Abstract
Multiple myeloma (MM) is preceded by the asymptomatic premalignant state, monoclonal gammopathy of undetermined significance (MGUS). Although MGUS patients may remain stable for years, they are at increased risk of progressing to MM. A better understanding of the relevant molecular changes underlying the transition from an asymptomatic to symptomatic disease state is urgently needed. Our studies show for the first time that the CD147 molecule (extracellular matrix metalloproteinase inducer) may be playing an important biological role in MM. We first demonstrate that CD147 is over-expressed in MM plasma cells (PCs) vs. normal and premalignant PCs. Next, functional studies revealed that the natural CD147 ligand, cyclophilin B, stimulates MM cell growth. Moreover, when MM patient PCs displaying bimodal CD147 expression were separated into CD147bright and CD147dim populations and analyzed for proliferation potential, we discovered that CD147bright PCs displayed significantly higher levels of cell proliferation than did CD147dim PCs. Lastly, CD147 silencing significantly attenuated MM cell proliferation. Taken together, these data suggest that the CD147 molecule plays a key role in MM cell proliferation and may serve as an attractive target for reducing the proliferative compartment of this disease.
Keywords: CD147, multiple myeloma, cyclophilin B, plasma cells
Introduction
Multiple myeloma (MM) is a malignancy characterized by the clonal expansion of malignant plasma cells (PC) that reside primarily within the bone marrow (BM) and is defined as having ≥ 10% PCs and ≥3g/dL of monoclonal immunoglobulin (Ig; M-protein), along with end organ damage which may include lytic bone lesions. MM is preceded by the asymptomatic, pre-malignant disorder monoclonal gammopathy of undetermined significance (MGUS),(1, 2) which is defined as presenting with <10% PCs in the BM, a serum M-protein of <3g/dL, and no end organ damage.(3) Although the clonal MGUS PC population may remain remarkably stable for years, MGUS patients have a lifelong, significantly increased risk of progressing to overt MM at a rate of 1% per year.(3, 4) Treatment of these patients is not initiated until transformation has occurred and serious end organ damage emerges.(5) Although there have been significant advances in treatment of this disease, MM patients continue to have a high mortality rate with a median survival rate of only 4–7 years.(6)
Although many groups have helped to define phenotypic markers that generally distinguish MM PCs from normal bone marrow PCs (BMPCs), e.g., CD19,(7) no such marker(s) is currently available which permits distinction between MGUS and MM clonal PCs or has the capability to discern those cells with proliferative potential. In the present study, we examine the possibility that the CD147 molecule may be playing an important role in MM disease biology. CD147, also known as extracellular matrix metalloproteinase (MMP) inducer (EMMPRIN), is a ubiquitously expressed transmembrane glycoprotein belonging to the Ig superfamily(8) and its various functions have been the topic of recent reviews.(8, 9) While CD147 is expressed widely on many cell types, albeit often at very low levels, there is extensive literature describing its aberrantly elevated expression in human cancer.(10–12) Moreover, CD147 levels have been shown to increase during progression from benign to malignant disease in a number of solid tumors.(13–15) Dysregulation of CD147 in tumor cells has been shown to stimulate increased angiogenesis(16) and production of MMPs resulting in degradation of the extracellular matrix, tumor growth, and metastasis.(17) CD147 has also been suggested to protect cancer cells from the apoptosis-like fate, anoikis. (18) Finally, CD147 binds to its natural ligands, cyclophilin A (CypA) and CypB(19, 20) together with heparin sulfate proteoglycans, thereby promoting cellular proliferation.(21) Cyps are cellular proteins which were once thought to play roles limited to intracellular functions. However, it is now universally accepted that these proteins can be secreted under inflammatory conditions and play a role as soluble mediators of intercellular communication.(22) Although extracellular CypA and CypB have been reported to support anti-apoptotic action and contribute to STAT3 signaling and survival in MM cells,(23) to our knowledge, the association of CD147 with cyclophilins in MM has not been previously demonstrated.
Because of the collective evidence in the literature concerning correlation of CD147 expression levels with malignant progression, in this study we investigated CD147 expression levels across the disease continuum of MGUS to MM and its potential role in malignant PC proliferation.
Material and Methods
Patient material
MGUS and MM patient bone marrow (BM) aspirates were collected as part of the routine clinical examination. BM aspirates obtained from patients undergoing spine surgeries without coincident B lineage malignancies served as a source of normal BMPCs. The Mayo Clinic Institutional Review Board approved the protocol to obtain samples from healthy donors as well as from individuals with monoclonal gammopathies. Written informed consent to participate in this research study was provided by all subjects from whom BM aspirates were drawn in accordance with the Declaration of Helsinki. Normal donor peripheral blood served as a source of normal B cells. BM aspirate mononuclear cells (MNCs) were isolated by Ficoll-Paque density gradient centrifugation. Both MM and normal PCs were isolated from the patient BM aspirates by magnetic bead separation using the human CD138 positive selection kit (StemCell Technologies, Vancouver, Canada) and a Robosep Cell Separator (StemCell Technologies).
Peripheral blood B (PB B) lymphocytes were enriched to >95% purity by negative selection using StemCell Technologies B cell enrichment cocktail. PB B cells were activated by culturing cells at 37°C in a 5% CO2 incubator for 5 days in the presence of 10 μg/ml CpG (oligodeoxynucleotide 2006 5′-TCGTCGTTTTGTCGTTTTGTCGTT), IL-2 (100 U/ml; Fitzgerald Industries International, Acton, MA), and IL-15 (10 ng/ml; Peprotech, Rocky Hill, NJ).
Cell lines and culture medium
The human myeloma cell lines (HMCL) ANBL-6,(24) ALMC-2,(25) and MCG were all derived in our laboratory. The IL-6 independent RPMI 8226 HMCL was purchased from ATCC (Manassas, VA). All HMCLs were maintained in IMDM supplemented with 50 U/ml penicillin G, 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA) and 5% heat-inactivated FCS (PAA Laboratories, Etobicoke, Ontario, Canada), Recombinant IL-6 was used at a final concentration of 1 ng/ml (Novartis, Basal, Switzerland). Recombinant IGF-I was used at a final concentration of 10 μg/ml (Sigma-Aldrich, St. Louis, MO).
DNA synthesis assays
DNA synthesis assays measuring [3H]-thymidine (TdR) (Perkin Elmer, Waltham, MA) incorporation were performed as previously described.(26) Cells were incubated in IMDM media plus 0.5% BSA (Celliane, Norcross, GA) with or without 1 ng/ml IL-6 and 10 nM CypB (Prospec, Rehovot, Israel) at 37°C in the presence of 5% CO2. For CD147 blocking studies, cells were incubated with or without 0.5 μg/ml of either an isotype-matched control Ab with irrelevant specificity (MOPC-21; Sigma-Aldrich) or an α-CD147 neutralizing antibody (Novus Biologicals, Littleton, CO) for 2h prior to stimulation with IL-6 or CypB. After the indicated length of incubation, DNA synthesis was measured. Data are presented as either the mean [3H]-TdR incorporation of triplicate samples or data from independent experiments +/− SEM.
Cell cycle and DNA content analysis
MNCs were incubated with complete IMDM media containing 10 μM bromodeoxyuridine (BrdU) overnight prior to staining the next day using a BrdU-APC flow kit (BD Biosciences, San Diego, CA) as previously described.(27) Cells were stained for CD147 followed by fixing and permeabilization before treating with 30 μg/ml DNAse for 1 hour at 37°C and staining with anti-BrdU. Finally, 7-AAD was added to the sample. For DNA content analysis, cells were cultured for 24 hrs before washing in cold PBS prior to the slow addition of 1 mL ice-cold ethanol while vortexing. Cells were incubated overnight at −20°C, washed twice in cold PBS, and incubated for 20 minutes with 50μL of RNase A (10μg/mL) at 37°C. 400uL of 50 μg/ml propidium iodide (PI) was added to samples prior to analysis on the FACStar (BD Biosciences). Data were analyzed using FlowJo (Tree Star, Ashland, OR).
