Abstract
Stem cell transplantation is being tested as a potential therapy for a number of diseases. Stem cells isolated directly from tissue specimens or generated via reprogramming of differentiated cells require rigorous testing for both safety and efficacy in preclinical models. The availability of mice with immune-deficient background that carry additional mutations in specific genes facilitates testing the efficacy of cell transplantation in disease models. The muscular dystrophies are a heterogeneous group of disorders, of which Duchenne muscular dystrophy is the most severe and common type. Cell-based therapy for muscular dystrophy has been under investigation for several decades, with a wide selection of cell types being studied, including tissue-specific stem cells and reprogrammed stem cells. Several immune-deficient mouse models of muscular dystrophy have been generated, in which human cells obtained from various sources are injected to assess their preclinical potential. After transplantation, the presence of engrafted human cells is detected via immunofluorescence staining, using antibodies that recognize human, but not mouse, proteins. Here we show that one antibody specific to human spectrin, which is commonly used to evaluate the efficacy of transplanted human cells in mouse muscle, detects myofibers in muscles of NOD/Rag1nullmdx5cv, NOD/LtSz-scid IL2Rγnull mice, or mdx nude mice, irrespective of whether they were injected with human cells. These “reactive” clusters are regenerating myofibers, which are normally present in dystrophic tissue and the spectrin antibody is likely recognizing utrophin, which contains spectrin-like repeats. Therefore, caution should be used in interpreting data based on detection of single human-specific proteins, and evaluation of human stem cell engraftment should be performed using multiple human-specific labeling strategies.
Introduction
Stem cells of human origin are considered of potential therapeutic value for a number of diseases. Stem cells directly isolated from either discarded or consented human tissue specimens, or generated via reprogramming of adult cells, require rigorous testing in preclinical models for both safety and efficacy. Preclinical testing is facilitated by the availability of immune-deficient murine models, such as nude (Flanagan, 1966; Pantelouris, 1968), NOD/Rag1null (Shultz et al., 2000), or NSG (NOD/LtSz-scid IL2Rγnull or NOD scid gamma) mice (Shultz et al., 2005, 2007). These immune-deficient mice are often crossed to immune-competent models that carry additional genetic mutations in specific genes. After several backcrosses into the immune-deficient background, immune-deficient models for a specific disease are generated and they are suitable for testing the safety and efficacy of stem cell transplantation.
The muscular dystrophies are a heterogeneous group of disorders, one of which, Duchenne muscular dystrophy (DMD), arises from primary mutations in the dystrophin gene (Monaco et al., 1986; Hoffman et al., 1987). Cell-based therapy for DMD has been under investigation for several decades (Partridge et al., 1989; Karpati, 1990; Gussoni et al., 1992; Huard et al., 1992; Karpati et al., 1993; Mendell et al., 1995; Morandi et al., 1995; Neumeyer et al., 1998). Although injection of expanded donor muscle cells into human muscle is safe, the overall efficacy has been low, partially because of cell death immediately after injection and host immune reaction to the donor cells (Huard et al., 1994; Gussoni et al., 1997; Beauchamp et al., 1999). Several additional cell types are currently being studied for cell-based therapy of muscular dystrophy, including mesenchymal stem cells (MSCs), mesoangioblasts/pericytes, CD133+ cells, induced pluripotent stem cells (iPSCs), and embryonic stem cells (ESCs) (Torrente et al., 2004; Dellavalle et al., 2007; Crisan et al., 2008; Negroni et al., 2009; Darabi et al., 2012; Goudenege et al., 2012; Tedesco et al., 2012). Human cells are routinely injected into immune-deficient mouse models of muscular dystrophy, such as the mdx4cv and mdx5cv models bred into the NSG or NOD/Rag1null background (Darabi et al., 2012; Goudenege et al., 2012; Lapan et al., 2012); or into immune-deficient mice in which the recipient muscle has been damaged with cardiotoxin or by cryoinjury before injection (Cooper et al., 2001, 2003; Brimah et al., 2004; Ehrhardt et al., 2007; Crisan et al., 2008; Negroni et al., 2009; Meng et al., 2010, 2011). After transplantation, the presence of engrafted human cells is detected via immunofluorescence staining using antibodies that recognize human, but not mouse, proteins. One antibody in particular, anti-human spectrin (SPEC1), has been used extensively to track the expression of human-derived protein after injection of human cells into the muscle of immune-deficient mice (Zheng et al., 2007; Crisan et al., 2008; Goudenege et al., 2012). In the present study, we tested the specificity with which individual antibodies that recognize proteins of human, not mouse, origin track the presence of transplanted human cells. Three immune-deficient mouse models were analyzed: NOD/Rag1nullmdx5cv and mdx/nude, which carry a mutation in the dystrophin gene (Partridge et al., 1989; Boldrin et al., 2012; Lapan et al., 2012); and the NSG immune-deficient model (Shultz et al., 2005, 2007), where the muscle was preinjured with cardiotoxin or by cryoinjury or irradiation followed by cryoinjury. It was found that detection of engrafted human cells, using anti-human-specific spectrin, results in visualization of false-positive myofibers in all three murine models. In particular, human spectrin-reactive myofibers were seen in animals that had never been injected with human cells, and this occurred after analyses of mice from all three immune-deficient background strains. The reactivity was seen irrespective of the type of fixation method used and it was specific to regenerating myofibers, as demonstrated by coexpression in these myofibers of embryonic myosin heavy chain (eMHC). Utrophin was identified as the candidate protein that might cross-react with the anti-human spectrin antibody, based on its subsarcolemmal position, upregulation in regenerating fibers (Gramolini et al., 1999), and the presence of a number of spectrin-like repeat domains. Therefore, to circumvent possible misinterpretation of transplant efficacy, detection of engrafted human stem cells in mouse dystrophic or injured muscle should be performed using multiple human-specific antigen labeling strategies, either on the same or on consecutive tissue sections.
