Abstract
The past few decades have seen an explosion in our understanding of the molecular basis of learning and memory. The majority of these studies in mammals focused on post-synaptic signal transduction cascades involved in post-synaptic long-lasting plasticity. Until recently, relatively little work examined the role of presynaptic proteins in learning and memory in complex systems. The synaptic cleft figuratively represents a “great divide” between our knowledge of post- versus presynaptic involvement in learning and memory. While great strides have been made in our understanding of presynaptic proteins, we know very little of how presynaptically expressed forms of short- and long-term plasticity participate in information processing and storage. The paucity of cognitive behavioral research in the area of presynaptic proteins, however, is in stark contrast to the plethora of information concerning presynaptic protein involvement in neuro-transmitter release, in modulation of release, and in both short- and long-term forms of presynaptic plasticity. It is now of great interest to begin to link the extensive literature on presynaptic proteins and presynaptic plasticity to cognitive behavior. In the future there is great promise with these approaches for identifying new targets in the treatment of cognitive disorders. This review article briefly surveys current knowledge on the role of presynaptic proteins in learning and memory in mammals and suggests future directions in learning and memory research on the presynaptic rim of the “great divide.”
Keywords: Presynaptic, Plasticity, Learning and memory, Short-term plasticity, Long-term potentiation, Synaptic vesicle, Behavior, Mossy fiber, Hippocampus
1. Introduction
Presynaptic proteins and presynaptic plasticity have been implicated in learning and memory through decades of study in highly tractable, “simpler” model systems, such as the giant marine mollusc Aplysia (Frost, Clark, & Kandel, 1988; Kandel, 2001; Roberts & Glanzman, 2003). In mammalian learning and memory, however, the role of presynaptically mediated short- and long-term plasticity, presynaptic release machinery, and presynaptic signal transduction has received much less attention. This is likely due to a focus on proteins involved in long-term potentiation (LTP) in area CA1 of the hippocampus, which is thought to be expressed largely through postsynaptic molecular changes (Chen & Tonegawa, 1997; Martin, Grimwood, & Morris, 2000; Silva, Smith, & Giese, 1997). Given the striking overlap in molecular mechanisms between invertebrate and mammalian learning and memory, it is likely that presynaptic mechanisms will also play a prominent role in memory formation in the mammalian brain.
While progress has been made in understanding which presynaptic proteins are involved in short-term forms of plasticity such as paired pulse facilitation (PPF) and post-tetanic potentiation (PTP) (Castillo, Schoch, Schmitz, Sudhof, & Malenka, 2002; Schoch et al., 2002; Sudhof, 2004; Zucker & Regehr, 2002), the role of short-term plasticity in information processing and storage remains to be determined (Abbott & Regehr, 2004). Is there a role for short-term plasticity in learning and memory? How could synaptic alterations lasting milliseconds to minutes play such a role? Does short-term plasticity play a role in rapid information processing such as sensorimotor gating or selective attention during learning? A complete picture of how the brain processes and stores information will depend on a fundamental understanding of these questions.
Similarly, the mechanisms involved in presynaptically expressed forms of long-term potentiation (LTP) are being elucidated (Castillo et al., 1997, 2002; Ferguson, Wang, Herschman, & Storm, 2004; Henze, Urban, & Barrionuevo, 2000; Huang et al., 1995; Kapur, Yeckel, Gray, & Johnston, 1998; Kumar, Baker, Storm, & Bowden, 2001; Lonart, 2002; Lonart, Janz, Johnson, & Sudhof, 1998; Mellor & Nicoll, 2001; Mellor, Nicoll, & Schmitz, 2002; Poser & Storm, 2001; Salin, Malenka, & Nicoll, 1996a; Salin, Scanziani, Malenka, & Nicoll, 1996b; Schmitz, Mellor, Breustedt, & Nicoll, 2003; Tong, Malenka, & Nicoll, 1996; Villacres, Wong, Chavkin, & Storm, 1998; Wang, Yeckel, Johnston, & Zucker, 2004; Weisskopf, Castillo, Zalutsky, & Nicoll, 1994; Weisskopf & Nicoll, 1995; Yeckel, Kapur, & Johnston, 1999). While present at cerebellar parallel fiber-Purkinje cell synapses and at corticothalamic synapses, presynaptically expressed LTP is most extensively studied at the dentate granule cell mossy fiber axon to CA3 pyramidal neuron synapse in the hippocampus (mfLTP). Recent advances in our understanding of how the CA3 region of the hippocampus may be involved in certain types of episodic memory, pattern completion, and pattern separation make understanding the role of this form of presynaptic plasticity particularly relevant to understanding hippocampal function.
Presynaptically expressed forms of plasticity are likely to be critical for information processing and storage in the brain. Here, I review recent studies using mammalian genetic models to link presynaptic proteins and presynaptic plasticity to cognitive function with an emphasis on learning and memory and the hippocampus. The reader is oriented with a brief overview of presynaptic neurotransmitter release and presynaptic plasticity. I then summarize recent behavioral studies of mice with altered short-term and long-term plasticity and directions for future study. This is followed by a discussion of the behavioral implications of additional presynaptic protein knockouts. Finally, I summarize the implications and directions for future study of presynaptic proteins with the goal of bridging the synaptic divide in mammalian cognitive function.
2. Neurotransmitter release and plasticity
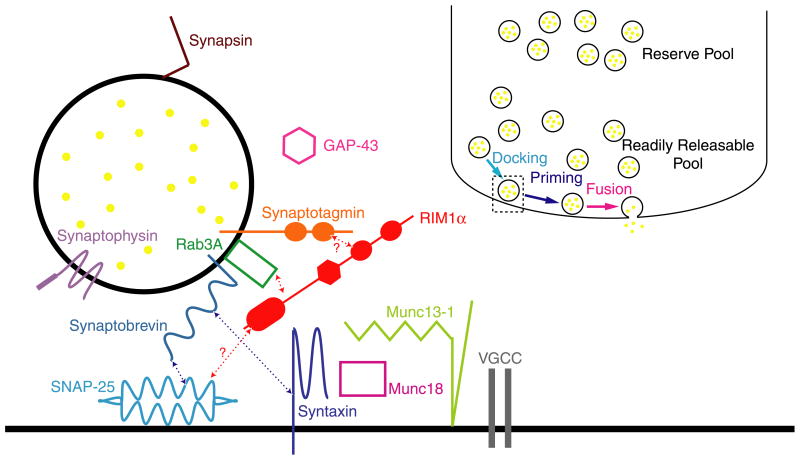
Release of neurotransmitter from presynaptic terminals requires the highly regulated fusion of synaptic vesicle membrane with the plasma membrane in a specialized, electron-dense region known as the active zone. The details of this process and its regulation have been thoroughly reviewed (Sudhof, 2004) and a brief overview is provided here to orient the reader. Synaptic vesicles first dock at the presynaptic active zone and then an ATP-dependent priming reaction makes them competent for exocytosis when an action potential triggers Ca2+ influx into the presynaptic terminal via voltage-gated Ca2+ channels. Multiple studies have identified several important components of the molecular machinery responsible for synaptic vesicle fusion, its regulation by Ca2+, and modulation of neurotransmitter release (Fig. 1).
Fig. 1.
Schematic diagram of a presynaptic terminal and presynaptic proteins (Sudhof, 2004). Inset depicts a presynaptic terminal with reserve pool and readily releasable pool of synaptic vesicles. Also depicted are three final steps in neurotransmitter release: docking, priming, and fusion. The dashed box represents the region magnified in the main figure to the left. Main figure to the left depicts a single synaptic vesicle, synaptic vesicle-associated proteins, active zone proteins, and other proteins. The SNARE complex proteins which interact to mediate fusion of the synaptic vesicle (synaptobrevin, SNAP-25, and syntaxin) are shown in shades of blue. Known interactions between proteins are depicted as bidirectional arrows. A question mark indicates that the interaction has not been completely validated. Additional possible interactions between the proteins depicted are described in the text.
Specific presynaptic proteins regulate coordinated, Ca2+-triggered neurotransmitter release. At the core of neuro-transmitter release are the SNARE proteins synaptobrevin/VAMP, syntaxin, and SNAP-25. Synaptobrevin is a synaptic vesicle protein containing an R-SNARE helix. Syntaxin contains a single Qa-SNARE helix and SNAP-25 contains both a Qb- and Qc-SNARE helices. The four SNARE helices of synaptobrevin, syntaxin, and SNAP-25 form a tight complex which is thought to pull the synaptic vesicle membrane in close proximity to the presynaptic membrane, creating an unstable conformation. In association with a variety of modulators, this unstable intermediate can be triggered by Ca2+ to form a fusion pore which allows the vesicle to fuse with the presynaptic plasma membrane (Sudhof, 2004). The main Ca2+ sensor for fast neurotransmitter release is synaptotagmin (Fernandez-Chacon et al., 2001; Geppert et al., 1994; Littleton, Stern, Schulze, Perin, & Bellen, 1993). Synaptotagmin associates constitutively with SNARE complexes and upon binding Ca2+ results in vesicle fusion and release of neurotransmitter (Sudhof, 2004).
Both synaptic vesicle, cytoplasmic, and active zone-associated proteins can regulate this process. These proteins can affect vesicle availability, docking, priming, Ca2+ triggering, and even Ca2+ entry, all of which can affect both the probability of neurotransmitter release and how neurotransmitter release changes as a function of patterns of presynaptic action potential input.
The probability of neurotransmitter release in response to a given action potential is robustly modulated by the pattern of presynaptic activation. Thus, the presynaptic terminal acts as a computational unit that can vary its output depending on the pattern of previous input. This type of presynaptic regulation of neurotransmitter release is generally referred to as short-term plasticity (Fig. 2). For the purposes of this review, short-term plasticity refers to alterations in presynaptic release lasting for at most a few minutes. A brief overview of short-term plasticity is provided here, while more detailed reviews are available (Fisher, Fischer, & Carew, 1997; Sudhof, 2004; Zucker & Regehr, 2002).