RNA isolation, cDNA synthesis, and sequence analysis of IGHV genes
Total cellular RNA was extracted from purified PCs from MGUS and MM patients and healthy donors using the Trizol reagent (Invitrogen). Two μg of RNA was converted to cDNA using the GE Healthcare (Piscataway, NJ) First Strand Synthesis kit and cDNA was amplified by PCR using the Qiagen (Valencia, CA) HotStarTaq MasterMix kit and primers specific for CD147 and β-actin as an internal control. The CD147 primers used were: forward 5′-ACATCAACGAGGGGGAGACG-3′ and reverse 5′-GGCTTCAGACAGGCAGGACA-3′. Amplification was carried out in a Perkin Elmer 9600 thermocycler using the following conditions: 94°C for 15 minutes and then 30 cycles of 94°C for 30 seconds; 57°C for 30 seconds; 72°C for 90 sec; and a final cycle of 72°C for 10 minutes. Samples were electrophoresed in 1.5% agarose gels containing ethidium bromide. IGHV analysis was performed as previously described.(28)
Immunophenotypic analysis
Using previously described methods,(25) cells were incubated with the following antibodies: PE-conjugated CD147 (Abcam, Cambridge, MA), PE-conjugated mouse IgG1 isotype control (R&D, Minneapolis, MN), APC- and FITC-conjugated-CD138, FITC- and APC-conjugated-CD19, and FITC- and APC-conjugated mouse IgG1 isotype-matched controls (BD Biosciences). CD19 was used to distinguish normal PCs (CD138+/CD19+) from malignant PCs (CD138+/CD19−).(7) Data were analyzed using FlowJo (Tree Star). In some analyses, the data are reported as delta mean fluorescence intensity (ΔCD147 MFI), which is calculated by dividing the CD147 MFI by the control antibody MFI. In those cases where there was bimodal expression of CD147, the ΔCD147 MFI was calculated by gating only on cells that were CD147bright.
Cell survival analysis
Trypan blue exclusion was used to measure cell viability after culturing cells for 24, 48 and 72 hours. For apoptosis studies, cells were cultured in a final volume of 2 ml in Costar 24-well plates for 72 hrs prior to Annexin-FITC (BD Biosciences) and 7-AAD staining using flow cytometry.
Western blot analysis
Cells were lysed for 45 min on ice in lysis buffer containing 10 mM Tris (pH 7.4), 150 mM NaCl, 0.5 mM MgCl2, 0.5 mM CaCl2, 10 mM NaF, 10mM Na4P2O7, 1% NP-40, 10% glycerol, 0.1% SDS, 2 mM EDTA, complete mini protease inhibitor cocktail, and phosSTOP phosphatase inhibitor cocktail (Roche, Indianapolis, IN). Lysates were cleared of insoluble material by centrifugation and subjected to 10% SDS-PAGE before transfer to a PVDF membrane (Millipore, Billerica, MA) and blocked with 25 mM Tris-HCl (pH 7.2), 150 mM NaCl, and 0.1% Tween 20 (TBST) plus 5.0% Blotto (ISC BioExpress, Kaysville, UT). The blot was probed with anti-CD147 antibody (GeneTex, Irvine, CA) at a 1:2500 dilution, anti-phospho MAPK or anti-total MAPK at a 1:1000 dilution (NEB, Ipswich, MA), or anti-β-actin antibody (Novus) at a 1:5000 dilution. HRP-conjugated secondary antibodies (GE Healthcare) were used at a 1:2000 dilution. SuperSignal (Pierce, Rockford, IL) chemiluminescent substrate was used to detect proteins.
siRNA transfection parameters
CD147/BSG siGenome siRNA duplexes (target sequences are proprietary) were synthesized and purchased from Dharmacon Research, Inc (Lafayette, CO). Transfection of the siRNA duplexes was achieved via electroporation as previously described.(29) Briefly, cells were transfected with CD147 siRNA and subsequently plated in normal growth media. Twenty-four hours after the first transfection, a second transfection with CD147 siRNA was performed and cells were plated in normal growth media. Twenty-four hours following the second transfection, cells were washed twice prior to culture (2.5×104 cells/well in a final volume of 200 μl in 96-well flat bottom microtiter plates and 2.5 × 105 cells/well in a final volume of 2 ml in 24-well flat bottom plates). All cultures were performed in triplicate in the presence of 0.5% BSA and IL-6 (1 ng/ml) for 3 days at 37°C in the presence of 5% CO2. At 48h following culture commencement in the 96-well microtiter plates, cultures were pulsed with 1 μCi [3H]-Tdr and 18h later cells were harvested and [3H]-Tdr incorporation was assessed. For cells cultured in 24-well plates, cells were counted and analyzed by flow cytometry at 24h, 48h and 72h following the second transfection.
Statistical Analysis
Statistical analysis was performed using a Wilcoxon rank test. Values of p less than 0.05 were considered significant.
Results
Elevated expression of CD147 in MM PCs
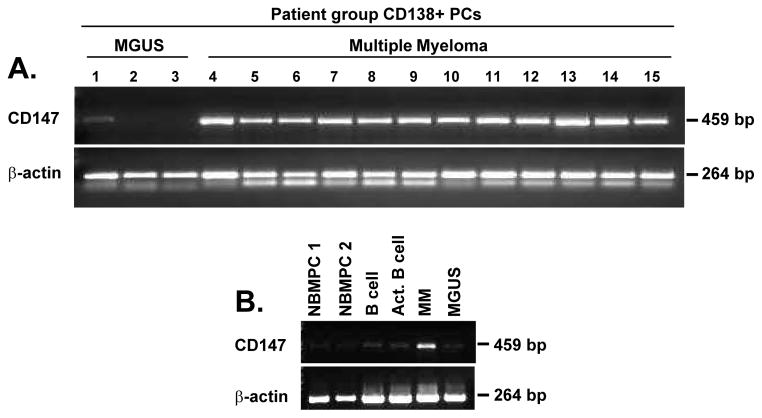
To determine whether there was a biological role for CD147 in MM, we assessed CD147 mRNA expression levels in purified PCs obtained from a series of MGUS and MM patients. We observed notably higher CD147 mRNA levels in MM PCs vs. MGUS PCs (Figure 1A). Figure 1B illustrates CD147 mRNA levels across a spectrum of normal resting and activated B cells and BMPCs compared to MGUS and MM patient PC samples. In contrast to MM PC CD147 mRNA levels, CD147 mRNA levels were considerably lower in MGUS PCs, normal BMPCs, and resting and activated normal B cells.
Figure 1. CD147 mRNA expression levels are elevated in primary MM patient PCs as compared to both MGUS patient PCs and normal samples.
A) RT-PCR was used to assess CD147 and β-actin mRNA levels in purified CD138+ MGUS (lanes 1–3) and MM PCs (lanes 4–15) as well as levels in (B) normal bone marrow PCs (NBMPC), CD19+ B cells and activated CD19+ B cells.
