Abstract
A group of antibiotic resistance genes (ARGs) (blaTEM, blaCTX-M-1, mecA, armA, qnrA, and qnrS) were analyzed by real-time quantitative PCR (qPCR) in bacteriophage DNA isolated from feces from 80 healthy humans. Seventy-seven percent of the samples were positive in phage DNA for one or more ARGs. blaTEM, qnrA, and, blaCTX-M-1 were the most abundant, and armA, qnrS, and mecA were less prevalent. Free bacteriophages carrying ARGs may contribute to the mobilization of ARGs in intra- and extraintestinal environments.
TEXT
Antibiotic resistance may be obtained by spontaneous mutations or acquired by the incorporation of antibiotic resistance genes (ARGs) (1). ARGs spread between cells by using genetic platforms known as mobile genetic elements (MGEs). The most commonly studied MGEs are plasmids, transposons, integrons, and, more recently, bacteriophages (2).
Bacteriophages or phage-related elements carry ARGs in Gram-positive (3–6) and Gram-negative (7–10) bacteria. Recently, some studies have suggested that the role of phages carrying ARGs in the environment is much more important than previously thought (2, 11–13). Abundant ARGs have been reported in the bacteriophage DNA fraction of fecally contaminated water (14–16), and metagenomic analyses indicate that there are abundant ARGs in viral DNA (17). As a result of their higher incidence in clinical settings, much effort has been devoted to the study of plasmids, integrons, and transposons. However, there is little information on phages carrying ARGs in clinical settings.
This study analyzes a group of ARGs in phage DNA isolated from stool samples. The ARGs studied include two groups of beta-lactamase genes from Gram-negative bacteria (blaTEM and blaCTX-M-1 group); mecA, responsible for resistance to methicillin in Staphylococcus spp.; armA, a gene which confers high-level resistance to aminoglycosides in Gram-negative bacteria; and qnrA and qnrS, plasmid-mediated genes that provide some degree of reduced quinolone susceptibility.
The study was performed using 80 human fecal samples from 46 females and 34 males from 6 months to 102 years of age who visited the Sant Pau Hospital (Barcelona, Spain) during a 6-month period. Stool samples were processed according to conventional protocols for the isolation of enteropathogenic bacteria, rotavirus, and adenovirus and were microscopically examined for protozoa. Only samples that were negative for these pathogens were included in the study. None of the patients selected was involved in a food-borne outbreak or showed any severe gastrointestinal pathology. To our knowledge, none of the patients were receiving antibiotic treatment during the time of the study, although previous antibiotic treatments could not be excluded.
Fecal samples were homogenized to a 1:5 (wt/vol) dilution in phosphate-buffered saline (PBS) by magnetic stirring for 15 min. Fifty milliliters of the homogenate was centrifuged at 3,000 ×g, and the phage lysate was purified and concentrated as described previously (15, 16). Phage suspensions were treated with DNase (100 U/ml) to eliminate free DNA outside the phage particles. To confirm total removal of nonencapsidated DNA, eubacterial 16S rRNA genes and the different ARGs (see Table S1 in the supplemental material) were evaluated in the sample after DNase treatment and before its disencapsidation.
Phage DNA was extracted from the suspension as previously described (16, 18). Total DNA (including Gram-positive and Gram-negative bacterial and viral DNA) was extracted from 200 μl of the homogenate by use of a QIAamp DNA stool minikit (Qiagen Inc., Valencia, CA) in accordance with the manufacturer's instructions.