Materials and Methods
Mouse strains and care
Recipient NOD/Rag1nullmdx5cv mice were generated by breeding the mdx5cv mutation into mice with a NOD/Rag1null background for more than 10 generations (Lapan et al., 2012). Homozygote females and hemizygote males were bred, and 100% of the progeny were used in cell transplantation studies as described. NSG mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and were also kindly donated by C. Kim (Boston Children's Hospital, Boston, MA). Dystrophin/utrophin double-null muscle tissue was kindly provided by the laboratory of L.M. Kunkel (Boston Children's Hospital). mdx nude mice were generated as previously described (Partridge et al., 1989). All procedures involving live animals were approved by the Boston Children's Hospital Institutional Animal Care and Use Committee (IACUC) or under U.K. Home Office license, in accordance with the Animals (Scientific Procedures) Act 1986.
Cell injections in recipient mice
For intramuscular injection of human fetal muscle cells, 1×105 human melanoma cell adhesion molecule (MCAM)+ cells were delivered to one tibialis anterior muscle of NOD/Rag1nullmdx5cv mice, as previously described (Lapan et al., 2012). Human fetal muscle cells were extracted from discarded, deidentified tissue according to a protocol approved by the Boston Children's Hospital Committee on Clinical Investigation and Internal Review Board. Inert green fluorescent beads (diluted 1:10,000; Life Technologies, Carlsbad, CA) were coinjected with the human cells to track the injection site after sectioning. Transplanted muscles and nontransplanted contralateral muscles were collected 1–2 months after transplantation and frozen in liquid nitrogen-cooled isopentane. For NSG mice, the tibialis anterior muscle was injured with 15 μl of cardiotoxin as previously described (Rivier et al., 2004). Regenerating muscles were collected 7 days after cardiotoxin injury.
For intramuscular injection of postnatal myoblasts, host mice were mdx nude mice, aged 4 weeks. Tibialis anterior muscles were cryoinjured, or irradiated with 18 Gy 3 days before cryoinjury and grafting as described previously (Brimah et al., 2004; Boldrin et al., 2012). Into some of these cryoinjured host muscles, either 1×106 donor human myoblasts, derived from a 2-year-old male patient with DMD, or 5×105 human skeletal muscle-derived CD133+ cells derived from the paraspinal muscle of a 16-year-old male with adolescent idiopathic scoliosis, or no cells, were grafted. Other muscles were noninjured and nongrafted. Human cells were supplied by the MRC CNMD Biobank London (REC reference number 06/Q0406/33). For all samples collected by the Biobank after January 9, 2006, broad written consent for research has been supplied by all patients or their parent/guardian. All samples have been supplied anonymously to the project. Copies of the consent forms are kept with the Biobank in a locked cabinet and are also available in the patient's hospital notes at Great Ormond Street Hospital for Children (London, UK) and Hammersmith Hospital (London, UK). Muscles were removed for analysis 4 weeks after cell transplantation. Vetergesic (buprenorphine hydrochloride; Alstoe Animal Health, Sheriff Hutton, UK) was administered as analgesic.
Immunofluorescence staining
For analysis of NOD/Rag1nullmdx5cv and NSG mice, consecutive skeletal muscle tissue sections (10 μm) were collected on Tissue Tack microscope slides (Polysciences, Warrington, PA). Various methods of fixation were tested: slides were fixed for 3 min in 100% methanol at room temperature; or fixed in 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) for 15 min at room temperature followed by 3 min of permeabilization with 0.5% Triton X-100; or left to air dry for 30 min. After fixation, sides were washed once in PBS and blocked in 10% fetal bovine serum (FBS)–PBS–0.1% Triton X-100 for 30 min at room temperature. Slides were stained with polyclonal rabbit anti-dystrophin (CAP6-10, diluted 1:2000) (Lidov et al., 1990; Byers et al., 1993), monoclonal anti-human dystrophin (clone Dy10/12B2, diluted 1:20; Vector Laboratories, Burlingame, CA), rabbit anti-human lamin A/C (2966-1, diluted 1:500, Epitomics, Burlingame, CA), rabbit anti-laminin (L9393, diluted 1:500; Sigma-Aldrich, St. Louis, MO), monoclonal mouse anti-human spectrin (NCL-SPEC-1, Leica Biosystems, Buffalo Grove, IL, or clone RBC2/3D5, diluted 1:100; Vector Laboratories, Burlingame, CA), monoclonal anti-human fetal embryonic myosin heavy chain (F1.652, diluted 1:100; Developmental Studies Hybridoma Bank [DSHB]) diluted in 10% FBS–PBS–0.1% Triton X-100 overnight at 4°C. For secondary antibody staining, DyLight 488-conjugated donkey anti-rabbit (diluted 1:1000, preadsorbed, minimal cross-reactivity with other species; Jackson Immunoresearch, West Grove, PA) and Alexa Fluor 568-conjugated donkey anti-mouse IgG (diluted 1:1000; Life Technologies) antibodies diluted in 1× PBS were used. Sections were coverslipped, using VECTASHIELD mounting medium with 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA). Images were taken using a ×20 objective lens on a Nikon Eclipse E1000 microscope fitted with a Hamamatsu ORCA-ER CCD camera.