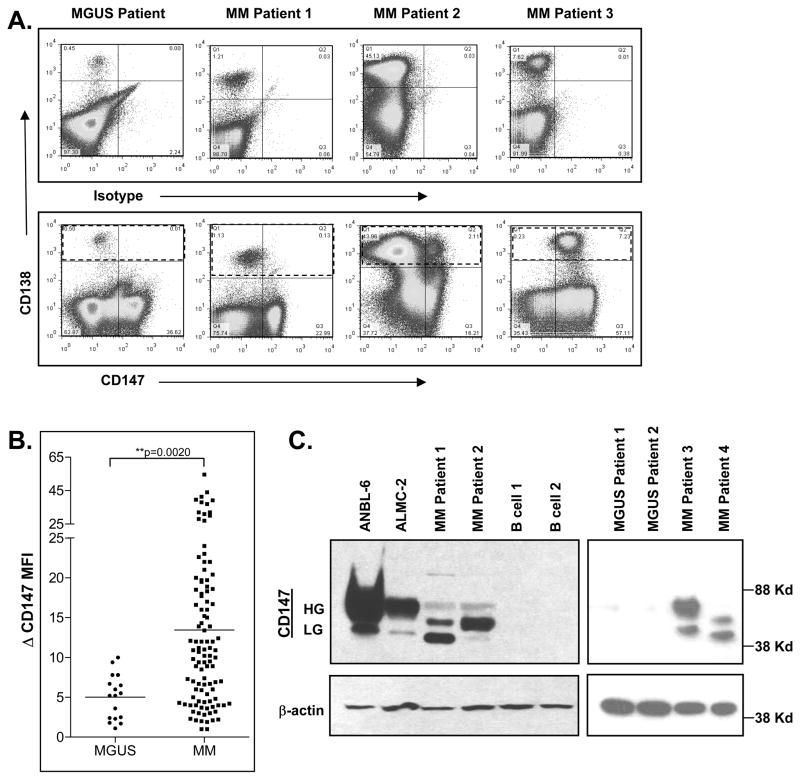
We next examined whether CD147 mRNA expression levels correlated with MGUS and MM PC cell surface CD147 expression. Flow cytometric analysis revealed several patterns (Figure 2A, bottom panels): 1) uniform lack of CD147 expression (MGUS Patient); 2) uniform CD147dim expression (MM Patient 1); 3) bimodal CD147 expression (MM Patient 2); and 4) uniform CD147bright expression (MM Patient 3). This methodology was used to assess CD147 levels on an additional 17 MGUS and 104 MM patients and Figure 2B shows that PCs from MM patients expressed significantly higher levels of CD147 than did PCs from MGUS patients. Finally, we performed western blot analysis to evaluate CD147 protein expression, as this method allowed us to visualize differences in glycosylation patterns. There is evidence that the higher glycosylated form of CD147 is the active form, as it induces MMP production(17, 30, 31) and metastasis.(32, 33) Figure 2C shows that CD147 was undetectable in normal B cells using this method, while MGUS PCs displayed extremely low levels of this molecule. By contrast, CD147 protein was clearly evident in the ANBL-6 and ALMC-2 HMCLs and in each of the four patient MM PC samples analyzed. Of interest, both high and low glycosylated forms of CD147 were observed in both HMCLs and MM patient samples, albeit to varying degrees. Collectively, these data demonstrate that MM cells on average express increased levels of CD147 mRNA and protein as compared with MGUS PCs.
Figure 2. CD147 protein levels are increased on MM patient PCs and HMCLs.
A) Variable patterns of CD147 surface expression levels are observed on CD138+ primary patient PCs (within dashed lines) from MGUS (left panel) and MM (middle and right panels) patients. B) Flow cytometry was used to determine the ΔMFI of CD147 expression. C) Western blot analysis validates increased protein expression levels of both high glycosylated (HG) and low glycosylated (LG) forms of CD147 in HMCLs and MM patient samples over that of normal CD19+ B cells and MGUS patient samples. (Patient samples independent of 2A).
Increased CD147 expression may accompany MM disease progression
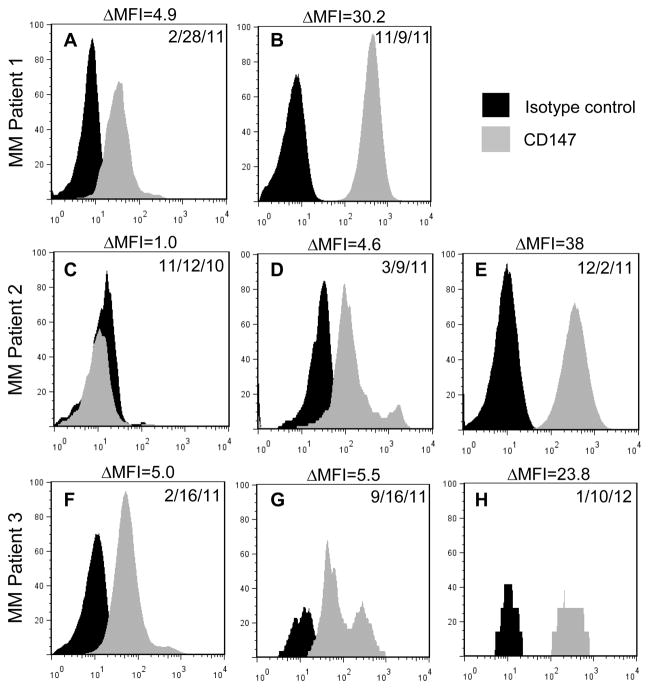
To determine whether CD147 expression levels correlate with MM disease progression, serial CD138+ MM PC samples from 3 patients were analyzed. At the time of initial assessment MM Patient 1 was clinically diagnosed with asymptomatic but progressive disease, and markedly elevated BM PCs (60%) and IgA levels (1610 mg/dL). Figure 3A demonstrates that CD147 was clearly detectable on the MM PCs at this stage. Upon reassessment nine months later, the level of BMPCs had risen to 70% and CD147 levels increased by over six-fold (Figure 3B). One month later, MM Patient 1 succumbed to disease. MM Patient 2 was initially assessed after having received an autologous peripheral blood stem cell transplant (PBSCT). At this time, CD147 was not detectable by flow cytometry (Figure 3C). At day 100 post-PBSCT, MM Patient 2 was reported to be responding well to treatment, however, CD147 expression could now be detected by flow cytometry (Figure 3D). One year post-PBSCT, the patient was determined to be relapsing with circulating PCs, 50% PCs in the BM and high IgA/kappa levels (1170 mg/dL, 5.4 mg/dL respectively). CD147 expression had significantly increased at this time (Fig. 3E). Finally, initial assessment of MM Patient 3 revealed the presence of CD147 (Figure 3F). At this time the patient was asymptomatic, yet concerns of an increasing malignant BM PC population (18%) and elevated kappa light chain (334 mg/dL) prompted the initiation of therapy. As therapy continued, the patient was determined to be responding well, with declining percentages of MM PCs and kappa light chain levels, however, the physician report stated a concern for possible progression. During this time, CD138+ MM PC staining revealed CD147 levels had increased to a ΔMFI of 5.5 (Figure 3G) and a ΔMFI of 23.8 (Figure 3H), respectively.
Figure 3. CD147 expression levels are increased on MM sequential patient PCs samples with progressive disease.
Surface expression levels of CD147 on primary CD138+ primary MM PCs (gray histogram) versus isotype-matched control (black histogram). A) CD147 levels on primary CD138+ primary MM PCs from a MM patient (Pt. 1) with asymptomatic but progressive disease (ΔMFI= 4.9) B) and eight months later (ΔMFI =30.2). C) CD147 levels on CD138+ cells from a MM patient (Pt. 2) at time of autologous stem cell transplant (ΔMFI =1.0) D) and 100 days post-PBSCT (ΔMFI =4.6), who eventually progressed to relapse E) 1 yr post-PBSCT (ΔMFI =38). F) CD147 levels on primary CD138+ primary MM PCs from an asymptomatic MM patient placed on initial treatment (Pt. 3)(ΔMFI =5) and CD147 levels measured 7 months post-treatment initiation G) (ΔMFI =5.5) and 11 months post-treatment initiation, at which time a very good partial response with concerns of progression were noted H) (ΔMFI =23.8).