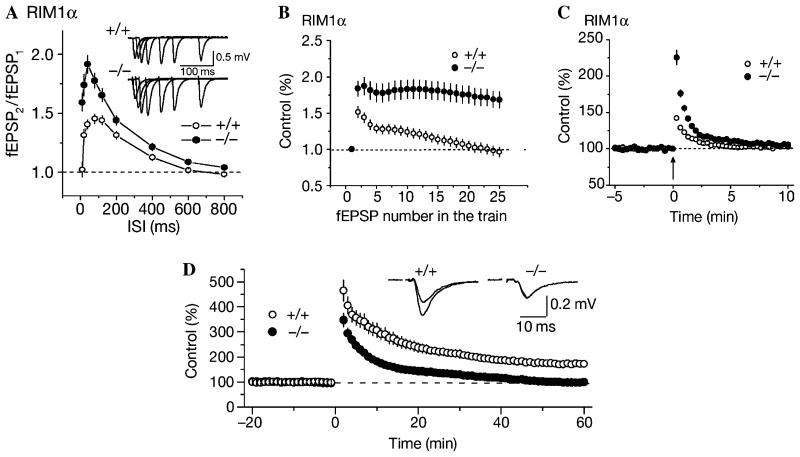
Fig. 2.
Examples of short- and long-term presynaptic plasticity. (A) Example of paired pulse facilitation (PPF) in wild-type (open circles, +/+) and RIM1α knockout (filled circles, −/−). The ratio of the second extracellularly recorded excitatory post-synaptic potential (fEPSP) to that of the first fEPSP elicited at different interstimulus intervals (ISI) is plotted. RIM1α knockout mice show increased PPF compared to wild-type. Inset shows examples of fEPSPs elicited at various ISIs after the initial fEPSP. (B) Example of synaptic depression. In wild-type mice, stimulation at 14 Hz elicits an initial facilitation followed by synaptic depression. Depression in RIM1α−/− mice is markedly decreased. (C) Example of post-tetanic potentiation (PTP). In the presence of the N-methyl-D-aspartate inhibitor 4-aminophosphonovaleric acid to block long-term potentiation in area CA1 of the hippocampus, a high frequency, tetanic stimulation (arrow) elicits immediate potentiation of the synaptic response lasting 2–3 min. PTP in RIM1α−/− mice is significantly enhanced. (D) Example of mossy fiber long-term potentiation (mfLTP) in area CA3 of the hippocampus. In the presence of 2-amino-5-phosphonovaleric acid (APV), high-frequency stimulation at time 0 elicits a robust, long-lasting potentiation in wild-type mice. RIM1α−/− mice do not exhibit significant mfLTP. A–C used with permission from Schoch et al. (2002). D used with permission from Castillo et al. (2002).
Paired pulse facilitation (PPF) is a form of short-term plasticity lasting on the order of hundreds of milliseconds (Fig. 2A). When two presynaptic action potentials occur within 25–400 ms, the post-synaptic response to the second action potential is increased dramatically relative to the first. This is widely held to be due to residual Ca2+ in the presynaptic terminal from the first action potential adding to the Ca2+ influx from the second pulse (Katz & Miledi, 1968; Zucker & Regehr, 2002). The larger presynaptic Ca2+ leads to facilitated or increased neurotransmitter release. PPF is not limited to only the second of two presynaptic activations, in fact responses to high-frequency stimulation beyond the second pulse can be facilitated even further. The role of this robust integration of presynaptic activity in information processing remains a mystery, though initial studies of how PPF might play a role in behavior are one focus of this review.
Prolonged high-frequency presynaptic activation eventually leads to a decrease in neurotransmitter release known as synaptic depression (Fig. 2B). This ultimately results in a lower steady-state level of neurotransmitter release that can take up to a few minutes to recover. Synaptic depression is commonly taken to be the result of a decrease in the readily releasable pool of synaptic vesicles, though the precise mechanisms are poorly understood (Zucker & Regehr, 2002). The readily releasable pool of vesicles is defined by examining release rates under various stimulation conditions including sustained high-frequency stimulation (Goda & Stevens, 1998; Rosenmund & Stevens, 1996; Stevens & Tsujimoto, 1995). The release obtained with high-frequency stimulation correlates well with that obtained by application of hypertonic sucrose and with the number of vesicles visualized as “docked” by electron microscopy (Zucker & Regehr, 2002).
Post-tetanic potentiation (PTP) refers to an increase in neurotransmitter release after a brief, high-frequency train of action potentials (Fig. 2C). This large enhancement may last on the order of one-half to several minutes and is typically measured after recovery from synaptic depression. While the precise mechanism of PTP remains to be determined, genetic manipulation of certain presynaptic proteins can clearly lead to altered PTP with or without changes in other forms of presynaptic plasticity (Zucker & Regehr, 2002). The potential behavioral implications of altering PTP are also a focus of this review.
In addition to these short-term forms of presynaptic plasticity, some long-term forms of synaptic plasticity appear to be expressed through lasting changes in presynaptic neurotransmitter release. These forms of long-term plasticity are discussed in detail below.
3. Difficulties in linking presynaptic plasticity to behavior
The use of mice with genetically altered presynaptic proteins to study behavior is subject to all of the usual caveats associated with knockout studies (Bilbo & Nelson, 2001; Crawley, 1999; Crawley et al., 1997; Crawley & Paylor, 1997; Keverne, 1997). Briefly, in traditional knockouts, regional specificity is lacking and developmental effects must be ruled out. Even in the best-case scenario of a conditional knockout in which cell-type specific, adult-inducible knockout can be achieved, there is still at least a few-day delay between the manipulation leading to conditional knockout and the behavioral tests. This allows the affected neurons to completely turnover the majority of their proteins, alter gene expression and protein translation, and potentially alter neuronal function in ways that are very indirect with respect to the protein of interest. These caveats are major considerations when trying to specifically link an individual protein to a particular cellular or behavioral abnormality.
If the goal is to map synaptic physiology onto behavior, then the underlying molecular changes are less important than the final alteration in synaptic physiology. For example, if one is interested in the role of PPF in memory, then the particular protein knocked out and any molecular accommodations are less critical than a thorough examination of other features of synaptic physiology. In this case, the physiologic abnormality is the experimental variable correlated with behavior. Assuming that all other electro-physiologic abnormalities are ruled out, then one can correlate the physiologic abnormality with any behavioral deficits. In the final analysis, one needs convergence of behavioral findings across multiple molecular manipulations causing similar or overlapping physiologic abnormalities.
There are several experimental hurdles specific to the study of presynaptic plasticity in behavior using knockout mice. One major problem is that alterations in short-term plasticity often coexist with alterations in baseline synaptic transmission (Zucker & Regehr, 2002) or altered long-term synaptic plasticity (Castillo et al., 1997, 2002; Schoch et al., 2002), which could themselves alter behavior. It is possible, for example, that a decrease in probability of neurotransmitter release (Pr) may accompany an increase in PPF. Subtle changes in Pr are difficult to detect with an input output curve from an extracellular recording or even by comparing whole cell excitatory post-synaptic current (EPSC) size across preparations (Zucker & Regehr, 2002). The relevance of an altered Pr setpoint to behavior is unclear, though a recent study of a synaptotagmin 1 point mutant suggests that a dramatic decrease in Pr does not necessarily lead to global behavioral abnormalities (Powell et al., 2004).
In the face of overlapping electrophysiologic deficits, one is left to look for common behavioral patterns in mice with similar short-term plasticity deficits but different alterations in other parameters of synaptic function. Interpretation of studies involving mice with short-term plasticity abnormalities, alterations in long-term plasticity, and Pr changes is difficult. It is certainly possible for such combinations of synaptic abnormalities to interact with one another in complex ways, causing behavioral abnormalities that might not occur if only a single synaptic parameter were affected. Direct interpretation is made even more difficult in mice where careful studies of baseline synaptic transmission have not been performed. Detailed analysis of synaptic transmission requires sophisticated electrophysiological analysis (Zucker & Regehr, 2002), which is outside the expertise of many behavioral and molecular biological laboratories.
With any mutant, it is impractical to examine synaptic physiology at more than a few central synapses. Alterations in synaptic physiology in the thalamus or sensorimotor cortex, for example, might lead to altered sensory perception and thus indirectly affect learning and memory. Behavioral controls for sensory abnormalities including tests of nociception (sensitivity to footshock) and of vision (visible platform version of the water maze) are often used. Unfortunately, more subtle visual processing abnormalities are difficult to rule out.
Behavioral studies in genetically altered mice must include appropriate controls. In fear conditioning experiments, for example, it is critical to show that all groups are equally sensitive to footshock and sound and that they are able to exhibit the behavioral measure of fear. In Morris water maze experiments, an appropriate test for vision such as the visible platform version of the water maze must be used and the tendency for mice to swim along the walls, known as thigmotaxis, must be measured. In addition, it is important to ensure that mice swim at similar speeds (if using latency to reach the hidden platform as a measure) or the learning data should be analyzed using the distance traveled prior to reaching the hidden platform. Subtle swim speed differences should not affect the spatial bias or “probe” test indications of spatial learning. For other learning tasks, similar controls should be in place to rule out performance deficits and sensory abnormalities. In this review, it can be assumed that appropriate behavioral control experiments were performed and these are discussed only in cases where there are important caveats to interpretation of the behavioral findings.
Despite the inherent complexity, a complete understanding of learning and memory in the mammalian brain will require a thorough understanding of how presynaptic proteins and presynaptic plasticity are involved in information processing and storage. Several laboratories have begun to scratch the surface of this critical problem.
The question of how presynaptic forms of synaptic plasticity are involved in information processing and storage in the brain has been difficult to answer. In the following section, I will review behavioral studies of knockout mice with altered short-term presynaptic plasticity.
