Despite decades of research on the effects of zinc in infectious diarrhea, there is surprisingly little consensus on how zinc works (or even if it works), on whether zinc’s effects are pathogen-specific, and what patient populations are most likely to benefit from zinc supplementation.1,2 Some recent articles focus on the growth-promoting effects of zinc on the pathogen itself, leading the reader to wonder if zinc supplements might make certain kinds of gastroenteritis worse, not better.3 Indeed, zinc is an essential mineral for all known forms of life, including bacteria, archea, fungi, protists, animals, and plants.
The article by Medeiros et al. in this issue of Virulence focuses on zinc and enteroaggregative E. coli (EAEC), strain 042.4 In this study, concentrations of zinc salts in the range of 0.05 to 0.2 mM inhibited several measures of EAEC virulence, including adherence to cultured host intestinal cells, formation of biofilm on polystyrene plastic, and inhibition of expression of several virulence factors, including aggR, virK, aatA, and so on. Furthermore, zinc-deficient mice challenged with EAEC orogastrically showed weight loss that was reversed by “rescue” with supplemental zinc. The study by Medeiros et al. was conducted with a single EAEC strain, and since EAEC are fairly diverse genetically, follow-up experiments with a collection of EAEC strains would help expand the generalizability of this study.
The article by Medeiros et al. on zinc and EAEC adds to the literature showing a beneficial effect of zinc on infection with diarrheagenic E. coli. For strains of enterotoxigenic E. coli (ETEC) producing the heat-labile toxin LT, there is a theoretical rationale for the use of zinc, based on zinc’s ability to inhibit the cyclic AMP-stimulated potassium channel located in the basolateral aspect of intestinal cells.5,6 Cholera toxin produced by Vibrio cholerae acts via cyclic AMP to stimulate the same K channel, which by K+ efflux maintains a negative intracellular potential in the epithelial cell, allowing chloride secretion via the apical side of the cell to be sustained.
In addition to the theoretical rationale for zinc in ETEC infection, zinc also has anti-virulence effects in enteropathogenic E. coli (EPEC) and Shiga toxigenic E. coli (STEC, also known as enterohemorrhagic E. coli, EHEC).7,8 In the case of EPEC and STEC we also have evidence that zinc supplementation is effective in vivo in rabbit infection models.
Although EPEC and STEC are quite dissimilar to EAEC in terms of genetics and pathogenesis, there is one intriguing parallel in the mode of action of zinc against all three pathogens, which is its ability to induce envelope stress in these E. coli strains.9 Figure 5E of the article by Medeiros et al. shows that degP was strongly induced in EAEC by low concentrations of zinc (0.01 to 0.05 mM). The degP protein is a dual-function protein with chaperone and protease activity involved in degradation of proteins that are misfolded in response to envelope stress. Mellies et al. showed that induction of the envelope stress response was associated with an inhibition of Type III secretion in EPEC, and others have shown that the envelope stress response downregulates other virulence factors.10-12
In his study, Mellies et al. used electron microscopy to visualize the cell surface blebbing and abnormal contour of E. coli cells that accompanied zinc-induced envelope stress. In our laboratory, we wondered if the same changes in the E. coli cells might be visible in the light microscope using structured illumination microscopy (SIM) which is one of several types of super-resolution microscopy techniques developed in the past few years.13 Figure 1 shows the effects of zinc on the size and shape of fluorescently labeled bacterial cells of EPEC strain E2348/69. After exposure to 0.4 mM zinc acetate, some EPEC bacterial cells maintained their normal shape, but many others were misshapen, appearing like deformed clubs, with a lumpy-bumpy outline or a moth-eaten appearance (asterisks in Fig. 1). Other zinc-treated bacteria took on even more bizarre, spheroidal shapes (dagger symbol in Fig. 1), which are atypical of E. coli. Clearly, the envelope stress response is worthy of further study in regard to bacterial pathogenesis.