Standard and quantitative PCR (qPCR) procedures for blaTEM, blaCTX-M-1 group, and mecA were performed as previously described (16). The armA qPCR assay was designed using the sequence of armA in plasmid pMUR050 (NC_007682.3) from an Escherichia coli pig isolate (19). pMUR050 was also used to generate standard curves (16). The armA qPCR assay has an average efficiency of 98.4% and a detection limit of 2.74 gene copies (GC). The qnrA qPCR assay detects seven variants (qnrA 1 to 7), and the qnrS qPCR assay detects six variants (qnrS 1 to 6) (20). The 565-bp fragment of qnrA was obtained from E. coli strain 266, and the 425-bp fragment of qnrS was obtained from the environmental strain Enterobacter cloacae 565 isolated from sewage. Both fragments were cloned in pGEM-T-Easy vector (Promega, Barcelona, Spain) to generate the standard curves (16). The qnrA qPCR assay showed 98.2% efficiency and a detection limit of 3.1 GC/μl, and the qnrS assay showed 99.4% efficiency and a detection limit of 8.3 GC/μl. All qPCR assays (see Table S1 in the supplemental material) were performed under standard conditions (15, 16). To screen for PCR inhibition, dilutions of the standard of each gene were spiked with the DNA isolated from the samples, and the results were compared to the true number of GC of the target genes in the standards. No inhibition of the PCR by the samples was detected. Sequencing was performed as previously described (16).
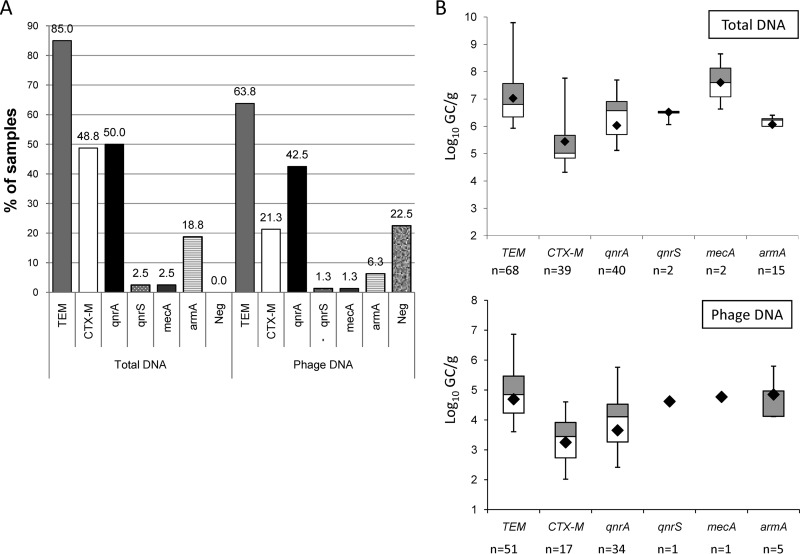
When the ARGs in the total DNA were analyzed, all samples were positive for one or more ARGs (Fig. 1A). When purified phage DNA was used, 22.5% of the samples were negative for all ARGs and 77.5% harbored one or more ARGs. All the ARGs analyzed were present in our samples, and the distribution of the ARGs in phage DNA was found to follow the same order of prevalence as in total DNA (Fig. 1A). No correlation was found between the patient's age or gender and the presence of ARGs in total or phage DNA.
FIG 1.
(A) Proportion of each ARG studied among 80 samples in total and phage DNA. In total DNA, values are expressed for 80 positive samples. In total DNA, all samples were positive for at least one ARG, while in phage DNA, 18 samples were negative for all ARGs (Neg). (B) Box plot chart with the averaged values obtained from all ARGs in positive samples for total and phage DNA. Within the box plot chart, the cross-pieces of each box plot represent (from top to bottom) maximum, upper quartile, median (horizontal black bar), lower quartile, and minimum values. A black diamond indicates the mean value. The gray boxes in the box plot chart include samples showing values within the 75th percentile, and white boxes include samples showing values within the 25th percentile. n, number of positive samples for each ARG.
When the different ARGs found were quantified per gram of fecal sample (Fig. 1B), the highest values were seen in total DNA with differences in log10 units between total and phage DNA ranging from 1.2 for armA to 2.8 for mecA. In phage DNA, blaTEM showed the highest prevalence and abundance, with values as high as 6.8 log10 GC/g. The second most prevalent gene was qnrA. blaCTX-M group1 was the third most prevalent gene, although the densities were lower than those of the two previous ARGs. armA showed a low prevalence of only five positive samples but remarkably high densities (up to 6 log10 units). There were a small number of samples positive for qnrS and mecA in phage DNA, which did not allow us to draw conclusions regarding their abundance.