4. Short-term presynaptic plasticity and memory
Short-term plasticity includes synaptic plasticity lasting on the order of a few minutes or less. The most commonly studied forms of short-term plasticity include paired pulse facilitation (PPF), paired pulse depression (PPD), synaptic depression, and post-tetanic potentiation (PTP) and augmentation. The physiologic characteristics and mechanisms of these forms of plasticity have been recently, extensively reviewed (Zucker & Regehr, 2002). Very few studies have specifically addressed the question of the role of short-term plasticity in learning and memory (see Table 1).
Table 1.
Summary of short-term plasticity mutants and behavioral results
| PPF | PTP | LTP | Fear conditioning | Water maze | Other | References | |
|---|---|---|---|---|---|---|---|
| α CaMKII+/− | ↓ | ↑ | Normal | ↓ | ↓ | Silva et al. (1996) | |
| Ataxin I−/− | ↓ | Normal | Normal | — | ↓ | Matilla et al. (1998) | |
| Synapsin II−/− | Normal | ↓ | Normal | ↓ | — | Silva et al. (1996) | |
| Synapsin I/II−/− | Normal | ↓ | Normal | ↓ | — | Silva et al. (1996) | |
| Synapsin I−/− | ↑ | Normal | Normal | Normal | Normal | Silva et al. (1996) | |
| Rab3A−/− | ↑ | Normal | Normal | Normal | Normal | ↓ mfLTP |
Castillo et al. (2002), Powell et al. (2004), Schoch et al. (2002) |
| Normal | Normal | ↓ mfLTP |
Hensbroek et al. (2003) | ||||
| Normal | ↓ Reversal learning |
D’Adamo et al. (2004) | |||||
| Synaptotagmin 1 R233Q+/+ | ↑ | — | — | Normal | Normal | ↓Pr CA1 |
Powell et al. (2004) |
| Synaptotagmin IV−/− | ↑ | ↑ | ↑ | ↓Passive avoidance (normal object recognition) | — | Ferguson, Anagnostaras, Silva, & Herschman (2000) | |
| RIM1α−/− | ↑ | ↑ | Normal | ↓ | ↓ | ↓ mfLTP |
Castillo et al. (2002), Powell et al. (2004), Schoch et al. (2002) |
PPF, paired pulse facilitation; PTP, post-tetanic potentiation; Pr, probability of evoked neurotransmitter release; mfLTP, mossy fiber long-term potentiation in area CA3; —, means not tested. All electrophysiological data are from area CA1 of the hippocampus unless otherwise specified.
Initial studies suggested that a decrease in PPF is correlated with impairments in hippocampus-dependent learning while an increase in PPF is not. Silva and colleagues began with a systematic study of the role of PPF and PTP in learning and memory using mice that were heterozygous for Ca2+/calmodulin-dependent protein kinase II (αCaM-KII+/−). αCaMKII-deficient mice exhibit increased PTP and decreased PPF in area CA1 of the hippocampus with normal CA1 LTP (Chapman, Frenguelli, Smith, Chen, & Silva, 1995), and were impaired in both fear conditioning and Morris water maze learning (Silva et al., 1996). This study makes no mention of assessing swim speeds, though this should not affect the differences in spatial memory demonstrated in the probe trial. Thus, increased PTP or decreased PPF was implicated in the learning deficits. In a study by Matilla et al., mice lacking ataxin-1 had decreased PPF with normal PTP and LTP in area CA1 (Matilla et al., 1998). These mice with an apparently isolated decrease in PPF exhibited severe spatial learning deficits in the Morris water maze (Matilla et al., 1998), though baseline Pr was not measured in these mice. These studies suggest that decreased PPF is correlated with impairments in hippocampus-dependent learning, while increased PPF is not.
In addition to decreased PPF, decreased PTP has also been correlated with learning and memory deficits. In the same study by Silva et al., mice deficient in synapsin II or synapsins I and II showed decreased PTP, with normal PPF and LTP in CA1, and were impaired in fear conditioning (Silva et al., 1996).
While decreases in PPF and PTP have been correlated with learning and memory deficits, it appears that increased PPF alone is not consistently correlated with altered learning and memory. A recent study by Powell et al., examined two presynaptic protein knockout mice that were previously extensively characterized electrophysiologically and that exhibited increased PPF in area CA1 of the hippocampus. Rab3A−/− mice had increased CA1 PPF with no change in baseline Pr (Castillo et al., 2002; Schoch et al., 2002), and exhibited completely normal water maze and fear conditioning learning (Powell et al., 2004). Two other studies confirmed this lack of learning and memory deficits in Rab3A−/− mice (D’Adamo et al., 2004; Hensbroek, Kamal, Baars, Verhage, & Spruijt, 2003). One of these studies of Rab3A−/− mice demonstrated more subtle deficits in working memory and episodic-like memory, though these abnormalities were attributed to altered long-term plasticity at mossy fiber synapses in area CA3 of the hippocampus (D’Adamo et al., 2004). Similarly, syn-aptotagmin 1 point mutant knockin mice (Syt1R233Q+/+) exhibited increased PPF along with an approximately 50% decrease in Pr in area CA1 (Fernandez-Chacon et al., 2001). Surprisingly, Syt1R233Q+/+ mice showed normal water maze and fear conditioning learning (Powell et al., 2004) in spite of the significant alteration in Pr in CA1 and likely at other central synapses. Similarly, synapsin I-deficient mice showed increased PPF, but no deficits in fear conditioning or water maze learning (Silva et al., 1996). One potential caveat to interpreting all of these negative findings is that weaker fear conditioning training paradigms were not tested, though a single pairing of footshock with context and tone was used. In synaptotagmin IV knockout mice, both PPF and PTP were increased and the mice showed normal novel object recognition, but impaired long-term passive avoidance memory (Ferguson et al., 2004). Importantly, no mention was made of controls for footshock or other pain sensitivity in this study. This disparate finding could also be due to the different behavioral paradigms compared to the other studies or due to an interaction between increased PPF and increased PTP. In summary, increased PPF alone was not consistently correlated with abnormalities in learning and memory in the Morris water maze or fear conditioning paradigms or with augmented learning.
These initial studies suggest the possibility that PPF and perhaps PTP are necessary for or involved in normal learning and memory. In fact, the majority of mutant mice mentioned in this article that display a behavioral phenotype have some abnormality in presynaptic short-term plasticity (Tables 1 and 2). While it is true that many others have similar short-term plasticity deficits with no behavioral abnormalities, this may be an issue of the degree of short-term plasticity deficit or associated physiologic deficits. Of course, given the widespread nature of the genetic manipulation in these studies, one must use caution in associating a single hippocampal synaptic abnormality with a behavioral phenotype. Convergence across multiple increasingly localized manipulations will help to resolve this issue.
Table 2.
Summary of long-term presynaptic plasticity mutants and behavioral results
| mfLTP | CA1 LTP | Fear conditioning | Spatial memory | Other | References | |
|---|---|---|---|---|---|---|
| PKA Cβ1−/− | ↓ | Normal E-LTP ↓L-LTP | Normal | Normal water maze and Barnes Maze | ↓CA1 LTD | Huang et al. (1995), Qi et al., 1996 |
| PKA R1β−/− | ↓ | Normal E-LTP normal L-LTP |
Normal | Normal water maze and Barnes maze | ↓CA1 LTD | Brandon et al. (1995), Huang et al. (1995) |
| Rab3A−/− | ↓ | Normal E-LTP | Normal | Normal water maze | ↑CA1 PPF | Castillo et al. (1997, 2002), Powell et al. (2004) |
| ↓ | Normal | Normal water maze | Hensbroek et al. (2003) | |||
| Normal delay and trace fear conditioning | Normal initial water maze ↓reversal learning |
D’Adamo et al. (2004) | ||||
| Type I adenylyl cyclase−/− | ↓ | ↓E-LTP | — | ↓Water maze | Villacres et al. (1998), Wu et al. (1995) | |
| MGluR1−/− | ↓ | Normal E-LTP | ↓Cerebellar LTP ↓corticostriatal plasticity, cerebellar synaptic abnormalities |
Bordi et al. (1997), Conquet et al. (1994), Gubellini et al. (2001), Lapointe et al. (2004) | ||
| PAC1−/− | ↓ | — | ↓Contextual normal cue | Normal water maze | ↓Anxiety ↑locomotor activity normal PP-dentate LTP |
Otto et al. (2001a, 2001b) |
| GAP-43+/− | — | — | ↓Contextual normal cue | — | Rekart et al. (2005) | |
| GAP-43 Tg | — | — | — | ↑Learning in 8-arm radial maze | ↑PP-dentate LTP in vivo | Routtenberg et al. (2000) |
| Complexin II−/− | ↓ | ↓ | — | ↓ | ↓CA1 LTP | Glynn et al. (2003), Takahashi et al. (1999) |
PKA Cβ1, catalytic subunit of protein kinase A; PKA R1β, regulatory subunit of protein kinase A. E-LTP refers to the early phase of long-term potentiation (lasting 1–3 h). L-LTP refers to the late, protein synthesis-dependent phase of long-term potentiation. MGluR1 refers to the metabotropic glutamate receptor type 1. PAC1 refers to pituitary adenylyl cyclase receptor type 1. PP-dentate LTP refers to LTP of the perforant path to dentate granule cell in the hippocampal formation.
Clearly, additional confirmation of the role of presynaptic plasticity in learning and memory is necessary. In particular, it will be important to examine additional mouse models in which short-term plasticity is affected in isolation of other synaptic physiologic abnormalities. Mice lacking various modulators of presynaptic plasticity and release are excellent candidates for such genetic manipulation. In future studies, it will be helpful to restrict the knockout of such targets to specific presynaptic neurons, perhaps in subregions of the hippocampal formation using conditional knockout approaches. Similarly, it will be important to expand the number of learning and memory paradigms used to characterize these mice. Working memory is one such understudied form of memory in which short-term plasticity might play a critical role. In addition to learning and memory paradigms, it will also be important to assess other aspects of behavior such as sensorimotor gating, selective attention, and impulsivity. Finally, given the caveats to interpretation of knockout studies, it will be critical to make use of other techniques to alter short-term plasticity to confirm findings in mutant mice.