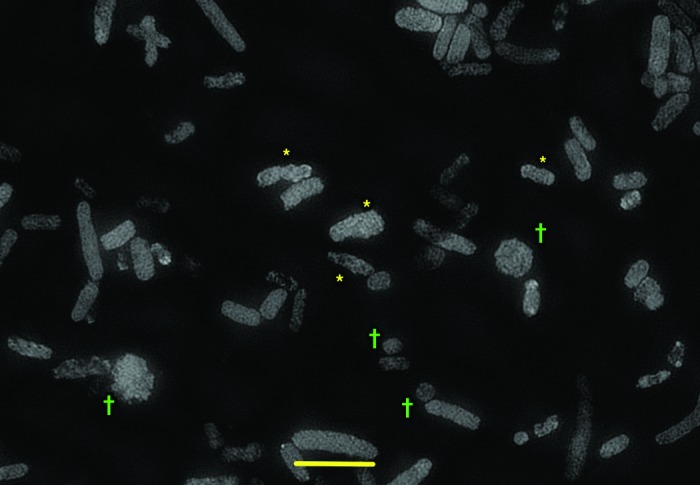
Figure 1. Visualization of the envelope stress response in Escherichia coli strain E2348/69. Classic enteropathogenic E. coli strain E2348/69 was grown in DMEM medium in the presence of 0.1 mg/L trimethoprim in order to increase the size of the E. coli cells to improve visualization. Trimethoprim alone did not cause any changes in the E. coli cell shape or contour (images not shown). After 1 h of growth, zinc acetate was added to 0.4 mM final concentration and growth was continued for 3 more h (4 h total). At this time the E. coli bacterial suspension was mixed with an equal volume of 0.2% acridine orange in ethanol and allowed to stain at room temperature for 10 min. Staining was followed by two cycles of washes consisting of centrifugation at 700 g x 10 min, followed by decanting and resuspension in water. After the second wash, 40 µl of bacterial suspension was spotted on an ordinary glass microscope slide and allowed to dry. The slides were sent to Adrian Quintanilla of the Applied Precision division of GE Healthcare, Issaquah, WA, who performed the imaging using the OMX instrument and the Structured Illumination Microscopy protocol. Color images were converted to black and white. *E. coli cells showing distorted shapes, bumpy cell outlines, and moth-eaten appearance. †E. coli cells showing spheroidal shapes. Size bar at bottom is approximately 4 µm.
The beneficial effects of zinc, however, are apparently not limited to diarrheagenic E. coli infections. Zinc oxide nanoparticles showed anti-virulence effects against Campylobacter jejuni in vitro.14,15 Zinc also seems to be effective for children with rotavirus as the etiology of the diarrheal illness.16
The emphasis on the theoretical rationale for zinc therapy for infectious diarrhea may seem like an esoteric, academic debate, but it has important public health policy implications. Zinc-oral rehydration solution (Zn-ORS) is officially recommended by the World Health Organization (WHO) for rehydration of patients with cholera, such as in the ongoing outbreaks of cholera in Haiti. Use of Zn-ORS is allowed for other etiologies of diarrhea, but use of zinc-ORS has not been encouraged in developed countries because of the misbelief that zinc is only beneficial in the setting of zinc deficiency. Diarrheal illness due to enteroaggregative E. coli infection is not uncommon even in developed countries such as the United States.17 In addition, the STEC O104:H4 strain that caused the large outbreak in Germany in May 2011, was an EAEC strain that had acquired the Stx toxin gene,18 reminding us that neglected tropical diseases can sometimes boomerang and show up in unexpected places (think also of dengue fever, West Nile virus, and chikungunya fever). The article by Medeiros et al., and others showing direct effects of zinc on pathogenic bacteria, should revive efforts to determine which pathogens are susceptible to zinc therapy, and which, if any, are not. In this context, what would be the effect of zinc against norovirus and Clostridium difficile? The latter two notorious pathogens are difficult to control and apparently on the increase in developed countries.19-21
Disclosures of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The author thanks Adrian Quintanilla of Applied Precision for performing the structured illumination microscopy and providing the images. This work was supported by NIH grant RO1 AI 81528.
