Abstract
Trichoderma viridescens is recognised as a species complex. Multigene analyses based on the translation elongation factor 1-alpha encoding gene (tef1), a part of the rpb2 gene, encoding the second largest RNA polymerase subunit and the larger subunit of ATP citrate lyase (acl1) reveals 13 phylogenetic species with little or no phenotypic differentiation. This is the first use of acl1 in Trichoderma phylogenetics. The typification of T. viridescens s.str. is clarified and Hypocrea viridescens is replaced by the new name T. paraviridescens. Besides these two species, eleven are phylogenetically recognised and T. olivascens, T. viridarium, T. virilente, T. trixiae, T. viridialbum, T. appalachiense, T. neosinense, T. composticola, T. nothescens and T. sempervirentis are formally described and illustrated. Several species produce yellow diffusing pigment on cornmeal dextrose agar, particularly after storage at 15 °C, while T. olivascens is characterised by the formation of an olivaceous pigment. The results are compared with earlier publications on this group of species.
Keywords: acl1, Hypocrea, Hypocreaceae, phylogenetic analysis, rpb2, systematics, tef1
INTRODUCTION
In contrast to its original morphological definition (Bissett 1991), Trichoderma sect. Trichoderma or the Viride clade is currently conceived in a phylogenetic sense (cf. Jaklitsch 2011). This group of species is one of the largest and most complex in Trichoderma, with varying and often subtle morphological differences that do not always correlate with the results from phylogenetic inferences (Jaklitsch et al. 2006, 2012, Samuels et al. 2006a, Jaklitsch 2009, 2011). In these works, the adopted principle of species recognition was based on a combination of phylogenetic and phenotypic traits. Species within the T. koningii species aggregate were rather narrowly defined (Samuels et al. 2006a), while Jaklitsch et al. (2006) focussed on the distinction between T. viride and the newly introduced T. viridescens, based on a concept that gave asexual morph morphology a dominant criterion for species definition and recognition. The phylogenetic study by Jaklitsch et al. (2006) was only based on tef1 introns, and T. viridescens was conceived in a phenotypically homogeneous sense, while the complex structure of the various subclades within the monophyletic T. viridescens complex remained taxonomically unresolved.
In recent years several new species (T. aeroaquaticum, T. asperelloides, Hypocrea caerulescens, T. evansii, H. hispanica, T. junci, T. martiale, T. lieckfeldtiae, T. paucisporum, T. samuelsii, T. subeffusum, T. theobromicola, T. valdunense and T. yunnanense) have been established in sect. Trichoderma (Samuels et al. 2006b, 2010, Yu et al. 2007, Hanada et al. 2008, Samuels & Ismaiel 2009, Jaklitsch 2011, Jaklitsch et al. 2012, Yamaguchi et al. 2012).
Since 2006 numerous strains referable to T. viridescens sensu Jaklitsch et al. (2006) have been collected in southern Europe and the Canary Islands. These are the primary source of the present work, in which the T. viridescens complex was analysed using three phylogenetic markers and several new species were resolved. The starting point of the present study was the detection of a diffuse olivaceous pigment in many strains of T. viridescens s.lat. after storage at 15 °C, which correlated with identical tef1 sequences of the respective strains. These strains are described below as T. olivascens. We include in addition some South American strains from the study by Hoyos-Carvajal et al. (2009) and others characterised at the USDA-ARS that were not included in Jaklitsch et al. (2006). Finally, we compare the newly delimited species with data of previous publications and evaluate all GenBank tef1 accessions labelled as T. viridescens.
MATERIALS AND METHODS
Isolates and specimens
The isolates used in this study originated from ascospores or conidia of Trichoderma sexual or asexual morph specimens collected mainly on dead plant material or obtained from soil or from living plant tissues as endophytes. Numbers of strains including NCBI GenBank accession numbers of gene sequences used to compute the phylogenetic trees are listed in Table 1. The following strain acronyms have been used: acronyms of official culture collections (ATCC, CBS, DAOM, ICMP, IMI: see WFCC homepage http://www.wfcc.info/ccinfo/home/); BBA (Biologische Bundesanstalt für Land- und Forstwirtschaft, Institute of Plant Virology, Microbiology, and Biosafety, Berlin, Germany; now at Julius Kühn Institut, Braunschweig); C.P.K. strains are maintained at the University of Technology Vienna; strain acronym abbreviations such as J.B. NZ, J.B. PER or PER are those of J. Bissett (Agriculture and Agri-Food Canada; see Jaklitsch et al. 2006, Hoyos-Carvajal et al. 2009), specimens and strains collected or received by W. Jaklitsch are Hypo, S and W.J., strains collected or received by G.J. Samuels are abbreviated by G.J.S., N.R., Tr or V.S.L. (V.S. Lopez); UNISS strains are from the University of Sassari, Sardinia, Italy, and the strain acronym VI was used by Hageskal et al. (2008). Representative isolates have been deposited at the Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands (CBS) or the American Type Culture Collection, Manassas, VA (ATCC). Specimens of G.J. Samuels have been deposited in BPI, those of W. Jaklitsch in the Herbarium of the Institute of Botany, University of Vienna (WU).
Table 1.
Strains and NCBI GenBank accessions used in phylogenetic analyses.
| Taxon | Clade | Strain | Country | Substrate | GenBank accessions |
||
|---|---|---|---|---|---|---|---|
| acl1 | rpb2 | tef1 | |||||
| Trichoderma olivascens | v1 | Hypo 273 = CBS 119322 = C.P.K. 2047 | UK | Fagus sylvatica, wood | KC285717 | KC285750 | DQ672609 |
| C.P.K. 998 | Russia | soil | AY665706 | ||||
| C.P.K. 999 | Russia | soil | AY665707 | ||||
| G.J.S. 05-185 | Iran | Vitis sylvestris | DQ841720 | ||||
| G.J.S. 05-466 | UK | Fagus sylvatica, stem endophyte | KC285598 | ||||
| G.J.S. 05-482 | UK | Fagus sylvatica, stem endophyte | DQ841728 | ||||
| S34 | Italy | Acer opalus | KC285718 | KC285751 | KC285615 | ||
| S46 | Italy | Alnus cordata | KC285623 | ||||
| S80 | Italy | deciduous wood | KC285629 | ||||
| S96 | Italy | Fagus sylvatica | KC285630 | ||||
| S105 | Italy | Fagus sylvatica | KC285599 | ||||
| S107 | Italy | Stereum subtomentosum / Acer obtusatum | KC285600 | ||||
| S110 | Italy | Anthostoma decipiens, Melogramma campylosporum/Carpinus betulus | KC285601 | ||||
| S115 | Italy | Steccherinum ochraceum / Carpinus betulus | KC285602 | ||||
| S135 | Italy | Carpinus betulus | KC285603 | ||||
| S140 | Italy | Carpinus betulus | KC285604 | ||||
| S166 | Spain (La Palma) | Ocotea foetens | KC285605 | ||||
| S185 | Spain (La Palma) | Castanea sativa | KC285606 | ||||
| S198 | Portugal (Madeira) | Ocotea foetens | KC285607 | ||||
| S225 | Spain (Tenerife) | Biscogniauxia sp. / Persea indica | KC285608 | ||||
| S233 | Spain (Tenerife) | Prunus lusitanica | KC285609 | ||||
| S289 | Croatia | Quercus cerris | KC285610 | ||||
| S290 | Croatia | Quercus pubescens | KC285611 | ||||
| S294 | Croatia | Fraxinus ornus | KC285612 | ||||
| S329 | Spain | Fagus sylvatica | KC285613 | ||||
| S339 | Spain | Fraxinus sp. | KC285614 | ||||
| S362 | France | Phyllostachys sp. | KC285616 | ||||
| S364 | France | Hymenochaete corrugata / Corylus avellana | KC285617 | ||||
| S405 | Spain (Mallorca) | Quercus ilex | KC285618 | ||||
| S407 | Spain (Mallorca) | Quercus ilex | KC285619 | ||||
| S412 | Spain (Mallorca) | Quercus ilex | KC285620 | ||||
| S428 | Spain (La Palma) | Laurus novocanariensis | KC285621 | ||||
| S457 | Spain (La Palma) | Laurus novocanariensis | KC285622 | ||||
| S475 = CBS 132574; type | Spain (Tenerife) | Ganoderma applanatum | KC285719 | KC285752 | KC285624 | ||
| S612 | Greece (Corfu) | Quercus pubescens | KC285625 | ||||
| S616 | Greece (Corfu) | Corticiaceae / Quercus ilex | KC285626 | ||||
| S631 | Greece (Corfu) | Quercus coccifera | KC285627 | ||||
| S632 | Greece (Corfu) | Quercus ilex | KC285628 | ||||
| Trichoderma viridescens | v2 | CBS 433.34 | UK | rotten apples | AF456905 | ||
| G.J.S. 99-18 | Japan | Pinus radiata, wood | DQ307519 | ||||
| S1 | Italy (Sardinia) | Cistus monspeliensis | KC285725 | KC285757 | KC285634 | ||
| S41 | Italy | Spartium junceum | KC285643 | ||||
| S47 | Italy | Alnus cordata | KC285647 | ||||
| S55a | Italy | Fraxinus ornus | KC285652 | ||||
| S70 | Italy | Quercus pubescens | KC285655 | ||||
| S74 | Italy | Olea europaea | KC285656 | ||||
| S188 | Spain (La Palma) | Castanea sativa | KC285635 | ||||
| S220 | Spain (Tenerife) | Erica arborea | KC285636 | ||||
| S226 | Spain (Tenerife) | Rumex lunaria | KC285637 | ||||
| S244 | Spain (Tenerife) | Eucalyptus globulus | KC285638 | ||||
| S323 | Spain | Ulex europaeus | KC285639 | ||||
| S350 | Spain | Phyllostachys sp. | KC285640 | ||||
| S382 | Spain | Hedera helix | KC285641 | ||||
| S406 | Spain (Mallorca) | Quercus ilex | KC285642 | ||||
| S436 | Spain (La Palma) | Stereum hirsutum / Malus sp. | KC285644 | ||||
| S438 | Spain (La Palma) | Chamaecytisus proliferus | KC285645 | ||||
| S452 = CBS 132573, epitype | Spain (La Palma) | Chamaecytisus proliferus | KC285726 | KC285758 | KC285646 | ||
| S471 | Spain (Tenerife) | Eucalyptus globulus | KC285648 | ||||
| S535 | Spain | Rosmarinus officinalis | KC285649 | ||||
| S538 | Spain | Populus nigra | KC285650 | ||||
| S559a | France | Sarothamnus scoparius | KC285651 | ||||
| S588 | Greece (Crete) | Platanus orientalis | KC285653 | ||||
| S607 | Greece (Crete) | Platanus orientalis | KC285654 | ||||
| Tr 4 | USA (OR) | Pseudotsuga menziesii, root | DQ307520 | ||||
| Trichoderma viridarium | v3 | G.J.S. 04-81 | Italy | soil | DQ841709 | ||
| G.J.S. 89-142 = CBS 120065 | USA (NC) | decorticated wood | AY376049 | ||||
| G.J.S. 94-118 = IMI 374788 | France | Carpinus bark | DQ307510 | ||||
| G.J.S. 98-129 = CBS 101928 | France | bark | DQ307542 | ||||
| G.J.S. 98-182 = W.J. 1223 = CBS 120067 | Austria | Carpinus | DQ307511 | ||||
| Hypo 234 = CBS 119323 = C.P.K. 2045 | Germany | Picea abies, wood | DQ672607 | ||||
| Hypo 246 = C.P.K. 2046 | UK | Fagus sylvatica, wood | KC285727 | KC285759 | DQ672608 | ||
| Hypo 340 = C.P.K. 2139 | Germany | Fagus sylvatica | KC285657 | ||||
| S54 | Italy | Ostrya carpinifolia | KC285663 | ||||
| S95 | Italy | Fagus sylvatica | KC285729 | KC285762 | KC285665 | ||
| S136 = CBS 132568; ex-type | Italy | Carpinus betulus | KC285728 | KC285760 | KC285658 | ||
| S326 | Spain | Carpinus betulus | KC285659 | ||||
| S345 | Spain | deciduous wood | KC285660 | ||||
| S351 | France | Quercus robur | KC285661 | ||||
| S492 | Spain | Acacia retinodes | KC285761 | KC285662 | |||
| S589 | Greece (Crete) | Castanea sativa | KC285664 | ||||
| Trichoderma paraviridescens | v4 | ATCC 20898 | USA (NY) | soil | DQ307518 | ||
| CBS 274.79 | Austria | wood | DQ307513 | ||||
| C.P.K. 891 | Austria | soil | AY665708 | ||||
| G.J.S. 04-202 | Switzerland | soil | DQ841710 | ||||
| G.J.S. 05-115 | Switzerland | soil | FJ463283 | ||||
| G.J.S. 05-144 | USA (WI) | soil | KC285666 | ||||
| G.J.S. 05-172 | USA (WI) | soil | KC285667 | ||||
| G.J.S. 05-464 | UK | Fagus sylvatica, trunk endophyte | DQ841714 | ||||
| Hypo 12 = CBS 119324 = C.P.K. 942 | Austria | Picea abies | KC285668 | ||||
| Hypo 38 = C.P.K. 947 | Austria | Picea abies | DQ672604 | ||||
| Hypo 156 = C.P.K. 2043 | Austria | Fagus sylvatica | DQ672605 | ||||
| Hypo 229 = C.P.K. 2044 | Germany | Picea abies | KC285669 | ||||
| Hypo 372 = CBS 119321 = C.P.K. 2140; type | Austria | Fagus sylvatica | KC285730 | KC285763 | DQ672610 | ||
| S16 | Italy (Sardinia) | Quercus virgiliana | KC285732 | KC285765 | KC285673 | ||
| S31 | Italy | Castanea sativa | KC285674 | ||||
| S32 | Italy | Castanea sativa | KC285675 | ||||
| S36 | Italy | Ostrya carpinifolia | KC285766 | KC285678 | |||
| S68 | Italy | Prunus dulcis | KC285688 | ||||
| S69 | Italy | Quercus pubescens | KC285689 | ||||
| S111 | Italy | Carpinus betulus | KC285670 | ||||
| S122 = CBS 132566 | Italy | Hippocrepis emerus | KC285731 | KC285764 | KC285671 | ||
| S151 | Italy | Quercus cerris | KC285672 | ||||
| S324 | Spain | ?Castanea sativa | KC285676 | ||||
| S327 | Spain | Datronia mollis / Carpinus betulus | KC285677 | ||||
| S374 | France | Salix caprea | KC285679 | ||||
| S375 | France | Picea abies | KC285680 | ||||
| S490 | Spain | Cytisus linifolius | KC285681 | ||||
| S497 | Spain | Eucalyptus globulus | KC285682 | ||||
| S598 | Greece (Crete) | Juglans regia | KC285683 | ||||
| S604 | Greece (Crete) | Platanus orientalis | KC285684 | ||||
| S613 | Greece (Corfu) | Juglans regia | KC285685 | ||||
| S619 | Greece (Corfu) | Rhytidhysteron rufulum / Hippocrepis emerus | KC285686 | ||||
| S626 | Greece (Corfu) | Ulmus minor | KC285687 | ||||
| UNISS104 | Italy (Sardinia) | soil | EF488108 | ||||
| UNISS179 | Italy (Sardinia) | soil | EF488122 | ||||
| UNISS376S = C.P.K. 2069 | Italy (Sardinia) | soil | DQ790657 | ||||
| UNISS3b7 = C.P.K. 2084 | Italy (Sardinia) | soil | DQ790658 | ||||
| VI03691 | Norway | drinking water | AM498517 | ||||
| VI03905 | Norway | drinking water | AM498523 | ||||
| VI03921 | Norway | drinking water | AM498503 | ||||
| VI03928 | Norway | drinking water | AM498505 | ||||
| VI03935 | Norway | drinking water | AM498510 | ||||
| VI03937 | Norway | drinking water | AM498511 | ||||
| VI03962 | Norway | drinking water | AM498513 | ||||
| VI03986 | Norway | drinking water | AM498520 | ||||
| VI03987 | Norway | drinking water | AM498514 | ||||
| VI03988 | Norway | drinking water | AM498515 | ||||
| VI03997 | Norway | drinking water | AM498506 | ||||
| VI03998 | Norway | drinking water | AM498507 | ||||
| Trichoderma virilente | v5 | DAOM 234234 | Guatemala | wood | EU280009 | ||
| S108 | Italy | Hedera helix | KC285690 | ||||
| S145 | Italy | Cistus salviifolius | KC285691 | ||||
| S281 = CBS 132569; ex-type | Croatia | Fraxinus ornus | KC285733 | KC285767 | KC285692 | ||
| S335 | Spain | Diatrypaceae / Ulex europaeus | KC285693 | ||||
| S517 | Spain | Calicotome spinosa | KC285734 | KC285768 | KC285694 | ||
| S520 | Spain | Pistacia lentiscus | KC285735 | KC285769 | KC285695 | ||
| S531 | Spain | Rhamnus frangula ssp. baetica | KC285696 | ||||
| S546 | Spain | Quercus cf. canariensis | KC285697 | ||||
| S570 | Italy | Robinia pseudacacia | KC285698 | ||||
| S585 | Greece (Crete) | Citrus sinensis | KC285699 | ||||
| S630 | Greece (Corfu) | Quercus coccifera | KC285700 | ||||
| S635 | Greece (Corfu) | Cercis siliquastrum | KC285701 | ||||
| Trichoderma sp. | v6 | DAOM 233967 | Peru | ?soil | EU280020 | ||
| J.B. PER43 | Peru | soil | DQ845420 | ||||
| J.B. PER52 | Peru | soil | DQ845421 | ||||
| PER15-1 | Peru | ?soil | EU280027 | ||||
| Trichoderma trixiae | v7 | ATCC 32630 = Tr 26 | Sweden | Fagus wood | KC285736 | KC285770 | DQ307526 |
| DAOM 172787 | Colombia? | ?soil | EU280016 | ||||
| G.J.S. 92-11 = ICMP 16297 | New Zealand? | Pinus radiata | KC285737 | KC285771 | DQ307524 | ||
| G.J.S. 99-11 = N.R. 6969 | Germany | soil | DQ841717 | ||||
| CBS 134702 = C.P.K. 2138 = Hypo 228; type | Germany | Picea abies | KC285738 | DQ672606 | |||
| Tr 5 | USA (OR) | Pseudotsuga menziesii, root infected with Phellinus weirii | DQ307525 | ||||
| Tr 6 | USA (OR) | see Tr 5 | AY376050 | ||||
| Trichoderma viridialbum | v8 | G.J.S. 07-138 | Mexico | soil | KC285702 | ||
| G.J.S. 07-139 | Mexico | soil | KC285703 | ||||
| G.J.S. 07-145 | Mexico | soil | KC285739 | KC285772 | KC285704 | ||
| S177 | Spain (La Palma) | Sideritis cf. canariensis | KC285740 | KC285773 | KC285705 | ||
| S250 = CBS 133495; type | Spain (Tenerife) | Descurainia bourgeauana | KC285741 | KC285774 | KC285706 | ||
| S429 | Spain (La Palma) | Erica arborea | KC285742 | KC285775 | KC285707 | ||
| TU Graz 6TSM1 = C.P.K. 3300 | Spain (Tenerife) | soil | EU871009 | ||||
| VI03958 | Norway | drinking water | AM498519 | ||||
| VI03963 | Norway | drinking water | AM498518 | ||||
| V.S.L. 11 | Mexico | soil | HM535602 | ||||
| V.S.L. 15 | Mexico | soil | HM535601 | ||||
| V.S.L. 16 | Mexico | soil | HM535603 | ||||
| Trichoderma appalachiense | v9 | G.J.S. 00-67 | USA (WV) | decorticated wood | KC285743 | DQ307502 | |
| G.J.S. 97-216 | USA (VA) | decorticated wood | KC285708 | ||||
| CBS 133558 = G.J.S. 97-243, ex-type | USA (GA) | decorticated wood | KC285744 | DQ307503 | |||
| Trichoderma neosinense | v9a | G.J.S. 94-9 | Taiwan | bark | DQ307507 | ||
| G.J.S. 94-10 | Taiwan | decorticated wood | KC285745 | KC285776 | DQ307506 | ||
| CBS 134884 = G.J.S. 94-11; ex-type | Taiwan | bark | KC285746 | KC285777 | DQ307508 | ||
| Trichoderma composticola | v10 | CBS 439.95 | Northern Ireland | mushroom compost | KC285720 | KC285753 | AY937413 |
| CBS 333.72 | Netherlands | unknown | DQ307523 | ||||
| G.J.S. 04-232 | Mexico | soil under Agave tequillensis | DQ841716 | ||||
| G.J.S. 97-274 = BBA 68432 | Russia | cardboard | DQ307505 | ||||
| CBS 133497 = S590; type | Greece (Crete) | Vitis vinifera | KC285721 | KC285754 | KC285631 | ||
| Trichoderma nothescens | v10a | G.J.S. 99-128 | Australia | bark | DQ307515 | ||
| CBS 134882 = G.J.S. 99-142; ex-type | Australia | bark | KC285722 | DQ307512 | |||
| G.J.S. 99-86 | Australia | Eucalyptus regnans | DQ307516 | ||||
| Trichoderma sp. | J.B. NZ61 | New Zealand | soil under fern | DQ845419 | |||
| Trichoderma sempervirentis | v11 | CBS 133498 = S599; type | Greece (Crete) | Acer sempervirens | KC285723 | KC285632 | KC285755 |
| S601 | Greece (Crete) | Acer sempervirens | KC285724 | KC285756 | KC285633 | ||
| Trichoderma sp. | DAOM 237554 = JB PER14-1 | Peru | soil | EU280026 | |||
| Trichoderma sp. VD1 | G.J.S. 02-87 | Sri Lanka | bark | DQ307544 | |||
| G.J.S. 03-151 = CBS 120068 | Ghana | pyrenomycete | DQ841711 | ||||
| Trichoderma vinosum | G.J.S. 02-54 = ICMP 16295= CBS 119086 | New Zealand | Nothofagus menziesii | DQ307528 | |||
| G.J.S. 99-156 = ICMP 16293 | Australia | bark | KC285747 | KC285778 | DQ307527 | ||
| G.J.S. 99-158 = ICMP 16294= CBS 119087, type | New Zealand | Nothofagus menziesii | KC285748 | KC285779 | AY376047 | ||
| G.J.S. 99-183 | Australia | bark | DQ841719 | ||||
| Trichoderma sp. | G.J.S. 97-241 | Thailand | bamboo | KC285709 | |||
| Hypocrea caerulescens | S2 | Italy (Sardinia) | Cistus monspeliensis | JN715602 | JN715615 | ||
| S8 | Italy (Sardinia) | Alnus glutinosa | JN715616 | ||||
| S195 = CBS 130011 | Spain (La Palma) | Erica arborea | KC285710 | JN715604 | JN715621 | ||
| S206 = CBS 130012 | Portugal (Madeira) | Clethra arborea | KC285711 | JN715605 | JN715624 | ||
| S232 | Spain (Tenerife) | Erica platycodon | KC285712 | JN715606 | JN715631 | ||
| Trichoderma koningii | Hypo 51 = CBS 119500 = C.P.K. 957 | Austria | Carpinus betulus | KC285713 | FJ860541 | KC285594 | |
| S22 | Italy (Sardinia) | Myrtus communis? | KC285714 | KC285749 | KC285595 | ||
| S227 | Spain (Tenerife) | Castanea sativa | JN715609 | KC285596 | |||
| Hypo 242 = C.P.K. 1937 | France | Fagus sylvatica | JN715611 | ||||
| Trichoderma neokoningii | G.J.S. 04-216 | Peru | Moniliophthora roreri / Theobroma cacao | DQ841718 | |||
| Trichoderma samuelsii | S5 = CBS 130537 | Italy (Sardinia) | Hymenochaete sp. / Cistus monspeliensis | KC285715 | JN715599 | JN715655 | |
| S42 | Italy | Quercus pubescens | JN715598 | JN715652 | |||
| S398 | Spain (Mallorca) | Stereum sp. / Quercus ilex | JN715654 | ||||
| S537 | Spain | Phyllostachys sp. | KC285716 | KC285597 | |||
| Trichoderma gamsii | G.J.S. 05-111 = CBS 120072 | Italy (Pisa) | Ricinus communis | DQ841722 | |||
| S488 | Spain | Cytisus linifolius | JN715613 | ||||
| Trichoderma sp. | VI03967 | Norway | drinking water | AM498521 | |||
| VI03968 | Norway | drinking water | AM498525 | ||||
| VI03925 | Norway | drinking water | AM498504 | ||||
| VI03945 | Norway | drinking water | AM498508 | ||||
Culture preparation, growth rate determination and analysis of phenotype
Cultures were prepared and maintained as described previously (Evans et al. 2003, Jaklitsch et al. 2006, Jaklitsch 2009). Cultures used for study of asexual morph micro-morphology were grown on CMD, PDA or SNA (Nirenberg 1976, omitting the filter paper), at 25 °C under alternating 12 h cool white fluorescent light and 12 h darkness.
