Abstract
Clathrin-independent endocytosis (CIE) mediates the internalization of many plasma membrane (PM) proteins involved in homeostasis, immune response, and signaling. CIE cargo molecules are internalized independent of clathrin, and dynamin, and modulated by the small G protein Arf6. After internalization the CIE cargo proteins either follow a default pathway of trafficking to lysosomes for degradation or follow a pathway where they are routed directly to the recycling endosomes for return to the PM. The selective endosomal sorting of molecules like CD44, CD98, and CD147, which are involved in cell-cell and cell-extracellular interactions, indicates that sorting mechanisms dictate the post-endocytic fate of CIE cargo proteins. In a recent study, we identified sorting signals that specify the endosomal trafficking of CIE cargo proteins and uncover a role for Hook1 as an endosomal cargo adaptor that routes CIE cargo to the recycling endosomes. Furthermore, we found that Hook1, microtubules, and Rab22a work in coordination to directly recycle the cargo and facilitate cell spreading. Here, we discuss our current view on the endosomal sorting of CIE cargo proteins and their molecular regulators.
Keywords: clathrin-independent endocytosis, Hook1, Rab22a, Rab22, microtubules, endosomal sorting, sorting signals, recycling, basigin, CD147
Introduction
Endocytosis is an essential process required by all eukaryotic cells for the maintenance of homeostasis and signaling regulation. Through this process cells internalize plasma membrane (PM) lipids and proteins, as well as nutrients, through the formation of vesicles that are excised from the PM into the cytoplasm. The cargo-loaded vesicles are delivered to early endosomes, and then to a degradation compartment or recycled back to the PM. Eukaryotic cells have engineered different modes of endocytosis that allow them to efficiently control the composition of the PM, downregulate signaling receptors, regulate cell migration and protein turnover.1
Endocytosis can be classified into two major classes based on their requirements for the clathrin coat for internalization. Clathrin-mediated endocytosis (CME) facilitates the internalization of PM proteins that harbor internalization signals in their cytoplasmic tails, which are recognized by clathrin-endocytic adaptors.1,2 These adaptors concentrate cargo proteins, clathrin, and other components at sites of endocytosis. The G protein dynamin then completes the severing of the coated endocytic vesicle. Once internalized, CME vesicles fuse with the classical early-endocytic endosomes from which the cargo proteins are directed to late endosomes for degradation or recycled back to the PM. On the other hand, clathrin-independent endocytosis (CIE) facilitates the internalization of integral membrane proteins devoid of clathrin-adaptor targeting sequences. CIE mechanisms do not require clathrin or any other coat to facilitate the internalization of the cargo proteins.3-6 CIE mechanisms are fundamental for many physiological processes, such as immune surveillance, cell signaling, cell migration, metastasis, and wound healing.
Our group has characterized a form of CIE that is associated with the small GTP-binding protein Arf6 that is independent of dynamin (Fig. 1).7-9 The conservation of the Arf6-associated CIE pathway has been supported by its prevalence in a variety of human cell lines, and in the model organism Caenorhabditis elegans.8,10 Significant progress has been made in our understanding of this form of CIE by studying the endocytic event and endosomal trafficking of endogenous proteins that use CIE as the mode of entry. The pool of CIE cargo proteins is very diverse including the major histocompatibility complex class I (MHCI), the α chain of the IL-2 receptor (Tac), glycosylphosphatidylinositol anchored proteins (GPI-APs), proteins involved in interactions with extracellular matrix (CD44, CD98, CD147, and β-integrins), ion channels (Mucolipin 2 and Kir3.4) and nutrient transporters (CD98 and Lat1).11 CIE cargo proteins enter the cells through vesicular structures associated with Arf6. Then, the CIE cargo-loaded vesicles mature into or fuse with Rab5 positive-early endosomes. Interestingly, from these endosomes the CIE cargo proteins segregate and take different trafficking routes to their final destinations.9,12,13 A group of CIE cargo proteins (MHCI, CD59, and Tac) travels along the typical CIE route, which is characterized by localization of the cargo with Rab5- and EEA1- positive endosomes, followed by co-localization with transferrin. From there the cargo is either degraded in late endosomes or recycled back to the PM.9,12 Another group of CIE cargo proteins (CD44, CD98, and CD147) avoids going to EEA1-positive endosomes and meeting with transferrin receptor, a CME cargo protein. Instead, it is directly recycled back to the PM in a process dependent on another G protein Rab22a.14,15 The ability to be sorted out of early endosomes and be directly targeted to recycling endosomes confers on these proteins a prolonged surface half-life of more than 24 h, whereas MHCI and Tac have a half-life of approximately 4 h in HeLa cells.16
