Abstract
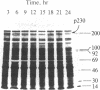
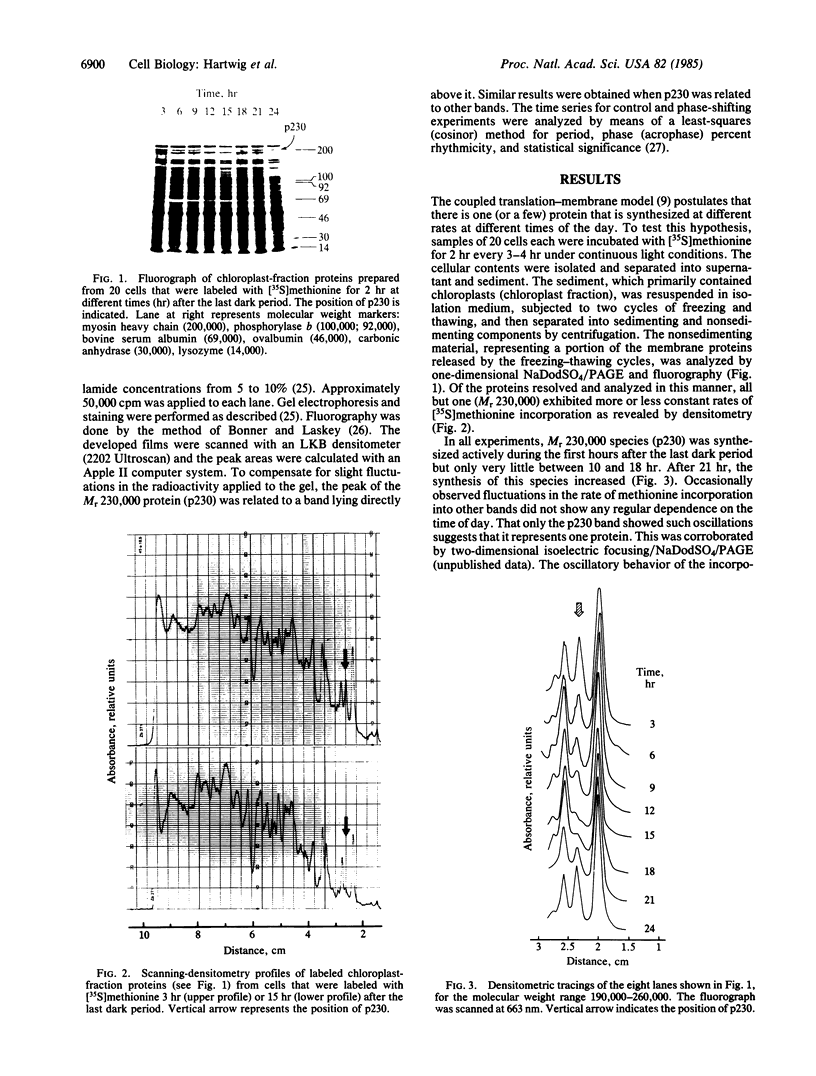
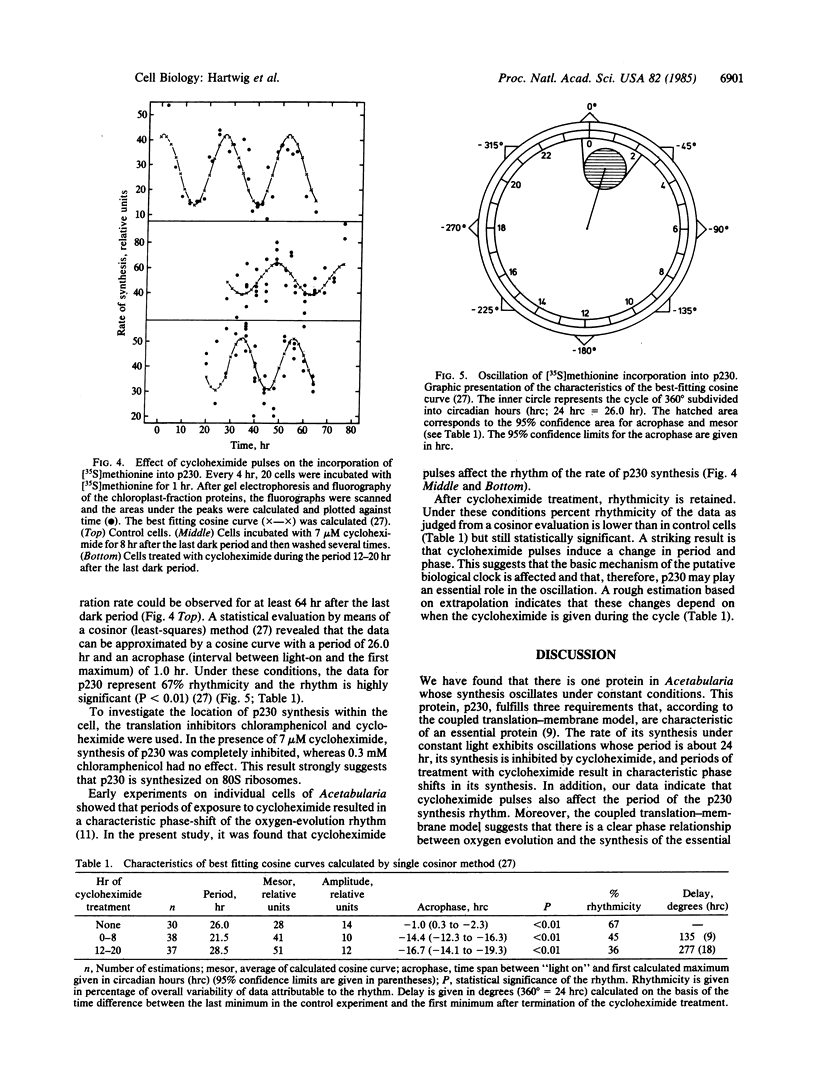
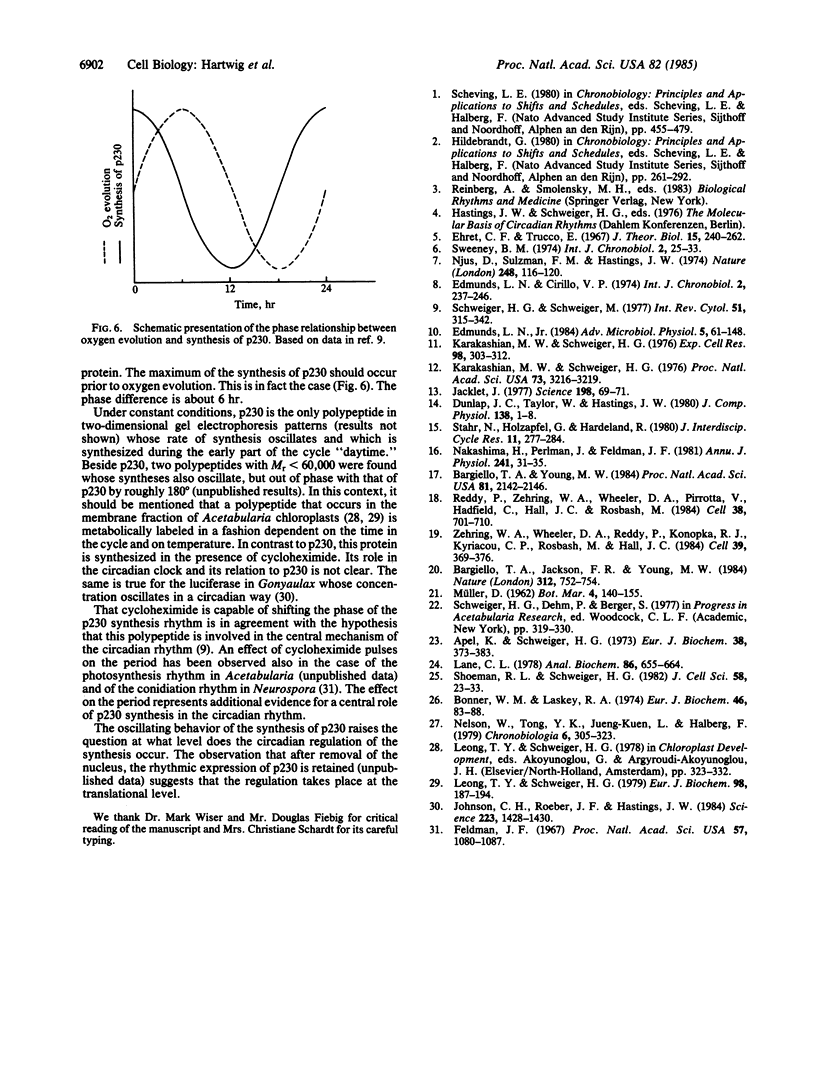
In the chloroplast fraction of the unicellular and uninucleate green alga Acetabularia, we have detected a Mr ≈230,000 protein (p230) whose synthesis exhibits a pronounced endogenous diurnal rhythm. As judged by scanning densitometry of fluorographs of NaDodSO4/polyacrylamide gels, the synthesis of other proteins in the same fraction was independent of the time in the cycle. The incorporation of [35S]methionine into p230 was completely inhibited by cycloheximide, whereas chloramphenicol had no effect. This strongly suggests that p230 is translated on 80S ribosomes. Eighthour periods of exposure to cycloheximide produced a shift in the phase of the oscillation of p230 synthesis. The results are consistent with the hypothesis that p230 is essential for expression of circadian rhythms in Acetabularia.
