bahmer FA. Biologic effects of topical calcipotriol (MC 903) treatment in psoriatic skin. J Am Acad Dermatol. 1997;36:19–28. doi: 10.1016/S0190-9622(97)70320-7. [DOI] [PubMed] [Google Scholar]
- 11.Grant WB, Cross HS, Garland CF, Gorham ED, Moan J, Peterlik M, et al. Estimated benefit of increased vitamin D status in reducing the economic burden of disease in western Europe. Prog Biophys Mol Biol. 2009;99:104–13. doi: 10.1016/j.pbiomolbio.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Grant WB. Solar ultraviolet irradiance and cancer incidence and mortality. Adv Exp Med Biol. 2008;624:16–30. doi: 10.1007/978-0-387-77574-6_2. [DOI] [PubMed] [Google Scholar]
- 13.Rass K, Reichrath J. UV damage and DNA repair in malignant melanoma and nonmelanoma skin cancer. Adv Exp Med Biol. 2008;624:162–78. doi: 10.1007/978-0-387-77574-6_13. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8–18. doi: 10.1016/S1011-1344(01)00198-1. [DOI] [PubMed] [Google Scholar]
- 15.Cust AE, Jenkins MA, Goumas C, Armstrong BK, Schmid H, Aitken JF, et al. Early-life sun exposure and risk of melanoma before age 40 years. Cancer Causes Control. 2011;22:885–97. doi: 10.1007/s10552-011-9762-3. [DOI] [PubMed] [Google Scholar]
- 16.English DR, Armstrong BK, Kricker A, Winter MG, Heenan PJ, Randell PL. Demographic characteristics, pigmentary and cutaneous risk factors for squamous cell carcinoma of the skin: a case-control study. Int J Cancer. 1998;76:628–34. doi: 10.1002/(SICI)1097-0215(19980529)76:5<628::AID-IJC3>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 17.Holman CD, Armstrong BK. Cutaneous malignant melanoma and indicators of total accumulated exposure to the sun: an analysis separating histogenetic types. J Natl Cancer Inst. 1984;73:75–82. [PubMed] [Google Scholar]
- 18.Majewski S, Skopinska M, Bollag W, Jablonska S. Combination of isotretinoin and calcitriol for precancerous and cancerous skin lesions. Lancet. 1994;344:1510–1. doi: 10.1016/S0140-6736(94)90330-1. [DOI] [PubMed] [Google Scholar]
- 19.Tang JY, Parimi N, Wu A, Boscardin WJ, Shikany JM, Chren MM, et al. Osteoporotic Fractures in Men (MrOS) Study Group Inverse association between serum 25(OH) vitamin D levels and non-melanoma skin cancer in elderly men. Cancer Causes Control. 2010;21:387–91. doi: 10.1007/s10552-009-9470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang G, Nan H, Qureshi AA, Han J. Pre-diagnostic plasma 25-hydroxyvitamin D levels and risk of non-melanoma skin cancer in women. PLoS One. 2012;7:e35211. doi: 10.1371/journal.pone.0035211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asgari MM, Tang J, Warton ME, Chren MM, Quesenberry CP, Jr., Bikle D, et al. Association of prediagnostic serum vitamin D levels with the development of basal cell carcinoma. J Invest Dermatol. 2010;130:1438–43. doi: 10.1038/jid.2009.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunter DJ, Colditz GA, Stampfer MJ, Rosner B, Willett WC, Speizer FE. Diet and risk of basal cell carcinoma of the skin in a prospective cohort of women. Ann Epidemiol. 1992;2:231–9. doi: 10.1016/1047-2797(92)90055-U. [DOI] [PubMed] [Google Scholar]
- 23.van Dam RM, Huang Z, Giovannucci E, Rimm EB, Hunter DJ, Colditz GA, et al. Diet and basal cell carcinoma of the skin in a prospective cohort of men. Am J Clin Nutr. 2000;71:135–41. doi: 10.1093/ajcn/71.1.135. [DOI] [PubMed] [Google Scholar]
- 24.Eide MJ, Johnson DA, Jacobsen GR, Krajenta RJ, Rao DS, Lim HW, et al. Vitamin D and nonmelanoma skin cancer in a health maintenance organization cohort. Arch Dermatol. 2011;147:1379–84. doi: 10.1001/archdermatol.2011.231. [DOI] [PubMed] [Google Scholar]
- 25.Flohil SC, de Vries E, van Meurs JB, Fang Y, Stricker BH, Uitterlinden AG, et al. Vitamin D-binding protein polymorphisms are not associated with development of (multiple) basal cell carcinomas. J Invest Dermatol. 2010;130:1438–43. doi: 10.1038/jid.2009.402. [DOI] [Google Scholar]
