Abstract
Purpose
To define a male and female pelvic normal tissue contouring atlas for Radiation Therapy Oncology Group (RTOG) trials.
Methods and Materials
One male pelvis computed tomography (CT) data set and one female pelvis CT data set were shared via the Image-Guided Therapy QA Center. A total of 16 radiation oncologists participated. The following organs at risk were contoured in both CT sets: anus, anorectum, rectum (gastrointestinal and genitourinary definitions), bowel NOS (not otherwise specified), small bowel, large bowel, and proximal femurs. The following were contoured in the male set only: bladder, prostate, seminal vesicles, and penile bulb. The following were contoured in the female set only: uterus, cervix, and ovaries. A computer program used the binomial distribution to generate 95% group consensus contours. These contours and definitions were then reviewed by the group and modified.
Results
The panel achieved consensus definitions for pelvic normal tissue contouring in RTOG trials with these standardized names: Rectum, AnoRectum, SmallBowel, Colon, BowelBag, Bladder, UteroCervix, Adnexa_R, Adnexa_L, Prostate, SeminalVesc, PenileBulb, Femur_R, and Femur_L. Two additional normal structures whose purpose is to serve as targets in anal and rectal cancer were defined: AnoRectumSig and Mesorectum. Detailed target volume contouring guidelines and images are discussed.
Conclusions
Consensus guidelines for pelvic normal tissue contouring were reached and are available as a CT image atlas on the RTOG Web site. This will allow uniformity in defining normal tissues for clinical trials delivering pelvic radiation and will facilitate future normal tissue complication research.
Keywords: Radiation therapy atlas, Pelvic contouring atlas, Normal tissue volumes, Male pelvis, Female pelvis
Introduction
The Image-Guided Therapy Quality Assurance Center (ITC) for Clinical Trials Quality Assurance collects and reviews the radiotherapy image—based planning and verification data for patients enrolled in cooperative group trials. Thanks to the collaboration between radiation therapy clinical trial-participating institutions and cooperative groups such as the Radiation Therapy Oncology Group (RTOG), the ITC has a rapidly growing collection of pelvic planning computed tomography (CT) scans with normal tissue contours. Because these clinical trials prospectively collect toxicity data, this represents an opportunity to advance radiation research in pelvic normal tissue complications.
One obstacle to effectively use these data is the lack of standardized definitions of normal pelvic structures, which hinders comparing complication rates between different protocols for a given organ at risk (OAR). Consequently, significant resources are needed to recontour all these normal structures for proper analysis.
The male and female pelvic atlas that follows was produced by a consensus panel of 16 radiation oncologists (H.J.B., J.M., S.A.R., C.L., W.R.L., H.S., A.Z., R.M., L.A.D., C.W., L.A.K., A.J., L.P., J.R., W.S., and D.G.) who were assigned by the RTOG Advanced Technology Integration Steering Committee. The formation of this panel was motivated by the pelvic normal organ contouring variability observed by some panel members when reviewing several RTOG trials, including those for prostate cancer, gynecologic cancers, and anorectal cancers. The panel believed it was important to standardize OAR definitions for clinical trials. This will allow researchers to improve normal tissue dose constraints.
This report provides the recommendations of this consensus panel and serves as a template for the definition of the male and female pelvic normal tissue structures for radiotherapy planning.
Methods and Materials
After the development of the RTOG Advanced Technology Integration Steering Committee, a pelvic contouring consensus panel of 4 gastrointestinal (GI), 5 gynecologic (GYN), and 7 genitourinary (GU) radiation oncologists was assembled. Multiple informal discussions were held by both telephone conference and e-mail to develop pelvic normal tissue volumes for the purpose of this atlas. Two sample sets of CT images, male pelvis and female pelvis, were anonymized and distributed to each collaborator by CD ROM or download from the ITC web site.
The male patient was simulated in a supine position with a full bladder and small bowel contrast. The male CT scan was obtained from the L4/L5 interspace to the anus in 2.9-mm slices. The female patient was simulated in a supine position with a full bladder, without any contrast. The CT scan was obtained from the L3/L4 interspace to approximately the mid femur in 2-mm slices.
Participants were initially asked to draw the following GI, GYN, GU, and other normal pelvic organs based on the following obsolete definitions:
Anus (GI definition)—Distal 3 cm of bowel from the distal rectum to the anal verge as marked with a radiopaque marker (BB) at the time of simulation.
Rectum (GU definition)—The rectum from the anus (at the level of the ischial tuberosities) to the rectosigmoid flexure. This generally is at or below the bottom of the sacroiliac joints.
Rectum (GI definition)—Superiorly from the rectosigmoid flexure (level where the rectal contour ends and the bowel structures move anteriorly away from the sacrum) to an inferior border 3 cm from the anal verge (level of the levators).
Anorectum (GI definition)—Combination of rectum and anus (GI definitions).
Bowel NOS (not otherwise specified) (non-GI definition)—Peritoneal space occupied or potentially occupied by bowel, large or small.
Small bowel—To distinguish from large bowel, the use of oral contrast, administered 30 minutes before scanning, is encouraged. The small bowel can be outlined as loops containing contrast.
Large bowel—All intestine seen above the rectum; usually delineated as the bowel starting with noncircular or oval structures or above 15 cm.
Uterus—The whole organ should be contoured from the dome to the cervix.
Cervix—As a normal structure, it is included as uterus and cervix because it is not consistently possible on a planning CT scan to differentiate fundus from cervix as separate avoidance targets.
Ovaries—Right and left ovaries should be identified separately along with fallopian tube if visible.
Prostate—As a normal structure, from its base superiorly to the apex inferiorly, excluding seminal vesicles. If the capsule is visible, the muscles and soft tissues abutting the capsule are not included as “prostate.”