Figure 1. General model for the endosomal sorting of CIE cargo proteins. CIE cargo proteins are internalized by a pathway independent of clathrin, dynamin and associated with the small G protein Arf6. Prototypical CIE cargo proteins (red bars) enter the cell in Arf6-positive endocytic vesicles that either fuse with or mature into Rab5-, EEA1- and transferrin- positive endosomes. Then, the cargo proteins are either targeted to late endosomes (LE) for degradation or recycled back to the PM in a Rab22/Rab11 dependent manner. CIE cargo proteins harboring cytoplasmic sorting motifs (green bars) avoid trafficking to EEA1- and transferrin- (TfR/Tf; black bars) associated endosomes and traffic directly to the recycling endosomes (RE). Hook1 facilitates the directed recycling of CIE cargo proteins, such as CD98 and CD147, through its interaction with microtubules and their cytoplasmic sequences on sorting endosomes.
The endosomal segregation of these CIE cargo proteins suggests that the process is not random and that sorting determinants may dictate their fate. Uncovering the mechanisms that govern the sorting of CIE cargo proteins will be crucial to understand regulatory aspects of CIE, including modes of cargo selection at the PM and at endosomes, determinants of the intracellular fate of the proteins and the spatial-temporal regulation of the CIE machinery. In our most recent work we uncovered cytoplasmic sorting signals responsible for the endosomal segregation and recycling of specific CIE cargo proteins.17 Moreover, we have identified Hook1 as an adaptor-tethering factor involved in the recycling of CIE cargo proteins through its interaction with cargo and microtubules.
CIE Endosomal Sorting Signals Provide Access to the Recycling Endosome
Sorting signals for the specific internalization of PM proteins have been extensively documented for CME cargo proteins in mammalian and yeast cells. Specifically, CME adaptors use short peptide motifs and/or post-transcriptional modifications to facilitate the selective internalization of proteins and their intracellular trafficking.18 The absence of known internalization signals in the cytoplasmic tail of CIE cargo proteins and the lack of specificity at the PM argues against the idea of this being a selective process like CME. Historically, CIE has been referred as “bulk endocytosis” of PM proteins that do not fulfill the requirements necessary to enter through CME.6,19 Currently, our knowledge about how CIE internalization takes place is very limited. Evidence shows that most CIE cargo proteins associate with lipid rafts and their internalization is cholesterol–dependent.7,9,12,20 However, mechanisms for membrane deformation, vesicle formation and scission of CIE cargo-loaded vesicles, remain to be elucidated.
Although, CIE cargo proteins do not have common cytoplasmic sequence information, the observation that they become segregated on endosomes and routed to different compartments suggested that a selective process takes place after internalization. This hypothesis led us to investigate whether CIE cargo proteins that directly recycle back to the PM and avoid lysosomal degradation had post-endocytic sorting information that would specifically facilitate their endosomal segregation. In our recent study, we found that indeed the cytoplasmic tails of the CIE cargo proteins CD44, CD98 and CD147, harbor sorting information that determines their preference for the CIE recycling route.17 Mutagenesis analysis of the CD147 cytoplasmic sequence revealed that two clusters of acidic amino acids are necessary for the trafficking of CD147 away from EEA1-associated endosomes. Normally CD147 will start to accumulate in a Lamp1-associated compartment after a 24 h chase.16 On the other hand, when the acidic clusters are mutated to alanine we observed CD147 accumulating in Lamp1-endosomes as early as 4 h, similar to CIE cargo proteins that follow the degradation route. The two acidic clusters are highly conserved among the different species that express CD147, which is also known as EMMPRIN or Basigin. Cytoplasmic acidic clusters are also observed in CD44 and CD98. Importantly, CIE cargo proteins that preferentially follow the default degradation route of the pathway are characterized by the lack of cytoplasmic di-acidic or acidic cluster residues (MHCI, Tac and GPI-APs).