For analysis of mdx/utrophin double-null muscle, frozen tissue sections were fixed for 3 min in 100% methanol followed by three washes with 1× PBS and 1 hr of blocking with 10% FBS and 0.1% Triton X-100, all at room temperature. Tissue sections were further stained according to the procedure described in the M.O.M. (mouse on mouse) kit protocol (BMK-2202; Vector Laboratories, Burlingame, CA). Anti-human spectrin (Vector Laboratories, Burlingame, CA) was diluted 1:100, anti-laminin (Sigma-Aldrich) was diluted 1:500, and anti-embryonic myosin heavy chain (DSHB) was diluted 1:100. The primary mouse antibody was visualized with fluorescein-conjugated streptavidin (SA-5001; Vector Laboratories, Burlingame, CA) diluted 1:50 and with Alexa Fluor 568-conjugated goat anti-rabbit (Life Technologies) diluted 1:500.
For analysis of mdx nude mice, muscles were frozen in isopentane chilled in liquid nitrogen and 7-μm cryosections were cut throughout the muscles. Sections were rehydrated in PBS for 5 min, and then incubated in M.O.M. blocking reagent (Vector Laboratories, Peterborough, UK) diluted in 10% normal goat serum (Vector Laboratories, Peterborough, UK) in PBS for 1 hr at room temperature, according to the manufacturer's instructions. Sections were then stained with primary antibodies to human spectrin (mouse monoclonal, diluted 1:20; Vector Laboratories, Peterborough, UK), human lamin A/C (mouse monoclonal, diluted 1:100; Vector Laboratories, Peterborough, UK), neonatal myosin (mouse monoclonal, diluted 1:50, BF34; Borrione et al., 1989), human dystrophin (Mandys 106, diluted 1:100; MDA Monoclonal Antibody Resource, Oswestry, UK), human neonatal myosin (mouse monoclonal, diluted 1:100; Vector Laboratories, Peterborough, UK), or utrophin (mouse monoclonal, diluted 1:50, Vector Laboratories, Peterborough, UK). All antibodies were diluted in 10% normal goat serum and used alone or in combination. Primary antibody incubation was for 1 hr at room temperature or 4°C overnight. Sections were washed in PBS before incubation with secondary antibodies, Alexa Fluor 488-conjugated goat anti-mouse IgG and Alexa Fluor 594-conjugated goat anti-rabbit IgG were both diluted 1:500 in PBS (Life Technologies) at room temperature for 1 hr. Sections were again washed with PBS and mounted in Fluoromount (Dako, Ely, UK) containing 4′,6-diamidino-2-phenylindole (DAPI, 100 ng/ml; Sigma-Aldrich). Sections were visualized with epifluorescence, using a Leica DMR microscope, and digitally captured with MetaMorph software (Universal Imaging, Downington, PA).
Results and Discussion
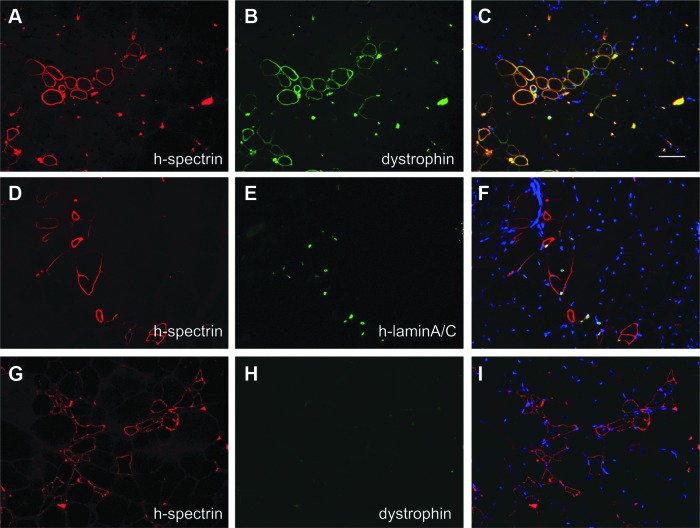
Human fetal MCAM+ muscle cells were isolated as previously described (Lapan and Gussoni, 2012; Lapan et al., 2012). MCAM+ cells were injected via the intramuscular route into NOD/Rag1nullmdx5cv recipient mice and the muscles were examined 1–2 months after injection. Immunostaining with mouse anti-human spectrin and rabbit anti-dystrophin (this antibody recognizes both human and mouse dystrophin) revealed the presence of myofibers positive for both proteins (Fig. 1A–C), which demonstrated that human donor cells had fused to these myofibers. In different sections, spectrin-reactive myofibers appeared to contain human-derived nuclei based on the expression of human-specific lamin A/C (Fig. 1D–F), confirming the presence of injected human cells. In addition, clusters or individual human spectrin-positive myofibers that were negative for dystrophin expression were also seen (Fig. 1G–I and Supplementary Fig. S1; supplementary data are available online at www.liebertpub.com/hum). Given the low abundance of the dystrophin transcript and an estimated time of 16 hr to transcribe the entire molecule (Tennyson et al., 1995), we initially thought that these human spectrin-reactive myofibers represented expression of successfully engrafted human cells that required additional time to display dystrophin expression.
FIG. 1.