Seminal vesicles—Entire seminal vesicles including those slices that also have prostate identified.
Penile bulb—That portion of the bulbous spongiosum of the penis immediately inferior to the GU diaphragm. Do not extend this structure anteriorly into the shaft or pendulous portion of the penis.
Bladder—The bladder should be contoured from its base to the dome.
Femurs—The ball of the femur, trochanters, and proximal shaft to the level of the bottom of ischial tuberosities.
Each participant used his or her own treatment planning system (TPS) to contour. A computer program developed by one of the coauthors used the binomial distribution to generate 95% confidence level group consensus contours. The contours were then reviewed by the group at various times through ATC-sponsored virtual meetings.
Statistical analysis
Contours from each investigator were imported into the Computerized Environment for Radiation Research (CERR), an open-source MATLAB (The MathWorks, Natick, MA)–based radiation therapy planning analysis tool (1). Contours were then compared for agreement by use of a MATLAB program for estimating consensus contours from given individual expert’s contours. The software is an imputation method using expected-maximum algorithms for simultaneous truth and performance level estimation (2). In this approach the true contouring decisions at each voxel are formulated as maximum likelihood estimates from the observed contours by optimizing sensitivity and specificity parameters of each expert’s performance using the expected-maximum algorithm, assuming a binomial distribution.
Results
A total of 20 expert radiation oncologists were asked to participate. Sixteen submitted contours for evaluation. The male patient was contoured by: H.J.B., J.M., S.R., C.L., W.R.L., H.S., A.Z., R.M., L.A.D., C.W., L.A.K., A.J., L.P., W.S., and D.G. The female patient was contoured by: H.J.B., J.M., S.A.R., C.L., H.S., A.Z., R.M., L.A.D., C.W., L.A.K., A.J., L.P., J.R., W.S., and D.G. The resulting 95% confidence level contours were reviewed by the panel and modified based on the comments. The structures and their definitions were further refined as in the Table. In some cases the contours were further adjusted to conform to the revised definition. The mesorectum and female bladder were subsequently contoured by H.A.G. and the sigmoid by H.A.G. and R.M. Given the variability in the contours of the adnexa, the Adnexa_R and Adnexa_L were contoured by A.N.V., who verified these with an experienced radiologist. The standardized target and OAR naming convention for use in radiation therapy proposed by Santanam et al. (3) was used whenever applicable (Table). Additional naming terms resulted from this pelvic atlas effort.
Table.
RTOG male and female pelvis normal tissue consensus definitions
| Organ | Standardized TPS name | Tumor category | Consensus definition |
|---|---|---|---|
| Rectum | Rectum | GU | Inferiorly from the lowest level of the ischial tuberosities (right or left). Contouring ends superiorly before the rectum loses its round shape in the axial plane and connects anteriorly with the sigmoid. The Rectum is used with the BowelBag. |
| Anus + rectum | AnoRectum | GYN | Inferiorly from the anal verge as marked with a radiopaque marker at the time of simulation. Contouring ends superiorly before the rectum loses its round shape in the axial plane and connects anteriorly with the sigmoid. The AnoRectum is used with the Sigmoid and BowelBag. |
| Sigmoid | Sigmoid | GYN | Bowel continuing where the AnoRectum contour ended. Stops before connecting to the ascending colon laterally. Contoured when a brachytherapy applicator rests in the uterus. Any sigmoid adjacent or above the uterus, as well as the brachytherapy applicator, should be contoured. |
| Bowel bag | BowelBag | GU, GYN | Inferiorly from the most inferior small or large bowel loop or above the Rectum (GU) or AnoRectum (GYN), whichever is most inferior.* If, when following the bowel loop rule, the Rectum or AnoRectum is present in that axial slice, it should be included as part of the bag; otherwise, it should be excluded. |
| Tips: Contour the abdominal contents excluding muscle and bones. Contour every other slice when the contour is not changing rapidly, and interpolate and edit as necessary. Finally, subtract any overlapping non-GI normal structures. If the TPS does not allow subtraction, leave as is. | |||
| Small bowel | SmallBowel | GI | To distinguish from large bowel, the use of oral contrast is encouraged.* After administration of contrast (e.g., 3 oz of Gastrografin (Bracco Diagnostics Inc., Princeton, NJ) and 3 oz of water–barium mixture) 30 minutes before scanning, the small bowel can be outlined as loops containing contrast. |
| Colon | Colon | GI | Large bowel continuing where the AnoRectumSig contour ended.* Depending on the volume treated, this will include portions or all of the ascending, transverse, descending, and sigmoid colon. |