- 26.Querings K, Reichrath J. A plea for the analysis of Vitamin-D levels in patients under photoprotection, including patients with xeroderma pigmentosum (XP) and basal cell nevus syndrome (BCNS) Cancer Causes Control. 2004;15:219. doi: 10.1023/B:CACO.0000019571.83095.97. [DOI] [PubMed] [Google Scholar]
- 27.Tang JY, Wu A, Linos E, Parimi N, Lee W, Aszterbaum M, et al. High prevalence of vitamin D deficiency in patients with basal cell nevus syndrome. Arch Dermatol. 2010;146:1105–10. doi: 10.1001/archdermatol.2010.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reichrath J. Will analogs of 1,25-dihydroxyvitamin D(3) (calcitriol) open a new era in cancer therapy? Onkologie. 2001;24:128–33. doi: 10.1159/000050299. [DOI] [PubMed] [Google Scholar]
- 29.Bikle DD. The vitamin D receptor: a tumor suppressor in skin. Discov Med. 2011;11:7–17. [PMC free article] [PubMed] [Google Scholar]
- 30.Uhmann A, Niemann H, Lammering B, Henkel C, Hess I, Nitzki F, et al. Antitumoral effects of calcitriol in basal cell carcinomas involve inhibition of hedgehog signaling and induction of vitamin D receptor signaling and differentiation. Mol Cancer Ther. 2011;10:2179–88. doi: 10.1158/1535-7163.MCT-11-0422. [DOI] [PubMed] [Google Scholar]
- 31.Jiang YJ, Teichert AE, Fong F, Oda Y, Bikle DD. 1α,25(OH)(2)-Dihydroxyvitamin D(3)/VDR protects the skin from UVB-induced tumor formation by interacting with the β-catenin pathway. J Steroid Biochem Mol Biol. 2012 Sep 28. pii S0960-0760(12)00190-2. 10.1016/j.jsbmb.2012.09.024 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 32.Reichrath J, Kamradt J, Zhu XH, Kong XF, Tilgen W, Holick MF. Analysis of 1,25-dihydroxyvitamin D(3) receptors (VDR) in basal cell carcinomas. Am J Pathol. 1999;155:583–9. doi: 10.1016/S0002-9440(10)65153-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitschele T, Diesel B, Friedrich M, Meineke V, Maas RM, Gärtner BC, et al. Analysis of the vitamin D system in basal cell carcinomas (BCCs) Lab Invest. 2004;84:693–702. doi: 10.1038/labinvest.3700096. [DOI] [PubMed] [Google Scholar]
- 34.Kamradt J, Rafi L, Mitschele T, Meineke V, Gärtner BC, Wolfgang T, et al. Analysis of the vitamin D system in cutaneous malignancies. Recent Results Cancer Res. 2003;164:259–69. doi: 10.1007/978-3-642-55580-0_19. [DOI] [PubMed] [Google Scholar]
- 35.Reichrath J, Rafi L, Rech M, Mitschele T, Meineke V, Gärtner BC, et al. Analysis of the vitamin D system in cutaneous squamous cell carcinomas. J Cutan Pathol. 2004;31:224–31. doi: 10.1111/j.0303-6987.2003.00183.x. [DOI] [PubMed] [Google Scholar]
- 36.Köstner K, Denzer N, Müller CSL, Klein R, Tilgen W, Reichrath J. The relevance of vitamin D receptor (VDR) gene polymorphisms for cancer: a review of the literature. Anticancer Res. 2009;29:3511–36. [PubMed] [Google Scholar]
- 37.Denzer N, Vogt Th, Reichrath J. Vitamin D receptor (VDR) polymorphisms and skin cancer: A systematic review. Dermatoendocrinol. 2011;3:205–10. doi: 10.4161/derm.3.3.16519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han J, Colditz GA, Hunter DJ. Polymorphisms in the MTHFR and VDR genes and skin cancer risk. Carcinogenesis. 2007;28:390–7. doi: 10.1093/carcin/bgl156. [DOI] [PubMed] [Google Scholar]
- 39.Ramachandran S, Fryer AA, Lovatt TJ, Smith AG, Lear JT, Jones PW, et al. Combined effects of gender, skin type and polymorphic genes on clinical phenotype: use of rate of increase in numbers of basal cell carcinomas as a model system. Cancer Lett. 2003;189:175–81. doi: 10.1016/S0304-3835(02)00516-5. [DOI] [PubMed] [Google Scholar]
- 40.Raimondi S, Johansson H, Maisonneuve P, Gandini S. Review and meta-analysis on vitamin D receptor polymorphisms and cancer risk. Carcinogenesis. 2009;30:1170–80. doi: 10.1093/carcin/bgp103. [DOI] [PubMed] [Google Scholar]
- 41.Köstner K, Denzer N, Koreng M, Reichrath S, Gräber S, Klein R, et al. Association of genetic variants of the vitamin D receptor (VDR) with cutaneous squamous cell carcinomas (SCC) and basal cell carcinomas (BCC): a pilot study in a German population. Anticancer Res. 2012;32:327–33. [PubMed] [Google Scholar]
