Abstract
Cu,Zn-superoxide dismutase (SOD) is known to be a locus of mutation in familial amyotrophic lateral sclerosis (FALS). Transgenic mice that express a mutant Cu,Zn-SOD, Gly-93--> Ala (G93A), have been shown to develop amyotrophic lateral sclerosis (ALS) symptoms. We cloned the FALS mutant, G93A, and wild-type cDNA of human Cu,Zn-SOD, overexpressed them in Sf9 insect cells, purified the proteins, and studied their enzymic activities for catalyzing the dismutation of superoxide anions and the generation of free radicals with H2O2 as substrate. Our results showed that both enzymes contain one copper ion per subunit and have identical dismutation activity. However, the free radical-generating function of the G93A mutant, as measured by the spin trapping method, is enhanced relative to that of the wild-type enzyme, particularly at lower H2O2 concentrations. This is due to a small, but reproducible, decrease in the value of Km for H2O2 for the G93A mutant, while the kcat is identical for both enzymes. Thus, the ALS symptoms observed in G93A transgenic mice are not caused by the reduction of Cu,Zn-SOD activity with the mutant enzyme; rather, it is induced by a gain-of-function, an enhancement of the free radical-generating function. This is consistent with the x-ray crystallographic studies showing the active channel of the FALS mutant is slightly larger than that of the wild-type enzyme; thus, it is more accessible to H2O2. This gain-of-function, in part, may provide an explanation for the association between ALS and Cu,Zn-SOD mutants.
Full text
PDF

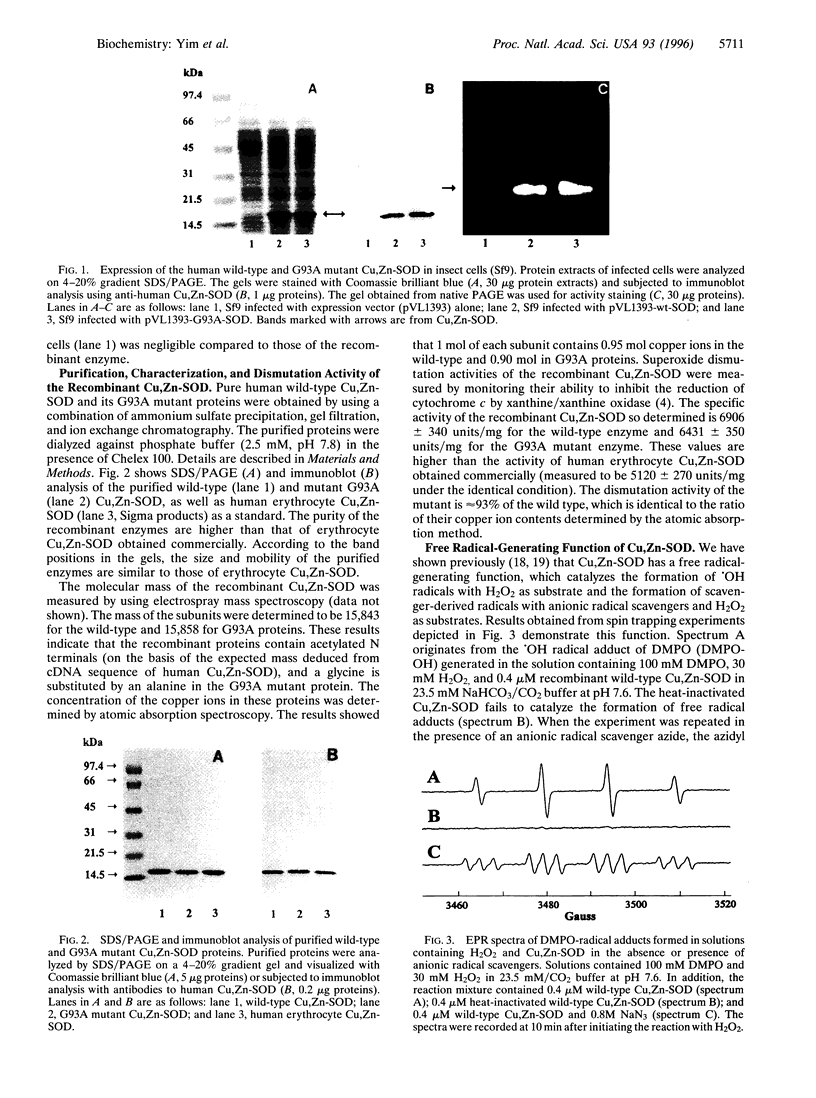
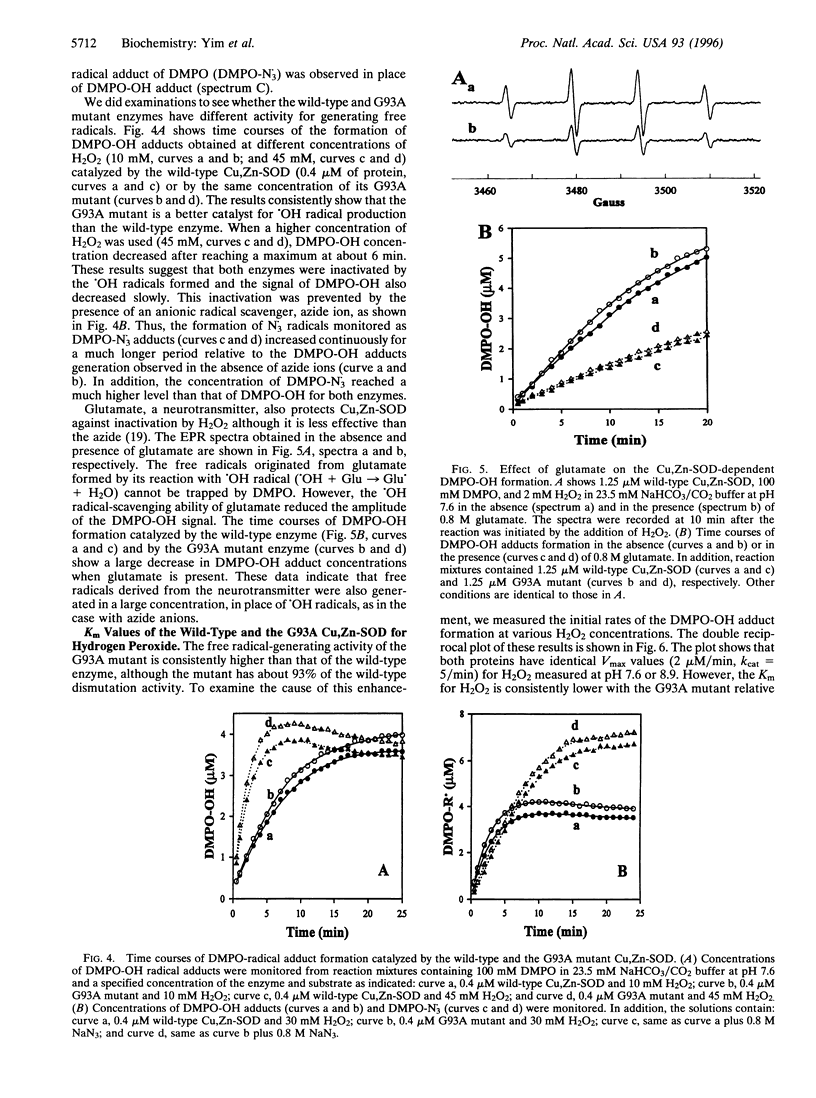
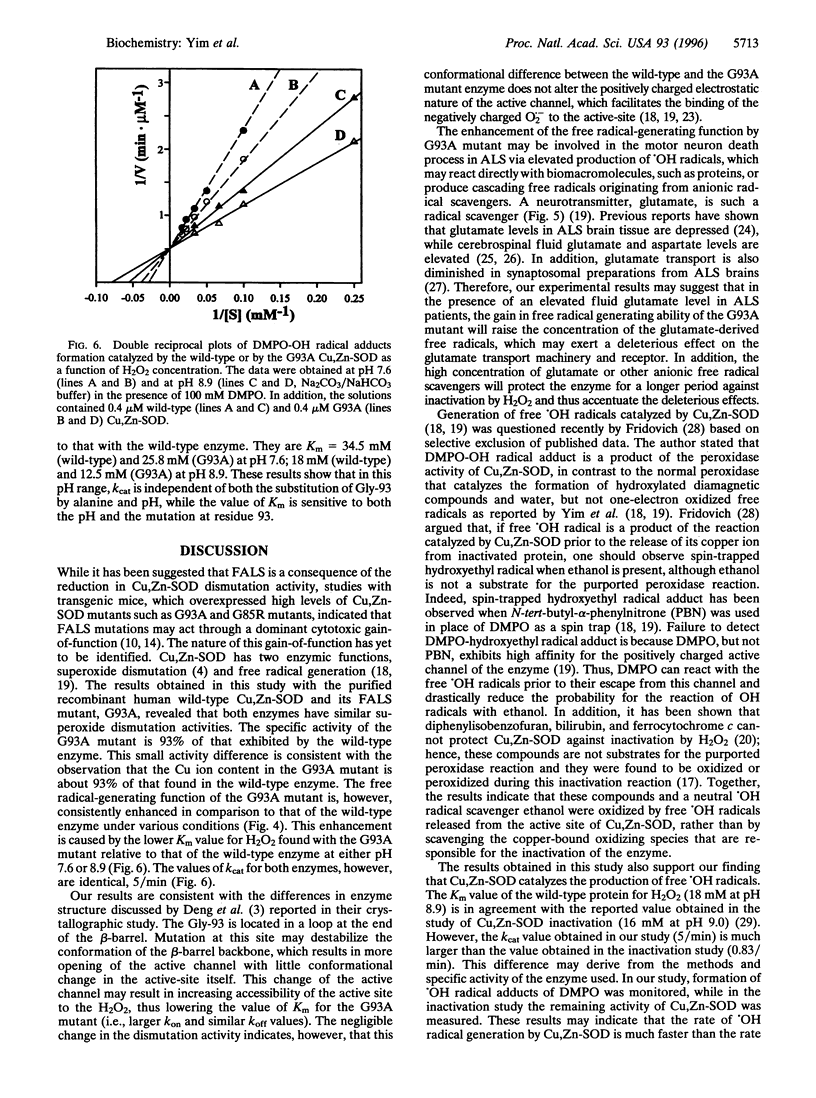

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki M., Ogasawara M., Matsubara Y., Narisawa K., Nakamura S., Itoyama Y., Abe K. Mild ALS in Japan associated with novel SOD mutation. Nat Genet. 1993 Dec;5(4):323–324. doi: 10.1038/ng1293-323. [DOI] [PubMed] [Google Scholar]