5. Long-term presynaptic plasticity and memory
The most-studied form of long-term synaptic plasticity is long-term potentiation (LTP) at the Schaffer-collateral to CA1 pyramidal neuron synapse in the hippocampus (Malenka & Bear, 2004). This form of LTP is induced post-synaptically via NMDA receptor activation and expressed, at least in part, post-synaptically via increased post-synaptic responsiveness to neurotransmitter release.
Work by several laboratories has revealed a clearly different form of LTP that is thought to be expressed presynaptically as an increase in presynaptic neurotransmitter release. This form of presynaptically expressed LTP generally can be induced without NMDA receptor activation and has been most-studied at the mossy fiber to area CA3 pyramidal neuron synapse (mfLTP) in the hippocampal formation (Fig. 2D). While the mossy fiber synapse is clearly a highly specialized synapse differing greatly from even other hippocampal synapses, presynaptically expressed LTP is also found at cerebellar parallel fiber-Purkinje cell synapses and at corticothalamic synapses. Garcia-Junco-Clemente et al., have eloquently reviewed the physiologic and molecular mechanisms of these presynaptically expressed forms of LTP most recently (Garcia-Junco-Clemente, Linares-Clemente, & Fernandez-Chacon, 2005). Here, I will focus on learning and memory in mice lacking presynaptically expressed LTP at the mossy fiber-CA3 synapse in learning and memory, though presynaptically expressed LTP at other synapses has generally not been tested in most of these mice.
Several molecular manipulations in mice have led to absent or reduced mfLTP with or without additional synaptic plasticity deficits. Results of behavioral analyses in these mice are mixed, but a relatively clear picture is emerging (see Table 2). The convergent evidence from several studies suggests that mfLTP is not required for traditional water maze, delay fear conditioning, or trace fear conditioning learning, but may necessary for episodic-like memory versions of the water maze (delayed matching to place) and perhaps working memory. This conclusion, however, is subject to alternative interpretations due to the limitations of traditional knockout studies mentioned above.
To better understand the implications of various molecular manipulations affecting mfLTP, it is important to have in mind one working molecular model for mfLTP expression. While it is generally agreed that mfLTP is expressed presynaptically, the mechanism and location of mfLTP induction is controversial. Some studies implicate a need for post-synaptic involvement in mfLTP induction (Contractor et al., 2002; Jaffe & Johnston, 1990; Wang et al., 2004; Williams & Johnston, 1989; Yeckel et al., 1999) including depolarization, increased Ca2+, and increased cAMP. Other studies suggest that post-synaptic activation is not necessary and that mfLTP can be induced purely by presynaptic activation (Castillo, Weisskopf, & Nicoll, 1994; Mellor & Nicoll, 2001; Nicoll & Malenka, 1995; Tong et al., 1996; Weisskopf et al., 1994; Weisskopf & Nicoll, 1995). Regardless of the induction mechanism, several studies have implicated increased presynaptic neurotransmitter release as the final common pathway for mfLTP expression (Nicoll & Malenka, 1995; Tong et al., 1996; Weisskopf & Nicoll, 1995). Indeed, post-synaptic induction of mfLTP does not preclude presynaptic expression. In fact, molecular mechanisms for retrograde signaling in mfLTP have been implicated and provide an explanation to reconcile presynaptic expression with post-synaptic induction mechanisms (Contractor et al., 2002).
Additional studies have implicated presynaptic Ca2+/cal-modulin sensitive adenylyl cyclases and presynaptic activation of protein kinase A as critical for induction of mfLTP (Huang et al., 1995; Villacres et al., 1998). This work led to a search for presynaptic PKA substrates that might be involved in modulating presynaptic release. Eventually it was determined that the active zone protein RIM1α, a PKA substrate, and its binding partner, the synaptic vesicle-associated small GTPase Rab3A, were required for mfLTP (Castillo et al., 1997, 2002).
Mossy fiber LTP seems to be required for more complex forms of spatial memory requiring cognitive flexibility or pattern recognition, a function consistent with current ideas about the role of plasticity in recurrent-collateral synapses in area CA3 of the hippocampus (Kesner, Lee, & Gilbert, 2004; Nakazawa et al., 2002, 2003). The behavioral ramifications of altering mfLTP have been studied in several lines of mice lacking mfLTP. An early study demonstrated that absence of the regulatory (R1β) or catalytic (Cβ1) subunit of PKA prevents mfLTP while sparing several learning and memory tasks including the Morris water maze, fear conditioning, and radial arm maze (Huang et al., 1995). Subsequently, Hensbroek and collaborators showed that absence of mfLTP, in Rab3A−/− mice tested with two different genetic backgrounds, did not affect fear conditioning or the Morris water maze (Hensbroek et al., 2003). Our own data and those of D’Adamo et al., confirmed this finding (D’Adamo et al., 2004; Powell et al., 2004). A more thorough behavioral characterization of the Rab3A−/− mice, however, indicated deficits in the delayed match to place version of the Morris water maze and in working memory (D’Adamo et al., 2004). These data indicate that mfLTP is only required for episodic-like spatial memory requiring cognitive flexibility but not for initial learning of fear conditioning or the Morris water maze.
On the contrary, other reports suggested a role for mfLTP in learning tasks. Mice lacking the metabotropic glutamate receptor (mGluR1) and the type I adenylyl cyclase (AC1) exhibit deficits in mfLTP and also show subtle deficits in the Morris water maze (Conquet et al., 1994; Villacres et al., 1998; Wu et al., 1995). AC1 knockout mice also exhibit abnormalities in area CA1 LTP, which could explain their learning and memory deficits (Wu et al., 1995). Similarly, mGluR1 knockouts had severe motor abnormalities (Conquet et al., 1994), cerebellar synaptic abnormalities (Conquet et al., 1994), and synaptic plasticity abnormalities at corticostriatal and other synapses (Bordi, Reggiani, & Conquet, 1997; Gubellini et al., 2001, 2003; Lapointe et al., 2004). Another line of mice, pituitary adenylyl cyclase activating peptide (PACAP) type I receptor knockout mice, have absent mfLTP and normal water maze learning but impaired fear conditioning (Otto et al., 2001a, 2001b). However, PACAP knockout mice also exhibit impairments in other emotional behaviors (Otto et al., 2001b), a common confound for behavioral studies of memory that is often neglected in other studies. Because these molecules are upstream of the likely final common effectors of mfLTP, there are many opportunities for molecular divergence and effects on neuronal functions unrelated to mfLTP, making direct interpretation difficult.
In linking any form of long-lasting synaptic plasticity to learning and memory, one must keep in mind the disconnect between the time course of memory testing compared to that of the plasticity. In most learning and memory experiments, memory is tested several hours to one day after learning. In contrast, most experiments on synaptic plasticity are performed in hippocampal slices in vitro, allowing at most a 3–4 h recording period. Most studies do not measure LTP beyond the first hour or two. This is not so much a caveat to interpretation, but rather a caution that one cannot say whether longer lasting forms of synaptic plasticity are required for lasting memory. Even in the best case scenario, one can only conclude that the first 1–3 h of synaptic plasticity are required for the expression of a lasting memory. The link between memories that last months to years cannot yet be linked to synaptic plasticity studied in vitro.
The three molecular manipulations closest to the presumed final effectors of mfLTP expression, Rab3A and regulatory (R1β) or catalytic (Cβ1) subunits of PKA, all lead to absent mfLTP with intact learning and memory on multiple paradigms. Three separate groups, in more than two different genetic backgrounds, have confirmed the results in Rab3A−/− mice (D’Adamo et al., 2004; Hensbroek et al., 2003; Powell et al., 2004). Among the remaining studies, only one of two learning and memory paradigms, either fear conditioning or water maze, was affected where both were tested and the affected paradigm varied across studies. Thus, while it is possible that specific molecular accommodations account for these differences, the simplest interpretation is that mfLTP is required for episodic-like spatial memory tasks that require “cognitive flexibility” in the sense that the subject is asked to learn a new strategy in the same task quite frequently. MfLTP does not appear to be consistently required for classic water maze or delay or trace fear conditioning. Still, additional studies will be helpful in both confirming the involvement of mfLTP in episodic-like memory as well as it is lack of involvement in fear conditioning and water maze learning.
6. Additional presynaptic proteins in learning and memory
6.1. RIM1α
RIM1α is an active zone protein primarily expressed in brain that is involved in several aspects of presynaptic function (Castillo et al., 2002; Schoch et al., 2002). RIM1α was identified as a putative Rab3A effector protein and found to be highly localized to the active zone region of central synapses (Wang, Okamoto, Schmitz, Hofmann, & Sudhof, 1997). RIM1α is known to regulate Pr in both Caenorhabditis elegans and mice (Castillo et al., 2002; Koushika et al., 2001; Schoch et al., 2002), most likely at a step between docking and release known as priming. The precise mechanism of this effect on Pr is not known, however it is thought to be related to the many interactions between RIM1α and multiple presynaptic active zone and synaptic vesicle proteins. RIM1α thereby acts as a molecular scaffolding protein involved in organizing and modulating synaptic release machinery (Fig. 1) (Betz et al., 2001; Coppola et al., 2001; Ohtsuka et al., 2002; Schoch et al., 2002; Takao-Rikitsu et al., 2004; Wang, Liu, Biederer, & Sudhof, 2002; Wang et al., 1997; Wang, Sugita, & Sudhof, 2000). Interactions in vitro occur between RIM1α and active zone components munc 13-1 (Betz et al., 2001), SNAP25, voltage-gated Ca2+ channels (Coppola et al., 2001), and CAST/ERC proteins (Takao-Rikitsu et al., 2004; Wang et al., 2002) as well as the synaptic vesicle-associated proteins Rab3A (Wang et al., 1997) and synaptotagmin 1 (Coppola et al., 2001). Additionally, RIM1α interacts with 14-3-3 (Sun, Bittner, & Holz, 2003), α-liprins (Schoch et al., 2002), and a family of RIM binding proteins (Wang et al., 2002). RIM1α binds to Rab3A and Munc13-1 via its N-terminal Zn2+-finger domain (Betz et al., 2001; Schoch et al., 2002; Wang et al., 1997, 2000), to CAST/ERC proteins via its PDZ domain (Takao-Rikitsu et al., 2004; Wang et al., 2002), and to α-liprins, synaptotagmin 1, SNAP25, and voltage-gated Ca2+ channels via its C2B domain (Coppola et al., 2001; Schoch et al., 2002). Its selective localization to presynaptic active zones (Wang et al., 1997) and its interactions with multiple presynaptic molecules (Wang et al., 2002) places RIM1α in a key position to modulate presynaptic release and plasticity.
