Abstract
Prior in vitro work demonstrated that PARP inhibition induces cell death in PTEN-null endometrial cancer cell lines, but the in vivo therapeutic efficacy of these agents against endometrial cancer remains unknown. Here we test the efficacy of AZD2281 (Olaparib), an oral PARP inhibitor, in therapy of PTEN-null endometrial tumors in a pre-clinical endometrial cancer mouse model. Primary endometrial tumors were generated by epithelial loss of PTEN using an in vivo model. This model recapitulates epithelial specific loss of PTEN seen in human tumors and histologically resembles endometrioid carcinomas, the predominant sub-type of human endometrial cancers. Olaparib was administered orally to tumor bearing mice in two hormonal extremes: high or low estrogen. Olaparib treatment achieved a significant reduction in tumor size in a low estrogenic milieu. In striking contrast, no response to Olaparib was seen in tumors exposed to high levels of estrogen. Two key observations were made when estrogen levels were dropped. Serum concentration of Olaparib was significantly increased resulting in sustained PARP inhibition at the tumor bed. The homologous recombination pathway was compromised evidenced by decreased Rad51 protein and function. These two mechanisms may account for the sensitization of PTEN-null tumors to Olaparib with estrogen deprivation. Results of this pre-clinical trial suggest that orally administered PARP inhibitors in a low estrogenic hormonal milieu can effectively target PTEN-null endometrial tumors. Extension of this work to clinical trials could personalize the therapy of women afflicted with advanced endometrial cancer using well tolerated orally administered therapeutic agents.
Introduction
A major challenge in advanced recurrent and metastatic endometrial cancer is the limited benefit of current treatments (1). Clinical trials comparing the efficacy of chemotherapy using platinums, taxanes, and anthracyclines have not shown any clear benefit for women with advanced disease (2). Instead, the focus is shifting toward the development of drugs targeting specific molecular pathways involved in endometrial carcinogenesis (3). The mechanism responsible for initiation of most endometrial cancers involves activation of the PI3-kinase pathway resulting from the loss of phosphatase and tensin homologue (PTEN) function (4). PTEN mutations have been identified in up to 80% of sporadic endometrioid cancers, the most common sub-type of endometrial tumors (5, 6). Given the prevalence of this mutation, strategies exploiting cellular defects associated with loss of PTEN function in endometrial tumor cells could have broad clinical application. Previous studies demonstrated that PTEN-null endometrial cancer cell lines can be targeted with PARP inhibitors in vitro (7). But to date, the in vivo anti-tumor effects of these agents in therapy of endometrial tumors have not been tested.
Poly(ADP-ribose) polymerase (PARP) inhibitors have emerged as targeted cancer therapeutic agents (8). While PARP inhibitors have proven efficacious in small trials (9, 10) their therapeutic utility remains limited based on less promising results in larger clinical series (11). Classically, these drugs cause cell death through synthetic lethality, a concept in which a substance not inherently detrimental to cells becomes cytotoxic when combined with another specific defect (12, 13). As a single agent, PARP inhibition blocks the base excision repair pathway (8). This leads to the accumulation of single-strand DNA breaks, which are converted to double-strand breaks during DNA replication. In normal cells, double-strand DNA breaks are repaired by the homologous recombination (HR) DNA repair pathway. However in cells with compromised HR, death ensues with PARP inhibition (8). As an example, PARP inhibitors exhibit antitumor activity in patients with BRCA-mutant cancers (9, 10). Since BRCA proteins are essential for homologous recombination, BRCA-deficient tumors regress because of their inability to repair double-strand DNA breaks induced by PARP inhibition (12, 13).
To extend the use of PARP inhibitors, non-traditional pathways that may interfere with HR have been investigated. Inactivation of PTEN in mouse embryonic fibroblasts induced chromosome instability due to defective Rad51 mediated HR DNA repair (14). Rad51 is a critical component of the HR pathway, functioning in the homology search and strand exchange phases of DNA repair (15). PTEN-null tumor cells were hypothesized to be sensitive to PARP inhibition as a result of Rad51 down-regulation and or loss of function. Two in vitro studies reported cytotoxic effects of PARP inhibition in PTEN-null cancer cell lines associated with altered Rad51 function (7, 16), but the in vivo capacity of these drugs to resolve PTEN-null tumors, particularly endometrial cancer, has not been fully investigated. The existence of Rad51 defects in PTEN-null tumors has been controversial as recent studies did not demonstrate defective Rad51 function in PTEN-null prostate cancer cell lines (17). While PTEN status may not predict response to PARP inhibition, Rad51 function may define sensitivity to this therapy [(18) and reviewed in (19)]. Decreased expression of Rad51 promoted sensitivity to PARP inhibition in vitro (17). Conversely, increased Rad51 levels have been implicated in resistance to PARP inhibitors (20, 21). Overall, mechanisms accounting for sensitivity or resistance to PARP inhibitors remain debated.
To test the efficacy of PARP inhibitors in therapy of endometrioid carcinomas driven by loss of PTEN, an in vivo mouse model established by our group was utilized (22). In this model, combinations of PTEN-null epithelia and wild type stroma regenerate giving rise to tumors that closely resemble human endometrial cancers based on two major criteria. In human endometrioid tumors PTEN expression is lost in the tumor epithelia but preserved in the stroma (23). Unlike existing PTEN-null mouse models (24–26) but similar to human endometrial cancers, tumors in our model harbor epithelial-specific loss of PTEN but maintain its expression in the tumor stroma. The histology of tumors established in this model recapitulates the histopathology of endometrioid carcinomas, the major subtype of human endometrial cancers. In this pre-clinical study Olaparib (AZD2281), an orally bioavailable PARP inhibitor tested in human trials (http://clinicaltrials.gov/show/NCT01237067), was administered to mice bearing PTEN-null endometrial tumors. The efficiency of this therapeutic agent was tested under two hormonal conditions, high or low estrogen. These hormonal milieus were examined as many patients with endometrial cancer have a hyper-estrogenic state due to obesity (27) that can be reduced pharmacologically.
Using this model we demonstrate PTEN-null endometrial tumors are sensitive to synthetic lethal effects of PARP inhibitors, but this response hinges on the hormonal milieu. When estrogen levels were high, a measurable response to PARP inhibition was not detected. In striking contrast, low levels of circulating estrogen sensitized these same tumors to PARP inhibition demonstrated by a reduction in tumor burden. Here we dissect cellular mechanisms that account for this dichotomous response. Our results show that the endogenous estrogen levels impact: (a) levels of circulating Olaparib modulating PARP inhibition at the tumor bed and, (b) levels and function of Rad51 a critical protein involved in homologous recombination. These results suggest that PARP inhibition should be tested in conjunction with hormonal ablation in clinical trials treating women with PTEN-null endometrial tumors.
Methods and Materials
Animals
Mouse strains Bl6 (C57BL/6), PTENloxPloxP (C;129S4-Ptentm1Hwu/J), and CB17Scid/Scid were from Jackson Laboratories. Mice were maintained in accordance with University of California Los Angeles (UCLA) Division of Laboratory Animal Medicine guidelines. All animal experiments were approved by the UCLA Animal Research Committee.
Isolation of PTEN-null endometrial epithelia and assays
(A) Preparation of PTEN-null epithelia: Trop1+CD90−CD45−CD31−Ter119− epithelia were isolated by FACS from the uteri of PTENloxPloxP adult female mice (22). Epithelia were infected with Cre-RFP (22) to delete PTEN. (B) In vivo tumor generation: PTEN-null tumors were prepared (22), details are available in Supplementary Methods. Cellular combinations were grown for 8 weeks to establish tumors prior to initiation of therapy. (C) In vitro generation of PTEN-null spheres: Cre-infected PTENloxP/loxP epithelia were plated in matrigel (BD Bioscience) and PrEGM media (Lonza) as previously described (28). WT mouse uterine stroma was added to each well. Cells were cultured for 2 weeks. PTEN deletion was confirmed by PCR as described (29).
