Abstract
Rapid pathogen detection is crucial for the timely introduction of therapeutics. Two groups (one in the United Kingdom and one in the United States) independently evaluated inhibitor-resistant PCR reagents for the direct testing of substrates. In the United Kingdom, a multiplexed Bacillus anthracis (target) and Bacillus subtilis (internal-control) PCR was used to evaluate 4 reagents against 5 PCR inhibitors and down-selected the TaqMan Fast Virus 1-Step master mix (Life Technologies Inc.). In the United States, four real-time PCR assays (targeting B. anthracis, Brucella melitensis, Venezuelan equine encephalitis virus [VEEV], and Orthopoxvirus spp.) were used to evaluate 5 reagents (plus the Fast Virus master mix) against buffer, blood, and soil samples and down-selected the KAPA Blood Direct master mix (KAPA Biosystems Inc.) with added Platinum Taq (Life Technologies). The down-selected reagents underwent further testing. In the United Kingdom experiments, both reagents were tested against seven contrived aerosol collector samples containing B. anthracis Ames DNA and B. subtilis spores from a commercial formulation (BioBall). In PCR assays with reaction mixtures containing 40% crude sample, an airfield-collected sample induced inhibition of the B. subtilis PCR with the KAPA reagent and complete failure of both PCRs with the Fast Virus reagent. However, both reagents allowed successful PCR for all other samples—which inhibited PCRs with a non-inhibitor-resistant reagent. In the United States, a cross-assay limit-of-detection (LoD) study in blood was conducted. The KAPA Blood Direct reagent allowed the detection of agent DNA (by four PCRs) at higher concentrations of blood in the reaction mixture (2.5%) than the Fast Virus reagent (0.5%), although LoDs differed between assays and reagent combinations. Across both groups, the KAPA Blood Direct reagent was determined to be the optimal reagent for inhibition relief in PCR.
INTRODUCTION
PCR is used to detect biological warfare agents (BWAs) from various sample types (1–5). In this context, multiple PCR inhibitors negatively affect agent detection; these are known to include compounds such as humic acids, hemoglobin, complex polysaccharides, hematin, and urea (6–11). The nature of inhibition due to these compounds is not always understood, although interference with the performance of the polymerase and with the degradation/capture of nucleic acids are thought to be common mechanisms (8).
Standard nucleic acid extraction and purification technologies (12) can impose high operative, logistical, and temporal burdens. Even with highly efficient extraction methods, some target nucleic acid is lost (13). Inhibitor carryover from the purification process can also produce false-negative results for PCR detection (14). In the context of BWA detection, the potential for false-negative results becomes increasingly important, since minimal overlap exists between the diagnostic and therapeutic windows for these highly pathogenic viruses and bacteria. To facilitate a faster time-to-answer and to minimize the operative and logistical burdens, PCR reagents with reported resistance to various PCR inhibitors have been developed or sold commercially (15–17). Modifications include N-terminal DNA polymerase truncation (15), the addition of betaine or protease inhibitors (16), or the addition of bovine serum albumin (BSA) (16, 18). These modified reagents offer the possibility of testing unprocessed or crude samples. All of the target nucleic acid would therefore be present in the reaction mixture, but at the cost of maintaining the PCR inhibitors found in the respective matrix.
In this paper, we present the results of two independent evaluations of commercially available inhibitor-resistant PCR reagents using real-time PCR assays for the detection of highly pathogenic bacteria and viruses. The best-performing reagent from each evaluation was then tested by the alternate institute by spiking BWA DNA into a panel of aerosol samples or whole blood in order to determine whether the down-selected PCR reagents could tolerate a variety of PCR inhibitor types.
MATERIALS AND METHODS
United Kingdom reagents and PCR conditions.
A real-time PCR assay (pXO1-MGB) designed to detect Bacillus anthracis (19) was multiplexed with an in-house real-time PCR (Bsub) designed to detect the nonpathogenic species Bacillus subtilis. With the addition of a commercially available, freeze-dried, soluble spore preparation—BioBall 10K B. subtilis (BTF, Sydney, Australia)—to the sample, the B. subtilis PCR acts as an internal (DNA extraction) control PCR, similar in principle to systems developed elsewhere (20). In this context, successful amplification by the B. subtilis PCR would demonstrate that thermal hold and cycling steps were enabling the detection of spore DNA. For this purpose, the primer concentrations of the Bsub PCR were limited to prevent adverse competition effects with the target pXO1-MGB PCR. Assay sequences and concentrations are summarized in Table S1 in the supplemental material.
A standard PCR master mix (containing 50 mM Tris-HCl, 50 μM EGTA, 1 μg/μl BSA, 4 mM MgCl2, 0.04 U/μl JumpStart Taq polymerase [Sigma, United Kingdom], and 200 μM deoxynucleoside triphosphates [dNTPs]) was used as a baseline (non-inhibitor-tolerant) reference. Four real-time PCR reagents were selected for testing on the basis of reported inhibitor resistance: TaqMan Environmental master mix 2.0 (Life Technologies), Path-ID qPCR master mix (Life Technologies), QuantiTect 1-step RT-PCR NoROX master mix (Qiagen), and TaqMan Fast Virus 1-step master mix (Life Technologies). The standard PCR master mix thermocycling profile consisted of 95°C for 3 min, followed by 45 cycles of 95°C for 15 s and 60°C for 30 s. The Environmental and Path-ID master mixes had the same cycling conditions except with an initial temperature (95°C) hold (hot start) of 10 min instead of 3 min. The Fast Virus and QuantiTect master mix profiles comprised 95°C for 3 min (15 min for QuantiTect) followed by 45 cycles of 95°C for 15 s and 60°C for 60 s. All PCRs were performed on a SmartCycler PCR platform (Cepheid), and results were analyzed in terms of the quantification cycle (Cq), the cycle number at which fluorescence is first detected.
Initial United Kingdom screen of inhibitor resistance chemistries.
Five commercially available compounds were chosen to represent different mechanisms of PCR inhibition: humic acid, colloidal silica, chlorophyll a, dextran sulfate, and urban dust. All compounds were purchased from the same supplier (Sigma-Aldrich, United Kingdom). The compounds were suspended in water or dimethyl sulfoxide (DMSO) (for chlorophyll a) and were diluted to appropriate concentrations. To test the inhibitor tolerances of the five master mixes, each PCR inhibitor was added to PCR mixtures containing 1 pg B. anthracis and 1 pg B. subtilis DNA. The concentration of the inhibitor was increased until complete PCR inhibition was observed (no Cq) in the majority of reactions/reagents. Twenty-five-microliter PCR mixtures comprised 12.5 μl 2× master mix (or 6.25 μl 4× master mix and 6.25 μl water for the Fast Virus master mix), 2.5 μl primer-probe mixture, 5 μl B. anthracis and B. subtilis DNA, and 5 μl inhibitor sample. All Cq data were recorded and analyzed in the United Kingdom study.
