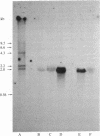
Abstract
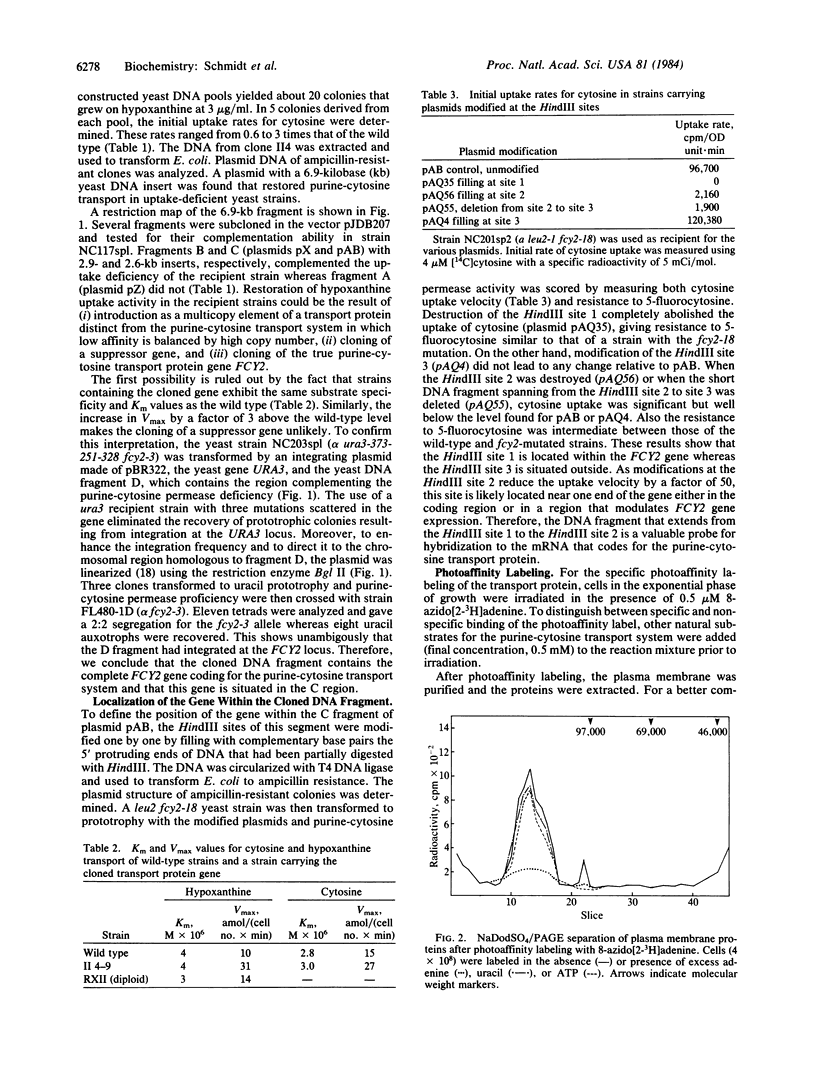
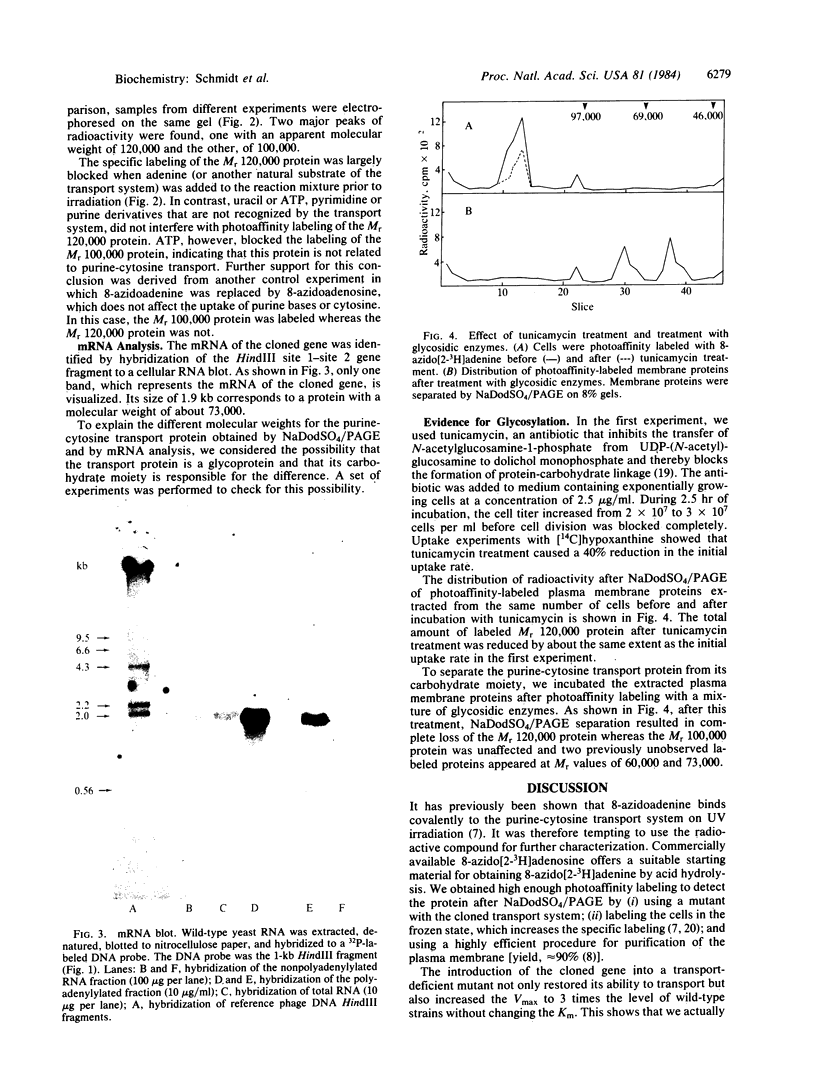
8-Azido[2-3H]adenine was used as a photoaffinity label for the purine-cytosine transport system. After irradiation in the presence of the photoaffinity label, the cells were converted into protoplasts, their plasma membranes were purified, and the membrane proteins were extracted and separated by NaDodSO4/PAGE. The radioactivity was specifically incorporated into a protein with a molecular weight of 120,000. Photoaffinity labeling of this protein could be blocked by irradiation in the presence of natural substrates for the transport system. The molecular weight as determined by NaDod-SO4/PAGE was found to be twice the value calculated from mRNA analysis of the cloned gene. Incubation of exponentially growing cells with tunicamycin, an antibiotic that inhibits glycosylation of proteins, resulted in a 40% decrease in the overall initial uptake rate, which correlates with the reduction of the labeled Mr 120,000 protein. Treatment of the extracted labeled plasma membrane proteins with glycosidic enzymes resulted in disappearance of the Mr 120,000 peak and the appearance of new peaks at Mr 60,000 and Mr 73,000. These findings indicate that the purine-cytosine transport protein is a glycoprotein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beggs J. D. Transformation of yeast by a replicating hybrid plasmid. Nature. 1978 Sep 14;275(5676):104–109. doi: 10.1038/275104a0. [DOI] [PubMed] [Google Scholar]
- Chevallier M. R. Cloning and transcriptional control of a eucaryotic permease gene. Mol Cell Biol. 1982 Aug;2(8):977–984. doi: 10.1128/mcb.2.8.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier M. R., Jund R., Lacroute F. Characterization of cytosine permeation in Saccharomyces cerevisiae. J Bacteriol. 1975 May;122(2):629–641. doi: 10.1128/jb.122.2.629-641.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson J. J., Jr Irradiation in the frozen state: a technique for direct photoaffinity labeling. Photochem Photobiol. 1980 Aug;32(2):137–142. doi: 10.1111/j.1751-1097.1980.tb04000.x. [DOI] [PubMed] [Google Scholar]
- Forêt M., Schmidt R., Reichert U. On the mechanism of substrate binding to the purine-transport system of Saccharomyces cerevisiae. Eur J Biochem. 1978 Jan 2;82(1):33–43. doi: 10.1111/j.1432-1033.1978.tb11994.x. [DOI] [PubMed] [Google Scholar]
- Hinnen A., Hicks J. B., Fink G. R. Transformation of yeast. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1929–1933. doi: 10.1073/pnas.75.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Losson R., Lacroute F. Cloning of a eukaryotic regulatory gene. Mol Gen Genet. 1981;184(3):394–399. doi: 10.1007/BF00352511. [DOI] [PubMed] [Google Scholar]