The prevalence of the genes in this study corroborates the descriptions found in the literature. blaTEM is probably the most prevalent ARG worldwide (21, 22) and in phage DNA in wastewater (15, 16). blaCTX-M-1 group includes blaCTX-M-15, which over the past decade has become one of the most prevalent extended-spectrum beta-lactamase genes (23). The horizontally transferable qnrA and qnrS genes (24, 25) are widely distributed in our region and clinically relevant (20), particularly qnrA, which was the first quinolone resistance gene described and the most commonly found (26, 27). armA is also highly prevalent in Enterobacteriaceae, and it is spreading worldwide (19, 28, 29). mecA was not prevalent in this study, perhaps because Staphylococcus spp. are not commonly found in the intestinal tract. The previous detection of mecA in phages from sewage (16) may be attributable to a nonfecal origin.
The qPCR assays produce a short amplicon, and to better confirm the identity of the ARGs detected in phage DNA by sequencing, we amplified longer fragments by conventional PCR. Sequencing was performed with forward and reverse primers and in duplicate. The consensus of all sequences generated fragments of different sizes for blaTEM, blaCTX-M group1, qnrA, and qnrS that matched 100% previously described sequences of the corresponding ARGs from different bacterial genera available in the GenBank database (http://www.ncbi.nlm.nih.gov/GenBank/index.html) (Table 1). armA was not amplified by conventional PCR in the few samples that tested positive for this gene. The specific variant of the ARG sequenced was not determined because of the length and location of some of these fragments and because the limited amount of DNA obtained from the samples did not allow the amplification of the complete ARGs.
TABLE 1.
Sequence homology of some of the ARGs amplified from phage DNA of fecal samplesa
| Fragment size (bp) | ARG assay result | First sequence homologue | GenBank accession no. |
|---|---|---|---|
| 574 | blaTEM | blaTEM-1 | GQ470444.1 |
| 571 | blaTEM | blaTEM-1 | JN002397.1 |
| AY832935.1 | |||
| 580 | blaTEM | blaTEM-116 | NZ_ADUR01000022 |
| NZ_ADFT01000030 | |||
| AY265885.1 | |||
| JF327796.1 | |||
| 576 | blaTEM | blaTEM-1 | JN002397.1 |
| AY832935.1 | |||
| 386 | blaCTX-M | blaCTX-M-15 | JX129219.1 |
| blaCTX-M-15-like | KC107824.1 | ||
| 380 | blaCTX-M | blaCTX-M-33 | AY238472.1 |
| blaCTX-M-15-like | KC107824.1 | ||
| blaCTX-M-114 | GQ351346.1 | ||
| 360 | blaCTX-M | blaCTX-M-15 | NC_013122.1 |
| 359 | blaCTX-M-33 | AY238472.1 | |
| blaCTX-M-15 | JX129219.1 | ||
| 359 | blaCTX-M | blaCTX-M-109 | JF274248.1 |
| blaCTX-M-33 | AY238472.1 | ||
| blaCTX-M-15 | EF158301.1 | ||
| 355 | blaCTX-M | blaCTX-M-15 | EU979556.1 |
| HQ256746.1 | |||
| blaCTX-M-15-like | JX268658.1 | ||
| 412 | mecA | mecA | KC243783.1 |
| 437 | JQ764731.1 | ||
| 466 | HE978800.1 | ||
| 436 | HE978798.1 | ||
| GU301100.1 | |||
| GU301101.1 | |||
| 452 | qnrA | qnrA1 | JN687470.1 |
| JF728153.1 | |||
| JF969163.1 | |||
| HQ184955.1 | |||
| GU324551.1 | |||
| 456 | qnrA | qnrA1 | JN687470.1 |
| JF728153.1 | |||
| JF969163.1 | |||
| GQ891753.1 | |||
| GU295955.1 | |||
| 352 | qnrS | qnrS2 | HE616910.2 |
| JN315883.1 | |||
| JF773350.1 | |||
| DQ485530.1 |
The fragment size corresponds to the length of the consensus sequence generated with the forward and reverse sequences of the PCR amplimer, performed in duplicate, and is the fragment used to search for homologies. No PCR amplimer was obtained for the armA gene with the samples that showed positive for this ARG.
