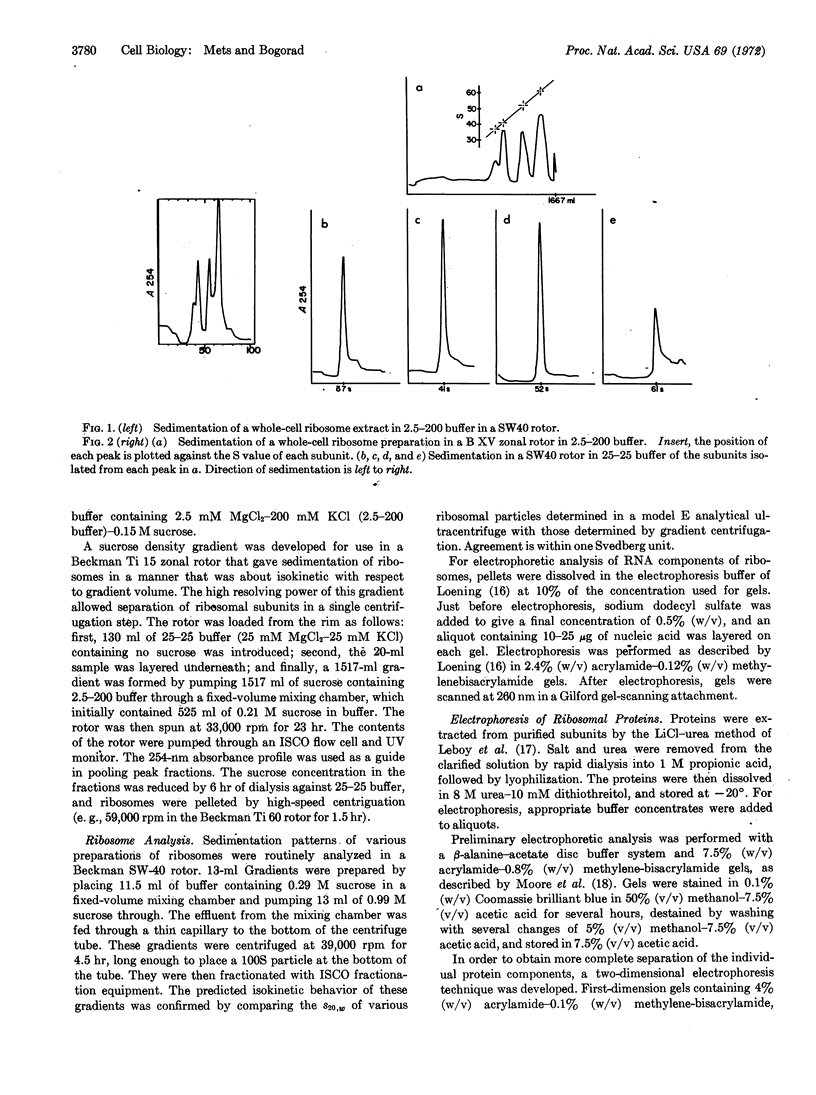
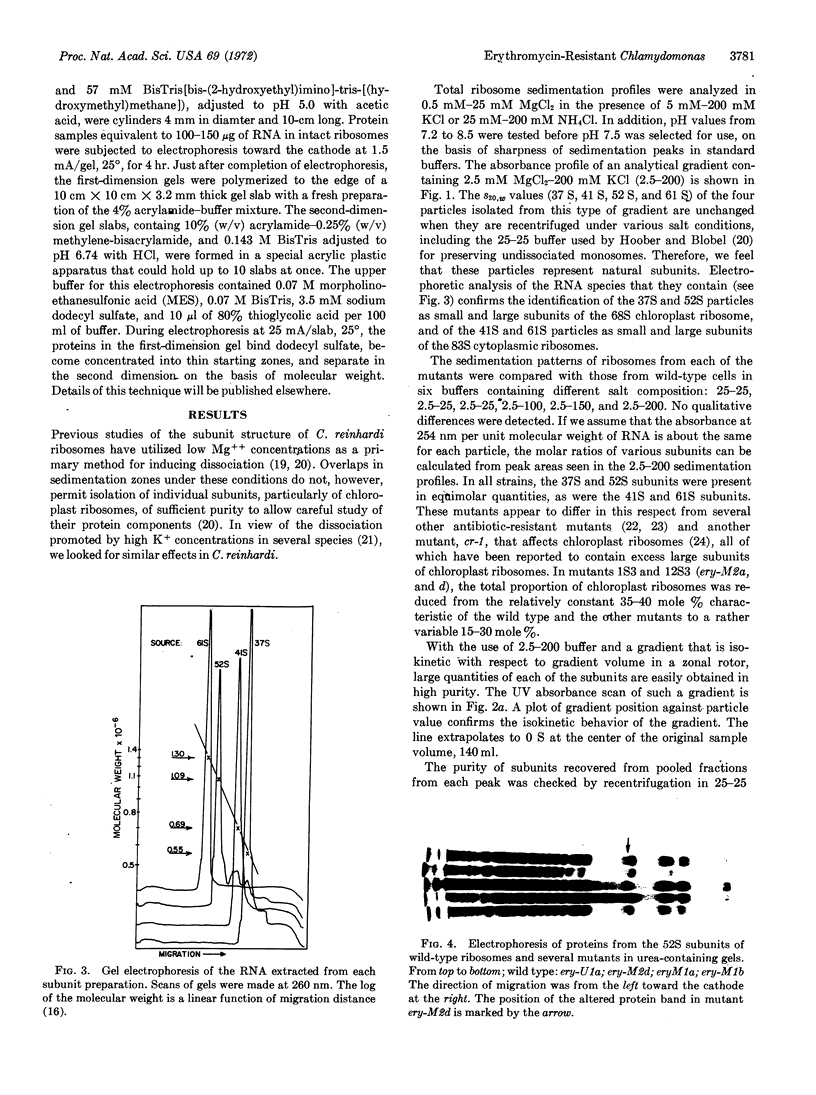
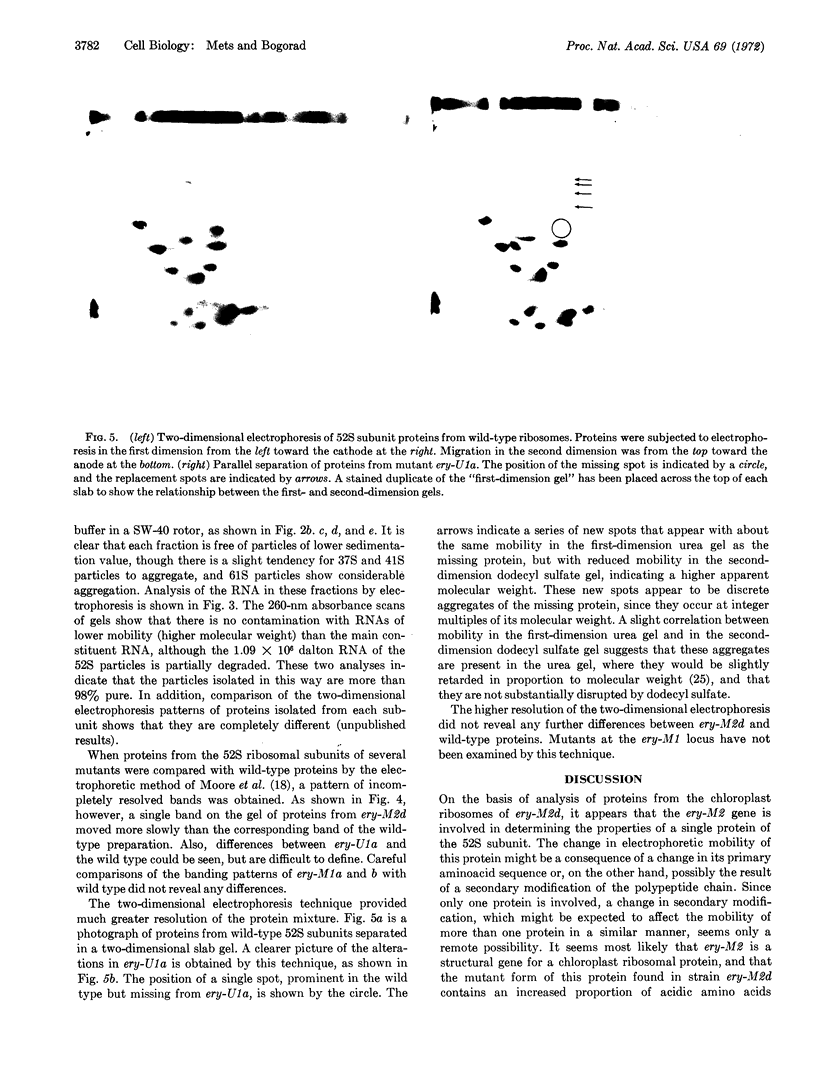
Abstract
The phenotype of several erythromycin-resistant mutants of Chlamydomonas reinhardi was further characterized in terms of the electrophoretic properties of their chloroplast ribosomal proteins. In mutant ery-M2d a single protein of the large (52 S) subunit has altered properties, which probably result from a change in its primary sequence. This mutation is inherited in a Meudelian manner. In mutant ery-U1a, which is inherited in a uniparental manner, a different single protein of the 52 S subunit is altered. This change might result from a change in either the primary sequence of the protein or in some form of secondary modification. These results indicate that these two distinct genetic systems must cooperate in the production of chloroplast ribosomes.