- 42.Albertson DG, Ylstra B, Segraves R, Collins C, Dairkee SH, Kowbel D, et al. Quantitative mapping of amplicon structure by array CGH identifies CYP24 as a candidate oncogene. Nat Genet. 2000;25:144–6. doi: 10.1038/75985. [DOI] [PubMed] [Google Scholar]
- 43.Maas RM, Reus K, Diesel B, Steudel WI, Feiden W, Fischer U, et al. Amplification and expression of splice variants of the gene encoding the P450 cytochrome 25-hydroxyvitamin D(3) 1,alpha-hydroxylase (CYP 27B1) in human malignant glioma. Clin Cancer Res. 2001;7:868–75. [PubMed] [Google Scholar]
- 44.Hart RW, Setlow RB, Woodhead AD. Evidence that pyrimidine dimers in DNA can give rise to tumors. Proc Natl Acad Sci U S A. 1977;74:5574–8. doi: 10.1073/pnas.74.12.5574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sutherland BM, Blackett AD, Feng NI, Freeman SE, Ogut ES, Gange RW, et al. Photoreactivation and other ultraviolet/visible light effects on DNA in human skin. Ann N Y Acad Sci. 1985;453:73–9. doi: 10.1111/j.1749-6632.1985.tb11799.x. [DOI] [PubMed] [Google Scholar]
- 46.Brash DE, Rudolph JA, Simon JA, Lin A, McKenna GJ, Baden HP, et al. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci U S A. 1991;88:10124–8. doi: 10.1073/pnas.88.22.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agar NS, Halliday GM, Barnetson RS, Ananthaswamy HN, Wheeler M, Jones AM. The basal layer in human squamous tumors harbors more UVA than UVB fingerprint mutations: a role for UVA in human skin carcinogenesis. Proc Natl Acad Sci U S A. 2004;101:4954–9. doi: 10.1073/pnas.0401141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Besaratinia A, Kim SI, Pfeifer GP. Rapid repair of UVA-induced oxidized purines and persistence of UVB-induced dipyrimidine lesions determine the mutagenicity of sunlight in mouse cells. FASEB J. 2008;22:2379–92. doi: 10.1096/fj.07-105437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Applegate LA, Ley RD, Alcalay J, Kripke ML. Identification of the molecular target for the suppression of contact hypersensitivity by ultraviolet radiation. J Exp Med. 1989;170:1117–31. doi: 10.1084/jem.170.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kripke ML, Cox PA, Alas LG, Yarosh DB. Pyrimidine dimers in DNA initiate systemic immunosuppression in UV-irradiated mice. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(16):7516-20 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deliconstantinos G, Villiotou V, Stravrides JC. Release by ultraviolet B (u.v.B) radiation of nitric oxide (NO) from human keratinocytes: a potential role for nitric oxide in erythema production. Br J Pharmacol. 1995;114:1257–65. doi: 10.1111/j.1476-5381.1995.tb13341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bruch-Gerharz D, Ruzicka T, Kolb-Bachofen V. Nitric oxide in human skin: current status and future prospects. J Invest Dermatol. 1998;110:1–7. doi: 10.1046/j.1523-1747.1998.00084.x. [DOI] [PubMed] [Google Scholar]
- 53.Cals-Grierson MM, Ormerod AD. Nitric oxide function in the skin. Nitric Oxide. 2004;10:179–93. doi: 10.1016/j.niox.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 54.Paunel A, Dejam A, Thelen S, Kirsch M, Horstjann M, Gharini P, et al. UVA induces immediate and enzyme-independent nitric oxide formation in healthy human skin leading to NO-specific signalling. J Invest Dermatol. 2005;125:A3–3. [Google Scholar]
- 55.Mowbray M, McLintock S, Weerakoon R, Lomatschinsky N, Jones S, Rossi AG, et al. Enzyme-independent NO stores in human skin: quantification and influence of UV radiation. J Invest Dermatol. 2009;129:834–42. doi: 10.1038/jid.2008.296. [DOI] [PubMed] [Google Scholar]
- 56.Wikonkal NM, Brash DE. Ultraviolet radiation induced signature mutations in photocarcinogenesis. J Investig Dermatol Symp Proc. 1999;4:6–10. doi: 10.1038/sj.jidsp.5640173. [DOI] [PubMed] [Google Scholar]