Acidic clusters have been implicated in the endocytic trafficking of two other CIE cargo proteins; the rectifying K channel, Kir3.4 and the lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1). The KAC motif, which is found in the cytoplasmic sequence of Kir3.4, consists of two different acidic residue arrangements, one containing the residues DEEEE and the other consisting of acidic and non-acidic residues organized in an alternate fashion (EAEKEAEAEH).21 Mutation of the KAC motif reduces the surface levels of the Kir3.4 channel and causes its accumulation in internal endosome-like structures. Moreover, these acidic motifs are necessary for the localization of the channel to the recycling endosomes, raising the possibility that these residues are involved in sorting Kir3.4 to the CIE recycling route. Further studies have to be conducted in order to decipher the specific role of the KAC motifs and to determine if Kir3.4 follows the same endocytic trafficking itinerary as CD44, CD98 and CD147. In the case of the LOX-1 receptor, the cytoplasmic acidic motif (DDL) serves as a signal for internalization through a clathrin-independent mechanism.22 Mutation of this tri-peptide motif impaired the constitutive internalization of the LOX-1 receptor and also the uptake of its ligand in HeLa cells.
The current evidence in the field indicates that acidic motifs could play multiple roles in the endocytic- and post-endocytic trafficking of CIE cargo.11 It is also possible that the acidic motifs function in coordination with other trafficking motifs to regulate the trafficking of specific cargo proteins. For instance, CD44, Glut1 and Syndecan-2, all CIE cargo proteins, contain a PDZ-ligand domain in their cytoplasmic sequences that could potentially be involved in their endosomal trafficking.23-25 The diversity governing the cytoplasmic sequences of CIE cargo provides to this pathway the ability to traffic multiple classes of PM proteins. It will be interesting to see how different sorting signals control the temporal and spatial distribution of CIE cargo proteins along the endosomal system.
Regulating the Endosomal Sorting of CIE Cargo Proteins
The protein-protein and protein-lipid interactions that mediate the endosomal sorting of CIE cargo proteins remain to be elucidated. Our most recent work identified Hook1 as the first sorting factor that binds to the cytoplasmic sequence of the CIE cargo proteins CD98 and CD147 and facilitates their recycling.17 Hook1 works together with Rab22a and the microtubule network to coordinate the recycling of CIE cargo proteins.
Hook1 Sorts CIE Cargo Proteins
Hook1 associates with microtubules through its N-terminal domain and to the cargo through its carboxyl-terminal region. Hook1 colocalizes with the CIE cargo proteins in endosomes devoid of EEA1 and in the tubular recycling endosomes also decorated with Rab22a (Fig. 2). Although the interaction of Hook1 with the cargo is independent of its association with the microtubules, both interactions are necessary for the segregation and direct recycling of the cargo. The perturbation of the microtubule network with nocodazole results in loss of endosomal segregation of all CIE cargo proteins and the accumulation of Hook1 and the cargo in scattered endosomes containing EEA1 and the trasferrin receptor. Therefore, the endosomal sorting of CIE cargo molecules away from a degradation route depends on an intact microtubule network and factors that connect the cargo-containing membranes to it.
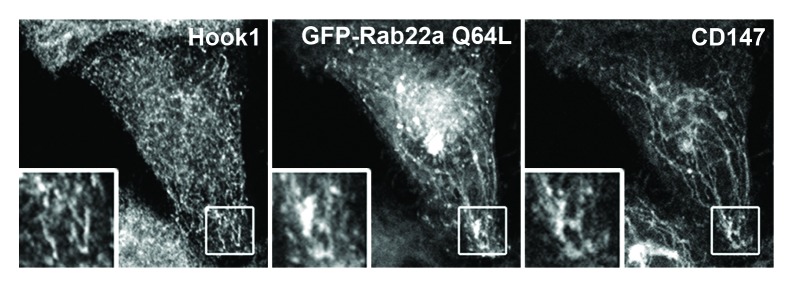
Figure 2. Hook1 and Rab22a colocalize with CIE cargo on recycling tubular endosomes. HeLa cells expressing endogenous levels of Hook1 and overexpressing the constitutively active Rab22a mutant (GFP-Rab22a Q64L) were incubated with anti-CD147 antibody for 30 min at 37°C to allow internalization of bound antibodies. Hook1, Rab22a and CD147 meet on puncta and tubular endosomal structures after internalization.