U.S. reagents and PCR conditions.
Sample buffer (phosphate-buffered saline [PBS; Life Technologies, Grand Island, NY]), whole blood (Bioreclamation, Westbury, NY), and soil (National Institute of Standards and Technology, Germantown, MD) were assessed with each chemistry for inhibition relief. A stock soil suspension (10%, wt/vol) was generated by suspending 5 g soil in a total of 50 ml PBS with 0.05% Tween 20 (Sigma-Aldrich, St. Louis, MO). Serial dilutions of genomic DNA (B. anthracis Ames, Brucella melitensis 16M, or vaccinia virus Lister) or RNA (Venezuelan equine encephalitis virus [VEEV] IA/IB Trinidad donkey) were added to the diluted matrix for a final concentration of 2.5% or 0.25% buffer, 2.5%, 0.5%, 0.25%, or 0.05% whole blood, or 0.25%, 0.005%, 0.025%, or 0.005% soil in the reaction mixture.
Five different inhibitor-resistant PCR chemistries were selected from a previous study (17). These included three separate chemistries: Phire Hot Start DNA polymerase (New England BioLabs, Ipswich, MA), the Phusion Blood Direct PCR kit (New England BioLabs), and the KAPA Blood PCR kit (KAPA Biosystems, Woburn, MA). In addition, two buffers (Ampdirect buffer [Rockland Immunochemicals, Gilbertsville, PA] and STRboost buffer [Biomatrica, San Diego, CA]) were run with the Phire Hot Start polymerase (Phire). As noted in previous studies, these polymerases were not designed for real-time (TaqMan) PCR detection, specifically 5′-to-3′ digestion of the labeled PCR probe (17). Therefore, 0.8 U of Platinum Taq (Life Technologies) was added to each reaction mixture to ensure adequate probe hydrolysis and fluorophore detection. The Fast Virus kit, down-selected in the United Kingdom evaluation, was also included; however, Platinum Taq was not added to this reagent, because it is specifically designed for real-time (TaqMan) PCR.
Each reagent was run with multiple real-time PCR assays in order to assess inhibition with varying reaction kinetics. These previously published assays were the BAPA and Omp2a (1), VEEV NSP4 (21), and orthopoxvirus hemagglutinin (OP HA) (22) assays, which target the genes of B. anthracis protective antigen (BAPA), Brucella melitensis outer membrane protein 2a, VEEV NSP4, and orthopoxvirus hemagglutinin, respectively.
The R.A.P.I.D. platform (BioFire Diagnostics, Salt Lake City, UT) was used for all real-time PCRs, with cycling conditions of 2 min at 95°C and 45 cycles of 95°C for 1 s and 60°C for 20 s, followed by a single fluorescence measurement after the 60°C amplification. For VEEV, the cycling conditions included a reverse transcriptase step of 50°C for 15 min, followed by a 5-min hold at 95°C and 45 cycles of 95°C for 1 s and 60°C for 20 s using the SuperScript One-Step RT-PCR system (Life Technologies, Grand Island, NY) supplemented with 0.25 mM BSA (Sigma-Aldrich, St. Louis, MO). A PCR was considered positive if the Cq value was less than 40 cycles.
Initial U.S. screen of inhibitor resistance chemistries.
A preliminary limit-of-detection (LoD) study was conducted with each of the six inhibitor-resistant master mixes involving two serial dilutions of nucleic acid: series 1 (10 pg, 1 pg, 100 fg, 50 fg, and 10 fg) and series 2 (2 pg, 200 fg, 20 fg, 10 fg, and 2 fg). Dilutions of the matrix were run with the nucleic acid dilution series. Series 1 was evaluated with 2.5% buffer, 2.5% and 0.25% whole blood, and 0.25% and 0.025% soil. Series 2 was evaluated with 0.25% buffer, 0.5% and 0.05% whole blood, and 0.05% and 0.005% soil. These combinations allowed for a preliminary estimation of the amount of target nucleic acid detectable within different dilutions of matrix. Each sample was run in duplicate, and both samples had to be positive for that DNA or RNA sample to be considered positive by real-time PCR.
United Kingdom evaluation of down-selected reagents against aerosol samples.
A horizontal wetted wall cyclone (HWWC) aerosol collector (23) was situated in one of seven different outdoor scenarios. Each 700-liter·min−1 HWWC was run for as long as 6 h depending on the scenario. The collection buffer (sterile distilled water with 0.01% Tween 80 [Sigma-Aldrich, United Kingdom]) was aspirated out of the collection zone. The scenarios were as follows: “farmyard,” within the environs of a working sheep farm; “airfield,” situated near the runway of an operational airfield; “burning combustibles,” situated downwind from burning straw, wood, and tires; “diesel generator,” situated next to an operating diesel generator; “burning fuel,” situated downwind from burning fuel; “firing range,” situated on an operational artillery range; “dockyard,” within the environs of a working dockyard. Buffer samples collected from the HWWC from each scenario are shown in Fig. S1 in the supplemental material.
Five hundred microliters of each aerosol sample was spiked with B. anthracis DNA to give a concentration of 100 fg·μl−1. To incorporate a DNA extraction control system into the test procedure, a single 10K BioBall (bioMérieux) containing 10,000 B. subtilis NCTC 10040 spores was added to each sample. Ten-microliter replicates of each sample (containing 1 pg B. anthracis DNA and approximately 200 B. subtilis spores) were then tested by this multiplexed PCR using the down-selected inhibitor-resistant master mixes (Fast Virus, United Kingdom; KAPA, United States) and the standard PCR master mix (United Kingdom). PCR mixtures comprised 2.5 μl of a primer-probe mixture, 10 μl cyclone sample, and either (i) 6.25 μl 4× master mix (Fast Virus), (ii) 12.5 μl 2× master mix (KAPA) and 0.8 U Platinum Taq, or (iii) 12.5 μl of the standard 2× master mix. Reaction volumes were adjusted to 25 μl with the addition of water. Eight replicates of each sample were tested with each PCR reagent. A blank cyclone buffer sample was also tested with each PCR reagent to act as a negative control (no PCR inhibitor).
U.S. evaluation of down-selected reagents against whole-blood samples spiked with BWA DNA.