| Anus + rectum + rectosigmoid (target) | AnoRectumSig | GI | Target structure. Inferiorly from the anal verge as marked with a radiopaque marker at time of simulation. Contouring ends superiorly at the rectosigmoid flexure after the mesorectum disappears. The AnoRectumSig is used with the SmallBowel and Colon. |
| Mesorectum (target) | Mesorectum | GI | Target structure for anal and rectal cancer. The rectum inferiorly below where the mesorectal fat disappears, continuing superiorly, and encompassing the mesorectal fat until the mesorectal fascia disappears. For these entities, the AnoRectoSig (anus + rectum + rectosigmoid), unlike the rest of the alimentary canal, is not an avoidance structure. In cases where it is difficult to visualize the mesorec tum, the anatomic borders of the mesorectum include the following: cranial, the level of the rectosigmoid junction; caudal, the anorectal junction defined by where the levator muscles fuse with the external sphincter muscles (or where the mesorectal fat/space can no longer be seen tapering inferiorly); posterior, pre-sacral space; anterior, GU/GYN organs with an internal margin of 10 mm to the anterior mesorectal border on the axial slices of the bladder to account for bladder volume variation on this boundary; and lateral, medial edge of the levator ani in the lower pelvis and pelvic brim in upper (excluding any non-target muscle). |
| Tip: Adjusting the windowing level may facilitate visualizing the mesorectum. | |||
| Bladder | Bladder | GU, GYN, GI | Inferiorly from its base and superiorly to the dome. |
| Uterus + cervix | UteroCervix | GYN | The uterus and cervix as one structure. |
| Tip: Fuse with MRI to help identify it. | |||
| Ovaries + fallopian tubes | Adnexa_R Adnexa_L | GYN | Right and left ovaries and fallopian tubes. |
| Tip: Fuse with MRI to help identify these. Refer to the article by Olson et al. | |||
| Prostate | Prostate | GU | Inferiorly from its apex and superiorly to its base. If the capsule is visible, the muscles and soft tissues abutting the capsule are not included as “prostate.” |
| Tips: The apex is above the hourglass or a slit shape that results from the in-bowing of the levator ani just below. Refer to the article by McLaughlin et al. (4) | |||
| Seminal vesicles | SeminalVesc | GU | Entire seminal vesicles including those slices that also have prostate identified. |
| Penile bulb | PenileBulb | GU | That portion of the bulbous spongiosum of the penis immediately inferior to the GU diaphragm. Do not extend this structure anteriorly into the shaft or pendulous portion of the penis. |
| Tips: The penile bulb is best identified with MRI (bright on T2) or CT scan when there is contrast in the urethra. On CT scan, the penile bulb will be posterior to the urethra and has a round shape. Refer to the article by Wallner et al. | |||
| Proximal femurs | Femur_R Femur_L | GU, GYN, GI | The proximal femur inferiorly from the lowest level of the ischial tuberosities (right or left) and superiorly to the top of the ball of the femur, including the trochanters. |
| Tips: Auto-contouring threshold parameters with bone can facilitate this process but requires editing any auto-contouring artifacts. |
Abbreviations: CT = computed tomography; GI = gastrointestinal; GU = genitourinary; MRI = magnetic resonance imaging; PTV = planning target volume; RTOG = Radiation Therapy Oncology Group; TPS = treatment planning software.
One should stop contouring the BowelBag, SmallBowel, and Colon 1 cm above the PTV for most coplanar beam plans, but the choice will depend on the treatment technique. One should stop these PTVs at distances much greater than 1 cm for non-coplanar beam plans depending on the beam angle and path. TomoTherapy plans will require stopping from 1 to 5 cm above the PTV, depending on the selected field size, which is often 2.5 cm.
Clinical target volume (CTV) consensus development
At the consensus conference, the contours were reviewed and discussed. The full CT data set with each contoured slice can be found on the RTOG web site (www.rtog.org) in the “Core Lab” section under “Contouring Atlases.”
The male atlas web page is http://www.rtog.org/CoreLab/ContouringAtlases/MaleRTOGNormalPelvisAtlas.aspx (Appendix E1). The female atlas web page is http://www.rtog.org/CoreLab/ContouringAtlases/FemaleRTOGNormalPelvisAtlas.aspx (Appendix E2).
The male and female atlases are available in Adobe Reader PDF format (Adobe Systems, San Jose, CA). Figure 1 shows some highlights from the male atlas, and Fig. 2 shows some highlights from the female atlas. Both data sets can also be interactively explored by installing the fulAccess viewer version of the atlases (Version 1.2.3; Fulcrum Medical, Chesterfield, MO). These Microsoft Windows–executable files (Microsoft, Redmond, WA) will display the CT data sets and allow manipulation of the images for enhanced visualization in the axial, sagittal, and coronal planes.
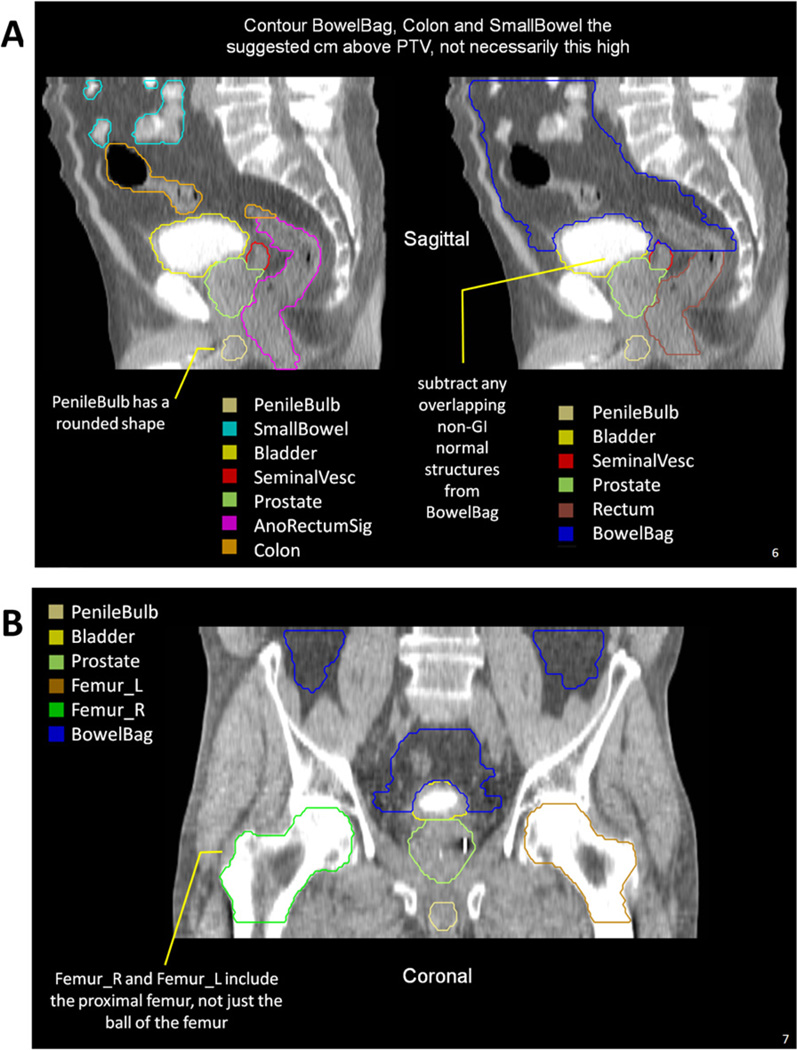
Fig. 1.