Depletion of Hook1 or the overexpression of the Hook1 C-terminal sequence (Hook1 dominant negative) affects the composition of the CIE endosomal system. These conditions affect the recycling of only CIE cargo proteins that avoid the default route to EEA1-associated endosomes and lysosomes (CD44, CD98, and CD147) and not that of MHCI and the transferrin receptor. Also, these treatments cause the fragmentation or loss of the recycling endosomes. These observations suggest that a continual segregation of CIE cargo-containing membranes is necessary to maintain the CIE recycling compartment. Altogether, our results led us to propose that Hook1 functions as an adaptor and tethering factor that selectively sorts CD98 and CD147 on endosomes to prevent their routing to the degradation route.
Hook1 was originally discovered in Drosophila melanogaster as an important factor for the delivery of PM receptors to late endosomal compartments.26,27 In mammals, three members of the Hook protein family have been described, defined as Hook1, 2, and 3. Hook2 localizes to the Golgi and centrosomes and plays a role in ciliogenesis.28,29 Hook3 also localizes to the Golgi and unidentified vesicular structures.30 Interestingly, Hook3 interacts with the cytoplasmic domain of the macrophage scavenger receptor A (SR-A).31 SR-A is a type II transmembrane protein that enters the cell trough CME.32,33 A region in the carboxyl-terminal domain of Hook3 that is enriched in basic amino acids and acidic residues in the cytoplasmic domain of SR-A mediate the interaction between these two proteins.31 We observed a similar relationship between Hook1 and CD147 in a direct yeast-two hybrid assay. Thus, Hook proteins may serve to coordinate the turnover of proteins after internalization using similar recognition mechanisms.
A Hook-related protein family (HkRP), has also been identified and is involved in the trafficking of the EGFR back to the PM.34 The HkRPs share with Hook proteins the conserved N-terminal microtubule association domain N-terminal domain sequence. Both families share the same domain organization, starting with the microtubule-association domain, followed by a coiled-coil region and the membrane/cargo-association domain at the carboxyl-terminus. The carboxyl-terminus of HkRP harbors a conserved HkRP domain, which is responsible for the membrane association of the protein. The cytoplasmic sequence of HkRP1 localizes to endosomal structures devoid of EEA1, Lamp1 or Golgi markers. However, the authors showed that under low levels of expression the cytoplasmic sequence of HkRP1 colocalizes with the sorting nexin 1 (SNX1). In contrast, expressing high levels of the carboxyl-terminal of HkRP causes the redistribution of SNX1 to the cytosol and loss of the EGFR from the plasma membrane. These findings further support a role for Hook and Hook-related proteins in the regulation of cargo recycling from early endosomes.
Hook1 interacts with components of the HOPs complex and the ubiquitinylation system, suggesting that Hook1 may function in coordination with other protein complexes to regulate protein sorting.35,36 It is not clear whether the Hook proteins initially recognize the cargo at the PM or if the interaction takes place after the cargo have reached the early endosomes. Furthermore, the conditions required to establish the interaction still remain to be uncovered.
Hook1 and Rab22 Sort CIE Cargo Protein into Recycling Endosomes
Hook1 works in coordination with microtubules and Rab22a to regulate the recycling of CIE cargo proteins back to the PM.17 Moreover, our work revealed that Rab22a depletion impairs the sorting and recycling of all CIE cargo proteins. These observations put Rab22a as a central component of the CIE sorting machinery. siRNA-mediated depletion of Rab22a or the expression of the constitutively inactive mutant form of Rab22a (Rab22a S19N) impair the recycling of CIE membranes by affecting the formation of the recycling endosomes and inhibiting its fusion with the PM.15,17 Intriguingly, overexpression of Hook1 rescues the Rab22a S19N dominant negative phenotype. Similarly, overexpression of Rab22a rescues the Hook1 dominant negative effect by restoring the endosomal sorting of CIE cargo. Although, we did not observe a physical interaction between Hook1 and Rab22a, the reciprocal rescue of their respective dominant negative phenotypes and their colocalization on CIE-cargo containing membranes (Fig. 2) suggest that both sorting factors work at the same step to control the direct recycling of CIE cargo proteins from early endosomes.