Following the preliminary LoD determination, the reagents selected by the U.S. (KAPA) and United Kingdom (Fast Virus) groups were assessed for LoD confirmation with each real-time PCR assay in whole blood. Confirmation of the LoD required 29/30 replicates to be positive. If fewer than 29 replicates yielded positive results, the LoD determination was repeated at the next higher target concentration until at least 29 replicates were positive. Using this metric, the LoD was confirmed with 85% success at ∼95% confidence at a specific concentration based on binomial sampling statistics. This analysis was performed at either 2.5% (1:10 dilution of whole blood) or 0.5% (1:50 dilution of whole blood) of the sample matrix.
RESULTS
Initial United Kingdom screen of inhibitor resistance chemistries.
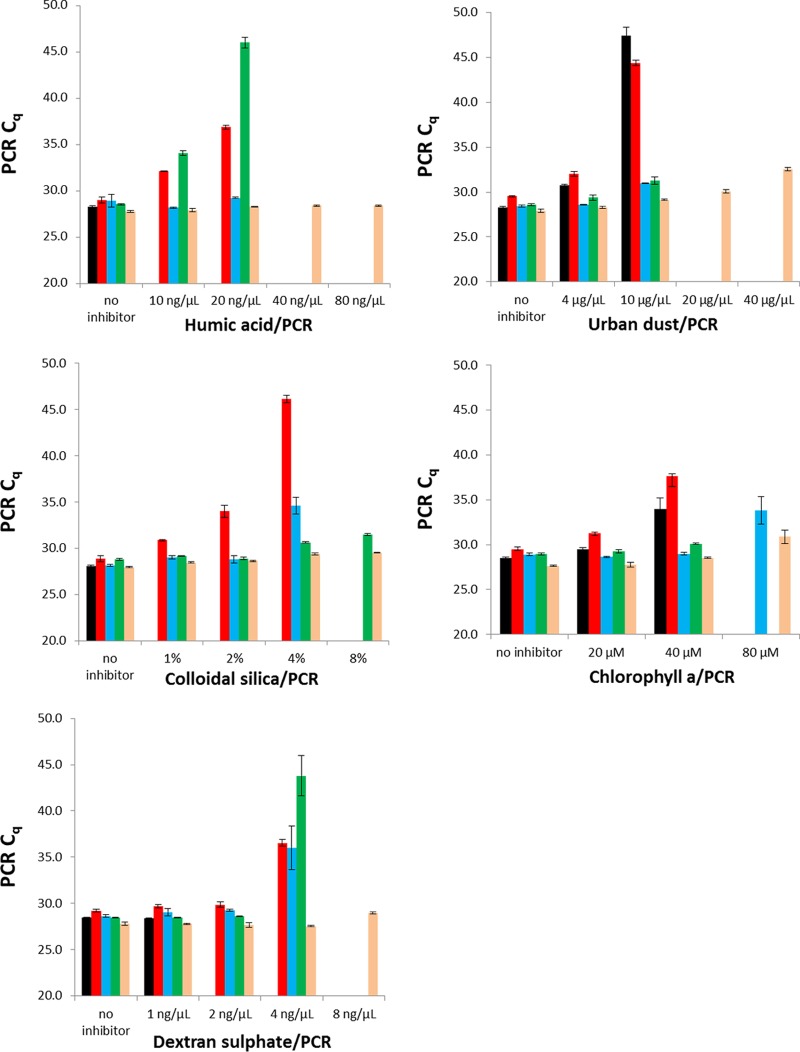
PCR (pXO1-MGB) results from each United Kingdom reagent-inhibitor combination are graphically represented in Fig. 1. Briefly, increasing the concentration of each inhibitor resulted in elevated Cq values and/or an increased number of negative replicates with nearly all reagents tested. At the highest concentration of each inhibitor, only the Fast Virus reagent supported effective PCR performance for both PCR assays in the multiplex PCR (Bsub PCR assay [data not shown]). The TaqMan Fast Virus 1-step master mix was therefore chosen as the down-selected reagent by the United Kingdom group.
FIG 1.
Inhibitor tolerances of five real-time PCR master mixes. Each PCR mixture contained 1 pg B. anthracis DNA (Ames) with increasing concentrations of humic acid, urban dust, colloidal silica, chlorophyll a, or dextran sulfate. Black, standard master mix; red, TaqMan Environmental master mix 2.0; blue, Path-ID qPCR master mix; green, QuantiTect 1-step RT-PCR NoROX master mix; beige, TaqMan Fast Virus 1-step master mix. Error bars represent standard deviations from three replicates.
Initial U.S. screen of inhibitor resistance chemistries.
We determined preliminary LoDs s (pLoDs) for 4 different real-time PCRs with each of the 5 U.S. formulations and the Fast Virus master mix in sample buffer, whole blood, and soil in order to determine the potential impact for inhibition relief across assays (Table 1). The addition of serial dilutions of nucleic acid to dilution series of the matrix showed that no single set of reactants performed optimally in all matrices or across different assays. Counterintuitively, the buffer (Dulbecco's PBS [DPBS]) induced some inhibition, based on observed pLoDs and dilution of the buffer matrix. On the basis of these and previous data (17), the U.S. group down-selected the KAPA Blood Direct reagent. Specifically, KAPA was the only chemistry that detected target nucleic acid for all assays at the highest concentrations of soil and whole blood.
TABLE 1.
