Abstract
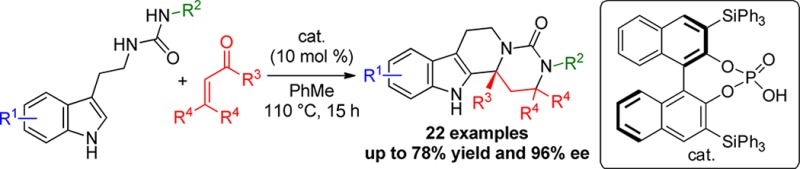
A Michael addition/iminium ion cyclization cascade of enones with tryptamine-derived ureas under BINOL phosphoric acid (BPA) catalysis is reported. The cascade reaction tolerates a wide variety of easily synthesized tryptamine-derived ureas, including those bearing substituents on the distal nitrogen atom of the urea moiety, affording polyheterocyclic products in good yields and good to excellent enantioselectivities.
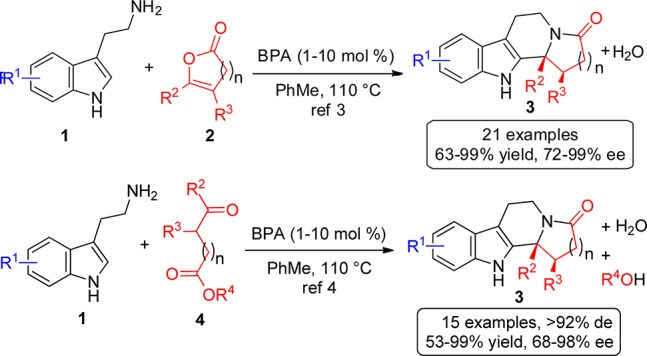
Chiral Brønsted acid-catalyzed cascade reaction sequences1 involving ring closure of carbon or heteroatom-centered nucleophiles onto reactive iminium ion intermediates2 are useful for the enantioselective synthesis of difficult-to-access and architecturally complex compounds. To this end, we recently described the enantioselective cascade reactions of various enol lactones 2(3) or ketoacids 4(4) with a range of tryptamine derivatives 1 under BINOL phosphoric acid (BPA)5−7 catalysis, affording the polycyclic products 3 in good yields and high enantiomeric excesses (Scheme 1).8 In both cases, the scope of the reaction was found to be broad with respect to the substitution pattern of the tryptamine 1, enol lactones 2, or ketoacids 4, and more than one stereocenter could be incorporated into the products with control via dynamic kinetic processes.
Scheme 1. Previous Enantioselective BPA-Catalyzed Cyclization Cascades to Access Indole-Derived Polycyclic Products.
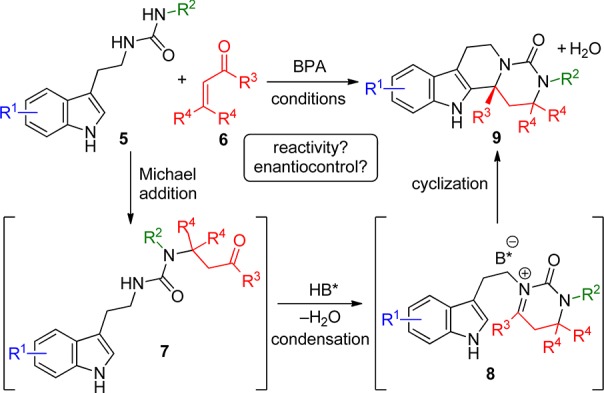
In a continuation of this research program, we were interested in extending our findings to other tryptamine-derived functionalities that would naturally enable enantioselective access to other polyheterocyclic structures. One such motif that caught our attention was the tryptamine-derived urea 5 (Scheme 2). These are known to be readily synthesized from the parent tryptamine by reaction with an isocyanate9 and are perfectly poised to undergo a cyclization cascade through an acid-catalyzed condensation with substrates possessing two electrophilic centers such as enones 6.10 Provided an initial Michael addition to the distal nitrogen atom of the urea occurred to generate intermediate 7, a subsequent acid-catalyzed condensation of the ketone with the tryptamine nitrogen atom would give the reactive iminium ion (intermediate 8) poised for enantiofacial attack by the pendant indole nucleophile under the control of the chiral conjugate base of the BPA.11,12 With four points of diversity, numerous polyheterocyclic structures 9 bearing additional stereogenic centers, functionalities and spectator groups could be readily accessed, making this methodology attractive for target or library synthesis. Herein we report our findings.