CypB stimulates myeloma cell DNA synthesis
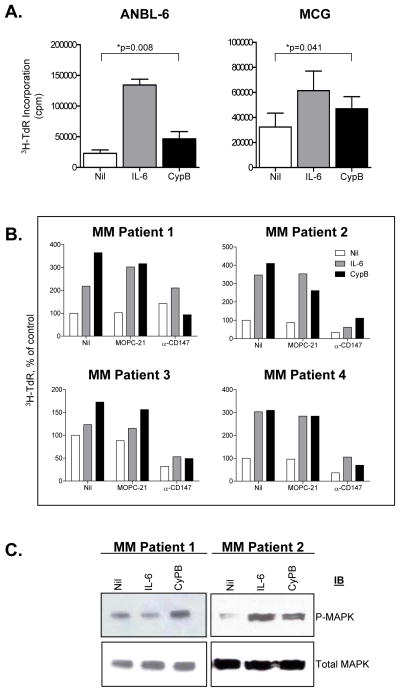
We next analyzed the ability of CypB, a natural CD147 ligand, to induce MM cell proliferation. Interestingly, in both IL-6 dependent HMCLs (Figure 4A), addition of exogenous CypB also reproducibly increased DNA synthesis in both HMCLs, albeit to a lesser degree than IL-6. To ensure Cyp stimulation required CD147, we determined whether a blocking mAb antibody to CD147 would inhibit Cyp-stimulated DNA synthesis. The data shown in Table 1 provide additional evidence that CypB stimulates HMCL DNA synthesis. Although the addition of an isotype-matched control mAb (MOPC-21) did not inhibit CypB-stimulated responsiveness, the addition of the CD147 blocking antibody almost completely abolished the CypB-stimulated response. Moreover, the anti-CD147 antibody also slightly inhibited the magnitude of IL-6 stimulated DNA synthesis suggesting a more general role for CD147 in MM cell proliferation. Lastly, cytokine independent background proliferation observed in all three HMCLs was also significantly inhibited by the addition of the blocking antibody. In experiments not shown, we determined that the anti-CD147 induced inhibition of MM cell proliferation was not secondary to induction of apoptosis. These results support the conclusion CypB stimulation of MM cell DNA synthesis requires CD147 expression. Taken together, these data identify extracellular cyclophilins as novel molecules that may contribute to the overall proliferation levels of MM cells.
Figure 4. CypB stimulates both HMCLs and CD147-expressing CD138+ primary myeloma patient cells.
A) Cells were starved overnight in IMDM+0.5% BSA followed by washing 2× in saline prior to culturing for 3 days in the presence of IL-6 or CypB before assessing DNA synthesis. Data represent values from independent experiments ± SEM using ANBL-6 (n=8) and MCG (n=4) HMCLs. ANBL-6 and MCG each displayed a statistically significant response to CypB (p=0.008 and 0.041, respectively). B) CD138+ primary MM patient cells were incubated for 2 hrs in the presence of a control antibody or anti-CD147 prior to stimulation with IL-6 or CypB for 5 days. C) Western blot analysis reveals phosphorylation of MAPK after CypB stimulation. IL-6 was used as a positive control. Beta actin used as loading control.
Table 1.
Effects of α-CD147 mAb on IL-6 and CypB stimulated HMCL DNA synthesis
| Cell Line | mAb | 3H-TdR Incorporation (cpm × 10−3 ± SEM) | ||
|---|---|---|---|---|
| Nil | IL-6 | CypB | ||
| ALMC-2 | -- | 6.4 ± 0.6 | 50.4 ± 3.1 | 11.3 ± 0.1* |
| MOPC | 7.3 ± 0.1 | 52.5 ± 0.8 | 12.9 ± 0.6 | |
| CD147 | 1.7 ± 0.1 | 35.9 ± 0.3 | 2.5 ± 0.1** | |
| ANBL-6 | -- | 28.6 ± 1.3 | 193.4 ± 4.1 | 41.5 ± 0.2* |
| MOPC | 35.5 ± 1.3 | 191.5 ± 2.4 | 50.0 ± 0.4 | |
| CD147 | 4.8 ± 0.3 | 131.1 ± 2.6 | 9.9 ± 0.6** | |
| MCG | -- | 47.3 ± 1.7 | 75.3 ± 3.1 | 67.6 ± 0.9* |
| MOPC | 48.3 ± 0.8 | 87.2 ± 3.5 | 74.8 ± 1.2 | |
| CD147 | 39.3 ± 0.3 | 71.1 ± 4.7 | 50.1 ± 0.1** | |
Myeloma cell lines were cultured in IMDM + 0.5% BSA in the presence or absence of 1 ng/ml IL-6 or 10 nM recombinant CypB, in combination with or without 0.5 μg/ml of control MOPC-21 or α-CD147. DNA synthesis was assayed on day 3. Values represent the mean of triplicate values and are representative of multiple experiments.
p<0.0040;
p<0.0003
CD147 is necessary for CypB mediated signaling in primary MM cells
We next investigated whether CD147-expressing primary MM patient PCs also respond to CypB stimulation. Figure 4B demonstrates that, along with IL-6, CypB can stimulate DNA synthesis of CD147-expressing patient MM cells. Furthermore, DNA synthesis resulting from both IL-6 and CypB stimulation was significantly reduced by the addition of a CD147 blocking antibody. Figure 4C also shows that CypB stimulation results in MAPK phosphorylation. IL-6 was used as a positive control, while total MAPK levels served as a loading control. These results clearly demonstrate that CypB can not only specifically stimulate DNA synthesis through CD147 in HMCLs, but can also stimulate primary patient MM cell DNA synthesis and MAPK phosphorylation.
CD147 expression is associated with cycling cells
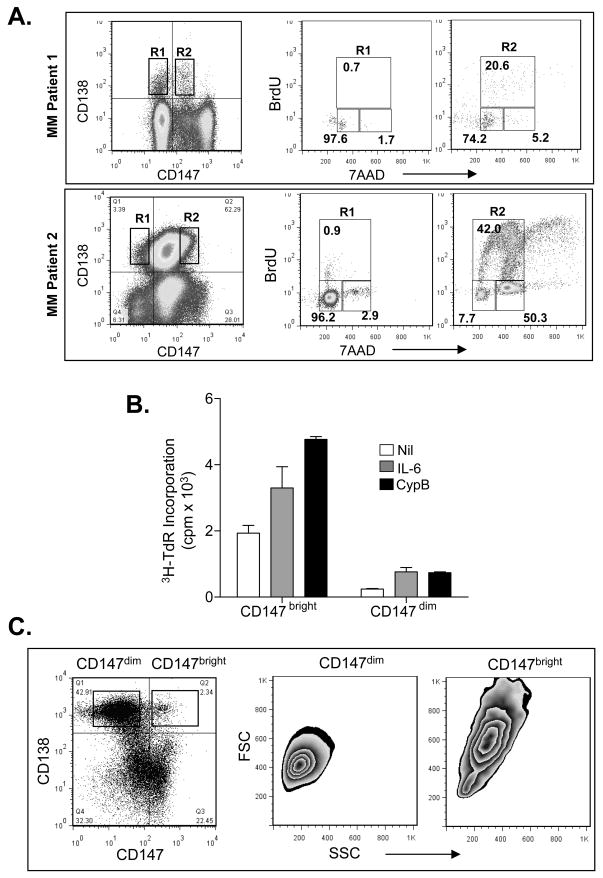
Next, we wished to more directly assess whether there is an association between MM cell CD147 expression and cell cycle transit. To do this, we performed cell cycle analysis on patient samples known to bimodally express CD147. After culturing cells overnight in the presence of BrdU, CD138+/CD147bright and CD138+/CD147dim cells were analyzed. Remarkably, the CD138+/CD147bright cells were greatly enriched for cells within the S and G2/M phases of the cell cycle (20.6% and 5.2%, respectively) while the CD138+/CD147dim cells resided almost exclusively within the G0/G1 phase (97.6%) (Figure 5A; upper panel).
Figure 5. CD147 expressing primary MM cells are transiting the cell cycle.
A) BM MNCs from two MM patients were cultured overnight at 37°C in the presence of BrdU. MM Patient 1 displayed a bimodal CD147 expression and the gated CD147bright subset (R2) was shown to be significantly enriched for cells in S and G2/M phases. MM patient 2, which exhibited more uniform CD147 expression, was analyzed by gating CD147 bright and dim populations. The CD147bright cells (R2) were greatly enriched for cells in S and G2/M. B) Sorted CD147bright and CD147dim populations from MM patient 2 were cultured for 5 days before assessing IL-6 and CypB responsiveness as measured by DNA synthesis. C) Forward and size scatter analysis of primary MM patient CD138+/CD147dim vs. CD138+/CD147bright cells.