Tumor Generation
Endometrial tumor generation was performed as previously described (22). Combinations of WT stroma and Cre-infected PTENloxP/loxP epithelia were suspended in collagen, implanted under the renal capsule of oophorectomized CB17Scid/Scid mice with a time-release estrogen pellet (60-d time release, 0.72-mg β-estradiol/pellet, SE-121 Innovative Research of America) and regenerated for 6–8weeks. Details are outlined in Supplementary methods.
Drug preparation and administration
(A) In vivo: Olaparib stocks were prepared as previously described (12, 13). Details are outlined in Supplementary Methods. Olaparib at 50 mg/kg or vehicle was orally administered to mice once daily for 3 weeks. Drug doses and schedules were selected based on published data (12, 13). (B) In vitro: Olaparib (10 μM) or equal volume of vehicle were added to each well.
Measurement of drug levels and activity
The in vitro efficacy of Olaparib was confirmed by examining drug induced DNA damage measured by formation of γH2AX foci as previously described (7). Details are outlined in Supplementary Methods. PARP enzyme activity in tumors was determined by measuring poly(ADP-ribose) levels using the PARP PDA II ELISA assay (Trevigen, 4520-096-K). Serum Estrogen concentrations were determined using the estradiol EIA kit with associated controls according to manufacturer protocol (Cayman Chemical). Serum Olaparib concentrations were determined by HPLC/MS. Details are given in Supplementary Methods. Three independent measurements were made for each time point.
Measurement of Rad51 foci formation
In vitro co-cultures of Cre-infected PTENloxP/loxP epithelia with WT stroma were treated with 10 μM Olaparib or vehicle for 3 days. Epithelial spheres were released from matrigel as previously described (28). RFP positive spheres were picked, dissociated to single cells (28) and transferred to slides by cytospin. Cells were fixed and co-stained for γH2AX and Rad51 (antibodies in Supplementary Table S1). Cells were visualized using a Zeiss Axiovert with Apotome, EXFO X-Cite series 120Q source, Zeiss McR camera and Axiovision software. Nuclear γH2AX foci with or without Rad51 were scored on 5 random high powered fields of view per sample.
Statistical analysis
Results are expressed as the mean of 3 or more measurements with standard errors. Normal distributions were verified using normal quantile plots. To determine if differences between two groups were significant, p values were calculated using unpaired Student’s t-test. For comparisons between 3 or more groups, one-way ANOVA was performed where all pairwise mean comparisons were assessed using the Tukey HSD criterion. ANOVA analysis of serum estrogen and CYP3A41 transcript levels was performed on log transformed values. All statistical calculations were performed using GraphPad Prism software.
Results
PTEN deficient endometrial tumors were resistant to Olaparib in a high estrogenic hormonal milieu
Many patients with endometrial cancer also suffer from obesity (27). In obese patients, aromatase enzyme in adipocytes converts androgen to estradiol resulting in high circulating estrogen levels (30). Therefore, in the clinic many patients with endometrial cancers also have high serum estrogen. Based on in vitro studies (7, 16), we hypothesized that endometrial tumors initiated by epithelial loss of PTEN would respond to PARP inhibition in vivo. To mimic the high estrogen levels in endometrial cancer patients, the response of PTEN-null tumors to PARP inhibition was initially examined in a high estrogenic milieu.
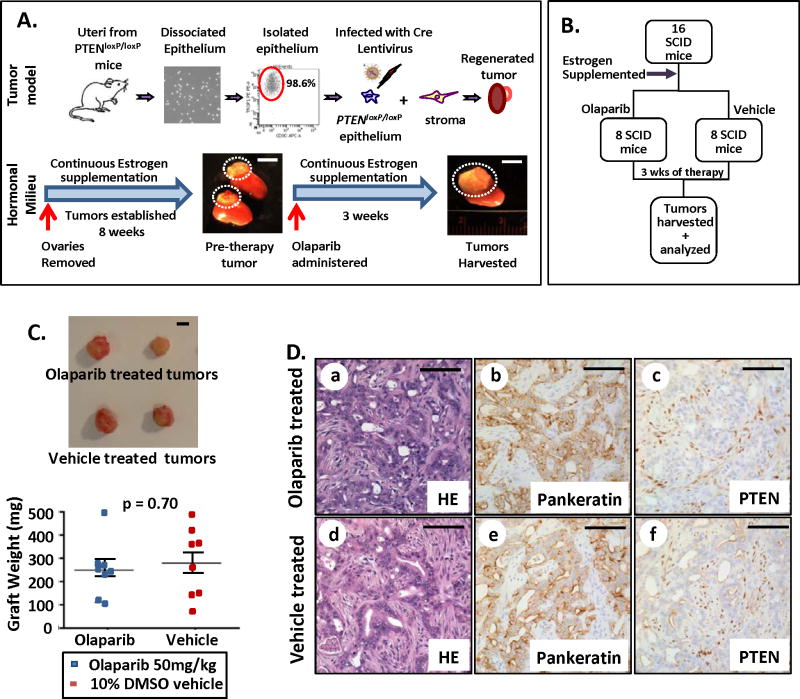
The effects of Olaparib were tested in vivo using an endometrial cancer model developed by our group (22). Endometrial epithelia FACS isolated from PTENloxP/loxP uteri were infected with a Cre-expressing lentivirus resulting in epithelial-specific deletion of PTEN. Combinations of PTEN-null epithelia and wild type (WT) stroma were placed under the kidney capsule of immunocompromised mice to prevent rejection of grafted tissue (22). Concurrent with cell implantation, mice were oophorectomized and supplemented with a 60 day time-release estradiol pellet to establish high and steady circulating estrogen levels. Within eight weeks, cell combinations gave rise to endometrial tumors in the context of cell-autonomous loss of PTEN evidenced by presence of tumor attached to the kidney (Fig. 1A and Supplementary Fig. S1A). This mouse tumor model is highly reproducible, resulting in formation of PTEN-null tumors that share histologic features and molecular markers of human endometrioid endometrial cancer with 100% efficiency (Supplementary Fig S1A) (22). Sixteen independent tumor bearing mice were supplemented with fresh estrogen pellets to maintain a high estrogen milieu then separated into two groups. The first 8 mice were treated daily with Olaparib at 50 mg/kg, while remaining 8 mice were treated on the same schedule with 10% DMSO vehicle for three weeks (Fig. 1B). All treatments were delivered by oral gavage to replicate the oral route of administration in patients. Prior to the initiation of therapy, drug efficacy was verified in vitro (Supplementary Fig. S1B). After 3 weeks of treatment, tumors were harvested and analyzed. Olaparib treatment had no effect on tumor size or mass in this hormonal milieu (Fig. 1C). Tumors treated with Olaparib or vehicle had similar histology, characterized by infiltrating glands mostly with lumens containing atypical and prominent nucleoli (Fig. 1D a&b vs. d&e). In these tumors only the epithelia was PTEN-null while the stroma expressed PTEN (Fig. 1D c&f and Supplementary Fig. S1C). The expression of estrogen receptor alpha (ER-α) was similar in Olaparib and vehicle treated controls (Supplementary Fig. S1D). To validate our results this experiment was performed on an additional cohort of mice confirming that olaparib treatment had no significant effect on the size of PTEN-null tumors in a high estrogenic hormonal milieu (Supplementary Fig S1E).