Scheme 2. Proposed Concept of a BPA-Catalyzed Cyclization Cascade Using Tryptamine-Derived Ureas 5 and Enones 6.
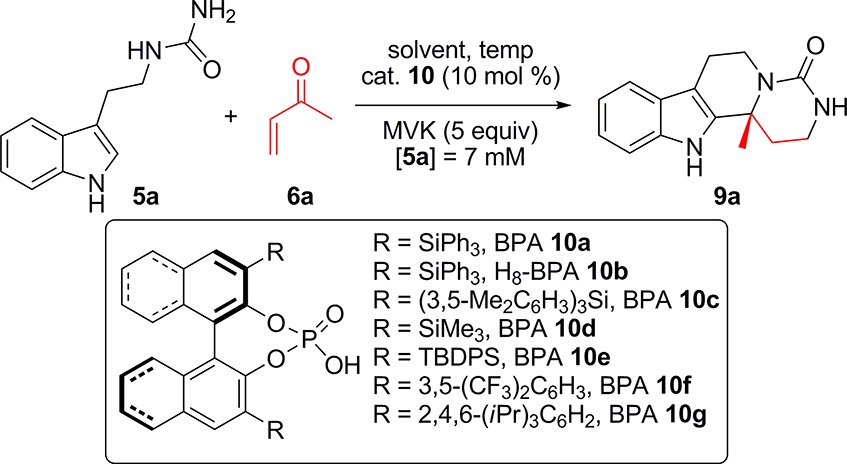
Preliminary investigations into the cascade were carried out on readily prepared urea 5a and methyl vinyl ketone (MVK) 6a. From the results of previous studies,3,4,8 toluene was selected as the initial solvent and 3,3′-bis-triphenylsilyl BPA 10a as the initial catalyst in a series of reactions to establish whether our proposed cascade reaction was viable. Disappointingly, with MVK 6a (5 equiv) at room temperature no reaction occurred after 120 h (Table 1, entry 1). However, heating at 50 °C for 120 h under the same conditions enabled product 9a to be isolated in 31% yield with a good level of stereocontrol (72% ee; Table 1, entry 2). Increasing the reaction temperature to 110 °C, pleasingly, afforded 9a in an improved 76% yield while maintaining the enantioselectivity (71% ee; Table 1, entry 4). Changing the solvent to xylene or THF led to no significant improvement in either the yield or the enantioselectivity of the cascade reaction (Table 1, entries 5 and 6), while using DMSO or ethanol resulted in no product formation (Table 1, entries 7 and 8). With these initial results in hand, a catalyst screen probing variation of the BINOL scaffold and the substituents at the 3 and 3′ positions was carried out (Table 1, entries 9–14). Pleasingly, all of the screened BPA catalysts efficiently facilitated the cyclization cascade when it was conducted in toluene at reflux for 15 h. Enantioselectivity was observed in all cases, but the optimal control arose when using BPA 10a and H8–BPA 10b, affording 9a in the same yield (76%) and enantioselectivity (71% ee; Table 1, entries 4 and 9) in both cases. The optimal conditions employed a substrate concentration of [5a] = 5 mM, MVK 6a (5 equiv) in toluene at 110 °C with BPA 10a (10 mol %). Under these conditions, 9a was obtained in 76% yield and 73% ee (Table 1, entry 15).