- Beyer W. F., Jr, Fridovich I., Mullenbach G. T., Hallewell R. Examination of the role of arginine-143 in the human copper and zinc superoxide dismutase by site-specific mutagenesis. J Biol Chem. 1987 Aug 15;262(23):11182–11187. [PubMed] [Google Scholar]
- Borchelt D. R., Guarnieri M., Wong P. C., Lee M. K., Slunt H. S., Xu Z. S., Sisodia S. S., Price D. L., Cleveland D. W. Superoxide dismutase 1 subunits with mutations linked to familial amyotrophic lateral sclerosis do not affect wild-type subunit function. J Biol Chem. 1995 Feb 17;270(7):3234–3238. doi: 10.1074/jbc.270.7.3234. [DOI] [PubMed] [Google Scholar]
- Borchelt D. R., Lee M. K., Slunt H. S., Guarnieri M., Xu Z. S., Wong P. C., Brown R. H., Jr, Price D. L., Sisodia S. S., Cleveland D. W. Superoxide dismutase 1 with mutations linked to familial amyotrophic lateral sclerosis possesses significant activity. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):8292–8296. doi: 10.1073/pnas.91.17.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling A. C., Schulz J. B., Brown R. H., Jr, Beal M. F. Superoxide dismutase activity, oxidative damage, and mitochondrial energy metabolism in familial and sporadic amyotrophic lateral sclerosis. J Neurochem. 1993 Dec;61(6):2322–2325. doi: 10.1111/j.1471-4159.1993.tb07478.x. [DOI] [PubMed] [Google Scholar]
- Brown R. H., Jr Amyotrophic lateral sclerosis: recent insights from genetics and transgenic mice. Cell. 1995 Mar 10;80(5):687–692. doi: 10.1016/0092-8674(95)90346-1. [DOI] [PubMed] [Google Scholar]