Keywords: electrophoresis, Mendelian and uniparental inheritance, sucrose density centrifugation
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bitte L., Kabat D. Phosphorylation of ribosomal proteins in sarcoma 180 tumor cells. J Biol Chem. 1972 Sep 10;247(17):5345–5350. [PubMed] [Google Scholar]
- Boynton J. E., Gillham N. W., Burkholder B. Mutations Altering Chloroplast Ribosome Phenotype in Chlamydomonas, II. A New Mendelian Mutation. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1505–1512. doi: 10.1073/pnas.67.3.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillham N. W., Boynton J. E., Burkholder B. Mutations altering chloroplast ribosome phenotype in Chlamydomonas. I. Non-mendelian mutations. Proc Natl Acad Sci U S A. 1970 Oct;67(2):1026–1033. doi: 10.1073/pnas.67.2.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Hoober J. K., Blobel G. Characterization of the chloroplastic and cytoplasmic ribosomes of Chlamydomonas reinhardi. J Mol Biol. 1969 Apr 14;41(1):121–138. doi: 10.1016/0022-2836(69)90130-2. [DOI] [PubMed] [Google Scholar]
- Kabat D. Phosphorylation of ribosomal proteins in rabbit reticulocytes. Characterization and regulatory aspects. Biochemistry. 1970 Oct 13;9(21):4160–4175. doi: 10.1021/bi00823a019. [DOI] [PubMed] [Google Scholar]
- LEBOY P. S., COX E. C., FLAKS J. G. THE CHROMOSOMAL SITE SPECIFYING A RIBOSOMAL PROTEIN IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1964 Dec;52:1367–1374. doi: 10.1073/pnas.52.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. J., Weisblum B. Altered methylation of ribosomal RNA in an erythromycin-resistant strain of Staphylococcus aureus. Proc Natl Acad Sci U S A. 1971 Apr;68(4):856–860. doi: 10.1073/pnas.68.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine R. P., Goodenough U. W. The genetics of photosynthesis and of the chloroplast in Chlamydomonas reinhardi. Annu Rev Genet. 1970;4:397–408. doi: 10.1146/annurev.ge.04.120170.002145. [DOI] [PubMed] [Google Scholar]
- Loening U. E. Molecular weights of ribosomal RNA in relation to evolution. J Mol Biol. 1968 Dec;38(3):355–365. doi: 10.1016/0022-2836(68)90391-4. [DOI] [PubMed] [Google Scholar]
- Martin T. E., Rolleston F. S., Low R. B., Wool I. G. Dissociation and reassociation of skeletal muscle ribosomes. J Mol Biol. 1969 Jul 14;43(1):135–149. doi: 10.1016/0022-2836(69)90084-9. [DOI] [PubMed] [Google Scholar]
- Maruta H., Tsuchiya T., Mizuno D. In vitro reassembly of functionally active 50 s ribosomal particles from ribosomal proteins and RNA's of Escherichia coli. J Mol Biol. 1971 Oct 14;61(1):123–134. doi: 10.1016/0022-2836(71)90210-5. [DOI] [PubMed] [Google Scholar]
- Mets L. J., Bogorad L. Mendelian and uniparental alterations in erythromycin binding by plastid ribosomes. Science. 1971 Nov 12;174(4010):707–709. doi: 10.1126/science.174.4010.707. [DOI] [PubMed] [Google Scholar]
- Moore P. B., Traut R. R., Noller H., Pearson P., Delius H. Ribosomal proteins of Escherichia coli. II. Proteins from the 30 s subunit. J Mol Biol. 1968 Feb 14;31(3):441–461. doi: 10.1016/0022-2836(68)90420-8. [DOI] [PubMed] [Google Scholar]
- Nomura M., Erdmann V. A. Reconstitution of 50S ribosomal subunits from dissociated molecular components. Nature. 1970 Nov 21;228(5273):744–748. doi: 10.1038/228744a0. [DOI] [PubMed] [Google Scholar]
- Otaka E., Itoh T., Osawa S., Tanaka K., Tamaki M. Peptide analyses of a protein component, 50-8, of 50s ribosomal subunit from erythromycin resistant mutants of Escherichia coli and Escherichia freudii. Mol Gen Genet. 1972;114(1):14–22. doi: 10.1007/BF00268742. [DOI] [PubMed] [Google Scholar]
- Otaka E., Teraoka H., Tamaki M., Tanaka K., Osawa S. Ribosomes from erythromycin-resistant mutants of Escherichia coli Q13. J Mol Biol. 1970 Mar;48(3):499–510. doi: 10.1016/0022-2836(70)90061-6. [DOI] [PubMed] [Google Scholar]
- Sager R., Hamilton M. G. Cytoplasmic and chloroplast ribosomes of Chlamydomonas: ultracentrifugal characterization. Science. 1967 Aug 11;157(3789):709–711. doi: 10.1126/science.157.3789.709. [DOI] [PubMed] [Google Scholar]
- Sager R., Ramanis Z. A genetic map of non-Mandelian genes in Chlamydomonas. Proc Natl Acad Sci U S A. 1970 Mar;65(3):593–600. doi: 10.1073/pnas.65.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueoka N. MITOTIC REPLICATION OF DEOXYRIBONUCLEIC ACID IN CHLAMYDOMONAS REINHARDI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):83–91. doi: 10.1073/pnas.46.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub P., Nomura M. Structure and function of E. coli ribosomes. V. Reconstitution of functionally active 30S ribosomal particles from RNA and proteins. Proc Natl Acad Sci U S A. 1968 Mar;59(3):777–784. doi: 10.1073/pnas.59.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]