Preliminary LoD determination by the U.S. group for four PCR assays with six inhibitor-resistant PCR reagents in varying total reaction concentrations of DPBS, soil, and whole blood
| Assay and reagent | LoDa with the indicated % of: |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1× DPBS |
Whole blood |
Soil |
||||||||
| 25.0 | 5.0 | 2.50 | 0.50 | 0.25 | 0.05 | 0.25 | 0.05 | 0.025 | 0.005 | |
| BAPA | ||||||||||
| Phire | 10 pg | 10 fg | ND | 20 fg | 50 fg | 10 fg | ND | 10 fg | 10 fg | 10 fg |
| Phusion | 50 fg | 2 pg | 50 fg | 2 pg | 50 fg | 2 fg | 50 fg | 2 fg | 10 fg | 2 fg |
| KAPA | 50 fg | 10 fg | 50 fg | 10 fg | 100 fg | 10 fg | 1 pg | 200 fg | 10 fg | 20 fg |
| Fast Virus | 100 fg | 20 fg | ND | 2 pg | 1 pg | 200 fg | 1 pg | 200 fg | 100 fg | 200 fg |
| Phire plus STRboost | 10 fg | 2 pg | ND | ND | 10 pg | 2 pg | 1 pg | 2 pg | 100 fg | 2 pg |
| Phire plus Ampdirect | 50 fg | 200 fg | ND | 10 fg | ND | 200 fg | ND | 200 fg | 100 fg | 200 fg |
| Omp2A | ||||||||||
| Phire | 50 fg | 200 fg | 1 pg | 10 fg | 100 fg | 200 fg | 10 pg | 10 fg | 100 fg | 200 fg |
| Phusion | 10 fg | 200 fg | ND | ND | ND | 200 fg | 10 fg | 2 fg | 50 fg | 2 pg |
| KAPA | 10 fg | 200 fg | 1 pg | 200 fg | 100 fg | 200 fg | 100 fg | 200 fg | 50 fg | 2 pg |
| Fast Virus | 1 pg | 200 fg | 10 pg | 2 pg | 100 fg | 200 fg | 50 fg | 20 fg | 10 fg | 200 fg |
| Phire plus STRboost | 1 pg | 200 fg | 10 pg | 2 pg | 50 fg | 200 fg | 50 fg | 200 fg | 50 fg | 200 fg |
| Phire plus Ampdirect | 50 fg | 200 fg | 100 fg | 200 fg | 10 pg | 200 fg | ND | 10 fg | 10 fg | 200 fg |
| VEEV NSP | ||||||||||
| Phire | 10 fg | 10 fg | ND | 200 fg | 10 fg | 20 fg | ND | 10 fg | 10 fg | 2 fg |
| Phusion | 10 fg | 2 fg | ND | 200 fg | ND | 2 fg | 1 pg | 2 fg | 10 fg | 10 fg |
| KAPA | 10 fg | 2 fg | 10 pg | ND | 50 fg | 2 fg | 10 fg | 2 fg | 10 fg | 2 fg |
| Fast Virus | 50 fg | 20 fg | ND | ND | 10 pg | 2 pg | 10 fg | 10 fg | 10 fg | 20 fg |
| Phire plus STRboost | 50 fg | 10 fg | 50 fg | 100 fg | 50 fg | 50 fg | 10 fg | 10 fg | 50 fg | 20 fg |
| Phire plus Ampdirect | 10 fg | 10 fg | ND | ND | 10 pg | 200 fg | ND | 2 pg | 10 fg | 10 fg |
| OP HA | ||||||||||
| Phire | 10 pg | 200 fg | ND | 2 pg | ND | 20 fg | ND | ND | 1 pg | 2 pg |
| Phusion | 100 fg | 200 fg | ND | ND | 1 pg | 20 fg | 1 pg | 200 fg | 10 fg | 20 fg |
| KAPA | 100 fg | 200 fg | 1 pg | 200 fg | 50 fg | 20 fg | 50 fg | 20 fg | 10 fg | 200 fg |
| Fast Virus | 1 pg | 2 pg | 10 pg | 2 pg | 1 pg | 200 fg | 50 fg | 20 fg | 50 fg | 200 fg |
| Phire plus STRboost | 100 fg | 200 fg | ND | 2 pg | 50 fg | 10 fg | 10 fg | 20 fg | 10 fg | 10 fg |
| Phire plus Ampdirect | 100 fg | 200 fg | ND | 200 fg | ND | 200 fg | ND | 2 pg | 50 fg | 200 fg |
The lowest concentration at which both replicates of duplicate samples yielded positive results. ND, not detected.
United Kingdom evaluation of down-selected reagents against aerosol samples spiked with BWA DNA and B. subtilis spores.
PCR results for each reagent–aerosol collector sample combination can be found in Table 2. Briefly, the U.S.-down-selected KAPA-Platinum Taq combination mix supported the activity of each PCR in the presence of each of the aerosol collector samples, except for the Bsub internal-control PCR with the airfield cyclone sample. The United Kingdom-selected Fast Virus reagent supported the activity of each PCR except for the airfield cyclone sample, where no PCR activity (either pXO1 or BSub2) was supported. Except for the firing-range cyclone sample, all of the aerosol collector samples inhibited PCRs with the standard PCR master mix. General linear models were fitted to the Cq data. In the majority of cases where Cq values could be compared, Fast Virus PCRs gave significantly lower Cq values than most of the KAPA PCRs (Table 2). Cq values and analysis differed across the aerosol samples. In general, the airfield sample induced more failures than the other samples, and the burning-combustibles and burning-fuels samples produced higher Cq values. The dockyard sample and the buffer control produced lower Cq values (analysis not shown).
TABLE 2.
Comparison by the United Kingdom group of the performances of down-selected inhibitor-resistant PCR reagents tested against aerosol collector samples spiked with B. anthracis DNA and B. subtilis spores
| Sample type | No. of positive PCRsa (mean Cqb) for: |
|||||
|---|---|---|---|---|---|---|
| Target pXO1-MGB (B. anthracis) PCR |
DNA extraction control Bsub (B. subtilis) PCR |
|||||
| Standard PCR master mix | Fast Virus 1-step master mix | KAPA Blood PCR master mix | Standard PCR master mix | Fast Virus 1-step master mix | KAPA Blood PCR master mix | |
| Airfield | 0/8 | 0/8 | 8/8 (32.37) | 0/8 | 0/8 | 0/8 |
| Sheep farm | 0/8 | 8/8 (31.30) | 8/8 (31.50) | 0/8 | 8/8 (37.62) | 8/8 (28.81e) |
| Burning combustibles | 0/8 | 8/8 (32.59d) | 8/8 (33.68) | 0/8 | 8/8 (29.92d) | 8/8 (30.85) |
| Diesel generator | 0/8 | 8/8 (31.22d) | 8/8 (32.58) | 0/8 | 8/8 (29.94d) | 8/8 (30.60) |
| Burning fuels | 0/8 | 8/8 (32.93d) | 8/8 (33.67) | 0/8 | 8/8 (29.81d) | 8/8 (30.59) |
| Firing range | 8/8 (33.91) | 8/8 (31.91) | 8/8 (32.34) | 7/8 (34.04) | 8/8 (28.59d) | 8/8 (29.79) |
| Dockyard | 0/8 | 8/8 (30.63d) | 8/8 (31.58) | 0/8 | 8/8 (28.30d) | 8/8 (29.13) |
| Sterile distilled water + 0.01% Tween 80 | 8/8 (33.64) | 8/8 (30.93c) | 8/8 (31.30c) | 8/8 (31.40) | 8/8 (29.46c) | 8/8 (29.94c) |
From 8 replicates.
Cq, PCR cycle number at which fluorescence was first detected in 45-cycle PCR. Means were calculated for positive PCRs only.
Significantly lower than the Cq value for the respective PCR with the standard PCR master mix (99% confidence level).
Significantly lower than the Cq value for the respective PCR with the KAPA Blood PCR master mix (99% confidence level).