Figure 1. PTEN deficient endometrial carcinomas were refractory to PARP inhibition with high levels of circulating estrogen.
(A) Schema for in vivo generation and therapy of endometrial tumors driven by epithelial loss of PTEN in a high estrogen milieu. (B) Design for therapy with hormonal supplementation. (C) No significant difference in tumor mass was detected between Olaparib (n=8) or vehicle (n=8) treated tumors (p = 0.70). (D) Endometrioid tumors (a&d) containing crowded pankeratin positive (b&e) PTEN-null (c&f) epithelial glands with lumens were observed in both vehicle and Olaparib treated group. Scale bars equal 5 mm in A&C and 100 μm in D.
These findings suggest that Olaparib was ineffective in treating PTEN-null tumors exposed to high levels of estrogen. One possibility is that PARP inhibition is not effective against PTEN-null tumors in an in vivo setting. Alternatively, high levels of estrogen may induce resistance to PARP inhibition.
Low levels of circulating estrogen sensitized PTEN deficient endometrial cancers to anti-tumor effects of Olaparib
Our results demonstrate that PTEN-null endometrial tumors are resistant to PARP inhibition in a high estrogenic milieu. Clinically, endogenous estrogen levels can be reduced by surgical removal of ovaries and co-administration of aromatase inhibitors. Aromatase inhibitors block the conversion of adrenal androgens to estrogen in adipose tissue (31). Given the feasibility of this clinical approach, we wanted to test the efficacy of PARP inhibitors in achieving tumor response in a low estrogen state. We hypothesized that reduced of estrogen levels might sensitize PTEN-null endometrial tumors to the synthetic lethal effects of PARP inhibitors.
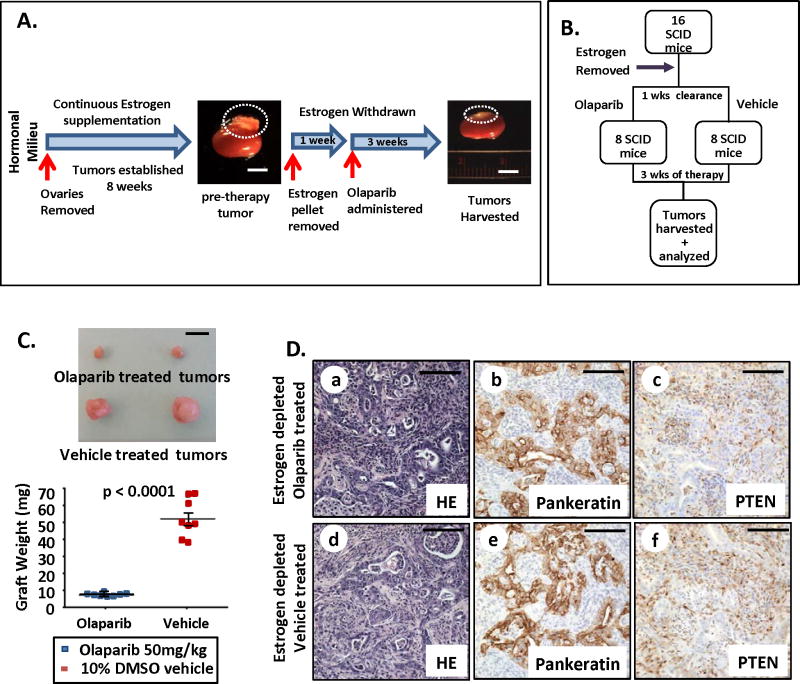
PTEN-null endometrial tumors were established in 17 independent oophorectomized, estrogen supplemented mice as outlined in the previous experiment (Fig. 2A). Tumor establishment was confirmed by sacrificing one random mouse (Fig 2A & Supplementary Fig. S2A). At this point, estrogen pellets were surgically removed from all mice after endometrial tumors were established (Fig. 2A). Prior to the initiation of Olaparib therapy, mice were rested one week to clear circulating estrogen (Fig. 2A). After establishing a low estrogen milieu, tumor-bearing mice were separated into equal groups. Half were treated with Olaparib (50 mg/kg daily) orally, while remaining mice received vehicle (Fig. 2B). After three weeks of therapy, all tumors were harvested and analyzed. Estrogen deprivation was confirmed in this experimental cohort as compared to estrogen supplemented mice (Supplementary Fig. S2B). The concentration of estrogen in our hormone depleted mice was within the range of plasma estradiol concentrations reported in postmenopausal women with or without aromatase therapy (32). Olaparib treatment resulted in a 6-fold decrease in tumor mass when administered in a low estrogen milieu (Fig. 2C). Despite the efficacy of this treatment in reducing tumor size, Olaparib did not eradicate the PTEN-null tumors demonstrated by evidence of residual cancer in these tissues (Fig. 2D a–c). The histology of Olaparib and vehicle-treated tumors was similar and resembled human endometrioid carcinomas (Fig. 2D and Supplementary Fig. S2C). Estrogen deprivation did not alter the histologic features or tumor grade of PTEN-null endometrioid carcinomas based on patterns of gland distribution and nuclear features (Fig. 2D vs. Fig. 1D). Administration of Olaparib in this hormonal milieu did not affect ER-α expression (Supplementary Fig. S2D). Importantly, estrogen depletion alone did not cause tumor regression (Supplementary Fig S2E).
Figure 2. PARP inhibition decreased tumor mass when administered under low levels of circulating estrogen.
(A) Strategy for therapy of PTEN-null tumors in a low estrogen milieu. (B) Treatment design. (C) PARP inhibition with low circulating estrogen levels significantly decreased tumor mass compared to vehicle treated controls (n=8 per group, p < 0.0001). (D) Both Olaparib and vehicle treated tumors contained neoplastic epithelial glands with lumens (a&d) that were pankeratin positive (b&e) but PTEN negative (c&f). Scale bars equal 5 mm in A&C and 100 μm in D.
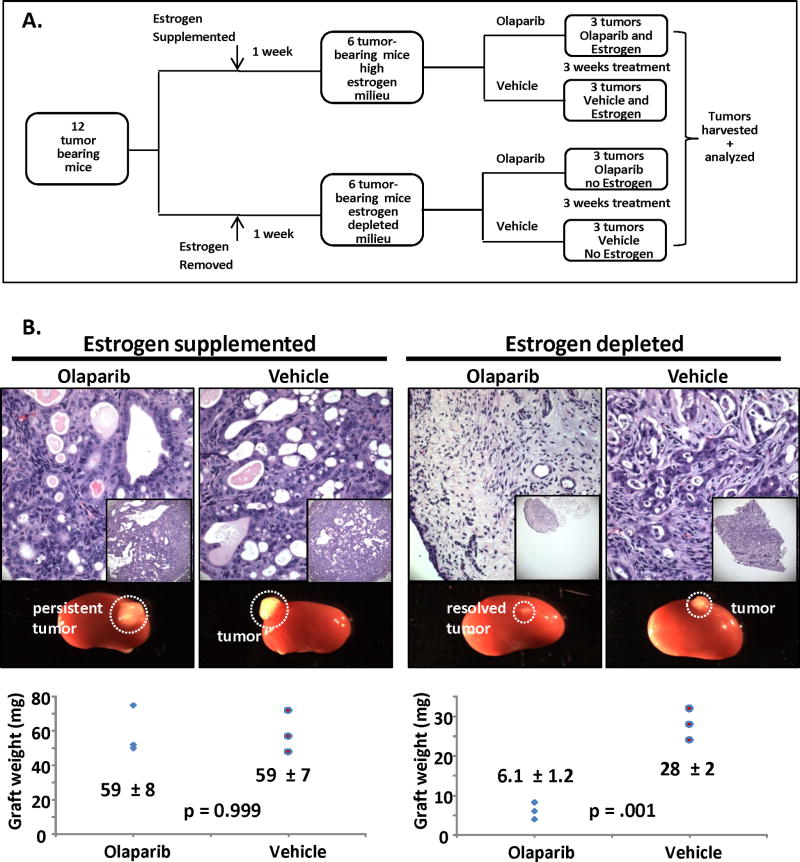
Given these results (Fig. 1&2), we wanted to directly compare the effects of estrogen depletion in modulating PARP sensitivity of PTEN-null endometrial tumors in a single experiment. To achieve this goal, estrogen depletion and supplementation was performed in the same cohort of experimental mice treated with Olaparib or vehicle (Fig. 3A). Also, to test if initial tumor burden would impact response to this combined therapy (Olaparib + estrogen depletion), the number of implanted epithelia and stroma were reduced approximately 2.5-fold. Results of this experiment confirmed previous findings by showing Olaparib resistance in a high estrogenic milieu (Fig 3B). In this experiment complete resolution of PTEN-null tumors treated with Olaparib in a low estrogenic milieu was found (Fig. 3B), suggesting that the initial tumor burden may impact response to this dual therapy.