- 57.Douki T, Court M, Sauvaigo S, Odin F, Cadet J. Formation of the main UV-induced thymine dimeric lesions within isolated and cellular DNA as measured by high performance liquid chromatography-tandem mass spectrometry. J Biol Chem. 2000;275:11678–85. doi: 10.1074/jbc.275.16.11678. [DOI] [PubMed] [Google Scholar]
- 58.Cooke MS, Podmore ID, Mistry N, Evans MD, Herbert KE, Griffiths HR, et al. Immunochemical detection of UV-induced DNA damage and repair. J Immunol Methods. 2003;280:125–33. doi: 10.1016/S0022-1759(03)00269-2. [DOI] [PubMed] [Google Scholar]
- 59.Courdavault S, Baudouin C, Charveron M, Favier A, Cadet J, Douki T. Larger yield of cyclobutane dimers than 8-oxo-7,8-dihydroguanine in the DNA of UVA-irradiated human skin cells. Mutat Res. 2004;556:135–42. doi: 10.1016/j.mrfmmm.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 60.Mouret S, Baudouin C, Charveron M, Favier A, Cadet J, Douki T. Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation. Proc Natl Acad Sci U S A. 2006;103:13765–70. doi: 10.1073/pnas.0604213103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ravanat JL, Douki T, Cadet J. Direct and indirect effects of UV radiation on DNA and its components. J Photochem Photobiol B. 2001;63:88–102. doi: 10.1016/S1011-1344(01)00206-8. [DOI] [PubMed] [Google Scholar]
- 62.Pattison DI, Davies MJ. Actions of ultraviolet light on cellular structures. EXS. 2006;96:131–57. doi: 10.1007/3-7643-7378-4_6. [DOI] [PubMed] [Google Scholar]
- 63.Applegate LA, Scaletta C, Panizzon R, Niggli H, Frenk E. In vivo induction of pyrimidine dimers in human skin by UVA radiation: initiation of cell damage and/or intercellular communication? Int J Mol Med. 1999;3:467–72. doi: 10.3892/ijmm.3.5.467. [DOI] [PubMed] [Google Scholar]
- 64.Rochette PJ, Therrien J-P, Drouin R, Perdiz D, Bastien N, Drobetsky EA, et al. UVA-induced cyclobutane pyrimidine dimers form predominantly at thymine-thymine dipyrimidines and correlate with the mutation spectrum in rodent cells. Nucleic Acids Res. 2003;31:2786–94. doi: 10.1093/nar/gkg402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Courdavault S, Baudouin C, Sauvaigo S, Mouret S, Candéias S, Charveron M, et al. Unrepaired cyclobutane pyrimidine dimers do not prevent proliferation of UV-B-irradiated cultured human fibroblasts. Photochem Photobiol. 2004;79:145–51. doi: 10.1562/0031-8655(2004)079<0145:UCPDDN>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 66.Jiang W, Ananthaswamy HN, Muller HK, Kripke ML. p53 protects against skin cancer induction by UV-B radiation. Oncogene. 1999;18:4247–53. doi: 10.1038/sj.onc.1202789. [DOI] [PubMed] [Google Scholar]
- 67.Kochevar IE, Pathak MA, Parrish JA. Photophysics, Photochemistry and Photobiology. In Fitzpatrick's Dermatology in General Medicine, Fifth Edition ed.; Freedberg, I. M., Eisen,A, Wolff K, Austen, K.F., Goldsmith, L, Katz S and Fitzpatrick, T.B., Ed. McGraw-Hill: New York, 1999; Vol. 1, pp 220-230. [Google Scholar]
- 68.Nishigori C, Yarosh DB, Ullrich SE, Vink AA, Bucana CD, Roza L, et al. Evidence that DNA damage triggers interleukin 10 cytokine production in UV-irradiated murine keratinocytes. Proc Natl Acad Sci U S A. 1996;93:10354–9. doi: 10.1073/pnas.93.19.10354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sequiera V. D. K., Mason RS, Resisting the sun with vitamin D. In special edition Newly recognized actions of vitamin D3. Immunology, Endocrine & Metabolic Agents in Medicinal Chemistry 2009;129-136. [Google Scholar]
- 70.Lamola AA. Production of pyrimidine dimers in DNA in the dark. Biochem Biophys Res Commun. 1971;43:893–8. doi: 10.1016/0006-291X(71)90701-7. [DOI] [PubMed] [Google Scholar]
- 71.Lhiaubet-Vallet V, Cuquerella MC, Castell JV, Bosca F, Miranda MA. Triplet excited fluoroquinolones as mediators for thymine cyclobutane dimer formation in DNA. J Phys Chem B. 2007;111:7409–14. doi: 10.1021/jp070167f. [DOI] [PubMed] [Google Scholar]