Absolute levels of CD147 may also impact the proliferative potential of MM cells. To address this possibility, we analyzed primary patient PCs that expressed CD147 in a unimodal manner, albeit over a wide, several-logs range. Of interest, the CD147bright PCs were greatly enriched within the S (42%) and G2/M (50.3%) phases, whereas CD147dim PCs resided almost exclusively within the G0/G1 phase (96.2%) (Figure 5A; lower panel). Given that some investigators have proposed a correlation between CD45+ PCs and the proliferative compartment of the MM clone,(34) CD147bright/dim populations were also analyzed for CD45 expression. However, CD45 expression was comparable in both the CD147bright and CD147dim populations (data not shown). We also sorted CD138+ PCs from MM Patient 2 into CD147dim and CD147bright populations and cultured these cells for 5 days before assessing DNA synthesis. Figure 5B clearly shows that the CD147bright PCs display high background proliferation and respond significantly to the addition of IL-6 and CypB. By comparison, proliferation levels displayed by the CD147dim PCs, including basal DNA synthesis, were significantly lower. Wright-Giemsa staining demonstrated that CD147dim PCs were morphologically smaller in size than CD147bright PCs (data not shown). This finding was corroborated by flow cytometry, which demonstrated the lower level of forward scatter displayed by the CD147dim PCs vs. the CD147bright PCs prior to sorting (Figure 5C).
Given the striking differences in proliferation rates of CD147bright and CD147dim PCs, it remained possible that the CD147dim PCs were not clonally related and might represent residual normal BMPCs. To test this possibility, RNA was isolated from the CD147bright and CD147dim PC populations from MM Patient 2 and subjected to IGHV analysis. However, Supplemental Figure 1 demonstrates that the IGHV region genes in both CD147bright and CD147dim populations were identical to one another, as well as to the original unsorted primary patient cells. We also assessed CD147 mRNA expression levels in each subpopulation by RT-PCR and verified diminished CD147 mRNA levels in the CD147dim PCs (Supplemental Figure 2). In results not shown, we also analyzed both subpopulations by FISH and both PC populations contained trisomies for 3, 7, 9 and 15 along with a monosomy 13.
siRNA induced down-regulation of CD147 decreases the proliferation of HMCLs
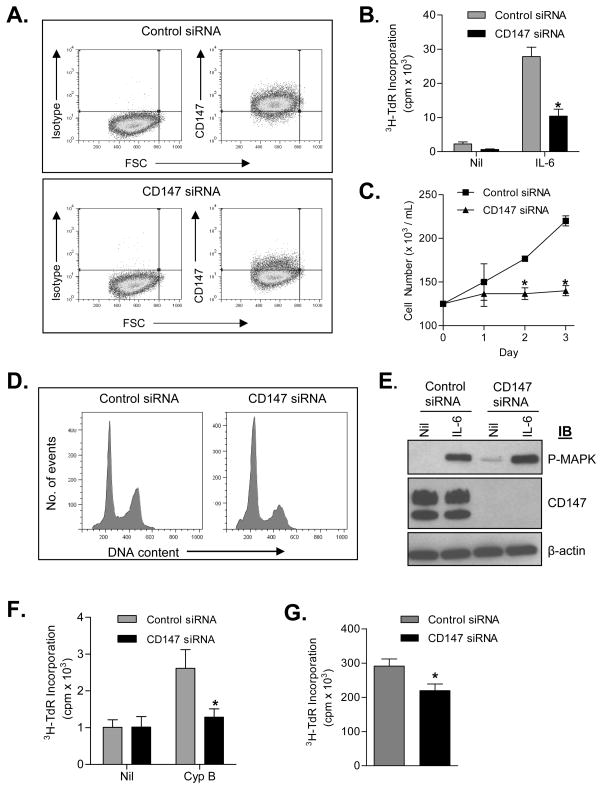
Finally, we employed siRNA knockdown strategies to definitively test the role of CD147 expression and MM cell proliferation. First, ALMC-2, ANBL-6, and KAS-6/1 cells were transfected with CD147 specific siRNA. Reduced CD147 expression levels were verified by flow cytometry (Figure 6A; AMLC-2 shown as a representative HMCL in all Figure 6 panels). Moreover, IL-6-induced DNA synthesis (Figure 6B) and cell proliferation (Figure 6C) was significantly compromised following CD147 down-regulation (p= 0.0001 and p= 0.0006 on day 3, respectively). We next determined whether CD147 down-regulation had an effect on the number of apoptotic cells present in our HMCLs. In data not shown, the number of apoptotic cells did not increase following down-regulation of CD147. Notably, cell cycle analysis revealed that CD147 down-regulation resulted in a significant increase in the number of cells in the G0/G1 phase of the cell cycle and a decrease in the number of cells in the G2/M phase of the cell cycle, as compared to cells transfected with control siRNA (Figure 6D). Although the IL-6-induced proliferative capacity of HMCLs was decreased following down-regulation of CD147, IL-6-mediated phosphorylation of MAPK remained robust (Figure 6E) suggesting that the IL-6 signaling pathway overall was not compromised in these cells. In order to determine whether our results following down-regulation of CD147 are specific to IL-6 induced signaling in MM cells or reflect an overall decrease in proliferative capacity we next examined the effect of CD147 downregulation on CypB-induced proliferation. As shown in Figure 6F, CypB induced DNA synthesis was significantly compromised following CD147 downregulation (p=0.033 on day 3). To further test whether the function of CD147 is independent of IL-6 signaling, we assessed CD147 expression in the IL-6 independent cell line RPMI 8226. This analysis revealed that these cells express very high levels of CD147 (ΔMFI=302, data not shown). Although CD147 siRNA only achieved a moderate reduction in the expression of CD147 (ΔMFI=85), DNA synthesis (FIG 6G) was significantly compromised in these cells (p=0.001 day 3, Figure 6G). Taken together, these data suggest that downregulation of CD147 decreases the overall proliferative capacity of MM cell lines, independent of the IL-6 signaling pathway.
Figure 6. Downregulation of CD147 decreases the proliferative capability of MM cells.
A) Flow cytometry was used to confirm downregulation of CD147 in ALMC-2 cells 72 hrs after transfection of control siRNA or CD147 specific siRNA. B) Downregulation of CD147 decreased both background and IL-6 induced DNA synthesis in ALMC-2 cells measured after 3 days of culture. Note, during the 4 hrs prior to commencement of the experiment cells were deprived of serum and IL-6. C) CD147 downregulation inhibited IL-6 stimulated ALMC-2 proliferation. D) Western blot verifying CD147 knockdown in ALMC-2 cells and lack of interference with IL-6 stimulated MAPK phosphorylation. Note, during the 4 hrs prior to commencement of the experiment cells were deprived of serum and IL-6. E) Downregulation of CD147 in ALMC-2 cells increases the number of cells in the G0/G1 cell cycle phase. F) CypB induced DNA synthesis in ALMC-2 cells is decreased following downregulation of CD147. Note, for 12 hrs prior to commencement of the experiment cells were cultured in media containing 0.5% BSA. G) Downregulation of CD147 results in decreased DNA synthesis of the IL-6 independent cell line RPMI 8226. (p<0.05)
Discussion
Previous studies have demonstrated the CD147 molecule is overexpressed in many carcinomas when compared to their normal and benign counterparts,(10, 11, 35, 36) and contributes to biological activities which foster tumor progression.(13, 15, 37). However, prior to our studies, CD147 expression in MM cells and its potential biological function were unknown. Herein, we provide the first evidence that CD147 expression levels may become dysregulated in MM and more importantly, that CD147 plays a biological role in this disease.
Regarding CD147 expression levels, our data support the conclusion that CD147 mRNA levels are increased in MM PCs relative to MGUS PCs and normal BMPCs. Analysis of publicly available gene expression profiling (GEP) data39 similarly revealed that CD147 mRNA levels were elevated on MM PCs (n=559) vs. MGUS PCs (n=44)(p <0.0001), while analysis of Mayo GEP data (kindly provided by Dr. Rafael Fonseca) also showed MM PCs (n=53 previously untreated MM patients) expressed higher levels of CD147 mRNA than MGUS PCs (n=24)(p <0.0034). CD147 protein levels, as seen by ΔMFI, were elevated on MM cells vs. MGUS cells (p=0.0020). We also analyzed CD147 expression in normal B cells, and whereas CD147 mRNA levels were low, CD147 was undetectable by western blot analysis. Moreover, intentional activation of normal B cells with polyclonal activation failed to upregulate CD147 expression (data not shown), suggesting that increased CD147 on MM cells may reflect genetic changes occurring during malignant transformation.