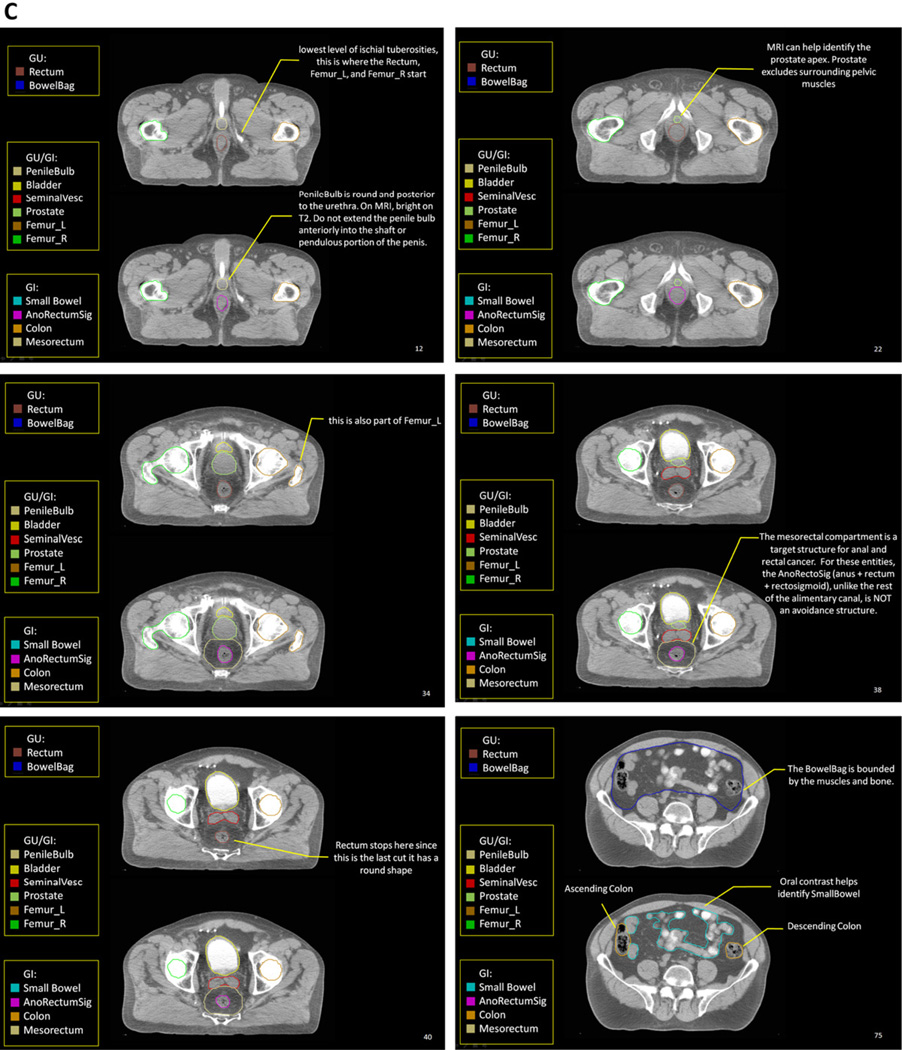
Highlights from the Radiation Therapy Oncology Group (RTOG) male pelvis normal tissue atlas: sagittal view (A), coronal view (B), and axial views (C). The full atlas is available on the RTOG Web site. GI = gastrointestinal; GU = genitourinary; MRI = magnetic resonance imaging; PTV = planning target volume.
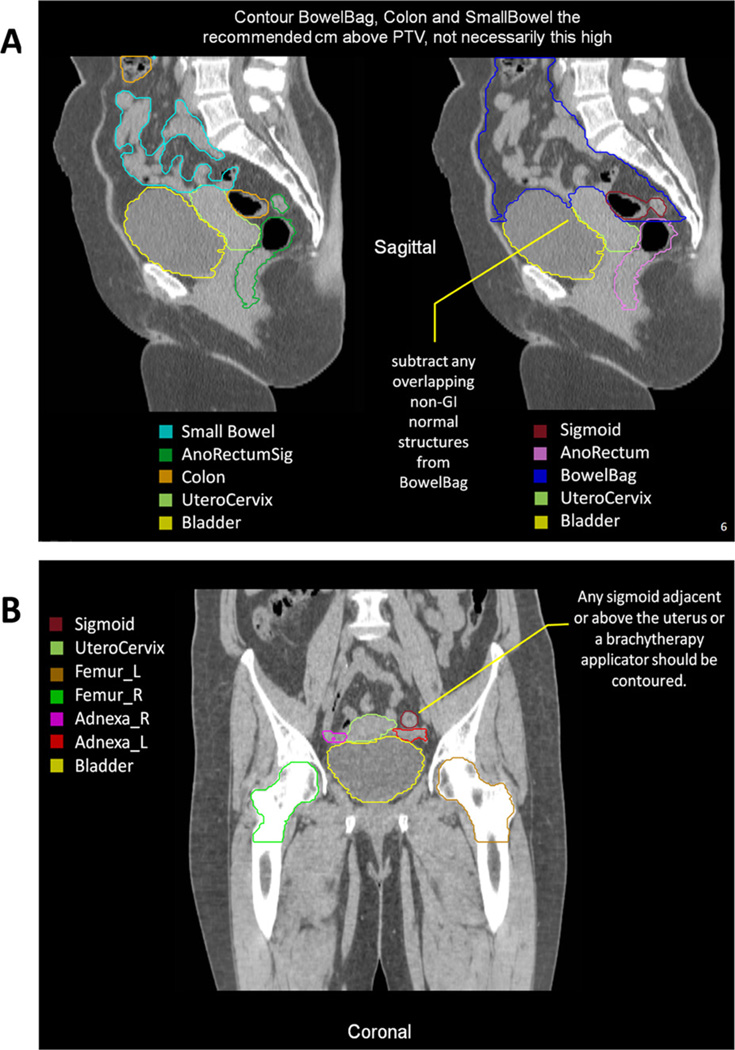
Fig. 2.