Clusters of human spectrin-positive myofibers are detected in mice injected with human cells. (A–I) Recipient NOD/Rag1nullmdx5cv muscles 45 days after injection of 1×105 MCAM+ human fetal cells. (A–C) Coexpression of human spectrin and dystrophin (antibody recognizes both mouse and human proteins) documents human cell engraftment in myofibers. (D–F) Human spectrin-positive myofibers contain nuclei expressing human-specific lamin A/C. (G–I) Clusters of small myofibers reactive to human spectrin but negative for dystrophin expression. H-spectrin, human spectrin; h-lamin A/C, human lamin A/C. Scale bar: 50 μm
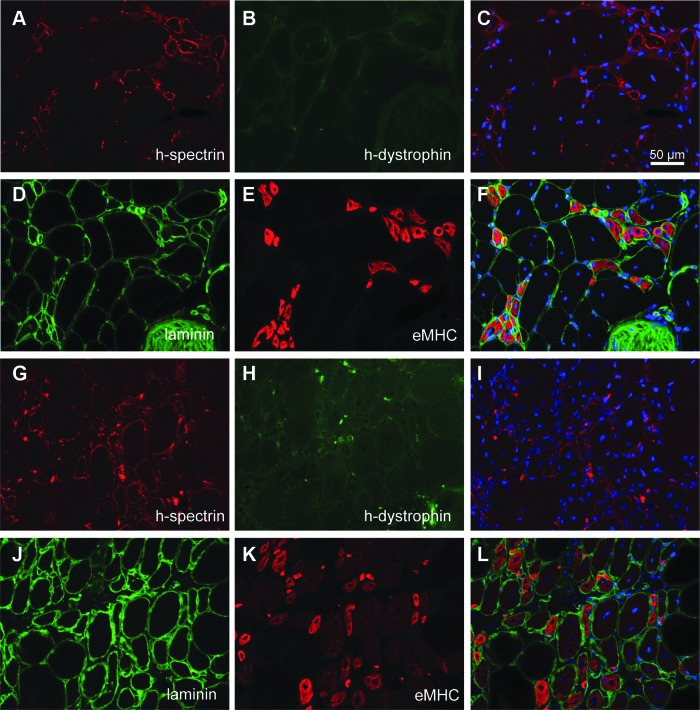
To further study the nature of these human spectrin-reactive myofibers and determine whether this expression unequivocally marks the engraftment of donor human cells, tissue sections of 2- to 4-month-old NOD/Rag1nullmdx5cv mice not injected with human cells were analyzed (Fig. 2). Consecutive muscle tissue sections were stained with mouse anti-human spectrin or with mouse anti-human dystrophin. Human spectrin-reactive fibers were detected in mice that had never been injected with human cells (Fig. 2A), whereas dystrophin was never detected in the same fibers (Fig. 2B). These human spectrin-reactive myofibers were detected irrespective of the fixation method used: methanol, PFA, or unfixed tissue (Supplementary Fig. S2).
FIG. 2.
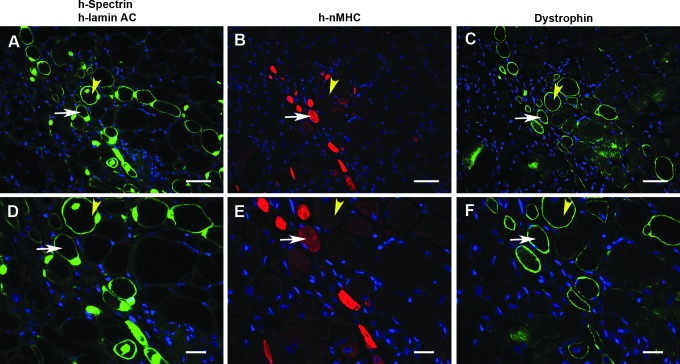
Clusters of human spectrin-positive myofibers are detected in mice never injected with human cells; these are regenerating myofibers. (A–C) NOD/Rag1nullmdx5cv muscle tissue sections from animals not injected with human cells. Myofibers reactive to the human-spectrin antibody are detected. (D–F) The human spectrin-reactive myofibers are regenerating myofibers. Consecutive sections stained for laminin and embryonic myosin heavy chain (eMHC) reveal the identity of the spectrin-reactive myofibers in NOD/Rag1nullmdx5cv mice. (G–L) NSG mouse muscles from 6-month-old animals never injected with human cells and injured with cardiotoxin show expression of human spectrin in regenerating myofibers. (G–I) Human spectrin-positive myofibers are negative for dystrophin. (J–L) In consecutive sections, the spectrin-reactive myofibers are within the injured area, as documented by coexpression of laminin and eMHC. Scale bar: 50 μm.
On the basis of the morphology, we suspected that these could be clusters of regenerating myofibers, which are normally present in dystrophic tissue. To confirm this, consecutive tissue sections of NOD/Rag1nullmdx5cv mice were costained with rabbit anti-laminin and mouse anti-embryonic myosin heavy chain antibodies (Fig. 2D and E). Positive staining for embryonic myosin heavy chain confirmed that the human spectrin-reactive clusters contained regenerating myofibers (Fig. 2E). To address whether other immune-deficient mouse strains that are used for human cell transplantation experiments exhibited similar findings, NSG mice (Shultz et al., 2005, 2007) were injured with cardiotoxin in one tibialis anterior muscle. The injured muscles, which were never injected with human cells, were collected 7 days after injury. Immunostaining of muscle tissue sections with anti-human spectrin again revealed reactivity of this antibody with small clusters of myofibers (Fig. 2G); however, they did not express human dystrophin (Fig. 2H). These myofibers were within the cardiotoxin-injured area, as indicated by the expression of eMHC (Fig. 2K). Thus, under all conditions tested, regenerating human spectrin-reactive myofibers were detected in mice that were never injected with human fetal cells.