Table 1. Proof of Principle and Optimization Study of the BPA-Catalyzed Cascade Reaction Using Tryptamine-Derived Urea 5a and MVK 6a.
| entrya | solvent | cat. | temp (°C) | time (h) | yieldb (%) | eec (%) |
|---|---|---|---|---|---|---|
| 1 | PhMe | 10a | rt | 120 | – | – |
| 2 | PhMe | 10a | 50 | 120 | 31 | 72 |
| 3 | PhMe | 10a | 80 | 15 | 60 | 70 |
| 4 | PhMe | 10a | 110 | 15 | 76 | 71 |
| 5 | Xylene | 10a | 140 | 15 | 48 | 72 |
| 6 | THF | 10a | 70 | 15 | 60 | 68 |
| 7 | DMSO | 10a | 110 | 60 | – | – |
| 8 | EtOH | 10a | 80 | 24 | – | – |
| 9 | PhMe | 10b | 110 | 15 | 76 | 71 |
| 10 | PhMe | 10c | 110 | 15 | 60 | 41 |
| 11 | PhMe | 10d | 110 | 15 | 40 | 35 |
| 12 | PhMe | 10e | 110 | 15 | 48 | 60 |
| 13 | PhMe | 10f | 110 | 15 | 60 | 56 |
| 14 | PhMe | 10g | 110 | 15 | 68 | 18 |
| 15d | PhMe | 10a | 110 | 15 | 76 | 73 |
| 16e | PhMe | 10a | 110 | 15 | 76 | 58 |
Reactions were performed on a 0.10 mmol scale.
Isolated yield after purification by flash column chromatography on silica gel.
Determined by HPLC analysis.
[5a] = 5 mM.
[5a] = 10 mM.
With optimal reactivity and enantiocontrol established, the scope of the reaction was surveyed by using an array of substituted ureas 5 and enones 6 (Scheme 3). Pleasingly, the cascade reaction was found to tolerate a variety of electron-rich and electron-poor tryptamine-derived ureas, furnishing the desired tetracycles 9c–f in good yields and enantioselectivities (73–77% yield, 60–70% ee). When tryptamine-derived ureas containing methyl or ethyl substituents in the 7-position were employed, a significant increase in the enantioenrichment of the products 9g–j was observed (54–78% yield, 90–92% ee). Using mesityl oxide as the enone coupling partner afforded 9k in 78% yield, but the enantioinduction was diminished to 43% ee. Similarly, the cyclization of a tryptamine-derived thiourea proceeded smoothly furnishing 9l in good yield, but a significant decrease in enantioselectivity was observed (72% yield, 31% ee). We next investigated the influence that substituents on the urea had on the cascade reaction. Gratifyingly, urea substrates bearing alkyl substituents on the distal nitrogen of the urea furnished the corresponding tetracycles 9m–q with good yields and enantioselectivities (64–76% yield, 71–90% ee). The cyclization of substituted ureas bearing aryl substituents was also tolerated in the cascade reaction using a variety of enones, affording products 9r–w in good to excellent enantioselectivities (55–76% yield, 81–96% ee).
Scheme 3. Scope of the BPA-Catalyzed Cyclization Cascade Using Substituted Ureas 5a–p and Enones 6a–e.