Growth rate experiments, recording of culture characteristics and morphological analyses of microscopic characters were carried out as described earlier (Jaklitsch et al. 2006, Jaklitsch 2009). These papers should also be consulted for the descriptive terminology used here. To avoid misunderstandings: if a colony is described as not zonate, then this refers to the mycelium in the agar and on the agar surface. Aerial hyphae and particularly conidiation structures may later on produce zonation. As different brands of PDA result in slightly different growth rates and particularly in different extent of conidiation and overall appearance of cultures, the brand (Difco: used by G.J. Samuels; Merck: used by W. Jaklitsch) is specified in the species descriptions. Microscopic observations were made in 3 % KOH, except for microtome sections that were examined in lactic acid. Chlamydospores were measured by observation of CMD culture plates on a compound microscope using a 40× objective. Data were gathered using a Nikon DXM1200 camera (Nikon ACT 1 software), a Nikon Coolpix 4500 or a Nikon DS-U2 digital camera and measured by using NIS-Elements D v. 3.0 or Scion Image Beta v. 4.0.2 (Scioncorp, Frederick, MD) software. Methods of microscopy included stereo-microscopy (stereo), Nomarski differential interference contrast (DIC), and epifluorescence (FL). The fluorescent brightener calcofluor (Sigma Fluorescent Brightener 28 C.I. 40622 Calcofluor white M2R) was used for FL. Scanning Electron Microscopy (SEM) was done as described by Jaklitsch et al. (2006). Kornerup & Wanscher (1978) was used as the colour standard.
DNA extraction and sequencing methods
The extraction of genomic DNA was performed as reported previously (Dodd et al. 2002, Jaklitsch et al. 2012) using either the DNeasy Plant Mini Kit (QIAgen GmbH, Hilden, Germany) or Puregene Genomic DNA Isolation Kit (Gentra Systems, Minneapolis, Minnesota). A part of the translation elongation factor 1 alpha (tef1) was amplified using the primers EF1-728F (Carbone & Kohn 1999) and TEF1 rev (Samuels et al. 2002) or TEF1LLErev (Jaklitsch et al. 2005). A c. 1 kb fragment of RNA polymerase II subunit B (rpb2) was amplified using the primer pair fRPB2-5f and fRPB2-7cr (Liu et al. 1999). A 0.9 kb fragment of the larger subunit of ATP citrate lyase (acl1) was amplified using the primers acl1-230up (5’-AGC CCG ATC AGC TCA TCA AG-3’) and acl1-1220low (5’-CCT GGC AGC AAG ATC VAG GAA GT-3’; Gräfenhan et al. 2011). DNA sequences were obtained after purification of the amplicons with a kit or an enzymatic PCR clean-up as described by Jaklitsch (2009) using the Big Dye Terminator cycle sequencing kit (Applied Biosystems, Foster City, California) and an automated DNA sequencer (ABI Genetic Analyzers, Applied Biosystems) with the same primers as in PCR.
Analyses of sequence data
Tef1 sequences obtained for numerous strains from southern Europe and the Canary Islands obtained in this study were aligned with sequences of the Large Viridescens Clade of Jaklitsch et al. (2006) using ClustalX, with all tef1 sequences labelled Hypocrea viridescens or Trichoderma viridescens retrieved from GenBank and with a set of sequences that were obtained at the USDA-ARS that had not been deposited in GenBank. After elimination of sequences that were too short or contained obvious errors, the alignment was used for further phylogenetic analyses.
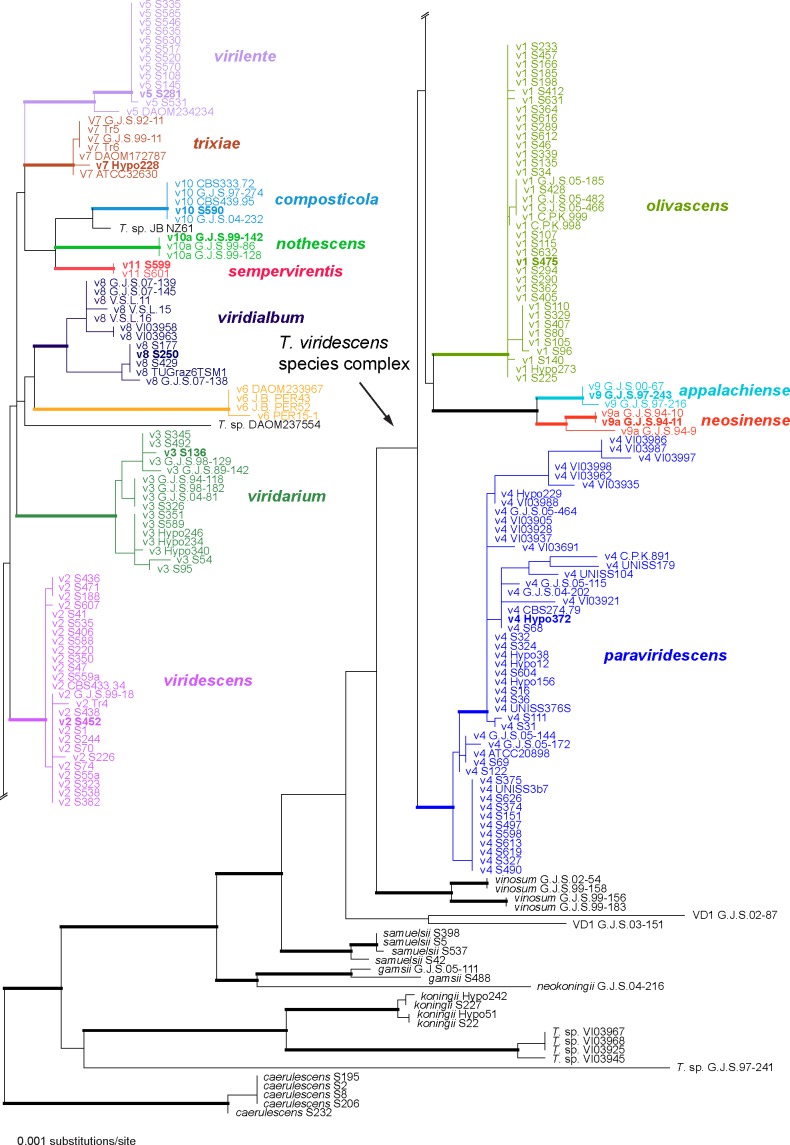
As tef1 introns are considered to have the highest potential of resolution within clades in Trichoderma (see e.g. Jaklitsch et al. 2006, Jaklitsch 2009), a limited number of members of each of the resulting clades, with the exception of Clade V6 (see Fig. 1), of which no cultures were available, were selected for the determination of rpb2 and acl1 sequences. Wherever possible, the ex-type strain and a second representative of each clade were selected.
Fig. 1.
Phylogram of the best maximum likelihood tree (lnL = −5182.0723) revealed by RAxML from an analysis of the tef1 sequence alignment. Branches formatted in bold had MP and ML bootstrap support above 80 % and 90 %, respectively, and Bayesian posterior probabilities above 90 %; accessions of type or ex-type strains of the respective clades are formatted in bold.
After selection of sequences to be included and the exclusion of excessive leading/trailing gap regions, the tef1 matrix used for phylogenetic analyses contained 210 sequences of 1 314 alignment positions. According to Jaklitsch et al. (2012), H. caerulescens was selected as outgroup. For ML analyses, 500 rounds of random addition of sequences as well as 500 fast bootstrap replicates were computed with RAxML (Stamatakis 2006a) as implemented in raxmlGUI 0.95 (Silvestro & Michalak 2012) using the GTRGAMMAI and GTRCATI substitution models, respectively. GTRGAMMAI corresponds to the well-known general time reversible model (Rodríguez et al. 1990) including estimation of invariant sites and assuming a discrete gamma distribution with six categories (GTR+I+G); GTRCATI efficiently approximates the GTR+I+G substitution model (Stamatakis 2006b). Maximum parsimony (MP) bootstrap analysis was performed with PAUP v. 4.0 b10 (Swofford 2002), using 1 000 replicates of heuristic search with 5 rounds of random addition of sequences and subsequent TBR branch swapping (MULTREES option in effect, COLLAPSE = MAXBRLEN, steepest descent option not in effect); each bootstrap replicate was limited to 1 million rearrangements. All molecular characters were unordered and given equal weight; analyses were performed with gaps treated as missing data. Bayesian analyses were performed with the computer program MrBayes (v. 3.1.2; Huelsenbeck & Ronquist 2001), implementing the GTR+I+G model of sequence substitutions. Three parallel runs of four incrementally heated, simultaneous Markov chains were performed over 5 million generations, of which every 500th tree was sampled in each run. The first 1 000 trees sampled were discarded, and a 90 % majority rule consensus of the remaining trees was computed to obtain posterior probabilities (PP). To test convergence of runs, the results were analysed using AWTY (Nylander et al. 2008); no indication of lack of convergence was detected.
The combined acl1-rpb2-tef1 sequence matrix contained 3 394 alignment positions (924, 1 167 and 1 303 characters from acl1, rpb2 and tef1, respectively). Prior to phylogenetic analyses, the approach of Wiens (1998) was applied to test for significant levels of localized incongruence among the three gene partitions, using the level of bootstrap support (Sung et al. 2007). For this, the 70 % maximum parsimony (MP) bootstrap consensus trees calculated for each individual partition were compared using the same parameters as for the combined analysis given below. No topological conflicts were observed between these bootstrap trees of acl1, rpb2 and tef1, indicating the absence of significant incongruence and combinability of the three matrices (Wiens 1998).
Maximum parsimony (MP), maximum Likelihood (ML) and Bayesian analyses (including selection of models of sequence substitution for the latter) were performed as described for tef1 and rpb2 in Jaklitsch et al. (2012). For MP analyses of the combined matrix, the transition/transversion (Ti/Tv) bias R was calculated with Mega v. 5.05 (Tamura et al. 2011), using the maximum composite likelihood (MCL) method and the substitution model of Tamura & Nei (1993). Because these values suggested a significant Ti/Tv bias (R = 2.5 for acl1, R = 3 for rpb2 and R = 1.8 for tef1), the transversions of the acl1, rpb2 and tef1 genes were weighted according to the R-values in the subsequent MP analyses (Birksa & Edwards 2002).
In ML and Bayesian analyses, substitution model parameters were calculated separately for the different gene regions included in the combined analyses. For ML analyses, 1 000 rounds of random addition of sequences as well as 1 000 thorough bootstrap replicates were computed with RAxML (Stamatakis 2006a) as implemented in raxmlGUI 0.95 (Silvestro & Michalak 2012) using the GTRGAMMA and GTRCAT algorithms, respectively. For Bayesian analyses using MrBayes v. 3.1.2 (Huelsenbeck & Ronquist 2001), three parallel runs of four incrementally heated simultaneous Markov chains were performed over 10 million generations, from which every 1 000th tree was sampled in each run. The models selected by Modeltest v. 3.6 (Posada & Crandall 1998) for acl1, rpb2 and tef1 using the hierarchical likelihood test were K80+G, TrNef+G and HKY+I+G, respectively. However, as the first two models could not be implemented in MrBayes, the following most similar models were chosen for the different data partitions: for rpb2, the general time reversible (GTR) model with gamma-distributed substitution rates; for acl1 and tef1 the HKY model of Hasegawa et al. (1985), assuming a proportion of invariant sites in tef1 and gamma-distributed substitution rates in both. The first 1 000 trees were discarded, and a 90 % majority rule consensus of the remaining trees was computed to obtain posterior probabilities. To test convergence of runs, the results were analysed using AWTY (Nylander et al. 2008); no indication of lack of convergence was detected. The sequence alignment files have been deposited in TreeBASE and are available at http://purl.org/phylo/treebase/phylows/study/TB2:S14013.
RESULTS
Phylogeny
Of the 1 314 characters included in the tef1 matrix, 237 were parsimony-informative. Fig. 1 shows the best ML tree (lnL = −5182.0723); the branches formatted in bold denote MP and ML bootstrap support above 80 % and 90 %, respectively, and Bayesian posterior probabilities above 90 %.
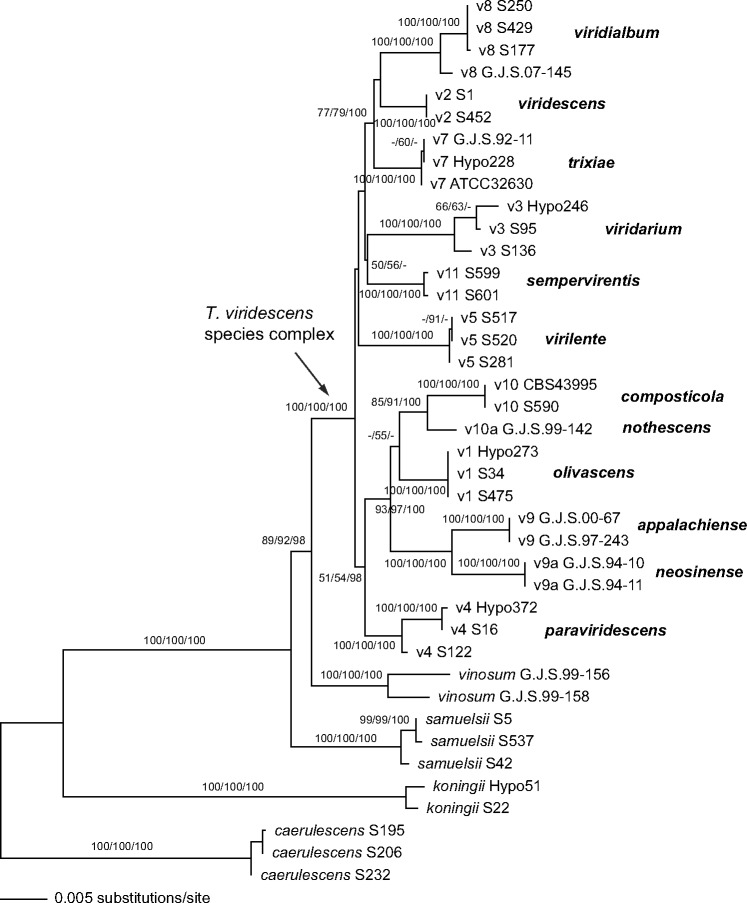
Of the 3 394 characters included in the combined matrix, 536 were parsimony informative (151 in acl1, 176 in rpb2, 209 in tef1). MP analyses revealed 18 MP trees with a score of 1082.7, which slightly differed in the position of the V5 clade relative to a V3–V11 clade at the base of the viridescens s.lat. clade (not shown). Fig. 2 shows the best ML tree (lnL = −9486.946), which is similar to the MP trees except for minor topological differences in few nodes with low or insignificant MP/ML support. Tree topologies of the Bayesian analyses were fully congruent with the ML tree. The three Bayesian runs revealed almost identical posterior probabilities. MP and ML bootstrap support above 50 % and Bayesian posterior probabilities above 90 % are given in Fig. 2 in this order above or below the branches.
Fig. 2.
Phylogram of the best maximum likelihood tree (lnL = −9486.946) revealed by RAxML from an analysis of the combined acl1-rpb2-tef1 alignment. MP and ML bootstrap support above 50 % and Bayesian posterior probabilities above 90 % are given in this order above or below the branches.
Much of the deeper tree backbone and all clades described here as new species were highly supported in both tef1 and multigene analyses (Fig. 1, 2). The clade containing the T. viridescens species complex (TVS clade) was highly supported in the multigene analyses, but in the tef1 tree received minor support in the MP bootstrap analysis (65 %) but high support in ML bootstrap and Bayesian analyses (89 % and 100 %, respectively). Within the TVS clade, much of the tree backbone was either unsupported or received only low support in both analyses (Fig. 1, 2).
Sexual morphs (‘teleomorphs’)
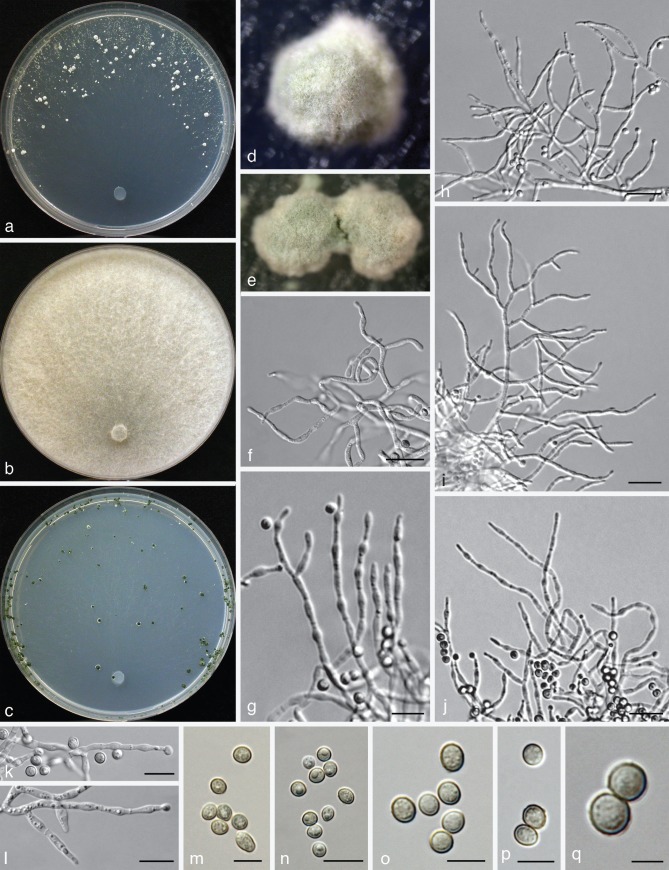
Sexual morphs are known for most of the clades of the T. viridescens species complex (cf. Large Viridescens Clade of Jaklitsch et al. 2006), but have so far not been found for the clades V6, V10 and V11. The frequency of sexual morph occurrence varies considerably among the clades: sexual morphs of Clade V1 (T. olivascens) are most common and widely distributed. They are found throughout Europe and most frequently in the Canary Islands. Clade V2 (T. viridescens s.str.) is common and has a wide distribution that includes southern Europe and Japan, but perithecial stromata of V2 have only been found in the Canary Islands. Clade V3 (T. viridarium) is common in southern Europe but we have only seen two sexual morph collections from Italy. Stromata of clade V4 (T. paraviridescens) have been found several times in central Europe (Austria, Germany), but in southern Europe only the asexual morph. Strains of V5 (T. virilente) are common in southern Europe but we have seen only one sexual morph collection, from Croatia. Members of clades V7 (T. trixiae) and V8 (T. virialbum) are apparently common and each has a wide distribution that includes Europe and America, but for V7 and V8 we have seen only immature collections of sexual morphs found once each, respectively, in Germany and the Canary island La Palma. Clades V9 (T. appalachiense), V9a (T. neosinense) and V10a (T. nothescens) are only known from sexual morph collections, respectively from eastern USA, Taiwan and south-eastern Australia.
Stromata of the different species of the T. viridescens species complex are morphologically and anatomically more or less indistinguishable. Due to this conservation of traits, which is also true for many other taxa of sect. Trichoderma, we omit stromal anatomy in most of the individual species descriptions, but give a generalised description here: Stromata are scattered to aggregated, sometimes developing from large effuse, first white to yellowish mycelial mats that can later break up into several discrete, individual part stromata. When fresh, stromata are typically 0.5–3 mm wide and 0.5–1.5 mm thick, pulvinate with varying outline and free, sometimes lobate margin; ostiolar dots lacking or inconspicuous, rarely appearing as dark dots or spots; ostioles only visible in longitudinal sections or from above at most as scarcely visible, minute, light dots; surface smooth, uneven or slightly tubercular, at first downy or velutinous, later often glabrous; in early stages in pale or light colours; yellow, pale orange to orange-brown or pale brown 5B5–6 to 6CD5–6, or reddish, later and at maturity typically dark reddish brown, 7–8EF5–8, 10CD7–8 to 9–10F7–8. Stromata when dry like the fresh ones, but distinctly flatter, mostly only up to 0.6 mm thick, discoid, flat pulvinate, placentiform or subeffuse; when young more distinctly downy than fresh, covered by brown hairs or finely velvety with short rust hairs, and surface more uneven, tubercular or rugose; colour medium to very dark reddish brown or nearly black, only rarely remaining orange-brown. Spore deposits white.
Stromal anatomy: Cortical tissue present around the entire stroma except for the point of attachment, comprising a textura angularis of rather indistinct and thin-walled cells with inhomogeneously deposited reddish brown to yellow- or orange-brown pigment. Hairs arising from surface cells usually abundant on young stromata, less common on mature stromata, 1- or several-celled, thin- or thick-walled, pale brownish, smooth to slightly verruculose. Subcortical tissue (below the cortex and between the perithecia) comprising mainly hyaline hyphae. Tissue below perithecia consisting of a more or less homogeneous, dense textura angularis–epidermoidea of hyaline to pale brownish, thin-walled cells, variably interspersed with hyphae; cells above the point of attachment sometimes arranged as a palisade. Ostiolar canal narrow, scarcely projecting beyond the stroma surface, periphysate, not containing differentiated apical cells. Perithecia flask-shaped, ellipsoidal or globose, 150–350 × 70–300 μm; peridium hyaline, not changing in 3 % KOH. Asci cylindrical, containing 8 uniseriate ascospores. Ascospores hyaline, verruculose or spinulose, disarticulating into two cells in the ascus; cells dimorphic, distal cells (sub)globose, ellipsoidal or cuneate, (3.5−)4–6(−7.5) × (3−)4–5(−6.5) μm, proximal cells oblong, ellipsoidal, cuneate or subglobose, (3.8−)4.5–6.5(−8.5) × (2.7−)3–4.5(−5.8) μm.
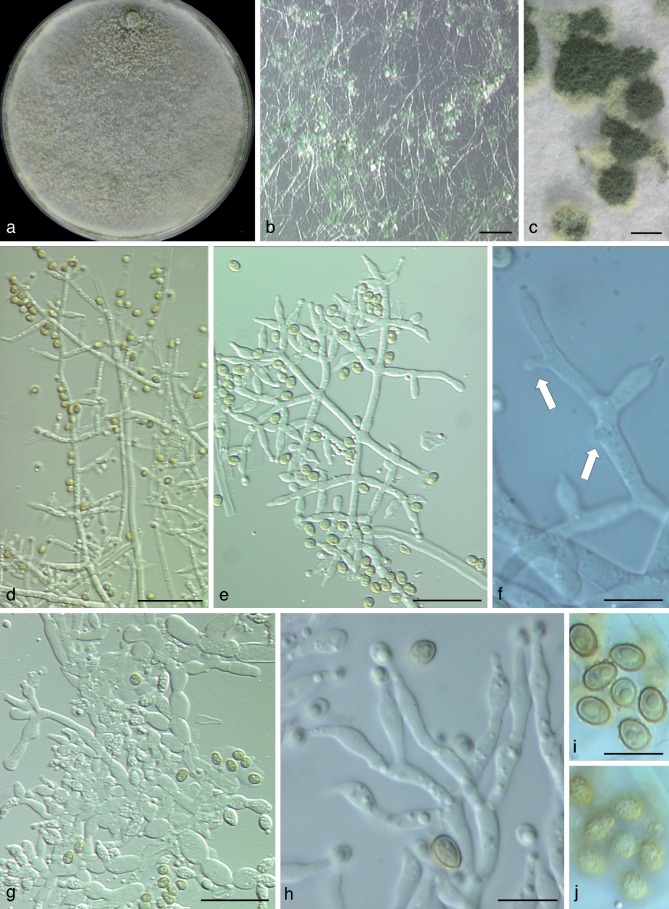
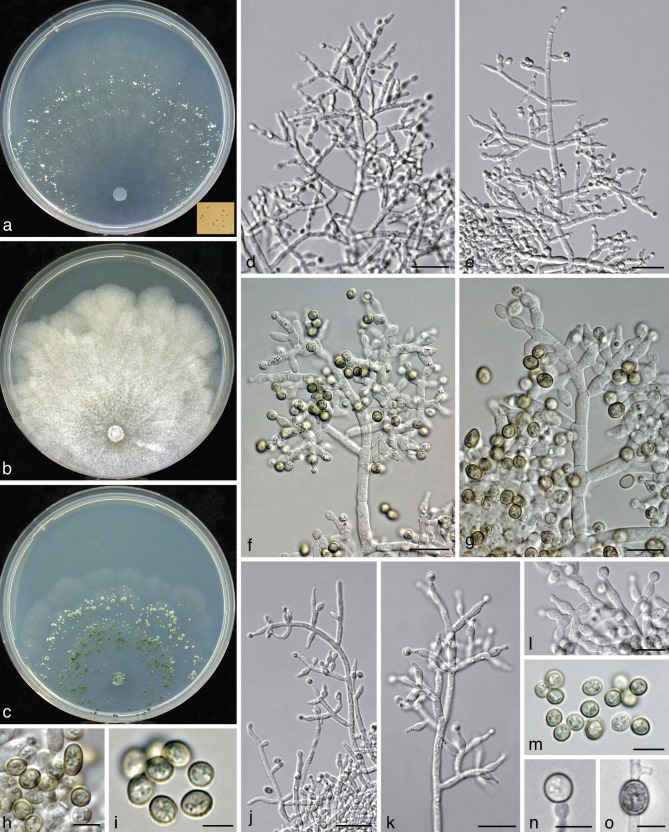
Asexual morph (‘anamorph’) and culture morphology
All species included in the Trichoderma viridescens species complex have the following traits in common: green, verruculose conidia, submoniliform terminal conidiophore branches (= ‘eidamia’-morphology = repetitive phialides = percurrently proliferating phialides) on CMD (and PDA) with considerable variations including greatly swollen cells, and distinct coconut-like odour on CMD (and PDA). Conidiation on PDA is generally absent or poor and then effuse, on SNA and CMD in pustules, which are usually larger and more abundant on SNA. As repetitive phialides usually do not occur on SNA, conidiophores are here mainly described from CMD. The arrangement of conidiation structures on CMD varies from effuse over loose shrubs to small compact pustules. Shrubs may be aggregations of simple conidiophores or a more complex reticulum arising from a single stipe and are up to 0.5 mm diam. Pustules are usually transparent under a 10× objective, only T. viridescens s.str. forms nearly opaque pustules.