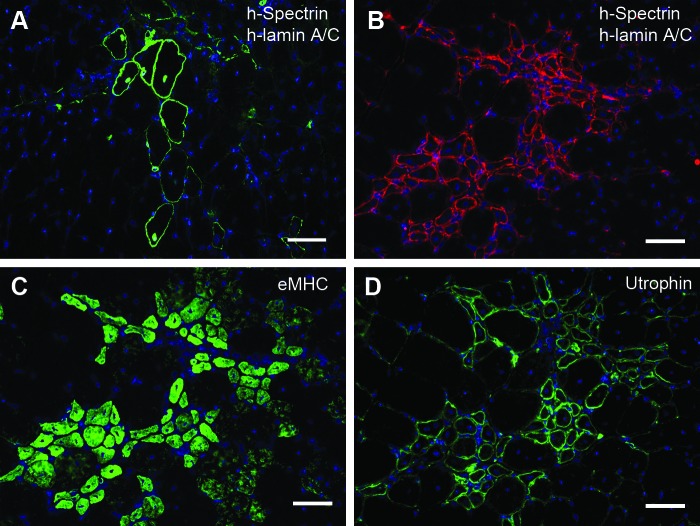
In mdx nude host mice, human postnatal myoblasts grafted into cryoinjured host muscles were also able to contribute to regenerated muscle fibers expressing human spectrin; many of these fibers also contained a nucleus expressing human lamin A/C 21 days after engraftment (Fig. 3A). As these donor myoblasts were derived from a patient with DMD, dystrophin could not be used as a marker of muscle of donor origin. In a muscle that had not been injured or grafted with human cells, clusters of small human spectrin-positive fibers were detected (Fig. 3B); however, no human nuclei (expressing human lamin A/C) were seen in or near these clusters. It was therefore suspected, because of their small size and central nuclei within the fibers, that these clusters were regenerating mouse muscle fibers, and did not contain nuclei of human origin. To confirm that these areas were in the process of regeneration, serial sections were stained with an antibody to neonatal myosin heavy chain, which is expressed in recently regenerated muscle fibers (Gross and Morgan, 1999). The vast majority of the fibers reactive to the human spectrin antibody were indeed regenerating myofibers as confirmed by neonatal myosin staining (Fig. 3B and C). The same myofibers were also reactive to the protein utrophin, which is upregulated and expressed in a subsarcolemmal position in regenerating fibers (Gramolini et al., 1999).
FIG. 3.
Examples of “true” human spectrin-positive fibers (A) and “false” human spectrin-positive fibers (B) on mdx nude muscle sections. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; blue). (A) Immunostaining of human lamin A/C and human spectrin (both green) on a transverse cryosection of an mdx nude tibialis anterior (TA) muscle that had been grafted with Duchenne muscular dystrophy (DMD) myoblasts 21 days earlier. Bona fide human spectrin-positive fibers were confirmed by the presence of human lamin A/C-positive nuclei within the fibers. (B) Immunostaining of human lamin A/C and human spectrin (both red) on a transverse cryosection from a noninjured, nongrafted mdx nude TA muscle. Fibers expressing human spectrin-positive fibers are not of human origin, as they do not contain human lamin A/C-positive nuclei. (C) Immunostaining of neonatal myosin heavy chain (nMHC; green) on a section serial to that shown in (B). The “false” human spectrin-reactive fibers are mostly regenerating muscle fibers, expressing nMHC. (D) Immunostaining of utrophin (green) on a section serial to that shown in (B) and (C). The “false” human spectrin-reactive fibers were also utrophin-positive, suggesting a cross-reaction of the human spectrin antibody with mouse utrophin. Scale bars: 25 μm.
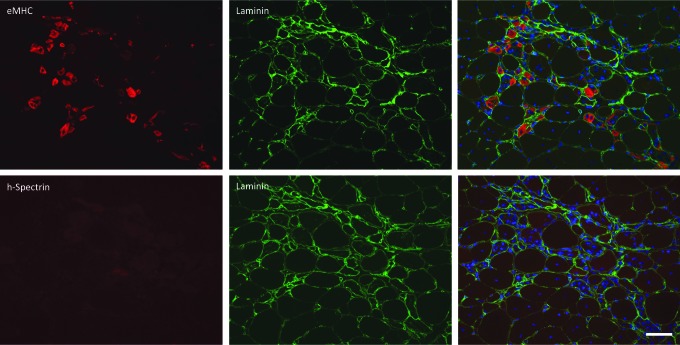
To investigate whether utrophin is the cause of this cross-reaction of human specific-spectrin antibody with recently regenerated mouse muscle fibers, a BLAST search was performed to find conserved amino acid sequences between human β-spectrin and mouse utrophin (Supplementary Fig. S3). Mouse utrophin contains a number of spectrin-like repeat domains that allow its binding to actin (Rybakova and Ervasti, 2005) (Supplementary Fig. S3). To confirm whether upregulation of utrophin was responsible for the cross-reaction of the human specific spectrin antibody with mouse muscle fibers, transverse cryosections of mdx/utrophin double-null tibialis anterior (TA) muscles were stained with anti-eMHC and laminin to detect regenerating muscle fibers and with human spectrin and laminin on consecutive sections (Fig. 4). In mdx/utrophin double-null tissue sections, the human-specific spectrin antibody did not display reactivity to regenerating fibers, supporting the conclusion that utrophin is the likely cross-reacting protein (Fig. 4).
FIG. 4.
Human spectrin does not recognize regenerating myofibers in mdx/utrophin double-null mice. Transverse cryosections of mdx/utrophin double-null mouse TA muscle. Sections were stained with antibodies to eMHC, laminin, and human spectrin. A group of regenerating fibers expressing neonatal myosin is not recognized by antibodies to human spectrin, confirming that utrophin is the likely cross-reactive protein. Scale bar: 50 μm.
