Abstract
Elevated levels of Ca2+-binding proteins are reported in transformed cells and thought to be involved in their uncontrolled proliferation and often increased motility. Therefore, three cell lines [LICR(Lond)-HN 1, -HN 2, and -HN 6] derived from human carcinomas displaying various degrees of locomotive activity were investigated for the presence of parvalbumin and related Ca2+-binding proteins. By applying different immunohistochemical methods in conjunction with a monospecific anti-parvalbumin antiserum, an intense staining was seen in cells displaying translocative motility. Often in these cells, an association with filamentous structures located in the nuclear region was observed. Unique Ca2+-binding proteins, absent from comparable normal tissue, were found in the malignant cell lines when analyzed by two-dimensional polyacrylamide gel electrophoresis and high-performance liquid chromatography. From these tumor cells a protein (Mr, 12,000; pI, 4.8) was isolated that crossreacted with antiparvalbumin antiserum. Peptide maps of this protein revealed a further structural homology to parvalbumin (Mr, 12,000; pI, 4.9). In analogy to muscle, where there is evidence for a regulatory role of parvalbumin in the contraction-relaxation cycle, we speculate that this protein is tumor-associated and connected to the motile behavior of carcinoma cells.
Full text
PDF

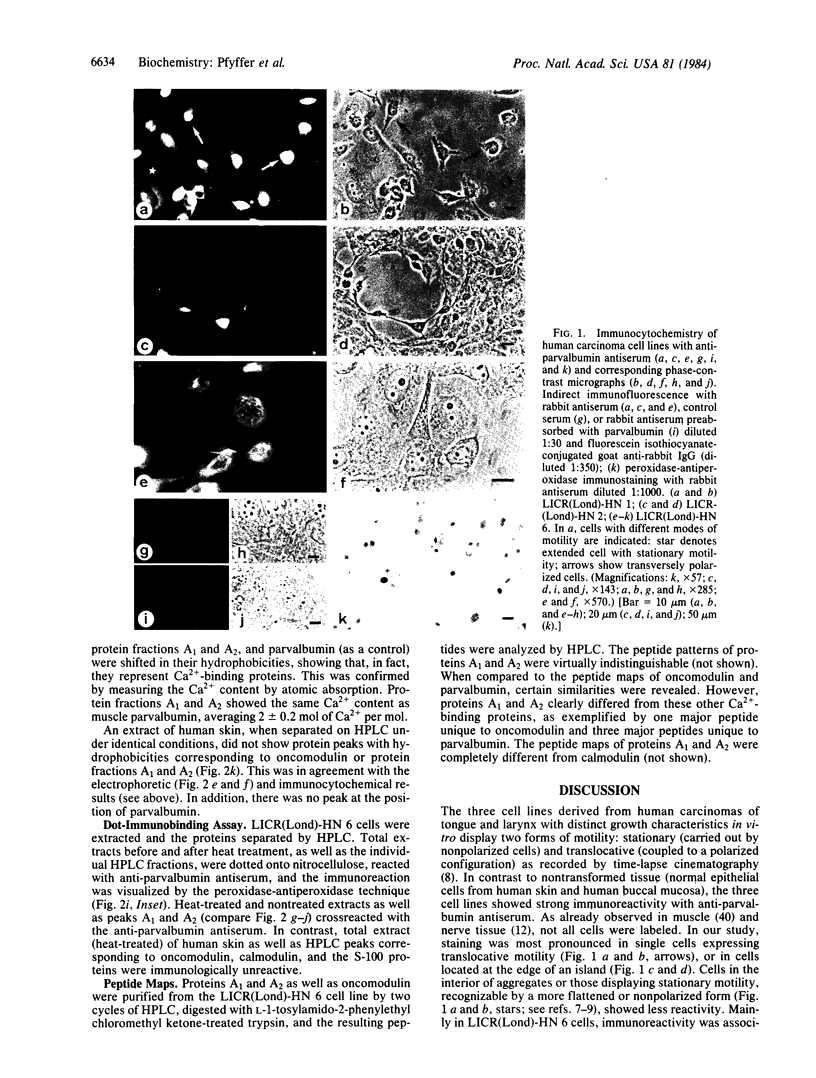
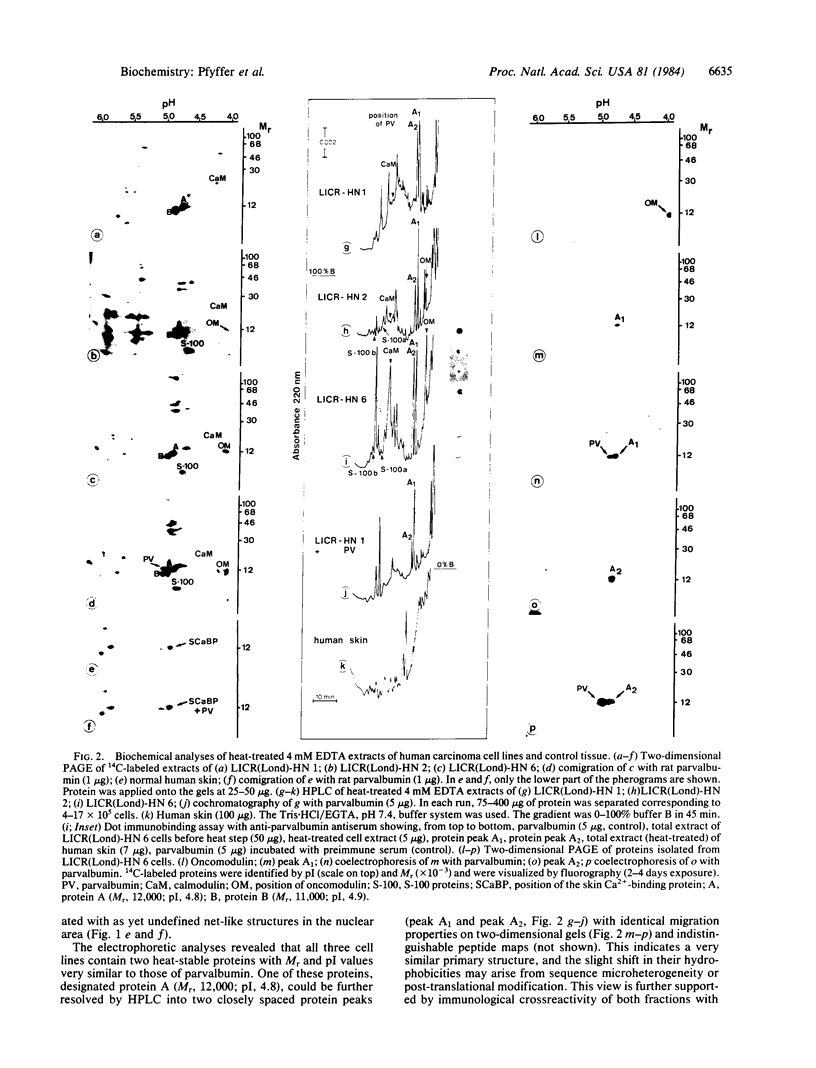

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anghileri L. J., Heidbreder M. Effects of increased intracellular Ca2+ on cyclic nucleotides production by liver tissue. Experientia. 1977 Apr 15;33(4):415–416. doi: 10.1007/BF01922183. [DOI] [PubMed] [Google Scholar]
- Baron G., Demaille J., Dutruge E. The distribution of parvalbumins in muscle and in other tissues. FEBS Lett. 1975 Aug 1;56(1):156–160. doi: 10.1016/0014-5793(75)80131-1. [DOI] [PubMed] [Google Scholar]
- Berchtold M. W., Celio M. R., Heizmann C. W. Parvalbumin in non-muscle tissues of the rat. Quantitation and immunohistochemical localization. J Biol Chem. 1984 Apr 25;259(8):5189–5196. [PubMed] [Google Scholar]