All three conidiophore types circumscribed by Jaklitsch et al. (2006) for the Large Viride and Large Viridescens Clades, occur in the T. viridescens species complex. Pustules typically comprise dense central clusters without clear main axes and loose and often regularly tree-like peripheral conidiophores. This structure of pustules is also typical of T. stilbohypoxyli, but that species does not form submoniliform branches. The dense central clusters usually contain ‘type 1’ conidiophores, which are typical of peripheral conidiophores of T. viride. They are characterised by highly irregular, unpaired and sinuous branches and typically do not have a well-defined main axis. Conidiophores of shrubs and at the periphery of pustules belong to ‘type 2’ or ‘type 3’. They are characterised by a more or less readily discernable, well-developed main axis, from which lateral branches arise at or near 90°; the lateral branches are longer with distance from the tip and secondary branches, i.e. those arising from the lateral branches are shorter with distance from the point of departure of the branch from the main axis. Branches arise in pairs at the upper levels and often asymmetrically near the base. Phialides tend to terminate branches in more or less cruciate whorls of 3–4. The phialides are mostly straight, cylindrical or somewhat swollen at or slightly above or below the middle. The ‘type 3’ (‘eidamia’-) conidiophore is a variant of ‘type 2’ and differs from it basically by the production of percurrently proliferating phialides, and a more variable branching system. In ‘type 3’ conidiophores branches may be distinctly inclined upwards. In their extreme form they produce irregularly swollen, subglobose cells in the centre of old pustules on CMD and PDA, particularly in the subclades V7 (T. trixiae), V8 (T. viridialbum) and V10a (T. nothescens). The ‘type 3’ conidiophore occurs also in other species of the section, including T. vinosum, T. gamsii and T. neokoningii. In some clades of the T. viridescens species complex, however, particularly V3, V4, V5, V9, V9a and sometimes in V10, this morphology is reduced and often only one or two cells below the terminal phialide are enlarged. Conidiophores of Clade V3 deviate from typical conidiophores by rather steeply ascending and mostly unpaired side branches. Clade V8 lacks a distinct main axis generally. Phialides are lageniform and vary little. Conidia of the T. viridescens species complex show a rather uniform size range, but have marked tendencies regarding their shape: while conidia in V4, V8, V9, V9a, V10a and V11 are mostly subglobose, those of V1, V2, V5 and V10 are mostly ellipsoidal, and those of V3 and V7 are varying between these shapes.
Pigments on CMD
While no pigment is noted on SNA and on PDA pigment formation is usually indistinct in the T. viridescens species complex, conspicuous formation of diffusing pigment has been noted on CMD, particularly after prolonged storage of plates at 15 °C. This trait has not been recorded for all species, but the following observations are evident: Clade V1 (T. olivascens) consistently produced a (yellowish-, greyish- or brownish-)olivaceous pigment after several months, while Clades V4 (T. paraviridescens) and V11 consistently formed a rich yellow pigment. A similar pigment is sometimes formed in CMD agar in V3 and V10 and also by other species of the section, most notably T. petersenii.
Growth rates
All species of the T. viridescens species complex have their optimal growth temperature at 25 °C and typically a marked decrease in growth rate at 30 °C. None of these species grows at 35 °C. Strains of the T. viridescens complex fall into two groups defined by their growth rates, and there is little overlap between the groups. After 72 h on PDA at 25 °C, the colony radius of members of the fast-growing group (V1–V4, V7–10 and V10a) is (25−)30–40(−45) mm with V1 and V9a being at the lower end of this range, while the colony radius of members of the slow-growing group (V5 and V11) was only 15–20(−25) mm. A clear difference in growth rates was also noted within the first group between the clades V9 and V9a that reached a growth radius of 40–45 mm vs 25–35 mm, respectively, under the conditions given above. Differences in the colony radius after 3 d were somewhat more striking at 30 °C on Difco-PDA: < 20 mm in the clades V1, V2, V3, V7, V9a and V10a, and > 20 mm in V8, V9 and V10.
Biogeography and ecology
Trichoderma viridescens sensu Jaklitsch et al. (2006) is one of the most common species of the genus Trichoderma in Europe. However, only southern Europe and the Canary Islands have been screened sufficiently to reach a more or less representative sampling that includes collection of asexual colonies from plant material. Diversity data from other parts of Europe are mostly based on sexual morph material (Jaklitsch et al. 2006, Jaklitsch 2011) and no intensive collection of asexual morphs has been undertaken. Material from other regions has been gathered randomly and does therefore not form a basis for sound interpretation. In southern Europe more than 100 mostly asexual morph specimens of the T. viridescens species complex have been gathered, amounting to c. 16 % of all specimens of the genus collected there. All clades of the T. viridescens species complex occur in Europe except V6, V9, V9a and V10a. V6 is only known from Peru, V9 from eastern USA, V9a from Taiwan, and V10a from south-eastern Australia. Clade V5 is only known from southern Europe except for one strain from Guatemala (DAOM 234234), which is associated with this clade. The commonest species of the T. viridescens species complex in Europe are V1 (T. olivascens), V4 (T. paraviridescens) and V2 (T. viridescens), followed by V3, V5 and V8. These species have been occasionally collected in other continents and may be cosmopolitan, particularly in temperate to subtropical climates. V11 is only known from elevations above 700 m in Crete, Greece.
Ecologically, species of this clade do not differ from others in sect. Trichoderma, but are also similar to members of other clades of the genus. Most specimens have been obtained from dead wood and bark of broad-leaved trees and on fungi growing on them, sometimes conifers, less commonly on bamboos, other plant material, e.g. apples, few as endophytes from deciduous trees, notably Fagus, few from mushroom compost and some from drinking water or soil. The highest numbers of isolates from soil are found in the clades V4 and V8, and the small clade V6 only contains strains from soil. Endophytes have been found in V1 and V4. Isolates from drinking water belong to the clades V4 and V8, those from compost or cardboard only represent V10, those from bamboo belong to V1 and V2. Clade V7 seems to prefer coniferous trees. Sexual and asexual morphs that have been found to grow directly on fungi of various kind (basidiomycetes, disco- and pyrenomycetous ascomycetes) belong mainly to V1 (e.g. on Anthostoma, Biscogniauxia, Ganoderma, Hymenochaete, Melogramma, Steccherinum, Stereum, Terana and other corticiaceous fungi) and V4 (e.g. on Datronia, Rhytidhysteron). Clade V11 has so far only been found on Acer sempervirens.
Taxonomy
Twelve of the terminal clades that are well supported in both phylogenetic trees (Fig. 1, 2) are formally described or emended as species below. Clade V6 was not available for a detailed study. An overview of the taxa, arranged by the clade numbers of the phylogenetic trees follows:
| V1 | Trichoderma olivascens |
| V2 | T. viridescens |
| V3 | T. viridarium |
| V4 | T. paraviridescens |
| V5 | T. virilente |
| V6 | not named |
| V7 | T. trixiae |
| V8 | T. viridialbum |
| V9 | T. appalachiense |
| V9a | T. neosinense |
| V10 | T. composticola |
| V10a | T. nothescens |
| V11 | T. sempervirentis |
Clade V1
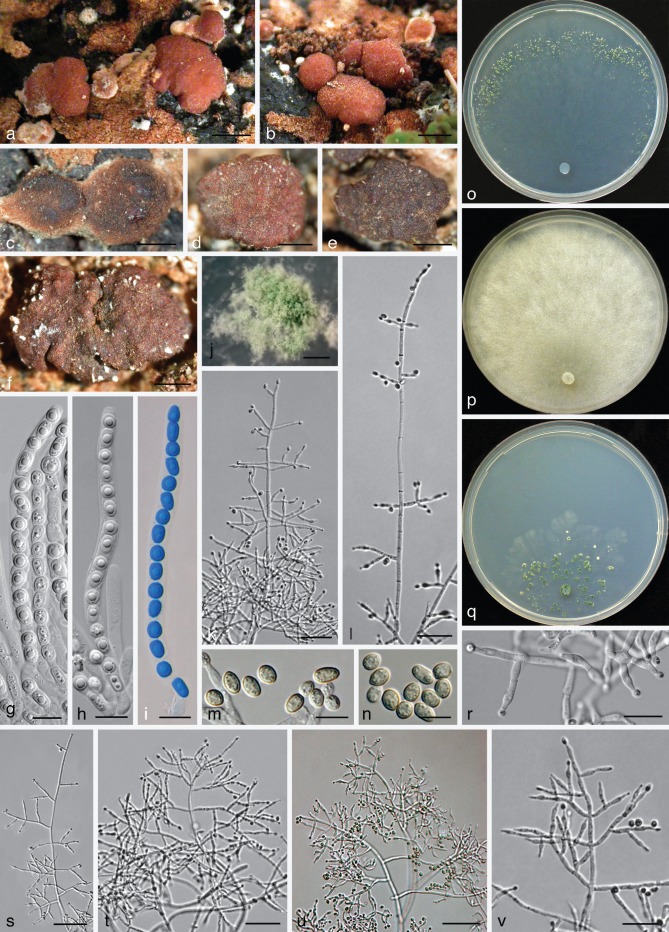
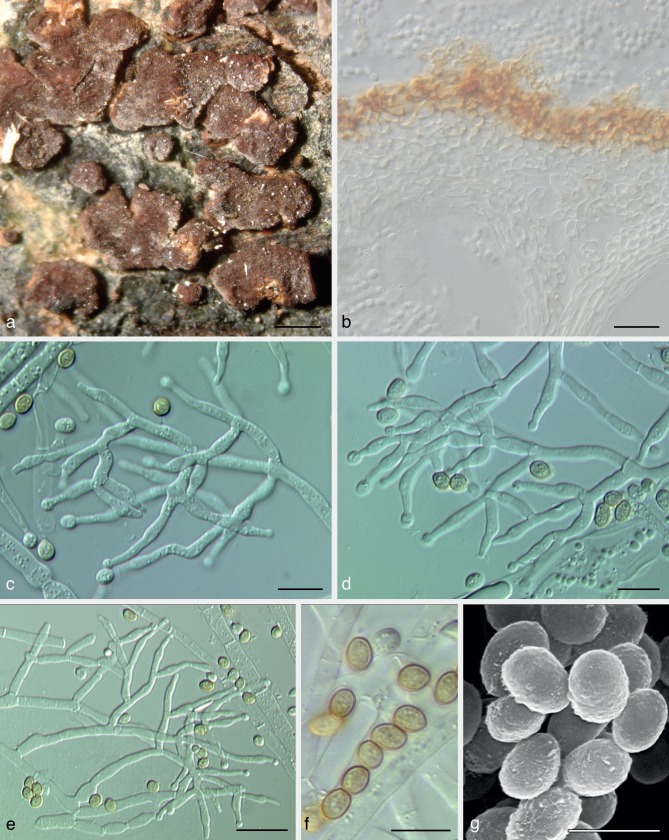
Trichoderma olivascens Jaklitsch, Samuels & Voglmayr, sp. nov. — MycoBank MB803621; Fig. 3
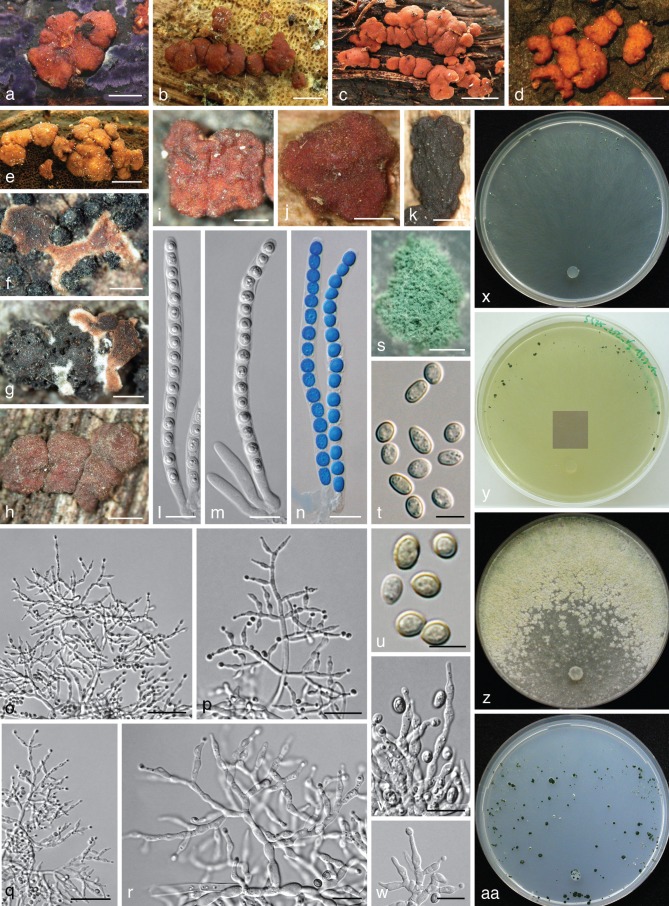
Fig. 3.
Trichoderma olivascens. a–n: Sexual morph. a–e. Fresh stromata (a. on Terana caerulea; b. on a resupinate polypore; e. on Ganoderma applanatum); f–k. dry stromata (f, g. immature; f. on Anthostoma decipiens; g. on Melogramma campylosporum); l–n. asci (n. in cottonblue/lactic acid). — o–aa: Asexual morph and cultures at 25 °C (CBS 132574 = S475). o–r. Conidiophores (after 10–11 d); s. conidiation pustule (12 d); t, u. conidia (10 d); v, w. phialides (10–11 d); x–aa. cultures (x. on CMD, 14 d; y. on CMD, 44 d; insert: S428 after 20 mo at 15 °C; z. on PDA, 14 d; aa. on SNA, 14 d); o–w: all from CMD (a: WU 31620; b, j: WU 24027; c, h: WU 31618; d, i: WU 31621; e, l–n: WU 31622; f, g, k: WU 31616). — Scale bars: a, b, d = 1.3 mm; c = 3 mm; e = 2 mm; f, j = 0.5 mm; g, h, k, s = 0.8 mm; i = 0.3 mm; l–n, r, v, w = 10 μm; o, q = 30 μm; p = 20 μm; t, u = 5 μm.
Etymology. For the olivaceous colouring of CMD cultures after extended storage at 15 °C.
Holotype. Spain, Islas Canarias, Tenerife, Macizo de Anaga, Las Carboneras, walking path to El Batán from the road to Taborno, N28°32′22″, W16°16′34″, elev. 845 m, on a basidioma of Ganoderma applanatum, holomorph, 16 Dec. 2010, W. Jaklitsch (WU 31622; ex-type culture S475 = CBS 132574; from ascospores).
Stromata scattered, gregarious or aggregated in various numbers, sometimes formed in larger clusters breaking up into up to 10 individual stromata, when fresh 1–3 mm diam, 0.5–1.5 mm thick, pulvinate with circular or angular outline, downy or velutinous and yellow, pale orange-brown or pale brown when young, when mature margin free, surface smooth or uneven, ostioles invisible or visible as very inconspicuous minute light dots; typically intensely reddish brown 10CD7–8 to 10F7–8. Stromata when dry (0.5−)0.7–2.1(−3.2) × (0.4−)0.6–1.5(−2.6) mm, 0.2–0.6(−1.1) mm thick (n = 32), pulvinate, discoid, placentiform or subeffuse; outline variable, circular to irregular; margin at first adnate, becoming free; ostioles typically invisible, sometimes appearing as inconspicuous diffuse dots; surface when immature velutinous due to brown or rust hyphae, rust, yellow-, orange- or red-brown, when mature smooth or slightly tubercular, rugose when old; typically dark reddish brown, dark purple to nearly black, rarely remaining orange-brown. Spore deposits white. Asci (88−)94–103(−109) × (5.2−)5.5–6.2(−6.8) μm, stipe (5−)9–16(−24) μm long (n = 50). Ascospores hyaline, spinulose, cells dimorphic, distal cells subglobose or ellipsoidal, (3.3−)4.0–5.0(−5.5) × (3.3−)3.8–4.5(−4.8) μm, l/w (0.9−)1.0–1.2(−1.5) (n = 90), proximal cells oblong, ellipsoidal or subglobose, (3.8−)4.5–6.0(−7.0) × (2.7−)3.2–4.0(−4.7) μm, l/w (1.0−)1.2–1.8(−2.6) (n = 90), proximal cell of basal ascospore typically more elongated.
Cultures and asexual morph — Optimal growth at 25 °C on all media, slow growth at 30 °C, no growth at 35 °C.
On CMD after 72 h colony radius 30–32 mm at 25 °C, 7–13 mm at 30 °C; mycelium covering the plate after 7–11 d at 25 °C. Colony hyaline, dense, circular, with distinct differences in hyphal width, not zonate; margin becoming wavy. Aerial hyphae inconspicuous. Autolytic excretions and coilings lacking. Odour strongly coconut-like after less than a wk. Agar turning yellowish 1B3–4, 2B2–3 from the centre after 5–15 d, after subsequent storage at 15 °C to c. 1 mo yellowish green to dull olivaceous-greenish, 29–30B3 to 30C4, 29–1CD4–6, after 8–23 mo dilute to dark or dull greyish olive or yellow-olive, 2–3E5–8. Chlamydospores uncommon, intercalary and terminal, subglobose, ellipsoidal or oblong, (6−)7–10.5(−12) × (4−)5.5–8.5(−9.5) μm, l/w (1.0−)1.1–1.5(−2.1) (n = 30). Conidiation at first effuse after 3–7 d on short erect conidiophores arising from surface hyphae; from c. 5 d on in small shrubs, aggregating tufts or pustules 0.5–2.5 mm diam, forming in 1–3(−5) usually diffuse, distal concentric zones; turning dark green 27–28F5–8 after 7–9 d. Pustules with a hairy or fluffy aspect, amorphous, loose or comprising a strongly condensed core and a loose periphery of loose, hairy conidiophores; pustules arising on often long, wide, thick-walled stipes that are asymmetrically branched into wide and long primary branches that can re-branch 2–3 times, forming often broad conidiophores without clearly defined or with short main axes, straight to slightly curved, 1.5–4 μm wide branches, inserted at right angles or inclined upwards, unpaired in lower regions and often paired towards their apices. Phialides arising from cells c. 2–3 μm wide, paired or in cruciform whorls of 3 or often solitary and repetitive, i.e. forming a submoniliform terminal branch of up to 4 cells in a chain including the terminal phialide. Phialides (5.0−)6.7–10.7(−15.5) × (2.0−)2.5–3.3(−4.0) μm, l/w (1.4−)2.2–3.9(−5.6), basal width (1.2−)1.5–2.2(−2.5) μm (n = 70), lageniform, often inequilateral, straight or slightly curved, with a more or less distinct widening below or at the middle; neck and often base narrow, neck often long; grading into nearly ampulliform within pustules. Conidia (3.4−)3.8–4.8(−5.7) × (2.7−)3–3.4(−3.9) μm, l/w (1−)1.2–1.6(−1.9) (n = 80), ellipsoidal or oval, often slightly attenuated toward one end, sometimes subglobose, green, at first smooth, becoming verruculose, scar sometimes distinctly truncate; sometimes forming chains when old.
Colony radius on PDA (Merck) after 72 h 30–32 mm at 25 °C, 6–8 mm at 30 °C; mycelium covering the plate after 1 wk at 25 °C. Colony circular, dense, not zonate, margin wavy. Autolytic excretions moderate to frequent, coilings sparse. Aerial hyphae abundant, forming a loose whitish mat with radial to substellate structure at its base; mat eventually becoming pustular. Conidiation noticeable after 3–5 d, at first effuse on aerial hyphae, later in broad, coalescing floccules or pustules with diffuse outline, to c. 3 mm diam, turning yellowish green to pale green 1B3–4, 2B3, 26–29B3 after 8–10 d. Diffusing pigment lacking, reverse yellowish 3–4B3, odour coconut-like.
Colony radius on SNA after 72 h 29–31 mm at 25 °C, 7–10 mm at 30 °C; mycelium covering the plate after 8–10 d at 25 °C. Colony hyaline, not zonate, dense; margin loose, becoming wavy or lobate. Aerial hyphae sparsely produced; autolytic excretions inconspicuous, coilings frequent; diffusing pigment lacking, odour indistinct; chlamydospores rare. Conidiation noticeable after 2–3 d, turning green after 4–7 d, at first effuse, later conidia forming in wet heads to 40 μm in pustules. Pustules spreading over the colony, to c. 2.5 mm diam, dark green 26–27F5–8; phialides non-repetitive, mostly in cruciform arrangement.
Distribution — Europe, particularly common in the south including the Canary Islands; also one isolate each known from Iran and Mexico.
Habitat — On fungi growing on and in dead, often well-decayed wood and bark, less commonly isolated from soil.