Our discovery that many primary patient MM cells express elevated levels of CD147 prompted further investigation into the biological role of this molecule in malignant PCs. Here, we took advantage of our discovery of patients whose MM cells expressed CD147 in a bimodal manner as it provided us with a tool to isolate CD147dim and CD147bright MM PCs from the same patient and ask whether CD147 plays a role in accelerated PC proliferation. In this regard, we clearly demonstrated that CD147bright PCs were greatly enriched for cells within the S and G2/M phases of the cell cycle, whereas CD147dim PCs largely resided within G0/G1. Taken together with our data demonstrating that CD147 knockdown decreases MM cell proliferation, these results support a critical role for CD147 in MM cell proliferation. These findings are clinically significant because the proliferative rate of PCs, measured by the PC labeling index (PCLI), is an important prognostic factor. Generally, MGUS PCs display an extremely low PCLI and PC numbers in these patients can remain stable for years. Elevations in PCLI are generally indicative of disease progression, whether it is from MGUS to MM or in progressing MM patients.(38, 39) Our observations that CD147 expression levels may increase with disease progression suggest a mechanism that may underlie elevations in PCLI.
Of note, CD45 expression has been suggested to identify the proliferative compartment within MM.(34) However, this conclusion remains controversial. For example, some have argued that the absence of this marker is associated with poor prognosis,(40, 41) while still others have contradicted this observation by stating that CD45 expression correlates with aggressiveness of the disease.(42) Finally, additional investigators have found no correlation or prognostic significance of CD45 within this disease.(43) Despite the controversy, to address the possibility that CD147 is a surrogate for CD45 expression, we also assessed CD45 expression on CD147bright and CD147dim PCs that had been pulsed with BrdU. However, we observed that CD45+ PCs were found to reside in both CD147bright and CD147dim PCs populations of the clone thereby suggesting that CD147 expression marks a distinctive cellular state (data not shown).
Given the demonstrated role of CD147 in MM cell proliferation, we also carried out studies to determine if its natural ligand, extracellular cyclophilins,(19) could stimulate MM cell proliferation. Of interest, the CD147-cyclophilin interaction has been implicated as playing an important role in inflammatory diseases by inducing MMP production.(9, 44, 45) Recent evidence also shows that the addition of extracellular cyclophilins to CD147-expressing tumor cells enhances both tumor cell invasion and cellular proliferation.(46) Here, we show that CypB indeed stimulates DNA synthesis of MM CD147bright but not CD147dim PCs demonstrating the specificity of the interaction. Moreover, addition of a blocking antibody to CD147 abrogated Cyp-stimulated MM cell responses. The Cyp responsiveness of MM cells is consistent with recent work by Bauer et al demonstrating that CypA and CypB support myeloma cell survival through STAT3 signaling.(23) It is important to note that the HMCLs differed in their level of responsiveness to CypB. Although this observation needs further study, we speculate that this may result from variable levels of CypB autocrine signaling, i.e., some HMCLs may secrete higher levels of CypB that would render the activity of exogenous CypB less obvious. Preliminary studies have demonstrated that not only is CypB found in the plasma of BM MM patient samples, but it is also secreted by both HMCLs and CD138+ primary patient samples (Arendt et al., unpublished observations). Collectively, our results taken together with reports in the literature that blocking CD147 induces cell death in cancer cells(47) and silencing CD147 inhibits tumor progression, proliferation and migration activity,(48–50) suggest CD147 may be an attractive molecule to target therapeutically in MM patients.
In addition to a role in MM cell proliferation, the CD147 molecule has a number of other activities with possible relevance to MM. For example, a characteristic feature of MM is that the tumor cell shares a reciprocal interaction with the BM microenvironment. The signals that are exchanged, causing immune suppression, angiogenesis and lytic bone lesions, play a critical role in maintaining tumor cell growth and survival. Many of these distinguishing features have been attributed to CD147 in other cancers and an attractive hypothesis currently under study is that this molecule also exerts these additional activities within the MM cell niche. There are two relevant examples. First, CD147 overexpression has been shown to promote increased angiogenesis(16) and an angiogenic phenomenon is also observed as MGUS evolves into MM.(51, 52) Second, it has been shown that CD147 stimulates production of MMPs not only by surrounding stromal cells, but by tumor cells themselves.(15) Importantly, the dysregulated presence of MMPs in the MM microenvironment has been implicated in extracellular matrix remodeling and osteolytic bone destruction.(53) Other investigators have suggested that the higher glycosylated form of CD147 is the active form, inducing both MMP production(17, 30, 31) and metastasis(32, 33) Therefore, our observations that the CD147 glycosylation patterns of primary MM cells were quite varied support the need for additional investigation into CD147 glycosylation status and bone disease in MM patients.
Finally, in a small cohort of patients from whom we had serial samples collected at various stages of disease, we obtained preliminary evidence that CD147 expression levels may correlate with disease progression (Figure 3). Taken together, these studies provide the rationale for future studies aimed at determining whether CD147 would function as a clinically useful biomarker of disease progression. We emphasize that larger studies utilizing serial samples are needed to formally test this possibility. Intriguingly, we observed some MGUS patients with higher CD147 levels and some MM patients with very low CD147 levels. Follow-up studies of both groups of patients over time are necessary to understand the significance of these observations. However, anecdotal review of the small subset of MM patients with CD147dim PCs indicated these patients were undergoing preconditioning for ASCT or were asymptomatic and/or lacked CT/PET positivity at the time of sample draw. Lastly, the patterns of CD147 expression observed in patient samples are intriguing and varied from patient PCs with uniform CD147 negative, CD147dim, or CD147bright expression to patient PCs that included only a small CD147bright subpopulation. Longitudinal studies are also needed to determine whether the emergence of a CD147bright PC population indeed correlates with progression across the disease continuum.
In summary, our studies demonstrate that CD147 is differentially expressed across the disease continuum both at the mRNA and protein levels and signaling via this molecule may be necessary for MM PCs to transit the cell cycle. As CD147 has been implicated as playing a significant role in other cancers, these results not only emphasize the possible importance of CD147 in the evolution of MGUS to MM tumor progression, but its potential role in tumor proliferation. Our studies provide an important novel foundation for future studies investigating the ability of CD147 to serve as an important biomarker of disease progression in the monoclonal gammopathies.
Supplementary Material
Acknowledgments
This work was supported in part by the Multiple Myeloma Research Foundation, a grant from the National Institutes of Health (CA164232; awarded to DFJ), and the JABBS and Predolin Foundations.
Footnotes
Authorship Contributions
BKA designed and performed research, analyzed data, and wrote the manuscript. DKW, XW, RCT, and KJH performed research and analyzed data. PMH and AD designed research and enrolled patients. DFJ designed research and wrote and approved the manuscript. All authors reviewed and gave final approval of the manuscript.
Disclosure of Conflicts of Interest
The authors declare no conflicts of interest.