- Deng H. X., Hentati A., Tainer J. A., Iqbal Z., Cayabyab A., Hung W. Y., Getzoff E. D., Hu P., Herzfeldt B., Roos R. P. Amyotrophic lateral sclerosis and structural defects in Cu,Zn superoxide dismutase. Science. 1993 Aug 20;261(5124):1047–1051. doi: 10.1126/science.8351519. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- Fuchs H. J., Borders C. L., Jr Affinity inactivation of bovine Cu,Zn superoxide dismutase by hydroperoxide anion, HO2-. Biochem Biophys Res Commun. 1983 Nov 15;116(3):1107–1113. doi: 10.1016/s0006-291x(83)80256-3. [DOI] [PubMed] [Google Scholar]
- Getzoff E. D., Tainer J. A., Weiner P. K., Kollman P. A., Richardson J. S., Richardson D. C. Electrostatic recognition between superoxide and copper, zinc superoxide dismutase. Nature. 1983 Nov 17;306(5940):287–290. doi: 10.1038/306287a0. [DOI] [PubMed] [Google Scholar]
- Gurney M. E., Pu H., Chiu A. Y., Dal Canto M. C., Polchow C. Y., Alexander D. D., Caliendo J., Hentati A., Kwon Y. W., Deng H. X. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994 Jun 17;264(5166):1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Hodgson E. K., Fridovich I. The interaction of bovine erythrocyte superoxide dismutase with hydrogen peroxide: chemiluminescence and peroxidation. Biochemistry. 1975 Dec 2;14(24):5299–5303. doi: 10.1021/bi00695a011. [DOI] [PubMed] [Google Scholar]
- Hodgson E. K., Fridovich I. The interaction of bovine erythrocyte superoxide dismutase with hydrogen peroxide: inactivation of the enzyme. Biochemistry. 1975 Dec 2;14(24):5294–5299. doi: 10.1021/bi00695a010. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Orrell R., de Belleroche J., Marklund S., Bowe F., Hallewell R. A novel SOD mutant and ALS. Nature. 1995 Apr 6;374(6522):504–505. doi: 10.1038/374504a0. [DOI] [PubMed] [Google Scholar]