Available evidence from acute hippocampal slices suggests that RIM1α modulates neurotransmitter release and presynaptically mediated plasticity. Mice lacking RIM1α (RIM1α−/−) display a ~50% reduction in probability of evoked neurotransmitter release (Pr) at the Schaffer-collateral to CA1 pyramidal neuron synapse in hippocampal slices (Schoch et al., 2002). This decrease in Pr is accompanied by an increase in PPF in the same synapses. Prolonged stimulation at 14 Hz reveals a lack of normal synaptic depression (Schoch et al., 2002). In addition, post-tetanic potentiation (PTP), another presynaptically expressed form of short-term plasticity, is augmented in RIM1α−/− mice (Schoch et al., 2002). Analysis of hippocampal synapses in RIM1α−/− mice at the electron microscopic level revealed no significant ultrastructural abnormalities. Additionally, Western blot data analyzing multiple other synaptic proteins revealed only a reduction in Munc13-1 levels. Reduction of Munc13-1 in Munc13-1 heterozygous mice, however, does not lead to any of the synaptic physiology abnormalities seen in RIM1−/− mice (Schoch et al., 2002), so decreased Munc13-1 is unlikely to contribute to the RIM1α knockout phenotype.
A subsequent study of RIM1α-deficient hippocampal autapses in culture revealed that the RIM1α−/− mice exhibit decreased Pr via a decrease in the readily releasable pool of synaptic vesicles (Calakos, Schoch, Sudhof, & Malenka, 2004). Curiously, the decreased Pr in RIM1α−/− hippocampal cultures is observed only during the initial 4–5 stimuli during sustained, 14 Hz stimulation (Calakos et al., 2004). Wild-type synapses exhibit depression of Pr during 14 Hz stimulation, while RIM1α−/− synapses do not. Thus, the relationship between Pr and frequency of synaptic activation in the RIM1α−/− mice is complex. In autaptic hippocampal cultures, at low frequencies, RIM1α−/− mice exhibit decreased Pr, while at 14 Hz, and presumably higher frequencies, the Pr of RIM1α−/− mice becomes similar to that of wild-type mice after the first 4–5 responses (Schoch et al., 2002). Nevertheless, loss of RIM1α affects presynaptically mediated forms of synaptic plasticity including PPF, PTP, and synaptic depression at 14 Hz.
RIM1α−/− mice were also deficient in presynaptically expressed, protein kinase A (PKA)-dependent forms of LTP. At the mossy fiber to CA3 pyramidal neuron synapse in the hippocampus, mfLTP is absent in RIM1α−/− mice (Castillo et al., 2002). Similarly, presynaptically expressed LTP at the parallel fiber-Purkinje cell synapse in the cerebellum is absent in these mice (Castillo et al., 2002; Lonart et al., 2003). RIM1α is a PKA substrate in vivo and is primarily phosphorylated by PKA at serine 413 (Lonart et al., 2003), and PKA is the only known kinase for this site, though other protein kinase consensus sites exist and remain unexplored (Lonart et al., 2003). Phosphorylation of RIM1α at serine 413 is also required for presynaptically expressed LTP in cerebellar neurons. RIM1α−/− cerebellar cultures lack presynaptic LTP, which can be rescued by expression of wild-type RIM1α. Expression of PKA phosphorylation site mutant RIM1α S413A fails to rescue presynaptic LTP in the same cultures (Lonart et al., 2003). Thus, phosphorylation of RIM1α by PKA is thought to be responsible for synaptic potentiation via increased Pr, or at least to be permissive for some other mechanism of increased Pr.
RIM1α’s interaction with 14-3-3 protein and the small, synaptic vesicle-associated GTPase Rab3A is also required for presynaptic LTP (Castillo et al., 2002; Lonart et al., 1998). Thus, presynaptically expressed forms of LTP are thought to require presynaptic activation of PKA and phosphorylation of RIM1α, which leads, via required interactions with 14-3-3 and Rab3A, to increased Pr (or RIM1α phosphorylation and its interactions are permissive for a parallel mechanism to increase Pr).
The abnormalities in both short- and long-term plasticity in RIM1α−/− mice led to the question of whether RIM1α might play a critical role in learning and memory. A broad behavioral analysis of RIM1α−/− mice revealed abnormalities in learning and memory in both Morris water maze and fear conditioning (Powell et al., 2004). Appropriate controls indicated normal footshock sensitivity and normal swim speed in the RIM1α−/− mice. In response to novelty, RIM1α−/− mice were hyperactive. This might confound their ability to exhibit freezing behavior in fear conditioning experiments, though two separate controls suggested that the RIM1α−/− mice were capable of normal freezing responses. In the Morris water maze, the RIM1α−/− mice were initially slightly more thigmotaxic than controls and did not alter this strategy during the task, indicating a difficulty in shifting learning strategies and not necessarily a difference in spatial learning. In spite of the various synaptic physiology abnormalities and the widespread expression of RIM1α throughout the brain, RIM1α−/− mice revealed normal motor coordination and anxiety-like behaviors (Powell et al., 2004).
The abnormalities in Morris water maze behavior imply abnormal hippocampus-dependent learning in the RIM1α−/− mice. While RIM1α−/− mice are deficient in mfLTP, Rab3A−/− mice with mfLTP deficits, increased PPF, and abnormal synaptic depression exhibit normal learning and memory (Powell et al., 2004). Since mfLTP, PPF, and depression alone are unlikely to play a role in the RIM1α learning and memory deficits, the decrease in Pr in area CA1 seems a likely candidate.
A decrease in Pr in isolation, however, does not necessarily alter learning and memory behavior. Syt1R233Q+/+ mice have a similar decrease in Pr in CA1 as the RIM1α−/− mice, but they exhibit normal learning and memory using the same tasks (Powell et al., 2004). Regardless of any molecular accommodations in these mice, the decreased Pr remains, suggesting that Syt1R233Q+/+ mice can somehow accommodate at the synaptic or circuit level to dramatic alterations in their Pr setpoint
Other possibilities remain to explain the behavioral abnormalities in RIM1α-deficient mice. These include increased PTP at excitatory synapses and altered paired pulse depression (PPD) at inhibitory synapses. Furthermore, the decreased Pr in RIM1α−/− mice may differ from that of Syt1R233Q+/+ mice. For example, it is possible that the synaptotagmin 1 mutation affects inhibitory and excitatory synapses equally, whereas the loss of RIM1α may affect excitatory more than inhibitory synapses. Such differential regulation of neurotransmitter release between excitatory and inhibitory synapses has been described for other presynaptic proteins such as munc13-1 and munc13-2. In addition, it may be that the multiple synaptic abnormalities in RIM1−/− mice interact to lead to the behavioral deficits, though this conclusion requires excluding individual synaptic abnormalities as a cause. Finally, while LTP in area CA1 of the hippocampus was reportedly normal in RIM1α−/− mice, this experiment was performed in the presence of GABAA antagonists using a very strong, 3 s, 100 Hz, LTP induction paradigm. Thus, it will be of interest to examine LTP and LTD in area CA1 of the hippocampus in RIM1α−/− mice more thoroughly before examining additional possible physiologic explanations for the hippocampus-dependent learning deficits.
In fear conditioning, RIM1α−/− mice were abnormal in both context-dependent and cue-dependent fear conditioning. The abnormal cue-dependent fear conditioning implicates RIM1α in amygdala function. Thus, it will be of particular interest to examine LTP in the amygdala, where some forms of LTP are thought to be NMDA receptor-independent. This is an interesting proposition in light of recent data suggesting that some forms of heterosynaptic LTP in amygdala are presynaptically induced and expressed.
While the loss of RIM1α leads to learning and memory deficits, many questions remain. Is RIM1α modulated during learning in vivo? Could RIM1α be phosphorylated at its PKA site in the hippocampus during learning? Preliminary data from our laboratory suggest that RIM1α is phosphorylated by PKA in the hippocampus during contextual fear conditioning (unpublished observation, C.M. Powell). Does phosphorylation of this PKA site play an active role in learning and memory in vivo or is some more basic function of RIM1α merely permissive for normal LTP? What RIM1α binding proteins might also be important for learning and memory? Does the loss of RIM1α in a particular brain region correlate with the learning and memory deficits? These are questions currently under active investigation.
6.2. GAP-43
Elegant experiments over the past several years were among the first to implicate the presynaptic growth-associated protein (GAP-43, F1, B50) in normal learning and memory. These studies by Routtenberg and colleagues identified GAP-43 as a presynaptic protein kinase C (PKC) substrate that is phosphorylated during LTP in the dentate gyrus. They showed that GAP-43 is required for normal learning and memory as assessed in GAP-43 heterozygous knockdown mice (Rekart, Meiri, & Routtenberg, 2005). Indeed, transgenic overexpression of GAP-43, but not the PKC phosphorylation site mutant form of GAP-43, has been shown to increase both dentate gyrus LTP and learning in an 8-arm radial maze (Routtenberg, Cantallops, Zaffuto, Serrano, & Namgung, 2000). It seems that PKC phosphorylation of presynaptic GAP-43 is required for normal learning in vivo and that GAP-43 can bidirectionally modulate learning and memory in vivo.
