Abstract
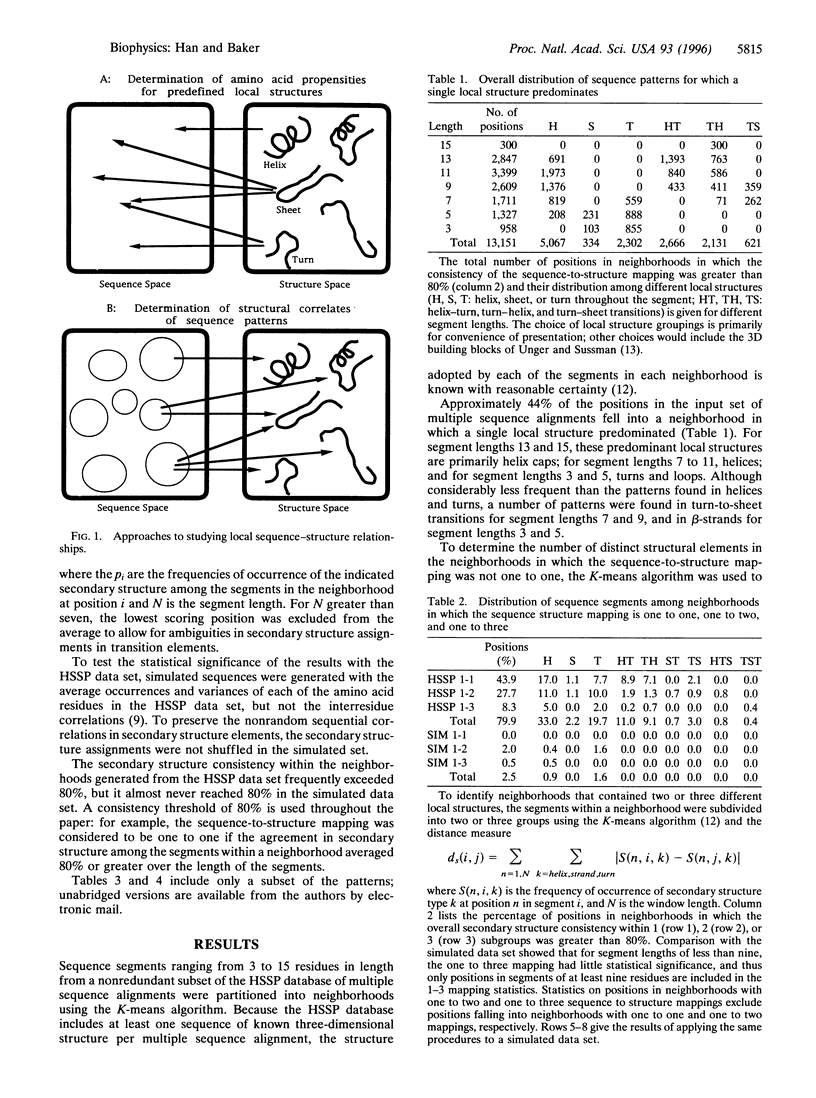
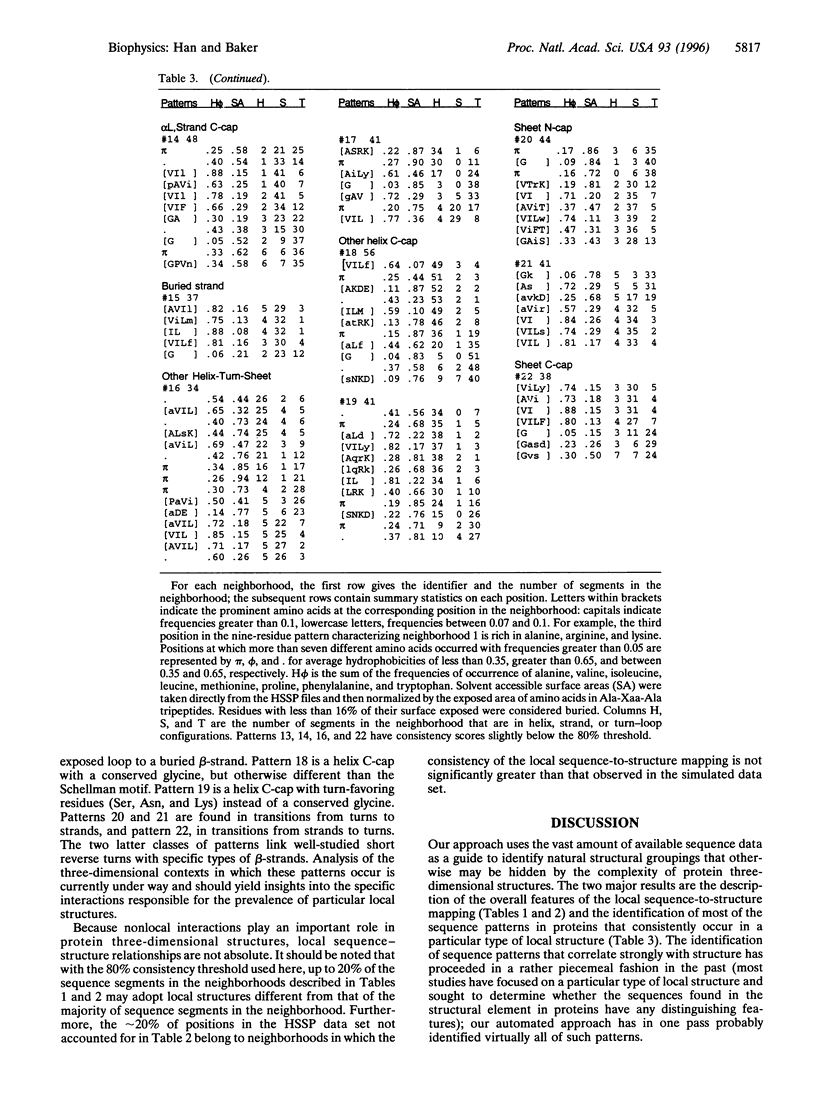
Local protein structure prediction efforts have consistently failed to exceed approximately 70% accuracy. We characterize the degeneracy of the mapping from local sequence to local structure responsible for this failure by investigating the extent to which similar sequence segments found in different proteins adopt similar three-dimensional structures. Sequence segments 3-15 residues in length from 154 different protein families are partitioned into neighborhoods containing segments with similar sequences using cluster analysis. The consistency of the sequence-to-structure mapping is assessed by comparing the local structures adopted by sequence segments in the same neighborhood in proteins of known structure. In the 154 families, 45% and 28% of the positions occur in neighborhoods in which one and two local structures predominate, respectively. The sequence patterns that characterize the neighborhoods in the first class probably include virtually all of the short sequence motifs in proteins that consistently occur in a particular local structure. These patterns, many of which occur in transitions between secondary structural elements, are an interesting combination of previously studied and novel motifs. The identification of sequence patterns that consistently occur in one or a small number of local structures in proteins should contribute to the prediction of protein structure from sequence.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aurora R., Srinivasan R., Rose G. D. Rules for alpha-helix termination by glycine. Science. 1994 May 20;264(5162):1126–1130. doi: 10.1126/science.8178170. [DOI] [PubMed] [Google Scholar]
- Cohen F. E., Abarbanel R. M., Kuntz I. D., Fletterick R. J. Turn prediction in proteins using a pattern-matching approach. Biochemistry. 1986 Jan 14;25(1):266–275. doi: 10.1021/bi00349a037. [DOI] [PubMed] [Google Scholar]
- Han K. F., Baker D. Recurring local sequence motifs in proteins. J Mol Biol. 1995 Aug 4;251(1):176–187. doi: 10.1006/jmbi.1995.0424. [DOI] [PubMed] [Google Scholar]