It is possible that Hook1 and Rab22a share a common interacting partner or that they interact with components of a sorting complex necessary to complete the segregation and recycling step of the pathway. Cytosolic proteins that could potentially fulfill this role are the Rab effectors, which modulate the downstream effects of activated Rab proteins by recruiting them to specific membranes, creating defined membrane domains on endosomes and recruiting other effectors.37 Even though the list of Rab effectors has drastically increased in the past ten years, little is known about specific effectors regulating the function of Rab proteins on CIE membranes.
Rab proteins and Rab effectors could also interact with scaffold proteins that bring cargo and sorting machinery together in specific domains of the sorting endosomes.37,38 Recently, it has been shown that Hook2 interacts with pericentriolar material protein 1 (PCM1), which is a Rab8a effector critical for the recruitment of Rab8 to primary cilium.28 Hook2 brings PCM1 to the base of the primary cilium. In turn, PCM1 activates Rab8a at that location to complete the maturation event during ciliogenesis. The absence of Hook2 or inactivation of Rab8 causes the arrest of ciliogenesis at a very early step. The coordinated function of Rab8a and Hook2 is reminiscent of the Hook1/Rab22a functional interaction. Therefore, it is tempting to speculate that Hook1 could be mediating the establishment of a specialized domain on the sorting endosomes for the action of Rab22a. This could be achieved through the recruitment of a Rab22a effector or a scaffold protein responsible to bring other sorting components to a specific membrane domain. Although, Hook1 interacts in vitro with other Rab proteins (Rab7, Rab9 and Rab11), the physiological significance and role of these interactions in vivo have not been examined.39 Alternatively, Hook1 and Rab22 might interact with a microtubule motor protein to facilitate the microtubule-based transport of the CIE cargo proteins back to the PM.
Endosomal Sorting of CIE Cargo Proteins Includes Multiple Sorting Factors
Considering the diversity of CIE cargo proteins and their unique cytoplasmic sequence composition it is conceivable to predict that multiple adaptors mediate the sorting of specific CIE cargo proteins after internalization. In agreement with this view, a recent study by Steinberg et al. revealed that the sorting nexin 27 (SNX27) in coordination with the VPS26-VPS29-VPS35 retromer trimer, performs a major role in the recycling of PM membrane proteins from endosomal compartments.40 Interestingly, depletion of SNX27 reduced the surface levels of known CIE cargo proteins (for example Glut1, CD147, and other ion and nutrient transporters) by deviating their trafficking to lysosomes. The authors also demonstrated that the PDZ-domain/PDZ-ligand interaction between SNX27 and Glut1 was required to maintain surface levels of the transporter and prevent its premature delivery to lysosomes. Whether SNX27 regulates the sorting of other CIE cargo proteins through the same PDZ-domain mediate interactions remains to be determined. However, the new role for the retromer in the sorting and recycling of multiple CIE membrane proteins emphasizes the versatile character of the CIE endosomal sorting network.
Additionally, other regulatory factors may contribute to the spatial and temporal-regulation of CIE cargo recycling. For instance, other Rab proteins localize to CIE endosomal membranes. In particular, Rab11 has been shown to be required for the recycling of MHCI although it is not necessary for the formation of the recycling endosomes.15 The EPS15 homology domain-containing (EHD) proteins EHD1, MICAL-L1 and phospholipase A2 function together to facilitate the endosomal trafficking and recycling of the GPI-APs, CD55 and CD59.41,42 The field of CIE waits for more studies to reveal the underlining mechanisms that help orchestrate the different components of this complex sorting system.