The high prevalence of ARGs in phage DNA isolated from fecally polluted environments (14–16) indicates that phages could play a role in the mobilization of ARGs. The question we address here is whether the origin of these phages could be free phage particles excreted in feces, free phages present in those environments, or phages induced from bacteria (allochthonous or autochthonous) occurring in those environments. The results of the present study clearly indicate that free phages encoding ARGs are directly excreted from healthy individuals via feces. The phage particles could be infectious or not to a given host, but as previously shown, the genes harbored by the phages are functional and able to confer resistance to a given antibiotic (16). This would make it likely that a phage harboring ARGs infects a new host and transfers the ARG that could be incorporated into the host genome by recombination.
The significant prevalence of phages in human feces has been shown by recent metagenomics studies (30–32). Among these, many remark on the high number of sequences of ARGs in the virome fraction of the human gut (17, 33). A recent report indicates that the number of ARGs in the “phageome” is significant and that the ARG content in the phage DNA fraction of the gut microbiome increases after antibiotic treatment (34). Specific phages could carry ARGs of Gram-positive (4–6) and Gram-negative (7–10) bacteria. Although these reports do not indicate the nature of the phage particles, some authors suggest that they could have been generated by means of generalized transducing phages that can mobilize chromosomal genes and plasmids (4, 8, 35).
As phages harboring ARGs are excreted in human feces from healthy individuals (or animals) (14–16), there must be many of these phages circulating in the population. These phages probably exist in some food and water, but they will not normally be detected by regular quality controls. They could be ingested as free particles and cause conversion of susceptible hosts within the gut that could be later selected by the presence of antimicrobial agents. At present, phages seem to be suitable vehicles for the mobilization and transmission of ARGs, and probably many other genes, in both intra- and extraintestinal environments.
Supplementary Material
ACKNOWLEDGMENTS
We thank B. González-Zorn for the construct containing the armA gene used for the qPCR standards.
This study was partially supported by the Spanish Ministry of Education and Science (AGL2012-30880), by the Generalitat de Catalunya (2009SGR1043), by a project of the RecerCaixa program (La Caixa), and by the Fundación Ramon Areces and was partially supported by the Spanish Network for the Research in Infectious Diseases (REIPI RD06/0008). Marta Colomer-Lluch has a grant FI from the Generalitat de Catalunya.
Footnotes
Published ahead of print 28 October 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01684-13.
REFERENCES
- 1.Lupo A, Coyne S, Berendonk TU. 2012. Origin and evolution of antibiotic resistance: the common mechanisms of emergence and spread in water bodies. Front. Microbiol. 3:18. 10.3389/fmicb.2012.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang XX, Zhang T, Fang HH. 2009. Antibiotic resistance genes in water environment. Appl. Microbiol. Biotechnol. 82:397–414. 10.1007/s00253-008-1829-z [DOI] [PubMed] [Google Scholar]
- 3.Ubukata K, Konno M, Fujii R. 1975. Transduction of drug resistance to tetracycline, chloramphenicol, macrolides, lincomycin and clindamycin with phages induced from Streptococcus pyogenes. J. Antibiot. (Tokyo) 28:681–688. 10.7164/antibiotics.28.681 [DOI] [PubMed] [Google Scholar]