- Pardo C. A., Xu Z., Borchelt D. R., Price D. L., Sisodia S. S., Cleveland D. W. Superoxide dismutase is an abundant component in cell bodies, dendrites, and axons of motor neurons and in a subset of other neurons. Proc Natl Acad Sci U S A. 1995 Feb 14;92(4):954–958. doi: 10.1073/pnas.92.4.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry T. L., Hansen S., Jones K. Brain glutamate deficiency in amyotrophic lateral sclerosis. Neurology. 1987 Dec;37(12):1845–1848. doi: 10.1212/wnl.37.12.1845. [DOI] [PubMed] [Google Scholar]
- Perry T. L., Krieger C., Hansen S., Eisen A. Amyotrophic lateral sclerosis: amino acid levels in plasma and cerebrospinal fluid. Ann Neurol. 1990 Jul;28(1):12–17. doi: 10.1002/ana.410280105. [DOI] [PubMed] [Google Scholar]
- Price D. L., Cleveland D. W., Koliatsos V. E. Motor neurone disease and animal models. Neurobiol Dis. 1994 Nov;1(1-2):3–11. doi: 10.1006/nbdi.1994.0002. [DOI] [PubMed] [Google Scholar]
- Rabizadeh S., Gralla E. B., Borchelt D. R., Gwinn R., Valentine J. S., Sisodia S., Wong P., Lee M., Hahn H., Bredesen D. E. Mutations associated with amyotrophic lateral sclerosis convert superoxide dismutase from an antiapoptotic gene to a proapoptotic gene: studies in yeast and neural cells. Proc Natl Acad Sci U S A. 1995 Mar 28;92(7):3024–3028. doi: 10.1073/pnas.92.7.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripps M. E., Huntley G. W., Hof P. R., Morrison J. H., Gordon J. W. Transgenic mice expressing an altered murine superoxide dismutase gene provide an animal model of amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 1995 Jan 31;92(3):689–693. doi: 10.1073/pnas.92.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robberecht W., Sapp P., Viaene M. K., Rosen D., McKenna-Yasek D., Haines J., Horvitz R., Theys P., Brown R., Jr Cu/Zn superoxide dismutase activity in familial and sporadic amyotrophic lateral sclerosis. J Neurochem. 1994 Jan;62(1):384–387. doi: 10.1046/j.1471-4159.1994.62010384.x. [DOI] [PubMed] [Google Scholar]