On the basis of this evidence, in order to avoid incorrectly identifying recently regenerated mouse muscle fibers as muscle fibers of the donor, human origin, multiple markers that detect the presence of engrafted human cells must be used. When host mouse muscles are injected with donor cells derived from an unaffected individual, costaining with dystrophin can provide a second marker for donor muscle. To distinguish between naturally occurring revertant fibers that express dystrophin in mdx mice (Hoffman et al., 1990; Lu et al., 2000; Yokota et al., 2006), a human-specific dystrophin antibody may be used (Fig. 5). For DMD donor cells, however, the use of dystrophin as a second marker of muscle fibers of donor origin is not possible. Therefore, if fibers are small, centrally nucleated, are found in a tight cluster, and have no human lamin A/C-positive nuclei, then they are likely to be regenerating mouse fibers. Four weeks after grafting, bona fide muscle fibers of human origin will be of normal size, in diffuse clusters and either contain, or be in close proximity, to human lamin A/C-positive nuclei (Fig. 5).
FIG. 5.
Human spectrin, human lamin A/C, and human dystrophin are coexpressed in myofibers of mdx nude mice 4 weeks after injection of human cells. (A and D) Immunostaining of human lamin A/C and human spectrin (both green); (B and E) human neonatal myosin (red); and (C and F) human dystrophin (Mandys 106, green) on serial sections of mdx nude muscles that had been cryoinjured and intramuscularly transplanted with human muscle-derived CD133+ cells 4 weeks previously. Yellow arrowheads: a human spectrin-positive fiber containing a human lamin A/C nucleus, which is also human dystrophin-positive, but neonatal myosin-negative; white arrows: a human spectrin-positive fiber containing a human lamin A/C nucleus, which is also human dystrophin-positive and human neonatal myosin-positive. Nuclei were counterstained with DAPI (blue). Scale bars: top, 25 μm; bottom, 10 μm.
Stem cell transplantation is one of the strategies being tested for the therapy of several human diseases, including muscular dystrophy. In addition to the promise held by the use of embryonic stem cells (ESCs), the field of cell-based therapy has gained increased interest with the development of strategies that generate induced pluripotent stem cells (iPSCs). iPSCs are derived from adult, committed cell types and have the potential to be expanded in unlimited numbers, making them attractive candidates for downstream translational applications. Preclinical testing of human-derived cells in immune-deficient mouse models of specific diseases is often the next step toward translation into the clinic. For DMD, several immune-deficient animal models are available that also carry a mutation in the dystrophin gene (Partridge et al., 1989; Boldrin et al., 2012; Darabi et al., 2012; Lapan et al., 2012). After transplantation of human cells, detection of engrafted myofibers expressing human proteins is necessary. Some of the most commonly used antibodies are as follows: anti-human dystrophin (Millipore, Bedford, MA) or Mandys 106 (G. Morris, MDA Monoclonal Antibody Resource); anti-human spectrin (SPEC1); anti-human lamin A/C (Epitomics); and anti-dystrophin (mouse and human). Although the use of multiple human-specific markers to track the presence of donor human cells seems standard practice, several reports have relied, in at least part of their work, on the detection of a single antigen, anti-human spectrin, to detect the expression of a human-derived protein in myofibers (Pye et al., 2004; Crisan et al., 2008; Goudenege et al., 2012). We found that the use of this antibody alone to detect the presence of engrafted human cells can give false positive results. Our data demonstrate that the antibody reacts with clusters of regenerating myofibers in dystrophic mice, even when the animals were never injected with human cells. Notably, these reactive myofibers were detected irrespective of the fixation method used and in all mouse strains tested, including NSG mice whose muscles were injured with cardiotoxin.
Therefore, caution should be used in interpreting immunofluorescence staining based on the detection of single human-specific proteins, and the evaluation of human cell engraftment using multiple human-specific antibodies is strongly recommended. One of the preferred combinations includes the use of an anti-dystrophin antibody, either human specific or that recognizes both mouse and human proteins, together with anti-human lamin A/C. This will allow the codetection of human-derived nuclei within the myofibers expressing dystrophin. Alternatively, the use of the human-specific spectrin together with an anti-dystrophin or anti-human lamin A/C antibody will also ensure coexpression of both proteins in the same myofibers, which is indicative of human cell engraftment. Conversely, the presence of human spectrin alone or in combination with anti-lamin A/C does not necessarily mark mouse myofibers engrafted with human cells. In fact, the presence of human lamin A/C-expressing nuclei can be due to individual cells residing near the plane of spectrin-reactive myofibers, but not necessarily fused within a myofiber (Meng et al., 2010). Thus, although there is considerable excitement over the prospect of developing new cell-based strategies for the therapy of many diseases, including muscular dystrophy, caution should be taken in the design of the evaluation steps and the possible limitations or pitfalls with specific assays.
Supplementary Material
Acknowledgments
The authors are grateful to the laboratories of Dr. Louis M. Kunkel and Dr. Carla Kim for providing frozen muscle tissue from mdx/utrophin double-null mice and muscle tissue from NSG mice, respectively. This work was supported by a grant from the National Institutes of Health (NINDS 2R01NS047727 to E.G.) and by a grant from the Muscular Dystrophy Association (grant 199642 to E.G.). This work was also supported by EU MYOAMP (contract number LSHB-CT-2006-037479). J.M. is supported by the MRC (grant G0900872, held by J.E.M.). J.E.M. is supported by a Wellcome Trust University Award (084241/Z/07/Z) and by the Great Ormond Street Hospital Children's Charity. The authors gratefully acknowledge the MRC Centre for Neuromuscular Diseases Biobank (http://www.cnmd.ac.uk/research/activities/biobanking) for providing donor muscle cells.