Significantly lower than the Cq value for the respective PCR with the Fast Virus 1-step master mix (99% confidence level).
U.S. evaluation of down-selected reagents against whole-blood samples spiked with BWA DNA.
The LoD confirmation procedure documented the abilities of both the Fast Virus and KAPA reagents to detect several BWA targets across a statistically relevant number of replicates in a blood matrix. We chose whole blood, as opposed to soil, for LoD confirmation, because the pLoD evaluation showed blood to be the more challenging of the matrices tested. The concentrations determined in the pLoD evaluation often did not reflect the confirmed LoD. This was due to the difficulty of achieving the necessary 29 of 30 positive replicates for confirmation. In cases where this issue arose, we increased the amount of nucleic acid and reran the 30 replicates. Based on the data, optimal inhibition relief depended greatly on the specific assay in question (Table 3). For example, the Fast Virus reagent detected 200 fg orthopoxvirus DNA (OP HA assay) while detecting 2 pg B. anthracis DNA (BAPA assay) in reaction mixtures with the same percentage of blood. Similarly, the KAPA reagent detected 100 fg BAPA and 1 pg OP HA DNA in reaction mixtures with the same percentage of blood. Overall, the KAPA reagent performed more consistently, as reflected in the consistently lower concentration of target material detected at a higher percentage of whole blood.
TABLE 3.
Determination by the U.S. group of LoDs for four PCRs with the KAPA and Fast Virus reagents within whole blood
| Assay | KAPA reagent |
Fast Virus reagent |
||
|---|---|---|---|---|
| LoD (% matrixa) | Cqb (CVc) | LoD (% matrix) | Cq (CV) | |
| BAPA | 100 fg (2.5) | 33.51 (3.01) | 2 pg (0.05) | 30.61 (0.82) |
| Omp2A | 200 fgd (0.5) | 33.93 (16.31) | 200 fg (0.05) | 34.51 (2.53) |
| VEEV NSP | 10 pg (0.25) | 26.75 (1.12) | 2 pg (0.05) | 30.56 (3.72) |
| OP HA | 1 pg (2.5) | 35.18 (6.28) | 200 fg (0.05) | 36.36 (2.23) |
% matrix, percentage of blood in the reaction mixture.
PCR cycle number at which fluorescence was first detected in 45-cycle PCR.
CV, coefficient of variation, calculated as [(standard deviation of the mean/mean) × 100] and expressed as a percentage.
Twenty-nine of 30 replicates were positive.
DISCUSSION
This study reports on the evaluations of nine commercially available PCR reagents for inhibitor resistance. The United Kingdom group evaluated reagents specifically designed to support real-time (TaqMan) PCR, while the U.S. group evaluated reagents selected from a previous study (17), which were not specifically designed to support TaqMan PCR chemistry. For reagents that did not support TaqMan chemistry, Platinum Taq was included in the reaction mixture to allow for probe hydrolysis and detection. Reagents were evaluated across a spectrum of environmental and clinical matrices for inhibition relief across a wide range of PCR inhibitors, many of which were not part of the individual manufacturers' intended application of their respective products. For example, the information supplied with the reagents indicates that the Environmental and Path-ID master mixes allow for real-time PCR with environmental and animal health samples, respectively, while the Fast Virus master mix is designed for real-time PCR in samples containing inhibitors found in blood and stool. All U.S. reagents were developed to facilitate PCR from blood samples except for the Phire reagent, which is marketed as an enhanced polymerase.
The overarching evaluation was conducted within the framework of two independent research programs. The United Kingdom study was conducted primarily to find inhibition relief in environmental samples, while the U.S. study was targeted at both clinical and environmental samples. No funding was available to plan and conduct a joint evaluation; there was only an avenue for exchanging information and preparing a report on the data generated. The decision that each institute would test the “best” reagent, as determined by the other, exposes a potential limitation to the evaluation. A given reagent tested at one institute may not have been tested against its optimal sample type at the other. For example, the United Kingdom-screened Path-ID reagent, designed for use against animal health samples, has not been tested against animal health (or similar clinical) sample types in the overall study.
United Kingdom evaluation and test of the Fast Virus and KAPA Blood Direct reagents in aerosol samples.
The United Kingdom evaluation utilized a multiplexed PCR assay comprising an assay for a BWA target (B. anthracis) and an internal-control PCR with a non-BWA target (B. subtilis). The initial study indicated that the Fast Virus reagent had a clear performance advantage over the other three inhibitor-resistant reagents when tested against increasing concentrations of five environmental PCR inhibitors. At the highest concentrations of three inhibitors (humic acid, urban dust, and dextran sulfate), only the Fast Virus reagent supported PCR activity, while for the other two, colloidal silica and chlorophyll a, the QuantiTect and Path-ID reagents, respectively, also allowed positive PCRs. The study was not extended to determine the concentrations at which the Fast Virus reagent would not support PCR, although for some compounds (urban dust and chlorophyll a), at the highest concentrations, the Fast Virus Cq values were higher than previous values.
The second round of the United Kingdom study focused on testing contrived aerosol samples from 7 different scenarios, which were generated in an attempt to gather as wide a range of inhibitors found in urban, rural, and military environments as possible. PCR mixtures (for both Fast Virus- and KAPA-supported PCRs) contained 40% crude sample (10 μl of a 25-μl total reaction mixture). The presence of undetermined PCR inhibitors in all samples was observed by the failure of the standard PCR master mix to generate any positive results for 6 sample types and by the elevated Bsub PCR Cq values from the firing-range sample. It was notable that the one sample with which the two inhibitor-resistant master mixes could not completely prevent inhibition was the airfield sample, which had no observable particulates. Although we have not determined what the PCR inhibitor was in the airfield sample, this should be considered if any biosurveillance system is to be run in the vicinity of jet aircraft. Where present, particulates were purposely left in the reaction mixtures, in order to give as robust a challenge as possible, and also because high levels of particulates can be associated with increased levels of aerosolized pathogens (24). Samples that did contain observable particulates (burning combustibles, burning fuels) did not cause total PCR inhibition, even though these samples had a dark black coloration. However, the Cq values with these two samples were higher than those with some of the other samples. The SmartCycler PCR system requires reaction tubes to be centrifuged before insertion into the platform, which pulls particulates to the bottom of the reaction chamber; this procedure appears not to have affected the optical measurement of fluorescence while still allowing sample lysis and the release of DNA from the sample (and nontarget B. subtilis control spores) by the thermal hold and cycling steps.
