Abstract
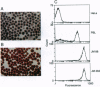
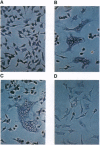
A diverse group of GPI-anchored protein structures are ubiquitously expressed on the external cell membranes of eukaryotes. Whereas the physiological role for these structures is usually defined by their protein component, the precise biological significance of the glycolipid anchors remains vague. In the course of producing a HeLa cell line (JM88) that contained a recombinant adeno-associated virus genome expressing a GPI-anchored CD4-GPI fusion protein on the surface of the cells, we noted the transfer of CD4-GPI to native HeLa cells. Transfer occurred after direct cell contact or exposure to JM88 cell supernatants. The magnitude of contact-mediated CD4-GPI transfer correlated with temperature. Supernatant CD4-GPI also attached to human red blood cells and could be cleaved with phosphatidylinositol-specific phospholipase C. The attached CD4-GPI remained biologically active after transfer and permitted the formation of syncytium when coated HeLa cells were incubated with glycoprotein 160 expressing H9 cells. JM88 cells provide a model for the production, release, and reattachment of CD4-GPI and may furnish insight into a physiologic role of naturally occurring GPI-anchored proteins. This approach may also allow the production of other recombinant GPI-anchored proteins for laboratory and clinical investigation.
Full text
PDF
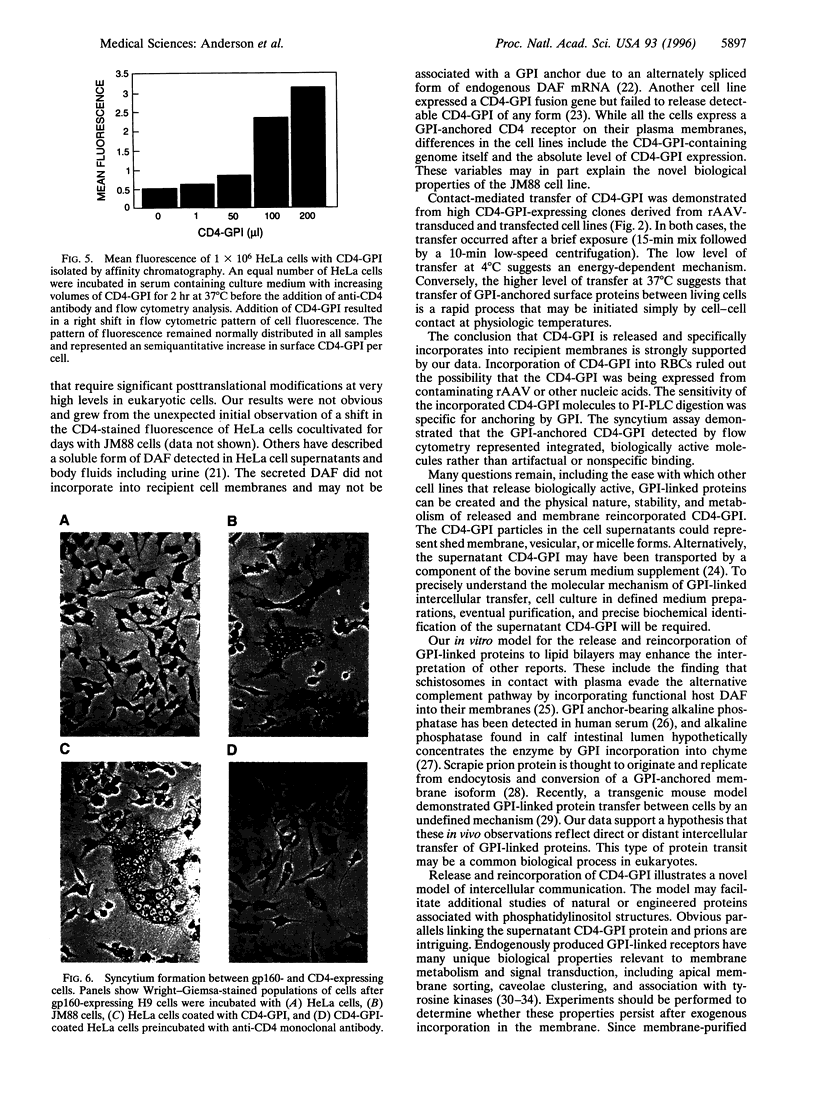

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashorn P. A., Berger E. A., Moss B. Human immunodeficiency virus envelope glycoprotein/CD4-mediated fusion of nonprimate cells with human cells. J Virol. 1990 May;64(5):2149–2156. doi: 10.1128/jvi.64.5.2149-2156.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky R. A., Jane S. M., Vanin E. F., Mitsuya H., Peters T. R., Shimada T., Medof M. E., Nienhuis A. W. Purified GPI-anchored CD4DAF as a receptor for HIV-mediated gene transfer. Hum Gene Ther. 1994 Oct;5(10):1231–1239. doi: 10.1089/hum.1994.5.10-1231. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Rose J. K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992 Feb 7;68(3):533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Caras I. W., Davitz M. A., Rhee L., Weddell G., Martin D. W., Jr, Nussenzweig V. Cloning of decay-accelerating factor suggests novel use of splicing to generate two proteins. Nature. 1987 Feb 5;325(6104):545–549. doi: 10.1038/325545a0. [DOI] [PubMed] [Google Scholar]
- Casey P. J. Protein lipidation in cell signaling. Science. 1995 Apr 14;268(5208):221–225. doi: 10.1126/science.7716512. [DOI] [PubMed] [Google Scholar]
- Diamond D. C., Finberg R., Chaudhuri S., Sleckman B. P., Burakoff S. J. Human immunodeficiency virus infection is efficiently mediated by a glycolipid-anchored form of CD4. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5001–5005. doi: 10.1073/pnas.87.13.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl P. L., Hügin A. W., Moss B. Removal of cryptic poxvirus transcription termination signals from the human immunodeficiency virus type 1 envelope gene enhances expression and immunogenicity of a recombinant vaccinia virus. J Virol. 1990 May;64(5):2448–2451. doi: 10.1128/jvi.64.5.2448-2451.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl P. L., Koenig S., Moss B. Biological and immunological properties of human immunodeficiency virus type 1 envelope glycoprotein: analysis of proteins with truncations and deletions expressed by recombinant vaccinia viruses. J Virol. 