- Losson R., Lacroute F. Interference of nonsense mutations with eukaryotic messenger RNA stability. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5134–5137. doi: 10.1073/pnas.76.10.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney W. C., Duksin D. Biological activities of the two major components of tunicamycin. J Biol Chem. 1979 Jul 25;254(14):6572–6576. [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W., Rothstein R. J. Yeast transformation: a model system for the study of recombination. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6354–6358. doi: 10.1073/pnas.78.10.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak A., Grenson M. Evidence for a common transport system for cytosine, adenine and hypoxanthine in Saccharomyces cerevisiae and Candida albicans. Eur J Biochem. 1973 Jan 15;32(2):276–282. doi: 10.1111/j.1432-1033.1973.tb02608.x. [DOI] [PubMed] [Google Scholar]
- Reichert U., Forêt M. Energy coupling in hypoxanthine transport of yeast. Potentiometric evidence for proton symport and potassium antiport. FEBS Lett. 1977 Nov 15;83(2):325–328. doi: 10.1016/0014-5793(77)81033-8. [DOI] [PubMed] [Google Scholar]
- Reichert U., Schmidt R., Foret M. A possible mechanism of energy coupling in purine transport of Saccharomyces cerevisiae. FEBS Lett. 1975 Mar 15;52(1):100–102. doi: 10.1016/0014-5793(75)80647-8. [DOI] [PubMed] [Google Scholar]
- Reichert U., Winter M. Uptake and accumulation of purine bases by stationary yeast cells pretreated with glucose. Biochim Biophys Acta. 1974 Jul 12;356(1):108–116. doi: 10.1016/0005-2736(74)90298-3. [DOI] [PubMed] [Google Scholar]
- Schmidt R., Ackermann R., Kratky Z., Wasserman B., Jacobson B. Fast and efficient purification of yeast plasma membranes using cationic silica microbeads. Biochim Biophys Acta. 1983 Jul 27;732(2):421–427. doi: 10.1016/0005-2736(83)90059-7. [DOI] [PubMed] [Google Scholar]
- Schmidt R., Manolson M. F., Angelides K. J., Poole R. J. 8-Azidoadenine: a photoaffinity label for the purine transport system in Saccharomyces cerevisiae. FEBS Lett. 1981 Jul 6;129(2):305–308. doi: 10.1016/0014-5793(81)80189-5. [DOI] [PubMed] [Google Scholar]
- Struhl K., Stinchcomb D. T., Scherer S., Davis R. W. High-frequency transformation of yeast: autonomous replication of hybrid DNA molecules. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1035–1039. doi: 10.1073/pnas.76.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]