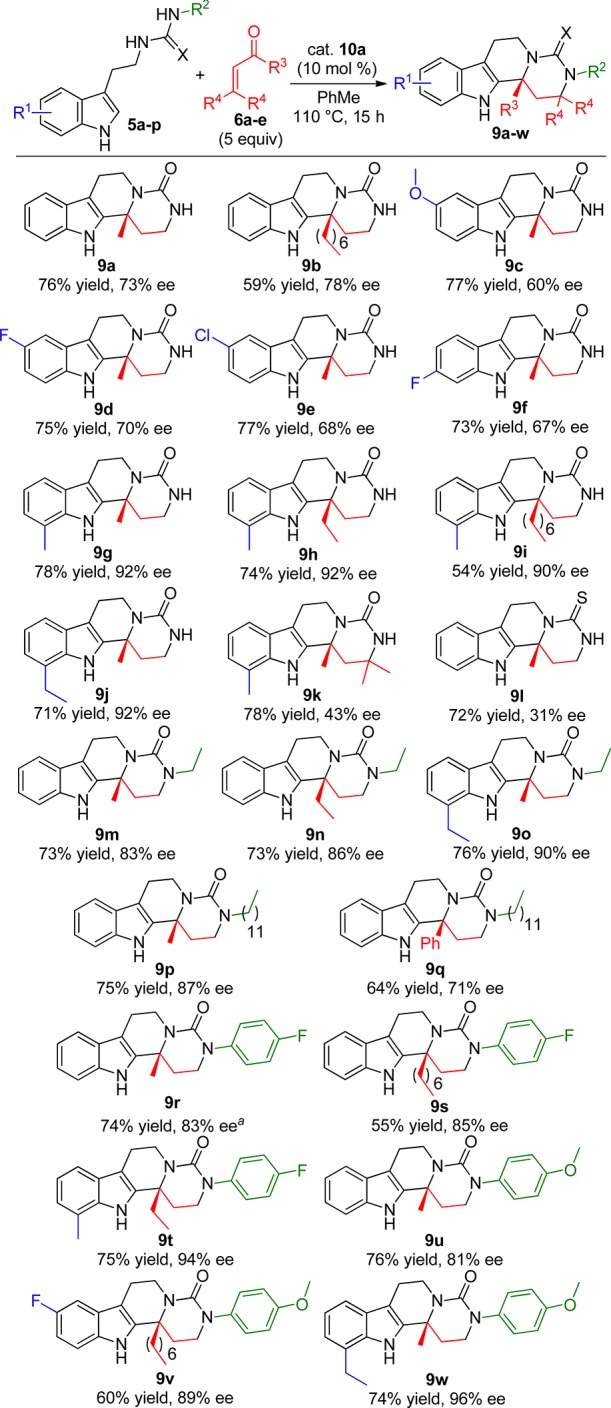
Absolute configuration determined to be (R) by single-crystal X-ray diffraction; all other compounds were assigned by analogy (see Figure 1 and the Supporting Information).
Figure 1.
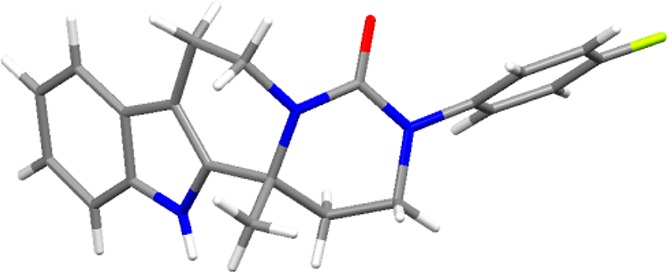
Structure of compound 9r determined from single-crystal X-ray diffraction data.13
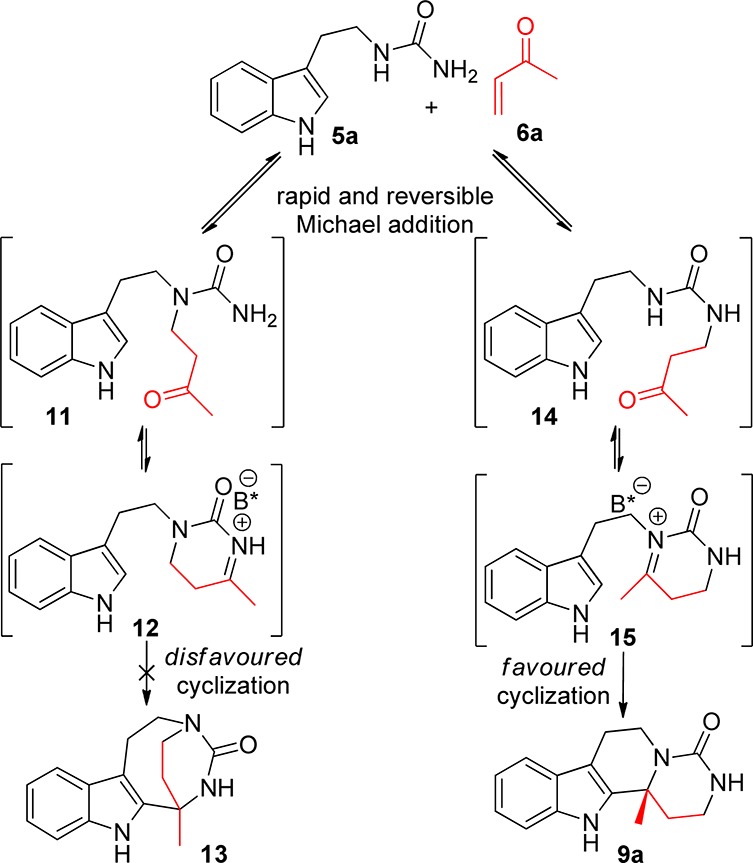
That the cascade affords the desired products in generally high yields (typically more than 50%) despite the presence of two potentially nucleophilic urea nitrogen atoms is intriguing and worthy of further comment (Scheme 4). Assuming that the synthesis of 9a results from an initial Michael addition to the nitrogen atom distal to the indole ring followed by iminium ion formation and an enantiodetermining carbocyclization, the high reaction yield is strongly suggestive of a rapid and reversible Michael addition step. Using the reaction of 5a with 6a as an example, we propose that a reversible Michael addition between these two starting materials can lead to the ketone intermediates 11 and 14. In the case of intermediate 11, condensation of the ketone onto the urea nitrogen creates an iminium ion that would need to undergo an 8-membered ring cyclization to furnish tetracycle 13. We postulate that this cyclization is unfavorable and, as a result, the reaction course follows the pathway of intermediate 14. Condensation of the urea nitrogen and the ketone affords intermediate 15, which then undergoes a more favorable 6-endo-trig cyclization to afford 9a in high yield.
Scheme 4. Proposed Mechanistic Pathway of the Michael Addition/Iminium Ion Cyclization Cascade.
In summary, an efficient and highly enantioselective Michael addition/iminium ion cyclization cascade of tryptamine-derived ureas and enones has been developed. The reaction is easy to perform, broad in scope and provides the desired tetracycles in good yields (up to 78%) and enantioselectivities (up to 96% ee). Work to expand and apply these findings is ongoing, and the results will be reported in due course.
Acknowledgments
We thank the European Commission [IEF to I.A. (PEIF-GA-2009-254053)], the EPSRC (studentship to D.M.B and Leadership Fellowship to D.J.D.), and AstraZeneca (studentship to D.M.B) for support.