Summary and Perspectives
The intracellular sorting mechanisms controlling the segregation of CIE cargo proteins could be cell specific or perhaps be determined by the cargo’s physiological function. We have previously shown that inactivation of Arf6 blocks the recycling of CIE membranes, which in turn inhibits cell spreading in HeLa cells.43 Our recent work underscores the importance of CIE membrane recycling for cell spreading by showing that Hook1 depleted cells have a delayed cell spreading phenotype. Specifically, we believe that cells require the recycling back to the PM of proteins like CD44, CD98 and CD147 to spread, adhere or migrate in response to developmental or environmental cues. These CIE cargo proteins have been extensively characterized as factors involved in cell-cell interactions and interactions with the extra-cellular matrix.11,44-46 Increased surface levels of these CIE cargo proteins are a common feature of several types of cancer.11 Due to their roles in cancer metastasis and cell migration, CD44 and CD147 have become therapeutic targets for cancer treatment and prevention.47 The sorting of cargo proteins involved in nutrient and ion transport may be modulated by environmental or growth conditions. It will be interesting to see whether starvation conditions could cause a change in the intracellular trafficking of proteins like Glut1, CD98 and Lat1. Further studies focusing on identifying the molecular players that control CIE will provide insight into the mechanisms that control the diverse trafficking itineraries followed by CIE cargo proteins and their impacts on cell physiology.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/BioArchitecture/article/26638
References
- 1.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 2.Traub LM. Tickets to ride: selecting cargo for clathrin-regulated internalization. Nat Rev Mol Cell Biol. 2009;10:583–96. doi: 10.1038/nrm2751. [DOI] [PubMed] [Google Scholar]
- 3.Howes MT, Mayor S, Parton RG. Molecules, mechanisms, and cellular roles of clathrin-independent endocytosis. Curr Opin Cell Biol. 2010;22:519–27. doi: 10.1016/j.ceb.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8:603–12. doi: 10.1038/nrm2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandvig K, Pust S, Skotland T, van Deurs B. Clathrin-independent endocytosis: mechanisms and function. Curr Opin Cell Biol. 2011;23:413–20. doi: 10.1016/j.ceb.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Prosser DC, Drivas TG, Maldonado-Báez L, Wendland B. Existence of a novel clathrin-independent endocytic pathway in yeast that depends on Rho1 and formin. J Cell Biol. 2011;195:657–71. doi: 10.1083/jcb.201104045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown FD, Rozelle AL, Yin HL, Balla T, Donaldson JG. Phosphatidylinositol 4,5-bisphosphate and Arf6-regulated membrane traffic. J Cell Biol. 2001;154:1007–17. doi: 10.1083/jcb.200103107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donaldson JG, Porat-Shliom N, Cohen LA. Clathrin-independent endocytosis: a unique platform for cell signaling and PM remodeling. Cell Signal. 2009;21:1–6. doi: 10.1016/j.cellsig.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naslavsky N, Weigert R, Donaldson JG. Convergence of non-clathrin- and clathrin-derived endosomes involves Arf6 inactivation and changes in phosphoinositides. Mol Biol Cell. 2003;14:417–31. doi: 10.1091/mbc.02-04-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balklava Z, Pant S, Fares H, Grant BD. Genome-wide analysis identifies a general requirement for polarity proteins in endocytic traffic. Nat Cell Biol. 2007;9:1066–73. doi: 10.1038/ncb1627. [DOI] [PubMed] [Google Scholar]