Due to the small amounts of sample available, we were unable to undertake limit-of-detection studies with the aerosol samples. Therefore, it was not possible to determine whether the significantly lower Cq values generally observed across most sample types when the Fast Virus reagent was used would have resulted in a greater sensitivity of PCRs with this reagent than with the KAPA Blood reagent. Each sample tested contained 1 pg of B. anthracis Ames DNA, an amount corresponding to approximately 175 genome equivalents (GEs) (3). With mean Cq values in the low 30s, it would appear possible that at least a 10-fold decrease in agent concentration could be detected by these assays and reagents. In context, 17.5 GEs in a 10-μl sample volume would equate to an agent concentration of 1.75 × 103 CFU·ml−1 (17.5 × 100) in an aerosol sample.
This extrapolation does not account for factors such as the efficiency of spore lysis within the PCR assay chamber and/or the presence or absence of DNA on spore surfaces (25). Although by adding B. subtilis spores into the PCR we have shown that direct detection of spore DNA is possible, it may be that in this instance, the PCR thermal hold (95°C; 2 or 3 min) and subsequent cycling steps are simply releasing DNA from the exospore and are not facilitating spore lysis and thus access to endospore DNA. Although reasonable amounts of exosporium-associated DNA (approximately 2 fg per spore) have been found on Bacillus spores (26), it has also been shown that PCR detection from B. anthracis spores can be enhanced by incorporating a pre-PCR sonication lysis step into the assay procedure (27). Therefore, depending on the target agent(s), it may be necessary to engineer a rapid lysis step into a collector or assay consumable, when an inhibitor-resistant polymerase is used in a biosurveillance system, in order to meet or exceed the potential LoD stated above. Of note, any such lysis steps may have to be mechanical, since we are aware that direct addition of lysis buffers containing guanidinium salts, as found in many DNA extraction kits, can inhibit PCRs conducted with the Fast Virus master mix (S. A. Weller, unpublished data).
Both the Fast Virus and KAPA reagents offer advantages and disadvantages in the testing of environmental samples for biosurveillance purposes. The KAPA reagent demonstrated inhibitor resistance across all samples. The Fast Virus reagent is a 4× master mix (including a reverse transcriptase enzyme), increasing by 6.25 μl the volume available for a crude sample in a 25-μl PCR mixture (over that with the KAPA 2× master mix). This would improve the probability of detection in samples with low titers of the agent. However, this reagent was completely inhibited by the airfield sample, indicating the importance of the internal-control assay in reducing the chance of false-negative results—as observed in other aerosol testing studies (28).
U.S. evaluation and testing of the Fast Virus and KAPA Blood Direct reagents in whole-blood samples.
For the initial U.S. evaluation, 5 different chemistries plus the Fast Virus master mix were tested for inhibition relief in clinical (whole-blood) and environmental (soil) samples. With the exception of the Fast Virus reagent, these chemistries were optimized for non-real-time PCR applications; Platinum Taq was added for efficient probe hydrolysis and detection. A preliminary LoD was determined with 4 different real-time PCR assays for detecting B. anthracis, B. melitensis, VEEV, and Orthopoxvirus spp. No single chemistry performed best across all assays and sample matrices. This was expected, because previous analyses had shown these reagents to be sensitive to perturbations of reactants in terms of the matrix tested (17). In these analyses, the KAPA reagent performed solidly in whole blood and soil across the multiple assays queried. Confirming a finding of the United Kingdom evaluation, the Fast Virus reagent relieved inhibition when the soil sample was tested. However, its poor performance in whole blood was surprising, since this reagent was recommended by the manufacturer for use with blood and stool samples. The poor performance of the Fast Virus reagent in blood may result from insufficient testing and development in a whole-blood matrix, as opposed to testing and development with specific inhibitors, such as hemoglobin, as noted in the manufacturer's literature.
The second round of the U.S. study consisted of more-detailed LoD determinations with the KAPA and Fast Virus reagents, conducted using a clinical sample (whole blood) spiked with pathogen nucleic acids and analyzed with cognate real-time PCR assays. This analysis confirmed the performance advantage observed across assays with the KAPA reagent in pLoD evaluations. Specifically, the KAPA reagent detected larger amounts of target nucleic acids in higher percentages of whole blood than the Fast Virus reagent. Typically, pLoD estimations are relatively good indicators of the statistically relevant LoD; in this analysis, however, it was often difficult to produce the minimum 29 of 30 positive replicates under pLoD conditions. The most logical explanation for this observation is variability in inhibitor concentrations. Because we performed these analyses with a complex clinical sample matrix as opposed to discrete inhibitors, the concentrations of the numerous inhibitors in whole blood could differ significantly from sample to sample. These observations suggest that testing inhibition in the endpoint context, such as the relevant sample matrix, rather than with discrete inhibitor compounds will yield a more accurate reflection of diagnostic assay performance.
Examination of real-time PCR results with the B. anthracis-specific BAPA PCR further characterizes the potential for clinical utility of inhibitor-resistant PCR. Specifically, the KAPA reagent generated the most-sensitive PCR results, with a LoD of 100 fg DNA in a reaction mixture containing 2.5% whole blood. One hundred femtograms of B. anthracis Ames strain DNA corresponds to 17.5 genome equivalents (3), and in a 20-μl PCR mixture, 2.5% equates to a volume of 0.5 μl. Extrapolating upward, 20 GEs in a 0.5-μl volume would equate to a detectable agent concentration of 3.5 × 104 CFU·ml−1 (17.5 × 2,000) in whole blood. Although this is an improvement over the 106-CFU·ml−1 level observed in a previous inhibitor resistance study with a Francisella tularensis PCR (17), an alternate study has demonstrated a point-of-care (PoC) PCR system, with integrated sample processing and DNA extraction, that is able to detect B. anthracis at sensitivities of 103 CFU·ml−1 and below in whole blood (29). Similarly, sample processing evaluations using the same PCR assays as those used here showed a relative sensitivity of 5 × 101 CFU·ml−1 in whole blood when the nucleic acid was extracted (13). B. anthracis concentrations of 103 to 101 CFU·ml−1 are comparable to the first culturable levels for bacteremia observed in individual animals in a B. anthracis primate infection model (30).
These results imply that systems with sample preparation can at present deliver better performance than direct analysis of a crude clinical sample with an inhibitor-resistant reagent. However, the current performance of the B. anthracis BAPA PCR with the KAPA Blood reagent showed detection of B. anthracis DNA at a range that was clinically relevant and has potential for clinical utility. Strategies that could improve the performance of this PCR-reagent combination could include increasing the volume of the existing PCR mixture to allow more unprocessed sample to be analyzed or developing reagents that can withstand higher concentrations of whole blood.
Summary.