Several possible mechanisms for GAP-43’s involvement in learning and memory have been proposed, including direct modulation of presynaptic neurotransmitter release (Routtenberg et al., 2000). GAP-43 interacts directly with components of the synaptic release machinery including the SNARE complex proteins (SNAP-25, syntaxin, and synaptobrevin) and synaptotagmin (depicted in Fig. 1) (Haruta, Takami, Ohmura, Misumi, & Ikehara, 1997). Some of these interactions seem to be dependent on PKC phosphorylation of GAP-43 (Haruta et al., 1997), making this interaction intriguing for the dynamic regulation of plasticity and memory. In fact, GAP-43 phosphorylation may lead to increased neurotransmitter release (Dekker et al., 1990; Heemskerk et al., 1990), while decreasing GAP-43 may decrease evoked neurotransmitter release (Hens et al., 1995; Ivins, Neve, Feller, Fidel, & Neve, 1993). While presynaptic effects of GAP-43 may serve to enhance plasticity and memory, another possible mechanism is GAP-43’s involvement in synaptic and circuit development, which might function to regulate plasticity and memory via developmental effects.
6.3. Complexin II
The complexin family of presynaptic proteins are small cytoplasmic proteins involved in modulating neurotransmitter release through their interactions with the SNARE complex (Chen et al., 2002; Marz & Hanson, 2002; Rizo & Sudhof, 2002). Complexin I/II double knockout mice die at birth, indicating their importance in neurotransmitter release (Reim et al., 2001). Complexin II knockout mice (CPLXII−/−), however, survive to maturity and exhibit deficits in both hippocampal LTP and learning and memory.
Takahashi and coworkers demonstrated normal synaptic transmission and PPF, but decreased CA1 LTP and mfLTPin CPLXII−/− mice (Takahashi et al., 1999). Unfortunately, their findings cannot be interpreted directly due to many experimental caveats. First, this study used extracellular field recordings and measured population spike amplitude as the main measure of synaptic strength for input/output curves, PPF, and LTP. Typically, one would measure the slope of the excitatory post-synaptic potential, a more direct measure of synaptic efficacy. When the population spike is used, alterations in cell excitability can account for any differences and could potentially confound the interpretation that LTP is decreased and that PPF is normal. Importantly, the authors make no mention of using NMDA receptor antagonists when examining mfLTP in area CA3 of the hippocampus. Thus, it is very difficult to exclude the possibility that their mfLTP is contaminated by recurrent-collateral LTP in area CA3. Nonetheless, their findings are consistent with the possibility that LTP is impaired in both CA1 and CA3 regions of the hippocampus, though further studies are necessary to reach a definitive conclusion.
Consistent with the hippocampal LTP data, CPLXII−/− mice exhibited learning deficits in the Morris water maze and two-choice swim tank reversal tasks as well as deficits of motor coordination on the rotating rod task (Glynn, Bortnick, & Morton, 2003). Interestingly, the water maze learning deficit increased with age of the animals, suggesting perhaps a neurodegenerative process or at least decreased mental reserve with age. It would be of great interest to examine a similar age-dependent relationship in additional knockout mice to better understand whether this is a feature specific to CPLXII−/− mice or a more general phenomenon.
6.4. The future of presynaptic proteins in learning and memory
These studies implicate presynaptic proteins and presynaptic function in learning and memory. Unfortunately, a complete picture of how presynaptic short-term plasticity, long-term plasticity, or presynaptic signaling is involved in learning and memory has not yet emerged. Ongoing studies will continue to examine existing presynaptic protein knockout mice that have been extensively characterized electrophysiologically. Future studies will focus on restricted, conditional knockout approaches, on modulation of presynaptic proteins during learning, and on examining how such dynamic regulation of presynaptic proteins is involved in learning and memory.
With some exceptions, the existing studies stop short of understanding how dynamic modulation of presynaptic proteins might play a role in both synaptic plasticity and learning. It will be important to understand how presynaptic proteins are modified during synaptic plasticity and during learning tasks in vivo. More precise molecular manipulations in mice including point mutations that alter targets of these modifications should also prove informative.
Also, it is likely that presynaptic proteins and presynaptic plasticity are critical for learning and memory in specific brain regions and indeed particular neuronal subtypes. Clearly, following screening studies on existing knockout mice, regionally targeted, conditional knockout approaches will be critical for understanding how specific molecules fit into various memory circuits. For example, the use of conditional knockout mice combined with focal, virally mediated cre recombinase expression is one novel way to approach this problem (Hommel, Sears, Georgescu, Simmons, & DiLeone, 2003).
While the focus to date has been on learning and memory systems in the hippocampus and amygdala, it is very likely that in formation processing in many other areas of the brain are intimately involved in learning and memory and other cognitive processes important in human disease. Presynaptic proteins and both the short- and long-term plasticity they subserve are likely to be critical in other cognitive domains such as sensorimotor gating, attention, executive functioning, impulsivity, olfactory learning, etc. Our understanding of cognitive function will be enhanced by broadening our behavioral horizons beyond a few simple learning tasks.
All of these issues must be approached in a multidisciplinary fashion combining targeted, conditional genetic manipulations in mice, broader behavioral screening, thorough electrophysiological characterization, biochemical studies, and correlation with effects on synaptic plasticity. We are now in an excellent position to link presynaptic proteins and presynaptic plasticity to cognitive behavior, thereby identifying potential new targets in the treatment of cognitive disorders. We must be willing to cross the synaptic divide in mammalian systems to gain a complete picture of the molecular, cellular, and physiologic basis of learning and memory.
Acknowledgments
This work was supported by NIMH MH065975-01A1 and a NARSAD Young Investigator Award, 2003 Lieber Investigator (C.M.P.). The author thanks Dr. Thomas Südhof for comments on portions of the manuscript.
References
- Abbott LF, Regehr WG. Synaptic computation. Nature. 2004;431:796–803. doi: 10.1038/nature03010. [DOI] [PubMed] [Google Scholar]
- Betz A, Thakur P, Junge HJ, Ashery U, Rhee JS, Scheuss V, et al. Functional interaction of the active zone proteins Munc13-1 and RIM1 in synaptic vesicle priming. Neuron. 2001;30:183–196. doi: 10.1016/s0896-6273(01)00272-0. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Nelson RJ. Behavioral phenotyping of transgenic and knockout animals: A cautionary tale. Lab Animal (NY) 2001;30:24–29. [PubMed] [Google Scholar]
- Bordi F, Reggiani A, Conquet F. Regulation of synaptic plasticity by mGluR1 studied in vivo in mGluR1 mutant mice. Brain Research. 1997;761:121–126. doi: 10.1016/s0006-8993(97)00320-x. [DOI] [PubMed] [Google Scholar]
- Brandon EP, Zhuo M, Huang YY, Qi M, Gerhold KA, Burton KA, et al. Hippocampal long-term depression and depotentiation are defective in mice carrying a targeted disruption of the gene encoding the RI beta subunit of cAMP-dependent protein kinase. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:8851–8855. doi: 10.1073/pnas.92.19.8851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calakos N, Schoch S, Sudhof TC, Malenka RC. Multiple roles for the active zone protein RIM1alpha in late stages of neurotransmitter release. Neuron. 2004;42:889–896. doi: 10.1016/j.neuron.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Janz R, Sudhof TC, Tzounopoulos T, Malenka RC, Nicoll RA. Rab3A is essential for mossy fibre long-term potentiation in the hippocampus. Nature. 1997;388:590–593. doi: 10.1038/41574. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Schoch S, Schmitz F, Sudhof TC, Malenka RC. RIM1alpha is required for presynaptic long-term potentiation. Nature. 2002;415:327–330. doi: 10.1038/415327a. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Weisskopf MG, Nicoll RA. The role of Ca2+ channels in hippocampal mossy fiber synaptic transmission and long-term potentiation. Neuron. 1994;12:261–269. doi: 10.1016/0896-6273(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Chapman PF, Frenguelli BG, Smith A, Chen CM, Silva AJ. The alpha-Ca2+/calmodulin kinase II: A bidirectional modulator of presynaptic plasticity. Neuron. 1995;14:591–597. doi: 10.1016/0896-6273(95)90315-1. [DOI] [PubMed] [Google Scholar]
- Chen C, Tonegawa S. Molecular genetic analysis of synaptic plasticity, activity-dependent neural development, learning, and memory in the mammalian brain. Annual Review of Neuroscience. 1997;20:157–184. doi: 10.1146/annurev.neuro.20.1.157. [DOI] [PubMed] [Google Scholar]
- Chen X, Tomchick DR, Kovrigin E, Arac D, Machius M, Sudhof TC, et al. Three-dimensional structure of the complexin/SNARE complex. Neuron. 2002;33:397–409. doi: 10.1016/s0896-6273(02)00583-4. [DOI] [PubMed] [Google Scholar]
- Conquet F, Bashir ZI, Davies CH, Daniel H, Ferraguti F, Bordi F, et al. Motor deficit and impairment of synaptic plasticity in mice lacking mGluR1. Nature. 1994;372:237–243. doi: 10.1038/372237a0. [DOI] [PubMed] [Google Scholar]