- 11.Maldonado-Báez L, Williamson C, Donaldson JG. Clathrin-independent endocytosis: A cargo-centric view. Exp Cell Res. 2013 doi: 10.1016/j.yexcr.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naslavsky N, Weigert R, Donaldson JG. Characterization of a nonclathrin endocytic pathway: membrane cargo and lipid requirements. Mol Biol Cell. 2004;15:3542–52. doi: 10.1091/mbc.E04-02-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radhakrishna H, Donaldson JG. ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J Cell Biol. 1997;139:49–61. doi: 10.1083/jcb.139.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eyster CA, Higginson JD, Huebner R, Porat-Shliom N, Weigert R, Wu WW, Shen RF, Donaldson JG. Discovery of new cargo proteins that enter cells through clathrin-independent endocytosis. Traffic. 2009;10:590–9. doi: 10.1111/j.1600-0854.2009.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weigert R, Yeung AC, Li J, Donaldson JG. Rab22a regulates the recycling of membrane proteins internalized independently of clathrin. Mol Biol Cell. 2004;15:3758–70. doi: 10.1091/mbc.E04-04-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eyster CA, Cole NB, Petersen S, Viswanathan K, Früh K, Donaldson JG. MARCH ubiquitin ligases alter the itinerary of clathrin-independent cargo from recycling to degradation. Mol Biol Cell. 2011;22:3218–30. doi: 10.1091/mbc.E10-11-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maldonado-Báez L, Cole NB, Krämer H, Donaldson JG. Microtubule-dependent endosomal sorting of clathrin-independent cargo by Hook1. J Cell Biol. 2013;201:233–47. doi: 10.1083/jcb.201208172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonifacino JS, Lippincott-Schwartz J. Coat proteins: shaping membrane transport. Nat Rev Mol Cell Biol. 2003;4:409–14. doi: 10.1038/nrm1099. [DOI] [PubMed] [Google Scholar]
- 19.Hansen CG, Nichols BJ. Molecular mechanisms of clathrin-independent endocytosis. J Cell Sci. 2009;122:1713–21. doi: 10.1242/jcs.033951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabharanjak S, Sharma P, Parton RG, Mayor S. GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev Cell. 2002;2:411–23. doi: 10.1016/S1534-5807(02)00145-4. [DOI] [PubMed] [Google Scholar]
- 21.Gong Q, Weide M, Huntsman C, Xu Z, Jan LY, Ma D. Identification and characterization of a new class of trafficking motifs for controlling clathrin-independent internalization and recycling. J Biol Chem. 2007;282:13087–97. doi: 10.1074/jbc.M700767200. [DOI] [PubMed] [Google Scholar]
- 22.Twigg MW, Freestone K, Homer-Vanniasinkam S, Ponnambalam S. The LOX-1 Scavenger Receptor and Its Implications in the Treatment of Vascular Disease. Cardiol Res Pract. 2012;2012:632408. doi: 10.1155/2012/632408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bunn RC, Jensen MA, Reed BC. Protein interactions with the glucose transporter binding protein GLUT1CBP that provide a link between GLUT1 and the cytoskeleton. Mol Biol Cell. 1999;10:819–32. doi: 10.1091/mbc.10.4.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thorne RF, Legg JW, Isacke CM. The role of the CD44 transmembrane and cytoplasmic domains in co-ordinating adhesive and signalling events. J Cell Sci. 2004;117:373–80. doi: 10.1242/jcs.00954. [DOI] [PubMed] [Google Scholar]
- 25.Zimmermann P, Zhang Z, Degeest G, Mortier E, Leenaerts I, Coomans C, Schulz J, N’Kuli F, Courtoy PJ, David G. Syndecan recycling [corrected] is controlled by syntenin-PIP2 interaction and Arf6. Dev Cell. 2005;9:377–88. doi: 10.1016/j.devcel.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Krämer H, Phistry M. Mutations in the Drosophila hook gene inhibit endocytosis of the boss transmembrane ligand into multivesicular bodies. J Cell Biol. 1996;133:1205–15. doi: 10.1083/jcb.133.6.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sunio A, Metcalf AB, Krämer H. Genetic dissection of endocytic trafficking in Drosophila using a horseradish peroxidase-bride of sevenless chimera: hook is required for normal maturation of multivesicular endosomes. Mol Biol Cell. 1999;10:847–59. doi: 10.1091/mbc.10.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baron Gaillard CL, Pallesi-Pocachard E, Massey-Harroche D, Richard F, Arsanto JP, Chauvin JP, Lecine P, Krämer H, Borg JP, Le Bivic A. Hook2 is involved in the morphogenesis of the primary cilium. Mol Biol Cell. 2011;22:4549–62. doi: 10.1091/mbc.E11-05-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szebenyi G, Hall B, Yu R, Hashim AI, Krämer H. Hook2 localizes to the centrosome, binds directly to centriolin/CEP110 and contributes to centrosomal function. Traffic. 2007;8:32–46. doi: 10.1111/j.1600-0854.2006.00511.x. [DOI] [PubMed] [Google Scholar]