- 72.Mason RS, Sequeira VB, Dixon KM, Gordon-Thomson C, Pobre K, Dilley A, et al. Photoprotection by 1alpha,25-dihydroxyvitamin D and analogs: further studies on mechanisms and implications for UV-damage. J Steroid Biochem Mol Biol. 2010;121:164–8. doi: 10.1016/j.jsbmb.2010.03.082. [DOI] [PubMed] [Google Scholar]
- 73.Gupta R, Dixon KM, Deo SS, Holliday CJ, Slater M, Halliday GM, et al. Photoprotection by 1,25 dihydroxyvitamin D3 is associated with an increase in p53 and a decrease in nitric oxide products. J Invest Dermatol. 2007;127:707–15. doi: 10.1038/sj.jid.5700597. [DOI] [PubMed] [Google Scholar]
- 74.Kvam E, Tyrrell RM. Induction of oxidative DNA base damage in human skin cells by UV and near visible radiation. Carcinogenesis. 1997;18:2379–84. doi: 10.1093/carcin/18.12.2379. [DOI] [PubMed] [Google Scholar]
- 75.Agar NS, Halliday GM, Barnetson RS, Ananthaswamy HN, Wheeler M, Jones AM. The basal layer in human squamous tumors harbors more UVA than UVB fingerprint mutations: a role for UVA in human skin carcinogenesis. Proc Natl Acad Sci U S A. 2004;101:4954–9. doi: 10.1073/pnas.0401141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McAndrew J, Patel RP, Jo HJ, Cornwell T, Lincoln T, Moellering D, et al. The interplay of nitric oxide and peroxynitrite with signal transduction pathways: implications for disease. Semin Perinatol. 1997;21:351–66. doi: 10.1016/S0146-0005(97)80002-X. [DOI] [PubMed] [Google Scholar]
- 77.Burney S, Caulfield JL, Niles JC, Wishnok JS, Tannenbaum SR. The chemistry of DNA damage from nitric oxide and peroxynitrite. Mutat Res. 1999;424:37–49. doi: 10.1016/S0027-5107(99)00006-8. [DOI] [PubMed] [Google Scholar]
- 78.Niles JC, Wishnok JS, Tannenbaum SR. Peroxynitrite-induced oxidation and nitration products of guanine and 8-oxoguanine: structures and mechanisms of product formation. Nitric oxide. Biol Chem. 2006;14:109–21. doi: 10.1016/j.niox.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 79.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wink DA, Kasprzak KS, Maragos CM, Elespuru RK, Misra M, Dunams TM, et al. DNA deaminating ability and genotoxicity of nitric oxide and its progenitors. Science. 1991;254:1001–3. doi: 10.1126/science.1948068. [DOI] [PubMed] [Google Scholar]
- 81.Nataraj AJ, Trent JC, 2nd, Ananthaswamy HN. p53 gene mutations and photocarcinogenesis. Photochem Photobiol. 1995;62:218–30. doi: 10.1111/j.1751-1097.1995.tb05262.x. [DOI] [PubMed] [Google Scholar]
- 82.Berg RJ, van Kranen HJ, Rebel HG, de Vries A, van Vloten WA, Van Kreijl CF, et al. Early p53 alterations in mouse skin carcinogenesis by UVB radiation: immunohistochemical detection of mutant p53 protein in clusters of preneoplastic epidermal cells. Proc Natl Acad Sci U S A. 1996;93:274–8. doi: 10.1073/pnas.93.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brash DE, Ziegler A, Jonason AS, Simon JA, Kunala S, Leffell DJ. Sunlight and sunburn in human skin cancer: p53, apoptosis, and tumor promotion. J Investig Dermatol Symp Proc. 1996;1:136–42. [PubMed] [Google Scholar]
- 84.Soehnge H, Ouhtit A, Ananthaswamy ON. Mechanisms of induction of skin cancer by UV radiation. Front Biosci. 1997;2:d538–51. doi: 10.2741/a211. [DOI] [PubMed] [Google Scholar]
- 85.Besaratinia A, Synold TW, Xi B, Pfeifer GP. G-to-T transversions and small tandem base deletions are the hallmark of mutations induced by ultraviolet a radiation in mammalian cells. Biochemistry. 2004;43:8169–77. doi: 10.1021/bi049761v. [DOI] [PubMed] [Google Scholar]
- 86.Murata M, Kawanishi S. Oxidative DNA damage induced by nitrotyrosine, a biomarker of inflammation. Biochem Biophys Res Commun. 2004;316:123–8. doi: 10.1016/j.bbrc.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 87.Ouhtit A, Gorny A, Muller HK, Hill LL, Owen-Schaub L, Ananthaswamy HN. Loss of Fas-ligand expression in mouse keratinocytes during UV carcinogenesis. Am J Pathol. 2000;157:1975–81. doi: 10.1016/S0002-9440(10)64836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Matsumura Y, Ananthaswamy HN. Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol. 2004;195:298–308. doi: 10.1016/j.taap.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 89.De Haes P, Garmyn M, Degreef H, Vantieghem K, Bouillon R, Segaert S. 1,25-Dihydroxyvitamin D3 inhibits ultraviolet B-induced apoptosis, Jun kinase activation, and interleukin-6 production in primary human keratinocytes. J Cell Biochem. 2003;89:663–73. doi: 10.1002/jcb.10540. [DOI] [PubMed] [Google Scholar]