Other material examined. Croatia, Cres, Prašče Brdo, between Orline and Dragozetići, N45°04′24″, E14°20′24″, elev. 450 m, on 6–7 cm thick, decorticated branch of Quercus cerris, and Quercus pubescens, asexual morph, soc. effuse Hypoxylon sp., corticiaceous fungi, 15 Oct. 2010, I. Kušan, W. Jaklitsch & N. Matočec (cultures 289, S290); Tramontana, Crna, N45°05′22″, E14°19′32″, elev. 390 m, on a 6 cm thick branch of Fraxinus ornus, asexual morph, 15 Oct. 2010, W. Jaklitsch (culture S294). – France, Aquitaine, Pyrénées-Atlantiques, S St. Palais, Château d’Uhart-Mixe, N43°16′51″, W1°01′10″, elev. 90 m, on a 3 cm thick culm of Phyllostachys sp., asexual morph, 4 Nov. 2010, W. Jaklitsch (culture S362); Ariège, Rimont, Las Muros, N43°00′48″, E1°17′21″, elev. 455 m, on Hymenochaete corrugata on Corylus avellana, asexual morph, 5 Nov. 2010, W. Jaklitsch & J. Fournier (culture S364). – Greece, Corfu, Kanakades, N39°39′25″, E19°45′35″, elev. 85 m, on a corticiaceous fungus on Quercus ilex 1.5–3 cm thick, asexual morph, 20 Apr. 2012, H. Voglmayr & W. Jaklitsch (culture S616); SE Liapades, N39°40′13″, E19°45′33″, elev. 70 m, on 1–3 cm thick twig of Quercus pubescens, asexual morph, 20 Apr. 2012, H. Voglmayr & W. Jaklitsch (culture S612); Prinilas, N39°42′06″, E19°41′28″, elev. 300 m, on a 1 cm thick twig of Quercus ilex, 23 Apr. 2012, W. Jaklitsch & H. Voglmayr (culture S632); Troumpettas, Agia Anna, N39°42′18″, E19°44′18″, elev. 400 m, on a 3–4 cm thick branch of Quercus coccifera, asexual morph, 23 Apr. 2012, W. Jaklitsch & H. Voglmayr (culture S631). – Iran, locality and collection date unknown, on Vitis sylvestris (G.J.S. 05-185). – Italy, Apulia, Foggia, Gargano, Monte Barone, N41°46′18″, E16°08′07″, elev. 350 m, on a 8 cm thick branch of a deciduous tree, holomorph, sexual morph immature, 20 Nov. 2009, H. Voglmayr & W. Jaklitsch (culture S80); SW from Mandrione, Foresta Umbra, Riserva biogenetica Falascone, N41°48′22″, E15°58′54″, elev. 760 m, on 5 cm thick, corticated branch of Fagus sylvatica, holomorph, sexual morph immature, 21 Nov. 2009, W. Jaklitsch & H. Voglmayr (culture S96); ibid., N41°50′37″, E16°02′53″, elev. 430 m, on a 8 cm thick, decorticated branch of Fagus sylvatica, holomorph, sexual morph immature, soc. corticiaceous fungus, 22 Nov. 2009, W. Jaklitsch & H. Voglmayr (culture S105); ibid., N41°50′35″, E16°02′54″, elev. 440 m, on Stereum subtomentosum/Acer obtusatum, asexual morph, 22 Nov. 2009, W. Jaklitsch & H. Voglmayr (culture S107); ibid., N41°50′37″, E16°02′55″, elev. 450 m, on 4 cm thick branch of Carpinus betulus, on bark, wood, old Melogramma campylosporum and Anthostoma decipiens, holomorph, 22 Nov. 2009, H. Voglmayr & W. Jaklitsch (WU 31616, culture S110); ibid., c. 100 m below the military station, heading to Peschici, N41°49′35″, E15°59′38″, elev. 760 m, on Steccherinum ochraceum/Carpinus betulus, asexual morph, 22 Nov. 2009, W. Jaklitsch & H. Voglmayr (culture S115); Basilicata, Parco Nazionale del Pollino, San Severino, Bosco Magnano, along the river Peschiera, N40°02′56″, E16°07′31″, elev. 630 m, on a 3–4 cm thick branch of Alnus cordata, asexual morph, soc. Datronia mollis, 17 Nov. 2009, W. Jaklitsch & H. Voglmayr (culture S46); Campania, Parco Nazionale del Cilento, left roadside of Via Provinciale del Corticato shortly after the highest point heading to Sacco, N40°23′04″, E15°25′08″, elev. 960 m, on a 2–3 cm thick branch of Acer obtusatum, asexual morph, 16 Nov. 2009, W. Jaklitsch & H. Voglmayr (culture S34); Lazio, Lago di Vico, west side, N42°19′47″, E12°09′08″, elev. 560 m, on branch of Carpinus betulus, holomorph, sexual morph immature, 26 Nov. 2009, W. Jaklitsch, H. Voglmayr & W. Gams (culture S135; WU 31617, culture S140). – Mexico, locality and collection date unknown, on decorticated wood (G.J.S. 98-86). – Portugal, Madeira, Seixal, Chao da Ribeira, N32°47′56″, W17°06′59″, elev. 500 m, on a 2 cm thick, corticated branch of Ocotea foetens, asexual morph, 17 Feb. 2010, W. Jaklitsch (culture S198). – Russia, Central State Forest Biosphere Reserve, South Taiga, from soil (C.P.K. 998, 999). – Spain, Euskadi, Bizkaia, Gorbeia, forest at the road A624 south of Ziorraga, N42°59′54″, W2°53′21″, elev. 400 m, on old pyrenomycete on a 4 cm thick, cut branch of Fagus sylvatica, asexual morph, 1 Nov. 2010, W. Jaklitsch (culture S329); Gipuzkoa, Aralar, GI2133, Larraitz, deciduous forest between Abaltzisketa and Amezketa, N43°02′30″, W2°06′05″, elev. 335 m, on 5 cm thick, corticated branch of Fraxinus sp., asexual morph, 2 Nov. 2010, W. Jaklitsch (culture S339); Islas Baleares, Mallorca, Escorca, N39°49′8–11″, E2°52′29–36″, elev. 660–690 m, on mostly decorticated, 5–7 cm thick branches of Quercus ilex, asexual morph, soc. Terana caerulea, Trametes sp., on/soc. corticiaceous fungi, 18 Nov. 2010, W. Jaklitsch (cultures S407, S412); road Ma-10 above Fornalutx, opposite the property Monnaber, N39°47′45″, E2°45′58″, elev. 650 m, on a decorticated, 8–10 cm thick branch of Quercus ilex, asexual morph, 17 Nov. 2010, W. Jaklitsch (culture S405); Islas Canarias, La Palma, Cumbre Nueva, chestnut plantation at LP 301, close to crossing with LP 3, N28°38′23″, W17°49′53″, elev. 1015 m, on 1–4 cm thick twigs of Castanea sativa, holomorph, 13 Dec. 2009, W. Jaklitsch (WU 31618, culture S185); ibid., N28°38′23″, W17°49′48″, elev. 1065 m, on branches to 10 cm thick of Castanea sativa, holomorph, on wood, on/soc. Terana caerulea, Annulohypoxylon multiforme, Stereum sp., 2 Dec. 2010, W. Jaklitsch (WU 31620, culture S433); Los Sauces, Los Tilos, N28°47′19″, W17°48′03″, elev. 520 m, on a 7–8 cm thick branch of Ocotea foetens, sexual morphs, 10 Dec. 2009, W. Jaklitsch (culture S166); Malgarida (above San Isidro), N28°37′26″, W17°49′14″, elev. 1070 m, on branch of Laurus novocanariensis, on bark, soc. Diatrype flavovirens, corticiaceous fungus, old sexual morph, 2 Dec. 2010, W. Jaklitsch (WU 31619, culture S428); Mazo, LP2062, beginning of the walking path Camino La Banda, N28°36′40″, W17°48′00″, elev. 950 m, on black, corticated, 4–8 cm thick branches of Laurus novocanariensis, on bark and on/soc. Hypoxylon cf. canariense, Creosphaeria sassafras, etc., sexual morph, 5 Dec. 2010, W. Jaklitsch (WU 31621, culture S457); Tenerife, Macizo de Anaga, walking path before descending to Batán de Arriba, N28°32′07″, W16°17′38″, elev. 900 m, on Biscogniauxia sp. on Persea indica, asexual morph, 13 Apr. 2010, W. Jaklitsch (culture S225); walking path to El Batán from the road to Taborno, N28°32′20″, W16°16′29″, elev. 870 m, on 3–4 cm thick, corticated branch of Prunus lusitanica, asexual morph, soc. effuse Hypoxylon sp., resupinate polypores, 15 Apr. 2010, W. Jaklitsch (culture S233). – United Kingdom, England, North East London, Epping Forest, between the Robin Hood Roundabout and Hill Wood, 43–34/1, N51°39′15″, E00°02′13″, elev. 40 m, on a branch of Fagus sylvatica on the ground in leaf litter, on/soc. a resupinate polypore, soc. Hypocrea lixii, Ascocoryne sarcoides, Diatrype decorticata, 16 Sept. 2004, H. Voglmayr & W. Jaklitsch, Hypo 273 (WU 24027; culture CBS 119322 = C.P.K. 2047); localities and collection dates unknown, stem endophytes of Fagus sylvatica, S. Thomas (G.J.S. 05-466 and G.J.S. 05-482).
Notes — This species is common in Europe and can be frequently found as sexual morph on other fungi. Drying of stromata is typically associated with a distinct change from pale orange-brown to dark red-brown. The asexual morph in nature is white to dark green, effuse to pulvinate, only rarely forming sulphur-yellow spots. Conidial pustules on CMD are dense, but less compact than in T. viridescens s.str. (as exemplified by strain S452). A CMD culture of S290 yielded a brown, tubercular stroma at 15 °C after 23 mo. The strain G.J.S. 98-86 from Mexico (tef1 accession DQ307509, as Hypocrea rufa), was not included in the tef1 tree because of errors in the sequence. Images of T. olivascens in addition to Fig. 3 are given in Fig. 4b, c and Fig. 7g, i of Jaklitsch et al. (2006) and in Fig. 26b, c and Fig. 27d, e, i, l–o of Jaklitsch (2011).
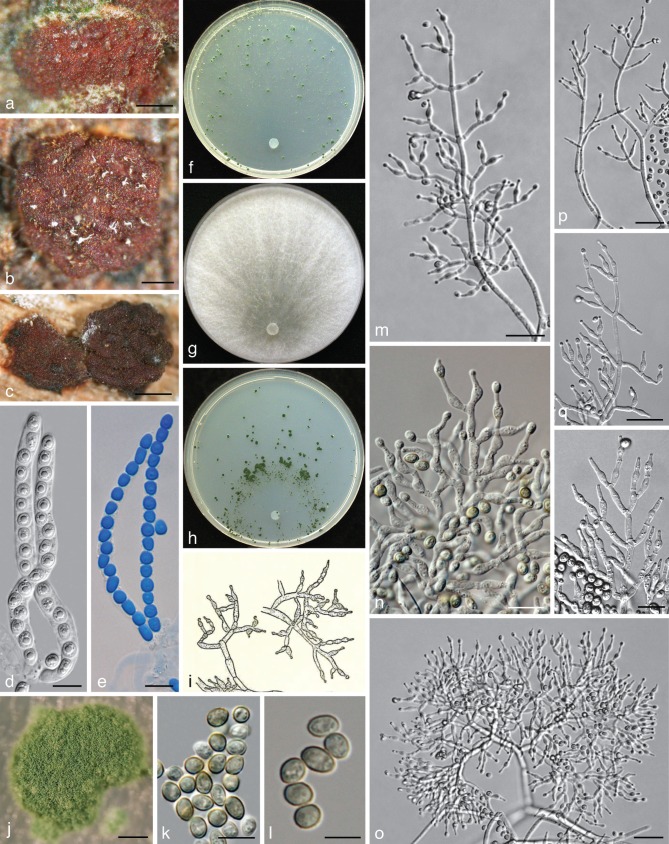
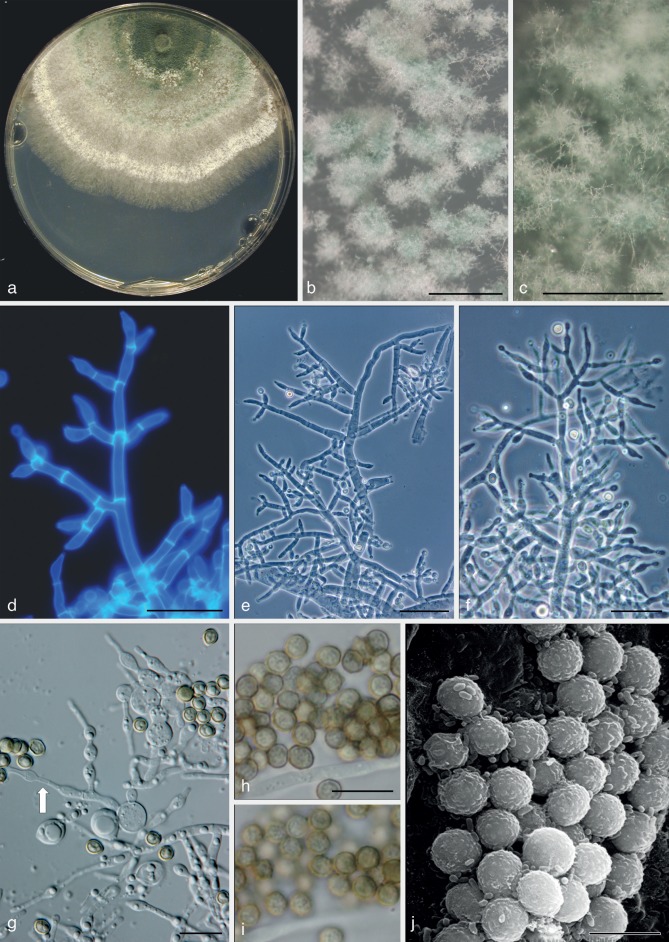
Fig. 4.
Trichoderma viridescens. a–e: Sexual morph. a–c. Dry stromata; d, e. asci (e. in cottonblue/lactic acid). — f–r: Asexual morph and cultures at 25 °C except i (20 °C). f–h. Cultures after 14 d (f. on CMD; g. on PDA; h. on SNA); i, m–r. conidiophores; j. conidiation pustule (9 d); k, l. conidia; j–r. all from CMD after 7 d except j (a, d, e: WU 31624; b: WU 31623; c: WU 31625; f–g, j–r: ex-type CBS 132573 = S452; i: lectotype). — Scale bars: a, b = 0.2 mm; c = 0.3 mm; d, e, n, r = 10 μm; j = 0.5 mm; k = 7 μm; l = 5 μm; m, o–q = 20 μm.
Fig. 7.
Trichoderma virilente. a–i: Sexual morph (WU 31628). a–b. Fresh stromata; c–f. dry stromata (c. immature); g–i. asci (i. in cottonblue/lactic acid). — j–v: Asexual morph and cultures at 25 °C (CBS 132569 = S281). j. Conidiation pustule (10 d); k, l, s–v. conidiophores; m, n. conidia; o–q. cultures (o. on CMD after 14 d; p. on PDA after 10 d; q. on SNA after 14 d); r. phialides; j–n, r–v. all from CMD after 8–10 d. — Scale bars: a, b = 1 mm; c–f, j = 0.5 mm; g–i, r = 10 μm; k, l, t, v = 25 μm; m, n = 7 μm; s, u = 40 μm.
Clade V2
The description of T. viridescens by Jaklitsch et al. (2006) and Jaklitsch (2011) comprised several species. For this reason an emended description of T. viridescens s.str. is given here.
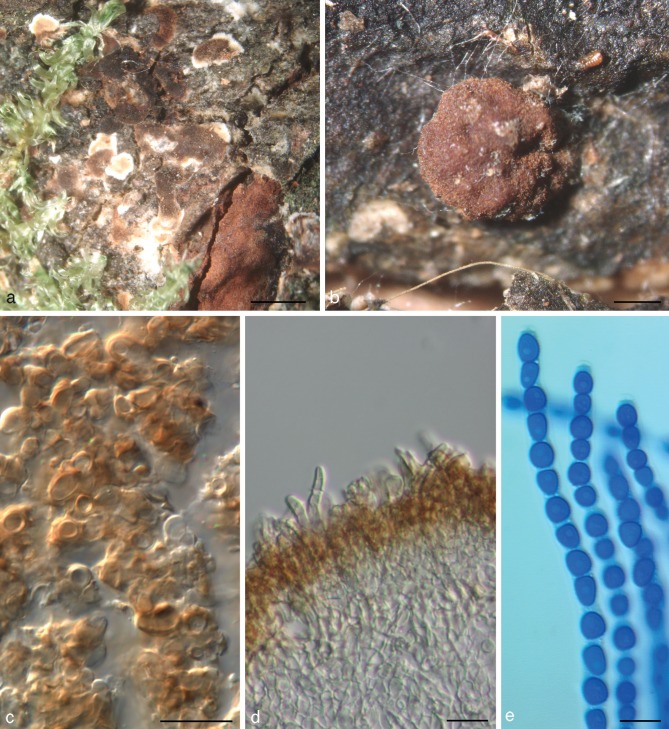
Trichoderma viridescens (A.S. Horne & H.S. Will.) Jaklitsch & Samuels, Stud. Mycol. 56: 156, emend. — Fig. 4
Basionym. Eidamia viridescens A.S. Horne & H.S. Will., Ann. Bot. 37: 396. 1923.
Stromata scattered to aggregated, when fresh orange to orange-brown; when dry (0.5−)0.6–1.8(−3) × (0.4−)0.6–1.1(−1.2) mm, 0.2–0.6 mm thick (n = 25), discoid or pulvinate with variable outline; margin free, tightly connected to the substrate, sometimes with rust basal mycelium; surface often tubercular or rugose, smooth or short-haired when mature. Ostioles invisible or, apparently after rehydration in nature, appearing as light, partly slightly projecting, watery dots (23−)32–55(−63) μm diam (n = 20). Colour medium- to very dark reddish brown. Spore deposits white. Asci (85−)90–103(−113) × (5.8−)6.0–6.8(−7.5) μm, stipe to 20 μm long (n = 31). Ascospores hyaline, spinulose; cells dimorphic, distal cells (4.0−)4.5–5.5(−6.0) × (3.5−)4.0–4.7(−5.2) μm, l/w (0.9−)1.0–1.3(−1.5) (n = 60), (sub)globose, ellipsoidal or wedge-shaped, proximal cells (4.8−)5.2–6.5(−8.5) × (3.0−)3.5–4.3(−5.5) μm, l/w (1.0−)1.3–1.8(−2.6) (n = 60), oblong, ellipsoidal or subglobose.
Cultures and asexual morph — Optimal growth at 25 °C on all media, slow and limited growth at 30 °C, no growth at 35 °C.
On CMD after 72 h colony radius 30–33 mm at 25 °C, 7–11 mm at 30 °C; mycelium covering the plate after 7–9 d at 25 °C. Colony hyaline, dense, circular, not zonate, with conspicuous differences in hyphal width and numerous finely reticulate secondary hyphae at the margin. Aerial hyphae nearly absent. Autolytic excretions and coilings lacking. No diffusing pigment formed, after 7–20 mo at 15 °C agar colourless or faintly yellowish; odour coconut-like within 1 wk. Chlamydospores infrequent, terminal, less commonly intercalary, (7−)8–10(−12) × (6−)7–10(−11) μm, l/w (0.9−)1.0–1.2(−1.4) (n = 21), globose to pyriform, sometimes 2-celled. Conidiation starting after 2–4 d, turning green after 5–7 d, at first scant and short-effuse, soon in loose shrubs or thick, semiglobose or pulvinate, rounded or oblong, compact pustules. Pustules (0.3−)0.4–1.8(−2.3) mm diam, medium to dark green 26–27F5–8, compact, usually at first forming in a median concentric zone, later in up to 5 indistinct zones, or irregularly distributed. Pustules comprising broad and dense internal clusters and loose, long and narrow, slightly projecting peripheral conidiophores. Peripheral conidiophores straight or slightly curved, narrowly tree-like, often with paired branches slightly inclined upward; terminal branches sometimes submoniliform with up to 4 widened cells including terminal phialide in a chain. Central body of pustules consisting of broad, dense, richly branched structures, formed on thick-walled, to 9 μm wide stipes with short, asymmetric, submoniliform branches and terminal whorls of up to 4 branches. Branches typically 1.5–3.5(−4.5) μm wide. Phialides solitary or divergent in whorls of 2–4; one often on an intercalary cell in the whorl. Phialides (6.8−)8.5–12.5(−13.8) × (2.3−)2.7–3.2(−3.5) μm, l/w (2.2−)2.8–4.5(−5.7), base (1.0−)1.5–2.2(−2.5) μm wide (n = 30), narrowly lageniform, symmetric or inaequilateral, often distinctly curved to sigmoid in dense structures, distinctly thickened at or below the middle, often with long neck, sometimes also an extended base. Conidia (4.0−)4.2–5.0(−5.8) × (2.7−)3.0–3.5(−3.8) μm, l/w (1.2−)1.3–1.5(−1.8) (n = 50), ellipsoidal, often attenuated toward one end, finely verruculose, green.
Colony radius on PDA (Merck) after 72 h 28–33 mm at 25 °C, 5–6 mm at 30 °C; mycelium covering the plate after 6–7 d at 25 °C. Colony circular, dense, comprising hyphae with conspicuous width differences. Aerial hyphae abundant, forming a thick, white, floccose, basally distinctly radial to substellate mat, collapsing from above. Autolytic excretions frequent, coilings inconspicuous. No diffusing pigment formed, reverse faintly yellowish 3A3, 4AB3, odour coconut-like to rancid. Conidiation starting after 4–8 d, scant, effuse on aerial hyphae, not turning green (observed for 2 wk).
Colony radius on SNA after 72 h 24–26 mm at 25 °C, 4–5 mm at 30 °C; mycelium covering the plate after 8–9 d at 25 °C. Colony hyaline, dense, circular, with conspicuous differences in hyphal width and numerous finely reticulate secondary hyphae. Aerial hyphae frequent, long and high at the colony margin. Autolytic excretions inconspicuous, coilings moderately occurring. No diffusing pigment, no distinct odour observed. Chlamydospores infrequent, terminal and intercalary. Conidiation starting after 3 d, turning green after 4 d, at first loose, effuse, then mainly in pustules with long radial tree-like conidiophores. Pustules dark green 26–27F5–8, to 2 mm diam, partly confluent into clusters of up to 8 mm, numerous, densely disposed, usually forming early in a central concentric zone, irregularly spreading and/or forming additional zones.
Distribution — Europe (Mediterranean, Canary Islands, UK), one isolate each from the USA and Japan.
Habitat — On dead wood and bark of broad-leaved and coniferous trees and shrubs and wood inhabiting fungi, also on other dead plant material like apples or larger herbaceous stems.
Typification — The authors of Eidamia viridescens (Horne & Williamson 1923) did not designate a holotype for their species nor did they mention strain numbers in the protologue. Jaklitsch et al. (2006) proposed a neotype for it, but did not consider the illustration of E. viridescens that was published as part of the protologue for typification. Accordingly, we designate here Fig. 5 (illustration of conidiophores) by Horne & Williamson in Ann. Bot. 37, 3: 397 (1923), as lectotype of E. viridescens, rendering the neotypification proposed by Jaklitsch et al. (2006) superfluous. In addition, the following epitype (MBT175076) is here designated in order to connect the asexual with the sexual morph and molecular phylogeny: Spain, Islas Canarias, La Palma, Garafía, at LP1 close to the junction to El Tablado, N28°47′48″, W17°53′39.6″, elev. 1110 m, on a 4–5 cm thick branch of Chamaecytisus proliferus, holomorph, on dark, welldecayed wood and a pyrenomycete, soc. Orbilia sp. and a resupinate polypore, 4 Dec. 2010, W. Jaklitsch (WU 31624, ex-epitype culture CBS 132573 = S452; from ascospores). This strain is identical in gene sequences with the authentic conidial strain CBS 433.34, which was isolated by the original authors Horne & Williamson from rotten apples in the UK and deposited as Eidamia viridescens.
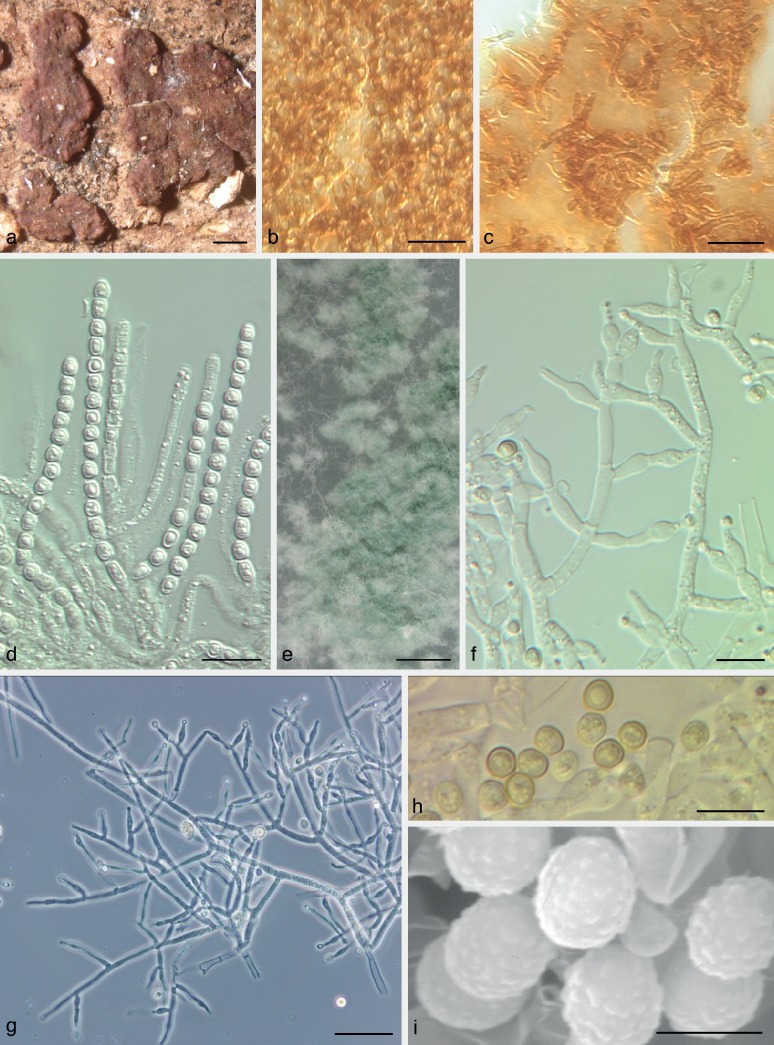
Fig. 5.
Trichoderma viridarium. a–k: Sexual morph. a–d. Fresh stromata (a. immature); e–h. dry stromata (e, f. immature); i. rehydrated stroma; j, k. asci (k. in cottonblue/lactic acid). — l–v: Asexual morph and cultures at 25 °C (CBS 132568 = S136). l, m, o, p. Conidiophores; n. conidiation pustule (9 d); q–s. conidia; t–v. cultures after 10 d (t. on CMD; u. on PDA; v. on SNA); l–s. all from CMD after 7 d except n (a, c, d, i, k: WU 24025; b, e, g, h, j: WU 31626; f: WU 31627). — Scale bars: a, b, d = 1 mm; c, f = 0.8 mm; e, g, n = 0.5 mm; h, i = 0.3 mm; j, q = 10 μm; k, p = 15 μm; l, m, o = 30 μm; r, s = 7 μm.