Figure 3. Levels of estrogen modulate the sensitivity of PTEN-null endometrial tumors to PARP inhibition in vivo.
(A) Schema for generation and treatment of tumors. (B) With estrogen supplementation tumors were resistant to PARP inhibition based on histology and tumor weight. Estrogen deprivation sensitized tumors to PARP inhibition demonstrated by reduction in graft size and resolution of tumor tissue.
Our results suggest that modulation of the hormonal milieu can sensitize PTEN-deficient endometrial carcinomas to Olaparib therapy. No response to Olaparib was noted when estrogen levels were high. The same dose of Olaparib, in an estrogen deprived milieu, resulted in significant decreases in tumor size. A number of factors could underpin this dichotomous response including effects of estrogen on drug metabolism or components of the HR DNA repair pathway.
Estrogen depletion maximized PARP inhibition at the tumor bed concomitant with a rise in circulating levels of Olaparib
To assess the bioavailability and activity of Olaparib in a high and low estrogenic milieu, we measured serum levels of Olaparib in experimental mice and its efficacy in inhibiting PARP activity in the tumors. Olaparib effects on tumor cell proliferation and apoptotic induced cell death were also examined in each hormonal condition.
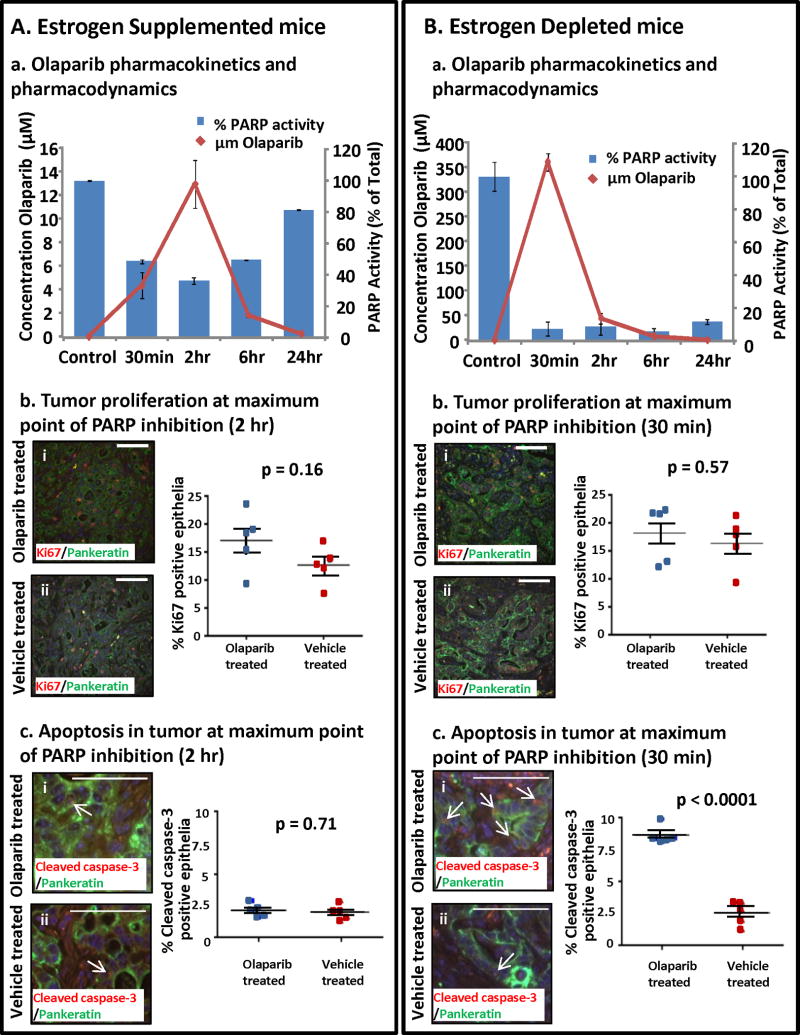
To measure kinetics of drug response, serum and tumors were collected from tumor bearing mice sacrificed at 30 minutes, 2, 6 and 24 hours after Olaparib administration. Serum Olaparib concentrations were measured using mass spectrometry. To assess inhibition of PARP enzyme, poly-ADP ribose levels, a surrogate marker for PARP activity (33), were measured in tumor extracts. In mice supplemented with estrogen, serum Olaparib concentrations peaked at 2 hours coincident with maximal PARP inhibition in the tumor (Fig. 4Aa). No differences were detected in the epithelial tumor proliferation (Fig. 4Ab) or apoptosis (Fig. 4Ac). After 2 hours, serum levels of Olaparib steadily declined, concomitant with decreased PARP inhibition in the tumor (Fig. 4Aa). The serum Olaparib concentration achieved with the dose in our study (50 mg/kg daily) was 5.2 μg/ml at peak concentration. This is comparable to serum concentrations of 5.9 μg/ml in patients treated with a commonly administered dose of Olaparib (400mg twice/day) (34). In tumor bearing mice subjected to low estrogen levels, serum Olaparib concentrations peaked earlier at 30 minutes and were 20-fold higher than in estrogen supplemented mice (Fig. 4Ba vs. 4Aa). An Olaparib induced increase in tumor apoptotic cell death (Fig. 4Bc) was detected in this hormonal milieu without a change in the proliferation (Fig. 4Bb). In this low estrogenic milieu, a profound and sustained inhibition of PARP enzyme activity was detected in the tumor bed (Fig. 4Ba) likely resulting in increased cell death and decreased tumor size.
Figure 4. Pharmacokinetics and pharmacodynamics of Olaparib varied with circulating estrogen levels.
(A) Olaparib was detected in the serum of estrogen supplemented mice with measurable inhibition of PARP enzyme activity but no apparent effects on tumor proliferation or apoptosis. (a) Olaparib serum concentrations were undetectable in vehicle treated mice, peaked at 2 h after drug ingestion and then slowly returned to baseline levels. Inhibition of PARP enzyme activity at the tumor bed correlated with the Olaparib serum concentration. (b) Epithelial proliferation (pankeratin & Ki67 dual positive cells) in Olaparib and vehicle treated tumors was similar (i vs. ii, p = 0.16). (c) PARP inhibition in a high estrogenic milieu did not increase apoptotic cell death in tumor PTEN-null epithelia (pankeratin & cleaved caspase 3 dual positive cells) (i vs ii, p=0.71). (B) Higher serum Olaparib levels concomitant with sustained PARP inhibition was detected in estrogen deprived tumor bearing mice. (a) In estrogen depleted mice peak concentrations of serum Olaparib were detected 30 min after dosing then dropped to baseline within 24 h. PARP activity at the tumor bed was sharply decreased compared to vehicle treated mice, and PARP inhibition was sustained throughout a 24h period. (b) PARP inhibition did not diminish epithelial proliferation in estrogen depleted mice (i vs. ii, p = 0.57). (c) Increased apoptosis was observed in tumors subjected to PARP inhibition with estrogen deprivation (I vs. ii, p<0.0001). Scale bars equal 100 μm.