Supplementary information is available at Leukemia’s website
References
- 1.Landgren O, Kyle RA, Pfeiffer RM, Katzmann JA, Caporaso NE, Hayes RB, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009 May 28;113(22):5412–5417. doi: 10.1182/blood-2008-12-194241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss BM, Abadie J, Verma P, Howard RS, Kuehl WM. A monoclonal gammopathy precedes multiple myeloma in most patients. Blood. 2009 May 28;113(22):5418–5422. doi: 10.1182/blood-2008-12-195008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Myeloma Working G. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003 Jun;121(5):749–757. [PubMed] [Google Scholar]
- 4.Kyle RA, Rajkumar SV. Monoclonal gammopathy of undetermined significance. Br J Haematol. 2006 Sep;134(6):573–589. doi: 10.1111/j.1365-2141.2006.06235.x. [DOI] [PubMed] [Google Scholar]
- 5.Kyle RA. “Benign” monoclonal gammopathy--after 20 to 35 years of follow-up. Mayo Clin Proc. 1993 Jan;68(1):26–36. doi: 10.1016/s0025-6196(12)60015-9. [DOI] [PubMed] [Google Scholar]
- 6.Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008 Mar 1;111(5):2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raja KR, Kovarova L, Hajek R. Review of phenotypic markers used in flow cytometric analysis of MGUS and MM, and applicability of flow cytometry in other plasma cell disorders. Br J Haematol. 2010 May;149(3):334–351. doi: 10.1111/j.1365-2141.2010.08121.x. [DOI] [PubMed] [Google Scholar]
- 8.Iacono KT, Brown AL, Greene MI, Saouaf SJ. CD147 immunoglobulin superfamily receptor function and role in pathology. Exp Mol Pathol. 2007 Dec;83(3):283–295. doi: 10.1016/j.yexmp.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yurchenko V, Constant S, Eisenmesser E, Bukrinsky M. Cyclophilin-CD147 interactions: a new target for anti-inflammatory therapeutics. Clin Exp Immunol. 2010 Jun;160(3):305–317. doi: 10.1111/j.1365-2249.2010.04115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Xu J, Chen L, Zhong WD, Zhang Z, Mi L, et al. HAb18G (CD147), a cancer-associated biomarker and its role in cancer detection. Histopathology. 2009 May;54(6):677–687. doi: 10.1111/j.1365-2559.2009.03280.x. [DOI] [PubMed] [Google Scholar]
- 11.van den Oord JJ, Paemen L, Opdenakker G, de Wolf-Peeters C. Expression of gelatinase B and the extracellular matrix metalloproteinase inducer EMMPRIN in benign and malignant pigment cell lesions of the skin. Am J Pathol. 1997 Sep;151(3):665–670. [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng HC, Takahashi H, Murai Y, Cui ZG, Nomoto K, Miwa S, et al. Upregulated EMMPRIN/CD147 might contribute to growth and angiogenesis of gastric carcinoma: a good marker for local invasion and prognosis. Br J Cancer. 2006 Nov 20;95(10):1371–1378. doi: 10.1038/sj.bjc.6603425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanekura T, Chen X. CD147/basigin promotes progression of malignant melanoma and other cancers. J Dermatol Sci. 2010 Mar;57(3):149–154. doi: 10.1016/j.jdermsci.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Nawroth R, Stohr R, Hartmann A, Gschwend JE, Retz M. EMMPRIN (CD147). A new key protein during tumor progression in bladder cancer. Urologe A. 2008 Sep;47(9):1152–1156. doi: 10.1007/s00120-008-1828-9. [DOI] [PubMed] [Google Scholar]
- 15.Yan L, Zucker S, Toole BP. Roles of the multifunctional glycoprotein, emmprin (basigin; CD147), in tumour progression. Thromb Haemost. 2005 Feb;93(2):199–204. doi: 10.1160/TH04-08-0536. [DOI] [PubMed] [Google Scholar]
- 16.Tang Y, Nakada MT, Kesavan P, McCabe F, Millar H, Rafferty P, et al. Extracellular matrix metalloproteinase inducer stimulates tumor angiogenesis by elevating vascular endothelial cell growth factor and matrix metalloproteinases. Cancer Res. 2005 Apr 15;65(8):3193–3199. doi: 10.1158/0008-5472.CAN-04-3605. [DOI] [PubMed] [Google Scholar]
- 17.Sun J, Hemler ME. Regulation of MMP-1 and MMP-2 production through CD147/extracellular matrix metalloproteinase inducer interactions. Cancer Res. 2001 Mar 1;61( 5):2276–2281. [PubMed] [Google Scholar]
- 18.Yang JM, O’Neill P, Jin W, Foty R, Medina DJ, Xu Z, et al. Extracellular matrix metalloproteinase inducer (CD147) confers resistance of breast cancer cells to Anoikis through inhibition of Bim. J Biol Chem. 2006 Apr 7;281(14):9719–9727. doi: 10.1074/jbc.M508421200. [DOI] [PubMed] [Google Scholar]
- 19.Yurchenko V, O’Connor M, Dai WW, Guo H, Toole B, Sherry B, et al. CD147 is a signaling receptor for cyclophilin B. Biochem Biophys Res Commun. 2001 Nov 9;288(4):786–788. doi: 10.1006/bbrc.2001.5847. [DOI] [PubMed] [Google Scholar]
- 20.Yurchenko V, Zybarth G, O’Connor M, Dai WW, Franchin G, Hao T, et al. Active site residues of cyclophilin A are crucial for its signaling activity via CD147. J Biol Chem. 2002 Jun 21;277(25):22959–22965. doi: 10.1074/jbc.M201593200. [DOI] [PubMed] [Google Scholar]
- 21.Pakula R, Melchior A, Denys A, Vanpouille C, Mazurier J, Allain F. Syndecan-1/CD147 association is essential for cyclophilin B-induced activation of p44/42 mitogen-activated protein kinases and promotion of cell adhesion and chemotaxis. Glycobiology. 2007 May;17( 5):492–503. doi: 10.1093/glycob/cwm009. [DOI] [PubMed] [Google Scholar]
- 22.Allain F, Vanpouille C, Carpentier M, Slomianny MC, Durieux S, Spik G. Interaction with glycosaminoglycans is required for cyclophilin B to trigger integrin-mediated adhesion of peripheral blood T lymphocytes to extracellular matrix. Proc Natl Acad Sci U S A. 2002 Mar 5;99(5):2714–2719. doi: 10.1073/pnas.052284899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bauer K, Kretzschmar AK, Cvijic H, Blumert C, Loffler D, Brocke-Heidrich K, et al. Cyclophilins contribute to Stat3 signaling and survival of multiple myeloma cells. Oncogene. 2009 Aug 6;28(31):2784–2795. doi: 10.1038/onc.2009.142. [DOI] [PubMed] [Google Scholar]
- 24.Jelinek DF, Ahmann GJ, Greipp PR, Jalal SM, Westendorf JJ, Katzmann JA, et al. Coexistence of aneuploid subclones within a myeloma cell line that exhibits clonal immunoglobulin gene rearrangement: clinical implications. Cancer Res. 1993 Nov 1;53(21):5320–5327. [PubMed] [Google Scholar]
- 25.Arendt BK, Ramirez-Alvarado M, Sikkink LA, Keats JJ, Ahmann GJ, Dispenzieri A, et al. Biologic and genetic characterization of the novel amyloidogenic lambda light chain-secreting human cell lines, ALMC-1 and ALMC-2. Blood. 2008 Sep 1;112(5):1931–1941. doi: 10.1182/blood-2008-03-143040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Westendorf JJ, Ahmann GJ, Greipp PR, Witzig TE, Lust JA, Jelinek DF. Establishment and characterization of three myeloma cell lines that demonstrate variable cytokine responses and abilities to produce autocrine interleukin-6. Leukemia. 1996 May;10(5):866–876. [PubMed] [Google Scholar]
- 27.Walters DK, Wu X, Tschumper RC, Arendt BK, Huddleston PM, Henderson KJ, et al. Evidence for ongoing DNA damage in multiple myeloma cells as revealed by constitutive phosphorylation of H2AX. Leukemia. 2011 Aug;25(8):1344–1353. doi: 10.1038/leu.2011.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tschumper RC, Geyer SM, Campbell ME, Kay NE, Shanafelt TD, Zent CS, et al. Immunoglobulin diversity gene usage predicts unfavorable outcome in a subset of chronic lymphocytic leukemia patients. J Clin Invest. 2008 Jan;118(1):306–315. doi: 10.1172/JCI32625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walters DK, Jelinek DF. The effectiveness of double-stranded short inhibitory RNAs (siRNAs) may depend on the method of transfection. Antisense Nucleic Acid Drug Dev. 2002 Dec;12(6):411–418. doi: 10.1089/108729002321082483. [DOI] [PubMed] [Google Scholar]