- 90.De Haes P, Garmyn M, Verstuyf A, De Clercq P, Vandewalle M, Vantieghem K, et al. Two 14-epi analogues of 1,25-dihydroxyvitamin D3 protect human keratinocytes against the effects of UVB. Arch Dermatol Res. 2004;295:527–34. doi: 10.1007/s00403-004-0451-x. [DOI] [PubMed] [Google Scholar]
- 91.De Haes P, Garmyn M, Carmeliet G, Degreef H, Vantieghem K, Bouillon R, et al. Molecular pathways involved in the anti-apoptotic effect of 1,25-dihydroxyvitamin D3 in primary human keratinocytes. J Cell Biochem. 2004;93:951–67. doi: 10.1002/jcb.20227. [DOI] [PubMed] [Google Scholar]
- 92.De Haes P, Garmyn M, Verstuyf A, De Clercq P, Vandewalle M, Degreef H, et al. 1,25-Dihydroxyvitamin D3 and analogues protect primary human keratinocytes against UVB-induced DNA damage. J Photochem Photobiol B. 2005;78:141–8. doi: 10.1016/j.jphotobiol.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 93.Trémezaygues L, Sticherling M, Pföhler C, Friedrich M, Meineke V, Seifert M, et al. Cutaneous photosynthesis of vitamin D: an evolutionary highly-conserved endocrine system that protects against environmental hazards including UV-radiation and microbial infections. Anticancer Res. 2006;26(4A):2743–8. [PubMed] [Google Scholar]
- 94.Mitchell DL, Cleaver JE, Epstein JH. Repair of pyrimidine(6-4)pyrimidone photoproducts in mouse skin. J Invest Dermatol. 1990;95:55–9. doi: 10.1111/1523-1747.ep12873312. [DOI] [PubMed] [Google Scholar]
- 95.Katiyar SK. Kinetics of UV light-induced cyclobutane pyrimidine dimers in human skin in vivo: an immunohistochemical analysis of both epidermis and dermis. Clin Cancer Res. 2000;6:3864–9. doi: 10.1562/0031-8655(2000)072<0788:koulic>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 96.Matsumura Y, Ananthaswamy HN. Short-term and long-term cellular and molecular events following UV irradiation of skin: implications for molecular medicine. Expert Rev Mol Med. 2002;4:1–22. doi: 10.1017/S146239940200532X. [DOI] [PubMed] [Google Scholar]
- 97.Schul W, Jans J, Rijksen YM, Klemann KH, Eker AP, de Wit J, et al. Enhanced repair of cyclobutane pyrimidine dimers and improved UV resistance in photolyase transgenic mice. EMBO J. 2002;21:4719–29. doi: 10.1093/emboj/cdf456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cleaver JE. Defective repair replication of DNA in xeroderma pigmentosum. Nature. 1968;218:652–6. doi: 10.1038/218652a0. [DOI] [PubMed] [Google Scholar]
- 99.McGregor JM, Hawk JLM. Acute Effects of Ultraviolet Radiation on the Skin. In Fitzpatrick's Dermatology in General Medicine, Fifth ed ed.; Freedberg, I. M., Eisen,A, Wolff K, Austen, K.F., Goldsmith, L, Katz S and Fitzpatrick, T.B., Ed. McGraw- Hill: 1999; Vol. 1, pp 1555-1561. [Google Scholar]
- 100.Mitra S, Boldogh I, Izumi T, Hazra TK. Complexities of the DNA base excision repair pathway for repair of oxidative DNA damage. Environ Mol Mutagen. 2001;38:180–90. doi: 10.1002/em.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nilsen H, Krokan HE. Base excision repair in a network of defence and tolerance. Carcinogenesis. 2001;22:987–98. doi: 10.1093/carcin/22.7.987. [DOI] [PubMed] [Google Scholar]
- 102.Zurer I, Hofseth LJ, Cohen Y, Xu-Welliver M, Hussain SP, Harris CC, et al. The role of p53 in base excision repair following genotoxic stress. Carcinogenesis. 2004;25:11–9. doi: 10.1093/carcin/bgg186. [DOI] [PubMed] [Google Scholar]