- Contractor A, Rogers C, Maron C, Henkemeyer M, Swanson GT, Heinemann SF. Trans-synaptic Eph receptor-ephrin signaling in hippocampal mossy fiber LTP. Science. 2002;296:1864–1869. doi: 10.1126/science.1069081. [DOI] [PubMed] [Google Scholar]
- Coppola T, Magnin-Luthi S, Perret-Menoud V, Gattesco S, Schiavo G, Regazzi R. Direct interaction of the Rab3 effector RIM with Ca2+ channels, SNAP-25, and synaptotagmin. The Journal of Biological Chemistry. 2001;276:32756–32762. doi: 10.1074/jbc.M100929200. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Behavioral phenotyping of transgenic and knockout mice: Experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Research. 1999;835:18–26. doi: 10.1016/s0006-8993(98)01258-x. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, et al. Behavioral phenotypes of inbred mouse strains: Implications and recommendations for molecular studies. Psychopharmacology (Berl) 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Paylor R. A proposed test battery and constellations of specific behavioral paradigms to investigate the behavioral phenotypes of transgenic and knockout mice. Hormones and Behavior. 1997;31:197–211. doi: 10.1006/hbeh.1997.1382. [DOI] [PubMed] [Google Scholar]
- D’Adamo P, Wolfer DP, Kopp C, Tobler I, Toniolo D, Lipp HP. Mice deficient for the synaptic vesicle protein Rab3a show impaired spatial reversal learning and increased explorative activity but none of the behavioral changes shown by mice deficient for the Rab3a regulator Gdi1. The European Journal of Neuroscience. 2004;19:1895–1905. doi: 10.1111/j.1460-9568.2004.03270.x. [DOI] [PubMed] [Google Scholar]
- Dekker LV, De Graan PN, Spierenburg H, De Wit M, Versteeg DH, Gispen WH. Evidence for a relationship between B-50 (GAP-43) and [3H]noradrenaline release in rat brain synaptosomes. European Journal of Pharmacology. 1990;188:113–122. doi: 10.1016/0922-4106(90)90046-z. [DOI] [PubMed] [Google Scholar]
- Ferguson GD, Anagnostaras SG, Silva AJ, Herschman HR. Deficits in memory and motor performance in synaptotagmin IV mutant mice. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:5598–5603. doi: 10.1073/pnas.100104597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson GD, Wang H, Herschman HR, Storm DR. Altered hippocampal short-term plasticity and associative memory in synaptotagmin IV (−/−) mice. Hippocampus. 2004;14:964–974. doi: 10.1002/hipo.20013. [DOI] [PubMed] [Google Scholar]
- Fernandez-Chacon R, Konigstorfer A, Gerber SH, Garcia J, Matos MF, Stevens CF, et al. Synaptotagmin I functions as a calcium regulator of release probability. Nature. 2001;410:41–49. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- Fisher SA, Fischer TM, Carew TJ. Multiple overlapping processes underlying short-term synaptic enhancement. Trends in Neuroscience. 1997;20:170–177. doi: 10.1016/s0166-2236(96)01001-6. [DOI] [PubMed] [Google Scholar]
- Frost WN, Clark GA, Kandel ER. Parallel processing of short-term memory for sensitization in Aplysia. Journal of Neurobiology. 1988;19:297–334. doi: 10.1002/neu.480190402. [DOI] [PubMed] [Google Scholar]
- Garcia-Junco-Clemente P, Linares-Clemente P, Fernandez-Chacon R. Active zones for presynaptic plasticity in the brain. Molecular Psychiatry. 2005;10:185–200. doi: 10.1038/sj.mp.4001628. [DOI] [PubMed] [Google Scholar]
- Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, et al. Synaptotagmin I: A major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- Glynn D, Bortnick RA, Morton AJ. Complexin II is essential for normal neurological function in mice. Human Molecular Genetics. 2003;12:2431–2448. doi: 10.1093/hmg/ddg249. [DOI] [PubMed] [Google Scholar]
- Goda Y, Stevens CF. Readily releasable pool size changes associated with long term depression. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:1283–1288. doi: 10.1073/pnas.95.3.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubellini P, Saulle E, Centonze D, Bonsi P, Pisani A, Bernardi G, et al. Selective involvement of mGlu1 receptors in corticostriatal LTD. Neuropharmacology. 2001;40:839–846. doi: 10.1016/s0028-3908(01)00021-1. [DOI] [PubMed] [Google Scholar]
- Gubellini P, Saulle E, Centonze D, Costa C, Tropepi D, Bernardi G, et al. Corticostriatal LTP requires combined mGluR1 and mGluR5 activation. Neuropharmacology. 2003;44:8–16. doi: 10.1016/s0028-3908(02)00214-9. [DOI] [PubMed] [Google Scholar]
- Haruta T, Takami N, Ohmura M, Misumi Y, Ikehara Y. Ca2+-dependent interaction of the growth-associated protein GAP-43 with the synaptic core complex. The Biochemical Journal. 1997;325(Pt 2):455–463. doi: 10.1042/bj3250455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heemskerk FM, Schrama LH, Gianotti C, Spierenburg H, Versteeg DH, De Graan PN, et al. 4-Aminopyridine stimulates B-50 (GAP43) phosphorylation and [3H]noradrenaline release in rat hippocampal slices. Journal of Neurochemistry. 1990;54:863–869. doi: 10.1111/j.1471-4159.1990.tb02331.x. [DOI] [PubMed] [Google Scholar]
- Hens JJ, De Wit M, Boomsma F, Mercken M, Oestreicher AB, Gispen WH, et al. N-terminal-specific anti-B-50 (GAP-43) antibodies inhibit Ca(2+)-induced noradrenaline release, B-50 phosphorylation and dephosphorylation, and calmodulin binding. Journal of Neurochemistry. 1995;64:1127–1136. doi: 10.1046/j.1471-4159.1995.64031127.x. [DOI] [PubMed] [Google Scholar]
- Hensbroek RA, Kamal A, Baars AM, Verhage M, Spruijt BM. Spatial, contextual and working memory are not affected by the absence of mossy fiber long-term potentiation and depression. Behavioural Brain Research. 2003;138:215–223. doi: 10.1016/s0166-4328(02)00243-7. [DOI] [PubMed] [Google Scholar]
- Henze DA, Urban NN, Barrionuevo G. The multifarious hippocampal mossy fiber pathway: A review. Neuroscience. 2000;98:407–427. doi: 10.1016/s0306-4522(00)00146-9. [DOI] [PubMed] [Google Scholar]
- Hommel JD, Sears RM, Georgescu D, Simmons DL, DiLeone RJ. Local gene knockdown in the brain using viral-mediated RNA interference. Nature Medicine. 2003;9:1539–1544. doi: 10.1038/nm964. [DOI] [PubMed] [Google Scholar]
- Huang YY, Kandel ER, Varshavsky L, Brandon EP, Qi M, Idzerda RL, et al. A genetic test of the effects of mutations in PKA on mossy fiber LTP and its relation to spatial and contextual learning. Cell. 1995;83:1211–1222. doi: 10.1016/0092-8674(95)90146-9. [DOI] [PubMed] [Google Scholar]
- Ivins KJ, Neve KA, Feller DJ, Fidel SA, Neve RL. Antisense GAP-43 inhibits the evoked release of dopamine from PC12 cells. Journal of Neurochemistry. 1993;60:626–633. doi: 10.1111/j.1471-4159.1993.tb03194.x. [DOI] [PubMed] [Google Scholar]
- Jaffe D, Johnston D. Induction of long-term potentiation at hippocampal mossy-fiber synapses follows a Hebbian rule. Journal of Neurophysiology. 1990;164:948–960. doi: 10.1152/jn.1990.64.3.948. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: A dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Kapur A, Yeckel MF, Gray R, Johnston D. L-Type calcium channels are required for one form of hippocampal mossy fiber LTP. Journal of Neurophysiology. 1998;79:2181–2190. doi: 10.1152/jn.1998.79.4.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Miledi R. The role of calcium in neuromuscular facilitation. The Journal of Physiology. 1968;195:481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Lee I, Gilbert P. A behavioral assessment of hippocampal function based on a subregional analysis. Reviews in the Neurosciences. 2004;15:333–351. doi: 10.1515/revneuro.2004.15.5.333. [DOI] [PubMed] [Google Scholar]
- Keverne EB. An evaluation of what the mouse knockout experiments are telling us about mammalian behaviour. Bioessays. 1997;19:1091–1098. doi: 10.1002/bies.950191208. [DOI] [PubMed] [Google Scholar]
- Koushika SP, Richmond JE, Hadwiger G, Weimer RM, Jorgensen EM, Nonet ML. A post-docking role for active zone protein Rim. Nature Neuroscience. 2001;4:997–1005. doi: 10.1038/nn732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar PA, Baker LP, Storm DR, Bowden DM. Expression of type I adenylyl cyclase in intrinsic pathways of the hippocampal formation of the macaque (Macaca nemestrina) Neuroscience Letters. 2001;299:181–184. doi: 10.1016/s0304-3940(01)01493-8. [DOI] [PubMed] [Google Scholar]
- Lapointe V, Morin F, Ratte S, Croce A, Conquet F, Lacaille JC. Synapse-specific mGluR1-dependent long-term potentiation in interneurones regulates mouse hippocampal inhibition. The Journal of Physiology. 2004;555:125–135. doi: 10.1113/jphysiol.2003.053603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littleton JT, Stern M, Schulze K, Perin M, Bellen HJ. Mutational analysis of Drosophila synaptotagmin demonstrates its essential role in Ca(2+)-activated neurotransmitter release. Cell. 1993;74:1125–1134. doi: 10.1016/0092-8674(93)90733-7. [DOI] [PubMed] [Google Scholar]
- Lonart G. RIM1: An edge for presynaptic plasticity. Trends in Neuroscience. 2002;25:329–332. doi: 10.1016/s0166-2236(02)02193-8. [DOI] [PubMed] [Google Scholar]
- Lonart G, Janz R, Johnson KM, Sudhof TC. Mechanism of action of rab3A in mossy fiber LTP. Neuron. 1998;21:1141–1150. doi: 10.1016/s0896-6273(00)80631-5. [DOI] [PubMed] [Google Scholar]
- Lonart G, Schoch S, Kaeser PS, Larkin CJ, Sudhof TC, Linden DJ. Phosphorylation of RIM1alpha by PKA triggers presynaptic long-term potentiation at cerebellar parallel fiber synapses. Cell. 2003;115:49–60. doi: 10.1016/s0092-8674(03)00727-x. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: An embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: An evaluation of the hypothesis. Annual Review of Neuroscience. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- Marz KE, Hanson PI. Sealed with a twist: Complexin and the synaptic SNARE complex. Trends in Neuroscience. 2002;25:381–383. doi: 10.1016/s0166-2236(02)02207-5. [DOI] [PubMed] [Google Scholar]