In this evaluation, the reagent with optimal inhibitor resistance was the KAPA Blood Direct master mix with added Platinum Taq. The manufacturers' information indicates that the polymerase within the KAPA product has been engineered specifically to allow direct amplification of DNA from blood, whereas the inhibitor tolerance of the Fast Virus reagent is conferred by additives in the reagent rather than by a reengineered polymerase. We do not know the unit polymerase number for either of these two reagents, since this information is not provided by the manufacturers. The addition of extra Taq has been shown to confer inhibitor resistance against some matrices (31); however, it is unlikely that the higher inhibitor resistance of the KAPA reagent (which had added Platinum Taq) than of the Fast Virus reagent is due to this. This is evidenced by the analysis of the aerosol samples, where the same assays with different reagents generally produced significantly lower Cq values for the Fast Virus master mix. This suggests that elevated levels of Taq did not increase PCR efficiency in the overall KAPA mix and that the inhibitor resistance was conferred by the engineered polymerase. This also suggests that a reagent with both an engineered polymerase and reagent additives could offer an increased level of inhibitor resistance.
This is not to say that PCR efficiency (the amount of DNA amplified during each PCR cycle) would not be important in direct PCR analysis of crude samples. The PCR assays used in this study were taken from a number of research programs conducted over the past decade and were used directly with the new master mixes, with only minor (the amount of additional Taq in U.S. PCRs) or no (United Kingdom assays) reoptimization over the previously optimized reaction conditions and reagents. It was striking to observe the different LoDs from individual PCRs conducted in different chemistries, as well as the effects of different inhibitors on LoDs. A recent study (32) in which seven PCR assays for the malaria parasite Plasmodium falciparum were tested under standardized conditions showed better performance (in terms of LoD, precision, and consistency) for higher-efficiency than for lower-efficiency PCRs. It would therefore seem intuitive that to fully exploit the inhibitor resistance of a given reagent, and to narrow the gap in performance between different assays, PCRs with high efficiency, which have also been fully optimized with the chosen master mix, should be used.
In general, inhibitor-resistant PCR master mixes offer potential for direct analysis of samples, without sample preparation, for both environmental and clinical substrates. Factors that should be considered include the use of optimized, highly efficient PCR assays, any requirement for preassay treatments to rapidly lyse recalcitrant cell types (i.e., spores), and the need to include as much crude sample in the PCR mixture as possible. Even in laboratories where sample preparation is still considered to be applicable (i.e., automated high-throughput clinical laboratories), the uptake of inhibitor-resistant master mixes could improve the overall diagnostic sensitivity of the laboratory by reducing the number of inhibited reactions, e.g., from inhibitor carryover in purified DNA preparations (14).
Supplementary Material
ACKNOWLEDGMENTS
The United Kingdom study was funded by the Ministry of Defense (MoD) Programme Directorate. The U.S. study was funded by the Defense Threat Reduction Agency (DTRA). Collaboration between the DSTL and USAMRIID was facilitated by the Technical Cooperation Program (TTCP), specifically Technical Panel 14 (Rapid Diagnostics).
Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the United Kingdom MoD or the U.S. Army.
The United Kingdom authors thank Victoria Cox for statistical analysis.
Footnotes
Published ahead of print 13 December 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03478-13.
REFERENCES
- 1.Christensen DR, Hartman LJ, Loveless BM, Frye MS, Shipley MA, Bridge DL, Richards MJ, Kaplan RS, Garrison J, Baldwin CD, Kulesh DA, Norwood DA. 2006. Detection of biological threat agents by real-time PCR: comparison of assay performance on the R.A.P.I.D., the LightCycler, and the Smart Cycler platforms. Clin. Chem. 52:141–145. 10.1373/clinchem.2005.052522 [DOI] [PubMed] [Google Scholar]
- 2.Mitchell JL, Chatwell N, Christensen D, Diaper H, Minogue TD, Parsons TM, Walker B, Weller SA. 2010. Development of real-time PCR assays for the specific detection of Francisella tularensis ssp. tularensis, holarctica and mediaasiatica. Mol. Cell. Probes 24:72–76. 10.1016/j.mcp.2009.10.004 [DOI] [PubMed] [Google Scholar]
- 3.Rachwal PA, Rose HL, Cox V, Lukaszewski RA, Murch AL, Weller SA. 2012. The potential of TaqMan Array Cards for detection of multiple biological agents by real-time PCR. PLoS One 7:e35971. 10.1371/journal.pone.0035971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skottman T, Piiparinen H, Hyytiainen H, Myllys V, Skurnik M, Nikkari S. 2007. Simultaneous real-time PCR detection of Bacillus anthracis, Francisella tularensis and Yersinia pestis. Eur. J. Clin. Microbiol. Infect. Dis. 26:207–211. 10.1007/s10096-007-0262-z [DOI] [PubMed] [Google Scholar]
- 5.Tomaso H, Jacob D, Eickhoff M, Scholz HC, Al Dahouk S, Kattar MM, Reischl U, Plicka H, Olsen JS, Nikkari S, Matero P, Beuret C, Ciammaruconi A, Lista F, Gala JL, Broll H, Appel B, Sellek Cano RE, Ybarra de Villavicencio MDC, Broekhuijsen M, Indra A, Petersen R, Neubauer H. 2008. Preliminary validation of real-time PCR assays for the identification of Yersinia pestis. Clin. Chem. Lab. Med. 46:1239–1244. 10.1515/CCLM.2008.251 [DOI] [PubMed] [Google Scholar]
- 6.Akane A, Matsubara K, Nakamura H, Takahashi S, Kimura K. 1994. Identification of the heme compound copurified with deoxyribonucleic acid (DNA) from bloodstains, a major inhibitor of polymerase chain reaction (PCR) amplification. J. Forensic Sci. 39:362–372 [PubMed] [Google Scholar]
- 7.Monteiro L, Bonnemaison D, Vekris A, Petry KG, Bonnet J, Vidal R, Cabrita J, Megraud F. 1997. Complex polysaccharides as PCR inhibitors in feces: Helicobacter pylori model. J. Clin. Microbiol. 35:995–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Radstrom P, Knutsson R, Wolffs P, Lovenklev M, Lofstrom C. 2004. Pre-PCR processing: strategies to generate PCR-compatible samples. Mol. Biotechnol. 26:133–146. 10.1385/MB:26:2:133 [DOI] [PubMed] [Google Scholar]