- Berchtold M. W., Heizmann C. W., Wilson K. J. Ca2+-binding proteins: a comparative study of their behavior during high-performance liquid chromatography using gradient elution on reverse-phase supports. Anal Biochem. 1983 Feb 15;129(1):120–131. doi: 10.1016/0003-2697(83)90060-x. [DOI] [PubMed] [Google Scholar]
- Berchtold M. W., Wilson K. J., Heizmann C. W. Isolation of neuronal parvalbumin by high-performance liquid chromatography. Characterization and comparison with muscle parvalbumin. Biochemistry. 1982 Dec 7;21(25):6552–6557. doi: 10.1021/bi00268a035. [DOI] [PubMed] [Google Scholar]
- Bock E. Nervous system specific proteins. J Neurochem. 1978 Jan;30(1):7–14. doi: 10.1111/j.1471-4159.1978.tb07028.x. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Celio M. R., Heizmann C. W. Calcium-binding protein parvalbumin as a neuronal marker. Nature. 1981 Sep 24;293(5830):300–302. doi: 10.1038/293300a0. [DOI] [PubMed] [Google Scholar]
- Celio M. R., Heizmann C. W. Calcium-binding protein parvalbumin is associated with fast contracting muscle fibres. Nature. 1982 Jun 10;297(5866):504–506. doi: 10.1038/297504a0. [DOI] [PubMed] [Google Scholar]
- Chafouleas J. G., Pardue R. L., Brinkley B. R., Dedman J. R., Means A. R. Regulation of intracellular levels of calmodulin and tubulin in normal and transformed cells. Proc Natl Acad Sci U S A. 1981 Feb;78(2):996–1000. doi: 10.1073/pnas.78.2.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criss W. E., Kakiuchi S. Calcium: calmodulin and cancer. Fed Proc. 1982 May;41(7):2289–2291. [PubMed] [Google Scholar]
- Easty D. M., Easty G. C., Carter R. L., Monaghan P., Butler L. J. Ten human carcinoma cell lines derived from squamous carcinomas of the head and neck. Br J Cancer. 1981 Jun;43(6):772–785. doi: 10.1038/bjc.1981.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easty D. M., Easty G. C., Carter R. L., Monaghan P., Pittam M. R., James T. Five human tumour cell lines derived from a primary squamous carcinoma of the tongue, two subsequent local recurrences and two nodal metastases. Br J Cancer. 1981 Sep;44(3):363–370. doi: 10.1038/bjc.1981.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor R., Irie R., Morton D., Herschman H. R. S100 protein is present in cultured human malignant melanomas. Nature. 1980 Jul 24;286(5771):400–401. doi: 10.1038/286400a0. [DOI] [PubMed] [Google Scholar]