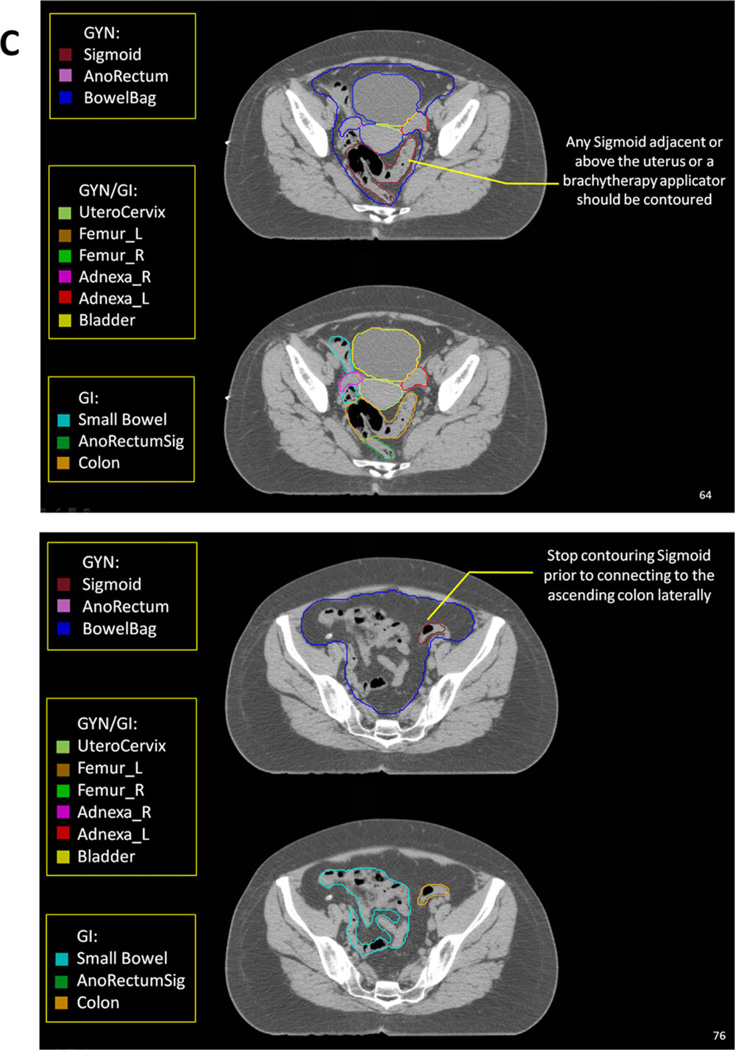
Highlights from the Radiation Therapy Oncology Group (RTOG) female pelvis normal tissue atlas: sagittal view (A), coronal view (B), and axial views (C). The full atlas is available on the RTOG Web site. GI = gastrointestinal; GU = genitourinary; GYN = gynecologic; PTV = planning target volume.
RTOG consensus definitions
Rectum, AnoRectum, AnoRectumSig, Sigmoid, and Mesorectum
Having simplicity in mind, three different structures encompassing the rectum were defined to meet the normal tissue or tumor target needs when treating GU, GYN, and GI tumors (see Table 1).
The Rectum is defined inferiorly from the lowest level of the ischial tuberosities (right or left) and ends superiorly before it loses its round shape in the axial plane and connects anteriorly with the sigmoid. Part of the anus may unintentionally be included when using this definition, which is of no concern. This definition should be used when treating GU tumors because it encompasses the rectal volume at risk with the minimum contouring effort, avoids placing an anal marker, and is arguably the most reproducible of the definitions. Common mistakes when using this definition are contouring inferiorly below the lowest level of the ischial tuberosities or continuing superiorly into the rectosigmoid, where the rectum has lost its round shape and becomes elongated.
The AnoRectum includes the Rectum as previously described and adds the anus inferiorly to the anal verge. Including the anus is most relevant when treating the distal vagina or vulva. When treating GYN tumors, the acute toxicities and quality of life (QOL) concerns are different for the anus and rectum. However, because there are no specific dose constraints for the anus at present, it is contoured as part of the AnoRectum. Future research may help define anal dose constraints, which may require contouring the anus and rectum separately.