Author Disclosure Statement
No competing financial interests exist.
References
- Beauchamp J.R., Morgan J.E., Pagel C.N., and Partridge T.A. (1999). Dynamics of myoblast transplantation reveal a discrete minority of precursors with stem cell-like properties as the myogenic source. J. Cell Biol. 144, 1113–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrin L., Neal A., Zammit P.S., et al. (2012). Donor satellite cell engraftment is significantly augmented when the host niche is preserved and endogenous satellite cells are incapacitated. Stem Cells 30, 1971–1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrione A.C., Zanellato A.M., Scannapieco G., et al. (1989). Myosin heavy-chain isoforms in adult and developing rabbit vascular smooth muscle. Eur. J. Biochem. 183, 413–417 [DOI] [PubMed] [Google Scholar]
- Brimah K., Ehrhardt J., Mouly V., et al. (2004). Human muscle precursor cell regeneration in the mouse host is enhanced by growth factors. Hum. Gene Ther. 15, 1109–1124 [DOI] [PubMed] [Google Scholar]
- Byers T.J., Lidov H.G., and Kunkel L.M. (1993). An alternative dystrophin transcript specific to the peripheral nerve. Nat. Genet. 4, 77–80 [DOI] [PubMed] [Google Scholar]
- Cooper R.N., Irintchev A., Di Santo J.P., et al. (2001). A new immunodeficient mouse model for human myoblast transplantation. Hum. Gene Ther. 12, 823–831 [DOI] [PubMed] [Google Scholar]
- Cooper R.N., Thiesson D., Furling D., et al. (2003). Extended amplification in vitro and replicative senescence: Key factors implicated in the success of human myoblast transplantation. Hum. Gene Ther. 14, 1169–1179 [DOI] [PubMed] [Google Scholar]
- Crisan M., Yap S., Casteilla L., et al. (2008). A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3, 301–313 [DOI] [PubMed] [Google Scholar]
- Darabi R., Arpke R.W., Irion S., et al. (2012). Human ES- and iPS-derived myogenic progenitors restore DYSTROPHIN and improve contractility upon transplantation in dystrophic mice. Cell Stem Cell 10, 610–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellavalle A., Sampaolesi M., Tonlorenzi R., et al. (2007). Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat. Cell Biol. 9, 255–267 [DOI] [PubMed] [Google Scholar]
- Ehrhardt J., Brimah K., Adkin C., et al. (2007). Human muscle precursor cells give rise to functional satellite cells in vivo. Neuromuscul. Disord. 17, 631–638 [DOI] [PubMed] [Google Scholar]
- Flanagan S.P. (1966). “Nude,” a new hairless gene with pleiotropic effects in the mouse. Genet. Res. 8, 295–309 [DOI] [PubMed] [Google Scholar]
- Goudenege S., Lebel C., Huot N.B., et al. (2012). Myoblasts derived from normal hESCs and dystrophic hiPSCs efficiently fuse with existing muscle fibers following transplantation. Mol. Ther. 20, 2153–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramolini A.O., Karpati G., and Jasmin B.J. (1999). Discordant expression of utrophin and its transcript in human and mouse skeletal muscles. J. Neuropathol. Exp. Neurol. 58, 235–244 [DOI] [PubMed] [Google Scholar]
- Gross J.G., and Morgan J.E. (1999). Muscle precursor cells injected into irradiated mdx mouse muscle persist after serial injury. Muscle Nerve 22, 174–185 [DOI] [PubMed] [Google Scholar]
- Gussoni E., Pavlath G.K., Lanctot A.M., et al. (1992). Normal dystrophin transcripts detected in Duchenne muscular dystrophy patients after myoblast transplantation. Nature 356, 435–438 [DOI] [PubMed] [Google Scholar]
- Gussoni E., Blau H.M., and Kunkel L.M. (1997). The fate of individual myoblasts after transplantation into muscles of DMD patients. Nat. Med. 3, 970–977 [DOI] [PubMed] [Google Scholar]
- Hoffman E.P., Brown R.H., Jr, and Kunkel L.M. (1987). Dystrophin: The protein product of the Duchenne muscular dystrophy locus. Cell 51, 919–928 [DOI] [PubMed] [Google Scholar]
- Hoffman E.P., Morgan J.E., Watkins S.C., and Partridge T.A. (1990). Somatic reversion/suppression of the mouse mdx phenotype in vivo. J. Neurol. Sci. 99, 9–25 [DOI] [PubMed] [Google Scholar]
- Huard J., Bouchard J.P., Roy R., et al. (1992). Human myoblast transplantation: Preliminary results of 4 cases. Muscle Nerve 15, 550–560 [DOI] [PubMed] [Google Scholar]
- Huard J., Roy R., Guérette B., et al. (1994). Human myoblast transplantation in immunodeficient and immunosuppressed mice: Evidence of rejection. Muscle Nerve 17, 224–234 [DOI] [PubMed] [Google Scholar]
- Karpati G. (1990). The principles and practice of myoblast transfer. Adv. Exp. Med. Biol. 280, 69–74 [DOI] [PubMed] [Google Scholar]
- Karpati G., Ajdukovic D., Arnold D., et al. (1993). Myoblast transfer in Duchenne muscular dystrophy. Ann. Neurol. 34, 8–17 [DOI] [PubMed] [Google Scholar]