Other material examined. France, Dept. Alpes-de-Haute-Provence, Rougon, walking path to Suech, c. 1080 m, on branch of Sarothamnus scoparius, asexual morph, 25 July 2011, H. Voglmayr (S559a). – Greece, Crete, between Sempronas and Vasiliana, N35°21′45″, E23°48′59″, elev. 830 m, on 7–8 cm thick branch of Platanus orientalis, asexual morph, 24 Nov. 2011, W. Jaklitsch (culture S588); Plemeniana, N35°19′31″, E23°43′25″, elev. 345 m, on 3 cm thick branch of Platanus orientalis, asexual morph, 27 Nov. 2011, W. Jaklitsch (culture S607). – Italy, Apulia, Andria, Parco Nazionale dell’Alta Murgia, Castel del Monte, between SP234 and Masseria Savignano, N41°02′36″, E16°18′01″, elev. 515 m, on branch of Quercus pubescens, holomorph, sexual morph immature, 19 Nov. 2009, W. Jaklitsch & H. Voglmayr (culture S70); Foggia, Gargano, Mattinata, N41°44′48″, E16°08′00″, elev. 120 m, on 3–4 cm thick branches of Olea europaea, asexual morph, soc. a pyrenomycete and a corticiaceous fungus, 20 Nov. 2009, H. Voglmayr & W. Jaklitsch (culture S74); Basilicata, Parco Nazionale del Pollino, San Severino, Bosco Magnano, along the river Peschiera, N40°02′59″, E16°07′21″, elev. 660 m, on a 4–5 cm thick branch of Alnus cordata, fluffy asexual morph on wood, 17 Nov. 2009, W. Jaklitsch & H. Voglmayr (culture S47); Calabria, Cosenza, Parco Nazionale del Pollino, above Morano Calabro, N39°50′48″, E16°06′47″, elev. 760 m, on a 1 cm thick branch of Fraxinus ornus, asexual morph, soc. Trichoderma polysporum, 18 Nov. 2009, W. Jaklitsch & H. Voglmayr (culture S55); Campania, prov. Salerno, Parco Nazionale del Cilento, Roscigno, N40°24′24″, E15°21′51″, elev. 730 m, on a decorticated, 2 cm thick twig of Spartium junceum, asexual morph, 16 Nov. 2009, W. Jaklitsch (culture S41); Sardinia, roadside of SP 38 at junction to Isalle Orrule, N40°21′49″, E9°31′00″, elev. 190 m, on twig of Cistus monspeliensis, asexual morph, soc. Hymenochaete sp., 2 Nov. 2009, W. Jaklitsch (culture S1). – Japan, locality and collection date unknown, on wood of Pinus radiata (G.J.S. 99-18). – Spain, Andalucia, Gaucín, roadside in village area, N36°31′14″, W5°19′07″, elev. 605 m, on 2–5 cm thick branch of Rosmarinus officinalis, asexual morph, soc. Stereum sp., Bisporella sulfurina, 22 Mar. 2011, W. Jaklitsch & H. Voglmayr (culture S535); El Colmenar, at Rio Guadiaro, N36°32′18″, W5°23′37″, elev. 210 m, on decorticated, 4 cm thick branch of Populus nigra, 23 Mar. 2011, H. Voglmayr & W. Jaklitsch (culture S538); Euskadi, Bizkaia, at the road BI2238 to Ibarrangelu, shortly after Basetxeta, N43°22′57″, W2°38′04.5″, elev. 120 m, on 2–4 cm thick branch of Ulex europaeus, asexual morph, 31 Oct. 2010, W. Jaklitsch (culture S323); Bengoetxea, at A2522/AP68, N43°07′50″, W2°55′33″, elev. 130 m, on 1.5–2 cm thick, corticated twig of Hedera helix, asexual morph, on wood and bark, 7 Nov. 2010, W. Jaklitsch (culture S382); Gipuzkoa, BI3440, at the golf course Justiz, N43°22′27″, W1°49′46″, elev. 130 m, on 1.5–2 cm thick culm of Phyllostachys sp., asexual morph, soc. black hyphomycete, 3 Nov. 2010, W. Jaklitsch (culture S350); Islas Baleares, Mallorca, Ma-10, at Refugi Gorg Blau, N39°48′02″, E2°48′40″, elev. 625 m, on recently dead, corticated, 1–5 cm thick twigs of Quercus ilex, asexual morph, 17 Nov. 2010, W. Jaklitsch (culture S406); Islas Canarias, La Palma, Cumbre Nueva, chestnut plantation at LP 301, close to crossing with LP 3, N28°38′23–27″, W17°49′49–50″, elev. 1040 m, on 2–3 cm thick branch of Castanea sativa, asexual morph on wood, 13 Dec. 2009, W. Jaklitsch (culture S188); ibid., on Stereum hirsutum on Malus sp., asexual morph, 2 Dec. 2010, W. Jaklitsch (culture S436); ibid., on 4–5 cm thick branch of Chamaecytisus proliferus, holomorph, soc. various ascomycetes, 2 Dec. 2010, W. Jaklitsch (WU 31623, culture S438); Tenerife, Bosque de La Esperanza, N28°25′38″, W16°23′55″, elev. 1330 m, on 5–6 cm thick branch of Eucalyptus globulus, holomorph, on wood, soc. old semi-resupinate polypore, white hyphomycete; 14 Dec. 2010, W. Jaklitsch (WU 31625, culture S471); above Farrobillo, N28°24′50″, W16°28′35″, elev. 650 m, on 0.5–1.5 cm thick twigs of Rumex lunaria, asexual morph, 14 Apr. 2010, W. Jaklitsch (culture S226); La Orotava, Pista de Benijos, above Aguamansa/La Orotava, N28°21′32″, W16°30′23″, elev. 1115 m, on 3 cm thick branch of Erica arborea, asexual morph, 12 Apr. 2010, W. Jaklitsch (culture S220); near Pista de Benijos and the camping place La Caldera, N28°21′27″, W16°30′22″, elev. 1145 m, on 4–5 cm thick branch of Eucalyptus globulus, asexual morph, soc. Hypoxylon sp., Diatrype sp., 16 Apr. 2010, W. Jaklitsch (culture S244). – USA, Oregon, locality and collection date unknown, from root of Pseudotsuga menziesii infected with Phellinus weirii, E. Nelson (Tr 4 = C.P.K. 1183).
Notes — Trichoderma viridescens s.str. is characterised by distinct and rather dense pustules on CMD, straight and regularly tree-like conidiophores at the periphery of pustules and distinctly enlarged cells in the submoniliform branches of the conidiophores. Bright yellow mycelium may occasionally occur around the green, often widely, up to 3 cm, effused asexual morph in nature. We did not observe a yellow pigment on CMD. Images of T. viridescens in addition to Fig. 4 are given in Fig. 6c, d, g–i, l, m of Jaklitsch et al. (2006).
Fig. 6.
Trichoderma paraviridescens. a–k: Sexual morph. a–d. Fresh stromata (a. immature; d. half dry); e–g. dry stromata; h. rehydrated stroma; i–k. asci (j, k. in cottonblue/lactic acid). — l–x: Asexual morph and cultures at 25 °C (CBS 132566 = S122). l, o, p, r. Conidiophores; m. conidiation pustule (9 d); n, t, u. conidia; q, s. phialides; v–x. cultures (v. on CMD after 7 d, insert: yellow pigment in strain S490 after 17 mo at 15 °C; w. on PDA after 10 d; x. on SNA after 10 d); l–u. all from CMD after 5 d (a, d, i, j: WU 24029; b: WU 24024; c, e, f, h, k: WU 24019; g: WU 24018). — Scale bars: a, e, m = 0.3 mm; b, d = 1.3 mm; c = 1 mm; f, h = 0.5 mm; g = 0.2 mm; i, j, s = 10 μm; k, q = 15 μm; l = 30 μm; n = 7 μm; o, p, r = 20 μm; t, u = 5 μm.
Clade V3
Trichoderma viridarium Jaklitsch, Samuels & Voglmayr, sp. nov. — MycoBank MB803622; Fig. 5
Etymology. A term based on green conidia to document the relationship to other species of the Viride clade.
Holotype. Italy, Lazio, Lago di Vico, west side, N42°19′48″, E12°09′08″, elev. 560 m, on 3–7 cm thick branches of Carpinus betulus, on wood and bark, soc. Steccherinum ochraceum, corticiaceous fungi, Nemania sp., holomorph, 26 Nov. 2009, W. Jaklitsch, H. Voglmayr & W. Gams (WU 31627, ex-type culture S136 = CBS 132568; from ascospores).
Stromata solitary, gregarious or aggregated, sometimes developing from large effuse, initially white mycelial mats, later breaking up into individual parts, when fresh pulvinate, downy when young, later smooth, sometimes tubercular or rugose; light (orange-)brown 5B5 to 6CD5–6, yellow-brown to mostly reddish brown 8CD5–6, 9F7–8, sometimes yellow in early stages; ostiolar dots lacking or inconspicuous, rarely appearing as dark dots or spots. Stromata when dry (0.6−)1.0–2.2(−3.0) × (0.5−)0.8–1.6(−1.9) mm, 0.3–0.7(−0.9) mm thick (n = 20), at first effuse, becoming pulvinate, or irregularly discoid; outline circular, oblong or irregular; margin adnate, becoming free; surface covered by brown hairs when young, later smooth; ostiolar dots lacking; at first white, then turning yellow-brown or olive-brown, later intensely red-brown, dark reddish brown, dark brown to nearly black. Spore deposits white to yellowish. Asci (78−)87–112(−127) × (4.5−)5.0–6.3(−7.3) μm, stipe (4−)6–22(−33) μm long (n = 30). Ascospores hyaline, spinulose, cells dimorphic, distal cells (sub)globose or ellipsoidal, (3.8−)4.3–5.5(−6.3) × (3.3−)3.7–4.7(−5.2) μm, l/w (0.9−)1.0–1.3(−1.8) (n = 70), proximal cells oblong, cuneate or ellipsoidal, (4.2−)4.8–6.5(−8.0) × (2.8−)3.2–4.2(−4.7) μm, l/w (1.1−)1.3–1.9(−2.7) (n = 70).
Cultures and asexual morph — Optimal growth at 25 °C on all media, slow growth at 30 °C, no growth at 35 °C.
On CMD after 72 h colony radius 42–46 mm at 25 °C, 3–5 mm at 30 °C; mycelium covering the plate after 5–7 d at 25 °C. Colony hyaline, circular, loose, radial, with conspicuous differences in hyphal width, not zonate. Aerial hyphae scant or inconspicuous. Autolytic excretions and coilings scant. Agar colourless, sometimes turning faintly yellowish; after 8–21 mo at 15 °C faintly to dull yellowish, less commonly straw-yellow (3B4–6). Odour coconut-like. Chlamydospores infrequent, terminal and intercalary, (6−)7.5–11(−13) × (6−)7–9.5(−10.5) μm, l/w (0.9−)1.0–1.2(−1.3) (n = 20), globose, subglobose or ellipsoidal. Conidiation first noticeable after 2 d, turning green after 5–6 d, at first scant, short-effuse, soon in minute downy or powdery shrubs eventually aggregating to form flat tufts up to 2.5(−5) mm diam of variable outline and surface in one broad marginal zone or two scarcely separated concentric zones, eventually dark green 27–28F5–8. Shrubs and tufts formed on up to 12 μm wide, thick-walled, verrucose stipes with unpaired, c. 6–10 μm wide, rather abruptly attenuated primary branches; the latter giving rise of a loose reticulum. Conidiophores more or less radially arranged, arising from the reticulum or directly from primary branches, tree-like, straight or slightly curved, often broad, comprising a distinct main axis, and steeply ascending, mostly unpaired, sometimes also paired or verticillate, 2–4(−5) μm wide side branches. Phialides solitary or divergent in whorls of 2–3, narrowly lageniform, scarcely widened, (7.3−)9.0–13.5(−16.8) × 2.5–3.2(−3.7) μm, l/w (2.2−)3–5(−6.7), (1.2−)1.5–2.5(−3.0) μm wide at the base (n = 35), often with long necks, only rarely repetitive with 1–2 slightly widened cells below the terminal phialide. Conidia initially forming in wet heads, subglobose-oval-ellipsoidal, (3.5−)4.0–4.7(−5.3) × (3.0−)3.3–3.7(−4.0) μm, l/w (1.0−)1.2–1.4(−1.5) (n = 55), green, nearly smooth to verruculose.
Colony radius on PDA (Merck) after 72 h 30–34 mm at 25 °C, 3–5 mm at 30 °C; mycelium covering the plate after 1 wk at 25 °C. Colony circular, dense, with inconspicuous difference in hyphal width. Aerial hyphae abundant but loosely disposed, forming a white mat, becoming floccose to granular in the centre and turning pale yellowish green to greyish yellow 28–30CD4–6, 30B3, 1B3–4, 30A4, 3B3–5 from the proximal margin or in a diffuse concentric zone due to conidiation. Conidiation first noticeable after 2 d, turning green after 5–6 d, effuse on aerial hyphae. Autolytic excretions numerous, coilings moderate. No diffusing pigment formed, reverse dull yellowish; odour coconut-like to unpleasant.
Colony radius on SNA after 72 h 35–40 mm at 25 °C, 3–5 mm at 30 °C; mycelium covering the plate after 6 d at 25 °C. Colony hyaline, circular, loose, radial, not zonate. Aerial hyphae frequent at the margin usually forming a broad downy or floccose zone. Autolytic excretions and coiling frequent. No diffusing pigment, no distinct odour formed. Chlamydospores infrequent, terminal and intercalary. Conidiation noticeable after 2 d, turning green after 4–5 d, effuse and in small loose shrubs in the downy zone. Shrubs aggregating to form irregular pustules or larger structures to 5 mm long, eventually dark green 26–27F5–8; conidia formed in wet heads to 40 μm diam; phialides non-repetitive.
Distribution — Europe, one isolate from the USA (North Carolina).
Habitat — On dead wood and bark and fungi growing on it; one strain from soil.
Other material examined. Austria, Vienna, 23rd district, Maurer Wald, N48°08′57″, E16°14′50″, elev. 360 m, on decorticated branch of Carpinus betulus on the ground, in mixed deciduous forest, asexual morph, soc. Tubeufia cerea, 3 Oct. 1998, W. Jaklitsch, W.J. 1223 (WU 24009, BPI 747557; culture G.J.S. 98-182 = CBS 120067). – France, Aquitaine, Pyrénées Atlantiques, Isle de Sauveterre de Bearn, elev. 100 m, on bark of recently dead tree, 25 Oct. 1998, G.J.S. & F. Candoussau (BPI 748308; culture G.J.S. 98-129 = CBS 101928); SE Salies de Bearn, road D430, deciduous forest at the junction to Quartier du Cout, N43°28′07″, W0°54′34″, elev. 85 m, on a 2–4 cm thick, corticated branch of Quercus robur, asexual morph, 3 Nov. 2010, W. Jaklitsch (culture S351); Midi-Pyrénées, Haute Garonne, Bord de la Garonne, 31000 Toulouse, on bark of Carpinus sp., 11 Dec. 1994, J.F. Magni, comm. F. Candoussau (BPI 737860; culture G.J.S. 94-118 = IMI 374788). – Germany, Baden-Württemberg, Landkreis Lörrach, Todtnau, at the crossing to St. Blasien, MTB 8113/4, N47°48′11″, E07°56′01″, elev. 490 m, on mostly decorticated, up to 35 cm thick cut logs of Picea abies, in pile, soc. effete Ophiostoma sp., white mould, 3 Sept. 2004, W. Jaklitsch & H. Voglmayr, Hypo 234 (WU 24025; culture CBS 119323 = C.P.K. 2045); Bavaria, Starnberg, Tutzing, Erling, at the Hartschimmel terrain, N47°56′41″, E11°10′37″, elev. 700 m, on partly decorticated branch of Fagus sylvatica 13–15 cm thick, on the ground in leaf litter, 3 Sept. 2005, W. Jaklitsch, Hypo 340 (WU 24028; culture C.P.K. 2139). – Greece, Crete, chestnut plantation near Sempronas, N35°22′31″, E23°49′18″, elev. 670 m, on a 7 cm thick, corticated branch of Castanea sativa, asexual morph, soc. corticiaceous fungi, 24 Nov. 2011, W. Jaklitsch (culture S589). – Italy, Apulia, Foggia, Gargano, SW from Mandrione, Foresta Umbra, Riserva biogenetica Falascone, N41°48′22″, E15°58′54″, elev. 760 m, on a branch of Fagus sylvatica, holomorph, on wood, soc. Mucidula mucida, black hyphomycete, Mollisia sp., 21 Nov. 2009, W. Jaklitsch & H. Voglmayr (WU 31626; culture S95); Calabria, Cosenza, Parco Nazionale del Pollino, above Morano Calabro, N39°50′48″, E16°06′47″, elev. 760 m, on a 4 cm thick branch of Ostrya carpinifolia, on bark, soc. Steccherinum ochraceum, asexual morph, 18 Nov. 2009, H. Voglmayr & W. Jaklitsch (culture S54); locality and collection date unknown, from soil (G.J.S. 04-81). – Spain, Andalucia, Alcalá de los Gazules, via de servicio south of the exit at km 54 off the A7 (A381), N36°21′57″, W5°38′36″, elev. 50 m, on a 7 cm thick branch of Acacia retinodes, on wood, asexual morph, soc. Steccherinum ochraceum and other corticiaceous fungi, 17 Mar. 2011, H. Voglmayr & W. Jaklitsch (culture S492); Bizkaia, 2.5 km north of Amorebieta, at roadside, N43°14′36″, W2°42′36″, elev. 190 m, on a 6–7 cm thick, corticated, cut branch of Carpinus betulus, asexual morph, soc. Exidia glandulosa, Hypoxylon fuscum, 31 Oct. 2010, W. Jaklitsch (culture S326); Gipuzkoa, Berastegi, at Irai, N43°08′14″, W1°59′16″, elev. 390 m, on wood of a deciduous tree, asexual morph, 2 Nov. 2010, W. Jaklitsch (culture S345). – United Kingdom, Devon, Exeter, Stoke Woods, close to the parking place Forest Walks, SX919959, N50°45′10″, W03°31′54″, elev. 30 m, on 4 cm thick branch of Fagus sylvatica, on the ground in leaf litter, 8 Sept. 2004, H. Voglmayr, W. Jaklitsch & J. Webster, Hypo 246 (WU 24026; culture C.P.K. 2046). – USA, North Carolina, Macon Co., Nantahala National Forest, W of Franklin, Wayah Bald, elev. 5340 ft, 26 Sept. 1989, on decorticated wood, G.J. Samuels, C.T. Rogerson, W.R. Buck & R.C. Harris (BPI 744478, culture G.J.S. 89-142 = CBS 120065).
Notes — On CMD T. viridarium does not form dense or compact pustules. Conidiophores are characterised by rather steeply ascending branches and only indistinctive and inconspicuous enlargement of cells in terminal branches. Conidia of T. viridarium show the entire shape range found in the T. viridescens species complex, from subglobose to oval to ellipsoidal. Images of T. viridarium in addition to Fig. 5 are given in Fig. 4a, i, k, l, o, Fig. 5d, i, Fig. 7j and Fig. 8h of Jaklitsch et al. (2006) and in Fig. 26a, i, l, o–q of Jaklitsch (2011).
Fig. 8.
Trichoderma trixiae. a. Culture on PDA after 5 d at 25 °C; b, c. pustules on CMD (in c. cottony aspect); d–g. conidiophores (d. fluorescence microscopy in 0.5 M calcofluor; g. ‘eidamia’ aspect with a percurrently proliferated phialide indicated by the arrow); h–j. conidia (h. optical section; i. surface view; j. SEM view) (a, d, e, g: Tr 26; b, c, f: Tr 5; h–j: G.J.S. 92-11). — Scale bars: b, c = 0.5 mm; d, e, f = 20 μm; g–i = 10 μm; j = 5 μm.
Based on the tef1 accession JX184102 in GenBank (as Hypocrea viridescens) also strain AN605 from Poland (Błaszczyk et al. 2011) belongs to T. viridarium.
Clade V4
Trichoderma paraviridescens Jaklitsch, Samuels & Voglmayr, nom. nov. — MycoBank MB803623; Fig. 6
Replaced synonym. Hypocrea viridescens Jaklitsch & Samuels, Stud. Mycol. 56: 156 (2006).
Etymology. Paraviridescens means next to or close to viridescens.
Holotype. Austria, Kärnten, Völkermarkt, Eisenkappel-Vellach, Vellacher Kotschna, MTB 9653/1, N46°24′02″, E14°34′06″, elev. 970 m, on 7–8 cm thick, split and partly decorticated branch of Fagus sylvatica on the ground, soc. Corticium roseum, 31 Oct. 2005, H. Voglmayr & W. Jaklitsch, W.J. 2881 (WU 24029, ex-type culture CBS 119321 = C.P.K. 2140; from ascospores). The dry culture WU 24029a, erroneously designated as ‘epitype’ of T. viridescens by Jaklitsch et al. (2006) is an integral part of the holomorphic holotype specimen of T. paraviridescens.
Stromata when fresh 0.5–3 mm diam, 0.5–1.5 mm thick, solitary, gregarious or aggregated, sometimes in lines, at first semi-effuse and white to yellowish, becoming discrete and pulvinate with free, sometimes lobate margins, with more or less circular outline; surface smooth or slightly tubercular, ostioles inconspicuous or invisible; light brownish or yellowish brown 5B6 when young, later reddish brown, often very dark, 7–8EF5–8. Stromata when dry (0.5−)0.8–1.9(−2.6) × (0.5−)0.7–1.6(−2) mm, (0.2−)0.3–0.5(−0.6) mm thick (n = 20), like fresh, but when young more distinctly downy or finely velvety with short rust hairs and surface more uneven, tubercular or rugose. Ostiolar dots inconspicuous, minute, hyaline, only rarely darker than the surrounding surface, (30−)42–70(−80) μm (n = 20). Asci (84−)92–110(−115) × (5.0−)5.5–7.0(−7.5) μm, stipe to 20 μm long (n = 25), apex up to 1.5 μm thick. Ascospores hyaline, finely spinulose, cells dimorphic, distal cells (3.3−)4.2–5.5(−7.5) × (3.2−)3.7–4.5(−5.0) μm, l/w 1.0–1.3(−1.7) (n = 60), (sub)-globose or cuneate, proximal cells (3.8−)4.5–6.5(−8.0) × (2.7−)3.0–4.0(−4.5) μm, l/w (1.1−)1.2–2.0(−2.7) (n = 60), oblong or subglobose.
Cultures and asexual morph — Optimal growth at 25 °C on all media, slow and restricted growth at 30 °C, no growth at 35 °C.
On CMD after 72 h colony radius 14–17 mm at 15 °C, 39–47 mm at 25 °C, 10–24 mm at 30 °C; mycelium covering the plate after 5–7 d at 25 °C. Colony hyaline, dense, circular, with inconspicuous differences in hyphal width, not zonate. Aerial hyphae generally inconspicuous, but common at the colony margin, which becomes downy. Autolytic excretions and coilings absent or sparsely formed. Agar turning yellowish, 1B2–6 to 2–3B2–3 within 1 wk or later, after extended storage at 15 °C distinctly yellow 4A3–5, 4B4–6. Odour distinctly coconut-like, recognizable in less than 1 wk. Chlamydospores formed sparsely, terminal and intercalary, globose or pyriform, (7−)8–11(−13) × (5.7−)6.5–9.0(−9.5) μm, l/w (0.9−)1.0–1.4(−1.7) (n = 21). Conidiation noticeable after 2–3 d, turning green after 4–5 d, eventually dark green 27–28F5–8 to nearly black, effuse, concentrated in shrubs in one or several, downy to farinose or granulose distal to marginal zones. Less commonly loose tufts to c. 1 mm diam formed after c. 2 wk, aggregating to flat amorphous pustular clusters up to 2–3 mm diam, translucent, never compact. Shrubs arising on up to 11 μm wide stipes with up to 2.5 μm thick walls and unpaired, 6–9 μm wide primary branches that give rise to a loose asymmetric reticulum. Conidiophores emerging from the reticulum, tree-like, comprising a distinct main axis with 1.5–4.5 μm wide side branches at right angles and often unpaired at lower levels, tending to be paired or in whorls and more inclined upwards at upper levels; with some cells widened to 5.5 μm. Some conidiophores broad and dense, with mostly symmetric branches. Phialides solitary or divergent in small whorls of 2–3, not commonly repetitive, sometimes with 1–2 enlarged supporting cells. Phialides (6.2−)7.3–10.3(−12.8) × (2.3−)2.7–3.3(−3.7) μm, l/w (2.0−)2.2–3.7(−5.1), base (1.3−)1.5–2.1(−2.7) μm wide (n = 40), lageniform, straight, curved or sinuous, mostly inaequilateral, neck not conspicuously long, but sometimes abruptly attenuated. Conidia globose to subglobose, rarely ellipsoidal, (3.3−)3.7–4.3(−4.7) × (3.0−)3.5–4.0(−4.2) μm, l/w (1.0−)1.1–1.2 (n = 50), green, sometimes forming chains.
Colony radius on PDA (Merck) after 72 h 10–13 mm at 15 °C, 32–45 mm at 25 °C, 7–20 mm at 30 °C; mycelium covering the plate after 5–6 d at 25 °C. Colony circular, dense, with inconspicuous differences in hyphal width, but marginal surface hyphae very wide, forming strands. Aerial hyphae abundant, forming a white, coarsely floccose, thick mat. Autolytic excretions often abundant, coiling moderate. No diffusing pigment observed, reverse faintly yellowish 4AB3, odour coconut-like to unpleasant. Conidiation typically absent.
Colony radius on SNA after 72 h 11–12 mm at 15 °C, 32–41 mm at 25 °C, 5–13 mm at 30 °C; mycelium covering the plate after 5–6 d at 25 °C. Colony hyaline, dense, circular, with distinct difference in hyphal width; broad marginal zone becoming downy to finely floccose due to aerial hyphae. Autolytic activity moderate, coilings frequent. No diffusing pigment produced; odour indistinct. Chlamydospores sparsely formed, terminal and intercalary. Conidiation starting after 2 d, green after 4 d, at first short-effuse and on aerial hyphae, later in numerous shrubs and pustules to 2 mm diam developing in several concentric zones that appear consecutively from the centre, eventually dark green 26–27F5–8.