The AnoRectumSig includes the anus, rectum, and rectosigmoid and is a target structure in anal and/or rectal cancer. The panel strongly believed that the AnoRectumSig target should be illustrated and clearly distinguished from the normal Rectum and AnoRectum. Similar to the AnoRectum, the AnoRectumSig starts at the anal verge. Of note, neither the male nor female CT data set had an anal marker, which is suboptimal, and the male data set did not go inferior enough to appreciate the full extent of the anus. The critical differences between the AnoRectumSig and the Rectum and AnoRectum are that the AnoRectumSig continues superiorly until the mesorectal fascia stops and that it is a target, not normal, structure. The Mesorectum is also a target structure in radiation planning for anal and rectal cancers. Using the Rectum or AnoRectum definitions to define the anal and/or rectal tumor target will likely result in underdosing the superior part of the mesorectal compartment and compromise treatment. The Sigmoid is contoured as a separate structure in cases where a brachytherapy applicator rests in the uterus because of the close proximity of the sigmoid and uterus.
SmallBowel, Colon, and BowelBag
The SmallBowel and Colon should be used in GI tumor cases along with the AnoRectumSig. In contrast, the BowelBag should be used in GU and GYN tumor cases, along with the Rectum and AnoRectum, respectively. The term “Colon” was used instead of large bowel because it excludes the rectosigmoid and better describes what is contoured. Optimally distinguishing the SmallBowel from the Colon necessitates the administration of contrast. The BowelBag, as described in Table, may provide a simpler and faster way of contouring the bowel, especially in the absence of small bowel contrast. A correctly contoured BowelBag will encompass all the Small-Bowel and Colon contours.
One should stop contouring the BowelBag, SmallBowel, and Colon 1 cm above the planning target volume (PTV) for most coplanar beam plans, but the choice will depend on the treatment technique. One should stop these PTVs at distances much greater than 1 cm for non-coplanar beam plans depending on the beam angle and path. TomoTherapy plans (TomoTherapy, Madison, WI) will require stopping from 1 to 5 cm above the PTV, depending on the selected field size, which is often 2.5 cm. The optimal distance depending on treatment technique should be formally studied.
Bladder
In general, contouring the Bladder is usually simple. A CT scan with contrast in the bladder or magnetic resonance imaging (MRI) may facilitate identifying the most inferior and superior extent of the bladder. In some cases when there is contrast in the bladder and small bowel, it may be difficult to distinguish the dome of the bladder from the bowel, and careful analysis of the coronal and sagittal views may be necessary.
UteroCervix and Adnexa
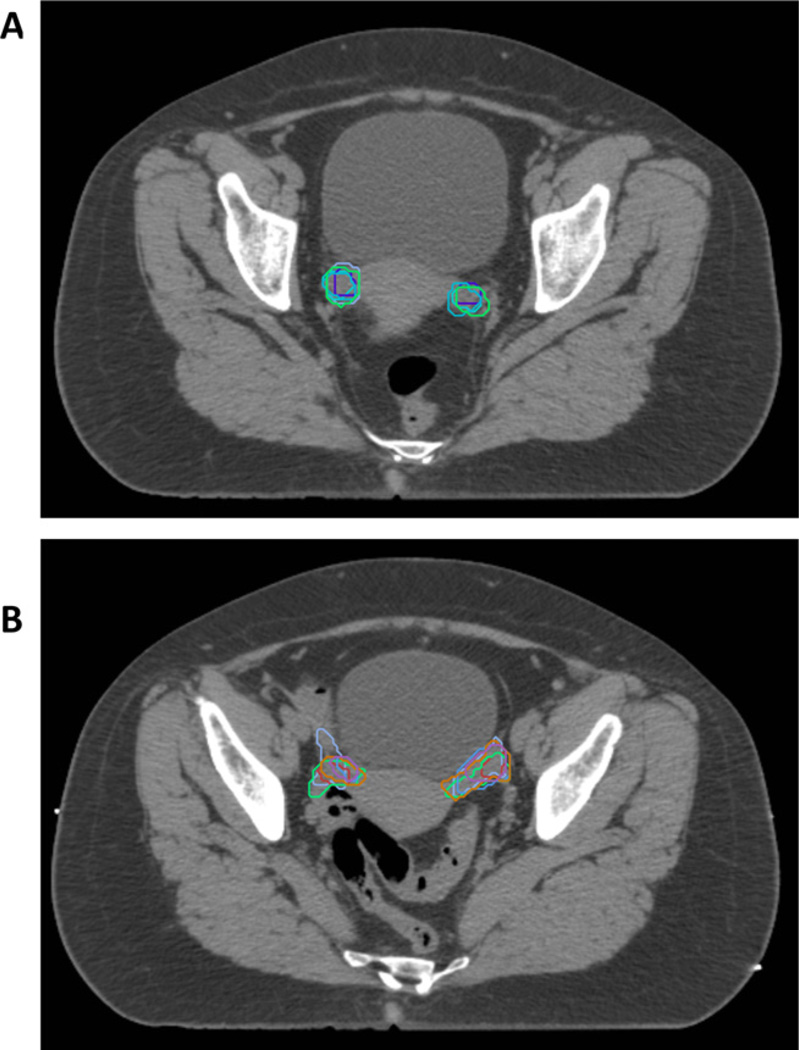
Contouring the UteroCervix and Adnexa can be challenging. Instead of contouring the uterus and cervix as separate contours, the panel believed that it would be simpler and most reproducible to contour both as a single structure, given the difficulty in distinguishing them. The same argument applies to the ovaries and fallopian tubes, which are contoured as Adnexa_R (right) and Adnexa_L (left). There was great variability between the members of the panel when contouring the adnexa (Fig. 3), which required fusion with MRI, recontouring, and verification with an experienced radiologist.
Fig. 3.
Variability and difficulty in contouring ovaries. Five experts contoured the ovaries more round and inferior (A) compared with six experts whose contours were more superior and elongated (B). It should be noted that in the final version of the atlas, the ovaries and fallopian tubes comprise the adnexa (Adnexa_R and Adnexa_L) and correlate with the contours in B, whereas the contours in A were not deemed part of the adnexa in the final version.