Supporting Information Available
Experimental procedures and spectral data for compounds 5a–p and 9a–w. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Supplementary Material
References
- For selected reviews of cascade reactions, see:; a Nicolaou K. C.; Chen J. S. Chem. Soc. Rev. 2009, 38, 2993–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Grondal C.; Jeanty M.; Enders D. Nat. Chem. 2010, 2, 167–178. [DOI] [PubMed] [Google Scholar]; c Lu L.-Q.; Chen J.-R.; Xiao W.-J. Acc. Chem. Res. 2012, 45, 1278–1293. [DOI] [PubMed] [Google Scholar]
- For selected reviews of reactions involving iminium ions, see:; a Speckamp W. N.; Moolenaar M. J. Tetrahedron 2000, 56, 3817–3856. [Google Scholar]; b Maryanoff B. E.; Zhang H.-C.; Cohen J. H.; Turchi I. J.; Maryanoff C. A. Chem. Rev. 2004, 104, 1431–1628. [DOI] [PubMed] [Google Scholar]; c Yazici A.; Pyne S. G. Synthesis 2009, 339–368. [Google Scholar]; d Shiri M. Chem. Rev. 2012, 112, 3508–3549. [DOI] [PubMed] [Google Scholar]
- Muratore M. E.; Holloway C. A.; Pilling A. W.; Storer R. I.; Trevitt G.; Dixon D. J. J. Am. Chem. Soc. 2009, 131, 10796–10797. [DOI] [PubMed] [Google Scholar]
- Holloway C. A.; Muratore M. E.; Storer R. I.; Dixon D. J. Org. Lett. 2010, 12, 4720–4723. [DOI] [PubMed] [Google Scholar]
- For selected reviews of asymmetric organocatalysis by H-bond donors and Brønsted acids, see:; a Akiyama T.; Itoh J.; Fuchibe K. Adv. Synth. Catal. 2006, 348, 999–1010. [Google Scholar]; b Akiyama T. Chem. Rev. 2007, 107, 5744–5758. [DOI] [PubMed] [Google Scholar]; c Doyle A.; Jacobsen E. N. Chem. Rev. 2007, 107, 5713–5743. [DOI] [PubMed] [Google Scholar]; d Terada M. Chem. Commun. 2008, 35, 4097–4112. [DOI] [PubMed] [Google Scholar]; e MacMillan D. W. C. Nature 2008, 455, 304–308. [DOI] [PubMed] [Google Scholar]; f Terada M. Synthesis 2010, 12, 1929–1982. [Google Scholar]; g Kampen D.; Reisinger C. M.; List B. Top. Curr. Chem. 2010, 291, 395–456. [DOI] [PubMed] [Google Scholar]; h Zamfir A.; Schenker S.; Freund M.; Tsogoeva S. B. Org. Biomol. Chem. 2010, 8, 5262–5276. [DOI] [PubMed] [Google Scholar]; i Rueping M.; Kuenkel A.; Atodiresei I. Chem. Soc. Rev. 2011, 40, 4539–4549. [DOI] [PubMed] [Google Scholar]; j Yu J.; Shi F.; Gong L.-Z. Acc. Chem. Res. 2011, 44, 1156–1171. [DOI] [PubMed] [Google Scholar]
- For seminal work on BINOL phosphoric acids, see:; a Uraguchi D.; Terada M. J. Am. Chem. Soc. 2004, 126, 5356–5357. [DOI] [PubMed] [Google Scholar]; b Akiyama T.; Itoh J.; Yokota K.; Fuchibe K. Angew. Chem., Int. Ed. 2004, 43, 1566–1568. [DOI] [PubMed] [Google Scholar]