- 30.Walenta JH, Didier AJ, Liu X, Krämer H. The Golgi-associated hook3 protein is a member of a novel family of microtubule-binding proteins. J Cell Biol. 2001;152:923–34. doi: 10.1083/jcb.152.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sano H, Ishino M, Krämer H, Shimizu T, Mitsuzawa H, Nishitani C, Kuroki Y. The microtubule-binding protein Hook3 interacts with a cytoplasmic domain of scavenger receptor A. J Biol Chem. 2007;282:7973–81. doi: 10.1074/jbc.M611537200. [DOI] [PubMed] [Google Scholar]
- 32.Goldstein JL, Ho YK, Basu SK, Brown MS. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979;76:333–7. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kosswig N, Rice S, Daugherty A, Post SR. Class A scavenger receptor-mediated adhesion and internalization require distinct cytoplasmic domains. J Biol Chem. 2003;278:34219–25. doi: 10.1074/jbc.M303465200. [DOI] [PubMed] [Google Scholar]
- 34.Simpson F, Martin S, Evans TM, Kerr M, James DE, Parton RG, Teasdale RD, Wicking C. A novel hook-related protein family and the characterization of hook-related protein 1. Traffic. 2005;6:442–58. doi: 10.1111/j.1600-0854.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- 35.Richardson SC, Winistorfer SC, Poupon V, Luzio JP, Piper RC. Mammalian late vacuole protein sorting orthologues participate in early endosomal fusion and interact with the cytoskeleton. Mol Biol Cell. 2004;15:1197–210. doi: 10.1091/mbc.E03-06-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu L, Sowa ME, Chen J, Li X, Gygi SP, Harper JW. An FTS/Hook/p107(FHIP) complex interacts with and promotes endosomal clustering by the homotypic vacuolar protein sorting complex. Mol Biol Cell. 2008;19:5059–71. doi: 10.1091/mbc.E08-05-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci U S A. 2006;103:11821–7. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–17. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 39.Luiro K, Yliannala K, Ahtiainen L, Maunu H, Järvelä I, Kyttälä A, Jalanko A. Interconnections of CLN3, Hook1 and Rab proteins link Batten disease to defects in the endocytic pathway. Hum Mol Genet. 2004;13:3017–27. doi: 10.1093/hmg/ddh321. [DOI] [PubMed] [Google Scholar]
- 40.Steinberg F, Gallon M, Winfield M, Thomas EC, Bell AJ, Heesom KJ, Tavaré JM, Cullen PJ. A global analysis of SNX27-retromer assembly and cargo specificity reveals a function in glucose and metal ion transport. Nat Cell Biol. 2013;15:461–71. doi: 10.1038/ncb2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cai B, Caplan S, Naslavsky N. cPLA2α and EHD1 interact and regulate the vesiculation of cholesterol-rich, GPI-anchored, protein-containing endosomes. Mol Biol Cell. 2012;23:1874–88. doi: 10.1091/mbc.E11-10-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai B, Katafiasz D, Horejsi V, Naslavsky N. Pre-sorting endosomal transport of the GPI-anchored protein, CD59, is regulated by EHD1. Traffic. 2011;12:102–20. doi: 10.1111/j.1600-0854.2010.01135.x. [DOI] [PubMed] [Google Scholar]
- 43.Song J, Khachikian Z, Radhakrishna H, Donaldson JG. Localization of endogenous ARF6 to sites of cortical actin rearrangement and involvement of ARF6 in cell spreading. J Cell Sci. 1998;111:2257–67. doi: 10.1242/jcs.111.15.2257. [DOI] [PubMed] [Google Scholar]
- 44.Cantor JM, Ginsberg MH. CD98 at the crossroads of adaptive immunity and cancer. J Cell Sci. 2012;125:1373–82. doi: 10.1242/jcs.096040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iacono KT, Brown AL, Greene MI, Saouaf SJ. CD147 immunoglobulin superfamily receptor function and role in pathology. Exp Mol Pathol. 2007;83:283–95. doi: 10.1016/j.yexmp.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zöller M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer. 2011;11:254–67. doi: 10.1038/nrc3023. [DOI] [PubMed] [Google Scholar]
- 47.Hao JL, Cozzi PJ, Khatri A, Power CA, Li Y. CD147/EMMPRIN and CD44 are potential therapeutic targets for metastatic prostate cancer. Curr Cancer Drug Targets. 2010;10:287–306. doi: 10.2174/156800910791190193. [DOI] [PubMed] [Google Scholar]