Prostate, SeminalVesc, and PenileBulb
The Prostate and SeminalVesc (seminal vesicles) are most often target structures but may be considered avoidance structures if uninvolved with cancer. Contouring of the prostate requires a clear understanding of the prostate anatomy. The reader should carefully study the prostate contouring article by McLaughlin et al. (4).
Optimal identification of the penile bulb requires MRI or a CT scan with contrast in the urethra. Without these, there is usually great variability in how the penile bulb is contoured. Some common errors include contouring the penile bulb anterior to the urethra or symphysis pubis; too small in diameter (about the size of the urethra); not as a round structure but as a large triangular, trapezoidal, or rectangular shape; too anteriorly; or too superior and close to the prostate apex.
Femurs
The proximal femurs are contoured as Femur_R and Femur_L. The definition of the proximal femur is very objective but is often ignored in protocols submitted to RTOG. Common errors include only contouring the ball of the femoral head, not contouring inferiorly to the lowest level of the ischial tuberosities (right or left), contouring unnecessarily below the ischial tuberosities, not contouring the trochanters, not conforming to the round shape of the ball of the femur or drawing it too large or small, and using the auto-threshold contouring tools in the TPS and not editing the resulting errors.
Discussion
Standardization of the contours of normal pelvic structures in radiotherapy is essential for the evaluation of normal tissue dose constraints. These normal tissue atlases may serve as an educational tool in assisting radiation oncologists in their contouring of normal avoidance as well as target structures depending on the primary site of cancer. Proper contouring may, in turn, promote enhanced normal tissue sparing as well as treatment efficacy.
The consensus panel could have defined the various normal structures many different ways. The proposed definitions took into account reproducibility and the expertise of those treating GI, GYN, and GU tumors. The standardized target and OAR naming convention for use in radiation therapy proposed by Santanam et al. (3) was used whenever applicable. As Santanam et al. stated, a standardized nomenclature is critical for interinstitutional data sharing, clinical trial repositories, integrated multi-institutional collaborative databases, and quality-control centers. It could also enable improved plan benchmarking among clinical institutions and vendors and facilitate automated treatment plan quality control in individual clinics.
Although the Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC) effort provided updated normal tissue dose constraints for various organs, guidance regarding normal organ contouring was absent (5). Analysis of the QUANTEC rectum recommendations shows great variability in how the rectum was defined in the quoted references (6). Examples are as follows:
The level of the ischial tuberosities to the rectosigmoid flexure (7).
The “(solid) rectum from the anal verge or ischial tuberosities (whichever was higher) to the sacroiliac joints or rectosigmoid junction (whichever was lower). Rectal wall was defined based on the solid rectum contours with 3- to 4-mm wall thickness” (8).
The “rectum (considered as a solid organ) starting just above the anal verge and continuing to the point at which it turns into the sigmoid colon” (9).
The “anorectum was defined as going from the anal verge until the lower GI tract was no longer adjacent to the sacrum. The inner contour of the anorectal wall was automatically constructed from the delineated outer wall surface, using the method of Meijer et al. (10). The anal canal was defined as the caudal 3 cm of the anorectum measured in craniocaudal direction. Relative dose–volume histograms (DVH) of the anorectal wall and anal wall were constructed for each patient in dose bins of 0.5 Gy” (11).
The “entire rectal volume was outlined to include the external rectal wall plus contents. The rectum was outlined about 11 cm in length starting at 2 cm below the inferior-most aspect of the ischial tuberosities” (12).
These contouring differences hinder the comparison of data and performance of meta-analyses. Another critical area where consensus is needed is in the choice of instruments to measure morbidity. For example, the rectal toxicity literature has used a plethora of toxicity scales, ranging from modifications of the RTOG scale (13–16) to the modified RTOG and Subjective, Objective, Management, Analytic (SOMA) scale (17), Gynecologic Oncology Group Common Toxicity Criteria (GOG CTC) scale (18, 19), institutional scales (20), and various versions of the Common Terminology Criteria for Adverse Events (CTCAE) (8), among others.
The 95% confidence level group consensus contours methodology was useful as a starting point but required fine tuning to resolve any resulting overlapping contours, as well as consistency with the final normal structure definitions and visible anatomy. Overall, there was consensus among the panelists regarding contouring definitions except for the optimal contouring strategy for the bowel. The GI panelists favored contouring the SmallBowel and Colon, whereas the GU and GYN panelists favored the BowelBag. There was no consensus as to which of these two strategies was best for a given clinical situation. The advantages and disadvantages of each of the strategies need to be formally studied, taking into account organ motion, bladder-filling variability, and intensity-modulated radiotherapy (IMRT) optimization strategy, among other variables. Regarding the delineation of the contours, there was general agreement among the panelists for most structures except for the adnexa, where there was great variability, and this subjectively posed the greatest difficulty in identification.
Conclusions
Consensus guidelines for pelvic normal tissue contouring were reached and are available as a CT image atlas on the RTOG Web site. This will allow uniformity in defining normal tissues for clinical trials delivering pelvic radiation and will facilitate future normal tissue complication research.
Supplementary Material
Summary.
The Radiation Therapy Oncology Group male and female pelvis contouring guidelines for radiotherapy are presented. Gynecologic, gastrointestinal, and genitourinary radiation oncologists collaborated in the creation of these guidelines.
Acknowledgment
The authors thank Cheryl A. Sadow, M.D., radiologist at Brigham and Women’s Hospital, for verifying the contours of the adnexa.
Supported by Grants CA81647, CA21661, CA32115, and CA37422 from the National Cancer Institute.
Footnotes
Conflict of interest: none.
Supplementary material for this article can be found at www.redjournal.org.