- 4.Schuch R, Fischetti VA. 2006. Detailed genomic analysis of the beta and gamma phages infecting Bacillus anthracis: implications for evolution of environmental fitness and antibiotic resistance. J. Bacteriol. 188:3037–3051. 10.1128/JB.188.8.3037-3051.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazaheri Nezhad Fard R, Barton MD, Heuzenroeder MW. 2011. Bacteriophage-mediated transduction of antibiotic resistance in Enterococci. Lett. Appl. Microbiol. 52:559–564. 10.1111/j.1472-765X.2011.03043.x [DOI] [PubMed] [Google Scholar]
- 6.Varga M, Kuntová L, Pantùček R, Mašlaňová I, Rùžičková V, Doškaø J. 2012. Efficient transfer of antibiotic resistance plasmids by transduction within methicillin-resistant Staphylococcus aureus USA300 clone. FEMS Microbiol. Lett. 332:146–152. 10.1111/j.1574-6968.2012.02589.x [DOI] [PubMed] [Google Scholar]
- 7.Blahová J, Hupková M, Krcméry V, Schäfer V. 1992. Imipenem and cefotaxime resistance: transduction by wild-type phages in hospital strains of Pseudomonas aeruginosa. J. Chemother. 4:335–337 [DOI] [PubMed] [Google Scholar]
- 8.Willi K, Sandmeier H, Kulik EM, Meyer J. 1997. Transduction of antibiotic resistance markers among Actinobacillus actinomycetemcomitans strains by temperate bacteriophages Aa phi 23. Cell. Mol. Life Sci. 53:904–910. 10.1007/s000180050109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmieger H, Schicklmaier P. 1999. Transduction of multiple drug resistance of Salmonella enterica serovar typhimurium DT104. FEMS Microbiol. Lett. 170:251–256. 10.1111/j.1574-6968.1999.tb13381.x [DOI] [PubMed] [Google Scholar]
- 10.Oliver A, Coque TM, Alonso D, Valverde A, Baquero F, Cantón R. 2005. CTX-M-10 linked to a phage-related element is widely disseminated among Enterobacteriaceae in a Spanish hospital. Antimicrob. Agents Chemother. 49:1567–1571. 10.1128/AAC.49.4.1567-1571.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Academy of Microbiology 2009. Antibiotic resistance: an ecological perspective on an old problem. A report from the American Academy of Microbiology. American Academy of Microbiology, Washington, DC [Google Scholar]
- 12.Cangelosi GA, Freitag NE, Buckley MR. 2004. From outside to inside: environmental microorganisms as human pathogens. American Academy of Microbiology, Washington, DC [Google Scholar]
- 13.Brabban AD, Hite E, Callaway TR. 2005. Evolution of foodborne pathogens via temperate bacteriophage-mediated gene transfer. Foodborne Pathog. Dis. 2:287–303. 10.1089/fpd.2005.2.287 [DOI] [PubMed] [Google Scholar]
- 14.Muniesa M, García A, Miró E, Mirelis B, Prats G, Jofre J, Navarro F. 2004. Bacteriophages and diffusion of beta-lactamase genes. Emerg. Infect. Dis. 10:1134–1137. 10.3201/eid1006.030472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colomer-Lluch M, Imamovic L, Jofre J, Muniesa M. 2011. Bacteriophages carrying antibiotic resistance genes in fecal waste from cattle, pigs, and poultry. Antimicrob. Agents Chemother. 55:4908–4911. 10.1128/AAC.00535-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colomer-Lluch M, Jofre J, Muniesa M. 2011. Antibiotic resistance genes in the bacteriophage DNA fraction of environmental samples. PLoS One 6:e17549. 10.1371/journal.pone.0017549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minot S, Sinha R, Chen J, Li H, Keilbaugh SA, Wu GD, Lewis JD, Bushman FD. 2011. The human gut virome: inter-individual variation and dynamic response to diet. Genome Res. 21:1616–1625. 10.1101/gr.122705.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 19.González-Zorn B, Catalan A, Escudero JA, Domínguez L, Teshager T, Porrero C, Moreno MA. 2005. Genetic basis for dissemination of armA. J. Antimicrob. Chemother. 56:583–585. 10.1093/jac/dki246 [DOI] [PubMed] [Google Scholar]
- 20.Lavilla S, González-López JJ, Sabaté M, García-Fernández A, Larrosa MN, Bartolomé RM, Carattoli A, Prats G. 2008. Prevalence of qnr genes among extended-spectrum beta-lactamase-producing enterobacterial isolates in Barcelona, Spain. J. Antimicrob. Chemother. 61:291–295. 10.1093/jac/dkm448 [DOI] [PubMed] [Google Scholar]