Distribution — Europe, widely distributed and common; also known from Colombia, Iran, Japan, Mexico and the USA (New York, Wisconsin).
Habitat — On and in wood and bark and fungi growing on it.
Other material examined. Austria, Oberösterreich, Grieskirchen, Natternbach, forest close to Gaisbuchen, MTB 7548/3, N48°24′39″, E13°41′40″, elev. 580 m, on branch of Fagus sylvatica on leaf litter in spruce forest, holomorph, 1 Aug. 2004, H. Voglmayr, Hypo 156 (WU 24022; culture C.P.K. 2043); Steiermark, Liezen, Kleinsölk, walking path between Schwarzensee 1170 m, on 100 cm thick log segment of Picea abies in grass, holomorph, soc. Neonectria fuckeliana, 6 Aug. 2003, H. Voglmayr & W. Jaklitsch, Hypo 12 (WU 24018; culture CBS 119324 = C.P.K. 942); (Ost-)Tirol, Lienz, Hopfgarten in Defereggen, Dölsach, at roadside between the current transformer and the beverage depot, MTB 9041/3, N46°55′23″, E12°32′41″, elev. 990 m, on 16 cm thick log of Picea abies stored in grass, holomorph, 4 Sept. 2003, W. Jaklitsch, Hypo 38 (WU 24019; culture C.P.K. 947). – France, Aquitaine, Pyrénées-Atlantiques, Forêt communale d’Hasparren, D22, near junction to Jatxou/Ustaritz, N43°23′08″, W1°23′05″, elev. 115 m, on bark of Salix caprea and on a 20 cm thick, cut branch of Picea abies, asexual morph, 6 Nov. 2010, W. Jaklitsch (cultures S374, S375). – Germany, Baden-Württemberg, Landkreis Breisgau-Hochschwarzwald, St. Märgen, shortly after Glashütte, coming from Hexenloch, on the right side of the road close to a bridge, MTB 8014/2, N47°59′37″, E08°07′32″, elev. 750 m, on 4 cm thick, cut branch of Picea abies on moist ground, holomorph, 2 Sept. 2004, H. Voglmayr & W. Jaklitsch, Hypo 229 (WU 24024; culture C.P.K. 2044). – Greece, Corfu, SE Liapades, shortly before Kanakades heading south, N39°39′36″, E19°45′58″, elev. 60 m, on a branch of Juglans regia, asexual morph, 20 Apr. 2012, W. Jaklitsch & H. Voglmayr (culture S613); shortly before Skripero heading north, NE Poulades, opposite of the marble quarry, N39°40′58″, E19°48′04″, elev. 70 m, on a branch of Hippocrepis emerus, on/soc. Rhytidhysteron rufulum, soc. Valsaria sp., asexual morph, 21 Apr. 2012, W. Jaklitsch & H. Voglmayr (culture S619); SSW Kouramaditika, N39°35′59″, E19°51′18″, elev. 30 m, on a 3 cm thick corticated branch of Ulmus minor, asexual morph, 22 Apr. 2012, asexual morph (culture S626); Crete, between Agioi Pantes and Vrysses, N35°23′31″, E24°10′07″, elev. 130 m, on a 7 cm thick branch of Juglans regia, asexual morph, soc. Mycoacia uda, 26 Nov. 2011, W. Jaklitsch (culture S598); south of Platanos, N35°26′49″, E23°35′03″, elev. 305 m, on a 4 cm thick branch of Platanus orientalis, asexual morph, 27 Nov. 2011, W. Jaklitsch (culture S604). – Italy, Abruzzo, Sulmona, Vallelarga, close to the village sign at the North of the village, N42°00′09″, E13°55′39″, elev. 600 m, submediterranean forest, on a 1 cm thick twig of Hippocrepis emerus, asexual morph, 24 Nov. 2009, W. Jaklitsch & H. Voglmayr (culture S122 = CBS 132566); Apulia, Andria, Parco Nazionale dell’Alta Murgia, Castel del Monte, between the road SP234 and Masseria Savignano, N41°02′35″, E16°18′01″, elev. 510 m, on Phellinus pomaceus/Prunus dulcis, and a 4 cm thick, decorticated branch of Crataegus laciniata, asexual morph, 19 Nov. 2009, W. Jaklitsch & H. Voglmayr (cultures S68, S69); Foggia, Gargano, SW from Mandrione, Foresta Umbra, N41°50′31″, E16°02′56″, elev. 460 m, on well-decayed wood of Carpinus betulus, asexual morph, 22 Nov. 2009, H. Voglmayr & W. Jaklitsch (culture S111); Campania, Teggiano, chestnut plantation at San Marco, N40°22′46″, E15°30′52″, elev. 520 m, on a stump and a stored trunk of Castanea sativa, asexual morph, 16 Nov. 2009, W. Jaklitsch & H. Voglmayr (cultures S31, S32); left roadside of Via Provinciale del Corticato shortly after the highest point heading to Sacco, Parco Nazionale del Cilento, N40°23′03″, E15°25′10″, elev. 970 m, on a branch of Ostrya carpinifolia, asexual morph, 16 Nov. 2009, W. Jaklitsch & H. Voglmayr (culture S36); Lazio, Bomarzo, at Pyramide/Santa Cecilia, on a branch of Quercus virgiliana, 22 Oct. 2012, H. Voglmayr, W. Jaklitsch & W. Gams (culture S645); Ponte di San Pietro near Farnese, N42°31′41″, E11°36′00″, elev. 125 m, on a 4–5 cm thick, corticated, cut branch of Ostrya carpinifolia, holomorph, sexual morph immature, 28 Nov. 2009, W. Jaklitsch, W. Gams & H. Voglmayr (culture S151); Sardinia, San Giusta/Pratobello, N40°07′55″, E9°16′27″, elev. 945 m, on a branch of Quercus virgiliana, asexual morph, 4 Nov. 2009, W. Jaklitsch (culture S16). – Spain, Andalucia, Alcalá de los Gazules, Via de Servicio south of the exit at km 54 off the A7 (A381), N36°22′10″, W5°38′42″, elev. 70 m, on a corticated twig of Cytisus linifolius, asexual morph, 17 Mar. 2011, W. Jaklitsch & H. Voglmayr (culture S490); Embalse de Charco Redondo, at the A7, N36°15′19″, W5°34′57″, elev. 90 m, on a 5–6 cm thick branch of Eucalyptus globulus, asexual morph, 18 Mar. 2011, W. Jaklitsch & H. Voglmayr (culture S497); Bizkaia, 2.5 km north of Amorebieta, at roadside, N43°14′36″, W2°42′36″, elev. 190 m, on Datronia mollis/Carpinus betulus, asexual morph, soc. Hypoxylon howeianum, 31 Oct. 2010, W. Jaklitsch (culture S327); at the road BI2238 to Ibarrangelu, shortly after Basetxeta, N43°22′55″, W2°38′07″, elev. 120 m, on nearly black wood of ?Castanea sativa, asexual morph, soc. corticiaceous fungus, 31 Oct. 2010, W. Jaklitsch (culture S324); Gipuzkoa, Aralar, east from San Martin/Ataun, at the road BI4151, N42°59′48.5″, W2°09′40″, elev. 410 m, on a 14 cm thick, corticated, cut branch of Alnus glutinosa, soc. Macrotyphula contorta, asexual morph, 2 Nov. 2010, W. Jaklitsch (culture S336). – Switzerland, soil along a road, G. Vanacci (G.J.S. 05-115). – USA, Wisconsin, soil in an orchard, S. Jeffers (G.J.S. 05-144, G.J.S. 05-172). See Table 1 for additional strains.
Notes — Trichoderma paraviridescens is the commonest species of the T. viridescens complex in Europe. In nature stromata or green asexual morphs are often accompanied by sulphur yellow mycelium that may sometimes be thickly pulvinate. Asexual morph colonies in nature are typically effuse and up to 1 cm long and to 3 mm thick. Also in CMD culture the conidiation is typically only effuse. At most, the conidiophores can form shrubs while somewhat larger, flat pustules develop only rarely and only after c. 2 wk. Conidia of T. paraviridescens are more subglobose and more distinctly warted than in T. viridescens s.str. Storage of CMD cultures at 15 °C, monitored for 10–22 mo, resulted in distinct warm yellow pigment diffusing through the entire agar. It is nearly as intense as in T. petersenii. After this period some whitish to yellowish mycelial spots or tufts or yellowish brownish stroma-like aggregations of mycelial tufts to c. 8 mm diam and several mm thick are common. Images of T. paraviridescens in addition to Fig. 6 are given in Fig. 4d–h, j, m, n, Fig. 5g, j, Fig. 6a, b, j and Fig. 7k, l of Jaklitsch et al. (2006) and in Fig. 26d–h, j, k, m, n, r and in Fig. 27a–c, f–h, j, k of Jaklitsch (2011).
The following strains also belong to T. paraviridescens, but their tef1 sequences from GenBank were not included in the phylogenetic tree, either due to short length or obvious errors in the sequence: strains G.J.S. 99-8 (= CBS 130676, N.R. 5541; tef1 accession DQ307517) from leaf litter in Japan; G.J.S. 97-272 (= BBA 66069; tef1 accession DQ307504) from soil in Germany and G.J.S. 99-10 (= N.R. 5510; tef1 accession DQ307514) from soil in the Czech Republic, all as Hypocrea rufa in GenBank. As Hypocrea viridescens in GenBank: CIB T10 (tef1 accession EU279999) from Colombia, AN241 (tef1 JX184103) and AN435 (tef1 JX184105) from Poland (Błaszczyk et al. 2011); VI03955 (tef1 AM498509), VI03961 (tef1 AM498512) and VI03988 (tef1 AM498515) from Norway (Hageskal et al. 2008). Also the tef1 accession EU871008 of the strain TU Graz 5TSM1 (= C.P.K. 3299) from soil in Tenerife may belong here, but the sequence is very short and possibly contains errors.
Clade V5
Trichoderma virilente Jaklitsch & Voglmayr, sp. nov. — MycoBank MB803624; Fig. 7
Etymology. Viri stands for the first two syllables of viridescens and lente means slowly, addressing slow growth and particularly slow development of conidiation.
Holotype. Croatia, Istrija, Fažana, forest at Valbandon, N44°54′53″, E13°50′21″, elev. 30 m, on a 2–3 cm thick, partly decorticated branch of Fraxinus ornus, on wood and bark, holomorph, on the stroma surface of an old Diaporthe sp. on wood and bark, soc. Cosmospora sp., Nitschkia grevillei, Exidia sp., corticiaceous fungi, 26 Sept. 2010, W. Jaklitsch & H. Voglmayr S281 (WU 31628, ex-type culture CBS 132569; from ascospores).
Stromata scattered to aggregated in small groups, when fresh flat pulvinate, 1–4 mm diam, typically with variable outline, at first white to yellow and hairy, later short-haired or glabrous, turning reddish to nearly violaceous-brown; ostioles invisible. Stromata when dry (0.9−)1.3–2.4(−3) × (0.8−)1.0–1.8(−2.1) mm, (0.3−)0.4–0.7(−0.9) mm thick (n = 20), flat pulvinate, sometimes turbinate, with irregular, oblong, triangular, circular or lobate outline; edge free; surface tubercular, rugose or smooth, ostioles invisible or extremely inconspicuous; when young hairy, at first white, turning yellowish brown from the centre, later dark reddish brown to violaceous, with scarce, light yellow to reddish brown hairs. Spore deposits white. Asci (93−)102–118(−130) × (5.0−)5.8–7.0(−7.7) μm, stipe to 18 μm long (n = 30). Ascospores hyaline, spinulose; cells dimorphic, distal cells (4.5−)4.7–5.7(−6.7) × (3.8−)4.2–5.0(−6.3) μm, l/w 1.0–1.3(−1.5) (n = 50), (sub)globose or ellipsoidal, proximal cells (4.8−)5.7–7.3(−8.2) × (3.0−)3.5–4.5(−5.8) μm, l/w (1.2−)1.4–2.0(−2.4) (n = 50), oblong or ellipsoidal.
Cultures and asexual morph — Optimal growth at 25 °C on all media, slow growth at 30 °C, no growth at 35 °C.
On CMD after 72 h colony radius 27–29 mm at 25 °C, 2–5 mm at 30 °C; mycelium covering the plate after 9–10 d at 25 °C. Colony hyaline, dense, with inconspicuous difference in hyphal width and often with conspicuously large curvatures and coiling in surface hyphae, not zonate, margin wavy to sublobate. Aerial hyphae scant, sometimes common and long at the margin. Autolytic activity inconspicuous, coilings lacking, sometimes large at the margin. Agar after storage at 15 °C for 1–24 mo either colourless, faintly yellowish or rarely more distinctly yellow. Odour coconut-like. Chlamydospores uncommon. Conidiation noticeable after 2–4 d, turning green after 7–8 d or later, at first short-effuse, slowly developing and often scant, later in shrubs or minute transparent pustules < 1 mm diam, rarely to 2 mm diam, in a broad distant concentric zone and/or irregularly distributed, eventually dark green 27E4–6. Shrubs comprising loose aggregations of broad conidiophores with paired branches at the upper levels tending to be asymmetric downwards; cells and phialides generally long and narrow. Pustules consisting of one or several dense and short central clusters with often conspicuously curved conidiophores and phialides, and of typically straight, projecting, peripheral conidiophores arising more or less radially from a loose, simple reticulum. Peripheral conidiophores often narrow and simple, with distinct, often long main axes bearing phialides on a single cell or with short unbranched or 1–2 times rebranching side branches perpendicular to the axis, slightly inclined upwards at the upper levels. Branches 1.5–3.5(−5) μm wide; submoniliform terminal branches uncommon, usually only the cell supporting the phialide enlarged. Phialides solitary or divergent in whorls of 2–3, (7.3−)8.5–12.2(−15.3) × (2.2−)2.5–3.0(−3.2) μm, l/w (2.5−)3.1–4.8(−6.3), base (1.2−)1.5–2.2(−2.3) μm wide (n = 30), narrowly lageniform, straight or often curved or sigmoid, symmetric or inaequilateral. Conidia (3.8−)4.2–5.2(−6.0) × (2.8−)3.0–3.5(−4.0) μm, l/w 1.2–1.6(−1.8) (n = 50), broadly ellipsoidal or subglobose, rarely oblong, pale green, thick-walled, nearly smooth to faintly verruculose, with some minute guttules; scar indistinct.
Colony radius on PDA (Merck) after 72 h 21–23 mm at 25 °C, 1–2 mm at 30 °C; mycelium covering the plate after 9–10 d at 25 °C. Colony circular, dense, with inconspicuous difference in hyphal width, not zonate, margin wavy, surface flat, white to yellowish, farinose. Aerial hyphae common, forming a loose, flat, continuous, unstructured mat. Autolytic activity moderate, coilings inconspicuous. No diffusing pigment produced; odour coconut-like to unpleasant. Conidiation noticeable after 2–3 d, not turning green within 2 wk, short-effuse.
Colony radius on SNA after 72 h 12–22 mm at 25 °C, 2–4 mm at 30 °C; mycelium not covering the plate after c. 2 wk at 25 °C. Colony hyaline, dense, irregularly lobed, with inconspicuous difference in hyphal width and often with conspicuous curvatures in surface hyphae. Aerial hyphae scant. Autolytic activity inconspicuous, coiling abundant. No diffusing pigment produced; odour indistinct. Chlamydospores uncommon, terminal and intercalary, (sub)globose or oblong or pyriform, (5−)6–11 (−12) × (4.5−)5.5–9.5(−11.5) μm, l/w 1.0–1.4(−1.9) (n = 16), apparently only formed in surface hyphae. Conidiation noticeable after 2–4 d, turning green after 5–7 d, effuse, later in shrubs or small medium green 28CD5–6, 29CD4 pustules growing to c. 2 mm diam. Pustules comprising a dense centre of short and broad conidiophores and a periphery of long and narrow tree-like conidiophores.
Distribution — Southern Europe, known from Croatia, Italy, Greece and Spain. One strain (DAOM 234234) from Guatemala is associated with this clade.
Habitat — On dead wood and bark and fungi growing on it.
Other material examined. Greece, Corfu, Troumpettas, Agia Anna, N39°42′14″, E19°44′17″, elev. 420 m, on a cut branch of Quercus coccifera, asexual morph, 23 Apr. 2012, W. Jaklitsch & H. Voglmayr (culture S630); Zygos, N39°43′28″, E19°48′05″, elev. 300 m, on a 4–5 cm thick branch of Cercis siliquastrum, asexual morph, 23 Apr. 2012, W. Jaklitsch & H. Voglmayr (culture S635); Crete, Fournés, citrus plantation along the river Keritis, N35°26′10″, W23°56′18″, elev. 90 m, on 1–3 cm thick branches of Citrus sinensis, soc. Orbilia sp., holomorph, sexual morph immature, 24 Nov. 2011, W. Jaklitsch (culture S585). – Italy, Apulia, Foggia, Gargano, SW from Mandrione, Foresta Umbra, N41°50′34″, E16°02′54″, elev. 440 m, on a corticated branch of Hedera helix, asexual morph, 22 Nov. 2009, W. Jaklitsch & H. Voglmayr (culture S108); Lazio, Corviano near Soriano, N42°28′30″, E12°11′50″, elev. 280 m, on a 1 cm thick, corticated twig of Cistus salviifolius, asexual morph, 27 Nov. 2009, W. Jaklitsch, W. Gams & H. Voglmayr (culture S145); Lombardia, Brescia, between Provaglio Valsabbia and Barghe, at the brook Reaclino, N45°40′55″, E10°25′18″, elev. 400 m, on a 1–2 cm thick twig of Robinia pseudoacacia, asexual morph, 21 Oct. 2011, W. Jaklitsch & H. Voglmayr (culture S570). – Spain, Andalucia, Castellar de la Frontera, northeast from the village at the road A2100, N36°17′55″, W5°21′53″, elev. 185 m, on a 2–5 cm thick, corticated branch of Calicotome spinosa, asexual morph, 20 Mar. 2011, H. Voglmayr & W. Jaklitsch (culture S517); ibid., N36°17′55″, W5°21′57″, elev. 180 m, on a 2–3 cm thick branch of Pistacia lentiscus, asexual morph, 20 Mar. 2011, W. Jaklitsch & H. Voglmayr (culture S520); between El Bosque and Algar, N36°40′21″, W5°36′28″, elev. 210 m, on a 5 cm thick branch of Quercus cf. faginea, on bark, soc. Hypoxylon sp., asexual morph, 24 Mar. 2011, H. Voglmayr & W. Jaklitsch (culture S546); San Pablo de Buceite, N36°27′59″, W5°24′09″, elev. 70 m, on a 5 cm thick branch of Rhamnus frangula ssp. baetica, soc. Daldinia sp., corticiaceous fungi, asexual morph, 22 Mar. 2011, W. Jaklitsch & H. Voglmayr (culture S531); Gipuzkoa, Aralar, east from San Martin/Ataun, at the road BI 4151, N42°59′49″, W2°09′58″, elev. 350 m, on a Diatrypaceae on Ulex europaeus, soc. corticiaceous fungus, asexual morph, 2 Nov. 2010, W. Jaklitsch (culture S335).
Notes — The asexual morph of this species on natural substrates is often effuse, and its colonies are several cm long. Sometimes they are associated with bright yellow mycelium. Trichoderma virilente is characterised by slow growth and slow development of conidiation in culture. CMD cultures may remain sterile, even for 1–2 years (monitored at 15 °C). Distinct curvatures in conidiophores on CMD may be an aging effect.
Clade V6
The strains of clade V6 comprise a phylogenetically well-delineated Trichoderma species currently known only from Peru. It is known to us only through sequences deposited in GenBank, including the culture DAOM 233968 (tef1 accession EU280022; see Hoyos-Carvajal et al. 2009).
Clade V7
Trichoderma trixiae Samuels & Jaklitsch, sp. nov. — MycoBank MB803625; Fig. 8
Etymology. Trixiae, in recognition of invaluable collaboration over many years by Beatrix ‘Trix’ Merkx (Centraalbureau voor Schimmelcultures).
Holotype. Germany, Baden-Württemberg, Freiburg, Landkreis Schwarzwald-Baar-Kreis, Furtwangen, MTB 8015/1, N47°59′36″, E8°10′50″, elev. 1000 m, on 20–40 cm thick cut logs of Picea abies in a pile at roadside, holomorph, sexual morph immature, culture from conidia, 2 Sept. 2004, W. Jaklitsch & H. Voglmayr (CBS 134702, culture permanently preserved in a metabolically inactive state; other culture numbers: Hypo 228, C.P.K. 2138; source: specimen WU 24023).
Sexual morph only known in immature condition.
Cultures and asexual morph — Optimum temperature for growth 25 °C. Colony radius after 72 h at 25 °C on PDA 34–37 mm, on SNA 23–32 mm. Not growing at 35 °C.
On CMD conidia forming within 1 wk in concentric rings of flocculent mycelium, often with pustules. Pustules pulvinate, 1–2 mm diam, discrete or confluent, cottony, lacking protruding sterile hairs or conidiophores. Conidiophores arising from aerial hyphae and terminating hyphae in pustules. On SNA and CMD conidiophores consisting of a more or less well-developed central axis, from which paired or unpaired, fertile branches arise at more or less widely spaced intervals; fertile branches typically terminating in a single phialide or a divergent whorl of 2–3 phialides; phialides also arising directly from the tip of the main axis. Phialides lageniform, somewhat swollen below the middle, (5−)7–9(−20) μm long, (2.0−)2.5–3.5(−5.0) μm at the widest point, l/w (1.4−)2.1–4.0(−7.5) (n = 120), arising from a (1.5−)2.2–3.5(−5.0) μm wide cell; ratio of phialide length to width of supporting cell (1.7−)2.3–4.3(−8.2); ratio of width of phialide to width of supporting cell (0.7−)0.9–1.3(−1.8). On CMD and PDA conidiophores often reduced, comprising a more or less sinuous rachis with phialides arising at the end and laterally, sometimes in chains of a few; rachis often arising from subglobose, thin-walled cells; phialides very narrow and abruptly swollen below the middle, often proliferating percurrently to form a moniliform chain of two or more phialides. Conidia ellipsoidal to subglobose, (3.0−)3.7–4.5(−5.2) × (2.5−)3.2–4.0(−4.7) μm, l/w (0.9−)1.0–1.3(−1.6) (n = 120), green, tuberculate. Chlamydospores few.
Cultures on PDA (Difco) within 1 wk felty, conidial production beginning in the middle, progressing toward the margin in more or less marked, narrow concentric rings, conidia grey-green, forming in aerial mycelium, pustules few; no diffusing pigment.
Distribution — Europe, USA; possibly also New Zealand.
Habitat — On dead wood and in soil; possibly fungicolous.
Other material examined. Germany, locality, date and collector unknown, isolated from soil (CBS 130678 = G.J.S. 99-11). – ?New Zealand, locality, date and collector unknown, isolated from wood of Pinus radiata, comm. M.E. Palm (CBS 123771 = ICMP 16297 = G.J.S. 92-11; GenBank accession for ITS: DQ315442, as Hypocrea rufa). – Sweden, locality, collector and date unknown, isolated from a Fagus pole (ATCC 32630 = Tr 26; GenBank: ITS DQ315445, as Hypocrea rufa). – USA, Oregon, locality and date unknown, isolated from roots of Pseudotsuga menziesii infected with Phellinus weirii, E. Nelson 11 (Tr 5; GenBank: ITS DQ315443, as Hypocrea rufa); ibid., E. Nelson 24 (Tr 6; GenBank: ITS DQ315444, as Hypocrea rufa).
Notes — The sexual morph of T. trixiae has been found only once in Germany (Hypo 228) in immature condition. The origin of strain CBS 123771 (G.J.S. 92-11) given as ?New Zealand is uncertain, as it was isolated from a shipment of wood from New Zealand that was intercepted at a port in California. The tef1 accession EU280016 of the strain DAOM 172787 represents T. trixiae. However, Hoyos-Carvajal et al. (2009) only mentioned the strain number in their phylogenetic tree, origin (possibly Colombia?) and habitat (probably soil) are thus uncertain. The strain AN248 (tef1 sequence JX184104; as Hypocrea viridescens) obviously also belongs to T. trixiae. Images of T. trixiae in addition to Fig. 8 are given in Fig. 5a, b, k and Fig. 8f of Jaklitsch et al. (2006).
Clade V8
Trichoderma viridialbum Jaklitsch, Samuels & Voglmayr, sp. nov. — MycoBank MB803626; Fig. 9
Fig. 9.
Trichoderma viridialbum, cultures and asexual morph at 25 °C. a–c. Cultures after 14 d (a. on CMD; b. on PDA; c. on SNA); d, e. conidiation pustules (13 and 22 d); f–j. conidiophores; k, l. phialides; m–q. conidia; d–q. all from CMD; f–q. after 7–8 d except m (after 11 d) (a–c, e–l, n–q: CBS 133495 = S250; d, m. S177). — Scale bars: d = 0.4 mm; e = 0.5 mm; f, j = 15 μm; g, k, l, n = 10 μm; h, i = 20 μm; m, o, p = 6 μm; q = 3 μm.