CYP3A4, the primary enzyme that metabolizes Olaparib in humans (35, 36), is reported to be regulated by estrogen (37). To account for difference in circulating levels of Olaparib in each hormonal milieu, we examined expression of CYP3A41, a mouse homologue of CYP3A4, in the livers of experimental mice. Significantly higher levels of CYP3A41 transcript was detected in estrogen treated compared to estrogen depleted mice (Supplementary Fig S3A). These findings were confirmed on western blot (Supplementary Fig S3B). This may explain why concentrations of Olaparib were lower in estradiol supplemented mice. Definitive testing of this hypothesis would require measurement of PARP metabolites in each hormonal condition, an approach that was not feasible as specific metabolites for Olaparib are not published or disclosed.
Results here demonstrate that the bioavailability and activity of Olaparib were enhanced in a low estrogenic hormonal milieu in our in vivo model. This may be one mechanism sensitizing estrogen deprived PTEN-null endometrial tumors to Olaparib therapy. However, Olaparib resistance was noted despite measurable inhibition of PARP enzyme activity in estrogen supplemented mice. Failure of Olaparib therapy in this cohort may be due to an intact HR pathway repairing Olaparib induced DNA damage.
Rad51 function in a high estrogen milieu may promote resistance of PTEN-null endometrial tumors to PARP inhibition
Homologous recombination requires the action of a large complement of proteins that normally safeguard the genome (15). If HR is defective, cells become susceptible to synthetic lethal effects of PARP inhibitors. Previous work suggests that estrogen may regulate expression of key components of the HR pathway (38–40). Given that expression and function of Rad51 has been implicated as a potential biomarker of response to PARP inhibition (17, 18, 41) we first examined the expression of Rad51 protein in PTEN-null tumors propagated in low and high estrogen environments.
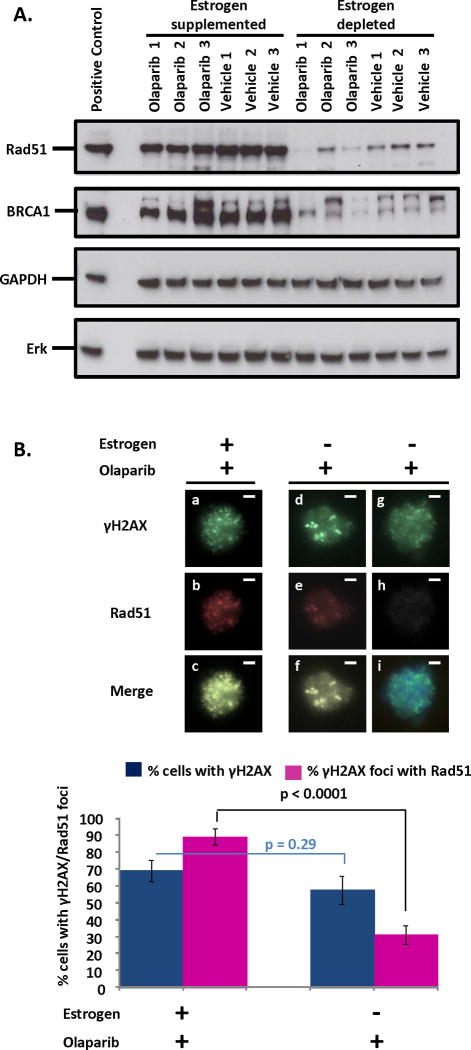
Western blotting was performed to measure levels of Rad51 in tumor lysates from 3 independent mice treated with Olaparib or vehicle in low or high estrogenic milieus. In the presence of estrogen significant amounts of tumor Rad51 were detected (Fig. 5A). Conversely, when estrogen was withdrawn a dramatic reduction in tumor Rad51 expression was noted (Fig. 5A). To test whether estrogen deprivation altered expression of other HR proteins, levels of BRCA1 were also measured in tumor lysates (Fig. 5A). Similar to Rad51, higher levels of BRCA1 were detected in estrogen supplemented vs. estrogen deprived PTEN-null tumors (Fig. 5A). Our findings demonstrate that the estrogenic hormonal milieu may affect expression of HR proteins in PTEN-null endometrial tumors.
Figure 5. Decreased levels and function of Rad51 may drive the sensitivity of PTEN-null tumors to PARP inhibition in estrogen depleted conditions.
(A) Western blot reveals diminished expression of both Rad51 and BRCA1 in lysates of tumors from estrogen deprived compared to estrogen supplemented mice regardless of treatment with Olaparib or vehicle. 3T3 and HeLa lysates were positive controls for Rad51 and BRCA1 respectively. Erk was used as a loading control. Expression of GAPDH was not altered by estrogen levels, suggesting that reduced proliferation with estrogen deprivation is not responsible for changes in Rad51 or BRCA1 levels. (B) Rad51 was efficiently recruited to Olaparib-induced sites of DNA damage (marked by γH2AX foci) in PTEN-null endometrial epithelial cells supplemented with estrogen (a–c). In estrogen deprived cells, low (d–f) or no (g–i) levels of Rad51 incorporation into γH2AX foci was observed compared to estrogen supplemented mice (p<0.0001). Olaparib induced γH2AX foci formation was unaffected by estrogen deprivation (p=0.29). Scale bars equal 10 μm.
To test the functional implications of reduced Rad51 levels, we examined recruitment of Rad51 to sites of DNA damage in Olaparib or vehicle treated PTEN-null endometrial epithelial cells exposed to high and low levels of estrogen. Addressing this question requires clear visualization of Rad51 and γH2AX co-localization in nuclei of Olaparib treated cells. Given that nuclei are tightly packed in the tumor, we chose an alternative in vitro experimental approach to measurement this co-localization. Primary endometrial epithelia harvested from PTENloxP/loxP mice were infected with a Cre-red fluorescent protein (RFP) tagged lentivirus and plated in a 3-D sphere assay (28) supplemented with estrogen or placebo and co-cultured with WT endometrial stroma to model the paracrine interactions between epithelium and stroma seen in tumors (Supplementary Fig. S4). Within 2 weeks spheroid epithelial structures outgrew, many of which were RFP positive indicating expression of Cre and deletion of PTEN. Established spheres were treated with Olaparib or vehicle for 36 hours. Following treatment, RFP positive spheres were handpicked and dissociated into single cells (Supplementary Fig. S4). Loss of PTEN was validated in this cell population (Supplementary Fig S5A). A 6-fold reduction in Rad51 transcript was detected in PTEN-null epithelia exposed to low levels of estrogen, suggesting estrogen modulation of Rad51 expression in vitro (Supplementary Fig. S5B) as well as in vivo (Fig. 5A). To assess if reduced Rad51 levels led to compromised HR, co-localization of Rad51 to γH2AX foci was examined in all experimental groups. Robust recruitment of Rad51 to sites of DNA damage was detected in the nuclei of Olaparib treated, estrogen exposed PTEN-null endometrial epithelia (Fig. 5B & Supplementary Fig. S5C). Conversely, in the absence of estrogen a significant reduction in Rad51 recruitment was noted despite Olaparib treatment (Fig. 5B & Supplementary Fig. S5C). In the absence of Olaparib γH2AX foci formation was minimal (Supplementary Fig. S5D). Findings demonstrate that recruitment of Rad51 is compromised in estrogen deprived conditions.