- 9.Tsai YL, Olson BH. 1992. Detection of low numbers of bacterial cells in soils and sediments by polymerase chain reaction. Appl. Environ. Microbiol. 58:754–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Widjojoatmodjo MN, Fluit AC, Torensma R, Verdonk GP, Verhoef J. 1992. The magnetic immuno polymerase chain reaction assay for direct detection of salmonellae in fecal samples. J. Clin. Microbiol. 30:3195–3199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson IG. 1997. Inhibition and facilitation of nucleic acid amplification. Appl. Environ. Microbiol. 63:3741–3751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rose HL, Dewey CA, Ely MS, Willoughby SL, Parsons TM, Cox V, Spencer PM, Weller SA. 2011. Comparison of eight methods for the extraction of Bacillus atrophaeus spore DNA from eleven common interferents and a common swab. PLoS One 6:e22668. 10.1371/journal.pone.0022668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shipley MA, Koehler JW, Kulesh DA, Minogue TD. 2012. Comparison of nucleic acid extraction platforms for detection of select biothreat agents for use in clinical resource limited settings. J. Microbiol. Methods 91:179–183. 10.1016/j.mimet.2012.06.008 [DOI] [PubMed] [Google Scholar]
- 14.Kramvis A, Bukofzer S, Kew MC. 1996. Comparison of hepatitis B virus DNA extractions from serum by the QIAamp blood kit, GeneReleaser, and the phenol-chloroform method. J. Clin. Microbiol. 34:2731–2733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kermekchiev MB, Kirilova LI, Vail EE, Barnes WM. 2009. Mutants of Taq DNA polymerase resistant to PCR inhibitors allow DNA amplification from whole blood and crude soil samples. Nucleic Acids Res. 37:e40. 10.1093/nar/gkn1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abu Al-Soud W, Radstrom P. 2000. Effects of amplification facilitators on diagnostic PCR in the presence of blood, feces, and meat. J. Clin. Microbiol. 38:4463–4470 http://jcm.asm.org/content/38/12/4463.long [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trombley Hall A, Zovanyi AM, Christensen DR, Koehler JW, Minogue TD. 2013. Evaluation of inhibitor-resistant real-time PCR methods for diagnostics in clinical and environmental samples. PLoS One 8:e73845. 10.1371/journal.pone.0073845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kreader CA. 1996. Relief of amplification inhibition in PCR with bovine serum albumin or T4 gene 32 protein. Appl. Environ. Microbiol. 62:1102–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parsons TM, Cox V, Essex-Lopresti A, Hartley MG, Lukaszewski RA, Rachwal PA, Stapleton HL, Weller SA. 2013. Development of three real-time PCR assays to detect Bacillus anthracis and assessment of diagnostic utility. J. Bioterr. Biodef. S3:009. 10.4172/2157-2526.S3-009 [DOI] [Google Scholar]
- 20.Picard FJ, Gagnon M, Bernier MR, Parham NJ, Bastien M, Boissinot M, Peytavi R, Bergeron MG. 2009. Internal control for nucleic acid testing based on the use of purified Bacillus atrophaeus subsp. globigii spores. J. Clin. Microbiol. 47:751–757. 10.1128/JCM.01746-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parker MD, Buckley MJ, Melanson VR, Glass PJ, Norwood D, Hart MK. 2010. Antibody to the E3 glycoprotein protects mice against lethal Venezuelan equine encephalitis virus infection. J. Virol. 84:12683–12690. 10.1128/JVI.01345-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kulesh DA, Baker RO, Loveless BM, Norwood D, Zwiers SH, Mucker E, Hartmann C, Herrera R, Miller D, Christensen D, Wasieloski LP, Jr, Huggins J, Jahrling PB. 2004. Smallpox and pan-orthopox virus detection by real-time 3′-minor groove binder TaqMan assays on the Roche LightCycler and the Cepheid smart Cycler platforms. J. Clin. Microbiol. 42:601–609. 10.1128/JCM.42.2.601-609.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Errington FP, Powell EO. 1969. A cyclone separator for aerosol sampling in the field. J. Hyg. (Lond.) 67:387–399. 10.1017/S0022172400041802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen P-S, Tsai FT, Lin CK, Yang C-Y, Chan C-C, Young C-Y, Lee C-H. 2010. Ambient influenza and avian influenza virus during dust storm days and background days. Environ. Health Perspect. 118:1211–1216. 10.1289/ehp.0901782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Almeida JL, Harper B, Cole KD. 2008. Bacillus anthracis spore suspensions: determination of stability and comparison of enumeration techniques. J. Appl. Microbiol. 104:1442–1448. 10.1111/j.1365-2672.2007.03684.x [DOI] [PubMed] [Google Scholar]
- 26.Kuske CR, Banton KL, Adorada DL, Stark PC, Hill KK, Jackson PJ. 1998. Small-scale DNA sample preparation method for field PCR detection of microbial cells and spores in soil. Appl. Environ. Microbiol. 64:2463–2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belgrader P, Hansford D, Kovacs GT, Venkateswaran K, Mariella R, Jr, Milanovich F, Nasarabadi S, Okuzumi M, Pourahmadi F, Northrup MA. 1999. A minisonicator to rapidly disrupt bacterial spores for DNA analysis. Anal. Chem. 71:4232–4236. 10.1021/ac990347o [DOI] [PubMed] [Google Scholar]
- 28.Usachev EV, Agranovski IE. 2012. Internally controlled PCR system for detection of airborne microorganisms. J. Environ. Monit. 14:1631–1637. 10.1039/c2em30019b [DOI] [PubMed] [Google Scholar]
- 29.Weller SA, Cox V, Essex-Lopresti A, Hartley MG, Parsons TM, Rachwal PA, Stapleton HL, Lukaszewski RA. 2012. Evaluation of two multiplex real-time PCR screening capabilities for the detection of B. anthracis, F. tularensis, and Y. pestis in blood samples generated from murine infection models. J. Med. Microbiol. 61:1546–1555. 10.1099/jmm.0.049007-0 [DOI] [PubMed] [Google Scholar]
- 30.Rossi CA, Ulrich M, Norris S, Reed DS, Pitt LM, Leffel EK. 2008. Identification of a surrogate marker for infection in the African green monkey model of inhalation anthrax. Infect. Immun. 76:5790–5801. 10.1128/IAI.00520-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Opel KL, Chung D, McCord BR. 2010. A study of PCR inhibition mechanisms using real-time PCR. J. Forensic Sci. 55:25–33. 10.1111/j.1556-4029.2009.01245.x [DOI] [PubMed] [Google Scholar]
- 32.Alemayehu S, Feghali KC, Cowden J, Komisar J, Ockenhouse CF, Kamau E. 2013. Comparative evaluation of published real-time PCR assays for the detection of malaria following MIQE guidelines. Malar. J. 12:277. 10.1186/1475-2875-12-277 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.