- 103.David SS, O’Shea VL, Kundu S. Base-excision repair of oxidative DNA damage. Nature. 2007;447:941–50. doi: 10.1038/nature05978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Javeri A, Huang XX, Bernerd F, Mason RS, Halliday GM. Human 8-oxoguanine-DNA glycosylase 1 protein and gene are expressed more abundantly in the superficial than basal layer of human epidermis. DNA Repair (Amst) 2008;7:1542–50. doi: 10.1016/j.dnarep.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 105.Hall PA, McKee PH, Menage HD, Dover R, Lane DP. High levels of p53 protein in UV-irradiated normal human skin. Oncogene. 1993;8:203–7. [PubMed] [Google Scholar]
- 106.Eller MS, Maeda T, Magnoni C, Atwal D, Gilchrest BA. Enhancement of DNA repair in human skin cells by thymidine dinucleotides: evidence for a p53-mediated mammalian SOS response. Proc Natl Acad Sci U S A. 1997;94:12627–32. doi: 10.1073/pnas.94.23.12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Saito S, Yamaguchi H, Higashimoto Y, Chao C, Xu Y, Fornace AJ, Jr., et al. Phosphorylation site interdependence of human p53 post-translational modifications in response to stress. J Biol Chem. 2003;278:37536–44. doi: 10.1074/jbc.M305135200. [DOI] [PubMed] [Google Scholar]
- 108.Zhu Q, Wani MA, El-Mahdy M, Wani AA. Decreased DNA repair efficiency by loss or disruption of p53 function preferentially affects removal of cyclobutane pyrimidine dimers from non-transcribed strand and slow repair sites in transcribed strand. J Biol Chem. 2000;275:11492–7. doi: 10.1074/jbc.275.15.11492. [DOI] [PubMed] [Google Scholar]
- 109.Amundson SA, Patterson A, Do KT, Fornace AJ., Jr. A nucleotide excision repair master-switch: p53 regulated coordinate induction of global genomic repair genes. Cancer Biol Ther. 2002;1:145–9. doi: 10.4161/cbt.59. [DOI] [PubMed] [Google Scholar]
- 110.Müllauer L, Gruber P, Sebinger D, Buch J, Wohlfart S, Chott A. Mutations in apoptosis genes: a pathogenetic factor for human disease. Mutat Res. 2001;488:211–31. doi: 10.1016/S1383-5742(01)00057-6. [DOI] [PubMed] [Google Scholar]
- 111.Carrier F, Georgel PT, Pourquier P, Blake M, Kontny HU, Antinore MJ, et al. Gadd45, a p53-responsive stress protein, modifies DNA accessibility on damaged chromatin. Mol Cell Biol. 1999;19:1673–85. doi: 10.1128/mcb.19.3.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McKay BC, Francis MA, Rainbow AJ. Wildtype p53 is required for heat shock and ultraviolet light enhanced repair of a UV-damaged reporter gene. Carcinogenesis. 1997;18:245–9. doi: 10.1093/carcin/18.2.245. [DOI] [PubMed] [Google Scholar]
- 113.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr., Butel JS, et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–21. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 114.Siliciano JD, Canman CE, Taya Y, Sakaguchi K, Appella E, Kastan MB. DNA damage induces phosphorylation of the amino terminus of p53. Genes Dev. 1997;11:3471–81. doi: 10.1101/gad.11.24.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Banin S, Moyal L, Shieh S, Taya Y, Anderson CW, Chessa L, et al. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;281:1674–7. doi: 10.1126/science.281.5383.1674. [DOI] [PubMed] [Google Scholar]
- 116.Maclaine NJ, Hupp TR. The regulation of p53 by phosphorylation: a model for how distinct signals integrate into the p53 pathway. Aging (Albany NY) 2009;1:490–502. doi: 10.18632/aging.100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bruins W, Zwart E, Attardi LD, Iwakuma T, Hoogervorst EM, Beems RB, et al. Increased sensitivity to UV radiation in mice with a p53 point mutation at Ser389. Mol Cell Biol. 2004;24:8884–94. doi: 10.1128/MCB.24.20.8884-8894.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kripke ML. Antigenicity of murine skin tumors induced by ultraviolet light. J Natl Cancer Inst. 1974;53:1333–6. doi: 10.1093/jnci/53.5.1333. [DOI] [PubMed] [Google Scholar]
- 119.Ullrich SE. Mechanisms underlying UV-induced immune suppression. Mutat Res. 2005;571:185–205. doi: 10.1016/j.mrfmmm.2004.06.059. [DOI] [PubMed] [Google Scholar]
- 120.Schwarz A, Schwarz T. UVR-induced regulatory T cells switch antigen-presenting cells from a stimulatory to a regulatory phenotype. J Invest Dermatol. 2010;130:1914–21. doi: 10.1038/jid.2010.59. [DOI] [PubMed] [Google Scholar]