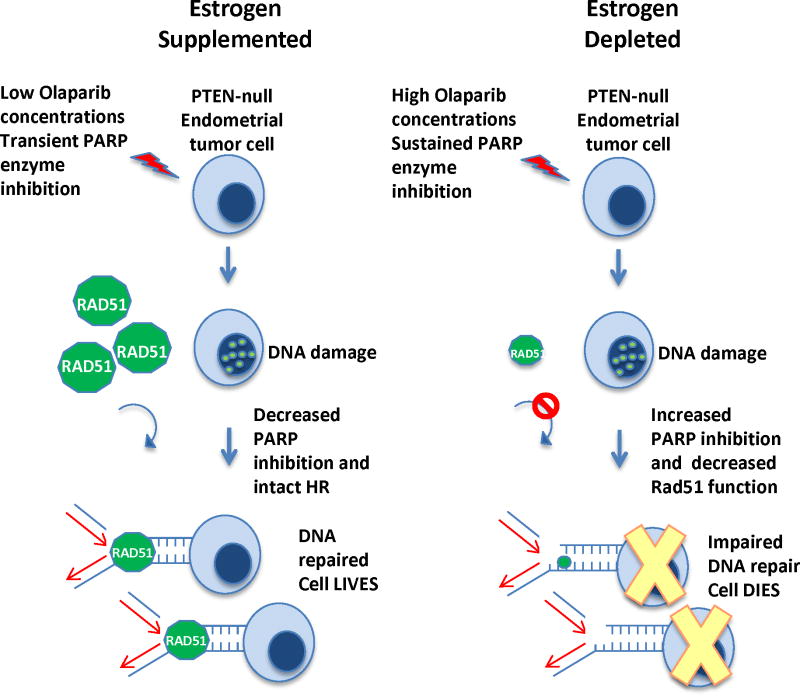
Based on our results PTEN-null endometrial tumors are sensitized to PARP inhibition in a low estrogenic milieu. When estrogen is depleted, increased amounts of DNA damage may occur due to sustained PARP enzyme inhibition at the tumor bed and impaired Rad51 mediated HR. High levels of estrogen may induce PARP resistance in PTEN-null endometrial tumors by lowering levels of circulating Olaparib in vivo resulting in less PARP inhibition in the tumor. Simultaneously robust expression and recruitment of Rad51 in this hormonal milieu may induce efficient tumor DNA repair. The net result is resistance to Olaparib therapy when estrogen levels are high (Fig. 6).
Figure 6. Model for the differential sensitivity of PTEN-null endometrial cells to PARP inhibition in high and low estrogen.
With a fixed dose of Olaparib, serum levels were higher in the estrogen depleted compared to estrogen supplemented mice. Elevated Olaparib concentrations in estrogen depleted mice may account for more sustained PARP inhibition and a measurable tumor response. Simultaneously levels of Rad51 were decreased in estrogen deprived compared to estrogen supplemented tumors. Diminution of Rad51 may compromise its recruitment to Olaparib induced sites of DNA damage. These two mechanisms may sensitize PTEN-null endometrial tumors to Olaparib in estrogen deprived conditions.
Discussion
PARP inhibitors have been tested in clinical trials as single-agent therapies against hormonally regulated solid tumors [reviewed in (20)]. While administration of this targeted therapy has been coupled with chemotherapeutic agents, no studies have examined efficacy of PARP inhibition in conjunction with hormonal blockade [reviewed in (20)]. Results of our experiments demonstrate that estrogen levels affect both the availability of Olaparib and efficiency of cell death induced by PARP inhibition. When estrogen levels were high resistance to Olaparib was noted in PTEN-null endometrial tumors. In contrast, a measurable tumor response to PARP inhibition was detected with the same dose of Olaparib in a low estrogenic milieu.
In clinical trials of patients with breast (42) or ovarian cancer (10), improved outcomes were reported with administration of higher Olaparib doses. An unexpected finding in our study was that estrogen significantly impacted serum concentrations of Olaparib. Despite administration of a fixed dose of Olaparib in our experiments, higher levels of circulating Olaparib coupled with greater and sustained PARP inhibition at the tumor bed was detected upon estrogen depletion. The cytochrome P450 superfamily of enzymes play a role in hepatic drug metabolism (43). A member of this family, CYP3A4, is thought to be the enzyme responsible for Olaparib metabolism in humans (35, 36). In our experiments an up-regulation of CYP3A41, a mouse homologue of CYP3A4, was observed with estrogen supplementation. Estrogen mediated induction of this enzyme could lead to decreased Olaparib bioavailability. These findings suggest that the patient’s hormonal status could alter the optimal dosing of Olaparib required to achieve a therapeutic response.
Mechanisms that induce resistance or sensitivity to PARP inhibition remain poorly understood. One challenge in interpreting human trials has been the variability of biologic response (44), which should be carefully taken into account in treatment. An advantage of our model is the use of genetically reproducible tissues from genetically identical backgrounds, which decrease variability of Olaparib response. A second challenge has been a dearth of data analyzing PARP resistant tumor tissue from patients treated with these agents (20). An advantage of our model is the ability to harvest both PARP sensitive and resistant tumors generated from the same genetic background. Analysis of this tissue revealed that levels of Rad51, a key modulator of HR, were significantly lower in estrogen deprived compared to estrogen supplemented tumors. Additionally, recruitment of Rad51 to sites of DNA damage was compromised in estrogen deprived PTEN-null endometrial cells. Our results suggest that estrogen modulation of HR pathway proteins such as Rad51 may regulate sensitivity to PARP inhibition. This relationship may also hold true for human endometrial tumors. Microarray analysis of control and estrogen treated normal human endometrial tissue demonstrated up-regulation of both Rad51 (1.5-fold) and BRCA1 (2.2-fold) message upon estrogen treatment (45).
Loss of PTEN function has been proposed as a potential biomarker for sensitivity to PARP inhibition (41). Prior studies examining the effects of PARP inhibition in a PTEN-null background have all utilized cancer cell lines (7, 16). In these cells lines, co-existing genetic defects in addition to PTEN loss may account for defective HR and sensitivity to PARP inhibition. An advantage of our model is that epithelial inactivation of PTEN is the sole genetic change, allowing for a definitive evaluation of PARP response in PTEN-null tumor cells. In this model, PTEN status was not an independent biomarker of sensitivity to PARP inhibition as endometrial tumors were resistant to Olaparib therapy in a high estrogenic milieu. However, a significant response to therapy was observed in PTEN-null endometrial tumors when serum estrogen was decreased. PTEN-null cells are proposed to have compromised HR DNA repair that stems from decreased activity of Rad51 (14), but the association between PTEN deletion and Rad51 function has been controversial (7, 14, 16, 17). Here we find that Rad51 expression and recruitment to sites of DNA damage was robust in PTEN-null epithelia exposed to high estrogen levels. With estrogen deprivation, Rad51 protein levels were diminished and its function was decreased in PTEN-null epithelia. We suspect that estrogen mediated regulation of Rad51 in PTEN-null endometrial epithelia is a regulator of PARP sensitivity in our model.