- 21.Rodríguez-Baño J, Alcalá JC, Cisneros JM, Grill F, Oliver A, Horcajada JP, Tórtola T, Mirelis B, Navarro G, Cuenca M, Esteve M, Peña C, Llanos AC, Cantón R, Pascual A. 2008. Community infections caused by extended-spectrum β-lactamase-producing Escherichia coli. Arch. Intern. Med. 168:1897–1902. 10.1001/archinte.168.17.1897 [DOI] [PubMed] [Google Scholar]
- 22.Díaz MA, Hernández-Bello JR, Rodríguez-Baño J, Martínez-Martínez L, Calvo J, Blanco J, Pascual A, Spanish Group for Nosocomial Infections (GEIH) 2010. The diversity of Escherichia coli producing extended-spectrum β-lactamases in Spain: second nationwide study. J. Clin. Microbiol. 48:2840–2845. 10.1128/JCM.02147-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cantón R, Coque TM. 2006. The CTX-M beta-lactamase pandemic. Curr. Opin. Microbiol. 9:466–475. 10.1016/j.mib.2006.08.011 [DOI] [PubMed] [Google Scholar]
- 24.Rodríguez-Martínez JM, Cano ME, Velasco C, Martínez-Martínez L, Pascual A. 2011. Plasmid-mediated quinolone resistance: an update. J. Infect. Chemother. 17:149–182. 10.1007/s10156-010-0120-2 [DOI] [PubMed] [Google Scholar]
- 25.Robicsek A, Jacoby GA, Hooper DC. 2006. The worldwide emergence of plasmid-mediated quinolone resistance. Lancet Infect. Dis. 6:629–640. 10.1016/S1473-3099(06)70599-0 [DOI] [PubMed] [Google Scholar]
- 26.Robicsek A, Strahilevitz J, Sahm DF, Jacoby GA, Hooper DC. 2006. qnr prevalence in ceftazidime-resistant Enterobacteriaceae isolates from the United States. Antimicrob. Agents Chemother. 50:2872–2874. 10.1128/AAC.01647-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nordmann P, Poirel L. 2005. Emergence of plasmid-mediated resistance to quinolones in Enterobacteriaceae. J. Antimicrob. Chemother. 56:463–469. 10.1093/jac/dki245 [DOI] [PubMed] [Google Scholar]
- 28.Galimand M, Sabtcheva S, Courvalin P, Lambert T. 2005. Worldwide disseminated armA aminoglycoside resistance methylase gene is borne by composite transposon Tn1548. Antimicrob. Agents Chemother. 49:2949–2953. 10.1128/AAC.49.7.2949-2953.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Granier SA, Hidalgo L, San Millan A, Escudero JA, Gutierrez B, Brisabois A, Gonzalez-Zorn B. 2011. ArmA methyltransferase in a monophasic Salmonella enterica isolate from food. Antimicrob. Agents Chemother. 55:5262–5266. 10.1128/AAC.00308-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breitbart M, Hewson I, Felts B, Mahaffy JM, Nulton J, Salamon P, Rohwer F. 2003. Metagenomic analyses of an uncultured viral community from human feces. J. Bacteriol. 185:6220–6223. 10.1128/JB.185.20.6220-6223.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reyes A, Haynes M, Hanson N, Angly FE, Heath AC, Rohwer F, Gordon JI. 2010. Viruses in the faecal microbiota of monozygotic twins and their mothers. Nature 466:334–338. 10.1038/nature09199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stern A, Mick E, Tirosh I, Sagy O, Sorek R. 2012. CRISPR targeting reveals a reservoir of common phages associated with the human gut microbiome. Genome Res. 22:1985–1994. 10.1101/gr.138297.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Kim Y, Ma Q, Hong SH, Pokusaeva K, Sturino JM, Wood TK. 2010. Cryptic prophages help bacteria cope with adverse environments. Nat. Commun. 1:147. 10.1038/ncomms1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Modi SR, Lee HH, Spina CS, Collins JJ. 2013. Antibiotic treatment expands the resistance reservoir and ecological network of the phage metagenome. 2013. Nature 499:219–222. 10.1038/nature12212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schicklmaier P, Moser E, Wieland T, Rabsch W, Schmieger H. 1998. A comparative study on the frequency of prophages among natural isolates of Salmonella and Escherichia coli with emphasis on generalized transducers. Antonie Van Leeuwenhoek 73:49–54. 10.1023/A:1000748505550 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.