- Harper E. T., Rose G. D. Helix stop signals in proteins and peptides: the capping box. Biochemistry. 1993 Aug 3;32(30):7605–7609. doi: 10.1021/bi00081a001. [DOI] [PubMed] [Google Scholar]
- Henikoff S., Henikoff J. G. Amino acid substitution matrices from protein blocks. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10915–10919. doi: 10.1073/pnas.89.22.10915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobohm U., Sander C. Enlarged representative set of protein structures. Protein Sci. 1994 Mar;3(3):522–524. doi: 10.1002/pro.5560030317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabsch W., Sander C. On the use of sequence homologies to predict protein structure: identical pentapeptides can have completely different conformations. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1075–1078. doi: 10.1073/pnas.81.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presnell S. R., Cohen B. I., Cohen F. E. A segment-based approach to protein secondary structure prediction. Biochemistry. 1992 Feb 4;31(4):983–993. doi: 10.1021/bi00119a006. [DOI] [PubMed] [Google Scholar]
- Rost B., Sander C. Prediction of protein secondary structure at better than 70% accuracy. J Mol Biol. 1993 Jul 20;232(2):584–599. doi: 10.1006/jmbi.1993.1413. [DOI] [PubMed] [Google Scholar]
- Sander C., Schneider R. Database of homology-derived protein structures and the structural meaning of sequence alignment. Proteins. 1991;9(1):56–68. doi: 10.1002/prot.340090107. [DOI] [PubMed] [Google Scholar]
- Unger R., Sussman J. L. The importance of short structural motifs in protein structure analysis. J Comput Aided Mol Des. 1993 Aug;7(4):457–472. doi: 10.1007/BF02337561. [DOI] [PubMed] [Google Scholar]
- Zhou H. X., Lyu P., Wemmer D. E., Kallenbach N. R. Alpha helix capping in synthetic model peptides by reciprocal side chain-main chain interactions: evidence for an N terminal "capping box". Proteins. 1994 Jan;18(1):1–7. doi: 10.1002/prot.340180103. [DOI] [PubMed] [Google Scholar]