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/26504
References
- 1.Patel AB, Mamtani M, Badhoniya N, Kulkarni H. What zinc supplementation does and does not achieve in diarrhea prevention: a systematic review and meta-analysis. BMC Infect Dis. 2011;11:122. doi: 10.1186/1471-2334-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel A, Mamtani M, Dibley MJ, Badhoniya N, Kulkarni H. Therapeutic value of zinc supplementation in acute and persistent diarrhea: a systematic review. PLoS One. 2010;5:e10386. doi: 10.1371/journal.pone.0010386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gielda LM, DiRita VJ. Zinc competition among the intestinal microbiota. MBio. 2012;3:e00171–12. doi: 10.1128/mBio.00171-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medeiros P, Bolick DT, Roche JK, Noronha F, Pinheiro C, Kolling GL, Lima A, Guerrant RL. The micronutrient zinc inhibits EAEC strain 042 adherence, biofilm formation, virulence gene expression, and epithelial cytokine responses benefiting the infected host. Virulence. 2013;4:624–33. doi: 10.4161/viru.26120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoque KM, Rajendran VM, Binder HJ. Zinc inhibits cAMP-stimulated Cl secretion via basolateral K-channel blockade in rat ileum. Am J Physiol Gastrointest Liver Physiol. 2005;288:G956–63. doi: 10.1152/ajpgi.00441.2004. [DOI] [PubMed] [Google Scholar]
- 6.Canani RB, Cirillo P, Buccigrossi V, Ruotolo S, Passariello A, De Luca P, Porcaro F, De Marco G, Guarino A. Zinc inhibits cholera toxin-induced, but not Escherichia coli heat-stable enterotoxin-induced, ion secretion in human enterocytes. J Infect Dis. 2005;191:1072–7. doi: 10.1086/428504. [DOI] [PubMed] [Google Scholar]
- 7.Crane JK, Byrd IW, Boedeker EC. Virulence inhibition by zinc in shiga-toxigenic Escherichia coli. Infect Immun. 2011;79:1696–705. doi: 10.1128/IAI.01099-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crane JK, Naeher TM, Shulgina I, Zhu C, Boedeker EC. Effect of zinc in enteropathogenic Escherichia coli infection. Infect Immun. 2007;75:5974–84. doi: 10.1128/IAI.00750-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mellies JL, Thomas K, Turvey M, Evans NR, Crane J, Boedeker EC, Benison GC. Zinc-induced envelope stress diminishes type III secretion in enteropathogenic Escherichia coli. BMC Microbiol. 2012;12:123. doi: 10.1186/1471-2180-12-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leuko S, Raivio TL. Mutations that impact the enteropathogenic Escherichia coli Cpx envelope stress response attenuate virulence in Galleria mellonella. Infect Immun. 2012;80:3077–85. doi: 10.1128/IAI.00081-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macritchie DM, Ward JD, Nevesinjac AZ, Raivio TL. Activation of the Cpx envelope stress response down-regulates expression of several locus of enterocyte effacement-encoded genes in enteropathogenic Escherichia coli. Infect Immun. 2008;76:1465–75. doi: 10.1128/IAI.01265-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vogt SL, Raivio TL. Just scratching the surface: an expanding view of the Cpx envelope stress response. FEMS Microbiol Lett. 2012;326:2–11. doi: 10.1111/j.1574-6968.2011.02406.x. [DOI] [PubMed] [Google Scholar]
- 13.Ehrenberg R. View to a Cell. Sci News. 2013;183:21–7. doi: 10.1002/scin.5591831217. [DOI] [Google Scholar]
- 14.Bratz K, Gölz G, Riedel C, Janczyk P, Nöckler K, Alter T. Inhibitory effect of high-dosage zinc oxide dietary supplementation on Campylobacter coli excretion in weaned piglets. J Appl Microbiol. 2013 doi: 10.1111/jam.12307. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 15.Xie Y, He Y, Irwin PL, Jin T, Shi X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl Environ Microbiol. 2011;77:2325–31. doi: 10.1128/AEM.02149-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel AB, Dibley MJ, Mamtani M, Badhoniya N, Kulkarni H. Influence of zinc supplementation in acute diarrhea differs by the isolated organism. Int J Pediatr. 2010;2010:671587. doi: 10.1155/2010/671587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen MB, Nataro JP, Bernstein DI, Hawkins J, Roberts N, Staat MA. Prevalence of diarrheagenic Escherichia coli in acute childhood enteritis: a prospective controlled study. J Pediatr. 2005;146:54–61. doi: 10.1016/j.jpeds.2004.08.059. [DOI] [PubMed] [Google Scholar]
- 18.Buchholz U, Bernard H, Werber D, Böhmer MM, Remschmidt C, Wilking H, Deleré Y, an der Heiden M, Adlhoch C, Dreesman J, et al. German outbreak of Escherichia coli O104:H4 associated with sprouts. N Engl J Med. 2011;365:1763–70. doi: 10.1056/NEJMoa1106482. [DOI] [PubMed] [Google Scholar]
- 19.Estes MK, Prasad BV, Atmar RL. Noroviruses everywhere: has something changed? Curr Opin Infect Dis. 2006;19:467–74. doi: 10.1097/01.qco.0000244053.69253.3d. [DOI] [PubMed] [Google Scholar]
- 20.Freeman J, Bauer MP, Baines SD, Corver J, Fawley WN, Goorhuis B, Kuijper EJ, Wilcox MH. The changing epidemiology of Clostridium difficile infections. Clin Microbiol Rev. 2010;23:529–49. doi: 10.1128/CMR.00082-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ricciardi R, Rothenberger DA, Madoff RD, Baxter NN. Increasing prevalence and severity of Clostridium difficile colitis in hospitalized patients in the United States. Arch Surg. 2007;142:624–31, discussion 631. doi: 10.1001/archsurg.142.7.624. [DOI] [PubMed] [Google Scholar]