Etymology. Viridialbum refers to green and white, due to the white surface of the conidiation pustules formed on CMD.
Holotype. Spain, Islas Canarias, Tenerife, Teide National Park, path 23 between Regatones Negros and Roques Blancos, UTM 28R 0338342; 3123629, elev. 2550 m, on a 5–7 mm thick twig of Descurainia bourgeauana on wet soil, asexual morph, 23 Apr. 2010, L. Quijada & J. Díaz (CBS 133495, culture permanently preserved in a metabolically inactive state; other culture number: S250).
Sexual morph only known in immature condition; stroma reddish brown, velutinous.
Cultures and asexual morph — Optimal growth at 25 °C on all media, slow and restricted growth at 30 °C, no growth at 35 °C.
On CMD after 72 h colony radius 40–45 mm at 25 °C, 8–12 mm at 30 °C; mycelium covering the plate after 5 d at 25 °C. Colony circular, hyaline, dense, with distinct differences in hyphal width. Aerial hyphae inconspicuous. Autolytic excretions and coilings scarce. Odour strongly coconut-like, no diffusing pigment formed, agar at most faintly dull yellowish after 17 mo at 15 °C. Chlamydospores uncommon, terminal and intercalary. Conidiation absent or microscopically perceptible after 3–4 d, at first effuse, simple, after 5–10 d in minute, often scarcely visible, loose shrubs or amorphous pustules < 1 mm diam, less commonly up to 2.5 mm diam, appearing in a broad marginal zone or few scarcely separated, distant to marginal zones. Pustule surface velutinous, remaining white for several weeks. Conidia developing within the pustules, resulting colour whitish to pale green 26–27BC3, 28BC3–4. Shrubs and pustules arising on a thick-walled, to 11 μm wide stipe, asymmetrically producing thick-walled, up to 7 μm wide primary branches abruptly narrowing into a loose or dense reticulum. Conidiophores rarely comprising a distinct main axis, often scarcely and typically asymmetrically branched at narrow angles, (1.0−)1.5–3.5(−4.5) μm wide, straight or conspicuously curved, forming broad structures and terminally long conspicuous submoniliform chains of up to 8 enlarged cells. Centre of aged pustules sometimes forming conspicuous, irregularly inflated cells and phialides. Pustule periphery often sterile or forming few phialides. Phialides solitary, less commonly in whorls of 2–3, (6.8−)8.8–12.7(−15.0) × (2.2−)2.5–3.0(−3.5) μm, l/w (2.0−)3.0–4.7(−5.8), (1.2−)1.5–2.0(−2.3) μm wide at the base (n = 44), narrowly lageniform with scarcely or distinctly swollen at or below the middle, straight, in age often conspicuously curved, often with long base or neck. Conidia globose to subglobose, less commonly broadly ellipsoidal, rarely oblong, often slightly attenuated towards one end, (3.2−)3.7–4.5(−5.5) × (3.0−)3.3–4.0(−4.5) μm, l/w 1.0–1.2(−1.5) (n = 90), green, verruculose or verrucose with loosely disposed warts, scar indistinct or truncate.
Colony radius on PDA (Merck) after 72 h 35–38 mm at 25 °C, 5–6 mm at 30 °C; mycelium covering the plate after 5 d at 25 °C. Colony circular, dense. Aerial hyphae abundant, forming a dense white mat, agglutinating in thick strands. Autolytic excretions frequent, coilings inconspicuous. No diffusing pigment produced, reverse yellowish 3B3–5 to 4B4; odour coconut-like to unpleasant, not persistent. Conidiation absent or noticeable after 4–5 d, scant, effuse, not turning green.
Colony radius on SNA after 72 h 34–37 mm at 25 °C, 4–6 mm at 30 °C; mycelium covering the plate after 5–6 d at 25 °C. Colony hyaline, dense, circular, not zonate, of more or less radiating hyphae with conspicuous differences in width. Colony surface becoming downy due to numerous long aerial hyphae except in the centre. Autolytic activity inconspicuous, coilings abundant. No diffusing pigment produced, agar sometimes faintly yellowish; odour indistinct. Chlamydospores common but loosely disposed, terminal and intercalary, globose to subglobose or pyriform, sometimes oblong, (6−)8–12(−17) × (5−)7–11(−13) μm, l/w 0.9–1.3(−1.8) (n = 30). Conidiation noticeable after 3 d, turning green after 5–6 d, eventually dark green 27F4–8, 26EF3–5, 28–29F4–8; at first effuse, mostly ascending on aerial hyphae in downy regions, later in small compact pustules, sometimes up to 3 mm diam, disposed along the margin and irregularly over the colony.
Distribution — Europe (Spain: Canary Islands, Norway) and Mexico.
Habitat — On dead wood and bark and in soil.
Other material examined. Spain, Islas Canarias, La Palma, Cumbre Nueva, branching off LP-301 at Area Recreativa del Pilar, N28°37′16″, W17°49′52″, elev. 1440 m, on a 1.5–2 cm thick stalk of Sideritis canariensis, holomorph, sexual morph immature, 11 Dec. 2009, W. Jaklitsch (culture S177); pine forest below Refugio El Pilar, N28°36′28.5″, W17°49′47″, elev. 1455 m, on a 2.5–3 cm thick branch of Erica arborea, asexual morph, 2 Dec. 2010, W. Jaklitsch & R.M. Dähncke (culture S429).
Notes — Conidial pustules on CMD remain externally white for a long time (e.g. 3 wk) and the greenish appearance is due to green internal conidia shining through the sterile surface layer. The submoniliform chains or repetitive phialides in conidiophores are a typical and integral part of the conidiogenous structures on CMD and are not age-related. The conspicuously curved or sinuous conidiophores and sometimes also phialides are reminiscent of T. viride.
Other material determined to belong to this species via tef1 sequences: Mexico, locality and date unknown, from soil (G.J.S. 07-138, G.J.S. 07-139, G.J.S. 07-145, V.S.L. 11, V.S.L. 15 and V.S.L. 16). The strains VI03958 and VI03963 were isolated from drinking water in Norway (Hageskal et al. 2008). Also strain TU Graz 6TSM1, reported from Tenerife, belongs to this species. However, the deposited tef1 sequence is extremely short (177 bp) and neither the strain number nor the accession number (EU871009) can be found in the corresponding publication of the submitters (Zachow et al. 2009). The strain UNISS 3.32_new from Sardinian soil also belongs here. However, its tef1 accession DQ790659 is extremely short.
Clade V9
Trichoderma appalachiense Samuels & Jaklitsch, sp. nov. — MycoBank MB803627; Fig. 10
Fig. 10.
Trichoderma appalachiense. a–d: Sexual morph. a. Dry stromata; b. cells at the stroma surface in face view; c. hairs arising from cells at the stroma surface; d. asci and ascospores (note the thickened ascal apex). — e–i: Asexual morph from CMD. e. Pustules; f, g. conidiophores; h, i. conidia (a: G.J.S. 97-243; b–i: G.J.S. 00-67). — Scale bars: a = 1 mm; b–d, f–h = 10 μm; e = 0.5 mm; i = 2.5 μm.
Etymology. Appalachiense refers to the Appalachian Mountains region of the eastern United States from which this species is described.
Holotype. USA, Georgia, Rabun County, vic. Clayton, forest at Chatahoochie Rd, Warwoman Dell, elev. 750 m, on dry decorticated wood, 14 Oct. 1997, G.J. Samuels, E. Lieckfeldt & X. Tong (BPI 746727; ex-type culture CBS 133558 = G.J.S. 97-243, from ascospores; GenBank ITS: DQ315419).
Stromata forming on decorticated wood, sometimes on top of black fungi, purplish brown, circular, 0.5–3.0 mm diam, becoming confluent, not more than 5 mm thick, margins free, plane, non-papillate, ostiolar openings not visible, young stromata lighter in colour, appearing velutinous from hairs at the stroma surface. Cells at the stroma surface in face view circular to irregular in outline, 2.5–7.5 μm diam, with unevenly pigmented walls. Hairs arising from the stroma surface cylindrical, septate, unbranched or infrequently branched, (7−)10–20(−25) μm long, (1.5−)3.0–4.5(−5.2) μm wide, with visibly thickened walls, disappearing at maturity. Perithecia fully immersed in the stroma, (175−)200–250(−290) μm tall, (65−)80–180(−280) μm wide, ostiolar canal 60–90 μm long; cells at the ostiolar opening not sharply differentiated from the surrounding stroma tissue. Surface region of stroma 15–30 μm thick, cells nearly circular to irregular in outline, 6–9 μm diam, walls thickened, pigmented. Tissue below the stroma surface and between perithecia hyphal, disorganized. Cells below perithecia arranged more or less in a textura epidermoidea or nearly hyphal, 9–30 × 7–10 μm, thin-walled. Asci cylindrical, (61−)80–100(−118) × (3.0−)4.5–6.5(−7.7) μm (n = 30), apex thickened and with a pore. Ascospores hyaline, finely spinulose; cells dimorphic, distal cells subglobose to nearly conical, (3.5−)4.0–5.0(−6.0) × (3.2−)3.7–4.5(−5.2) μm (n = 30); proximal cells wedge-shaped to ellipsoidal, (4.0−)4.5–5.7(−6.5) × (3.0−)3.5–4.0(−4.5) μm (n = 30).
Cultures and asexual morph — Optimum temperature for growth 25 °C. Colony radius after 72 h at 25 °C on PDA 40–45 and SNA 30–35 mm. Not growing at 35 °C.
On CMD pustules beginning to form in concentric rings within 1 wk; pustules confluent, cottony, lacking sterile hairs or protruding terminally fertile conidiophores. Conidiophores terminating hyphae that compose pustules; a main axis more or less well developed; phialides arising directly from the main axis or terminating more or less widely spaced lateral branches, solitary or in pairs in divergent whorls, lageniform, at most only slightly swollen below the middle, (5.0−)5.7–9.5(−13.2) μm long, (1.7−)2.5–3.5(−4.0) μm at the widest point, l/w (1.5−)1.4–3.4(−6.0) (n = 60), arising from 1.7–3.5(−6.0) μm wide supporting cells, ratio of phialide length to width of supporting cell (1.5−)2.2–4.0(−6.2), ratio of phialide width to width of supporting cell (0.7−)1.0–1.5(−1.9). Conidia broadly ellipsoidal, 3.5–4.0(−4.5) × (2.5−)3.0–3.5(−4.0) μm, l/w (0.9−)1.1–1.3(−1.5) (n = 60), green, appearing smooth by light microscopy, finely warted in SEM. Chlamydospores not observed on CMD.
Cultures on PDA within 1 wk cottony with pustules beginning to form in the centre and in the aerial mycelium elsewhere, no diffusing pigment observed.
Distribution — Mid Atlantic states of the USA (Georgia, Virginia, West Virginia).
Habitat — On decorticated wood.
Additional specimens examined. USA, Virginia, Giles Co., Mt. Lake Biological Station, trail to Rhododendrons, elev. 1100 m, on decorticated wood, 29 Sept. 1997, G.J. Samuels & E. Lieckfeldt (BPI 746718; culture G.J.S. 97-216); West Virginia: Tucker Co., experimental forest, Big Springs, N39°30′, W79°39′, 13 June 2000, K. Põldmaa 377 (BPI 877338; culture CBS 122569 = G.J.S. 00-67; GenBank ITS: DQ315418).
Notes — Trichoderma appalachiense is among the fastest growing species in the T. viridescens species complex, reaching a colony radius of 62 mm and nearly completely filling a 9-cm-diam Petri plate after 96 h at 25 °C on PDA. Its closest relative, T. neosinense, in contrast, reaches a radius of only 40 mm after 96 h at 25 °C and the difference in growth rate is even more marked at 30 °C. An additional image of T. appalachiense is given in Fig. 8g of Jaklitsch et al. (2006).
Clade V9a
Trichoderma neosinense Samuels & Jaklitsch, sp. nov. — MycoBank MB803628; Fig. 11
Fig. 11.
Trichoderma neosinense. a, b: Sexual morph. a. Dry stromata; b. surface region of a stroma with deteriorating hairs. — c–g: Asexual morph from CMD. c–e. Conidiophores; f, g. conidia (g. note surface ornamentation in SEM view) (a: G.J.S. 94-9; b–f: G.J.S. 94-10; g: G.J.S. 94-11). — Scale bars: a = 1 mm; b = 20 μm; c–f = 10 μm; g = 5 μm.
Etymology. Neosinense refers to the type locality, Taiwan.
Holotype. Taiwan, NTU Experimental Farm, on decorticated wood, 22 Apr. 1994, A.Y. Rossman s.n. (BPI 749315, ex-type strain CBS 134884 = G.J.S. 94-11, from ascospores; GenBank ITS: DQ315422).
Stromata forming on decorticated wood, sometimes on top of black fungi, purplish brown, circular to irregular in outline, 0.5–2.0 mm diam, becoming confluent, not more than 5 mm thick, margins free, surface plane, non-papillate, ostiolar openings not visible, young stromata appearing velutinous from hairs at the stroma surface. Cells at the stroma surface in face view circular, 2.5–6.5 μm diam, with unevenly pigmented walls. Hairs arising from the stroma surface cylindrical, septate, unbranched or infrequently branched, 25–30 μm long, c. 3.5 μm wide, with visibly thickened walls, lacking at maturity. Perithecia fully immersed in the stroma, 200–300 μm tall, 120–230 μm wide, ostiolar canal 60–90 μm long; cells at the ostiolar opening not sharply differentiated from the surrounding stroma tissue. Surface region of stroma c. 20 μm thick, cells nearly circular or irregular in outline, 4–7 μm diam, walls thickened, pigmented. Tissue below the stroma surface and between perithecia hyphal. Cells below perithecia arranged in a textura epidermoidea, 6–15 × 5–10 μm, thin-walled. Asci cylindrical, (60−)80–100(−110) × (3.7−)4.2–6.0(−7.0) μm (n = 30), apex thickened and with a pore. Ascospores hyaline, finely spinulose, cells dimorphic, distal cells subglobose to nearly conical, (3.5−)4.2–5.2(−6.0) × (3.2−)3.7–4.5(−5.0) μm (n = 30); proximal cells wedge-shaped to ellipsoidal, (3.2−)4.7–5.7(−6.5) × (3.0−)3.2–4.0(−4.5) μm (n = 30).
Cultures and asexual morph — Optimum temperature for growth 25 °C. Colony radius after 72 h at 25 °C on PDA 25–35 and SNA 15–20 mm. Not growing at 35 °C.
On CMD conidia beginning to form within 1 wk in the aerial mycelium without obvious pustules. Conidiophores lacking a well-developed main axis or at most central axis weakly developed; sterile hairs or projecting, terminally fertile conidiophores lacking; fertile branches arising at odd intervals and rebranching once or a few times, each branch terminating in 1–2 phialides in more or less divergent whorls. Phialides lageniform, constricted above the middle, (5.5−)8.5–13.5(−20) μm long, (1.5−)2.2–2.7(−3.0) μm at the widest point, l/w (1.9−)3.3–5.7(−9.5) (n = 81), arising from 1.5–5.0(−11) μm wide cells, ratio of phialide length to width of supporting cell (1.2−)3.0–5.8(−8.9), ratio of phialide width to width of supporting cell (0.2−)0.7–1.3(−1.9). Conidia broadly ellipsoidal, (2.7−)3.5–4.5(−5.0) × (2.2−)2.7–3.5(−4.0) μm, l/w (1.0−)1.2–1.5(−1.6) (n = 90), green, finely warted but appearing smooth by light microscopy. Chlamydospores sparsely formed on CMD, forming within hyphae, subglobose, 6–11 μm diam.
Cultures on PDA within 1 wk felty, distinctly zonate, sterile or conidial production scant, no diffusing pigment formed.
Distribution — Taiwan.
Habitat — On dead wood and bark.
Other specimens examined. Taiwan, NTU Experimental Farm, on bark, 22 Apr. 1994, A.Y. Rossman s.n. (BPI 749314; culture G.J.S. 94-9; GenBank rpb2: FJ442703; ITS: DQ315421); same place and date (BPI 749315; culture G.J.S. 94-10; GenBank ITS DQ315420).
Notes — Within the T. viridescens species complex, T. neosinense has one of the slowest rates of growth on SNA and is in this respect similar to T. virilente and T. sempervirentis. Conidiation was poor on CMD and PDA and at most only very minute pustules were formed. Conidia appear smooth by light microscopy, but are minutely asperate when observed with SEM, consistent with other species in the T. viridescens complex.
Clade V10
Trichoderma composticola Samuels & Jaklitsch, sp. nov. — MycoBank MB803629; Fig. 12
Fig. 12.
Trichoderma composticola. a–c. Cultures after 10 d at 25 °C (a. on CMD, insert: yellow pigment in strain S590 after 10 mo at 15 °C on white background; b. on Merck-PDA; c. on SNA); d. loose conidiation pustules at the plate margin on CMD; e, g–i. conidiophores (e. from SNA, g–i. from CMD; h, i. note the ‘eidamia’ development with chains of phialides and abrupt constriction above and below the middle in some phialides); f, j, k. conidia; l, m. chlamydospores; j–m. from CMD after 10 d (a–c, j–m: S590; d, e: G.J.S. 04-232; f, g: CBS 438.95; h, i: CBS 333.72). — Scale bars: d = 1 mm; e = 20 μm; f, j, k = 5 μm; g–i, l, m = 15 μm.
Etymology. Composticola refers to the typical habitats like compost and agricultural waste.
Holotype. Greece, Crete, south of Kakopetros, roadside, N35°24′46″, E23°45′49″, elev. 495 m, on a 4–5 cm thick, cut branch segments of Vitis vinifera, on wood and bark, asexual morph, 25 Nov. 2011, W. Jaklitsch (CBS 133497, culture permanently preserved in a metabolically inactive state; other culture number: S590).
Sexual morph not known.
Cultures and asexual morph — Optimal growth at 25 °C on all media, not growing at 35 °C.
On CMD after 72 h colony radius c. 27 mm at 15 °C, c. 40 mm at 25 °C, c. 18 mm at 30 °C; mycelium covering the plate after 6 d at 25 °C. Colony circular, hyaline, dense, mycelium with radial arrangement. Aerial hyphae scant, autolytic excretions and coilings absent, diffusing pigment absent, but after extended storage (e.g. 10 mo) at 15 °C sometimes present, distinctly yellow, odour coconut-like. Chlamydospores only visible in surface hyphae, uncommon, terminal and intercalary, (7−)8–13(−18) × (7−)8–11(−15) μm, l/w (0.9−)1.0–1.4(−1.6) (n = 60), subglobose, less commonly oblong. Conidiation noticeable after 3 d, turning greyish green 26CD4, 27CD4–5 after 4–6 d, eventually dark green 25–27F4–8, at first effuse, simple, scant, soon in shrubs or small finely granular pustules disposed in up to 4 concentric zones. Shrubs and pustules lacking sterile hairs or protruding terminally fertile conidiophores, being formed on a thick-walled, verrucose, up to 11 μm wide stipe, loose, comprising broad regularly tree-like peripheral conidiophores and dense central clusters. Peripheral conidiophores comprising a more or less well-developed main axis, phialides often arising directly from the main axis, and fertile branches tending to arise at uniform intervals, often paired, rebranching, the secondary branches producing phialides directly or in divergent whorls of up to four at the ends of branches. Branches at right angles or slightly inclined upwards, paired or not, 2–4.5(−5) μm wide, terminal branches sometimes submoniliform with up to five swollen cells in a chain. Phialides lageniform, swollen or not, often inaequilateral and abruptly constricted above and below the middle, straight, less commonly distinctly curved, (5.5−)6.5–11.2(−19.5) μm long, (2.2−)2.7–3.7(−5.0) μm at the widest point, l/w (1.4−)2.2–3.8(−5.3), (1.5−)1.7–2.2(−2.5) μm wide at the base (n = 60), arising from (2.0−)2.5–3.7(−5.0) μm wide cells, ratio of phialide length to width of supporting cell (1.4−)2.3–3.9(−5.7), ratio of phialide width to width of supporting cell (0.7−)0.9–1.3(−1.8). Conidia ellipsoidal, often attenuated toward one end, less commonly subglobose, (3.5−)4.0–5.2(−8.5) × (2.7−)3.0–3.7(−4.5) μm, l/w (1.0−)1.1–1.5(−2.0) (n = 100), green, verruculose, warts irregularly and loosely distributed.
Colony radius on PDA (Merck) after 72 h c. 25 mm at 15 °C, 35–45 mm at 25 °C, c. 15 mm at 30 °C, mycelium covering the plate after 6 d at 25 °C. Colony circular, dense, not zonate; margin wavy, surface whitish and radial because of numerous strands of surface hyphae and abundant aerial hyphae. Autolytic excretions frequent, coiling moderate. No diffusing pigment formed, reverse turning slightly yellowish 3C4; odour weakly coconut-like. Conidiation noticeable after 3 d, turning greenish after 6 d, eventually bluish green or grey-green, mostly 25DE4–6, effuse, mainly on aerial hyphae, spreading from the centre, evenly (Merck-PDA) or in broad concentric rings (Difco-PDA).
Colony radius on SNA after 72 h c. 26 mm at 15 °C, 30–37 mm at 25 °C, c. 8 mm at 30 °C; mycelium covering the plate after 6 d at 25 °C. Colony circular, hyaline, dense. Aerial hyphae scant, autolytic excretions scant, coiling abundant, diffusing pigment absent, odour indistinct. Chlamydospores absent. Conidiation noticeable after 2–3 d, turning green after 3–4 d, in broad transparent whitish to bluish green shrubs, floccules or tufts to c. 1.5 mm diam; the latter comprising slender branches, long radially emergent regularly tree-like conidiophores and several dense clusters; similar to CMD, but without submoniliform terminal branches. Shrubs disposed in one broad or several hardly separated concentric zones; eventually dark green 26–27F4–8.
Distribution — Europe, Russia, UK, Mexico; probably cosmopolitan north temperate to subtropical.
Habitat — Mushroom compost, soil, found once on cut branch segments of grape lying on the ground.
Other material examined. Mexico, isolated from soil below Agave tequillensis, 2004, V.S. Lopez, VSL 143 (G.J.S. 04-232). – Netherlands, locality and substrate not known, Apr. 1972, T.Y. Schepp-Stolk, CL 430 (CBS 333.72). – Russia, locality not known, isolated from paper board in a cellar, date and collector unknown, BBA 68432 (G.J.S. 97-274). – United Kingdom, Northern Ireland, Mourne, on mushroom compost, 1993, D.A. Seaby, D8 (CBS 439.95); same data, 1993, J. Fletcher, T55FJ (CBS 438.95).
Notes — The closest relative of T. composticola is T. nothescens, from which it differs in quicker and more abundant production of conidia on PDA and CMD at 25 °C. Trichoderma composticola is one of the fast-growing members of the T. viridescens species complex, and is in this respect similar to T. appalachiense and T. paraviridescens. The closest relative of T. composticola, T. nothescens, grows considerably slower. The ‘eidamia’ morphology, comprising chains of enlarged cells with a phialide at their end, cannot always be observed in T. composticola and may therefore be an aging effect in this species. Images of T. composticola in addition to Fig. 12 are given in Fig. 5e, f, h, l, Fig. 6e, f, k and Fig. 7h of Jaklitsch et al. (2006). The strain CBS 127109 (= GJS 99-175) belongs to T. composticola; however, its source (Victoria, Australia, from Hypoxylon sp. on Nothofagus cunninghamii) is doubtful.
Clade V10a
Trichoderma nothescens Samuels & Jaklitsch, sp. nov. — MycoBank MB803630; Fig. 13, 14
Fig. 13.
Trichoderma nothescens. Sexual morph. a, b. Stromata (a. pale, semi-effuse, immature; b. velvety stroma surface, ostiolar openings invisible); c. cells of the stroma surface in face view; d. surface region of a stroma with hairs arising from the cells at the surface; e. asci and ascospores, stained in cotton blue (note the thickened ascal apex with a pore) (a, c, d: G.J.S. 99-142; b: G.J.S. 99-86; e: G.J.S. 99-128). — Scale bars: a = 2 mm; b = 0.5 mm; c, d = 20 μm; e = 10 μm.
Fig. 14.
Trichoderma nothescens, culture and asexual morph. a. Culture on PDA after 17 d at 25 °C; b. shrubs on CMD; c. pustules on SNA (note yellow colour); d, e. conidiophores on CMD; f. phialides and intercalary phialides (arrows); g. ‘eidamia’ development in a pustule with swollen cells on CMD; h. phialides within a pustule on CMD (some abruptly narrowed above and below the middle); i, j. conidia (i. optical section; j. surface view) (a, c, f: G.J.S. 99-142; b, e, g: G.J.S. 99-86; d, h–j: G.J.S. 99-128. — Scale bars: b = 0.5 mm; c = 0.25 mm; d, e, g = 20 μm; f, h–j = 10 μm.
Etymology. Nothescens is an abbreviation of nothoviridescens, a false T. viridescens.