- Rosen D. R., Bowling A. C., Patterson D., Usdin T. B., Sapp P., Mezey E., McKenna-Yasek D., O'Regan J., Rahmani Z., Ferrante R. J. A frequent ala 4 to val superoxide dismutase-1 mutation is associated with a rapidly progressive familial amyotrophic lateral sclerosis. Hum Mol Genet. 1994 Jun;3(6):981–987. doi: 10.1093/hmg/3.6.981. [DOI] [PubMed] [Google Scholar]
- Rosen D. R., Siddique T., Patterson D., Figlewicz D. A., Sapp P., Hentati A., Donaldson D., Goto J., O'Regan J. P., Deng H. X. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993 Mar 4;362(6415):59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Rothstein J. D., Martin L. J., Kuncl R. W. Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. N Engl J Med. 1992 May 28;326(22):1464–1468. doi: 10.1056/NEJM199205283262204. [DOI] [PubMed] [Google Scholar]
- Rothstein J. D., Tsai G., Kuncl R. W., Clawson L., Cornblath D. R., Drachman D. B., Pestronk A., Stauch B. L., Coyle J. T. Abnormal excitatory amino acid metabolism in amyotrophic lateral sclerosis. Ann Neurol. 1990 Jul;28(1):18–25. doi: 10.1002/ana.410280106. [DOI] [PubMed] [Google Scholar]
- Wiedau-Pazos M., Goto J. J., Rabizadeh S., Gralla E. B., Roe J. A., Lee M. K., Valentine J. S., Bredesen D. E. Altered reactivity of superoxide dismutase in familial amyotrophic lateral sclerosis. Science. 1996 Jan 26;271(5248):515–518. doi: 10.1126/science.271.5248.515. [DOI] [PubMed] [Google Scholar]
- Yim M. B., Chock P. B., Stadtman E. R. Copper, zinc superoxide dismutase catalyzes hydroxyl radical production from hydrogen peroxide. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5006–5010. doi: 10.1073/pnas.87.13.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim M. B., Chock P. B., Stadtman E. R. Enzyme function of copper, zinc superoxide dismutase as a free radical generator. J Biol Chem. 1993 Feb 25;268(6):4099–4105. [PubMed] [Google Scholar]