Keywords: biological clock, translation
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apel K., Schweiger H. G. Sites of synthesis of chloroplast-membrane proteins. Evidence for three types of ribosomes engaged in chloroplast-protein synthesis. Eur J Biochem. 1973 Oct 5;38(2):373–383. doi: 10.1111/j.1432-1033.1973.tb03070.x. [DOI] [PubMed] [Google Scholar]
- Bargiello T. A., Jackson F. R., Young M. W. Restoration of circadian behavioural rhythms by gene transfer in Drosophila. Nature. 1984 Dec 20;312(5996):752–754. doi: 10.1038/312752a0. [DOI] [PubMed] [Google Scholar]
- Bargiello T. A., Young M. W. Molecular genetics of a biological clock in Drosophila. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2142–2146. doi: 10.1073/pnas.81.7.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Edmunds L. N., Jr, Cirillo V. P. On the interplay among cell cycle, biological clock and membrane transport control systems. Int J Chronobiol. 1974;2(3):233–246. [PubMed] [Google Scholar]
- Edmunds L. N., Jr Physiology of circadian rhythms in micro-organisms. Adv Microb Physiol. 1984;25:61-148, 301-3. doi: 10.1016/s0065-2911(08)60291-x. [DOI] [PubMed] [Google Scholar]
- Ehret C. F., Trucco E. Molecular models for the circadian clock. I. The chronon concept. J Theor Biol. 1967 May;15(2):240–262. doi: 10.1016/0022-5193(67)90206-8. [DOI] [PubMed] [Google Scholar]
- Feldman J. F. Lengthening the period of a biological clock in Euglena by cycloheximide, an inhibitor of protein synthesis. Proc Natl Acad Sci U S A. 1967 Apr;57(4):1080–1087. doi: 10.1073/pnas.57.4.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacklet J. W. Neuronal circadian rhythm: phase shifting by a protein synthesis inhibitor. Science. 1977 Oct 7;198(4312):69–71. doi: 10.1126/science.897685. [DOI] [PubMed] [Google Scholar]
- Johnson C. H., Roeber J. F., Hastings J. W. Circadian changes in enzyme concentration account for rhythm of enzyme activity in gonyaulax. Science. 1984 Mar 30;223(4643):1428–1430. doi: 10.1126/science.223.4643.1428. [DOI] [PubMed] [Google Scholar]
- Karakashian M. W., Schweiger H. G. Evidence for a cycloheximide-sensitive component in the biological clock of Acetabularia. Exp Cell Res. 1976 Mar 15;98(2):303–312. doi: 10.1016/0014-4827(76)90442-0. [DOI] [PubMed] [Google Scholar]
- Karakashian M. W., Schweiger H. G. Temperature dependence of cycloheximide-sensitive phase of circadian cycle in Acetabularia mediterranea. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3216–3219. doi: 10.1073/pnas.73.9.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane L. C. A simple method for stabilizing protein-sulfhydryl groups during SDS-gel electrophoresis. Anal Biochem. 1978 Jun 1;86(2):655–664. doi: 10.1016/0003-2697(78)90792-3. [DOI] [PubMed] [Google Scholar]
- Leong T. Y., Schweiger H. G. The role of chloroplast-membrane-protein synthesis in the circadian clock. Purification and partial characterization of a polypeptide which is suggested to be involved in the clock. Eur J Biochem. 1979 Jul;98(1):187–194. doi: 10.1111/j.1432-1033.1979.tb13176.x. [DOI] [PubMed] [Google Scholar]
- Nelson W., Tong Y. L., Lee J. K., Halberg F. Methods for cosinor-rhythmometry. Chronobiologia. 1979 Oct-Dec;6(4):305–323. [PubMed] [Google Scholar]
- Njus D., Sulzman F. M., Hastings J. W. Membrane model for the circadian clock. Nature. 1974 Mar 8;248(5444):116–120. doi: 10.1038/248116a0. [DOI] [PubMed] [Google Scholar]
- Reddy P., Zehring W. A., Wheeler D. A., Pirrotta V., Hadfield C., Hall J. C., Rosbash M. Molecular analysis of the period locus in Drosophila melanogaster and identification of a transcript involved in biological rhythms. Cell. 1984 Oct;38(3):701–710. doi: 10.1016/0092-8674(84)90265-4. [DOI] [PubMed] [Google Scholar]
- Schweiger H. G., Schweiger M. Circadian rhythms in unicellular organisms: an endeavor to explain the molecular mechanism. Int Rev Cytol. 1977;51:315–342. doi: 10.1016/s0074-7696(08)60230-2. [DOI] [PubMed] [Google Scholar]
- Shoeman R. L., Schweiger H. G. Gene expression in Acetabularia. I. Calibration of wheat germ cell-free translation system proteins as internal references for two-dimensional electrophoresis. J Cell Sci. 1982 Dec;58:23–33. doi: 10.1242/jcs.58.1.23. [DOI] [PubMed] [Google Scholar]
- Sweeney B. M. A physiological model for circadian rhythms derived from the acetabularia rhythm paradoxes. Int J Chronobiol. 1974;2(1):25–33. [PubMed] [Google Scholar]
- Zehring W. A., Wheeler D. A., Reddy P., Konopka R. J., Kyriacou C. P., Rosbash M., Hall J. C. P-element transformation with period locus DNA restores rhythmicity to mutant, arrhythmic Drosophila melanogaster. Cell. 1984 Dec;39(2 Pt 1):369–376. doi: 10.1016/0092-8674(84)90015-1. [DOI] [PubMed] [Google Scholar]