Holotype. Australia, Victoria, vic. Healesville, Toolangi State Forest, Myers Creek Rd., Wirrawalla rainforest walk and myrtle walking track in Myrtle Gully, elev. 575 m, on bark of recently dead tree, 23 Aug 1999, G.J. Samuels 8600 (BPI 746813; ex-type culture CBS 134882 = G.J.S. 99-142, from ascospores; GenBank: ITS DQ315427).
Stromata forming on bark, usually associated with other fungi, at first semi-effuse, brown with a white margin, 0.5–3.5 mm diam, darker brown or remaining light, ostiolar openings typically not visible, surface plane, non papillate, velvety at first, cells at the surface of the stroma in face view nearly circular in outline, 8–12 μm diam, walls thickened, pigmented; hairs arising from cells of the stroma surface, cylindrical, septate, unbranched, 5–50 μm long, c. 5 μm wide, walls thickened. Perithecia fully immersed in the stroma, (170−)230–320(−340) μm high, (70−)130–280 μm wide, ostiolar canal 70–120 μm long; stroma surface region 20–25 μm wide, cells 3–7 μm diam, walls thickened, pigmented, cells of the ostiolar region of the perithecia merging with the cells of the surrounding stroma, cells immediately below the stroma surface more or less hyphal, loosely disposed, thin-walled, non pigmented. Cells below perithecia of textura epidermoidea with short hyphal elements, 3–15(−40) × 2–10 μm wide, thin-walled. Asci cylindrical, (56−)75–104(−110) × 4.0–6.5(−8.0) μm (n = 30), apex thickened and with a ring. Ascospores hyaline, spinulose, cells dimorphic, proximal cells subglobose to broadly conical, (4.0−)4.5–5.5(−6.7) × (3.5−)4.0–5.0(−5.5) μm (n = 30); proximal cells wedge-shaped to oval, (3.5−)4.5–6.5(−7.5) × (2.7−)3.5–4.2(−4.7) μm (n = 30).
Cultures and asexual morph — Optimum temperature for growth 25 °C. Colony radius after 72 h on PDA c. 40 mm at 25 °C, c. 10 mm at 30 °C; on SNA c. 30 mm at 25 °C, c. 10 mm at 30 °C. Not growing at 35 °C. Cultures on PDA within 1 wk felty, sterile or at most sparsely conidial; within 2 wk conidial production beginning in the middle, subsequently in broad concentric zones, grey green but darker and lighter coloured rings alternating, conidia forming in minute, confluent, cottony pustules arranged in obscure concentric rings; no diffusing pigment. On CMD conidia forming within 2 wk at the margin of the colony. Pustules cottony, lacking sterile hairs or protruding terminally fertile conidiophores; on SNA pustules often with greenish yellow colouration due to pigmented conidia. Conidiophores forming in aerial mycelium and terminating the hyphae comprising pustules; of two types. The first type of conidiophores formed in aerial mycelium on CMD and SNA, comprising a more or less well-developed main axis, phialides often arising directly from the main axis, and fertile branches tending to arise at uniform intervals, often paired, rebranching, the secondary branches producing phialides directly or in divergent whorls of up to three at the ends of branches. The second type of conidiophores formed on CMD and PDA, in pustules as on SNA or much branched and appearing fasciculate or lacking a repeating pattern of organization, often arising from moniliform chains of swollen cells, then phialides usually solitary, often proliferating percurrently. Phialides lageniform, swollen or not below the middle, phialides formed on CMD and PDA often constricted above and below the middle, (5−)7–10(−14) μm long, (1.7−)2.5–3.2(−3.5) μm at the widest point, l/w (1.6−)2.0–4.0(−7.4) (n = 120), arising from (1.5−)2.0–3.0(−4.5) μm wide cells, ratio of phialide length to width of supporting cell (1.2−)2.3–4.7(−7.0), ratio of phialide width to width of supporting cell (0.7−)0.9–1.4(−1.7). Conidia subglobose to broadly ellipsoidal, (3.2−)3.5–4.5(−4.7) × (2.7−)3.0–3.7(−4.2) μm, l/w 1.0–1.3(−1.4) (n = 120), grey-green, tuberculate. Chlamydospores not observed.
Distribution — Australia (Victoria).
Habitat — On dead hardwood bark.
Additional specimens examined: Australia, Victoria, same locality as the type, on bark of Eucalyptus regnans, 23 Aug. 1999, G.J. Samuels 8595 (BPI 746807; culture CBS 134883 = ICMP 16290 = G.J.S. 99-86); between Yarram and Turaralgia, Balook, Tarras Bulga National Park visitor centre. Forest trail, elev. 550 m, on bark, 22 Aug. 1999, G.J. Samuels 8582 and J. Dyke (BPI 746793; culture G.J.S. 99-128; GenBank: ITS DQ315431).
Notes — The closest relative of T. nothescens is T. composticola from which it differs most conspicuously in its greatly diminished growth rate at 30 °C (after 72 h on Difco-PDA colony radius c. 10 and 30–35 mm, respectively). Trichoderma nothescens is known in nature only as its sexual morph whereas T. composticola is only known as an asexual morph. The sexual morph of T. nothescens is completely consistent with sexual morphs of other members of the Viridescens Clade.
Clade V11
Trichoderma sempervirentis Jaklitsch & Voglmayr, sp. nov. — MycoBank MB803631; Fig. 15
Fig. 15.
Trichoderma sempervirentis (S599 = CBS 133498). a–c. Cultures after 10 d at 25 °C (a. on CMD, insert: yellow pigment in strain S601 after 9 mo at 15 °C on white background; b. on PDA; c. on SNA); d–g, j, k. conidiophores; h, i, m. conidia; l. phialides; n, o. chlamydospores (17 d); d–o. all from CMD after 6–7 d except n and o. — Scale bars: d, j = 20 μm; e, f, k = 15 μm; g, l, n = 10 μm; h, i = 5 μm; m, o = 7 μm.
Etymology. Named after its host, Acer sempervirens.
Holotype. Greece, Crete, road to Askyfou, N35°18′06″, E24°12′13″, elev. 720 m, on 3–4 and 7 cm thick, corticated branches of Acer sempervirens lying in wet grass, asexual morph, soc. Terana caerulea and other corticioid fungi, 26 Nov. 2011, W. Jaklitsch (CBS 133498, culture permanently preserved in a metabolically inactive state; other culture number: S599).
Sexual morph not known.
Cultures and asexual morph — Optimal growth at 25 °C on all media, at 30 °C slow and restricted growth associated with hyper-branching and autolysing hyphae; no growth at 35 °C.
On CMD after 72 h colony radius 24–26 mm at 25 °C, 1–2 mm at 30 °C; mycelium covering the plate after 12–17 d at 25 °C. Colony hyaline, dense, centre loose, comprising conspicuously curved hyphae with variable width, indistinctly or irregularly zonate; margin wavy. Aerial hyphae inconspicuous. Autolytic excretions scant, coilings absent. Agar turning pale yellowish 3B3, more intensely (brownish) yellow after 9 mo at 15 °C, odour strongly coconut-like within 1 wk. Chlamydospores common but loosely disposed, terminal and intercalary, (6−)8–12(−13.5) × (5−)7–11(−12.5) μm, l/w (0.9−)1.0–1.2(−1.6) (n = 33), globose or subglobose, sometimes pyriform or ellipsoidal. Conidiation noticeable after 3–4 d, green after 5–7 d, at first short-effuse, scant, soon in small green 28CF4–6, 27–28F4–8 shrubs or loose, polymorphic pustules forming at each end of up to seven concentric zones, sometimes growing to 2 mm diam. Pustules comprising dense central clusters with submoniliform branches (up to 6 thickened cells in a chain) and peripheral conidiophores, arising on usually long, 6–12 μm wide, thick-walled stipes; unpaired primary branches forming a reticulum, from which numerous main axes originate more or less radially. Peripheral conidiophores straight or slightly curved, regularly tree-like, i.e. with a distinct main axis; branches 1.5–4.5 μm wide, at right angles or slightly inclined upward, typically unpaired at lower levels, tending to be paired or in whorls at upper levels; moniliform branches uncommon in the periphery. Phialides solitary or in whorls of 2–4, (5.3−)5.5–9.3(−12.8) × (2.8−)3.0–3.5(−4.0) μm, l/w 1.6–2.9(−4.0), (1.5−)1.7–2.3(−2.6) μm wide at the base (n = 35), lageniform, inaequilateral, often slightly curved, distinctly enlarged, rather plump with mostly short neck and base, sometimes narrower with a long neck on young conidiophores. Conidia (3.5−)4.0–5.0(−6.0) × (3.2−)3.5–4.0(−4.7) μm, l/w 1.0–1.3(−1.6) (n = 42), green, subglobose, sometimes oval, rarely oblong, smooth to faintly verruculose.
Colony radius on PDA (Merck) after 72 h 19–21 mm at 25 °C, 0.5–1 mm at 30 °C; mycelium covering the plate after c. 2 wk at 25 °C. Colony circular, dense, of uniform hyphae; margin wavy to irregularly sublobate. Aerial hyphae frequent, of widely spaced fascicles, forming a more or less radial flat whitish farinose to floccose mat. Autolytic excretions and coilings common. No diffusing pigment formed, agar slightly yellowish 3B4, odour transient, coconut-like to unpleasant. Conidiation starting after c. 2 wk in central floccules, effuse, remaining white.
Colony radius on SNA after 72 h 16–19 mm at 25 °C, 1–2 mm at 30 °C; mycelium covering the plate after c. 3 wk at 25 °C. Colony hyaline, dense, circular, of conspicuously curved hyphae with variable width, propagating discontinuously, with up to 5 new initially ill-defined, irregularly lobate growth zones. Aerial hyphae common. Autolytic excretions scant, coilings frequent. Diffusing pigment absent, odour indistinct. Chlamydospores common, terminal and intercalary. Conidiation noticeable after 3 d, turning green after 5 d, eventually dark green 27–28F4–8, at first short-effuse from the centre, soon in pustules. Pustules to c. 2 mm diam, at first forming in the central zone and subsequently in each new growth zone. Conidia forming on loose, regularly tree-like conidiophores.
Distribution — Europe, only known from Crete, Greece.
Habitat — On dead wood and bark of Acer sempervirens and on fungi growing on it.
Other material examined. Greece, Crete, Askyfou, road to the Halara peak, N35°17′48″, E24°12′29″, elev. 780 m, on a 5–6 cm thick, decorticated branch of Acer sempervirens, soc. Hypoxylon howeanum, corticiaceous fungi, asexual morph, 26 Nov. 2011, W. Jaklitsch (culture S601).
Notes — This species is so far only known as asexual morph from two isolates. It features the slowest growth of all here involved species, conspicuously curved hyphae and macroscopically visible conidiation in narrow zones starting before the colony covers the plate in a central area.
DISCUSSION
The Large Viridescens Clade as defined by Jaklitsch et al. (2006) accommodated phylogenetically diverse taxa. With only one molecular marker and an apparent paucity of phenotypic characters, Jaklitsch et al. (2006) did not divide that clade taxonomically. In the present work we have re-evaluated the taxonomy of the Large Viridescens Clade by including many more strains and by adding additional molecular markers, thus adopting the Genealogical Concordance Phylogenetic Species Recognition approach (GCPSR, Taylor et al. 2000). With the exceptions of T. virilente (clade V5) and Trichoderma sp. (clade V6), which were not included in the original study of Jaklitsch et al. (2006), the clades revealed by GCPSR conform exactly to those delimited by tef1, confirming once again (see e.g. Samuels et al. 2012) the utility of this marker in tracking phylogeny and species recognition in Trichoderma. However, the 12 species accepted here are difficult to delimit and recognise on the basis of their morphological characters. Species are defined as complex cocktails of traits. Although there are differences, most notably in pigment formation, growth rates, conidial shape and conidiophore structure, the phenotypic characters overlap to such an extent that it is unlikely that they will enable one to determine species in this complex. We therefore refrain from providing a determinative key. A certain identification is provided only by tef1 sequences, ideally in alignments with other species of the clade and ideally in combination with rpb2 and/or acl1 sequences, which should be always determined, if a new species in this clade is to be recognized.
For the first time we tested acl1 in Trichoderma phylogenetics. Sequences of this marker are easily obtainable and well suitable for this task. The number of parsimony informative sites (151 vs 176 in rpb2 and 209 in tef1) and thus the phylogenetic resolution obtained using this marker is comparable to rpb2 and tef1 and superior to other markers recently used in Trichoderma, such as cal1 and chi18-5 (Druzhinina et al. 2012, Jaklitsch et al. 2008). Acl1 is also promising for other clades of Trichoderma. Unlike rpb2, of which no intron is used in phylogenetic analyses, but similar to tef1 introns, parts of acl1 are highly variable (unpubl. data), which suggest that this marker is useful for resolving fine-tuned taxonomic units within species complexes of the larger clades of Trichoderma, rather than in an overall phylogeny of the genus.
Earlier publications on the Trichoderma viridescens species complex
Jaklitsch et al. (2006) erred in linking Hypocrea viridescens to the asexual morph T. viridescens s.str. The latter name was based on Eidamia viridescens and is supported by the authentic strain CBS 433.34 (dried culture specimen CBS H-7868). Hypocrea viridescens and CBS 433.34, however, fall in different phylogenetic subclades of the T. viridescens species complex. In the current work clade V2 is recognised as T. viridescens s.str. based on strain CBS 433.34, here epitypified with strain CBS 132573 (= S452). The holotype of H. viridescens resides in clade V4 and its epithet cannot be combined in Trichoderma. Therefore the new name T. paraviridescens was given for members of clade V4. The ex-type strain of H. viridescens (Hypo 372 = CBS 119321 = C.P.K. 2140) cannot serve as the epitype of T. viridescens, and the epitypification of T. viridescens by Jaklitsch et al. (2006) is cancelled herewith.
The description and illustrations by Jaklitsch et al. (2006) of Hypocrea viridescens/Trichoderma viridescens comprised several species. For this reason, an emended description of T. viridescens s.str. (clade V2) is given above.
Some clades that were not formally established in Jaklitsch et al. (2006) are re-assessed: Clade Vd 1 included two isolates from Sri Lanka and Ghana that have ellipsoidal, warted conidia and lack an eidamia-like morphology. These isolates cluster together again (see Fig. 1), but show long branches, which suggest single lineages rather than a well-defined clade.
Jaklitsch et al. (2012) described T. samuelsii and reported four collections that belong to this species. In addition to these, strains included by Jaklitsch et al. (2006) as Trichoderma Vd 2 are also T. samuelsii, including J.B. NZ151 (tef1 accession DQ845422), J.B. PER23 (tef1 accession DQ845423), C.P.K. 1488 (= UNISS 4-15, from Sardinian soil, tef1 accession DQ845416) and G.J.S. 05-186 (tef1 accession DQ841713). They are currently labelled Trichoderma viride or Hypocrea rufa in GenBank. However, the tef1 accession DQ845429 given by Jaklitsch et al. (2006) in Table 1 for the strain C.P.K. 2071 (a duplicate of C.P.K. 1488) represents in fact T. gamsii and is currently labelled in GenBank Trichoderma sp. MKZ-2006 strain JB RSA75, while the accession DQ845430 erroneously given for J.B. RSA75 in that table represents T. viride strain J.B. NSW13, as given in the same table and correctly included in GenBank.
Clade Vd3 of Jaklitsch et al. (2006) is recognised here as the geographically separated species T. appalachiense (clade V9) and T. neosinense (clade V9a).
A new clade clearly representing an undescribed species containing accessions from GenBank labelled T. viridescens was revealed in the tef1 tree. This clade is situated outside the clade of the T. viridescens species complex, close to T. dingleyae and comprises the four strains VI03925, VI03967, VI03968, VI03945 (Fig. 1) and probably also VI03960 (tef1 accession AM498524; Hageskal et al. 2008).
Acknowledgments
We thank Begoña Aguirre-Hudson, Esperanza Beltran-Tejera, Liliana Bernardo, Marco Contu, Rose Marie Dähncke, Walter Gams, Quirico Migheli, Neven Matocec, Ivana Kušan, Barbara Scherm, Olga Sükösd and Luis Quijada for excursion support; Trix Merkx and Gerard Verkley (CBS) for patience with our cultures and Anton Hausknecht for insertion of specimens into WU. James Plaskowitz, USDA-ARS, undertook the SEM work. Financial support by the Austrian Science Fund (FWF; project P22081-B17) is gratefully acknowledged.
REFERENCES
- Birksa SM, Edwards SV. 2002. A phylogeny of the megapodes (Aves: Megapodiidae) based on nuclear and mitochondrial DNA sequences. Molecular Phylogenetics and Evolution 23: 408–421 [DOI] [PubMed] [Google Scholar]
- Bissett J. 1991. A revision of the genus Trichoderma. II. Infrageneric classification. Canadian Journal of Botany 69: 2357–2372 [Google Scholar]
- Błaszczyk L, Popiel D, Chełkowski J, Koczyk G, Samuels GJ, et al. 2011. Species diversity of Trichoderma in Poland. Journal of Applied Genetics 52: 233–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone I, Kohn LM. 1999. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556 [Google Scholar]
- Dodd SL, Lieckfeldt E, Chaverri P, Overton BE, Samuels GJ. 2002. Taxonomy and phylogenetic relationships of two species of Hypocrea with Trichoderma anamorphs. Mycological Progress 1: 409–428 [Google Scholar]
- Druzhinina IS, Komoń-Zelazowska M, Ismaiel A, Jaklitsch W, Mullaw T, et al. 2012. Molecular phylogeny and species delimitation in the section Longibrachiatum of Trichoderma. Fungal Genetics and Biology 49: 358–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans HC, Holmes KA, Thomas SE. 2003. Endophytes and mycoparasites associated with an indigenous forest tree, Theobroma gileri, in Ecuador and a preliminary assessment of their potential as biocontrol agents of cocoa diseases. Mycological Progress 2: 149–160 [Google Scholar]
- Gräfenhan T, Schroers HJ, Nirenberg HI, Seifert KA. 2011. An overview of the taxonomy, phylogeny, and typification of nectriaceous fungi in Cosmospora, Acremonium, Fusarium, Stilbella, and Volutella. Studies in Mycology 68: 79–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageskal G, Vrålstad T, Knutsen AK, Skaar I. 2008. Exploring the species diversity of Trichoderma in Norwegian drinking water systems by DNA barcoding. Molecular Ecology Resources 8: 1178–1188 [DOI] [PubMed] [Google Scholar]
- Hanada RE, Jorge Souza T de, Pomella AW, Hebbar KP, Pereira JO, et al. 2008. Trichoderma martiale sp. nov., a new endophyte from sapwood of Theobroma cacao with a potential for biological control. Mycological Research 112: 1335–1343 [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Kishino H, Yano T. 1985. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. Journal of Molecular Evolution 22: 160–174 [DOI] [PubMed] [Google Scholar]
- Horne AS, Williamson HS. 1923. The morphology and physiology of the genus Eidamia. Annals of Botany 37: 393–432 [Google Scholar]
- Hoyos-Carvajal L, Orduz S, Bissett J. 2009. Genetic and metabolic biodiversity of Trichoderma from Colombia and adjacent neotropic regions. Fungal Genetics and Biology 46: 615–631 [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F. 2001. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755 [DOI] [PubMed] [Google Scholar]
- Jaklitsch WM. 2009. European species of Hypocrea I. The green-spored species. Studies in Mycology 63: 1–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM. 2011. European species of Hypocrea Part II: species with hyaline ascospores. Fungal Diversity 48: 1–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM, Komon M, Kubicek CP, Druzhinina IS. 2005. Hypocrea voglmayrii sp. nov. from the Austrian Alps represents a new phylogenetic clade in Hypocrea / Trichoderma. Mycologia 97: 1365–1378 [DOI] [PubMed] [Google Scholar]
- Jaklitsch WM, Kubicek CP, Druzhinina IS. 2008. Three green-spored species of Hypocrea with reddish-brown stromata. Mycologia 100: 790–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM, Samuels GJ, Dodd SL, Lu B-S, Druzhinina IS. 2006. Hypocrea rufa / Trichoderma viride : a reassessment and description of five closely related species with and without warted conidia. Studies in Mycology 56: 135–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM, Stadler M, Voglmayr H. 2012. Blue pigment in Hypocrea caerulescens sp. nov. and two additional new species in sect. Trichoderma. Mycologia 104: 925–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornerup A, Wanscher JH. 1978. Methuen handbook of colour; 3rd ed.Sankt Jørgen Tryk, Copenhagen [Google Scholar]
- Liu YL, Whelen S, Hall BD. 1999. Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Molecular Biology and Evolution 16: 1799–1808 [DOI] [PubMed] [Google Scholar]
- Nirenberg HI. 1976. Untersuchungen über die morphologische und biologische Differenzierung in der Fusarium-Sektion Liseola. Mitteilungen aus der Biologischen Bundesanstalt für Land- und Forstwirtschaft Berlin-Dahlem 169: i–v, 1–117 [Google Scholar]
- Nylander JA, Wilgenbusch JC, Warren DL, Swofford DL. 2008. AWTY (are we there yet?): a system for graphical exploration of MCMC convergence in Bayesian phylogenetics. Bioinformatics 24: 581–583 [DOI] [PubMed] [Google Scholar]
- Posada D, Crandall KA. 1998. Modeltest: testing the model of DNA substitution. Bioinformatics 14: 817–818 [DOI] [PubMed] [Google Scholar]
- Rodríguez F, Oliver JF, Martín A, Medina JR. 1990. The general stochastic model of nucleotide substitution. Journal of Theoretical Biology 142: 485–501 [DOI] [PubMed] [Google Scholar]
- Samuels GJ, Dodd SL, Gams W, Castlebury LA, Petrini O. 2002. Trichoderma species associated with the green mold epidemic of commercially grown Agaricus bisporus. Mycologia 94: 146–170 [PubMed] [Google Scholar]
- Samuels GJ, Dodd SL, Lu B-S, Petrini O, Schroers H-J, Druzhinina IS. 2006a. The Trichoderma koningii aggregate species. Studies in Mycology 56: 67–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels GJ, Ismaiel A. 2009. Trichoderma evansii and T. lieckfeldtiae: two new T. hamatum-like species. Mycologia 101: 142–156 [DOI] [PubMed] [Google Scholar]
- Samuels GJ, Ismaiel A, Bon MC, Respinis S de, Petrini O. 2010. Trichoderma asperellum sensu lato consists of two cryptic species. Mycologia 102: 944–966 [DOI] [PubMed] [Google Scholar]
- Samuels GJ, Ismaiel A, Souza J de, Chaverri P. 2012. Trichoderma stromaticum and its overseas relatives. Mycological Progress 11: 215–254 [Google Scholar]
- Samuels GJ, Suarez C, Solis K, Holmes KA, Thomas SE, et al. 2006b. Trichoderma theobromicola and T. paucisporum: two new species isolated from cacao in South America. Mycological Research 110: 381–392 [DOI] [PubMed] [Google Scholar]
- Silvestro D, Michalak I. 2012. RaxmlGUI: a graphical front-end for RAxML. Organisms Diversity & Evolution 12: 335–337 [Google Scholar]
- Stamatakis E. 2006a. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690 [DOI] [PubMed] [Google Scholar]
- Stamatakis E. 2006b. Phylogenetic models of rate heterogeneity: a high performance computing perspective. In: Proceedings of the 20th International Parallel and Distributed Processing Symposium 2006, Rhodos, Greece. 10.1109/IPDPS.2006.1639535. [Google Scholar]
- Sung GH, Sung JM, Hywel-Jones NL, Spatafora JW. 2007. A multigene phylogeny of Clavicipitaceae (Ascomycota, Fungi): identification of localized incongruence using a combinational bootstrap approach. Molecular Phylogenetics and Evolution 44: 1204–1223 [DOI] [PubMed] [Google Scholar]
- Swofford DL. 2002. PAUP* 4.0b10: Phylogenetic analysis using parsimony (*and other methods). Sunderland, Massachusetts, Sinauer Associates [Google Scholar]
- Tamura K, Nei M. 1993. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution 10: 512–526 [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance and maximum parsimony methods. Molecular Biology and Evolution 28: 2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JW, Jacobson DJ, Kroken S, Kasuga T, Geiser DM, et al. 2000. Phylogenetic species recognition and species concepts in Fungi. Fungal Genetics and Biology 31: 21–31 [DOI] [PubMed] [Google Scholar]
- Wiens JJ. 1998. Combining datasets with different phylogenetic histories. Systematic Biology 47: 568–581 [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Tsurumi Y, Suzuki R, Chuaseeharonnachai C, Sri-Indrasutdhi V, et al. 2012. Trichoderma matsushimae and T. aeroaquaticum: two aero-aquatic species with Pseudaegerita-like propagules. Mycologia 104: 1109–1120 [DOI] [PubMed] [Google Scholar]
- Yu Z-F, Qiao M, Zhang Y, Zhang K-Q. 2007. Two new species of Trichoderma from Yunnan, China. Antonie van Leeuwenhoek 92: 101–108 [DOI] [PubMed] [Google Scholar]
- Zachow C, Berg C, Müller H, Meincke R, Komon-Zelazowska M, et al. 2009. Fungal diversity in the rhizosphere of endemic plant species of Tenerife (Canary Islands): relationship to vegetation zones and environmental factors. ISME Journal 3: 79–92 [DOI] [PubMed] [Google Scholar]