Collectively, our findings suggest that the use of PARP inhibitors in combination with hormonal therapy could provide an exciting novel approach to the treatment of PTEN-null endometrial cancers. Pre-clinical studies have demonstrated the chemo- and radio-sensitizing activity of PARP inhibitors, but despite these positive results, a major limiting factor in translation to clinical trials has been the untoward toxicities of chemotherapy and radiation (46). Aromatase inhibitors and estrogen receptor antagonists are well tolerated medications that can decrease the production and block the effects of estrogen (31, 47). While they have been unsuccessful as mono-therapy against endometrial cancer (48, 49), they could be used to sensitize estrogen dependent tumors to PARP inhibition. Our pre-clinical findings support this combined approach and merit progression into clinical trials investigating the synergistic effects of PARP inhibitors and hormonal ablation in patients with PTEN-null endometrial cancers. We recognize that there are limitations in extrapolating the data from these experiments to human patients, as the hormonal manipulations in our mouse model do no completely represent the hormonal milieu of endometrial cancer patients. However, some similarities do exist. Similar to our model, almost all endometrial cancer patients are oophorectormized as part of their treatment. A recent case report showed that administration of Olaparib was efficacious in therapy of a patient with a PTEN-null endometrial tumor and a predicted low estrogen level due to removal of her ovaries (50). Based on our findings, the combined use of PARP inhibition and hormonal ablation should also be tested in breast and prostate cancer tumor models. We demonstrate that the hormonal milieu can profoundly affect sensitivity to PARP inhibition in this pre-clinical in vivo endometrial cancer model. Future work by our group will focus on testing this therapeutic strategy in endometrial tumors driven by alternative genetic pathways.
Supplementary Material
Acknowledgments
Funding: S. Memarzadeh is supported by the Concern foundation, CDU/UCLA NIH/NCI Grant #U54-CA-143931, VA CDA-2 Award, Scholars in Translational Medicine Program, Mary Kay Foundation Award, STOP Cancer Award, Broad Stem Cell Research Center Research Award, Sidney Kimmel Foundation award, National Center for Research Resources Grant UL1TR000124 and the University of California Cancer Research Coordinating Committee grant. O.N. Witte is an Investigator of the Howard Hughes Medical Institute.
We thank Drs. Jeffery Gornbein, Xianyu Rao and Yuri Buminovich for helpful discussions of this manuscript.
Footnotes
Competing interest: The authors declare that they have no competing interests.
References
- 1.Rauh-Hain JA, Del Carmen MG. Treatment for advanced and recurrent endometrial carcinoma: combined modalities. Oncologist. 2010;15:852–61. doi: 10.1634/theoncologist.2010-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Humber CE, Tierney JF, Symonds RP, Collingwood M, Kirwan J, Williams C, et al. Chemotherapy for advanced, recurrent or metastatic endometrial cancer: a systematic review of Cochrane collaboration. Ann Oncol. 2007;18:409–20. doi: 10.1093/annonc/mdl417. [DOI] [PubMed] [Google Scholar]
- 3.Salvesen HB, Haldorsen IS, Trovik J. Markers for individualised therapy in endometrial carcinoma. Lancet Oncol. 2012;13:e353–61. doi: 10.1016/S1470-2045(12)70213-9. [DOI] [PubMed] [Google Scholar]
- 4.Chalhoub N, Baker SJ. PTEN and the PI3-kinase pathway in cancer. Annu Rev Pathol. 2009;4:127–50. doi: 10.1146/annurev.pathol.4.110807.092311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudd ML, Price JC, Fogoros S, Godwin AK, Sgroi DC, Merino MJ, et al. A unique spectrum of somatic PIK3CA (p110alpha) mutations within primary endometrial carcinomas. Clin Cancer Res. 2011;17:1331–40. doi: 10.1158/1078-0432.CCR-10-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dedes KJ, Wetterskog D, Mendes-Pereira AM, Natrajan R, Lambros MB, Geyer FC, et al. PTEN deficiency in endometrioid endometrial adenocarcinomas predicts sensitivity to PARP inhibitors. Sci Transl Med. 2010;2:53ra75. doi: 10.1126/scitranslmed.3001538. [DOI] [PubMed] [Google Scholar]
- 8.Underhill C, Toulmonde M, Bonnefoi H. A review of PARP inhibitors: from bench to bedside. Ann Oncol. 2011;22:268–79. doi: 10.1093/annonc/mdq322. [DOI] [PubMed] [Google Scholar]
- 9.Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–34. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 10.Audeh MW, Carmichael J, Penson RT, Friedlander M, Powell B, Bell-McGuinn KM, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376:245–51. doi: 10.1016/S0140-6736(10)60893-8. [DOI] [PubMed] [Google Scholar]
- 11.Ledford H. Drug candidates derailed in case of mistaken identity. Nature. 2012;483:519. doi: 10.1038/483519a. [DOI] [PubMed] [Google Scholar]
- 12.Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–7. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 13.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–21. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 14.Shen WH, Balajee AS, Wang J, Wu H, Eng C, Pandolfi PP, et al. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell. 2007;128:157–70. doi: 10.1016/j.cell.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 15.Krejci L, Altmannova V, Spirek M, Zhao X. Homologous recombination and its regulation. Nucleic Acids Res. 2012;40:5795–818. doi: 10.1093/nar/gks270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mendes-Pereira AM, Martin SA, Brough R, McCarthy A, Taylor JR, Kim JS, et al. Synthetic lethal targeting of PTEN mutant cells with PARP inhibitors. EMBO Mol Med. 2009;1:315–22. doi: 10.1002/emmm.200900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraser M, Zhao H, Luoto KR, Lundin C, Coackley C, Chan N, et al. PTEN deletion in prostate cancer cells does not associate with loss of RAD51 function: implications for radiotherapy and chemotherapy. Clin Cancer Res. 2012;18:1015–27. doi: 10.1158/1078-0432.CCR-11-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukhopadhyay A, Elattar A, Cerbinskaite A, Wilkinson SJ, Drew Y, Kyle S, et al. Development of a functional assay for homologous recombination status in primary cultures of epithelial ovarian tumor and correlation with sensitivity to poly(ADP-ribose) polymerase inhibitors. Clin Cancer Res. 2010;16:2344–51. doi: 10.1158/1078-0432.CCR-09-2758. [DOI] [PubMed] [Google Scholar]
- 19.Rehman FL, Lord CJ, Ashworth A. Synthetic lethal approaches to breast cancer therapy. Nat Rev Clin Oncol. 2010;7:718–24. doi: 10.1038/nrclinonc.2010.172. [DOI] [PubMed] [Google Scholar]
- 20.Basu B, Sandhu SK, de Bono JS. PARP inhibitors: mechanism of action and their potential role in the prevention and treatment of cancer. Drugs. 2012;72:1579–90. doi: 10.2165/11635510-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 21.Schild D, Wiese C. Overexpression of RAD51 suppresses recombination defects: a possible mechanism to reverse genomic instability. Nucleic Acids Res. 2010;38:1061–70. doi: 10.1093/nar/gkp1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Memarzadeh S, Zong Y, Janzen DM, Goldstein AS, Cheng D, Kurita T, et al. Cell-autonomous activation of the PI3-kinase pathway initiates endometrial cancer from adult uterine epithelium. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1012548107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mutter GL, Lin MC, Fitzgerald JT, Kum JB, Baak JP, Lees JA, et al. Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. J Natl Cancer Inst. 2000;92:924–30. doi: 10.1093/jnci/92.11.924. [DOI] [PubMed] [Google Scholar]