- 121.De Fabo EC, Noonan FP. Mechanism of immune suppression by ultraviolet irradiation in vivo. I. Evidence for the existence of a unique photoreceptor in skin and its role in photoimmunology. J Exp Med. 1983;158:84–98. doi: 10.1084/jem.158.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Beissert S, Rühlemann D, Mohammad T, Grabbe S, El-Ghorr A, Norval M, et al. IL-12 prevents the inhibitory effects of cis-urocanic acid on tumor antigen presentation by Langerhans cells: implications for photocarcinogenesis. J Immunol. 2001;167:6232–8. doi: 10.4049/jimmunol.167.11.6232. [DOI] [PubMed] [Google Scholar]
- 123.Vink AA, Strickland FM, Bucana C, Cox PA, Roza L, Yarosh DB, et al. Localization of DNA damage and its role in altered antigen-presenting cell function in ultraviolet-irradiated mice. J Exp Med. 1996;183:1491–500. doi: 10.1084/jem.183.4.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Halliday GM, Muller HK. Sensitization through carcinogen-induced Langerhans cell-deficient skin activates specific long-lived suppressor cells for both cellular and humoral immunity. Cell Immunol. 1987;109:206–21. doi: 10.1016/0008-8749(87)90305-4. [DOI] [PubMed] [Google Scholar]
- 125.Caceres-Dittmar G, Ariizumi K, Xu S, Tapia FJ, Bergstresser PR, Takashima A. Hydrogen peroxide mediates UV-induced impairment of antigen presentation in a murine epidermal-derived dendritic cell line. Photochem Photobiol. 1995;62:176–83. doi: 10.1111/j.1751-1097.1995.tb05255.x. [DOI] [PubMed] [Google Scholar]
- 126.Devary Y, Gottlieb RA, Smeal T, Karin M. The mammalian ultraviolet response is triggered by activation of Src tyrosine kinases. Cell. 1992;71:1081–91. doi: 10.1016/S0092-8674(05)80058-3. [DOI] [PubMed] [Google Scholar]
- 127.Tobin D, van Hogerlinden M, Toftgård R. UVB-induced association of tumor necrosis factor (TNF) receptor 1/TNF receptor-associated factor-2 mediates activation of Rel proteins. Proc Natl Acad Sci U S A. 1998;95:565–9. doi: 10.1073/pnas.95.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stapelberg MP, Williams RB, Byrne SN, Halliday GM. The alternative complement pathway seems to be a UVA sensor that leads to systemic immunosuppression. J Invest Dermatol. 2009;129:2694–701. doi: 10.1038/jid.2009.128. [DOI] [PubMed] [Google Scholar]
- 129.Halliday GM. Common links among the pathways leading to UV-induced immunosuppression. J Invest Dermatol. 2010;130:1209–12. doi: 10.1038/jid.2009.374. [DOI] [PubMed] [Google Scholar]
- 130.Halliday GM, Byrne SN, Kuchel JM, Poon TS, Barnetson RS. The suppression of immunity by ultraviolet radiation: UVA, nitric oxide and DNA damage. Photochem Photobiol Sci. 2004;3:736–40. doi: 10.1039/b313199h. [DOI] [PubMed] [Google Scholar]
- 131.Damian DL, Barnetson RS, Halliday GM. Low-dose UVA and UVB have different time courses for suppression of contact hypersensitivity to a recall antigen in humans. J Invest Dermatol. 1999;112:939–44. doi: 10.1046/j.1523-1747.1999.00610.x. [DOI] [PubMed] [Google Scholar]
- 132.Poon TS, Barnetson RS, Halliday GM. Sunlight-induced immunosuppression in humans is initially because of UVB, then UVA, followed by interactive effects. J Invest Dermatol. 2005;125:840–6. doi: 10.1111/j.0022-202X.2005.23894.x. [DOI] [PubMed] [Google Scholar]
- 133.Damian DL, Matthews YJ, Phan TA, Halliday GM. An action spectrum for ultraviolet radiation-induced immunosuppression in humans. Br J Dermatol. 2011;164:657–9. doi: 10.1111/j.1365-2133.2010.10161.x. [DOI] [PubMed] [Google Scholar]
- 134.Tyrrell RM, Reeve VE. Potential protection of skin by acute UVA irradiation--from cellular to animal models. Prog Biophys Mol Biol. 2006;92:86–91. doi: 10.1016/j.pbiomolbio.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 135.Matthews YJ, Halliday GM, Phan TA, Damian DL. Wavelength dependency for UVA-induced suppression of recall immunity in humans. J Dermatol Sci. 2010;59:192–7. doi: 10.1016/j.jdermsci.2010.07.005. [DOI] [PubMed] [Google Scholar]