References
- 1.Deasy JO, Blanco AI, Clark VH. CERR: A computational environment for radiotherapy research. Med Phys. 2003;30:979–985. doi: 10.1118/1.1568978. [DOI] [PubMed] [Google Scholar]
- 2.Warfield SK, Zou KH, Wells WM. Simultaneous truth and performance level estimation (STAPLE): An algorithm for the validation of image segmentation. IEEE Trans Med Imaging. 2004;23:903–921. doi: 10.1109/TMI.2004.828354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santanam L, Hurkmans C, Mutic S, et al. Standardizing naming conventions in radiation oncology. Int J Radiat Oncol Biol Phys. 2012 Jan 13; doi: 10.1016/j.ijrobp.2011.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McLaughlin PW, Evans C, Feng M, et al. Radiographic and anatomic basis for prostate contouring errors and methods to improve prostate contouring accuracy. Int J Radiat Oncol Biol Phys. 2010;76:369–378. doi: 10.1016/j.ijrobp.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 5.Marks LB, Yorke ED, Jackson A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76:S10–S19. doi: 10.1016/j.ijrobp.2009.07.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michalski JM, Gay H, Jackson A, et al. Radiation dose-volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys. 2010;76(Suppl.):S123–S129. doi: 10.1016/j.ijrobp.2009.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tucker SL, Dong L, Bosch WR, et al. Fit of a generalized Lyman normal-tissue complication probability (NTCP) model to grade ≥ 2 late rectal toxicity data from patients treated on protocol RTOG 94-06. Int J Radiat Oncol Biol Phys. 2007;69:S8–S9. [Google Scholar]
- 8.Sohn M, Yan D, Liang J, et al. Incidence of late rectal bleeding in high-dose conformal radiotherapy of prostate cancer using equivalent uniform dose-based and dose-volume-based normal tissue complication probability models. Int J Radiat Oncol Biol Phys. 2007;67:1066–1073. doi: 10.1016/j.ijrobp.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rancati T, Fiorino C, Gagliardi G, et al. Fitting late rectal bleeding data using different NTCP models: Results from an Italian multi-centric study (AIROPROS0101) Radiother Oncol. 2004;73:21–32. doi: 10.1016/j.radonc.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 10.Meijer GJ, van den Brink M, Hoogeman MS, et al. Dose-wall histograms and normalized dose-surface histograms for the rectum: A new method to analyze the dose distribution over the rectum in conformal radiotherapy. Int J Radiat Oncol Biol Phys. 1999;45:1073–1080. doi: 10.1016/s0360-3016(99)00270-9. [DOI] [PubMed] [Google Scholar]
- 11.Peeters ST, Hoogeman MS, Heemsbergen WD, et al. Rectal bleeding, fecal incontinence, and high stool frequency after conformal radiotherapy for prostate cancer: Normal tissue complication probability modeling. Int J Radiat Oncol Biol Phys. 2006;66:11–19. doi: 10.1016/j.ijrobp.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 12.Cheung R, Tucker SL, Ye JS, et al. Characterization of rectal normal tissue complication probability after high-dose external beam radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2004;58:1513–1519. doi: 10.1016/j.ijrobp.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Cozzarini C, Fiorino C, Ceresoli GL, et al. Significant correlation between rectal DVH and late bleeding in patients treated after radical prostatectomy with conformal or conventional radiotherapy (66.6-70.2 Gy) Int J Radiat Oncol Biol Phys. 2003;55:688–694. doi: 10.1016/s0360-3016(02)04117-2. [DOI] [PubMed] [Google Scholar]
- 14.Fiorino C, Sanguineti G, Cozzarini C, et al. Rectal dose-volume constraints in high-dose radiotherapy of localized prostate cancer. Int J Radiat Oncol Biol Phys. 2003;57:953–962. doi: 10.1016/s0360-3016(03)00665-5. [DOI] [PubMed] [Google Scholar]
- 15.Huang EH, Pollack A, Levy L, et al. Late rectal toxicity: Dose-volume effects of conformal radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2002;54:1314–1321. doi: 10.1016/s0360-3016(02)03742-2. [DOI] [PubMed] [Google Scholar]
- 16.Pollack A, Zagars GK, Starkschall G, et al. Prostate cancer radiation dose response: Results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys. 2002;53:1097–1105. doi: 10.1016/s0360-3016(02)02829-8. [DOI] [PubMed] [Google Scholar]
- 17.Boersma LJ, van den Brink M, Bruce AM, et al. Estimation of the incidence of late bladder and rectum complications after high-dose (70–78 GY) conformal radiotherapy for prostate cancer, using dose-volume histograms. Int J Radiat Oncol Biol Phys. 1998;41:83–92. doi: 10.1016/s0360-3016(98)00037-6. [DOI] [PubMed] [Google Scholar]
- 18.Tseng CJ, Chang CT, Lai CH, et al. A randomized trial of concurrent chemoradiotherapy versus radiotherapy in advanced carcinoma of the uterine cervix. Gynecol Oncol. 1997;66:52–58. doi: 10.1006/gyno.1997.4721. [DOI] [PubMed] [Google Scholar]
- 19.Wolfson AH, Brady MF, Rocereto T, et al. A gynecologic oncology group randomized phase III trial of whole abdominal irradiation (WAI) vs. cisplatin-ifosfamide and mesna (CIM) as post-surgical therapy in stage I-IV carcinosarcoma (CS) of the uterus. Gynecol Oncol. 2007;107:177–185. doi: 10.1016/j.ygyno.2007.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peeters ST, Lebesque JV, Heemsbergen WD, et al. Localized volume effects for late rectal and anal toxicity after radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2006;64:1151–1161. doi: 10.1016/j.ijrobp.2005.10.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.