- Lapan A.D., and Gussoni E. (2012). Isolation and characterization of human fetal myoblasts. Methods Mol. Biol. 798, 3–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapan A.D., Rozkalne A., and Gussoni E. (2012). Human fetal skeletal muscle contains a myogenic side population that expresses the melanoma cell-adhesion molecule. Hum. Mol. Genet. 21, 3668–3680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidov H.G.W., Byers T.J., Watkins S.C., and Kunkel L.M. (1990). Localization of dystrophin to postsynaptic regions of central nervous system cortical neurons. Nature 348, 725–728 [DOI] [PubMed] [Google Scholar]
- Lu Q.L., Morris G.E., Wilton S.D., et al. (2000). Massive idiosyncratic exon skipping corrects the nonsense mutation in dystrophic mouse muscle and produces functional revertant fibers by clonal expansion. J. Cell Biol. 148, 985–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell J.R., Kissel J.T., Amato A.A., et al. (1995). Myoblast transfer in the treatment of Duchenne's muscular dystrophy. N. Engl. J. Med. 333, 832–838 [DOI] [PubMed] [Google Scholar]
- Meng J., Adkin C.F., Arechavala-Gomeza V., et al. (2010). The contribution of human synovial stem cells to skeletal muscle regeneration. Neuromuscul. Disord. 20, 6–15 [DOI] [PubMed] [Google Scholar]
- Meng J., Adkin C.F., Xu S.W., et al. (2011). Contribution of human muscle-derived cells to skeletal muscle regeneration in dystrophic host mice. PLoS One 6, e17454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco A.P., Neve R.L., Colletti-Feener C., et al. (1986). Isolation of candidate cDNAs for portions of the Duchenne muscular dystrophy gene. Nature 323, 646–650 [DOI] [PubMed] [Google Scholar]
- Morandi L., Bernasconi P., Gebbia M., et al. (1995). Lack of mRNA and dystrophin expression in DMD patients three months after myoblast transfer. Neuromuscul. Disord. 5, 291–295 [DOI] [PubMed] [Google Scholar]
- Negroni E., Riederer I., Chaouch S., et al. (2009). In vivo myogenic potential of human CD133+ muscle-derived stem cells: A quantitative study. Mol. Ther. 17, 1771–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumeyer A.M., Cros D., McKenna-Yasek D., et al. (1998). Pilot study of myoblast transfer in the treatment of Becker muscular dystrophy. Neurology 51, 589–592 [DOI] [PubMed] [Google Scholar]
- Pantelouris E.M. (1968). Absence of thymus in a mouse mutant. Nature 217, 370–371 [DOI] [PubMed] [Google Scholar]
- Partridge T.A., Morgan J.E., Coulton G.R., et al. (1989). Conversion of mdx myofibers from dystrophin negative to positive by injection of normal myoblasts. Nature 337, 176–179 [DOI] [PubMed] [Google Scholar]
- Pye D., Watt D.J., Walker C., et al. (2004). Identification of the RAG-1 as a suitable mouse model for mitochondrial DNA disease. Neuromuscul. Disord. 14, 329–336 [DOI] [PubMed] [Google Scholar]
- Rivier F., Alkan O., Flint A.F., et al. (2004). Role of bone marrow cell trafficking in replenishing skeletal muscle SP and MP cell populations. J. Cell Sci. 117, 1979–1988 [DOI] [PubMed] [Google Scholar]
- Rybakova I.N., and Ervasti J.M. (2005). Identification of spectrin-like repeats required for high affinity utrophin-actin interaction. J. Biol. Chem. 280, 23018–23023 [DOI] [PubMed] [Google Scholar]
- Shultz L.D., Lang P.A., Christianson S.W., et al. (2000). NOD/LtSz-Rag1null mice: An immunodeficient and radioresistant model for engraftment of human hematolymphoid cells, HIV infection, and adoptive transfer of NOD mouse diabetogenic T cells. J. Immunol. 164, 2496–2507 [DOI] [PubMed] [Google Scholar]
- Shultz L.D., Lyons B.L., Burzenski L.M., et al. (2005). Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2Rγnull mice engrafted with mobilized human hemopoietic stem cells. J. Immunol. 174, 6477–6489 [DOI] [PubMed] [Google Scholar]
- Shultz L.D., Ishikawa F., and Greiner D.L. (2007). Humanized mice in translational biomedical research. Nat. Rev. Immunol. 7, 118–130 [DOI] [PubMed] [Google Scholar]
- Tedesco F.S., Gerli M.F., Perani L., et al. (2012). Transplantation of genetically corrected human iPSC-derived progenitors in mice with limb-girdle muscular dystrophy. Sci. Transl. Med. 4, 140ra89. [DOI] [PubMed] [Google Scholar]
- Tennyson C.N., Klamut H.J., and Worton R.G. (1995). The human dystrophin gene requires 16 hours to be transcribed and is cotranscriptionally spliced. Nat. Genet. 9, 184–190 [DOI] [PubMed] [Google Scholar]
- Torrente Y., Belicchi M., Sampaolesi M., et al. (2004). Human circulating AC133+ stem cells restore dystrophin expression and ameliorate function in dystrophic skeletal muscle. J. Clin. Invest. 114, 182–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T., Lu Q.L., Morgan J.E., et al. (2006). Expansion of revertant fibers in dystrophic mdx muscles reflects activity of muscle precursor cells and serves as an index of muscle regeneration. J. Cell Sci. 119, 2679–2687 [DOI] [PubMed] [Google Scholar]
- Zheng B., Cao B., Crisan M., et al. (2007). Prospective identification of myogenic endothelial cells in human skeletal muscle. Nat. Biotechnol. 25, 1025–1034 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.