- 24.Stambolic V, Tsao MS, Macpherson D, Suzuki A, Chapman WB, Mak TW. High incidence of breast and endometrial neoplasia resembling human Cowden syndrome in pten+/- mice. Cancer Res. 2000;60:3605–11. [PubMed] [Google Scholar]
- 25.Kim TH, Wang J, Lee KY, Franco HL, Broaddus RR, Lydon JP, et al. The Synergistic Effect of Conditional Pten Loss and Oncogenic K-ras Mutation on Endometrial Cancer Development Occurs via Decreased Progesterone Receptor Action. J Oncol. 2010:139087. doi: 10.1155/2010/139087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joshi A, Ellenson LH. Adenovirus mediated homozygous endometrial epithelial Pten deletion results in aggressive endometrial carcinoma. Exp Cell Res. 2011;317:1580–9. doi: 10.1016/j.yexcr.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cramer DW. The epidemiology of endometrial and ovarian cancer. Hematology/oncology clinics of North America. 2012;26:1–12. doi: 10.1016/j.hoc.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janzen DM, Cheng D, Schafenacker AM, Paik DY, Goldstein AS, Witte ON, et al. Estrogen and progesterone together expand murine endometrial epithelial progenitor cells. Stem Cells. 2013;31:808–22. doi: 10.1002/stem.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiao J, Wang S, Qiao R, Vivanco I, Watson PA, Sawyers CL, et al. Murine cell lines derived from Pten null prostate cancer show the critical role of PTEN in hormone refractory prostate cancer development. Cancer Res. 2007;67:6083–91. doi: 10.1158/0008-5472.CAN-06-4202. [DOI] [PubMed] [Google Scholar]
- 30.Cleland WH, Mendelson CR, Simpson ER. Effects of aging and obesity on aromatase activity of human adipose cells. J Clin Endocrinol Metab. 1985;60:174–7. doi: 10.1210/jcem-60-1-174. [DOI] [PubMed] [Google Scholar]
- 31.Umar A, Dunn BK, Greenwald P. Future directions in cancer prevention. Nat Rev Cancer. 2012;12:835–48. doi: 10.1038/nrc3397. [DOI] [PubMed] [Google Scholar]
- 32.Kunovac Kallak T, Baumgart J, Stavreus Evers A, Sundstrom Poromaa I, Moby L, Kask K, et al. Higher than expected estradiol levels in aromatase inhibitor-treated, postmenopausal breast cancer patients. Climacteric : the journal of the International Menopause Society. 2012;15:473–80. doi: 10.3109/13697137.2011.642427. [DOI] [PubMed] [Google Scholar]
- 33.Kinders RJ, Hollingshead M, Khin S, Rubinstein L, Tomaszewski JE, Doroshow JH, et al. Preclinical modeling of a phase 0 clinical trial: qualification of a pharmacodynamic assay of poly (ADP-ribose) polymerase in tumor biopsies of mouse xenografts. Clin Cancer Res. 2008;14:6877–85. doi: 10.1158/1078-0432.CCR-08-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamamoto N, Nokihara H, Yamada Y, Goto Y, Tanioka M, Shibata T, et al. A Phase I, dose-finding and pharmacokinetic study of olaparib (AZD2281) in Japanese patients with advanced solid tumors. Cancer Sci. 2012;103:504–9. doi: 10.1111/j.1349-7006.2011.02179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samol J, Ranson M, Scott E, Macpherson E, Carmichael J, Thomas A, et al. Safety and tolerability of the poly(ADP-ribose) polymerase (PARP) inhibitor, olaparib (AZD2281) in combination with topotecan for the treatment of patients with advanced solid tumors: a phase I study. Investigational new drugs. 2012;30:1493–500. doi: 10.1007/s10637-011-9682-9. [DOI] [PubMed] [Google Scholar]
- 36.Dean E, Middleton MR, Pwint T, Swaisland H, Carmichael J, Goodege-Kunwar P, et al. Phase I study to assess the safety and tolerability of olaparib in combination with bevacizumab in patients with advanced solid tumours. Br J Cancer. 2012;106:468–74. doi: 10.1038/bjc.2011.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi SY, Koh KH, Jeong H. Isoform-specific regulation of cytochromes p450 expression by estradiol and progesterone. Drug metabolism and disposition: the biological fate of chemicals. 2013;41:263–9. doi: 10.1124/dmd.112.046276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gudas JM, Nguyen H, Li T, Cowan KH. Hormone-dependent regulation of BRCA1 in human breast cancer cells. Cancer Res. 1995;55:4561–5. [PubMed] [Google Scholar]
- 39.Buterin T, Koch C, Naegeli H. Convergent transcriptional profiles induced by endogenous estrogen and distinct xenoestrogens in breast cancer cells. Carcinogenesis. 2006;27:1567–78. doi: 10.1093/carcin/bgi339. [DOI] [PubMed] [Google Scholar]
- 40.Wang W, Dong L, Saville B, Safe S. Transcriptional activation of E2F1 gene expression by 17beta-estradiol in MCF-7 cells is regulated by NF-Y-Sp1/estrogen receptor interactions. Mol Endocrinol. 1999;13:1373–87. doi: 10.1210/mend.13.8.0323. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, Weaver DT. The ups and downs of DNA repair biomarkers for PARP inhibitor therapies. American journal of cancer research. 2011;1:301–27. [PMC free article] [PubMed] [Google Scholar]
- 42.Tutt A, Robson M, Garber JE, Domchek SM, Audeh MW, Weitzel JN, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235–44. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 43.Rendic S, Di Carlo FJ. Human cytochrome P450 enzymes: a status report summarizing their reactions, substrates, inducers, and inhibitors. Drug metabolism reviews. 1997;29:413–580. doi: 10.3109/03602539709037591. [DOI] [PubMed] [Google Scholar]
- 44.Bundred N, Gardovskis J, Jaskiewicz J, Eglitis J, Paramonov V, McCormack P, et al. Evaluation of the pharmacodynamics and pharmacokinetics of the PARP inhibitor olaparib: a Phase I multicentre trial in patients scheduled for elective breast cancer surgery. Investigational new drugs. 2013;31:949–58. doi: 10.1007/s10637-012-9922-7. [DOI] [PubMed] [Google Scholar]
- 45.Hanifi-Moghaddam P, Boers-Sijmons B, Klaassens AH, van Wijk FH, den Bakker MA, Ott MC, et al. Molecular analysis of human endometrium: short-term tibolone signaling differs significantly from estrogen and estrogen + progestagen signaling. Journal of molecular medicine. 2007;85:471–80. doi: 10.1007/s00109-006-0146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Curtin NJ, Szabo C. Therapeutic applications of PARP inhibitors: Anticancer therapy and beyond. Molecular aspects of medicine. 2013 doi: 10.1016/j.mam.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller WR, Anderson TJ, Evans DB, Krause A, Hampton G, Dixon JM. An integrated view of aromatase and its inhibition. The Journal of steroid biochemistry and molecular biology. 2003;86:413–21. doi: 10.1016/s0960-0760(03)00352-2. [DOI] [PubMed] [Google Scholar]
- 48.Ma BB, Oza A, Eisenhauer E, Stanimir G, Carey M, Chapman W, et al. The activity of letrozole in patients with advanced or recurrent endometrial cancer and correlation with biological markers--a study of the National Cancer Institute of Canada Clinical Trials Group. Int J Gynecol Cancer. 2004;14:650–8. doi: 10.1111/j.1048-891X.2004.14419.x. [DOI] [PubMed] [Google Scholar]
- 49.Rose PG, Brunetto VL, VanLe L, Bell J, Walker JL, Lee RB. A phase II trial of anastrozole in advanced recurrent or persistent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2000;78:212–6. doi: 10.1006/gyno.2000.5865. [DOI] [PubMed] [Google Scholar]
- 50.Forster MD, Dedes KJ, Sandhu S, Frentzas S, Kristeleit R, Ashworth A, et al. Treatment with olaparib in a patient with PTEN-deficient endometrioid endometrial cancer. Nat Rev Clin Oncol. 2011;8:302–6. doi: 10.1038/nrclinonc.2011.42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.