- Haemmerli G., Felix H. Interrelation of motility, shape, and surface architecture in human carcinoma cells. Cell Biol Int Rep. 1981 Sep;5(9):929–936. doi: 10.1016/0309-1651(81)90208-3. [DOI] [PubMed] [Google Scholar]
- Haemmerli G., Sträuli P. In vitro motility of cells from human epidermoid carcinomas. A study by phase-contrast and reflection-contrast cinematography. Int J Cancer. 1981 May 15;27(5):603–610. doi: 10.1002/ijc.2910270506. [DOI] [PubMed] [Google Scholar]
- Haglid K. G., Stavrou D., Rönnbäck L., Carlsson C. A., Weidenbach W. The S-100 protein in water-soluble and pentanol-extractable form in normal human brain and tumours of the human nervous system. A quantitative study. J Neurol Sci. 1973 Sep;20(1):103–111. doi: 10.1016/0022-510x(73)90122-6. [DOI] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Heizmann C. W., Berchtold M. W., Rowlerson A. M. Correlation of parvalbumin concentration with relaxation speed in mammalian muscles. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7243–7247. doi: 10.1073/pnas.79.23.7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heizmann C. W. Parvalbumin, an intracellular calcium-binding protein; distribution, properties and possible roles in mammalian cells. Experientia. 1984 Sep 15;40(9):910–921. doi: 10.1007/BF01946439. [DOI] [PubMed] [Google Scholar]
- ITZHAKI R. F., GILL D. M. A MICRO-BIURET METHOD FOR ESTIMATING PROTEINS. Anal Biochem. 1964 Dec;9:401–410. doi: 10.1016/0003-2697(64)90200-3. [DOI] [PubMed] [Google Scholar]
- Jarrett H. W., Penniston J. T. Purification of the Ca2+-stimulated ATPase activator from human erythrocytes. Its membership in the class of Ca2+-binding modulator proteins. J Biol Chem. 1978 Jul 10;253(13):4676–4682. [PubMed] [Google Scholar]
- Jentoft N., Dearborn D. G. Labeling of proteins by reductive methylation using sodium cyanoborohydride. J Biol Chem. 1979 Jun 10;254(11):4359–4365. [PubMed] [Google Scholar]
- MacManus J. P., Watson D. C., Yaguchi M. The complete amino acid sequence of oncomodulin--a parvalbumin-like calcium-binding protein from Morris hepatoma 5123tc. Eur J Biochem. 1983 Oct 17;136(1):9–17. doi: 10.1111/j.1432-1033.1983.tb07698.x. [DOI] [PubMed] [Google Scholar]