- For selected examples of reactions catalyzed by BINOL phosphoric acids, see:; a Storer R. I.; Carrera D. E.; Ni Y.; MacMillan D. W. C. J. Am. Chem. Soc. 2006, 128, 84–86. [DOI] [PubMed] [Google Scholar]; b Rueping M.; Theissmann T.; Raja S.; Bats J. W. Adv. Synth. Catal. 2008, 350, 1001–1006. [Google Scholar]; c Cheon C. H.; Yamamoto H. J. Am. Chem. Soc. 2008, 130, 9246–9247. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Liang T.; Zhang Z.; Antilla J. C. Angew. Chem., Int. Ed. 2010, 49, 9734–9736. [DOI] [PubMed] [Google Scholar]; e Yu J.; Chen W.-J.; Gong L.-Z. Org. Lett. 2010, 12, 4050–4053. [DOI] [PubMed] [Google Scholar]; f Müller S.; Webber M. J.; List B. J. Am. Chem. Soc. 2011, 133, 18534–18537. [DOI] [PubMed] [Google Scholar]; g Momiyama N.; Konno T.; Furiya Y.; Iwamoto T.; Terada M. J. Am. Chem. Soc. 2011, 133, 19294–19297. [DOI] [PubMed] [Google Scholar]; h Henseler A.; Kato M.; Mori K.; Akiyama T. Angew. Chem., Int. Ed. 2011, 50, 8180–8183. [DOI] [PubMed] [Google Scholar]; i Lee J.-W.; List B. J. Am. Chem. Soc. 2012, 134, 18245–18248. [DOI] [PubMed] [Google Scholar]; j Saito K.; Akiyama T. Chem. Commun. 2012, 48, 4573–4575. [DOI] [PubMed] [Google Scholar]; k Feng J.; Yan W.; Wang D.; Li P.; Sun Q.; Wang R. Chem. Commun. 2012, 48, 8003–8005. [DOI] [PubMed] [Google Scholar]; l He L.; Bekkaye M.; Retailleau P.; Masson G. Org. Lett. 2012, 14, 3158–3161. [DOI] [PubMed] [Google Scholar]; m Shi F.; Xing G.-J.; Tao Z.-L.; Luo S.-W.; Tu S.-J.; Gong L.-Z. J. Org. Chem. 2012, 77, 6970–6979. [DOI] [PubMed] [Google Scholar]; n Rueping M.; Maji M. S.; Küçük H. B.; Atodiresei I. Angew. Chem., Int. Ed. 2012, 51, 12864–12868. [DOI] [PubMed] [Google Scholar]; o Čorić I.; List B. Nature 2012, 483, 315–319. [DOI] [PubMed] [Google Scholar]; p Guo C.; Song J.; Huang J.-Z.; Chen P.-H.; Luo S.-W.; Gong L.-Z. Angew. Chem., Int. Ed. 2012, 51, 1046–1050. [DOI] [PubMed] [Google Scholar]
- For related cascade reactions, see:; a Pilling A. W.; Boehmer J.; Dixon D. J. Angew. Chem., Int. Ed. 2007, 46, 5428–5430. [DOI] [PubMed] [Google Scholar]; b Yang T.; Campbell L.; Dixon D. J. J. Am. Chem. Soc. 2007, 129, 12070–12071. [DOI] [PubMed] [Google Scholar]; c Pilling A. W.; Böhmer J.; Dixon D. J. Chem. Commun. 2008, 832–834. [DOI] [PubMed] [Google Scholar]; d Muratore M. E.; Shi L.; Pilling A. W.; Storer R. I.; Dixon D. J. Chem. Commun. 2012, 48, 6351–6353. [DOI] [PubMed] [Google Scholar]
- a Ho B. T.; An R.; Noel M. B.; Tansey L. W. J. Med. Chem. 1971, 14, 553–554. [DOI] [PubMed] [Google Scholar]; b Zhang W.; Chen C. H.-T.; Nagashima T. Tetrahedron Lett. 2003, 44, 2065–2068. [Google Scholar]; c Figlus M.; Tarruella A. C.; Messer A.; Sollis S. L.; Hartley R. C. Chem. Commun. 2010, 46, 4405–4407. [DOI] [PubMed] [Google Scholar]
- a Fisyuk A. S.; Mukanov A. Y.; Novikova E. Y. Mendeleev Commun. 2003, 13, 278–279. [Google Scholar]; b Fisyuk A. S.; Mukanov A. Y. Zh. Org. Khim. 2006, 42, 1291–1296. [Google Scholar]
- For examples of chiral counterion induced enantioselection in reactions involving iminium ions, see:; a Terada M.; Machioka K.; Sorimachi K. Angew. Chem., Int. Ed. 2009, 48, 2553–2556. [DOI] [PubMed] [Google Scholar]; b Rueping M.; Lin M.-Y. Chem.