- Matilla A, Roberson ED, Banfi S, Morales J, Armstrong DL, Burright EN, et al. Mice lacking ataxin-1 display learning deficits and decreased hippocampal paired-pulse facilitation. The Journal of Neuroscience. 1998;18:5508–5516. doi: 10.1523/JNEUROSCI.18-14-05508.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor J, Nicoll RA. Hippocampal mossy fiber LTP is independent of postsynaptic calcium. Nature Neuroscience. 2001;4:125–126. doi: 10.1038/83941. [DOI] [PubMed] [Google Scholar]
- Mellor J, Nicoll RA, Schmitz D. Mediation of hippocampal mossy fiber long-term potentiation by presynaptic Ih channels. Science. 2002;295:143–147. doi: 10.1126/science.1064285. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Quirk MC, Chitwood RA, Watanabe M, Yeckel MF, Sun LD, et al. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science. 2002;297:211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Sun LD, Quirk MC, Rondi-Reig L, Wilson MA, Tonegawa S. Hippocampal CA3 NMDA receptors are crucial for memory acquisition of one-time experience. Neuron. 2003;38:305–315. doi: 10.1016/s0896-6273(03)00165-x. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Malenka RC. Contrasting properties of two forms of long-term potentiation in the hippocampus. Nature. 1995;377:115–118. doi: 10.1038/377115a0. [DOI] [PubMed] [Google Scholar]
- Ohtsuka T, Takao-Rikitsu E, Inoue E, Inoue M, Takeuchi M, Matsubara K, et al. Cast: A novel protein of the cytomatrix at the active zone of synapses that forms a ternary complex with RIM1 and munc13-1. The Journal of Cell Biology. 2002;158:577–590. doi: 10.1083/jcb.200202083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto C, Kovalchuk Y, Wolfer DP, Gass P, Martin M, Zuschratter W, et al. Impairment of mossy fiber long-term potentiation and associative learning in pituitary adenylate cyclase activating polypeptide type I receptor-deficient mice. The Journal of Neuroscience. 2001a;21:5520–5527. doi: 10.1523/JNEUROSCI.21-15-05520.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto C, Martin M, Paul Wolfer D, Lipp H, Maldonado R, Schutz G. Altered emotional behavior in PACAP-type-I-receptor-deficient mice. Brain Research Molecular Brain Research. 2001b;92:78–84. doi: 10.1016/s0169-328x(01)00153-x. [DOI] [PubMed] [Google Scholar]
- Poser S, Storm DR. Role of Ca2+-stimulated adenylyl cyclases in LTP and memory formation. International Journal of Developmental Neuroscience. 2001;19:387–394. doi: 10.1016/s0736-5748(00)00094-0. [DOI] [PubMed] [Google Scholar]
- Powell CM, Schoch S, Monteggia L, Barrot M, Matos MF, Feldmann N, et al. The presynaptic active zone protein RIM1alpha is critical for normal learning and memory. Neuron. 2004;42:143–153. doi: 10.1016/s0896-6273(04)00146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi M, Zhuo M, Skalhegg BS, Brandon EP, Kandel ER, McK-night GS, et al. Impaired hippocampal plasticity in mice lacking the Cβ1 catalytic subunit of cAMP-dependent protein kinase. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:1571–1576. doi: 10.1073/pnas.93.4.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reim K, Mansour M, Varoqueaux F, McMahon HT, Sudhof TC, Brose N, et al. Complexins regulate a late step in Ca2+-dependent neurotransmitter release. Cell. 2001;104:71–81. doi: 10.1016/s0092-8674(01)00192-1. [DOI] [PubMed] [Google Scholar]
- Rekart JL, Meiri K, Routtenberg A. Hippocampal-dependent memory is impaired in heterozygous GAP-43 knockout mice. Hippocampus. 2005;15:1–7. doi: 10.1002/hipo.20045. [DOI] [PubMed] [Google Scholar]
- Rizo J, Sudhof TC. Snares and Munc18 in synaptic vesicle fusion. Nature Reviews Neuroscience. 2002;3:641–653. doi: 10.1038/nrn898. [DOI] [PubMed] [Google Scholar]
- Roberts AC, Glanzman DL. Learning in Aplysia: Looking at synaptic plasticity from both sides. Trends in Neuroscience. 2003;26:662–670. doi: 10.1016/j.tins.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Stevens CF. Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron. 1996;16:1197–1207. doi: 10.1016/s0896-6273(00)80146-4. [DOI] [PubMed] [Google Scholar]
- Routtenberg A, Cantallops I, Zaffuto S, Serrano P, Namgung U. Enhanced learning after genetic overexpression of a brain growth protein. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:7657–7662. doi: 10.1073/pnas.97.13.7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin PA, Malenka RC, Nicoll RA. Cyclic AMP mediates a presynaptic form of LTP at cerebellar parallel fiber synapses. Neuron. 1996a;16:797–803. doi: 10.1016/s0896-6273(00)80099-9. [DOI] [PubMed] [Google Scholar]
- Salin PA, Scanziani M, Malenka RC, Nicoll RA. Distinct short-term plasticity at two excitatory synapses in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 1996b;93:13304–13309. doi: 10.1073/pnas.93.23.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz D, Mellor J, Breustedt J, Nicoll RA. Presynaptic kainate receptors impart an associative property to hippocampal mossy fiber long-term potentiation. Nature Neuroscience. 2003;6:1058–1063. doi: 10.1038/nn1116. [DOI] [PubMed] [Google Scholar]
- Schoch S, Castillo PE, Jo T, Mukherjee K, Geppert M, Wang Y, et al. RIM1alpha forms a protein scaffold for regulating neuro-transmitter release at the active zone. Nature. 2002;415:321–326. doi: 10.1038/415321a. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Rosahl TW, Chapman PF, Marowitz Z, Friedman E, Frankland PW, et al. Impaired learning in mice with abnormal short-lived plasticity. Current Biology. 1996;6:1509–1518. doi: 10.1016/s0960-9822(96)00756-7. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Smith AM, Giese KP. Gene targeting and the biology of learning and memory. Annual Review of Genetics. 1997;31:527–546. doi: 10.1146/annurev.genet.31.1.527. [DOI] [PubMed] [Google Scholar]
- Stevens CF, Tsujimoto T. Estimates for the pool size of releasable quanta at a single central synapse and for the time required to refill the pool. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:846–849. doi: 10.1073/pnas.92.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC. The synaptic vesicle cycle. Annual Review of Neuroscience. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- Sun L, Bittner MA, Holz RW. Rim, a component of the presynaptic active zone and modulator of exocytosis, binds 14-3-3 through its N terminus. The Journal of Biological Chemistry. 2003;278:38301–38309. doi: 10.1074/jbc.M212801200. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Ujihara H, Huang GZ, Yagyu KI, Sanbo M, Kaba H, et al. Reduced hippocampal LTP in mice lacking a presynaptic protein: Complexin II. The European Journal of Neuroscience. 1999;11:2359–2366. doi: 10.1046/j.1460-9568.1999.00652.x. [DOI] [PubMed] [Google Scholar]
- Takao-Rikitsu E, Mochida S, Inoue E, Deguchi-Tawarada M, Inoue M, Ohtsuka T, et al. Physical and functional interaction of the active zone proteins, CAST, RIM1, and Bassoon, in neurotransmitter release. The Journal of Cell Biology. 2004;164:301–311. doi: 10.1083/jcb.200307101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong G, Malenka RC, Nicoll RA. Long-term potentiation in cultures of single hippocampal granule cells: A presynaptic form of plasticity. Neuron. 1996;16:1147–1157. doi: 10.1016/s0896-6273(00)80141-5. [DOI] [PubMed] [Google Scholar]
- Villacres EC, Wong ST, Chavkin C, Storm DR. Type I adenylyl cyclase mutant mice have impaired mossy fiber long-term potentiation. The Journal of Neuroscience. 1998;18:3186–3194. doi: 10.1523/JNEUROSCI.18-09-03186.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Yeckel MF, Johnston D, Zucker RS. Photolysis of postsynaptic caged Ca2+ can potentiate and depress mossy fiber synaptic responses in rat hippocampal CA3 pyramidal neurons. Journal of Neurophysiology. 2004;91:1596–1607. doi: 10.1152/jn.01073.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liu X, Biederer T, Sudhof TC. A family of RIM-binding proteins regulated by alternative splicing: Implications for the genesis of synaptic active zones. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:14464–14469. doi: 10.1073/pnas.182532999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Okamoto M, Schmitz F, Hofmann K, Sudhof TC. Rim is a putative Rab3 effector in regulating synaptic-vesicle fusion. Nature. 1997;388:593–598. doi: 10.1038/41580. [DOI] [PubMed] [Google Scholar]
- Wang Y, Sugita S, Sudhof TC. The RIM/NIM family of neuronal C2 domain proteins. Interactions with Rab3 and a new class of Src homology 3 domain proteins. The Journal of Biological Chemistry. 2000;275:20033–20044. doi: 10.1074/jbc.M909008199. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Castillo PE, Zalutsky RA, Nicoll RA. Mediation of hippocampal mossy fiber long-term potentiation by cyclic AMP. Science. 1994;265:1878–1882. doi: 10.1126/science.7916482. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Nicoll RA. Presynaptic changes during mossy fibre LTP revealed by NMDA receptor-mediated synaptic responses. Nature. 1995;376:256–259. doi: 10.1038/376256a0. [DOI] [PubMed] [Google Scholar]
- Williams S, Johnston D. Long-term potentiation of hippocampal mossy fiber synapses is blocked by postsynaptic injection of calcium chelators. Neuron. 1989;3:583–588. doi: 10.1016/0896-6273(89)90268-7. [DOI] [PubMed] [Google Scholar]
- Wu ZL, Thomas SA, Villacres EC, Xia Z, Simmons ML, Chavkin C, et al. Altered behavior and long-term potentiation in type I adenylyl cyclase mutant mice. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:220–224. doi: 10.1073/pnas.92.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeckel MF, Kapur A, Johnston D. Multiple forms of LTP in hippocampal CA3 neurons use a common postsynaptic mechanism. Nature Neuroscience. 1999;2:625–633. doi: 10.1038/10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annual Review of Physiology. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]