- MacManus J. P., Whitfield J. F., Boynton A. L., Durkin J. P., Swierenga S. H. Oncomodulin--a widely distributed, tumour-specific, calcium-binding protein. Oncodev Biol Med. 1982;3(2-3):79–90. [PubMed] [Google Scholar]
- Means A. R., Tash J. S., Chafouleas J. G. Physiological implications of the presence, distribution, and regulation of calmodulin in eukaryotic cells. Physiol Rev. 1982 Jan;62(1):1–39. doi: 10.1152/physrev.1982.62.1.1. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pfyffer G. E., Bologa L., Herschkowitz N., Heizmann C. W. Parvalbumin, a neuronal protein in brain cell cultures. J Neurochem. 1984 Jul;43(1):49–57. doi: 10.1111/j.1471-4159.1984.tb06677.x. [DOI] [PubMed] [Google Scholar]
- Rinaldi M. L., Haiech J., Pavlovitch J., Rizk M., Ferraz C., Derancourt J., Demaille J. G. Isolation and characterization of a rat skin parvalbumin-like calcium-binding protein. Biochemistry. 1982 Sep 14;21(19):4805–4810. doi: 10.1021/bi00262a044. [DOI] [PubMed] [Google Scholar]
- Stavrou D., Haglid K. G., Weidenbach W. The brain specific proteins S 100 and 14.3.2 in experimental brain tumors of the rat. Z Gesamte Exp Med. 1971;156(3):237–242. doi: 10.1007/BF02047353. [DOI] [PubMed] [Google Scholar]
- Suzuki F., Nakajima T., Kato K. Peripheral distribution of nervous system-specific S-100 protein in rat. J Biochem. 1982 Sep;92(3):835–838. doi: 10.1093/oxfordjournals.jbchem.a133996. [DOI] [PubMed] [Google Scholar]
- Van Eldik L. J., Watterson D. M. Characterization of a calcium-modulated protein from transformed chicken fibroblasts. J Biol Chem. 1979 Oct 25;254(20):10250–10255. [PubMed] [Google Scholar]
- Van Rossum G. D., Galeotti T., Morris H. P. The mineral content and water compartments of liver and of Morris hepatomata 5123tc and 3924A and the changes of composition occurring during necrosis in hepatoma 3924A. Cancer Res. 1973 May;33(5):1078–1085. [PubMed] [Google Scholar]
- Veigl M. L., Sedwick W. D., Vanaman T. C. Calmodulin and Ca2+ in normal and transformed cells. Fed Proc. 1982 May;41(7):2283–2288. [PubMed] [Google Scholar]
- Wei J. W., Hickie R. A. Increased content of calmodulin in Morris hepatoma 5123 t.c. (h). Biochem Biophys Res Commun. 1981 Jun;100(4):1562–1568. doi: 10.1016/0006-291x(81)90697-5. [DOI] [PubMed] [Google Scholar]