–Eur. J. 2010, 16, 4169–4172. [DOI] [PubMed] [Google Scholar]; c Li G.; Kaplan M. J.; Wojtas L.; Antilla J. C. Org. Lett. 2010, 12, 1960–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For organocatalyzed enantioselective additions of indoles to iminium ions, see:; a Seayad J.; Seayad A. M.; List B. J. Am. Chem. Soc. 2006, 128, 1086–1087. [DOI] [PubMed] [Google Scholar]; b Raheem I. T.; Thiara P. S.; Peterson E. A.; Jacobsen E. N. J. Am. Chem. Soc. 2007, 129, 13404–13405. [DOI] [PubMed] [Google Scholar]; c Sewgobind N. V.; Wanner M. J.; Ingemann S.; De Gelder R.; Van Maarseveen J. H.; Hiemstra H. J. Org. Chem. 2008, 73, 6405–6408. [DOI] [PubMed] [Google Scholar]; d Klausen R. S.; Jacobsen E. N. Org. Lett. 2009, 11, 887–890. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Peterson E. A.; Jacobsen E. N. Angew. Chem., Int. Ed. 2009, 48, 6328–6331. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Rueping M.; Nachtsheim B. J. Synlett 2010, 1, 119–122. [Google Scholar]; g Cai Q.; Liang X.-W.; Wang S.-G.; Zhang J.-W.; Zhang X.; You S.-L. Org. Lett. 2012, 14, 5022–5025. [DOI] [PubMed] [Google Scholar]; For related additions, see:; h Raheem I. T.; Thiara P. S.; Jacobsen E. N. Org. Lett. 2008, 10, 1577–1580. [DOI] [PubMed] [Google Scholar]; i Wanner M. J.; Van Der Haas R. N. S.; De Cuba K. R.; Van Maarseveen J. H.; Hiemstra H. Angew. Chem., Int. Ed. 2007, 46, 7485–7487. [DOI] [PubMed] [Google Scholar]; j Sun F.-L.; Zheng X.-J.; Gu Q.; He Q.-L.; You S.-L. Eur. J. Org. Chem. 2010, 47–50. [Google Scholar]
- Single-crystal X-ray diffraction data were collected at 150 K with an Oxford Diffraction SuperNova diffractometer and processed with CrysAlisPro as per the Supporting Information (CIF). The structure was solved with SIR9214 and refined with CRYSTALS15 including the Flack x parameter16 which refined to −0.05(12) (unrestrained) and −0.001(8) with the application of Bijvoet difference restraints.16d Bayesian analysis of the Bijvoet pairs gave the Hooft y parameter as −0.03(3), G of 1.06(6), and the probability that the structure was the correct hand of >99.99% given that the crystal is enantiopure or a racemic twin.17 Full crystallographic data (in CIF format) are available as Supporting Information and have been deposited with the Cambridge Crystallographic Data Centre (reference code 921344).
- Altomare A.; Cascarano G.; Giacovazzo C.; Guagliardi A.; Burla M. C.; Polidori G.; Camalli M. J. Appl. Crystallogr. 1994, 27, 435–436. [Google Scholar]
- a Betteridge P. W.; Carruthers J. R.; Cooper R. I.; Prout K.; Watkin D. J. J. Appl. Crystallogr. 2003, 36, 1487. [Google Scholar]; b Cooper R. I.; Thompson A. L.; Watkin D. J. J. Appl. Crystallogr. 2010, 43, 1100–1107. [Google Scholar]
- a Flack H. D. Acta Crystallogr. 1983, A39, 876–881. [Google Scholar]; b Flack H. D.; Bernardinelli G. J. Appl. Crystallogr. 2000, 33, 1143–1148. [Google Scholar]; c Thompson A. L.; Watkin D. J. Tetrahedron: Asymmetry 2009, 20, 712–717. [Google Scholar]; d Thompson A. L.; Watkin D. J. J. Appl. Crystallogr. 2011, 44, 1017–1022. [Google Scholar]
- Hooft R. W. W.; Straver L. H.; Spek A. L. J. Appl. Crystallogr. 2008, 41, 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