1991 Jan;65(1):31–41. doi: 10.1128/jvi.65.1.31-41.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl P. L., Koenig S., Moss B. Biological and immunological properties of human immunodeficiency virus type 1 envelope glycoprotein: analysis of proteins with truncations and deletions expressed by recombinant vaccinia viruses. J Virol. 1991 Jan;65(1):31–41. doi: 10.1128/jvi.65.1.31-41.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund P. T. The structure and biosynthesis of glycosyl phosphatidylinositol protein anchors. Annu Rev Biochem. 1993;62:121–138. doi: 10.1146/annurev.bi.62.070193.001005. [DOI] [PubMed] [Google Scholar]
- Fujita T., Inoue T., Ogawa K., Iida K., Tamura N. The mechanism of action of decay-accelerating factor (DAF). DAF inhibits the assembly of C3 convertases by dissociating C2a and Bb. J Exp Med. 1987 Nov 1;166(5):1221–1228. doi: 10.1084/jem.166.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann-Blume E., Garcia Marenco M. B., Ehle H., Bublitz R., Schulze M., Horn A. Evidence for glycosylphosphatidylinositol anchoring of intralumenal alkaline phosphatase of the calf intestine. Eur J Biochem. 1991 Jul 15;199(2):305–312. doi: 10.1111/j.1432-1033.1991.tb16125.x. [DOI] [PubMed] [Google Scholar]
- Jasin M., Page K. A., Littman D. R. Glycosylphosphatidylinositol-anchored CD4/Thy-1 chimeric molecules serve as human immunodeficiency virus receptors in human, but not mouse, cells and are modulated by gangliosides. J Virol. 1991 Jan;65(1):440–444. doi: 10.1128/jvi.65.1.440-444.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller G. A., Siegel M. W., Caras I. W. Endocytosis of glycophospholipid-anchored and transmembrane forms of CD4 by different endocytic pathways. EMBO J. 1992 Mar;11(3):863–874. doi: 10.1002/j.1460-2075.1992.tb05124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihn L., Dinwoodie A., Stinson R. A. High-molecular-weight alkaline phosphatase in serum has properties similar to the enzyme in plasma membranes of the liver. Am J Clin Pathol. 1991 Oct;96(4):470–478. doi: 10.1093/ajcp/96.4.470. [DOI] [PubMed] [Google Scholar]
- Kost T. A., Kessler J. A., Patel I. R., Gray J. G., Overton L. K., Carter S. G. Human immunodeficiency virus infection and syncytium formation in HeLa cells expressing glycophospholipid-anchored CD4. J Virol. 1991 Jun;65(6):3276–3283. doi: 10.1128/jvi.65.6.3276-3283.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G. The glycosyl-phosphatidylinositol anchor of membrane proteins. Biochim Biophys Acta. 1989 Dec 6;988(3):427–454. doi: 10.1016/0304-4157(89)90014-2. [DOI] [PubMed] [Google Scholar]
- Lublin D. M., Atkinson J. P. Decay-accelerating factor: biochemistry, molecular biology, and function. Annu Rev Immunol. 1989;7:35–58. doi: 10.1146/annurev.iy.07.040189.000343. [DOI] [PubMed] [Google Scholar]
- Lublin D. M. Glycosyl-phosphatidylinositol anchoring of membrane proteins. Curr Top Microbiol Immunol. 1992;178:141–162. doi: 10.1007/978-3-642-77014-2_9. [DOI] [PubMed] [Google Scholar]
- McHugh R. S., Ahmed S. N., Wang Y. C., Sell K. W., Selvaraj P. Construction, purification, and functional incorporation on tumor cells of glycolipid-anchored human B7-1 (CD80). Proc Natl Acad Sci U S A. 1995 Aug 15;92(17):8059–8063. doi: 10.1073/pnas.92.17.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Kinoshita T., Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med. 1984 Nov 1;160(5):1558–1578. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Walter E. I., Rutgers J. L., Knowles D. M., Nussenzweig V. Identification of the complement decay-accelerating factor (DAF) on epithelium and glandular cells and in body fluids. J Exp Med. 1987 Mar 1;165(3):848–864. doi: 10.1084/jem.165.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. L., Donahue R. E., Sellers S. E., Samulski R. J., Young N. S., Nienhuis A. W. Recombinant adeno-associated virus (rAAV)-mediated expression of a human gamma-globin gene in human progenitor-derived erythroid cells. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):10183–10187. doi: 10.1073/pnas.91.21.10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzyczka N. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr Top Microbiol Immunol. 1992;158:97–129. doi: 10.1007/978-3-642-75608-5_5. [DOI] [PubMed] [Google Scholar]
- Okada N., Harada R., Okada H. Erythrocytes of patients with paroxysmal nocturnal haemoglobinuria acquire resistance to complement attack by purified 20-kD homologous restriction factor. Clin Exp Immunol. 1990 Apr;80(1):109–113. doi: 10.1111/j.1365-2249.1990.tb06449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce E. J., Hall B. F., Sher A. Host-specific evasion of the alternative complement pathway by schistosomes correlates with the presence of a phospholipase C-sensitive surface molecule resembling human decay accelerating factor. J Immunol. 1990 Apr 1;144(7):2751–2756. [PubMed] [Google Scholar]
- Pratt J. C., Gaulton G. N. Multifunctional roles of glycosyl-phosphatidylinositol lipids. DNA Cell Biol. 1993 Dec;12(10):861–869. doi: 10.1089/dna.1993.12.861. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Genetic and infectious prion diseases. Arch Neurol. 1993 Nov;50(11):1129–1153. doi: 10.1001/archneur.1993.00540110011002. [DOI] [PubMed] [Google Scholar]
- Saltiel A. R., Osterman D. G., Darnell J. C., Chan B. L., Sorbara-Cazan L. R. The role of glycosylphosphoinositides in signal transduction. Recent Prog Horm Res. 1989;45:353–382. doi: 10.1016/b978-0-12-571145-6.50011-x. [DOI] [PubMed] [Google Scholar]
- Samulski R. J., Chang L. S., Shenk T. Helper-free stocks of recombinant adeno-associated viruses: normal integration does not require viral gene expression. J Virol. 1989 Sep;63(9):3822–3828. doi: 10.1128/jvi.63.9.3822-3828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy-Scaria A. M., Kwong J., Fujita T., Olszowy M. W., Shaw A. S., Lublin D. M. Signal transduction through decay-accelerating factor. Interaction of glycosyl-phosphatidylinositol anchor and protein tyrosine kinases p56lck and p59fyn 1. J Immunol. 1992 Dec 1;149(11):3535–3541. [PubMed] [Google Scholar]
- Shimada T., Fujii H., Mitsuya H., Nienhuis A. W. Targeted and highly efficient gene transfer into CD4+ cells by a recombinant human immunodeficiency virus retroviral vector. J Clin Invest. 1991 Sep;88(3):1043–1047. doi: 10.1172/JCI115365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyng S. L., Heuser J. E., Harris D. A. A glycolipid-anchored prion protein is endocytosed via clathrin-coated pits. J Cell Biol. 1994 Jun;125(6):1239–1250. doi: 10.1083/jcb.125.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanová I., Horejsí V., Ansotegui I. J., Knapp W., Stockinger H. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science. 1991 Nov 15;254(5034):1016–1019. doi: 10.1126/science.1719635. [DOI] [PubMed] [Google Scholar]
- Väkevä A., Jauhiainen M., Ehnholm C., Lehto T., Meri S. High-density lipoproteins can act as carriers of glycophosphoinositol lipid-anchored CD59 in human plasma. Immunology. 1994 May;82(1):28–33. [PMC free article] [PubMed] [Google Scholar]