- 30.Tang W, Chang SB, Hemler ME. Links between CD147 function, glycosylation, and caveolin-1. Mol Biol Cell. 2004 Sep;15(9):4043–4050. doi: 10.1091/mbc.E04-05-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang W, Hemler ME. Caveolin-1 regulates matrix metalloproteinases-1 induction and CD147/EMMPRIN cell surface clustering. J Biol Chem. 2004 Mar 19;279(12):11112–11118. doi: 10.1074/jbc.M312947200. [DOI] [PubMed] [Google Scholar]
- 32.Voigt H, Vetter-Kauczok CS, Schrama D, Hofmann UB, Becker JC, Houben R. CD147 impacts angiogenesis and metastasis formation. Cancer Invest. 2009 Mar;27(3):329–333. doi: 10.1080/07357900802392675. [DOI] [PubMed] [Google Scholar]
- 33.Xu J, Xu HY, Zhang Q, Song F, Jiang JL, Yang XM, et al. HAb18G/CD147 functions in invasion and metastasis of hepatocellular carcinoma. Mol Cancer Res. 2007 Jun;5(6):605–614. doi: 10.1158/1541-7786.MCR-06-0286. [DOI] [PubMed] [Google Scholar]
- 34.Robillard N, Pellat-Deceunynck C, Bataille R. Phenotypic characterization of the human myeloma cell growth fraction. Blood. 2005 Jun 15;105(12):4845–4848. doi: 10.1182/blood-2004-12-4700. [DOI] [PubMed] [Google Scholar]
- 35.Caudroy S, Polette M, Tournier JM, Burlet H, Toole B, Zucker S, et al. Expression of the extracellular matrix metalloproteinase inducer (EMMPRIN) and the matrix metalloproteinase-2 in bronchopulmonary and breast lesions. J Histochem Cytochem. 1999 Dec;47(12):1575–1580. doi: 10.1177/002215549904701209. [DOI] [PubMed] [Google Scholar]
- 36.Muraoka K, Nabeshima K, Murayama T, Biswas C, Koono M. Enhanced expression of a tumor-cell-derived collagenase-stimulatory factor in urothelial carcinoma: its usefulness as a tumor marker for bladder cancers. Int J Cancer. 1993 Aug 19;55(1):19–26. doi: 10.1002/ijc.2910550105. [DOI] [PubMed] [Google Scholar]
- 37.Nabeshima K, Iwasaki H, Koga K, Hojo H, Suzumiya J, Kikuchi M. Emmprin (basigin/CD147): matrix metalloproteinase modulator and multifunctional cell recognition molecule that plays a critical role in cancer progression. Pathol Int. 2006 Jul;56(7):359–367. doi: 10.1111/j.1440-1827.2006.01972.x. [DOI] [PubMed] [Google Scholar]
- 38.Greipp PR, Lust JA, O’Fallon WM, Katzmann JA, Witzig TE, Kyle RA. Plasma cell labeling index and beta 2-microglobulin predict survival independent of thymidine kinase and C-reactive protein in multiple myeloma. Blood. 1993 Jun 15;81(12):3382–3387. [PubMed] [Google Scholar]
- 39.Steensma DP, Gertz MA, Greipp PR, Kyle RA, Lacy MQ, Lust JA, et al. A high bone marrow plasma cell labeling index in stable plateau-phase multiple myeloma is a marker for early disease progression and death. Blood. 2001 Apr 15;97(8):2522–2523. doi: 10.1182/blood.v97.8.2522. [DOI] [PubMed] [Google Scholar]
- 40.Kumar S, Rajkumar SV, Kimlinger T, Greipp PR, Witzig TE. CD45 expression by bone marrow plasma cells in multiple myeloma: clinical and biological correlations. Leukemia. 2005 Aug;19(8):1466–1470. doi: 10.1038/sj.leu.2403823. [DOI] [PubMed] [Google Scholar]
- 41.Moreau P, Robillard N, Avet-Loiseau H, Pineau D, Morineau N, Milpied N, et al. Patients with CD45 negative multiple myeloma receiving high-dose therapy have a shorter survival than those with CD45 positive multiple myeloma. Haematologica. 2004 May;89(5):547–551. [PubMed] [Google Scholar]
- 42.Menke DM, Horny HP, Griesser H, Atkinson EJ, Kaiserling E, Kyle RA. Immunophenotypic and genotypic characterisation of multiple myelomas with adverse prognosis characterised by immunohistological expression of the T cell related antigen CD45RO (UCHL-1) J Clin Pathol. 1998 Jun;51(6):432–437. doi: 10.1136/jcp.51.6.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mateo G, Montalban MA, Vidriales MB, Lahuerta JJ, Mateos MV, Gutierrez N, et al. Prognostic value of immunophenotyping in multiple myeloma: a study by the PETHEMA/GEM cooperative study groups on patients uniformly treated with high-dose therapy. J Clin Oncol. 2008 Jun 1;26(16):2737–2744. doi: 10.1200/JCO.2007.15.4120. [DOI] [PubMed] [Google Scholar]
- 44.Yang Y, Lu N, Zhou J, Chen ZN, Zhu P. Cyclophilin A up-regulates MMP-9 expression and adhesion of monocytes/macrophages via CD147 signalling pathway in rheumatoid arthritis. Rheumatology (Oxford) 2008 Sep;47(9):1299–1310. doi: 10.1093/rheumatology/ken225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yurchenko V, Constant S, Bukrinsky M. Dealing with the family: CD147 interactions with cyclophilins. Immunology. 2006 Mar;117(3):301–309. doi: 10.1111/j.1365-2567.2005.02316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li M, Zhai Q, Bharadwaj U, Wang H, Li F, Fisher WE, et al. Cyclophilin A is overexpressed in human pancreatic cancer cells and stimulates cell proliferation through CD147. Cancer. 2006 May 15;106(10):2284–2294. doi: 10.1002/cncr.21862. [DOI] [PubMed] [Google Scholar]
- 47.Baba M, Inoue M, Itoh K, Nishizawa Y. Blocking CD147 induces cell death in cancer cells through impairment of glycolytic energy metabolism. Biochem Biophys Res Commun. 2008 Sep 12;374(1):111–116. doi: 10.1016/j.bbrc.2008.06.122. [DOI] [PubMed] [Google Scholar]
- 48.Chen X, Lin J, Kanekura T, Su J, Lin W, Xie H, et al. A small interfering CD147-targeting RNA inhibited the proliferation, invasiveness, and metastatic activity of malignant melanoma. Cancer Res. 2006 Dec 1;66(23):11323–11330. doi: 10.1158/0008-5472.CAN-06-1536. [DOI] [PubMed] [Google Scholar]
- 49.Chen X, Su J, Chang J, Kanekura T, Li J, Kuang YH, et al. Inhibition of CD147 gene expression via RNA interference reduces tumor cell proliferation, activation, adhesion, and migration activity in the human Jurkat T-lymphoma cell line. Cancer Invest. 2008 Aug;26(7):689–697. doi: 10.1080/07357900701867892. [DOI] [PubMed] [Google Scholar]
- 50.Wang B, Xu YF, He BS, Pan YQ, Zhang LR, Zhu C, et al. RNAi-mediated silencing of CD147 inhibits tumor cell proliferation, invasion and increases chemosensitivity to cisplatin in SGC7901 cells in vitro. J Exp Clin Cancer Res. 2010;29:61. doi: 10.1186/1756-9966-29-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rajkumar SV, Mesa RA, Fonseca R, Schroeder G, Plevak MF, Dispenzieri A, et al. Bone marrow angiogenesis in 400 patients with monoclonal gammopathy of undetermined significance, multiple myeloma, and primary amyloidosis. Clin Cancer Res. 2002 Jul;8(7):2210–2216. [PubMed] [Google Scholar]
- 52.Ribatti D, Vacca A, Nico B, Quondamatteo F, Ria R, Minischetti M, et al. Bone marrow angiogenesis and mast cell density increase simultaneously with progression of human multiple myeloma. Br J Cancer. 1999 Feb;79(3–4):451–455. doi: 10.1038/sj.bjc.6690070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kelly T, Borset M, Abe E, Gaddy-Kurten D, Sanderson RD. Matrix metalloproteinases in multiple myeloma. Leuk Lymphoma. 2000 Apr;37(3–4):273–281. doi: 10.3109/10428190009089428. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.